Abstract
Objective:
High comorbidity among psychiatric disorders suggests that they may share underlying neurobiological deficits. Abnormalities in cortical thickness and volume have been demonstrated in clinical samples of adults, but less is known when these structural differences emerge in youth. The purpose of this study was to examine the association between dimensions of psychopathology and brain structure.
Method:
We studied 1,394 youth imaged as part of the Philadelphia Neurodevelopmental Cohort. Dimensions of psychopathology were constructed using a bifactor model of symptoms. Cortical thickness and volume were quantified using high-resolution MRI at 3T. Structural covariance networks were derived using non-negative matrix factorization and analyzed using generalized additive models with penalized splines to capture both linear and nonlinear age-related effects.
Results:
Fear symptoms were associated with reduced cortical thickness in most networks, while overall psychopathology was associated with globally reduced gray matter volume across all networks. Lastly, structural covariance networks predicted psychopathology symptoms above and beyond demographic characteristics and cognitive performance.
Conclusions:
Our results suggest a dissociable relationship whereby fear is most strongly linked to reduced cortical thickness and overall psychopathology is most strongly linked to global reductions in gray matter volume. Such results have implications for understanding how abnormalities of brain development may be associated with divergent dimensions of psychopathology.
INTRODUCTION
Psychiatric disorders have high rates of co-morbidity, with many symptoms being continuous, showing non-specificity, cutting across disorders, and being hierarchically arranged (1-3). Additionally, there is substantial heterogeneity within psychiatric disorders (1, 3). It is increasingly recognized that such clinical co-morbidity is mirrored by neurobiological nonspecificity, with similar abnormalities of brain structure being described in multiple disorders. For example, abnormalities in cortical thickness have been reported in anxiety, depression, psychosis, and behavioral disorders (4-7). Likewise, a meta-analysis by Goodkind et al. reported common gray matter volume loss in the dorsal anterior cingulate and insula across disorders as varied as schizophrenia, bipolar disorder, major depressive disorder, addiction, obsessive-compulsive disorder, and anxiety (8). Genetic studies suggest that while cortical thickness influences volume measurements of cortical grey matter, these two measures can provide unique information (9).
However, within this broad literature, several current limitations are notable. First, the specific effects of each structural neuroimaging study are quite heterogeneous. Inconsistent results may be due to small sample sizes and selective reporting of regions of interest. Second, most studies are restricted to adults. This is an important caveat, as most psychiatric disorders first manifest during childhood, adolescence, or young adulthood (10, 11), suggesting that large studies of brain structure in youth are needed.
Third and perhaps most importantly, prior studies usually employ a case-control approach that applies restrictive inclusion criteria that limits comorbidity and fails to take into account the dimensional nature of psychopathology. Prior factor analytic work commonly reveals four dimensions of psychiatric symptoms: anxious-misery/distress, psychosis, behavioral/externalizing symptoms, and fear (1, 3, 12). However, dimensions derived from traditional factor analytic models are often highly correlated with each other, suggesting the importance of considering the overall burden of psychopathology in an individual. Akin to the overall “g” intelligence factor in cognition research, the “p” psychopathology factor quantifies the overall level of psychopathology present across clinical domains (2, 3). This p factor can be measured using bifactor models, which yield both the p factor and orthogonal factors for each specific dimension. Prior work relates higher p factor scores with reduced gray matter volume in adults (13) and children (14); further studies are needed to evaluate the relationship between p and diverse measures of brain structure in youth.
In response to these gaps in the field, we investigated associations between brain structure and psychopathology using a large sample of 1,394 youth imaged as part of the Philadelphia Neurodevelopmental Cohort (PNC) (15, 16). We quantified psychopathology dimensions using a bifactor analysis of dimensional clinician ratings that were assessed for every individual. We then delineated structural covariance networks using non-negative matrix factorization (NMF), a multivariate analysis technique developed in the context of computer vision research that has been recently adapted for neuroimaging data (17). Using these networks, we investigated how abnormalities of two different measures of brain structure (cortical thickness and volume) were associated with each dimension of psychopathology. As described below, we found that specific dimensions of psychopathology were dissociably linked to distinct abnormalities of brain structure.
METHODS
Participants
1,601 participants completed multimodal neuroimaging as part of the PNC (15, 16), a large-scale community-based study of brain development. The institutional review boards of the University of Pennsylvania and the Children's Hospital of Philadelphia approved the study procedures. All participants provided written informed consent after receiving a complete description of the study. The final sample consisted of 1,394 youth; demographics of the sample are summarized in Table 1; see Supplement for details. Among this final sample, 155 participants (11%) were taking psychiatric psychoactive medications at the time of imaging and were evaluated in sensitivity analyses, as described below.
Table 1.
