Abstract
Rationale: Rare genetic variants in telomere-related genes have been identified in familial, idiopathic, and rheumatoid arthritis–associated pulmonary fibrosis. Short peripheral blood leukocyte (PBL) telomere length predicts poor outcomes in chronic hypersensitivity pneumonitis (CHP).
Objectives: Determine the prevalence and clinical relevance of rare protein-altering variants in telomere-related genes in patients with CHP.
Methods: Next-generation sequences from two CHP cohorts were analyzed to identify variants in TERT (telomerase reverse transcriptase), TERC (telomerase RNA component), DKC1 (dyskerin pseudouridine synthase 1), RTEL1 (regulator of telomere elongation helicase 1), PARN (poly[A]-specific RNase), and TINF2 (TERF1-interacting nuclear factor 2). To qualify, variants were required to have a minor allele frequency less than 0.005 and be predicted to be damaging to protein function. Variant status (binary variable) was used in statistical association tests, including Cox proportional hazard models for transplant-free survival. PBL telomere length was measured using quantitative PCR.
Measurements and Main Results: Qualifying variants were identified in 16 of 144 patients (11.1%; 95% confidence interval [CI], 6.5–17.4) in the discovery cohort and 17 of 209 patients (8.1%; 95% CI, 4.8–12.7) in the replication cohort. Age- and ancestry-adjusted PBL telomere length was significantly shorter in the presence of a variant in both cohorts (discovery: −561 bp; 95% CI, −933 to −190; P = 0.003; replication: −612 bp; 95% CI, −870 to −354; P = 5.30 × 10−6). Variant status was significantly associated with transplant-free survival in both cohorts (discovery: age-, sex-, and ancestry-adjusted hazard ratio, 3.73; 95% CI, 1.92–7.28; P = 0.0001; replication: hazard ratio, 2.72; 95% CI, 1.26–5.88; P = 0.011).
Conclusions: A substantial proportion of patients diagnosed with CHP have rare, protein-altering variants in telomere-related genes, which are associated with short peripheral blood telomere length and significantly reduced transplant-free survival.
Keywords: alveolitis, extrinsic allergic, telomere, prognosis
At a Glance Summary
Scientific Knowledge on the Subject
Rare variants in telomere-related genes (TERT [telomerase reverse transcriptase], TERC [telomerase RNA component], DKC1 [dyskerin pseudouridine synthase 1], RTEL1 [regulator of telomere elongation helicase 1], PARN [poly[A]-specific RNase], and TINF2 [TERF1-interacting nuclear factor 2]) have been identified in families with pulmonary fibrosis and sporadic idiopathic pulmonary fibrosis. Although most patients with familial pulmonary fibrosis are diagnosed with IPF, up to 12% are diagnosed with chronic hypersensitivity pneumonitis (CHP). Short peripheral blood telomere length has been associated with reduced transplant-free survival in patients with CHP.
What This Study Adds to the Field
This is the first study to demonstrate that a substantial proportion of patients diagnosed with CHP have rare protein-altering variants in telomere-related genes, which are associated with short peripheral blood telomere length and significantly reduced transplant-free survival. These findings support the role of telomere dysfunction in the pathogenesis and prognosis of a subset of patients with CHP.
Hypersensitivity pneumonitis (HP) is an interstitial lung disease caused by an abnormal immune response to a variety of inhaled antigens (1). A subset of patients with HP develops significant lung fibrosis, the hallmark of chronic HP (CHP) (1). Lung fibrosis in CHP is believed to develop from chronic or repetitive lung injury resulting from persistent interstitial lung inflammation, possibly due to ongoing antigen exposure. The presence and extent of lung fibrosis are strong predictors of mortality in CHP (2). However, it remains poorly understood why only a subset of patients with HP develops progressive lung fibrosis.
Telomere dysfunction is a core mechanism in the pathogenesis of idiopathic pulmonary fibrosis (IPF), and emerging evidence suggests it may also be important in a subset of patients with CHP (3, 4). For example, rare variants in six telomere-related genes (TERT [telomerase reverse transcriptase], TERC [telomerase RNA component], DKC1 [dyskerin pseudouridine synthase 1], RTEL1 [regulator of telomere elongation helicase 1], PARN [poly[A]-specific RNase], and TINF2 [TERF1-interacting nuclear factor 2]) have been identified in families with pulmonary fibrosis. Although the majority of affected family members are diagnosed with IPF, up to 12% are diagnosed with CHP (5–13). In addition, shorter telomere length measured in peripheral blood leukocytes (PBLs) of patients with CHP is associated with more severe lung fibrosis, features of lung remodeling typical of usual interstitial pneumonia (UIP), and reduced survival (3). Whether telomere shortening in patients with CHP is due to defects in genes responsible for telomere maintenance is unknown.
Using next-generation sequencing in two well-described cohorts of patients with CHP, our objective was to determine the prevalence of otherwise rare, protein-altering variants in the six telomere-related genes that have previously been identified in familial pulmonary fibrosis and IPF as well as their associations with clinical features and outcomes.
Methods
Study Participants
The discovery cohort included patients diagnosed with CHP at the University of California San Francisco (UCSF) who were prospectively enrolled into a database and biorepository between September 2003 and March 2016. The replication cohort consisted of patients diagnosed with CHP at three interstitial lung disease (ILD) centers: University of California Davis (March 2011 to December 2017), the University of Chicago (October 2001 to August 2016), and additional patients from UCSF not included in the discovery cohort (May 2006 to November 2017). Local institutional review boards approved these registries and biorepositories (UCSF 10-01592 and 10-00198; University of California Davis 585448-7 and 875917-2, University of Chicago 14163A), and all subjects provided written informed consent at the time of enrollment, including consent for use of samples in future genetics studies. All patients who had a confident diagnosis of CHP made by in-person multidisciplinary team discussion and who provided peripheral blood were included. At each institution, a confident diagnosis of CHP generally required either 1) well-established exposure and classic high-resolution computed tomography findings (i.e., mixed pattern of fibrosis and air trapping in three or more lobes with or without ground-glass opacities or centrilobular nodules), or 2) surgical lung biopsy most consistent with CHP. These criteria are consistent with those reported in a recent international Delphi survey identifying diagnostic criteria for CHP (14). All patients with a multidisciplinary diagnosis of IPF enrolled into the UCSF database over the same time period were used for survival comparisons. Internal whole-genome sequencing datasets from patients with asthma and age-related macular degeneration were used for comparison to the discovery cohort, and the Exome Aggregation Consortium (v0.3.1) dataset (ExAC) was used for comparison to the replication cohort. See online supplement for additional details, including clinical, radiographic, and histopathologic data collection.
