Abstract
We aimed to test whether predefined dietary patterns that are inversely related to risk of type 2 diabetes (T2D) in Western populations were similarly associated with lower T2D risk in an Asian population. We included 45,411 middle-aged and older participants (ages 45–74 years) in the Singapore Chinese Health Study who were free of diabetes, cancer, and cardiovascular disease at baseline (1993–1998). Participants were followed up for T2D diagnosis through 2010. Dietary information was collected using a validated food frequency questionnaire. Dietary pattern scores were calculated for the alternate Mediterranean diet (aMED), Alternate Healthy Eating Index 2010 (AHEI-2010), the Dietary Approaches to Stop Hypertension (DASH) diet, an overall plant-based diet index, and a healthful plant-based diet index. During a median of 11.1 years of follow-up, 5,207 incident cases of T2D occurred. After adjustment for multiple potential confounders, the 5 dietary pattern scores were significantly associated with 16% (for aMED) to 29% (for DASH) lower risks of T2D when comparing the highest score quintiles with the lowest (all P-for-trend values < 0.001). These associations did not vary substantially by baseline age, sex, body mass index, or hypertension status but were limited to nonsmokers (aMED: P for interaction < 0.001; AHEI-2010: P for interaction = 0.03). Adherence to a high-quality diet, as reflected by several predefined diet quality indices derived in Western populations, was significantly associated with lower T2D risk in an Asian population.
Keywords: alternate Mediterranean diet, Asians, diet quality, Dietary Approaches to Stop Hypertension diet, dietary patterns, Healthy Eating Index, plant-based diet index, type 2 diabetes mellitus
A dietary pattern approach that takes into account the complexity of people’s diets has emerged as a valuable tool for examining the relationship between overall diet and health outcomes (1). Findings derived from such a dietary approach are less subject to confounding by other dietary behaviors, and they may be more practical for nutritional recommendations. Dietary patterns could be either data-derived or hypothesis-derived. Hypothesis-derived dietary patterns are constructed on the basis of preliminary knowledge on diet-related disease risks; common examples include the Mediterranean diet (2) and its international adaptation, the alternate Mediterranean diet (aMED) (3); the Alternate Healthy Eating Index (AHEI) (4) and its updated version, Alternate Healthy Eating Index 2010 (AHEI-2010) (5); and the Dietary Approaches to Stop Hypertension (DASH) diet (6).
The aforementioned common dietary patterns have been extensively studied for their potential cardiovascular benefits (7–9), but their relationships with type 2 diabetes (T2D) have been examined less, and only in Western countries (10). Moreover, previous studies have indicated possible racial/ethnic discrepancies in the associations of these dietary patterns with T2D risk (11, 12), raising the question of whether the dietary patterns derived in Western populations are also associated with T2D risk in Asians, who have varying diet cultures and divergent genetic, metabolic, and lifestyle backgrounds (13).
In addition, a recent pooled analysis of data from 3 large US cohorts showed that adherence to dietary patterns defined by low intake of animal foods and high intake of plant foods (overall plant-based diet index (PDI)), especially intake of certain healthful plant foods (healthful plant-based diet index (hPDI)), was associated with a substantially lower risk of T2D (14). Given that plant products in Asian diets (e.g., abundant refined grains such as white rice and noodles, soy products, green leafy vegetables, and green tea) differ from those in Western diets in terms of both quantity and quality, it may be of particular interest to evaluate these dietary patterns in relation to T2D risk in Asians. Therefore, we investigated several predetermined diet quality indices, including the aMED, AHEI-2010, DASH diet, PDI, and hPDI, in relation to risk of T2D in an Asian population.
METHODS
Study population
We analyzed data from the Singapore Chinese Health Study (SCHS), the design of which has been described elsewhere (15). Briefly, a total of 63,257 Chinese persons aged 45–74 years were enrolled in the SCHS between 1993 and 1998. Participants belonged to one of the major dialect groups (Hokkien or Cantonese) of Chinese in Singapore and were citizens or permanent residents of government-built housing estates, where 86% of the general population resided during the enrollment period. In-person interviews were conducted at recruitment to collect subjects’ information on habitual diet, demographic factors, height, weight, tobacco use, physical activity, female menstrual and reproductive history, and medical history. The first and second follow-up interviews were conducted by telephone calls made during 1999–2004 and 2006–2010, respectively, with a participation rate of 89.9% for the first follow-up and 81.9% for the second follow-up. Informed consent was obtained from all enrolled participants. The study was approved by the institutional review boards of the National University of Singapore (Singapore, Republic of Singapore) and the University of Pittsburgh (Pittsburgh, Pennsylvania).
Dietary assessment and dietary pattern scores
Baseline information on habitual dietary intake over the past year was collected using a 165-item, semiquantitative food frequency questionnaire that was administered by a trained interviewer. For major food items, 8 food-frequency categories (ranging from ‘‘never or hardly ever’’ to ‘‘2 or more times a day’’) and 3 portion sizes (with accompanying photographs) were available for respondents to denote their habitual intake. For alcoholic beverages, the same 8 frequency categories were followed by 4 serving sizes. The daily intake values were derived by multiplying intake frequency with portion sizes. For nonalcoholic beverages, there were 9 intake frequency options (ranging from “never or hardly ever” to “6 or more times a day”) and the standard serving size was assigned as 1 cup or 1 glass. Daily nutrient and energy intakes were computed from the Singapore Food Composition Table, developed in conjunction with establishment of the cohort (15). In our previous validation study of 810 randomly selected cohort participants that compared food frequency questionnaire data with data from two 24-hour diet recalls, correlations between the 2 different methods ranged from 0.24 to 0.79 for dietary energy and nutrients, with most mean intakes from the food frequency questionnaire and diet recalls being within 10% of each other’s values (15). Furthermore, several components of the dietary patterns were associated with plasma fatty acid concentrations, as expected based on the fatty acid composition of these foods in a subsample of the SCHS (16).
