Summary
Precise optogenetic control, ideally down to single cells in dense cell populations, is essential in understanding the heterogeneity of cell networks. Devices with such capability, if built in a chip scale, will advance optogenetic studies at cellular levels in a variety of experimental settings. Here we demonstrate optogenetic control of intracellular Ca2+ dynamics at the single cell level using a 16-μm pitched micro-light emitting diode (LED) array that features high brightness, small spot size, fast response, and low voltage operation. Individual LED pixels are able to reliably trigger intracellular Ca2+ transients, confirmed by fluorescence microscopy and control experiments and cross-checked by two genetically coded Ca2+ indicators. Importantly, our array can optogenetically address individual cells that are sub-10 μm apart in densely packed cell populations. These results suggest the possible use of the micro-LED array toward a lab-on-a-chip for single-cell optogenetics, which may allow for pharmaceutical screening and fundamental studies on a variety of cell networks.
Subject Areas: Techniques in Genetics, Cellular Neuroscience, Techniques in Neuroscience, Bioelectronics, Electronic Materials
Graphical Abstract
Highlights
-
•
Precise optogenetic control of Ca2+ signaling down to the single cell level
-
•
Bright, localized optogenetic stimulus with a high-density micro-LED array
-
•
Advancing micro-LED arrays toward a lab-on-a-chip for single-cell optogenetics
Techniques in Genetics; Cellular Neuroscience; Techniques in Neuroscience; Bioelectronics; Electronic Materials
Introduction
Over the past decade, optogenetics has emerged as a powerful method to interrogate specific cell types in complex tissues (Boyden, 2015, Deisseroth, 2015). In this method, cells expressed with light-sensitive proteins can be either excited or silenced, when exposed to light pulses at specific wavelengths (Boyden et al., 2005, Prakash et al., 2012). Such bidirectional optical control over the activity of cells enables minimally invasive assessment of their roles at the levels of cells, circuits, and behavior. For these reasons, optogenetics is widely used to modulate the activity of neurons (Chow et al., 2010, Hochbaum et al., 2014), cardiomyocytes (Wang et al., 2017, Johnston et al., 2017), C2C12 myotubes (Asano et al., 2015, Sebille et al., 2017), and human embryonic kidney 293 cells (i.e., HEK 293) (Baaske et al., 2018, Miyazaki et al., 2019), which deepens the understanding of brain/heart/muscle functions and gene expression.
To make the full impact of optogenetics, research has now focused on advancing the hardware to enable precise optogenetic control (McGovern et al., 2010, Nakajima et al., 2012, Steude et al., 2016), ideally down to single cells in dense cell populations. Such single-cell precision is essential in understanding the heterogeneity of cell networks, which is challenging to achieve by electrical stimulation methods using micro/nanoelectrodes (Hierlemann et al., 2011, Abbott et al., 2017, Lu et al., 2018). If successful, the resulting hardware will help scientists find precise connections within and between different tissue regions at an unprecedented cellular level. However, it is technically difficult to express optogenetic actuators in targeted single cells; in fact, densely packed cells often express them simultaneously, thereby all becoming light sensitive. Thus, to achieve single-cell optogenetic control one should employ high-density light sources that can address individual cells by localized light output.
Among available light sources, micron-sized light-emitting diode arrays (i.e., micro-light emitting diode [LED] arrays) are suitable for high-precision optogenetic control (Steude et al., 2016, Poher et al., 2008, Grossman et al., 2010, McGovern et al., 2010, Nakajima et al., 2012, Pisanello et al., 2016). These devices are recognized for their scalability, good lifetime in biological environments, and medium power dissipation for in vivo use. To date, GaN-based micro-LED arrays with 50- to 200-μm pitches have been built into the microscope optics for multi-site light illumination at a variety of settings, using a patch clamp or microelectrode arrays to record optogenetically induced action potentials (Grossman et al., 2010, Nakajima et al., 2012). Recently, organic micro-LED arrays with sub-10-μm pitches have been applied to HEK 293 cell culture, employing a patch clamp to monitor the photocurrent in single cells that were illuminated by three LED pixels (Steude et al., 2016). Although these studies showcased single-cell optogenetics using high-density micro-LEDs, studying cell activity in the electrical domain only is insufficient to fully understand cellular network dynamics. In fact, cell circuits often not only involve the transmission of electrical signals among cells but also associate with complex synaptic chemistry (Garris, 2010, Andrews, 2013). These chemistries correlate with each other, play key roles in regulating cell activity, and add to high-content analysis of cell signaling. For instance, intracellular calcium concentration (i.e., [Ca2+]) is an essential biochemical signal in the regulation of muscle contraction, neurotransmitter release, and gene expression (Berridge et al., 2003, Clapham, 2007, Tu et al., 2016, Grewe et al., 2010, Seta et al., 2004).
To this end, here we demonstrate precise optogenetic control of intracellular Ca2+ dynamics at the single cell level using a 100%-yield, 16-μm pitched micro-LED array that can output bright, localized, and fast-switching light in low-voltage operation. Single LED pixels are able to reliably trigger intracellular Ca2+ transients, evidenced by fluorescence microscopy, control groups, and comparative studies using two complementary Ca2+ indicators. Importantly, our array can optogenetically address individual cells that are sub-10 μm apart in densely packed cell populations. Our results suggest the promise of the high-density micro-LED array toward a lab-on-a-chip for single-cell optogenetics. Combined with its highly scalable structure, this device may enable exciting opportunities in pharmaceutical screening and cell signaling studies in a variety of cell networks.
Results
To conduct Ca2+ imaging under optogenetic stimulus, the activation spectrum of the optogenetic actuator and the excitation spectrum of the Ca2+ indicator need to be well separated from each other. This way we can minimize the optical cross talk between the excitation light (used for Ca2+ imaging) and the activation light (used for optogenetic control). Therefore, we selected two genetically coded Ca2+ indicators, jRCaMP1a and NIR-GECO1 (Dana et al., 2016, Qian et al., 2019), each paired with an optogenetic actuator, ChR2 (Zhang et al., 2007), and co-expressed them (either ChR2 with jRCaMP1a or ChR2 with NIR-GECO1) in HEK 293 cells seeded on a polydimethylsiloxane (PDMS) piece (Figures 1A and S1). We note that NIR-GECO1 is an inverse response indicator to [Ca2+] change, which is opposite to jRCaMP1a. Therefore, a comparative study using these two complementary Ca2+ indicators can cross-check the effectiveness of the optogenetic stimulus applied in the cell experiment.