DNA Analysis
Whole-genome sequencing (30× coverage) and whole-exome sequencing (50× coverage) were performed on DNA isolated from peripheral blood samples in the discovery and replication cohorts, respectively. Variant discovery was performed following the Genome Analysis Toolkit best practices (15–18). See supplemental methods in the online supplement for more detailed sequencing and variant discovery methods. Qualifying variants in six telomere-related genes (TERT, TERC, DKC1, RTEL1, PARN, and TINF2) were defined as 1) rare (minor allele frequency [MAF] less than 0.005 in ExAC and the Exome Sequencing Project), and 2) protein altering (loss-of-function variants or missense variants predicted to be at least “possibly damaging” by Polyphen-2 [19, 20] or “damaging” by Sorting Intolerant from Tolerant [SIFT]; 21–25]). Combined Annotation Dependent Depletion (CADD) version 1.5 scores were calculated for each qualifying variant but were not used as selection criteria for qualifying variants (26). PBL telomere length was measured using quantitative PCR in triplicate as previously described (27). The intra-assay intraclass correlation coefficient for triplicate measurements was 0.901.
Statistical Analysis
For all analyses, a simple burden test was applied in which patients were coded as possessing or not possessing (a binary predictor variable) at least one qualifying telomere-related gene variant (no patients had more than one qualifying variant). Variant burden (per individual) in cases was compared with control subjects using the odds ratio (OR) and Fisher exact test (see additional methodologic details in the online supplement). Principal component analysis was performed in Plink version 1.9 (see Figures E3–E6 in the online supplement). The association of variant status with clinical, radiographic, and histopathologic features was evaluated using the t test or Wilcoxon rank sum test, where appropriate, for continuous features, and the Fisher exact test for categorical features. Difference in telomere length by variant status was evaluated using a linear regression model adjusted for age and the first two principal components. Transplant-free survival (the primary outcome) was defined as time from initial diagnosis to either death or lung transplantation, with right-censoring at the date of last vital status/transplant status verification. Transplant-free survival was compared by variant status using Kaplan-Meier plots and the log-rank test. Three Cox proportional hazards models were constructed, including 1) an unadjusted model including only variant status; 2) an adjusted model including the key confounders of age, sex, and first two principal components; and 3) a model additionally adjusted for baseline FVC % predicted. All analyses were performed in the whole cohort and then restricted to the European subgroup (as defined by principal component analysis). Pooled analyses were then performed combining all patients from both cohorts to evaluate transplant-free survival stratified by MUC5B (mucin 5B gene) genotype, variant status, and PBL telomere length. In these analyses, short PBL telomere length was defined as the lowest quartile of telomere length. Pooled survival analyses were adjusted for age, sex, FVC % predicted, and self-reported race/ethnicity (non-Hispanic white vs. other).
Results
Cohort Comparisons and Qualifying Telomere-related Variants
A total of 144 and 209 patients were included in the discovery and replication cohorts, respectively (Table 1). The prevalence of a qualifying telomere-related variant was 16 of 144 (11.1%; 95% confidence interval [CI], 6.5–17.4) in the discovery cohort and 17 of 209 (8.1%; 95% CI, 4.8–12.7) in the replication cohort (P = 0.345). In patients without a family history of ILD (i.e., sporadic CHP), the prevalence was 11 of 132 (8.3%; 95% CI, 4.2–14.4) and 15 of 195 (7.7%; 95% CI, 4.4–12.4), respectively. The burden of qualifying variants was significantly increased in the discovery (OR, 4.49; P = 0.0017) and replication cohorts (OR, 4.46; P = 1.94 × 10−8) compared with control subjects (Tables E1–E3). This association was primarily driven by the increased frequency of qualifying variants in TERT and RTEL1 in both cohorts. TERT and RTEL1 variants were most common among qualifying variants, followed by PARN variants (Table 2; see also Tables E4 and E5). PBL telomere length was significantly shorter in those with a variant than those without a variant in both cohorts after adjustment for age and the first two principal components: difference in bp, −561 (95% CI, −933 to −190; P = 0.003) in the discovery cohort and −612 (95% CI, −870 to −354; P = 5.30 × 10−6) in the replication cohort.
Table 1.
Baseline Characteristics
| Characteristic | Discovery Cohort | Replication Cohort |
|---|---|---|
| N | 144 | 209 |
| Enrollment date range | Sept. 10, 2003, to Feb. 18, 2016 | Oct. 28, 2001, to Dec. 14, 2017 |
| Age, mean (SD), yr | 63.5 (11.2) | 65.2 (10.3) |
| Sex, M, n (%) | 59 (41.0) | 91 (43.5) |
| Self-reported race/ethnicity, n (%) | ||
| African American | 3 (2.1) | 10 (4.8) |
| Asian | 4 (2.8) | 4 (1.9) |
| European | 121 (84.0) | 169 (80.9) |
| Hispanic | 13 (9.0) | 18 (8.6) |
| Other or unknown | 3 (2.1) | 8 (3.8) |
| Family history, n (%) | 12 (8.3) | 14 (6.7) |
| Ever-smoker, n (%) | 73 (52.5) | 109 (52.2) |
| Exposure type, n (%) | ||
| Avian | 69 (48.0) | 88 (42.1) |
| Mold | 26 (18.1) | 45 (21.5) |
| Other | 4 (2.8) | 9 (4.3) |
| Unknown | 45 (31.2) | 67 (32.1) |
| FVC % predicted, mean (SD) | 67.3 (19.0) | 65.5 (18.2) |
| DlCO % predicted, mean (SD) | 47.2 (16.6) | 59.0 (21.2) |
| UIP pattern, n/total (%) | ||
| Definite | 5/134 (3.7) | 13/204 (6.4) |
| Possible | 12/134 (9.0) | 16/204 (7.9) |
| Inconsistent | 117/134 (87.3) | 173/204 (85.6) |
| CT fibrosis, n/total (%) | 110/134 (82.1) | 172/202 (85.1) |
| Surgical lung biopsy, n (%) | 91 (63.2) | 116 (55.5) |
| MUC5B genotype, n (%) | ||
| G/G | 81 (59.6) | 114 (54.8) |
| G/T | 50 (36.8) | 86 (41.3) |
| T/T | 5 (3.7) | 8 (3.8) |
| Telomere length, median (IQR), bp | 6,075 (5,579–6,563) | 6,060 (5,627–6,369) |
Definition of abbreviations: CT = computed tomography of the chest; IQR = interquartile range; MUC5B = mucin 5B gene; UIP = usual interstitial pneumonia.
Table 2.