Components of the dietary patterns included in this study and standards for scoring are presented in Web Table 1 (available at https://academic.oup.com/aje). A higher score represents better adherence. Scores for the aMED (3), AHEI-2010 (5), and DASH (6) indices were calculated on the basis of previously proposed methods. For AHEI-2010, we omitted the original trans- fat component, and for the DASH index, we used total dairy foods instead of low-fat dairy foods because data on dietary trans- fat and low-fat dairy foods were not collected in the SCHS. The PDI and hPDI, which were recently developed by Satija et al. (14), are both based on food quintiles and reverse-code intakes of animal foods, such that higher intakes provide fewer points. While the PDI further awards points for all plant food intakes, the hPDI only awards points for intake of “healthy plant foods” (e.g., coffee) and reverse-codes intake of “less healthy plant foods” (e.g., sugar-sweetened beverages). In our study, both indices included 15 nonoverlapping food groups with statistically meaningful intake levels for the analyses. More detailed procedures for calculation of the 5 dietary pattern scores are reported in the Web Appendix.
Ascertainment of incident diabetes
Diabetes was assessed through telephone interviews by the following question: “Have you been told by a doctor that you have diabetes (high blood sugar)?” If the response was yes, the participant was asked, “Please also tell me the age at which you were first diagnosed.” We counted as incident T2D cases those participants who reported receiving a physician’s diagnosis of diabetes at any time between the initial enrollment interview and the first or second follow-up telephone interview. Among participants who reported diabetes at follow-up 1 and participated in follow-up 2 (n = 4,588), 95.3% (n = 4,374) also reported diabetes at follow-up 2. In a subsample of 1,651 participants who reported physician-diagnosed diabetes in the follow-up 1 interview, incident diabetes was validated through linkage with a nationwide hospital-based discharge database (n = 949) or administration of a supplementary questionnaire about symptoms, diagnostic tests, and hypoglycemic therapy (n = 702). The validation study showed a positive predictive value of 98.8% for self-reported diabetes (17). Among participants who did not report a diagnosis of diabetes and provided blood samples at the first follow-up, 2,625 were randomly selected and hemoglobin A1c concentration was measured in their blood samples; 2,477 of them had a hemoglobin A1c level less than 6.5% (17), yielding a high negative predictive value (94.4%).
Statistical analysis
We excluded from the present analysis those participants who had known diabetes (n = 5,696) or cancer, stroke, or heart disease (n = 4,108) at baseline; had an unrealistic daily energy intake (<700 kcal/day or >3,700 kcal/day for men and <600 kcal/day or >3,000 kcal/day for women; n = 869); or were lost to follow-up or died before diabetes diagnosis (n = 7,173). This left 45,411 eligible subjects.
We calculated Pearson partial correlation coefficients for correlations between dietary pattern scores with adjustment for total energy intake. Person-years of follow-up time were computed from the year of the baseline questionnaire to the year of reported initial diagnosis of diabetes or the date of the second follow-up interview, whichever came first. Hazard ratios and 95% confidence intervals for T2D risk were estimated by means of Cox proportional hazards models, with dietary pattern scores being analyzed both categorically in quintiles (using the lowest quintile as the reference group) and continuously in 1–standard-deviation increments. Two models were constructed to account for potential confounders. The first model included age at baseline, sex, dialect group, year of baseline interview, and daily energy intake. The second model further included body mass index (BMI; weight (kg)/height (m)2), physical activity (no moderate or vigorous activity; 0.5–3.9 hours/week of moderate activity or 0.5–1.9 hours/week of vigorous activity; or ≥4.0 hours/week of moderate activity or ≥2.0 hours/week of vigorous activity), education (no formal education, primary school, or secondary school or higher), smoking (never smoker, former smoker, current smoker of 1–12 cigarettes/day, or current smoker of ≥13 cigarettes/day), and self-reported hypertension status (physician-diagnosed). Model 2 also included coffee and/or alcohol intake for the dietary patterns not including the coffee and/or alcohol item.
We conducted stratified analyses and tested interactions between these dietary pattern scores and baseline age (<55 years vs. ≥55 years), sex, BMI (<25.0 vs. ≥25.0), hypertension (yes vs. no), and smoking status (current smokers vs. nonsmokers). We included multiplicative interaction terms in the fully adjusted models to compute P values for interaction. To account for potential bias due to undiagnosed diabetes or reverse causality, we performed sensitivity analyses by excluding T2D cases diagnosed during the first 4 years of follow-up. To verify how scoring criteria may have affected the results, we carried out additional analyses to reconstruct the aMED and AHEI-2010 indices according to quintiles of their components.
To determine potential contributors to any significant association between the dietary patterns and risk of T2D, we further investigated the components of each dietary pattern in relation to T2D risk, using the full multivariable model described above and mutual adjustment for other components within the respective dietary pattern. In this analysis, components that were substantially correlated with dietary energy intake (Pearson’s r ≥ 0.30) were adjusted for total energy intake using the residual method (18). All analyses were performed using Stata 14.0 (StataCorp LLC, College Station, Texas). All P values were 2-sided, and the level of significance was set at less than 0.05.
RESULTS
Study population characteristics
Baseline characteristics of the study population are presented in Table 1 according to quintiles of the dietary pattern scores. For each of the 5 dietary patterns, participants with higher scores were less likely to be smokers and more likely to be from the Cantonese dialect group, to have higher educational achievement, and to report diagnosed hypertension. Higher dietary pattern scores also tended to be associated with female sex (except for PDI) and being physically active (except for hPDI). Adherence to the aMED, AHEI-2010, and PDI dietary patterns was also associated with younger age. The 5 dietary pattern scores showed moderate-to-strong correlations with each other, ranging from 0.41 for correlation between AHEI-2010 and PDI to 0.72 for correlation between AHEI-2010 and DASH (Web Table 2).
Table 1.
