Abstract
Background
Infantile colic has an effect on both infants and their parents, who become exhausted and concerned as they attempt to comfort their child. Common approaches have focused upon physical treatments to reduce symptoms, with inconclusive evidence as to their effectiveness. An alternative approach seeks to provide training, support and psychological interventions for parents. This approach is known as parent training programmes. Programmes can include soothing techniques, advice on feeding or normalisation material in any form. The teaching format can vary including face‐to‐face courses, online learning, printed materials, home visits and remote support and counselling. Here, we aim to collate the evidence on the effectiveness of these interventions and examine their effectiveness at reducing infantile colic symptoms and parental anxiety levels, and their safety.
Objectives
1. To evaluate the effectiveness and safety of parent training programmes for managing colic in infants under four months of age. 2. To identify the educational content and attributes of such published programmes.
Search methods
In June 2019 we searched CENTRAL, MEDLINE, Embase, 13 other databases and two trials registers. We also handsearched conference abstracts, inspected the references of included studies and contacted leaders in the field for more trials.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs investigating the effectiveness of any form of parental training programmes, alone or in combination, versus another intervention(s) or control, on infantile colic.
Data collection and analysis
Two authors independently selected studies for inclusion, extracted data, and assessed the risk of bias within the included studies. We used Review Manager 5 to analyse the data. We assessed the certainty of the evidence using GRADE methodology.
Main results
Our search found 6064 records from which we selected 20 for full‐text review. From these, we identified seven studies with 1187 participants that met our inclusion criteria. All of the studies included infants under the age of four months suffering from infantile colic. Four studies were conducted in the USA, one in Canada, one in the Netherlands and one in Iran. Four studies stated their funding sources, which included national research institutes, foundations and nutritional companies. Five studies assessed parent training versus a control group that received reassurance or routine care; and of these, one study was three‐armed and also examined the effectiveness of using a specialised baby seat. One study examined parent training programmes against a milk‐exclusion diet and one study assessed a parent training programme versus the same parent training programme plus swaddling. The duration of the interventions varied, with the shortest being six days and the longest being three months.
Generally, most studies had low participant numbers and were at high risk of bias, prone to selection bias, performance bias, and the placebo effect.
We could not complete the planned qualitative analysis (objective 2) due to lack of data in study reports and no further information being supplied by authors on request. Instead, we completed a descriptive content analysis with the limited information available. The parent training interventions were found to focus on one or a combination of the following: soothing techniques for crying infants (six studies); general care advice, including sleep (four studies); feeding advice (two studies); stress reduction and empathic programme for parents (two studies); and positive play interaction advice (one study). One study taught 'kangaroo care', a specific form of skin‐to‐skin cuddling. The control groups consisted of reassurance (two studies), advice to rock the infant in the crib (one study), or no intervention (two studies).
Parent training versus control We conducted a meta‐analysis using data from three studies (157 infants) that assessed the primary outcome of 'crying time at completion of study period'. Parent training was more effective than control: mean difference (MD) −113.58 m/d, 95% confidence interval (CI) −144.19 m/d to −82.96 m/d; low‐certainty evidence (downgraded due to imprecision and some concerns with risk of bias).
Parent training versus specialised baby seat One study (38 participants) found no difference in mean crying time at completion between the parent training group and the specialised baby seat group, but did not report specific figures.
Parent training versus a milk‐exclusion/soy milk formula One study (20 participants) comparing parent training with a milk‐exclusion/soy milk formula found crying time at completion of the study to be 2.03 hours versus 1.08 hours, respectively.
Parent training versus parent training plus swaddling One study (398 participants) comparing parental training with the same intervention plus training on how to swaddle an infant did not report separate data for each group.
No adverse effects were reported, but these were not explicitly reported in any study.