Summary of demographic data
| M | SD | |
|---|---|---|
| Age (years) | 14.98 | 3.64 |
| N | Percent | |
| Gender | ||
| Male | 663 | 48% |
| Female | 731 | 52% |
| Race | ||
| Caucasian | 618 | 44% |
| Non-Caucasian | 776 | 56% |
| Maternal Level of Education | ||
| 12 years or less | 509 | 37% |
| Greater than 12 years | 868 | 62% |
| Missing | 17 | 1% |
| Lifetime Prevalence* | ||
| Typically Developing | 428 | 31% |
| ADHD | 230 | 16% |
| Agoraphobia | 81 | 6% |
| Anorexia | 16 | 1% |
| Bulimia | 5 | 0.4% |
| Conduct Disorder | 121 | 9% |
| Generalized Anxiety Disorder | 27 | 2% |
| Major Depression | 193 | 14% |
| Mania | 16 | 1% |
| Obsessive-Compulsive Disorder | 43 | 3% |
| Oppositional Defiant Disorder | 458 | 33% |
| Panic | 14 | 1% |
| Psychosis-spectrum | 399 | 29% |
| PTSD | 172 | 12% |
| Separation Anxiety | 63 | 5% |
| Social Anxiety | 328 | 24% |
| Specific Phobia | 426 | 31% |
Note.
Due to comorbidity, some participants met criteria for more than one disorder and are counted in multiple categories for lifetime prevalence.
Clinical and cognitive assessment
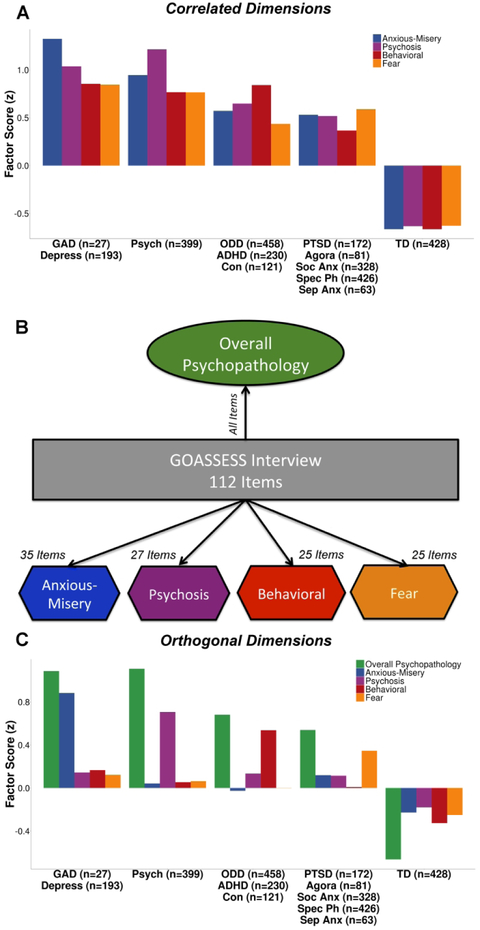
As described previously (15, 16), psychiatric symptoms were assessed using a structured screening interview (GOASSESS) based on a modified version of the Kiddie-Schedule for Affective Disorders and Schizophrenia (Supplement). The lifetime prevalence of each disorder is summarized in Table 1. An exploratory factor analysis of 112 item-level symptoms (Supplement) identified four correlated dimensions of psychopathology: anxious-misery, psychosis, behavioral, and fear (Figure 1A), which show a high degree of overlap across dimensions and diagnostic screening categories. To increase specificity, we then used a confirmatory item-bifactor analysis which yielded five orthogonal dimensions of psychopathology: anxious-misery, psychosis, behavioral/externalizing, fear, and overall psychopathology (symptom burden across all psychiatric disorders; Figure 1B and 1C). Additionally, three cognitive factors (executive function/complex reasoning, social cognition, and episodic memory) derived from factor analysis (Supplement) were included as predictors in the multivariate analyses described below.
Figure 1. Correlated dimensions of psychopathology show a high degree of overlapping symptoms.
A) An exploratory factor analysis of 112 psychiatric symptoms identified four correlated dimensions of psychopathology: anxious-misery, psychosis, behavioral, and fear, which show a high degree of overlap across dimensions and diagnostic screening categories. Here we show the mean factor scores of each dimension (anxious-misery, psychosis, behavioral, and fear) in the related screening diagnoses. B) A confirmatory bifactor analysis constrained the dimensions of psychopathology (anxious-misery, psychosis, behavioral, and fear) to be orthogonal, and extracted a common factor (overall psychopathology). C) The orthogonal factors load more specifically onto the relevant disorders. Sample sizes for each diagnostic screening category are shown in parentheses. GAD = generalized anxiety disorder; Depress = depressive disorders; Psych = psychosis; ODD = oppositional defiant disorder; ADHD = attention-deficit/hyperactivity disorder; Con = conduct disorder; PTSD = posttraumatic stress disorder; Agora = agoraphobia; Soc Anx = social anxiety disorder; Spec Ph = specific phobia; Sep Anx = separation anxiety disorder; TD = typically developing.
Image acquisition, quality assurance, and image processing
Image acquisition and processing are reported in detail elsewhere (15). Briefly, imaging data were acquired on the same MRI scanner using the same imaging sequences for all participants (Supplement). Three highly trained image analysts independently assessed structural image quality control using manually derived ratings (18) (Supplement); and average quality rating across the three raters was included as a covariate in all models in order to control for the confounding influence of variation in image quality.
