Abstract
Gender-affirming or cross-sex hormone therapy is integral to the management of transgender individuals yet our appreciation of the effects of such hormones on cardiovascular health is limited. Insights into vascular pathophysiology and outcomes in transgender people receiving sex steroids could be fundamental in providing better care for this population through the management of cardiovascular risk and more broadly advance our understanding of the role of sex and gender in vascular health and disease. In addition, there is a need to understand how gender-affirming hormone therapy impacts cardiovascular disease risk and events as transgender individuals age. This review explores the available evidence on the associations between gender-affirming hormones and cardiovascular events such as coronary artery disease, stroke, hypertension, thrombosis, lipid abnormalities, and diabetes mellitus. Current research about vascular outcomes in adults receiving hormonal therapy is limited by the absence of large cohort studies, lack of appropriate control populations, and inadequate data acquisition from gender identity services. Existing epidemiological data suggest that the use of estrogens in transgender females confers an increased risk of myocardial infarction and ischemic stroke. Conversely, transgender males receiving testosterone lack any consistent or convincing evidence of increased risk of cardiovascular or cerebrovascular disease. Further studies are required to confirm whether such risk exists and the mechanisms by which they occur.
Keywords: cardiovascular, disease, diabetes mellitus, dyslipidemias, hypertension, stroke, thrombosis, transgender persons
Transgender people experience gender dysphoria due to incongruence between their gender identity and the sex they were assigned at birth (Table 1).1 Due to lack of accurate census data, barriers to healthcare such as social stigma and variable engagement with gender-affirming healthcare, the size of the transgender population is uncertain, however, it is estimated to represent ≈0.5% of the US population.1–5 An expansion in population prevalence and the utilization of transgender health services has been observed in recent years.6–8
Gender-affirming hormone therapy (GHT) aims to align the characteristics of an individual with their gender identity. The mainstay of this lifelong treatment in transgender males (TGM) is testosterone, typically delivered as intramuscular testosterone undecanoate or ester formulation. Transgender females (TGF) receive oral or transdermal estrogen preparations (eg, estradiol valerate or hemihydrate) often in conjunction with either a gonadotropin-releasing hormone analog (eg, Goserelin) or an anti-androgen (eg, cyproterone acetate).9,10
A deeper understanding of the alterations in vascular pathology and outcomes in transgender people receiving GHT could be fundamental in informing the management of cardiovascular risk. This review outlines the current evidence relating to the effects of GHT, and in particular exogenous testosterone and estrogen, on vascular health outcomes in transgender adults.
Transgender Health
Ischemic Heart Disease
The first observational study relating to GHT and cardiovascular disease (CVD) was published in 1989 (Table 2). This did not demonstrate any difference in the crude incidence of myocardial infarction (MI) or mortality relating to MI over a 4-year follow-up period when comparing 303 TGF to cisgender males (CGM). No cases of MI were noted within the TGM group.11
In 1997, van Kesteren et al12 undertook a retrospective observational study in 816 TGFs and 293 TGMs receiving GHT with a respective follow-up of 7734 and 2418 patient-years. This study demonstrated a decreased standardized incidence ratio (SIR) of MI in TGFs (SIR, 0.5 [95% CI, 0.24–0.91]) compared with CGMs. There was no difference in MI standardized mortality ratio (SMR, 0.71 [95% CI, 0.26–1.55]). SIR was no different in TGMs compared with cisgender females (CGFs).
In 966 TGFs and 365 TGMs with a median follow-up of 18.5 years, mortality was 51% higher compared with the general population.13 This was attributed to increased suicide, HIV infection, CVD, and substance abuse. TGFs had a SMR of 1.64 (95% CI, 1.43–1.87) relating to ischemic heart disease. The adjusted hazard ratio (HR) for cardiovascular mortality was 3.12 (95% CI, 1.28–7.63) in those using ethinylestradiol compared to former or never-users. TGM demonstrated a nonsignificant SMR of 1.19 (95% CI, 0.39–2.74) for ischemic heart disease mortality.
A population-based matched cohort study of 324 transgender people who had undergone gender reassignment surgery with mean follow-up of 11 years demonstrated an increased risk of cardiovascular death (HR, 2.5; 95% CI, 1.2–5.3) compared with cisgender people.14 In a cohort study of 100 transgender people retrospectively followed-up for 10 years, 2 cases of MI were identified in TGFs and no cases in TGM.15 A cross-sectional study in 138 TGM and 214 TGFs receiving GHT over an average of 7.7 years (range 4–13 years) demonstrated significantly increased prevalence of MI in TGFs (18.7 per 1000) compared with CGFs but not CGMs.16
Studies using data from the Behavioral Risk Factor Surveillance System showed that transgender individuals are at greater risk of MI than cisgender individuals.17–19 Meyer et al17 reported a higher odds ratio (OR) of MI in transgender (OR, 1.82 [95% CI, 1.22–2.72]) compared with cisgender people, with no increased risk of angina or coronary heart disease (OR, 1.37 [95% CI, 0.83–2.25]). Nokoff et al18 demonstrated an increased risk of MI (OR, 2.9 [95% CI, 1.6–5.3]) in TGFs compared with CGFs, but not CGMs (OR, 1.09 [95% CI, 0.59–2.03]). There was no increased risk comparing TGMs to CGMs or CGFs. The risk of angina or coronary heart disease was not increased in either TGFs or TGMs. Conversely, Alzahrani et al19 demonstrated that TGM but not TGF had an associated higher risk of MI compared with their natal sex comparators after adjusting for traditional CVD risk factors. Importantly these studies are cross-sectional, do not confirm the use of GHT and rely on the self-reporting of health issues such as MI and are, therefore, at risk of significant bias.
In a retrospective analysis of electronic medical records between 2006 and 2014, 2842 TGFs and 2118 TGMs receiving GHT were followed up for 4.0 and 3.6 years, respectively. A higher rate of MI in TGFs was observed compared with CGFs (HR, 1.8 [95% CI, 1.1–2.9]).20 However, this was not increased when compared with CGMs (HR, 0.9 [95% CI, 0.6–1.5]). Again, no differences were observed between TGMs, CGMs, or CGFs.
Overall, evidence derived from age-adjusted population comparisons and age-matched cohort studies suggest that TGFs have a higher risk of CVD and ischemic events than CGFs, however, in only one study was this higher than CGMs.13 Importantly, most studies use TGF populations under the age of 50 years, and little is known of the impact of GHT on vascular health in the longer term. As discussed in recent meta-analysis, there is a paucity of data about important patient outcomes, such as MI, and the ability to draw conclusion from studies where control groups and age-matched cohorts are not clearly defined impedes any meaningful assessment.21 No consensus exists on whether a transgender person’s cardiovascular risk should be compared with their natal sex or gender.
