Abstract
Covalent modifications of histone proteins regulate a wide variety of cellular processes. Methylation of histone H3K79 and H3K4 is associated with active transcription and is catalyzed by Dot1L and Set1, respectively. Both Dot1L and Set1 are activated by prior ubiquitination of histone H2B on K120 in a process termed “histone-crosstalk”. Recent structures of Dot1L bound a ubiquitinated nucleosome reveled how Dot1L is activated by ubiquitin and how Dot1L distorts the nucleosome to access its substrate. Structures of Dot1L-interacting proteins have provided insight into how Dot1L is recruited to sites of active transcription. Cryo-EM and crystallographic studies of the complex of proteins associated with Set1 (COMPASS) uncovered the architecture of COMPASS and how Set1 is activated upon complex assembly.
Introduction
The packaging of eukaryotic DNA into chromatin governs all cellular processes requiring access to DNA, such as transcription [1], DNA replication [2], and DNA repair [3]. The minimal organizational unit of chromatin is the nucleosome, which comprises 147 base pairs of DNA wrapped around an octameric core of histone proteins H2A, H2B, H3 and H4 [4]. The cell regulates the higher-order organization and position of nucleosomes through post-translational modification of histone proteins. These modifications modulate the packaging of DNA into chromatin and recruit a variety of protein complexes and enzymes [5,6]. Most posttranslational histone marks are small chemical modifications such as phosphorylation, methylation, and acetylation, which alter the steric bulk or charge of the modified histone sidechain. Histones can also be modified with the 76-amino acid protein, ubiquitin [7], a comparatively large modification that regulates a wide array of cellular processes including transcription activation, silencing, DNA repair and chromatin compaction [8,9]. The structural motifs that recognize ubiquitin [10] and ubiquitinated nucleosomes [11] have been reviewed recently. The precise patterning of histone modifications – termed the “histone code” [6] – is therefore required to properly regulate processes that access DNA. Dysregulation of histone modifications can lead to a variety of human diseases.
Some histone marks trigger modification of other histone residues. This interdependence of histone modifications, where recognition or deposition of one mark depends on the presence of another, is called “histone crosstalk” [12]. A well-established example of histone crosstalk is the dependence of histone H3K4 and H3K79 methylation on prior monoubiquitination of histone H2B K120 (H2B-Ub) (in humans; H2B K123 in yeast) [13-16]. H3K79 and H3K4 methylation are catalyzed by two enzymes: disruptor of telomeric silencing-like protein (Dot1L) [17], which methylates H3K79 in humans, and the complex of proteins associated with Set1 (COMPASS), which methylates H3K4 [18]. Dot1L contains a catalytic domain that resembles arginine methyltransferases [19] and the catalytic subunit of COMPASS (Set1) contains a SET lysine methyltransferase domain [20-22], [23]**, [24]**. Dot1L and COMPASS are evolutionarily unrelated enzymes that are conserved from yeast to humans. Recent structures of Dot1L bound to a ubiquitinated nucleosome [25]**, [26]**, [27]**, [28]*, [29]*, Dot1L-binding partners, and COMPASS [23]**, [24]** have provided crucial insights into how Dot1L recognizes and is activated by ubiquitin and how the COMPASS subunits activate Set1
Activation of Dot1L by H2B-Ub nucleosomes
In contrast to most sites of histone modifications, which occur on flexible histone tails, H3K79 is located in the folded histone octamer core of the nucleosome. Methylation of H3K79 by Dot1L is broadly associated with transcription [30], telomeric silencing [31] and the DNA damage response [32-34], although the mechanism by which H3K79 methylation promotes or regulates these processes remains unknown. The dependence of H3K79 methylation by human Dot1L and yeast Dot1p on H2B-Ub has been well established in vivo [15,16] and, in the case of Dot1L, in vitro [35]. Muir and colleagues have shown that a surface on ubiquitin centered on L73 and L71 is critical for Dot1L activation [36,37]. Dot1L activity also depends on a patch of basic residues in the flexible N-terminal tail of histone H4 [38-40], but a structural explanation for these observations had been lacking. In addition, although structures of yeast Dot1p [41] and human Dot1L [19] have been available for quite some time, it had not been possible to construct a model for how the relatively inaccessible H3K79 side chain enters the enzyme active site.
