Abstract
Exploitation of the immune system has emerged as an important therapeutic strategy for acute lymphoblastic leukemia (ALL). However, the mechanisms of immune evasion during leukemia progression remain poorly understood. We sought to understand the role of calcineurin in ALL and observed that depletion of calcineurin B (CnB) in leukemia cells dramatically prolongs survival in immune-competent but not immune-deficient recipients. Immune-competent recipients were protected from challenge with leukemia if they were first immunized with CnB-deficient leukemia, suggesting robust adaptive immunity. In the bone marrow, recipients of CnB-deficient leukemia harbored expanded T cell populations as compared to controls. Gene expression analyses of leukemia cells extracted from the bone marrow identified Cn-dependent significant changes in the expression of immunoregulatory genes. Increased secretion of IL-12 from CnB-deficient leukemia cells was sufficient to induce T cell activation ex vivo, an effect that was abolished when IL-12 was neutralized. Strikingly, recombinant IL-12 prolonged survival of mice challenged with highly aggressive B-ALL. Moreover, gene expression analyses from children with ALL showed that patients with higher expression of either IL-12A or IL-12B exhibited prolonged survival. These data suggest that leukemia cells are dependent upon calcineurin for immune evasion by restricting the regulation of pro-inflammatory genes, particularly IL-12.
Introduction
Despite dramatic improvements in survival for children with acute lymphoblastic leukemia (ALL) over the last few decades, there remain groups of patients for whom novel therapeutic strategies are urgently needed (1). Data implicating the graft vs. leukemia effect (2), and more recent clinical advances in cell-based and antibody therapy for B cell leukemias highlight the potent anti-leukemia activity of the immune system that can be exploited as a treatment option (3–6).
Hematological malignancies exhibit unique mechanisms of immune evasion that are not shared with solid tumors (7). Although antigen-specific T cells proliferate early in the tumor course in lymphoid and myeloid tumors (8,9), these cells are unable to trigger the effector functions necessary for tumor rejection (9), and in some cases differentiate into regulatory T cells (Tregs) (8). Several mechanisms have been observed that lead to the development of T cell tolerance. In a murine B cell lymphoma model in which the tumor antigen presentation was restricted to the host antigen presenting cells (APCs), T cells were unresponsive to antigen re-stimulation despite showing a phenotype consisting with antigen recognition (9). Furthermore, activation of the host APCs via CD40 (10) or by inducing the production of Type I interferon (11) improved both the levels of active cytotoxic T cells and mouse survival after myeloid leukemia challenge (10,11). In a murine B-ALL model, direct interaction between the tumor and CD4+ T cells led to the differentiation into Tregs, while abrogating this interaction by knocking down MHC-II in the leukemia cells led to an increase of Th1 CD4+ T cells (8) responsible for the promotion of cell-mediated immunity. However, the mechanisms by which pre-leukemia cells are eliminated by the immune system and how they eventually evade the immune surveillance during leukemia progression are incompletely understood.
Calcineurin is a serine/threonine phosphatase that plays a critical role in coupling Ca2+ signals to cellular responses (12). In all tissues except testes, its function is dependent upon the regulatory calcineurin B subunit encoded by Ppp3r1 (CnB). Although calcineurin’s function is best defined in T cells, it has also been studied for its role in oncogenesis and drug resistance in leukemia and lymphoma. For example, BCR-ABL1+ leukemia cells become dependent upon calcineurin when exposed to tyrosine kinase inhibition (13,14). In addition, calcineurin and one of its downstream substrates, NFAT, are activated in lymphoma and leukemia suggesting a role in pathogenesis (15,16). Furthermore, calcineurin was demonstrated to be essential in the development and maintenance of NOTCH and ETV6-JAK2 induced T cell ALL (15,16). The authors of the study concluded that calcineurin is required for leukemia stem cell function in T ALL (15). They and others have recently demonstrated a key role for CXCR4, downstream of calcineurin, in leukemia cell homing and engraftment (17).
While investigating the role of leukemia-cell calcineurin in resistance to tyrosine kinase inhibition (13,14), we made the striking observation that calcineurin-deficient leukemia cells engraft and progress in immune-competent recipients, but later regress to below the level of detection, resulting in long-term survival. This is in stark contrast to the Cn-expressing leukemia which rapidly and uniformly progresses to fatal disease within 14–21 days. Due to its role in the regulation of immunomodulatory cytokines in B cells and T cells (18,19), we reasoned that calcineurin in leukemia cells might influence the development of leukemia by altering the bone marrow immune microenvironment, thereby evading immune-mediated elimination. Indeed, transplantation of calcineurin-deficient leukemia into immune-compromised recipients eliminated spontaneous remissions, indicating that leukemia-cell calcineurin is critical for immune evasion. Examination of the bone marrow during leukemogenesis revealed differences in immune cell subsets, with recipients of calcineurin-deficient leukemia having more T cells than recipients of the control, calcineurin-expressing leukemia. Analysis of gene expression indicates that calcineurin-deficient leukemia cells express higher levels of pro-inflammatory genes. Mechanistically, calcineurin-deficient leukemia cells secrete more cytokines and chemokines than the control leukemia, including IL-12, a potent T cell activator. Neutralization of calcineurin-dependent secretion of IL-12 abrogated leukemia-cell T cell activation ex vivo, and treatment of mice with leukemia with recombinant IL-12 significantly prolonged survival. Gene expression analyses in children with B cell ALL (B-ALL) demonstrates that higher expression of the IL-12 subunits is associated with improved survival. To our knowledge, this is the first report to suggest that leukemia cells are dependent upon calcineurin specifically for evasion of immunity, due to restricted expression of pro-inflammatory genes and the secretion of IL-12.
Methods
Mice
C57BL/6, Tcrα−/− (B6.129S2-Tcratm1Mom/J) and Rag1−/− (B6.129S7-Rag1tm1Mom/J) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Aged C57BL/6 mice (20 month C57BL/6 mice) were provided by the National Institute on Aging (Bethesda, MD). Mice were housed in micro-isolators in standard conditions in the Center for Comparative Medicine at the University of Colorado School of Medicine or the Division of Animal Resources Facility in the Health Sciences Research Building at Emory University. All animal studies were approved by the Emory University or University of Colorado Institutional Animal Care and Use Committee.
Leukemia Model
The luciferase expressing, BCR-ABL1+ Arf−/− B-cell acute lymphoblastic leukemia line was originally provided by Dr. Richard Williams (20–23). Leukemia cells were transduced with lentiviruses expressing non-silencing control shRNA (shNS) or shRNA against Ppp3r1, which encodes the essential regulatory subunit of calcineurin (shCnB), with over 90% knockdown as previously described (14). A total of 5×105 cells were transferred via tail vein injection into un-irradiated, 6–8 week-old, female wild type (WT) or immune compromised recipients. After intraperitoneal injection of luciferin and anesthesia with inhaled isoflurane, leukemia burden was measured by the In Vivo Imaging System (IVIS) manufactured by Perkin Elmer (Waltham, MA). Mice were removed from the study and euthanized when ill-appearing or the luciferase signal exceeded 108 photons/second, whichever came first. Cyclosporine was administered via oral gavage at 25mg/kg/dose daily, as previously described (13). Anti-CD8 (clone 2.43) and anti-CD4 (clone GK1.5) were purchased from Bio X Cell (West Lebanon, NH). Recombinant murine IL-12-p70 was purchased from Peprotech (Rocky Hill, NJ).
