Abstract
Background
Establishing whether patients are exposed to a ‘known cause’ is a key element in both the diagnostic assessment and the subsequent management of hypersensitivity pneumonitis (HP).
Objective
This study surveyed British interstitial lung disease (ILD) specialists to document current practice and opinion in relation to establishing causation in HP.
Methods
British ILD consultants (pulmonologists) were invited by email to take part in a structured questionnaire survey, to provide estimates of demographic data relating to their service and to rate their level of agreement with a series of statements. A priori ‘consensus agreement’ was defined as at least 70% of participants replying that they ‘Strongly agree’ or ‘Tend to agree’.
Results
54 consultants took part in the survey from 27 ILD multidisciplinary teams. Participants estimated that 20% of the patients in their ILD service have HP, and of these, a cause is identifiable in 32% of cases. For patients with confirmed HP, an estimated 40% have had a bronchoalveolar lavage for differential cell counts, and 10% a surgical biopsy. Consensus agreement was reached for 25 of 33 statements relating to causation and either the assessment of unexplained ILD or management of confirmed HP.
Conclusions
This survey has demonstrated that although there is a degree of variation in the diagnostic approach for patients with suspected HP in Britain, there is consensus opinion for some key areas of practice. There are several factors in clinical practice that currently act as potential barriers to identifying the cause for British HP patients.
Keywords: Allergic Alveolitis, Interstitial Fibrosis, Occupational Lung Disease
Key messages.
Identifying causation in hypersensitivity pneumonitis is a common challenge for multidisciplinary teams, and there is little published research to inform the optimal approach.
Interstitial lung disease specialists estimated that a cause is identifiable in around 32% of British HP cases, and agreed that there are several barriers that prevent this for most patients.
This paper provides the first British data on causation in hypersensitivity pneumonitis and presents a series of agreed Consensus Statements for the investigation and management of these patients.
Introduction
Hypersensitivity pneumonitis (HP) is a common form of interstitial lung disease (ILD), with widely varying causes and clinical outcomes.1 Differentiating HP from other forms of fibrotic ILD is a common diagnostic dilemma for multidisciplinary teams (MDTs),2 resulting in a low level of diagnostic agreement internationally.3
Identifying exposure to a ‘known cause’ of HP remains a key element, both in the diagnostic approach for patients with unexplained ILD and in the management of confirmed HP.4–8 Despite this, the optimal approach for establishing causation remains to be determined,9 with up to 63% of cases having no identifiable aetiological agent.10–17
The aim of this study was to document current practice and opinion among British ILD specialists (pulmonologists) in relation to establishing causation in HP.
Methods
A structured questionnaire was developed by a Steering Committee (n=8) of clinicians taken from recent or current members of the British Thoracic Society Specialist Advisory Groups for Interstitial or Occupational and Environmental Lung Disease with experience of managing HP. The series of statements for agreement were developed to gather expert opinion and identify areas of best practice at a national level.
Once the content of the questionnaire was agreed by the Steering Committee, the lead respiratory consultants of all British ILD MDTs were contacted via email and invited to participate in the ‘Great British HP Survey’. Participants were asked to provide the email address of the other respiratory consultant members of their regional ILD MDT, and these individuals were also invited to participate by email. The number of participants was not restricted, and reminder emails were sent to improve participation. Data were collected between March and August 2018. Participants completed the questionnaire via a Web link (SurveyMonkey) provided in the email and were required to answer all questions. Responses required were predominantly numerical estimates or level of agreement/disagreement with statements devised by the Steering Committee (Strongly Agree, Tend to Agree, Neither Agree nor Disagree, Tend to Disagree and Strongly Disagree). A priori, ‘Consensus Agreement’ was defined as 70% or greater of participants replying that they ‘Agree’ (Strongly or Tend to). Participants were asked to rate how commonly their ILD service suspected a range of known domestic and occupational causes. For statements relating to causation, agreement that an exposure was a ‘common cause’ was defined as participants reporting that a cause was ‘Commonest’ or ‘Common’. Median and IQR for numerical estimates were calculated using the online IQR calculator (EasyCalculation.com).
