Abstract
The prominent antibacterial and quorum sensing (QS) inhibition activity of aromatic plants can be used as a novel intervention strategy for attenuating bacterial pathogenicity. In the present work, a total of 29 chemical components were identified in the essential oil (EO) of Melaleuca bracteata leaves by gas chromatography-mass spectrometry (GC-MS). The principal component was methyleugenol, followed by methyl trans-cinnamate, with relative contents of 90.46% and 4.25%, respectively. Meanwhile, the antibacterial activity and the QS inhibitory activity of M. bracteata EO were first evaluated here. Antibacterial activity assay and MIC detection against seven pathogens (Dickeya dadantii Onc5, Staphylococcus aureus ATCC25933, Pseudomonas spp., Escherichia coli ATCC25922, Serratia marcescens MG1, Pseudomonas aeruginosa PAO1 and Chromobacterium violaceum ATCC31532) demonstrated that S. aureus ATCC25933 and S. marcescens MG1 had the higher sensitivity to M. bracteata EO, while P. aeruginosa PAO1 displayed the strongest resistance to M. bracteata EO. An anti-QS (anti-quorum sensing) assay revealed that at sub-minimal inhibitory concentrations (sub-MICs), M. bracteata EO strongly interfered with the phenotype, including violacein production, biofilm biomass, and swarming motility, as well as N-hexanoyl-L-homoserine lactone (C6-HSL) production (i.e., a signaling molecule in C. violaceum ATCC31532) of C. violaceum. Detection of C6-HSL indicated that M. bracteata EO was capable of not only inhibiting C6-HSL production in C. violaceum, but also degrading the C6-HSL. Importantly, changes of exogenous C6-HSL production in C. violaceum CV026 revealed a possible interaction between M. bracteata EO and a regulatory protein (cviR). Additionally, quantitative real-time polymerase chain reaction (RT-qPCR) analysis demonstrated that the expression of QS-related genes (cviI, cviR, vioABCDE, hmsNR, lasA-B, pilE1, pilE3, and hcnB) was significantly suppressed. Conclusively, these results indicated that M. bracteata EO can act as a potential antibacterial agent and QS inhibitor (QSI) against pathogens, preventing and controlling bacterial contamination.
Keywords: Melaleuca bracteata, essential oil, gas chromatography-mass spectrometry (GC-MS), chemical components, antibacterial activity, pathogens, sub-minimal inhibitory concentrations (sub-MICs), quorum sensing (QS), bacterial contamination
1. Introduction
It is well documented that the large-scale use of chemical antimicrobials and antibiotics causes resistance in pathogenic microorganisms. Bacteria rapidly mutate and adapt in response to new hostile environments [1]. Therefore, it is not a wise solution that the use of these antimicrobials be kept to a minimum or until pathogens are halted. Recently, natural antimicrobials from aromatic plants have attracted attention as alternatives to chemical ones [2]. Essential oils (EOs), the secondary metabolites of aromatic plants, are used to prevent bacterial infections due to their prominent antibacterial activity and quorum sensing (QS) inhibition. They are also safe and nontoxic compounds, meeting the requirements for green antibacterial agents [3].
QS is a cell-density-dependent mechanism used by bacteria to regulate gene expression [4]. Bacteria release autoinducers (AIs) or signals that realize cell-to-cell communication, and AIs have been identified as oligopeptides and N-acyl-homoserine lactones (AHLs) in Gram-positive and Gram-negative bacteria, respectively [5]. Numerous studies have shown that bacteria rely on QS systems to orchestrate the synchronous secretion of virulence factors (VFs) and biofilm formation [6]. Potential QS inhibitors (QSIs) can reduce the bacterial pathogenicity and target bacterial QS systems rather than killing cells, which can reduce or slow the selective pressure for developing resistance [7,8]. Chromobacterium violaceum ATCC31532, a well-documented Gram-negative bacterium, has been used widely in screening QS inhibitors and in researching the QS inhibitory mechanism, due to its visible violacein [9] and clear QS regulatory system. N-hexanoyl-l-homoserine lactone (C6-HSL), which is modulated by the cviI gene, binds to the transcriptional regulator to regulate biofilm formation, swarming movement, and the secretion of virulence factors such as violacein and exopolysaccharide (EPS) [10]. It was previously found that sub-minimal inhibitory concentration (sub-MIC) EO levels of green cardamom, rose, clove, and chamomile are capable of blocking the network in C. violaceum [11,12]. In this light, plant EOs are expected to be emerging QSIs to attenuate the virulence of pathogens and control bacterial infections and drug resistance.
Melaleuca is a genus of plants in the Myrtle family Myrtaceae, mainly in Australia, and several species have been introduced and cultivated in China [13]. Species from this genus are known to be good sources of antibacterial agents and medicinal materials. For example, “tea tree oil” derived from M. alternifolia is used in food processing to extend product shelf life [14]. Melaleuca bracteata is popularly exploited as an ornamental plant and is well known for its aromatic properties, as well as its vast medicinal properties. It is used to treat heart attack, stroke, infected wounds, skin disorders, and fungal infection. Furthermore, M. bracteata is also used to aid in stimulating glandular secretions and to reduce congestion in the veins, and its leaves constitute a component of an anti-HIV concoction. In addition, the stem bark extract of M. bracteata possesses antisecretory and antiulcerogenic activities [15]. The antibacterial and antioxidant activity of M. bracteata EO has recently been reported [16,17], but its anti-QS ability has never been described. Thus, we tested its antibacterial ability against pathogenic bacteria and anti-QS activity against C. violaceum ATCC31532 in this work, providing a theoretical basis for the development of M. bracteata EO as an antibacterial agent and QS inhibitor to prevent and control bacterial contamination.
2. Results
2.1. Analysis of the Components in M. bracteata EO by GC-MS
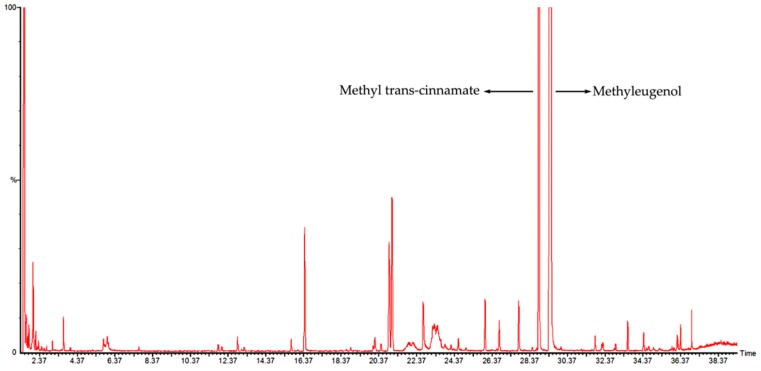
A total ion flow chromatogram of the M. bracteata EO analyzed by GC-MS is shown in Figure 1. The correlations of the peak area normalization method with the mass spectrometry database were determined to qualitatively and quantitatively analyze the components of the EO. Table 1 shows that 29 components were identified from the M. bracteata EO, accounting for 96.49% of the total contents, among which methyleugenol displayed the largest proportion, up to 90.46%, followed by methyl trans-cinnamate (relative content of 4.25%), and the relative content of other components was less than 1%.
Figure 1.
The GC-MS total ion chromatogram of Melaleuca bracteata essential oil (EO).
Table 1.
Chemical composition of volatile compounds in the M. bracteata EO.