Authors' conclusions
There is limited evidence on the effectiveness and safety of parent training programmes for managing infantile colic. Despite a single meta‐analysis showing that parent training may reduce crying times for infants, compared to control, the certainty of the evidence was low. Evidence for other comparisons was sparse. We were unable to identify comprehensively the educational content and attributes of the included programmes due to a lack of information in study reports. Further RCTs are needed: they should define interventions clearly to ensure replicability, address all appropriate outcome measures, and minimise risk of bias in order to assess definitively the role of parent training programmes in managing infantile colic.
Plain language summary
Parent training programmes for managing infantile (baby) colic
What is infantile colic? Infantile colic is a condition where a seemingly healthy infant up to the age of four months has periods of inconsolable and unexplained crying. These periods of crying tend to last more than three or more hours per day, and occur three days per week for at least three weeks. This can cause parents to become exhausted and concerned whilst attempting to comfort their child.
What are parent training programmes? Parent training programmes involve providing training, support and psychological interventions to parents, to help reduce their infant’s symptoms and parents' anxiety levels.
What did the researchers investigate? This Cochrane Review aimed to assess the effectiveness and safety of parent training programmes for managing infantile colic, and to identify the educational content and attributes of such programmes.
Included studies This review found seven relevant, small, randomised controlled trials (a type of experiment in which participants are randomly allocated to two or more groups), with 1187 participants. All studies included infants younger than four months of age. The duration of the interventions varied, with the shortest being six days and the longest being three months. Four studies were conducted in the USA, one in Canada, one in the Netherlands and one in Iran. Four studies stated their funding sources, which included national research institutes, foundations and nutritional companies.
Five of the studies compared a parent training programme with a control (reassurance, no intervention or rocking the baby in their crib). One of these studies also compared training programmes to a third group who received a specialised baby seat. One study compared a parent training programme against a milk‐exclusion diet. The last study compared a parent training programme to the same parent training programme plus swaddling. Details of the content of training was limited, but most interventions commonly focused on one or a combination of the following: soothing techniques for crying infants (six studies); general care advice including sleep (four studies); feeding advice (two studies); stress reduction and empathic counselling for parents (two studies); and positive play interaction advice (one study).
Results We found evidence to suggest that parent training programmes may reduce infants' crying time compared to control. However, the evidence for this comes from just three small studies (157 infants) that suffered from a range of weaknesses. We therefore considered the overall certainty of these findings to be low. We found evidence from one small study (20 infants) that crying time at completion was shorter for infants on a milk‐exclusion/soy milk formula than for parent training. For the comparisons ʻParent training versus specialised baby seatʼ and ʻParent training plus swaddlingʼ we found no useable data on which to draw conclusions.
None of the included studies explicitly reported the occurrence of adverse effects of parent training.
Authors conclusions Although our analyses suggest that crying times may be reduced amongst infants whose parents attend parent training programmes, the certainty of the evidence is low. Further, better‐designed randomised controlled trials are needed to answer questions on the effectiveness and safety of parent training programmes for managing infantile colic.
Summary of findings
Summary of findings for the main comparison. Parent training versus control for managing infantile colic.
| Parent training versus control for managing infantile colic | ||||||
| Patient or population: infants with colic Setting: outpatients Intervention: parent training Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with parent training | |||||
| Change in duration of crying (not reported) | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
|
Crying time at completion Measured in: minutes/day Follow‐up: 6 days to 3 months |
The mean crying time in the control groups ranged from 176.4 to 372 minutes per day | The mean crying time in the intervention groups was, on average, 113.58 minutes per day lower (144.19 lower to 82.96 lower) | 157 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | ‐ | |
| Adverse effects (not reported) | ‐ | ‐ | ‐ | ‐ | Not reported | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; №: Number; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded 1 level due to risk of bias: concerns with unclear allocation concealment in 2 of 3 of studies. bDowngraded 1 level due to low participants numbers leading to imprecision.
Background
Description of the condition
Infantile colic can be defined as periods of inconsolable, unexplained and incessant crying in a seemingly healthy infant that, quite understandably, leads to exhausted, frustrated and concerned parents seeking to comfort their child (Landgren 2010a).