Qualifying Telomere-related Gene Variants Identified in the Discovery and Replication Cohorts and Detailed Clinical Information of Patients with Variants
| Gene | dbSNP | CHR | Position* | REF | ALT | Genome Effect | Exon† | Effect on Protein | ExAC AF | ESP AF | PP2‡ | SIFT§ | CADD|| | Cohort¶ | Race/Ethnicity** | Exposure†† | FH | TL (bp) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TERT | rs370445231 | 5 | 1260593 | G | A | Missense | 12/16 | R951W | 8.26 × 10−6 | Absent | D (0.999) | T (0.106) | 17.5 | Replication | Hispanic | Mold | Yes | 5,559 |
| TERT | rs775014633 | 5 | 1268538 | G | A | Missense | 9/16 | A855V | 8.24 × 10−6 | Absent | P (0.522) | T (0.199) | 7.4 | Discovery | White | Down | No | 5,266 |
| TERT | rs387907249 | 5 | 1278781 | G | A | Missense | 6/16 | A716T | Absent | Absent | D (0.998) | D (0.0) | 23.1 | Discovery | White | Bird | Yes | 5,055 |
| TERT | rs866282352 | 5 | 1279329 | G | A | Missense | 5/16 | R698W | Absent | Absent | D (0.981) | D (0.008) | 23.1 | Discovery | White | Mold | No | 4,248 |
| TERT | N/A | 5 | 1280334 | G | T | Missense | 4/16 | H592N | Absent | Absent | D (0.996) | D (0.005) | 23.7 | Replication | White | Unknown | No | 4,312 |
| TERT | N/A | 5 | 1282488 | C | A | Missense | 3/16 | K570N | Absent | Absent | D (0.965) | D (0.0) | 25.0 | Discovery | White | Bird | Yes | 4,832 |
| TERT | N/A | 5 | 1282507 | G | A | Missense | 3/16 | T564M | Absent | Absent | D (1.0) | D (0.0) | 26.5 | Replication | African American | Unknown | No | 5,380 |
| TERT | N/A | 5 | 1293394 | C | T | Missense | 2/16 | G498R | Absent | Absent | D (1.0) | D (0.0) | 25.5 | Replication | Hispanic | Down | No | 4,783 |
| TERT | rs34094720 | 5 | 1293652 | G | A | Missense | 2/16 | H412Y | 0.0022 | 0.0043 | D (0.998) | D (0.007) | 20.2 | Discovery | Hispanic | Unknown | No | 6,511 |
| Discovery | White | Unknown | Yes | 5,782 | ||||||||||||||
| Discovery | White | Unknown | No | 5,486 | ||||||||||||||
| Replication | White | Unknown | No | 5,516 | ||||||||||||||
| TERT | N/A | 5 | 1294393 | T | C | Missense | 2/16 | S165G | Absent | Absent | P (0.734) | D (0.005) | 21.1 | Replication | White | Mold | No | 5,415 |
| TERT | rs1060502990 | 5 | 1294549 | C | CG | Frameshift | 2/16 | 112-113X | Absent | Absent | N/A | N/A | 15.9 | Replication | White | Mold | No | 4,762 |
| TERT | N/A | 5 | 1294656 | A | G | Missense | 2/16 | L77P | Absent | Absent | D (0.0997) | D (0.028) | 23.8 | Replication | White | Unknown | No | 4,493 |
| TERT | N/A | 5 | 1294829 | C | T | Missense | 1/16 | C54Y | Absent | Absent | D (1.0) | D (0.011) | 23.1 | Replication | White | Bird | No | 5,010 |
| TERC | rs552679780 | 3 | 169765027 | C | T | N/A | N/A | N/A | Absent | Absent | N/A | N/A | 10.4 | Discovery | Asian | Unknown | Yes | 4,850 |
| PARN | rs377199187 | 16 | 14432695 | C | G | Missense | 22/24 | R538P | 8.28 × 10−5 | 3.63 × 10−4 | P (0.928) | T (0.055) | 4.1 | Discovery | White | Unknown | No | 5,863 |
| PARN | N/A | 16 | 14554142 | T | C | Missense | 20/24 | K443R | Absent | Absent | P (0.755) | T (0.167) | 25.5 | Replication | White | Bird | No | 5,284 |
| PARN | rs774170618 | 16 | 14604184 | G | A | Missense | 11/24 | R249C | 3.31 × 10−5 | Absent | D (0.998) | D (0.016) | 31 | Discovery | White | Mold | No | 5,683 |
| PARN | N/A | 16 | 14610777 | G | A | Stop gained | 7/24 | Q141* | Absent | Absent | N/A | N/A | 31 | Replication | White | Bird | No | 5,788 |
| RTEL1 | rs151214675 | 20 | 63661882 | G | A | Missense | 4/35 | A112T | 2.22 × 10−4 | 2.33 × 10−4 | D (0.998) | D (0.005) | 27.3 | Discovery | White | Down | No | 5,767 |
| Replication | White | MAC | No | 6,216 | ||||||||||||||
| RTEL1 | N/A | 20 | 63672587 | G | T | Missense | 9/35 | G244V | Absent | Absent | D (0.965) | D (0.003) | 27.8 | Discovery | White | Down | Yes | 5,386 |
| RTEL1 | N/A | 20 | 63688042 | T | A | Missense | 18/35 | F529L | Absent | Absent | P (0.85) | D (0.01) | 22.6 | Discovery | White | Mold | No | 6,630 |
| RTEL1 | rs1035926074 | 20 | 63689829 | G | A | Missense | 24/35 | R702H | Absent | Absent | D (1.0) | D (0.005) | 26.0 | Replication | White | Mold/Bird | No | 6,490 |
| RTEL1 | rs775121139 | 20 | 63690303 | C | G | Missense | 26/35 | P759A | 1.66 × 10−5 | Absent | P (0.751) | D (0.021) | 9.5 | Replication | White | Unknown | No | 6,139 |
| RTEL1 | rs772872062 | 20 | 63691764 | C | T | Missense | 28/35 | S860F | 3.31 × 10−5 | Absent | P (0.848) | T (0.137) | 19.8 | Replication | White | Unknown | Yes | 5,273 |
| RTEL1 | rs754727644 | 20 | 63692883 | A | T | Missense | 23/35 | N911Y | Absent | Absent | B (0.271) | D (0.013) | 22.4 | Discovery | White | Unknown | No | 4,839 |
| RTEL1 | rs753270617 | 20 | 63693161 | G | A | Missense | 30/35 | R957Q | 8.28 × 10−6 | Absent | D (0.996 | D (0.014) | 25.9 | Discovery | White | Down | No | 5,091 |
| RTEL1 | rs759564073 | 20 | 63693208 | G | A | Missense | 30/35 | G973R | 8.29 × 10−6 | Absent | D (1.0) | D (0.003) | 25.9 | Replication | Unknown | Unknown | No | 5,478 |
| RTEL1 | rs367598119 | 20 | 63695356 | G | C | Missense | 34/35 | K1176N | 1.67 × 10−5 | Absent | P (0.501) | D (0.012) | 17.0 | Replication | White | Mold | No | 6,636 |
| DKC1 | rs146700772 | X | 154769233 | A | C | Missense | 9/15 | S280R | 2.80 × 10−4 | 1.50 × 10−4 | P (0.519) | D (0.042) | 23.3 | Discovery | White | Unknown | No | 5,243 |
Definition of abbreviations: ALT = alternate allele; CADD = Combined Annotation Dependent Depletion score; CHR = chromosome number; dbSNP = database of short genetic variations identifiers, build 150, released July 10, 2017; DKC1 = dyskerin pseudouridine synthase 1; ESP AF = exome sequencing project allele frequency; ExAC AF = Exome Aggregation Consortium allele frequency; FH = family history of interstitial lung disease; MAC = Mycobacterium avium complex; N/A = not applicable; PARN = poly(A)-specific RNase; PP2 = polyphen-2 prediction of effect on protein function; REF = reference genome allele; RTEL1 = regulator of telomere elongation helicase 1; SIFT = Sorting Intolerant from Tolerant prediction of effect on protein function; TERC = telomerase RNA component; TERT = telomerase reverse transcriptase; TL = telomere length in base pairs.