| NO. | Compounds Name | Molecular Formula | Molecular Weight | Relative Content | Retention Time/min | Retention Index |
|---|---|---|---|---|---|---|
| 1 | Methyleugenol | C11H14O2 | 178.23 | 90.46% | 29.472 | 1399 |
| 2 | Methyl trans-cinnamate | C10H10O2 | 162.1852 | 4.25% | 28.864 | 1382 |
| 3 | Estragole | C10H12O | 148.2 | 0.32% | 21.064 | 1194 |
| 4 | alpha-Terpineol | C10H18O | 154.25 | 0.23% | 20.905 | 1190 |
| 5 | 3,7-Dimethyl-1,6-octadien-3-yl acetate 3,7 2-aminobenzoate | C17H23NO2 | 273.37 | 0.22% | 16.42 | 1098 |
| 6 | 2,7-Dimethyl-2,6-octadien-1-ol | C10H18O | 154.2493 | 0.18% | 23.219 | 1240 |
| 7 | Citronellol | C10H20O | 156.27 | 0.14% | 22.719 | 1229 |
| 8 | 2,2-Dimethoxybutane | C6H14O2 | 118.17 | 0.06% | 3.626 | - |
| 9 | 3-Hexen-1-ol | C6H12O | 100.16 | 0.05% | 5.735 | 838 |
| 10 | Z-Methyl geranate | C11H18O2 | 182.26 | 0.05% | 26.75 | 1321 |
| 11 | Myrcene | C10H16 | 136.23 | 0.05% | 23.411 | 1244 |
| 12 | Elemicin | C12H16O3 | 208.25 | 0.04% | 33.566 | 1544 |
| 13 | Citral | C10H16O | 152.23 | 0.04% | 24.591 | 1269 |
| 14 | Terpinen-4-ol | C10H18O | 154.25 | 0.03% | 20.159 | 1175 |
| 15 | Methyl propionate | C4H8O2 | 88.11 | 0.03% | 2.154 | - |
| 16 | Espatulenol | C15H24O | 220.3505 | 0.03% | 34.417 | 1575 |
| 17 | (3aS,3bR,4S,7R,7aR)-7-methyl-3 | C15H24 | 204.3511 | 0.03% | 31.836 | 1481 |
| 18 | Methyl 3,4,5-trimethoxybenzoate | C11H14O5 | 226.23 | 0.03% | 36.955 | 1718 |
| 19 | 4-epi-cubedol | C15H26O | 222 | 0.03% | 36.201 | 1664 |
| 20 | epi-a-Cadinol | C15H26O | 222.3663 | 0.03% | 36.368 | 1673 |
| 21 | (R)-Lavandulyl acetate | C12H20O2 | 196 | 0.02% | 21.881 | 1211 |
| 22 | 1,3,8-p-Menthatriene | C10H14 | 134.2182 | 0.02% | 12.847 | 1100 |
| 23 | 2-Carene(7CI,8CI) | C10H16 | 136.234 | 0.02% | 15.707 | 1083 |
| 24 | Methyl butyrate | C5H10O2 | 102.13 | 0.02% | 3.038 | - |
| 25 | Decane | C10H22 | 142.28 | 0.02% | 11.826 | 999 |
| 26 | Dispiro[2.0.2.5]undecane, 8-methylene | C12H18 | 162.27132 | 0.02% | 23.878 | 1254 |
| 27 | Copaene(6CI) | C15H24 | 204.3511 | 0.02% | 32.245 | 1495 |
| 28 | 2-(4-Methylphenyl)propan-2-ol | C10H14O | 150.22 | 0.02% | 20.484 | 1182 |
| 29 | trans-α-Bergamotene | C15H24 | 204.35106 | 0.02% | 23.382 | 1243 |
| Total | 96.49% |
2.2. Determination of the Antimicrobial Activity and MIC of M. bracteata EO
The antimicrobial activities of the M. bracteata EO were tested against the common pathogens D. dadantii Onc5, S. aureus ATCC25933, Pseudomonas spp., E. coli ATCC25922, S. marcescens MG1, P. aeruginosa PAO1, and C. violaceum ATCC31532 using the plate perforation method. M. bracteata EO exhibited an effective concentration-dependent inhibitory effect against all tested bacteria. M. bracteata EO showed stronger inhibition against S. aureus ATCC25933 and S. marcescens MG1 with higher inhibition ability (15.28 ± 1.083 mm and 14.11 ± 0.789 mm, respectively) than other test bacteria at the concentration of 80‰. M. bracteata EO exhibited antibacterial activity against D. dadantii Onc5, P. aeruginosa PAO1, E. coli ATCC25922, Pseudomonas spp., and C. violaceum ATCC31532 with inhibition zone diameters of 11.89 ± 0.246 mm, 10.47 ± 0.186 mm, 11.38 ± 0.286 mm, 13.42 ± 0.715 mm, and 11.55 ± 0.34 mm, respectively (Table 2).
Table 2.
Antibacterial activities of M. bracteata EO.
| Bacterial Strains | Concentration/Antimicrobial Diameters (mm) | MIC | |||||
|---|---|---|---|---|---|---|---|
| 80‰ | 40‰ | 20‰ | 10‰ | Methanol | Kanamycin (250 µg/mL) | ||
| Dickeya dadantii Onc5 | 11.89 ± 0.246 ah | 10.70 ± 0.291 abg | 10.03 ± 0.303 bcfgh | 9.00 ± 0.518 cfg | 6.00 ± 0.00 | 20.21 ± 0.11 | 10‰ |
| Staphylococcus aureus ATCC25933 | 15.28 ± 1.083 ae | 13.05 ± 0.323 be | 10.98 ± 0.520 cef | 9.21 ± 0.078 def | 6.00 ± 0.00 | 26.01 ± 0.131 | 2.5‰ |
| Escherichia coli ATCC25922 | 11.38 ± 0.286 ai | 10.15 ± 0.451 bgh | 9.325 ± 0.343 bh | 8.33 ± 0.354 cgh | 6.00 ± 0.00 | 18.78 ± 1.032 | 10‰ |
| Pseudomonas aeruginosa PAO1 | 10.47 ± 0.186 aj | 9.82 ± 0.279 bh | 9.45 ± 0.236 ch | 8.15 ± 0.193 dh | 6.00 ± 0.00 | 17.23 ± 0.187 | 20‰ |
| Serratia marcescens MG1 | 14.11 ± 0.789 af | 11.81 ± 0.363 bf | 10.57 ± 0.191 cefg | 9.87 ± 0.484 de | 6.00 ± 0.00 | 25.08 ± 1.31 | 2.5‰ |
| Pseudomonas spp. | 13.42 ± 0.715 ag | 12.49 ± 0.308 bef | 11.50 ± 0.236 ce | 9.69 ± 0.315 def | 6.00 ± 0.00 | 23.17 ± 0.33 | 5‰ |
| Chromobacterium violaceum ATCC31532 | 11.55 ± 0.34 ai | 10.86 ± 0.49 ag | 9.80 ± 0.27 bgh | 8.03 ± 0.26 ch | 6.00 ± 0.00 | 21.61 ± 1.029 | 10‰ |
Note: Different letters (a–d) within the same row represent significant differences at the different concentrations (p < 0.05). Different letters (e–j) within the same line represent significant differences at the different concentrations (p < 0.05).
The MIC of M. bracteata EO was assessed for all test pathogens using the double dilution method with concentrations varying from 80‰ to 0.625‰. The MIC of M. bracteata EO was 2.5‰ for S. aureus ATCC25933 and S. marcescens MG1, 5‰ for Pseudomonas spp., 10‰ for D. dadantii Onc5, E. coli ATCC25922, and C. violaceum ATCC31532, and 20‰ for P. aeruginosa PAO1 (Table 2).
Collectively, the results indicated that S. aureus ATCC25933 and S. marcescens MG1 had higher sensitivity to M. bracteata EO, while P. aeruginosa PAO1 showed the strongest resistance to M. bracteata EO.
2.3. Quorum Sensing Inhibition (QSI) assays of M. bracteata EO
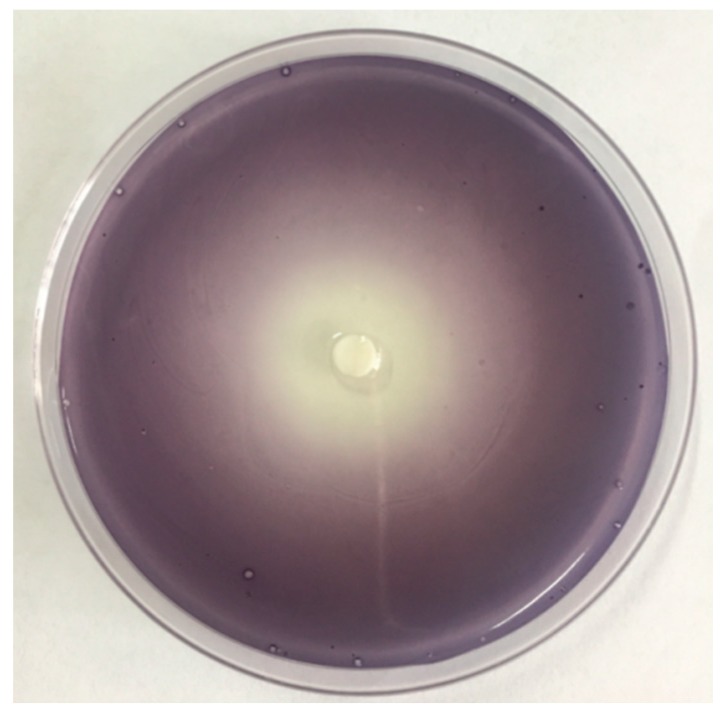
The zone of non-purple pigment on agar plates and the extent of the inhibition of purple pigment in C. violaceum CV026 by M. bracteata EO were observed as shown in Figure 2, indicating that M. bracteata EO showed good inhibition for the QS-mediated violacein production of CV026. Hence, in the present study, M. bracteata EO at sub-MICs (5‰, 2.5‰, 1.25‰, and 0.625‰) was used for the further experiments.
