Abstract
With the significant financial burden of chronic cutaneous wounds on the healthcare system, not to the personal burden mention on those individuals afflicted, it has become increasingly essential to improve our clinical treatments. This requires the translation of the most recent benchtop approaches to clinical wound repair as our current treatment modalities have proven insufficient. The most promising potential treatment options rely on stem cell-based therapies. Stem cell proliferation and signaling play crucial roles in every phase of the wound healing process and chronic wounds are often associated with impaired stem cell function. Clinical approaches involving stem cells could thus be utilized in some cases to improve a body's inhibited healing capacity. We aim to present the laboratory research behind the mechanisms and effects of this technology as well as current clinical trials which showcase their therapeutic potential. Given the current problems and complications presented by chronic wounds, we hope to show that developing the clinical applications of stem cell therapies is the rational next step in improving wound care.
Keywords: Chronic inflammation, Chronic wounds, Growth factors, Personalized medicine, Skin, Stem cells, Wound healing
Introduction
The skin is the largest organ in the body and the body's first line of immune and physical defense. Types of skin wounds include abrasions, lacerations, punctures, surgical wounds, ulcers, and burn and burn-like wounds, each accompanied by their own set of complications. Cutaneous wounds can be broadly classed in two categories; acute, which heal uneventfully, and chronic, which do not heal for prolonged periods of time. Although not officially defined, this time period without healing is usually between 4 weeks1 to 3 months.2 The frequency of chronic wounds continues to rise with the increasing frequency of diabetes, vascular disease, and obesity, all known to be significant risk factors for chronic wound development.3 At present, chronic wounds cost the U.S. healthcare system about $25 billion dollars annually.4 Given the variety of wounds and their prevalence, it is imperative that functional improvements be made in compensating for inhibited aspects of the healing process.
Cutaneous wound complications include clinical morbidity and mortality, economic burden, and emotional damage. Here, we seek to elucidate current methods for treatment and explore the promising options on the horizon presented by cell-based therapies.
Normal anatomic and histologic features of the skin
Skin is the largest organ, accounting for about 15% of the human body by mass. It serves as a protective barrier against biological and chemical agents, as well as moderating temperature and retaining fluids.5 To fulfill these functions, skin is composed of numerous cell types organized into three layers: epidermis, dermis, and hypodermis.
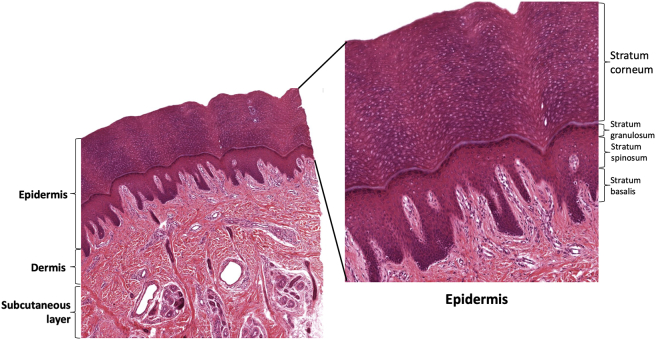
The epidermis is primarily composed of keratinocytes,6 Keratinocytes are epithelial cells of ectodermal origin which produce keratin, the major extracellular protein of the epidermis.6 The epidermis may itself be subdivided into four layers according to keratinocyte morphology. The cornified or horny layer (stratum corneum) is the most superficial layer, and is made up of dead anucleated horny cells surrounded by dense keratin extracellular matrix.7 This layer decreases water permeability and acts as a mechanical barrier. Deep to the stratum corneum is the granular layer (stratum granulosum), which consists of flattened cells containing abundant intracellular proteins, that renew the contents of the stratum corneum.8 Beneath the stratum granulosum, the spinous layer (stratum spinosum) also produces some keratin, but its primary role is strong mechanical adhesion through desmosomes, increasing the structural integrity of skin.6 The deepest layer of the epidermis is the basal layer (stratum basalis), consisting of a single layer of columnar or cuboidal keratinocytes sitting atop the basement membrane.9 The cells of the stratum basalis are actively dividing and provide replacement cells for all the more superficial layers of the epidermis.9 Consistent epithelial thickness is maintained through apoptosis and mitotic control, regulated via numerous cell signaling molecules, and dysregulated in a number of pathologic conditions.6 Sweat glands and hair follicles in the epidermis serve thermoregulatory and signaling purposes.
Between the epidermis and the dermis is the basement membrane, a layer of basal cells interconnected by hemidesmosomes and type IV collagen. This structure maintains adherence of the dermis and epidermis in the face of mechanical shear and tensile stress and also allows exchange of cells and fluids between the layers.10, 11 It also functions as a master regulator for the epidermis, providing developmental signals and establishing cell polarity and directionality for keratinocyte migration.10
The dermis is the thick layer beneath the basement membrane, consisting of fibroblasts, smooth muscle, nerves, blood vessels, and mast cells. The dermis provides most of the key physical properties of skin, including tensile strength, elasticity, and pliability.6 It also provides immune and nutritional support through circulation and somatic sensation via dense innervation. Many of the physical properties of the dermis are provided by type I collagen, the dominant extracellular protein in this layer, synthesized by fibroblasts.12 Following synthesis, collagen is crosslinked with elastin fibrils. This crosslinked structure gives this layer its unique strength and flexibility.
Most of the cells in the dermis are mesenchymal in origin, with the exception of nerves and nerve plexuses which are derived from neural crest.13 Though the dermis is organized in a less obvious manner than the epidermis, there are consistent depth dependent changes in cell structure and composition.6 The dermis contains mitotically capable cells, such as the stem cells present in hair follicles, which renew cells lost to senescence or injury.14
The hypodermis is a layer of subcutaneous tissue deep to the dermis. This fat provides thermal insulation, buoyancy, and acts as a store of energy.5 It also has endocrine functions, producing leptin and converting androstenedione to estrone.15 The skin has numerous important protective and regulatory functions, and cutaneous wounds can be associated with significant morbidity and mortality. In health, skin is a highly labile tissue, able to undergo regeneration and repair (Fig. 1).
Figure 1.
Layers of the skin. Skin consists of numerous cell types organized into 3 primary layers: the epidermis, dermis, and the hypodermis. The epidermis can further be sub-stratified into stratum corneum, granulosum, spinosum, and basalis. The cornified or horny layer (stratum corneum) is the most superficial layer, consisting of dead cells and keratin matrix. Deep to the stratum corneum is the granular layer (stratum granulosum), made up of cells in the process of anucleation and keratin production. Beneath the stratum granulosum lies the spinous layer (stratum spinosum) which anchors the upper and lower layers of the epidermis. The deepest layer of the epidermis is the basal layer (stratum basalis), consisting of a single layer of rapidly dividing columnar or cuboidal keratinocytes sitting atop the basement membrane. Between the epidermis and the dermis is the basement membrane (unlabeled) which maintains adherence between the dermis and epidermis. The dermis is the thick layer beneath the basement membrane. The dermis contains fibroblasts, smooth muscle, nerves, and blood vessels, and provides most of the key physical properties of skin. The hypodermis is the subcutaneous fat layer lying below the dermis.
Physiologic cutaneous wound healing
Wound healing is a delicate and complicated process, involving an intricate interplay of cell migration and proliferation, all orchestrated by numerous cytokines. Successful cutaneous wound healing restores the barrier function and tensile integrity of skin. Under normal, healthy circumstances, wound healing proceeds through an inflammatory stage, a proliferative phase, and a remodeling stage.16 Though somewhat chronological, the different phases overlap, and each phase has important implications for the others. Small disturbances can have major consequences.
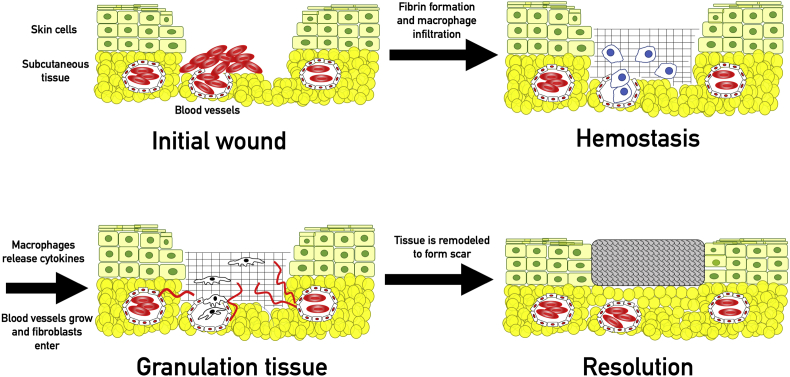
Hemostasis is the first step in wound healing.17 Platelets exposed to extracellular matrix proteins are activated, causing conformational change and aggregation, as well as release of pro-thrombotic and pro-inflammatory mediators.17, 18 Formation of a strong and stable cross-linked insoluble fibrin-platelet clot is the end result of secondary hemostasis. Fibrin also has a pro-inflammatory role mediated through activation of monocytes and neutrophils,19 while platelets are involved in cellular signaling through release of platelet derived growth factor (PDGF) and tumor necrosis factor-alpha (TNF- α).18 The insoluble clot is a key element for later stages of repair, acting as a scaffold for the migration of fibroblasts, leukocytes, keratinocytes, and endothelial cells.20
Chemotactic factors produced during hemostasis attract neutrophils and monocytes to the wound. The cellular response is initially dominated by neutrophils, which are later replaced by macrophages. Macrophages play an instrumental role in the inflammatory response: they phagocytize pathogenic organisms and cellular debris, produce regulatory cytokines, influence angiogenesis, and recruit fibroblasts with chemotactic chemicals.21 Macrophages produce vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), fibroblast growth factor (FGF), and tumor growth factors α and β.22 The inflammatory phase usually resolves in 2 weeks. A persistent inflammatory response is considered pathologic and is called chronic inflammation. Chronic inflammation is not always characterized by the cardinal signs of inflammation such as redness, pain, or swelling.21
The proliferative phase follows the inflammatory phase, though there is significant overlap between the beginning of proliferation and the end of inflammation. Angiogenesis and fibroplasia are the principle components of this phase. The cytokines released during the inflammatory phase recruit vascular endothelial cells, fibroblasts, and keratinocytes.23 3–5 days following the initial injury, fibroblasts begin to secrete type III collagen, proteoglycans, and elastin, which forms the granulation tissue.24 Angiogenesis occurs in concert with granulation tissue formation. Vascular endothelial cells, attracted by VEGF, PDGF, and TGF-β, create new capillaries and carry circulating cells and nutrients to the wound.22
Tissue remodeling follows closure of the wound and can take a year or longer.25 Macrophages, fibroblasts, and endothelial cells are eliminated from the wound via apoptosis, leaving a mostly acellular collagenous matrix, consisting primarily of type III collagen, which is replaced with type I collagen over the next 6–12 months. Fully healed tissue provides a barrier with 60–80% of the tensile strength of the original epithelium (Fig. 2).26
Figure 2.