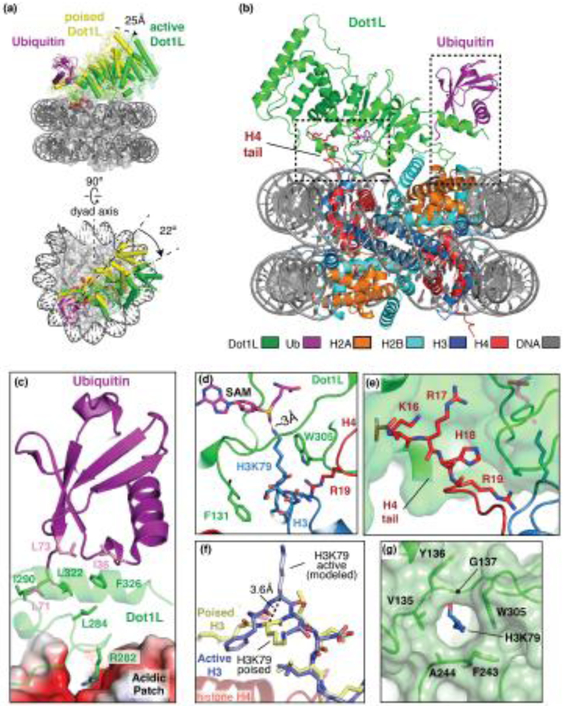
Recent cryo-EM structures of Dot1L bound to an H2B-Ub nucleosome have revealed how Dot1L recognizes ubiquitin and the acidic patch of the nucleosome [25]**, [26]**,[27]**, [29]* Four recent studies captured Dot1L in a state in which the active site is oriented far from the H3K79 sidechain, a configuration that would presumably exist either directly before or after methyl transfer (Figure 1a). In this “poised” state, Dot1L is anchored to one side of the nucleosome though direct interactions with the H2B-linked ubiquitin (Figure 1 b, c). Dot1L binds ubiquitin using a C-terminal helix and loop, which bears no resemblance to any previously identified ubiquitin-binding motifs. This region of Dot1L binds to a hydrophobic patch on ubiquitin that includes I36, L71 and L73 (Figure 1c), the latter two of which had previously been shown to be important for activating Dot1L [36]. There is no connecting density between Ubiquitin and H2B in any of the structures, indicating that this connection is not ordered and thus explaining how ubiquitin modifications near H2BK120 can also stimulate Dot1L [37]. In addition, Dot1L contacts the conserved H2A/H2B acidic patch with a single arginine, R282, which is clearly visible in all poised state structures (Figure 1b, c) [25]**, [26]**, [27]**, [29]*. This provides yet another example of a so-called “arginine anchor” contacting the nucleosome acidic patch, which is a hot spot of interactions with nucleosome binding proteins [42]. A fifth structure reported at 6.8 A resolution shows a similar overall orientation of Dot1L on the nucleosome [28]*. In all poised state structures, weak density at the N-terminal end of Dot1L, which is the most distant from the ubiquitin anchor point, indicates that the enzyme can access multiple positions while tethered at its C-terminus by ubiquitin and the acidic patch. Interestingly, classification of the poised state structure by Anderson et. al. [26]** isolated several states of Dot1L that are rotated to varying degrees about the arginine anchor and ubiquitin contact, showing that Dot1L can sample a wide area of the nucleosome surface. Furthermore, structures of Dot1L bound to an unmodified nucleosome, [27]** and [29]*, showed that Dot1L binds to essentially the same nucleosome surface with or without ubiquitin. However, the Dot1L density in these structures is very weak, indicating that the enzyme is much more mobile without the H2B-Ub modification.
Figure 1. Dot1L recognition of an H2B-Ubiquitinated nucleosome.
(a) Dot1L (2-416) switching from the poised (yellow) to the active (green) state as shown from the side (top) and top (bottom). The nucleosome is shown as a semi-transparent gray surface. (b) Structure of the active state complex between Dot1L and the H2B-Ub nucleosome (PDB: 6NJ9). (c) Close up view of Dot1L interactions with ubiquitin and the acidic patch. Interface residues are shown as sticks. (d) Formation of the Dot1L active site enclosure. Dot1L, H3 and H4 are shown as green, blue and red cartoons respectively. The SAM cofactor is shown as magenta sticks. (e) Dot1L interactions with the H4 tail. Dot1L is depicted as a green cartoon surrounded by a semi-transparent green surface. (f) The conformational change of H3K79. The position of H3K79 in the poised state is depicted with yellow sticks and the active state H3K79 is shown with blue sticks. (g) The Dot1L hydrophobic lysine binding channel, with residues that compose the channel shown as sticks.
Source: Adapted from E. Worden et. al, [25]**
The structure of Dot1L bound to an H2B-Ub nucleosome in a catalytically competent, “active” state was determined at 3.0 Å resolution by cryo-EM (Figure 1a, b) [25]**. This active conformation was trapped by replacing H3K79 with norleucine, a non-native amino acid lacking the ε-amino group. Peptides containing norleucine in place of the substrate lysine have been shown to bind tightly to SET domain methyltransferases, mimicking the tight binding of Lysine-to-methionine mutations seen in pontine gliomas [43-45]. As compared to the poised state, the N-terminal domain of Dot1L moves toward the nucleosome by ~25Å and rotates by 22° to adopt an active state (Figure 1 a, b) in which the SAM methyl group is within 3 Å of the attacking lysine (Figure 1d). In addition to providing details on the catalytically relevant complex, the structure revealed an unexpected role for the tail of histone H4 and explained how Dot1L modifies the relatively inaccessibly side chain of H3K79.
In the active state structure, the tail of histone H4 inserts into a groove formed in the Dot1L N-terminal domain (Figure 1 e), thus explaining previous observations about the critical role of a basic patch in this histone for Dot1L and Dot1p activity [38-40]. The dual interactions at the Dot1L N-terminus with the H4 tail and the C-terminus with ubiquitin orient Dot1L over the H3K79 substrate lysine [25]**. In addition to binding to Dot1L, the H4 tail assists Dot1L in inducing a conformational change in histone H3 that alters the backbone flanking H3K79 and reorients the lysine sidechain by 90°, inserting it into the hydrophobic Dot1L lysine binding channel (Figure 1e - g). This unprecedented conformational distortion in the globular core histone is stabilized by a tripartite set of interactions involving Dot1L residues F131 and W305 and the H4 tail residue R19, which contacts backbone of H3 residues 77, 79 and 80 (Figure 1d). Together, these contacts by Dot1L and histone H4 “pinch” H3K79 from all sides and force it from its inaccessible position into the Dot1L active site. The conformational change in core histone fold of H3 and the participation of the H4 tail in stabilizing the altered conformation of H3 have never been observed previously, although recent studies have suggested that the core histone fold can be deformed due to the action of chromatin remodeling enzymes [46]* and during DNA unwrapping and translocation [47]*, [48]*. The intriguing implication of the conformational distortion induced by Dot1L is that there may be other histone-modifying enzymes that similarly deform the core histone fold to access their substrate sidechain in the context of the fully formed nucleosome.
