Abstract
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immunosuppressive cells of the myeloid lineage upregulated by mediators of inflammation such as IL-2, GCSF, and S100A8/A9. These cells have been studied extensively by tumor biologists. Because of their robust immunosuppressive potential, MDSCs have stirred recent interest among transplant immunologists as well. MDSCs inhibit T cell responses through, among other mechanisms, the activity of arginase-1 and iNOS, and the expansion of T regulatory (Treg) cells. In the context of transplantation, MDSCs have been studied in several animal models, and to a lesser degree in humans. Here, we will review the immunosuppressive qualities of this important cell type and discuss the relevant studies of MDSCs in transplantation. It may be possible to exploit the immunosuppressive capacity of MDSCs for the benefit of transplant patients.
1. Characteristics of MDSCs:
MDSCs have been investigated extensively by tumor biologists seeking to determine factors which suppress a host’s immune response to cancer.1–4 A brief review of this important literature is important in order to understand the potential value of MDSCs in transplantation.4
Hematopoietic stem cells (HSC)s in the bone marrow differentiate into common myeloid precursors (CMPs) (figure 1), a developmental stage marked by DNA demethylation. 5,6 CMPs give rise to immature myeloid cells (IMCs). In nonpathologic conditions IMCs migrate to lymphoid organs where they can differentiate into dendritic cells, macrophages, and neutrophils.7 In pathological conditions, however, such as tumor, stress, or infection, IMCs become activated and differentiate into MDSCs with an immunosuppressive phenotype. MDSC development appears to be associated with downregulation of IRF7 and IRF8 and upregulation of IRF1.8–10 Recent data have illustrated that MDSCs can develop from monocytes, and that this transition occurs in response to GM-CSF (produced by T cells endogenously or administered exogenously). Importantly, it appears that GM-CSF exposure must preempt exposure to inflammatory mediators, such as IFN-gamma.10
Figure 1.
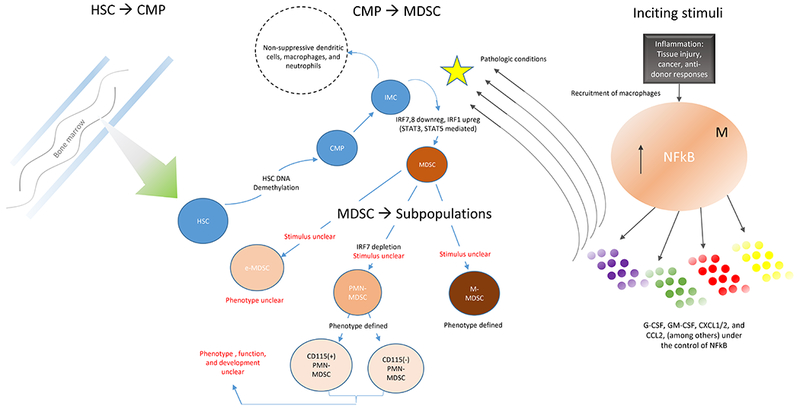
Development of MDSCs based on a synthesis of the literature
Endogenous and/or exogenous signals generated by chronic inflammation, including auto-immunity, cancer, and infection11,12 among others are the stimuli for MDSCs’ activation and expansion (table 1).1,5,13–15 The drivers of MDSC activation have been reviewed elsewhere, but include G-CSF, GM-CSF, IL-2, TGF-beta, CXCL1/2, S100A8/A9, and PGE2.16–18 This MDSC expansion is observed clinically in healthy human bone marrow transplant donors with G-CSF mobilization. After G-CSF administration, donors demonstrate a 3-fold increase in peripheral blood MDSCs.19 Once developed within the bone marrow, MDSCs can be sustained by T cells through the release of IL-10.20
Table 1:
Phenotype of MDSCs for humans and mice
| Molecules known to upregulate MDSC proliferation and/or lead to MDSC activation | ||
|---|---|---|
| • IL-1B | • CXCL8 | • S100A8/A9 |
| • IL-2 | • CXCL12 | • HMGB1 |
| • IL-4 | • CXCR4 | • PGE2 |
| • IL-6 | • SDF-1 | |
| • CCL2 | • TGF-beta | |
| • CCL5 | • IFN-gamma | |
| • CXCL1/2 | • G-CSF | |
| • CXCL6 | • GM-CSF | |
Upon exiting the bone marrow, MDSCs migrate to sites of inflammation, and this migration is associated with (among others) CCR2 as well as and L and E-selectin expression.21–25 For example, in a mouse islet transplantation model, MDSCs homed to the allograft in a CCR2-dependent fashion and MDSC homing was enhanced by the presence of proinflammatory IFN-gamma.26 Further, MDSCs generated in mice which did not express CCR2 failed to exit the bone marrow.25 In other models, MDSC migration was dependent on expression of CXCL-1 and CD62L (L-selectin).27–29
There are 2 primary sub-populations of MDSCs: monocytic MDSCs (M-MDSCs) and polymorphonuclear MDSCs (PMN-MDSCs).30 While M-MDSCs are similar phenotypically and morphologically to monocytes, PMN-MDSCs are more similar to polymorphonuclear cells (PMN)s.3,31 A third, very small group (<3%) of MDSCs are characterized as “early” or having myeloid colony-forming activity.30,32–34 Complicating their identification, MDSCs are a heterogeneous group of cells and expression of MDSC cell surface markers may change depending on the environment.35 As an example, tumor-derived MDSCs in environments devoid of tumor-derived growth factors may differentiate into macrophages or dendritic cells.35,36 Further, it has been hypothesized that M-MDSC and PMN-MDSC may represent sequential developmental stages in the life of an individual MDSC. This potential MDSC plasticity may have important implications for transplantation.
