Abstract
Aims
To evaluate population-based electronic health record (EHR) definitions of atrial fibrillation (AF) and valvular heart disease (VHD) subtypes, time trends in prevalence and prognosis.
Methods and results
A total of 76 019 individuals with AF were identified in England in 1998–2010 in the CALIBER resource, linking primary and secondary care EHR. An algorithm was created, implemented, and refined to identify 18 VHD subtypes using 406 diagnosis, procedure, and prescription codes. Cox models were used to investigate associations with a composite endpoint of incident stroke (ischaemic, haemorrhagic, and unspecified), systemic embolism (SSE), and all-cause mortality. Among individuals with AF, the prevalence of AF with concomitant VHD increased from 11.4% (527/4613) in 1998 to 17.6% (7014/39 868) in 2010 and also in individuals aged over 65 years. Those with mechanical valves, mitral stenosis (MS), or aortic stenosis had highest risk of clinical events compared to AF patients with no VHD, in relative [hazard ratio (95% confidence interval): 1.13 (1.02–1.24), 1.20 (1.05–1.36), and 1.27 (1.19–1.37), respectively] and absolute (excess risk: 2.04, 4.20, and 6.37 per 100 person-years, respectively) terms. Of the 95.2% of individuals with indication for warfarin (men and women with CHA2DS2-VASc ≥1 and ≥2, respectively), only 21.8% had a prescription 90 days prior to the study.
Conclusion
Prevalence of VHD among individuals with AF increased from 1998 to 2010. Atrial fibrillation associated with aortic stenosis, MS, or mechanical valves (compared to AF without VHD) was associated with an excess absolute risk of stroke, SSE, and mortality, but anticoagulation was underused in the pre-direct oral anticoagulant (DOAC) era, highlighting need for urgent clarity regarding DOACs in AF and concomitant VHD.
Keywords: Valvular heart disease, Atrial fibrillation, Electronic health records, Stroke, Systemic embolism, Mortality
What’s new?
In the first large-scale electronic health record (EHR) study of atrial fibrillation (AF) and valvular heart disease (VHD), different subtypes of VHD had high prevalence and high risk of adverse events.
The burden of VHD increased from 1998 to 2010 among individuals with AF, possibly due to increased diagnostic sensitivity, increased reporting of milder VHD or longevity with VHD.
Among VHD subtypes, AF with mechanical valves, mitral stenosis, and aortic stenosis had greatest thromboembolic risk, with worst outcomes in aortic stenosis.
There was low oral anticoagulant utilization in the pre-direct oral anticoagulant (DOAC) (direct anticoagulant) era, despite high predicted and actual stroke/mortality risks, and particularly in the highest risk patients (e.g. aortic stenosis).
Our EHR VHD phenotype provides transparent, reproducible and interoperable definitions for future EHR analyses, including international datasets.
Prognostic differences across AF/VHD subtypes support targeted DOAC trials in specific VHD subpopulations.
Introduction
Varying definitions across practice, guidelines, observational studies, and trials1 make ‘valvular’ atrial fibrillation (AF) an obsolete term. European AF guidelines consider valvular heart disease (VHD) as mechanical heart valves (MechV) or mitral stenosis (MS),2 which direct oral anticoagulant (DOAC) trials excluded.1
Atrial fibrillation3 and VHD4 are increasing globally, occurring together in 2–31% of AF.5 Despite consistent data for MechV and MS,6 AF studies to-date are neither representative,1,7,8 nor include all VHD subtypes6 (e.g. aortic stenosis, AS1) nor time trends (e.g. increases in prevalence of AF with AS or mitral regurgitation, MR, vs. reductions in rheumatic AF).
Compared to AF without VHD,6 stroke and systemic embolism (SSE) risk is increased in AF with MechV and MS,9 with similar risk factor profiles.5 Costs of not anticoagulating are likely to be high10 in VHD. Warfarin is recommended in all VHD subtypes with AF, based on stroke and bleeding risk,1 whereas DOACs lack conclusive trial evidence. However, the full range of VHD remains unstudied in the pre-DOAC era.
Electronic health records (EHRs) could address these uncertainties with greater sample size and generalizability than other study designs.11 Valvular heart disease has been studied in EHR,12 but without distinguishing subtypes in AF. We investigated: (i) feasibility of using EHR to identify AF with VHD, (ii) temporal trends in prevalence of VHD subtypes with AF 1998–2010 (prior to routine DOAC use), and (iii) prognosis of VHD subtypes of AF.
Methods
Data sources
CALIBER,13 connecting mortality (Office of National Statistics, ONS), primary (Clinical Practice Research Datalink, CPRD), and secondary care (Hospital Episode Statistics) data, is representative of the UK population by age, sex, ethnicity,14 and mortality,15 providing valid risk estimates associated with cardiovascular diseases16–20 (SR1). Coding involves four controlled clinical terminologies: Read (primary care diagnoses/procedures, mapping to SNOMED-CT) (SR2), prescriptions (British National Formulary, BNF, and primary care) (SR3), ICD-10 (secondary care diagnoses/mortality) (SR4), and OPCS-4 (secondary care procedures) (SR5).
Study population
A validated EHR AF phenotype in primary and/or secondary care was used (1998–2010) (SR6), including individuals aged ≥18 years, with any AF pattern and ≥1 year of primary care follow-up prior to earliest coded AF during the study period (‘baseline’).