Ex vivo leukemia cell culture
Leukemia cells were cultured in RPMI medium + 10% FBS + 1% penicillin/streptomycin + 0.1% 2-ME in a 37°C incubator. Cells were plated at 0.5–2 × 105 cells/ml and split every 48–72 hours. For the cytokine array experiments, leukemia cells were plated at 2×105 cells/ml and cultured for 48hrs before collection of supernatant. Cells were counted at 48 hrs to ensure similar numbers and viability of cells at the time of supernatant harvest. A general protein quantification assay with Bradford reagents was used to allow adjustment of supernatant volumes for equal overall protein concentration. The Proteome Array Mouse XL Cytokine Array Kit (R&D Systems, Minneapolis, MN) was used according to manufacturer’s instructions. Arrays were quantified as signal over background using ImageJ , with normalization to reference dots.
Flow Cytometry
Bone marrow was harvested from mice seven days after leukemia transplantation and stained with CD3e-APC, CD4-PE-Cy7, CD8b-PE, B220-eFluor 450, and Fixable Viability Dye eFluor450 (eBioscience, San Diego, CA). Samples were analyzed on a Gallios561 flow cytometer. Leukemia cells cultured ex vivo were stained for MHCI-FITC, MHCII-FITC, PD-L2 FITC, CD40-FITC, CTLA-4-PE, CD80 PECy-7, CD86-FITC, CXCR4-Alexa Fluor 488 (eBioscience) or PD-L1 PE-Cy7 (Life Technologies, Frederick, MD) and analyzed on a Guava Easy Cyte Plus. FlowJo software (TreeStar) was used for data analysis.
Quantitative gene expression analysis
Control and shCnB leukemia cells were transduced with MSCV expressing green fluorescence protein (GFP). Mouse bone marrow was harvested seven days after transplantation and sorted for GFP+ leukemia cells. RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) and transcriptome sequencing (RNA-seq) was performed on an Illumina HiSEQ 2000 (Illumina, San Diego, CA) using single 50bp reads by the University of Colorado Genomics and Microarray Core Laboratory. Transcript sequences were mapped to the mouse genome (GRCm38) using Bowtie (24) and normalized with the Reads per Kilobase per Million (RPKM) algorithm (25). Significant differences in expression were determined after correction for False Discovery Rate (26). DAVID and Gene Set Enrichment Analysis were used to identify pathways with significantly different gene expression (27,28). RNA-seq data are deposited with GEO (GSE130272). Publicly available mRNA-Seq data from the TARGET Phase II ALL project (https://ocg.cancer.gov/programs/target; accessed June, 2017) was normalized (29) and queried for gene expression analysis. The tumor samples were divided into high- and low- expression groups using the median for each gene as a cut-off. Clinical outcome analyses were performed using CASAS (30).
Cytokine ELISAs
Parental, control, and shCnB leukemia cells were cultured in vitro at a cell density of 1×105 or 5×105 cells/well and supernatants were collected after 24 hours. The concentration of IL-12p40 in the supernatants was determined using the Mouse IL-12 (p40) ELISA Set (BD Biosciences; cat. no. 555165) per the manufacturer’s instructions. Absorbance at 450 nm was recorded using the SPECTRA Max Plus 384 microplate reader (Molecular Devices). IL-1β and CXCL5 ELISA kits were performed according to the manufacturers’ recommendations (RayBiotech and R&D Systems, respectively).
T cell Activation Assays
U-bottom plates (Corning) were coated with αCD3 (10 ug/mL; eBioscience), sealed with parafilm, and kept overnight at 4o. Plates were washed twice with cold 1X PBS to remove unbound antibodies using a multichannel pipette. Splenocytes were harvested from WT C57BL/6 mice and plated at 5×104 cells/well after red blood cell lysis with ACK lysing buffer (ThermoFisher Scientific). Plated cells were then stimulated with αCD28 (2 ug/mL; eBioscience) prior to adding fresh 10% RPMI media (control), conditioned media from shNS leukemia cells (generated from 1×105 or 5×105 leukemia cells), or conditioned media from shCN leukemia cells (generated from 1×105 or 5×105 leukemia cells). Control IgG (10 μg/mL; ThermoFisher Scientific) or αIL-12p40 neutralizing antibodies (10 μg/mL; ThermoFisher Scientific) were added to the appropriate wells. On day 3 of culture, cells were harvested and stained with αCD4-PE-Cy7 (Biolegend) and αCD8 eFluor 450 (ThermoFisher Scientific) on ice for 1 hour. After staining for surface antigens, intracellular cytokine staining for IFN-γ (αIFN-γ-APC; Biolegend) and TNF-α (αTNF-α-FITC; BD Biosciences) was performed as previously described (31,32) using the BD Optima Cytofix/Cytoperm Kit with Golgi Plug (BD Biosciences). Flow cytometric analysis was performed on the LSR II (BD Biosciences) to delineate IFN-γ and TNF-α producing CD4+ and CD8+ T-cells. FlowJo software (TreeStar) was used for data analysis.
Statistics
Except for gene expression analyses, statistical analyses were performed using GraphPad Prism software. Statistical significance between 2 groups was determined by Student’s t test, while Analysis of Variance (ANOVA) with Tukey’s multiple comparison test was used to test significance between 3 or more groups. Error bars in figures represent the standard deviation and may be obscured when narrow. Animal experiments included at least 3 mice/group, and all mice are included in survival analyses. To minimize animal use, in vivo experiments were repeated only once, unless results were inconclusive, in which case the experiment was repeated a third time. The Mantel-Cox (log-rank) test was used to test for significant differences in survival.
Results
Depletion of leukemia-cell calcineurin impairs leukemia progression in immune-competent mice
To study the function of leukemia-cell calcineurin in B cell ALL, we knocked down calcineurin B (CnB), the essential subunit of calcineurin in an aggressive, luciferase-expressing mouse model of B cell ALL (14). This well-characterized model recapitulates the genetics and behavior of human BCR-ABL1+ ALL, and has been demonstrated to be immunogenic (20–23). Driven by BCR-ABL1 in an Arf−/− background, the leukemia homes quickly to the bone marrow in un-irradiated, immune competent recipients, and disseminates diffusely thereafter (Supplemental Figure 1A). We found that the luciferase signal correlates highly with the percentage of leukemia cells in the BM (R2=0.97, P<0.001; Supplemental Figure 1B), making luciferase activity a reliable measure of leukemia burden. As compared to control leukemia cells expressing a non-silencing shRNA sequence (shNS), knockdown of CnB (shCnB) had very little effect on the proliferation rate, background levels of apoptosis, or sensitivity to DNA damage induced apoptosis (Supplemental Figure 1C,D,E), suggesting no major cell-autonomous effects of calcineurin deficiency in BCR-ABL1+ ALL cells.
We first tested the effect of CnB knockdown in leukemia cells injected into un-irradiated, wild-type B6 recipients. IVIS imaging 7 days after transfer indicated engraftment of leukemia in recipients of both shNS and shCnB leukemia, although significantly less in recipients of shCnB leukemia (Figure 1A and Supplemental Figure 1F). Furthermore, by day 10, while all but one recipient of shNS leukemia continued to have rapid disease progression, the shCnB recipients had low or no disease burden, as measured by luciferase activity (Fig. 1A,B,C; Supplemental Figure 1F). All recipients of shNS leukemia necessitated euthanasia by day 17 due to leukemia progression. In contrast, recipients of shCnB leukemia remained well appearing. A cohort of these shCnB recipients was observed long-term, during which leukemia burden remained below background signal level for weeks, and demonstrated significantly prolonged survival (Figure 1C; Supplemental Figure 1G). Importantly, in this experiment, all of the shCnB recipients eventually showed evidence of leukemia progression and were euthanized due to high leukemia burden, indicating that leukemia cells had engrafted, but remained dormant for weeks in each of the recipients. Together, these data indicate that the BCR-ABL1+ leukemia cells are dependent upon CnB for progression in immune competent mice.
Figure 1. Leukemia-cell calcineurin is critical for leukemia progression in immune-competent mice.
