Abstract
Background:
Membrane contact sites (MCS) are fundamental for transmission and translation of signals in multicellular organisms. A prime example is junctional membrane complexes (JMC) in the cardiac dyads, where T-tubules are juxtaposed to the sarcoplasmic reticulum (SR). T-tubule uncoupling and remodeling are well-known features of cardiac disease and heart failure. Even subtle alterations in the association between T-tubules and the Junctional SR can cause serious cardiac disorders. Nexilin (NEXN) has been identified as an actin-binding protein and multiple mutations in the NEXN gene are associated with cardiac diseases, but the precise role of NEXN in heart function and disease is still unknown.
Methods:
Nexn global and cardiomyocyte specific KO mice were generated. Comprehensive phenotypic and RNA-seq and mass spectrometry analyses were performed. Heart tissue samples and isolated single cardiomyocytes were analyzed by electron and confocal microscopy.
Results:
Global and cardiomyocyte specific loss of Nexn in mice resulted in a rapidly progressive dilated cardiomyopathy. In vivo and in vitro analyses revealed that NEXN interacted with junctional SR proteins, was essential for optimal calcium transients, and was required for initiation of T-tubule invagination and formation.
Conclusion:
These results demonstrated that NEXN is a pivotal component of the JMC, and is required for initiation and formation of T-tubules, thus providing insight into mechanisms underlying cardiomyopathy in patients with mutations in NEXN.
Keywords: Cardiomyopathy, T-Tubule, Membrane Contact Sites, Junctional Membrane Complexes
Introduction
Recent findings have highlighted the critical role of membrane contact sites (MCSs), regions of close apposition between membranes of distinct intracellular organelles, in mediating intra-organelle communication, upending the traditional view of intracellular organelles floating freely within the cytoplasm like boats in a pond 1-3. It has become progressively clear that endoplasmic reticulum-plasma membrane (ER-PM) contact sites are crucial for many different biological processes involved in cell homeostasis and organismal health4-8.
In cardiomyocytes, specialized MCSs with an average gap size of 12nm between the plasma membrane (also known as sarcolemma in muscle cells) and the highly specialized endoplasmic reticulum (sarcoplasmic reticulum/SR), termed junctional membrane complexes (JMCs), are essential for calcium ion (Ca2+)-induced Ca2+ release(CICR), an amplification of Ca2+ signaling required to generate and maintain the rhythmic contraction of the heart 9-12. In fetal cardiomyocytes the formation of the JMCs is dependent on the junctional protein Junctophilin2 (Jph2)13. In adult cardiomyocytes, amplification of Ca2+ release is possible owing to a highly specialized network of sarcolemmal invaginations, Transverse (T)-tubules that are closely associated with the SR in a dyad configuration14. CICR occurs when sarcolemmal depolarization activates L-type calcium channels (LTCCs), causing an influx of Ca2+ that triggers a much higher Ca2+ release from the SR via type 2 Ryanodine Receptors (RyR2s) 9. In cardiomyocytes, these highly specialized JMCs, composed of clusters of LTCCs in the T-tubules, and RyR2 on the junctional SR are specifically localized in the dyads15, 16. Optimal CICR requires proper formation of dyads and association of other regulatory proteins with LTCC and RyR2. The dyads of the adult cardiomyocyte are formed gradually during development in a highly orchestrated manner17. Seminal to dyad establishment, T-tubule formation is initiated around postnatal day 5 (P5), as portions of the sarcolemma enriched in LTCC start to invaginate to become closely associated with the RyR2-enriched SR.
T-tubule uncoupling and remodeling are well-known features of cardiac disease and heart failure18-22. Even subtle alterations in the association between T-tubules and the Junctional SR can cause serious cardiac disorders23. Despite the key role of T-tubule and SR coupling for cardiac function, proteins required to initiate T-tubule formation are unknown. Here, we identify Nexilin (NEXN) as a previously unsuspected component of the JMC and show that it is essential for initiating the sarcolemma invaginations that lead to T-tubule formation in postnatal cardiomyocytes.
Multiple mutations in NEXN are associated with cardiomyopathy in humans, and deficiency of NEXN in zebrafish results in perturbed Z-disk stability and heart failure, highlighting a significant role in cardiac function24, 25. Global loss of NEXN in mice leads to severe cardiomyopathy and a reported endomyocardial fibroelastosis, resulting in perinatal death26. In contradiction to the zebrafish model, no sarcomeric alterations in cardiomyocytes of Nexn global knockout (gKO) mice were noted26. However, little is known as to specific role(s) of NEXN in cardiomyocytes, or mechanism(s) by which loss of NEXN results in rapidly progressive cardiomyopathy. Here, our findings reveal that NEXN, rather than being a Z-disk protein as previously thought, interacts with SR proteins and is required for initiation of T-tubule invagination and overall T-tubule formation. Our results demonstrate that NEXN is required to form this structure that is critical for optimal contraction of cardiomyocytes and normal heart function.
Methods
The data, analytical methods, and study materials will be made available to other researchers for the purposes of reproducing the results or replicating the procedure. The sources of reagents and detailed methods and any associated references are provided in the online-only Data Supplement. List of primers and antibodies used are reported in Supplemental Tables 1 and 2. All animal studies were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals27 and approved by the Institutional Animal Care and Use Committee of the University of California, San Diego. The mouse line was generated as previously described (Supplemental Figure 1)28. Neonatal and adult mice cardiomyocytes were prepared as previously described29, 30. RNA-seq reads were mapped against the Ensembl v91 mouse gene model (GRCm38) for counting reads and estimating gene expression as described by Salmon31. The DEseq2 package32 was used to evaluate the reproducibility and perform differential gene expression analysis.
Statistics
The survival of Nexn mice was estimated using the Kaplan-Meier method, and survival curves were compared using the log-rank test. For all other statistics, values were expressed as mean +/− SEM and statistical differences were tested by 2-tailed Student’s t-test. A value of P <0.05 was considered statistically significant. All the analyses were performed with GraphPad Prism software.