The hydrophobic nature of the lysine binding channel may also explain how H3K79 is deprotonated for the attack on the SAM methyl group during catalysis. Dot1L lacks ionizable residues in the active site that could potentially abstract a proton from the charged lysine side chain (Figure 1g) [19], [25]**. Burying the charged H3K79 sidechain within the hydrophobic environment of the lysine binding channel would lower the pKa of the ε-amino group and favor deprotonation [49].
Recruitment of Dot1L to genomic loci
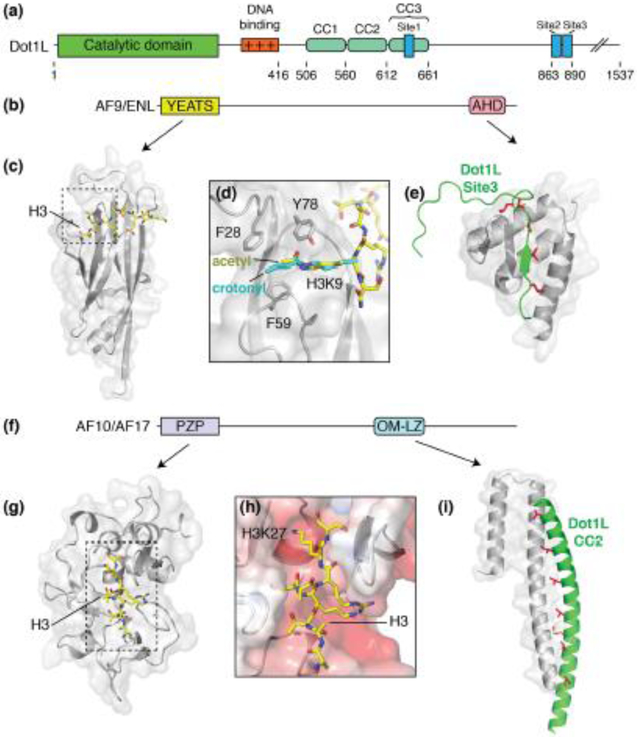
Dot1L is recruited to specific genomic loci by the partner proteins AF9, ENL, AF10 and AF17, which contain C-terminal Dot1L interaction domains and N-terminal chromatin binding domains [50]*, [51]*, [52]*, [53]*, (Figure 2b-i). The precise composition of Dot1L complexes with its various partner proteins, as well as the role each partner proteins plays in recruiting Dot1L to different genomic loci, remains an open question. Chromosomal translocations that fuse Dot1L binding proteins to the human Set1 methyltransferase homolog, MLL, lead to aberrant recruitment of Dot1L to MLL target genes, which is a causative factor in many leukemias [54] and other cancers [55,56].
Figure 2. Dot1L partner proteins.
(a) Domain architecture schematic of Dot1L. (b) Domain architecture schematic for Dot1L partners, AF9 and ENL. (c) Structure of the AF9 YEATS domain (PDB: 4TMP, gray) bound to acetylated H3K9 (yellow sticks). (d) Close up view of the acetylated H3K9 binding site. Crotonylated H3K9 (PDB: 6MIM) is shown in cyan. (e) Structure of the AF9 AHD (PDB: 2MV7, gray) domain bound to Dot1L site3 (green). Buried hydrophobic residues are colored red and shown as sticks. (f) Generalized domain architecture schematic for AF10 and AF17. (g) Structure of the AF10 PZP domain (PDB: 5DAH, gray) bound to an unmodified H3 peptide (yellow sticks). (h) Close up view of the AF10 PZP H3 binding groove. The surface of AF10 is colored according to electrostatic potential. (i) Structure of the AF10 OM-LZ motif (PDB: 6CKO, gray) bound to the CC2 of Dot1L (green). Buried hydrophobic residues are colored red and shown as sticks.
While there are, as yet, no structures of complete complexes containing intact Dot1L or its partner proteins, structural studies of several Dot1L and chromatin-interacting domains have shed light on key aspects of these complexes [50]*, [51]*, [52]*, [53]* . Dot1L partners, AF9 and ENL, contain an N-terminal YEATS (Yaf9, ENL, AF9, Taf14, Sas5) domain that binds to acetylated or crotonylated H3K9, H3K27 and H3K18 (Figure 2b-d) [50]*, [57]*. The YEATS domain recognizes the acetyl-,[50]* or crotonyl-lysine [57]* in a surface tunnel that terminates in an aromatic cage composed of F28, F59 and Y78 (Figure 2c,d). AF9 and ENL also contain a C-terminal ANC1 homology domain (AHD) that interact with one of three binding sites (Site1-Site3) in the C-terminal part of Dot1L and recruit the enzyme to specific genomic loci (Figure 2a,e) [51]*. Mutations in the AHD that disrupt Dot1L binding decrease H3K79 methylation at targets of the MLL-AF9 fusion protein [51]*.
AF10 and AF17 have an N-terminal PDH finger-Zn knuckle-PDH finger (PZP) domain that binds to the unmodified tail of histone H3 between residues 22-28 (Figure 2f-h) [52]*. The structure of the AF10 PZP domain shows how the H3 tail binds in an acidic groove and makes specific contacts with multiple charged residues (Figure 2h). Binding of the H3 tail to the PZP domain is abolished upon methylation of H3K27, which is a hallmark of silenced chromatin [58], consistent with the role of Dot1L in transcription activation [30]. AF10 and AF17 also contain an octapeptide motif leucine zipper (OM-LZ) domain which can form a coiled-coil with any one of three sequences (CC1-CC3) in C-terminal part of Dot1L (Figure 2a,i) [53]*. Mutations in the Dot1L binding interface of AF10 disrupt Dot1L binding and lead to a reduction in the leukemogenic transformation potential of MLL-AF10 cell lines [53]*.