Structural image processing utilized Advanced Normalization Tools (ANTs; Supplement). Prior large-scale evaluation studies have shown that this procedure is highly accurate and more sensitive to individual differences over the lifespan than comparable techniques. To avoid registration bias and maximize sensitivity, a custom adolescent template and tissue priors were used. This procedure yielded two maps for each subject: a cortical thickness image and a volume image (log transformed determinant of the Jacobian of the deformation field).
Non-negative matrix factorization (NMF)
We used NMF to identify networks where brain structure co-varies consistently across participants (17). Details regarding the implementation of NMF have been presented elsewhere (17, 19), and are also described in the Supplement (see Supplemental Figure 1 for a schematic of the NMF procedure). NMF yields networks that are highly interpretable and have improved statistical power compared to standard mass-univariate analyses (see Supplement for details).
NMF networks were calculated from cortical thickness maps; to allow for correspondence across image types, the loadings for these networks were then applied to the volume maps, resulting in only cortical regions being analyzed. Results were similar when networks were calculated directly from volume maps. NMF networks were visualized on the inflated Population-Average, Landmark-, and Surface-based (PALS) cortical surfaces using Caret software.
Group-level statistical analyses
After identifying networks, we conducted analyses to identify associations between brain structure and dimensions of psychopathology. Given that structural maturation is a non-linear process, we modeled both linear and nonlinear age effects using penalized splines within generalized additive models (GAM) using the R package voxel which relies upon mgcv (20). GAMs assess a penalty on nonlinearity in order to avoid over-fitting and capture both linear and non-linear effects in a data-driven fashion. All models used the restricted maximum likelihood (REML) framework, which produces estimates of variance and covariance parameters. Based on prior work documenting sex differences in cortical thickness and volume (21), we included sex in the model. In addition, we added mean image quality ratings (described above) as an additional model covariate to ensure that image quality did not drive the observed associations (18). For each network, we examined associations between each dimension of psychopathology and cortical thickness or volume:
| (1) |
Interactions between fear and age, fear and sex, and age and sex were evaluated and found to be non-significant for cortical thickness. The same interactions for overall psychopathology and anxious-misery were non-significant for volume; significant age by sex interactions are reported below. To control for multiple testing across networks, we controlled the False Discovery Rate (FDR, q<0.05).
Sensitivity analyses
We conducted sensitivity analyses to ensure that our results were robust to methodological choices and were not influenced by confounding variables. First, we repeated the analyses described above using standard anatomical regions. Anatomic regions were delineated using a top-performing, highly-accurate multi-atlas labeling tool with joint label fusion implemented in ANTs (Supplement). Second, we included maternal level of education as an additional covariate and excluded the minority (11%, n=155) of participants who were taking psychiatric psychotropic medications at the time of imaging (included n=1,226). Third, we evaluated whether global differences in total gray matter volume or average cortical thickness were driving the observed effects. Fourth, we used a traditional case-control approach to illustrate the usefulness of the bifactor model in increasing the specificity of the results by taking into account the overlapping variance between disorders.
NMF networks as predictors of psychopathology
In addition to univariate associations between each structural covariance network and each dimension of psychopathology, we also conducted a multivariate analysis to examine whether structural networks predicted psychopathology above and beyond demographic characteristics and cognitive performance. To do this, we compared a reduced model including age, sex, and three cognitive performance factors to a full model predicting fear with age, sex, cognitive factors, and all 18 cortical thickness networks using an F-test. Similarly, we also performed the same analyses with the volume networks as predictors of overall psychopathology and anxious-misery. Adjusted R2 is reported for the models.
Data and code availability
See https://github.com/PennBBL/KaczkurkinPark_BifactorStructure/wiki/KaczkurkinPark_BifactorStructure for code and a companion wiki detailing analytic procedures used in this manuscript. Data from the PNC can be accessed at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000607.v3.p2. The NMF code can be found at https://github.com/asotiras/brainlets.
