Abstract
The pathophysiology of malarial anemia is multifactorial and incompletely understood. We assessed mechanistic and risk factors for post-malarial anemia in Ghanaian and Gabonese children with severe P. falciparum malaria treated with parenteral artesunate followed by an oral artemisinin-combination therapy. We analyzed data from two independent studies in which children were followed on Days 7,14, and 28 after treatment with artesunate. Specific hematological parameters included the presence of hemoglobinopathies and erythropoietin. Presence of once-infected erythrocytes was assessed by flow cytometry in a sub-population. Of 143 children with a geometric mean parasitemia of 116,294/µL (95% CI: 95,574–141,505), 91 (88%) had anemia (Hb < 10 g/dL) at presentation. Hemoglobin increased after Day 7 correlating with increased erythropoiesis through adequate erythropoietin stimulation. 22 children (24%) remained anemic until Day 28. Post-artesunate delayed hemolysis was detected in 7 children (5%) with only minor differences in the dynamics of once-infected erythrocytes. Hyperparasitemia and hemoglobin at presentation were associated with anemia on Day 14. On Day 28 only lower hemoglobin at presentation was associated with anemia. Most children showed an adequate erythropoiesis and recovered from anemia within one month. Post-artesunate delayed hemolysis (PADH) and hyperparasitemia are associated with early malarial anemia and pre-existing anemia is the main determinant for prolonged anemia.
Subject terms: Paediatric research, Malaria
Introduction
The superior efficacy of artesunate over quinine in the treatment of severe malaria has been shown in different clinical and geographical settings1–4. Its use is not restricted by adverse events typical of quinine, such as QT prolongation, visual and auditory disturbances, and severe hypoglycemia5. Consequently parenteral artesunate is recommended as the treatment of choice for severe malaria by the World Health Organization6. The clinically most relevant adverse event of parenteral artesunate is delayed hemolysis occurring approximately two weeks after treatment7,8. Hemolysis affects between 20 and 30% of adult travelers from Europe with no or waning semi-immunity against malaria and high parasitemia treated with artesunate9. Delayed hemolysis has been reported in 5% of African children exposed to artesunate10,11.
Anemia is highly prevalent in African children under the age of 5 years12. The causes of anemia in this population are manifold and range amongst others from iron-deficiency to parasitic infections and genetic traits12,13. Additionally, anemia is major clinical hallmark of malaria and related to direct destruction of red blood cells by the parasite but also indirect effects, such as disruption of erythropoiesis and immune mechanisms14. Here we describe the recovery from malarial anemia in children with severe malaria treated with artesunate and assess predictive and mechanistic factors contributing to prolonged anemia.
Material and Methods
Patient population
This analysis is based on individual patient data from two studies: between April and September 2012 patients were recruited into a sub-study of the “Comparative, Open Label, Dose and Regimen Optimization Follow-up Study of Intravenous and Intramuscular Artesunate in African Children with Severe Malaria” (PACTR201102000277177) at the Komfo Anokye Teaching Hospital in Kumasi, Ghana, and at the Centre de Recherches Médicales de Lambaréné, Gabon, conducted by the Severe Malaria in African Children Network (SMAC)15,16. The primary endpoint of this study was the incidence of post-artesunate delayed hemolysis. Exploratory results of this sub-study have been published10.
Between January and August 2015 patients were recruited into a second study at the St. Michael’s Hospital in Pramso and the Komfo Anokye Teaching Hospital in Kumasi, Ghana.
Study procedures
Children aged 6 months to 10 years were included in both studies if they presented to a study site with a primary diagnosis of P. falciparum malaria (>5000 parasites/µL on a thick blood smear) with signs or symptoms requiring hospitalization as judged by the treating physician as per the definitions of the Severe Malaria in African Children (SMAC) network reflecting standard practice in most African settings17,18. In both studies, patients were treated with parenteral artesunate followed by a course of weight-adapted oral artemether/lumefantrine. Within the first study, all patients received a total dose of 12 mg/kg artesunate according to three randomly assigned regimens (3 doses of 4 mg/kg body weight intravenously, 3 doses of 4 mg/kg body weight intramuscularly, or 5 doses of 2.4 mg/kg body weight intramuscularly). Within the second study, patients received at least three weight-adapted doses of parenteral (i.m. or i.v.) artesunate (2.4 mg/kg). If the patient was able to tolerate oral medication, treatment was switched to artemether/lumefantrine at this time point – else parenteral artesunate was continued. In both studies patients received folic acid supplementation for at least 14 days after treatment initiation.
Procedures have been described before10. Visits were scheduled on Day 0 (first day of treatment), Days 7 (±2), 14 (±2), and 28 (±4). The second study included an additional visit on Day 2.
Reticulocytes were assessed microscopically: after supravital staining with brilliant cresyl blue, reticulocytes per 1,000 erythrocytes were counted. Haptoglobin and erythropoietin was measured from serum stored at −80 °C after the end of recruitment by a commercial quality-controlled laboratory in Hamburg, Germany.
Genotyping of red cell polymorphisms was performed by high-throughput genotyping using fluorescent melting curve assays on 384-well microplate formats in a homogenous system (LightTyper, Roche Diagnostics)10,19,20.
Samples from the second study were assessed for the presence of once-infected (“pitted”) erythrocytes21,22. Erythrocytes were analyzed by flow cytometry (FACSCalibur™, BD Biosciences). Erythrocytes were incubated with monoclonal mouse anti-RESA (ring-infected erythrocyte surface antigen) antibodies (mAb 28/2, Walter + Eliza Hall Institute of Medical Research, Australia) followed by Peridinin chlorophyll protein (PerCP)-labelled anti-mouse-IgG to label RESA-positive erythrocytes. Nucleic acid staining was performed with Syto 16 (life technologies™). RBCs were plotted in two-dimensional scattergrams and gated according to their logarithmic forward and side scatter properties. Syto16 (DNA staining) was detected in the FL1 channel, PerCP (RESA-staining) was detected in the FL3 channel.
Double-positive (PerCP and Syto 16) erythrocytes were defined as infected, PerCP-positive, SYTO 16-negative erythrocytes as once-infected erythrocytes. Flow-cytometry data analyses was performed using FlowJo v10 software (Tree Star, Inc. Ashland, OR, USA). Two representative plots of one patient showing the switch from infected to once-infected RBCs are available as Supplementary Fig. 1.
Definition of anemia
We defined anemia as a hemoglobin level lower than 10 g/dL, corresponding to at least a moderate anemia as defined by the World Health Organization23,24.