Baseline Characteristics of Participants According to Extreme Quintiles of Dietary Pattern Scores in the Singapore Chinese Health Study, 1993–2010
| Characteristic | Dietary Pattern Index and Score Quintile | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aMED Index | AHEI-2010 | DASH Index | PDI | hPDI | ||||||||||||||||
| Q1 (Lowest) (n = 8,916) | Q5 (Highest) (n = 9,358) | Q1 (Lowest) (n = 9,082) | Q5 (Highest) (n = 9,083) | Q1 (Lowest) (n = 6,415) | Q5 (Highest) (n = 9,820) | Q1 (Lowest) (n = 7,980) | Q5 (Highest) (n = 9,555) | Q1 (Lowest) (n = 8,982) | Q5 (Highest) (n = 10,352) | |||||||||||
| Mean | % | Mean | % | Mean | % | Mean | % | Mean | % | Mean | % | Mean | % | Mean | % | Mean | % | Mean | % | |
| Age, years | 56.3 | 54.2 | 55.1 | 54.8 | 54.1 | 55.6 | 56.0 | 54.6 | 54.2 | 55.7 | ||||||||||
| Male sex | 44.8 | 41.3 | 52.1 | 39.6 | 59.3 | 30.8 | 41.6 | 46.0 | 57.0 | 32.5 | ||||||||||
| Cantonese dialect | 40.1 | 55.4 | 44.3 | 53.0 | 41.5 | 55.3 | 40.5 | 53.0 | 47.3 | 48.5 | ||||||||||
| Higher educationa | 20.2 | 42.3 | 26.9 | 39.8 | 26.8 | 36.6 | 21.8 | 39.3 | 30.9 | 32.5 | ||||||||||
| Body mass indexb | 23.0 | 23.0 | 23.0 | 23.0 | 23.1 | 22.9 | 22.9 | 23.0 | 23.0 | 23.1 | ||||||||||
| Current smoker | 25.8 | 11.8 | 27.0 | 11.5 | 32.9 | 8.2 | 23.3 | 15.0 | 25.6 | 12.3 | ||||||||||
| Current alcohol drinker | 18.1 | 23.3 | 16.8 | 28.2 | 30.0 | 14.1 | 20.6 | 21.2 | 27.0 | 14.5 | ||||||||||
| Higher physical activityc | 27.1 | 42.6 | 31.3 | 40.5 | 30.4 | 41.0 | 27.4 | 41.3 | 36.4 | 34.9 | ||||||||||
| Hypertensiond | 18.6 | 19.6 | 17.9 | 20.4 | 16.8 | 20.3 | 17.5 | 20.9 | 17.6 | 20.9 | ||||||||||
| Dietary intakee | ||||||||||||||||||||
| Energy, kcal/day | 1,312.5 | 1,832.1 | 1,578.8 | 1,675.2 | 1,607.8 | 1,580.5 | 1,333.4 | 1,827.1 | 1,866.8 | 1,372.7 | ||||||||||
| Whole grains | 0.09 | 0.76 | 0.14 | 0.79 | 0.04 | 0.96 | 0.10 | 0.78 | 0.16 | 0.66 | ||||||||||
| Refined grains | 2.50 | 2.78 | 2.85 | 2.53 | 2.83 | 2.54 | 2.28 | 2.97 | 3.20 | 2.82 | ||||||||||
| Fruit | 0.63 | 1.95 | 0.77 | 2.05 | 0.65 | 1.90 | 0.69 | 1.91 | 0.98 | 1.13 | ||||||||||
| Vegetables | 0.92 | 2.12 | 1.09 | 2.11 | 1.07 | 1.92 | 1.05 | 2.01 | 1.36 | 1.70 | ||||||||||
| Nuts | 0.04 | 0.31 | 0.06 | 0.32 | 0.07 | 0.26 | 0.04 | 0.32 | 0.08 | 0.27 | ||||||||||
| Legumes | 0.23 | 0.76 | 0.26 | 0.77 | 0.29 | 0.65 | 0.29 | 0.71 | 0.41 | 0.58 | ||||||||||
| Red and processed meats | 0.28 | 0.34 | 0.38 | 0.26 | 0.44 | 0.20 | 0.32 | 0.31 | 0.46 | 0.20 | ||||||||||
| Poultry | 0.18 | 0.28 | 0.25 | 0.23 | 0.29 | 0.19 | 0.23 | 0.23 | 0.33 | 0.16 | ||||||||||
| Fish | 0.43 | 0.80 | 0.49 | 0.74 | 0.61 | 0.60 | 0.62 | 0.62 | 0.73 | 0.51 | ||||||||||
| Dairy foods | 0.23 | 0.34 | 0.26 | 0.33 | 0.11 | 0.51 | 0.34 | 0.27 | 0.36 | 0.21 | ||||||||||
| Eggs | 0.23 | 0.29 | 0.30 | 0.24 | 0.32 | 0.21 | 0.30 | 0.23 | 0.40 | 0.15 | ||||||||||
| Vegetable oils | 0.30 | 0.81 | 0.29 | 0.85 | 0.39 | 0.68 | 0.24 | 0.89 | 0.25 | 0.90 | ||||||||||
| Desserts | 0.60 | 1.10 | 0.88 | 0.86 | 0.79 | 0.86 | 0.43 | 1.28 | 1.16 | 0.59 | ||||||||||
| SSBs | 0.10 | 0.07 | 0.27 | 0.02 | 0.28 | 0.02 | 0.04 | 0.13 | 0.23 | 0.02 | ||||||||||
| Fruit juices | 0.04 | 0.12 | 0.12 | 0.06 | 0.10 | 0.06 | 0.02 | 0.17 | 0.13 | 0.05 | ||||||||||
| Tea | 0.43 | 0.64 | 0.52 | 0.61 | 0.51 | 0.54 | 0.32 | 0.76 | 0.47 | 0.63 | ||||||||||
| Coffee | 1.56 | 1.25 | 1.58 | 1.22 | 1.73 | 1.13 | 1.18 | 1.57 | 1.30 | 1.50 | ||||||||||
| MUFA:SFA ratio | 0.90 | 1.07 | 0.92 | 1.07 | 0.97 | 1.01 | 0.92 | 1.06 | 0.93 | 1.07 | ||||||||||
| n-3 PUFA, mg/day | 225.9 | 418.6 | 256.2 | 386.5 | 321.3 | 314.7 | 322.7 | 328.7 | 387.5 | 268.3 | ||||||||||
| PUFA, % of energy | 4.03 | 6.34 | 4.04 | 6.56 | 4.56 | 5.69 | 4.45 | 5.93 | 4.45 | 6.03 | ||||||||||
| Sodium, mg/day | 890.9 | 1,348.1 | 1,186.0 | 1,137.1 | 1,268.2 | 1,021.3 | 988.5 | 1,277.4 | 1,454.9 | 885.9 | ||||||||||
Abbreviations: AHEI, Alternate Healthy Eating Index; aMED, alternate Mediterranean diet; DASH, Dietary Approaches to Stop Hypertension; hPDI, healthful plant-based diet index; MUFA, monounsaturated fatty acids; PDI, plant-based diet index; PUFA, polyunsaturated fatty acids; Q, quintile; SFA, saturated fatty acids; SSBs, sugar-sweetened beverages.