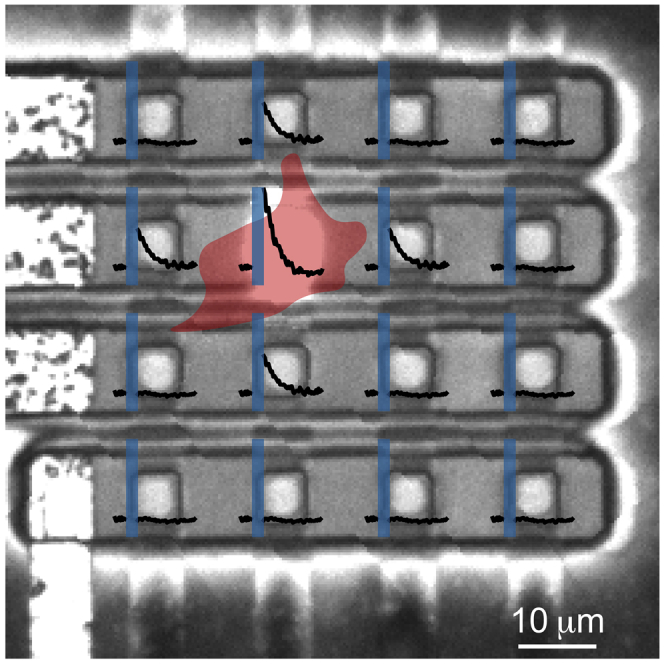
Figure 1.
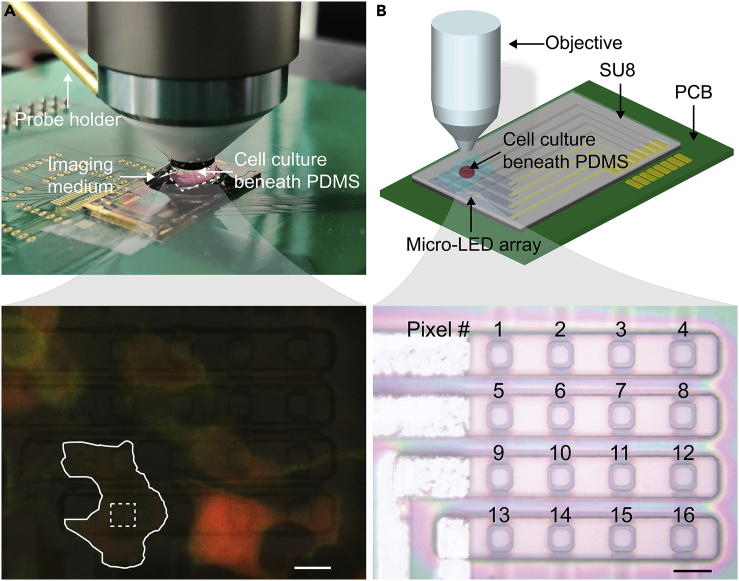
Experimental Setup
(A) One micro-LED array wired bonded onto a printed circuit board (PCB) under an upright fluorescence microscope configured for cell imaging. A flipped PDMS piece, seeded with cells on its top surface, was aligned to LED pixels by a probe holder; this way ChR2 (green)-jRCaMP1a (red) co-expressed cell (outlined) spatially overlapped with the LED pixel (squared). Scale bar, 10 μm.
(B) Illustration of the experimental setup using a 4-by-4 micro-LED array. Scale bar, 10 μm.
See also Figures S1–S4.
On the hardware side, we fabricated a GaN-based, 4-by-4 micro-LED array that can output 462/19 nm light to activate ChR2 (Figure S2). This device was built on commercial epitaxial GaN-on-Si wafers, formed by sequentially growing multiple GaN-based layers on top of a (111) Si substrate (see Figure S3 and Transparent Methods). Using reactive-ion etching steps, a total of 16 LED pixels, each 6.5 μm-by-6.5 μm in size, were patterned in a cross-bar structure with a 16-μm pitch (Figure 1B). We noted that this pitch size is three times smaller than previous GaN-based LED arrays used for optogenetics (Poher et al., 2008, Grossman et al., 2010, McGovern et al., 2010) and close to the typical diameter of a HEK 293 cell. The column and row select lines of the array were formed by Ni/indium tin oxide (5/120 nm for p-GaN contacts) and Ti/Al/Ti/Au layers (10/70/10/120 nm for n-GaN contacts), respectively, and passivated by plasma-enhanced chemical vapor deposition-based SiO2 (PECVD- SiO2) layers. The array was then encapsulated by another PECVD-SiO2 layer (∼200 nm) with a cross-linked SU8 layer on top and wire-bonded onto a printed circuit board for pixel selection (Figure S4). During cell experiments, we flipped a PDMS piece seeded with cells and placed it onto the encapsulated array (Figure 1B). This way, cells faced the LED pixels and could be aligned to the pixel of interest by moving the PDMS piece with a micro-manipulator (see the probe holder in Figure 1A).