Electronic health record phenotype algorithm
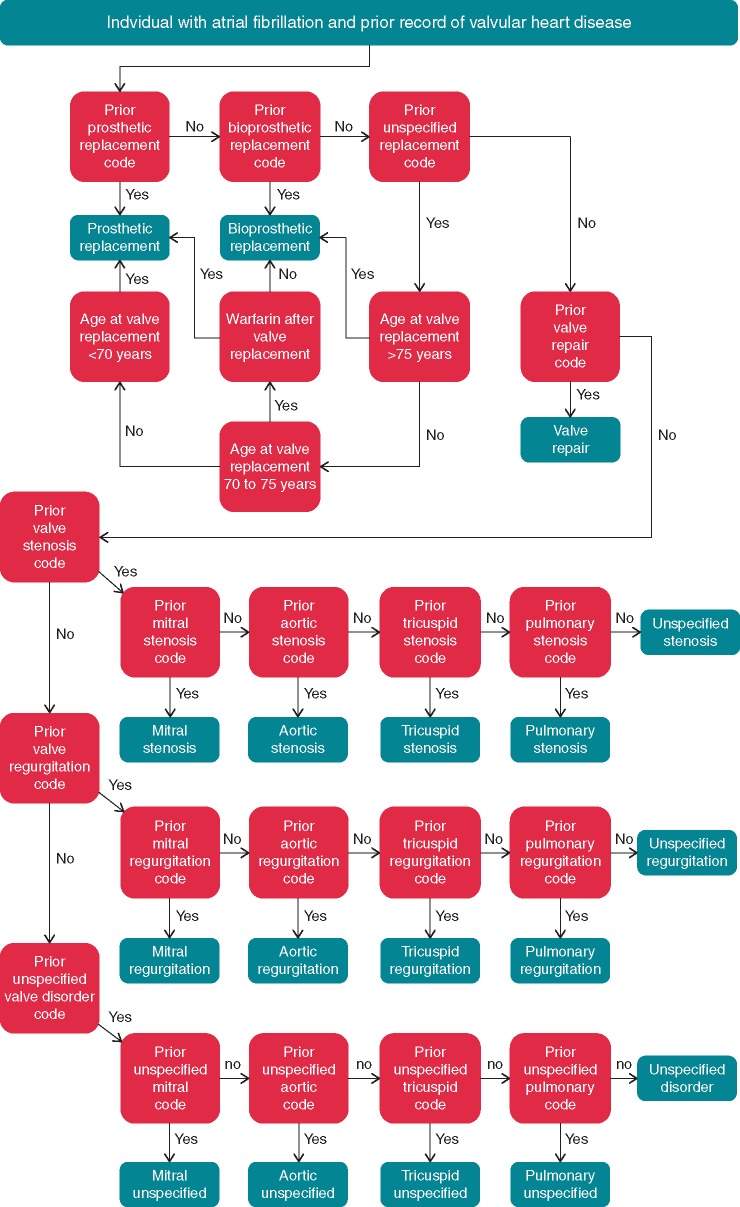
We followed CALIBER guidelines for algorithm development (SR7); combining informatics (with re-usable scripts), clinical review of codes/algorithms, and validation. The final algorithm for acquired VHD (excluding congenital) combined 406 diagnosis, procedure, prescription, and valve replacement codes (Figure 1; Supplementary material online, Appendix), classifying the single most relevant VHD, in order of replacements, repairs, and stenosis/regurgitation (supported by ROCKET-AF) (SR8). Recurrences were unnecessary for confirming events since individuals with less frequently captured data may be systematically excluded.
Figure 1.
Electronic health record algorithm to classify AF and concomitant VHD. Flow diagram illustrates electronic health record algorithm for classifying individuals with AF and prevalent valvular disease at study index into one of 18 valvular AF subtypes. The algorithm considers first valve replacements, followed by valve repairs and then valve diseases, which are recorded at any prior time point in an individual’s medical history. The algorithm is underpinned by 406 diagnosis, procedure, and prescription codes from the Read, ICD-10, OPCS-4, and BNF systems. Full code list and electronic health record algorithm provided in Supplementary material online, Appendix. AF, atrial fibrillation; VHD, valvular heart disease.
Risk factors
CHADS2 (SR9) and CHA2DS2VASc variables (SR10) were extracted: age and sex16: primary care registration information; heart failure (HF) (SR11), diabetes mellitus (DM type I, II, and unclassified),17 SSE/transient ischaemic attack (TIA), and vascular disease (myocardial infarction19 or peripheral artery disease): Read/ICD-10; hypertension18: Read/ICD-10, ≥2 blood pressure measurements ≥140/90 mmHg or repeat anti-hypertensive prescriptions. Eligibility for oral anticoagulant (OAC) was determined by CHADS2/CHA2DS2VASc. Oral anticoagulant use was based on BNF warfarin codes or International Normalized Ratio (INR) tests ≤90 days before study entry. Bleeding risk was ascertained: hypertension, age >75 years, prior haemorrhagic stroke, and labile INR (≥5 at least once).
Primary outcomes
Primary composite outcome was SSE (including ischaemic, haemorrhagic, and unspecified stroke) or all-cause mortality; a common trial primary endpoint (SR12-SR15). Follow-up was until transfer/last visit in primary care, when secondary care was censored to align data sources, avoiding missing events and immortal follow-up time.
Statistical analysis
Analysis was by baseline VHD: (i) replacement; (ii) repair; (iii) MS, (iv) AS, (v) MR, or (vi) aortic regurgitation (AR). Tricuspid and pulmonary valve disorders were analysed together due to limited numbers (n = 277) (Table 1). Baseline characteristics were analysed by VHD. Prevalence was calculated at monthly/yearly intervals 1998–2010, dividing total number with prevalent VHD by total number at risk. LOESS (LOcally wEighted Scatterplot Smoothing) lines were fitted to identify temporal trends in prevalence, making no assumption about data distribution (SR16). Incident cases were included in prevalence calculation over time, not in risk modelling which considered baseline VHD.
Table 1.