Luciferase expressing BCR-ABL1+/Arf−/− leukemia cells (B-ALL) were transduced with either control shRNA (shNS) or shRNA against Ppp3r1, which encodes CnB (shCnB). Un-irradiated recipient mice were injected with 5×105 leukemia cells. A. Representative IVIS imaging of WT recipients at days 7 and 10. B. Quantification of change in luciferase signal between days seven and ten (P<0.0001, unpaired T test). C. Kaplan-Meier curve of overall survival of mice in C (n=7/group from a single experiment).
Leukemia cells are dependent upon CnB to evade immune-mediated suppression
We next tested the hypothesis that calcineurin is required for immune evasion during leukemia progression. Control and shCnB leukemia cells were injected into un-irradiated WT and immune compromised Rag1−/− (lacking mature T and B cells) or Tcra−/− (lacking mature T cells) recipients, which were monitored for leukemia progression over time. The CnB-deficient leukemia progressed in genetically immune compromised recipients, albeit variably and somewhat slower than the rates of control leukemia in WT or immune compromised recipients (Figure 2A,B; Supplemental Figure 2A,B). As before, the WT recipients of shCnB again had progression, then regression of leukemia burden, and prolonged survival, including some recipients that never demonstrated relapse. Similar results were found using a second single-cell clonal population of both the shCnB and shNS leukemias (Supplemental Figure 2C). Importantly, we also observed calcineurin-dependent immune evasion in a distinct model of murine AML, indicating that this phenomenon is not limited to murine B ALL (Figure 2C). From a cohort of long-term survivors of calcineurin-deficient ALL, BCR-ABL1 transcript was not detected by real-time, reverse transcriptase PCR in the bone marrow (Supplemental Figure 2D). Although leukemia isolated from Tcra−/− mice at time of sacrifice still showed knockdown of calcineurin, most WT recipients of shCnB leukemia that later relapsed had levels of CnB in leukemia cells at time of relapse similar to that of the shNS control leukemia (Figure 2D, Supplemental Figure 2E), representing either re-expression of CnB or outgrowth of a minor population of cells in which CnB was not efficiently knocked down. This emergence of CnB expressing cells at the time of relapse suggests that CnB is essential for leukemia progression in immune-competent recipients.
Figure 2. Leukemia-cell calcineurin is essential for immune evasion during progression of lymphoid and myeloid leukemia.
A. Luciferase signal over time (left) and overall survival (right) in WT or Rag1−/− recipients of shNS or shCnB B-ALL (n=6/group from 2 independent experiments). B. Luciferase signal over time (left) and overall survival (right) in WT or Tcra−/− recipients of shNS or shCnB B-ALL (n=9/group from 2 independent experiments). C. c1498 acute myeloid leukemia cells were transduced with shNS or shCnB and injected into un-irradiated WT or Tcra−/− recipients. Overall survival is demonstrated. (n=15 WT and n=13 Tcra−/− recipients for each group from 3 independent experiments.) D. Western blot for CnB from B-ALL cells cultured in vitro or recovered from recipient mice at the time of euthanasia due to leukemia progression.
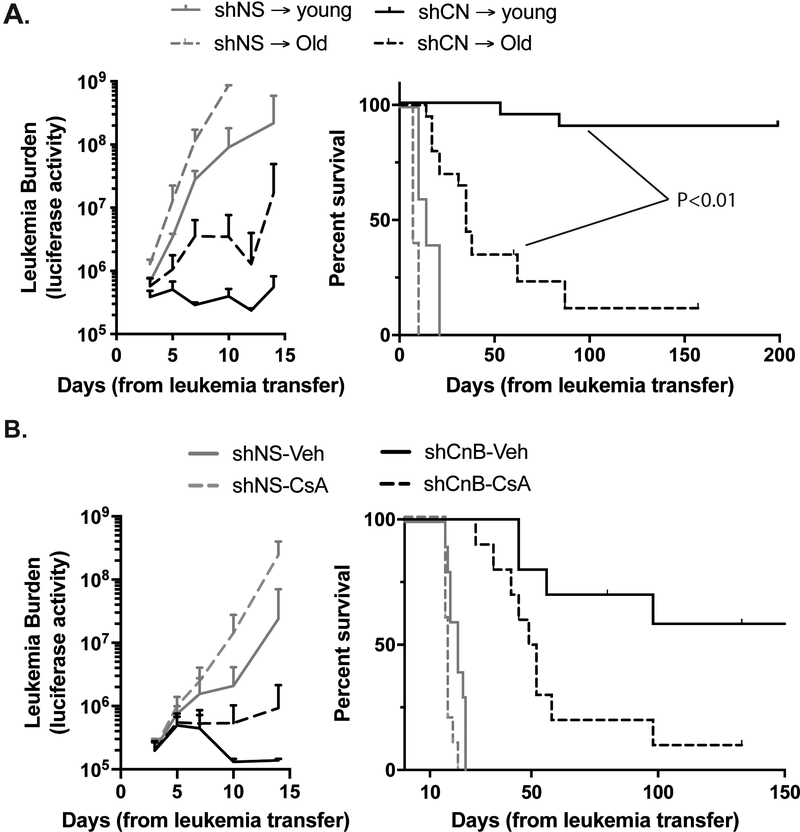
To model a more physiologic state of immune dysfunction, we transferred leukemia into aged mice (20 months old), which have dysfunction of most components of the immune system (33) and compared leukemia progression and survival to young recipients (2 months old). Aged recipients of calcineurin deficient leukemia demonstrated slower leukemia progression and had prolonged survival compared to aged or young recipients of control leukemia (Figure 3A). Some of the recipients even seemed to enter remission, with leukemia burden below the level of detection after 7–10 days (Supplemental Figure 2F). However, almost all of the aged recipients of calcineurin-deficient leukemia eventually relapsed, and their survival was significantly shorter than young recipients of the same leukemia (Figure 3A, Supplemental Figure 2F).
Figure 3. Physiologic and pharmacologic immune deficiency accelerates leukemia progression.
Un-irradiated recipients were injected with 2×105 luciferase expressing shNS or shCnB B ALL cells A. Luciferase signal over time (left) and overall survival (right) in young or old WT recipients of shNS (n=5/group) or shCnB (n=20/group, from 2 independent experiments) B-ALL. B. Recipient mice were treated with 25mg/kg CsA or vehicle control (n=10/group from 2 independent experiments).
In this model, calcineurin-deficiency is limited to the leukemia cells expressing the shRNA. However, systemic inhibition of calcineurin, such as with cyclosporine A (CsA), a calcineurin inhibitor used clinically to prevent organ rejection, suppresses T cell function. To determine whether pharmacologic calcineurin inhibition would slow shNS leukemia or enable calcineurin-deficient leukemia to progress, we treated wild-type recipients of shNS or shCnB leukemia with. Despite immune suppression, many recipients of calcineurin-deficient leukemia had some early decrease in leukemia burden, similar to vehicle treated recipients of calcineurin-deficient leukemia, albeit slower and more variable (Figure 3B, Supplemental Figure 2G). However, CsA treated recipients had earlier and more frequent relapses, leading to significantly shorter survival, as compared to vehicle treated recipients (Figure 3B, Supplemental Figure 2G). Importantly, systemic pharmacologic inhibition of calcineurin with CsA did not impair the progress of the control leukemia (shNS) and may have even accelerated its progression, suggesting that an intact immune system slows, but cannot fully control leukemia progression in this model. Taken together, these data indicate that the immune system plays a critical role in suppressing the development of leukemia, and suggest that leukemia-cell calcineurin plays a key role in evading the immune system during leukemia progression.