Architecture and activation of COMPASS
Methylation of H3K4 is highly enriched at gene promotors and transcription start sites and is dependent on prior ubiquitination of H2B [13,14,58]. H3K4 methylation is catalyzed by COMPASS, which is composed of 8 subunits: the Set1 methyltransferase, Cps60, Cps50, Cps40, Cps35, Cps30, Cps25 and Cps15 [18,59]. The H3K4 methyltransferase activity of the Set1 catalytic domain on its own is low, but is significantly stimulated when incorporated into COMPASS [60,61]. Studies of the contribution of each subunit to the activity of the catalytic subunit Set1 have identified a minimal sub-complex of COMPASS composed of Set1, Cps60, Cps50, Cps40, Cps30, and Cps25 that is sufficient for H2B-Ub dependent stimulation of Set1 [62].
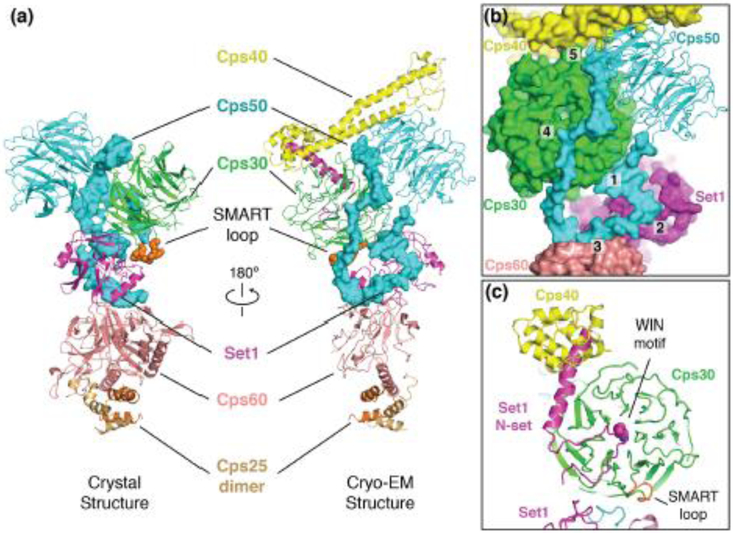
Two structures of the minimal COMPASS subcomplex were recently determined [23]**, [24]**. One structure was determined by cryo-EM and comprises all 6 subunits of the minimal ubiquitin-sensing complex [23]** and the other was determined by X-ray crystallography and comprises a smaller complex lacking Cps40, which is referred to as the WRAD complex, after its human homologs (WDR5/RbBP5/ASH2L/DPY30) (Figure 3a) [24]**. The structures show that COMPASS forms a Y-shaped, highly intertwined, structure with the Set1 catalytic domain at its center (Figure 3a). An interesting feature of these structures is the C-terminal extension of Cps50, which winds around the complex and contacts almost every other subunit except the Cps25 dimer (Figure 3a and 3b, contacts 1-5). Disruption of many of these contacts greatly decreases the activity of Set1, explaining the role of Cps50 in organizing the complex and in activating the catalytic activity of Set1 [23]**.
Figure 3. Architecture of yeast COMPASS.
(a) Structures of the yeast COMPASS complex determined by X-ray crystallography (left) and Cryo-EM (right). The C-terminal extension of Cps50 is shown as a blue surface. (b) Detailed view of the contacts made by the Cps50 C-terminal extension. Distinct contacts are labeled 1-5. (c) View of the N-set motif in Set1.
The N-Set domain of Set1 is required for the ubiquitin-depended activation of H3K4 methylation [62]. The cryo-EM structure of COMPASS [23]** shows that a single lysine of the WIN motif within the N-Set domain of Set1 docks into the WD40 domain of Cps30 in a similar arrangement to the WIN motif from human homologs of Set1 (Figure 3c) [63]. In addition, the N-set domain of Set1 forms a long, helix that inserts between Cps40 and Cps30 (Figure 3c). Flowever, it is still not clear how the N-set motif of Set1 can sense ubiquitinated H2B and the location of this motif within COMPASS does not clarify this mechanism.
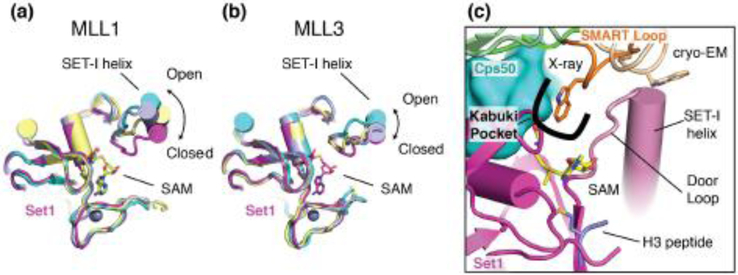
Crystal structures of the human homologs of Set1, MLL1 [64], [65]* and MLL3 [65]*, have established that the critical SET-I helix in these enzymes is mobile and can interconvert between “open” and “closed” states and that this interconversion can be suppressed by binding to proteins in the WRAD complex, leading to activation of the SET domain (Figure 4a,b) [64], [65]*. In both recent structures of COMPASS [23]**, [24]**, the SET-I helix is stabilized in the closed state, which explains how incorporation into COMPASS activates the enzyme (Figure 4a,b). Furthermore, the closed state of the Set-I helix in COMPASS explains the previous observation that C3882 in MLL1 can be auto-methylated in the absence of H3 peptide: closure of the Set-I helix would position C3882 close to the SAM cofactor. Interestingly the Set1 methyltransferase activity regulator (SMART) loop of Cps30 directly contacts the SET-I helix of Set1 in both structures (Figure 4c). However, the two structures differ with respect to a tryptophan residue (W183 in S. cerevisiae) on the tip of the SMART motif that points away from Set1 in the Cryo-EM structure [23]** and is completely buried in the crystal structure within a hydrophobic pocket termed the “kabuki pocket” (Figure 4c) [24]**. Because the cryo-EM structure was determined in the absence of SAM cofactor and H3 peptide [23]**, which are present in the crystal structure [24]**, the different conformations of the SMART loop may correspond to important conformational changes that occur during substrate binding and release. In agreement with its location next to the critical Set-I helix, mutations in the SMART loop of Cps30 lead to defects in H3K4 methylation by COMPASS [23]**, [24]**. Furthermore, the close contacts between the Set-I helix of Set1, the Cps30 SMART loop and the Cps50 C-terminal extension likely help determine the product specificity of different Set1 homologs for mono-, di-, or tri-methylation of H3K4 by restricting motion of the Set-I helix to varying degrees [24]**. Remarkably, re-engineering the “door loop” C-terminal to the Set-I motif in MLL3 to more closely resemble yeast Set1 converted MLL3 from a mono-methylase into a multi-methylase [24]**.