Physiologic wound healing. Following the initial wound, platelets and coagulation factors enter the wound bed from blood vessels and produce a fibrin clot through primary and secondary hemostasis. The fibrin clot is infiltrated by macrophages which function to phagocytose cellular debris and pathogens as well as releasing cytokines. These cytokines (including VEGF, FGF, and PDGF) summon fibroblasts and stimulate angiogenesis. Fibroblasts produce type III collagen for tissue repair. Angiogenesis allows sufficient transport of nutrients to the healing wound bed. Eventually the type III collagen is remodeled into type I collagen, which restores 60–80% of the original strength.
Chronic wounds
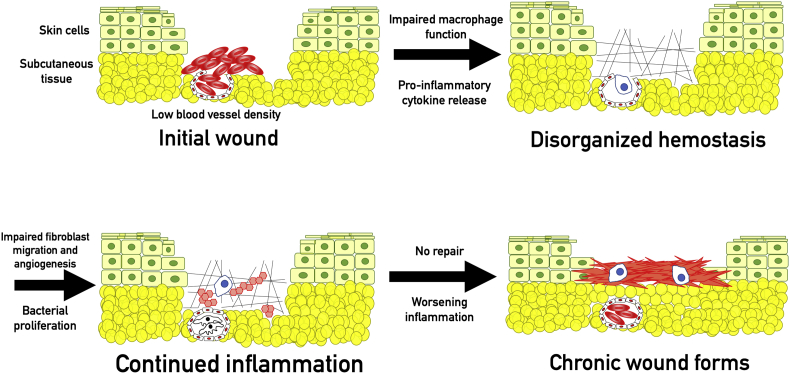
Chronic wounds form when the healing process stalls. Mechanisms underlying chronic wound formation vary but can include metabolic diseases (diabetes), failures of blood supply (peripheral vascular disease, radiation injury), medications, or altered immune function. Non-healing wounds are a major issue in healthcare today, an issue which will likely be exacerbated with an aging population. Though prevalence in the general population is about 3:1000, this worsens to about 1:50 for patients older than 75.27 An estimated 500,000 U.S. citizens are afflicted with chronic ulcers.28 Chronic wounds cost the U.S. healthcare system about $25 billion dollars annually,4 about 5.5% of NHS spending.29 On an individual level, chronic wounds are painful, isolating, and emotionally distressing.30 Chronic wounds often lead to further health complications with chronic ulcers preceding 85% of amputations and providing a direct cause for 70%.31 Current treatments may also not be effective long term: though treatment for ulcers improves mobility and energy in the short term, one study showed that positive effects were not maintained over a 48 week period.32
Wound healing is a highly metabolic activity and requires abundant nutrients and oxygen.33 This necessitates good circulation and explains why chronic wounds are often associated with conditions which decrease blood flow. Non-healing wounds may have less than a quarter of the oxygen tension seen in healing wounds.34 Hypoxemia impairs tissue repair by decreasing metabolic activity of repair cells. It also increases risk of infection by impairing oxygen-dependent killing of microbes.4 Ischemia caused by decreased blood flow is often enough to prevent wound healing even in the absence of comorbid risk factors.
In the US alone, an estimated 24.7 million people have been diagnosed with type II diabetes mellitus (T2DM), about 7.6% of the total population.35 Diabetes impacts every step of the wound healing process. Peripheral neuropathy and vascular disease increase risk of skin breakdown. Ulcers in diabetic patients show abnormal cytokine signaling, resulting in decreased immune cell presence.36 Decreased immune cells increase prevalence of secondary infections, a serious complication of diabetic ulcers.37 Cytokine signaling creates complex feedback loops. Diminished macrophage presence and impaired macrophage function in diabetic wounds is both a cause and result of altered signaling.38
Diabetic macrophages release significantly less VEGF than macrophages from healthy individuals.39 Decreased VEGF expression may be a major factor contributing to defective diabetic healing, supported by the fact that administration of topical VEGF has been shown to improve healing time in diabetic, but not control, subjects.40 Insulin receptors (InsR), downregulated in T2DM, may also play a role in wound healing. InsR expression levels were inversely correlated to protein expression levels in diabetic wounds and upregulation of InsR receptors improved healing time.41 Adipose tissue derived stem cells (ASCs) play an important role in cutaneous wound healing, and diabetic patients have functionally defective ASCs resulting in inadequate growth factor release and decreased presence of ASCs in wound sites.42 Diabetic patients also show an inadequate cellular response to hypoxia. In normal wound healing, hypoxia summons endothelial progenitor cells (EPCs) which participate in neovascularization to increase blood flow and oxygen delivery. EPCs harvested from diabetic patients showed decreased hypoxia-induced adhesion, migration, and proliferation, resulting in impaired capillary formation and persistent ischemia.43 Finally, diabetic keratinocytes function abnormally, leading to decreased re-epithelization and persistence of wounds.44
Tissue repair is a carefully coordinated process, organized through a communication system of growth factors, cytokines, and chemokines. It is therefore unsurprising that disruptions in the cytokine profile may be a primary issue in chronic wounds. The role of VEGF in wound healing has already been mentioned. In fact, decreased VEGF has been noted in many chronic wound beds.45
Wound healing requires a balance of inflammation and repair, and increased release of pro-inflammatory cytokines may be as damaging as decreased production of growth factors. Inflammation is a key step in the healing process but is usually self-limited. The micro-environment within nonhealing wounds (regardless of underlying pathology) is one of uncontrolled inflammation. Nonhealing wounds show significantly increased levels of the pro-inflammatory cytokines IL-1, IL-6, and TNF- α.46 Fibroblasts seeded with pro-inflammatory wound fluid demonstrated impaired proliferative response compared to those seeded with fluid from healing wounds.46, 47 Increased inflammation upregulates elastase in the wound, resulting in the degradation of exogenously added therapeutic growth factors TGF-β and PDGF.48 Decreasing the concentration of the pro-inflammatory cytokines in a wound has been shown to enhance speed of healing.49
Cellular response to cytokines may also impact wound healing. Senescent cells are often unable to respond adequately to growth signals.50 Increasing senescent cells in wound beds decreased rate of healing.51 All these findings suggest that altered cell signaling, predominantly increased pro-inflammatory cytokines and decreased anti-inflammatory and angiogenic cytokines, plays a key role in chronic wound formation. The intricacy of the healing process complicates attempts to pinpoint a specific clinical target, and the great range of possible underlying pathologies makes it difficult to cover the entire spectrum with current medical techniques (Fig. 3).
Figure 3.
Chronic wounds. Impaired macrophage function impairs phagocytosis, resulting in continued pathogenic colonization of the wound. In chronic wounds, macrophages also malfunction in their signaling role, resulting in increased pro-inflammatory cytokine production. Impaired communication and persistent infection lead to a prolonged inflammatory state, which inhibits fibroblast migration and angiogenesis. Eventually, persistent infection, hypoxemia, and insufficient tissue repair results in a chronic inflammatory state. Chronic inflammation creates a self-reinforcing positive feedback loop that leads to the formation of a chronic wound.
Standard therapy for chronic wounds
Standard wound care attempts to prevent complications arising from epithelial breakdown (such as infection or fluid loss) as well as to create an environment that is conducive to healing. Wound care and dressings are a mainstay of treatment. Debridement (removal of necrotic or otherwise nonviable tissue) is important for exposure of healthy tissue and reduction in the inflammatory effects of necrotic tissue.4 Dressings are used to prevent infection and promote healing processes. Different dressings, such as moist occlusive dressings, dry gauze dressings, semi-permeable films, and hydrogels, have various beneficial effects including improved re-epithelialization, increased moisture, thermal insulation, or fluid absorption.52 Offloading of pressure and management of secondary infections are also important aspects of treatment.53
Skin substitutes are biologic substances that act as synthetic skin. They have been widely and effectively used in surgical defects and large surface area burns. High cost has prevented their adoption for chronic wound care, though an economic analysis showed long term cost savings when used to treat recalcitrant chronic wounds.54
Vacuum assisted closure, or sub-atmospheric pressure dressing, has been shown to increase blood flow and decrease swelling by siphoning excess fluid from the wound bed.55 It also facilitates removal of bacteria from wounds which decreases rates of infection.56 Treatment with hyperbaric oxygen has been proposed to enhance angiogenesis and immune function, but is associated with serious adverse oxygen toxicity.57 Furthermore, studies have shown only an incremental improvement in diabetic ulcer healing over a 1 year period with hyperbaric oxygen therapy.58
As previously discussed, altered cytokine profile is a critical issue in many chronic wounds. Efforts have been made to normalize the micro-environment of the wound bed via application of exogenous growth factors. Platelet derived growth factor (PDGF) attracts and activates fibroblasts, endothelial cells, and epithelial cells.59 Application of high dose (1.0 μG/cm2 ulcer area) topical PDGF improved healing of pressure ulcers in diabetic patients, though lower doses showed no effect.60 A large randomized control study supported this finding, as 50% of ulcers treated with high dose PDGF healed over a 20 week period compared to 36% in the control group.61 Fibroblast growth factor (FGF) enhances angiogenesis and promotes migration and activation of fibroblasts.59 FGF has been shown to markedly accelerate wound healing in diabetic mice,62 though clinical trials showed no benefit to topical FGF over placebo.63, 64 Vascular endothelial growth factor (VEGF) increases vascular permeability to allow migration of endothelial cells and lymphocytes as well as stimulating angiogenesis.65 Mice treated with an anti-VEGF antibody almost completely lacked granulation tissue formation and had severely decreased vessel density.66 Conversely, wounds on diabetic mice treated with high dose topical VEGF showed increased granulation tissue formation and increased vessel density. Topical VEGF also corrected other cytokine levels, normalizing FGF and PDGF levels to that of non-diabetic mice.40 VEGF and FGF may also have synergistic effects. Co-stimulation with VEGF and FGF significantly increased angiogenesis in vivo when compared to either growth factor alone.67 However, the wound models used in laboratory trials may not replicate clinical conditions. In general, laboratory wounds are new, small, and sterile, differing significantly from the environment in most clinical cases.