RESULTS
NMF identifies structural covariance networks
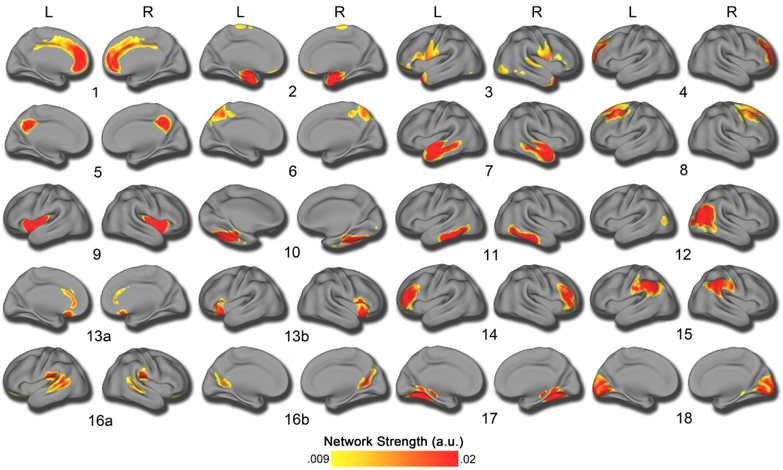
Structural covariance networks were delineated using NMF at multiple resolutions. The final 18-network solution was chosen on the basis of two considerations. First, we evaluated the gradient of reconstruction error (Supplementary Figure 2), which shows only nominal decrements in error beyond 14 networks. Second, we checked the split-half reliability at this resolution, which revealed an ARI of .93 for the 18-network solution, suggesting that this solution is highly reproducible. This resolution is also consistent with previous reports (19). Accordingly, the 18-network solution was used for all subsequent analyses. As in prior work using NMF (17, 19), the structural covariance networks identified were highly symmetric bilaterally (Figure 2).
Figure 2. Structural covariance networks delineated by NMF.
Structural covariance networks are shown for the 18-network solution, with the spatial distribution of each network indicated by loadings at each voxel in arbitrary units (shown with the color bar, where warmer colors correspond to higher values). High symmetry can be seen between the left (L) and right (R) hemispheres. The anatomical coverage of each structural covariance network was as follows: 1) cingulate cortex; 2) medial temporal cortex; 3) temporal pole; 4) dorsolateral prefrontal cortex; 5) posterior cingulate cortex; 6) superior parietal cortex; 7) superior temporal cortex; 8) dorsal prefrontal cortex; 9) insular cortex; 10) fusiform cortex; 11) inferior temporal cortex; 12) right lateral occipital cortex; 13) subgenual cingulate, anterior cingulate, and anterior insula; 14) inferior prefrontal cortex; 15) inferior parietal cortex; 16a) precuneus and 16b) temporoparietal junction; 17) lingual gyrus; 18) medial occipital cortex.
Psychopathology dimensions are associated with structural differences in multiple networks
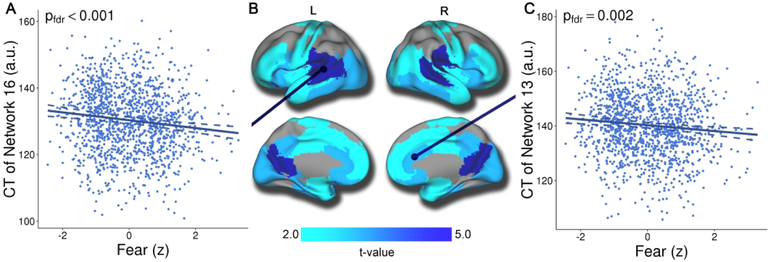
Having identified 18 cortical thickness covariance networks using NMF, we next examined associations with the dimensions of psychopathology summarized by the bifactor analysis. Results revealed that increased fear was associated with reduced cortical thickness in 13 networks after FDR correction (Supplemental Table 1 and Figure 3), with the relationship between fear and CT showing small effect sizes (partial r ≤ −.12). Regions impacted included the posterior cingulate and temporal-parietal junction (network 16, see Figure 3A), the anterior and subgenual cingulate cortex, and the anterior insula (network 13, see Figure 3C). Furthermore, there were relatively widespread associations across temporal, orbitofrontal, and occipital cortex. Importantly, the association between fear and cortical thickness was specific, and cortical thickness was only weakly associated with other dimensions of psychopathology. The anxiousmisery and behavioral dimensions were not associated with cortical thickness in any network, whereas psychosis and overall psychopathology were associated with diminished thickness in only a single network (networks 5 and 16, respectively). However, the results for psychosis and overall psychopathology did not remain significant during sensitivity analyses (described below). Interactions between fear and age, fear and sex, and age and sex were non-significant for cortical thickness.
Figure 3. Fear is associated with reduced cortical thickness in multiple structural covariance networks.
Mass-univariate analyses using GAMs that controlled for linear and nonlinear age, sex, and image quality revealed that fear symptoms were associated with reduced cortical thickness in multiple networks. This association was maximal in networks such as the temporal-parietal junction and posterior cingulate cortex (Figure 3A; network 16). Significant associations were also present in networks that included the anterior cingulate, anterior insula, and subgenual cingulate cortex (Figure 3C; network 13). Composite network boundaries were obtained by assigning each voxel to one of the 18 networks with the highest loading for that voxel. Multiple comparisons were accounted for using the False Discovery Rate (Q<0.05). Dotted lines on scatterplots represent the 95% confidence interval.