The full survey comprised 126 questions or statements covering background demographic information, causation, clinical assessment, prognosis and management. Where appropriate, participants were also given the opportunity to provide free text comments for certain questions. The results from the relevant sections of the survey relating to demographic data and causation are presented in this manuscript.
Patient and public involvement
This research was unfunded and was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Results
Fifty-four ILD specialist consultants (online supplementary appendix 1) took part in the survey (45 from England, 6 from Scotland and 3 from Wales) representing 27 ILD MDTs. Participants had worked in ILD MDTs for a median of 10 years (IQR 5–15 years), representing a cumulative total of 537 years of clinical practice. Thirty-five (65%) reported that they have routine access to bronchoalveolar lavage (BAL) differential cell counts, and nine (17%) routine access to cryobiopsy. Access to occupational lung disease (OLD) services varied between centres, with 39 (72%) participants agreeing that they have an established route of referral to a regional OLD service, and 22 (41%) agreeing that they work in an ILD MDT that has a consultant with expertise in OLD.
bmjresp-2019-000469supp001.pdf (39.6KB, pdf)
Participants estimated that 20% (IQR 15%–30%) of the patients in their ILD service have HP as a final diagnosis, and of these, 40% (IQR 3%–75%) have had a BAL and 10% (IQR 5%–20%) a surgical biopsy. Thirty (56%) of those taking part in the survey stated that their preferred classification for HP is based on the predominant radiological feature on high resoltion CT scan (inflammatory, mixed or fibrotic HP), whereas nine (17%) prefer classification based on duration of symptoms (acute, subacute or chronic HP).
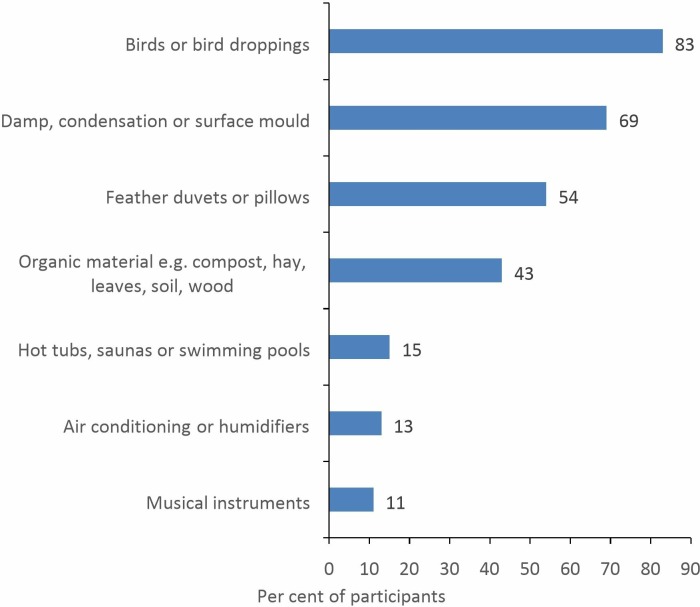
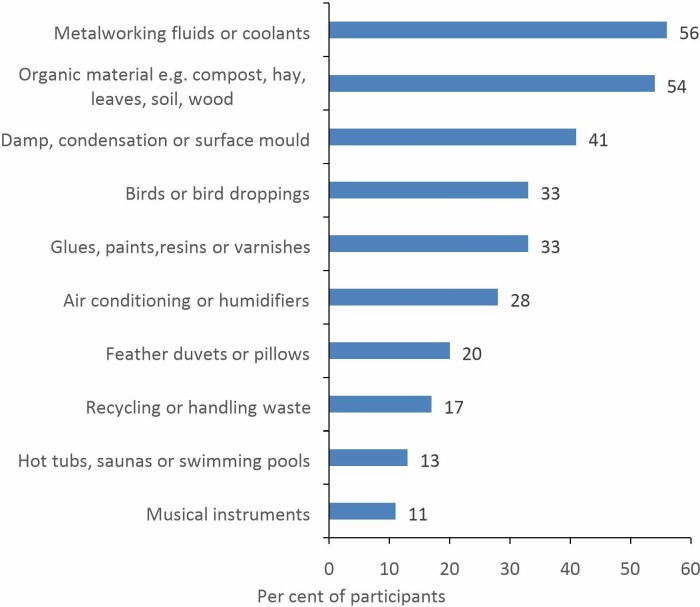
Participants estimated that they are able to identify a cause in 32% (IQR 20%–50%) of patients diagnosed with HP. Thirty-two (59%) respondents agreed that in the ILD service they worked in, HP is commonly attributed to domestic exposures in the home or garden, whereas the corresponding figure for workplace exposures was only 21 (39%). The most commonly suspected domestic and occupational causes, respectively, are exposure to avian proteins (figure 1) and metalworking fluids (MWFs)/coolants (figure 2).