Results
Cardiomyocyte specific ablation of Nexn results in a rapidly progressive severe dilated cardiomyopathy
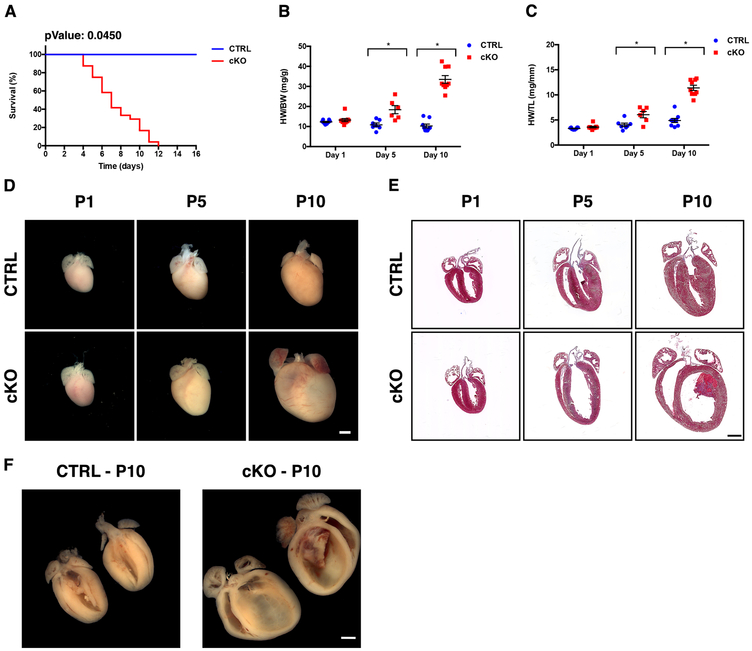
To investigate the role of NEXN in cardiac function, we generated Nexn gKO mice by crossing targeted mice containing Nexn floxed alleles (Nexnf/f) with global deleter Sox2-cre mice, resulting in complete loss of the protein (Supplemental Figure 1A-C). Global deficiency of NEXN resulted in perinatal lethality. All Nexn gKO mice died before postnatal day 12 (P12) exhibiting decreased body weight starting at 5 days after birth (P5). Heart weight to body weight (HW/BW) and heart weight to tibial length (HW/TL) ratios were significantly increased in gKO mice compared to WT littermate controls (CTRL) starting from P5 (Supplemental Figure 1D-F).
Transthoracic echocardiography revealed that global loss of NEXN resulted in increased left ventricular diameter (Supplemental Figure 1H, I). Abnormal dimensions in the heart were accompanied by reduced systolic cardiac function, as evidenced by a significant decrease in fractional shortening percentage (%FS) in gKO mice. Although left ventricular dimensions in Nexn gKO mice were not significantly changed compared to CTRLs at postnatal day 1 (P1), %FS was already reduced by 20% (Supplemental Figure 1G). To determine the starting point of abnormal heart function, echocardiography at embryonic day 18.5 (E18.5) mice was performed. No significant changes in %FS were observed between Nexn−/− mice and WT littermates at E18.5(Supplemental Figure 1G), indicating that defects in heart function consequent to loss of NEXN began to develop after E18.5.
Gross anatomical characterization of KO mice confirmed severe cardiac enlargement starting from P5 (Supplemental Figure 1J). Histological analysis of gKO hearts showed severe left ventricular dilation starting from P5 with a progressive phenotype, leading to thinning of the cardiac walls, clearly visible at postnatal day 10 (P10) (Supplemental Figure 1K).
Aherrahrou and colleagues reported that dilated hearts from Nexn gKO mice evidence endomyocardial fibroelastosis, a thickening of the endocardium characterized by elastic fibers and collagen 26. Although we similarly observed, from P7 onward, collagen positive “mural masses” in left ventricles of Nexn gKO mice, further histological analyses revealed an absence of elastic fibers, and presence of fibrin in these structures, suggesting they were most likely intracardiac mural thrombi (Supplemental Figure 2, Supplemental Movie).
To confirm that the Nexn gKO cardiac phenotype resulted from specific loss of NEXN in cardiomyocytes, we crossed Nexnf/f mice with cardiomyocyte-specific Xenopus laevis myosin light-chain 2-Cre(XMLC2) transgenic mice 33. This XMLC2-Cre line specifically drives Cre recombinase activity in myocardial cells (Supplemental Figure 3), our echocardiography studies also showed that this XMLC2-Cre mouse line in the same genetic background as that of our mouse models has normal cardiac function (Supplemental Figure 4). All Nexn cardiomyocytes specific KO (cKO) mice died before P12, with phenotypes recapitulating those of gKOs (Fig. 1, 2; Supplemental Table 3). Thus, reduced body size and weight, altered cardiac function, and resulting dilated cardiomyopathy (DCM) were all a direct consequence of NEXN loss in cardiomyocytes. Similar results were also obtained crossing Nexnf/f mice to cardiomyocyte-specific troponin T2 (cTNT) cre transgenic mice (Supplemental Figure 5).
Figure 1. Loss of NEXN in cardiomyocytes results in a progressive dilated cardiomyopathy.
(A) Kaplan-Meier survival curves for NEXN CTRL (n = 35) and cKO (n = 24) mice; (B, C) Graphs showing (B) HW/BW (n=7-9 mice per time point) and (C) HW/TL (n=5-6 mice per time point) in CTRL and cKO mice. (*) Statistically significant differences with P value < 0.05. (D) Representative whole heart images from postnatal day 1 (P1), 5 (P5) or 10 (P10) mice. (E) Representative 4-chambers view Masson’s trichrome staining images of longitudinal histological sections from the same stages. (F) Butterfly cut gross anatomy showing the tridimensional organization of the organized thrombus in the left ventricle of the cKO heart and the relative CTRL. (D-F) Scale bars 1mm.
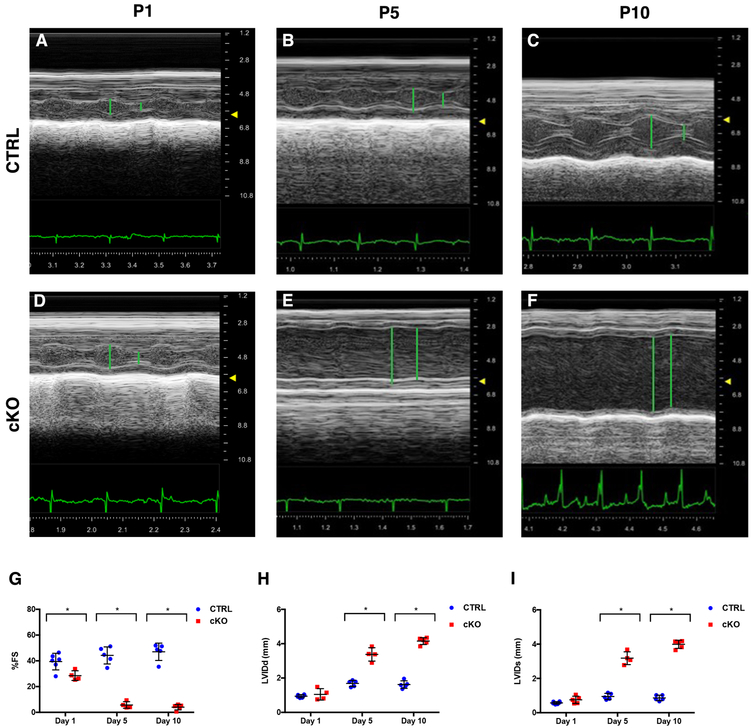
Figure 2. Cardiomyocytes-specific KO of NEXN results in reduced cardiac function.