The distinction between M-MDSCs and PMN-MDSCs is phenotypically, functionally, and anatomically important.29 In cancers, the ratio of PMN-MDSCs to M-MDSCs in each compartment of the tumor microenvironment depends on the type of cancer.37 In human bone marrow transplant recipients, MDSC subtype is also important as it may predict risk of graft versus host disease.19 Little is known about the MDSC subtypes which develop after organ transplantation, but a single study in humans suggested that M-MDSCs were the predominant MDSC subtype to develop in the peripheral blood of kidney transplant recipients.38
Table 2 shows some of the accepted phenotypes for mouse and human M-MDSCs and PMN-MDSCs.30,39 In mice, the accepted phenotype of total MDSCs is defined as the dual expression of CD11b, also known as alpham-integrin, and the myeloid lineage marker Gr1.36 CD11b is expressed on myeloid cells, as well as small subpopulations of natural killer (NK) cells, T cells, and B cells.40,41 CD11b binds noncovalently with CD18 to form the leukocyte integrin Mac-1 complex which regulates inflammatory cell recruitment.42 MDSC sub-classification is determined by expression of either Ly6G (PMN-MDSCs) or Ly6C (M-MDSCs). Mouse M-MDSCs also express CD49d, whereas PMN-MDSCs do not.43 This is important because CD49d is a subunit of the alpha-4 integrin receptor which is critical for lymphocyte homing to sites of inflammation.44,45
Table 2:
Inflammatory signals which activate MDSCs and lead to their chemotaxis
| Accepted phenotyping of MDSCs in mice and humans | ||
|---|---|---|
| Mice | ||
| M-MDCS | PMN-MDSC | e-MDSC |
| CD11b+Gr-1midLy6ChiLy6G−CD49d+ | CD11b+Gr-1hiLy6ClowLy6G+CD49d− | poorly defined |
| Human | ||
| M-MDCS | PMN-MDSC | e-MDSC |
| CD11b+CD14+HLA-DRlow/−CD15− | CD11b+CD14−CD15+(or CD66b+) | Lin-(CD3/14/15/19/56)/HLA-DR−/CD33+ |
Human PMN-MDSCs express CD15 with or without the expression of CD66b, but they do not express CD14.29,30,32 CD33, a cell surface marker for myeloid cells, can be used in place of CD11b. CD15 is an adhesion molecule important for chemotaxis, phagocytosis, and cell-cell contact expressed on (but not restricted to) immature myeloid cells.46,47 CD15 is upregulated during development of granulocytes and is highly expressed by human neutrophils and eosinophils. CD66b is also known as carcinoembryonic antigen-related cell adhesion molecule 8 (CEACAM8), and is an activation marker for human granulocytes. Under nonpathologic conditions myeloid cells express minimal CD66b.48 Human M-MDSCs are defined as CD11b+CD14+HLA-DRlow/−, but they do not express CD15. CD14 is a co-receptor of Toll Like Receptor 4 (TLR4), and TLR4 (as well as CD14) binds cytosolic calcium binding proteins S100A8 and S100A9, leading to NF-kB upregulation and subsequent proinflammatory cytokine release.17 Importantly, classic human monocytes are CD33+CD11b+CD14+CD15- and HLA-DR− expression in monocytes is likely influenced by immunosuppression. Thus, the cell surface markers for MDSCs in the context of transplantation require further investigation.
Despite the importance of MDSC subtype, distinguishing human M-MDSCs from PMN-MDSCs by flow cytometry remains challenging. Recent data suggest that MDSC expression of S100A9, may be useful in making this distinction.23,49,50 Human flow cytometry studies comparing PMBC from healthy controls and patients with malignancies have helped to clarify gating strategies which can help differentiate between MDSC subtypes.51 This was shown nicely by Bronte et al.30 Specifically, expression of CD14+HLA-DR−/lo on human PBMC can identify the M-MDSC population while CD14−CD15+CD11b+ appear to distinguish PMN-MDSC.30 Differentiating between PMN-MDSCs and non-MDSC PMNs is also challenging and controversial.33 This difficulty is due, in part, to the heterogeneous nature of this immature cell type (eg not all MDSCs express the same cell surface markers). PMN-MDSCs and non-MDSC PMNs have similar morphology and are difficult to distinguish with Wright-Giemsa staining.2,52 In mice, cell surface expression of Ly6C and CD11 are slightly lower for PMN-MDSC than non-MDSC PMNs.2,3 Further, CD115 and CD224 are expressed at much higher levels in mouse MDSCs versus non-MDSC PMNs.2,33 In humans, density gradient centrifugation is used to separate PMN-MDSCs from non-MDSC PMNs. However, this technique is suboptimal because it precludes the separation of PMN-MDSC from other myeloid cells and it inconsistently separates activated, nonsuppressive PMNs, from immunosuppressive PMN-MDSCs.33 New data, however, suggests that, lectin-type oxidized LDL receptor 1 (LOX-1), may reliably distinguish human PMN-MDSC from non-MDSC PMNs by flow cytometry, allowing for cellular separation.33
2. Function:
A key function of MDSCs is T cell suppression.3,36,53–55 MDSC mediated T cell suppression has been shown nicely in co-culture models. For example, when Gr1+ MDSCs isolated from C57Bl/6 mice bearing Lung Lewis carcinoma were co-cultured with CD3+ stimulated T cells, T cell proliferation was markedly suppressed. This effect was most pronounced when the MDSC:T cell ratio was 1:1. In this model, PMN-MDSCs were more potent suppressors than M-MDSCs.53,54
One of the primary mechanisms by which MDCSs mediate their immunosuppressive effects is through the action of arginase-1.32,56,57 Arginase-1 reduces local levels of L-arginine, starving lymphocytes of this critical amino-acid and inhibiting their ability to proliferate.57–61 MDSCs also deplete local levels of cysteine through sequestration. MDSC depletion of cysteine also leads to T cell suppression because T cells cannot produce cysteine independently and because cysteine is required for T cell activation. 60,62
MDSC mediated immunosuppression also occurs through oxidative stress. MDSCs express both inducible nitric oxide synthase (iNOS) as well as well as NADPH oxidase-2 (Nox2) which lead to the production of nitric oxide (NO) and reactive oxygen species (ROS), respectively.29 NO and ROS suppress proliferating cells.29,63 MDSCs also inhibit lymphocyte trafficking through the downregulation of L-selectin, as well as through the production of peroxynitrite (PNT). PNT-associated nitrosylation of the T cell receptor inhibits binding to the antigen-MHC complex. 31,32,43,58
Additionally, MDSCs inhibit T cell responses through the expansion of T regs.38,43 MDSCs express programmed death ligand-1 (PDL-1) and bind PD-1 expressed by Treg, upregulating Treg responses.25,64,65 Indeed, MDSCs enhance PDL-1 mediated T cell suppression and blockade of PDL-1 inhibits MDSC-mediated suppression.25,64–66
Both cell-cell contact in addition to soluble factors are important for MDSC effector function. Isolation and analysis of MDSCs generated in animals with hepatocellular carcinoma revealed expression of membrane-bound TGF-beta. Transwell assays showed that soluble factors (ie soluble TGF-beta, other factors) produced by MDSCs could inhibit T cells responses (CD4 and NKT).13 In an investigation of human umbilical cord blood-derived MDSCs (largely PMN-MDSCs), inhibition of Th1 responses was dependent on cell-cell interaction. In subsequent transwell assays, MDSC-mediated control of Th2 responses was mediated by soluble factors including ROS.67,68 Taken together, the effector function of MDSCs involves both cell-cell contact and the release of soluble inhibitory factors. However, MDSC effector function may vary depending on the stimulus for MDSC activation, the MDSC sub-type, and target cell type.68 This important topic requires further investigation.