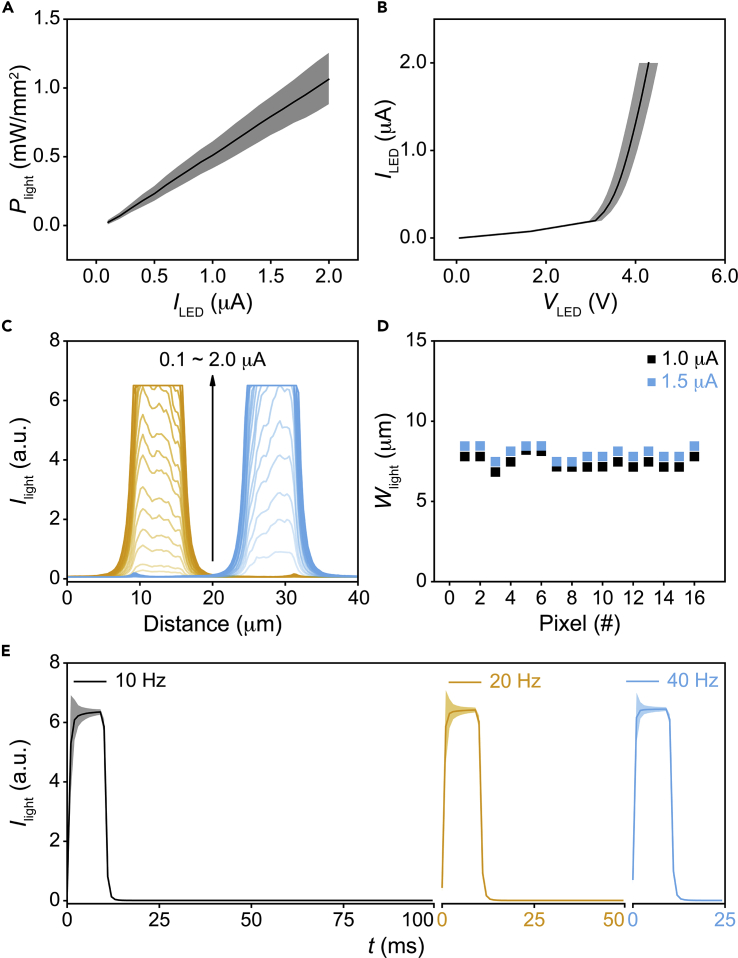
To enable optogenetic control at the single cell level, the micro-LED array is required to output bright, localized, and fast-switching light, ideally in a low-voltage operation. To this end, we first measured the optical power density (Plight) and the spatial profile of the illumination spot (Ilight) of each LED pixel, using an optical power meter and a fluorescence microscope, respectively. When biased at injection currents (ILED) ranging from 0.1 to 2.0 μA, all 16 pixels show high brightness with Plight ∼ 0.1–1.0 mW/mm2 (Figure 2A), which falls into the range required for optogenetic control in HEK 293 cells and mammalian neurons (Boyden et al., 2005, Steude et al., 2016, Morton et al., 2019). We note that this high brightness is achieved with the driving voltage across each pixel (VLED) being less than 4.8 V (Figure 2B). This low-voltage operation leads to sub-10-μW electrical power dissipation per pixel, which may ultimately allow for in vivo use (Marblestone et al., 2013, Mao et al., 2018). Moreover, at ILED = 1.0 or 1.5 μA, all 16 pixels output small light spots with the full width at half maximum (FWHM) < 10 μm at the array surface (Figures 2C and 2D, the FWHM is overestimated here as the center of the light spot in some pixels saturates the camera). Importantly, this localized pixel output with Plight ∼ 0.5–0.8 mW/mm2 is encouraging for optogenetic control over single cells close to the array surface, as HEK 293 cells are ∼10 μm in size. Finally, we found that such bright, localized pixel output (i.e., Plight ∼ 0.5 mW/mm2, FWHM <10 μm) can be pulsed with a 10-ms duration at up to 40-Hz pulsing frequencies (Figure 2E). After 3 ms in each pulse, the pulsed light intensity approached to its steady state value, with <3% variation among all pulses during 1-s recording. These results suggest that our LEDs meet the brightness, resolution, and speed requirement for optogenetic studies at cellular levels.
Figure 2.
Array Characterization
(A) Plight versus ILED for all 16 pixels.
(B) ILED-VLED curves for all 16 pixels.
(C) Spatial profile of the pixel output (from two neighboring pixels) at the array surface with ILED ranging from 0.1 to 2.0 μA.
(D) FWHM values of all 16 pixels with ILED = 1.0 and 1.5 μA.
(E) Pixel output pulsed with a 10-ms pulse duration at 10- to 40-Hz pulsing frequencies. The pixel was biased at ILED = 2.0 μA.
In (A), (B), and (E), shaded areas represent ±1 SD.
To prepare the optogenetic experiments, we flipped the PDMS piece—seeded with HEK 293 cells on its top surface—and placed it onto the array to let cells face the LED pixels. This PDMS-flipping approach adds to single-cell optogenetics in two ways. First, by getting cells closer to the array, this approach enhances the amount of light each cell receives and thus the strength of optogenetic control. Second, since LEDs emit light omnidirectionally, the spatial resolution they can offer is better close to the array surface, where the light spot is smaller. On the biology side, we cultured HEK 293 cells on sterilized PDMS pieces at 37°C in a humidified incubator, added the co-factor all-trans retinal to enhance light transduction of ChR2 (Steude et al., 2016, Cho et al., 2019), and transfected one of the two Ca2+ indicators with (co-transfection) or without (single transfection) ChR2 into these cells, all following the manufacturers' recommended protocols (see Transparent Methods). Before optogenetic experiments, we added an imaging solution containing 80 mM CaCl2 to cell culture, which serves to enhance cell responses to optogenetic stimulus as more extracellular Ca2+ would flush into the cell when ChR2 gets activated (Cho et al., 2019, Lin et al., 2009).
With these preparation steps, we next conducted optogenetic experiments using a standard Ca2+ imaging configuration by a fluorescence microscope. During each experiment, we pulsed 575/25-nm excitation light using the microscope with 0.5 frame per second and 100-ms exposure time per frame to alleviate the photo-bleaching effect. Meanwhile, the cell of interest was optogenetically stimulated by LED pixels in three consecutive recording periods. In each period, we illuminated the select LED pixel 20 s after the Ca2+ signal of the cell reached the steady state, with the excitation light being shut off at the same time. Here we chose not to collect Ca2+ imaging data during the optogenetic stimulation since the LED light would otherwise leak through the emission filter, introduce an artifact in the Ca2+ imaging data, and obscure the analysis. In parallel, we also conducted experiments with the optogenetic stimulus being provided by the microscope (3.92 mW at 470/24 nm), in which case all cells in the field of view were illuminated simultaneously.