Figure 2.
Quorum sensing inhibition (QSI) effect of M. bracteata EO on biosensor CV026.
2.4. Growth Curve
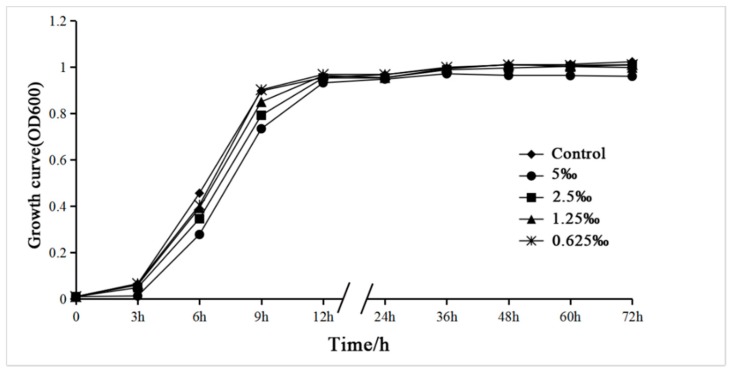
To confirm the non-antibacterial activity of M. bracteata EO at sub-MICs (5‰, 2.5‰, 1.25‰, and 0.625‰), the activities of C. violaceum treated with or without different concentrations of M. bracteata EO were determined for 0–72 h. The growth curve showed that C. violaceum treated with M. bracteata EO at sub-MICs began to enter the logarithmic phase later than the control. Nevertheless, the growth of C. violaceum did not differ between the control and treated groups in the stationary phase (>12 h) (Figure 3). These results revealed that M. bracteata EO was inefficient at inhibiting growth under the test conditions. We therefore assessed the specific effect of M. bracteata EO at sub-MICs (5‰, 2.5‰, 1.25‰, and 0.625‰) on QS in C. violaceum.
Figure 3.
Effect of M. bracteata EO on the growth of Chromobacterium violaceum. Growth curves of C. violaceum treated with varying concentrations of EO: 5‰, 2.5‰, 1.25‰, and 0.625‰ (the control had no M. bracteata EO).
2.5. Determination of Violacein
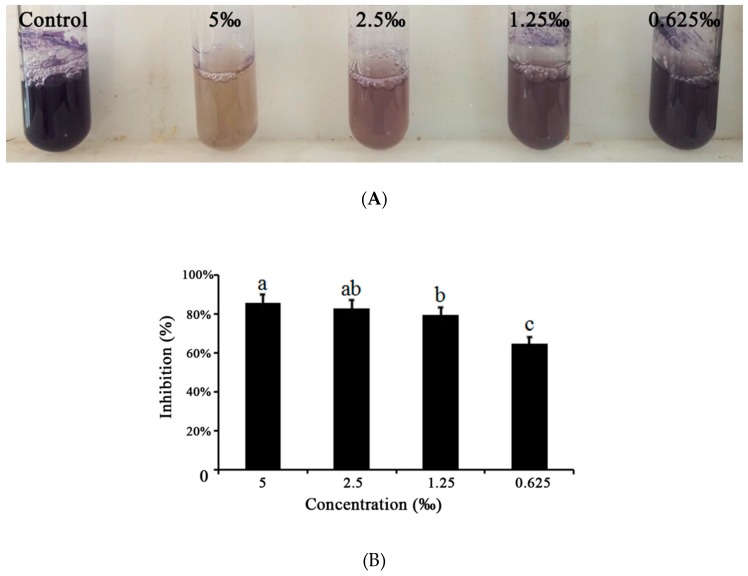
Violacein is the important metabolite of C. violaceum which is regulated by the QS system [18]. Therefore, we detected the effect of M. bracteata EO on the violacein in C. violaceum. In the quantitative assay, violacein inhibition reached a maximum of 85.47% in C. violaceum when treated with M. bracteata EO at 5‰ (the highest tested concentration) (Figure 4).
Figure 4.
(A) Effect of M. bracteata EO on violacein in C. violaceum. (B) Quantitative analysis of violacein inhibition in C. violaceum by EO (5‰, 2.5‰, 1.25‰, and 0.625‰). Mean values of triplicate independent experiments and SD are shown. Bars indicate standard errors and different letters (a–c) above the bars represent significant differences (p < 0.05).
2.6. Biofilm Determination
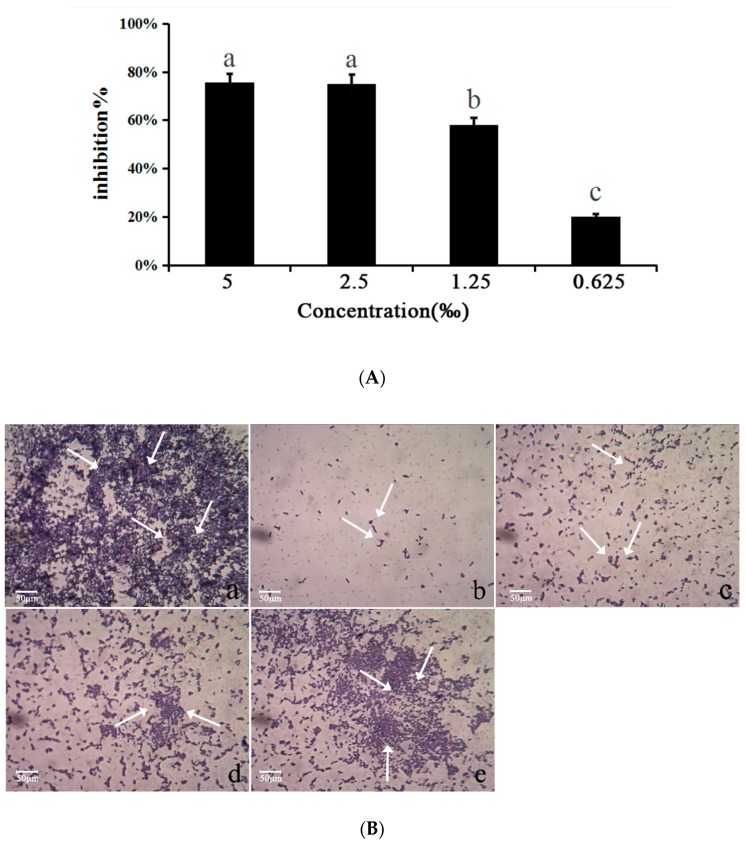
The quantitative biofilm assay demonstrated that treatment with M. bracteata EO (5‰, 2.5‰, 1.25‰, and 0.625‰) inhibited the biofilm biomass of C. violaceum in a concentration-dependent manner, and the inhibition of biofilm biomass were 75.56%, 75.03%, 58.16%, and 20.2%, respectively (Figure 5A). In addition, M. bracteata EO was found to be very effective in inhibiting biofilm formation of C. violaceum based on observations by light microscope (Figure 5B).
Figure 5.
M. bracteata EO reduced biofilm formation of C. violaceum. (A) Quantitative assessment of biofilm biomass inhibition. Mean values of eight independent experiments and SD are shown. Bars indicate standard errors and different letters (a–c) above the bars represent significant differences (p < 0.05). (B) Light microscope images (a–e) under a light microscope at a magnification of 40×. a: untreated; b: 5‰; c: 2.5‰; d: 1.25‰; e: 0.625‰. The arrows indicate the dyed biofilm.
2.7. Swarming Motility Assay
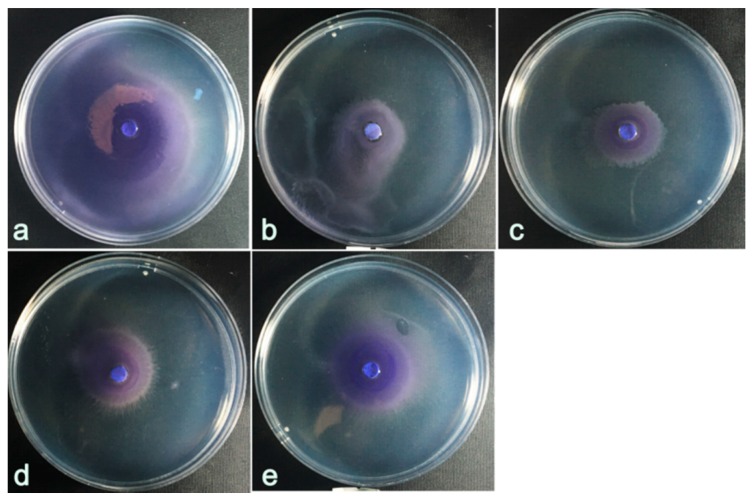
Swarming migration plays an important role in QS-regulated biofilm formation in pathogens [19,20]. An effort was made to examine the anti-QS potential of M. bracteata EO against swarming motility in C. violaceum. The results showed that M. bracteata EO disturbed the swarming behavior of C. violaceum, and the maximum inhibition was recorded at the highest concentration (5‰) (Figure 6).