Early reports in the tumor biology literature suggested that MDSC-mediated CD8 T cell suppression was antigen specific.69 CD8 T cell antigen specificity was restricted to MHC class I and required cell-cell contact.70 In contrast, more recent work showed that MDSC-mediated CD4 T cell regulation could also be achieved, however this was only possible when MDSCs expressed substantial MHC class II.71 MDSCs which develop after transplantation do not appear to demonstrate the same degree of antigen specificity, however limited work has been done to address this important topic (for caveats see evidence for a role of MDSCs in transplantation).69,72
While MDSCs’ immunosuppressive effects are typically described as “local” MDSCs also downregulate CD62L on T and B cells.73 This is important because MDSC-mediated loss of CD62L expression on naïve T cells allows for more far-reaching MDSC-dependent immunosuppressive effects. 73
3. Evidence for MDSC control of semi-allogeneic responses at the maternal fetal interface:
MDSCs can control immune responses at the maternal-fetal interface.74 An immunologic environment supportive of the semi-allogeneic fetus is provided by the mother at the level of the placenta.58 MDSCs within the placenta sustain pregnancy by preventing T cell influx into the uterus and reducing T cell activation. Loss or disruption of this tolerogenic interface may lead to spontaneous abortion or miscarriage.58,75
Tregs also help to protect pregnancies against allogeneic responses, and proliferation of placental Tregs is driven, at least in part, by MDSCs.58,74,76 Adoptive transfer of normal mouse T regulatory cells can prevent murine miscarriage.77 At the maternal-fetal interface of successful pregnancies, CD4+ T cells are oriented towards the regulatory Th2 phenotype as suggested by the production of TGF-beta and IL-4 and IL-10.58,78 In contrast, Th1 responses mediated pregnancy loss.76 This Th2 regulatory response was reliant on the presence of MDSCs, and placental MDSCs readily polarized T cells towards the Th2 phenotype.67
Kostlin et al demonstrated that PMN-MDSCs were expanded in fetal cord blood. A similar population of MDSCs was expanded in the peripheral blood of healthy pregnant patients. These PMN-MDSCs demonstrated a regulatory phenotype as reflected by their expression of Arg1 and iNOS.79 After delivery, the PMN-MDSC population was reduced to levels of nonpregnant controls.79 Suppressive macrophages within the placenta were found to be important for fetal tolerance and successful gestation.80 Further, these macrophages were induced by G-CSF, produced anti-inflammatory cytokines, and induced FoxP3+ T reg.80 While these macrophages were not described as expressing the classical MDSC cell-surface markers, they appeared functionally similar. Given the heterogeneity of the MDSC population it is possible that these placental macrophages represented a unique group of MDSCs. The Tregs induced by maternal-fetal interface macrophages expressed CTLA-4, CD39 and IL-10. Further, placental-macrophage associated Tregs were inducible with IL-10 and TGF-beta and they were functionally suppressive.80 Taken together, it appears that myeloid derived cells present at the maternal fetal interface are important for fetal success, largely through their ability to suppress T cell responses.
4. Evidence for a role of MDSCs in transplantation:
There is important evidence to suggest that MDSCs are important in transplantation. The same proinflammatory factors which characterize the development and chemotaxis of MDSCs in cancer and infection also characterize anti-donor responses.29,36,81–86 Accordingly, it may be possible to exploit the regulatory capacity of MDSCs following transplantation to either supplement or eliminate the need for immunosuppressive drugs.38,85,87
Corneal transplantation:
MDSCs can prolong graft survival in corneal transplantation models.88 An exciting recent report published in Transplantation described a mouse model in which anti-donor T cell infiltration of corneal grafts could be inhibited through the adoptive transfer of MDSCs induced by either tumor or inflammation.89 T cell inhibition led to a reduction in the histopathological changes in the corneal allograft. In vitro studies demonstrated suppression of allogeneic responses with both tumor derived and inflammation induced MDSCs. These data suggest that transplantation may lead to recipient derived MDSCs capable of suppressing anti-donor responses.89,90
Islet transplantation:
A recent study of mouse islet transplantation showed that peri-transplantation MDSC infusion prolonged allograft survival. Prolongation of islet survival was due to MDSC-mediated inhibition of T cell responses. Further, administration of MDSCs increased the number of Tregs within the graft.26 These data corroborated findings by Marigo et al in which adoptive transfer of MDSCs, generated from treating BM cells in vitro with GM-CSF + IL-6, induced long-term acceptance of islet allografts in mice.72 In Marigo’s protocol, animals were given 4 weekly injections of syngeneic MDSCs beginning on the day of islet transplantation. At 200 days after transplantation, 75% of mice were euglycemic.72 Importantly, these MDSCs were not globally suppressive, but rather they suppressed antigen-specific responses.72 It is also important to note that various combinations of GM-CSF, G-CSF, and IL6 in this model yielded MDSCs with differing suppressive potentials.
Skin transplantation:
Skin grafts can readily promote the accumulation of MDSCs in recipient spleens.91 In a separate model, investigators evaluated the ability of MDSCs from immunoglobulin-like transcript 2 (ILTR2) transgenic mice to prolong skin graft survival.92 Uniquely, ILT2R mice MDSCs express higher levels of Arg-1 when compared with wild type mice. In a separate study, administration of 1 million syngeneic MDSCs significantly prolonged skin graft survival. The suppressive in vivo effects of the adoptively transferred MDSCs were dose-dependent, and graft survival was prolonged by 50% when the dose of MDSCs was increased to 3 million cells.93 Other groups found that skin graft survivals were markedly prolonged with the adoptive transfer of syngeneic MDSCs.94 MDSCs were given prior to transplantation and on posttransplantion day 6. Prolongation could be enhanced with weekly injections of syngeneic MDSCs.94 When recipient skin grafts and spleens were analyzed 2 weeks after adoptive transfer, no MDSCs were identified, suggesting rapid elimination. Authors suggested that techniques to improve the suppressive ability after adoptive transfer could decrease the frequency of MDSC injections.94
Kidney transplantation:
In rats, MDSCs accumulate in the peripheral blood and within grafts after kidney transplantation. However, these MDSCs were identified only after treatment with anti-CD28 antibodies. These MDSCs were suppressive in vitro of both donor-derived and recipient-derived CD3-stimulated recipient T cells.95 The suppressive function of MDSCs relied on iNOS, and appeared to be cell contact dependent.95 Importantly, MDSCs in this model were suppressive of anti-donor as well as 3rd party responses, suggesting a lack of antigen specificity.95
Cardiac transplantation:
Transcoronary adoptive transfer of MDSCs led to a 2-fold increased graft survival following cardiac transplantation in mice.96 In this model, animals were treated with rapamycin as well. When MDSCs were depleted using anti-Gr1 antibodies, graft survival was reduced, suggesting a synergy in the mechanisms of action for rapamycin and MDSCs.96 These rapamycin-induced MDSCs expressed high levels of iNOS and induced Tregs.96 In a separate model of mouse cardiac transplantation, co-stimulatory blockade-induced MDSCs also suppressed anti-donor responses through the action of iNOS.25 Unlike the above-cited islet cell models, heart transplants did not survive in the long term with MDSC infusion alone (ie without pharmacologic immunosuppression).25,72 Co-stimulatory blockade-induced MDSCs accumulated in the blood, bone marrow, as well as in the transplanted cardiac allograft.25
Human experience:
There is a paucity of human data addressing the role of MDSCs in transplantation. In an important study of 29 kidney transplant patients, separation of peripheral blood mononuclear cells revealed an upregulation in M-MDSCs over the course of 1 year after transplantation.38 Increases in MDSCs were observed as early as 3 months after transplantation. Further, CD11b+CD33+DR- MDSCs lead to a robust increase in FoxP3+ Treg cells in vitro. Notably, the MDSCs which accumulated in the peripheral blood of transplanted patients were largely M-MDSCs. It remains unknown if the M-MDSCs identified in the kidney transplant population home to the transplanted graft.38 Further, the human MDSC response to other organs such as livers and lungs (which may themselves carry many donor-derived MDSCs), hearts, and pancreata is unstudied.38
In a report of 31 renal transplant recipients, investigators observed a higher percentage of CD14+ and CD14(−) MDSCs in the peripheral blood, when compared with healthy volunteers (n=34).97 Nontransplanted patients with chronic kidney disease also demonstrated higher percentages of MDSCs, but only of the CD14(−) subset.97 MDSCs from these human renal transplant recipients were suppressive of anti-donor T cell responses in vitro. This important study included patients on varied immunosuppressive protocols transplanted over a long period of time and MDSC profiles were measured at a single time point. Nonetheless, authors concluded that renal failure alone did not lead to MDSC mobilization, and that the combination of transplantation and immunosuppression influenced peripheral blood MDSC populations.97
Immunosuppression effects on MDSCs:
Regarding the use of immunosuppressive agents and their effects on MDSCs, there is also a paucity of data. In vitro, G-CSF + cyclosporine A (CyA) led to increased differentiation of MDSCs. MDSCs cultured with CyA demonstrated increased suppressive activity against T cells activated in an anti-CD3/anti-CD28 system, and this increased suppressive activity was attributed to expression of iNOS.98 While there are no investigations of tacrolimus’ effect on MDSCs there are data suggesting that FK binding protein (FKBP; target of tacrolimus) is upregulated in MDSCs, and that blocking FKBP reduces MDSCs’ immunosuppressive capacity.99,100 These data imply that tacrolimus negatively affects MDSC function, but this remains unclear.