(A-F) Representative echocardiographic M-mode images of NEXN mice: (A) P1 CTRL, %FS 40, LVIDd 0.84mm, LVIDs 0.50mm, heart rate (HR) 297; (B) P5 CTRL, %FS 46, LVIDd 1.42mm, LVIDs 0.76mm, HR 455; (C) P10 CTRL, %FS 49, LVIDd 1.96mm, LVIDs 1.03mm, HR 484; (D) P1 cKO, %FS 25, LVIDd 1.15mm, LVIDs 0.81mm, HR 410; (E) P5 cKO, %FS 9, LVIDd 3.40mm, LVIDs 3.08mm, HR 492; (F) P10 cKO, %FS 5, LVIDd 4.29mm, LVIDs 4.14mm, HR 422. (G-I) graphs representing echocardiographic measurements from CTRL and cKO mice (n = 6-7 mice per time point): (G) % FS, (H) LVIDd and (I) LVIDs. Echocardiographic parameters reported in Supplemental Table 3. (*) Statistically significant differences with P value < 0.05.
Loss of Nexilin alters Ca2+-handling gene and protein expression in the heart
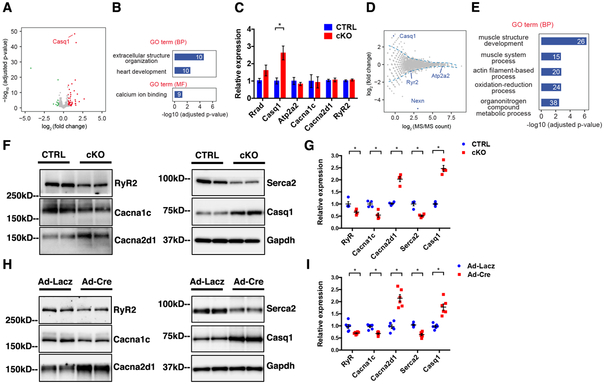
To gain a better understanding of how loss of NEXN in cardiomyocytes leads to this rapidly progressing severe DCM, we constructed RNA-Sequencing (RNA-Seq) libraries from cardiac ventricles of mice at P1, a time prior to Nexn cKO mice develop DCM and found that 74 genes were significantly altered in Nexn cKOs (Supplemental Figure 6, Fig. 3A). Gene Ontology (GO) enrichment analysis identified the top biological processes or molecular functions as extracellular structure organization, heart development, and Ca2+-binding (Fig. 3B). Quantitative PCR (q-PCR) on samples from hearts of E18.5 mice confirmed that expression of a set of Ca2+-handling proteins was altered in Nexn cKO mice with respect to littermate CTRLs, suggesting SR stress. Among these genes, calsequestrin, the main Ca2+-buffering protein of the SR, were significantly upregulated (Fig. 3C). To further investigate possible involvement of Ca2+-handling machinery in the phenotype resulting from cardiomyocyte-specific ablation of Nexn, we also performed mass spectrometry on lysates from P1 cardiac ventricles. A total of 2456 proteins were identified and quantified, with NEXN itself showing the most significant fold-change (Fig. 3D), suggesting the feasibility of this strategy for identifying changes at the proteome level. 185 proteins were substantially altered in cKO hearts compared to those of CTRLs (Fig. 3D). Consistent with results from RNA-seq, GO term enrichment analysis revealed proteins involved primarily in regulation of muscle system (Fig. 3E), including 12 Ca2+-binding proteins (Supplemental Table 4).
Figure 3. Abnormal expression of Ca2+-handling genes in NEXN cKO heart.
(A) Volcano plot of gene expression changes between CTRL and NEXN cKO P1 ventricles (n = 3). Genes with adjusted P value < 0.05 and fold change > 1.5 or < 2/3 are considered as significantly up- or down-regulated genes, and highlighted in red and green, respectively. (B) GO enrichment analysis of differentially expressed genes by RNAs-seq. Number of genes belonging to each category is indicated. BP: biological process, MF: molecular function. (C) qPCR analysis of Ca2+-handling genes from E18.5 mouse ventricles (n = 4). (D) MA plot showing the fold change of protein abundance against average MS/MS count assayed by mass spectrometry from P1 mouse ventricles (n = 4). Blue dotted line: fold-change cutoff selecting differentially expressed proteins. (E) GO enrichment analysis of differentially expressed proteins by mass spectrometry. (F-H) Western blot representative images and (G-I) quantification graphs of Ca2+-handling proteins from (F-G) E18.5 mouse ventricles (n = 4) and from (H-I) isolated neonatal NEXNf/f cardiomyocytes treated with Ad-Lacz or Ad-cre virus (n=6). (*) Statistically significant differences with P value < 0.05.
To investigate whether observed altered levels in Ca2+-binding proteins detected at P1 in Nexn cKO hearts could be a primary cause leading to DCM, western blot analyses for some of the major Ca2+-related proteins was performed on lysates from E18.5 hearts, a time just prior to evident defects in cardiac function. Results demonstrated that RyR2, LTCC subunits Cacna1c and Cacna2d1, Searc2, and Casq1 had altered expression levels in cKO hearts relative to those in CTRLs (Fig. 3F, G). Interestingly, although mRNA levels of Casq2, the major calsequestrin isoform in the heart, were slightly upregulated, its protein levels were not changed (Supplemental Figure 7) To further demonstrate that abnormal Ca2+-handling protein expression preceded functional and structural changes in Nexn cKOs, an in vitro model of acute Nexn ablation was utilized, treating primary neonatal Nexnf/f cardiomyocytes with a Cre-expressing adenovirus. Subsequent western blot analyses revealed changes in Ca2+-handling proteins consistent with those obtained from E18.5 ventricles (Fig. 3H, I). These results suggested a mechanistic link between Ca2+-handling and DCM caused by loss of NEXN.