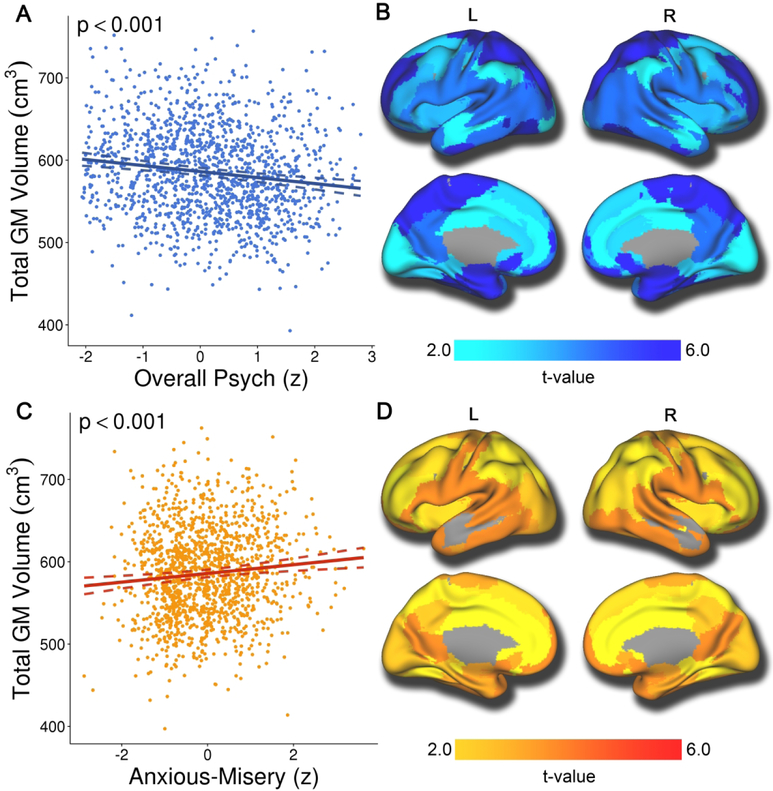
Next, we repeated these analyses, but instead quantified volume (rather than cortical thickness) within each structural covariance network. These analyses revealed that overall psychopathology was associated with reduced volume in all networks, suggesting a global association with gray matter volume (partial r ≤ −.14; Supplemental Table 2). As illustrated in Figure 4A and 4B, total gray matter volume was negatively associated with overall psychopathology symptoms. In contrast, anxious-misery symptoms were associated with increased volume in most networks (partial r ≤ −.12; Supplemental Table 2). Specifically, total gray matter volume (Figure 4C) was positively associated with anxious-misery symptoms, as were all networks except one (network 7; Figure 4D). Furthermore, fear symptoms were associated with reduced volume in multiple regions (Supplemental Table 2), and the behavioral dimension showed reduced volume in the superior parietal cortex (network 6) and the fusiform cortex (network 10). However, associations with the fear and behavioral dimensions did not remain significant during sensitivity analyses (see below). Psychopathology by age and psychopathology by sex interactions were non-significant for volume. Significant age by sex interactions were apparent in all volume networks (pfdr-values ≥ .027) except networks 4 and 8.
Figure 4. Overall psychopathology is associated with reduced volume globally, while anxious-misery is associated with greater volume in multiple structural covariance networks.
Mass-univariate analyses using GAMs that controlled for linear and nonlinear age, sex, and image quality revealed that overall psychopathology was associated with reduced volume across the brain (Figure 4A and 4B). Conversely, anxious-misery symptoms were associated with increased volume in most networks (Figure 4C and 4D). Composite network boundaries were obtained by assigning each voxel to one of the 18 networks with the highest loading for that voxel. Multiple comparisons were accounted for using the False Discovery Rate (Q<0.05). Dotted lines on scatterplots represent the 95% confidence interval.
Sensitivity analyses provide convergent results
To ensure that our results were not specific to NMF networks, we evaluated associations using a highly-accurate anatomic brain parcellation. Parcellation results aligned with analyses using NMF networks for all dimensions including fear, overall psychopathology, and anxiousmisery (Supplementary Figure 3). In addition, we conducted sensitivity analyses to evaluate potentially confounding variables. Nearly all associations between cortical thickness and fear and between volume and overall psychopathology or anxious-misery remained significant after excluding participants taking psychotropic medications (11%) and including maternal education as an additional covariate (Supplemental Table 3). However, the associations between network thickness and fear were no longer significant when average cortical thickness was included as an additional covariate (pfdr-values ≥ .485), suggesting a distributed effect. Similarly, the associations between network volume and either overall psychopathology or anxious-misery were no longer significant when total gray matter volume was added as a covariate, consistent with a global effect (pfdr-values ≥ .229). Finally, using a traditional case-control approach, we found that cortical thickness and volume were reduced in most diagnostic categories (Table S4), illustrating the lack of specificity when using this approach.
Structural covariance networks predict dimensions of psychopathology
Next, we tested whether structural covariance networks provided information about psychopathology above and beyond demographic characteristics and cognitive performance. We found a significant difference between a reduced model with only age, sex, and the three cognitive factors and a full model where fear was predicted by age, sex, cognitive factors, and the 18 cortical thickness networks (F(1367, 1385) = 2.32, p = .001). The correlation between the actual fear scores and the predicted fear scores in the full model was r(1389) = .28, p < .001 (Supplemental Figure 4A). However, while the proportion of variance in fear explained by the predictors improved in the full model, it was still relatively modest (adjusted R2 = .06). For volume networks, there was a significant difference between the reduced and full models for overall psychopathology (F(1367, 1385) = 1.78, p = .023) but not anxious-misery (p = .218). The correlation between the actual and predicted overall psychopathology scores in the full model was r(l389) = .35, p < .001 (Supplemental Figure 4B). Compared to the reduced model, the proportion of variance in overall psychopathology explained by the predictors also showed a small improvement in the full model (adjusted R2 = .11).