CnB-deficient leukemia cells elicit an adaptive immune response
The progression of calcineurin-deficient leukemia in Tcra−/− recipients led us to hypothesize that T cells play an essential role in the calcineurin-dependent leukemia suppression. To further explore the role of T cells during leukemogenesis, WT mice were injected with either GFP expressing control or calcineurin-deficient leukemia, and bone marrow was harvested seven days later. A group of mice without leukemia was included as controls. Bone marrow from each of the three groups of mice was stained for hematopoietic lineage markers and analyzed via flow cytometry (Supplemental Figure 3A). Consistent with data from measurement of leukemia burden by IVIS imaging, despite injection of the same number of leukemia cells, the percentage of leukemia cells in the bone marrow after 7 days was significantly less in recipients of shCnB leukemia (Supplemental Figure 3B). In addition, the percentage of CD3+ T cells was higher in the bone marrow of shCnB recipients compared to both the shNS recipients and the non-leukemic mice (Figure 4A). CD8+ subsets of T cells were significantly higher in recipients of shCnB leukemia compared to shNS recipients, in which CD8+ T cells were nearly absent in the bone marrow (Figure 4A, Supplemental Figure 3A). The ratio of CD8/CD4 cells was significantly higher in recipients of shCnB leukemia compared to shNS, and increased ratios highly correlated with lower leukemia burden in vivo (Figure 4A, Supplemental Figure 3C).
Figure 4. Depletion of leukemia-cell calcineurin elicits an adaptive immune response to leukemia cells.
A. Bone marrow was harvested and analyzed by flow cytometry 7 days after injection of GFP+ B ALL cells into un-irradiated WT recipient mice as in Figure 1. Data are representative of 2 independent experiments with 5 mice/group. (ANOVA with Tukey’s multiple comparison test). B. WT mice were injected with neutralizing antibodies directed against CD4 and/or CD8 followed by injection of shCnB ALL. (n=6/group from 2 independent experiments) C. WT mice were injected with shCnB B ALL or PBS 14 days prior to challenge with a second injection of PBS, shNS or shCnB leukemia. (n=6–8/group from 2 independent experiments.)
To determine if either CD4+ or CD8+ T cell subsets are sufficient for surveillance and suppression of calcineurin deficient leukemia cells, we used monoclonal antibodies to selectively deplete these populations in WT recipients prior to challenging them with leukemia. Consistent, with a critical role of both CD4+ and CD8+ cells in adaptive immune responses, depletion of either population resulted in progression of calcineurin-deficient leukemia at rates similar to depletion of both and similar to the rate in Tcra−/− recipients (Figure 4B).
In order to confirm that calcineurin-dependent, long-term disease control was due to contributions from the adaptive immune system, mice were first “immunized” by injection of either vehicle (PBS) or shCnB cells; fourteen days later, 18 of the 20 shCnB recipients had signal levels below background, and were challenged with shNS or shCnB leukemia. Mice initially given PBS had regression of the shCnB leukemia but not the shNS leukemia (Supplemental Figure 3D), as seen in previous experiments. The 2 mice that still had detectable disease at the time of second injection (PBS or shCnB) had relatively early disease progression, as compared to those in which leukemia was undetectable (Supplemental Figure 3D). In contrast, mice that had experienced remission of the shCnB leukemia had regression of a second transplantation of shCnB leukemia or a challenge with shNS leukemia, suggesting that protective T cell immunity was established after immunization with shCnB leukemia. Although relapses occurred more quickly in the shCnB-shNS recipients, they still experienced a significant increase in survival time (Figure 4C). Together, these data suggest that adaptive immune memory plays a critical role in long-term repression of leukemia.
Leukemia-cell CnB regulates the expression and secretion of inflammatory cytokines
We next sought to determine the molecular mechanisms of leukemia-cell calcineurin mediated immune evasion by first examining the expression of immunomodulatory molecules on the surface of the leukemia cells. Flow cytometry was used to examine the cell surface expression of MHC-I, MHC-II, PD-L1, PD-L2, CD80, CD40, and CD86 in vitro (Supplemental Fig. 4A), all of which demonstrated similar mean fluorescent intensities on shNS and shCnB leukemia cells. As amino acids and eicosanoids are potent regulators of T cells (34,35), several of each were quantified using tandem mass spectrometry, and no difference was found in arginine, its metabolites urea and ornithine, or PGE2 (Supplemental Figure 4B).
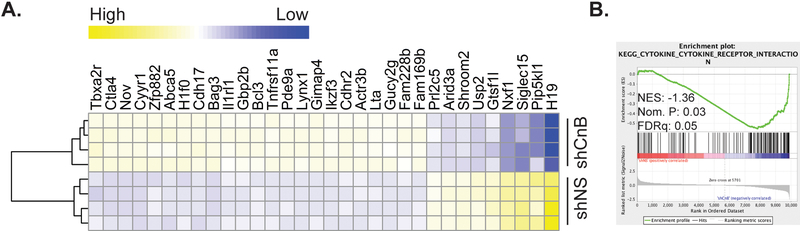
To evaluate the effect of CnB knockdown on global gene expression patterns in leukemia cells, we performed transcriptome sequencing (RNA-Seq) on leukemia cells isolated from the bone marrow of mice transplanted with either shNS or shCnB B-ALL cells. Bone marrow was harvested seven days after transplant, and GFP+ leukemia cells were recovered via FACS. Unexpectedly, the pattern of gene expression changes in shCnB cells suggested that calcineurin plays an important role in restricting, rather than promoting the expression of genes in leukemia cells, as more genes were expressed at higher levels than lower levels in shCnB cells compared to shNS. For example, at a false discovery threshold of p<0.1, 385 genes were significantly expressed higher in shCnB cells, whereas 213 were expressed at significantly lower levels (Supplemental Table 1). A subset of the most significantly differentially expressed genes is represented in Figure 5A. Pathways analysis using DAVID revealed that the KEGG Pathway “cytokine-cytokine receptor interaction” was the most over-represented among the differentially expressed genes (Supplemental Table 2). Analysis of the entire data set using Gene Set Enrichment Analysis (GSEA) confirms that genes in the cytokine-cytokine receptor interaction gene set are differentially expressed in shCnB leukemia cells compared to shNS controls (Figure 5B). Taken together, these data implicate a novel immune evasion program mediated by leukemia-cell calcineurin in which the expression of pro-inflammatory genes is restricted.
Figure 5. Leukemia-cell calcineurin regulates an immuno-modulatory gene expression program.
A. GFP+ leukemia cells were sorted from the bone marrow of recipients of shNS or shCnB leukemia 7 days after injection, and subject to transcriptome sequencing. A subset of the most significantly differentially-expressed genes is depicted (Fold change >2 with false discovery rate<5%). B. Gene set enrichment analysis (GSEA) of the transcriptome using the KEGG cytokine-cytokine receptor interaction gene-set demonstrates significant upregulation of genes in this set in shCnB B-ALL cells.
IL-12 is secreted from CnB deficient leukemia cells, activates T cells ex vivo, and promotes immune clearance of leukemia in vivo.
To determine whether differential expression of immune signaling genes is associated with enhanced secretion of cytokines and/or chemokines from the leukemia cells, we performed a cytokine/chemokine antibody array. We found higher levels of most cytokines, chemokines and other molecules in the supernatant of shCnB leukemia cells as compared to shNS (Figure 6A and Supplemental Figure 5A). To validate these results, we performed ELISA for a subset of these molecules, including IL-12p40, IL-1β and CXCL5 and confirmed that there is significantly more IL-12 secreted from shCnB leukemia cells (Figure 6B). IL-1β and CXCL5 were below the level of detection of the ELISA assays. We next tested whether secreted factors, and specifically IL-12, from shCnB leukemia cells could stimulate the proliferation and/or activation of T cells more than those from shNS, implicating a mechanism for enhanced immune response and survival in vivo. Consistent with higher levels of pro-inflammatory cytokines in the supernatant of shCnB B-ALL cells, we saw enhanced T cell activation with shCnB cell supernatant as compared to that from shNS cells, as measured by intracellular IFN-γ and TNF-α production. Importantly, the addition of an IL-12 neutralizing antibody to supernatant from shCnB B-ALL cells abrogated the activation of CD4+ and CD8+ T cells ex vivo (Figure 6C, D, E, F and Supplemental Figure 5B).