Nexilin is crucial for Ca2+ homeostasis in cardiomyocytes
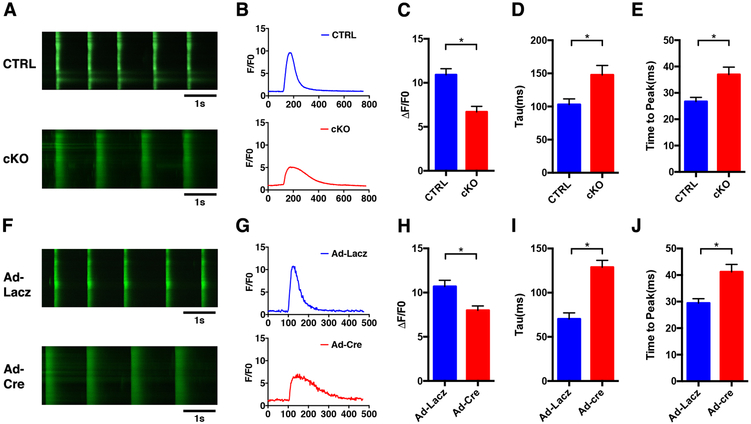
To determine functional consequences of altered Ca2+-handling gene and protein expression, we tested Ca2+ flux in cardiomyocytes isolated from E18.5 Nexn cKO mice and littermate CTRLs stained with the Fluo4 Ca2+ indicator. Results from time-lapse confocal microscopy showed that the amplitude of Ca2+-transients was significantly reduced, and the decay of the Ca2+ transient (Tau) was slower in cKO cardiomyocytes (Fig. 4A-E). Since alterations in Ca2+ transients can either be a cause or consequence of heart failure34, we also studied Ca2+ transients in cardiomyocytes following acute loss of NEXN. Cardiomyocytes were isolated from E18.5 Nexnf/f mice and transfected with adenovirus expressing Cre (Ad-Cre) or control adenovirus expressing LacZ (Ad-LacZ). 24 hours following transfection, cells were stained with Fluo4 and time-lapse confocal imaging performed. Similar to results obtained with Nexn cKO cardiomyocytes, Ca2+ transients in Ad-Cre Nexnf/f cardiomyocytes were reduced in amplitude, had increased duration, and prolonged decay relative to control Ad-LacZ Nexnf/f cardiomyocytes (Fig. 4F-J). These data supported the hypothesis that aberrant Ca2+-homeostasis in cKO cardiomyocytes was most likely the cause, and not a consequence of subsequently reduced cardiac output and progressive DCM.
Figure 4. Alteration of Ca2+ dynamics in NEXN cKO cardiomyocytes.
(A) Representative time-lapse confocal images (scale bars 1 sec), Ca2+ transients measuraments were performed at a pace of 0.5Hz; (B) representative Ca2+ transient curve; and (C-E) histograms showing results from quantifications of Ca2+ transient (C) amplitude (ΔF/F0) (D)transient decay (Tau) and (E) time to peak of live cardiomyocytes isolated from E18.5 CTRL and cKO ventricles were labeled with Fluo4 (n=6 mice). (F) Representative time-lapse confocal images (scale bars 1 sec); (G) representative Ca2+ transient curve and (G-J) histograms showing results from quantification of Ca2+ transient (H) ΔF/F0 and (I) Tau and (J) time to peak of E18.5 isolated neonatal cardiomyocytes Nexnf/f treated with Ad-Lacz and Ad-cre virus labeled with Fluo4 (n=6 mice). (*) Statistically significant differences with P value < 0.05.
Nexilin is a newly identified component of junctional membrane complexes in the cardiac dyad
NEXN has been characterized in previous studies as a Z-disc protein and loss of NEXN in zebrafish results in perturbed Z-disc stability 24. However, more recently it has been reported that gKO of Nexn in mice does not alter sarcomere integrity or Z-disc structure 26. Likewise, in our gKO and cKO models, these structures were not altered (Supplemental Figure 8A). In adult heart, the terminal cisternae of the SR and T-tubules are localized to the Z-disc 35, and we observed abnormal Ca2+-homeostasis in Nexn cKO cardiomyocytes. Following upon these observations, we decided to further investigate NEXN’s precise localization in cardiomyocytes, and its potential association with dyad proteins. To do this, we generated three antibodies from three different epitopes. One of these antibodies, generated utilizing a peptide encoded by exon 6, included in all NEXN isoforms, exhibited a signal in wildtype, but not cKO tissue, either by western blot or by immunofluorescence (Supplemental Figure 1C, Supplemental Figure 8B). Utilizing this antibody, confocal microscopy revealed that NEXN co-localized with the junctional SR protein Jph2 in both isolated neonatal cardiomyocytes (when the Z-disc is already formed, but T-tubules and junctional SR are not) and adult cardiomyocytes (when mature T-tubules and junctional SR localize along the Z-discs), (Fig. 5A, B).
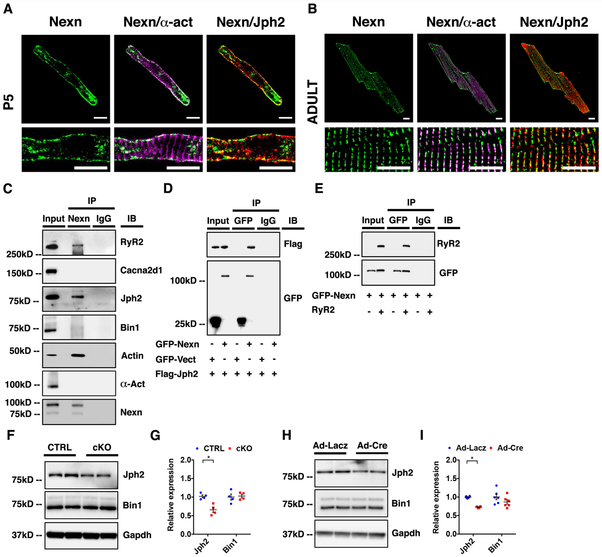
Figure 5. NEXN localizes to the JMC and interacts with junctional SR proteins.
(A) Representative confocal images of a P5 CTRL cardiomyocyte showing how NEXN in green do not co-localize with α-actinin (α-act) in magenta, while co-localizing with Jph2 in red; scale bars 10μm. (B) Representative confocal images of an adult CTRL cardiomyocyte in the lower panels showing dyadic localization of NEXN; scale bars 10μm.Fig (C) Co-immunoprecipitation of NEXN with putative binding partners using heart tissue lysates. (D) Immunoprecipitation of GFP-tagged NEXN and Flag-tagged Jph2 co-expressed in HEK293 cells. (E) Immunoprecipitation of GFP-tagged NEXN and tetracycline (10 ug/ml, 24 hours) induced RyR2 co-expressed in a stable tetracycline inducible HEK293 cell line expressing RyR2. (F-H) Western blot representative images and (G-I) quantification graphs of Ca2+-handling proteins from (F-G) E18.5 mouse ventricles (n = 4) and from (H-I) isolated neonatal NEXNf/f cardiomyocytes treated with Ad-Lacz or Ad-cre virus (n=6). (*) Statistically significant differences with P value < 0.05.
