Abstract
The transcription factor c-MYC (MYC thereafter) controls diverse transcription programs and plays a key role in the development of many human cancers. Cells develop multiple mechanisms to ensure that MYC levels and activity are precisely controlled in normal physiological context. As a short half-lived protein, MYC protein levels are tightly regulated by the ubiquitin proteasome system. Over a dozen of ubiquitin ligases have been found to ubiquitinate MYC whereas a number of deubiquitinating enzymes counteract this process. Recent studies show that SUMOylation and deSUMOylation can also regulate MYC protein stability and activity. Interestingly, evidence suggests an intriguing crosstalk between MYC ubiquitination and SUMOylation. Deregulation of the MYC ubiquitination-SUMOylation regulatory network may contribute to tumorigenesis. This review is intended to provide the current understanding of the complex regulation of the MYC biology by dynamic ubiquitination and SUMOylation and their crosstalk.
Keywords: deSUMOylating enzymes, Deubiquitinating enzymes, MYC, SUMO, SUMOylation, Ubiquitination
Introduction
The MYC oncoprotein regulates the expression of a large body of genes implicated in diverse cellular processes, including cell cycle progression, nucleotide biosynthesis, metabolism, RNA processing, ribosomal biogenesis/protein translation as well as apoptosis, cell senescence and differentiation.1, 2, 3 It functions as a pleiotropic transcription factor in association with its primary partner MAX4 through a C-terminal basic HLH/Leu-zipper domain (bHLH-LZ).5, 6 MYC/MAX heterodimers activate many target genes by binding to canonical E-box (CACGTG) elements within gene promoters and enhancers.6, 7, 8 The N-terminal first 143 amino acids of MYC constitute the transactivation domain (TAD) that is essential for both transcriptional activation and repression5, 9, 10, 11 and contains the highly conserved MYC box I (MBI) and MBII. Two important phosphorylation sites, Threonine 58 (T58) and Serine 62 (S62) lie in MBI, involved in regulation of MYC stability and activity in response to cell growth signals.1, 2, 12, 13 The central region of MYC contains the MBIII and MBIV which are both important for MYC's pro-apoptotic activity as well as for transcriptional repression and activation respectively.14, 15, 16
While MYC is essential for normal cell growth and proliferation, deregulated MYC overexpression and activation contribute to the development of most human cancers. Thus, it is not surprising that MYC levels and activity are under tight scrutiny and regulation during normal cell homeostasis.2, 17 Besides transcriptional regulation,18, 19, 20 there are various post-translational modifications of MYC that regulate its stability and activity, including phosphorylation, acetylation, glycosylation, proline isomerization, methylation, ubiquitination and SUMOylation. In this review, we will focus on the regulation of MYC stability and activity by dynamic ubiquitination and SUMOylation and their potential crosstalk.
MYC ubiquitination regulates MYC stability and activity
As one of the most prevalent protein posttranslational modifiers, ubiquitin is attached to substrates by a three-step enzymatic process, consisting of the ubiquitin-activating enzyme (E1), the ubiquitin-conjugating enzyme (E2) and the ubiquitin ligase (E3).21 The human genome encodes two E1 enzymes (UBE1 and UBA6), approximately 40 E2s, and more than 600 E3s that are classified into three groups based on their general mode of catalysis. The HECT (homologous to E6-AP carboxy terminus) domain containing E3s have an identifiable catalytic site, whereas the RING (really interesting new gene) domain-containing E3s are thought to mediate Ub transfer by positioning E2s in close proximity to targets. The RBR (RING-IBR-RING) E3s' action is still unclear, but likely similar to the RING E3s. RING proteins, and E3s in general, are regulated by a number of mechanisms that modulate either their activity or interactions.22, 23 Ub is first activated by ATP-dependent formation of a thioester bond between the active-Cys residue of the E1 and the C-terminus di-glycine motif of Ub. The activated Ub is then transferred to the active-Cys residue of one of the E2s. Finally, the E3 ubiquitin ligase mediates the isopeptide bond formation between the lysine residue of the substrate and C-terminal carboxyl group of ubiquitin by interacting with ubiquitin-charged E2 and a specific substrate. Proteins can be modified predominately by Lys48 linked ubiquitin chains for proteasome degradation, or by a single ubiquitin or a polyubiquitin chain with other or mixed linkages (Lys63, Lys6, Lys11, Lys27, Lys29, Lys33 or Met1), serving as signals for multifaceted regulatory functions.24 In general, the E2s determine the type of ubiquitin chain assembled while the E3s contribute to the efficiency and substrate specificity of the ubiquitination reaction.21, 25
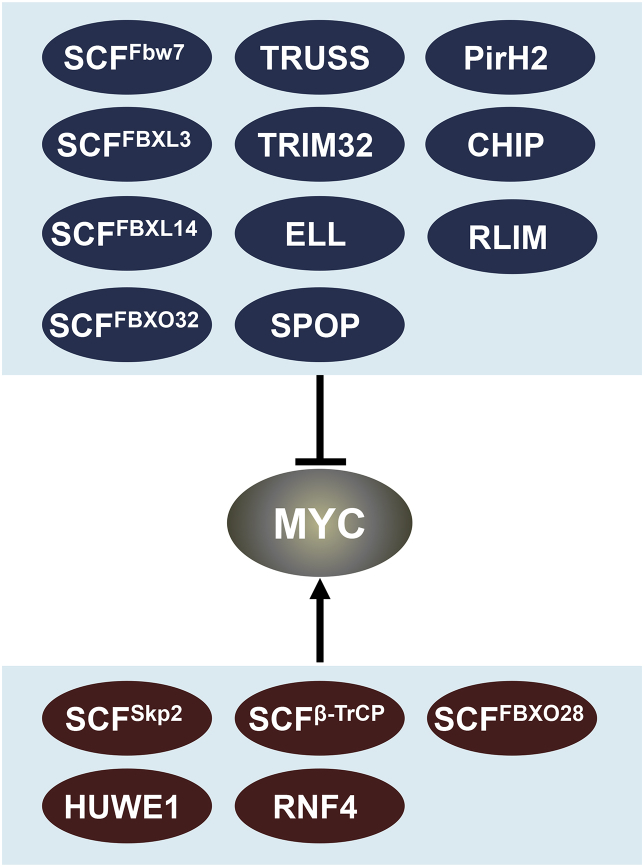
MYC is an unstable protein with a half-life of less than 30 min in non-transformed cells13, 26, 27 due to rapid turnover through the ubiquitin-proteasome system (UPS). Intriguingly, ubiquitination has been found to regulate MYC activity without affecting its protein levels or by promoting its turnover. To date, at least 16 ubiquitin E3s have been identified to ubiquitinate MYC, most of which were reported as negative regulators targeting MYC for degradation while the rest of them positively regulate MYC activity (Fig. 1).
Figure 1.
MYC ubiquitin ligases (E3s). Shown are the ubiquitin E3s reported to mediate MYC ubiquitination. The arrow indicates the activation of MYC activity whereas the bar indicates the inhibition of MYC activity.
SCFFbw7
SCFFbw7 is the best characterized ubiquitin E3 for MYC. The core components of SCF complex include an adaptor protein Skp1, a scaffold protein Cul1 and a ring-finger protein Rbx1/Roc1. Fbw7 is a F-box protein which is the variable component of the complex that determines specificity for substrates.28 There are three Fbw7 isoforms that differ in subcellular localization: Fbw7α (nucleoplasm), Fbw7β (cytoplasm) and Fbw7γ (nucleolus).28, 29 Both Fbw7α and Fbw7γ have been shown to target MYC for degradation. Fbw7 mediated MYC turnover is dependent on sequential MYC phosphorylation at S62 and T58. Following growth signals, MYC is stabilized upon S62 phosphorylation mediated by Ras-Raf-MEK-ERK kinase cascade and/or CDKs.13, 30, 31 S62 phosphorylation is a prerequisite for subsequent phosphorylation at T58 by GSK3β, which is inhibited by PI(3)K/Akt signaling, but activated when Ras signaling is down.10, 30, 31, 32, 33 Phosphorylation of T58 facilitates Pin1-mediated isomerization at proline 63 of MYC, allowing dephosphorylation of MYC at S62 by PP2A.34 Consequently, p-T58-MYC is recognized by SCFFbw7 for K48-linked ubiquitination and degradation through the proteasome system.29, 35, 36, 37 Consistently, as one of the “hot spots” for mutations in a subset of Burkitt's lymphomas, T58 mutation causes stabilization and enhanced activity of MYC.10, 38, 39, 40 MYCT58A transgenic mice show higher penetrance and reduced latency in cancer development compared with MYCWT transgenic mice.41, 42 Moreover, mutations and deletions of FBW7 have been identified in multiple human cancers, consistent with its role in negatively regulating MYC.28
SCFSkp2
Skp2 (S-phase Kinase-associated protein2), also known as FBXL1, has been well characterized as an oncoprotein.43, 44 As the substrate-binding subunit of the SCFSkp2 ubiquitin ligase complex, Skp2 was shown to bind to the MBII and bHLH-LZ domains of MYC and can mediate its ubiquitination and degradation during the G1/S transition of the cell cycle.45, 46, 47 Since both MYC and Skp2 are oncoproteins critical for G1/S transition, the finding that Skp2 targets MYC for degradation seems to be contradictory, but has given rise to the concept of a transcription-coupled proteasomal degradation mechanism for transcription: Skp2 mediates MYC ubiquitination at the site of its activity (chromatin), first promoting its activity and subsequently its degradation. Indeed, Skp2 increases transcriptional activity of MYC and promotes MYC-induced S phase entry.45, 47 In support of this transcription-coupled proteasomal degradation of MYC, we found that PIN1 also promotes the turnover of MYC at target gene promoters while increasing its transactivation activity.48, 49 ARF and SNIP1 were shown to compete with Skp2 to regulate MYC.50, 51 ARF inhibits Skp2-mediated ubiquitination and degradation, resulting in the switch of MYC from a canonical oncogenic protein towards an inducer of apoptosis,51 whereas SNIP1 stabilizes MYC by competing for binding with Skp2 and possibly switches MYC into another mode of transcription activation through recruitment of p300.50 In addition, Skp2 induces cell cycle genes and enhances the proliferative response of human β-cells in a MYC-dependent manner, suggesting MYC as a central Skp2 target for the induction of cell cycle entry, expansion and regeneration of human T2D β-cells.52 However, by using the Skp2-LRR mutant unable to form a SCF complex, several studies argued that Skp2 promotes MYC activity independently of MYC ubiquitination and SCFSkp2 E3 ligase activity. One report showed that Skp2 recruits p300 and Miz1 to the MYC transcriptional complex to promote RhoA transcription, cell migration and invasion in an E3 independent manner.53 Another group showed that the Skp2-LLR mutant restores MYC activity downregulated by hepatocyte growth factor (HGF), suggesting that Skp2 functions as a transcriptional activator of MYC rather than a component of the SCF complex in HGF signaling in HepG2 cells.54 Together, these studies clearly reveal a critical crosstalk between Skp2 and MYC in regulating cell cycle progression and cell transformation involving protein-protein interaction and protein turnover that impact on the MYC transcriptional output.55
SCFFBOX32
FBXO32, also known as Atrogin-1, was originally identified as the muscle-specific gene for muscle atrophy.56, 57 Recent evidence suggests that it has a role in regulating tumorigenesis, where it is downregulated.58, 59 SCFFBXO32 targets MYC for proteasome degradation by promoting MYC ubiquitination at K326 via K48-linked ubiquitin chains independently of T58 or S62 phosphorylation. FBXO32 interacts with MYC at MBII, MBIV and a PEST (rich in proline, glutamic acid, serine and threonine) domain. Functionally, FBXO32 suppresses MYC transactivation activity and inhibits cancer cell proliferation. Interestingly, FBXO32 is found to be a MYC target gene, thereby forming a negative regulatory loop with MYC.60