Figure 6.
Effect of M. bracteata EO at different concentrations (5‰, 2.5‰, 1.25‰ and 0.625‰) on the swarming motility of C. violaceum. a: control, untreated with EO. b–e: treated with EO concentrations of 5‰, 2.5‰, 1.25‰, and 0.625‰.
2.8. Detection of the Production of C6-HSL Signal Molecules
The C6-HSL production was measured through a well-diffusion assay using CVO26 as the monitor strain, and the concentration of C6-HSL extracts was estimated by measuring the diameter of the violacein induced zone. The extracts from C. violaceum treated with or without M. bracteata EO (5‰, 2.5‰, 1.25‰, and 0.625‰) suggested that M. bracteata EO is able to reduce the C6-HSL production in a concentration-dependent manner (Figure S1).
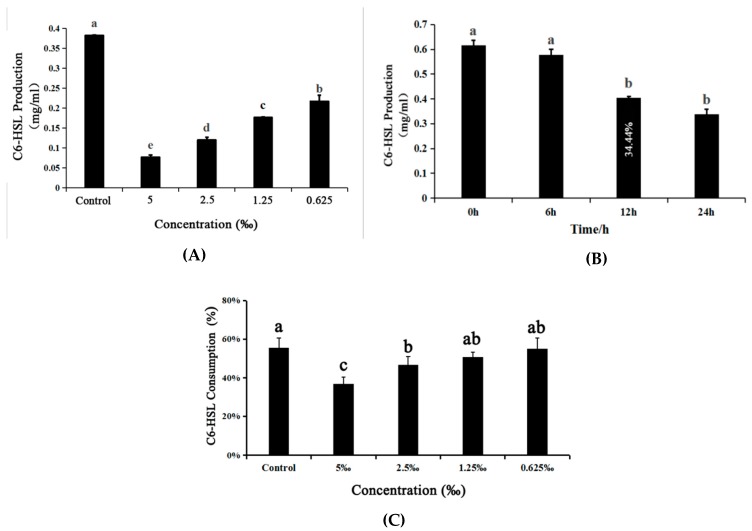
To verify the biosensor screening results, the C6-HSL extracts were analyzed quantitatively by GC technology. According to the retention time and the standard curve, the concentration of C6-HSL in the extracted samples was calculated (Figure S2). In agreement with observations in the CV026 biosensor, the concentration of C6-HSL in the treated groups decreased significantly compared with the control. In addition, the concentrations in the control group and the treatment group (5‰, 2.5‰, 1.25‰, and 0.625‰ M. bracteata EO) were 0.38, 0.08, 0.12, 0.18, and 0.22 mg/mL, respectively (Figure 7A and Figure S3). Our data confirmed that M. bracteata EO was able to repress C6-HSL production in C. violaceum, which resulted in the attenuation of bacterial virulence.
Figure 7.
(A) Effect of M. bracteata EO at different concentrations (5‰, 2.5‰, 1.25‰ and 0.625‰) on C6-HSL of C. violaceum. (B) Effect of M. bracteata EO (5‰) on C6-HSL treated for 0, 6, 12, and 24 h. The degradation of N-hexanoyl-l-homoserine lactone (C6-HSL) was 34.44% at 12 h. (C) Effect of M. bracteata EO at different concentrations (5‰, 2.5‰, 1.25‰, and 0.625‰) on C6-HSL of C. violaceum CV026. The results are the mean (n = 3) ± standard deviation. Bars indicate standard errors and different letters (a–c) above the bars represent significant differences (p < 0.05).
The capacity of M. bracteata EO to target C6-HSL directly was determined by adding exogenous C6-HSL to LB broth supplemented with M. bracteata EO extracts at the highest tested concentration of 5‰. Compared to the control, the extract had no significant effect at 6 h, and then a significant drop in the C6-HSL content was observed with the increase in incubation time (at 12 h and 24 h) (Figure 7B and Figure S4). These data suggest that M. bracteata EO can directly degrade C6-HSL.
In addition, the C6-HSL extracts from C. violaceum CV026 treated with M. bracteata EO (5‰, 2.5‰, 1.25‰, and 0.625‰) showed that the consumption of exogenous C6-HSL with M. bracteata EO treatment was lower than that of the control. Among the exogenous C6-HSL with the lowest consumption was at the highest tested concentration of 5‰, independent of a direct effect on growth (Figure 7C and Figure S5). The results indicate that M. bracteata EO may be able to interact with the cviR protein.
Collectively, we speculated that M. bracteata EO not only degraded the C6-HSL directly and inhibited C6-HSL production, but also interacted with the cviR protein.
2.9. M. bracteata EO Reduced the Expression of the QS-Related Genes
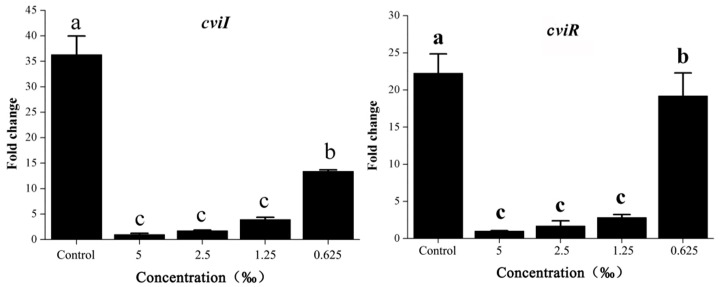
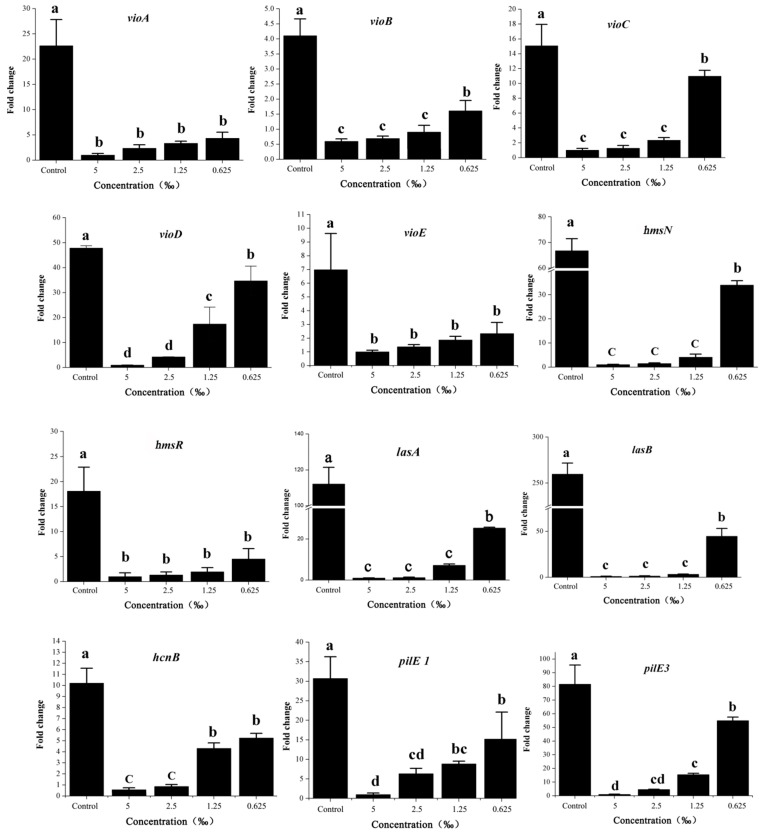
cviI/cviR are important regulatory factors in the QS system of C. violaceum [18]. Accordingly, we assessed the effects of M. bracteata EO on the main regulatory factors cviI and cviR in C. violaceum ATCC 31532 by RT-qPCR. As expected, the expression of cviI and cviR revealed a dose-dependent downregulation in response to M. bracteata EO (5‰, 2.5‰, 1.25‰, and 0.625‰) in comparison with the control (Figure 8). Furthermore, we detected the effect of methyleugenol (ME) on the expression of important virulence factors, including violacein production (via vioA–E), biofilm formation (via hmsNRHF), and elastase production (via lasA and lasB) [21]. These genes are downstream of the QS cascade and controlled by the QS system. In the presence of M. bracteata EO, the expression of vioA, vioB, vioC, vioD, vioE, hmsN, hmsR, lasA, and lasB were clearly suppressed relative to the control group (Figure 9). However, the expression of hmsH and hmsF was not affected by M. bracteata EO (Figure S6).