DISCUSSION
Leveraging a large sample of youth and multivariate analysis techniques, we provide novel evidence that dimensions of psychopathology that cross clinical diagnostic categories are dissociably linked to abnormalities in brain structure. The fear dimension was associated with diminished cortical thickness in the majority of networks. Furthermore, higher levels of overall psychopathology were associated with global reductions in gray matter volume, while anxiousmisery symptoms were associated with increased volume in most networks. Results were highly convergent when accounting for a range of covariates and when different image analysis methods were used. Finally, structural networks predicted psychopathology symptoms above and beyond demographic characteristics and cognitive performance.
Advantages of a dimensional approach to fear
We found that cortical thickness was reduced in association with fear symptoms in most networks. This result is consistent with case-control studies in adults showing cortical thinning in posttraumatic stress disorder, specific phobia, and social anxiety disorder (4, 22, 23). Our results are also broadly convergent with fear and anxiety networks identified using task-based functional MRI including the salience and ventral attention networks, which are critical for processing of emotionally salient information and attention bias to threat (24). These networks include regions that were impacted in this study, including the anterior insula, anterior cingulate cortex, temporoparietal junction, and ventrolateral prefrontal cortex. The anterior cingulate has been associated with self-regulation including emotional processing (25), salience processing (26), and attention (27), and has demonstrated functional connectivity with the insula (25, 26), which is implicated in fear. However, our results also extend beyond these salience and emotion regulation networks, suggesting a more widespread effect. While our results are generally consistent with previous case-control research, they build upon this literature by showing that global reduced cortical thickness is associated with the spectrum of fear symptoms across disorders in a community-based developmental sample with substantial co-morbidity.
Overall psychopathology across disorders is associated with reduced gray matter volume
It is increasingly recognized that psychopathology exists on a continuum. In a dimensional framework, individuals may be characterized by a profile of symptoms that span categorical boundaries (1, 3). Critically, the bifactor model captures co-morbidity and individual variation in the overall level of psychopathology through the general p factor, which may contribute to the non-specificity of biomarkers found across disorders (2). Recent research has demonstrated that p is linked to individual variation in cognition (28), executive function (29), cerebral blood flow (30), and genomics (31). Here, we add to this growing literature by demonstrating that higher levels of p were associated with global reductions in gray matter volume in youth. This finding is consistent with work by Goodkind et al. reporting common gray matter volume loss across multiple psychiatric disorders in adults (8) and with prior work showing reduced volume associated with p (13, 14). In contrast to the reduction of gray matter volume seen with higher levels of overall psychopathology, higher levels of anxious-misery symptoms were associated with significant increases in global gray matter volume. Although the magnitude of these effects were small, they are consistent with effect sizes reported for other variables associated with brain structure, such as IQ (32) and for other forms of psychopathology (5).
Interpreting specificity within the bifactor model
Associations with specific model dimensions, such as fear or anxious-misery, must be understood within the context of the bifactor model. Notably, scores from the bifactor model are uncorrelated, and represent the burden of a specific dimension while accounting for the overall burden of general psychopathology. In contrast, dimensions derived from traditional factor-analytic models can be highly correlated with each other, which reduces the specificity of observed associations (1, 3). As in any factor model, an individual receives a score from each dimension of the bifactor model, and groups can be compared on said scores. For example, while patients with depression on average have high scores on the anxious-misery dimension, due to prominent co-morbidity, they also tend to have high levels of overall psychopathology. Accordingly, even though the specific anxious-misery subfactor is associated with greater gray matter volume, this effect may be overwhelmed by the countervailing impact of the overall psychopathology dimension. Indeed, such an example illustrates the advantages of a hierarchical dimensional framework for parsing heterogeneous categorical clinical diagnoses. In contrast, case-control analyses in each diagnostic category yielded non-specific results, further illustrating the advantage of this approach. Given that traditional diagnostic categories do not take into account the substantial heterogeneity within and comorbidity among disorders, reliance on categorical diagnoses may impede the development of clinically useful neurobiological markers (33-35).