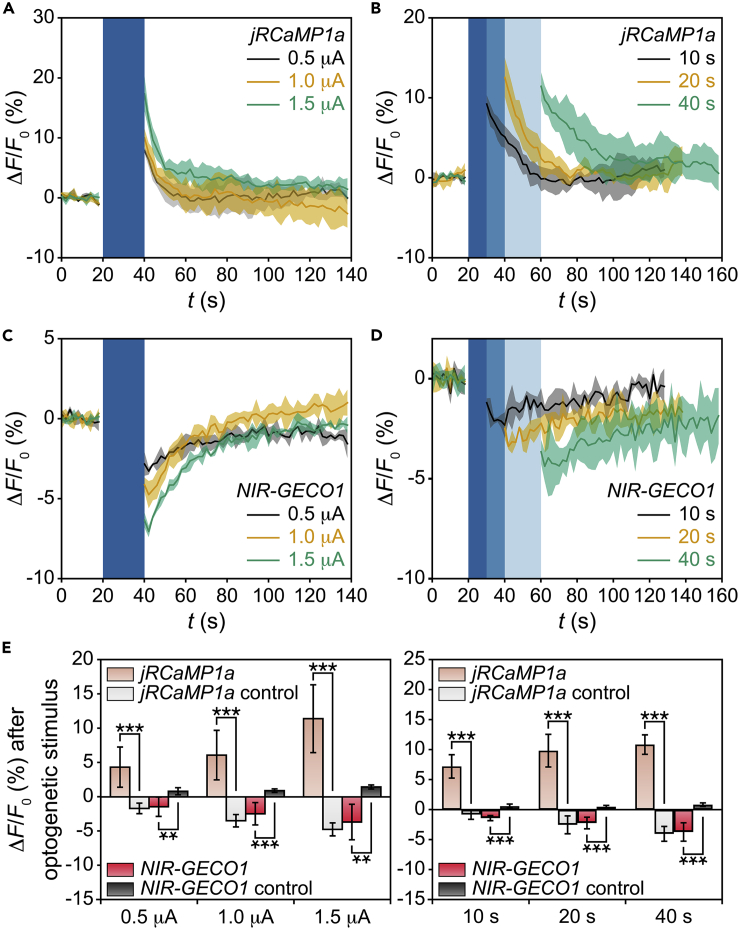
After each optogenetic stimulation with ILED ranging from 0.5 to 1.5 μA and the duration (TLED) ranging from 10 to 40 s, ChR2-jRCaMP1a co-expressed cells were found to reliably increase their emitted fluorescence intensities (Figures 3A and 3B). Here we define the F0 value as the 20-s average before each stimulation, subtracted by the background measured at the dark region in the field of view. The resulting positive ΔF/F0 values after each stimulation suggest an increase of intracellular Ca2+ level, coming from the optogenetically triggered Ca2+ influx to the illuminated cell (Dana et al., 2016, Morton et al., 2019). To examine if such Ca2+ increase was specific to the optogenetic activation of ChR2, we conducted control experiments with cells that are transfected with jRCaMP1a only (i.e., control cells). Indeed, these cells did not increase their ΔF/F0 values after optogenetic stimulus since no ChR2 were expressed to assist the Ca2+ influx (Figure S5). We confirmed these results by additional experiments with cells being optogenetically stimulated by the microscope (i.e., microscope-based stimulus), which yielded qualitatively similar ΔF/F0 traces in both co-expressed and control cells (Figure S6).
Figure 3.
Cell Responses to Optogenetic Stimulus Offered by LEDs
(A) ΔF/F0 traces from a ChR2-jRCaMP1a co-expressed cell with TLED = 20 s and ILED ranging from 0.5 to 1.5 μA.
(B) ΔF/F0 traces from a ChR2-jRCaMP1a co-expressed cell with TLED ranging from 10 to 40 s and ILED = 1.5 μA.
(C) ΔF/F0 traces from a ChR2-NIR-GECO1 co-expressed cell with TLED = 20 s and ILED ranging from 0.5 to 1.5 μA.
(D) Representative ΔF/F0 traces from a ChR2-NIR-GECO1 co-expressed cell with TLED ranging from 10 to 40 s and ILED = 1.5 μA. In (A), (B), (C), and (D), blue windows represent the periods of optogenetic stimulus; solid lines represent the mean values from three consecutive recording periods; shaded areas represent ±1 SD.
(E) ΔF/F0 signals versus ILED (left) and TLED (right) in both co-expressed and control cells. Error bars represent ±1 SD (n = 9 from three independent cells in each group, three recording periods from each cell); **p < 0.01, ***p < 0.001 based on Student's t test.
See also Figures S5 and S6.
On the other hand, we found that ChR2-NIR-GECO1 co-expressed cells reliably decreased their emitted fluorescence intensities by optogenetic stimulus (Figures 3C and 3D). The resulting negative ΔF/F0 values are opposite to that in ChR2-jRCaMP1a co-expressed cells, because NIR-GECO1 is an inverse response indicator to the optogenetically triggered Ca2+ influx (Qian et al., 2019). In the control experiments, cells only transfected with NIR-GECO1 did not decrease ΔF/F0 values after optogenetic stimulus since no ChR2 were expressed to assist the Ca2+ influx (Figure S5). Likewise, we confirmed these results by additional experiments with microscope-based optogenetic stimulus, which yielded similar ΔF/F0 traces (Figure S6). These data with NIR-GECO1 being the Ca2+ indicator further validate the effectiveness of the optogenetic stimulus offered by LEDs.
To quantify the strength of the applied optogenetic stimulus, here we define the ΔF/F0 value right after each optogenetic stimulus as our signal. To perform statistical analysis, we collected data from three independent ChR2-jRCaMP1a co-expressed cells, ChR2-NIR-GECO1 co-expressed cells, or their corresponding control cells. Since each cell was tested in three consecutive recording periods, our statistics is based on n = 9 such periods from three independent cells (Figure 3E).