Cerebrovascular Disease
In an observational study of 303 TGFs followed up for a median of 4.4 years, there were no differences in the crude incidence of transient ischemic attack compared with CGMs.11 Similar results were observed by van Kesteren et al,12 who did not demonstrate any significant difference in SIR for cerebrovascular disease in TGFs. In a study of 966 TGFs and 375 TGMs with a median follow-up of 18.5 years, there was no difference in mortality associated with stroke in TGFs and no cases at all in TGMs.13
More recently, Wierckx et al16 undertook a case-control study in 214 TGFs and 138 TGMs with an average of 7.7 years GHT and mean age of 43 years. TGFs demonstrated a higher prevalence of transient ischemic attack and cerebrovascular disease (23.4 per 1000), which, although no different from age-matched CGFs (14.9 per 1000), was significantly higher than age-matched CGMs (9.4 per 1000). No cases were observed in CGMs.
Survey data demonstrated no difference in cerebrovascular disease risk in transgender compared with cisgender populations.17 When stratified between TGFs and TGMs there was no increased risk in comparison to CGMs or CGF.18 The use of GHT was uncertain in these populations.
Getahun et al20 undertook an electronic medical record-based cohort study in 2842 TGFs and 2118 TGMs. TGFs had higher rates of ischemic stroke compared with corresponding rates in CGM and CGF. Ischemic stroke rates were highest in a sub-analysis of 853 TGFs who initiated GHT during the study period. These rates did not differ compared with cisgender populations during the first 6 years of follow-up but thereafter increased substantially compared with both CGMs (HR, 9.9 [95% CI, 3.0–33.1]) and CGFs (HR, 4.1 [95% CI, 1.5–11.4]).
Although uncertainty remains, TGFs appear to be at a higher risk of developing ischemic stroke compared with cisgender populations. This risk is most evident in longer-term estrogen use. Conversely, TGMs do not have increased cerebrovascular disease risk.
Hypertension
Numerous studies have suggested an increased incidence of hypertension in transgender populations. Asscheman et al11 observed an increase in the crude incidence of hypertension in 303 TGFs. However, no increase was demonstrated in TGMs. The SIR for hypertension (defined as >160/95 mm Hg) was not significantly higher in TGFs or TGMs compared with CGMs and CGFs, respectively. In a cohort study comparing 63 untreated TGMs to 48 TGMs undergoing GHT, the resting systolic and diastolic brachial blood pressures (BPs) in the treated group were significantly higher.22 After 2 years of GHT in 79 TGFs and 43 TGMs, the mean of 3 consecutive systolic BPs increased significantly by 17.8 mm Hg and 13.4 mm Hg, respectively.23 The TGF group also demonstrated a 3.17 mm Hg increase in diastolic BP, which was not noted in the TGM group.23 Lastly, self-reported hypertension in 369 TGFs and 239 TGMs was no higher when compared with cisgender populations.18
Furthermore, prospective studies demonstrated alteration in BP following GHT initiation. In a study of 20 TGFs commencing GHT an increase in both mean systolic (7 mm Hg) and diastolic (5.7 mm Hg) BP (measured every 5 minutes for 2 hours using an automatic device) was noted at one year.24 No significant changes in BP were noted in 17 TGMs in this timeframe. Oral estradiol valerate has been shown to reduce systolic BP by 6.3 mm Hg after 1 year in 40 TGFs. This was not observed in a transdermal estrogen cohort (n=13) or in TGMs receiving testosterone undecanoate.25 TGMs treated with intramuscular testosterone have demonstrated a 3.2 mm Hg increase in systolic but not diastolic BP.26 In 2019, van Velzen et al27 reported a prospective observational study of 242 TGFs and 188 TGMs, where BP was measured via an automated device 12 months after commencing GHT. In TGMs, no change was observed in systolic BP. However, diastolic BP significantly increases by 3 mm Hg in the pooled analysis. In TGFs, in whom 59.5% used oral estrogen valerate and 40.5% were prescribed transdermal estrogen patches, systolic and diastolic BP decreased by 3 and 2 mm Hg, respectively.
Results from these studies are inconsistent, and alterations in BP may be dependent on factors such as GHT duration and age. GHT appears to elevate BP in both TGMs and TGFs in most studies. However, this may only be apparent with longer-term use.
Venous Thromboembolism
GHT may also promote CVD by altering the thrombotic phenotype. Early observational studies demonstrated a 20 to 45 fold increase in the rates of venous thromboembolism (VTE) in GHT-exposed TGF.11,12 In a more recent retrospective analysis, VTE developed in 1.2% of TGF receiving oral therapy for 2 years and was more common in those exposed to conjugated equine estrogen (4%) compared with estrogen valerate (0.6%) or ethinylestradiol (0.7%).28 Subsequently, a case-control study of 214 TGFs with a mean estrogen duration of 7.7 years VTE occurred in 5.1%.16 The higher risk of VTE observed by Getahun increased with time, with 2- and 8- year risk differences of 4.1 (95% CI, 1.6–6.7) and 16.7 (95% CI, 6.4–27.5) per 1000 persons relative to CGM.20 Although the majority of studies demonstrate an increased risk in TGFs, this finding is not uniform.29,30 To our knowledge, no study has demonstrated an increased risk of VTE in TGMs.
Cardiometabolic Risk
GHT may also alter cardiometabolic parameters through which it may modify CVD risk. In a recent systematic review and meta-analysis 29 studies, including 4731 transgender individuals (68% TGFs) with follow-up ranging from 3 months to 41 years, were assessed.21 Testosterone therapy in TGMs, at greater than 2 years follow up was associated with an elevation in triglycerides (21.4 mg/dL; 95% CI, 0.14–42.6) and LDL (low-density lipoprotein; 17.8 mg/dL; 95% CI, 3.5–32.1) with reductions in HDL (high-density lipoprotein; −8.5 mg/dL; 95% CI, −13 to −3.9) with no significant change in total serum cholesterol. Conversely, no significant differences were apparent in LDL, HDL, or total cholesterol in TGFs, but an increase in triglycerides was observed (31.9 mg/dL; 95% CI, 3.9–59.9). Although this assessment is limited by low-quality evidence, it raises questions as to why lipids do not reflect their adopted and more favorable gender profile in TGF and why this shift in TGM does not confer an increased risk of CVD.
It has also been suggested that an increased risk of type 2 diabetes mellitus may exist in this population. In an observational study of 966 TGFs receiving cyproterone acetate and either oral or transdermal estrogen, there was no significant difference in diabetes mellitus-related SMR compared with the cisgender population (SMR 0.85; 95% CI, 0.41–1.32), while no cases were identified in 365 TGMs.13 In a cross-sectional study completed in 214 TGFs and 138 TGMs, there were higher rates of diabetes mellitus in TGFs compared with CGFs or CGMs (42 per 1000 cases).16 TGMs demonstrated a higher incidence (36.2 per 1000 cases) compared with CGFs but not CGMs. Increases in homeostatic model assessment for insulin resistance index in TGFs has been demonstrated after 2 years of transdermal estrogen, but not after 1 year or in TGM.23,31 Fasting blood glucose in TGF is higher with transdermal estrogen but not oral ethinylestradiol.23,24 Studies using the self-reporting Behavioral Risk Factor Surveillance system with no GHT confirmation did not demonstrate a higher risk of diabetes mellitus.17,18 Consequently, the relationship between GHT and altered glucose metabolism remains uncertain.