SCFFBXL3
An unexpected finding shows that the circadian repressor CRY2 recruits T58-phosphorylated MYC to the SCFFBXL3 ubiquitin E3 ligase complex for MYC ubiquitination and degradation.61 In fact, The SCFFBXL3 has been identified to regulate the clock by promoting the degradation of circadian repressor CRYs.62, 63 In addition to being a substrate of SCFFBXL3, CRY2, but not CRY1, acts as a cofactor associating with phospho-T58 (p-T58) MYC near its binding interface with FBXL3. Evidence shows that CRY2-FBXL3-mediated MYC turnover is independent of the FBW7 pathway. Overexpression of WT CRY2, but not the F428D mutant incapable of binding to FBXL3, reduced MYC levels. Importantly, significant low expression of CRY2 was observed in tumor samples from a variety of types of cancers compared to normal controls. Also, CRY2 deletion enhances MYC-driven Lymphoma in vivo. These findings suggest a critical CRY2-FBXL3-MYC pathway, which could explain how clock output affects cancer susceptibility due to circadian disruption.61
SCFFBXL14
FBXL14 is highly expressed in non-stem glioma cells and neural progenitors, but its expression is low in glioma stem cells (GSCs) that contribute to tumor initiation and malignant progression.64 Indeed, it has been shown that FBXL14 mediates MYC polyubiquitination for degradation, suggesting its role in controlling MYC at the posttranslational level in glioma cells, but not in GSCs. FBXL14 mediated ubiquitination of MYC appears to be T58 and S62dependent and the MYCT58A mutant shows less binding affinity to FBXL14. Consistently, overexpression of FBXL14 induces GSC differentiation and inhibits GSC tumor growth, which can be rescued by the ubiquitination-resistant MYCT58A mutant.64
SCFβ−TrCP
Emerging evidence has implicated that β-TrCP, also known as Fbxw1, plays an oncogenic role in human cancers.43 In line with this, SCFβ−TrCP has been found to antagonize SCFFbw7-mediated MYC degradation, leading to MYC stabilization that is required for MYC-dependent recovery from S phase arrest. β-TrCP binds to phosphorylated MYC at amino acids 278–283 that is highly similar to the phospodegron (DpSGXXpS) recognized by β-TrCP.65 Unlike Fbw7 which mediates K48-linked polyubiquitination by using Cdc34, β-TrCP requires UbcH5 to form K33/K63/K48 heterotypic polyubiquitin chains on MYC.65 Of note, ubiquitination by SCFβ−TrCP occurs preferentially in G2 phase, and Plk1, which is most active in G2 and M phases,66 phosphorylates MYC to enhance the binding of SCFβ−TrCP, suggesting a model in which phosphorylation of MYC by Plk1 recruits SCFβ−TrCP to stabilize MYC. Furthermore, the reduction of MYC levels is associated with the reduced levels of β-TrCP1 by PI3K/mTOR inhibitor treatment in triple-negative breast cancer cells (TNBCs).67
SCFFBOX28
As a less characterized F-box protein, FBXO28 was found to control MYC-dependent transcription by non-proteolytic ubiquitination that transmits CDK activity to MYC function during cell cycle progression.68 In S and G2/M phases, FBOX28 is phosphorylated at Ser 344 by CDK1/2. Although this phosphorylation is not required for FBOX28 binding to MYC, it positively affects SCFFBXO28 E3 activity. Further, SCFFBXO28 promotes ubiquitination on specific lysine residues within the 294–367 region of MYC, where the HUWE1-mediated MYC-p300 interaction occurs (see below).69 Indeed, SCFFBXO28 stimulates MYC activity by facilitating recruitment of p300 to target gene promoters. Consequently, FBXO28 promotes MYC-driven tumorigenesis and high expression of FBXO28 (and high Ser 344 phosphorylation) is associated with poor patient outcomes in human breast cancer.68 It would be interesting to further study how SCFFBXO28 interplays with other E3s during the cell cycle progression.
SCFSPOP
Recent studies highlight SPOP as an important regulator of cell proliferation and MYC expression in the prostate luminal epithelium.70, 71 SPOP mediates ubiquitination and degradation of MYC by directly binding to MYC, while this binding is attenuated by the prostate cancer-associated mutations, SPOPF102C and SPOPF133V. Consistently, analysis of transcriptomic signatures associated with mutant SPOP reveals the enrichment of MYC target genes.72, 73 These data suggest mutant SPOP- induced stabilization of MYC as another mechanism that can raise MYC levels in prostate cancer cells, in addition to MYC locus amplification, and support a role for SPOP's frequent inactivation in prostate cancer patients.70 Recently, LINC01638 lncRNA has been shown to stabilize MYC by blocking the binding between SPOP and MYC and thus inhibiting SPOP- induced MYC ubiquitination and degradation in TNBCs,71 indicating a new RNA-mediated mechanism utilized by cancer cells to stabilize MYC protein.
TRIM32
TRIM32 is a Ring-finger ubiquitin E3 ligase that targets MYC for degradation.74, 75 TRIM32WT, but not the RING finger mutant TRIM32C24A, can ubiquitinate and destabilize MYC. As a cell-fate determinant in neural stem cells, TRIM32 is required and sufficient for suppressing self-renewal and inducing neuronal differentiation partially by degrading MYC. Accordingly, it has been shown that TRIM32 is upregulated during neuronal differentiation of mouse neural stem cells, accompanied by subcellular translocation of TRIM32 from the cytoplasm of progenitor cells to the nucleus of mature neurons, which is in further agreement to its role in regulating MYC. In addition, TRIM32 has also been shown to ubiquitinate N-MYC and facilitate its degradation during mitosis, thereby inducing asymmetric cell division in human neuroblastoma cells.76
RLIM
RNF12/RLIM is a RING domain-containing E3 ubiquitin ligase that promotes MYC ubiquitination without affecting MYC levels, independently of S62 and T58 phosphorylation.77 However, RLIM attenuates MYC transcriptional activity towards at least some of its target genes such as E2F2 and nucleolin. MYC depletion abolished the effects of RLIM on cell growth, suggesting that RLIM may restrain cell proliferation by regulating MYC activity. It remains to be determined whether MYC is a direct substrate of RLIM and whether RLIM-mediated polyubiquitination directly suppresses MYC transactivation activity.
Pirh2
Pirh2 was initially identified as a p53 ubiquitin ligase that targets p53 for proteasomal degradation78 and was later shown to target CHK2 for ubiquitination and degradation.79 Pirh2 deletion mice consistently exhibit increased levels of p53 and increased p53-dependent apoptosis in response to DNA damage,80 indicating that Pirh2 possesses an oncogenic potential. However, subsequent studies showed that Pirh2 binds to MYC and mediates MYC polyubiquitination and proteasome degradation, suggesting that Pirh2 is a bona fide ubiquitin E3 ligase for MYC.80 Moreover, approximately 25% of Pirh2−/− and 17% of Pirh2+/− mice developed solid tumors including liver, testes, mammary and lung cancers and sarcomas. This is associated with increased levels of MYC in various tissues from the Pirh2−/− mice. Downregulation of Pirh2 is associated with poor outcome of a number of human cancers including breast, ovarian, and lung cancers.80 Thus, Pirh2 may promote cell proliferation, tumorigenesis and tumor progression in tissue and cell context-dependent manners. Indeed, Pirh2 promotes cell proliferation in the absence of p5381 and the lifespan of Pirh2−/−;p53−/− mice was significantly reduced compared to Pirh2−/− or p53−/− mice.80
DCXTRUSS
TRUSS (tumor necrosis factor receptor-associated ubiquitous scaffolding and signaling protein),82 also called TRPC4AP (transient receptor potential cation channel, subfamily C, member 4-associated protein),83 is the substrate-specific adaptor of a DCX (DDB1-CUL4-X-box) ubiquitin E3 ligase complex.84 TRUSS interacts with both MYC and N-MYC at their C-terminal bHLH-LZ domain, thus mediating DCX-dependent MYC ubiquitination and degradation.84 Moreover, TRUSS suppresses MYC transactivation and transformation activity, and was also shown to be downregulated in a number of cancers.84 Interestingly, TRUSS is primarily expressed in G1 phase and negatively correlated with the expression of S-phase specific Skp2 during cell cycle progression. TRUSS itself has been revealed as a substrate of SCFSkp2 for ubiquitination and degradation, implying the interplay between the ubiquitin E3s in regulating MYC stability and activity during cell cycle progression.85
HUWE1
HUWE1 (also known as ARF-BP1, HectH9, MULE), a member of the HECT-domain containing ubiquitin E3 ligases, has complex roles in regulating MYC function. It was originally reported that HUWE1 enhances MYC activity by catalyzing K63-linked polyubiquitin chains on MYC in a region containing 6 lysine residues near the nuclear localization signal (NLS).69 By using a MYC mutant with all the 6 lysines mutated to arginine, it was found that ubiquitination by HUWE1 enhances the transcriptional activity of MYC by recruiting the coactivator p300. Consistently, HUWE1 is overexpressed in multiple human tumors and is essential for proliferation of a subset of tumor cells.69 This oncogenic function of HUWE1 was further confirmed by a later study, showing that HUWE1 is required for growth of colorectal cancer cells both in cell culture and xenograft models.86 Consistent with other published data showing that HUWE1 destabilizes Miz1, a negative regulator of MYC,87, 88 inhibition of HUWE1 by small molecule inhibitors leads to Miz1's global accumulation on MYC target genes and contributes to the repression of MYC-activated target genes.86 However, an in vivo study shows that HUWE1 deletion accelerates skin carcinogenesis, suggesting a tumor suppressor role.87 Mechanistically, HUWE1 deficiency resulted in the accumulation of MYC/Miz1 complexes at promoters of p21 and p15 associated with decreased expression. In addition, inactivating HUWE1 mutations were found in human colorectal cancers (CRC) and deletion of HUWE1 in mouse CRC models leads to marked increase in tumor initiation that is partially associated with MYC stabilization,89 further supporting a tumor suppressor role of HUWE1. Thus, it will be interesting to examine whether HUWE1 can directly target MYC for degradation by mediating K48-linked polyubiquitination in these specific tissues. Further investigation of HUWE1 may shed light on the complexity of its E3 ubiquitin-mediated regulation of MYC.