Figure 4. Activation of Set1 in COMPASS.
(a) Superposition of the Set1 catalytic domain (PDB: 6CHG, magenta) with MLL1 in different states (PDB: 5F6L, 5F5E, 2W5Y) showing different conformations of the SET-I helix. (b) Superposition of the Set1 catalytic domain (PDB: 6CHG, magenta) with MLL3 in different states (PDB: 5F6K chain C, E and 5F59) showing different conformations of the SET-I helix. (c) details of the SMART loop interactions with the SET-I helix of Set1 in COMPASS. The SMART loop in the crystal structure is colored orange and the SMART loop in the cryo-EM structure is colored tan.
Conclusions
While great strides have been made in understanding the mechanistic underpinnings of cross-talk between H2B monoubiquitination and histone methylation, several outstanding questions remain to be addressed. It is still not clear how COMPASS binds to the nucleosome or is able to sense ubiquitinated H2B. A structure of the complex between COMPASS and a ubiquitinated nucleosome will be required to understand the crosstalk between H3K4 methylation and H2B-ubiquitination. In addition, the specific mechanism by which H3K79 methylation stimulates transcription is unknown, nor have any bona fide H3K79-methyl binding proteins been identified. Because of the relatively inaccessible position of H3K79 on the nucleosome, it is likely that any protein that recognizes modifications of this side chain would need to deform the structure of the nucleosome in a fashion similar to Dot1L. Furthermore, it is not clear how Dot1L establishes the correct mono-, di- or tri-methylation state of H3K79 in vivo. Recruitment by Dot1L binding partners clearly plays a role in governing the methylation state [51], while in vitro methylation assays have shown that Dot1L preferentially mono- and di-methylates of H3K79 [35]. An interesting possibility is that Dot1L-interacting proteins may activate the enzyme for tri-methylation or make Dot1L processive through multivalent interactions with chromatin. Multivalent binding of chromatin marks has, for example, been shown to promote processive hyperacetylation by the SAGA HAT module [66]. We can look to a future of exciting structures and biochemical studies to sort out these fascinating and important questions.
Highlights.
Structures of Dot1L bound to ubiquitinated nucleosomes show the mechanism of crosstalk between histone H3K79 methylation and H2B monoubiquitination
Structural insights into how Dot1L-interacting proteins recruit Dot1L to genomic loci through bivalent contacts with Dot1L and chromatin
Architecture of the COMPASS complex shows how the H3 K4 methyltransferase subunit, Set1, is activated
Acknowledgements
Supported by a grant from the National Institute of General Medical Sciences (R35 GM130393). E.J.W. is supported by a fellowship from the Damon Runyon Cancer Research Foundation.
Footnotes
Conflict of Interest Statement
C.W. is a member of the scientific advisory board of Thermo Fisher Scientific.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smolle M, Venkatesh S: Transcription Through Chromatin In Fundamentals of Chromatin. Edited by Workman J, Abmayr S: Springer; 2014:427–489. [Google Scholar]
- 2.MacAlpine DM, Almouzni G: Chromatin and DNA replication. Cold Spring Harb Perspect Biol 2013, 5:a010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapoor P, Shen X: Chromatin Remodeling in DNA Repair and Replication In Fundamentals of Chromatin. Edited by Workman J, Abmayr S: Springer; 2014:491–527. [Google Scholar]
- 4.Andrews AJ, Luger K: Nucleosome structure(s) and stability: variations on a theme. Annu Rev Biophys 2011,40:99–117. [DOI] [PubMed] [Google Scholar]
- 5.Clapier CR, Iwasa J, Cairns BR, Peterson CL: Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat Rev Mol Cell Biol 2017, 18:407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenuwein T, Allis CD: Translating the histone code. Science 2001, 293:1074–1080. [DOI] [PubMed] [Google Scholar]
- 7.Busch H, Goldknopf IL: Ubiquitin - protein conjugates. Mol Cell Biochem 1981, 40:173–187. [DOI] [PubMed] [Google Scholar]
- 8.Uckelmann M, Sixma TK: Histone ubiquitination in the DNA damage response. DNA Repair (Amst) 2017, 56:92–101. [DOI] [PubMed] [Google Scholar]
- 9.Weake VM: Histone Ubiquitylation Control of Gene Expression In Fundamentals of Chromatin. Edited by Workman J, Abmayr S: Springer; 2014:257–307. [Google Scholar]
- 10.Komander D, Rape M: The Ubiquitin Code. Annual Review of Biochemistry 2012, 81:203–229. [DOI] [PubMed] [Google Scholar]
- 11.Morgan MT, Wolberger C: Recognition of ubiquitinated nucleosomes. Current Opinion in Structural Biology 2017, 42:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JS, Smith E, Shilatifard A: The language of histone crosstalk. Cell 2010, 142:682–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Z-W, Allis CD: Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 2002, 418:104–108. [DOI] [PubMed] [Google Scholar]