Glucocorticoids’ effect on MDSCs have been studied in a mouse trauma model.101,102 When a glucocorticoid antagonist was administered, the MDSC response to trauma was abrogated. Glucocorticoids did not appear to affect expression of arginase-1, or other important MDSC regulatory mechanisms.102
Rapamycin has garnered interest among investigators of regulatory myeloid cells.96,103,104 Rapamycin administration significantly decreased both MDSC number and suppressive activity in an allogeneic skin transplant model.91 In vitro studies suggested that rapamycin directly inhibited MDSCs.91 In a model of multi-organ inflammation, molecular target of rapamycin (mTOR) signaling pathway transcripts were upregulated, particularly in the PMN-MDSC sub-population, suggesting that mTOR inhibitors may suppress the regulatory function of MDSCs.105,106 Paradoxically, after cardiac transplantation, rapamycin-treated mice produced MDSCs which were suppressive of T cell proliferation.96 In the same cardiac transplant model, rapamycin administration enhanced MDSC recruitment and activity via iNOS.96 An additional report addressing the murine MDSC response to acute kidney injury included an in vitro analysis of rapamycin on MDSC function.107 Rapamycin upregulated the expression of Arg-1 and iNOS, as well as Treg populations.107 Taken together, the evidence on mTOR inhibitors is mixed, and further work is needed to determine which MDSC populations demonstrate enhanced versus suppressed function in response to rapamycin.
MDSCs in transplantation tolerance:
MDSCs are important not only for self-tolerance108 (and cancer), but also for transplantation tolerance.109 The combination of M-CSF and TNF-alpha induced M-MDSCs and allograft tolerance of mouse skin transplants.110 Further, the suppressive function of the induced MDSCs required iNOS expression.110 Depletion of iNOS after tolerance induction in this model abrogated the tolerant state.95
An important study from Garcia et al (2010) showed in mice that suppressive monocytes (CD11b+ Gr1+CD115+), which were functionally and phenotypically similar to MDSCs, were required for the establishment of tolerance of cardiac allografts. The investigators used an established tolerance induction protocol of donor specific transfusion in combination with co-stimulatory blockade via anti CD40L. When animals were depleted of these suppressive monocytes, tolerance could not be induced.25 Monocytes were suppressive in vitro and functioned through the production of iNOS, in a manner quite similar to classical MDSCs.25 In a subsequent study using the same model, the authors showed that the suppressive monocytes which developed in tolerant animals homed to the cardiac allograft.111
Mixed chimerism has also been used to establish tolerance of heterotopic cardiac transplants.109 Following cardiac transplantation, mice were given 5 days of anti-thymocyte serum (ATS) and 10 days of total lymphocyte irradiation.109,112,113 This model, which is similar in design to human transplant tolerance protocols, yielded long-lasting chimerism and donor-specific tolerance to the transplanted heart.109 However, when recipients were depleted of MDSCs using an antibody to Gr1, chimerism and subsequent tolerance were lost. Add back of MDSCs to the transplanted recipients increased cardiac graft survival.109 MDSCs derived from the transplanted recipient were sufficient to control anti-donor T cell responses in vitro. It was hypothesized that tolerance was induced by MDSC-mediated T cell suppression via arginase-1 and Treg expansion via PD-L1 mediated pathways.25,65,109 MDSCs played an important role in the establishment and maintenance of both chimerism and tolerance.
In a rat model of kidney transplantation, MDSCs were critical for tolerance establishment. When tolerance was induced using anti-CD28 antibodies, peripheral blood MDSCs were increased 2-fold and this increase was not due solely to the use of anti-CD28.95 Adoptive transfer of MDSCs which were generated as a result of anti-CD28 administration was, however, not successful in tolerance establishment,95 suggesting that anti-CD28 generated MDSCs may not be sufficient for controlling anti-donor responses in vivo.
Tregs are important in many studies of transplantation tolerance.114–117 Recent evidence has suggested robust pathways of “crosstalk” between MDSC and Tregs.70,118,119 As discussed above, Tregs are one of the primary mechanisms by which MDSCs mediate T cell suppression.25,65,118 Depletion of MDSCs in cardiac tolerance models led to a failure of Treg development and tolerance.25 This link between cell types is important because in many tolerance studies the stimulus for Treg upregulation remains unclear.117,120–123 It is plausible that Treg responses in transplant tolerance models are at least partly driven by an “upstream” MDSC response.