CHIP
CHIP (carboxyl terminus of Hsc70-interacting protein) binds to Hsp70 and Hsp90 chaperones through its tetratricopeptide repeat (TPR) domain and functions as an E3 ubiquitin ligase containing a modified RING finger domain (U-box), allowing CHIP to network chaperone complexes to the ubiquitin-proteasome system.90, 91 It has been shown that CHIP mediates polyubiquitination of MYC for degradation and suppresses MYC activity.92 Indeed, CHIP interacts with MYC through its TPR domain, and the association between CHIP and MYC is dependent on the chaperone, particularly Hsp70. However, CHIP lacking the U-box domain was still able to promote MYC ubiquitination, indicating there may be E3 unrelated effects of CHIP on MYC ubiquitination possibly via involving other ubiquitin E3s. Further study needs to determine whether CHIP is a bona fide ubiquitin E3 for MYC and whether CHIP regulates MYC levels under physiological conditions.92
ELL
ELL (eleven–nineteen lysine-rich leukaemia), a key regulator of transcriptional elongation,93, 94 has been identified as a novel ubiquitin ligase, and MYC was found to be the very first substrate for ELL.95 ELL mediates MYC ubiquitination and degradation independent of MYC phosphorylation and UbcH8 was found to be the cognate ubiquitin-conjugating enzyme. Although no obvious conserved domains of typical ubiquitin ligases were found in ELL, Cys 595 was identified as an active cysteine required for its E3 activity. ELL-mediated MYC degradation inhibits MYC-dependent transcriptional activity and cell proliferation and suppresses MYC-dependent xenograft tumor growth.95 Consistently, ELL expression was decreased in human colon cancer specimens, negatively correlating with the expression of MYC, highlighting the tumor suppressor function of ELL that is at least partially associated with its role in downregulating MYC.95
RNF4
RNF4 is a well-characterized SUMO targeted ubiquitin E3 ligase (StubL) that recognizes SUMOylated substrates and mediates substrate ubiquitination and proteasome degradation.96, 97, 98 However, RNF4 was recently shown to bind and stabilize a group of substrates in a manner that depends on phosphorylation but not SUMOylation.99 RNF4 binds to MYC phosphorylated at Ser 62 and mediates its K11- and K33- linked ubiquitination that leads to MYC stabilization and activation. In line with its positive regulation of MYC, RNF4 is upregulated in multiple cancers correlating with poor patient outcomes.99 This finding presents a novel mechanism underlying the role for RNF4 in tumorigenesis wherein it promotes MYC activity via different types of ubiquitin linkages in specific cell contexts.
Erasing MYC ubiquitination by deubiquitinating enzymes
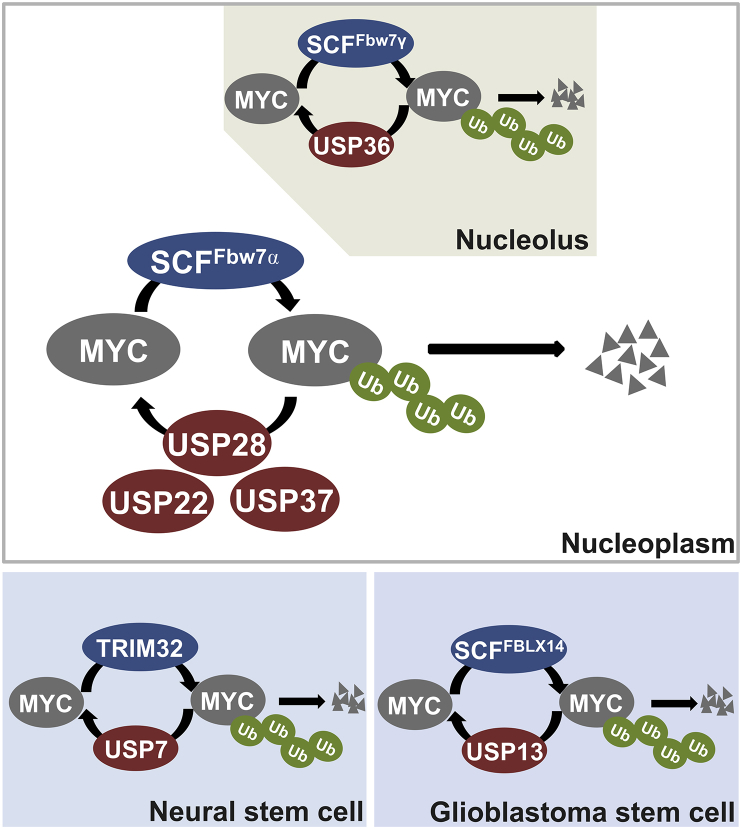
Like other posttranslational modifications, ubiquitination of MYC is dynamic and can be reversed by deubiquitinating enzymes (DUBs). Approximately 99 DUBs are encoded by the human genome that are classified into 7 families: ubiquitin C-terminal hydrolases (UCHs), ubiquitin specific protease (USPs), ovarian tumor associated proteases (OTUs), Machado-Josephin domain proteins (MJDs), JAB1/MPN/Mov34 metalloenzymes (JAMMs), motif interacting with Ub-containing novel DUB family (MINDY) and ZUP1.100, 101, 102 Emerging evidence reveals that deubiquitination plays an equally important role in regulating MYC protein stability and activity as ubiquitination. So far, six DUBs have been reported to act on MYC, including USP28, USP36, USP37, USP22, USP13 and USP7 (Fig. 2).
Figure 2.
MYC deubiquitinating enzymes. Shown are the DUBs known to deubiquitinate and stabilize MYC. Top: USP36 deubiquitinates MYC and counteracts Fbw7γ in the nucleolus whereas USP28 deubiquitinates MYC and counteracts Fbw7α in the nucleoplasm. USP22 and USP37 may also deubiquitinate MYC in the nucleoplasm. Bottom: the TRIM32-USP7 and FBLX14-USP13 axis regulate MYC stability and activity in neural stem cells and glioblastoma stem cells, respectively.
USP28
USP28 is the first DUB discovered to deubiquitinate and stabilize MYC.103 USP28 interacts with MYC via Fbw7α and reverses Fbw7α-mediated ubiquitination of MYC in the nucleoplasm. Consistently, USP28 overexpression was found in colon and breast cancers and USP28- mediated stabilization of MYC is essential for the cancer cell proliferation.103 Interestingly, a recent study showed that Usp28 deletion results in the accumulation of SCFFbw7 substrates, including NICD1, c-Jun, and MYC in both Fbw7-deficient mouse fibroblasts and intestinal tissues, suggesting that USP28 also functions independently of Fbw7. In fact, it was subsequently found that USP28 binds to MYC at the same motif recognized by Fbw7, but only when it is unphosphorylated, and promotes deubiquitination of MYC in the absence of Fbw7.104 Moreover, it has been reported that USP28 dissociates from Fbw7α in response to DNA damage, allowing MYC to be degraded by Fbw7α.105 Adding another layer of complexity in regulating MYC, USP28 was reported to control the stability of Fbw7 by antagonizing its auto-ubiquitination and degradation in specific tissues such as lung, pancreas and liver, leading to destabilization of Fbw7 targets and to dose-dependent effects in Usp28 knockout mice. Therefore, while heterozygousity can destabilize MYC, complete deletion of USP28 triggers Fbw7 auto-destruction, resulting in the stabilization of Fbw7 substrates including MYC in specific tissues.106 Thus, the USP28-Fbw7α interplay dictates MYC stability and activity in the nucleoplasm and involves fine stoichiometry of the two opposing enzymes.