Figure 1.
Commonly suspected domestic causes of HP (n=54). HP, hypersensitivity pneumonitis.
Figure 2.
Commonly suspected occupational causes of HP (n=54). HP, hypersensitivity pneumonitis.
Twenty-five statements reached consensus agreement for either the assessment of patients with unexplained ILD or management of confirmed HP (tables 1 and 2, respectively). Eight statements did not reach consensus agreement (table 3).
Table 1.
Agreed consensus statements for the assessment of HP diagnosis and/or cause in patients with unexplained ILD (n=54)
| Statements | % agree |
| All patients with suspected HP on clinical or radiological grounds should be referred for a regional ILD MDT opinion. | 83 |
| In patients with ILD of unknown cause, the following clinical features increase the likelihood of a final diagnosis of HP: | |
|
96 89 78 |
| The following tests should be requested for all patients with unexplained ILD: | |
|
81 |
| In day-to-day practice, the following tests are useful in helping to differentiate HP from other forms of ILD: | |
|
89 70 |
HP, hypersensitivity pneumonitis; ILD, interstitial lung disease; MDT, multidisciplinary team.
Table 2.
Agreed consensus statements for the management of patients with confirmed HP (n=54)
| Statements | % agree |
| The main aim of HP management is (where possible) to identify a cause and assist patients in avoiding further exposure. | 96 |
| A domestic cause of HP should be suspected if patients report that symptoms occur a few hours after a specific exposure in the home environment, or improve away from home, for example, following a 1- to 2-week holiday. | 94 |
| An occupational cause of HP should be suspected if patients report that symptoms occur a few hours after a specific exposure in the workplace, or improve away from work, for example, on rest days or holidays. | 94 |
| In many cases of confirmed HP it is difficult to identify a cause. | 98 |
| HP is commonly attributed to ‘no identifiable exposure’. | 93 |
| Identifying the cause of HP is difficult in some cases: | |
|
85 85 78 78 76 74 |
| Specific IgG titres to the cause (where available) may remain elevated in the blood following cessation of exposure and are not a reliable method of identifying ongoing exposure. | 85 |
| Prognosis in HP: | |
|
91 87 84 76 |
| The following features are associated with ‘reversible disease’, that is, the potential for some degree of clinical improvement with cessation of exposure and/or immunosuppression: | |
|
96 |
| In a proportion of biopsy proven HP, fibrosis progresses despite cessation of exposure to the cause. | 98 |
HP, hypersensitivity pneumonitis.
Table 3.
Statements not reaching consensus agreement for the assessment of HP diagnosis and/or cause in patients with unexplained ILD (n=54)
| Statements | % agree |
| In day-to-day practice, the following tests are useful in helping to differentiate HP from other forms of ILD: | |
|
19 48 35 |
| In patients where HP is the first choice clinical and radiological diagnosis, a BAL differential cell count: | |
|
59 61 |
| HP is commonly attributed to ‘an idiopathic disease’. | 54 |
| Normal levels of specific IgG to avian proteins effectively exclude bird or feather down/duvet exposures as the cause of HP in exposed individuals. | 15 |
| BAL lymphocytosis may persist following cessation of exposure and is not a reliable method of identifying ongoing exposure. | 43 |
BAL, bronchoalveolar lavage; HP, hypersensitivity pneumonitis; ILD, interstitial lung disease.