The role of MDSCs in models of transplantation tolerance induced by bone marrow transfusion is also unclear. While speculative, it is possible that intra-bone marrow MDSCs transplanted along with CD34+ HSCs may be important for tolerance establishment.124,125 Further data to support this theory come from a nonhuman primate model of tolerance induction where, although chimerism was lost after bone marrow transfusion induced mixed chimerism, animals remained tolerant.82,114 This result was puzzling. The mechanism for tolerance maintenance after loss of chimerism in this experiment was hypothesized to be the generation of peripheral Treg subsequent to a transient state of mixed chimerism. While unproven, it is reasonable to suggest that MDSCs which were present in the donor bone marrow, led to a Treg response in the recipient which aided in tolerance to donor antigens.84,124,125
Future Directions:
MDSCs are an important group of immunosuppressive cells, which may have the potential to benefit transplanted patients.126 Biologic therapies which act to harness the immunosuppressive capacity of a recipient’s own immune system may be game-changing for the field of transplantation. Critical questions remain ahead of the attempted clinical use of MDSCs, but these questions are indeed answerable. Cell surface markers which more clearly define MDSC sub-populations will be important for our understanding of MDSC’s in the blood, bone marrow, and allografts, following transplantation. Beyond the need for improved MDSC sub-population identification is the need for improved ability to distinguish PMNs from non-PMN MDSCs. The lifespan and migration patterns of MDSCs which develop after transplantation are poorly described. As an example, while MDSCs (particularly in cancer) are thought not to migrate to lymph nodes, it is possible that MDSCs which develop following transplantation do enter the lymphatic circulation. The effector function of MDSCs which develop after transplantation is largely unstudied. It may be that, analogous to cancer, different types of organ transplants (eg, kidneys versus hearts, etc.) induce different types of MDSCs, which have varied effector function.
Regarding transplantation, there are many unanswered questions from the standpoint of MDSCs. An important issue which requires clarification is that of antigen-specificity. Because MDSCs generated in tumor models can suppress anti-donor responses, and because limited data suggests that transplantation-induced MDSCs can suppress 3rd party T cell responses, it does not appear that MDSCs are antigen-specific. However, very little work has been done to address this question specifically. It is also not clear if tacrolimus affects MDSC function. Further, the data on rapamycin’s effect on MDSCs requires clarification. Whether or not MDSCs generated as a result of transplantation function similarly to those MDSCs generated after various tumors is also not clear. In addition, it remains unknown if the type of organ transplanted (eg kidney versus liver) leads to MDSCs with different suppressive capacities. Regarding transplantation tolerance, different induction protocols (eg co-stimulatory blockade versus mixed-chimerism) likely lead to the development of phenotypically, anatomically, and functionally different MDSCs, but this too remains unclear.
Not all MDSCs have equal suppressive potential.72 This may explain the varied approaches and outcomes associated with MDSCs and transplantation tolerance induction. For example, in some studies of transplantation, MDSC infusions are administered pretransplantation, and others are given posttransplantation. Some infusions are given one time, whereas others are given at multiple time points. Similarly, MDSC lifespan may differ depending on the sub-type of MDSC infused. An improved understanding of which treatments yield optimally suppressive MDSCs that remain in circulation (or in the transplanted graft) for the desired duration will be required for the adoption of clinical MDSC protocols. The authors’ laboratory has begun investigations designed to address each of these important questions.
Additional MDSC studies in large animals and following human transplantation are important for our understanding of both tolerance induction and immunosuppression minimization. The effects of immunosuppression on MDSCs should be studied further as well, as this may partly explain why previous attempts at tolerance induction in humans have been inconsistent.115,127–129 Indeed, it may be possible to augment naturally occurring recipient MDSCs after transplantation, such that immunosuppression dosing can be reduced or eliminated altogether.130–133
Acknowledgments
Grant support: This work was supported by a grant (to JRS) from the American Surgical Association Foundation and by the Living Legacy Foundation of Maryland.
Abbreviations:
- CMP
Common myeloid precursor
- IMC
Immature myeloid cell
- HSC
Hematopoietic stem cell
- IL
Interleukin
- LOX-1
lectin-type oxidized LDL receptor 1
- M
Monocyte
- MDSC
Myeloid derived suppressor cell
- PMN
polymorphonuclear cell
- TGF-b
transforming growth factor beta
- Treg
T Regulatory cell
Footnotes
Disclosure: The authors have no financial disclosures.
References:
- 1.Bronte V, Chappell DB, Apolloni E, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol 1999;162:5728–37. [PMC free article] [PubMed] [Google Scholar]
- 2.Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol 2012;91:167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 2010;40:2969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lees JR, Azimzadeh AM, Bromberg JS. Myeloid derived suppressor cells in transplantation. Curr Opin Immunol 2011;23:692–7. [DOI] [PubMed] [Google Scholar]
- 5.Mossadegh-Keller N, Sarrazin S, Kandalla PK, et al. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature 2013;497:239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez-Errico D, Vento-Tormo R, Sieweke M, Ballestar E. Epigenetic control of myeloid cell differentiation, identity and function. Nat Rev Immunol 2015;15:7–17. [DOI] [PubMed] [Google Scholar]
- 7.Ribechini E, Greifenberg V, Sandwick S, Lutz MB. Subsets, expansion and activation of myeloid-derived suppressor cells. Med Microbiol Immunol 2010;199:273–81. [DOI] [PubMed] [Google Scholar]
- 8.Waight JD, Netherby C, Hensen ML, et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest 2013;123:4464–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Hofer MJ, Jung SR, Lim SL, Campbell IL. IRF7-dependent type I interferon production induces lethal immune-mediated disease in STAT1 knockout mice infected with lymphocytic choriomeningitis virus. J Virol 2014;88:7578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eliana Ribechini JAH, Hergovits Sabine, Heuer Marion, Lucas Jorg, Schleicher Ulrike, Garrote Ana-Laura Jordán, Potte Sarah J., Riquelme Paloma, Brackmann Heike, Muller Nora, Raifer Hartmann, Berberich Ingolf, Huber Magdalena, Beilhack Andreas, Lohoff Michael, Bogdan Christian, Eyrich Matthias, Hermanns Heike M., Geissle Edward K., and Lutz Manfred B.. Novel GM-CSF signals via IFN-gR/IRF-1 and AKT/mTOR license monocytes for suppressor function. Blood Advances 2017;1:947–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ost M, Singh A, Peschel A, Mehling R, Rieber N, Hartl D. Myeloid-Derived Suppressor Cells in Bacterial Infections. Front Cell Infect Microbiol 2016;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goh C, Narayanan S, Hahn YS. Myeloid-derived suppressor cells: the dark knight or the joker in viral infections? Immunol Rev 2013;255:210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Li Z, Wang L, et al. Critical Role of Myeloid-Derived Suppressor Cells in Tumor-Induced Liver Immune Suppression through Inhibition of NKT Cell Function. Front Immunol 2017;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood 2009;113:3190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribechini E, Leenen PJ, Lutz MB. Gr-1 antibody induces STAT signaling, macrophage marker expression and abrogation of myeloid-derived suppressor cell activity in BM cells. Eur J Immunol 2009;39:3538–51. [DOI] [PubMed] [Google Scholar]