Though topical growth factors have shown promise for the treatment of chronic wounds, the difficulty of achieving correct dosages may limit clinical efficacy. Furthermore, multiple growth factors may be required to achieve optimal results, further increasing the complexity of treatment. Topically applied growth factors may also not penetrate to the wound base in deeper wounds.68 Growth factors often need to be changed daily, which interrupts standard compression treatment.68 A further complication is that the environment in chronic wounds is actively hostile to exogenous growth factors. Increased neutrophil activity in nonhealing wounds boosts elastase activity, which has been shown to degrade exogenously added TGF-β and PDGF-β.48 The difficulties associated with growth factor application have made clinical translation problematic, despite promising lab findings. Though current therapies are efficacious in preventing secondary complications of chronic wounds, treatments are often unable to correct micro-imbalances and achieve true resolution.
Endogenous stem cells in cutaneous wound healing
Adult stem cells play a crucial role in all stages of cutaneous wound healing. The inflammatory stage is characterized by migration of neutrophils and macrophages. While some of these leukocytes are pulled directly from circulating blood, studies have shown that bone marrow-derived stem cells (BMSCs) also play an important role,69 homing to injured tissues before proliferating and differentiating into required lineages.70 Mast cells, important directors of the inflammatory phase, have also been shown to arise from precursor stem cells present in the skin.71
The role of stem cells in the proliferative phase is more obvious. Division and differentiation of tissue-specific adipocyte stem cells (AdSCs) regenerate damaged or lost tissue.72 Interfollicular and hair follicle bulge epithelial stem cells proliferate and differentiate into cell lineages of keratinocytes for re-epithelialization.73 BMSCs may also contribute to fibroblast populations in wounds: up to 20% of fibroblasts may be of migratory BMSC lineage.74
Revascularization can occur via angiogenesis, the proliferation of endothelial cells in pre-existing blood vessels, or through vasculogenesis, which is the de novo creation of blood vessels by differentiation of endothelial progenitor cells (EPCs). Interestingly, angiogenesis is major mechanism of revascularization. Only 4% of vascular cells in wound sites arise from EPCs.75 Endothelial progenitor cells are still critical to wound healing but exert their effects primarily through secretion of growth factors, rather than proliferation.76 Hematopoietic stem cells (HSCs), derived from bone marrow, also play a role in production of new endothelial cells.77
Many pathologies related to chronic wounds impact stem cell functioning. EPCs from diabetic patients displayed impaired migration to wound sites and adhesion to TNF-activated endothelial cells.78 Diabetic EPCs also demonstrated decreased response to hypoxia, resulting in decreased vascularity in wound sites. Hypoxia usually induces vessel growth in control animals, but actually decreased vessel growth in diabetic animals.43 This feedback loop reinforces and chronically prolongs the hypoxic state, which alters the functions of many cells required for tissue repair. Fibroblasts in diabetic patients are also less responsive to growth factors, showing decreased proliferation when stimulated with PDGF, IGF, or EGF.79 Age is another risk factor for non-healing wounds. An epigenetic study in mice revealed upregulation of pro-inflammatory genes in the HSCs of older subjects.80
Every phase in wound healing is mediated by stem cell proliferation and signaling. Impaired stem cell functioning therefore leads to chronic wounds. As stem cells directly interact with the wound environment in a complex and multifactorial manner, clinical approaches which utilize them could theoretically be very beneficial. Cell based treatments are a clear and rational next step in chronic wound care.
Growth factors in stem cell-based skin repair
Stem cells both produce and are regulated by growth factors. Growth factors have variable roles, but are also highly specific, with some growth factors only acting on a single receptor of a single cell type.81 Activated growth factors can have a number of different effects, including direct protein activation, upregulation of genes regulating protein production, or migration and chemotaxis.82 Growth factor signaling is necessary for stem cell response in wound healing.
A number of different growth factors are known to have important roles in cutaneous wound healing. Hypoxic wound environments induce expression of HIF-1α, which upregulates FGF, and HIF-2α, which upregulates VEGF expression. VEGF plays numerous roles in revascularization. VEGF concentration directs and guides differentiating angioblasts to cause budding of vessels.83 FGF increase recruitment and replication of mesenchymal stem cells to support the structure of new capillaries.84 Growth factors control keratinocyte migration and de-differentiation in ECM remodeling in epithelial wounds, allowing them to multiply and fill in the wounded area.85 Lack of growth factors in chronic wounds has been associated with decreased stem cell function of many lineages. One strategy to mimic or restore growth factor signaling in the wound to promote improved stem cell function is to enable the slow release of the growth factor or an inducer molecule from a hydrogel or dressing A citrate-based thermoresponsive hydrogel was shown to protect the bioactivity of stromal cell-derived factor 1 alpha entrapped within the hydrogel and allow its slow release for at least 21 days with significant improvement in wound closure rate in diabetic mice.86 In another study, the slow release of copper ions from the same material demonstrated increased blood vessel formation, likely mediated via VEGF release.87
Therefore, although delivery of growth factors can be difficult, due to depth of penetration, concentration, or degradation by the wound environment, carefully designed biomaterials and small growth factor inducing molecules can be used to harness their potential. Viral transduction to temporarily over-express growth factors may also potentially solve many of the aforementioned issues.88 Viral transduction may present an efficient strategy to obtain maximum benefit from both stem cell and growth factor therapy.
Growth factors and stem cells have a complimentary relationship. Without adequate production of and response to growth factors, stem cells are not able to perform their tasks in wound healing. It is therefore insufficient to examine only the direct effects of stem cells in cutaneous wound healing; local growth factors must be examined for their role in reinforcing and directing stem cell activity.
Stem cell sources for clinical use
Numerous stem cell lines have been investigated for their clinical potential. Embryonic stem cells (ESCs) were initially investigated for skin healing for their ability to self-renew and divide into keratinocytes.89 Though ESCs have the potential to differentiate into many cell types including skin progenitors,90 studies have shown that they can generate tumors, and their use remains controversial.89
Human induced pluripotent stem cells (iPSCs) are created from differentiated adult somatic cells. Multiple terminally differentiated cells are capable of being reverse-engineered to pluripotency. iPSCs can be derived from adult mouse fibroblasts by treatment with Oct3/4, Sox2, c-Myc, and Klf4. iPSCs also can differentiate into tissues from all 3 germ layers in vivo.91 Keratinocytes have proven even more effective than fibroblasts for production of iPSCs: keratinocytes transduced with OCT4, SOX2, KLF4, and c-MYC produced iPSCs more quickly and efficiently than fibroblasts.92
The ability to induce pluripotency is only half the challenge. To be therapeutically effective, researchers must be able to direct stem cell differentiation into specific cell types and lineages. iPSCs have shown promise here as well. Researchers have induced differentiation of iPSCs into fibroblasts93 and keratinocytes94 that are functionally indistinguishable from embryologically derived cells or adult cells of the same lineage. iPSCs also demonstrate promising immunogenicity. Human iPSCs introduced to foreign lymphocytes elicited IL-10 (anti-inflammatory) production.95 In addition to their ease of harvest and favorable immune properties, iPSCs avoid the ethical controversy surrounding ESCs.
Tissues which continue to divide throughout life (labile) must maintain stem cell populations even in adulthood. These adult stem cells can be harvested and demonstrate regenerative potential. One such population is hematopoietic stem cells (HSCs), blood cell progenitors expressing the CD34 marker, usually residing in the bone marrow. Though HSCs mostly produce blood cells, they have also been shown to produce epithelial cells and hepatocytes.96 HSCs may also be a major source of fibroblasts during tissue repair.97 HSCs express a specialized form of CD44 which binds very strongly to E-selectin, resulting in powerful homing to sites of inflammation.98 It may be possible to deliver HSCs to the site of injury simply by injection into the bloodstream, simplifying clinical treatment.
Mesenchymal stem cells (MSCs) are the progenitor cells for connective tissue and can be found in nerves, adipocytes, umbilical cord blood, and bone marrow.89 They have a variety of favorable characteristics for use in tissue engineering, including rapid proliferation and wide differentiation capacity.99 Similar to iPSCs, MSCs lack immunological reactivity.100 Injected bone marrow derived MSCs have been shown to improve ulcer healing rate in diabetic patients compared to HSCs.101 MSCs derived from adipose tissue (AdSCs) are of particular clinical interest, as they share many of the properties of bone marrow derived MSCs (BMMSCs) but can be obtained less invasively.102 AdSCs display a similar cytokine profile to BMMSCs.99 Furthermore, in vitro results suggest that AdSCs also play a pro-angiogenic role.99 However, mice treated with BMMSCs in a toxic shock model showed a survival advantage over those treated with AdSCs, suggesting a comparative advantage for MSCs in resolution of inflammation.103
One of the least invasive potential sources of pluripotent stem cells is urine. Zhang et al discovered that select cells isolated from urine (USCs) were capable of forming progenitor-like cells with the ability to differentiate into urothelial, endothelial, smooth muscle and interstitial lineages.104 Human USCs have been shown to be efficacious for chronic wound repair. hUSCs cultured in bacterial cellulose significantly improved wound healing in rats, with stem cell treated wounds showing increased epithelialization and depth of healing within one week.105 Additionally, hUSCs significantly improved angiogenesis. All these effects (wound healing, collagen formation, and vessel formation) could be improved even further with application of silicate biomaterial (bioglass).106 Further innovation in hybrid cell and biomaterial based technologies could ensure adequate wound regeneration even with stem cells harvested in an entirely non-invasive manner, such as these urine derived stem cells. Interestingly, with additional reprogramming, USCs could even be reprogrammed to form human neural progenitor cells which have showed promise for repair of devastating spinal cord injuries.107 Although studies of USCs have showed extreme promise, there are multiple problems still associated with their use, including varying efficiency in cultures and problems with stem cell isolation,108 heterogeneity in cell markers potentially implying decreased purity of cell lines,109 and most importantly, their functional differentiation and long term effectiveness has not yet been carefully studied.110
In the next section, we will argue that the major mechanism by which stem cells improve wound healing is through cytokine signaling. Cytokine signaling takes place via production of extracellular vesicles, called exosomes. Recent studies have investigated removing stem cells from the picture entirely, and replacing them with exosomes, with promising results.111, 112, 113 A recent study showed that exosomes isolated from USCs effectively improved diabetic wound healing in rats through increased angiogenesis.114 Exosome treatments have the advantage over stem cells of not being living, replicating cells, which makes their application potentially simpler and safer. In addition, using exosomes could allow mass production of treatment as they may not need to be personalized to prevent immune rejection. However, exosomes face additional challenges including more difficult production and harvesting and full understanding of exosome effects has still not been completely elucidated.115
There have been many advances in the field of stem cells in the last 20 years, including harvest from adult tissues, induction of pluripotency, or even completely non-invasive harvesting or urine derived stem cells. Exosomes take this one step further by removing the cells from the equation entirely and treating directly with the secreted structures which impact wound healing. With the increased availability of various cell lines, in vivo experiments have begun to demonstrate the mechanisms and benefits of stem cell treatments for chronic wounds (Table 1).