Definition of post-artesunate delayed hemolysis (PADH)
PADH was defined as previously reported by our group10. Patients had to present both any decrease in hemoglobin and any increase in LDH between Days 7 (±2) and 14 (±2) in combination with both an elevated LDH (>350 IU/L) and low haptoglobin (<0.3 mg/dL) on Day 14.
Statistical analyses
Statistical analyses were performed with Stata IC 15 (StataCorp, College Station, TX, USA). Descriptive characteristics were reported using absolute and relative frequency for categorical variables, mean and 95% confidence interval for normally distributed, and median and interquartile range for non-normally distributed continuous variables. Continuous variables were represented by their mean and standard error of the mean in figures. The association between anemia level and transfusion status was assessed by a chi-square test. The difference in once-infected pitted erythrocytes between patients with PADH and those without was tested by the Mann-Whitney-U-test. To build models predicting anemia on Day 14 (±2) and 28 (±4) variables which were associated in the univariable analysis with anemia at a p-level of 0.1 were included in a multivariable logistic regression model.
Ethical considerations
Study protocols have been reviewed and approved by the respective institutional review boards (Committee on Human Research Publication and Ethics of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, and the institutional review board of the Centre de Recherches Médicales in Lambaréné, Gabon). Children were only included if informed consent was provided by the parent or legal guardian. All study procedures were performed in concordance with the Declaration of Helsinki.
Results
143 patients were eligible for this analysis, as represented in the patient flow diagram (Fig. 1). Baseline characteristics of the study population are described in Table 1. Children had a median age of 3.3 years (IQR: 2–5.5) and 88 (62%) were boys (Table 1). Geometric mean parasite density at presentation was 116,294/µL (95% CI: 95,574–141,505). 83 children (58%) fulfilled WHO criteria for complicated malaria, the most common being prostration (n = 69), hyperparasitemia (>200,000/µL; n = 47), and jaundice (n = 19). Three children died during the follow up period due to severe malaria.
Figure 1.
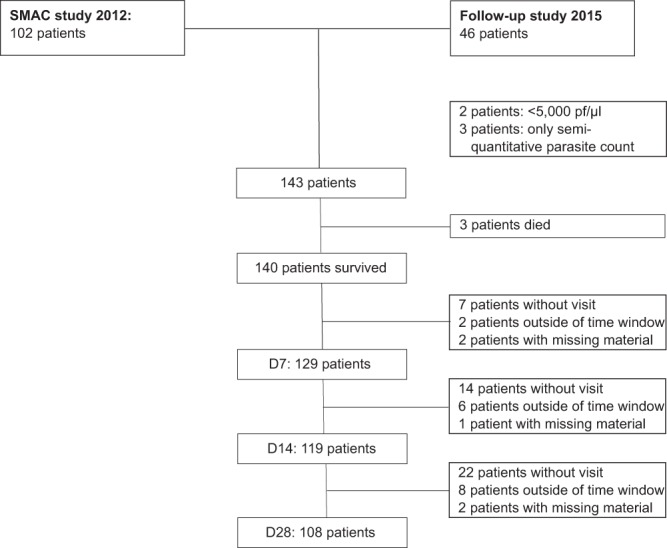
Patient diagram of the analysis population.
Table 1.
Baseline characteristics of children at presentation.
| Variable | Total (n = 143) | Ghana (n = 96) | Gabon (n = 47) |
|---|---|---|---|
| Female Sex, n (%) | 55 (38%) | 40 (42%) | 15 (32%) |
| Median Age, years (IQR) | 3.3 (2.0–5.5) | 3.2 (2.0–5.1) | 4.0 (2.0–5.6) |
| Mean weight, kg (95% CI) | 14 (13–15) | 14 (13–15) | 15 (13–17) |
| Mean height, cm (95% CI) | 98 (94–101) | 95 (91–99) | 103 (96–111) |
| Mean temperature at admission (95% CI) | 38.2 (38.0–38.4) | 38.2 (38.0–38.4) | 38.3 (38.0–38.6) |
| Hb Day 0, g/dL (95% CI) | 8.7 (8.3–9.1) | 8.6 (8.1–9.2) | 8.8 (8.3–9.3) |
| RBC Day 0, ×106/µL (95% CI) | 3.6 (3.4–3.7) | 3.4 (3.2–3.6) | 3.9 (3.6–4.1) |
| WBC Day 0, ×103/µL (95% CI) | 9.9 (9.3–10.6) | 10.1 (9.3–10.9) | 9.6 (8.5–10.7) |
| Platelets Day 0, ×103/µL (95% CI) | 103 (90–116) | 90 (76–103) | 130 (103–158) |
| Geometric mean parasite density, /µL (95% CI) | 116,294 (95,574–141,505) | 140,912 (111,933–177,391) | 78,565 (55,174–111,871) |
| Complicated malaria, n (%) | 83 (58%) | 69 (72%) | 14 (30%) |
| - Cerebral malaria | − 19 | − 5 | − 14 |
| - Repeated seizures | − 15 | − 14 | − 1 |
| - Severe anemia (≤5 g/dL) | − 16 | − 15 | − 1 |
| - Shock | − 3 | − 3 | − 0 |
| - Hyperparasitemia (≥200,000/µL) | − 47 | − 37 | − 10 |
| - Hypoglycaemia | − 10 | − 6 | − 4 |
| - Jaundice | − 19 | − 15 | − 4 |
| - Acute renal failure | − 1 | − 1 | − 0 |
| - Significant spontaneous bleeding | − 2 | − 2 | − 0 |
| - Prostration | − 69 | − 65 | − 4 |
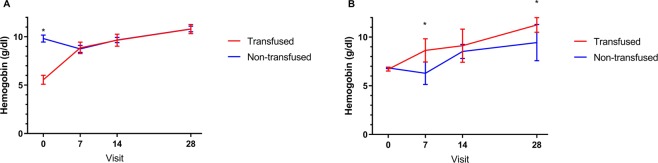
Upon presentation, 91 children (64%) were anemic (Hb <10 g/dL). 37 children (26%) with a mean hemoglobin of 5.5 g/dL (95% CI 5.1–6.0) received a blood transfusion. Children without transfusion had a mean hemoglobin of 9.8 g/dL (95% CI: 9.4–10.2). Figure 2A depicts the hemoglobin course over time in all children with data available: mean hemoglobin levels increased from Day 7 onwards and did not differ between patients who had and who had not received a transfusion. Highest hemoglobin levels were seen on Day 28 with a mean value of 10.8 g/dL (95% CI: 10.5–11.1) in those without a transfusion and of 10.7 g/dL (95% CI: 10.3–11.3) in those who had received a transfusion.