a Secondary school or higher educational level.
b Calculated as weight (kg)/height (m)2.
c At least 0.5 hours/week of moderate physical activity, vigorous work, or strenuous sports.
d Self-reported physician-diagnosed hypertension.
e Food intakes are reported as servings/day. The following standard local portion sizes were used for conversion: whole grains—16 g; refined grains—200 g (e.g., 1 bowl of white rice); fruit—1 medium piece; vegetables—67 g (0.5 cup of typical local vegetables); nuts—28 g of nuts or 1 tablespoon of peanut butter; legumes—1 medium-sized tofu item; red and processed meats—113.4 g of fresh red meat or 42.5 g of processed red meat; poultry—90 g (1 palm-sized piece); fish—90 g of fish or shellfish; dairy foods—250 mL or 1 cup of milk; eggs—50 g of egg or 1 whole egg; vegetable oils (nontropical)—1 tablespoon of corn oil, soybean oil, peanut oil, or sesame oil; desserts—50 g of pastries, Western cakes, or local Chinese desserts; SSBs—1 glass of soda; fruit juices—1 glass, packet, or typical local portion of fruit juice; tea—1 cup or glass of green tea or black tea; coffee—1 cup of coffee; alcohol—10 g of alcohol.
Dietary patterns and risk of T2D
During 475,458 person-years of follow-up (median, 11.1 years), 5,207 cases of incident T2D were documented. With basic adjustment for demographic variables and energy intake, participants in the highest quintiles of the 5 dietary pattern scores had statistically significant 15%–30% lower risks of T2D than those in the lowest quintiles (all P-for-trend values < 0.001) (Table 2), with the greatest risk reduction observed for a higher DASH score. All inverse associations remained significant and largely unchanged after further adjustment for BMI, hypertension, and lifestyle factors. The multivariable-adjusted hazard ratios for comparison of the highest quintiles of the dietary pattern scores with the lowest were 0.84 (95% confidence interval (CI): 0.77, 0.92) for aMED, 0.79 (95% CI: 0.73, 0.87) for AHEI-2010, 0.71 (95% CI: 0.65, 0.79) for DASH, 0.83 (95% CI: 0.76, 0.92) for PDI, and 0.81 (95% CI: 0.75, 0.89) for hPDI (all P-for-trend values < 0.001) (Table 2). Furthermore, each 1–standard-deviation increment in the dietary pattern scores was significantly associated with a 6% (for PDI) to 10% (for DASH) lower risk of T2D (Table 2).
Table 2.
Risk of Type 2 Diabetes Mellitus According to Quintile of Dietary Pattern Score in the Singapore Chinese Health Study, 1993–2010
| Dietary Pattern Index and Score Quintile | No. of Cases | No. of Person-Yearsa | Median Score (Range) | Model 1b | Model 2c | ||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| aMED index | |||||||
| Q1 | 1,097 | 91,711 | 2 (0–2) | 1.00 | Referent | 1.00 | Referent |
| Q2 | 1,042 | 91,352 | 3 (3–3) | 0.95 | 0.87, 1.03 | 0.96 | 0.88, 1.05 |
| Q3 | 1,109 | 100,298 | 4 (4–4) | 0.91 | 0.84, 0.99 | 0.92 | 0.84, 1.00 |
| Q4 | 951 | 92,828 | 5 (5–5) | 0.84 | 0.77, 0.92 | 0.83 | 0.76, 0.91 |
| Q5 | 1,008 | 99,269 | 6 (6–9) | 0.82 | 0.75, 0.90 | 0.84 | 0.77, 0.92 |
| P for trendd | <0.001 | <0.001 | |||||
| Per SD incremente | 0.93 | 0.90, 0.96 | 0.93 | 0.91, 0.96 | |||
| AHEI-2010 | |||||||
| Q1 | 1,115 | 92,739 | 41.1 (17.0–44.1) | 1.00 | Referent | 1.00 | Referent |
| Q2 | 1,060 | 94,067 | 46.3 (44.2–48.1) | 0.94 | 0.86, 1.02 | 0.93 | 0.86, 1.01 |
| Q3 | 1,081 | 95,111 | 50.0 (48.2–51.7) | 0.94 | 0.87, 1.03 | 0.93 | 0.86, 1.01 |
| Q4 | 1,017 | 96,330 | 53.7 (51.8–56.0) | 0.87 | 0.80, 0.95 | 0.86 | 0.79, 0.94 |
| Q5 | 934 | 97,211 | 59.4 (56.1–81.0) | 0.79 | 0.73, 0.87 | 0.79 | 0.73, 0.87 |
| P for trend | <0.001 | <0.001 | |||||
| Per SD increment | 0.93 | 0.90, 0.95 | 0.93 | 0.90, 0.95 | |||
| DASH index | |||||||
| Q1 | 830 | 66,373 | 18 (8–19) | 1.00 | Referent | 1.00 | Referent |
| Q2 | 1,266 | 105,326 | 21 (20–22) | 0.95 | 0.87, 1.04 | 0.95 | 0.87, 1.03 |
| Q3 | 981 | 86,822 | 23 (23, 24) | 0.89 | 0.81, 0.98 | 0.87 | 0.79, 0.96 |
| Q4 | 1,200 | 113,034 | 26 (25–27) | 0.83 | 0.76, 0.91 | 0.83 | 0.76, 0.91 |
| Q5 | 930 | 103,901 | 30 (28–39) | 0.70 | 0.64, 0.77 | 0.71 | 0.65, 0.79 |
| P for trend | <0.001 | <0.001 | |||||
| Per SD increment | 0.89 | 0.87, 0.92 | 0.90 | 0.87, 0.93 | |||
| PDI | |||||||
| Q1 | 960 | 82,502 | 32 (22–34) | 1.00 | Referent | 1.00 | Referent |
| Q2 | 948 | 83,904 | 36 (35–37) | 0.97 | 0.89, 1.06 | 0.96 | 0.87, 1.05 |
| Q3 | 1,082 | 96,277 | 39 (38–40) | 0.96 | 0.88, 1.04 | 0.92 | 0.85, 1.01 |
| Q4 | 1,184 | 111,365 | 42 (41–44) | 0.90 | 0.83, 0.98 | 0.87 | 0.79, 0.95 |
| Q5 | 1,033 | 101,411 | 47 (45–65) | 0.85 | 0.78, 0.94 | 0.83 | 0.76, 0.92 |
| P for trend | <0.001 | <0.001 | |||||
| Per SD increment | 0.95 | 0.92, 0.98 | 0.94 | 0.92, 0.97 | |||
| hPDI | |||||||
| Q1 | 1,100 | 91,739 | 38 (24–40) | 1.00 | Referent | 1.00 | Referent |
| Q2 | 947 | 83,660 | 42 (41–43) | 0.94 | 0.86, 1.02 | 0.93 | 0.85, 1.01 |