The prevalence of excessive crying varies according to the definition used, although it usually peaks during the second month of life with a prevalence of 1.5% to 11.9% (Reijneveld 2001). Traditionally, the definition of the condition was based on the ʻrule of threeʼ: unexplained episodes of paroxysmal crying for more than three hours per day, for three days per week, for at least three weeks (Wessel 1954). More recently a new definition has been proposed. It refers to a clinical condition of fussing and crying for at least one week in an otherwise healthy infant (Hyman 2006). Rome III includes infantile colic, with diagnostic criteria including all of the following in infants from birth to four months of age: paroxysms of irritability, fussing or crying that starts and stops without obvious cause; episodes lasting three or more hours per day and occurring at least three days per week for at least three weeks; and no failure to thrive (Mostafa 2008). Colic is a symptom rather than a condition or diagnosis in and of itself.
Between 10% and 30% of all infants are estimated to experience colic (Clifford 2002; Rosen 2007). Paroxysms of inconsolable crying are often accompanied by flushing of the face, meteorism (excessive gas in the intestinal tract with distention of the abdomen), drawing up of the legs, and flatulence (Savino 2010). Symptoms have historically typically started in the second week of life in both breast‐fed and formula‐fed infants and resolved by three months of age (Lucas 1998). Generally speaking, these symptoms are not indicative of disease and thus hospital admission for these infants is generally unnecessary, even detrimental, and should not be encouraged (Savino 2007). However, about 5% of colicky crying infants do have a serious, underlying medical problem (Freedman 2009; Savino 2005; Savino 2007), and there is evidence that older children presenting with migraine are more likely to have been babies who have suffered colic (Romanello 2013). Therefore, all colicky infants should undergo a complete medical assessment in order to exclude underlying medical conditions that require investigation and treatment (Savino 2010).
The aetiopathogenesis of infantile colic as a symptom remains undefined and is most likely multifactorial. Despite the common nature of the condition, and the large amount of research investigating this area, there have been no breakthroughs in terms of the real mechanisms underlying infant colic.
It has been suggested that a number of behavioural factors (psychological and social) and biological components (food hypersensitivity, allergy, gut microflora, bloating from trapped gas and dysmotility) can contribute to its manifestation (Gupta 2007). These include the following.
First, lactose intolerance — due to a relative lactase deficiency — has been identified as a possible causative factor in infant colic. Carbohydrate malabsorption leads to the colonic fermentation of sugars and an increase in the levels of hydrogen gas (Infante 2011). The rapid production of hydrogen in the lower bowel distends the colon, sometimes causing pain, whereas the osmotic pressures generated by lactose and lactic acid in the colon cause an influx of water, leading to further distension of the bowel. Although studies evaluating the degree of hydrogen in the breath of colicky infants have produced inconsistent results, increases in breath hydrogen levels have been reported (Hyams 1989; Miller 1990; Moore 1998).
Second, the immunological model, which focuses on possible allergens, has been suggested as a cause of colic. A key allergen is cows' milk proteins in breast milk or infant formula. Intact proteins from the mother's diet can sometimes cross over into the breast milk and provoke an allergic response and symptoms of colic in her infants. Consequently, a low‐allergen maternal diet, or hypoallergenic infant formula (Iacovou 2012), has been proposed as a form of treatment (Hill 2005; Schach 2002). The possibility that infantile colic could be related to allergens was first described by Shannon 1921. Since then a number of studies have evaluated the possible association between colic and food hypersensitivity (Heine 2013; Heine 2014; Hill 1995; Iacono 1991; Lothe 1982; Merras‐Salmio 2013; Saps 2011).