- 14.Dover J, Schneider J, Tawiah-Boateng MA, Wood A, Dean K, Johnston M, Shilatifard A: Methylation of histone H3 by COMPASS requires ubiquitination of histone H2B by Rad6. J Biol Chem 2002, 277:28368–28371. [DOI] [PubMed] [Google Scholar]
- 15.Briggs SD, Xiao T, Sun Z-W, Caldwell JA, Shabanowitz J, Hunt DF, Allis CD, Strahl BD: Trans-histone regulatory pathway in chromatin. Nature 2002, 418:498. [DOI] [PubMed] [Google Scholar]
- 16.Ng HH, Xu RM, Zhang Y, Struhl K: Ubiquitination of histone H2B by Rad6 is required for efficient Dot1-mediated methylation of histone H3 lysine 79. Journal of Biological Chemistry 2002, 277:34655–34657. [DOI] [PubMed] [Google Scholar]
- 17.Feng Q, Wang H, Ng HH, Erdjument-Bromage H, Tempst P, Struhl K, Zhang Y: Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr Biol 2002, 12:1052–1058. [DOI] [PubMed] [Google Scholar]
- 18.Miller T, Krogan NJ, Dover J, Erdjument-Bromage H, Tempst P, Johnston M, Greenblatt JF, Shilatifard A: COMPASS: a complex of proteins associated with a trithorax-related SET domain protein. Proceedings of the National Academy of Sciences of the United States of America 2001,98:12902–12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min J, Feng Q, Li Z, Zhang Y, Xu RM: Structure of the catalytic domain of human Dot1L, a non-SET domain nucleosomal histone methyltransferase. Cell 2003, 112:711–723. [DOI] [PubMed] [Google Scholar]
- 20.Min J, Zhang X, Cheng X, Grewal SIS, Xu R-M: Structure of the SET domain histone lysine methyltransferase Clr4. Nature structural biology 2002, 9:828–832. [DOI] [PubMed] [Google Scholar]
- 21.Wilson JR, Jing C, Walker PA, Martin SR, Howell SA, Blackburn GM, Gamblin SJ, Xiao B: Crystal structure and functional analysis of the histone methyltransferase SET7/9. Cell 2002, 111:105–115. [DOI] [PubMed] [Google Scholar]
- 22.Trievel RC, Beach BM, Dirk LMA, Houtz RL, Hurley JH: Structure and catalytic mechanism of a SET domain protein methyltransferase. Cell 2002, 111:91–103. [DOI] [PubMed] [Google Scholar]
- **23.Qu Q, Takahashi Y-h, Yang Y, Hu H, Zhang Y, Brunzelle JS, Couture J-F, Shilatifard A, Skiniotis G: Structure and Conformational Dynamics of a COMPASS Histone H3K4 Methyltransferase Complex. Cell 2018, 0:1–10.** This Cryo-EM study reveled the structure of the minimal ubiquitin-sensing subcomplex of COMPASS at 4.0 Å resolution and showed that the C-terminal extension of Cps50 arranges the complex and the position of the Cps40 subunit.
- **24.Hsu PL, Li H, Lau H-T, Leonen C, Dhall A, Ong S-E, Chatterjee C, Zheng N: Crystal Structure of the COMPASS H3K4 Methyltransferase Catalytic Module. Cell 2018, 0:1–11.** The 3.0 Å resolution x-ray crystal structure of the WRAD subcomplex of COMPASS shows that the C-terminal extension of Cps50 and that the SMART loop of Cps30 regulates Set1.
- **25.Worden EJ, Hoffmann NA, Hicks CW, Wolberger C: Mechanism of Cross-talk between H2B Ubiquitination and H3 Methylation by Dot1L. Cell 2019, 176:1490–1501.** This Cryo-EM study showed how Dot1L contacts the nucleosome and is activated by H2B-Ub. Structures of Dot1L bound to H2B-Ub nucleosomes were determined at 3.0 A resolution (active state) and 3.9 Å resolution (poised state). This study also showed that Dot1L collaborates with the tail of histone H4 to distort the fold of histone H3 in the nucleosome, thereby inserting K79 into the active site.
- **26.Anderson CJ, Baird MR, Hsu A, Barbour EH, Koyama Y, Borgnia MJ, McGinty RK: Structural Basis for Recognition of Ubiquitylated Nucleosome by Dot1L Methyltransferase. Cell Reports 2019, 26:1681–1690.** Cryo-EM structures of Dot1L bound to H2B-Ub nucleosome in the poised state, determined at 3.9 Å resolution, showed how H2B-Ub positions Dot1L on the nucleosome face. This study captured several orientations of Dot1L, showing the degrees of freedom the enzyme can adopt when bound to ubiquitin.
- **27.Valencia-Sanchez MI, De loannes P, Wang M, Vasilyev N, Chen R, Nudler E, Armache JP, Armache KJ: Structural Basis of Dot1L Stimulation by Histone H2B Lysine 120 Ubiquitination. Mol Cell 2019, 74:1010–1019.** Structures of Dot1L bound to H2B-Ub and unmodified nucleosomes were determined by cryo-EM at 3.5 Å and 4.9 Å resolution, respectively. This study showed that Dot1L binds to the nucleosome in the poised state even in the absence of H2B-Ub.
- *28.Jang S, Kang C, Yang H-S, Jung T, Hebert H, Jung KY, Kim SJ, Hohng S, Song J-J: Structural basis of recognition and destabilization of histone H2B ubiquitinated nucleosome by DOT1L histone H3 Lys79 methyltransferase. Genes Dev 2019, 33:620–625.* This lower resolution Cryo-EM study showed the general features of how Dot1L contacts the nucleosome and H2B-Ub.