| Q3 | 1,121 | 99,700 | 45 (44–46) | 0.93 | 0.85, 1.01 | 0.93 | 0.85, 1.01 |
| Q4 | 908 | 89,585 | 48 (47–49) | 0.83 | 0.76, 0.91 | 0.82 | 0.75, 0.90 |
| Q5 | 1,131 | 110,775 | 52 (50–67) | 0.83 | 0.76, 0.91 | 0.81 | 0.75, 0.89 |
| P for trend | <0.001 | <0.001 | |||||
| Per SD increment | 0.93 | 0.90, 0.96 | 0.93 | 0.90, 0.95 | |||
Abbreviations: AHEI, Alternate Healthy Eating Index; aMED, alternate Mediterranean diet; CI, confidence interval; DASH, Dietary Approaches to Stop Hypertension; hPDI, healthful plant-based diet index; HR, hazard ratio; PDI, plant-based diet index; Q, quintile; SD, standard deviation.
a For some categories, the numbers of person-years total 475,459 rather than 475,458 because of rounding.
b Model 1 adjusted for age at baseline interview (years), sex, dialect group (Hokkien or Cantonese), year of baseline interview (1993–1995 or 1996–1998), and energy intake (kcal/day).
c Model 2 included the potential confounders in model 1 and additionally adjusted for body mass index (weight (kg)/height (m)2), physical activity (no moderate or vigorous activity; 0.5–3.9 hours/week of moderate activity or 0.5–1.9 hours/week of vigorous activity; or ≥4.0 hours/week of moderate activity or ≥2.0 hours/week of vigorous activity), education (no formal education, primary school, or secondary school or higher), smoking (never smoker, former smoker, current smoker of 1–12 cigarettes/day, or current smoker of ≥13 cigarettes/day), and self-reported history of physician-diagnosed hypertension. Analyses for the aMED, AHEI-2010, and DASH indices additionally adjusted for coffee consumption (cups/day), and analyses for the DASH, PDI, and hPDI indices additionally adjusted for alcohol consumption (none, light (<0.5 servings/day), moderate (0.5–1.9 servings/day for men and 0.5–1.4 servings/day for women), moderate to heavy (2.0–3.4 servings/day for men and 1.5–2.4 servings/day for women), or heavy (≥3.5 servings/day for men and ≥2.5 servings/day for women)).
dP values for trend were calculated by fitting median scores for quintiles as continuous variables in statistical models.
e SDs were 1.68 for aMED, 7.30 for AHEI-2010, 4.30 for DASH, 5.71 for PDI, and 5.67 for hPDI.
Stratified analyses
Results were consistent in the analyses stratified by age, sex, BMI, and hypertension status (Web Table 3). However, the significant inverse associations with T2D risk were only observed in nonsmokers for all dietary pattern scores, and the interaction with smoking status was significant for the aMED (P for interaction < 0.001) and AHEI-2010 (P for interaction = 0.03). For example, the multivariable-adjusted hazard ratio for extreme quintiles of aMED score was 0.79 (95% CI: 0.72, 0.88) for nonsmokers and 1.05 (95% CI: 0.83, 1.33) for smokers. We performed additional analyses using more specific smoking categories. For each of the dietary pattern scores, the inverse associations with risk of T2D were broadly similar in never smokers and former smokers (though the associations were nonsignificant for PDI and hPDI in former smokers), whereas no significant association was found either in lighter smokers (1–12 cigarettes/day) or in heavier smokers (≥13 cigarettes/day) (Web Table 4).
Sensitivity analyses
We conducted several sensitivity analyses. First, we examined whether differences in the strength of associations between dietary pattern scores and T2D might be due to differences in the categorization of the score components (i.e., predefined cutoffs for AHEI-2010, median cutoffs for aMED, and quintiles for the other dietary patterns). However, associations of the aMED and AHEI-2010 scores with T2D risk were essentially unchanged when the scores were reconstructed according to quintiles of their components (Web Table 5). Second, we examined the likelihood of reverse causation by repeating the analyses after excluding T2D cases that occurred within the first 4 years of follow-up. This exclusion did not substantially change the results (Web Table 6).
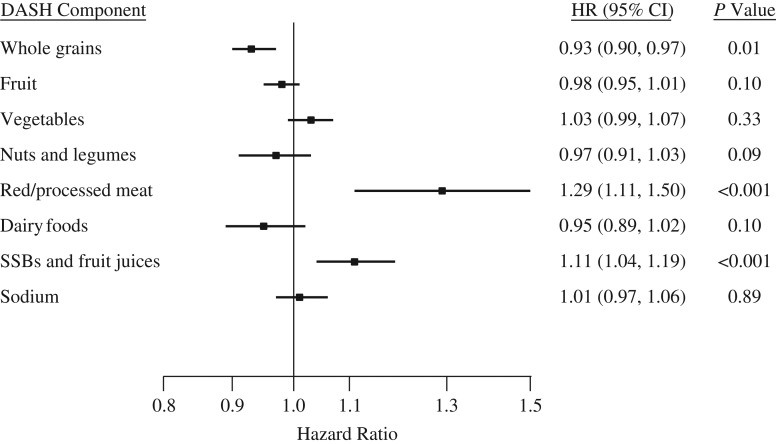
In addition, we examined individual components of the dietary patterns to identify potential contributors to the observed associations between dietary patterns and T2D. Taking components of the DASH index as an example, the hazard ratios for a 1-serving/day increment in intake were 0.93 (95% CI: 0.90, 0.97; P for trend = 0.01) for whole grains, 1.29 (95% CI: 1.11, 1.50; P < 0.001) for red and processed meats, and 1.11 (95% CI: 1.04, 1.19; P < 0.001) for the combination of sugar-sweetened beverages and fruit juices in the full multivariable model (Figure 1). Coffee consumption, which was included in the PDI and hPDI indices, was also inversely associated with T2D risk (per 2-cups/day increment, hazard ratio = 0.94, 95% CI: 0.90, 0.99; P = 0.004). No significant associations were found for other components of the examined dietary patterns (data not shown).