Table 1.
Stem cell sources in pre-clinical trials.
| Stem cell source | References | Advantages and disadvantages |
|---|---|---|
| Embryonic stem cells | 90 | Advantages: Ability to differentiate into any cell line Disadvantages: Controversial harvesting, potentially tumorigenic |
| Induced pluripotent fibroblasts | 91, 93, 95 | Advantages: No ethical problem with harvesting, immunogenicity. Disadvantages: Induction of pluripotency may take longer and not as efficient. |
| Induced pluripotent keratinocytes | 92, 94 | Advantages: No ethical problems with harvesting, improved production of IPSCs compared to fibroblasts. Disadvantages: May be difficult to harvest and isolate from samples, slower growth and replication. |
| Hematopoeitic stem cells | 96, 97, 98 | Advantages: CD44 allows homing to sites of inflammation, potentially allowing injection rather than topical application. Disadvantages: Have only shown ability to differentiate into blood cells, hepatocytes, and fibroblasts |
| Bone marrow mesenchymal stem cells | 99, 100, 101 | Advantages: Rapid proliferation, wide differentiation capacity, anti-inflammatory effects. Disadvantages: More difficult to harvest than AMSCs. |
| Adipocyte mesenchymal stem cells | 99, 100, 102 | Advantages: Ease of harvest, rapid proliferation, wide differentiation capacity, angiogenic properties. Disadvantages: Decreased proliferation and density compared to BMMSCs. May have poorer anti-inflammatory effects. |
| Urine derived stem cells | 104, 105, 106, 107, 108 | Advantages: Extreme ease of harvest, ability to form urothelial, endothelial, smooth muscle, or even neural lineages. Disadvantages: Varying isolation efficiency, increased heterogeneity of cell lines, lack of data on long term effectiveness of USC grafts |
In vivo experiments and mechanisms of stem cell treatment
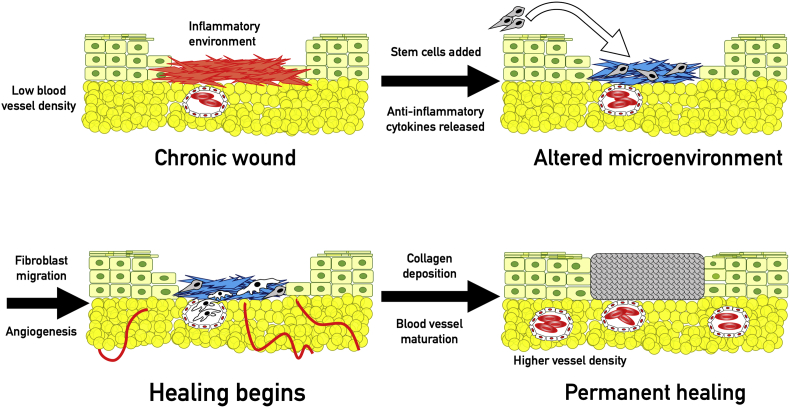
Since stem cells are involved in all stages of wound healing, it is logical that stem cell treatments exert their effects through divergent mechanisms (Fig. 4).
Figure 4.
Effects of stem cells. Stem cells improve the conditions in chronic wounds primarily through alteration of the microenvironment. Stem cells have been shown to produce pro-angiogenic and anti-inflammatory cytokines, especially IL-10, TGF-β, and VEGF. They also reduce the concentration of pro-inflammatory cytokines, mainly IL-1β, TNF-alpha and IL-6. Resolution of inflammation allows proliferation of fibroblasts and blood vessels, eventually resulting in repair as previously detailed.
Angiogenesis
Ischemia is often one of the major pathologies underlying impaired wound healing. Recent studies have shown that stem cell treatments can improve blood vessel formation and healing in ischemic settings. Injection of CD34 + EPCs into ischemic wounds on diabetic mice significantly improved vascular density and healing time.116 Another study using topically applied fetal-derived CD133 + cells showed similarly accelerated wound closure and increased capillarization.117 Stem cells also showed the potential to improve underlying ischemia. CD133 + cells injected into mice with ligated femoral arteries enhanced blood flow to the ischemic limbs via increased neovascularization.118 Injected AdSCs in irradiated mice improved skin perfusion and vessel density.119 Interestingly, vessel density in AdSC treated mice nearly doubled as compared to control mice, but only 2.6% of the vessels in the wound site expressed AdSC markers, implying that the major mechanism of increased angiogenesis was not based on proliferation of stem cells. This conclusion is supported by another study which demonstrated that new vascular structures were not derived from transplanted stem cells, though stem cells were seen to migrate to the wound bed and increase rate of healing.75
Cytokine signaling has been suggested to be the major mechanism of improved angiogenesis in stem cell treatments. One study found that CD133 + stem cells applied to wounds secreted large amounts of VEGF-A and IL-8 and increased rate of healing, while application of anti-VEGF-A or IL-8 antibodies rendered treatment ineffective.117 ASCs also increased capillary density in diabetic mice via augmented expression of HIF-1α and VEGF-A.120 Paracrine signaling from exogenous stem cell treatment improves vascularization and healing even in the anti-angiogenic environment of chronic wounds.
Inflammation
Ongoing inflammation is another trait of the non-healing wound. Although controlled inflammation is an important and necessary phase of wound healing, chronic inflammation damages the wound bed and inhibits the proliferative phase and tissue remodeling.121 Chronic inflammation also increases protease activity, degrading collagen and preventing repair.89 Stem cell treatments have been proven to be immunomodulatory and aid in resolving the inflammatory state. In vitro, MSCs secreted the anti-inflammatory cytokine IL-10, which decreased T-cell reactivity to antigen presenting cells.122 The role of IL-10 was further supported by a study which showed that addition of anti-IL-10 antibodies inhibited MSC immunosuppression.123 In addition to their own secretions, stem cells also alter the signaling of nearby cells: MSCs cultured with dendritic cells decreased secretion of the pro-inflammatory cytokine TNF-α and increased secretion of IL-10. T-cells cultured with MSCs produced less IFN-γ and IL-4.124 Macrophages can be activated to either pro- or anti-inflammatory phenotypes, and MSCs induce differentiation into the M2 (anti-inflammatory) form.125 ASCs also promoted M2 differentiation and decreased inflammatory response to lipopolysaccharides.126
Injection of CD-34 + umbilical cord blood cells into a wound decreased expression of the pro-inflammatory factors IL-1β, TNF-α, and IL-6 and increased IL-10 expression and products.49 ASCs produce both IL-10 and TGF-β, which decreases inflammation by inducing differentiation of regulatory T-cells.127, 128 Infusion of ASCs into a graft-versus-host mouse model decreased GVHD, an immunogenic reaction.129
Chronic inflammation can also be due to persistent infection. Stem cell treatment may paradoxically improve pathogen clearance even while modulating the inflammatory response. MSC treatment promoted clearance of bacterial products via increased phagocytic ability of host immune cells and improved survival in septic mice.130 ASCs have been shown to produce macrophage colony stimulating factor which recruits phagocytic cells.131
One of the mechanisms by which chronic inflammation prevents wound healing is through overproduction of reactive oxygen species, causing excessive free radical stress. An imbalance of free radical production can overwhelm local stem cells and prevent wound healing.132 Exogenously added stem cells can help to return balance to the system and promote healing. A recent study showed that injection of MSCs helped alleviate lung injury in paraquat poisoning in rats.133 Paraquat is an herbicide which causes cell damage through production of reactive oxygen species. Reduction in lung injury was accomplished through previously discussed signaling mechanisms, such as decreased TNF-α, IL-1β, and IL-6 production.
Stem cell treatment can reduce inflammation through a myriad of different effects. Direct IL-10 secretion modulates the inflammatory response and increases differentiation of anti-inflammatory cells. Alterations of local cell signaling decrease inflammatory cytokine secretion from leukocytes. Though stem cell treatment decreases inflammatory signaling, pathogen clearance is actually enhanced, decreasing mortality in a sepsis model. Reduction of inflammation modifies the chronic wound environment and promotes healing.
Differentiation and fibrosis
Proliferation of endogenous stem cells is a critical step in cutaneous wound healing. Exogenously added MSCs have shown the ability to differentiate into keratinocytes134 and epithelial cells.135 However, numerous studies have found that stem cell treatments show low levels of engraftment in vivo, often making up less than 1% of differentiated cells.136 Yet functional improvements have been observed despite minimal exogenous stem cell differentiation in various modalities.137
Though stem cell treatments do not contribute significantly to wound healing via direct differentiation, multiple studies have demonstrated stem cell mediated improvements in function of nearby cells. MSCs co-cultured with dermal fibroblasts increased fibroblast proliferation and migration.138 Another study demonstrated increased fibroblast collagen deposition when cultured with CD34 + umbilical cord blood derived cells.49 MSC conditioned medium applied to an excisional wound increased keratinocyte migration and proliferation.139 Stem cells also improved tissue remodeling: a study found that repeated topical application of MSCs yielded a basket-weave collagen organization very similar to healthy skin.140 Enhanced fibrosis and tissue reorganization are crucial to preventing recurrence and reversion of chronic wounds.
Decreased scar formation
Though fibrosis and scar formation is considered a normal part of physiologic wound healing, excessive or early fibrosis can actually prevent proper healing. An obvious example would be keloid or hypertrophic scars,141 however, a similar process can happen with large burns, trauma, or significant surgical interventions. Even large chronic wounds can cause problematic scars after healing, which has been shown to increase recurrence.142 These large scars sometimes require surgical correction, which triggers additional inflammatory reactions and potentially increased scar or chronic wound formation. Stem cells have been proven efficacious as a non-surgical intervention for scar formation by reducing the ratio of type I to type III collagen. Topical application of BMMSCs overexpressing TGF-β significantly reduced scar depth and density in a rabbit model.143 Further work in this area may demonstrate that cell-based therapies have a possible role in prevention or treatment of scar formation as well as epithelial breakdown.