USP36
We recently identified the nucleolar USP36 as a bono fide MYC DUB controlling MYC's nucleolar degradation pathway.107 USP36 directly interacts with and deubiquitinates MYC, leading to the stabilization of MYC. Notably, USP36 interacts with the nucleolar Fbw7γ but not the nucleoplasmic Fbw7α. Yet, it can abolish MYC degradation mediated both by Fbw7γ and by Fbw7α, suggesting that USP36 controls the end step of Fbw7-mediated MYC degradation in the nucleolus in concert with the role of USP28 in the nucleoplasm. Supporting this notion is that USP36 and USP28 cooperatively further increase MYC levels.108 Thus, it is likely that the Fbw7α-USP28 and Fbw7γ-USP36 axis dynamically control the life cycle of MYC in different cell compartments (Fig. 2). Interestingly, USP36 itself is a MYC target gene, suggesting that USP36 and MYC form a positive feedback regulatory loop.107 High expression levels of USP36 were found in a subset of human breast and lung cancers, indicating its oncogenic role. The finding of MYC as a USP36 target also supports the critical role for USP36 in ribosome biogenesis.109, 110, 111
USP37
USP37 was shown to directly interact with MYC at the MBIII region and deubiquitinate MYC.112 Although USP37-mediated deubiquitination and stabilization of MYC is independent of either Fbw7 or the phosphorylation of MYC at T58, overexpression of USP37 can block the Fbw7-mediated degradation of MYC. Functionally, overexpression of USP37 promotes cell proliferation and metabolism. USP37 is also upregulated in human lung cancer tissues that positively correlates with MYC expression.112
USP22
USP22 was recently found as another MYC DUB that deubiquitinates and stabilizes MYC. USP22 interacts with MYC through its N-terminal region containing zinc finger motif and abrogates Fbw7-mediated polyubiquitination and degradation of MYC. Consistently, overexpression of USP22 stimulates while downregulation of USP22 suppresses breast cancer cell growth, migration and tumorigenesis in a MYC dependent manner.113
USP13
As mentioned above, FBXL14 ubiquitinates and destabilizes MYC. In the same study, USP13 was found to stabilize MYC by antagonizing FBXL14-mediated ubiquitination to maintain GSC self-renewal and tumorigenic potential.64 USP13 was preferentially expressed in GSCs, and its depletion reduces MYC protein levels and potently impairs GSC proliferation and tumor growth. The expression of the ubiquitination-insensitive MYCT58A mutant rescued these effects caused by USP13 depletion. In gliobalstoma (GBM) patients, USP13 expression inversely correlates with the patient survival whereas FBXL14 expression displays a positive correlation. Thus, USP13 is implied as an attractive therapeutic target for disrupting GSCs and improving GBM treatment.64
USP7
USP7 has recently been shown to stabilize MYC by antagonizing TRIM32-mediated polyubiquitination and this function is required to maintain neural stem cell fate.74 USP7 directly interacts with MYC and TRIM32. The balanced activity of TRIM32 and USP7 to dynamically control MYC ubiquitination provides a novel mechanism of cell fate determination of neural stem cells. Interestingly, USP7 has also been shown to interact with, deubiquitinate and stabilize N-MYC, a driver of neuroblastoma tumorigenesis, but not MYC in neuroblastoma, which might be explained by cell type specific difference or the different spatial and temporal expression patterns for N-MYC and MYC.114
MYC is modified by SUMO
SUMOylation, a posttranslational modification of proteins by small ubiquitin-like modifiers (SUMOs), plays a crucial role in the regulation of diverse cellular processes, including transcription, chromatin dynamics, DNA replication and repair, RNA splicing and processing, cell cycle control, as well as ribosome biogenesis.115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125 Mammals express three main SUMO isoforms: SUMO2 and SUMO3 are 97% identical (referred to as SUMO2/3) and each shares 45% sequence identity with SUMO1.121, 126, 127, 128, 129 Like ubiquitination, SUMOylation is ATP-dependent and occurs through sequential reactions involving a heterodimeric SUMO-activating enzyme SAE1/SAE2 (E1), a single SUMO-conjugating enzyme Ubc9 (E2) and one of a few SUMO ligases (E3).121, 129, 130 Ubc9 transfers SUMO to substrate acceptor lysine residues via an isopeptide linkage, which is facilitated by but not necessarily dependent on a small number of SUMO E3s, such as the PIAS family members and RanBP2.121, 129, 130 The SUMO acceptor Lys is often present within a conserved ΨKxE motif, where Ψ is a large hydrophobic amino acid and x is any amino acid.121, 131, 132, 133 SUMOylation can interfere with protein-protein interactions through steric hindrance134 or compete with other lysine-directed modifications like acetylation or ubiquitination.135 Consequently, SUMOylation can regulate protein localization, trafficking, stability and activity.122, 131
Recently, several studies showed that MYC is subjected to SUMOylation in cells.136, 137, 138, 139 It was initially shown that MYC is modified by SUMO at the C-terminal Lys residues K323 and K326. Mass spectrometry analysis further showed that MYC can be SUMOlyated on at least 10 SUMO acceptor Lys residues, including K323 and K326.136, 137, 139 However, mutating K323 and K326 or all the 10 Ks did not abolish MYC SUMOylation and did not significantly alter its levels or activity,136, 137, 139 suggesting that c-MYC SUMOylation can act promiscuously and leaving the question of whether SUMOylation regulates MYC stability and activity still open. Yet, evidence has suggested that MYC SUMOylation may regulate MYC protein stability.136 We have shown that proteasome inhibition markedly induces the levels of MYC SUMOylation by either SUMO1 or SUMO2.140 The SUMO ligase PIAS1 was initially suggested to play a role in MYC SUMOylation as its knockdown reduced MYC SUMOylation and enhanced MYC transcriptional activity.136 Recently, PIAS1 was shown to SUMOylate MYC, mainly at K51 and K52.138 However, PIAS1-mediated SUMOylation augmented MYC stability and transactivation activity by recruiting JNK1 to phosphorylate MYC at S62. Consequently, PIAS1 overexpression promotes MYC-driven tumorigenesis.138 Therefore, it is still not clear how SUMOylation directly regulates MYC activity, largely because SUMO acceptor Lysines in MYC act promiscuously136, 137, 139 and SUMO-defective MYC mutants are not available, making it difficult to evaluate the direct effects of SUMO modification on MYC.
Erasing MYC SUMOylation by SENP1
SUMO modification is highly dynamic and reversible. SUMO can be removed from substrates via deSUMOylation by deSUMOylating enzymes (also called SUMO proteases). Studying MYC deSUMOylation allows us to learn about the role of SUMOylation in MYC regulation that was not possible through MYC mutagenesis studies. To this end, we screened a group of 9 known deSUMOylating enzymes in human, including SENP1-3 and SENP5-7, USPL1, DESI-1, and DESI-2,122, 141, 142 for their ability to bind to MYC and found that SENP1 binds to MYC and is a bona fide MYC deSUMOylase.140
SENP1 is a nuclear SUMO-specific protease that can cleave SUMO1, -2, and -3 conjugates although an in vivo study of MEF cells with mutant SENP1 suggests it mainly regulates SUMO1 modifications.143 SENP1 is enriched at the nuclear envelope through its interaction with components of the nuclear pore complex (NPC) and it is also present in nuclear foci that partially overlap with PML nuclear bodies.142 SENP1 has been reported to deSUMOylate a variety of proteins such as HIF-1α, PML, Ets-1, Elk1, Gli1, c-JUN, PTEN, Pin1, etc,144, 145, 146, 147, 148, 149, 150, 151 thus playing critical roles in diverse cellular processes including cell cycle control, transcription, immune response, DNA repair, and metabolism as well as in animal development. Homozygous deletion of the SENP1 gene is embryonic lethal due to impaired erythropoiesis.146 Consistent with the tight connection between dysregulation of the SUMO pathway and tumorigenesis, SENP1 has been suggested to have oncogenic function152, 153 and is overexpressed in many types of human cancers including prostate,154 breast,140, 145 and thyroid cancers.155 However, molecular mechanisms underlying SENP1's oncogenic function remain understudied.
SENP1 directly interacts with MYC, deconjugates MYC SUMOylation with either SUMO1 or SUMO2 and stimulates MYC transactivation activity. Interestingly, wild-type (WT) SENP1, but not its catalytically inactive C603S mutant (SENP1C603S), stabilizes MYC, demonstrating that SENP1-mediated deSUMOylation suppresses MYC turnover. Consistently, knockdown of SENP1 increases MYC SUMOylation with either SUMO1 or SUMO2, reduces MYC levels, induces cell cycle arrest, and markedly inhibits cell proliferation and anchorage-independent colony formation of cancer cells.140 Compared to MYC-independent breast cancer MCF7 and SKBR3 cells, MYC-dependent MDA-MB-231 and SUM159 cells exhibited stronger inhibition of proliferation and transformation upon SENP1 knockdown. These results suggest that the role of SENP1 in cell growth is at least partially through positively regulating MYC, and the indispensability of SENP1 in cell proliferation and transformation of the breast cancer cells correlates with their MYC dependency. Consistent with the oncogenic function, SENP1 is frequently overexpressed in human breast cancer tissues, correlating with the high expression of MYC.140
Crosstalk between MYC ubiquitination and SUMOylation
To understand how deSUMOylation of MYC increases its levels, we found that overexpression of SENP1WT, but not SENP1C603S, inhibits MYC ubiquitination and degradation. MG132 treatment significantly increased MYC SUMOylation by either SUMO1 or SUMO2 in various tested cells.140 Thus, SUMOylation promotes MYC ubiquitination and proteasome degradation. SUMOylation may regulate protein stability through several possible mechanisms: (1) Competing lysines for ubiquitination; (2) SUMOylation-directed ubiquitination by SUMO-targeted ubiquitin ligase (StubL) RNF4 or RNF111122, 131, 134, 135, 136; or (3) SUMOylation-regulated protein ubiquitination independently of StubLs. The StubL RNF111 does not play a role in MYC ubiquitination136 whereas RNF4-mediated ubiquitination has been reported to modestly affect c-MYC stability136 or promote MYC function, but not degradation.99 Given these unclear results, SUMOylated MYC may be ubiquitinated independently of StubLs. We have shown that SENP1 interacts with Fbw7 and suppresses Fbw7-mediated MYC ubiquitination and degradation dependent on its deSUMOylating enzyme activity, suggesting that SENP1 regulates growth signal-controlled MYC turnover at least in part via counteracting Fbw7-mediated MYC ubiquitination and degradation.140 Together, our data demonstrate that SENP1 positively regulates MYC activity and protein stability by counteracting MYC ubiquitination and proteasome degradation. Supporting this regulation, it has been shown that SENP1 also stabilizes hypoxia-inducible factor (HIF)- 1α stability by deSUMOylating HIF-1α.146
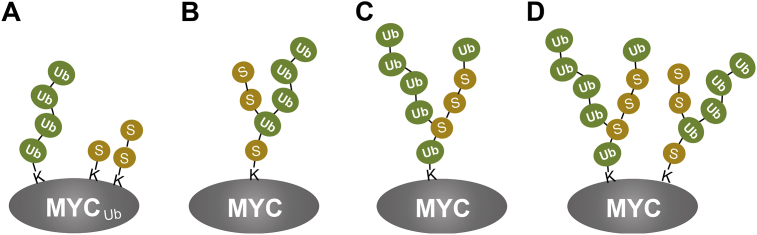
Intriguing questions arise as to how MYC SUMOylation cross talks with ubiquitination. One possibility is that SUMOylated MYC is a better Fbw7 substrate than non-SUMOylated MYC. In support of this, SUMOylation enables MYCT58A, a mutant normally not targeted by Fbw7, to be a Fbw7 substrate as we reported.140 It will be interesting in future studies to test whether and how SENP1 directly suppresses Fbw7 ubiquitin E3 activity given that SENP1 also physically interacts with Fbw7 in cells,140 including whether SENP1 deSUMOylates Fbw7 and suppresses its activity. It is also important to determine whether SUMOylation-directed MYC ubiquitination occurs directly at Lys residue(s) on MYC (Fig. 3A) or indirectly on SUMO attached to MYC (Fig. 3B–D). Supporting the latter possibility is that recent proteomic analyses identified ubiquitination of SUMO as well as SUMO-conjugation to multiple lysines of ubiquitin.156, 157 Our sequential co-IP assays revealed that MYC can be co-modified by both SUMO and ubiquitin, indicating that SUMOylated MYC can be targeted for proteasome degradation possibly due to co-modification with ubiquitin and SENP1 stabilizes MYC by removing ubiquitination via deSUMOylation (indirect deubiquitination). Interestingly, we show that overexpression of SENP1 in the absence of proteasome inhibition resulted in the accumulation of a mono-ubiquitinated form of MYC in cells, suggesting the SUMOylation of the ubiquitin attached to MYC (Fig. 3C), forming the mixed SUMO-ubiquitin chain that can be removed along with SUMO by SENP1, leaving the single ubiquitin attached to MYC. Future studies are warranted to further analyze the SUMO-ubiquitin mixed chains (Fig. 3) and their role in regulating MYC turnover and function. By the same token, it is also interesting to test whether SENP1 cross talks with other ubiquitin E3s and DUBs such as USP28 to co-regulate MYC ubiquitination and SUMOylation.