Position = genomic position, human genome version 38.
Exon number/total exons.
PP2 format: D = probably damaging, P = possibly damaging, B = benign (hdiv score).
SIFT format: D = damaging, T = tolerated (score).
CADD score 10 or greater indicates top 10% of most deleterious in genome, and score 20 or greater indicates top 1%.
Cohort: Cohort in which variant was identified.
Race/Ethnicity: Self-reported race/ethnicity.
Exposure: Exposure clinically suspected to be causing hypersensitivity pneumonitis.
Association of Variant Status with Clinical, Radiographic, and Pathologic Features
There were no statistically significant differences by variant status, in either cohort, in several baseline clinical features, including age at diagnosis, sex, self-reported race/ethnicity, smoking history, whether an exposure was identified, exposure type, or MUC5B genotype (Table E6). In the discovery cohort, patients with a variant were significantly more likely to have a family history of ILD than patients without a variant (31.2% vs. 5.5%, respectively; P = 0.004). In the replication cohort, patients with a variant had a nonsignificant trend toward lower mean baseline FVC % predicted (66.2 vs. 57.5; P = 0.057) and DlCO % predicted (59.8 vs. 49.7; P = 0.077) than patients without a variant. Variant status was not associated with any of the measured baseline computed tomography morphologic features in either cohort, including 2011 American Thoracic Society/European Respiratory Society UIP pattern classification (28), extent of fibrosis, honeycombing, traction bronchiectasis, craniocaudal or axial distribution, ground-glass opacities, or mosaic perfusion/air trapping (Table E7). The subgroup of patients drawn from UCSF who had prospectively scored surgical lung histopathology included 75 from the discovery cohort and 35 from the replication cohort (total n = 110; 12 variant-positive and 98 variant-negative). In this subset, there was a statistically significant association of variant status with increased profusion of fibroblast foci, adjusting for age and ancestry (P = 0.004). This analysis was exploratory and not corrected for multiple comparisons. There were no significant associations with other histopathologic features typical of UIP (microscopic honeycombing, heterogeneous distribution, and subpleural fibrosis) or HP (bronchiolocentric fibrosis, interstitial granulomas or giant cells, or lymphocytic inflammation; Table E8).
Association of Variant Status with Transplant-Free Survival
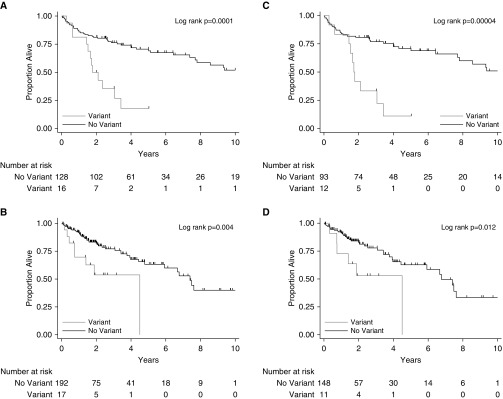
The median follow-up time was 3.3 years (interquartile range, 2.0–6.0 yr) and 1.6 years (interquartile range, 1.0–3.2 yr) in the discovery and replication cohorts, respectively. In the discovery cohort, 45 of 144 (31%) patients died and 11 of 144 (7.6%) underwent lung transplantation, for a total of 56 events occurring in 38.6% of the cohort. In the replication cohort, 46 of 209 (22%) patients died and 8 of 209 (3.8%) underwent lung transplantation, for a total of 54 events occurring in 25.8% of the cohort. Median transplant-free survival for those with versus without a variant was 1.8 years (95% CI, 1.44–3.42) versus 10.7 (95% CI, 7.7 to infinity) in the discovery cohort (log-rank P = 0.0001) and 4.5 years (95% CI, 0.7 to infinity) versus 7.4 (95% CI, 5.9 to infinity) in the replication cohort (log-rank P = 0.004) (Figures 1A and 1B). After adjusting for age, sex, and the first two principal components, there was a significantly increased risk of death or lung transplantation for patients with a variant versus without a variant in the discovery (hazard ratio [HR], 3.73; 95% CI, 1.92–7.28; P = 0.0001) and replication cohorts (HR, 2.72; 95% CI, 1.26–5.88; P = 0.011) (Table 3). This effect was slightly attenuated in the replication cohort, but not the discovery cohort, after additional adjustment for baseline FVC % predicted. The observed effects were similar in the analysis restricted to the European subgroup (Table 3 and Figures 1C and 1D). There was no evidence for a cohort difference in the effect of variant status on transplant-free survival (P value for interaction = 0.57).
Figure 1.
Kaplan-Meier plots of transplant-free survival by telomere-related gene variant status in patients with chronic hypersensitivity pneumonitis, including all patients in the (A) discovery and (B) replication cohorts, and within the genetically defined subgroup of European patients in the (C) discovery and (D) replication cohorts.
Table 3.
Association of Telomere-related Gene Variant Status with Transplant-Free Survival in Patients with Chronic Hypersensitivity Pneumonitis
| Model/Variables | Discovery |
Replication |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | HR | LCL | UCL | P Value | N | HR | LCL | UCL | P Value | |
| All patients | 144 | 209 | ||||||||
| Unadjusted: telomere variant | 3.38 | 1.76 | 6.49 | 0.0002 | 2.93 | 1.36 | 6.30 | 0.006 | ||
| Adjusted, model 1*: telomere variant | 3.73 | 1.92 | 7.28 | 0.0001 | 2.72 | 1.26 | 5.88 | 0.011 | ||
| Adjusted, model 2†: telomere variant | 3.28 | 1.66 | 6.46 | 0.001 | 2.10 | 0.97 | 4.58 | 0.061 | ||
| European | 105 | 159 | ||||||||
| Unadjusted: telomere variant | 4.26 | 2.01 | 9.01 | 0.0002 | 2.92 | 1.21 | 7.03 | 0.017 | ||
| Adjusted, model 1*: telomere variant | 4.17 | 1.94 | 8.98 | 0.0003 | 2.74 | 1.12 | 6.72 | 0.028 | ||
| Adjusted, model 2†: telomere variant | 3.06 | 1.39 | 0.74 | 0.006 | 2.08 | 0.83 | 5.22 | 0.117 | ||
Definition of abbreviations: HR = hazard ratio; LCL = lower 95% confidence limit; UCL = upper 95% confidence limit.
Model 1 adjusted for age, sex, and first two principal components.
Model 2 adjusted for age, sex, FVC % predicted, and first two principal components.