Baseline characteristics in atrial fibrillation with valvular heart disease
| No VHD | Valve replacement |
Valve repair | Mitral |
Aortic |
Overall cohorta | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mechanical | Bioprosthetic | Stenosis | Regurgitation | Other | Stenosis | Regurgitation | Other | ||||
| Individuals | 67 396 (88.7) | 1207 (1.6) | 695 (0.9) | 434 (0.6) | 527 (0.7) | 2374 (3.1) | 974 (1.3) | 1494 (2.0) | 444 (0.6) | 197 (0.3) | 76 019 |
| Follow-up | 2.3 (4.3) | 3.1 (5.0) | 2.0 (3.3) | 2.7 (4.5) | 2.5 (4.7) | 2.1 (3.8) | 1.8 (3.6) | 1.2 (2.7) | 1.9 (4.3) | 1.9 (3.7) | 2.2 (4.2) |
| Risk factors | |||||||||||
| Age | 77.7 (15.2) | 70.5 (13.4) | 78.7 (10.2) | 71.8 (13.8) | 75.5 (15.1) | 77.8 (13.3) | 78.2 (14.0) | 82.2 (11.3) | 78.9 (13.4) | 80.3 (11.8) | 77.7 (15.0) |
| Age ≥75 | 40 041 (59.4) | 385 (31.9) | 462 (66.5) | 157 (36.2) | 273 (51.8) | 1465 (61.7) | 601 (61.7) | 1171 (78.4) | 283 (63.7) | 146 (74.1) | 45 145 (59.4) |
| Female gender | 32 809 (48.7) | 537 (44.5) | 298 (42.9) | 223 (51.4) | 397 (75.3) | 1240 (52.2) | 547 (56.2) | 794 (53.1) | 208 (46.8) | 108 (54.8) | 37 299 (49.1) |
| Heart failure | 15 896 (23.6) | 539 (44.7) | 287 (41.3) | 187 (43.1) | 237 (45) | 1164 (49.0) | 432 (44.4) | 684 (45.8) | 196 (44.1) | 74 (37.6) | 19 840 (26.1) |
| Hypertension | 55 110 (81.8) | 1083 (89.7) | 647 (93.1) | 388 (89.4) | 454 (86.1) | 2129 (89.7) | 845 (86.8) | 1377 (92.2) | 405 (91.2) | 181 (91.9) | 62 849 (82.7) |
| Diabetes mellitus | 9568 (14.2) | 152 (12.6) | 101 (14.5) | 49 (11.3) | 81 (15.4) | 327 (13.8) | 139 (14.3) | 255 (17.1) | 51 (11.5) | 26 (13.2) | 10 798 (14.2) |
| Stroke/TIA/SE | 12 201 (18.1) | 224 (18.6) | 111 (16.0) | 62 (14.3) | 129 (24.5) | 426 (17.9) | 193 (19.8) | 326 (21.8) | 89 (20.0) | 46 (23.4) | 13 862 (18.2) |
| Vascular disease | 13 019 (19.3) | 202 (16.7) | 146 (21.0) | 75 (17.3) | 84 (15.9) | 608 (25.6) | 256 (26.3) | 432 (28.9) | 97 (21.8) | 46 (23.4) | 15 036 (19.8) |
| Prior haemorrhagic stroke | 975 (1.4) | 24 (2.0) | 7 (1.0) | 8 (1.8) | 6 (1.1) | 31 (1.3) | 21 (2.2) | 12 (0.8) | 11 (2.5) | 0 (0.0) | 1097 (1.4) |
| Prior INR >5 | 1162 (1.7) | 204 (16.9) | 38 (5.5) | 49 (11.3) | 33 (6.3) | 82 (3.5) | 31 (3.2) | 33 (2.2) | 8 (1.8) | 12 (6.1) | 1661 (2.2) |
| CHADS2 | |||||||||||
| 0 | 5822 (8.6) | 73 (6.0) | 29 (6.7) | 35 (6.6) | 87 (3.7) | 61 (6.3) | 29 (6.7) | 22 (1.5) | 17 (3.8) | 4 (2.0) | 6192 (8.1) |
| 1 | 16 778 (24.9) | 322 (26.7) | 126 (29.0) | 107 (20.3) | 430 (18.1) | 184 (18.9) | 126 (29.0) | 179 (12.0) | 81 (18.2) | 29 (14.7) | 18 411 (24.2) |
| ≥2 | 44 796 (66.5) | 812 (67.3) | 543 (78.1) | 279 (64.3) | 385 (73.1) | 1857 (78.2) | 729 (74.8) | 1293 (86.5) | 346 (77.9) | 164 (83.2) | 51 416 (67.6) |
| Mean | 2.2 | 2.2 | 2.5 | 2.1 | 2.5 | 2.5 | 2.5 | 2.8 | 2.5 | 2.6 | 2.2 |
| CHA2DS2-VASc | |||||||||||
| 0 | 2474 (3.7) | 34 (2.8) | 8 (1.2) | 14 (3.2) | 6 (1.1) | 27 (1.1) | 25 (2.6) | 5 (0.3) | 10 (2.3) | 2 (1.0) | 2609 (3.4) |
| 1 | 5578 (8.3) | 120 (9.9) | 30 (4.3) | 43 (9.9) | 28 (5.3) | 126 (5.3) | 48 (4.9) | 29 (1.9) | 15 (3.4) | 6 (3.0) | 6047 (8.0) |
| ≥2 | 59 344 (88.1) | 1053 (87.2) | 657 (94.5) | 377 (86.9) | 493 (93.5) | 2221 (93.6) | 901 (92.5) | 1460 (97.7) | 419 (94.4) | 189 (95.9) | 67 363 (88.6) |
| Mean | 3.7 | 3.5 | 4.0 | 3.5 | 4.2 | 4.1 | 4.1 | 4.5 | 4.1 | 4.3 | 3.7 |
| Bleeding risk ≥2 factors | 35 373 (52.5) | 500 (41.4) | 451 (64.9) | 176 (40.6) | 268 (50.9) | 1404 (59.1) | 569 (58.4) | 1102 (73.8) | 272 (61.3) | 139 (70.6) | 40 406 (53.2) |
| Anticoagulation | |||||||||||
| Total | 13 098 (19.4) | 829 (68.7) | 190 (27.3) | 236 (54.4) | 314 (59.6) | 839 (35.3) | 310 (31.8) | 306 (20.5) | 91 (20.5) | 47 (23.9) | 16 339 (21.5) |
| Use when indicated by | |||||||||||
| CHADS2 | 9176 (20.5) | 577 (71.1) | 157 (28.9) | 162 (58.1) | 232 (60.3) | 652 (35.1) | 243 (33.3) | 266 (20.6) | 70 (20.2) | 39 (23.8) | 11 637 (22.6) |