In further support of NEXN being localized to the JMC, western blot analyses of proteins co-immunoprecipitated with NEXN from mouse heart lysates identified two major junctional SR proteins (RyR2, Jph2), as possible binding partners of NEXN (Fig. 5C). Actin was also co-immunoprecipitated with NEXN, consistent with previous studies36, but T-tubule proteins Cacna2d1 and Bin1, or the major Z-line protein alpha actinin, were not co-immunoprecipitated with NEXN. To further validate these associations, we co-expressed GFP-tagged NEXN and Flag-tagged Jph2 in HEK293 cells and expressed GFP-tagged NEXN in a stable tetracycline inducible Ryr2-expressing HEK293 cell line37 and performed pull-down experiments on cell protein extracts. Western blot analyses of pull-down results confirmed that NEXN was co-immunoprecipitated with both Jph2 and RyR2 (Fig. 5D, E). Western blot analyses also demonstrated that levels of Jph2, but not Bin1, were significantly reduced both in Nexn cKO heart tissue samples, and in the in vitro model of acute Nexn ablation (Fig. 5F-I). These findings indicated that NEXN was not a Z-disc protein and its localization at the JMC reflected its association with junctional SR-proteins.
Nexilin is essential for initiation of cardiac T-tubule formation
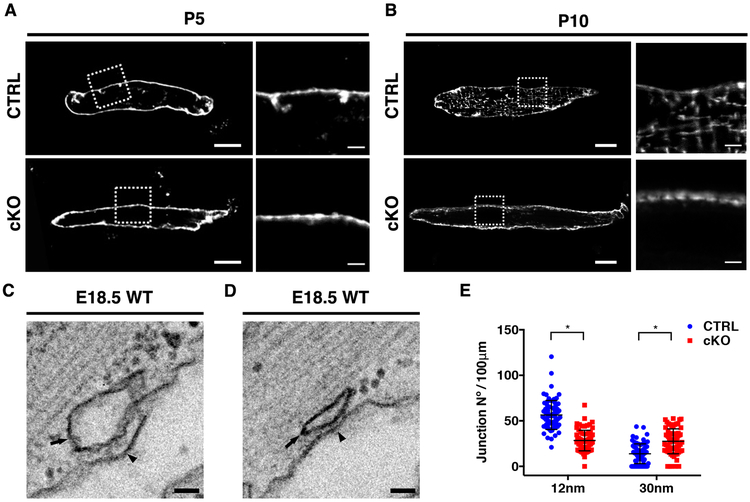
Considering NEXN association with JMC proteins, abnormal junctional protein expression and impaired Ca2+ transients in Nexn cKO cardiomyocytes, we investigated T-tubule morphology. Cardiomyocytes were isolated from Nexn cKO and littermate CTRL mice at P5 and P10 and stained them with the T-tubule marker Di-8-ANNEPS 20. Live-imaging confocal microscopy showed that membrane invaginations typical of initial T-tubule formation were evident in P5 CTRL cardiomyocytes but were not observed in Nexn cKO cardiomyocytes (Fig. 6A). Furthermore, in contrast to CTRL samples, organized T-tubules were completely absent in P10 Nexn cKO cardiomyocytes (Fig. 6B). We also analyzed formation of peripheral junctional membrane complexes at E18.5. At this stage, two types of peripheral SR/Sarcolemma associations are present, one separated by a space of 30nm (when the junctional membrane complex is forming), and the other by a space of 12nm (when the junctional membrane complex is established) 13. Electron microscopy analyses showed that in E18.5 Nexn cKO cardiomyocytes, when compared to CTRLS, 12nm junctions were significantly decreased, while 30nm junctions were significantly increased (Fig. 6C-E). These findings suggested impaired associations between the SR and sarcolemma, and failure of sarcolemmal invaginations required to initiate T-tubule formation in Nexn cKO cardiomyocytes.
Figure 6. NEXN is indispensable for MCS stability and T-tubule formation.
(A-B) Representative live confocal images of (A) P5 and (B) P10 CTRL and Nexn cKO cardiomyocytes stained with Di-8 Anepps; low magnification scale bars 10μm, high magnification scale bars 2μm. (C-D) Representative electron microscopy (EM) images of E18.5 mouse heart ventricles showing the two different types of junctional complexes between the sarcolemma and the SR membrane with gap sizes of about (C) 12 and (D) 30 nm; scale bars 50nm. (E) MCS occurrence quantification. Data were obtained from EM analysis of heart ventricle cardiomyocytes from Nexn cKOs and CTRLs E18.5 (n=3); for the Nexn cKO, 689μm of sarcolemma length were analyzed; for the CTRL 876μm of sarcolemma length were analyzed. (*) Statistically significant differences with P value < 0.05.
Discussion
Mutations in NEXN are associated with cardiomyopathies and a first animal model in zebrafish suggested NEXN as a sarcomeric protein localized to the Z-disc and involved in its stability 24, 25. More recently, Aherrahrou and colleagues reported that global KO of Nexn in mice did not alter sarcomeric structure of cardiomyocytes, but resulted in severe DCM, with aspects of endocardial fibroelastosis 26. Here, similarly, we found that Nexn−/− mice developed a severe, progressive DCM, with cardiac dysfunction first evident at P1, and collagen positive “mural masses” evident by P7. However, absence of elastic fibers and presence of fibrin suggested these masses were thrombi, rather than endocardial fibroelastosis. Considering the early onset of DCM against the later onset of the thrombi, the latter could not be considered a primary cause of altered cardiac function (Supplemental Figure 1, 2). We found that cKO of Nexn in cardiomyocytes totally recapitulated phenotypes observed in Nexn−/− mice (Fig. 1, 2), demonstrating that the DCM was a direct consequence of NEXN loss in cardiomyocytes.