In ChR2-jRCaMP1a co-expressed cells, the ΔF/F0 value after optogenetic stimulus is positive and mildly increases with ILED and TLED. This dependence is likely because more LED stimulus, by increasing either ILED or TLED, would increase the amount of Ca2+ influx by opening more ChR2-related Ca2+ channels. In contrast, among jRCaMP1a control cells, the ΔF/F0 value after optogenetic stimulus becomes negative (confirmed by additional experiments using microscope-based stimulus, see Figure S6) and mildly increases its amplitude (i.e., absolute value) with ILED and TLED. This negative ΔF/F0 value in jRCaMP1a control cells is likely due to the temporary photobleaching of jRCaMP1a by the 10- to 40-s constant LED illumination, which was later recovered at the end of each recording period. Another possible reason is that such constant LED illumination may temporarily increase the local temperature (Marblestone et al., 2013) and thus decrease the local pH next to the cell of interest. This local pH decrease can temporarily lower the fluorescence intensity of jRCaMP1a as reported before (Oliver et al., 2000, Kerruth et al., 2019, Zhao et al., 2011).
On the other hand, in ChR2-NIR-GECO1 co-expressed cells, the ΔF/F0 value after optogenetic stimulus is negative and mildly increases its amplitude (i.e., absolute value) with ILED and TLED. Again, this dependence is likely because the Ca2+ influx increases with the strength of the optogenetic stimulus offered by LEDs. In contrast, among NIR-GECO1 control cells, the ΔF/F0 value after optogenetic stimulus is found to be positive (confirmed by additional experiments using microscope-based stimulus, see Figure S6) and slightly increases with ILED and TLED. This positive ΔF/F0 value in NIR-GECO1 control cells is also likely because the 10- to 40-s constant LED illumination temporarily increased the local temperature and thus decreased the local pH next to the cell of interest. Such pH decrease (∼7.3 in the imaging solution) can temporarily enhance the fluorescence intensity of NIR-GECO1 as reported before (Qian et al., 2019). Another possibility is that such constant LED illumination may get NIR-GECO1 photoisomerized to a metastable brighter state, which was later overwhelmed by the photobleaching effect at the end of each recording period.
In comparison, we found that ChR2-jRCaMP1a co-expressed cells were overall brighter and had larger ΔF/F0 signals. ChR2-NIR-GECO1 co-expressed cells were overall inferior in these two aspects, but provided robust reverse response to Ca2+ changes, and helped cross-check if the optogenetic stimulus offered by LEDs was effective. In addition, our LEDs can typically generate ΔF/F0 values that are on par with—if not larger than—those generated by microscope-based stimulus (Figure S6). This fact re-affirms that our LEDs can indeed provide reliable optogenetic control of Ca2+ signaling.
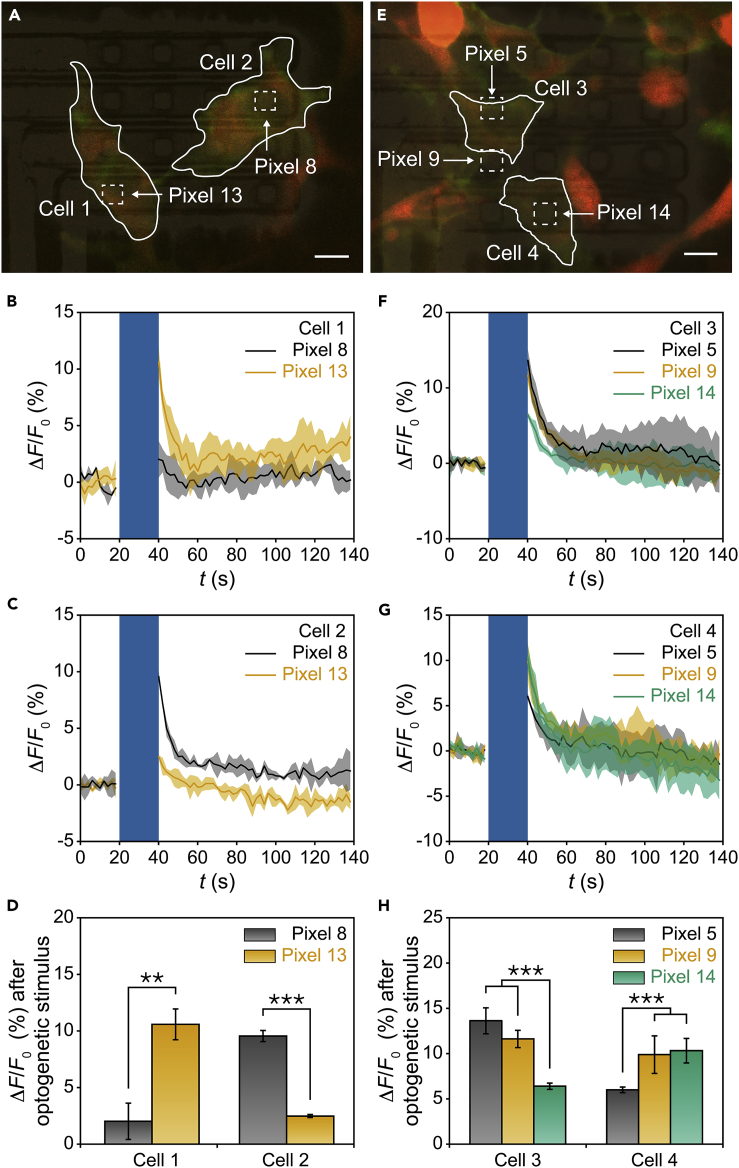
After validating the performance of our micro-LEDs, we now examine if they can provide precise optogenetic control at the single cell level in dense cell populations. To achieve this, we chose to optogenetically stimulate one pair of neighboring ChR2-jRCaMP1a co-expressed cells using different LED pixels (Figure 4), all with TLED = 20 s and Plight ∼ 0.71 mW/mm2 to compare their evoked ΔF/F0 signals (note: different pixels were biased at different ILED owing to pixel-to-pixel variation).
Figure 4.
Spatial Resolution of the Optogenetic Stimulus Offered by LEDs
(A) One pair of cells (outlined, overlapped with pixels 13 and 8) that were sub-10 μm apart. Scale bar, 10 μm.
(B) ΔF/F0 traces of cell 1 stimulated by pixels 8 and 13 with TLED = 20 s.
(C) ΔF/F0 traces of cell 2 stimulated by pixels 8 and 13 with TLED = 20 s.
(D) Statistical analysis of ΔF/F0 signals from cell 1 and cell 2.
(E) Another pair of cells (outlined, overlapped with pixels 5, 9, and 14) that were sub-5 μm apart. Scale bar, 10 μm.