Figure 3.
Possible models for the crosstalk between MYC ubiquitination and MYC SUMOylation. MYC may be ubiquitinated and SUMOylated directly at different Lys residues (A). MYC may be co-modified by mixed SUMO-Ub chain(s) via ubiquitination of SUMO conjugates on MYC (B) or via SUMOylation of ubiquitin conjugates on MYC (C), or the combination of B and C (D).
Conclusion
It is clear that MYC protein stability and activity are tightly regulated by ubiquitination. Recent studies further reveal the critical role of the concerted action of SUMOylation in regulating MYC stability and activity and highlight the intriguing function of MYC co-modification by ubiquitin and SUMO. The dynamic nature of MYC post-translational modifications has also been seen in its ubiquitination, which can either positively or negatively regulate MYC stability and activity in a ligase-dependent manner.12, 108 It is therefore conceivable that dynamic balance of SUMOylation and its crosstalk with ubiquitination are equally required for properly regulated MYC turnover and function in cells. Therefore, it is imperative in future studies to understand how SUMOylation interplays with ubiquitination to coordinately regulate MYC and in what conditions they may do so. As SENP1 promotes MYC binding to target gene promoters, it would be important to test whether SENP1 plays a role in regulating MYC SUMOylation and turnover on chromatin. We recently also found that posttranslational modification (Ser 62 phosphorylation and PIN1-mediated isomerization) targets MYC to the inner basket of the nuclear pore complex to regulate a subset of target genes involved in proliferation in response to growth signals.49 Interestingly, SENP1 is enriched at the nuclear envelop158, 159 and SENP1-stabilized MYC is phosphorylated at S62,140 suggesting that SENP1 may play a critical role in regulating MYC subnuclear localization and its control of target genes at the nuclear pore. Taken together, MYC SUMOylation plays a key role in regulating MYC stability and function. Further studies are warranted to reveal the temporal and spatial regulation of MYC SUMOylation and its physiological significance as well as the role of deregulation of MYC SUMOylation in cancer.
Conflicts of interest
These authors declare no conflicts of interest.
Acknowledgements
We thank members of the Dai and Sears laboratories for active discussion. This work was supported by NIH/NCI grant R01 CA186241 to M-S. D. and R. S.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Rosalie C. Sears, Email: searsr@ohsu.edu.
Mu-Shui Dai, Email: daim@ohsu.edu.
References
- 1.Adhikary S., Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6(8):635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 2.Meyer N., Penn L.Z. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8(12):976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 3.van Riggelen J., Yetil A., Felsher D.W. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer. 2010;10(4):301–309. doi: 10.1038/nrc2819. [DOI] [PubMed] [Google Scholar]
- 4.Blackwood E.M., Eisenman R.N. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991;251(4998):1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- 5.Amati B., Dalton S., Brooks M.W., Littlewood T.D., Evan G.I., Land H. Transcriptional activation by the human c-Myc oncoprotein in yeast requires interaction with Max. Nature. 1992;359(6394):423–426. doi: 10.1038/359423a0. [DOI] [PubMed] [Google Scholar]
- 6.Mao D.Y., Watson J.D., Yan P.S. Analysis of Myc bound loci identified by CpG island arrays shows that Max is essential for Myc-dependent repression. Curr Biol. 2003;13(10):882–886. doi: 10.1016/s0960-9822(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 7.Bieda M., Xu X., Singer M.A., Green R., Farnham P.J. Unbiased location analysis of E2F1-binding sites suggests a widespread role for E2F1 in the human genome. Genome Res. 2006;16(5):595–605. doi: 10.1101/gr.4887606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez P.C., Frank S.R., Wang L. Genomic targets of the human c-Myc protein. Genes Dev. 2003;17(9):1115–1129. doi: 10.1101/gad.1067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenman R.N. Deconstructing myc. Genes Dev. 2001;15(16):2023–2030. doi: 10.1101/gad928101. [DOI] [PubMed] [Google Scholar]
- 10.Oster S.K., Ho C.S., Soucie E.L., Penn L.Z. The myc oncogene: MarvelouslY Complex. Adv Cancer Res. 2002;84:81–154. doi: 10.1016/s0065-230x(02)84004-0. [DOI] [PubMed] [Google Scholar]
- 11.Wanzel M., Herold S., Eilers M. Transcriptional repression by myc. Trends Cell Biol. 2003;13(3):146–150. doi: 10.1016/s0962-8924(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 12.Farrell A.S., Sears R.C. MYC degradation. Cold Spring Harbor Perspect Medicine. 2014;4(3) doi: 10.1101/cshperspect.a014365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hann S.R. Role of post-translational modifications in regulating c-Myc proteolysis, transcriptional activity and biological function. Semin Cancer Biol. 2006;16(4):288–302. doi: 10.1016/j.semcancer.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Cowling V.H., Chandriani S., Whitfield M.L., Cole M.D. A conserved Myc protein domain, MBIV, regulates DNA binding, apoptosis, transformation, and G2 arrest. Mol Cell Biol. 2006;26(11):4226–4239. doi: 10.1128/MCB.01959-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst A., Hemann M.T., Tworkowski K.A., Salghetti S.E., Lowe S.W., Tansey W.P. A conserved element in Myc that negatively regulates its proapoptotic activity. EMBO Rep. 2005;6(2):177–183. doi: 10.1038/sj.embor.7400333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurland J.F., Tansey W.P. Myc-mediated transcriptional repression by recruitment of histone deacetylase. Cancer Res. 2008;68(10):3624–3629. doi: 10.1158/0008-5472.CAN-07-6552. [DOI] [PubMed] [Google Scholar]
- 17.Nesbit C.E., Tersak J.M., Prochownik E.V. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18(19):3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 18.Brooks T.A., Hurley L.H. Targeting MYC expression through G-quadruplexes. Genes Cancer. 2010;1(6):641–649. doi: 10.1177/1947601910377493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurley N.E., Schildmeyer L.A., Bosworth K.A. Modulating the functional contributions of c-Myc to the human endothelial cell cyclic strain response. J Vasc Res. 2010;47(1):80–90. doi: 10.1159/000235928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levens D. You don't muck with MYC. Genes Cancer. 2010;1(6):547–554. doi: 10.1177/1947601910377492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickart C.M. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 22.Jin J., Li X., Gygi S.P., Harper J.W. Dual E1 activation systems for ubiquitin differentially regulate E2 enzyme charging. Nature. 2007;447(7148):1135–1138. doi: 10.1038/nature05902. [DOI] [PubMed] [Google Scholar]
- 23.Stewart M.D., Ritterhoff T., Klevit R.E., Brzovic P.S. E2 enzymes: more than just middle men. Cell Res. 2016;26(4):423–440. doi: 10.1038/cr.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swatek K.N., Komander D. Ubiquitin modifications. Cell Res. 2016;26(4):399–422. doi: 10.1038/cr.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye Y., Rape M. Building ubiquitin chains: E2 enzymes at work. Nat Rev Mol Cell Biol. 2009;10(11):755–764. doi: 10.1038/nrm2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciechanover A., DiGiuseppe J.A., Bercovich B. Degradation of nuclear oncoproteins by the ubiquitin system in vitro. Proc Natl Acad Sci U S A. 1991;88(1):139–143. doi: 10.1073/pnas.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flinn E.M., Busch C.M., Wright A.P. Myc boxes, which are conserved in myc family proteins, are signals for protein degradation via the proteasome. Mol Cell Biol. 1998;18(10):5961–5969. doi: 10.1128/mcb.18.10.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welcker M., Clurman B.E. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8(2):83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 29.Welcker M., Orian A., Grim J.E., Eisenman R.N., Clurman B.E. A nucleolar isoform of the Fbw7 ubiquitin ligase regulates c-Myc and cell size. Curr Biol. 2004;14(20):1852–1857. doi: 10.1016/j.cub.2004.09.083. [DOI] [PubMed] [Google Scholar]
- 30.Lutterbach B., Hann S.R. Hierarchical phosphorylation at N-terminal transformation-sensitive sites in c-Myc protein is regulated by mitogens and in mitosis. Mol Cell Biol. 1994;14(8):5510–5522. doi: 10.1128/mcb.14.8.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sears R., Nuckolls F., Haura E., Taya Y., Tamai K., Nevins J.R. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14(19):2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregory M.A., Qi Y., Hann S.R. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem. 2003;278(51):51606–51612. doi: 10.1074/jbc.M310722200. [DOI] [PubMed] [Google Scholar]