Understanding the interplay between psychopathology and brain development
While the majority of translational psychiatric imaging studies have considered adults, in this study we document associations with dimensions of psychopathology in a community-based sample of youth. The PNC is a quasi-epidemiological sample; however, the clinical screening diagnoses were globally consistent with population rates (36). It is important to interpret our results in the context of normative cortical maturation, where reductions in cortical thickness and volume due to both myelination and pruning follow distinct trajectories throughout development (21, 37). Reduced volume or thickness associated with psychopathology is consistent with at least three potential developmental aberrations. First, our findings could represent a structural abnormality that is present from early life (or even in utero) and fixed throughout development. Such a deficit could potentially be linked to maternal infections during pregnancy or obstetric complications at birth (38). Second, these results could alternatively stem from a flattened trajectory of cortical expansion in early childhood, with reduced peak cortical volume and thickness. Finally, these results may be consistent with an accelerated course (or earlier onset) of the normative process of cortical thinning and gray matter volume loss. Intriguingly, recent evidence from multiple lines of research including epigenetics and translational neuroimaging suggests that childhood adversity may accelerate the process of cortical development (39). To disambiguate these possibilities, it will be necessary to follow large samples of youth longitudinally from early in life, and acquire detailed data regarding pregnancy and the childhood environment.
Conclusions
This study provides novel evidence that transdiagnostic fear symptoms are associated with reduced cortical thickness during development, while reduction in gray matter volume scales with the overall level of psychopathology present. Moving forward, longitudinal designs may allow researchers to determine whether these changes precede and predict the onset or worsening of psychopathology over time. Additionally, the use of bi-directional translational models that integrate multiple modalities will allow us to better probe for causal relationships (40).
Supplementary Material
DISCLOSURES AND ACKNOWLEDGEMENTS:
We thank the acquisition and recruitment team, including Karthik Prabhakaran. Thanks to Chad Jackson for data management and systems support. Dr. Shinohara has received legal consulting and advisory board income from Genentech/Roche. All other authors (Dr. Kaczkurkin, Ms. Park, Dr. Sotiras, Dr. Moore, Dr. Calkins, Dr. Cieslak, Mr. Rosen, Mr. Ciric, Mr. Xia, Dr. Cui, Dr. Sharma, Dr. Wolf, Ms. Ruparel, Dr. Pine, Dr. Roalf, Dr. R.C. Gur, Dr. Davatzikos, Dr. R.E. Gur, and Dr. Satterthwaite) report no competing interests.
FUNDING: This work was supported by grants from the National Institute of Mental Health (NIMH; grant numbers: K99MH117274 to ANK, R01MH107703 and R01MH113550 to TDS, R01NS085211 to RTS, R01MH112847 to RTS and TDS, R01MH107235 to RCG, and R01MH112070 to CD); the Dowshen Program for Neuroscience, and the Lifespan Brain Institute at the Children’s Hospital of Philadelphia and Penn Medicine. The PNC was funded by RC2 grants MH089983 and MH089924 to REG from the NIMH. Support for developing statistical analyses (RTS & TDS) was provided by a seed grant by the Center for Biomedical Computing and Image Analysis (CBICA) at Penn. Support for developing multivariate pattern analysis software (AS & TDS) was provided by a seed grant by the Center for Biomedical Computing and Image Analysis (CBICA) at Penn. Support was also provided by a NARSAD Young Investigator Award (ANK) as well as a Penn PROMOTES Research on Sex and Gender in Health grant (ANK) awarded as part of the Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) grant (K12 HD085848) at the University of Pennsylvania.
References
- 1.Conway CC, Forbes MK, Forbush KT, et al. : A hierarchical taxonomy of psychopathology can transform mental health research. Perspect Psychol Sci 2019; in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caspi A, Moffitt TE: All for one and one for all: Mental disorders in one dimension. Am J Psychiatry 2018; 175:831–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zald DH, Lahey BB: Implications of the Hierarchical Structure of Psychopathology for Psychiatric Neuroimaging. Biol Psychiatry Cogn Neurosci Neuroimaging 2017; 2:310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindemer ER, Salat DH, Leritz EC, et al. : Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF Veterans and the impact of comorbid TBI. NeuroImage Clin 2013; 2:601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmaal L, Hibar DP, Sämann PG, et al. : Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2017; 22:900–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon TD, Chung Y, He G, et al. : Progressive reduction in cortical thickness as psychosis develops: A multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry 2015; 77:147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makris N, Biederman J, Valera EM, et al. : Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex 2007;17:1364–1375 [DOI] [PubMed] [Google Scholar]