Discussion
This study presents the first British data relating to establishing HP causation, based on the opinion of ILD specialists with a cumulative total of over 500 years of clinical practice. There was a high level of consensus agreement among participants that identifying exposure to a known cause of HP is important, both for the assessment of patients with unexplained ILD and the management of patients with confirmed disease. There was also a clear consensus view that in many cases of HP in Britain it is not possible to identify a cause, and that there are identifiable barriers that contribute to this in day-to-day clinical practice.
The main limitations of this study are twofold. First, as there is no national database for HP, the survey results are reliant on estimates from practicing clinicians, rather than routinely recorded British outcome data. The second consideration is that as the overall participation rate was <50% of those invited by email, the results may not be truly representative of the full range of national practice and opinion.
Participants in the GB HP survey estimated that on average, 20% of all ILD cases in their service have HP as a final diagnosis, a figure that is slightly higher than the 2%–15% range reported from ILD registries or database studies from other countries.15 17–23 It is not possible to determine whether this difference is real, or due to the limitation of our study design. Participants also reported that on average, 40% of patients diagnosed with HP have had a BAL, although this estimate varied widely (between 0% and 100%), suggesting that the British diagnostic approach varies markedly between centres.
British specialists were also asked to estimate what proportion of the patients diagnosed with HP, have an identifiable causative exposure. Although the median value for this estimate was 32%, there was again a wide range of opinion (between 5% and 76%). This degree of variability is perhaps not surprising, considering the lack of a standardised national approach to establishing HP causation. While the true epidemiology of HP in Britain remains unknown, the range of estimates from the GB HP survey were broadly consistent with the findings from other studies, where the proportion of HP patients without an identifiable cause was: 0% in Japan14; 4% in Brazil15; 6% in Poland16; 28% in China17; and 25%–63% in the USA.10–13
Although consensus agreement was not reached, 54% of participants agreed that they commonly attribute HP to be an ‘idiopathic disease’. It is not possible from the survey results to determine whether this term is used to reflect the difficulties clinicians encounter in identifying the cause, or a true belief that HP can occur spontaneously (ie, without there being a cause to identify). Notably, 43% of participants recognised that BAL lymphocytosis in HP can persist following cessation of exposure, and in some cases it may therefore be impossible to identify the cause, if it is no longer present in the work or home environment.
Causation in HP is likely to vary between countries due to a wide range of factors, including differences in geography, climate, housing and industry. In terms of identifying possible causes, GB HP survey participants reported that they more commonly attribute the disease to domestic exposures in the home or garden, than occupational exposures in the workplace. For domestic HP, the most commonly suspected exposures are to birds, bird droppings or feathers. This is in keeping with the majority of studies from other countries, where avian exposure has been the most commonly identified cause, accounting for 17%–66% of all cases.10–12 14 16 17 24 A recent retrospective single-centred US study also noted a high prevalence of contact with avian protein in biopsy proven chronic HP cases (29% to bird and 58% to down), and incorporated a history of exposure into a prediction model.4 A slightly different pattern of causation for chronic fibrotic HP was reported from a retrospective single-centred Brazilian study, as exposure to mould (29% of cases) was a more common cause than contact with birds/feathers (23% of cases).15
Occupational exposures are also an important cause of HP,25 accounting for an estimated 19% of all cases.26 The GB HP survey identified variable access to specialist OLD services nationally, with only 41% of participants reporting that their ILD MDT has a consultant with expertise in OLD, and 72% an established route of referral. For occupational causes, the most commonly suspected exposures are to MWF or organic material (eg, compost, hay, leaves, soil, wood). This is entirely in keeping with data from the UK reporting scheme for occupational HP between 1996 and 2015, where exposure to MWF (35% of cases) and farming (17% of cases) were the most common causes.27 The reporting data demonstrated that over this time period, ‘Metalworking fluid HP’ has become the most commonly reported cause of occupational HP in the UK, a change in epidemiology that merits further research.28
Given the poor level of diagnostic agreement for HP between MDTs, the GB HP survey selected a relatively low (70%) level for consensus agreement.7 29 Utilising this threshold, the survey found consensus for 25 of 33 statements relating to different aspects of HP and causation, with many having much higher levels of agreement. Although methodological differences do not allow exact comparisons, the results from an international Delphi for HP diagnosis offer a valuable comparator for some of the British consensus statements. Of note, the international Delphi comprised three rounds, required 80% agreement for consensus, and sought views from 45 experts from 14 countries (22 from North America and 3 from the UK).7 Despite these differences, the international Delphi and GB HP surveys found similar consensus agreement for the importance of certain aspects of HP diagnosis, including MDT case discussion, identifying exposure to a known cause and recognising a temporal relation between symptoms and exposure. Another common view shared by participants of the two studies related to the use of specific inhalation challenges (SIC) to confirm causation in HP. Although these are routinely carried out as part of the diagnostic approach in some centres,8 SIC was only rated as diagnostically useful by 35% of British ILD specialists and as diagnostically important by 42% in the first round of the international Delphi.