Figure 8.
Effect of M. bracteata EO on the expression of cviI and cviR. Expression of the house-keeping gene rpoD was used as the internal control for each sample. Bars indicate standard errors and different letters (a–c) above the bars represent significant differences (p < 0.05).
Figure 9.
Effect of M. bracteata EO on the expression of genes regulated by LuxI–LuxR system. vioA, vioB, vioC, vioD, vioE, hmsN, hmsR, lasA, lasB, hcnB, pilE1, and pilE3 were detected in response to M. bracteata EO treatment. Expression of the house-keeping gene rpoD was used as the internal control for each sample. The M. bracteata EO treatment concentrations were 5‰, 2.5‰, 1.25‰, and 0.625‰. Control was untreated. Bars indicate standard errors, and different letters (a–c) above the bars represent significant differences (p < 0.05).
Studies have revealed that pilus (pilE1–3) and cyanide production (hcnA–C) were associated with the QS system in C. violaceum [22]. Considering our observation that the cviI/cviR system was significantly suppressed in response to EO, we decided to monitor the change in pilE1–3 and hcnA–C. Although other genes were significantly repressed by M. bracteata EO, hcnA, hcnC, and pilE2 were not (Figure 9 and Figure S6). It is possible that these genes were not directly or independently controlled by the QS system. Collectively, our data suggest that M. bracteata EO can be used to inhibit the expression of key virulence genes of C. violaceum, independent of a direct effect on growth rate.
3. Discussion
A variety of aromatic plants have been reported to have antibacterial activities and anti-quorum sensing activities [7,23]. M. bracteata, as an excellent medicinal aromatic plant with colorful leaves, has great development and utilization value. In this paper, we evaluated the antimicrobial activity of M. bracteata EO against seven pathogens, and its potential anti-QS activity was detected for the first time with C. violaceum ATCC31532.
M. bracteata EO was capable of inhibiting pathogens, including: D. dadantii Onc5, S. aureus ATCC25933, Pseudomonas spp., E. coli ATCC25922, S. marcescens MG1, P. aeruginosa PAO1, and C. violaceum ATCC31532. Among them, D. dadantii Onc5 and Pseudomonas spp. are pathogenic bacteria that cause plant soft rot. The results showed that M. bracteata EO had stronger inhibition against S. aureus ATCC25933 and S. marcescens MG1, and P. aeruginosa PAO1 was generally more resistant than the other organisms tested in this paper, which were well-matched with the MIC results. A study by Siddique et al. [16] showed that M. bracteata EO exhibited antimicrobial activities against pathogens including Gram-positive (Bacillus subtilis subsp spizizenii, Staphylococcus aureus) and Gram-negative bacteria (Enterobacter aerogenes, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, and Salmonella enterica). Collectively, M. bracteata EO has the potential to act as a natural antibacterial agent due to its outstanding and broad-spectrum antimicrobial activity. Some antimicrobial agents with broad-spectrum antimicrobial activity have QS inhibitory activities under sub-MICs [23,24]. Thus, we are very interested in whether M. bracteata EO is capable of suppressing bacterial pathogenicity based on interfering with QS systems instead of having a direct effect on growth.
In the present study, we have systematically studied the inhibitory effect of M. bracteata EO (sub-MICs) on the QS system of C. violaceum from the QS phenotype and at the molecular level. We detected the QS inhibitory potential of M. bracteata EO based on its ability to inhibit the production of AHL-dependent virulence factors such as violacein in C. violaceum. It has previously been confirmed that the compound, which has the ability to inhibit violacein production without influencing the growth of C. violaceum, is considered to be a promising QS inhibitor [25,26]. Biofilm formation plays an important role in bacterial pathogenicity and decreases drug sensitivity. Thus, interfering with biofilm formation might be a preferable and convenient way to attenuate the virulence of disease pathogens as well as drug resistance [27,28]. A biofilm detection assay showed that M. bracteata EO not only reduced the biofilm biomass but also influenced the colony formation, as evidenced by the light microscope images in Figure 5B. The inhibition response of natural products as found in our study is also supported by the findings on EO of Cuminum cyminum [29] and Mentha piperita [30], which also influenced the biofilm biomass and formation of Pseudomonas aeruginosa, C. violaceum, and Aeromonas hydrophila. Swarming involves the differentiation of vegetative cells into hyper-flagellated swarm cells that undergo rapid and coordinated population migration across solid surfaces, and swimming and swarming behavior essentially determine the biofilm formation [20]. Thus, the inhibition of swarming motility by M. bracteata EO partially accounted for the reduction in the biofilm biomass and disruption of biofilm architecture in C. violaceum. We next employed RT-qPCR to evaluate the expression of QS-regulated genes (vioA-E, hmsNRHF) in C. violaceum. The RT-qPCR results did not correlate well with biofilm results. The expression of hmsHF did not decrease in response to M. bracteata EO in a concentration-dependent manner compared with that of control. The above data indicate that biofilm formation is a sophisticated process and that QS is a vital regulatory mechanism for biofilm formation, but not the only one.
According to the working model of QS regulation, the LuxR receptors are unstable, and rapidly degraded at low AI concentrations. As the cell density increases, the accumulated AI binds the LuxR-type receptor, leading to stabilization of the LuxR–AI complex, which subsequently binds DNA in promoters, driving genes regulated by QS [31]. Thus, QS quenching can be achieved either by interruption of AI signal generation, inhibition of AI signal dissemination, or inhibition of AHL signal reception [32]. The reduction in C6-HSL concentrations in C. violaceum culture treated with M. bracteata EO could be the result of either (1) inhibition of cell growth, (2) degradation of C6-HSL, or (3) inhibition of the C6-HSL production. With regard to the first hypothesis, it was observed that the growth tendencies of C. violaceum treated and untreated with M. bracteata EO were similar, without obvious differences after 12 h. The second hypothesis refers to the potential degradation of C6-HSL by M. bracteata EO. The quantification of C6-HSL in inoculated broth treated with M. bracteata EO (the highest tested concentration of 5‰) revealed that M. bracteata EO was able to directly degrade C6-HSL (Figure 7B). Regarding the third hypothesis, a dose-dependent reduction of C6-HSL production in C. violaceum showed that M. bracteata EO interfered with the accumulation of C6-HSL (Figure 7A, Figure S2). When it is taken into account that C6-HSL degradation was approximately 34.44% in the presence of 5‰ M. bracteata EO for 12 h (Figure 7B), the inhibition of QS by M. bracteata EO could attributed to its capacity to inhibit C6-HSL production.
Moreover, the antagonists targeting the LuxR receptors also have huge potential for anti-QS development. Chloro thiolactone (CTL) and chloro lactone (CL) were shown to be cviR antagonists that prevent cviR from binding the promoter DNA of regulated genes [33]. Interestingly, we found that the consumption of exogenous C6-HSL was lowest in CV026 upon treatment with M. bracteata EO (at the highest tested concentration of 5‰); we inferred that M. bracteata EO can interact with cviR. Collectively, the findings in this work strongly support that M. bracteata EO acts through the QS machinery to inhibit specific virulence determinants in C. violaceum. The possibility of QS suppression by M. bracteata EO consists mainly of interactions with cviR and/or cviI proteins, directly resulting in the suppression of C6-HSL production, and is partially related to the degradation of C6-HSL production.
This finding is consistent with previous studies [34] which indicated that methyleugenol (ME) was the major constituent of M. bracteata EO, with a relative content of up to 90.46%. A study by Packiavathy et al. showed that ME exhibited violacein inhibition without growth inhibition [29]. Consistent with the above findings, ME also exhibited QSI activity toward the biosensor CV026 in the present study (Figure S7). Thus, we inferred that ME may play a leading role in the suppression of the QS system by M. bracteata EO. However, more work is clearly required in order to ascertain the exact binding site and molecular mechanism of action.
4. Materials and Methods
4.1. Essential Oil, Bacterial Strains, Medium, and Growth Conditions
The leaves of M. bracteata were collected from Fujian Agriculture and Forestry University (Fujian, China). Essential oil (EO) was extracted by steam distillation and then stored at −20 °C.
The microorganisms researched in this study were Dickeya dadantii Onc5, Staphylococcus aureus ATCC25933, Pseudomonas spp. (isolated from Capsicum annuum L), Escherichia coli ATCC25922, Serratia marcescens MG1, Pseudomonas aeruginosa PAO1, Chromobacterium violaceum ATCC31532, and Chromobacterium violaceum CV026, which were stocked in our laboratory. All tested bacteria were incubated in LB broth and grown under conditions of 30 °C, 150 rpm, and 12 h. The swarming motility medium consisted of 1% tryptone, 0.5% NaCl, 0.5% agar, and 0.5% d-glucose. N-Hexanoyl-l-homoserine lactone (C6-HSL) was purchased from Sigma-Aldrich (Shanghai, China). n-Alkanes (C8–C20) standard solution was purchased from Fluka (Shanghai, China).