Investigating mechanisms underlying cardiac dysfunction in Nexn cKO mice, mass spectrometry and western blot analysis of cardiac extracts showed that loss of NEXN resulted in altered expression of Ca2+-handling proteins such as RyR2, the LTCC pore-forming subunit Cacna1c, and Jph2. These data were confirmed utilizing an in vitro model of acute NEXN loss (Fig. 3). Consistent with these observations, loss of NEXN in cardiomyocytes resulted in abnormal Ca2+ transients, characterized by reduced amplitude, increased duration, and prolonged decay with respect to those of CTRLs (Fig. 4). Nexn cKO mice also exhibited diminished cardiac contractility and rapidly progressive cardiomyopathy and died before P12. Abnormal expression of SR proteins and changes in Ca2+ transients are manifestations of defects in EC-coupling and a hallmark of cardiac JMC dysfunction 38, 39, suggesting a potential role for NEXN in the specialized SR/T-tubule association necessary for dyad formation.
Development of the dyads of the adult cardiomyocyte occurs in a gradual, highly orchestrated manner. At embryonic day 9 (E9), early associations between the sarcolemma and ER membranes with gap sizes of either 12nm or 30 nm are progressively observed at the cell periphery13. At perinatal stages, newly formed SR cisternae enriched in RyR2 at the cell periphery are periodically arranged in association with the Z-disc40, 41. Around postnatal day 5 (P5), initiation of T-tubule formation occurs, as portions of the sarcolemma enriched in LTCC start to invaginate to become closely associated with the RyR2-enriched SR. From P10, these sarcolemmal invaginations penetrate more deeply within the cytoplasm, forming mature T-tubules that are juxtaposed to the junctional SR with an average gap size of 12nm, likely reflecting a requisite distance for physiological cross-talk between LTCCs and RyR2s 13.
Proper dyad formation is essential for cardiac function, with even subtle alterations in association between T-tubules and the Junctional SR causing serious cardiac disorders23. However, we know relatively little as to proteins orchestrating the development of this highly specialized architecture. Initial formation of early ER-PM associations requires the transmembrane ER/SR protein Jph242, 43, as global deficiency of Jph2 results in 90% loss of the 12nM ER-PM associations at E9.5, with mutants dying around E10.5 13. In mature cardiomyocytes, T-tubule architecture is modified by bridging integrator 1(Bin1). In cardiomyocyte-specific Bin1 mutants, T-tubule distribution is not changed, but individual T-tubules exhibit fewer folds and overall less membrane content, resulting in susceptibility to arrhythmias in adult mice44.
Although initiation of T-tubule development is a pivotal moment in mature dyad development, little is known as to proteins required for this key event. Here, we demonstrated through a series of observations that NEXN was a JMC protein required for initiation of T-tubule development. Immunostaining with an antibody specific to NEXN at P5 demonstrated that NEXN co-localized with Jph2 at the periphery, but was not present throughout the Z-disc, as observed for the Z-disc protein α-actinin (Fig. 5), suggesting that NEXN localized in the forming JMC and was not a Z-disc protein. Consistent with this, proteomics and pull-down assays demonstrated that NEXN interacted with junctional SR proteins Jph2 and RyR2 (Fig. 5C-E). Confocal analyses at P5 and P10 demonstrated that loss of NEXN in cardiomyocytes resulted in absence of sarcolemmal invaginations required to initiate T-tubule formation, and lack of T-tubule formation (Fig. 6A, B). It should also be pointed out that, although we did not detect alterations in JPH2 immunostaining by confocal microscopy in NEXN P5 cKO cardiomyocytes (Supplemental Figure 8), implying normal SR structure, more in depth studies would be required to unequivocally determine whether SR structures were changed in cKO cardiomyocytes.
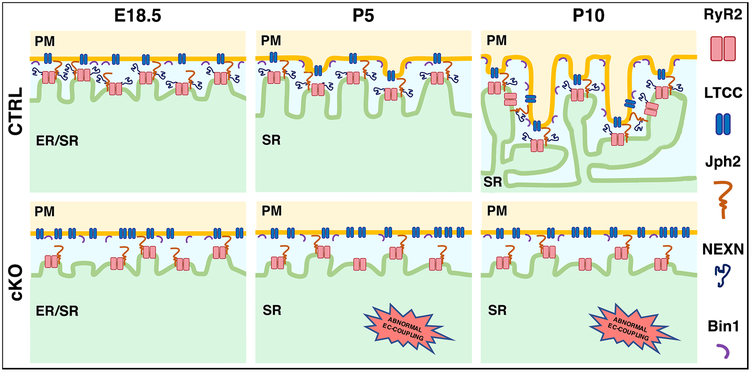
Our data suggests a model in which NEXN is critical for two aspects of JMC formation in cardiomyocytes. At E18.5, relative to CTRLs, Nexn mutant cardiomyocytes exhibited a reduced proportion of the more closely apposed 12nm associations between the ER and sarcolemma at the cell periphery (Fig. 7). At P5, NEXN mutants failed to develop sarcolemmal invaginations initiating T-tubule development, resulting in absence of T-tubule development at P10 (Fig. 7).
Figure 7. Model for NEXN role in cardiomyocytes.
At prenatal stage, NEXN is essential for the establishment of the JMCs. During neonatal stage (around P5), NEXN stabilization of the JMC allow the PM/SR tethering and PM buds start to invaginate inside the cell. Later in the development (around P10), the proper formation of JMCs leads to the deeper invagination of the forming T-tubules and the maturation of the dyads. Indeed, in cardiomyocytes lacking NEXN, the decrease of 12nm gap size junctions is observed. Furthermore, absence of NEXN prevent the sarcolemmal invaginations at P5 followed by a non-formation of T-tubules at P10. Finally, as a result of the altered membrane network, loss of NEXN in cardiomyocytes induce EC-coupling defects accompanied by severe DCM.
Altogether, results presented in this study identify NEXN as an unexpected component of JMCs in cardiomyocytes that is essential for T-tubule initiation and formation. Furthermore, our studies given mechanistic insight into molecular pathways leading to cardiomyopathy in patients with mutations in NEXN. It remains to be determined whether NEXN also plays a central role in T-tubule/JMC remodeling during heart failure. Of note, NEXN protein levels are not significantly altered in human cardiomyopathy or heart failure patients45, or in mouse heart two weeks following TAC (Supplemental Figure 9). However, these observations do not exclude an essential role of NEXN in T-tubule/JMC remodeling during heart failure. In fact, protein levels of RyR2, a major JMC protein and an interacting partner of NEXN, are also not altered in heart failure, but RyR2 function is significantly diminished due to post translational modifications 46-48. Further studies are needed to determine the potential role of NEXN in T-tubule/JMC remodeling during heart failure, and whether NEXN may have roles other than T-tubule/JMC formation.