Figure 6. Calcineurin-deficient leukemia cells secrete IL-12 and activate T-cells.
A. Supernatant from shNS and shCnB leukemia cells cultured ex vivo was analyzed by cytokine array. Secreted cytokines and chemokines are quantified as the relative fold-change of shCnB over shNS. (Graphs represent the mean +/− the SEM of 2 independent experiments.) B. ELISA was used to measure IL12-p40 levels in the supernatant of parental, shNS and shCnB leukemia cells cultured ex vivo. (n=3 independent experiments; *P<0.05, **P<0.01, ****P<0.0001, ANOVA with Tukey’s multiple comparisons test.) C, D, E, F. Murine splenocytes were stimulated ex vivo with CD3/CD28 antibodies and cultured with supernatant from shNS or shCnB leukemia cells with or without anti-IL12 neutralizing antibodies and analyzed by flow cytometry. C, D. Representative dot-plots of flow cytometry for intracellular TNF-α and IFN-γ in CD8+ (C) and CD4+ (D) cells. E. The percentage of double positive CD8+ cells. F. The percentage of double positive CD4+ cells. (n=3 experiments; *P<0.05, ***P<0.001, ANOVA with Tukey’s multiple comparisons test).
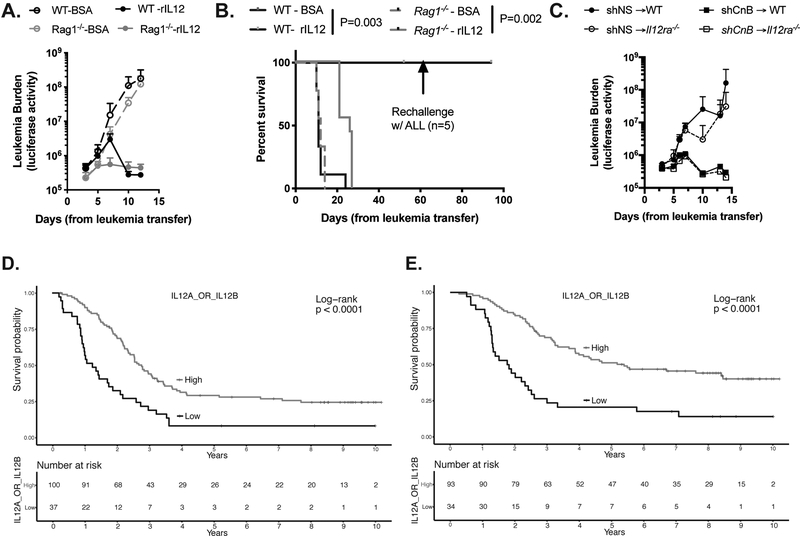
To determine whether higher levels of IL-12 is sufficient to slow the progression of B ALL in vivo, we treated immune-competent and immune-deficient recipients of parental luciferase-expressing BCR-ABL1+ B ALL cells with recombinant IL-12 (rIL-12). Wild-type mice treated with rIL-12 demonstrated a reduction in leukemia burden after 4 days of treatment, and entered a durable remission with prolonged survival and no evidence of disease 3 weeks after completion of treatment (Figure 7A, B). Immune competent recipients of BCR-ABL1+ B ALL cells and rIL-12 were re-challenged with leukemia cells 60 days after the first injection, and all survived another 30 days without evidence of disease (Figure 7B). In addition, Rag1−/− mice treated with rIL-12 also demonstrated a reduction in disease burden after treatment initiation and prolonged survival, but eventually relapsed after treatment cessation (Figure 7A, B). To address the possibility that IL-12 secreted from the leukemia microenvironment may contribute to the clearance of shCnB leukemia cells, we used Il12a−/− mice as recipients of shNS and shCnB leukemia. Il12a−/− mice do not produce IL-12 p35 and therefore have no endogenous IL-12 . The Il12a−/− recipients of shCnB leukemia had regression of leukemia similar to WT recipients (Figure 7C), indicating that micro-environment derived IL-12 is not responsible for the immune response to Cn-deficient leukemia.
Figure 7. Recombinant IL-12 prolongs survival of both immune competent and immune deficient mice with leukemia and its gene expression is correlated with survival in children with B ALL.
A, B. Parental BCR-ABL1+/Arf−/− leukemia cells were injected into un-irradiated WT or Rag1−/− recipients. On day 3, treatment with either rIL12 or BSA was begun (1μg/dose, intraperitoneal, on days 3–7, 10–14 and 17; n=9/group from 2 independent experiments). Leukemia burden (A) and survival (B) were measured over time. C. shNS and shCnB leukemia cells were injected into un-irradiated WT or Il12a−/− recipients. Leukemia burden was measured over time. (D) Event-free and (E) overall survival among children with high-risk B-ALL by high v. low expression of either of the IL-12 gene subunits (IL12A or IL12B).
To begin to address the clinical relevance of expression of IL-12 in leukemia cells we queried publicly available data from the TARGET ALL Study. We observed that higher expression of either one of the subunits that comprise IL-12 (IL12A or IL12B) is significantly correlated with longer event free (EFS) and overall survival (OS) in children with ALL (Figure 7D, E). Taken together, these data indicate that CnB-deficient leukemia cells secrete pro-inflammatory molecules, and IL-12 in particular, which induces the activation of CD4+ and CD8+ T cells to promote an effective immune response against leukemia, which may have some clinical relevance in children with ALL.
Discussion
Leukemia remains a leading cause of death in children, but harnessing the potency of the immune system is emerging as a tractable strategy for improving outcomes. Herein, we have uncovered a calcineurin-dependent mechanism of immune evasion in models of ALL and AML. ALL cells deficient in calcineurin are able to proliferate in immune competent mice for several days, but are then repressed to the point of being undetectable thereafter, and in about 50% of recipients, never relapse. Systemic inhibition of calcineurin with cyclosporine inhibits T cell function, and negated any benefit of pharmacologic calcineurin inhibition in leukemia cells. Our subsequent findings demonstrate at least one mechanism of calcineurin-dependent immune evasion, as calcineurin-deficient leukemia cells have higher expression of pro-inflammatory genes. Calcineurin-deficient leukemia cells secrete higher levels of IL-12, which is responsible for differential activation of T-cells ex vivo driving robust IFN-γ production consistent with a Th1 response. Consistent with its previously demonstrated role in anti-tumor immunity, treatment with rIL-12 promoted long-term survival in immune competent mice with ALL.
While not complete, there is emerging understanding of the mechanisms of immune evasion during leukemogenesis (7), not all of which are shared with solid tumors. To our knowledge, though, this is the first report of an intracellular signaling molecule in leukemia cells with such a profound role in immune evasion during leukemia progression. Our results are analogous to the finding that melanoma-intrinsic β-catenin signaling similarly promotes immune evasion (36). In that model, melanoma with active β-catenin fails to recruit T cells to the tumor microenvironment, at least in part due to impaired expression of CCL4. Similarly, in our model, leukemia cells with active calcineurin fail to elicit an adaptive immune response, at least in part due to impaired secretion of IL-12. These findings highlight an understudied aspect of immune evasion, the repression of danger signals that would otherwise elicit an effective immune response.