- *29.Yao T, Jing W, Hu Z, Tan M, Cao M, Wang Q, Li Y, Yuan G, Lei M, Huang J: Structural basis of the crosstalk between histone H2B monoubiquitination and H3 lysine 79 methylation on nucleosome. Cell Res 2019, 29:330–333.* A 4.1 Å resolution cryo EM structure of Dot1L bound in a poised state to ubiquitinated nucleosomes.
- 30.Steger DJ, Lefterova Ml, Ying L, Stonestrom AJ, Schupp M, Zhuo D, Vakoc AL, Kim J-e, Chen J, Lazar MA, et al. : DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells. Molecular and cellular biology 2008, 28:2825–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng HH, Feng Q, Wang H, Erdjument-Bromage H, Tempst P, Zhang Y, Struhl K: Lysine methylation within the globular domain of histone H3 by Dot1 is important for telomeric silencing and Sir protein association. Genes Dev 2002, 16:1518–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giannattasio M, Lazzaro F, Plevani P, Muzi-Falconi M: The DNA damage checkpoint response requires histone H2B ubiquitination by Rad6-Bre1 and H3 methylation by Dot1. J Biol Chem 2005, 280:9879–9886. [DOI] [PubMed] [Google Scholar]
- 33.Huyen Y, Zgheib O, Ditullio Ra, Gorgoulis VG, Zacharatos P, Petty TJ, Sheston Ea, Mellert HS, Stavridi ES, Halazonetis TD: Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature 2004, 432:406–411. [DOI] [PubMed] [Google Scholar]
- 34.Wysocki R, Javaheri A, Allard S, Sha F, Cote J, Kron SJ: Role of Dot1-dependent histone H3 methylation in G1 and S phase DNA damage checkpoint functions of Rad9. Mol Cell Biol 2005, 25:8430–8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGinty RK, Kim J, Chatterjee C, Roeder RG, Muir TW: Chemically ubiquitylated histone H2B stimulates hDot1L-mediated intranucleosomal methylation. Nature 2008, 453:812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holt MT, David Y, Pollock S, Tang Z, Jeon J, Kim J, Roeder RG, Muir TW: Identification of a functional hotspot on ubiquitin required for stimulation of methyltransferase activity on chromatin. Proceedings of the National Academy of Sciences of the United States of America 2015, 112:10365–10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatterjee C, McGinty RK, Fierz B, Muir TW: Disulfide-directed histone ubiquitylation reveals plasticity in hDot1L activation. Nature chemical biology 2010, 6:267–269. [DOI] [PubMed] [Google Scholar]
- 38.Altaf M, Utley RT, Lacoste N, Tan S, Briggs SD, Côté J: Interplay of Chromatin Modifiers on a Short Basic Patch of Histone H4 Tail Defines the Boundary of Telomeric Heterochromatin. Molecular Cell 2007, 28:1002–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fingerman IM, Li HC, Briggs SD: A charge-based interaction between histone H4 and Dot1 is required for H3K79 methylation and telomere silencing: Identification of a new trans-histone pathway. Genes and Development 2007, 21:2018–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGinty RK, Köhn M, Chatterjee C, Chiang KP, Pratt MR, Muir TW: Structure-activity analysis of semisynthetic nucleosomes: Mechanistic insights into the stimulation of Dot1L by ubiquitylated histone H2B. ACS Chemical Biology 2009, 4:958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawada K, Yang Z, Horton JR, Collins RE, Zhang X, Cheng X: Structure of the conserved core of the yeast Dot1p, a nucleosomal histone H3 lysine 79 methyltransferase. The Journal of biological chemistry 2004, 279:43296–43306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGinty RK, Tan S: Histone, Nucleosome, and Chromatin Structure In Fundamentals of Chromatin. Edited by Workman J, Abmayr S: Springer; 2014:1–28. [Google Scholar]
- 43.Brown ZZ, Müiler MM, Jain SU, Allis CD, Lewis PW, Muir TW: Strategy for "Detoxification" of a cancer-derived histone mutant based on mapping its interaction with the methyltransferase PRC2. Journal of the American Chemical Society 2014, 136:13498–13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jayaram H, Hoelper D, Jain SU, Cantone N, Lundgren SM, Poy F, Allis CD, Cummings R, Bellon S, Lewis PW, et al. : S-adenosyl methionine is necessary for inhibition of the methyltransferase G9a by the lysine 9 to methionine mutation on histone H3. Pnas 2016, 113:6182–6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis PW, Müller MM, Koletsky MS, Cordero F, Lin S, Banaszynski La, Garcia Ba, Muir TW, Becher OJ, Allis CD: Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science (New York, N.Y.) 2013, 340:857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *46.Sinha KK, Gross JD, Narlikar GJ: Distortion of histone octamer core promotes nucleosome mobilization by a chromatin remodeler. Science 2017, 355: pii: eaaa3761. doi: 10.1126/science.aaa3761* Crosslinking and methyl-trosy NMR was used to show that the folded histone core of the nucleosome can be distorted during the action of nucleosome remodelers.
- *47.Bilokapic S, Strauss M, Halic M: Histone octamer rearranges to adapt to DNA unwrapping. Nat Struct Mol Biol 2018, 25:101–108.* This Cryo-EM study (along with reference 48) showed that reconstituted nucleosome exist in many different structural states. This study showed that structural plasticity in the nucleosome core and the wrapped DNA distortions may have implications for the action of chromatin remodelers.
- *48.Bilokapic S, Strauss M, Halic M: Structural rearrangements of the histone octamer translocate DNA. Nat Commun 2018, 9:1330.* This Cryo-EM study (along with reference 47) showed that reconstituted nucleosomes exist in many different structural states. This study showed that structural plasticity in the nucleosome and in DNA can explain how DNA is able to translocate around the nucleosome during remodeling.