Surrogate Markers of Cardiovascular Risk in Transgender People
Few studies have assessed the effects of GHT on surrogate markers of cardiovascular risk in transgender people. TGFs prescribed either ethinylestradiol or conjugated estrogen for an average 61 months demonstrated improvements in flow-mediated and nitric oxide-induced vasodilation compared with cisgender individuals. No improvements in exercise-induced metabolic vasodilation were observed.32 Oral estrogen in 23 TGFs resulted in increased levels of IL (interleukin)-6, IL-1, and IL-8 in the first few months of GHT and fell thereafter, while levels of the anti-oxidant enzyme superoxide dismutase were elevated throughout the 6-month study period, which was not apparent in those prescribed transdermal estrogen.33 After 4 months of GHT endothelin levels increased in TGM and fell in TGF.34 Oral estrogen may also increase nitric oxide and decrease tissue plasminogen activator levels.35,36
Brachial-ankle pulse wave velocity was higher in GHT-exposed versus untreated TGM with no difference in carotid augmentation index.22 GHT in TGMs results in higher brachial artery diameter and reduced nitrate-induced vascular response, but similar endothelial function compared with that of age-matched controls.37 Reductions in estradiol in TGM treated with the aromatase inhibitor, anastrazole, in addition to parenteral testosterone esters, resulted in no change in flow-mediated dilation, however, a significant positive correlation in common carotid compliance and distensibility coefficients were observed.38 In 56 transgender individuals, carotid intima-media thickness and flow-mediated dilation of the brachial artery was measured 2 to 3 months after gender reassignment procedures and compared with those receiving GHT alone.39 Those undergoing surgery experience a reduction in flow-mediated dilation and increased carotid intima-media thickness.
Overall data describing the influence of GHT on vascular structure and physiology is limited. The GETS (Gender Dysphoria Treatment in Sweden) study primarily aims to assess the physiological and epigenetic effects of initiating GHT on skeletal muscle and adipose tissue, and if such changes influence metabolism and body composition.40 Secondary outcome measures include the assessment of carotid augmentation index, echocardiograms, coronary flow reserve, and carotid intima-media thickness.
Sex Steroids and the Vasculature
A compressive review of complexity of sex hormone receptor expression, signaling and regulation of vascular function is beyond the scope of this article. However, excellent reviews on this topic are available.41–44 In short, the vascular effects of estrogen are mediated via the estrogen receptors (ER-α, ER-β, and the GPER [G protein-coupled estrogen receptor]). These are expressed in endothelial, vascular smooth muscle, and myocardial cells (Figure 1). In mice, ER-α mediates protective effects of estrogen in response to vascular injury and atherosclerosis.45,46 In endothelial cells, ER-α has been shown to activate endothelium-dependent vasodilatation via endothelial nitric oxide synthase, endothelial proliferation, and migration, and promotes carotid artery re-endothelialization.47,48 Mouse models have also demonstrated an important role of ER-β in the regulation of vascular function and blood pressure 49 while loss of GPER action augments endothelium-dependent vasoconstriction and promotes atheroma formation and inflammation.50
Figure 1.
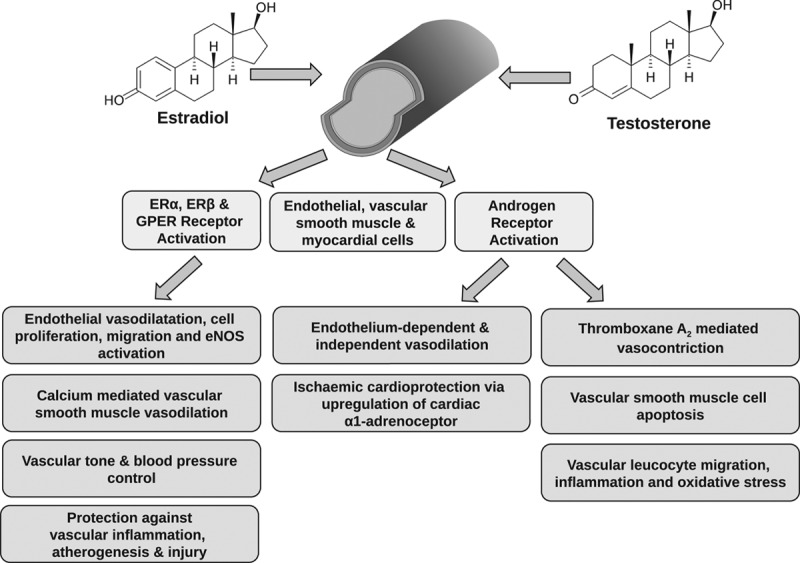
Vascular effects of sex steroids via estrogen and androgen receptors expressed in endothelial, vascular smooth muscle, and myocardial cells.
A paradoxical relationship, therefore, exists between the observed role of estrogen in the vasculature and the clinical outcomes observed in TGF. In postmenopausal CGF, estrogen’s age and exposure-dependent vasoprotective effects suggest a beneficial role in early, but not later stages, of atherogenesis.51–53 Similarly, in apolipoprotein E-deficient mice, exogenous estrogen precludes atheroma development but not the progression of established lesions.54 In addition to the greater atheroma burden associated with their natal sex, TGFs are deprived of the protection of endogenous life-long estrogen exposure (Figure 2).55 Alterations to estrogen receptor subtype expression, which is sex and tissue-specific, receptor sensitivity or signaling may contribute to increases in oxidative stress and inflammation in this relatively dyslipidemic, pro-hypertensive, and pro-thrombotic phenotype.33,56,57 Mechanistic studies exploring this relationship have yet to be performed.
Figure 2.
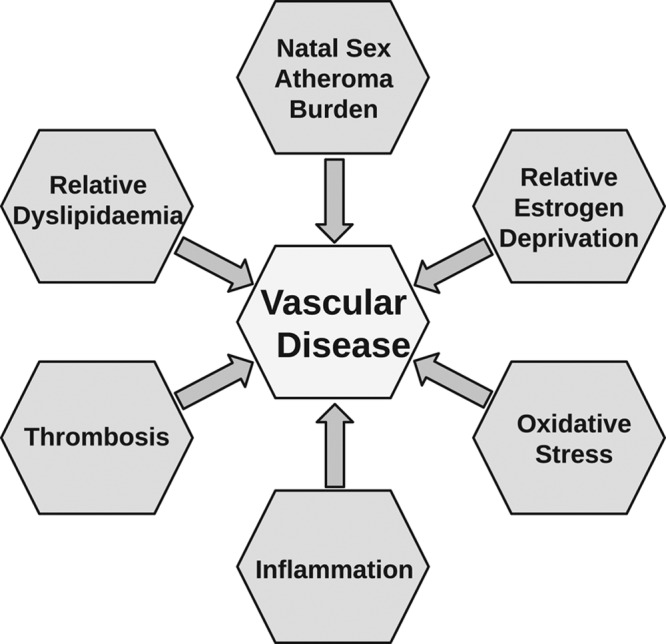
Putative model for increased vascular risk in transgender women.