Clinical applications
Stem cell treatments have proven efficacious in rodent models. A few representative examples include the following: bone marrow in a collagen matrix improved rate of wound healing in acute wounds on rats,144 ASCs injected into diabetic rats improved skin graft survival and decreased wound size within 1 week,145 ASCs seeded on a dermal matrix improved wound healing and blood vessel density in a full thickness wound model,146 and a recent study found that subcutaneous injection of ASCs significantly improved wound healing in diabetic rats through decreased pro-inflammatory factors.147
One of the earliest clinical applications, in 2003, examined topical application of BMMSCs for chronic wounds. They demonstrated complete closure of wounds in all 3 patients, despite wounds previously persisting for more than 1 year with standard therapy.148 A 2005 case study had similar results: topical treatment with autologous bone marrow cells reduced wound size and improved vascularity in a recalcitrant ulcer in a patient with T2DM.149 BMMSCs delivered in a novel fibrin spray improved chronic wound healing in patients as well as in diabetic mice.150
One of the challenges of stem cell therapy is growing sufficient numbers of cells for treatment. A follow up to the fibrin spray study found that nanofiber-expanded bone marrow cells, which increased number of cells, were still effective for treatment of patients with chronic venous ulcers.151 Administration of these cultured cells improved the cytokine profile of the wound bed, increasing pro-angiogenic markers and vascularity. Cultured BMMSCs applied to patients with intractable dermatopathies of various origins resulted in complete healing for 18 of 20 patients.152 Intramuscular injections of ASCs improved vascularity, pain, and walking distance in 10/15 diabetic patients with non-healing ulcers.153
Pressure wounds are often persistent and difficult to treat with current practices, in addition to frequent recurrence. In 3 patients with non-healing sacral pressure ulcers, treatment with CD34 + stem cells decreased wound volume by 60% from baseline in 3 weeks.154 In another study, topical application of bone marrow derived stem cells fully healed pressure ulcers in 19 of 22 treated patients within 3 weeks, despite prior persistence for more than 4 months. Remarkably, none of the ulcers recurred for at least 1 year following treatment.155
Though the number of positive results is encouraging, most of these studies have lacked effective randomization, positive placebos, or, in some cases, any control groups at all. A few larger randomized control trials have showed promise as well. Application of ASCs seeded in fibrin glue to patients with chronic perianal fistulas resulted in healing for 17 out of 24 patients, while only 4 out of 25 patients in the control group (fibrin glue without ASCs) displayed full healing in the same time frame.156 In a study of 96 diabetic patients with critical limb ischemia (CLI) and non-healing foot ulcers, treatment with BMMSCs (treatment groups were randomly assigned) showed improved brachial pressure index and significantly decreased limb amputations within 3 months of treatment.157 Another study of CLI in diabetic patients demonstrated 100% healing in patients treated with bone marrow derived MSCs, as well as significantly improved quantitative and qualitative measures including painless walking time, ankle brachial index results, and MRA analysis when compared to controls.101 Injection of BMMSCs into nonhealing foot ulcers of 12 diabetic patients significantly improved wound size, walking distance, and fibroblast proliferation over a 3 month period compared to those injected with saline.158 A recent study using bone marrow derived mesenchymal stem cells on diabetic foot ulcers showed significantly improved re-epithelization within a week of a single topical treatment with a much lower cell density by using single pass stem cells.159 This finding has important ramifications for one of the major problems involved in stem cell clinical treatments: the time required to amplify the initial sample. Because stem cells lose some of their stromal characteristics during the amplification process, cells harvested early during the expansion process are potent enough to improve wound healing even at lower density. Further research into this quality vs. quantity issue will need to be done to elucidate optimal in vivo amplification of stem cells for clinical treatments.
A double blinded, randomized clinical trial with 97 participants examining the long term efficacy of bone marrow aspirate for CLI recently closed, though results have not yet been made publicly available.160 Another interventional trial examining the efficacy of ASCs for treatment of diabetic ulcers, venous ulcers, and pressure ulcers has concluded, but results have not yet been published.161 A study investigating the effects of allogeneic stem cell treatment on burn wounds and the maximum safe dosage for treatment is still ongoing.162 Experimental results from case reports and randomized control trials have demonstrated the efficacy of stem cell techniques for chronic wound treatment (Table 2).
Table 2.
Clinical stem cell treatments for chronic wounds.
| Year | Reference | Study design (number of participants) | Stem cell source and modality of treatment | Result |
|---|---|---|---|---|
| 2003 | 109 | Case report, proof-of-principle (n = 3) | Bone marrow cells applied topically in isotonic NaCl solution | Complete healing of previously recalcitrant ulcer in 3/3 participants |
| 2005 | 110 | Case report (n = 1) | Bone marrow cells applied topically in isotonic NaCl solution | Improvement in epithelization and vascularization within 1 week |
| 2007 | 111 | Case report, experimental treatment (n = 8) | Cultured MSCs delivered in collagen sponge artificial dermis | Complete healing of previously recalcitrant ulcer in 8/8 participants |
| 2008 | 113 | Case report, experimental treatment (n = 20) | Cultured MSCs delivered in fibrin spray | Improvement of ulcer in 18/20 patients, healing positively correlated with number of cells applied |
| 2009 | 117 | Randomized control trial (n = 35) | Cultured ASCs in fibrin glue | Perianal fistula healed in 17/24 patients in treatment group, 4/25 in control |
| 2009 | 119 | Randomized control trial (n = 24) | Cultured BMMSCs | Significant improvements in ulcer size in experimental group |
| 2010 | 118 | Randomized control trial (n = 96) | Concentrated BMMSCs applied directly to wound bed | Significantly decreased limb amputations among patients in treatment group at 90 days post treatment |
| 2011 | 116 | Case report, experimental treatment (n = 22) | BM-MNCs suspended in saline solution injected into “wound pocket” | 19/22 pressure ulcers healed in 21 days, no recurrence in 19 months follow-up |
| 2012 | 114 | Case report, experimental treatment (n = 15) | ASCs injected intramuscularly | Improved vascularity and pain in ischemic ulcers at 6 months follow up |
| 2014 | 115 | Case report, experimental treatment (n = 3) | CD34 + BMCs injected into wound bed | Improvement in wound size in 3/3 patients |
| 2015 | 121 | Phase 2 randomized clinical trial (n = 25) | ASCs applied topically to wound | Results not yet published |
| 2016 | 120 | Phase 2 randomized clinical trial (n = 97) | Bone marrow aspirate injected into ischemic tissue | Results not yet published |
| 2018 | 157 | Case report, proof of principle (n = 3) | Early passage MSCs applied topically | Significantly improved healing within 1 week of application in 3/3 participants |
| 2018 | 122 | Phase 1 safety trial (n = 20) | Allogeneic stem cells topically added to burns | Study still ongoing |
Limitations and future directions
The difficulties associated with clinical stem cell treatment are significant. Stem cells are heterogeneous. The technology to standardize or monitor standardization on a large scale is not currently available. Cells from different sources or different hosts can vary in proliferative capacity, differentiation, and even mRNA expression levels.163 For minimal immunogenicity, stem cells should be harvested from the patient. However, stem cell function is frequently impaired in diabetic or elderly patients.164 Though expansion procedures allow production of large quantities of effective stem cells from a small original sample, these procedures are costly and time consuming.
Economic barriers to the adoption of these therapies are also formidable. The classic industry-led drug development model may not be an appropriate paradigm for stem cell-based therapies. Current pharmaceutical manufacturing techniques for small molecules are difficult to adapt to cellular manufacturing.165 Venture capitalists, viewing the field as too young to have profitable short term returns, have been unwilling to invest heavily in regenerative technology.166 The costs associated with cell-based therapy are frontloaded, so return on investment requires patience.167 Finally, intellectual property and patent law surrounding stem cells present new challenges that many companies are unable or unwilling to navigate.165 Governmental regulations for cell-based therapies are still in flux and are further complicated by the heterogeneity of the subject matter.168
Nonhealing wounds are a major challenge in healthcare today and will continue to become more problematic with an aging population. Many factors, including impaired macrophage function, fibroblast function, or altered cytokine signaling, lead to chronic wound formation. Current treatments fail to address the magnitude and complexity of the problem.
Stem cell treatments are a novel solution. Stem cells exert their effects primarily through cytokine signaling, creating an anti-inflammatory and pro-angiogenic environment. This environment contributes to healing and improves underlying pathologies, decreasing recurrence. In laboratory and clinical studies, stem cells obtained from various sources improved healing of stubborn wounds. The effects have often been startling: wounds which had previously persisted for more than a year healed within a matter of weeks. Though widespread adoption of these techniques has been complicated by the costs and complications associated with large-scale production of cell products, cell-based therapy for non-healing wounds is a field with great potential. In fact, as the population ages and the costs associated with chronic wound care increase, can we afford not to improve our treatments?
Conflict of interests
The authors declare no conflict of interests.