One notable difference between the findings of the two studies related to the utility of specific IgG blood testing to known causes of HP. The diagnostic value of specific IgG testing did not reach consensus for importance in the international Delphi, but in the first round, the majority (53%) rated it as important.7 In contrast, the GB HP survey participants did reach consensus agreement that specific IgG blood tests: are useful in helping to differentiate HP from other forms of ILD; should be requested for all patients with unexplained ILD; and act as a barrier to establishing causation in some cases due to the limited panel available. The diagnostic importance of IgG testing was further highlighted in the survey as the majority of British ILD clinicians (61%) agreed that in the context of a clear exposure history, typical radiology and an elevated level of specific IgG to a known cause, a BAL differential cell count is not required.
There was also consensus agreement among British ILD specialists that the lack of routine provision for home/workplace visits acts as a barrier to identifying the cause of HP in some cases. In Britain, ILD MDTs operate within a government funded National Health Service, and there is no standardised approach for investigating possible causes of HP, and no routinely available funding for home/workplace visits for environmental sampling. The current approach to identifying HP causation in Britain is therefore often heavily reliant on patient history and the results of specific IgG testing. Alternative strategies to antigen identification have been suggested in other countries, based either on the results of bespoke IgG testing or SIC to extracts of microorganisms cultured directly from the home or workplace.30 31 While this type of approach is promising, it requires further validation, and is not likely to impact on HP practice in Britain in the near future.
In conclusion, the GB HP survey has demonstrated national variation in the utilisation of invasive diagnostic tests in HP, but consensus opinion for some of the key aspects of practice relating to establishing causation. The survey has highlighted that ILD specialists believe this to be an important area of practice, affecting clinical outcomes, but that there are identifiable barriers preventing this for most British HP patients.
Acknowledgments
The authors are grateful to the GB HP survey participants for taking time to complete the survey.
Footnotes
Collaborators: Huzaifa Adamali, Suresh Babu, Shaney Barrat, Alexander Basran, Paul Beirne, Stephen Bianchi, George Chalmers, Nazia Chaudhuri, Sarah Davies, Owen Dempsey, Sinan Eccles, Christine Fiddler, Noleen Foley, Ian Forrest, Sophie Fletcher, Peter George, Salman Ghani, Michael Gibbons, Mike Greenstone, Simon Hart, Nick Hirani, Jennifer Hoyle, Rachel Hoyles, John Hutchinson, Gisli Jenkins, Eoin Judge, Ajay Kamath, Maria Kokosi, Candy Lee, Toby Maher, Ben Marshall, Neil McAndrew, Philip Molyneux, Douglas Morrison, Steve O’Hickey, Joanna Porter, Steve Renshaw, Charles Sharp, Nicky Simler, Mark Spears, Alexander Spiers, Katherine Spinks, Monica Spiteri, Chris Stenton, Sharon Sturney, Chris Warburton, Sarah Wiscombe, Felix Woodhead.