(F) ΔF/F0 traces of cell 3 stimulated by pixels 5, 9, and 14 with TLED = 20 s.
(G) ΔF/F0 traces of cell 4 stimulated by pixels 5, 9, and 14 with TLED = 20 s.
(H) Statistical analysis of ΔF/F0 signals from cell 3 and cell 4.
In (B), (C), (F), and (G), blue windows represent the periods of optogenetic stimulus; solid lines represent the mean values from three consecutive recording periods; shaded areas represent ±1 SD. The ILED values applied to output Plight ~ 0.71 mW/mm2 were 1.2 μA for pixel 5, 1.6 μA for pixel 8, 1.5 μA for pixel 9, 1.0 μA for pixel 13, and 1.1 μA for pixel 14. In (D) and (H), error bars represent ±1 SD (n = 3 recording periods); **p < 0.01, ***p < 0.001 based on Student's t test. See also Figure S7.
Specifically, cell 1 (overlapped with pixel 13) and cell 2 (overlapped with pixel 8) were sub-10 μm apart with an ∼50 μm center-to-center distance (Figure 4A). Our data in Figures 4B–4D show that (1) cell 1 had significantly larger ΔF/F0 signals when it got stimulated by pixel 13 than when it got stimulated by pixel 8 and (2) cell 2 had significantly larger ΔF/F0 signals when it got stimulated by pixels 8 than when it got stimulated by pixel 13. These results show that ChR2-expressed cells indeed responded more to LED pixels that were overlapped with them. Moreover, either pixel 8 or pixel 13 introduced low cross talk in the cell that was not overlapped with the pixel; the ΔF/F0 signal in the cell that was overlapped with the pixel was more than three times that in the cell that was not overlapped with the pixel (i.e., selectivity >3). Importantly, these data suggest that our array can indeed address individual cells that are sub-10 μm apart with low cross talk; pixels 13 and 8 can provide such precise optogenetic control over cell 1 and cell 2, respectively.
To examine the limit of spatial resolution our array can achieve, we conducted another experiment (Figure 4E) with two cells even closer to each other. Specifically, cell 3 (overlapped with pixels 5 and 9) and cell 4 (overlapped with pixel 14, spatially closer to pixel 9 than pixel 5) were sub-5 μm apart with an ∼30-μm center-to-center distance. Our data in Figures 4F–4H show that (1) cell 3 had significantly larger ΔF/F0 signals when it got stimulated by pixels 5 and 9 than when it got stimulated by pixel 14 and (2) cell 4 had significantly larger ΔF/F0 signals when it got stimulated by pixels 14 and 9 than when it got stimulated by pixel 5. These results reaffirm that ChR2-expressed cells responded more to LED pixels that were overlapped with or closer to them. The fact that cell 4 responded similarly to pixels 9 and 14 is likely because cell 4 was far away from the array surface, where the pixel 9 output was less confined (i.e., spot size increased to >10 μm). However, it is noted that the selectivity in this experiment was less than 3, suggesting that our array cannot address individual cells that are sub-5 μm apart with low cross talk. Taking one step further, we observed that the selectivity was even lower (consistently <2) when cells were sub-1 μm apart (see additional two experiments in Figure S7). We thus conclude that our array can currently achieve sub-10-μm resolution.
Discussion
In sum, we demonstrated optogenetic control of Ca2+ signaling at the single-cell level using a 100%-yield high-density micro-LED array. Our array was found to output bright, localized, and fast-switching light in a low-voltage operation, which can precisely address individual HEK 293 cells that were sub-10 μm apart. Importantly, our results were confirmed by epifluorescence microscopy, control experiments, and cross-checked by two complementary Ca2+ indicators, all of which showed statistical significance. This work suggests the promise of the high-density micro-LED array toward a lab-on-a-chip for single-cell optogenetics, which can add to high-content cell signaling studies. Combined with its highly scalable structure, this device may provide a cost-effective platform for pharmaceutical screening and fundamental studies on a variety of cell networks. Leveraging standard semiconductor fabrication steps, our LED arrays can, for instance, readily extend to a medium number of pixels (∼100) to study computational algorithms of the neural network at the in vitro setting. On the other hand, we can create alternative versions of the array to output different wavelengths in the visible spectrum. For example, AlGaInP-based micro-LED arrays can be similarly built to output 600–630 nm light, which can be applied to actuate red-shifted opsins (e.g., Chrimson) and monitor intracellular Ca2+ dynamics using green Ca2+ indicators (e.g., GCaMP7) at the same time (Klapoetke et al., 2014, Dana et al., 2019).
Finally, we remark that our high-performance array can be used to study single-cell optogenetics in other cell types (e.g., neurons or cardiomyocytes) and provide precise optogenetic control over other cellular signals (e.g., intracellular potassium concentration). For ex-vivo or in vivo applications our arrays will need to be encapsulated by biocompatible and transparent films (e.g., SU8 or epoxy). Furthermore, if built along a solid-state shank similar to that of the implantable silicon microelectrode arrays (Scholvin et al., 2016), the resulting device would be typically sub-100 μm wide, sub-100 μm thick, and 3–5 mm long, which may ultimately enable single-cell optogenetics in deep tissues. By sequentially illuminating individual pixels, such device would routinely consume sub-10 mW electrical power, which is suitable for long term in vivo use. If successful, for instance, one may implant such devices to trigger intracellular [Ca2+] change during muscle recovery from injury (van Bremen et al., 2017) or to offer precise modulation of the neurocircuitry in deep brain (Yawo et al., 2013).
Limitations of the Study
The energy conversion efficiency of our LED pixels, defined as the value of Plight/(ILED·VLED) here, suffered from voltage drop across the contact wires. To solve this issue, our array layout will need to be further optimized to reduce the series resistance from these contact wires. On the other hand, we may be able to change the constant LED illuminations to pulsed LED illuminations. We expect such change would alleviate the local heating effect to the cell of interest. Furthermore, by alternatingly pulsing LED illuminations and the excitation light (i.e., not turning them on at the same time), we might be able to monitor intracellular Ca2+ dynamics during the period of optogenetic stimulus; we did not do this with constant LED illuminations in this work, because the bright LED output was found to partially leak through the emission filters of the microscope and act as the background noise for Ca2+ imaging. In terms of the biocompatibility, our LEDs passivated with an SU8 layer were able to monitor cell activity for ca. 1.5 h at room temperature. We expect that this period can be further extended if cells on the array could be kept at ∼37°C by a fixed heating stage. Last but not least, the spatial resolution of our array is currently limited by the omnidirectional emission from LEDs and can be improved by adding light guide or microlens layers in the future.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Science Foundation under ECCS-1835268 and CMMI-1662835. The authors thank A. Arbabi, Y. Zhao, K. D. Piatkevich, T. Xie, and H. Tan for scientific discussions and technical assistance.