Figure 2.
Hemoglobin values over time stratified by transfusion status (mean ± SEM) (A) all children. (B) Children with hemoglobin values between 6 g/dL and 7 g/dL at presentation *p < 0.05.
All patients with a hemoglobin lower than 5 g/dl at presentation received a transfusion, while no patient with a hemoglobin equal or higher than 9 g/dl received a transfusion. Transfusion rates gradually decreased between these values (88% for Hb ≥5 g/dL and <6 g/dL; 59% for Hb ≥6 g/dL and <7 g/dL; 20% for Hb ≥7 g/dL and <8 g/dL; 5% for Hb ≥8 g/dL and <9 g/dL). To explore the impact of transfusion on hemoglobin during follow-up, a restricted analysis was performed in children with hemoglobin levels between 6 g/dL and 7 g/dL (n = 13 transfused children and 19 non-transfused children). This restriction was chosen for three reasons: (a) to match hemoglobin levels at presentation; (b) as in this group there was the largest variability in transfusion patterns; (c) transfusions are not generally recommended over 7 g/dL. Children who received a transfusion had higher hemoglobin levels at follow-up with a mean hemoglobin at Day 28 of 11.2 g/dL (95% CI: 10.5–12.0) compared to those without a transfusion (9.4 g/dL; 95% CI: 7.6–11.3; Fig. 2B).
To assess risk factors of prolonged anemia on Day 14 and 28, patients who received a transfusion were not further considered (Table 2). After adjusting for hyperparasitemia and age, hemoglobin at presentation was associated with anemia on Day 14 (adjusted odds ratio 0.27 (0.16–0.47) for each unit increase in hemoglobin; n = 92). Children with hyperparasitemia had 8.76 (1.64–46.82; p = 0.011) higher odds of anemia on day 14 than those without hyperparasitemia, while age was not associated with anemia on day 14 in the multivariable analysis. Only hemoglobin at presentation remained predictive for anemia on Day 28 (OR 0.52; 95% CI: 0.36–0.76 per unit increase; n = 91) after adjusting for hyperparasitemia, and age. Hyperparasitemia and age were not associated with anemia on Day 28 in the multivariable analysis. In the absence of a valid measure of total parasite burden (such as HRP2), the determination of peripheral parasitemia may have underestimated the impact of parasitemia.
Table 2.
Risk factors for prolonged anemia at Day 14 (n = 92) and Day 28 (n = 91) calculated by logistic regression.
| Risk factor | OR for anemia on Day 14 | p-value | AOR for anemia on Day 14* | p-value | OR for anemia on Day 28 | p-value | AOR for anemia on Day 28* | p-value |
|---|---|---|---|---|---|---|---|---|
| Hb Day 0, per unit increase | 0.27 (0.16–0.45) | <0.001 | 0.27 (0.16–0.47) | <0.001 | 0.50 (0.35–0.72) | <0.001 | 0.52 (0.36–0.76) | 0.001 |
| Hyperparasitemia | 10.00 (2.72–36.79) | 0.001 | 8.76 (1.64–46.82) | 0.011 | 2.98 (1.05–8.46) | 0.040 | 1.43 (0.43–4.75) | 0.559 |
| Age, per year increase | 0.84 (0.70–1.00) | 0.047 | 0.98 (0.75–1.28) | 0.872 | 0.84 (0.68–1.03) | 0.091 | 0.92 (0.73–1.16) | 0.464 |
| Sex (boys compared to girls) | 1.64 (0.71–3.79) | 0.250 | — | — | 0.93 (0.35–2.47) | 0.882 | — | — |
*AORs were adjusted for hemoglobin on Day 0, presence of hyperparasitemia on Day 0 and age.
No patient in the study had sickle cell anemia, of those with genotyping available (n = 108) only 4 were heterozygous for Hemoglobin S, 9 for hemoglobin C, and one had hemoglobin C disease. Due to the ensuing low power, hemoglobinopathies were not further assessed as risk factors for anemia. Similarly, the sample size was too low to detect any association between G6PD status in boys and anemia.
7 patients (5%) developed PADH, 5 of which have been described before10. The two new cases of PADH were only moderately affected with a nadir in hemoglobin of 10.0 g/dL in both patients on Day 14. For these two patients, pitting data were available. Compared to 17 children with a similar parasitemia range (50,000/µL to 110,000/µL) without PADH, there was some evidence that the median number of pitted erythrocytes was higher on Day 7 in children with PADH (78,269/µL vs 37,524/µL; p = 0.063) but not on Day 2 (69,068/µL vs 52,882/µL, p = 0.768). There was some evidence for a larger decrease in pitted erythrocytes between Days 7 and 14 in children with PADH (Δ pitted erythrocytes: 48,617/µL vs. 6,423/µL; p = 0.084).
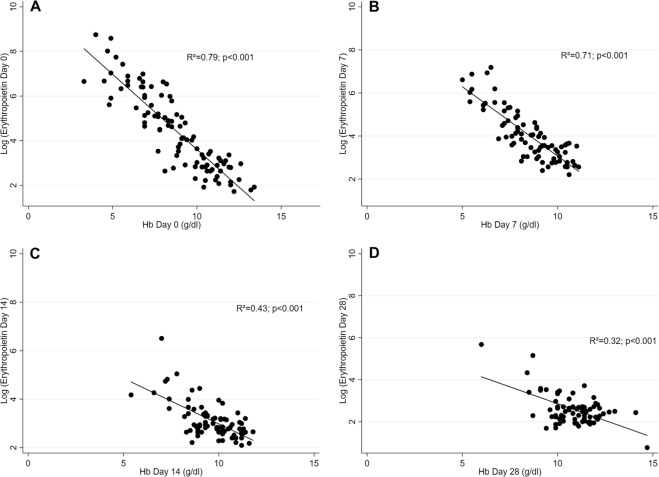
Figure 3 shows the association between hemoglobin levels and erythropoietin levels for the different visits. For analysis of Day 0 data, all patients with enough blood sample volume for erythropoietin measurements (n = 96) were included, for follow-up visits only those not having received blood transfusions were analyzed. On all days there is a strong log-linear association of erythropoietin with hemoglobin (p < 0.001), with hemoglobin explaining more than half of the variance of erythropoietin on Days 0 and 7 (R² = 0.79 and 0.71, respectively). Erythropoietin levels are highly elevated in response to severe anemia (Table 3). Reticulocytes show a time lag in the response to anemia, with reticulocytes count still being low on Day 0 while erythropoietin is already elevated at presentation (Fig. 4). After adjusting for hemoglobin at presentation, age, and hyperparasitemia, the logarithm of erythropoietin at presentation strongly predicts the increase in reticulocyte count between Day 0 and Day 7 (increase in the absolute reticulocyte count of 27.8 × 109/L per unit increase in the logarithm of erythropoietin; 95% CI: 8.8–46.9; p = 0.005).