| CHA2DS2VASc | 12 621 (19.7) | 807 (69.4) | 189 (27.6) | 226 (54.7) | 305 (60.3) | 827 (35.5) | 304 (32.4) | 303 (20.4) | 88 (20.3) | 47 (24.1) | 15 794 (21.8) |
| Endpoints | |||||||||||
| Ischaemic stroke | 2606 (3.9) | 44 (3.6) | 33 (4.7) | 8 (1.8) | 32 (6.1) | 88 (3.7) | 32 (3.3) | 52 (3.5) | 21 (4.7) | 9 (4.6) | 2930 (3.9) |
| Unspecified stroke | 4030 (6.0) | 43 (3.6) | 22 (3.2) | 12 (2.8) | 33 (6.3) | 103 (4.3) | 50 (5.1) | 77 (5.2) | 18 (4.1) | 9 (4.6) | 4405 (5.8) |
| Systemic embolism | 479 (0.7) | 6 (0.5) | 4 (0.6) | 1 (0.2) | 6 (1.1) | 14 (0.6) | 12 (1.2) | 10 (0.7) | 5 (1.1) | 0 (0) | 540 (0.7) |
| Haemorrhagic stroke | 626 (0.9) | 19 (1.6) | 10 (1.4) | 6 (1.4) | 5 (0.9) | 20 (0.8) | 8 (0.8) | 8 (0.5) | 7 (1.6) | 1 (0.5) | 711 (0.9) |
| Mortality | 20 428 (30.3) | 353 (29.2) | 165 (23.7) | 95 (21.9) | 174 (33) | 825 (34.8) | 319 (32.8) | 647 (43.3) | 164 (36.9) | 75 (38.1) | 23 348 (30.7) |
Continuous values are presented as median (interquartile range). Categorical variables are presented as N (%). Vascular disease (myocardial infarction and/or peripheral artery disease).
CHA2DS2-VASc score, congestive heart failure, hypertension, age (≥75 years), diabetes mellitus, stroke/TIA/SE, vascular disease, age (65–75 years) and sex (female); INR, International Normalized Ratio; SE, systemic embolism; TIA, transient ischaemic attack; VHD, valvular heart disease.
Total of 76 019 includes 277 not included as a subgroup due to small numbers: tricuspid stenosis (n = 2, 0.0%), tricuspid regurgitation (n = 167, 0.2%), tricuspid disorder not otherwise specified (n = 18, 0.0%), pulmonary stenosis (n = 19, 0.0%), pulmonary regurgitation (n = 18, 0.0%), pulmonary disorder not otherwise specified (n = 2, 0.0%), unspecified stenosis (n = 2, 0.0%), unspecified regurgitation (n = 1, 0.0%), and unspecified disorder (n = 48, 0.1%).
For prognostic validation of AF with VHD subtypes, we modelled associations (vs. AF without VHD) with the primary endpoint, expecting higher SSE risk with MechV and MS.6 Incrementally adjusted Cox regression with model assumptions and goodness-of-fit were assessed graphically, confirming proportionality over time with scaled Schoenfeld residuals. Adjustment was for age and sex (Model 1); Model 1 and baseline warfarin prescriptions (Model 2); and Model 2 and CHA2DS2VASc factors (Model 3) (Supplementary material online, Table S2). Interaction testing was conducted between baseline VHDs and key confounders: age, sex, warfarin, and prior SSE/TIA. All models stratified by primary care practice, accounting for potential local differences in the application of coding or management. Stata/SE 13.1 was used for data analyses and R 3.2.0 for figures.
Ethics
The study was approved by the MHRA (UK) Independent Scientific Advisory Committee (12_165), under Section 251 (NHS Social Care Act 2006).
Results
Among 76 019 individuals with AF, we used 165 diagnosis and 205 procedure codes (370 total) for VHD across Read (235; 63.5%), ICD-10 (49; 13.2%), and OPCS-4 (86; 23.2%) (Supplementary material online, Table S1). A total of 12 751 (16.8%) had AF and VHD; 8623 (11.3%) had prevalent VHD, median (interquartile range, IQR) 3.1 (8.9) years before study entry; and 4128 (5.4%) had incident VHD with median 1.4 (3.4) years of follow-up. A total of 2578 (3.3%) had valve replacements; 1902 (2.5%) prevalent at baseline; and 676 (0.9%) over follow-up.
A total of 67 396 (88.7%) had no VHD at baseline, 1207 (1.6%) had MechV, 695 (0.9%) bioprosthetic valve replacement, 434 (0.6%) valve repair, 527 (0.7%) MS, 2374 (3.1%) MR, 974 (1.3%) other mitral disorders, 1494 (2.0%) AS, 444 (0.6%) AR, and 197 (0.3%) other aortic disorders. Among 4128 with incident VHD, the affected valve was mitral in 2700 (65.4%), aortic in 1288 (31.2%), tricuspid in 398 (9.6%), pulmonary in 48 (1.2%), and unspecified in 63 (1.5%) (Supplementary material online, Figure S1).