- 16.Ochando JC, Chen SH. Myeloid-derived suppressor cells in transplantation and cancer. Immunol Res 2012;54:275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Z, Riva M, Bjork P, et al. CD14 Is a Co-Receptor for TLR4 in the S100A9-Induced Pro-Inflammatory Response in Monocytes. PLoS One 2016;11:e0156377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greifenberg V, Ribechini E, Rossner S, Lutz MB. Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol 2009;39:2865–76. [DOI] [PubMed] [Google Scholar]
- 19.Vendramin A, Gimondi S, Bermema A, et al. Graft monocytic myeloid-derived suppressor cell content predicts the risk of acute graft-versus-host disease after allogeneic transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood stem cells. Biol Blood Marrow Transplant 2014;20:2049–55. [DOI] [PubMed] [Google Scholar]
- 20.Pinton L, Solito S, Damuzzo V, et al. Activated T cells sustain myeloid-derived suppressor cell-mediated immune suppression. Oncotarget 2016;7:1168–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y, Yang X, Liu W, et al. Chemerin has a protective role in hepatocellular carcinoma by inhibiting the expression of IL-6 and GM-CSF and MDSC accumulation. Oncogene 2017. [DOI] [PubMed] [Google Scholar]
- 22.Mondanelli G, Bianchi R, Pallotta MT, et al. A Relay Pathway between Arginine and Tryptophan Metabolism Confers Immunosuppressive Properties on Dendritic Cells. Immunity 2017;46:233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Q, Li X, Chen H, et al. IRF7 regulates the development of granulocytic myeloid-derived suppressor cells through S100A9 transrepression in cancer. Oncogene 2017. [DOI] [PubMed] [Google Scholar]
- 24.Leon B, Ardavin C. Monocyte migration to inflamed skin and lymph nodes is differentially controlled by L-selectin and PSGL-1. Blood 2008;111:3126–30. [DOI] [PubMed] [Google Scholar]
- 25.Garcia MR, Ledgerwood L, Yang Y, et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest 2010;120:2486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin J, Arakawa Y, Morita M, Fung JJ, Qian S, Lu L. C-C Chemokine Receptor type 2 (CCR2)-Dependent Migration of Myeloid-Derived Suppressor Cells in Protection of Islet Transplants. Transplantation 2017. ;101(8):1793–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med 2000;192:899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grewal IS, Foellmer HG, Grewal KD, et al. CD62L is required on effector cells for local interactions in the CNS to cause myelin damage in experimental allergic encephalomyelitis. Immunity 2001;14:291–302. [DOI] [PubMed] [Google Scholar]
- 29.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol 2016;37:208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016;7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabrilovich DI, Bronte V, Chen SH, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res 2007;67:425; author reply 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer Immunol Res 2017;5:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas Condamine GAD, Youn Je-In, Kossenkov Andrew V., Mony Sridevi, Alicea-Torres Kevin, Tcyganov Evgenii, Hashimoto Ayumi, Nefedova Yulia, Lin Cindy, Partlova Simona, Garfall Alfred, Vogl Dan T., Xu Xiaowei, Knight Stella C., Malietzis George, Lee Gui Han, Eruslanov Evgeniy, Albelda Steven M., Wang Xianwei, Mehta Jawahar L., , Meenakshi Bewtra AR, Hockstein Neil, Witt Robert, Masters Gregory, Nam Brian, Smirnov Denis, Sepulveda Manuel A. and Gabrilovich Dmitry I.. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Science Immunology 2016:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol 2012;144:250–68. [DOI] [PubMed] [Google Scholar]
- 35.Narita Y, Wakita D, Ohkur T, Chamoto K, Nishimura T. Potential differentiation of tumor bearing mouse CD11b+Gr-1+ immature myeloid cells into both suppressor macrophages and immunostimulatory dendritic cells. Biomed Res 2009;30:7–15. [DOI] [PubMed] [Google Scholar]
- 36.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hochst B, Mikulec J, Baccega T, et al. Differential induction of Ly6G and Ly6C positive myeloid derived suppressor cells in chronic kidney and liver inflammation and fibrosis. PLoS One 2015;10:e0119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luan Y, Mosheir E, Menon MC, et al. Monocytic myeloid-derived suppressor cells accumulate in renal transplant patients and mediate CD4(+) Foxp3(+) Treg expansion. Am J Transplant 2013;13:3123–31. [DOI] [PubMed] [Google Scholar]
- 39.Mandruzzato S, Brandau S, Britten CM, et al. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunol Immunother 2016;65:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McFarland HI, Nahill SR, Maciaszek JW, Welsh RM. CD11b (Mac-1): a marker for CD8+ cytotoxic T cell activation and memory in virus infection. J Immunol 1992;149:1326–33. [PubMed] [Google Scholar]
- 41.Fiorentini S, Licenziati S, Alessandri G, et al. CD11b expression identifies CD8+CD28+ T lymphocytes with phenotype and function of both naive/memory and effector cells. J Immunol 2001;166:900–7. [DOI] [PubMed] [Google Scholar]
- 42.Wolf D, Bukosza N, Engel D, et al. Inflammation, but not recruitment, of adipose tissue macrophages requires signalling through Mac-1 (CD11b/CD18) in diet-induced obesity (DIO). Thromb Haemost 2017;117:325–38. [DOI] [PubMed] [Google Scholar]
- 43.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012;12:253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu S, Thomas SM, Woodside DG, et al. Binding of paxillin to alpha4 integrins modifies integrin-dependent biological responses. Nature 1999;402:676–81. [DOI] [PubMed] [Google Scholar]
- 45.Liu S, Kiosses WB, Rose DM, et al. A fragment of paxillin binds the alpha 4 integrin cytoplasmic domain (tail) and selectively inhibits alpha 4-mediated cell migration. J Biol Chem 2002;277:20887–94. [DOI] [PubMed] [Google Scholar]
- 46.Kenney-Herbert E, Al-Mayhani T, Piccirillo SG, et al. CD15 Expression Does Not Identify a Phenotypically or Genetically Distinct Glioblastoma Population. Stem Cells Transl Med 2015;4:822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gadhoum SZ, Sackstein R. CD15 expression in human myeloid cell differentiation is regulated by sialidase activity. Nat Chem Biol 2008;4:751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoon J, Terada A, Kita H. CD66b regulates adhesion and activation of human eosinophils. J Immunol 2007;179:8454–62. [DOI] [PubMed] [Google Scholar]
- 49.Zhao F, Hoechst B, Duffy A, et al. S100A9 a new marker for monocytic human myeloid-derived suppressor cells. Immunology 2012;136:176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol 2008;181:4666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brimnes MK, Vangsted AJ, Knudsen LM, et al. Increased level of both CD4+FOXP3+ regulatory T cells and CD14+HLA-DR(−)/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol 2010;72:540–7. [DOI] [PubMed] [Google Scholar]