- 33.Chang D.W., Claassen G.F., Hann S.R., Cole M.D. The c-Myc transactivation domain is a direct modulator of apoptotic versus proliferative signals. Mol Cell Biol. 2000;20(12):4309–4319. doi: 10.1128/mcb.20.12.4309-4319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeh E., Cunningham M., Arnold H. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6(4):308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 35.Moberg K.H., Mukherjee A., Veraksa A., Artavanis-Tsakonas S., Hariharan I.K. The Drosophila F box protein archipelago regulates dMyc protein levels in vivo. Curr Biol. 2004;14(11):965–974. doi: 10.1016/j.cub.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 36.Yada M., Hatakeyama S., Kamura T. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 2004;23(10):2116–2125. doi: 10.1038/sj.emboj.7600217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welcker M., Orian A., Jin J. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci U S A. 2004;101(24):9085–9090. doi: 10.1073/pnas.0402770101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahram F., von der Lehr N., Cetinkaya C., Larsson L.G. c-Myc hot spot mutations in lymphomas result in inefficient ubiquitination and decreased proteasome-mediated turnover. Blood. 2000;95(6):2104–2110. [PubMed] [Google Scholar]
- 39.Gregory M.A., Hann S.R. c-Myc proteolysis by the ubiquitin-proteasome pathway: stabilization of c-Myc in Burkitt's lymphoma cells. Mol Cell Biol. 2000;20(7):2423–2435. doi: 10.1128/mcb.20.7.2423-2435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salghetti S.E., Kim S.Y., Tansey W.P. Destruction of Myc by ubiquitin-mediated proteolysis: cancer-associated and transforming mutations stabilize Myc. EMBO J. 1999;18(3):717–726. doi: 10.1093/emboj/18.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hemann M.T., Bric A., Teruya-Feldstein J. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature. 2005;436(7052):807–811. doi: 10.1038/nature03845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X., Cunningham M., Zhang X. Phosphorylation regulates c-Myc's oncogenic activity in the mammary gland. Cancer Res. 2011;71(3):925–936. doi: 10.1158/0008-5472.CAN-10-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frescas D., Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8(6):438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gstaiger M., Jordan R., Lim M. Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci U S A. 2001;98(9):5043–5048. doi: 10.1073/pnas.081474898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S.Y., Herbst A., Tworkowski K.A., Salghetti S.E., Tansey W.P. Skp2 regulates Myc protein stability and activity. Mol Cell. 2003;11(5):1177–1188. doi: 10.1016/s1097-2765(03)00173-4. [DOI] [PubMed] [Google Scholar]
- 46.von der Lehr N., Johansson S., Larsson L.G. Implication of the ubiquitin/proteasome system in Myc-regulated transcription. Cell Cycle. 2003;2(5):403–407. [PubMed] [Google Scholar]
- 47.von der Lehr N., Johansson S., Wu S. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol Cell. 2003;11(5):1189–1200. doi: 10.1016/s1097-2765(03)00193-x. [DOI] [PubMed] [Google Scholar]
- 48.Farrell A.S., Pelz C., Wang X. Pin1 regulates the dynamics of c-Myc DNA binding to facilitate target gene regulation and oncogenesis. Mol Cell Biol. 2013;33(15):2930–2949. doi: 10.1128/MCB.01455-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su Y., Pelz C., Huang T. Post-translational modification localizes MYC to the nuclear pore basket to regulate a subset of target genes involved in cellular responses to environmental signals. Genes Dev. 2018;32(21–22):1398–1419. doi: 10.1101/gad.314377.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujii M., Lyakh L.A., Bracken C.P. SNIP1 is a candidate modifier of the transcriptional activity of c-Myc on E box-dependent target genes. Mol Cell. 2006;24(5):771–783. doi: 10.1016/j.molcel.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q., Spears E., Boone D.N., Li Z., Gregory M.A., Hann S.R. Domain-specific c-Myc ubiquitylation controls c-Myc transcriptional and apoptotic activity. Proc Natl Acad Sci U S A. 2013;110(3):978–983. doi: 10.1073/pnas.1208334110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiwari S., Roel C., Tanwir M. Definition of a Skp2-c-myc pathway to expand human beta-cells. Sci Rep. 2016;6:28461. doi: 10.1038/srep28461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan C.H., Lee S.W., Li C.F. Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis. Nat Cell Biol. 2010;12(5):457–467. doi: 10.1038/ncb2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X., Bian Y., Takizawa Y. ERK-dependent downregulation of Skp2 reduces Myc activity with HGF, leading to inhibition of cell proliferation through a decrease in Id1 expression. Mol Cancer Res. 2013;11(11):1437–1447. doi: 10.1158/1541-7786.MCR-12-0718. [DOI] [PubMed] [Google Scholar]
- 55.Hydbring P., Castell A., Larsson L.G. MYC Modulation around the CDK2/p27/SKP2 Axis. Genes (Basel) 2017;8(7) doi: 10.3390/genes8070174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bodine S.C., Latres E., Baumhueter S. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294(5547):1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 57.Gomes M.D., Lecker S.H., Jagoe R.T., Navon A., Goldberg A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98(25):14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ciarapica R., De Salvo M., Carcarino E. The Polycomb group (PcG) protein EZH2 supports the survival of PAX3-FOXO1 alveolar rhabdomyosarcoma by repressing FBXO32 (Atrogin1/MAFbx) Oncogene. 2014;33(32):4173–4184. doi: 10.1038/onc.2013.471. [DOI] [PubMed] [Google Scholar]
- 59.Guo W., Zhang M., Shen S. Aberrant methylation and decreased expression of the TGF-beta/Smad target gene FBXO32 in esophageal squamous cell carcinoma. Cancer. 2014;120(16):2412–2423. doi: 10.1002/cncr.28764. [DOI] [PubMed] [Google Scholar]
- 60.Mei Z., Zhang D., Hu B., Wang J., Shen X., Xiao W. FBXO32 targets c-myc for proteasomal degradation and inhibits c-myc activity. J Biol Chem. 2015;290(26):16202–16214. doi: 10.1074/jbc.M115.645978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huber A.L., Papp S.J., Chan A.B. CRY2 and FBXL3 cooperatively degrade c-MYC. Mol Cell. 2016;64(4):774–789. doi: 10.1016/j.molcel.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Busino L., Bassermann F., Maiolica A. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007;316(5826):900–904. doi: 10.1126/science.1141194. [DOI] [PubMed] [Google Scholar]
- 63.Godinho S.I., Maywood E.S., Shaw L. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316(5826):897–900. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- 64.Fang X., Zhou W., Wu Q. Deubiquitinase USP13 maintains glioblastoma stem cells by antagonizing FBXL14-mediated Myc ubiquitination. J Exp Med. 2017;214(1):245–267. doi: 10.1084/jem.20151673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Popov N., Schulein C., Jaenicke L.A., Eilers M. Ubiquitylation of the amino terminus of Myc by SCF(beta-TrCP) antagonizes SCF(Fbw7)-mediated turnover. Nat Cell Biol. 2010;12(10):973–981. doi: 10.1038/ncb2104. [DOI] [PubMed] [Google Scholar]
- 66.Macurek L., Lindqvist A., Lim D. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455(7209):119–123. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 67.Yi Y.W., Kang H.J., Bae E.J., Oh S., Seong Y.S., Bae I. beta-TrCP1 degradation is a novel action mechanism of PI3K/mTOR inhibitors in triple-negative breast cancer cells. Exp Mol Med. 2015;47:e143. doi: 10.1038/emm.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cepeda D., Ng H.F., Sharifi H.R. CDK-mediated activation of the SCF(FBXO) (28) ubiquitin ligase promotes MYC-driven transcription and tumourigenesis and predicts poor survival in breast cancer. EMBO Mol Med. 2013;5(7):999–1018. doi: 10.1002/emmm.201202341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adhikary S., Marinoni F., Hock A. The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell. 2005;123(3):409–421. doi: 10.1016/j.cell.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 70.Geng C., Kaochar S., Li M. SPOP regulates prostate epithelial cell proliferation and promotes ubiquitination and turnover of c-MYC oncoprotein. Oncogene. 2017;36(33):4767–4777. doi: 10.1038/onc.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo L., Tang H., Ling L. LINC01638 lncRNA activates MTDH-Twist1 signaling by preventing SPOP-mediated c-Myc degradation in triple-negative breast cancer. Oncogene. 2018;37(47):6166–6179. doi: 10.1038/s41388-018-0396-8. [DOI] [PubMed] [Google Scholar]
- 72.Cancer Genome Atlas Research N The molecular taxonomy of primary prostate cancer. Cell. 2015;163(4):1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geng C., Rajapakshe K., Shah S.S. Androgen receptor is the key transcriptional mediator of the tumor suppressor SPOP in prostate cancer. Cancer Res. 2014;74(19):5631–5643. doi: 10.1158/0008-5472.CAN-14-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nicklas S., Hillje A.L., Okawa S. A complex of the ubiquitin ligase TRIM32 and the deubiquitinase USP7 balances the level of c-Myc ubiquitination and thereby determines neural stem cell fate specification. Cell Death Differ. 2019;26(4):728–740. doi: 10.1038/s41418-018-0144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwamborn J.C., Berezikov E., Knoblich J.A. The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell. 2009;136(5):913–925. doi: 10.1016/j.cell.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Izumi H., Kaneko Y. Trim32 facilitates degradation of MYCN on spindle poles and induces asymmetric cell division in human neuroblastoma cells. Cancer Res. 2014;74(19):5620–5630. doi: 10.1158/0008-5472.CAN-14-0169. [DOI] [PubMed] [Google Scholar]