4.2. Determination of Components of M. bracteata EO by GC-MS
The M. bracteata EO was subjected to GC-MS analysis using a GC (Clarus®680) equipped with a mass-selective detector (SQ8T) in electronic ionization (EI) mode and Turbomass Ver 6.1.0 software (Perkin Elmer Company, MA, America). Sample injection was performed in split mode (20:1) into a DB-5MS capillary column (30 m × 25 mm × 0.25 µm). Helium was used as the carrier gas at 1 mL/min. The GC injector temperature was set at 250 °C. The oven temperature program was optimized to hold at 50 °C for 2 min), finally increasing by 50 °C/min up to 250 °C (maintained for 2 min). The transfer line temperature was adjusted to 250 °C. Mass spectrometry conditions were as follows: electron ionization source set to 70 eV, MS transmission line to 250 °C, and MS source to 230 °C. The mass spectrometer was run in full-scan mode (m/z 45–550). The essential oil production was analyzed by peak area.
The essential compounds were identified on the basis of a comparison of their retention index (RI) relative to n-alkanes (C8–C20), standard substance, as well as published data and EI mass spectra from the literature. The relative mass fraction of the M. bracteata EO was calculated using the peak area normalization method.
The formula for the retention index (RI) is shown in Equation (1) [35,36]:
| RI = 100Z + 100[RT(x) − RT(z)]/[RT(Z + 1) − RT(z)] | (1) |
where Z is the number of carbons© in the smaller alkane; RT(x) is the retention time of the unknown compound; RT(z) is the retention time of the smaller alkane; and RT(Z + 1) is the retention time of the larger alkane.
4.3. Determination of Antimicrobial Activity and MIC of M. bracteata EO
The test strains, D. dadantii Onc5, S. aureus ATCC25933, Pseudomonas spp., E. coli ATCC25922, S. marcescens MG1, P. aeruginosa PAO1, and C. violaceum, were cultured in LB broth at 150 rpm and 30 °C for 12 h. All of the tested strains were adjusted to a microbial suspension of 109 CFU/mL with distilled water. The M. bracteata EO was serially diluted to 80‰, 40‰, 20‰, and 10‰ with methanol. The plate perforation method was performed using the following procedure with some modifications [37]. Briefly, 1% test bacterial suspension (109 CFU/mL) was added to the heated LB medium (containing 2% agar) at a temperature of 50 °C. After blending, the mixture was quickly poured into the Petri dishes. Then, 6-mm holes were punched in the solidified LB medium in the Petri dishes. A 35-µL volume of M. bracteata EO at different concentrations (80‰, 40‰, 20‰, and 10‰) was added to the holes. The culture plates were incubated at 30 °C. The antimicrobial activity was determined by measuring the antimicrobial diameters. Methanol and kanamycin (250 µg/mL) solutions was used as the negative control and positive control, respectively.
Double dilution method was applied to test the minimum inhibitory concentrations (MICs) of M. bracteata EO. The assay was performed using 1.5-mL microcentrifuge tubes and consisted of a gradient of M. bracteata EO—80‰, 40‰, 20‰, 10‰, 5‰, 2.5‰, 1.25‰, and 0.625‰—with the same final volume (150 μL), and 150 μL of bacterial suspension (1% of tested bacteria (OD600, 0.9) were added to LB medium). After mixing, the tubes were incubated at 30 °C and 150 rpm for 24 h and the OD600 was measured. Each assay was performed in triplicate.
4.4. Quorum Sensing Inhibition Assays
The QSI assay was performed on agar plates employing the biosensor strain C. violaceum CV026, which produces a purple pigment only in response to added exogenous AHLs [38].
The quorum sensing inhibition assay was performed according to the procedure described by Zhang et al. [39], with some modifications. An overnight culture of 1% CV026 was spread on LB plates (20 mL), and then exogenous C6-HSL solution was added to the plates. Filter paper (6 mm in diameter) was then placed on the center of the plate. Next, 15 µL of the M. bracteata EO and methyleugenol (ME) was added to filter paper, and the plates were incubated for 24 h at 30 °C. QSI was assessed from the formation of a ring of inhibition, as a result of violacein production, around the filter paper.
4.5. Growth Curve Analysis
The 1% C. violaceum (OD600, 0.9) was incubated in a 250 mL Erlenmeyer flask containing 20 mL of LB broth supplemented with M. bracteata EO (sub-MICs, 6% v/v), and the culture was mixed at 30 °C with shaking at 150 rpm in a rotatory shaker. The growth of bacteria was determined by UV–Visible spectrophotometry (U-290, Hitachi Company, Tokyo, Japan ) at OD600 from 0 to 72 h.
4.6. Violacein Detection Assay
Overnight culture of 1% C. violaceum (OD600, 0.9) was added into a glass tube containing 5 mL LB broth supplemented with M. bracteata EO (sub-MICs, 6% v/v), and the liquid was mixed and incubated at 30 °C for 12 h. The specific methods were as follows: 1 mL of the cultured solution was centrifuged in a 1.5 mL centrifuge tube at 4 °C and 12,000× g for 20 min; the bacteria and violacein were collected, and 1 mL of dimethyl sulfoxide (DMSO) was added to the centrifuge tube. The mixed liquid was vortexed at room temperature and centrifuged again for 3 min to obtain a purple-colored solution, and the violacein inhibition was measured by UV–Visible spectrophotometry at OD595. Each assay was performed in triplicate.
4.7. Effect of Essential Oil on Biofilm Development
The effect of M. bracteata EO on biofilm was assessed by staining and quantifying the biofilm biomass using a microtiter dish with crystal violet (CV), as previously described [40]. The biofilm biomass was quantified by measuring the absorbance at 550 nm in a microplate reader and recording the absorbance of CV dye bound to the biofilm. Each assay was performed in eight replicates.
To determine the ability of M. bracteata EO to disrupt the biofilm, a biofilm disruption assay was performed by following the revised method described previously [27]. Briefly, the test pathogens treated with different concentrations of EO (sub-MICs) were developed in six-well plates with cover glasses 1 cm × 1cm for 12 h, and the biofilms were stained with crystal violet. Then, the biofilm was observed under a light microscope (Bimuyiqi Company, Shanghai, China).
4.8. Swarming Motility
An effort was made to examine the effects of M. bracteata EO on the swarming motility in C. violaceum. The specific method is described briefly as follows [27]. First, 5 μL of overnight bacterial cultured bacterium (OD600, 0.9) was placed on 6-mm filter paper at the center of a plate containing swarming motility medium supplemented with M. bracteata EO (sub-MICs, 6% v/v) and incubated. The ability of swarming motility was measured by the migration distance.
4.9. Extraction and Detection of AHL
The AHL production was obtained from bacterial culture supernatant using acidified ethyl acetate (0.1% glacial acetic acid in ethyl acetate) as previously described [41]. Cell-free supernatants were centrifugated at 12,000× g for 15 min and extracted with acidified ethyl acetate (0.1% glacial acetic acid in ethyl acetate) three times. The AHL extracts were concentrated by rotary evaporation. The AHL extracts were prepared for further assay.
The detection of AHL was determined by the CV026 biosensor and GC. The qualitative assay was performed following the methods of Joshi et al. [42] with some modifications. Briefly, agar plates were prepared by adding an overnight culture of the CV026 biosensor (1%) to the LB medium containing 0.4% agar supplemented with 100 µg/mL kanamycin. Then, 6-mm holes were punched into the agar, which were filled with the 50 µL AHL extracts. The test plates were incubated at 30 °C for 24 h. The AHL production was monitored by the size of diameter of the violacein. The experiments were replicated three times. For quantitative analysis, the C6-HSL was determined using a GC system (Acme 6100 GC, Korea YoungLin, Beijing, China) with a flame ionization detector (FID), both of which were controlled by a computer equipped with Autochro-2000 Chromatography Data System software (YoungLin, Korea). Sample (AHL extracts) injection was performed in split mode (20:1) into an HP-5MS capillary column (30 m × 25 mm × 0.25 µm). Hydrogen was used as the carrier gas at 1 mL/min. The GC injector temperature was set at 200 °C. The oven temperature program was optimized to hold at 100 °C for 1 min and then increased by 25 °C/min up to 280 °C. A standard curve was made with different concentrations of the C6-HSL standard and its corresponding peak areas. Then the C6-HSL concentration in C. violaceum was analyzed quantitatively.