- 52.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci 2013;70:3813–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raber P, Ochoa AC, Rodriguez PC. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol Invest 2012;41:614–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raber PL, Thevenot P, Sierra R, et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int J Cancer 2014;134:2853–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ostrand-Rosenberg S. Tolerance and immune suppression in the tumor microenvironment. Cell Immunol 2016;299:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 2009;69:1553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bronte V, Serafini P, De Santo C, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol 2003;170:270–8. [DOI] [PubMed] [Google Scholar]
- 58.Zhao AM, Xu HJ, Kang XM, Zhao AM, Lu LM. New insights into myeloid-derived suppressor cells and their roles in feto-maternal immune cross-talk. J Reprod Immunol 2016;113:35–41. [DOI] [PubMed] [Google Scholar]
- 59.Fu S, Zhang N, Yopp AC, et al. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 - precursors. Am J Transplant 2004;4:1614–27. [DOI] [PubMed] [Google Scholar]
- 60.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res 2010;70:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu J, Du W, Yan F, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol 2013;190:3783–97. [DOI] [PubMed] [Google Scholar]
- 62.Yang B, Wang X, Ren X. Amino acid metabolism related to immune tolerance by MDSCs. Int Rev Immunol 2012;31:177–83. [DOI] [PubMed] [Google Scholar]
- 63.Redd PS, Ibrahim ML, Klement JD, et al. SETD1B Activates iNOS Expression in Myeloid-Derived Suppressor Cells. Cancer Res 2017;77:2834–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y, Zeng B, Zhang Z, Zhang Y, Yang R. B7-H1 on myeloid-derived suppressor cells in immune suppression by a mouse model of ovarian cancer. Clin Immunol 2008;129:471–81. [DOI] [PubMed] [Google Scholar]
- 65.Ochando JC, Turnquist HR. Innate immune cell collaborations instigate transplant tolerance. Am J Transplant 2014;14:2441–3. [DOI] [PubMed] [Google Scholar]
- 66.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A 2008;105:9331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kostlin N, Hofstadter K, Ostermeir AL, et al. Granulocytic Myeloid-Derived Suppressor Cells Accumulate in Human Placenta and Polarize toward a Th2 Phenotype. J Immunol 2016;196:1132–45. [DOI] [PubMed] [Google Scholar]
- 68.Kostlin N, Vogelmann M, Spring B, et al. Granulocytic myeloid derived suppressor cells from human cord blood modulate T-helper-cell response towards an anti-inflammatory phenotype. Immunology 2017. ;152(1):89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol 2004;172:989–99. [DOI] [PubMed] [Google Scholar]
- 70.Nagaraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med 2007;13:828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagaraj S, Nelson A, Youn JI, Cheng P, Quiceno D, Gabrilovich DI. Antigen-specific CD4(+) T cells regulate function of myeloid-derived suppressor cells in cancer via retrograde MHC class II signaling. Cancer Res 2012;72:928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marigo I, Bosio E, Solito S, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity 2010;32:790–802. [DOI] [PubMed] [Google Scholar]
- 73.Ku AW, Muhitch JB, Powers CA, et al. Tumor-induced MDSC act via remote control to inhibit L-selectin-dependent adaptive immunity in lymph nodes. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ostrand-Rosenberg S, Sinha P, Figley C, et al. Frontline Science: Myeloid-derived suppressor cells (MDSCs) facilitate maternal-fetal tolerance in mice. J Leukoc Biol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ismail AQT. Does placental MDSC-mediated modulation of arginine levels help protect the foetus from auxotrophic pathogens? J Matern Fetal Neonatal Med 2017:1–3. [DOI] [PubMed] [Google Scholar]
- 76.Raghupathy R Th1-type immunity is incompatible with successful pregnancy. Immunol Today 1997;18:478–82. [DOI] [PubMed] [Google Scholar]
- 77.Alijotas-Reig J, Llurba E, Gris JM. Potentiating maternal immune tolerance in pregnancy: a new challenging role for regulatory T cells. Placenta 2014;35:241–8. [DOI] [PubMed] [Google Scholar]
- 78.Raghupathy R Maternal anti-placental cell-mediated reactivity and spontaneous abortions. Am J Reprod Immunol 1997;37:478–84. [DOI] [PubMed] [Google Scholar]
- 79.Kostlin N, Kugel H, Spring B, et al. Granulocytic myeloid derived suppressor cells expand in human pregnancy and modulate T-cell responses. Eur J Immunol 2014;44:2582–91. [DOI] [PubMed] [Google Scholar]
- 80.Svensson-Arvelund J, Mehta RB, Lindau R, et al. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol 2015;194:1534–44. [DOI] [PubMed] [Google Scholar]
- 81.Booth AJ, Grabauskiene S, Wood SC, Lu G, Burrell BE, Bishop DK. IL-6 promotes cardiac graft rejection mediated by CD4+ cells. J Immunol 2011;187:5764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nadazdin O, Boskovic S, Murakami T, et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med 2011;3:86ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Starzl TE, Zinkernagel RM. Transplantation tolerance from a historical perspective. Nat Rev Immunol 2001;1:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scalea JR, Bromberg J, Bartlett ST, Scalea TM. Mechanistic similarities between trauma, atherosclerosis, and other inflammatory processes. J Crit Care 2015;30:1344–8. [DOI] [PubMed] [Google Scholar]
- 85.LaRosa DF, Rahman AH, Turka LA. The innate immune system in allograft rejection and tolerance. J Immunol 2007;178:7503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Delano MJ, Scumpia PO, Weinstein JS, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med 2007;204:1463–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hock BD, McKenzie JL. Suppression of CD3/CD28 antibody stimulated responses by human granulocytic myeloid-derived suppressor cells: fact or artefact? Immunol Lett 2013;152:151–2. [DOI] [PubMed] [Google Scholar]
- 88.He Y, Bei J, Zeng H, Pan Z. The roles of sepsis-induced myeloid derived suppressor cells in mice corneal, skin and combined transplantation. Transpl Immunol 2016;34:8–13. [DOI] [PubMed] [Google Scholar]
- 89.He Y, Wang B, Jia B, Guan J, Zeng H, Pan Z. Effects of Adoptive Transferring Different Sources of Myeloid-Derived Suppressor Cells in Mice Corneal Transplant Survival. Transplantation 2015;99:2102–8. [DOI] [PubMed] [Google Scholar]
- 90.Guillonneau C Efficacy of Myeloid Derived Suppressor Cells on Transplant Survival. Transplantation 2015;99:2017–9. [DOI] [PubMed] [Google Scholar]
- 91.Wu T, Zhao Y, Wang H, et al. mTOR masters monocytic myeloid-derived suppressor cells in mice with allografts or tumors. Sci Rep 2016;6:20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang W, Liang S, Wu J, Horuzsko A. Human inhibitory receptor immunoglobulin-like transcript 2 amplifies CD11b+Gr1+ myeloid-derived suppressor cells that promote long-term survival of allografts. Transplantation 2008;86:1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carretero-Iglesia L, Bouchet-Delbos L, Louvet C, et al. Comparative Study of the Immunoregulatory Capacity of In Vitro Generated Tolerogenic Dendritic Cells, Suppressor Macrophages, and Myeloid-Derived Suppressor Cells. Transplantation 2016;100:2079–89. [DOI] [PubMed] [Google Scholar]