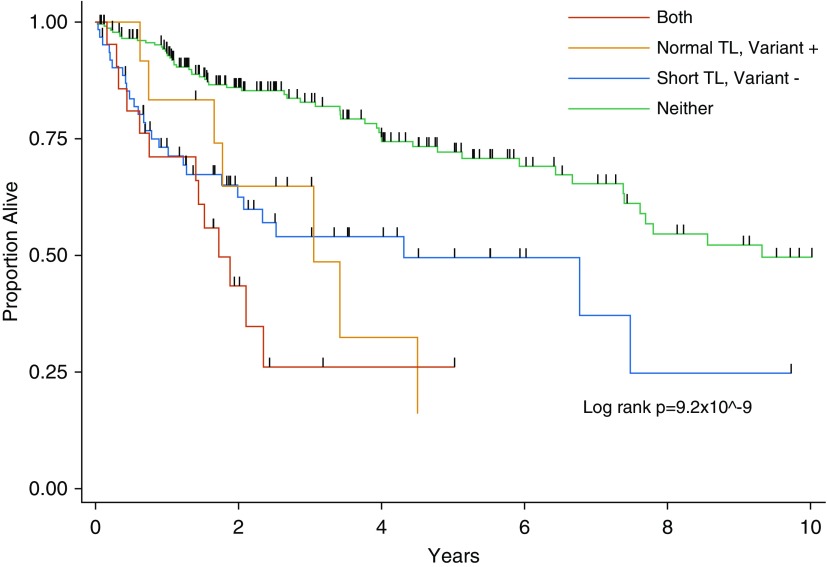
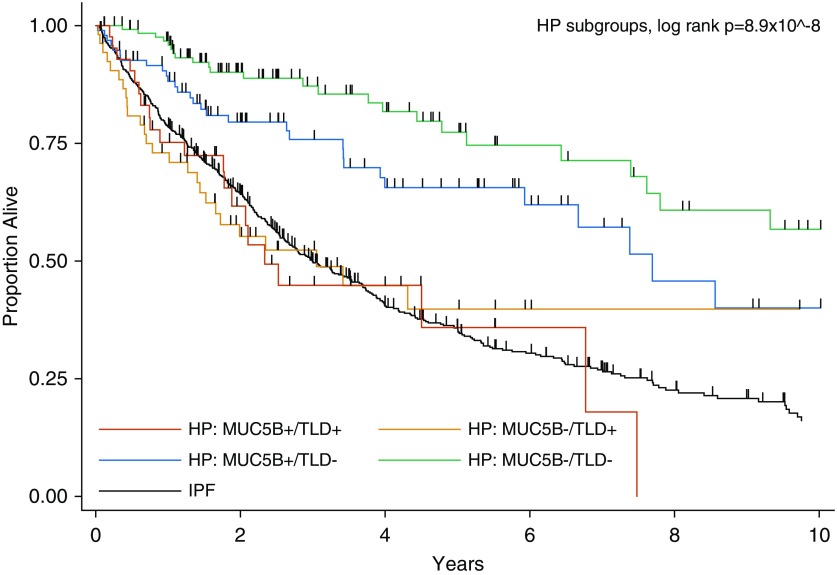
In the pooled cohort, transplant-free survival was similarly reduced for patients with any evidence of telomere dysfunction (short PBL telomeres and/or telomere-related gene variant) compared with those with longer PBL telomeres and no variant (Figure 2 and Table 4). Patients were then categorized by presence or absence of telomere dysfunction (defined as short PBL telomeres and/or a telomere-related gene variant) and MUC5B minor allele status. Compared with patients who were MUC5B homozygous wild type and had no evidence of telomere dysfunction, patients with at least one MUC5B minor allele but no evidence of telomere dysfunction had intermediate transplant-free survival (HR, 1.59; 95% CI, 0.91–2.77; P = 0.103), and patients with telomere dysfunction (regardless of MUC5B genotype) had the worst transplant-free survival (HRs, 3.38–3.52; both P < 0.0001), which was overall similar to the average patient with IPF (Figure 3 and Table 5).
Figure 2.
Kaplan-Meier plot of transplant-free survival in the pooled cohort of patients with chronic hypersensitivity pneumonitis categorized by peripheral blood leukocyte telomere length (TL) and telomere-related gene variant status.
Table 4.
Transplant-Free Survival in the Pooled Cohort of Patients with Chronic Hypersensitivity Pneumonitis Categorized by Peripheral Blood Leukocyte Telomere Length and Telomere-related Gene Variant Status
| Variable* | HR | 95% CI | P Value | n (%) |
|---|---|---|---|---|
| Normal TL/no variant | Ref | Ref | Ref | 234 (71.1) |
| Short TL/no variant | 2.37 | 1.44–3.90 | 0.0007 | 62 (18.8) |
| Normal TL/variant | 2.56 | 1.15–5.68 | 0.021 | 12 (3.6) |
| Short TL/variant | 3.94 | 2.08–7.48 | 0.00003 | 21 (7.3) |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; Ref = reference; TL = telomere length.
Adjusted for age, sex, FVC % predicted, and self-reported race/ethnicity (non-Hispanic white vs. other). Normal TL indicates upper three quartiles of peripheral blood leukocyte TL for the cohort; short TL indicates lowest quartile of peripheral blood leukocyte TL for the cohort; no variant indicates absence of a qualifying telomere-related gene variant; and variant indicates presence of a qualifying telomere-related gene variant. Comparison of “normal TL/variant” group to “short TL/no variant group,” P = 0.86; “short TL/variant” to “short TL/no variant group,” P = 0.15; and “short TL/variant” to “normal TL/variant group,” P = 0.36.
Figure 3.
Kaplan-Meier plot of transplant-free survival in the pooled cohort of patients with chronic hypersensitivity pneumonitis (HP) categorized by MUC5B (mucin 5B gene) minor allele status and telomere dysfunction (TLD). IPF = idiopathic pulmonary fibrosis.
Table 5.
Transplant-Free Survival in the Pooled Cohort of Patients with Chronic Hypersensitivity Pneumonitis Categorized by MUC5B Minor Allele Status and Telomere Dysfunction
| Variable* | HR | 95% CI | P Value | n (%) |
|---|---|---|---|---|
| MUC5B−/TLD− | Ref | Ref | Ref | 129 (40.2) |
| MUC5B+/TLD− | 1.59 | 0.91–2.77 | 0.103 | 97 (30.2) |
| MUC5B−/TLD+ | 3.38 | 1.87–6.08 | 0.00005 | 53 (16.5) |
| MUC5B+/TLD+ | 3.52 | 1.87–6.62 | 0.00009 | 42 (13.1) |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; MUC5B = mucin 5B gene; Ref = reference; TLD = telomere dysfunction.
Adjusted for age, sex, FVC % predicted, and self-reported race/ethnicity (non-Hispanic white vs. other). MUC5B− = homozygous wild-type (GG) for MUC5B rs35705950 SNP; MUC5B+ = at least one copy of the minor allele (GT or TT) for MUC5B rs35705950 SNP; TLD− = normal telomere length and absence of a qualifying telomere-related gene variant; TLD+ = short telomere length and/or presence of a qualifying telomere-related gene variant; comparison of MUC5B−/TLD+ to MUC5B+/TLD−, P = 0.006; MUC5B+/TLD+ to MUC5B+/TLD−, P = 0.0075; and MUC5B+/TLD+ to MUC5B−/TLD+, P = 0.89.