Acknowledgements
The contributing authors' laboratories were supported in part by research grants from the National Institutes of Health (CA226303, DE020140 to TCH and RRR), the U.S. Department of Defense (OR130096 to JMW), the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust (R.R.R., T.C.H., and G.A.A.), the Scoliosis Research Society (TCH and MJL), and the National Key Research and Development Program of China (2016YFC1000803 and 2011CB707906). This project was also supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number UL1 TR000430. EC was supported by the Summer Research Program of The University of Chicago Pritzker School of Medicine. TCH was also supported by the Mabel Green Myers Research Endowment Fund and The University of Chicago Orthopaedic Alumni Fund. Funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Izadi K., Ganchi P. Chronic wounds. Clin Plast Surg. 2005;32(2):209–222. doi: 10.1016/j.cps.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Demidova-Rice T.N., Hamblin M.R., Herman I.M. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, Part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care. 2012;25(7):304–314. doi: 10.1097/01.ASW.0000416006.55218.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ojeh N., Pastar I., Tomic-Canic M., Stojadinovic O. Stem cells in skin regeneration, wound healing, and their clinical applications. Int J Mol Sci. 2015;16(10):25476–25501. doi: 10.3390/ijms161025476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han G., Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599–610. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol. 2002;12(4):390–399. quiz 400–401. [PubMed] [Google Scholar]
- 6.Kolarsick P.A.J., Kolarsick M.A., Goodwin C. Anatomy and physiology of the skin. J Dermatol Nurses' Assoc. 2011;3(4):203. [Google Scholar]
- 7.Jackson S.M., Williams M.L., Feingold K.R., Elias P.M. Pathobiology of the stratum corneum. West J Med. 1993;158(3):279–285. [PMC free article] [PubMed] [Google Scholar]
- 8.Matoltsy A.G. Keratinization. J Investig Dermatol. 1976;67(1):20–25. doi: 10.1111/1523-1747.ep12512473. [DOI] [PubMed] [Google Scholar]
- 9.McGraith J., Eady R., Pope F. 7th ed. Blackwell Publishing; 2004. Rook's Textbook of Dermatology. [Google Scholar]
- 10.Aumailley M., Krieg T. Laminins: a family of diverse multifunctional molecules of basement membranes. J Investig Dermatol. 1996;106(2):209–214. doi: 10.1111/1523-1747.ep12340471. [DOI] [PubMed] [Google Scholar]
- 11.Stepp M.A., Spurr-Michaud S., Tisdale A., Elwell J., Gipson I.K. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci. 1990;87(22):8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3(1) doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Exan R.J., Hardy M.H. The differentiation of the dermis in the laboratory mouse. Am J Anat. 1984;169(2):149–164. doi: 10.1002/aja.1001690204. [DOI] [PubMed] [Google Scholar]
- 14.Rahmani W., Abbasi S., Hagner A. Hair follicle dermal stem cells regenerate the dermal sheath, repopulate the dermal papilla, and modulate hair type. Dev Cell. 2014;31(5):543–558. doi: 10.1016/j.devcel.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 15.James W., Berger T., Elston D. 10th ed. Elsevier Saunders; 2006. Andrews' Diseases of the Skin: Clinical Dermatology. [Google Scholar]
- 16.Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 17.Kirsner R.S., Eaglstein W.H. The wound healing process. Dermatol Clin. 1993;11(4):629–640. [PubMed] [Google Scholar]
- 18.Szpaderska A.M., Egozi E.I., Gamelli R.L., DiPietro L.A. The effect of thrombocytopenia on dermal wound healing. J Investig Dermatol. 2003;120(6):1130–1137. doi: 10.1046/j.1523-1747.2003.12253.x. [DOI] [PubMed] [Google Scholar]
- 19.Mosesson M.W., Siebenlist K.R., Meh D.A. The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936(1):11–30. doi: 10.1111/j.1749-6632.2001.tb03491.x. [DOI] [PubMed] [Google Scholar]
- 20.Grinnell F., Billingham R.E., Burgess L. Distribution of fibronectin during wound healing in vivo. J Investig Dermatol. 1981;76(3):181–189. doi: 10.1111/1523-1747.ep12525694. [DOI] [PubMed] [Google Scholar]
- 21.Li J., Chen J., Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Falanga V. Growth factors and wound healing. J Dermatol Surg Oncol. 1993;19(8):711–714. doi: 10.1111/j.1524-4725.1993.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 23.Maurer M., Theoharides T., Granstein R.D. What is the physiological function of mast cells? Exp Dermatol. 2003;12(6):886–910. doi: 10.1111/j.0906-6705.2003.0109a.x. [DOI] [PubMed] [Google Scholar]
- 24.Kurkinen M., Vaheri A., Roberts P.J., Stenman S. Sequential appearance of fibronectin and collagen in experimental granulation tissue. Lab Investig. 1980;43(1):47–51. [PubMed] [Google Scholar]
- 25.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008 doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 26.Lovvorn H.N., Cheung D.T., Nimni M.E., Perelman N., Estes J.M., Adzick N.S. Relative distribution and crosslinking of collagen distinguish fetal from adult sheep wound repair. J Pediatr Surg. 1999;34(1):218–223. doi: 10.1016/s0022-3468(99)90261-0. [DOI] [PubMed] [Google Scholar]
- 27.Nelzen O., Bergqvist D., Lindhagen A., Hallbook T. Chronic leg ulcers: an underestimated problem in primary health care among elderly patients. J Epidemiol Community Health. 1991;45(3):184–187. doi: 10.1136/jech.45.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paquette D., Falanga V. Leg ulcers. Clin Geriatr Med. 2002;18(1):77–88. doi: 10.1016/s0749-0690(03)00035-1. [DOI] [PubMed] [Google Scholar]
- 29.Phillips C.J., Humphreys I., Fletcher J., Harding K., Chamberlain G., Macey S. Estimating the costs associated with the management of patients with chronic wounds using linked routine data. Int Wound J. 2016;13(6):1193–1197. doi: 10.1111/iwj.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walshe C. Living with a venous leg ulcer: a descriptive study of patients' experiences. J Adv Nurs. 1995;22(6):1092–1100. doi: 10.1111/j.1365-2648.1995.tb03110.x. [DOI] [PubMed] [Google Scholar]
- 31.Järbrink K., Ni G., Sönnergren H. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev. 2017;6 doi: 10.1186/s13643-016-0400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franks P.J., Moffatt C.J., Doherty D.C., Smithdale R., Martin R. Longer-term changes in quality of life in chronic leg ulceration. Wound Repair Regen. 2006;14(5):536–541. doi: 10.1111/j.1743-6109.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- 33.MacKay D. Nutritional support for wound healing. Wound Heal. 2003;8(4):19. [PubMed] [Google Scholar]
- 34.Gottrup F. Oxygen in wound healing and infection. World J Surg. 2004;28(3):312–315. doi: 10.1007/s00268-003-7398-5. [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association Economic costs of diabetes in the U.S. In 2017. Diabetes Care. 2018;41(5):917–928. doi: 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galkowska H., Wojewodzka U., Olszewski W.L. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. 2006;14(5):558–565. doi: 10.1111/j.1743-6109.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 37.Jeffcoate W.J., Harding K.G. Diabetic foot ulcers. Lancet. 2003;361(9368):1545–1551. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- 38.Maruyama K., Asai J., Ii M., Thorne T., Losordo D.W., D'Amore P.A. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170(4):1178–1191. doi: 10.2353/ajpath.2007.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zykova S.N., Jenssen T.G., Berdal M., Olsen R., Myklebust R., Seljelid R. Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes. 2000;49(9):1451–1458. doi: 10.2337/diabetes.49.9.1451. [DOI] [PubMed] [Google Scholar]
- 40.Galiano R.D., Tepper O.M., Pelo C.R. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164(6):1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goren I., Müller E., Pfeilschifter J., Frank S. Severely impaired Insulin signaling in chronic wounds of diabetic ob/ob mice: a potential role of tumor necrosis factor-α. Am J Pathol. 2006;168(3):765–777. doi: 10.2353/ajpath.2006.050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cianfarani F., Toietta G., Rocco G.D., Cesareo E., Zambruno G., Odorisio T. Diabetes impairs adipose tissue–derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013;21(4):545–553. doi: 10.1111/wrr.12051. [DOI] [PubMed] [Google Scholar]
- 43.Capla J.M., Grogan R.H., Callaghan M.J. Diabetes impairs endothelial progenitor cell–mediated blood vessel formation in response to hypoxia. Plast Reconstr Surg. 2007;119(1):59. doi: 10.1097/01.prs.0000244830.16906.3f. [DOI] [PubMed] [Google Scholar]
- 44.Sundaram G.M., Common J.E.A., Gopal F.E. “See-saw” expression of microRNA-198 and FSTL1 from a single transcript in wound healing. Nature. 2013;495(7439):103–106. doi: 10.1038/nature11890. [DOI] [PubMed] [Google Scholar]
- 45.Graiani G., Emanueli C., Desortes E. Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of Type 1 diabetic mice. Diabetologia. 2004;47(6):1047–1054. doi: 10.1007/s00125-004-1414-7. [DOI] [PubMed] [Google Scholar]
- 46.Trengove N.J., Bielefeldt-Ohmann H., Stacey M.C. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8(1):13–25. doi: 10.1046/j.1524-475x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 47.Bennett N.T., Schultz G.S. Growth factors and wound healing: Part II. Role in normal and chronic wound healing. Am J Surg. 1993;166(1):74–81. doi: 10.1016/s0002-9610(05)80589-6. [DOI] [PubMed] [Google Scholar]
- 48.Yager D.R., Chen S.M., Ward S.I., Olutoye O.O., Diegelmann R.F., Kelman Cohen I. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen. 1997;5(1):23–32. doi: 10.1046/j.1524-475X.1997.50108.x. [DOI] [PubMed] [Google Scholar]
- 49.Kanji S., Das M., Aggarwal R. Nanofiber-expanded human umbilical cord blood-derived CD34 + cell therapy accelerates murine cutaneous wound closure by attenuating pro-inflammatory factors and secreting IL-10. Stem Cell Res. 2014;12(1):275–288. doi: 10.1016/j.scr.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schultz G.S., Sibbald R.G., Falanga V. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11(Suppl 1):S1–S28. doi: 10.1046/j.1524-475x.11.s2.1.x. [DOI] [PubMed] [Google Scholar]
- 51.Stanley A., Osler T. Senescence and the healing rates of venous ulcers. J Vasc Surg. 2001;33(6):1206–1211. doi: 10.1067/mva.2001.115379. [DOI] [PubMed] [Google Scholar]
- 52.Jones V., Grey J.E., Harding K.G. Wound dressings. BMJ. 2006;332(7544):777–780. doi: 10.1136/bmj.332.7544.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keyser J.E. Diabetic wound healing and limb salvage in an outpatient wound care program. South Med J. 1993;86(3):311–317. doi: 10.1097/00007611-199303000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Langer A., Rogowski W. Systematic review of economic evaluations of human cell-derived wound care products for the treatment of venous leg and diabetic foot ulcers. BMC Health Serv Res. 2009;9:115. doi: 10.1186/1472-6963-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venturi M.L., Attinger C.E., Mesbahi A.N., Hess C.L., Graw K.S. Mechanisms and clinical applications of the vacuum-assisted closure (VAC) Device: a review. Am J Clin Dermatol. 2005;6(3):185–194. doi: 10.2165/00128071-200506030-00005. [DOI] [PubMed] [Google Scholar]