4.10. Effect of M. bracteata EO on Signaling Molecules (C6-AHL)
The C. violaceum (1%, OD600, 0.9) was grown in 20 mL LB broth with or without EO (sub-MICs, 6% v/v) for 12 h at 30 °C and 150 rpm. Then, C6-HSL extracts were prepared for further assay by GC and CV026 biosensor.
In order to study the potential degradation of C6-HSL by M. bracteata EO, a known synthetic standard (C6-HSL) was added to 20 mL of LB broth untreated and treated with M. bracteata EO (sub-MICs, 6% v/v) and incubated for 0, 6, 12, and 24 h. The C6-HSL concentration was detected by GC.
The CV026 was grown in 20 mL of LB broth for 12 h with or without M. bracteata EO (sub-MICs, 6% v/v) supplemented with the exogenous C6-HSL. The C6-HSL extracts were prepared for further assay by GC.
4.11. Gene Expression Analysis
RT-qPCR was used to monitor the expression of QS genes of C. violaceum. The primers, which were used to amplify the cviI, cviR and other QS-regulated genes, are shown in Table S1. A total of 1% C. violaceum (OD600, 0.9) was used to incubate 20 mL of LB with or without a range of M. bracteata EO concentrations (sub-MICs, 6% v/v). Cultures were grown for 12 h, the cells were harvested by centrifugation (12,000× g, 2 min), and supernatants were discarded. Total RNA was extracted using an RNAprep Pure Cell/Bacteria Kit (Code No. DP430, TIANGEN, Beijing, China). The RNA was used for reverse-transcription using Transcript One-step gDNA Removal and cDNA Synthesis SuperMix (Transgen, Beijing, China). Quantitative RT-qPCR was performed using Real-time PCR Master Mix SYBR Green (Transgen, China) in a Bio-Rad C1000 Manager sequence detector system. The conditions were as follows: two steps of 30 s at 94 °C and 40 cycles of 94 °C for 5 s, 60 °C for 30 s. The calculated cycle threshold (CT) of each gene was normalized to the CT for rpoD amplified from the corresponding sample. The RT-qPCR was performed in a LightCycler 96. Fold changes in gene expression were calculated according the 2−ΔΔCT method.
4.12. Statistical Analysis
All experiments were performed at least in triplicate and all data were analyzed by SPSS 19.0 software and presented as the mean values. Differences with p < 0.05 were considered statistically significant.
5. Conclusions
A total of 29 components were identified in M. bracteata EO, accounting for 96.49% of the total contents, among which methyleugenol displayed the largest proportion (90.46%), followed by methyl cinnamate (4.25%), and the relative content of other components was less than 1%.
The M. bracteata EO demonstrated significant inhibitory effects against seven pathogens, including Dickeya dadantii Onc5, Staphylococcus aureus ATCC25933, Pseudomonas spp., Escherichia coli ATCC25922, Serratia marcescens MG1, Pseudomonas aeruginosa PAO1, and C. violaceum ATCC31532. S. aureus ATCC25933 and S. marcescens MG1 had the highest sensitivity to M. bracteata EO, while P. aeruginosa PAO1 displayed the strongest resistance to M. bracteata EO.
The MIC of M. bracteata EO against C. violaceum was 10‰. The M. bracteata EO also interfered with the QS phenotype behaviors of C. violaceum without inhibiting its growth, primarily as follows: the violacein and biofilm were reduced with the maximum of inhibition rates of 85.47% and 75.56%, respectively. Biofilm formation and the swarming movement of C. violaceum were also inhibited after treatment with M. bracteata EO. The data showed that M. bracteata EO was capable of directly degrading C6-HSL and inhibiting the C6-HSL production. Furthermore, treatment with M. bracteata EO significantly repressed QS gene expression at sub-MIC concentrations.
Collectively, the anti-QS activity observed here lays a foundation for the development of M. bracteata EO into a new QS-inhibitor to prevent and control bacterial contamination. Further study of the interaction mechanism between the components of M. bracteata EO and bacteria should be analyzed in detail.
Abbreviations
| QS | Quorum sensing |
| Anti-QS | Anti-quorum sensing |
| AHLs | N-acyl-homoserine lactones |
| C6-HSL | N-hexanoyl-l-homoserine lactone |
| MIC | Minimum inhibitory concentration |
| Sub-MIC | Sub-minimal inhibitory concentration |
| AI | Autoinducer |
| VF | virulence factor |
| EO | Essential oil |
| QSI | Quorum-sensing inhibitor |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/22/5696/s1.
Author Contributions
Conceptualization, W.W., S.W., and Y.L.; methodology, W.W., C.Y.; software, W.W.; validation, W.W., H.Y., and X.H.; formal analysis, W.W.; investigation, D.L., H.Y., and X.H.; resources, L.L., L.Z., Z.Q., and X.N.; writing—original draft preparation, W.W.; writing—review and editing, W.W., S.W., and Y.L.; funding acquisition, C.Y. and Y.L.
Funding
This study was financially supported by the National Natural Science Foundation of China (Grant Nos. 31501694 and 31902067), the Foundation of Fujian Science and Technology Committee (Grant No. 2018N0003), and the Foundation of Fuzhou Science and Technology Committee (Grant No. 2017NF30048).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Iñiguez-Moreno M. Resistance of pathogenic and spoilage microorganisms to disinfectants in the presence of organic matter and their residual effect on stainless steel and polypropylene. J. Glob. Antimicrob. Resist. 2018;14:197–201. doi: 10.1016/j.jgar.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Savoia D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012;7:979–990. doi: 10.2217/fmb.12.68. [DOI] [PubMed] [Google Scholar]
- 3.Wang L., Hu W., Deng J., Liu X., Zhou J., Li X. Antibacterial activity of Litsea cubeba essential oil and its mechanism against Botrytis cinerea. RSC Adv. 2019;9:28987–28995. doi: 10.1039/C9RA05338G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang C.Y., Krishnan T., Wang H., Chen Y., Yin W.F., Chong Y.M., Tan L.Y., Chong T.M., Chan K.G. Non-antibiotic quorum sensing inhibitors acting against N-acyl homoserine lactone synthase as druggable target. Sci. Rep. 2014;4:7245. doi: 10.1038/srep07245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J.W., Luo H.Z., Jiang H., Jian T.K., Chen Z.Q., Jia A.Q. Hordenine, a novel quorum sensing inhibitor and anti-biofilm agent against Pseudomonas aeruginosa. J. Agric. Food Chem. 2018;66:1620–1628. doi: 10.1021/acs.jafc.7b05035. [DOI] [PubMed] [Google Scholar]
- 6.Haque S., Ahmad F., Dar S.A., Jawed A., Mandal R.K., Wahid M., Lohani M., Khan S., Singh V., Akhter N. Developments in strategies for Quorum Sensing virulence factor inhibition to combat drug resistant bacteria. Microb. Pathog. 2018;121:293–302. doi: 10.1016/j.micpath.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 7.Kerekes E.B., Deák É., Takó M., Tserennadmid R., Petkovits T., Vágvölgyi C., Krisch J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 2013;115:933–942. doi: 10.1111/jam.12289. [DOI] [PubMed] [Google Scholar]
- 8.Jamuna B.A., Vittal R.R. Quorum Sensing Inhibitory and Anti-Biofilm Activity of Essential Oils and Their in vivo Efficacy in Food Systems. Food Biotechnol. 2014;28:269–292. [Google Scholar]
- 9.Kothari V., Sharma S., Padia D. Recent research advances on Chromobacterium violaceum. Asian Pac. J. Trop. Med. 2017;10:810–818. doi: 10.1016/j.apjtm.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Durán N., Justo G.Z., Durán M., Brocchi M., Cordi L., Tasic L., Castro G.R., Nakazato G. Advances in Chromobacterium violaceum and properties of violacein-Its main secondary metabolite: A review. Biotechnol. Adv. 2016;34:1030–1045. doi: 10.1016/j.biotechadv.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Eris R., Ulusoy S. Rose, clove, chamomile essential oils and pine turpentine inhibit quorum sensing in Chromobacterium violaceum and Pseudomonas aeruginosa. J. Essent. Oil Bear. Plants. 2013;16:126–135. doi: 10.1080/0972060X.2013.794026. [DOI] [Google Scholar]