Much like ERs, the AR (androgen receptor) is expressed in vascular endothelial and smooth muscle cells.44 In vitro studies demonstrate that testosterone acts as a vasodilator via both endothelium-dependent and independent mechanisms.58,59 This action is mediated, in part, by inhibition of voltage-operated calcium channels and the activation of potassium channels on vascular smooth muscle cells.60 Conversely, vasoconstrictive properties of testosterone have been observed in isolated and perfused rat coronary arteries.61 Testosterone has been shown to confer cardioprotection after ischemia in isolated perfused hearts and ventricular myocytes from orchidectomized rats via upregulation of the cardiac α1-adrenoceptor.62 In rats, testosterone also induces leukocyte migration via NADPH oxidase cyclooxygenase-dependent mechanisms and may contribute to inflammatory processes and oxidative stress in the vasculature, thereby potentially increasing cardiovascular risk.63 Testosterone induces apoptosis in vascular smooth muscle cells via the extrinsic apoptotic pathway with the involvement of AR activation and mitochondria-generated reactive oxygen species.64
Consequently, testosterone has both vasoprotective and vasoinjurious actions. It is unclear why the latter does not precipitate cardiovascular risk in TGMs or if androgen deprivation is responsible for an increased cardiovascular risk in TGF. This is further complicated by the use of gonadotropin-releasing hormone agonists, which receptor is expressed in the cardiovascular system and may increase cardiovascular risk in the treatment of prostate cancer, albeit in the context of hypogonadism.65,66
Conclusions
Sexual dimorphism exists in cisgender people across all spectrums of diseases including immune response, inflammatory disorders, malignancy, clinical pharmacology, and psychiatry.67–71 Although this review focuses on vascular disease, GHT impacts other aspects of health such as bone metabolism and the risk of malignancy. Therefore a cross-disciplinary approach is required to provide transgender people with optimal care.9,10
Although, GHT is integral to the management of transgender individuals the majority of studies identified by this review are retrospective and do not offer insight into the mechanisms by which sex steroids may alter vascular physiology and sex hormone receptor status in transgender people.72,73 The dose, formulation and mode of hormone therapy are heterogeneous, and achievement and sustainment of target hormone levels are often omitted. Their interpretation is also confounded by rates of mental health disorders, substance abuse, and health inequalities in transgender populations, which will contribute to the burden of cardiovascular risk.74 Furthermore, the long-term effects of pubertal suppression and subsequent introduction of GHT on vascular health in adolescents is unknown. Lastly, a consensus must be reached as to whether transgender individuals should be compared with their natal sex or their adopted gender.
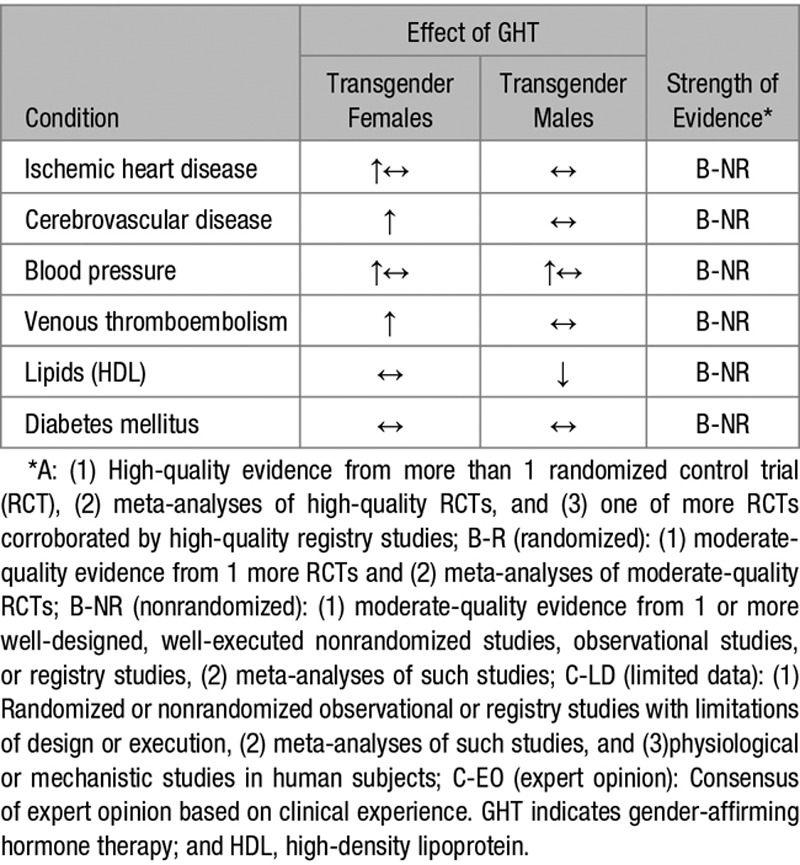
Current evidence suggests that the use of estrogen by TGFs confers an increased risk of MI and ischemic stroke (Table 3). Whether this is a consequence of GHT or legacy effect of natal sex remains unclear as not all studies demonstrate increased risk compared with CGMs. Conversely, TGMs lack any consistent or convincing evidence of increased risk of cardiovascular or cerebrovascular disease despite blood pressure elevations and dyslipidemia. The study of cardiovascular health and disease in transgender people is urgently required to implement better clinical care and evidence-based guidance.
Table 1.
Terms Used in Transgender Medicine
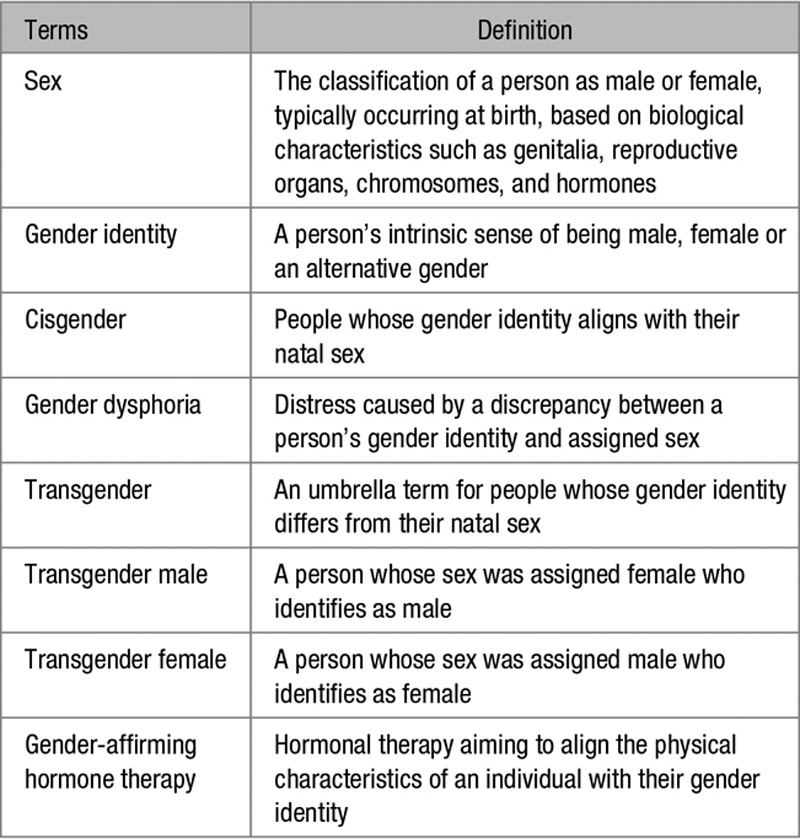
Table 2.