Figure 1.
Relationship between components of the Dietary Approaches to Stop Hypertension (DASH) diet index and risk of type 2 diabetes mellitus in the Singapore Chinese Health Study, 1993–2010. Foods and beverages were analyzed in 1-serving/day increments and sodium in 500-mg/day increments. Hazard ratios (HRs) were adjusted for the potential confounders listed for model 2 in Table 2, and results were mutually adjusted for other DASH components in quintiles. Results for several dietary components (fruit, vegetables, nuts and legumes, red/processed meat, and sodium) that were substantially correlated with dietary energy intake (correlation coefficients ≥0.30) were further adjusted for total energy intake using the residual method. P-for-trend values were calculated by fitting median intakes for quintiles as continuous variables in statistical models. Bars, 95% confidence intervals (CIs). SSBs, sugar-sweetened beverages.
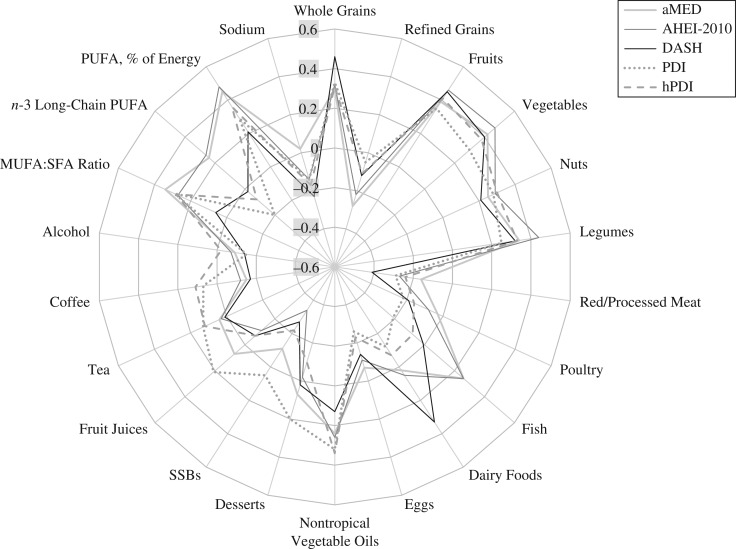
Given the observed effect modification by smoking status, we further evaluated correlations between the examined dietary patterns and their components in the whole study population and by smoking status, adjusting for total energy intake. The results showed that whole grains and red and processed meats were more strongly correlated with the DASH score than with other scores (Figure 2 and Web Table 7), and both also were more strongly associated with the evaluated dietary pattern scores in nonsmokers than in smokers (Web Table 7). For example, the correlation between red and processed meat consumption and DASH score was −0.41 and the correlations between red and processed meat consumption and other scores ranged from −0.16 to −0.29. The correlations for sugary beverages and coffee were generally weak across these subsets.
Figure 2.
Radar chart showing energy-adjusted Pearson correlation coefficients for correlations between dietary pattern scores and their components in the Singapore Chinese Health Study, 1993–2010. AHEI, Alternate Healthy Eating Index; aMED, alternate Mediterranean diet; DASH, Dietary Approaches to Stop Hypertension; hPDI, healthful plant-based diet index; MUFA, monounsaturated fatty acids; PDI, plant-based diet index; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; SSBs, sugar-sweetened beverages.
DISCUSSION
In this population-based prospective study of ethnic Chinese men and women, all 5 predetermined dietary pattern scores originally derived in Western populations were inversely associated with risk of T2D. Participants in the highest quintiles of the scores had a statistically significant 16% (for aMED) to 29% (for DASH) lower risk of T2D than those in the lowest quintiles. The observed inverse associations did not differ substantially according to baseline age, sex, BMI, or hypertension status, but they appeared to be limited to nonsmokers. Among components of the examined dietary patterns, higher intakes of whole grains and coffee and lower intakes of sugary beverages and red and processed meats were significantly associated with a lower T2D risk.
Our findings of a lower T2D risk associated with high-quality dietary patterns agree with recent results from prospective studies of Chinese populations. In 2 cohorts from Shanghai, China, adherence to a newly derived healthy diet score based on 8 food groups (high intakes of vegetables, fruit, dairy food, fish and seafood, and nuts and legumes and low intakes of red meat, processed meat, and refined grains) was significantly associated with lower risk of T2D; a 26% lower risk of T2D was observed for participants maintaining a high diet score as compared with those consistently having a low score during follow-up (19). In another large Chinese cohort, a low-risk dietary pattern defined by daily consumption of vegetables, fruit, and wheat and less-than-daily consumption of red meat was significantly associated with 26% lower risk of T2D (20). Our findings for individual components of the dietary patterns also concur with results of a recent systematic review and meta-analysis, in which Schwingshackl et al. (21) examined intakes of 12 major food groups (not including coffee consumption) in relation to T2D risk and concluded that there was a “high” level of evidence supporting a detrimental impact of red meat, processed meat, and sugar-sweetened beverages and a beneficial impact of whole grains on risk of T2D.