Discussion
This study demonstrates for the first time that a subset of patients diagnosed with CHP harbors rare protein-altering variants in telomere-related genes at an increased frequency compared with control populations and that these variants are associated with shorter peripheral blood telomere length and significantly reduced transplant-free survival. These findings were reproduced independently in two cohorts of patients drawn from three expert ILD centers using two different sequencing methods. They provide further evidence for the role of telomere dysfunction in the pathogenesis and prognosis of CHP.
Although criteria for qualifying variants have varied among studies, the prevalence of otherwise rare protein-altering telomere-related gene variants found in this study of CHP (8.1–11.1%) is remarkably similar to that identified in previous studies of sporadic IPF (9.0–11.3%) (29, 30) and rheumatoid arthritis–associated ILD (RA-ILD) (9.9%) (31). To compare more directly, we determined the frequency of qualifying variants in TERT, RTEL1, and PARN (the genes common across all three prior studies) in our study among non-Hispanic white patients that would have also met the inclusion criteria for these prior studies (Table E10). Petrovski and colleagues applied the strictest variant criteria in terms of MAF and predicted effects on protein function in patients with sporadic IPF. Variant frequency in our CHP cases (3.3–3.6%) is intermediate between that found in IPF (9.9%) and control subjects (0.26%) in the Petrovski and colleagues study (29). Juge and colleagues applied less strict criteria in terms of MAF but similar criteria for predicted effects on protein function, compared with our study, in patients with RA-ILD (31). The frequency of variants in our CHP cases that would have met these criteria was again intermediate (7.7–8.3%) between RA-ILD (12.3%) and control subjects (3.8%) in the Juge and colleagues study (31). Finally, the Dressen and colleagues study applied less strict criteria for MAF and predicted protein effects, compared with our study, to whole-genome sequence datasets of patients with IPF included in clinical trials and internal control subjects and found a qualifying variant frequency (8.5%) that is similarly increased in our CHP cases (8.3–9.9%) compared with their control subjects (2.9%) (30). Similar to these prior studies, we found that rare, protein-altering variants in telomere-related genes are increased in both CHP cohorts compared with available control datasets.
We selected, a priori, criteria for qualifying variants that were less strict than those applied in the Petrovski and colleagues study (29) on the basis of the hypothesis that “more common” and “less deleterious” variants that may have lower penetrance for causing disease responsible for idiopathic and familial cases would impact survival through disease modification in the context of established hypersensitivity pneumonitis. The reduced transplant-free survival observed in patients with CHP harboring rare telomere-related gene variants in our study is consistent with the poor prognosis previously observed in patients with familial pulmonary fibrosis with rare telomere variants, regardless of clinical diagnosis (13). In the context of these prior studies, our findings contribute to the mounting evidence that telomere dysfunction contributes to the pathogenesis and poor prognosis of multiple clinical subtypes of fibrotic interstitial lung disease.
This genetic association study did not explore the biochemical impact of the identified variants. However, 26 of 29 identified variants had a CADD score of 10 or greater, and 20 of 29 had a score of 20 or greater, indicating they are predicted to be in the 10% and 1%, respectively, most deleterious substitutions possible in the human genome. Furthermore, PBL telomere length was significantly shorter in individuals with a variant than those without a variant in both cohorts, suggesting that the variants influence telomere length. Many of the same variants identified in this study or variants resulting in amino acid changes at the same position (e.g., TERT R951W [32], H412Y [33], A716T [34], and H592N [35]; RTEL1 R957Q [36]; and DKC1 S280R [37]) have been reported in patients with diseases recognized to be caused by telomere dysfunction (see also Table E5). In addition, many of the identified RTEL1 variants are in key regions of the gene (38). The identified TERC variant occurs in the 5′ end of the pseudoknot/template domain of its secondary structure near where three SNPs and a small deletion have been previously associated with short telomere syndromes (6, 34, 39–43). Interestingly, the identified DKC1 variant, a gene associated with X-linked dyskeratosis congenita and adult-onset pulmonary fibrosis, was found in a female patient without a family history. However, the identified variant is known to cause X-linked dyskeratosis congenita, and heterozygous female carriers in DKC1 families have been reported to develop mucocutaneous manifestations typical of short telomere syndromes and early-onset emphysema (8). To our knowledge, this would be the first reported case of a female heterozygous for a pathogenic DKC1 variant without a family history to develop a fibrotic ILD, although this finding does not definitively prove causation. Why the dominant organ affected by the telomere dysfunction varies between patients, or is not found in others carrying the variant, requires further investigation (34).
Interestingly, short peripheral blood telomere length and rare telomere-related gene variants did not demonstrate complete overlap among patients with CHP, and yet both measures of telomere dysfunction appear to be prognostic. This suggests that identification of patients with clinically relevant telomere dysfunction and poor prognosis may require both measurement of peripheral blood telomere length and genetic analysis for rare protein-altering telomere-related gene variants. Future prospective studies are needed to evaluate the ability of clinical-grade telomere length measurement and telomere gene sequencing to stratify patients with CHP into prognostic subgroups. Importantly, it does not appear that any individual clinical or radiographic information will reliably predict which individuals with CHP harbor telomere variants. In addition to predicting prognosis, it will be important to determine whether the presence of short telomeres and/or telomere-related gene variants also predict differential response to or harm from the two major classes of medications currently available for treatment of fibrotic ILDs (immune-suppressing medications and IPF medications). If so, genetic classification could inform personalized treatment strategies for patients with CHP, and similar studies should be conducted across all fibrotic ILDs.
We hypothesize that two conceptual etiologic models could explain our findings. In one model, telomere-related gene variants act as genetic modifiers of established hypersensitivity pneumonitis. In this scenario, disease begins with exposure and abnormal immune response to antigens that cause HP. Persistent inflammation and injury to the alveolar–bronchiolar epithelium in the context of dysfunctional maintenance of telomeres then results in critical telomere shortening and senescence of alveolar–bronchiolar epithelial cells leading to progressive fibrosis and lung remodeling. In model two, telomere-related gene variants are the primary cause of disease, resulting in disease that is pathobiologically similar to IPF. In this scenario, additional genetic factors, the cellular subtype preferentially affected by telomere shortening, comorbid diseases, and/or environmental exposures may influence formation of morphologic features that are atypical of IPF and similar to that of the classification used to define CHP, which are difficult to distinguish from one another on the basis of the current clinical diagnostic framework. Potential cofactors in this scenario could include other genetic risk factors, smoking, air pollution, gastroesophageal reflux disease, or chitin inhalation (44–46). In either scenario, telomere dysfunction is the central driver of disease progression. Larger case–control studies that include detailed exposure evaluation will be needed to determine the relative contribution of telomere-related gene variations and their interaction with other genetic variants and environmental exposures in the pathogenesis of CHP.