Ischemic Heart Disease in Transgender Populations
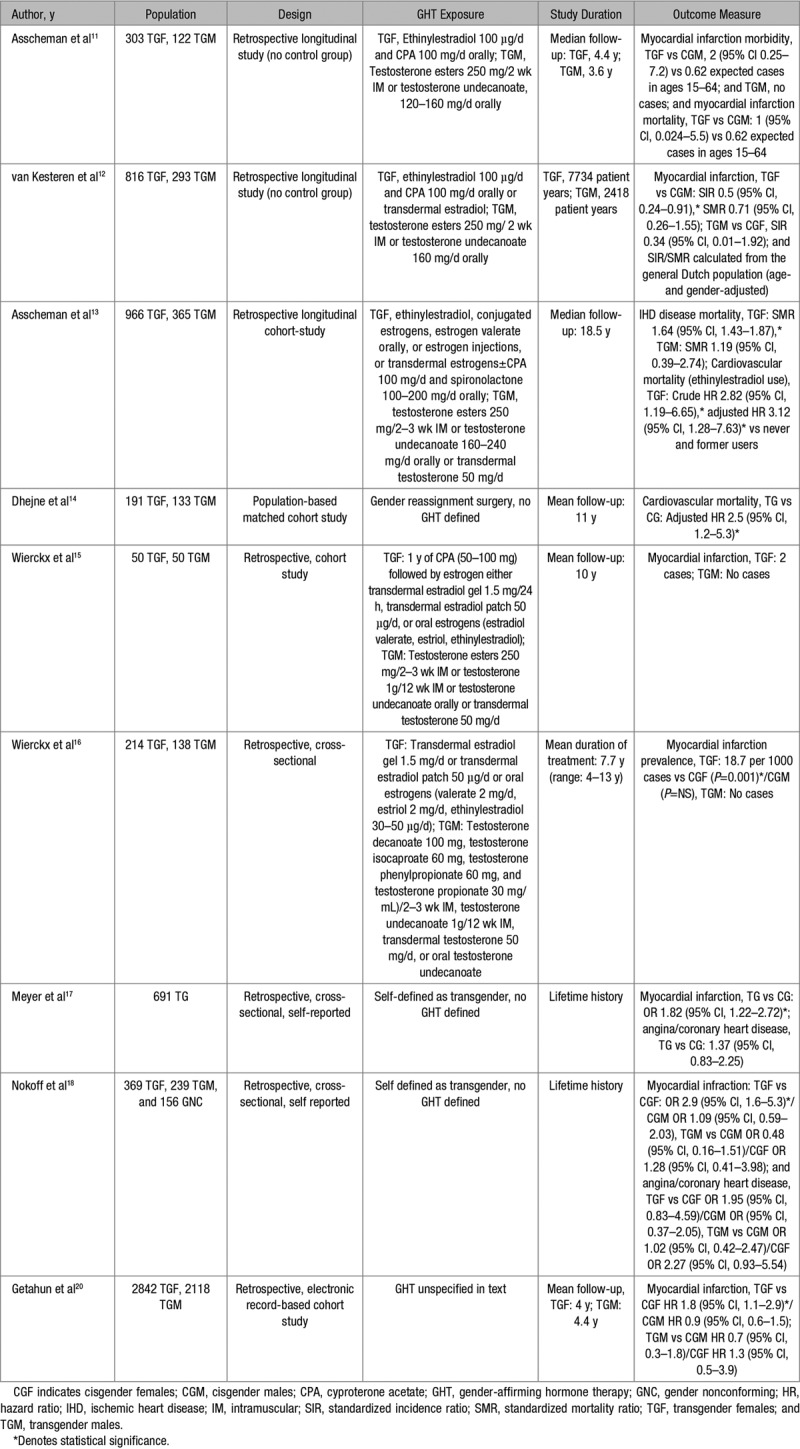
Table 3.
Summary of the Effects of GHT in the Vascular Health of Transgender Females and Males
Sources of Funding
This work was supported by the British Heart Foundation (Center of Research Excellence Awards RE/13/5/30177 and RE/18/6/34217).
Disclosures
None.
Footnotes
This paper was sent Robert M. Carey, Consulting Editor, for review by expert referees, editorial decision, and final disposition.
References
- 1.T’Sjoen G, Arcelus J, Gooren L, Klink DT, Tangpricha V. Endocrinology of Transgender Medicine. Endocr Rev. 2019;40:97–117. doi: 10.1210/er.2018-00011. doi: 10.1210/er.2018-00011. [DOI] [PubMed] [Google Scholar]
- 2.Winter S, Diamond M, Green J, Karasic D, Reed T, Whittle S, Wylie K. Transgender people: health at the margins of society. Lancet. 2016;388:390–400. doi: 10.1016/S0140-6736(16)00683-8. doi: 10.1016/S0140-6736(16)00683-8. [DOI] [PubMed] [Google Scholar]
- 3.Conron KJ, Scott G, Stowell GS, Landers SJ. Transgender health in Massachusetts: results from a household probability sample of adults. Am J Public Health. 2012;102:118–122. doi: 10.2105/AJPH.2011.300315. doi: 10.2105/AJPH.2011.300315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meerwijk EL, Sevelius JM. Transgender population size in the United States: a meta-regression of population-based probability samples. Am J Public Health. 2017;107:e1–e8. doi: 10.2105/AJPH.2016.303578. doi: 10.2105/AJPH.2016.303578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downing JM, Przedworski JM. Health of transgender adults in the U.S 2014–2016. Am J Prev Med. 2018;55:336–344. doi: 10.1016/j.amepre.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 6.Arcelus J, Bouman WP, Van Den Noortgate W, Claes L, Witcomb G, Fernandez-Aranda F. Systematic review and meta-analysis of prevalence studies in transsexualism. Eur Psychiatry. 2015;30:807–815. doi: 10.1016/j.eurpsy.2015.04.005. doi: 10.1016/j.eurpsy.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Zucker KJ. Epidemiology of gender dysphoria and transgender identity. Sex Health. 2017;14:404–411. doi: 10.1071/SH17067. doi: 10.1071/SH17067. [DOI] [PubMed] [Google Scholar]
- 8.Dhejne C, Öberg K, Arver S, Landén M. An analysis of all applications for sex reassignment surgery in Sweden, 1960-2010: prevalence, incidence, and regrets. Arch Sex Behav. 2014;43:1535–1545. doi: 10.1007/s10508-014-0300-8. doi: 10.1007/s10508-014-0300-8. [DOI] [PubMed] [Google Scholar]
- 9.Tangpricha V, den Heijer M. Oestrogen and anti-androgen therapy for transgender women. Lancet Diabetes Endocrinol. 2017;5:291–300. doi: 10.1016/S2213-8587(16)30319-9. doi: 10.1016/S2213-8587(16)30319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwig MS. Testosterone therapy for transgender men. Lancet Diabetes Endocrinol. 2017;5:301–311. doi: 10.1016/S2213-8587(16)00036-X. doi: 10.1016/S2213-8587(16)00036-X. [DOI] [PubMed] [Google Scholar]
- 11.Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross-gender hormone treatment. Metabolism. 1989;38:869–873. doi: 10.1016/0026-0495(89)90233-3. doi: 10.1016/0026-0495(89)90233-3. [DOI] [PubMed] [Google Scholar]
- 12.van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol (Oxf) 1997;47:337–342. doi: 10.1046/j.1365-2265.1997.2601068.x. doi: 10.1046/j.1365-2265.1997.2601068.x. [DOI] [PubMed] [Google Scholar]
- 13.Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164:635–642. doi: 10.1530/EJE-10-1038. doi: 10.1530/EJE-10-1038. [DOI] [PubMed] [Google Scholar]
- 14.Dhejne C, Lichtenstein P, Boman M, Johansson AL, Långström N, Landén M. Long-term follow-up of transsexual persons undergoing sex reassignment surgery: cohort study in Sweden. PLoS One. 2011;6:e16885. doi: 10.1371/journal.pone.0016885. doi: 10.1371/journal.pone.0016885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wierckx K, Mueller S, Weyers S, Van Caenegem E, Roef G, Heylens G, T’Sjoen G. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med. 2012;9:2641–2651. doi: 10.1111/j.1743-6109.2012.02876.x. doi: 10.1111/j.1743-6109.2012.02876.x. [DOI] [PubMed] [Google Scholar]
- 16.Wierckx K, Elaut E, Declercq E, Heylens G, De Cuypere G, Taes Y, Kaufman JM, T’Sjoen G. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case-control study. Eur J Endocrinol. 2013;169:471–478. doi: 10.1530/EJE-13-0493. doi: 10.1530/EJE-13-0493. [DOI] [PubMed] [Google Scholar]
- 17.Meyer IH, Brown TN, Herman JL, Reisner SL, Bockting WO. Demographic characteristics and health status of transgender adults in select US regions: behavioral risk factor surveillance system, 2014. Am J Public Health. 2017;107:582–589. doi: 10.2105/AJPH.2016.303648. doi: 10.2105/AJPH.2016.303648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nokoff NJ, Scarbro S, Juarez-Colunga E, Moreau KL, Kempe A. Health and cardiometabolic disease in transgender adults in the United States: behavioral risk factor surveillance system 2015. J Endocr Soc. 2018;2:349–360. doi: 10.1210/js.2017-00465. doi: 10.1210/js.2017-00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alzahrani T, Nguyen T, Ryan A, Dwairy A, McCaffrey J, Yunus R, Forgione J, Krepp J, Nagy C, Mazhari R, Reiner J. Cardiovascular disease risk factors and myocardial infarction in the transgender population. Circ Cardiovasc Qual Outcomes. 2019;12:e005597. doi: 10.1161/CIRCOUTCOMES.119.005597. doi: 10.1161/CIRCOUTCOMES.119.005597. [DOI] [PubMed] [Google Scholar]