The aMED, AHEI-2010, and DASH dietary patterns have similarities, including an abundance of minimally processed plant foods such as vegetables, fruit, nuts, and legumes; low red and processed meat intake; and, for AHEI-2010 and DASH, restricted intake of sugary beverages and added sodium. As a result, these dietary patterns are associated with a dietary profile that is thought to be beneficial for T2D prevention—for example, high intakes of cereal fiber, magnesium, plant protein, and polyphenols and low intakes of heme iron and animal protein (22). Evidence from prospective cohort studies (23, 24) and randomized trials (25, 26) indicates that adherence to these dietary patterns may improve glycemic control and insulin sensitivity. A recent meta-analysis pooling data from previous cohort studies, all of which were conducted in Western countries, suggested 13%, 18%, and 21% lower risks of T2D associated with greater adherence to the Mediterranean diet, the AHEI or AHEI-2010, and DASH-style diets, respectively (10). Our findings confirmed these inverse associations and suggested associations of similar strength in an Asian population.
As to the association of the Mediterranean diet with T2D risk, most previous cohort studies (11, 27–31), though not all (32), have supported an inverse association, in both Mediterranean (30, 31) and non-Mediterranean (11, 27, 28) geographic regions, as well as in a study of various European countries (29). The antidiabetic effect of the Mediterranean diet is also evidenced by a 4-year intervention trial (33), which showed that a Mediterranean diet supplemented with either olive oil or nuts, when compared with a low-fat diet, resulted in an approximately 50% reduction in T2D risk.
The original AHEI was inversely associated with T2D risk in 2 US cohorts (28, 34) but not in a European study (22). For the AHEI-2010, 4 studies in 3 publications (5, 11, 27) found significant 12% (11) to 35% (5) lower risks of T2D associated with greater adherence. The addition of low sugar-sweetened beverage consumption as a beneficial component in the updated AHEI-2010 may have improved its performance in predicting T2D risk. While other examined indices were generated from relative intake (e.g., median values or quintiles) of the components, making comparison of diet quality scores across populations difficult, the AHEI-2010 is largely based on absolute intake of food groups and nutrients. Our previous work showed that total AHEI-2010 score in the SCHS population was similar to the scores in 2 studies of US health professionals; however, individual components of AHEI-2010 contributed differentially to the total score across these populations (35). For example, the scores for vegetables and whole grains were much lower and those for nuts and legumes, sugary beverages, and red and processed meats were much higher in the SCHS population than in the US population (35).
The DASH index has been the strongest predictor of T2D risk among dietary pattern indices assessed in our study and several previous studies (11, 27, 28). It is possible that such a relatively simple index that captures key food groups relevant for T2D risk is more indicative of diet quality. In our study, the DASH index, as compared with other indices, was more strongly associated with certain dietary components (whole grains and red and processed meats) shown to be significantly associated with T2D risk. Therefore, the difference in the strength of the associations may be explained, at least in part, by the diverse distributions of the dietary constituents making up these indices. A similar explanation may account for the stronger associations observed in nonsmokers as compared with smokers, because of the stronger correlations between the evaluated dietary pattern indices and the aforementioned “significant contributors” in nonsmokers than in smokers. However, it is still possible that the difference in the associations by smoking status was due to residual confounding or other variations (e.g., preparation methods) in components of the dietary patterns among smokers and nonsmokers.
In a pooled analysis of data from 3 cohorts of US health professionals (14), higher PDI and hPDI scores were associated with 20% and 34% lower risks of T2D, respectively, after adjustment for multiple potential confounders. Our findings confirmed these inverse associations but showed similar estimates for the PDI and hPDI, which may be attributed to a lower relevance of certain “less healthy plant foods” for risk of T2D in our study population. For example, potatoes are more likely to be consumed as French fries and eaten in much greater amounts in US populations than in Asian populations, and earlier analyses of these US cohorts suggested that consumption of French fries was associated with a substantially higher risk of T2D (36).
Strengths of our study included the large sample size, the study’s population-based and prospective nature, and the use of a detailed food frequency questionnaire developed for local dietary habits. Our study also had several limitations. First, measurement error in dietary patterns resulting from using only a single dietary assessment at baseline may have attenuated the true associations between the dietary pattern scores and risk of T2D. Second, the trans- fat component in the original AHEI-2010 was omitted and total dairy food consumption was used as an alternative measure of low-fat dairy food consumption for the DASH index. In our study population, however, biomarker analyses indicated extremely low intake of trans- fat (35) as compared with the intake in the US population (37), and consumption of low-fat dairy foods was uncommon during the enrollment period. Third, while identifying incident diabetes according to participants’ self-reports may have resulted in a small proportion of patients being classified as noncases, validation studies in our study population indicated high accuracy of diabetes reporting (17). Fourth, the problem of residual confounding is of concern for any observational study. However, we carefully adjusted for various potential confounders, and the minimally and maximally adjusted estimates were similar in our study, which reduced the likelihood of residual confounding as a full explanation for our results. Finally, our findings may be most generalizable to East Asian populations and should be confirmed for other ethnic groups.
In summary, our findings showed that adherence to several predetermined dietary patterns inversely associated with risk of T2D in Western populations was similarly associated with lower risk of T2D in an Asian population.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Nutrition and Food Hygiene, School of Public Health, Soochow University, Suzhou, China (Guo-Chong Chen, Li-Qiang Qin); Saw Swee Hock School of Public Health, National University of Singapore and National University Health System, Singapore, Republic of Singapore (Guo-Chong Chen, Woon-Puay Koh, Nithya Neelakantan, Rob M. van Dam); Health Services and Systems Research, Duke-NUS Medical School, Singapore, Republic of Singapore (Woon-Puay Koh); Division of Cancer Control and Population Sciences, UPMC Hillman Cancer Center, University of Pittsburgh, Pittsburgh, Pennsylvania (Jian-Min Yuan); Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania (Jian-Min Yuan); Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore, Republic of Singapore (Rob M. van Dam); and Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Rob M. van Dam).
This work was supported by the US National Institutes of Health (grants R01 CA144034 and UM1 CA182876). W.-P.K. was supported by the National Medical Research Council, Singapore (grant NMRC/CSA/0055/2013). G.-C.C. received an award from the China Scholarship Council.
We express our gratitude to Dr. Siew-Hong Low for overseeing the fieldwork, Dr. Renwei Wang for the development and maintenance of the SCHS database, and Dr. Mimi C. Yu as the founding Principal Investigator of the SCHS.