Author Contributions
G.X. conceived and supervised the project. D.M. designed, fabricated, and characterized the arrays. N.L., D.M., and Y.S. prepared cell culture and developed the protocols for cell experiments. D.M. and Z.X. performed the cell experiments. Z.X., D.M., and G.X. analyzed the data and wrote the paper. All authors discussed the results and reviewed the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: November 22, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.10.024.
Contributor Information
Yubing Sun, Email: ybsun@umass.edu.
Guangyu Xu, Email: guangyux@umass.edu.
Data and Code Availability
All data generated or analyzed during this study are included in this published article and its Supplemental Information.
Supplemental Information
References
- Abbott J., Ye T., Qin L., Jorgolli M., Gertner R.S., Ham D., Park H. CMOS nanoelectrode array for all-electrical intracellular electrophysiological imaging. Nat. Nanotechnol. 2017;12:460–466. doi: 10.1038/nnano.2017.3. [DOI] [PubMed] [Google Scholar]
- Andrews A.M. The BRAIN initiative: toward a chemical connectome. ACS Chem. Neurosci. 2013;4:645. doi: 10.1021/cn4001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T., Ishizuka T., Morishima K., Yawo H. Optogenetic induction of contractile ability in immature C2C12 myotubes. Sci. Rep. 2015;5:8317. doi: 10.1038/srep08317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baaske J., Gonschorek P., Engesser R., Dominguez-Monedero A., Raute K., Fischbach P., Müller K., Cachat E., Schamel W.W., Minguet S. Dual-controlled optogenetic system for the rapid down-regulation of protein levels in mammalian cells. Sci. Rep. 2018;8:15024. doi: 10.1038/s41598-018-32929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Boyden E.S. Optogenetics and the future of neuroscience. Nat. Neurosci. 2015;18:1200–1201. doi: 10.1038/nn.4094. [DOI] [PubMed] [Google Scholar]
- Boyden E.S., Zhang F., Bamberg E., Nagel G., Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Cho Y.K., Park D., Yang A., Chen F., Chuong A.S., Klapoetke N.C., Boyden E.S. Multidimensional screening yields channelrhodopsin variants having improved photocurrent and order-of-magnitude reductions in calcium and proton currents. J. Biol. Chem. 2019;294:3806–3821. doi: 10.1074/jbc.RA118.006996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow B.Y., Han X., Dobry A.S., Qian X., Chuong A.S., Li M., Henninger M.A., Belfort G.M., Lin Y., Monahan P.E. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Dana H., Mohar B., Sun Y., Narayan S., Gordus A., Hasseman J.P., Tsegaye G., Holt G.T., Hu A., Walpita D. Sensitive red protein calcium indicators for imaging neural activity. Elife. 2016;5:e12727. doi: 10.7554/eLife.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana H., Sun Y., Mohar B., Hulse B.K., Kerlin A.M., Hasseman J.P., Tsegaye G., Tsang A., Wong A., Patel R. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods. 2019;16:649–657. doi: 10.1038/s41592-019-0435-6. [DOI] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 2015;18:1213–1225. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris P.A. Advancing neurochemical monitoring. Nat. Methods. 2010;7:106–108. doi: 10.1038/nmeth0210-106. [DOI] [PubMed] [Google Scholar]
- Grewe B.F., Langer D., Kasper H., Kampa B.M., Helmchen F. High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision. Nat. Methods. 2010;7:399–405. doi: 10.1038/nmeth.1453. [DOI] [PubMed] [Google Scholar]
- Grossman N., Poher V., Grubb M.S., Kennedy G.T., Nikolic K., McGovern B., Palmini R.B., Gong Z., Drakakis E.M., Neil M.A. Multi-site optical excitation using ChR2 and micro-LED array. J. Neural Eng. 2010;7:016004. doi: 10.1088/1741-2560/7/1/016004. [DOI] [PubMed] [Google Scholar]
- Hierlemann A., Frey U., Hafizovic S., Heer F. Growing cells atop microelectronic chips: interfacing electrogenic cells in vitro with CMOS-based microelectrode arrays. Proc. IEEE. 2011;99:252–284. [Google Scholar]
- Hochbaum D.R., Zhao Y., Farhi S.L., Klapoetke N., Werley C.A., Kapoor V., Zou P., Kralj J.M., Maclaurin D., Smedemark-Margulies N. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods. 2014;11:825–833. doi: 10.1038/nmeth.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C.M., Rog-Zielinska E.A., Wülfers E.M., Houwaart T., Siedlecka U., Naumann A., Nitschke R., Knöpfel T., Kohl P., Schneider-Warme F. Optogenetic targeting of cardiac myocytes and non-myocytes: tools, challenges and utility. Prog. Biophys. Mol. Biol. 2017;130:140–149. doi: 10.1016/j.pbiomolbio.2017.09.014. [DOI] [PubMed] [Google Scholar]
- Kerruth S., Coates C., Dürst C.D., Oertner T.G., Török K. The kinetic mechanisms of fast-decay red-fluorescent genetically encoded calcium indicators. J. Biol. Chem. 2019;294:3934–3946. doi: 10.1074/jbc.RA118.004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapoetke N.C., Murata Y., Kim S.S., Pulver S.R., Birdsey-Benson A., Cho Y.K., Morimoto T.K., Chuong A.S., Carpenter E.J., Tian Z. Independent optical excitation of distinct neural populations. Nat. Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.Y., Lin M.Z., Steinbach P., Tsien R.Y. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J. 2009;96:1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Liu X., Kuzum D. Graphene-based neurotechnologies for advanced neural interfaces. Curr. Opin. Biomed. Eng. 2018;6:138–147. [Google Scholar]