- 20.Getahun D, Nash R, Flanders WD, Baird TC, Becerra-Culqui TA, Cromwell L, Hunkeler E, Lash TL, Millman A, Quinn VP, Robinson B, Roblin D, Silverberg MJ, Safer J, Slovis J, et al. Cross-sex hormones and acute cardiovascular events in transgender persons. Ann Intern Med. 2018;169:205–213. doi: 10.7326/M17-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maraka S, Singh Ospina N, Rodriguez-Gutierrez R, Davidge-Pitts CJ, Nippoldt TB, Prokop LJ, Murad MH. Sex steroids and cardiovascular outcomes in transgender individuals: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2017;102:3914–3923. doi: 10.1210/jc.2017-01643. doi: 10.1210/jc.2017-01643. [DOI] [PubMed] [Google Scholar]
- 22.Emi Y, Adachi M, Sasaki A, Nakamura Y, Nakatsuka M. Increased arterial stiffness in female-to-male transsexuals treated with androgen. J Obstet Gynaecol Res. 2008;34:890–897. doi: 10.1111/j.1447-0756.2008.00857.x. doi: 10.1111/j.1447-0756.2008.00857.x. [DOI] [PubMed] [Google Scholar]
- 23.Colizzi M, Costa R, Scaramuzzi F, Palumbo C, Tyropani M, Pace V, Quagliarella L, Brescia F, Natilla LC, Loverro G, Todarello O. Concomitant psychiatric problems and hormonal treatment induced metabolic syndrome in gender dysphoria individuals: a 2 year follow-up study. J Psychosom Res. 2015;78:399–406. doi: 10.1016/j.jpsychores.2015.02.001. doi: 10.1016/j.jpsychores.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Elbers JM, Giltay EJ, Teerlink T, Scheffer PG, Asscheman H, Seidell JC, Gooren LJ. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol (Oxf) 2003;58:562–571. doi: 10.1046/j.1365-2265.2003.01753.x. doi: 10.1046/j.1365-2265.2003.01753.x. [DOI] [PubMed] [Google Scholar]
- 25.Wierckx K, Van Caenegem E, Schreiner T, Haraldsen I, Fisher AD, Fisher A, Toye K, Kaufman JM, T’Sjoen G. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. J Sex Med. 2014;11:1999–2011. doi: 10.1111/jsm.12571. doi: 10.1111/jsm.12571. [DOI] [PubMed] [Google Scholar]
- 26.Mueller A, Haeberle L, Zollver H, Claassen T, Kronawitter D, Oppelt PG, Cupisti S, Beckmann MW, Dittrich R. Effects of intramuscular testosterone undecanoate on body composition and bone mineral density in female-to-male transsexuals. J Sex Med. 2010;7:3190–3198. doi: 10.1111/j.1743-6109.2010.01912.x. doi: 10.1111/j.1743-6109.2010.01912.x. [DOI] [PubMed] [Google Scholar]
- 27.van Velzen DM, Paldino A, Klaver M, Nota NM, Defreyne J, Hovingh GK, Thijs A, Simsek S, T’Sjoen G, den Heijer M. Cardiometabolic effects of testosterone in transmen and estrogen plus cyproterone acetate in transwomen. J Clin Endocrinol Metab. 2019;104:1937–1947. doi: 10.1210/jc.2018-02138. doi: 10.1210/jc.2018-02138. [DOI] [PubMed] [Google Scholar]
- 28.Seal LJ, Franklin S, Richards C, Shishkareva A, Sinclaire C, Barrett J. Predictive markers for mammoplasty and a comparison of side effect profiles in transwomen taking various hormonal regimens. J Clin Endocrinol Metab. 2012;97:4422–4428. doi: 10.1210/jc.2012-2030. doi: 10.1210/jc.2012-2030. [DOI] [PubMed] [Google Scholar]
- 29.Ott J, Kaufmann U, Bentz EK, Huber JC, Tempfer CB. Incidence of thrombophilia and venous thrombosis in transsexuals under cross-sex hormone therapy. Fertil Steril. 2010;93:1267–1272. doi: 10.1016/j.fertnstert.2008.12.017. doi: 10.1016/j.fertnstert.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Arnold JD, Sarkodie EP, Coleman ME, Goldstein DA. Incidence of venous thromboembolism in transgender women receiving oral estradiol. J Sex Med. 2016;13:1773–1777. doi: 10.1016/j.jsxm.2016.09.001. doi: 10.1016/j.jsxm.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Gava G, Cerpolini S, Martelli V, Battista G, Seracchioli R, Meriggiola MC. Cyproterone acetate vs leuprolide acetate in combination with transdermal oestradiol in transwomen: a comparison of safety and effectiveness. Clin Endocrinol (Oxf) 2016;85:239–246. doi: 10.1111/cen.13050. doi: 10.1111/cen.13050. [DOI] [PubMed] [Google Scholar]
- 32.New G, Duffy SJ, Harper RW, Meredith IT. Estrogen improves acetylcholine-induced but not metabolic vasodilation in biological males. Am J Physiol. 1999;277:H2341–H2347. doi: 10.1152/ajpheart.1999.277.6.H2341. doi: 10.1152/ajpheart.1999.277.6.H2341. [DOI] [PubMed] [Google Scholar]
- 33.Wilson R, Spiers A, Ewan J, Johnson P, Jenkins C, Carr S. Effects of high dose oestrogen therapy on circulating inflammatory markers. Maturitas. 2009;62:281–286. doi: 10.1016/j.maturitas.2009.01.009. doi: 10.1016/j.maturitas.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Polderman KH, Stehouwer CD, van Kamp GJ, Dekker GA, Verheugt FW, Gooren LJ. Influence of sex hormones on plasma endothelin levels. Ann Intern Med. 1993;118:429–432. doi: 10.7326/0003-4819-118-6-199303150-00006. doi: 10.7326/0003-4819-118-6-199303150-00006. [DOI] [PubMed] [Google Scholar]