There are several strengths of this study. First, the inclusion of patients from expert ILD centers increases confidence in the CHP diagnosis and allowed for detailed clinical-radiographic-pathologic description of the cohorts. These cohorts included patients with sporadic CHP and are distinct from prior reports of CHP occurring in patients with familial pulmonary fibrosis (13). Second, all analyses were first conducted in a discovery cohort, followed by reproduction of findings in an independent replication cohort that included patients from separate ILD centers and that used different sequencing methods. The findings were also consistent when adjusting or restricting for ancestry in statistical models. Third, criteria for rare protein-altering telomere-related gene variants were established a priori, and sequences from both cohorts were processed using an identical bioinformatics pipeline.
This study has limitations that should be noted. Control subjects for the case–control analyses were not sequenced in randomized fashion along with cases (for the discovery cohort) or were drawn from a public database (for the replication cohort), and therefore results of these analyses should be considered preliminary. This was an epidemiologic association study and does not prove biologic causality of the identified telomere gene variants. However, the consistent association of identified telomere variants in both cohorts with telomere length and transplant-free survival strongly supports the biologic relevance of the majority of identified variants. The effect of telomere variants on transplant-free survival was slightly attenuated (but not significantly different) in the replication cohort, compared with the discovery cohort, which may be at least partially explained by the shorter follow-up time in the replication cohort. Also, the effect of telomere variants on transplant-free survival was attenuated (but not substantially different) in the replication cohort, but not the discovery cohort, after adjustment for baseline FVC percent predicted. However, this is to be expected, because disease severity, as reflected by lung function parameters, is likely a mediator in the causal pathway between a genetic variant and death. The minor differences in prevalence and outcome associations between the discovery and replication cohorts could also be partly due to differences in sequencing methods, which could result in differences in coverage of the candidate telomere genes. In any case, these differences were not statistically significant. There is well-recognized lack of agreement on CHP diagnosis among expert ILD centers, likely due to the absence of accepted international CHP diagnostic criteria (47). This could result in the misclassification of some IPF cases as CHP, resulting in biased prevalence and transplant-free survival estimates. However, given the similarity of telomere variant prevalence in this study compared with studies of IPF, the misclassification rate would have to be nearly 100% to explain our findings, which we believe is unlikely, considering that the large majority of patients did not meet radiographic or pathologic criteria for IPF. Because nearly all patients were treated with immune-suppressing medications, we were unable to evaluate the possibility of effect modification on the survival effect by treatment with immune suppression. Future prospective studies should evaluate for differential outcomes and adverse events of immune suppression in patients with CHP by telomere length and/or telomere variant status. Finally, telomere length was measured using quantitative PCR, which some researchers find to provide variable measures (48). In our experience however, the method provides reproducible measures when carefully applied (49). Furthermore, poor test characteristics would only increase the probability of not finding the associations reported herein.
In conclusion, a substantial subset of patients diagnosed with CHP harbors rare variants in telomere-related genes, even among nonfamilial (i.e., sporadic) cases. These variants appear to be biologically and clinically relevant, given their associations with shorter PBL telomere length and reduced transplant-free survival. Prospective studies are needed in CHP, and across fibrotic ILD subtypes, to determine the contribution of telomere gene variation to disease risk, progression, and response to treatments.
Supplementary Material
Footnotes
Genentech funded the whole-genome sequencing of DNA from patients in the discovery cohort. The Nina Ireland Program for Lung Health funded whole-exome sequencing of DNA from patients in the replication cohort. Supported by NIH grants KL2TR001870 (B.L.) and HL130796 (I.N.).
Author Contributions: B.L. and P.J.W. conceived of the study and study design. B.L., J.M.O., A.A., S.L., B.M.E., T.S.H., J.A.G., K.D.J., B.L.Y., J.R.A., and I.N. collected data for the study. B.L., D.G.T., J.L., A.D., B.L.Y., and T.J.H. performed data analysis. All authors contributed to data interpretation. B.L. wrote the original draft of the manuscript. All authors contributed to manuscript review, editing, and final approval for submission.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201902-0360OC on July 3, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Selman M, Pardo A, King TE., Jr Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186:314–324. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 2.Mooney JJ, Elicker BM, Urbania TH, Agarwal MR, Ryerson CJ, Nguyen MLT, et al. Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest. 2013;144:586–592. doi: 10.1378/chest.12-2623. [DOI] [PubMed] [Google Scholar]
- 3.Ley B, Newton CA, Arnould I, Elicker BM, Henry TS, Vittinghoff E, et al. The MUC5B promoter polymorphism and telomere length in patients with chronic hypersensitivity pneumonitis: an observational cohort-control study. Lancet Respir Med. 2017;5:639–647. doi: 10.1016/S2213-2600(17)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolters PJ, Blackwell TS, Eickelberg O, Loyd JE, Kaminski N, Jenkins G, et al. Time for a change: is idiopathic pulmonary fibrosis still idiopathic and only fibrotic? Lancet Respir Med. 2018;6:154–160. doi: 10.1016/S2213-2600(18)30007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 6.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kropski JA, Mitchell DB, Markin C, Polosukhin VV, Choi L, Johnson JE, et al. A novel dyskerin (DKC1) mutation is associated with familial interstitial pneumonia. Chest. 2014;146:e1–e7. doi: 10.1378/chest.13-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alder JK, Parry EM, Yegnasubramanian S, Wagner CL, Lieblich LM, Auerbach R, et al. Telomere phenotypes in females with heterozygous mutations in the dyskeratosis congenita 1 (DKC1) gene. Hum Mutat. 2013;34:1481–1485. doi: 10.1002/humu.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alder JK, Stanley SE, Wagner CL, Hamilton M, Hanumanthu VS, Armanios M. Exome sequencing identifies mutant TINF2 in a family with pulmonary fibrosis. Chest. 2015;147:1361–1368. doi: 10.1378/chest.14-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuart BD, Choi J, Zaidi S, Xing C, Holohan B, Chen R, et al. Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat Genet. 2015;47:512–517. doi: 10.1038/ng.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cogan JD, Kropski JA, Zhao M, Mitchell DB, Rives L, Markin C, et al. Rare variants in RTEL1 are associated with familial interstitial pneumonia. Am J Respir Crit Care Med. 2015;191:646–655. doi: 10.1164/rccm.201408-1510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kannengiesser C, Borie R, Ménard C, Réocreux M, Nitschké P, Gazal S, et al. Heterozygous RTEL1 mutations are associated with familial pulmonary fibrosis. Eur Respir J. 2015;46:474–485. doi: 10.1183/09031936.00040115. [DOI] [PubMed] [Google Scholar]