The evidence shows that about 25% of infants with moderate or severe symptoms have cows' milk protein‐dependent colic (Axelsson 1986; Hill 2000; Lindberg 1999), which improves after some days of a hypoallergenic diet (Campbell 1989; Dupont 2010; Estep 2000; Iacono 1991; Iacono 2005; Jakobsson 1983; Jakobsson 2000; Lothe 1989; Savino 2001). For these infants, infantile colic could be the first manifestation of atopic disease and for this reason dietetic treatment should be the first therapeutic approach (Gupta 2007; Hall 2012; Savino 2010). Indeed dietary changes, such as eliminating cows' milk proteins, are particularly indicated in cases of suspected intolerance to cows' milk proteins (e.g. in infants with a positive family history, eczema or onset after the first month of life, and colic associated with other gastrointestinal symptoms such as vomiting or diarrhoea) (Hill 1995; Hill 2005; Jakobsson 1983; Lucassen 2000; Savino 2014a). However, UpToDate 2016 grades the introduction of hydrolysate formula for formula‐fed infants or hypoallergenic diet for mothers of breast‐fed infants as a "2C": "a very weak recommendation; other alternatives may be equally reasonable"; "benefits and risks may be finely balanced, or the benefits and risks may be uncertain" and "the evidence comes from observational studies, unsystematic clinical experience, or from randomised, controlled trials with serious flaws". A Cochrane Review found no evidence to support the use of any dietary intervention in practice (Gordon 2018).
Third, there is growing evidence that the intestinal microbiota in colicky infants differ from those in non‐colicky controls, since higher levels of anaerobic bacteria such as coliform and Escherichia coli, microaerophilic bacteria such as Helicobacter pylori (Ali 2012), and a lower concentration of Lactobacilli have been reported in infants with colic (Savino 2010). Human milk naturally contains these prebiotics; they are defined as indigestible oligosaccharides that could selectively enhance the proliferation of certain probiotic bacteria in the colon, especially Bifidobacterium species (Thomas 2010). Some studies have failed to find a protective effect of breast feeding on the development of colic in breast‐fed infants (Clifford 2002); it is unclear, however, if these studies compared exclusively breast‐fed‐from‐birth infants with exclusively artificially‐fed‐from‐birth infants, and so it is still not known whether any breastfeeding has some protective effect or whether any artificial feeding compromises the infant gut microbiome in some way. Evidence suggests that oligosaccharide prebiotics (a mixture of galacto‐oligosaccharides and fructo‐oligosaccharides) to encourage growth of the positive bacteria in the gut may be effective treatments for allergy and food intolerance in general (Arslanoglu 2012), and for crying in formula‐fed infants with colic in particular (Savino 2006). Microbiota diversity is significantly lower in colicky infants and seems to decrease after birth rather than being that way from birth (De Weerth 2013). Evidence is building around the effectiveness of supplementing the infant's diet with probiotics to prevent colic and other symptoms (Oozeer 2013).
These three pathophysiological models indicate implicit treatment modalities; however, various therapeutic interventions have been used for infant colic that take a symptom‐reduction, focused approach. These include pain relief (Savino 2002; Savino 2012); probiotic supplementation (Indiro 2014); complementary and alternative medicines and nutritional supplements such as fennel extract (Harb 2015) and camomile (Perry 2011); sucrose and glucose solutions (Markestad 1997); and physical treatments such as manipulation (Dobson 2012; Olafdottir 2001), and massage and reflexology (Huhtala 2000; Perry 2011). Although systematic reviews have failed to provide evidence of its efficacy in reducing colicky symptoms by reducing trapped gas in the liquid of the stomach, simethicone is still often used (Metcalf 1994). Various other physical treatments have been studied to reduce symptoms, including carrying (Barr 1991), which may affect the baby in psychological or social ways, or to address mechanical aspects such as crib vibration (Huhtala 2000) and acupuncture (Landgren 2010a; Landgren 2010b; Reinthal 2008; Skjeie 2013). Evidence of efficacy is not comprehensive (Garrison 2000).
Description of the intervention
An alternate approach that has been investigated is to focus on training, support and psychologically‐underpinned interventions for parents of infants with colic.
There is recognition of the role of parental anxiety in the reported incidence of colic, and evidence that parental reassurance is successful in reducing reports of distress (Furlong 2012; Hiscock 2014; Taubman 1984; Taubman 1988; Wolfe 1994; Zwi 2011).