- 12.Asghar A., Butt M.S., Shahid M., Huang Q. Evaluating the antimicrobial potential of green cardamom essential oil focusing on quorum sensing inhibition of Chromobacterium violaceum. J. Food Sci. Technol. 2017;54:2306–2315. doi: 10.1007/s13197-017-2668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C., Liu H., Zhao L., Zhang W., Qiu S., Yang X., Tan H. Antibacterial neolignans from the leaves of Melaleuca bracteata. Fitoterapia. 2017;120:171–176. doi: 10.1016/j.fitote.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Hou W., Zhang W., Chen G., Luo Y. Optimization of Extraction Conditions for Maximal Phenolic, Flavonoid and Antioxidant Activity from Melaleuca bracteata Leaves Using the Response Surface Methodology. PLoS ONE. 2016;11:e0162139. doi: 10.1371/journal.pone.0162139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adesanwo K.J., Shode F.O., Aiyelaagbe O.O., Rabiu O.O., Oyede R.T., Oluwole F.S. Antisecretory and antiulcerogenic activities of the stem bark extract of Melaleuca bracteata and isolation of principles. J. Med. Plants Res. 2009;3:822–824. [Google Scholar]
- 16.Siddique S., Parveen Z., Mazhar S. Chemical composition, antibacterial and antioxidant activities of essential oils from leaves of three Melaleuca species of Pakistani flora. Arab. J. Chem. 2017;18:1–8. doi: 10.1016/j.arabjc.2017.01.018. [DOI] [Google Scholar]
- 17.Li Y., Ye Z., Wang W., Yang C., Liu J., Zhou L., Shen Y., Wang Z., Chen J., Wu S. Composition Analysis of Essential Oil from Melaleuca bracteata Leaves Using Ultrasound-assisted Extraction and its Antioxidative and Antimicrobial Activities. BioResources. 2018;13:8488–8504. doi: 10.15376/biores.13.4.8488-8504. [DOI] [Google Scholar]
- 18.Durán M., Faljoni-Alario A., Durán N. Chromobacterium violaceum and its important metabolites--review. Folia Microbiol. 2010;55:535–547. doi: 10.1007/s12223-010-0088-4. [DOI] [PubMed] [Google Scholar]
- 19.Husain F.M., Ahmad I., Althubiani A.S., Abulreesh H.H., Alhazza I.M., Aqil F. leaves Extracts of Mangifera indicaL. Inhibit Quorum Sensing—Regulated Production of Virulence Factors and Biofilm in Test Bacteria. Front. Microbiol. 2017;8:727. doi: 10.3389/fmicb.2017.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verstraeten N., Braeken K., Debkumari B., Fauvart M., Fransaer J., Vermant J., Michiels J. Living on a surface: Swarming and biofilm formation. Trends Microbiol. 2008;16:496–506. doi: 10.1016/j.tim.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh R., Tiwary B.K., Kumar A., Chakraborty R. Guava leaves Extract Inhibits Quorum-Sensing and Chromobacterium violaceum Induced Lysis of Human Hepatoma Cells: Whole Transcriptome Analysis Reveals Differential Gene Expression. PLoS ONE. 2014;9:e107703. doi: 10.1371/journal.pone.0107703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brito C.F., Carvalho C.B., Santos F., Gazzinelli R.T., Oliveira S.C., Azevedo V., Teixeira S.M. Chromobacterium violaceum genome: Molecular mechanisms associated with pathogenicity. Genet. Mol. Res. 2004;3:148–161. [PubMed] [Google Scholar]
- 23.Bacha K., Tariku Y., Gebreyesus F., Zerihun S., Mohammed A., Weiland-Bräuer N., Schmitz R.A., Mulat M. Antimicrobial and anti-Quorum Sensing activities of selected medicinal plants of Ethiopia: Implication for development of potent antimicrobial agents. BMC Microbiol. 2016;16:139. doi: 10.1186/s12866-016-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truchado P., López-Gálvez F., Gil M.I., Tomás-Barberán F.A., Allende A. Quorum sensing inhibitory and antimicrobial activities of honeys and the relationship with individual phenolics. Food Chem. 2009;115:1337–1344. doi: 10.1016/j.foodchem.2009.01.065. [DOI] [Google Scholar]
- 25.Taganna J.C., Quanico J.P., Perono R.M.G., Amor E.C., Rivera W.L. Tannin-rich fraction from Terminalia catappa inhibits quorum sensing (QS) in Chromobacterium violaceum and the QS-controlled biofilm maturation and LasA staphylolytic activity in Pseudomonas aeruginosa. J. Ethnopharmacol. 2011;134:865–871. doi: 10.1016/j.jep.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Zhu H., He C.C., Chu Q.H. Inhibition of quorum sensing in Chromobacterium violaceum by pigments extracted from Auricularia auricular. Lett. Appl. Microbiol. 2011;52:269–274. doi: 10.1111/j.1472-765X.2010.02993.x. [DOI] [PubMed] [Google Scholar]
- 27.Packiavathy I.A.S.V., Priya S., Pandian S.K., Ravi A.V. Inhibition of biofilm development of uropathogens by curcumin—An anti-quorum sensing agent from Curcuma longa. Food Chem. 2014;148:453–460. doi: 10.1016/j.foodchem.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Adhavan P., Kaur G., Princy A., Murugan R. Essential oil nanoemulsions of wild patchouli attenuate multi-drug resistant gram-positive, gram-negative and Candida albicans. Ind. Crops Prod. 2017;100:106–116. doi: 10.1016/j.indcrop.2017.02.015. [DOI] [Google Scholar]
- 29.Packiavathy I.A.S.V., Agilandeswari P., Musthafa K.S., Pandian S.K., Ravi A.V. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res. Int. 2012;45:85–92. doi: 10.1016/j.foodres.2011.10.022. [DOI] [Google Scholar]
- 30.Husain F.M., Ahmad I., Khan M.S., Ahmad E., Tahseen Q., Khan M.S., Alshabib N.A. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front. Microbiol. 2015;6:420. doi: 10.3389/fmicb.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stauff D.L., Bassler B.L. Quorum sensing in Chromobacterium violaceum: DNA recognition and gene regulation by the cviR receptor. J. Bacteriol. 2011;193:3871–3878. doi: 10.1128/JB.05125-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morten H., Michael G. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J. Clin. Investig. 2003;112:1300–1307. doi: 10.1172/JCI20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swem L.R., Swem D.L., O’Loughlin C.T., Gatmaitan R., Zhao B., Ulrich S.M., Bassler B.L. A Quorum-Sensing Antagonist Targets Both Membrane-Bound and Cytoplasmic Receptors and Controls Bacterial Pathogenicity. Mol. Cell. 2009;35:143–153. doi: 10.1016/j.molcel.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goswami P., Verma S.K., Chauhan A., Venkatesha K., Verma R.S., Singh V.R., Darokar M.P., Chanotiya C.S., Padalia R.C. Chemical Composition and Antibacterial Activity of Melaleuca bracteata Essential Oil from India: A Natural Source of Methyl Eugenol. Nat. Prod. Commun. 2017;12:1934578X1701200633. doi: 10.1177/1934578X1701200633. [DOI] [Google Scholar]
- 35.Lucero M., Estell R., Tellez M., Fredrickson E. A retention index calculator simplifies identification of plant volatile organic compounds. Phytochem. Anal. 2010;20:378–384. doi: 10.1002/pca.1137. [DOI] [PubMed] [Google Scholar]
- 36.Dool H.V.D., Kratz P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 37.Andotra S., Kalgotra N., Pandey S.K. Syntheses, Characterization, Thermal, and Antimicrobial Studies of Lanthanum(III) Tolyl/Benzyldithiocarbonates. Bioinorg. Chem. Appl. 2014;2014:780631. doi: 10.1155/2014/780631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasavi H.S., Arun A.B., Rekha P.D. Anti-quorum sensing potential of Adenanthera pavonina. Pharmacogn. Res. 2015;7:105. doi: 10.4103/0974-8490.147220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., Kong J., Huang F., Xie Y., Guo Y., Cheng Y., Qian H., Yao W. Hexanal as a QS inhibitor of extracellular enzyme activity of Erwinia carotovora and Pseudomonas fluorescens and its application in vegetables. Food Chem. 2018;255:1–7. doi: 10.1016/j.foodchem.2018.02.038. [DOI] [PubMed] [Google Scholar]
- 40.O’Toole G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. Jove. 2011;47:e2437. doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blosser R.S., Gray K.M. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J. Microbiol. Methods. 2000;40:47–55. doi: 10.1016/S0167-7012(99)00136-0. [DOI] [PubMed] [Google Scholar]
- 42.Joshi J.R., Khazanov N., Senderowitz H., Burdman S., Lipsky A., Yedidia I. Plant phenolic volatiles inhibit quorum sensing in pectobacteria and reduce their virulence by potential binding to ExpI and ExpR proteins. Sci. Rep. 2016;6:38126. doi: 10.1038/srep38126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.