Baseline characteristics
Median (IQR) age was 77.7 (15.0) years, 49.1% female with median (IQR) 2.2 (4.2) years follow-up. Comorbidities were common, e.g. 26.1% HF, 82.7% hypertension, 14.2% DM, and 19.8% vascular disease. About 67.6% of the population had CHADS2 ≥2 and 88.6% had CHA2DS2-VASc ≥2. Warfarin prescription ≤90 days prior to the study was low: 22.6% and 21.8% (indicated by CHADS2 and CHA2DS2-VASc, respectively) (Table 1). When indicated regardless of AF (i.e. MechV and MS), warfarin use was 65.9%.
In AF and VHD, rates of HF (45.7% vs. 23.6%), hypertension (89.8% vs. 81.8%), and CHA2DS2-VASC scores [mean (standard deviation, SD) 4.1 (1.7) vs. 3.7 (1.8)] were higher, compared to AF without VHD. Individuals with MechV were youngest [median (IQR): 70.5 (13.4) years] with highest warfarin use (68.7%). Individuals with MS were 75.3% female, had highest SSE/TIA prevalence (24.5%), and warfarin use was 59.6%. Individuals with AS were older [median (IQR) 82.2 (11.3) years], had high DM (17.1%), and vascular disease (28.9%) prevalence, high CHA2DS2VASC score [mean (SD): 4.5 (1.6)], and lowest warfarin use (20.5%).
Prevalence and incidence
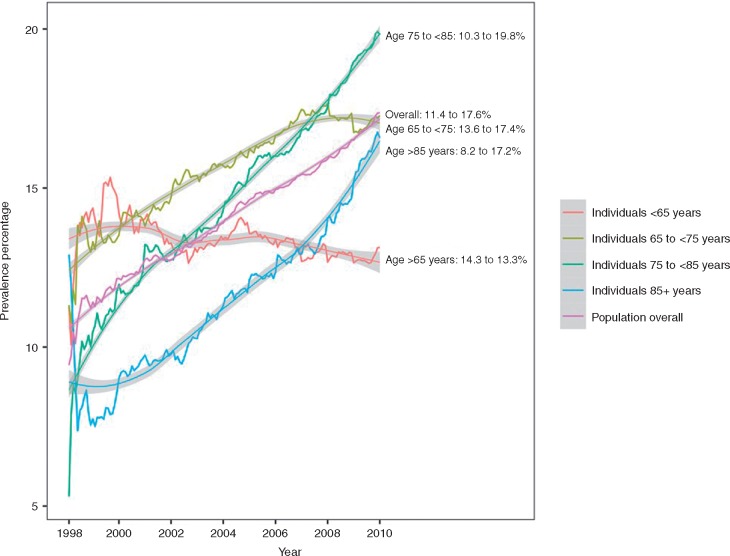
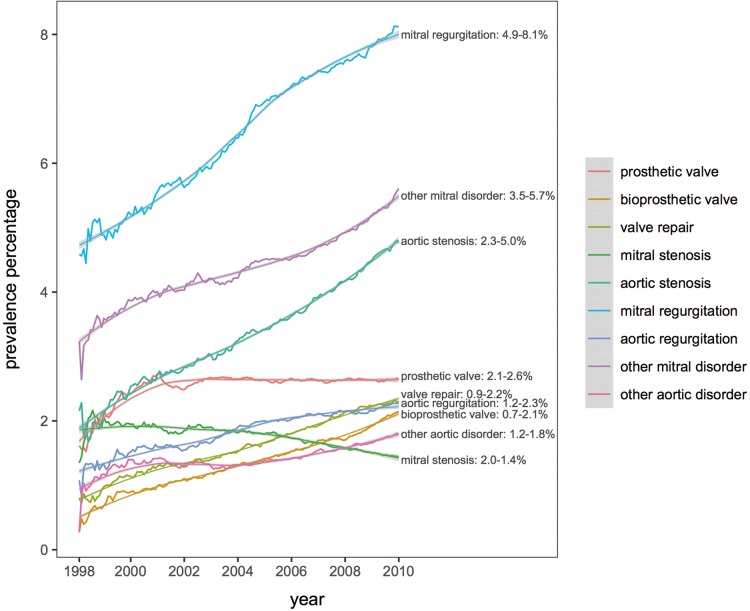
Valvular heart disease prevalence increased from 11.4% to 17.6% (1998–2010), particularly age >65 years (Figure 2). Prevalence increased for bioprosthetic replacements (0.7% in 1998–99 to 2.1% in 2009–10), valve repairs (0.9–2.2%), MR (4.9–8.1%), AS (2.3–5.0%), and AR (1.2–2.3%). Prevalence increased for MechV from 2.1% to 2.6% (1998–99 to 2002–03), then plateaued, and decreased for MS from 2.0% to 1.4% (1998–99 to 2009–10) (Figure 3).
Figure 2.
Trends in prevalence of valvular heart disease among 76 019 cases of AF (1998–2010) by age groups. AF, atrial fibrillation.
Figure 3.
Trends in prevalence of valvular AF subtypes (1998–2010). AF, atrial fibrillation.