Figure 3.
Association between hemoglobin levels and erythropoietin levels at different visits. (A) Visit Day 0: all children with measurements included (n = 96). (B) Visit Day 7: only non-transfused children (n = 82). (C) Visit Day 14: only non-transfused children (n = 81). (D) Visit Day 28: only non-transfused children (n = 78).
Table 3.
Erythropoietin levels stratified by hemoglobin at presentation.
| Hemoglobin at presentation (g/dL) | Erythropoietin (mIU/mL), median (range) |
|---|---|
| <5 | 963 (273–6297) |
| 5–7.4 | 609 (104–2303) |
| 7.5–9.9 | 88 (10–763) |
| ≥10 | 16 (6–34) |
Figure 4.
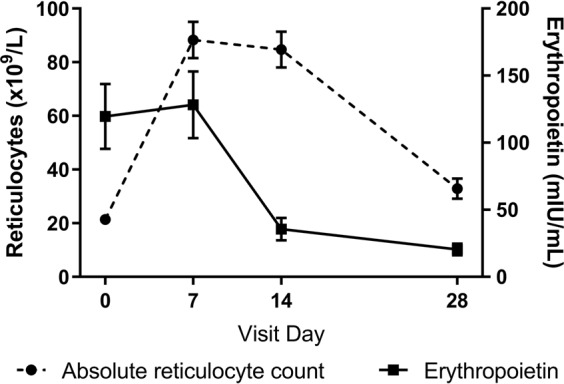
Absolute reticulocyte count and erythropoietin over time (mean ± SEM).
Discussion
In this population, children show a satisfactory hematological recovery after treatment with parenteral artesunate for malaria with a steady increase of hemoglobin after Day 7. The initial decline in hemoglobin up to the nadir on Day 7 is explained by the destruction of infected and uninfected red blood cells in acute malaria25. Additionally, malaria itself as well as the artemisinins impair erythropoiesis and early erythrocytic stages and reticulocytes are destroyed by erythrophagocytosis14,26,27. In combination with the potential destruction of infected reticulocytes, this translates to an inadequately low reticulocyte count at presentation, while erythropoietin is already elevated in response to anemia. There is a strong association between erythropoietin levels and hemoglobin levels at all time points, showing an adequate erythropoietin response. There are contradictory reports on whether the erythropoietin response is adequate in symptomatic malaria patients mainly due to methodological issues in older studies28–30. Our study showed an initially blunted response to erythropoietin but not a reduction in erythropoietin itself. Accordingly, after the initial lag phase, children showed a strong increase in reticulocytes and the erythropoietic response leads to a hematological recovery.
The major risk factor for prolonged anemia is a low hemoglobin level on presentation with an influence of baseline parasitemia on Day 14 but not on Day 28. Baseline causes of anemia, such as iron deficiency, malnutrition, hemoglobinopathies, and chronic infections prevail in malaria-endemic countries12. To explore the interplay of these covariates on malarial anemia and its recovery, larger cohort studies are needed. However, our data show that children recover from the early post-malarial decline in hemoglobin within one month after treatment. By raising the baseline hemoglobin through population-based measures, such as iron supplementation or mass treatment of helminthic infections, the duration and severity of malarial anemia on the individual child could be reduced. Similarly through reductions in malaria incidence and the subsequent reduction in malaria episodes per child, baseline hemoglobin values are likely to increase and the impact of each episode could be minimized31.
All children presenting with a hemoglobin level of less or equal than 5 g/dL received a transfusion, as WHO recommends6. Similarly, all children except one with a hemoglobin of less or equal than 6 g/dL have been transfused. There was no consensus for transfusing those children with a hemoglobin level between 6 and 7 g/dL, showing that the indication for transfusion in this group is mainly guided by individual patient factors. A conservative approach to transfusion has been historically propagated to ensure availability of blood transfusions and in the light of potential blood safety issues in African countries. However, there has been no solid evidence guiding transfusion thresholds in African children – especially not in severe malaria32. In our sample, transfusing children increased their hemoglobin up to 28 days after malaria compared to those without transfusion with the same starting hemoglobin. Whether this increase in hemoglobin also directly or indirectly reduces morbidity (such as fatigue or susceptibility to infections) is unclear. A recent randomized controlled trial in African children (of which 63% had malaria) did not show a difference in clinical outcomes after 6 months between aggressive and conservative transfusion approaches33,34.
In addition to five previously reported patients with PADH, we identified two additional patients with signs of PADH in the follow-up study10. The overall prevalence and severity of PADH seems to be lower in patients living in malaria-endemic settings than in returning European travelers with severe malaria4,5,11. Available evidence supports an overall frequency of PADH in African children treated with artesunate of 5% - with severe PADH occurring in 1% of children10,11,35. While these results provide some reassurance, an adverse reaction with a frequency of 1% would still be defined as “common” by the standard categories as recommended by the Council for International Organizations of Medical Sciences (CIOMS)36. Children reported in our studies had only moderately severe malaria; only a small proportion had very high parasite counts. We cannot rule out a significantly higher frequency of PADH in children with parasite counts over 250,000/µL. More evidence in this patient population with high parasitemias is critical, especially as the life-saving benefit of artesunate is highest in this patient group1.
With a lower than expected incidence of PADH (linked to lower than expected parasite counts), pathophysiological analyses can only remain exploratory due to a very low sample size.
Conclusion
Most children with malaria show an adequate erythropoietic response to malarial anemia and show a full recovery one month after treatment with parenteral artesunate. The incidence of PADH was lower than expected, but it should be investigated as a risk factor for anemia in a higher risk population with higher parasitemia. The main risk factor for prolonged anemia in our cohort of African children treated with artesunate is pre-existing anemia upon presentation.
Supplementary information
Acknowledgements
This work was supported by the German Center for Infectious Diseases Research (DZIF) [TI07.001_Rolling to TR and TI007.003_Scheu to K.S.]; the Werner-Otto-Stiftung [7/81 to T.R.]; and intramural funding by the Faculty of Medicine at the University Medical Center Hamburg-Eppendorf.