- 35.Wilson R, Jenkins C, Miller H, Carr S. The effect of oestrogen on cytokine and antioxidant levels in male to female transsexual patients. Maturitas. 2006;55:14–18. doi: 10.1016/j.maturitas.2006.01.001. doi: 10.1016/j.maturitas.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Giltay EJ, Gooren LJ, Emeis JJ, Kooistra T, Stehouwer CD. Oral, but not transdermal, administration of estrogens lowers tissue-type plasminogen activator levels in humans without affecting endothelial synthesis. Arterioscler Thromb Vasc Biol. 2000;20:1396–1403. doi: 10.1161/01.atv.20.5.1396. [DOI] [PubMed] [Google Scholar]
- 37.Credie RJMC, Bs C, Crohon JAMC, Turner LEO, Griffiths KA, Handelsman DJ, Celermajer DS. Vascular reactivity is impaired in genetic females taking high-dose androgens. J Am Coll Cardiol. 1998;32:1331–1335. doi: 10.1016/s0735-1097(98)00416-1. [DOI] [PubMed] [Google Scholar]
- 38.Bunck MC, Toorians AW, Lips P, Gooren LJ. The effects of the aromatase inhibitor anastrozole on bone metabolism and cardiovascular risk indices in ovariectomized, androgen-treated female-to-male transsexuals. Eur J Endocrinol. 2006;154:569–575. doi: 10.1530/eje.1.02126. doi: 10.1530/eje.1.02126. [DOI] [PubMed] [Google Scholar]
- 39.Ciccone MM, Loverro G, Scicchitano P, et al. Surgical and pharmacological reassignment: influence on transsexual cardiovascular risk profile. Intern Med J. 2017;47:1255–1262. doi: 10.1111/imj.13597. doi: 10.1111/imj.13597. [DOI] [PubMed] [Google Scholar]
- 40.Wiik A, Andersson DP, Brismar TB, et al. Metabolic and functional changes in transgender individuals following cross-sex hormone treatment: design and methods of the GEnder Dysphoria Treatment in Sweden (GETS) study. Contemp Clin Trials Commun. 2018;10:148–153. doi: 10.1016/j.conctc.2018.04.005. doi: 10.1016/j.conctc.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morselli E, Santos RS, Criollo A, Nelson MD, Palmer BF, Clegg DJ. The effects of oestrogens and their receptors on cardiometabolic health. Nat Rev Endocrinol. 2017;13:352–364. doi: 10.1038/nrendo.2017.12. doi: 10.1038/nrendo.2017.12. [DOI] [PubMed] [Google Scholar]
- 42.Pabbidi MR, Kuppusamy M, Didion SP, Sanapureddy P, Reed JT, Sontakke SP. Sex differences in the vascular function and related mechanisms: role of 17β-estradiol. Am J Physiol Heart Circ Physiol. 2018;315:H1499–H1518. doi: 10.1152/ajpheart.00194.2018. doi: 10.1152/ajpheart.00194.2018. [DOI] [PubMed] [Google Scholar]
- 43.Menazza S, Murphy E. The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ Res. 2016;118:994–1007. doi: 10.1161/CIRCRESAHA.115.305376. doi: 10.1161/CIRCRESAHA.115.305376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucas-Herald AK, Alves-Lopes R, Montezano AC, Ahmed SF, Touyz RM. Genomic and non-genomic effects of androgens in the cardiovascular system: clinical implications. Clin Sci (Lond) 2017;131:1405–1418. doi: 10.1042/CS20170090. doi: 10.1042/CS20170090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ Res. 2002;90:1087–1092. doi: 10.1161/01.res.0000021114.92282.fa. doi: 10.1161/01.res.0000021114.92282.fa. [DOI] [PubMed] [Google Scholar]
- 46.Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor alpha is a major mediator of 17beta-estradiol’s atheroprotective effects on lesion size in Apoe-/- mice. J Clin Invest. 2001;107:333–340. doi: 10.1172/JCI11320. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chambliss KL, Wu Q, Oltmann S, et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kypreos KE, Zafirovic S, Petropoulou PI, Bjelogrlic P, Resanovic I, Traish A, Isenovic ER. Regulation of endothelial nitric oxide synthase and high-density lipoprotein quality by estradiol in cardiovascular pathology. J Cardiovasc Pharmacol Ther. 2014;19:256–268. doi: 10.1177/1074248413513499. doi: 10.1177/1074248413513499. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–508. doi: 10.1126/science.1065250. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- 50.Barton M, Prossnitz ER. Emerging roles of GPER in diabetes and atherosclerosis. Trends Endocrinol Metab. 2015;26:185–192. doi: 10.1016/j.tem.2015.02.003. doi: 10.1016/j.tem.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143. doi: 10.1002/14651858.CD004143.pub5. doi: 10.1002/14651858.CD004143.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keck C, Taylor M. Emerging research on the implications of hormone replacement therapy on coronary heart disease. Curr Atheroscler Rep. 2018;20:57. doi: 10.1007/s11883-018-0758-2. doi: 10.1007/s11883-018-0758-2. [DOI] [PubMed] [Google Scholar]
- 53.Lobo RA. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol. 2017;13:220–231. doi: 10.1038/nrendo.2016.164. doi: 10.1038/nrendo.2016.164. [DOI] [PubMed] [Google Scholar]
- 54.Billon-Galés A, Fontaine C, Douin-Echinard V, Delpy L, Berges H, Calippe B, Lenfant F, Laurell H, Guéry JC, Gourdy P, Arnal JF. Endothelial estrogen receptor-alpha plays a crucial role in the atheroprotective action of 17beta-estradiol in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:2567–2576. doi: 10.1161/CIRCULATIONAHA.109.898445. doi: 10.1161/CIRCULATIONAHA.109.898445. [DOI] [PubMed] [Google Scholar]
- 55.Han SH, Bae JH, Holmes DR, Jr, Lennon RJ, Eeckhout E, Barsness GW, Rihal CS, Lerman A. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J. 2008;29:1359–1369. doi: 10.1093/eurheartj/ehn142. doi: 10.1093/eurheartj/ehn142. [DOI] [PubMed] [Google Scholar]