Supplementary Material
Clinical Perspective.
What is new?
Cardiomyocyte specific KO of Nexn results in a rapidly progressive severe dilated cardiomyopathy.
NEXN interacts with SR proteins, and is a previously unsuspected member of the SR-sarcolemma junctional complex
NEXN is required for initiation of T-tubule invagination and overall T-tubule formation, with loss of NEXN leading to impaired calcium handling.
What are the clinical implications?
Identification of NEXN as a new possible target for T-Tubule remodeling.
Providing mechanistic insight into molecular pathways leading to cardiomyopathy in patients with mutations in NEXN.
Acknowledgments
We thank Dr. Paolo Toti, (UNISI, Siena, Italy), Dr. Michael C. Fishbein (UCLA, Los Angeles, CA, USA) and Dr. Robert Ross (UCSD, La Jolla, CA, USA) for outstanding scientific discussions on histology results. We are grateful for their invaluable technical assistance to Dr. Alex Rosa Campos (SBP Proteomics Core of UCSD, La Jolla, CA, USA), Dr. Tea Jashashvili (MIC of USC, Los Angeles, CA, USA), Dr. Jennifer Santini (Microscopy Core of UCSD, La Jolla, CA, USA), Dr. Mark Ellisman and all the members of NCMIR (UCSD, La Jolla, CA, USA). We also thank Dr. Chao Chen and Dr. Robert Ross (UCSD, La Jolla, CA, USA) for providing the Cre-adenoviral vector used for the in vitro acute loss of Nexn. We thank Dr. Adam Pollak (UCSD, La Jolla, CA, USA) and Dr. S. R. Wayne Chen (University of Calgary, Canada) for the tetracycline inducible Ryr2-expressing HEK293 cell line.
Sources of Funding:
JC is funded by grants from NIH, the National Heart, Lung, and Blood Institute and holds an American Heart Association Endowed Chair in Cardiovascular Research. XF is supported by a NIH grant K99HL143210. OK is supported by the Shenzhen Basic Research Foundation (KCYJ20160428154108239, KQJSCX20170330155020267). We also thank National Center for Microscopy and Imaging Research grant support: 5P41GM103412 to Mark Ellisman.
Footnotes
Disclosures
None
References
- 1.Prinz WA. Bridging the gap: membrane contact sites in signaling, metabolism, and organelle dynamics. J Cell Biol. 2014;205:759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valm AM, Cohen S, Legant WR, Melunis J, Hershberg U, Wait E, Cohen AR, Davidson MW, Betzig E and Lippincott-Schwartz J. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature. 2017;546:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helle SC, Kanfer G, Kolar K, Lang A, Michel AH and Kornmann B. Organization and function of membrane contact sites. Biochim Biophys Acta. 2013;1833:2526–2541. [DOI] [PubMed] [Google Scholar]
- 4.Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N and De Camilli P. PI(4,5)P(2)-dependent and Ca(2+)-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell. 2013;153:1494–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lees JA, Messa M, Sun EW, Wheeler H, Torta F, Wenk MR, De Camilli P and Reinisch KM. Lipid transport by TMEM24 at ER-plasma membrane contacts regulates pulsatile insulin secretion. Science. 2017;355:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y and Emr SD. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell. 2011;144:389–401. [DOI] [PubMed] [Google Scholar]
- 7.Caldieri G, Barbieri E, Nappo G, Raimondi A, Bonora M, Conte A, Verhoef L, Confalonieri S, Malabarba MG, Bianchi F, Cuomo A, Bonaldi T, Martini E, Mazza D, Pinton P, Tacchetti C, Polo S, Di Fiore PP and Sigismund S. Reticulon 3-dependent ER-PM contact sites control EGFR nonclathrin endocytosis. Science. 2017;356:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips MJ and Voeltz GK. Structure and function of ER membrane contact sites with other organelles. Nat Rev Mol Cell Biol. 2016;17:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. [DOI] [PubMed] [Google Scholar]
- 10.Endo M Calcium-induced calcium release in skeletal muscle. Physiol Rev. 2009;89:1153–1176. [DOI] [PubMed] [Google Scholar]
- 11.Berridge MJ, Bootman MD and Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. [DOI] [PubMed] [Google Scholar]
- 12.Venetucci L, Denegri M, Napolitano C and Priori SG. Inherited calcium channelopathies in the pathophysiology of arrhythmias. Nat Rev Cardiol. 2012;9:561–575. [DOI] [PubMed] [Google Scholar]
- 13.Takeshima H, Komazaki S, Nishi M, Iino M and Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. [DOI] [PubMed] [Google Scholar]
- 14.Scriven DR, Asghari P and Moore ED. Microarchitecture of the dyad. Cardiovasc Res. 2013;98:169–176. [DOI] [PubMed] [Google Scholar]
- 15.Franzini-Armstrong C, Protasi F and Ramesh V. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys J. 1999;77:1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW and Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci U S A. 2006;103:4305–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louch WE, Koivumaki JT and Tavi P. Calcium signalling in developing cardiomyocytes: implications for model systems and disease. J Physiol. 2015;593:1047–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang HB, Li RC, Xu M, Xu SM, Lai YS, Wu HD, Xie XJ, Gao W, Ye H, Zhang YY, Meng X and Wang SQ. Ultrastructural uncoupling between T-tubules and sarcoplasmic reticulum in human heart failure. Cardiovasc Res. 2013;98:269–276. [DOI] [PubMed] [Google Scholar]
- 19.Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H and Song LS. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res. 2010;107:520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner E, Lauterbach MA, Kohl T, Westphal V, Williams GS, Steinbrecher JH, Streich JH, Korff B, Tuan HT, Hagen B, Luther S, Hasenfuss G, Parlitz U, Jafri MS, Hell SW, Lederer WJ and Lehnart SE. Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of T-tubule membrane structures after myocardial infarction. Circ Res. 2012;111:402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie YP, Chen B, Sanders P, Guo A, Li Y, Zimmerman K, Wang LC, Weiss RM, Grumbach IM, Anderson ME and Song LS. Sildenafil prevents and reverses transverse-tubule remodeling and Ca(2+) handling dysfunction in right ventricle failure induced by pulmonary artery hypertension. Hypertension. 2012;59:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Chen B, Guo A, Zhu Y, Miller JD, Gao S, Yuan C, Kutschke W, Zimmerman K, Weiss RM, Wehrens XH, Hong J, Johnson FL, Santana LF, Anderson ME and Song LS. Microtubule-mediated defects in junctophilin-2 trafficking contribute to myocyte transverse-tubule remodeling and Ca2+ handling dysfunction in heart failure. Circulation. 