Author contributions
T.R., J.P.C. and P.G.K. had the idea for the study and designed the study. A.A.A. and T.A. were the local principal investigators with D.A. and J.S. being local investigators. Together with K.S. and S.F. they were responsible for recruitment and study procedures. J.M. performed genotyping. T.J. and K.S. developed the FACS protocol. T.R. primarily analyzed the data with contributions by F.K., K.S., S.F., J.P.C., M.M.A., M.R. and C.D.V. All authors reviewed the manuscript critically and approved the final version.
Data availability
The datasets analyzed during the current study are not publicly available due to them containing information that could compromise research participant privacy/consent but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Katrin Scheu, Ayola Akim Adegnika, Tsiri Agbenyega and Thierry Rolling.
Supplementary information
is available for this paper at 10.1038/s41598-019-54639-4.
References
- 1.Dondorp A, et al. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet (London, England) 2005;366:717–725. doi: 10.1016/s0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 2.Dondorp AM, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet (London, England) 2010;376:1647–1657. doi: 10.1016/S0140-6736(10)61924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinclair, D., Donegan, S. & Lalloo, D. G. Artesunate versus quinine for treating severe malaria. The Cochrane database of systematic reviews, CD005967, 10.1002/14651858.CD005967.pub3 (2011). [DOI] [PubMed]
- 4.Kurth F, et al. Intravenous Artesunate Reduces Parasite Clearance Time, Duration of Intensive Care, and Hospital Treatment in Patients With Severe Malaria in Europe: The TropNet Severe Malaria Study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;61:1441–1444. doi: 10.1093/cid/civ575. [DOI] [PubMed] [Google Scholar]
- 5.Rolling T, et al. Artesunate versus quinine in the treatment of severe imported malaria: comparative analysis of adverse events focussing on delayed haemolysis. Malar. J. 2013;12:241. doi: 10.1186/1475-2875-12-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO). Guidelines for the treatment of malaria, third edition (2015).
- 7.Rolling T, Agbenyega T, Krishna S, Kremsner PG, Cramer JP. Delayed haemolysis after artesunate treatment of severe malaria - review of the literature and perspective. Travel Med Infect Dis. 2015;13:143–149. doi: 10.1016/j.tmaid.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Rehman K, Lötsch F, Kremsner PG, Ramharter M. Haemolysis associated with the treatment of malaria with artemisinin derivatives: a systematic review of current evidence. International Journal of Infectious Diseases. 2014;29:268–273. doi: 10.1016/j.ijid.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Jauréguiberry S, et al. Delayed-Onset Hemolytic Anemia in Patients with Travel-Associated Severe Malaria Treated with Artesunate, France, 2011–2013. Emerging Infectious Diseases. 2015;21:804–812. doi: 10.3201/eid2105.141171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolling T, et al. Delayed hemolysis after treatment with parenteral artesunate in African children with severe malaria–a double-center prospective study. The Journal of infectious diseases. 2014;209:1921–1928. doi: 10.1093/infdis/jit841. [DOI] [PubMed] [Google Scholar]
- 11.Fanello C, et al. Post-treatment haemolysis in African children with hyperparasitaemic falciparum malaria; a randomized comparison of artesunate and quinine. BMC Infect Dis. 2017;17:575. doi: 10.1186/s12879-017-2678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kassebaum NJ, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–624. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vos Theo, Barber Ryan M, Bell Brad, Bertozzi-Villa Amelia, Biryukov Stan, Bolliger Ian, Charlson Fiona, Davis Adrian, Degenhardt Louisa, Dicker Daniel, Duan Leilei, Erskine Holly, Feigin Valery L, Ferrari Alize J, Fitzmaurice Christina, Fleming Thomas, Graetz Nicholas, Guinovart Caterina, Haagsma Juanita, Hansen Gillian M, Hanson Sarah Wulf, Heuton Kyle R, Higashi Hideki, Kassebaum Nicholas, Kyu Hmwe, Laurie Evan, Liang Xiofeng, Lofgren Katherine, Lozano Rafael, MacIntyre Michael F, Moradi-Lakeh Maziar, Naghavi Mohsen, Nguyen Grant, Odell Shaun, Ortblad Katrina, Roberts David Allen, Roth Gregory A, Sandar Logan, Serina Peter T, Stanaway Jeffrey D, Steiner Caitlyn, Thomas Bernadette, Vollset Stein Emil, Whiteford Harvey, Wolock Timothy M, Ye Pengpeng, Zhou Maigeng, Ãvila Marco A, Aasvang Gunn Marit, Abbafati Cristiana, Ozgoren Ayse Abbasoglu, Abd-Allah Foad, Aziz Muna I Abdel, Abera Semaw F, Aboyans Victor, Abraham Jerry P, Abraham Biju, Abubakar Ibrahim, Abu-Raddad Laith J, Abu-Rmeileh Niveen ME, Aburto Tania C, Achoki Tom, Ackerman Ilana N, Adelekan Ademola, Ademi Zanfina, Adou Arsène K, Adsuar Josef C, Arnlov Johan, Agardh Emilie E, Al Khabouri Mazin J, Alam Sayed Saidul, Alasfoor Deena, Albittar Mohammed I, Alegretti Miguel A, Aleman Alicia V, Alemu Zewdie A, Alfonso-Cristancho Rafael, Alhabib Samia, Ali Raghib, Alla Francois, Allebeck Peter, Allen Peter J, AlMazroa Mohammad AbdulAziz, Alsharif Ubai, Alvarez Elena, Alvis-Guzman Nelson, Ameli Omid, Amini Heresh, Ammar Walid, Anderson