- 13.Newton CA, Batra K, Torrealba J, Kozlitina J, Glazer CS, Aravena C, et al. Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. Eur Respir J. 2016;48:1710–1720. doi: 10.1183/13993003.00308-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morisset J, Johannson KA, Jones KD, Wolters PJ, Collard HR, Walsh SLF, et al. HP Delphi Collaborators. Identification of diagnostic criteria for chronic hypersensitivity pneumonitis: an international modified Delphi survey. Am J Respir Crit Care Med. 2018;197:1036–1044. doi: 10.1164/rccm.201710-1986OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43:11.10.1–11.10.33. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;76:7.20.1–7.20.41. doi: 10.1002/0471142905.hg0720s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 22.Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng PC, Henikoff S. Accounting for human polymorphisms predicted to affect protein function. Genome Res. 2002;12:436–446. doi: 10.1101/gr.212802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng PC, Henikoff S. Predicting the effects of amino acid substitutions on protein function. Annu Rev Genomics Hum Genet. 2006;7:61–80. doi: 10.1146/annurev.genom.7.080505.115630. [DOI] [PubMed] [Google Scholar]
- 26.Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019;47:D886–D894. doi: 10.1093/nar/gky1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faust HE, Golden JA, Rajalingam R, Wang AS, Green G, Hays SR, et al. Short lung transplant donor telomere length is associated with decreased CLAD-free survival. Thorax. 2017;72:1052–1054. doi: 10.1136/thoraxjnl-2016-209897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrovski S, Todd JL, Durheim MT, Wang Q, Chien JW, Kelly FL, et al. An exome sequencing study to assess the role of rare genetic variation in pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196:82–93. doi: 10.1164/rccm.201610-2088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dressen A, Abbas AR, Cabanski C, Reeder J, Ramalingam TR, Neighbors M, et al. Analysis of protein-altering variants in telomerase genes and their association with MUC5B common variant status in patients with idiopathic pulmonary fibrosis: a candidate gene sequencing study. Lancet Respir Med. 2018;6:603–614. doi: 10.1016/S2213-2600(18)30135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juge PA, Borie R, Kannengiesser C, Gazal S, Revy P, Wemeau-Stervinou L, et al. FREX Consortium. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur Respir J. 2017;49:1602314. doi: 10.1183/13993003.02314-2016. [DOI] [PubMed] [Google Scholar]
- 32.Diaz de Leon A, Cronkhite JT, Katzenstein AL, Godwin JD, Raghu G, Glazer CS, et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS One. 2010;5:e10680. doi: 10.1371/journal.pone.0010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 34.Du HY, Pumbo E, Ivanovich J, An P, Maziarz RT, Reiss UM, et al. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood. 2009;113:309–316. doi: 10.1182/blood-2008-07-166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coghlan MA, Shifren A, Huang HJ, Russell TD, Mitra RD, Zhang Q, et al. Sequencing of idiopathic pulmonary fibrosis-related genes reveals independent single gene associations. BMJ Open Respir Res. 2014;1:e000057. doi: 10.1136/bmjresp-2014-000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borie R, Bouvry D, Cottin V, Gauvain C, Cazes A, Debray MP, et al. Regulator of telomere length 1 (RTEL1) mutations are associated with heterogeneous pulmonary and extra-pulmonary phenotypes. Eur Respir J. 2019;53:1800508. doi: 10.1183/13993003.00508-2018. [DOI] [PubMed] [Google Scholar]
- 37.Knight SW, Vulliamy TJ, Morgan B, Devriendt K, Mason PJ, Dokal I. Identification of novel DKC1 mutations in patients with dyskeratosis congenita: implications for pathophysiology and diagnosis. Hum Genet. 2001;108:299–303. doi: 10.1007/s004390100494. [DOI] [PubMed] [Google Scholar]
- 38.Speckmann C, Sahoo SS, Rizzi M, Hirabayashi S, Karow A, Serwas NK, et al. Clinical and molecular heterogeneity of RTEL1 deficiency. Front Immunol. 2017;8:449. doi: 10.3389/fimmu.2017.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calado RT, Brudno J, Mehta P, Kovacs JJ, Wu C, Zago MA, et al. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology. 2011;53:1600–1607. doi: 10.1002/hep.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han B, Liu B, Cui W, Wang X, Lin J, Zhao Y. Telomerase gene mutation screening in Chinese patients with aplastic anemia. Leuk Res. 2010;34:258–260. doi: 10.1016/j.leukres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Ly H, Schertzer M, Jastaniah W, Davis J, Yong SL, Ouyang Q, et al. Identification and functional characterization of 2 variant alleles of the telomerase RNA template gene (TERC) in a patient with dyskeratosis congenita. Blood. 2005;106:1246–1252. doi: 10.1182/blood-2005-01-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vulliamy TJ, Kirwan MJ, Beswick R, Hossain U, Baqai C, Ratcliffe A, et al. Differences in disease severity but similar telomere lengths in genetic subgroups of patients with telomerase and shelterin mutations. PLoS One. 2011;6:e24383. doi: 10.1371/journal.pone.0024383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xin ZT, Beauchamp AD, Calado RT, Bradford JW, Regal JA, Shenoy A, et al. Functional characterization of natural telomerase mutations found in patients with hematologic disorders. Blood. 2007;109:524–532. doi: 10.1182/blood-2006-07-035089. [DOI] [PubMed] [Google Scholar]
- 44.Van Dyken SJ, Liang HE, Naikawadi RP, Woodruff PG, Wolters PJ, Erle DJ, et al. Spontaneous chitin accumulation in airways and age-related fibrotic lung disease. Cell. 2017;169:497–509. doi: 10.1016/j.cell.2017.03.044. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johannson KA, Vittinghoff E, Morisset J, Wolters PJ, Noth EM, Balmes JR, et al. Air pollution exposure is associated with lower lung function, but not changes in lung function, in patients with idiopathic pulmonary fibrosis. Chest. 2018;154:119–125. doi: 10.1016/j.chest.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johannson KA, Strâmbu I, Ravaglia C, Grutters JC, Valenzuela C, Mogulkoc N, et al. Erice ILD Working Group. Antacid therapy in idiopathic pulmonary fibrosis: more questions than answers? Lancet Respir Med. 2017;5:591–598. doi: 10.1016/S2213-2600(17)30219-9. [DOI] [PubMed] [Google Scholar]
- 47.Walsh SLF, Wells AU, Desai SR, Poletti V, Piciucchi S, Dubini A, et al. Multicentre evaluation of multidisciplinary team meeting agreement on diagnosis in diffuse parenchymal lung disease: a case-cohort study. Lancet Respir Med. 2016;4:557–565. doi: 10.1016/S2213-2600(16)30033-9. [DOI] [PubMed] [Google Scholar]
- 48.Alder JK, Hanumanthu VS, Strong MA, DeZern AE, Stanley SE, Takemoto CM, et al. Diagnostic utility of telomere length testing in a hospital-based setting. Proc Natl Acad Sci USA. 2018;115:E2358–E2365. doi: 10.1073/pnas.1720427115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newton CA, Oldham JM, Ley B, Anand V, Adegunsoye A, Liu G, et al. Telomere length and genetic variant associations with interstitial lung disease progression and survival. Eur Respir J. 2019;53:1801641. doi: 10.1183/13993003.01641-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.