- 56.Hodges YK, Tung L, Yan XD, Graham JD, Horwitz KB, Horwitz LD. Estrogen receptors alpha and beta: prevalence of estrogen receptor beta mRNA in human vascular smooth muscle and transcriptional effects. Circulation. 2000;101:1792–1798. doi: 10.1161/01.cir.101.15.1792. doi: 10.1161/01.cir.101.15.1792. [DOI] [PubMed] [Google Scholar]
- 57.Montague CR, Hunter MG, Gavrilin MA, Phillips GS, Goldschmidt-Clermont PJ, Marsh CB. Activation of estrogen receptor-alpha reduces aortic smooth muscle differentiation. Circ Res. 2006;99:477–484. doi: 10.1161/01.RES.0000238376.72592.a2. doi: 10.1161/01.RES.0000238376.72592.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deenadayalu VP, White RE, Stallone JN, Gao X, Garcia AJ. Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol. 2001;281:H1720–H1727. doi: 10.1152/ajpheart.2001.281.4.H1720. doi: 10.1152/ajpheart.2001.281.4.H1720. [DOI] [PubMed] [Google Scholar]
- 59.Jones RD, English KM, Jones TH, Channer KS. Testosterone-induced coronary vasodilatation occurs via a non-genomic mechanism: evidence of a direct calcium antagonism action. Clin Sci (Lond) 2004;107:149–158. doi: 10.1042/CS20030386. doi: 10.1042/CS20030386. [DOI] [PubMed] [Google Scholar]
- 60.Tambo A, Roshan MH, Pace NP. Testosterone and cardiovascular disease. Open Cardiovasc Med J. 2016;10:1–10. doi: 10.2174/1874192401610010001. doi: 10.2174/1874192401610010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ceballos G, Figueroa L, Rubio I, Gallo G, Garcia A, Martinez A, Yañez R, Perez J, Morato T, Chamorro G. Acute and nongenomic effects of testosterone on isolated and perfused rat heart. J Cardiovasc Pharmacol. 1999;33:691–697. doi: 10.1097/00005344-199905000-00003. doi: 10.1097/00005344-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Tsang S, Wu S, Liu J, Wong TM. Testosterone protects rat hearts against ischaemic insults by enhancing the effects of alpha(1)-adrenoceptor stimulation. Br J Pharmacol. 2008;153:693–709. doi: 10.1038/sj.bjp.0707624. doi: 10.1038/sj.bjp.0707624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chignalia AZ, Oliveira MA, Debbas V, Dull RO, Laurindo FR, Touyz RM, Carvalho MH, Fortes ZB, Tostes RC. Testosterone induces leucocyte migration by NADPH oxidase-driven ROS- and COX2-dependent mechanisms. Clin Sci (Lond) 2015;129:39–48. doi: 10.1042/CS20140548. doi: 10.1042/CS20140548. [DOI] [PubMed] [Google Scholar]
- 64.Lopes RA, Neves KB, Pestana CR, Queiroz AL, Zanotto CZ, Chignalia AZ, Valim YM, Silveira LR, Curti C, Tostes RC. Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement. Am J Physiol Heart Circ Physiol. 2014;306:H1485–H1494. doi: 10.1152/ajpheart.00809.2013. doi: 10.1152/ajpheart.00809.2013. [DOI] [PubMed] [Google Scholar]
- 65.Kakar SS, Jennes L. Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor mRNAs in various non-reproductive human tissues. Cancer Lett. 1995;98:57–62. [PubMed] [Google Scholar]
- 66.Bhatia N, Santos M, Jones LW, Beckman JA, Penson DF, Morgans AK, Moslehi J. Cardiovascular effects of androgen deprivation therapy for the treatment of prostate cancer: ABCDE steps to reduce cardiovascular disease in patients with prostate cancer. Circulation. 2016;133:537–541. doi: 10.1161/CIRCULATIONAHA.115.012519. doi: 10.1161/CIRCULATIONAHA.115.012519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 68.Weyand CM, Schmidt D, Wagner U, Goronzy JJ. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum. 1998;41:817–822. doi: 10.1002/1529-0131(199805)41:5<817::AID-ART7>3.0.CO;2-S. doi: 10.1002/1529-0131(199805)41:5<817::AID-ART7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 69.Jemal A, Miller KD, Ma J, Siegel RL, Fedewa SA, Islami F, Devesa SS, Thun MJ. Higher lung cancer incidence in young women than young men in the United States. N Engl J Med. 2018;378:1999–2009. doi: 10.1056/NEJMoa1715907. doi: 10.1056/NEJMoa1715907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Legato MJ, Johnson PA, Manson JE. Consideration of sex differences in medicine to improve health care and patient outcomes. JAMA. 2016;316:1865–1866. doi: 10.1001/jama.2016.13995. doi: 10.1001/jama.2016.13995. [DOI] [PubMed] [Google Scholar]
- 71.Rubinow DR, Schmidt PJ. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology. 2019;44:111–128. doi: 10.1038/s41386-018-0148-z. doi: 10.1038/s41386-018-0148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinez C, Rikhi R, Haque T, Fazal A, Kolber M, Hurwitz BE, Schneiderman N, Brown TT. Gender identity, hormone therapy, and cardiovascular disease risk [published online September 20, 2019]. Current Probl Cardiol. doi: 10.1016/j.cpcardiol.2018.09.003. DOI: 10.1016/j.cpcardiol.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Streed CG, Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Intern Med. 2017;167:256–267. doi: 10.7326/M17-0577. doi: 10.7326/M17-0577. [DOI] [PubMed] [Google Scholar]
- 74.Reisner SL, Poteat T, Keatley J, Cabral M, Mothopeng T, Dunham E, Holland CE, Max R, Baral SD. Global health burden and needs of transgender populations: a review. Lancet. 2016;388:412–436. doi: 10.1016/S0140-6736(16)00684-X. doi: 10.1016/S0140-6736(16)00684-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