Benjamin O, Anderson H. Ross, Antonio Carl Abelardo T, Anwari Palwasha, Apfel Henry, Arsenijevic Valentain S Arsic, Artaman Al, Asghar Rana J, Assadi Reza, Atkins Lydia S, Atkinson Charles, Badawi Alaa, Bahit Maria C, Bakfalouni Talal, Balakrishnan Kalpana, Balalla Shivanthi, Banerjee Amitava, Barker-Collo Suzanne L, Barquera Simon, Barregard Lars, Barrero Lope H, Basu Sanjay, Basu Arindam, Baxter Amanda, Beardsley Justin, Bedi Neeraj, Beghi Ettore, Bekele Tolesa, Bell Michelle L, Benjet Corina, Bennett Derrick A, Bensenor Isabela M, Benzian Habib, Bernabe Eduardo, Beyene Tariku J, Bhala Neeraj, Bhalla Ashish, Bhutta Zulfiqar, Bienhoff Kelly, Bikbov Boris, Abdulhak Aref Bin, Blore Jed D, Blyth Fiona M, Bohensky Megan A, Basara Berrak Bora, Borges Guilherme, Bornstein Natan M, Bose Dipan, Boufous Soufiane, Bourne Rupert R, Boyers Lindsay N, Brainin Michael, Brauer Michael, Brayne Carol EG, Brazinova Alexandra, Breitborde Nicholas JK, Brenner Hermann, Briggs Adam DM, Brooks Peter M, Brown Jonathan, Brugha Traolach S, Buchbinder Rachelle, Buckle Geoffrey C, Bukhman Gene, Bulloch Andrew G, Burch Michael, Burnett Richard, Cardenas Rosario, Cabral Norberto L, Nonato Ismael R Campos, Campuzano Julio C, Carapetis Jonathan R, Carpenter David O, Caso Valeria, Castaneda-Orjuela Carlos A, Catala-Lopez Ferran, Chadha Vineet K, Chang Jung-Chen, Chen Honglei, Chen Wanqing, Chiang Peggy P, Chimed-Ochir Odgerel, Chowdhury Rajiv, Christensen Hanne, Christophi Costas A, Chugh Sumeet S, Cirillo Massimo, Coggeshall Megan, Cohen Aaron, Colistro Valentina, Colquhoun Samantha M, Contreras Alejandra G, Cooper Leslie T, Cooper Cyrus, Cooperrider Kimberly, Coresh Josef, Cortinovis Monica, Criqui Michael H, Crump John A, Cuevas-Nasu Lucia, Dandona Rakhi, Dandona Lalit, Dansereau Emily, Dantes Hector G, Dargan Paul I, Davey Gail, Davitoiu Dragos V, Dayama Anand, De la Cruz-Gongora Vanessa, de la Vega Shelley F, De Leo Diego, del Pozo-Cruz Borja, Dellavalle Robert P, Deribe Kebede, Derrett Sarah, Des Jarlais Don C, Dessalegn Muluken, deVeber Gabrielle A, Dharmaratne Samath D, Diaz-Torne Cesar, Ding Eric L, Dokova Klara, Dorsey E R, Driscoll Tim R, Duber Herbert, Durrani Adnan M, Edmond Karen M, Ellenbogen Richard G, Endres Matthias, Ermakov Sergey P, Eshrati Babak, Esteghamati Alireza, Estep Kara, Fahimi Saman, Farzadfar Farshad, Fay Derek FJ, Felson David T, Fereshtehnejad Seyed-Mohammad, Fernandes Jefferson G, Ferri Cluesa P, Flaxman Abraham, Foigt Nataliya, Foreman Kyle J, Fowkes F Gerry R, Franklin Richard C, Furst Thomas, Futran Neal D, Gabbe Belinda J, Gankpe Fortune G, Garcia-Guerra Francisco A, Geleijnse Johanna M, Gessner Bradford D, Gibney Katherine B, Gillum Richard F, Ginawi Ibrahim A, Giroud Maurice, Giussani Giorgia, Goenka Shifalika, Goginashvili Ketevan, Gona Philimon, de Cosio Teresita Gonzalez, Gosselin Richard A, Gotay Carolyn C, Goto Atsushi, Gouda Hebe N, Guerrant Richard l, Gugnani Harish C, Gunnell David, Gupta Rajeev, Gupta Rahul, Gutierrez Reyna A, Hafezi-Nejad Nima, Hagan Holly, Halasa Yara, Hamadeh Randah R, Hamavid Hannah, Hammami Mouhanad, Hankey Graeme J, Hao Yuantao, Harb Hilda L, Haro Josep Maria, Havmoeller Rasmus, Hay Roderick J, Hay Simon, Hedayati Mohammad T, Pi Ileana B Heredia, Heydarpour Pouria, Hijar Martha, Hoek Hans W, Hoffman Howard J, Hornberger John C, Hosgood H. Dean, Hossain Mazeda, Hotez Peter J, Hoy Damian G, Hsairi Mohamed, Hu Howard, Hu Guoqing, Huang John J, Huang Cheng, Huiart Laetitia, Husseini Abdullatif, Iannarone Marissa, Iburg Kim M, Innos Kaire, Inoue Manami, Jacobsen Kathryn H, Jassal Simerjot K, Jeemon Panniyammakal, Jensen Paul N, Jha Vivekanand, Jiang Guohong, Jiang Ying, Jonas Jost B, Joseph Jonathan, Juel Knud, Kan Haidong, Karch Andre, Karimkhani Chante, Karthikeyan Ganesan, Katz Ronit, Kaul Anil, Kawakami Norito, Kazi Dhruv S, Kemp Andrew H, Kengne Andre P, Khader Yousef S, Khalifa Shams Eldin AH, Khan Ejaz A, Khan Gulfaraz, Khang Young-Ho, Khonelidze Irma, Kieling Christian, Kim Daniel, Kim Sungroul, Kimokoti Ruth W, Kinfu Yohannes, Kinge Jonas M, Kissela Brett M, Kivipelto Miia, Knibbs Luke, Knudsen Ann Kristin, Kokubo Yoshihiro, Kosen Soewarta, Kramer Alexander, Kravchenko Michael, Krishnamurthi Rita V, Krishnaswami Sanjay, Defo Barthelemy Kuate, Bicer Burcu Kucuk, Kuipers Ernst J, Kulkarni Veena S, Kumar Kaushalendra, Kumar G Anil, Kwan Gene F, Lai Taavi, Lalloo Ratilal, Lam Hilton, Lan Qing, Lansingh Van C, Larson Heidi, Larsson Anders, Lawrynowicz Alicia EB, Leasher Janet L, Lee Jong-Tae, Leigh James, Leung Ricky, Levi Miriam, Li Bin, Li Yichong, Li Yongmei, liang Juan, Lim Stephen, Lin Hsien-Ho, Lind Margaret, Lindsay M Patrice, Lipshultz Steven E, Liu Shiwei, Lloyd Belinda K, Ohno Summer Lockett, Logroscino Giancarlo, Looker Katharine J, Lopez Alan D, Lopez-Olmedo Nancy, Lortet-Tieulent Joannie, Lotufo Paulo A, Low Nicola, Lucas Robyn M, Lunevicius Raimundas, Lyons Ronan A, Ma Jixiang, Ma Stefan, Mackay Mark T, Majdan Marek, Malekzadeh Reza, Mapoma Christopher C, Marcenes Wagner, March Lyn M, Margono Chris, Marks Guy B, Marzan Melvin B, Masci Joseph R, Mason-Jones Amanda J, Matzopoulos Richard G, Mayosi Bongani M, Mazorodze Tasara T, McGill Neil W, McGrath John J, McKee