- Mao D., Morley J., Zhang Z., Donnelly M., Xu G. High-yield passive Si photodiode array towards optical neural recording. IEEE Electron Device Lett. 2018;39:524–527. [Google Scholar]
- Marblestone A.H., Zamft B.M., Maguire Y.G., Shapiro M.G., Cybulski T.R., Glaser J.I., Amodei D., Stranges P.B., Kalhor R., Dalrymple D.A. Physical principles for scalable neural recording. Front. Comput. Neurosci. 2013;7:137. doi: 10.3389/fncom.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern B., Palmini R.B., Grossman N., Drakakis E.M., Poher V., Neil M.A.A., Degenaar P. A new individually addressable micro-LED array for photogenetic neural stimulation. IEEE Trans. Biomed. Circuits Syst. 2010;4:469–476. doi: 10.1109/TBCAS.2010.2081988. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Chowdhury S., Yamashita T., Matsubara T., Yawo H., Yuasa H., Yamanaka A. Large timescale interrogation of neuronal function by fiberless optogenetics using lanthanide micro-particles. Cell Rep. 2019;26:1033–1043. doi: 10.1016/j.celrep.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Morton A., Murawski C., Deng Y., Keum C., Miles G.B., Tello J.A., Gather M.C. Photostimulation for in vitro optogenetics with high-power blue organic light-emitting diodes. Adv. Biosyst. 2019;3:1800290. doi: 10.1002/adbi.201800290. [DOI] [PubMed] [Google Scholar]
- Nakajima A., Kimura H., Sawadsaringkarn Y., Maezawa Y., Kobayashi T., Noda T., Sasagawa K., Tokuda T., Ishikawa Y., Shiosaka S. CMOS image sensor integrated with micro-LED and multielectrode arrays for the patterned photostimulation and multichannel recording of neuronal tissue. Opt. Express. 2012;20:6097–6108. doi: 10.1364/OE.20.006097. [DOI] [PubMed] [Google Scholar]
- Oliver A.E., Baker G.A., Fugate R.D., Tablin F., Crowe J.H. Effects of temperature on calcium-sensitive fluorescent probes. Biophys. J. 2000;78:2116–2126. doi: 10.1016/S0006-3495(00)76758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanello F., Sileo L., De Vittorio M. Micro-and nanotechnologies for optical neural interfaces. Front. Neurosci. 2016;10:70. doi: 10.3389/fnins.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poher V., Grossman N., Kennedy G.T., Nikolic K., Zhang H.X., Gong Z., Drakakis E.M., Gu E., Dawson M.D., French P.M.W. Micro-LED arrays: a tool for two-dimensional neuron stimulation. J. Phys. D Appl. Phys. 2008;41:094014. [Google Scholar]
- Prakash R., Yizhar O., Grewe B., Ramakrishnan C., Wang N., Goshen I., Packer A.M., Peterka D.S., Yuste R., Schnitzer M.J. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat. Methods. 2012;9:1171–1179. doi: 10.1038/nmeth.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Piatkevich K.D., Mc Larney B., Abdelfattah A.S., Mehta S., Murdock M.H., Gottschalk S., Molina R.S., Zhang W., Chen Y. A genetically encoded near-infrared fluorescent calcium ion indicator. Nat. Methods. 2019;16:171–174. doi: 10.1038/s41592-018-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvin J., Kinney J.P., Bernstein J.G., Moore-Kochlacs C., Kopell N., Fonstad C.G., Boyden E.S. Close-packed silicon microelectrodes for scalable spatially oversampled neural recording. IEEE Trans. Biomed. Eng. 2016;63:120–130. doi: 10.1109/TBME.2015.2406113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebille S., Ayad O., Chapotte-Baldacci C.A., Cognard C., Bois P., Chatelier A. Optogenetic approach for targeted activation of global calcium transients in differentiated C2C12 myotubes. Sci. Rep. 2017;7:11108. doi: 10.1038/s41598-017-11551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seta K.A., Yuan Y., Spicer Z., Lu G., Bedard J., Ferguson T.K., Pathrose P., Cole-Strauss A., Kaufhold A., Millhorn D.E. The role of calcium in hypoxia-induced signal transduction and gene expression. Cell Calcium. 2004;36:331–340. doi: 10.1016/j.ceca.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Steude A., Witts E.C., Miles G.B., Gather M.C. Arrays of microscopic organic LEDs for high-resolution optogenetics. Sci. Adv. 2016;2:e1600061. doi: 10.1126/sciadv.1600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu M.K., Levin J.B., Hamilton A.M., Borodinsky L.N. Calcium signaling in skeletal muscle development, maintenance and regeneration. Cell Calcium. 2016;59:91–97. doi: 10.1016/j.ceca.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bremen T., Send T., Sasse P., Bruegmann T. Spot light on skeletal muscles: optogenetic stimulation to understand and restore skeletal muscle function. J. Muscle Res. Cell. Motil. 2017;38:331–337. doi: 10.1007/s10974-017-9481-9. [DOI] [PubMed] [Google Scholar]
- Wang Y., Lin W.K., Crawford W., Ni H., Bolton E.L., Khan H., Shanks J., Bub G., Wang X., Paterson D.J. Optogenetic control of heart rhythm by selective stimulation of cardiomyocytes derived from Pnmt+ cells in murine heart. Sci. Rep. 2017;7:40687. doi: 10.1038/srep40687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H., Asano T., Sakai S., Ishizuka T. Optogenetic manipulation of neural and non-neural functions. Dev. Growth Differ. 2013;55:474–490. doi: 10.1111/dgd.12053. [DOI] [PubMed] [Google Scholar]
- Zhang F., Wang L.P., Brauner M., Liewald J.F., Kay K., Watzke N., Wood P.G., Bamberg E., Nagel G., Gottschalk A. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Araki S., Wu J., Teramoto T., Chang Y.F., Nakano M., Abdelfattah A.S., Fujiwara M., Ishihara T., Nagai T. An expanded palette of genetically encoded Ca2+ indicators. Science. 2011;333:1888–1891. doi: 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplemental Information.