- 77.Gao R., Wang L., Cai H., Zhu J., Yu L. E3 ubiquitin ligase RLIM negatively regulates c-myc transcriptional activity and restrains cell proliferation. PLoS One. 2016;11(9):e0164086. doi: 10.1371/journal.pone.0164086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Logan I.R., Sapountzi V., Gaughan L., Neal D.E., Robson C.N. Control of human PIRH2 protein stability: involvement of TIP60 and the proteosome. J Biol Chem. 2004;279(12):11696–11704. doi: 10.1074/jbc.M312712200. [DOI] [PubMed] [Google Scholar]
- 79.Bohgaki M., Hakem A., Halaby M.J. The E3 ligase PIRH2 polyubiquitylates CHK2 and regulates its turnover. Cell Death Differ. 2013;20(6):812–822. doi: 10.1038/cdd.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hakem A., Bohgaki M., Lemmers B. Role of Pirh2 in mediating the regulation of p53 and c-Myc. PLoS Genet. 2011;7(11):e1002360. doi: 10.1371/journal.pgen.1002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Daks A., Petukhov A., Fedorova O. E3 ubiquitin ligase Pirh2 enhances tumorigenic properties of human non-small cell lung carcinoma cells. Genes Cancer. 2016;7(11–12):383–393. doi: 10.18632/genesandcancer.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soond S.M., Terry J.L., Colbert J.D., Riches D.W. TRUSS, a novel tumor necrosis factor receptor 1 scaffolding protein that mediates activation of the transcription factor NF-kappaB. Mol Cell Biol. 2003;23(22):8334–8344. doi: 10.1128/MCB.23.22.8334-8344.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poduslo S.E., Huang R., Huang J., Smith S. Genome screen of late-onset Alzheimer's extended pedigrees identifies TRPC4AP by haplotype analysis. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(1):50–55. doi: 10.1002/ajmg.b.30767. [DOI] [PubMed] [Google Scholar]
- 84.Choi S.H., Wright J.B., Gerber S.A., Cole M.D. Myc protein is stabilized by suppression of a novel E3 ligase complex in cancer cells. Genes Dev. 2010;24(12):1236–1241. doi: 10.1101/gad.1920310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jamal A., Swarnalatha M., Sultana S., Joshi P., Panda S.K., Kumar V. The G1 phase E3 ubiquitin ligase TRUSS that gets deregulated in human cancers is a novel substrate of the S-phase E3 ubiquitin ligase Skp2. Cell Cycle. 2015;0 doi: 10.1080/15384101.2015.1056946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peter S., Bultinck J., Myant K. Tumor cell-specific inhibition of MYC function using small molecule inhibitors of the HUWE1 ubiquitin ligase. EMBO Mol Med. 2014;6(12):1525–1541. doi: 10.15252/emmm.201403927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Inoue S., Hao Z., Elia A.J. Mule/Huwe1/Arf-BP1 suppresses Ras-driven tumorigenesis by preventing c-Myc/Miz1-mediated down-regulation of p21 and p15. Genes Dev. 2013;27(10):1101–1114. doi: 10.1101/gad.214577.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang Y., Do H., Tian X. E3 ubiquitin ligase Mule ubiquitinates Miz1 and is required for TNFalpha-induced JNK activation. Proc Natl Acad Sci U S A. 2010;107(30):13444–13449. doi: 10.1073/pnas.0913690107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Myant K.B., Cammareri P., Hodder M.C. HUWE1 is a critical colonic tumour suppressor gene that prevents MYC signalling, DNA damage accumulation and tumour initiation. EMBO Mol Med. 2017;9(2):181–197. doi: 10.15252/emmm.201606684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ballinger C.A., Connell P., Wu Y. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19(6):4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murata S., Minami Y., Minami M., Chiba T., Tanaka K. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep. 2001;2(12):1133–1138. doi: 10.1093/embo-reports/kve246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paul I., Ahmed S.F., Bhowmik A., Deb S., Ghosh M.K. The ubiquitin ligase CHIP regulates c-Myc stability and transcriptional activity. Oncogene. 2013;32(10):1284–1295. doi: 10.1038/onc.2012.144. [DOI] [PubMed] [Google Scholar]
- 93.Eissenberg J.C., Ma J., Gerber M.A., Christensen A., Kennison J.A., Shilatifard A. dELL is an essential RNA polymerase II elongation factor with a general role in development. Proc Natl Acad Sci U S A. 2002;99(15):9894–9899. doi: 10.1073/pnas.152193699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shilatifard A., Lane W.S., Jackson K.W., Conaway R.C., Conaway J.W. An RNA polymerase II elongation factor encoded by the human ELL gene. Science. 1996;271(5257):1873–1876. doi: 10.1126/science.271.5257.1873. [DOI] [PubMed] [Google Scholar]
- 95.Chen Y., Zhou C., Ji W. ELL targets c-Myc for proteasomal degradation and suppresses tumour growth. Nat Commun. 2016;7:11057. doi: 10.1038/ncomms11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lallemand-Breitenbach V., Jeanne M., Benhenda S. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol. 2008;10(5):547–555. doi: 10.1038/ncb1717. [DOI] [PubMed] [Google Scholar]
- 97.Sriramachandran A.M., Dohmen R.J. SUMO-targeted ubiquitin ligases. Biochim Biophys Acta. 2014;1843(1):75–85. doi: 10.1016/j.bbamcr.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 98.Tatham M.H., Geoffroy M.C., Shen L. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat Cell Biol. 2008;10(5):538–546. doi: 10.1038/ncb1716. [DOI] [PubMed] [Google Scholar]
- 99.Thomas J.J., Abed M., Heuberger J. RNF4-Dependent oncogene activation by protein stabilization. Cell Rep. 2016;16(12):3388–3400. doi: 10.1016/j.celrep.2016.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clague M.J., Urbe S., Komander D. Breaking the chains: deubiquitylating enzyme specificity begets function. Nat Rev Mol Cell Biol. 2019;20(6):338–352. doi: 10.1038/s41580-019-0099-1. [DOI] [PubMed] [Google Scholar]
- 101.Harrigan J.A., Jacq X., Martin N.M., Jackson S.P. Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat Rev Drug Discov. 2018;17(1):57–78. doi: 10.1038/nrd.2017.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nijman S.M., Luna-Vargas M.P., Velds A. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123(5):773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 103.Popov N., Wanzel M., Madiredjo M. The ubiquitin-specific protease USP28 is required for MYC stability. Nat Cell Biol. 2007;9(7):765–774. doi: 10.1038/ncb1601. [DOI] [PubMed] [Google Scholar]
- 104.Diefenbacher M.E., Chakraborty A., Blake S.M. Usp28 counteracts Fbw7 in intestinal homeostasis and cancer. Cancer Res. 2015;75(7):1181–1186. doi: 10.1158/0008-5472.CAN-14-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Popov N., Herold S., Llamazares M., Schulein C., Eilers M. Fbw7 and Usp28 regulate myc protein stability in response to DNA damage. Cell Cycle. 2007;6(19):2327–2331. doi: 10.4161/cc.6.19.4804. [DOI] [PubMed] [Google Scholar]
- 106.Schulein-Volk C., Wolf E., Zhu J. Dual regulation of Fbw7 function and oncogenic transformation by Usp28. Cell Rep. 2014;9(3):1099–1109. doi: 10.1016/j.celrep.2014.09.057. [DOI] [PubMed] [Google Scholar]
- 107.Sun X.X., He X., Yin L., Komada M., Sears R.C., Dai M.S. The nucleolar ubiquitin-specific protease USP36 deubiquitinates and stabilizes c-Myc. Proc Natl Acad Sci U S A. 2015;112(12):3734–3739. doi: 10.1073/pnas.1411713112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun X.X., Sears R.C., Dai M.S. Deubiquitinating c-Myc: USP36 steps up in the nucleolus. Cell Cycle. 2015;14(24):3786–3793. doi: 10.1080/15384101.2015.1093713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Endo A., Kitamura N., Komada M. Nucleophosmin/B23 regulates ubiquitin dynamics in nucleoli by recruiting deubiquitylating enzyme USP36. J Biol Chem. 2009;284(41):27918–27923. doi: 10.1074/jbc.M109.037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fraile J.M., Campos-Iglesias D., Rodriguez F. Loss of the deubiquitinase USP36 destabilizes the RNA helicase DHX33 and causes preimplantation lethality in mice. J Biol Chem. 2018;293(6):2183–2194. doi: 10.1074/jbc.M117.788430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thevenon D., Engel E., Avet-Rochex A. The Drosophila ubiquitin-specific protease dUSP36/Scny targets IMD to prevent constitutive immune signaling. Cell Host Microbe. 2009;6(4):309–320. doi: 10.1016/j.chom.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 112.Pan J., Deng Q., Jiang C. USP37 directly deubiquitinates and stabilizes c-Myc in lung cancer. Oncogene. 2015;34(30):3957–3967. doi: 10.1038/onc.2014.327. [DOI] [PubMed] [Google Scholar]
- 113.Kim D., Hong A., Park H.I. Deubiquitinating enzyme USP22 positively regulates c-Myc stability and tumorigenic activity in mammalian and breast cancer cells. J Cell Physiol. 2017;232(12):3664–3676. doi: 10.1002/jcp.25841. [DOI] [PubMed] [Google Scholar]
- 114.Tavana O., Li D., Dai C. HAUSP deubiquitinates and stabilizes N-Myc in neuroblastoma. Nat Med. 2016;22(10):1180–1186. doi: 10.1038/nm.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bergink S., Jentsch S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature. 2009;458(7237):461–467. doi: 10.1038/nature07963. [DOI] [PubMed] [Google Scholar]
- 116.Chymkowitch P., Nguea P.A., Enserink J.M. SUMO-regulated transcription: challenging the dogma. Bioessays. 2015;37(10):1095–1105. doi: 10.1002/bies.201500065. [DOI] [PubMed] [Google Scholar]