Guidance and informal education are often delivered by healthcare professionals to accompany any intervention for infantile colic so that parents and carers may better understand the potential aetiologies and pursue various management and treatment options (see, for example, Cook 2012). Parental behavioural modifications have often been suggested both for breast‐fed and formula‐fed infants, including advice to carry the infant (Barr 1991), not to carry the infant (McKenzie 1991), and to try to understand the infant's needs (Taubman 1984).
Whilst there have been some attempts to synthesise evidence‐informed pathways for infantile colic that do include some parental resources, for example NICE (National Institute for Health and Care Excellence) Clinical Knowledge Summaries and UpToDate Patient Information Tips/health professional information. it is well documented that the evidence base is poor and inconclusive. Indeed, NICE CKS 2014 states that their recommendations are forced to be pragmatic. There is currently no national or international consensus on best practice for such interventions, unlike pharmacological interventions, where the intervention itself may be understood and the issue is the effectiveness of such interventions. In this case, it is as important to clarify the make‐up of the intervention itself.
How the intervention might work
There is a substantial infant population suffering from colic with no clearly identifiable cause, who are known to have a natural history that will lead to symptom reduction by six months of age (Parkin 1993; St James‐Roberts 1991). Parents have little firm idea of what will and what will not work (Oshikoya 2009), and so they try lots of potential, non‐medical, solutions with variable results, which is extremely stressful for both baby and parents.
Many parent training programmes are focused on reassurance that colic symptoms will reduce in time and explanation of some soothing strategies to reduce parental anxiety. This is believed to be important as parental anxiety can, in turn, reduce the effectiveness of soothing strategies and increase the perceived impact of symptoms. Additionally, they seek to offer education on such soothing strategies or on understanding baby's potential needs in a manner that is consistent and in line with best practice to reduce conflicting messages, and provide information that is not always readily available to parents: baby often needs soothing or comfort rather than more feed, different feed, medicating, etc., and parents should be aware of the stress of a crying baby and seek to soothe themselves to avoid harming the child (Bryanton 2013; Chandran 2014; Levy 2015; Reijneveld 2001; Schmitt 1987). This combination of reassurance as to the natural history of symptom resolution, a consistent single set of messages regarding management advice, and explanation that a less anxious parent can reduce colic in the infant will have two actions. First, it will ensure the infant gets the most appropriate soothing techniques in a consistent manner to reduce their symptoms. Second, it will reduce anxiety in parents, enhancing their satisfaction, quality of life and potentially improving their ability to deliver the soothing strategies.
Why it is important to do this review
There is no clarity as to the extent different approaches contribute to the overall efficacy of symptom reduction strategies or to parental anxiety levels.
Established studies and reports may now be outdated (e.g. Schmitt 1987; Taubman 1984), and more recently reported approaches are based on different approaches (e.g. Hiscock 2014 is based on an intervention described in Cook 2012; Keefe 2006). Some interventions have been ineffective, such as the McRury 2010 study, which is based on techniques found in a popular parenting book — The Happiest Baby — by Karp 2003. Given the clinical and methodological heterogeneity of studies on these interventions, the efficacy of these interventions in reducing infant colic remains inconclusive at present.
It is also important to note that focus within the published body of work often seeks to assess 'whether' such training is effective (e.g. Hiscock 2014), and this can be considered of limited educational research value (Norcini 2011). This is important as, in this context, the intervention being considered is educational, and if this cannot be defined it cannot be reliably and validly reproduced and disseminated in a systematic fashion. Therefore, equally relevant questions are 'how' it achieves this outcome, 'why' the teaching is effective and 'for whom and when' such training can be effective. A review and synthesis of the evidence must also address these items and, from an educational stance, identify a relevant theory from this evidence base (Haji 2013). This will support future professionals in understanding and delivering such an intervention in a reliable and reproducible manner. Even if such data are not explicit within primary studies, synthesis can highlight such outcomes, as has been increasingly shown in the field of health education (Gordon 2011; Gordon 2013).
This review sets out to consider the effectiveness of parent training programmes (when compared to other interventions), the safety of such programmes, and to identify the content and attributes underpinning such programmes.