Conflict of interest: none declared.
Abbreviations
- AHEI
Alternate Healthy Eating Index
- aMED
alternate Mediterranean diet
- BMI
body mass index
- CI
confidence interval
- DASH
Dietary Approaches to Stop Hypertension
- hPDI
healthful plant-based diet index
- PDI
plant-based diet index
- SCHS
Singapore Chinese Health Study
- T2D
type 2 diabetes
REFERENCES
- 1. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 2. Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, et al. Diet and overall survival in elderly people. BMJ. 1995;311(7018):1457–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fung TT, Hu FB, McCullough ML, et al. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J Nutr. 2006;136(2):466–472. [DOI] [PubMed] [Google Scholar]
- 4. McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76(6):1261–1271. [DOI] [PubMed] [Google Scholar]
- 5. Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fung TT, Chiuve SE, McCullough ML, et al. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–720. [DOI] [PubMed] [Google Scholar]
- 7. Martinez-Gonzalez MA, Bes-Rastrollo M. Dietary patterns, Mediterranean diet, and cardiovascular disease. Curr Opin Lipidol. 2014;25(1):20–26. [DOI] [PubMed] [Google Scholar]
- 8. Medina-Remón A, Kirwan R, Lamuela-Raventós RM, et al. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and mental health problems. Crit Rev Food Sci Nutr. 2018;58(2):262–296. [DOI] [PubMed] [Google Scholar]
- 9. Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2015;115(5):780–800.e5. [DOI] [PubMed] [Google Scholar]
- 10. Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr. 2017;147(6):1174–1182. [DOI] [PubMed] [Google Scholar]
- 11. Jacobs S, Harmon BE, Boushey CJ, et al. A priori-defined diet quality indexes and risk of type 2 diabetes: the Multiethnic Cohort. Diabetologia. 2015;58(1):98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liese AD, Nichols M, Sun X, et al. Adherence to the DASH diet is inversely associated with incidence of type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2009;32(8):1434–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qi L, Hu FB, Hu G. Genes, environment, and interactions in prevention of type 2 diabetes: a focus on physical activity and lifestyle changes. Curr Mol Med. 2008;8(6):519–532. [DOI] [PubMed] [Google Scholar]
- 14. Satija A, Bhupathiraju SN, Rimm EB, et al. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13(6):e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hankin JH, Stram DO, Arakawa K, et al. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001;39(2):187–195. [DOI] [PubMed] [Google Scholar]
- 16. Seah JY, Gay GM, Su J, et al. Consumption of red meat, but not cooking oils high in polyunsaturated fat, is associated with higher arachidonic acid status in Singapore Chinese adults. Nutrients. 2017;9(2):pii:E101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Odegaard AO, Koh WP, Arakawa K, et al. Soft drink and juice consumption and risk of physician-diagnosed incident type 2 diabetes: the Singapore Chinese Health Study. Am J Epidemiol. 2010;171(6):701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 suppl):1220S–1228S. [DOI] [PubMed] [Google Scholar]
- 19. Yu D, Zheng W, Cai H, et al. Long-term diet quality and risk of type 2 diabetes among urban Chinese adults. Diabetes Care. 2018;41(4):723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lv J, Yu C, Guo Y, et al. Adherence to a healthy lifestyle and the risk of type 2 diabetes in Chinese adults. Int J Epidemiol. 2017;46(5):1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schwingshackl L, Hoffmann G, Lampousi AM, et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ley SH, Hamdy O, Mohan V, et al. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383(9933):1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. AlEssa HB, Malik VS, Yuan C, et al. Dietary patterns and cardiometabolic and endocrine plasma biomarkers in US women. Am J Clin Nutr. 2017;105(2):432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacobs S, Boushey CJ, Franke AA, et al. A priori-defined diet quality indices, biomarkers and risk for type 2 diabetes in five ethnic groups: the Multiethnic Cohort. Br J Nutr. 2017;118(4):312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kastorini CM, Milionis HJ, Esposito K, et al. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57(11):1299–1313. [DOI] [PubMed] [Google Scholar]
- 26. Shirani F, Salehi-Abargouei A, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta-analysis on controlled clinical trials. Nutrition. 2013;29(7-8):939–947. [DOI] [PubMed] [Google Scholar]
- 27. Cespedes EM, Hu FB, Tinker L, et al. Multiple healthful dietary patterns and type 2 diabetes in the Women’s Health Initiative. Am J Epidemiol. 2016;183(7):622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Koning L, Chiuve SE, Fung TT, et al. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care. 2011;34(5):1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. InterAct Consortium, Romaguera D, Guevara M, et al. Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study: the InterAct Project. Diabetes Care. 2011;34(9):1913–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rossi M, Turati F, Lagiou P, et al. Mediterranean diet and glycaemic load in relation to incidence of type 2 diabetes: results from the Greek cohort of the population-based European Prospective Investigation into Cancer and Nutrition (EPIC). Diabetologia. 2013;56(11):2405–2413. [DOI] [PubMed] [Google Scholar]
- 31. Martinez-González MA, de la Fuente-Arrillaga C, Nunez-Cordoba JM, et al. Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ. 2008;336(7657):1348–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abiemo EE, Alonso A, Nettleton JA, et al. Relationships of the Mediterranean dietary pattern with insulin resistance and diabetes incidence in the Multi-Ethnic Study of Atherosclerosis (MESA). Br J Nutr. 2013;109(8):1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salas-Salvadó J, Bulló M, Babio N, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34(1):14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Otto MC, Padhye NS, Bertoni AG, et al. Everything in moderation—dietary diversity and quality, central obesity and risk of diabetes. PLoS One. 2015;10(10):e0141341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neelakantan N, Naidoo N, Koh WP, et al. The Alternative Healthy Eating Index is associated with a lower risk of fatal and nonfatal acute myocardial infarction in a Chinese adult population. J Nutr. 2016;146(7):1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muraki I, Rimm EB, Willett WC, et al. Potato consumption and risk of type 2 diabetes: results from three prospective cohort studies. Diabetes Care. 2016;39(3):376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Allison DB, Egan SK, Barraj LM, et al. Estimated intakes of trans fatty and other fatty acids in the US population. J Am Diet Assoc. 1999;99(2):166–174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.