Risks of SSE and mortality
A total of 31 934 endpoints (9.2% ischaemic stroke, 13.8% unspecified stroke, 1.7% SE, 2.3% haemorrhagic stroke, 73.1% mortality) occurred in 3764 (11.8%) individuals with AF and VHD. Absolute SSE/mortality risk was high, compared to AF without VHD. Only AF with bioprosthetic replacements or valve repair had lower risk (13.9 and 15.3 per 100 person-years, respectively) compared to AF without VHD (18.2 per 100 person-years) (Supplementary material online, Table S2). The highest risk was in AS, MS, AR, and MechV (24.6, 22.4, 21.4, and 20.2 per 100 person-years respectively). Compared to AF without VHD, MechV, MS, and AS carried excess risk of the composite endpoint of 2.04, 4.20, and 6.37 per 100 person-years, respectively.
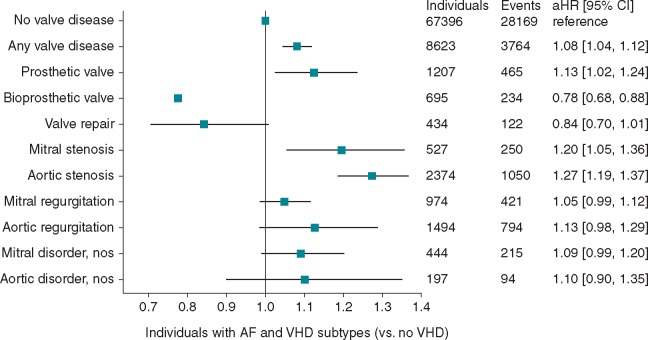
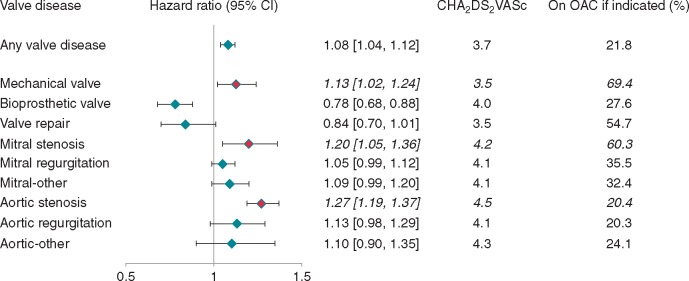
Compared with AF without VHD, MechV, MS, and AS carried greatest risk for the primary endpoint [hazard ratio (HR) (95% confidence interval): 1.13 (1.02–1.24); 1.20 (1.05–1.36); and 1.27 (1.19–1.37), respectively] after adjustment for age, sex, warfarin use, and CHA2DS2VASc risk factors, while bioprosthetic replacements carried lower risk [HR 0.78 (0.68–0.88)] (Table 2, Figure 4, Supplementary material online, Table S2). For MechV, there was no difference between mitral and aortic (HR 1.08, 0.91–1.29 and HR 1.08, 0.94–1.24, respectively], but increased risk with ‘unspecified’ (HR 1.26, 1.05–1.51). For bioprosthetic replacements, aortic appeared favourable (HR 0.93, 0.66–1.33 for mitral; HR 0.76, 0.65–0.90 for aortic; HR 0.77, 0.59–1.00 for unspecified, respectively).
Table 2.
Subtypes of AF with concomitant valvular heart disease and risk of incident stroke, systemic embolism, and all-cause mortality
| Subtypes of AF | Individuals | Events | HR (95% CI)a | HR (95% CI)b | HR (95% CI)c |
|---|---|---|---|---|---|
| No heart valve disease | 67 396 | 28 169 | Reference | Reference | Reference |
| Any heart valve disease | 8623 | 3764 | 1.17 (1.13–1.21) | 1.20 (1.16–1.25) | 1.08 (1.04–1.12) |
| Mechanical valve replacement | 1207 | 465 | 1.17 (1.07–1.28) | 1.26 (1.14–1.38) | 1.13 (1.02–1.24) |
| Bioprosthetic valve replacement | 695 | 234 | 0.82 (0.72–0.94) | 0.84 (0.74–0.95) | 0.78 (0.68–0.88) |
| Valve repair | 434 | 122 | 0.88 (0.74–1.06) | 0.93 (0.78–1.12) | 0.84 (0.70–1.01) |
| Mitral stenosis | 527 | 250 | 1.25 (1.10–1.42) | 1.34 (1.18–1.52) | 1.20 (1.05–1.36) |
| Mitral regurgitation | 2374 | 1050 | 1.14 (1.07–1.21) | 1.17 (1.10–1.25) | 1.05 (0.99–1.12) |
| Other mitral disorder | 974 | 421 | 1.19 (1.08–1.32) | 1.22 (1.11–1.35) | 1.09 (0.99–1.20) |
| Aortic stenosis | 1494 | 794 | 1.41 (1.32–1.52) | 1.42 (1.32–1.53) | 1.27 (1.19–1.37) |
| Aortic regurgitation | 444 | 215 | 1.23 (1.07–1.41) | 1.23 (1.08–1.41) | 1.13 (0.98–1.29) |
| Other aortic disorder | 197 | 94 | 1.14 (0.93–1.39) | 1.15 (0.94–1.41) | 1.10 (0.90–1.35) |
All stratified on primary care practice.
AF, atrial fibrillation; HR (95% CI), hazard ratio (95% confidence interval).
Adjusted for age and sex.
Adjusted for Model 1 + baseline warfarin prescription.
Model 2 + heart failure, hypertension, diabetes mellitus, stroke, transient ischaemic attack, or system embolism, vascular disease.
Figure 4.
AF and VHD subtypes and adjusted risk of incident stroke, systemic embolism, and all-cause mortality. Reference category: patients with AF and no record of valvular heart disease. Hazard ratios adjusted for age, sex, warfarin, heart failure, hypertension, diabetes mellitus, stroke, transient ischaemic attack or systemic embolism, and vascular disease. AF, atrial fibrillation; aHR (95% CI), adjusted hazard ratio (95% confidence interval); VHD, valvular heart disease; nos, not otherwise specified.