- 49.Isom DG, Castaneda CA, Cannon BR, Garcia-Moreno B: Large shifts in pKa values of lysine residues buried inside a protein. Proc Natl Acad Sci U S A 2011, 108:5260–5265.* This study identified that the YEATS domain was a histone reader domain and that it bound preferentially to acetylated H3K9. A structure of the YEATS domain showed how this reader recognized lysine acetylation.
- *50.Li Y, Wen H, Xi Y, Tanaka K, Wang H, Peng D, Ren Y, Jin Q, Dent SYR, Li W, et al. : AF9 YEATS domain links histone acetylation to DOT1L-mediated H3K79 methylation. Cell 2014, 159:558–571.* This study identified that the YEATS domain was a histone reader domain and that it bound preferentially to acetylated H3K9. A structure of the YEATS domain showed how this reader recognized lysine acetylation.
- *51.Kuntimaddi A, Achille NJ, Thorpe J, Lokken AA, Singh R, Hemenway CS, Adli M, Zeleznik-Le NJ, Bushweller JH: Degree of Recruitment of DOT1L to MLL-AF9 Defines Level of H3K79 Di- and Tri-methylation on Target Genes and Transformation Potential. Cell Reports 2014, 11:808–820.* NMR study of the structure of the AHD domain and its interaction with Dot1L. The authors also established that the binding affintiy of the AHD domain for Dot1L changes recruitment of Dot1L to genomic loci and the level of H3K79 mehtylation.
- *52.Chen S, Yang Z, Wilkinson AW, Deshpande AJ, Sidoli S, Krajewski K, Strahl BD, Garcia BA, Armstrong SA, Patel DJ, et al. : The PZP Domain of AF10 Senses Unmodified H3K27 to Regulate DOT1L-Mediated Methylation of H3K79. Molecular Cell 2015, 60:319–327.* Study showing that the PZP domain of AF10 binds to unmodified H3 near H3K27 and that methylation or acetylation of H3K27 disrupts that binding interaction.
- *53.Zhang H, Zhou B, Qin S, Xu J, Harding R, Tempel W, Nayak V, Li Y, Loppnau P, Dou Y, et al. : Structural and functional analysis of the DOT1L-AF10 complex reveals mechanistic insights into MLL-AF10-associated leukemogenesis. Genes Dev 2018, 32:341–346.* Structure of the OM-LZ domain of AF10 interacting with the coiled coil sequences of Dot1L.
- 54.Okada Y, Feng Q, Lin Y, Jiang Q, Li Y, Coffield VM, Su L, Xu G, Zhang Y: hDOT1L links histone methylation to leukemogenesis. Cell 2005, 121:167–178. [DOI] [PubMed] [Google Scholar]
- 55.Vlaming H, van Leeuwen F: The upstreams and downstreams of H3K79 methylation by DOT1L. Chromosoma 2016, 125:593–605. [DOI] [PubMed] [Google Scholar]
- 56.Chen CW, Armstrong SA: Targeting DOT1L and HOX gene expression in MLL-rearranged leukemia and beyond. Experimental Hematology 2015, 43:673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Sabari BR, Panchenko T, Wen H, Zhao D, Guan H, Wan L, Huang H, Tang Z, Zhao Y, et al. : Molecular Coupling of Histone Crotonylation and Active Transcription by AF9 YEATS Domain. Mol Cell 2016, 62:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hyun K, Jeon J, Park K, Kim J: Writing, erasing and reading histone lysine methylations. Exp Mol Med 2017, 49:e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krogan NJ, Dover J, Khorrami S, Greenblatt JF, Schneider J, Johnston M, Shilatifard A: COMPASS, a histone H3 (lysine 4) methyltransferase required for telomeric silencing of gene expression. Journal of Biological Chemistry 2002, 277:10753–10755. [DOI] [PubMed] [Google Scholar]
- 60.Dou Y, Milne Ta, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, Allis CD, Roeder RG: Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nature structural & molecular biology 2006, 13:713–719. [DOI] [PubMed] [Google Scholar]
- 61.Patel A, Dharmarajan V, Vought VE, Cosgrove MS: On the mechanism of multiple lysine methylation by the human mixed lineage leukemia protein-1 (MLL1) core complex. J Biol Chem 2009, 284:24242–24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim J, Kim JA, McGinty RK, Nguyen UTT, Muir TW, Allis CD, Roeder RG: The n-SET Domain of Set1 Regulates H2B Ubiquitylation-Dependent H3K4 Methylation. Molecular Cell 2013, 49:1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dharmarajan V, Lee JH, Patel A, Skalnik DG, Cosgrove MS: Structural basis for WDR5 interaction (Win) motif recognition in human SET1 family histone methyltransferases. Journal of Biological Chemistry 2012, 287:27275–27289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Southall SM, Wong PS, Odho Z, Roe SM, Wilson JR: Structural Basis for the Requirement of Additional Factors for MLL1 SET Domain Activity and Recognition of Epigenetic Marks. Molecular Cell 2009, 33:181–191. [DOI] [PubMed] [Google Scholar]
- *65.Li Y, Han J, Zhang Y, Cao F, Liu Z, Li S, Wu J, Hu C, Wang Y, Shuai J, et al. : Structural basis for activity regulation of MLL family methyltransferases. Nature 2016, 530:447–452.* Structural and functional study showing that binding to other subunits in the WRAD complex activates MLL1 and MLL3 by restricting motion of the Set-I helix.
- 66.Ringel AE, Cieniewicz AM, Taverna SD, Wolberger C: Nucleosome competition reveals processive acetylation by the SAGA HAT module. Proceedings of the National Academy of Sciences of the United States of America 2015, 112:E5461–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]