- 56.Streubel P.N., Stinner D.J., Obremskey W.T. Use of negative-pressure wound therapy in orthopaedic trauma. JAAOS – J Am Acad Orthop Surg. 2012;20(9):564. doi: 10.5435/JAAOS-20-09-564. [DOI] [PubMed] [Google Scholar]
- 57.Wu S.C., Marston W., Armstrong D.G. Wound care: the role of advanced wound-healing technologies. J Am Podiatr Med Assoc. 2010;100(5):385–394. doi: 10.7547/1000385. [DOI] [PubMed] [Google Scholar]
- 58.Kranke P., Bennett M., Roeckl-Wiedmann I., Debus S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004;(2):CD004123. doi: 10.1002/14651858.CD004123.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Bennett S.P., Griffiths G.D., Schor A.M., Leese G.P., Schor S.L. Growth factors in the treatment of diabetic foot ulcers. BJS. 2003;90(2):133–146. doi: 10.1002/bjs.4019. [DOI] [PubMed] [Google Scholar]
- 60.Robson M.C., Phillips L.G., Robson L.E., Thomason A., Pierce G.F. Platelet-derived growth factor BB for the treatment of chronic pressure ulcers. Lancet. 1992;339(8784):23–25. doi: 10.1016/0140-6736(92)90143-q. [DOI] [PubMed] [Google Scholar]
- 61.Wieman T.J., Smiell J.M., Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21(5):822–827. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- 62.Okumura M., Okuda T., Nakamura T., Yajima M. Acceleration of wound healing in diabetic mice by basic fibroblast growth factor. Biol Pharm Bull. 1996;19(4):530–535. doi: 10.1248/bpb.19.530. [DOI] [PubMed] [Google Scholar]
- 63.Richard J.L., Parer-Richard C., Daures J.P. Effect of topical basic fibroblast growth factor on the healing of chronic diabetic neuropathic ulcer of the foot. A pilot, randomized, double-blind, placebo-controlled study. Diabetes Care. 1995;18(1):64–69. doi: 10.2337/diacare.18.1.64. [DOI] [PubMed] [Google Scholar]
- 64.Robson M.C., Phillips L.G., Lawrence W.T. The safety and effect of topically applied recombinant basic fibroblast growth factor on the healing of chronic pressure sores. Ann Surg. 1992;216(4):401–408. doi: 10.1097/00000658-199210000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 66.Howdieshell T.R., Callaway D., Webb W.L. Antibody neutralization of vascular endothelial growth factor inhibits wound granulation tissue formation. J Surg Res. 2001;96(2):173–182. doi: 10.1006/jsre.2001.6089. [DOI] [PubMed] [Google Scholar]
- 67.Kano M.R., Morishita Y., Iwata C. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B–PDGFRβ signaling. J Cell Sci. 2005;118(16):3759–3768. doi: 10.1242/jcs.02483. [DOI] [PubMed] [Google Scholar]
- 68.Barrientos S., Brem H., Stojadinovic O., Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22(5):569–578. doi: 10.1111/wrr.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu Y., Wang J., Scott P.G., Tredget E.E. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen. 2007;15(s1):S18–S26. doi: 10.1111/j.1524-475X.2007.00221.x. [DOI] [PubMed] [Google Scholar]
- 70.Asahara T., Masuda H., Takahashi T. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 71.Meindl S., Schmidt U., Vaculik C., Elbe-Bürger A. Characterization, isolation, and differentiation of murine skin cells expressing hematopoietic stem cell markers. J Leukoc Biol. 2006;80(4):816–826. doi: 10.1189/jlb.0106015. [DOI] [PubMed] [Google Scholar]
- 72.Lau K., Paus R., Tiede S., Day P., Bayat A. Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol. 2009;18(11):921–933. doi: 10.1111/j.1600-0625.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- 73.Liang L., Bickenbach J.R. Somatic epidermal stem cells can produce multiple cell lineages during development. Stem Cells. 2002;20(1):21–31. doi: 10.1634/stemcells.20-1-21. [DOI] [PubMed] [Google Scholar]
- 74.Fathke C., Wilson L., Hutter J. Contribution of bone marrow–derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22(5):812–822. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bluff J.E., Ferguson M.W.J., O'Kane S., Ireland G. Bone marrow–derived endothelial progenitor cells do not contribute significantly to new vessels during incisional wound healing. Exp Hematol. 2007;35(3):500–506. doi: 10.1016/j.exphem.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 76.Rehman J., Li J., Orschell C.M., March K.L. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107(8):1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 77.Jiang S., Walker L., Afentoulis M. Transplanted human bone marrow contributes to vascular endothelium. Proc Natl Acad Sci U S A. 2004;101(48):16891–16896. doi: 10.1073/pnas.0404398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tepper O.M., Galiano R.D., Capla J.M. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106(22):2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 79.Loots M.A.M., Kenter S.B., Au F.L. Fibroblasts derived from chronic diabetic ulcers differ in their response to stimulation with EGF, IGF-I, bFGF and PDGF-AB compared to controls. Eur J Cell Biol. 2002;81(3):153–160. doi: 10.1078/0171-9335-00228. [DOI] [PubMed] [Google Scholar]
- 80.Chambers S.M., Shaw C.A., Gatza C., Fisk C.J., Donehower L.A., Goodell M.A. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5(8):e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miki T., Bottaro D.P., Fleming T.P. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci USA. 1992;89(1):246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koria P. Delivery of growth factors for tissue regeneration and wound healing. BioDrugs. 2012;26(3):163–175. doi: 10.2165/11631850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 83.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Madeddu P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp Physiol. 2005;90(3):315–326. doi: 10.1113/expphysiol.2004.028571. [DOI] [PubMed] [Google Scholar]
- 85.Streuli C. Extracellular matrix remodelling and cellular differentiation. Curr Opin Cell Biol. 1999;11(5):634–640. doi: 10.1016/s0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 86.Zhu Y., Hoshi R., Chen S. Sustained release of stromal cell derived factor-1 from an antioxidant thermoresponsive hydrogel enhances dermal wound healing in diabetes. J Control Release. 2016;238:114–122. doi: 10.1016/j.jconrel.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 87.Xiao J., Chen S., Yi J., Zhang H.F., Ameer G.A. A cooperative copper metal–organic framework-hydrogel system improves wound healing in diabetes. Adv Funct Mater. 2017;27(1):1604872. doi: 10.1002/adfm.201604872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davidson J.M. First-class delivery: getting growth factors to their destination. J Investig Dermatol. 2008;128(6):1360–1362. doi: 10.1038/jid.2008.128. [DOI] [PubMed] [Google Scholar]
- 89.Kanji S., Das H. Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediat Inflamm. 2017;2017 doi: 10.1155/2017/5217967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aberdam D. Derivation of keratinocyte progenitor cells and skin formation from embryonic stem cells. Int J Dev Biol. 2004;48(2–3):203–206. doi: 10.1387/ijdb.15272386. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 92.Aasen T., Raya A., Barrero M.J. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26(11):1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 93.Hewitt K.J., Shamis Y., Hayman R.B. Epigenetic and phenotypic profile of fibroblasts derived from induced pluripotent stem cells. PLoS One. 2011;6(2) doi: 10.1371/journal.pone.0017128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bilousova G., Chen J., Roop D.R. Differentiation of mouse induced pluripotent stem cells into a multipotent keratinocyte lineage. J Investig Dermatol. 2011;131(4):857–864. doi: 10.1038/jid.2010.364. [DOI] [PubMed] [Google Scholar]
- 95.Lu Q., Yu M., Shen C. Negligible immunogenicity of induced pluripotent stem cells derived from human skin fibroblasts. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0114949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Körbling M., Katz R.L., Khanna A. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002;346(10):738–746. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 97.Fu X., Sun X. Can hematopoietic stem cells be an alternative source for skin regeneration? Ageing Res Rev. 2009;8(3):244–249. doi: 10.1016/j.arr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 98.Sackstein R. The bone marrow is akin to skin: HCELL and the biology of hematopoietic stem cell homing. J Investig Dermatol. 2004;122(5):1061–1069. doi: 10.1111/j.0022-202X.2004.09301.x. [DOI] [PubMed] [Google Scholar]
- 99.Hsiao S.T.-F., Asgari A., Lokmic Z. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012;21(12):2189–2203. doi: 10.1089/scd.2011.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mansilla E., Marin G.H., Sturla F. Human mesenchymal stem cells are tolerized by mice and improve skin and spinal cord injuries. Transplant Proc. 2005;37(1):292–294. doi: 10.1016/j.transproceed.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 101.Lu D., Chen B., Liang Z. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92(1):26–36. doi: 10.1016/j.diabres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 102.Strioga M., Viswanathan S., Darinskas A., Slaby O., Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21(14):2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 103.Elman J.S., Li M., Wang F., Gimble J.M., Parekkadan B. A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflamm (Lond) 2014;11:1. doi: 10.1186/1476-9255-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y., McNeill E., Tian H. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180(5):2226–2233. doi: 10.1016/j.juro.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 105.Cao Y.-M., Liu M.-Y., Xue Z.-W. Surface-structured bacterial cellulose loaded with hUSCs accelerate skin wound healing by promoting angiogenesis in rats. Biochem Biophys Res Commun. 2019;516(4):1167–1174. doi: 10.1016/j.bbrc.2019.06.161. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y., Niu X., Dong X., Wang Y., Li H. Bioglass enhanced wound healing ability of urine-derived stem cells through promoting paracrine effects between stem cells and recipient cells. J Tissue Eng Regenerat Med. 2018;12(3):e1609–e1622. doi: 10.1002/term.2587. [DOI] [PubMed] [Google Scholar]
- 107.Liu Y., Zheng Y., Li S. Human neural progenitors derived from integration-free iPSCs for SCI therapy. Stem Cell Res. 2017;19:55–64. doi: 10.1016/j.scr.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kang H.S., Choi S.H., Kim B.S. Advanced properties of urine derived stem cells compared to adipose tissue derived stem cells in terms of cell proliferation, immune modulation and multi differentiation. J Korean Med Sci. 2015;30(12):1764–1776. doi: 10.3346/jkms.2015.30.12.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chun S.Y., Kim H.T., Lee J.-S. Characterization of urine-derived cells from upper urinary tract in patients with bladder cancer. Urology. 2012;79(5):1186.e1–1186.e7. doi: 10.1016/j.urology.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 110.Pavathuparambil Abdul Manaph N., Al-Hawwas M., Bobrovskaya L., Coates P.T., Zhou X.-F. Urine-derived cells for human cell therapy. Stem Cell Res Ther. 2018;9(1):189. doi: 10.1186/s13287-018-0932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang H.-C., Liu X.-B., Huang S. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012;21(18):3289–3297. doi: 10.1089/scd.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang B., Yin Y., Lai R.C., Tan S.S., Choo A.B.H., Lim S.K. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233–1244. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- 113.Mokarizadeh A., Delirezh N., Morshedi A., Mosayebi G., Farshid A.-A., Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147(1–2):47–54. doi: 10.1016/j.imlet.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 114.Chen C.-Y., Rao S.-S., Ren L. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics. 2018;8(6):1607–1623. doi: 10.7150/thno.22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De Jong O.G., Van Balkom B.W.M., Schiffelers R.M., Bouten C.V.C., Verhaar M.C. Extracellular vesicles: potential roles in regenerative medicine. Front Immunol. 2014;5 doi: 10.3389/fimmu.2014.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sivan-Loukianova E., Awad O.A., Stepanovic V., Bickenbach J., Schatteman G.C. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. JVR. 2003;40(4):368–377. doi: 10.1159/000072701. [DOI] [PubMed] [Google Scholar]