2014;129:1742–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, Korchev YE, Harding SE and Gorelik J. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci U S A. 2009;106:6854–6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassel D, Dahme T, Erdmann J, Meder B, Huge A, Stoll M, Just S, Hess A, Ehlermann P, Weichenhan D, Grimmler M, Liptau H, Hetzer R, Regitz-Zagrosek V, Fischer C, Nurnberg P, Schunkert H, Katus HA and Rottbauer W. Nexilin mutations destabilize cardiac Z-disks and lead to dilated cardiomyopathy. Nat Med. 2009;15:1281–1288. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Li Z, Wang J, Sun K, Cui Q, Song L, Zou Y, Wang X, Liu X, Hui R and Fan Y. Mutations in NEXN, a Z-disc gene, are associated with hypertrophic cardiomyopathy. Am J Hum Genet. 2010;87:687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aherrahrou Z, Schlossarek S, Stoelting S, Klinger M, Geertz B, Weinberger F, Kessler T, Aherrahrou R, Moreth K, Bekeredjian R, Hrabe de Angelis M, Just S, Rottbauer W, Eschenhagen T, Schunkert H, Carrier L and Erdmann J. Knock-out of nexilin in mice leads to dilated cardiomyopathy and endomyocardial fibroelastosis. Basic Res Cardiol. 2016;111:6. [DOI] [PubMed] [Google Scholar]
- 27.Guide for the Care and Use of Laboratory Animals National Research Council National Academies Press, Washington, DC; 2011. [Google Scholar]
- 28.Liang X, Zhou Q, Li X, Sun Y, Lu M, Dalton N, Ross J Jr. and Chen J. PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol Cell Biol. 2005;25:3056–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang X, Stroud MJ, Ouyang K, Fang L, Zhang J, Dalton ND, Gu Y, Wu T, Peterson KL, Huang HD, Chen J and Wang N. Adipocyte-specific loss of PPARgamma attenuates cardiac hypertrophy. JCI Insight. 2016;1:e89908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang X, Bogomolovas J, Wu T, Zhang W, Liu C, Veevers J, Stroud MJ, Zhang Z, Ma X, Mu Y, Lao DH, Dalton ND, Gu Y, Wang C, Wang M, Liang Y, Lange S, Ouyang K, Peterson KL, Evans SM and Chen J. Loss-of-function mutations in co-chaperone BAG3 destabilize small HSPs and cause cardiomyopathy. J Clin Invest. 2017;127:3189–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patro R, Duggal G, Love MI, Irizarry RA and Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14:417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Love MI, Huber W and Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breckenridge R, Kotecha S, Towers N, Bennett M and Mohun T. Pan-myocardial expression of Cre recombinase throughout mouse development. Genesis. 2007;45:135–144. [DOI] [PubMed] [Google Scholar]
- 34.Lou Q, Janardhan A and Efimov IR. Remodeling of calcium handling in human heart failure. Adv Exp Med Biol. 2012;740:1145–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong T and Shaw RM. Cardiac T-Tubule Microanatomy and Function. Physiol Rev. 2017;97:227–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohtsuka T, Nakanishi H, Ikeda W, Satoh A, Momose Y, Nishioka H and Takai Y. Nexilin: a novel actin filament-binding protein localized at cell-matrix adherens junction. J Cell Biol. 1998;143:1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H and Chen SR. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR). Proc Natl Acad Sci U S A. 2004;101:13062–13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorski PA, Ceholski DK and Hajjar RJ. Altered myocardial calcium cycling and energetics in heart failure--a rational approach for disease treatment. Cell Metab. 2015;21:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Oort RJ, Garbino A, Wang W, Dixit SS, Landstrom AP, Gaur N, De Almeida AC, Skapura DG, Rudy Y, Burns AR, Ackerman MJ and Wehrens XH. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123:979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziman AP, Gomez-Viquez NL, Bloch RJ and Lederer WJ. Excitation-contraction coupling changes during postnatal cardiac development. J Mol Cell Cardiol. 2010;48:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korhonen T, Rapila R, Ronkainen VP, Koivumaki JT and Tavi P. Local Ca2+ releases enable rapid heart rates in developing cardiomyocytes. J Physiol. 2010;588:1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo A, Wang Y, Chen B, Wang Y, Yuan J, Zhang L, Hall D, Wu J, Shi Y, Zhu Q, Chen C, Thiel WH, Zhan X, Weiss RM, Zhan F, Musselman CA, Pufall M, Zhu W, Au KF, Hong J, Anderson ME, Grueter CE and Song LS. E-C coupling structural protein junctophilin-2 encodes a stress-adaptive transcription regulator. Science. 2018;362:1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynolds JO, Chiang DY, Wang W, Beavers DL, Dixit SS, Skapura DG, Landstrom AP, Song LS, Ackerman MJ and Wehrens XH. Junctophilin-2 is necessary for T-tubule maturation during mouse heart development. Cardiovasc Res. 2013;100:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong T, Yang H, Zhang SS, Cho HC, Kalashnikova M, Sun B, Zhang H, Bhargava A, Grabe M, Olgin J, Gorelik J, Marban E, Jan LY and Shaw RM. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nat Med. 2014;20:624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen CY, Caporizzo MA, Bedi K, Vite A, Bogush AI, Robison P, Heffler JG, Salomon AK, Kelly NA, Babu A, Morley MP, Margulies KB and Prosser BL. Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nat Med. 2018;24:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sainte Beuve C, Allen PD, Dambrin G, Rannou F, Marty I, Trouve P, Bors V, Pavie A, Gandgjbakch I and Charlemagne D. Cardiac calcium release channel (ryanodine receptor) in control and cardiomyopathic human hearts: mRNA and protein contents are differentially regulated. J Mol Cell Cardiol. 1997;29:1237–1246. [DOI] [PubMed] [Google Scholar]
- .Meyer M, Schillinger W, Pieske B, Holubarsch C, Heilmann C, Posival H, Kuwajima G, Mikoshiba K, Just H, Hasenfuss G and et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation. 1995;92:778–784. [DOI] [PubMed] [Google Scholar]
- 48.Schillinger W, Meyer M, Kuwajima G, Mikoshiba K, Just H and Hasenfuss G. Unaltered ryanodine receptor protein levels in ischemic cardiomyopathy. Mol Cell Biochem. 1996;160–161:297–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.