Calcineurin has been demonstrated to play a critical role in lymphoid malignancies. It is active in primary B cell and T cell lymphomas, as well as mouse models of T cell leukemia (16). Furthermore, calcineurin-dependent CXCR4 expression is required for leukemia-initiating cell activity in T cell leukemia (15,17). In contrast, calcineurin has been reported to be dispensable in B cell ALL (37). A major distinction between that model and ours, though, is their use of irradiation of recipient mice, thereby effectively suppressing any immune response to the transplanted leukemia cells. Therefore, no conclusions can be made regarding the contributions of leukemia-cell calcineurin to immune evasion from their studies. Our findings are strengthened by the use of two different models of both lymphoblastic and myeloid leukemia (BCR-ABL1+/Arf−/− and c1498, respectively) that can be injected into un-manipulated recipients, allowing the study of fully intact immunity in response to leukemia. However, the survival advantage in WT recipients of Cn-deficient myeloid leukemia, as compared to immune deficient recipients, is not nearly as dramatic as that in recipients of lymphoid leukemia, suggesting that the extent to which hematologic malignancies rely on calcineurin for immune evasion may not be generalizable across all subtypes.
Pharmacologic inhibition of calcineurin is used clinically for immune suppression after organ transplantation due to T cell dependence on its phosphatase activity for activation . Systemic immune suppression with cyclosporine in our model is sufficient to abrogate the effects of calcineurin inhibition within the leukemia cell. Thus, in order to eventually exploit our observations therapeutically, it was critical to identify downstream mediators of calcineurin-dependent immune evasion. In T cells and neural glial cells, Cn/NFAT signaling can have both pro- and anti-inflammatory effects, depending on the cell type and binding partners of NFAT (38–41). Nfatc1−/− B cells less effectively stimulate T cells ex vivo, at least in part due to enhanced IL-10 production (42). Furthermore, NFATc1 has been very recently found to upregulate IL-10 in diffuse large B-cell lymphoma (43). However, in contrast to our expectations, and despite near eradication of CD8+ T cells in the bone marrow of recipients of Cn-expressing leukemia, we did not uncover a clear mechanism of immune suppression in leukemia cells with active calcineurin. Rather, our studies implicated IL-12 as a downstream mediator that promotes an effective immune response to calcineurin-deficient leukemia cells. IL-12 is produced by monocytes, dendritic cells, and other antigen presenting cells, promotes the secretion of IFN-γ and activation of NK and T cells (31,32), and has long been recognized to promote immune responses to cancers, including hematologic malignancies (44–46).
Our data support the development of strategies to promote the differentiation and activation of T cells. IL-12 functions as a third signal, along with antigenic and co-stimulatory signals to promote activation of naïve CD8+ T cells (47). In addition, IL-12 enhances the differentiation of effector and memory T cells, at least in part through regulation of T-box transcription factors T-bet and Eomes (48,49). In addition, IL-12 is responsible for the differentiation of CD4+ T cells into Th1 cells (50). We were able to confirm this effect as CD4+ T cells exposed to the factors secreted by the CnB-deficient leukemia cells differentiated into IFN-γ and TNF-α secreting cells, a phenotype consistent with Th1 cells. This effect was abrogated by IL-12 blocking antibodies. Our findings are consistent with the data from other groups that have shown that Th1 cells are critical for the control of B-ALL, as the differentiation of antigen-specific leukemia CD4+ T cells into Th1 cells correlates with increased survival of a leukemia challenge (8). Furthermore, in preclinical models of breast cancer, IL-12 mediated CD4+ T cells Th1 differentiation correlated with higher infiltration of CD8+ T cells into tumor tissues and better cytolytic tumor response (51). The effects of IL-12 go beyond CD4+ T cells, as we also saw the induction of effector CD8+ T cells by calcineurin deficient leukemia cells, as evidenced by increased production of IFN-γ and TNF-α induced by the shCnB leukemia. Our data are similar to observations by other groups in models of melanoma and breast cancer, in which IL-12 was required for the generation of IFN-γ producing effector CD8+ T cells and anti-tumoral responses (52,53). Moreover, in the B-16 melanoma model it was observed that IL-12 mediated the downregulation of PD-1 (52), which is consistent with the finding that the IL-12 expression from dendritic cells derived from AML blasts not only activates T cells but is able to revert T cells from unresponsive to activated (54). More broadly, these data are a reminder that strategies to stimulate immune responses may be considered as alternative or complementary to strategies that dis-inhibit immune responses, such as checkpoint inhibition.
Notably, we also observed prolonged survival in rIL-12 treated Rag1−/− mice with leukemia, suggesting the importance of components of the innate immune system in immune surveillance during leukemia progression. We hypothesize that NK cells are involved in the protection seen in Rag1−/− mice as IL-12 enhances the cytotoxicity of NK cells (55). This hypothesis is supported by murine models that have shown that dendritic cell production of IL-12 is necessary to control metastasis in which the main effector cells are NK cells (56), and that exogenous IL-12 is able to rescue the cytotoxicity of NK cells previously rendered anergic by the tumor (57). A complete characterization of the role of NK cells in calcineurin-dependent immune evasion is ongoing.
Our finding that there is higher expression of pro-inflammatory genes in calcineurin-deficient leukemia and the fact that the expression of IL-12 gene subunits is correlated with survival in children with B-ALL strongly suggests that our findings have clinical relevance. Cytokine and cytokine receptor expression seems to be a crucial component in the immune responses against ALL, as higher expression of IL-15Rα by lymphoblasts was associated with better survival in children with relapsed ALL (58). Furthermore, a “T cell inflamed microenvironment” has been identified in melanoma and is associated with better responses to immunomodulatory therapy with checkpoint inhibition (59). Whether the higher expression of the pro-inflammatory genes in ALL can be translated into a biomarker of protective inflammation and treatment outcome remains to be determined.
The clinical efficacy of IL-12 at tolerated doses has generally been limited, with the exceptions of cutaneous T cell lymphoma, mycosis fungoides and non-Hodgkin lymphoma (55,60–62). Systemic administration is associated with toxicity, precluding dose escalation, and prompting the study of local delivery of IL-12 via gene therapy for solid tumors and leukemia (63,64), including in an ongoing clinical trial (). Other methods of targeted delivery (i.e. oncolytic adenovirus and nanoparticles) have been tested in preclinical models demonstrating high anti-tumor efficacy with significantly lower systemic reactions (51,65), and in light of the dramatic success of CAR-T cell therapy for ALL (3–5), it may be reasonable to explore strategies for local delivery of IL-12 to activate endogenous NK or T cells or to enhance the activity of CAR-T cells.
In conclusion, we have found that leukemia-cell calcineurin prevents an effective immune response in immune-competent mice during leukemogenesis. Conversely, calcineurin depletion in leukemia cells induces changes in gene expression and cytokine secretion, particularly IL-12 that promote an effective immune response. These data suggest that modulating the leukemia microenvironment beyond checkpoint inhibition may be an effective strategy to further improve leukemia therapy.
Supplementary Material
Statement of Significance.
This report implicates calcineurin as an intracellular signaling molecule responsible for immune evasion during leukemia progression and raises the prospect of re-examining IL-12 as a therapeutic in leukemia.
Acknowledgments
This work was supported by the Cancer League of Colorado (CCP & JES), the National Cancer Institute through Cancer Center Support Grants to the University of Colorado (CA046934) and Emory Winship Cancer Institute (CA138292), the University of Colorado Predoctoral Training Program in Molecular Biology (GM008730) and the Aflac Cancer & Blood Disorders Center of Children’s Healthcare of Atlanta.