- 117.Barcelos L.S., Duplaa C., Kränkel N. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res. 2009;104(9):1095–1102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Das H., Abdulhameed N., Joseph M., Sakthivel R., Mao H.-Q., Pompili V.J. Ex vivo nanofiber expansion and genetic modification of human cord blood-derived progenitor/stem cells enhances vasculogenesis. Cell Transplant. 2009;18(3):305–318. doi: 10.3727/096368909788534870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ebrahimian T.G., Pouzoulet F., Squiban C. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol. 2009;29(4):503–510. doi: 10.1161/ATVBAHA.108.178962. [DOI] [PubMed] [Google Scholar]
- 120.Gao W., Qiao X., Ma S., Cui L. Adipose-derived stem cells accelerate neovascularization in ischaemic diabetic skin flap via expression of hypoxia-inducible factor-1α. J Cell Mol Med. 2011;15(12):2575–2585. doi: 10.1111/j.1582-4934.2011.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tarnuzzer R.W., Schultz G.S. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996;4(3):321–325. doi: 10.1046/j.1524-475X.1996.40307.x. [DOI] [PubMed] [Google Scholar]
- 122.Beyth S., Borovsky Z., Mevorach D. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 123.Yang S.-H., Park M.-J., Yoon I.-H. Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Exp Mol Med. 2009;41(5):315–324. doi: 10.3858/emm.2009.41.5.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 125.Cho D.-I., Kim M.R., Jeong H. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu Y., Qin C., Zheng G. Mesenchymal stem cell-educated macrophages ameliorate LPS-induced systemic response. Mediat Inflamm. 2016;2016:3735452. doi: 10.1155/2016/3735452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kokai L.E., Marra K., Rubin J.P. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res. 2014;163(4):399–408. doi: 10.1016/j.trsl.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 128.Li M.O., Flavell R.A. Contextual regulation of inflammation: a duet by transforming growth factor-β and interleukin-10. Immunity. 2008;28(4):468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 129.Yañez R., Lamana M.L., García-Castro J., Colmenero I., Ramírez M., Bueren J.A. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24(11):2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 130.Mei S.H.J., Haitsma J.J., Dos Santos C.C. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182(8):1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 131.Kilroy G.E., Foster S.J., Wu X. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212(3):702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 132.Lee J., Cho Y.S., Jung H., Choi I. Pharmacological regulation of oxidative stress in stem cells. Oxidative Med Cell Longev. 2018 doi: 10.1155/2018/4081890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang L., Li Q., Liu W., Liu Z., Shen H., Zhao M. Mesenchymal stem cells alleviate acute lung injury and inflammatory responses induced by paraquat poisoning. Med Sci Monit. 2019;25:2623–2632. doi: 10.12659/MSM.915804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sasaki M., Abe R., Fujita Y., Ando S., Inokuma D., Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 135.Spees J.L., Olson S.D., Ylostalo J. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci. 2003;100(5):2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Phinney D.G., Prockop D.J. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25(11):2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 137.Prockop D.J. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clin Pharmacol Ther. 2007;82(3):241–243. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- 138.Smith A.N., Willis E., Chan V.T. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res. 2010;316(1):48–54. doi: 10.1016/j.yexcr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen L., Tredget E.E., Wu P.Y.G., Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3(4) doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu Y., Huang S., Enhe J. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. Int Wound J. 2014;11(6):701–710. doi: 10.1111/iwj.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mari W., Alsabri S.G., Tabal N., Younes S., Sherif A., Simman R. Novel insights on understanding of keloid scar: article review. J Am Coll Clin Wound Spec. 2016;7(1–3):1–7. doi: 10.1016/j.jccw.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gohel M.S., Taylor M., Earnshaw J.J., Heather B.P., Poskitt K.R., Whyman M.R. Risk factors for delayed healing and recurrence of chronic venous leg ulcers—an analysis of 1324 legs. Eur J Vasc Endovasc Surg. 2005;29(1):74–77. doi: 10.1016/j.ejvs.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 143.Li M., Qiu L., Hu W. Genetically-modified bone mesenchymal stem cells with TGF-β3 improve wound healing and reduce scar tissue formation in a rabbit model. Exp Cell Res. 2018;367(1):24–29. doi: 10.1016/j.yexcr.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 144.Ichioka S., Kouraba S., Sekiya N., Ohura N., Nakatsuka T. Bone marrow-impregnated collagen matrix for wound healing: experimental evaluation in a microcirculatory model of angiogenesis, and clinical experience. Br J Plast Surg. 2005;58(8):1124–1130. doi: 10.1016/j.bjps.2005.04.054. [DOI] [PubMed] [Google Scholar]
- 145.Zografou A., Papadopoulos O., Tsigris C. Autologous transplantation of adipose-derived stem cells enhances skin graft survival and wound healing in diabetic rats. Ann Plast Surg. 2013;71(2):225–232. doi: 10.1097/SAP.0b013e31826af01a. [DOI] [PubMed] [Google Scholar]
- 146.Huang S.-P., Hsu C.-C., Chang S.-C. Adipose-derived stem cells seeded on acellular dermal matrix grafts enhance wound healing in a murine model of a full-thickness defect. Ann Plast Surg. 2012;69(6):656–662. doi: 10.1097/SAP.0b013e318273f909. [DOI] [PubMed] [Google Scholar]
- 147.Kuo Y.-R., Wang C.-T., Cheng J.-T., Kao G.-S., Chiang Y.-C., Wang C.-J. Adipose-derived stem cells accelerate diabetic wound healing through the induction of autocrine and paracrine effects. Cell Transplant. 2016;25(1):71–81. doi: 10.3727/096368915X687921. [DOI] [PubMed] [Google Scholar]
- 148.Badiavas E.V., Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139(4):510–516. doi: 10.1001/archderm.139.4.510. [DOI] [PubMed] [Google Scholar]
- 149.Humpert P.M., Bärtsch U., Konrade I. Locally applied mononuclear bone marrow cells restore angiogenesis and promote wound healing in a type 2 diabetic patient. Exp Clin Endocrinol Diabetes. 2005;113(9):538–540. doi: 10.1055/s-2005-872886. [DOI] [PubMed] [Google Scholar]
- 150.Falanga V., Iwamoto S., Chartier M. Autologous bone marrow–derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13(6):1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 151.Badiavas E.V., Ford D., Liu P. Long-term bone marrow culture and its clinical potential in chronic wound healing. Wound Repair Regen. 2007;15(6):856–865. doi: 10.1111/j.1524-475X.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 152.Yoshikawa T., Mitsuno H., Nonaka I. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121(3):860–877. doi: 10.1097/01.prs.0000299922.96006.24. [DOI] [PubMed] [Google Scholar]
- 153.Lee H.C., An S.G., Lee H.W. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J. 2012;76(7):1750–1760. doi: 10.1253/circj.cj-11-1135. [DOI] [PubMed] [Google Scholar]
- 154.Wettstein R., Savic M., Pierer G. Progenitor cell therapy for sacral pressure sore: a pilot study with a novel human chronic wound model. Stem Cell Res Ther. 2014;5(1):18. doi: 10.1186/scrt407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sarasúa J.G., López S.P., Viejo M.Á. Treatment of pressure ulcers with autologous bone marrow nuclear cells in patients with spinal cord injury. J Spinal Cord Med. 2011;34(3):301–307. doi: 10.1179/2045772311Y.0000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Garcia-Olmo D., Herreros D., Pascual I. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52(1):79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 157.Procházka V., Gumulec J., Jalůvka F. Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant. 2010;19(11):1413–1424. doi: 10.3727/096368910X514170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dash N.R., Dash S.N., Routray P., Mohapatra S., Mohapatra P.C. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009;12(5):359–366. doi: 10.1089/rej.2009.0872. [DOI] [PubMed] [Google Scholar]
- 159.Maksimova N., Krasheninnikov M., Zhang Y. Early passage autologous mesenchymal stromal cells accelerate diabetic wound re-epithelialization: a clinical case study. Cytotherapy. 2017;19(12):1548–1550. doi: 10.1016/j.jcyt.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 160.Bone marrow aspirate concentrate (BMAC) for treatment of critical limb ischemia (CLI) - No study results posted - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT01245335
- 161.Adipose derived regenerative cellular therapy of chronic wounds - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02092870
- 162.Stem cell therapy to improve burn wound healing - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02104713
- 163.Tremain N., Korkko J., Ibberson D., Kopen G.C., DiGirolamo C., Phinney D.G. MicroSAGE analysis of 2,353 expressed genes in a single cell-derived colony of undifferentiated human mesenchymal stem cells reveals mRNAs of multiple cell lineages. Stem Cells. 2001;19(5):408–418. doi: 10.1634/stemcells.19-5-408. [DOI] [PubMed] [Google Scholar]
- 164.Schatteman G.C., Ma N. Old bone marrow cells inhibit skin wound vascularization. Stem Cells. 2006;24(3):717–721. doi: 10.1634/stemcells.2005-0214. [DOI] [PubMed] [Google Scholar]
- 165.Rao M.S. Funding translational work in cell-based therapy. Cell Stem Cell. 2011;9(1):7–10. doi: 10.1016/j.stem.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 166.Harvey O. Human embryonic stem cell research in the United States: some policy options for industry development. Polit Policy. 2009;37(1):51–71. [Google Scholar]
- 167.Mason C., Brindley D.A., Culme-Seymour E.J., Davie N.L. Cell therapy industry: billion dollar global business with unlimited potential. Regen Med. 2011;6(3):265–272. doi: 10.2217/rme.11.28. [DOI] [PubMed] [Google Scholar]
- 168.Sipp D., Turner L.U.S. Regulation of stem cells as medical products. Science. 2012;338(6112):1296–1297. doi: 10.1126/science.1229918. [DOI] [PubMed] [Google Scholar]