Contributors: CMB planned the study, conducted the survey, drafted the manuscript and is responsible for guaranteeing the overall content of the research. SB, JRF, HP, EAR, LGS, GIW and REW contributed to designing the content of the survey, interpreting the results and had input into the writing of the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
Contributor Information
the GB HP Survey Participants:
Huzaifa Adamali, Suresh Babu, Shaney Barrat, Alexander Basran, Paul Beirne, Stephen Bianchi, George Chalmers, Nazia Chaudhuri, Sarah Davies, Owen Dempsey, Sinan Eccles, Christine Fiddler, Noleen Foley, Ian Forrest, Sophie Fletcher, Peter George, Salman Ghani, Michael Gibbons, Mike Greenstone, Simon Hart, Nick Hirani, Jennifer Hoyle, Rachel Hoyles, John Hutchinson, Gisli Jenkins, Eoin Judge, Ajay Kamath, Maria Kokosi, Candy Lee, Toby Maher, Neil McAndrew Ben Marshall, Philip Molyneux, Douglas Morrison, Steve O’Hickey, Joanna Porter, Steve Renshaw, Charles Sharp, Nicky Simler, Mark Spears, Alexander Spiers, Katherine Spinks, Monica Spiteri, Chris Stenton, Sharon Sturney, Chris Warburton, Sarah Wiscombe, and Felix Woodhead
References
- 1. Kouranos V, Jacob J, Nicholson A, et al. . Fibrotic hypersensitivity pneumonitis: key issues in diagnosis and management. J Clin Med 2017;6 10.3390/jcm6060062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. HE J, Corte TJ, Moodley Y, et al. . Evaluating the interstitial lung disease multidisciplinary meeting: a survey of expert centres. BMC Pulm Med 2016;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walsh SLF, Wells AU, Desai SR, et al. . Multicentre evaluation of multidisciplinary team meeting agreement on diagnosis in diffuse parenchymal lung disease: a case-cohort study. Lancet Respir Med 2016;4:557–65. 10.1016/S2213-2600(16)30033-9 [DOI] [PubMed] [Google Scholar]
- 4. Johannson KA, Elicker BM, Vittinghoff E, et al. . A diagnostic model for chronic hypersensitivity pneumonitis. Thorax 2016;71:951–4. 10.1136/thoraxjnl-2016-208286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vasakova M, Morell F, Walsh S, et al. . Hypersensitivity pneumonitis: perspectives in diagnosis and management. Am J Respir Crit Care Med 2017;196:680–9. 10.1164/rccm.201611-2201PP [DOI] [PubMed] [Google Scholar]
- 6. Salisbury ML, Myers JL, Belloli EA, et al. . Diagnosis and treatment of fibrotic hypersensitivity pneumonia. where we stand and where we need to go. Am J Respir Crit Care Med 2017;196:690–9. 10.1164/rccm.201608-1675PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morisset J, Johannson KA, Jones KD, et al. . Identification of diagnostic criteria for chronic hypersensitivity pneumonitis: an international modified Delphi survey. Am J Respir Crit Care Med 2018;197:1036–44. 10.1164/rccm.201710-1986OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morell F, Ojanguren I, Cruz MJ, et al. . Hypersensitivity pneumonitis. toward a less invasive diagnostic procedure. Archivos de Bronconeumología 2018;54:445–6. 10.1016/j.arbr.2018.02.018 [DOI] [PubMed] [Google Scholar]
- 9. Molyneaux PL, Maher TM. Time for an international consensus on hypersensitivity pneumonitis. A call to arms. Am J Respir Crit Care Med 2017;196:665–6. [DOI] [PubMed] [Google Scholar]
- 10. Hanak V, Golbin JM, Ryu JH. Causes and presenting features in 85 consecutive patients with hypersensitivity pneumonitis. Mayo Clin Proc 2007;82:812–6. 10.4065/82.7.812 [DOI] [PubMed] [Google Scholar]
- 11. Mooney JJ, Elicker BM, Urbania TH, et al. . Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest 2013;144:586–92. 10.1378/chest.12-2623 [DOI] [PubMed] [Google Scholar]
- 12. Fernández Pérez ER, Swigris JJ, Forssén AV, et al. . Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest 2013;144:1644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adegunsoye A, Oldham JM, Fernández Pérez ER, et al. . Outcomes of immunosuppressive therapy in chronic hypersensitivity pneumonitis. ERJ Open Res 2017;3. doi: 10.1183/23120541.00016-2017. [Epub ahead of print: 17 08 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dobashi K, Akiyama K, Usami A, et al. . Committee for Japanese guideline for diagnosis and management of occupational allergic diseases; Japanese Society of Allergology. Japanese guideline for occupational allergic diseases 2014. Allergol Int 2014;63:421–42. [DOI] [PubMed] [Google Scholar]