Martin, McLain Abby, McMahon Brian J, Meaney Peter A, Mehndiratta Man Mohan, Mejia-Rodriguez Fabiola, Mekonnen Wubegzier, Melaku Yohannes A, Meltzer Michele, Memish Ziad A, Mensah George, Meretoja Atte, Mhimbira Francis A, Micha Renata, Miller Ted R, Mills Edward J, Mitchell Philip B, Mock Charles N, Moffitt Terrie E, Ibrahim Norlinah Mohamed, Mohammad Karzan A, Mokdad Ali H, Mola Glen L, Monasta Lorenzo, Montico Marcella, Montine Thomas J, Moore Ami R, Moran Andrew E, Morawska Lidia, Mori Rintaro, Moschandreas Joanna, Moturi Wilkister N, Moyer Madeline, Mozaffarian Dariush, Mueller Ulrich O, Mukaigawara Mitsuru, Murdoch Michele E, Murray Joseph, Murthy Kinnari S, Naghavi Paria, Nahas Ziad, Naheed Aliya, Naidoo Kovin S, Naldi Luigi, Nand Devina, Nangia Vinay, Narayan K.M. Venkat, Nash Denis, Nejjari Chakib, Neupane Sudan P, Newman Lori M, Newton Charles R, Ng Marie, Ngalesoni Frida N, Nhung Nguyen T, Nisar Muhammad I, Nolte Sandra, Norheim Ole F, Norman Rosana E, Norrving Bo, Nyakarahuka Luke, Oh In Hwan, Ohkubo Takayoshi, Omer Saad B, Opio John Nelson, Ortiz Alberto, Pandian Jeyaraj D, Panelo Carlo Irwin A, Papachristou Christina, Park Eun-Kee, Parry Charles D, Caicedo Angel J Paternina, Patten Scott B, Paul Vinod K, Pavlin Boris I, Pearce Neil, Pedraza Lilia S, Pellegrini Carlos A, Pereira David M, Perez-Ruiz Fernando P, Perico Norberto, Pervaiz Aslam, Pesudovs Konrad, Peterson Carrie B, Petzold Max, Phillips Michael R, Phillips David, Phillips Bryan, Piel Frederic B, Plass Dietrich, Poenaru Dan, Polanczyk Guilherme V, Polinder Suzanne, Pope C A, Popova Svetlana, Poulton Richie G, Pourmalek Farshad, Prabhakaran Dorairaj, Prasad Noela M, Qato Dima, Quistberg D A, Rafay Anwar, Rahimi Kazem, Rahimi-Movaghar Vafa, Rahman Sajjad ur, Raju Murugesan, Rakovac Ivo, Rana Saleem M, Razavi Homie, Refaat Amany, Rehm Jurgen, Remuzzi Giuseppe, Resnikoff Serge, Ribeiro Antonio L, Riccio Patricia M, Richardson Lee, Richardus Jan Hendrik, Riederer Anne M, Robinson Margot, Roca Anna, Rodriguez Alina, Rojas-Rueda David, Ronfani Luca, Rothenbacher Dietrich, Roy Nobhojit, Ruhago George M, Sabin Nsanzimana, Sacco Ralph L, Ksoreide Kjetil, Saha Sukanta, Sahathevan Ramesh, Sahraian Mohammad Ali, Sampson Uchechukwu, Sanabria Juan R, Sanchez-Riera Lidia, Santos Itamar S, Satpathy Maheswar, Saunders James E, Sawhney Monika, Saylan Mete I, Scarborough Peter, Schoettker Ben, Schneider Ione JC, Schwebel David C, Scott James G, Seedat Soraya, Sepanlou Sadaf G, Serdar Berrin, Servan-Mori Edson E, Shackelford Katya, Shaheen Amira, Shahraz Saeid, Levy Teresa Shamah, Shangguan Siyi, She Jun, Sheikhbahaei Sara, Shepard Donald S, Shi Peilin, Shibuya Kenji, Shinohara Yukito, Shiri Rahman, Shishani Kawkab, Shiue Ivy, Shrime Mark G, Sigfusdottir Inga D, Silberberg Donald H, Simard Edgar P, Sindi Shireen, Singh Jasvinder A, Singh Lavanya, Skirbekk Vegard, Sliwa Karen, Soljak Michael, Soneji Samir, Soshnikov Sergey S, Speyer Peter, Sposato Luciano A, Sreeramareddy Chandrashekhar T, Stoeckl Heidi, Stathopoulou Vasiliki Kalliopi, Steckling Nadine, Stein Murray B, Stein Dan J, Steiner Timothy J, Stewart Andrea, Stork Eden, Stovner Lars J, Stroumpoulis Konstantinos, Sturua Lela, Sunguya Bruno F, Swaroop Mamta, Sykes Bryan L, Tabb Karen M, Takahashi Ken, Tan Feng, Tandon Nikhil, Tanne David, Tanner Marcel, Tavakkoli Mohammad, Taylor Hugh R, Te Ao Braden J, Temesgen Awoke Misganaw, Have Margreet Ten, Tenkorang Eric Yeboah, Terkawi Abdullah Sulieman, Theadom Alice M, Thomas Elissa, Thorne-Lyman Andrew L, Thrift Amanda G, Tleyjeh Imad M, Tonelli Marcello, Topouzis Fotis, Towbin Jeffrey A, Toyoshima Hideaki, Traebert Jefferson, Tran Bach X, Trasande Leonardo, Trillini Matias, Truelsen Thomas, Trujillo Ulises, Tsilimbaris Miltiadis, Tuzcu Emin M, Ukwaja Kingsley N, Undurraga Eduardo A, Uzun Selen B, van Brakel Wim H, van de Vijver Steven, Dingenen Rita Van, van Gool Coen H, Varakin Yuri Y, Vasankari Tommi J, Vavilala Monica S, Veerman Lennert J, Velasquez-Melendez Gustavo, Venketasubramanian Narayanaswamy, Vijayakumar Lakshmi, Villalpando Salvador, Violante Francesco S, Vlassov Vasiliy V, Waller Stephen, Wallin Mitchell T, Wan Xia, Wang Linhong, Wang JianLi, Wang Yanping, Warouw Tati S, Weichenthal Scott, Weiderpass Elisabete, Weintraub Robert G, Werdecker Andrea, Wessells K. Ryan R, Westerman Ronny, Wilkinson James D, Williams Hywel C, Williams Thomas N, Woldeyohannes Solomon M, Wolfe Charles DA, Wong John Q, Wong Haidong, Woolf Anthony D, Wright Jonathan L, Wurtz Brittany, Xu Gelin, Yang Gonghuan, Yano Yuichiro, Yenesew Muluken A, Yentur Gokalp K, Yip Paul, Yonemoto Naohiro, Yoon Seok-Jun, Younis Mustafa, Yu Chuanhua, Kim Kim Yun, Zaki Maysaa El Sayed, Zhang Yong, Zhao Zheng, Zhao Yong, Zhu Jun, Zonies David, Zunt Joseph R, Salomon Joshua A, Murray Christopher JL. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White NJ. Anaemia and malaria. Malaria Journal. 2018;17:371. doi: 10.1186/s12936-018-2509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kremsner PG, et al. Intramuscular Artesunate for Severe Malaria in African Children: A Multicenter Randomized Controlled Trial. PLoS medicine. 2016;13:e1001938. doi: 10.1371/journal.pmed.1001938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kremsner PG, et al. Prognostic value of circulating pigmented cells in African children with malaria. The Journal of infectious diseases. 2009;199:142–150. doi: 10.1086/595295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kremsner PG, et al. A simplified intravenous artesunate regimen for severe malaria. The Journal of infectious diseases. 2012;205:312–319. doi: 10.1093/infdis/jir724. [DOI] [PubMed] [Google Scholar]