Discussion
In the first large-scale EHR study of AF and VHD, different VHD subtypes had high prevalence and high risk of adverse events, yet low OAC utilization. Among 76 019 AF cases, there was high VHD burden (11.3% at baseline), increasing over time (11.4% in 1998 to 17.6% in 2010). Among VHD subtypes, AF with MechV, MS, and AS had greatest thromboembolic risk, with worst outcomes in AS (Figure 5).
Figure 5.
Subtypes of AF with concomitant valvular heart disease and risk of incident stroke, systemic embolism, and all-cause mortality. OAC, oral anticoagulation.
Cohorts [e.g. Framingham: 1544 incident AF cases (SR17)] and registries [e.g. EuroHeart Survey: 5333 AF patients (SR18)] lack scale to investigate VHD subtypes. Registries and trials support the observed high risk with AF and AS.8 Our algorithm’s validity is implied by replication of known associations of higher SSE/mortality risk with MechV and MS,6 consistent with guideline definitions2 (SR19), DOAC trials (SR12-SR15), and recent reviews.6
There was sufficient resolution to distinguish between specific valve(s) (98.5%) and between MechV and bioprosthetic replacements (84.3%). For ‘unspecified’, ‘mechanical’, or ‘bioprosthetic’ could be inferred by differences in age and warfarin use. Codes for rheumatic VHDs were seldom used, perhaps reflecting disease reductions in industrialized countries (SR20). As previously shown (SR21, SR22), MR and AS, associated with ageing, were most common with annual increases in prevalence. MS was the only VHD with decreasing prevalence. Echocardiography rates (Supplementary material online, Table S3) increased 1998–2010 at all ages (5.5–65.4% in <65 years, 1.8–48.3% in >85 years), arguing against age-specific diagnostic approaches. Increased VHD prevalence (e.g. MR) may reflect increased diagnostic sensitivity, increased reporting of milder VHD or longevity with VHD.
Implications
Clinical
First, there was pre-DOAC underuse of warfarin, despite high predicted and actual stroke/mortality risks, particularly with AS, MS, and MechV. Oral anticoagulant use has increased in recent years (SR23), but sufficiently powered DOAC trials in AF with VHD are lacking. In the absence of contraindications, warfarin should be initiated and continued lifelong1 (SR24). Second, increased mortality risk in aortic and mitral VHD suggest alternative VHD/AF patient pathways, e.g. VHD surveillance should incorporate regular AF screening and echocardiography in AF should emphasize VHD exclusion. Third, prognostic differences across AF/VHD subtypes support targeted DOAC trials in specific VHD subpopulations.
Research
First, given disparate exclusion criteria in DOAC trials (SR12-SR15), the significant thromboembolic risk with MechV and MS informs future trials. Second, our VHD phenotype provides transparent, reproducible, and interoperable definitions for future EHR analyses, including international datasets. The code list is useful, not only for AF, but also VHD, which is timely, given that new 2017 European VHD guidelines pinpoint extensive evidence gaps in risk stratification and comparative effectiveness of different surgical interventions (SR25). Third, underuse of rheumatic and non-rheumatic VHD codes warrants further study.
Strengths
Major strengths are generalizability, proven validity of EHRs in CALIBER,13 considerably larger sample size than prior studies (SR26), and replication of observed associations between VHD/AF and poorer prognosis6 (SR27).
Limitations
EHR data: AF and VHD cases may have been missed/misclassified due to unreliable coding for VHD severity, electrocardiogram, and echocardiography, despite GP questionnaire (positive predictive value = 96%) (SR28) and external (median 89% CPRD diagnoses) validation (SR29). Given poor prognosis in MS, all degrees of severity are likely to be recorded, whereas for MR or AS, capture of severe forms is more likely, leading to overestimation of impact. Electronic health record (including imaging) validation at scale (e.g. GP/patient re-contact studies) is required in the UK, but currently impractical.
Study design: Unmeasured confounding is possible in observational analyses. Relative importance of AF vs. VHD was not assessed since individuals without AF were excluded.
VHD severity: Our algorithm prioritized stenosis before regurgitation, based on prior data (SR8), which may be inappropriate (e.g. mild AS vs. severe MR), and omits ≥one affected valve, e.g. the most prevalent baseline VHD was MR (4.4%), decreasing to 3.1% with our algorithm.
Rheumatic disease: For rheumatic VHD, if MS is more common than MR, it is probably associated with higher mortality risk, independent of AF.
Prescriptions: Low OAC rates may reflect lack of secondary care prescription capture, or our more representative cohort (older, higher risk and less likely to receive OAC). Antiplatelet use was unavailable but likely to be significant, given high vascular disease and stroke/TIA rates, and guideline recommendations in low/intermediate-risk individuals. However, event rates in low/intermediate-risk patients were low (5.7 and 2.9 per 100 person-years for CHADS2 <2 and CHA2DS2VASc <2, respectively).
Conclusion
High prevalence of VHD in AF patients, associated with increased risk of adverse events, is a major and growing burden of disease, yet patients are sub-optimally treated with oral anticoagulation. We report a transparent and reproducible EHR algorithm, providing new insights into VHD subtypes in AF and key implications for future research.