Footnotes
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
References
- 1.Hunger SP, Raetz EA, Loh ML, and Mullighan CG (2011) Improving outcomes for high-risk ALL: translating new discoveries into clinical care. Pediatr Blood Cancer 56, 984–993 [DOI] [PubMed] [Google Scholar]
- 2.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringden O, Rozman C, Speck B, and et al. (1990) Graft-versus-leukemia reactions after bone marrow transplantation. Blood 75, 555–562 [PubMed] [Google Scholar]
- 3.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, and June CH (2013) Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368, 1509–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DC, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, and Brentjens R (2014) Efficacy and Toxicity Management of 19–28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci Transl Med 6, 224ra225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, Shalabi H, Fountaine TJ, Shern JF, Majzner RG, Stroncek DF, Sabatino M, Feng Y, Dimitrov DS, Zhang L, Nguyen S, Qin H, Dropulic B, Lee DW, and Mackall CL (2018) CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 24, 20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klinger M, Brandl C, Zugmaier G, Hijazi Y, Bargou RC, Topp MS, Gokbuget N, Neumann S, Goebeler M, Viardot A, Stelljes M, Bruggemann M, Hoelzer D, Degenhard E, Nagorsen D, Baeuerle PA, Wolf A, and Kufer P (2012) Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood 119, 6226–6233 [DOI] [PubMed] [Google Scholar]
- 7.Curran EK, Godfrey J, and Kline J (2017) Mechanisms of Immune Tolerance in Leukemia and Lymphoma. Trends Immunol 38, 513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manlove LS, Berquam-Vrieze KE, Pauken KE, Williams RT, Jenkins MK, and Farrar MA (2015) Adaptive Immunity to Leukemia Is Inhibited by Cross-Reactive Induced Regulatory T Cells. J Immunol 195, 4028–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sotomayor EM, Borrello I, Rattis FM, Cuenca AG, Abrams J, Staveley-O’Carroll K, and Levitsky HI (2001) Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood 98, 1070–1077 [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Chen X, Liu X, Kline DE, Teague RM, Gajewski TF, and Kline J (2013) CD40 ligation reverses T cell tolerance in acute myeloid leukemia. J Clin Invest 123, 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curran E, Chen X, Corrales L, Kline DE, Dubensky TW Jr., Duttagupta P, Kortylewski M, and Kline J (2016) STING Pathway Activation Stimulates Potent Immunity against Acute Myeloid Leukemia. Cell Rep 15, 2357–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klee CB, Ren H, and Wang X (1998) Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem 273, 13367–13370 [DOI] [PubMed] [Google Scholar]
- 13.Gregory MA, Phang TL, Neviani P, Alvarez-Calderon F, Eide CA, O’Hare T, Zaberezhnyy V, Williams RT, Druker BJ, Perrotti D, and Degregori J (2010) Wnt/Ca2+/NFAT signaling maintains survival of Ph+ leukemia cells upon inhibition of Bcr-Abl. Cancer Cell 18, 74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner LA, Klawitter J, Gregory MA, Zaberezhnyy V, Baturin D, Pollyea DA, Takebe N, Christians U, Gore L, DeGregori J, and Porter CC (2014) Inhibition of calcineurin combined with dasatinib has direct and indirect anti-leukemia effects against BCR-ABL1(+) leukemia. Am J Hematol 89, 896–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gachet S, Genesca E, Passaro D, Irigoyen M, Alcalde H, Clemenson C, Poglio S, Pflumio F, Janin A, Lasgi C, Dodier S, Soyer M, Dumenil G, and Ghysdael J (2013) Leukemia-initiating cell activity requires calcineurin in T-cell acute lymphoblastic leukemia. Leukemia 27, 2289–2300 [DOI] [PubMed] [Google Scholar]
- 16.Medyouf H, Alcalde H, Berthier C, Guillemin MC, dos Santos NR, Janin A, Decaudin D, de The H, and Ghysdael J (2007) Targeting calcineurin activation as a therapeutic strategy for T-cell acute lymphoblastic leukemia. Nat Med 13, 736–741 [DOI] [PubMed] [Google Scholar]
- 17.Passaro D, Irigoyen M, Catherinet C, Gachet S, Da Costa De Jesus C, Lasgi C, Tran Quang C and Ghysdael J (2015) CXCR4 Is Required for Leukemia-Initiating Cell Activity in T Cell Acute Lymphoblastic Leukemia. Cancer Cell 27, 769–779 [DOI] [PubMed] [Google Scholar]
- 18.Winslow MM, Gallo EM, Neilson JR, and Crabtree GR (2006) The calcineurin phosphatase complex modulates immunogenic B cell responses. Immunity 24, 141–152 [DOI] [PubMed] [Google Scholar]
- 19.Zhang BW, Zimmer G, Chen J, Ladd D, Li E, Alt FW, Wiederrecht G, Cryan J, O’Neill EA, Seidman CE, Abbas AK, and Seidman JG (1996) T cell responses in calcineurin A alpha-deficient mice. J Exp Med 183, 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulos N, Mulder HL, Calabrese CR, Morrison JB, Rehg JE, Relling MV, Sherr CJ, and Williams RT (2011) Chemotherapeutic agents circumvent emergence of dasatinib-resistant BCR-ABL kinase mutations in a precise mouse model of Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 117, 3585–3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams RT, den Besten W, and Sherr CJ (2007) Cytokine-dependent imatinib resistance in mouse BCR-ABL+, Arf-null lymphoblastic leukemia. Genes Dev 21, 2283–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams RT, Roussel MF, and Sherr CJ (2006) Arf gene loss enhances oncogenicity and limits imatinib response in mouse models of Bcr-Abl-induced acute lymphoblastic leukemia. Proc Natl Acad Sci U S A 103, 6688–6693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manlove LS, Berquam-Vrieze KE, Pauken KE, Williams RT, Jenkins MK, and Farrar MA (2015) Adaptive Immunity to Leukemia Is Inhibited by Cross-Reactive Induced Regulatory T Cells. J Immunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langmead B, Trapnell C, Pop M, and Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortazavi A, Williams BA, McCue K, Schaeffer L, and Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5, 621–628 [DOI] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y (1995) Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J. R. Statist. Soc. B 57, 289–300 [Google Scholar]
- 27.Huang DW, Sherman BT, and Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4, 44–57 [DOI] [PubMed] [Google Scholar]
- 28.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, and Mesirov JP (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102, 15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anders S, and Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11, R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rupji M, Zhang X, and Kowalski J (2017) CASAS: Cancer Survival Analysis Suite, a web based application. F1000Res 6, 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry CJ, Ornelles DA, Mitchell LM, Brzoza-Lewis KL, and Hiltbold EM (2008) IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J Immunol 181, 8576–8584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry CJ, Grayson JM, Brzoza-Lewis KL, Mitchell LM, Westcott MM, Cook AS, and Hiltbold EM (2010) The roles of IL-12 and IL-23 in CD8+ T cell-mediated immunity against Listeria monocytogenes: Insights from a DC vaccination model. Cell Immunol 264, 23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henry CJ, Casas-Selves M, Kim J, Zaberezhnyy V, Aghili L, Daniel AE, Jimenez L, Azam T, McNamee EN, Clambey ET, Klawitter J, Serkova NJ, Tan AC, Dinarello CA, and DeGregori J (2015) Aging-associated inflammation promotes selection for adaptive oncogenic events in B cell progenitors. J Clin Invest 125, 4666–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bronte V, and Zanovello P (2005) Regulation of immune responses by L-arginine metabolism. Nature reviews. Immunology 5, 641–654 [DOI] [PubMed] [Google Scholar]