- 15. Gimenez A, Storrer K, Kuranishi L, et al. . Change in FVC and survival in chronic fibrotic hypersensitivity pneumonitis. Thorax 2018;73:391–2. [DOI] [PubMed] [Google Scholar]
- 16. Szturmowicz M, Barańska I, Jędrych ME, et al. . Hypersensitivity pneumonitis recognised in a single pulmonary unit, between 2005 and 2015 - comparison with recently proposed diagnostic criteria. Adv Respir Med 2019;87:83–9. 10.5603/ARM.2019.0014 [DOI] [PubMed] [Google Scholar]
- 17. Wang LJ, Cai HR, Xiao YL, et al. . Clinical characteristics and outcomes of hypersensitivity pneumonitis: a population-based study in China. Chin Med J 2019;132:1283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thomeer MJ, Costabe U, Rizzato G, et al. . Comparison of registries of interstitial lung diseases in three European countries. Eur Respir J Suppl 2001;32:114s–8. [PubMed] [Google Scholar]
- 19. Xaubet A, Ancochea J, Morell F, et al. . Spanish group on interstitial lung diseases, SEPAR. Report on the incidence of interstitial lung diseases in Spain. Sarcoidosis Vasc Diffuse Lung Dis 2004;21:64–70. [PubMed] [Google Scholar]
- 20. Kornum JB, Christensen S, Grijota M, et al. . The incidence of interstitial lung disease 1995–2005: a Danish nationwide population-based study. BMC Pulm Med 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karakatsani A, Papakosta D, Rapti A, et al. . Hellenic interstitial lung diseases group. epidemiology of interstitial lung diseases in Greece. Respir Med 2009;103:1122–9. [DOI] [PubMed] [Google Scholar]
- 22. Kundu S, Mitra S, Ganguly J, et al. . Spectrum of diffuse parenchymal lung diseases with special reference to idiopathic pulmonary fibrosis and connective tissue disease: an eastern India experience. Lung India 2014;31:354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hyldgaard C, Hilberg O, Muller A, et al. . A cohort study of interstitial lung diseases in central Denmark. Respir Med 2014;108:793–9. 10.1016/j.rmed.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 24. Lacasse Y, Selman M, Costabel U, et al. . Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med 2003;168:952–8. 10.1164/rccm.200301-137OC [DOI] [PubMed] [Google Scholar]
- 25. Quirce S, Vandenplas O, Campo P, et al. . Occupational hypersensitivity pneumonitis: an EAACI position paper. Allergy 2016;71:765–79. 10.1111/all.12866 [DOI] [PubMed] [Google Scholar]
- 26. Blanc PD, Annesi-Maesano I, Balmes JR, et al. . The occupational burden of nonmalignant respiratory diseases. An official American thoracic Society and European respiratory Society statement. Am J Respir Crit Care Med 2019;199:1312–34. 10.1164/rccm.201904-0717ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barber CM, Wiggans RE, Carder M, et al. . Epidemiology of occupational hypersensitivity pneumonitis; reports from the SWORD scheme in the UK from 1996 to 2015. Occup Environ Med 2017;74:528–30. 10.1136/oemed-2016-103838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. James PL, Cannon J, Barber CM, et al. . Metal worker’s lung: spatial association with Mycobacterium avium. Thorax 2018;73:151–6. 10.1136/thoraxjnl-2017-210226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schoenberg NC, Barker AF, Bernardo J, et al. . A comparative analysis of pulmonary and critical care medicine Guideline development methodologies. Am J Respir Crit Care Med 2017;196:621–7. 10.1164/rccm.201705-0926OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Millerick-May ML, Mulks MH, Gerlach J, et al. . Hypersensitivity pneumonitis and antigen identification – an alternate approach. Respir Med 2016;112:97–105. 10.1016/j.rmed.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 31. Bellanger A-P, Reboux G, Rouzet A, et al. . Hypersensitivity pneumonitis: a new strategy for serodiagnosis and environmental surveys. Respir Med 2019;150:101–6. 10.1016/j.rmed.2019.02.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2019-000469supp001.pdf (39.6KB, pdf)