Supplementary Material
Funding
H.H. is a NIHR Senior Investigator, supported by Health Data Research UK [funded by MRC, EPSRC, ESRC, Department of Health and Social Care (England); Chief Scientist Office, Scottish Government Health and Social Care Directorates; Health and Social Care Research and Development Division (Welsh Government); Public Health Agency (Northern Ireland); BHF and Wellcome Trust] and NIHR UCLH BRC. The Farr Institute of Health Informatics Research was funded by MRC, Arthritis Research UK, BHF, CRUK, Chief Scientist Office, ESRC, EPSRC, NIHR, National Institute for Social Care and Health Research and Wellcome Trust (MR/K006584/1). V.A. was a Farr-funded PhD student. D.K. is funded by a NIHR Career Development Fellowship (CDF-2015–08-074), BHF (PG/17/55/33087) and ESC. L.H.L. is funded by Swedish Research Council [2013-23897-104604-23 and 523-2014-2336] and Swedish Heart Lung Foundation [20120321 and 20150557]. A.B., S.D., H.H., D.K., and L.H.L. are funded by the BigData@Heart Consortium, under the Innovative Medicines Initiative-2 [116074, supported by the European Union’s Horizon 2020 programme and EFPIA (Chairs: DE Grobbee, SD Anker)].
Conflict of interest: A.B. has been an advisory board member for Boehringer-Ingelheim, Astra-Zeneca, Novo-Nordisk and Pfizer outside the submitted work. L.L. reports funding from Novartis, Merck, Boehringer Ingelheim, Sanofi, Vifor Pharma, AstraZeneca, Relypsa, Bayer and Boston Scientific, outside the submitted work. All other authors have no conflicts of interest.
References
- 1. Lip GYH, Collet JP, Caterina R, Fauchier L, Lane DA, Larsen TB. et al. Antithrombotic therapy in atrial fibrillation associated with valvular heart disease: a joint consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology Working Group on Thrombosis, endorsed by the ESC Working Group on Valvular Heart Disease, Cardiac Arrhythmia Society of Southern Africa (CASSA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), South African Heart (SA Heart) Association and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE). Europace 2017;19:1757–8. [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 3. Chugh SS, Roth GA, Gillum RF, Mensah GA.. Global burden of atrial fibrillation in developed and developing nations. Glob Heart 2014;9:113–9. [DOI] [PubMed] [Google Scholar]
- 4. Iung B, Vahanian A.. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol 2011;8:162–72. [DOI] [PubMed] [Google Scholar]
- 5. Oldgren J, Healey JS, Ezekowitz M, Commerford P, Avezum A, Pais P. et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry. Circulation 2014;129:1568–76. [DOI] [PubMed] [Google Scholar]
- 6. De Caterina R, Camm AJ.. What is ‘valvular’ atrial fibrillation? A reappraisal. Eur Heart J 2014;35:3328–35. [DOI] [PubMed] [Google Scholar]
- 7. Philippart R, Brunet-Bernard A, Clementy N, Bourguignon T, Mirza A, Babuty D. et al. Prognostic value of CHA2DS2-VASc score in patients with ‘non-valvular atrial fibrillation’ and valvular heart disease: the Loire Valley Atrial Fibrillation Project. Eur Heart J 2015;36:1822–30. [DOI] [PubMed] [Google Scholar]
- 8. Thomas KL, Jackson LR, Shrader P, Ansell J, Fonarow GC, Gersh B. et al. Prevalence, characteristics, and outcomes of valvular heart disease in patients with atrial fibrillation: insights from the ORBIT-AF (Outcomes Registry for Better Informed Treatment for Atrial Fibrillation). J Am Heart Assoc 2017;6:e006475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP. et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1–23; quiz 101–2. [DOI] [PubMed] [Google Scholar]
- 10. Casciano JP, Dotiwala ZJ, Martin BC, Kwong WJ.. The costs of warfarin underuse and nonadherence in patients with atrial fibrillation: a commercial insurer perspective. J Manag Care Pharm 2013;19:302–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hemingway H, Asselbergs FW, Danesh J, Dobson R, Maniadakis N, Maggioni A. et al. Big data from electronic health records for early and late translational cardiovascular research: challenges and potential. Eur Heart J 2018;39:1481–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andell P, Li X, Martinsson A, Andersson C, Stagmo M, Zoller B. et al. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart 2017;103:1696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Denaxas SC, George J, Herrett E, Shah AD, Kalra D, Hingorani AD. et al. Data resource profile: cardiovascular disease research using linked bespoke studies and electronic health records (CALIBER). Int J Epidemiol 2012;41:1625–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mathur R, Bhaskaran K, Chaturvedi N, Leon DA, vanStaa T, Grundy E. et al. Completeness and usability of ethnicity data in UK-based primary care and hospital databases. J Public Health (Oxf) 2014;36:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T. et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol 2015;44:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. George J, Rapsomaniki E, Pujades-Rodriguez M, Shah AD, Denaxas S, Herrett E. et al. How does cardiovascular disease first present in women and men? Incidence of 12 cardiovascular diseases in a contemporary cohort of 1,937,360 people. Circulation 2015;132:1320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pujades-Rodriguez M, George J, Shah AD, Rapsomaniki E, Denaxas S, West R. et al. Heterogeneous associations between smoking and a wide range of initial presentations of cardiovascular disease in 1937360 people in England: lifetime risks and implications for risk prediction. Int J Epidemiol 2015;44:129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S. et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014;383:1899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alatorre CI, Hoogwerf BJ, Deeg MA, Nelson DR, Hunter TM, Ng WT. et al. Factors associated with stroke, myocardial infarction, ischemic heart disease, unstable angina, or mortality in patients from real world clinical practice with newly-diagnosed type 2 diabetes and early glycemic control. Curr Med Res Opin 2018;34:337–43. [DOI] [PubMed] [Google Scholar]
- 20. Archangelidi O, Pujades-Rodriguez M, Timmis A, Jouven X, Denaxas S, Hemingway H.. Clinically recorded heart rate and incidence of 12 coronary, cardiac, cerebrovascular and peripheral arterial diseases in 233,970 men and women: a linked electronic health record study. Eur J Prev Cardiol 2018;25:1485–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.