Objectives
To evaluate the effectiveness and safety of parent training programmes for managing colic in infants under four months of age.
To identify the educational content and attributes of such published programmes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), quasi‐RCTs, including cluster‐randomised trials.
Types of participants
Infants younger than four months of age who were already suffering from infantile colic, as defined by the study using, for example, Rome III criteria (Mostafa 2008) or the Wessel definition of colic (Wessel 1954). We included both breast‐fed and formula‐fed infants.
Types of interventions
Any form of parental training programmes, alone or in combination, versus another intervention(s), control or placebo.
Examples of programme content included:
normalisation material in any form;
soothing techniques; and
feeding management advice.
Teaching forms included:
face‐to‐face courses;
online and e‐learning;
printed materials;
home visits and coaching; and
remote support and counselling.
Types of outcome measures
For all proposed outcomes, we used the final outcomes and final outcomes accounting for baseline at the end of the interventions, and we recorded the timings of these outcomes since these could guide the subgroup analyses (see Subgroup analysis and investigation of heterogeneity).
Primary outcomes
Change in duration of crying (post‐intervention versus baseline). Data could have been continuous (e.g. hours per day), or dichotomous (e.g. reduction under a predefined threshold, as determined by the study authors)
Crying time at completion of study period (endpoint). Data could have been continuous (e.g. hours per day), or dichotomous (e.g. reduction under a predefined threshold, as determined by the trial authors)
Adverse effects, including choking, apparent life‐threatening events (dichotomous outcome)
Secondary outcomes
Number of responders in each group post‐intervention: change in frequency of crying episodes per 24 hours (post‐intervention versus baseline) (dichotomous outcome, as defined by the primary studies)
Parental or family quality of life, including measures of parental stress, anxiety or depression, as proposed by the primary studies (and so no single scale could be possible) (continuous outcome)
Infant sleep duration per 24 hours at seven, 14, and 21 days (post‐intervention versus baseline) (continuous outcome)
Search methods for identification of studies
We identified relevant trials by searching the sources described below.
Electronic searches
We searched the sources listed below in September 2016, February 2018 and June 2019.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 6) in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register (searched 4 June 2019).
MEDLINE Ovid (1946 to May week 4 2019).
MEDLINE In‐Process and Other Non‐Indexed Citations Ovid (searched 4 June 2019).
MEDLINE Epub Ahead of Print Ovid (searched 4 June 2019).
Embase Ovid (1974 to June week 3 2019).
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to 4 June 2019).
PsycINFO Ovid (1967 to May week 4 2019).
Science Citation Index ‒ Expanded Web of Science (SCI; 1970 to 4 June 2019).
Social Sciences Citation Index Web of Science (SSCI; 1970 to 4 June 2019).
Conference Proceedings Citation Index ‒ Science Web of Science (CPCI‐S; 1990 to 4 June 2019).
Conference Proceedings Citation Index ‒ Social Science and Humanities Web of Science (CPCI‐SSH; 1990 to 4 June 2019).
LILACS (Latin American and Caribbean Health Science Information database; lilacs.bvsalud.org/en; searched 5 June 2019).
Cochrane Database of Systematic Reviews (CDSR; 2018, Issue 2), part of the Cochrane Library (searched 4 June 2019).
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2), part of the Cochrane Library (searched 2 September 2016).
Epistemonikos (limited to systematic reviews; epistemonikos.org; searched 5 June 2019).
WorldCat (limited to theses; worldcat.org; searched 5 June 2019).
ClinicalTrials.gov (clinicaltrials.gov; searched 5 June 2019).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch; searched 5 June 2019).
The search strategies for each source are reported in Appendix 1. We did not impose any date or language restrictions.
Searching other resources
Handsearching
To identify studies that have not yet been published in full, we handsearched abstracts presented at relevant international meetings, including the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN), published from 2010 onwards. However, as there is some evidence that data from abstracts can be inconsistent with data in published articles (Pitkin 1999), we only included abstract publications if sufficient data were presented to judge inclusion and assess quality.