- 18.Taylor T, et al. Standardized data collection for multi-center clinical studies of severe malaria in African children: establishing the SMAC network. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100:615–622. doi: 10.1016/j.trstmh.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreuels B, et al. Differing effects of HbS and HbC traits on uncomplicated falciparum malaria, anemia, and child growth. Blood. 2010;115:4551–4558. doi: 10.1182/blood-2009-09-241844. [DOI] [PubMed] [Google Scholar]
- 20.May J, et al. Red cell glucose-6-phosphate dehydrogenase status and pyruvate kinase activity in a Nigerian population. Tropical medicine & international health: TM & IH. 2000;5:119–123. doi: 10.1046/j.1365-3156.2000.00529.x. [DOI] [PubMed] [Google Scholar]
- 21.Chotivanich K, et al. The mechanisms of parasite clearance after antimalarial treatment of Plasmodium falciparum malaria. The Journal of infectious diseases. 2000;182:629–633. doi: 10.1086/315718. [DOI] [PubMed] [Google Scholar]
- 22.Ndour Papa Alioune, Larréché Sébastien, Mouri Oussama, Argy Nicolas, Gay Frédérick, Roussel Camille, Jauréguiberry Stéphane, Perillaud Claire, Langui Dominique, Biligui Sylvestre, Chartrel Nathalie, Mérens Audrey, Kendjo Eric, Ghose Aniruddha, Hassan Md. Mahtab Uddin, Hossain Md. Amir, Kingston Hugh W. F., Plewes Katherine, Dondorp Arjen M., Danis Martin, Houzé Sandrine, Bonnefoy Serge, Thellier Marc, Woodrow Charles J., Buffet Pierre A. Measuring thePlasmodium falciparumHRP2 protein in blood from artesunate-treated malaria patients predicts post-artesunate delayed hemolysis. Science Translational Medicine. 2017;9(397):eaaf9377. doi: 10.1126/scitranslmed.aaf9377. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. World Malaria Report 2018. (World Health Organization, 2018).
- 24.Organization, W. H. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity (2011).
- 25.Looareesuwan S, et al. Erythrocyte survival in severe falciparum malaria. Acta tropica. 1991;48:263–270. doi: 10.1016/0001-706X(91)90014-B. [DOI] [PubMed] [Google Scholar]
- 26.Hien TT, White NJ. Qinghaosu. Lancet (London, England) 1993;341:603–608. doi: 10.1016/0140-6736(93)90362-K. [DOI] [PubMed] [Google Scholar]
- 27.Fendel R, et al. Hemolysis is associated with low reticulocyte production index and predicts blood transfusion in severe malarial anemia. PLoS One. 2010;5:e10038. doi: 10.1371/journal.pone.0010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamikanra AA, et al. Malarial anemia: of mice and men. Blood. 2007;110:18–28. doi: 10.1182/blood-2006-09-018069. [DOI] [PubMed] [Google Scholar]
- 29.Hassan AMAE, Saeed AM, Fandrey J, Jelkmann W. Decreased erythropoietin response in Plasmodium falciparum malaria-associated anaemia. European Journal of Haematology. 1997;59:299–304. doi: 10.1111/j.1600-0609.1997.tb01690.x. [DOI] [PubMed] [Google Scholar]
- 30.Burgmann H, et al. Serum Levels of Erythropoietin in Acute Plasmodium falciparum Malaria. The American journal of tropical medicine and hygiene. 1996;54:280–283. doi: 10.4269/ajtmh.1996.54.280. [DOI] [PubMed] [Google Scholar]
- 31.Korenromp EL, Armstrong-Schellenberg JR, Williams BG, Nahlen BL, Snow RW. Impact of malaria control on childhood anaemia in Africa–a quantitative review. Tropical medicine & international health: TM & IH. 2004;9:1050–1065. doi: 10.1111/j.1365-3156.2004.01317.x. [DOI] [PubMed] [Google Scholar]
- 32.Mpoya A, et al. Transfusion and Treatment of severe anaemia in African children (TRACT): a study protocol for a randomised controlled trial. Trials. 2015;16:593. doi: 10.1186/s13063-015-1112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maitland K, et al. Transfusion Volume for Children with Severe Anemia in Africa. New England Journal of Medicine. 2019;381:420–431. doi: 10.1056/NEJMoa1900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maitland K, et al. Immediate Transfusion in African Children with Uncomplicated Severe Anemia. New England Journal of Medicine. 2019;381:407–419. doi: 10.1056/NEJMoa1900105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burri C, et al. Delayed anemia after treatment with injectable artesunate in the Democratic Republic of the Congo: a manageable issue. The American journal of tropical medicine and hygiene. 2014;91:821–823. doi: 10.4269/ajtmh.14-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Council for International Organisations of Medical Sciences (CIOMS). Guidelines for preparing core clinical safety information on drugs. (Council for International Organisations of Medical Sciences (CIOMS), 1995).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to them containing information that could compromise research participant privacy/consent but are available from the corresponding author on reasonable request.