- 35.Kalinski P (2012) Regulation of immune responses by prostaglandin E2. J Immunol 188, 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spranger S, Bao R, and Gajewski TF (2015) Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 [DOI] [PubMed] [Google Scholar]
- 37.Rocchetti F, Tran Quang C, Maragno AL, Nguyen J, Lasgi C, and Ghysdael J (2017) The calcineurin protein phosphatase is dispensable for BCR-ABL-induced B-ALL maintenance, propagation and response to dasatinib. Leukemia 31, 248–251 [DOI] [PubMed] [Google Scholar]
- 38.Furman JL, and Norris CM (2014) Calcineurin and glial signaling: neuroinflammation and beyond. Journal of neuroinflammation 11, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Im SH, and Rao A (2004) Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol Cells 18, 1–9 [PubMed] [Google Scholar]
- 40.Yaseen NR, Maizel AL, Wang F, and Sharma S (1993) Comparative analysis of NFAT (nuclear factor of activated T cells) complex in human T and B lymphocytes. J Biol Chem 268, 14285–14293 [PubMed] [Google Scholar]
- 41.Tsai EY, Yie J, Thanos D, and Goldfeld AE (1996) Cell-type-specific regulation of the human tumor necrosis factor alpha gene in B cells and T cells by NFATp and ATF-2/JUN. Mol Cell Biol 16, 5232–5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharyya S, Deb J, Patra AK, Thuy Pham DA, Chen W, Vaeth M, Berberich-Siebelt F, Klein-Hessling S, Lamperti ED, Reifenberg K, Jellusova J, Schweizer A, Nitschke L, Leich E, Rosenwald A, Brunner C, Engelmann S, Bommhardt U, Avots A, Muller MR, Kondo E, and Serfling E (2011) NFATc1 affects mouse splenic B cell function by controlling the calcineurin--NFAT signaling network. J Exp Med 208, 823–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Zhang J, Chen J, Xu-Monette ZY, Miao Y, Xiao M, Young KH, Wang S, Medeiros LJ, Wang M, Ford RJ, and Pham LV (2018) B-cell receptor-mediated NFATc1 activation induces IL-10/STAT3/PD-L1 signaling in diffuse large B-cell lymphoma. Blood 132, 1805–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimura T, Watanabe K, Lee U, Yahata T, Ando K, Kimura M, Hiroyama Y, Kobayashi M, Herrmann SH, and Habu S (1995) Systemic in vivo antitumor activity of interleukin-12 against both transplantable and primary tumor. Immunol Lett 48, 149–152 [DOI] [PubMed] [Google Scholar]
- 45.Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, Gately MK, Wolf SF, Schreiber RD, Storkus WJ, and et al. (1994) Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol 153, 1697–1706 [PubMed] [Google Scholar]
- 46.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, and Gately MK (1993) Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med 178, 1223–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, and Mescher MF (1999) Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol 162, 3256–3262 [PubMed] [Google Scholar]
- 48.Chang J, Cho JH, Lee SW, Choi SY, Ha SJ, and Sung YC (2004) IL-12 priming during in vitro antigenic stimulation changes properties of CD8 T cells and increases generation of effector and memory cells. J Immunol 172, 2818–2826 [DOI] [PubMed] [Google Scholar]
- 49.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, and Reiner SL (2006) Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol 177, 7515–7519 [DOI] [PubMed] [Google Scholar]
- 50.Trinchieri G (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature reviews. Immunology 3, 133–146 [DOI] [PubMed] [Google Scholar]
- 51.Oh E, Choi IK, Hong J, and Yun CO (2017) Oncolytic adenovirus coexpressing interleukin-12 and decorin overcomes Treg-mediated immunosuppression inducing potent antitumor effects in a weakly immunogenic tumor model. Oncotarget 8, 4730–4746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin P, Liu X, Mansfield AS, Harrington SM, Li Y, Yan Y, and Dong H (2016) CpG-induced antitumor immunity requires IL-12 in expansion of effector cells and down-regulation of PD-1. Oncotarget 7, 70223–70231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang SX, Wei WS, Ouyan QW, Jiang QH, Zou YF, Qu W, Tu JH, Zhou ZB, Ding HL, Xie CW, Lei QM, and Zhong CR (2016) Interleukin-12 activated CD8(+) T cells induces apoptosis in breast cancer cells and reduces tumor growth. Biomed Pharmacother 84, 1466–1471 [DOI] [PubMed] [Google Scholar]
- 54.Curti A, Pandolfi S, Aluigi M, Isidori A, Alessandrini I, Chiodoni C, Testoni N, Colombo MP, Baccarani M, and Lemoli RM (2005) Interleukin-12 production by leukemia-derived dendritic cells counteracts the inhibitory effect of leukemic microenvironment on T cells. Exp Hematol 33, 1521–1530 [DOI] [PubMed] [Google Scholar]
- 55.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, and Anichini A (2007) Interleukin-12: biological properties and clinical application. Clin Cancer Res 13, 4677–4685 [DOI] [PubMed] [Google Scholar]
- 56.Mittal D, Vijayan D, Putz EM, Aguilera AR, Markey KA, Straube J, Kazakoff S, Nutt SL, Takeda K, Hill GR, Waddell N, and Smyth MJ (2017) Interleukin-12 from CD103(+) Batf3-Dependent Dendritic Cells Required for NK-Cell Suppression of Metastasis. Cancer Immunol Res 5, 1098–1108 [DOI] [PubMed] [Google Scholar]
- 57.Ohs I, Ducimetiere L, Marinho J, Kulig P, Becher B, and Tugues S (2017) Restoration of Natural Killer Cell Antimetastatic Activity by IL12 and Checkpoint Blockade. Cancer Res 77, 7059–7071 [DOI] [PubMed] [Google Scholar]
- 58.Wu S, Gessner R, von Stackelberg A, Kirchner R, Henze G, and Seeger K (2005) Cytokine/cytokine receptor gene expression in childhood acute lymphoblastic leukemia: correlation of expression and clinical outcome at first disease recurrence. Cancer 103, 1054–1063 [DOI] [PubMed] [Google Scholar]
- 59.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, and Ribas A (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rook AH, Wood GS, Yoo EK, Elenitsas R, Kao DM, Sherman ML, Witmer WK, Rockwell KA, Shane RB, Lessin SR, and Vonderheid EC (1999) Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses. Blood 94, 902–908 [PubMed] [Google Scholar]
- 61.Duvic M, Sherman ML, Wood GS, Kuzel TM, Olsen E, Foss F, Laliberte RJ, Ryan JL, Zonno K, and Rook AH (2006) A phase II open-label study of recombinant human interleukin-12 in patients with stage IA, IB, or IIA mycosis fungoides. J Am Acad Dermatol 55, 807–813 [DOI] [PubMed] [Google Scholar]
- 62.Younes A, Pro B, Robertson MJ, Flinn IW, Romaguera JE, Hagemeister F, Dang NH, Fiumara P, Loyer EM, Cabanillas FF, McLaughlin PW, Rodriguez MA, and Samaniego F (2004) Phase II clinical trial of interleukin-12 in patients with relapsed and refractory non-Hodgkin’s lymphoma and Hodgkin’s disease. Clin Cancer Res 10, 5432–5438 [DOI] [PubMed] [Google Scholar]
- 63.Pegram HJ, Purdon TJ, van Leeuwen DG, Curran KJ, Giralt SA, Barker JN, and Brentjens RJ (2015) IL-12-secreting CD19-targeted cord blood-derived T cells for the immunotherapy of B-cell acute lymphoblastic leukemia. Leukemia 29, 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang J, Liu Y, Au BC, Barber DL, Arruda A, Schambach A, Rothe M, Minden MD, Paige CJ, and Medin JA (2016) Preclinical validation: LV/IL-12 transduction of patient leukemia cells for immunotherapy of AML. Mol Ther Methods Clin Dev 3, 16074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu X, Gao X, Zheng S, Wang B, Li Y, Zhao C, Muftuoglu Y, Chen S, Li Y, Yao H, Sun H, Mao Q, You C, Guo G, and Wei Y (2017) Modified nanoparticle mediated IL-12 immunogene therapy for colon cancer. Nanomedicine 13, 1993–2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.