- 94.Drujont L, Carretero-Iglesia L, Bouchet-Delbos L, et al. Evaluation of the therapeutic potential of bone marrow-derived myeloid suppressor cell (MDSC) adoptive transfer in mouse models of autoimmunity and allograft rejection. PLoS One 2014;9:e100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dugast AS, Haudebourg T, Coulon F, et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol 2008;180:7898–906. [DOI] [PubMed] [Google Scholar]
- 96.Nakamura T, Nakao T, Yoshimura N, Ashihara E. Rapamycin Prolongs Cardiac Allograft Survival in a Mouse Model by Inducing Myeloid-Derived Suppressor Cells. Am J Transplant 2015;15:2364–77. [DOI] [PubMed] [Google Scholar]
- 97.Hock BD, Mackenzie KA, Cross NB, et al. Renal transplant recipients have elevated frequencies of circulating myeloid-derived suppressor cells. Nephrol Dial Transplant 2012;27:402–10. [DOI] [PubMed] [Google Scholar]
- 98.Han C, Wu T, Na N, Zhao Y, Li W, Zhao Y. The effect of immunosuppressive drug cyclosporine A on myeloid-derived suppressor cells in transplanted mice. Inflamm Res 2016;65:679–88. [DOI] [PubMed] [Google Scholar]
- 99.Kim YS, Kim YJ, Lee JM, et al. Functional changes in myeloid-derived suppressor cells (MDSCs) during tumor growth: FKBP51 contributes to the regulation of the immunosuppressive function of MDSCs. J Immunol 2012;188:4226–34. [DOI] [PubMed] [Google Scholar]
- 100.Rosborough BR, Raich-Regue D, Turnquist HR, Thomson AW. Regulatory myeloid cells in transplantation. Transplantation 2014;97:367–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol 2006;176:2085–94. [DOI] [PubMed] [Google Scholar]
- 102.Zhang K, Bai X, Li R, et al. Endogenous glucocorticoids promote the expansion of myeloid-derived suppressor cells in a murine model of trauma. Int J Mol Med 2012;30:277–82. [DOI] [PubMed] [Google Scholar]
- 103.Macedo C, Turquist H, Metes D, Thomson AW. Immunoregulatory properties of rapamycin-conditioned monocyte-derived dendritic cells and their role in transplantation. Transplant Res 2012;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol 2007;178:7018–31. [DOI] [PubMed] [Google Scholar]
- 105.Qu P, Shelley WC, Yoder MC, Wu L, Du H, Yan C. Critical roles of lysosomal acid lipase in myelopoiesis. Am J Pathol 2010;176:2394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ding X, Du H, Yoder MC, Yan C. Critical role of the mTOR pathway in development and function of myeloid-derived suppressor cells in lal−/− mice. Am J Pathol 2014;184:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang C, Wang S, Li J, et al. The mTOR signal regulates myeloid-derived suppressor cells differentiation and immunosuppressive function in acute kidney injury. Cell Death Dis 2017;8:e2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wegner A, Verhagen J, Wraith DC. Myeloid-derived suppressor cells mediate tolerance induction in autoimmune disease. Immunology 2017;151:26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hongo D, Tang X, Baker J, Engleman EG, Strober S. Requirement for interactions of natural killer T cells and myeloid-derived suppressor cells for transplantation tolerance. Am J Transplant 2014;14:2467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang F, Li Y, Wu T, et al. TNFalpha-induced M-MDSCs promote transplant immune tolerance via nitric oxide. J Mol Med (Berl) 2016;94:911–20. [DOI] [PubMed] [Google Scholar]
- 111.Conde P, Rodriguez M, van der Touw W, et al. DC-SIGN(+) Macrophages Control the Induction of Transplantation Tolerance. Immunity 2015;42:1143–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. Am J Transplant 2012;12:1133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scandling JD, Busque S, Shizuru JA, et al. Chimerism, graft survival, and withdrawal of immunosuppressive drugs in HLA matched and mismatched patients after living donor kidney and hematopoietic cell transplantation. Am J Transplant 2015;15:695–704. [DOI] [PubMed] [Google Scholar]
- 114.Kawai T, Sogawa H, Boskovic S, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant 2004;4:1391–8. [DOI] [PubMed] [Google Scholar]
- 115.Sachs DH, Kawai T, Sykes M. Induction of tolerance through mixed chimerism. Cold Spring Harb Perspect Med 2014;4:a015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Okumi M, Scalea JR, Gillon BC, et al. The induction of tolerance of renal allografts by adoptive transfer in miniature swine. Am J Transplant 2013;13:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scalea JR, Okumi M, Villani V, et al. Abrogation of renal allograft tolerance in MGH miniature swine: the role of intra-graft and peripheral factors in long-term tolerance. Am J Transplant 2014;14:2001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang C, Wang S, Yang C, Rong R. The Crosstalk between Myeloid Derived Suppressor Cells and Immune Cells: To Establish Immune Tolerance in Transplantation. J Immunol Res 2016;2016:4986797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 2006;66:1123–31. [DOI] [PubMed] [Google Scholar]
- 120.Fishbein JM, Rosengard BR, Gianello P, et al. Development of tolerance to class II-mismatched renal transplants after a short course of cyclosporine therapy in miniature swine. Transplantation 1994;57:1303–8. [DOI] [PubMed] [Google Scholar]
- 121.Gianello PR, Fishbein JM, Rosengard BR, et al. Tolerance to class I-disparate renal allografts in miniature swine. Maintenance of tolerance despite induction of specific antidonor CTL responses. Transplantation 1995;59:772–7. [DOI] [PubMed] [Google Scholar]
- 122.Pescovitz MD, Lunney JK, Sachs DH. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol 1984;133:368–75. [PubMed] [Google Scholar]
- 123.Scalea JR, Villani V, Gillon BC, et al. Development of antidonor antibody directed toward non-major histocompatibility complex antigens in tolerant animals. Transplantation 2014;98:514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scalea JR, Tomita Y, Lindholm CR, Burlingham W. Transplantation Tolerance Induction: Cell Therapies and Their Mechanisms. Front Immunol 2016;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scalea JR, Hickman JB, Moore DJ, Brayman KL. An overview of the necessary thymic contributions to tolerance in transplantation. Clin Immunol 2016. [DOI] [PubMed] [Google Scholar]
- 126.Hutchinson JA, Geissler EK. Now or never? The case for cell-based immunosuppression in kidney transplantation. Kidney Int 2015;87:1116–24. [DOI] [PubMed] [Google Scholar]
- 127.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2008;358:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kawai T, Sachs DH, Sprangers B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant 2014;14:1599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Leventhal J, Levitsky J, Abecassis MM, et al. Spontaneous operational tolerance in kidney transplant recipients. Am J Transplant 2012;12:1350; author reply 1-2. [DOI] [PubMed] [Google Scholar]
- 130.Chhabra D, Alvarado A, Dalal P, et al. Impact of calcineurin-inhibitor conversion to mTOR inhibitor on renal allograft function in a prednisone-free regimen. Am J Transplant 2013;13:2902–11. [DOI] [PubMed] [Google Scholar]
- 131.Kirk AD. The cam-path forward. Am J Transplant 2013;13:9–10. [DOI] [PubMed] [Google Scholar]
- 132.Kirk AD, Guasch A, Xu H, et al. Renal transplantation using belatacept without maintenance steroids or calcineurin inhibitors. Am J Transplant 2014;14:1142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McShane PJ, Garrity ER Jr. Minimization of immunosuppression after lung transplantation: current trends. Transpl Int 2009;22:90–5. [DOI] [PubMed] [Google Scholar]