- 117.Cubenas-Potts C., Matunis M.J. SUMO: a multifaceted modifier of chromatin structure and function. Dev Cell. 2013;24(1):1–12. doi: 10.1016/j.devcel.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eifler K., Vertegaal A.C. SUMOylation-mediated regulation of cell cycle progression and cancer. Trends Biochem Sci. 2015;40(12):779–793. doi: 10.1016/j.tibs.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Enserink J.M. Sumo and the cellular stress response. Cell Div. 2015;10:4. doi: 10.1186/s13008-015-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Finkbeiner E., Haindl M., Raman N., Muller S. SUMO routes ribosome maturation. Nucleus. 2011;2(6):527–532. doi: 10.4161/nucl.2.6.17604. [DOI] [PubMed] [Google Scholar]
- 121.Geiss-Friedlander R., Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8(12):947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 122.Jentsch S., Psakhye I. Control of nuclear activities by substrate-selective and protein-group SUMOylation. Annu Rev Genet. 2013;47:167–186. doi: 10.1146/annurev-genet-111212-133453. [DOI] [PubMed] [Google Scholar]
- 123.Nuro-Gyina P.K., Parvin J.D. Wiley Interdiscip Rev RNA; 2015. Roles for SUMO in Pre-mRNA Processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Raman N., Nayak A., Muller S. The SUMO system: a master organizer of nuclear protein assemblies. Chromosoma. 2013;122(6):475–485. doi: 10.1007/s00412-013-0429-6. [DOI] [PubMed] [Google Scholar]
- 125.Sarangi P., Zhao X. SUMO-mediated regulation of DNA damage repair and responses. Trends Biochem Sci. 2015;40(4):233–242. doi: 10.1016/j.tibs.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kamitani T., Kito K., Nguyen H.P., Fukuda-Kamitani T., Yeh E.T. Characterization of a second member of the sentrin family of ubiquitin-like proteins. J Biol Chem. 1998;273(18):11349–11353. doi: 10.1074/jbc.273.18.11349. [DOI] [PubMed] [Google Scholar]
- 127.Kamitani T., Nguyen H.P., Kito K., Fukuda-Kamitani T., Yeh E.T. Covalent modification of PML by the sentrin family of ubiquitin-like proteins. J Biol Chem. 1998;273(6):3117–3120. doi: 10.1074/jbc.273.6.3117. [DOI] [PubMed] [Google Scholar]
- 128.Kamitani T., Nguyen H.P., Yeh E.T. Preferential modification of nuclear proteins by a novel ubiquitin-like molecule. J Biol Chem. 1997;272(22):14001–14004. doi: 10.1074/jbc.272.22.14001. [DOI] [PubMed] [Google Scholar]
- 129.Muller S., Hoege C., Pyrowolakis G., Jentsch S. SUMO, ubiquitin's mysterious cousin. Nat Rev Mol Cell Biol. 2001;2(3):202–210. doi: 10.1038/35056591. [DOI] [PubMed] [Google Scholar]
- 130.Hay R.T. Decoding the SUMO signal. Biochem Soc Trans. 2013;41(2):463–473. doi: 10.1042/BST20130015. [DOI] [PubMed] [Google Scholar]
- 131.Gareau J.R., Lima C.D. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol. 2010;11(12):861–871. doi: 10.1038/nrm3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rodriguez M.S., Dargemont C., Hay R.T. SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J Biol Chem. 2001;276(16):12654–12659. doi: 10.1074/jbc.M009476200. [DOI] [PubMed] [Google Scholar]
- 133.Sampson D.A., Wang M., Matunis M.J. The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J Biol Chem. 2001;276(24):21664–21669. doi: 10.1074/jbc.M100006200. [DOI] [PubMed] [Google Scholar]
- 134.Moldovan G.L., Pfander B., Jentsch S. PCNA controls establishment of sister chromatid cohesion during S phase. Mol Cell. 2006;23(5):723–732. doi: 10.1016/j.molcel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 135.Desterro J.M., Rodriguez M.S., Hay R.T. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell. 1998;2(2):233–239. doi: 10.1016/s1097-2765(00)80133-1. [DOI] [PubMed] [Google Scholar]
- 136.Gonzalez-Prieto R., Cuijpers S.A., Kumar R., Hendriks I.A., Vertegaal A.C. c-Myc is targeted to the proteasome for degradation in a SUMOylation-dependent manner, regulated by PIAS1, SENP7 and RNF4. Cell Cycle. 2015;14(12):1859–1872. doi: 10.1080/15384101.2015.1040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kalkat M., Chan P.K., Wasylishen A.R. Identification of c-MYC SUMOylation by mass spectrometry. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0115337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rabellino A., Melegari M., Tompkins V.S. PIAS1 promotes lymphomagenesis through MYC upregulation. Cell Rep. 2016;15(10):2266–2278. doi: 10.1016/j.celrep.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sabo A., Doni M., Amati B. SUMOylation of Myc-family proteins. PLoS One. 2014;9(3):e91072. doi: 10.1371/journal.pone.0091072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sun X.X., Chen Y., Su Y. SUMO protease SENP1 deSUMOylates and stabilizes c-Myc. Proc Natl Acad Sci U S A. 2018;115(43):10983–10988. doi: 10.1073/pnas.1802932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hickey C.M., Wilson N.R., Hochstrasser M. Function and regulation of SUMO proteases. Nat Rev Mol Cell Biol. 2012;13(12):755–766. doi: 10.1038/nrm3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nayak A., Muller S. SUMO-specific proteases/isopeptidases: SENPs and beyond. Genome Biol. 2014;15(7):422. doi: 10.1186/s13059-014-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sharma P., Yamada S., Lualdi M., Dasso M., Kuehn M.R. Senp1 is essential for desumoylating Sumo1-modified proteins but dispensable for Sumo2 and Sumo3 deconjugation in the mouse embryo. Cell Rep. 2013;3(5):1640–1650. doi: 10.1016/j.celrep.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bawa-Khalfe T., Yang F.M., Ritho J., Lin H.K., Cheng J., Yeh E.T. SENP1 regulates PTEN stability to dictate prostate cancer development. Oncotarget. 2017;8(11):17651–17664. doi: 10.18632/oncotarget.13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen C.H., Chang C.C., Lee T.H. SENP1 deSUMOylates and regulates Pin1 protein activity and cellular function. Cancer Res. 2013;73(13):3951–3962. doi: 10.1158/0008-5472.CAN-12-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cheng J., Kang X., Zhang S., Yeh E.T. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell. 2007;131(3):584–595. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheng J., Perkins N.D., Yeh E.T. Differential regulation of c-Jun-dependent transcription by SUMO-specific proteases. J Biol Chem. 2005;280(15):14492–14498. doi: 10.1074/jbc.M412185200. [DOI] [PubMed] [Google Scholar]
- 148.Fasci D., Anania V.G., Lill J.R., Salvesen G.S. SUMO deconjugation is required for arsenic-triggered ubiquitylation of PML. Sci Signal. 2015;8(380):ra56. doi: 10.1126/scisignal.aaa3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ji Z., Degerny C., Vintonenko N. Regulation of the Ets-1 transcription factor by sumoylation and ubiquitinylation. Oncogene. 2007;26(3):395–406. doi: 10.1038/sj.onc.1209789. [DOI] [PubMed] [Google Scholar]
- 150.Liu H., Yan S., Ding J., Yu T.T., Cheng S.Y. DeSUMOylation of Gli1 by SENP1 attenuates sonic Hedgehog signaling. Mol Cell Biol. 2017;37(18) doi: 10.1128/MCB.00579-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Witty J., Aguilar-Martinez E., Sharrocks A.D. SENP1 participates in the dynamic regulation of Elk-1 SUMOylation. Biochem J. 2010;428(2):247–254. doi: 10.1042/BJ20091948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Driscoll J.J., Pelluru D., Lefkimmiatis K. The sumoylation pathway is dysregulated in multiple myeloma and is associated with adverse patient outcome. Blood. 2010;115(14):2827–2834. doi: 10.1182/blood-2009-03-211045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hoefer J., Schafer G., Klocker H. PIAS1 is increased in human prostate cancer and enhances proliferation through inhibition of p21. Am J Pathol. 2012;180(5):2097–2107. doi: 10.1016/j.ajpath.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 154.Wang Q., Xia N., Li T. SUMO-specific protease 1 promotes prostate cancer progression and metastasis. Oncogene. 2013;32(19):2493–2498. doi: 10.1038/onc.2012.250. [DOI] [PubMed] [Google Scholar]
- 155.Jacques C., Baris O., Prunier-Mirebeau D. Two-step differential expression analysis reveals a new set of genes involved in thyroid oncocytic tumors. J Clin Endocrinol Metab. 2005;90(4):2314–2320. doi: 10.1210/jc.2004-1337. [DOI] [PubMed] [Google Scholar]
- 156.Hendriks I.A., Vertegaal A.C. A comprehensive compilation of SUMO proteomics. Nat Rev Mol Cell Biol. 2016;17(9):581–595. doi: 10.1038/nrm.2016.81. [DOI] [PubMed] [Google Scholar]
- 157.Lamoliatte F., McManus F.P., Maarifi G., Chelbi-Alix M.K., Thibault P. Uncovering the SUMOylation and ubiquitylation crosstalk in human cells using sequential peptide immunopurification. Nat Commun. 2017;8:14109. doi: 10.1038/ncomms14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chow K.H., Elgort S., Dasso M., Powers M.A., Ullman K.S. The SUMO proteases SENP1 and SENP2 play a critical role in nucleoporin homeostasis and nuclear pore complex function. Mol Biol Cell. 2014;25(1):160–168. doi: 10.1091/mbc.E13-05-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Duheron V., Nilles N., Pecenko S., Martinelli V., Fahrenkrog B. Localisation of Nup153 and SENP1 to nuclear pore complexes is required for 53BP1-mediated DNA double-strand break repair. J Cell Sci. 2017;130(14):2306–2316. doi: 10.1242/jcs.198390. [DOI] [PubMed] [Google Scholar]