- 8.Goodkind M, Eickhoff SB, Oathes DJ, et al. : Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 2015; 72:305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkler AM, Kochunov P, Blangero J, et al. : Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 2010; 53:1135–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler RC, Amminger GP, Aguilar-Gaxiola S, et al. : Age of onset of mental disorders: A review of recent literature. Curr Opin Psychiatry 2007; 20:359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessler RC, Petukhova M, Sampson NA, et al. : Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res 2012; 21:169–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahey BB, Applegate B, Hakes JK, et al. : Is There a general factor of prevalent psychopathology during adulthood? J Abnorm Psychol 2012; 121:971–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romer AL, Knodt AR, Houts R, et al. : Structural alterations within cerebellar circuitry are associated with general liability for common mental disorders. Mol Psychiatry 2017; 1084–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder HR, Hankin BL, Sandman CA, et al. : Distinct Patterns of Reduced Prefrontal and Limbic Gray Matter Volume in Childhood General and Internalizing Psychopathology. Clin Psychol Sci 2017; 5:1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satterthwaite TD, Elliott MA, Ruparel K, et al. : Neuroimaging of the Philadelphia Neurodevelopmental Cohort. Neuroimage 2014; 86:544–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satterthwaite TD, Connolly JJ, Ruparel K, et al. : The Philadelphia Neurodevelopmental Cohort: A publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage 2016; 124:1115–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sotiras A, Resnick SM, Davatzikos C: Finding imaging patterns of structural covariance via Non-Negative Matrix Factorization. Neuroimage 2015; 108:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen AFG, Roalf DR, Ruparel K, et al. : Quantitative assessment of structural image quality. Neuroimage 2018; 169:407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sotiras A, Toledo JB, Gur RE, et al. : Patterns of coordinated cortical remodeling during adolescence and their associations with functional specialization and evolutionary expansion. Proc Natl Acad Sci 2017; 114:3527–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood SN: mgcv: GAMs and generalized ridge regression for R. R News 2001; 1:20–25 [Google Scholar]
- 21.Kaczkurkin AN, Raznahan A, Satterthwaite TD: Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology 2018; 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linares I, Jackowski A, Trzesniak C, et al. : Cortical thinning of the right anterior cingulate cortex in spider phobia: A magnetic resonance imaging and spectroscopy study. Brain Res 2014; 1576:35–42 [DOI] [PubMed] [Google Scholar]
- 23.Syal S, Hattingh CJ, Fouché J-P, et al. : Grey matter abnormalities in social anxiety disorder: A pilot study. Metab Brain Dis 2012; 27:299–309 [DOI] [PubMed] [Google Scholar]
- 24.Sylvester CM, Corbetta M, Raichle ME, et al. : Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci 2012; 35:527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bush G, Luu P, Posner MI: Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000; 4:215–222 [DOI] [PubMed] [Google Scholar]
- 26.Seeley WW, Menon V, Schatzberg AF, et al. : Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen S, Posner M: The attention system of the human brain: 20 years after. Annu Rev Neurosci 2012; 35:73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castellanos-Ryan N, Briere FN, O’Leary-Barrett M, et al. : The structure of psychopathology in adolescence and its common personality and cognitive correlates. J Abnorm Psychol 2016; 125:1039–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shanmugan S, Wolf DH, Calkins ME, et al. : Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. Am J Psychiatry 2016; 173:517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaczkurkin AN, Moore TM, Calkins ME, et al. : Common and dissociable regional cerebral blood flow differences associate with dimensions of psychopathology across categorical diagnoses. Mol Psychiatry 2017; 1981–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selzam S, Coleman JRI, Caspi A, et al. : A polygenic p factor for major psychiatric disorders. Transl Psychiatry 2018; 8:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw P, Greenstein D, Lerch J, et al. : Intellectual ability and cortical development in children and adolescents. Nature 2006; 440:676–679 [DOI] [PubMed] [Google Scholar]
- 33.Shackman AJ, Fox AS: Getting Serious about Variation: Lessons for Clinical Neuroscience (A Commentary on ‘The Myth of Optimality in Clinical Neuroscience’). Trends Cogn Sci 2018; 22:368–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olbert CM, Gala GJ, Tupler LA: Quantifying heterogeneity attributable to polythetic diagnostic criteria: Theoretical framework and empirical application. J Abnorm Psychol 2014; 123:452–462 [DOI] [PubMed] [Google Scholar]
- 35.Regier DA, Narrow WE, Clarke DE, et al. : DSM-5 Field Trials in the United States and Canada, Part II: Test-Retest Reliability of Selected Categorical Diagnoses. Am J Psychiatry 2013; 170:59–70 [DOI] [PubMed] [Google Scholar]
- 36.Merikangas KR, Calkins ME, Burstein M, et al. : Comorbidity of Physical and Mental Disorders in the Neurodevelopmental Genomics Cohort Study. Pediatrics 2015;135:e927–e938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamnes CK, Herting MM, Goddings A-L, et al. : Development of the Cerebral Cortex across Adolescence: A Multisample Study of Inter-Related Longitudinal Changes in Cortical Volume, Surface Area, and Thickness. J Neurosci 2017; 37:3402–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebner F, Tepest R, Dani I, et al. : The hippocampus in families with schizophrenia in relation to obstetric complications. Schizophr Res 2008; 104:71–78 [DOI] [PubMed] [Google Scholar]
- 39.Belsky J, De Haan M: Annual research review: Parenting and children’s brain development: The end of the beginning. J Child Psychol Psychiatry Allied Discip 2011; 52:409–428 [DOI] [PubMed] [Google Scholar]
- 40.Fox AS, Kalin NH: A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. Am J Psychiatry 2014; 171:1162–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
See https://github.com/PennBBL/KaczkurkinPark_BifactorStructure/wiki/KaczkurkinPark_BifactorStructure for code and a companion wiki detailing analytic procedures used in this manuscript. Data from the PNC can be accessed at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000607.v3.p2. The NMF code can be found at https://github.com/asotiras/brainlets.