Reference searching
We inspected the references of included studies for more studies, and for more papers relating to the included studies.
Personal contacts
We contacted leaders in the field to try to identify other published and unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (Morris Gordon (MG) and Shel Banks (SB)) independently screened titles, abstracts, and full‐text reports for eligibility against the inclusion criteria (see Criteria for considering studies for this review). Specifically, they:
merged search results using reference management software and removed duplicate records of the same report;
examined titles and abstracts to remove irrelevant records;
retrieved the full texts of potentially relevant reports;
linked together multiple reports of the same study;
examined full‐text reports for studies that met the eligibility criteria;
corresponded with investigators, when appropriate, to clarify study eligibility;
at all stages, noted reasons for inclusion and exclusion of reports on a study flow spreadsheet, resolving any disagreements through consensus;
made final decisions on study inclusions and resolved any discrepancies through a process of consensus; and
proceeded to data collection.
We recorded our selection process in a PRISMA diagram (Figure 1; Moher 2009).
1.
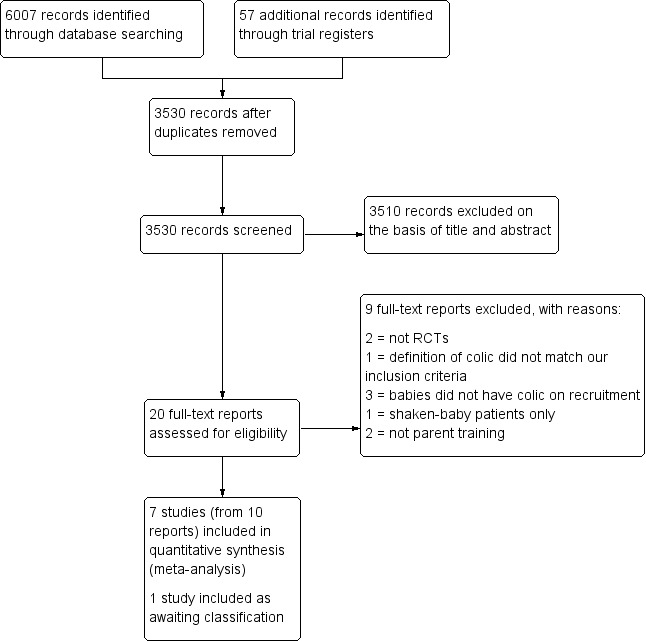
Study flow diagram.
Data extraction and management
We developed data extraction forms a priori, as per the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We extracted the information described below.
Characteristics of participants: source of participants, inclusion and exclusion criteria, total number at baseline, total number at study completion, setting, definition of 'colic' applied, diagnostic criteria applied, type of feeding (breast feeding, formula feeding), age at onset of colic, age at commencement of intervention, and evaluation of potential effect modifiers (e.g. age, gender).
Characteristics of intervention: content of training, pedagogical methods employed, context, resources and educator details, any theoretical underpinning described.
Interventions and controls: number of groups, intervention(s) applied, frequency and duration of intervention, total number of interventions, permitted co‐interventions.
Methods: study design, duration, sequence generation, allocation concealment, blinding of outcome assessors, evaluation of success of blinding.
Outcomes: list of outcomes assessed, definitions used, values of means and standard deviations (SDs) at baseline and at time points defined by the study protocol (or change from baseline measures, if given).
Results: measures at end of protocol, follow‐up data (including means and SDs, standard errors, or confidence intervals (CIs) for continuous data, and summary tables for dichotomous data), withdrawals, and losses to follow‐up.
Other: references to other relevant studies, points to follow up with authors, comments from study authors, key conclusions from the study (by the study authors), other comments from the review authors.
Two review authors (MG and SB) extracted the data independently using the data extraction form. A third review author (Megan Thomas (MT)) resolved any disagreements. We collated data in the latest version of Review Manager 5 (RevMan 5) (Review Manager 2014).
Assessment of risk of bias in included studies
Two review authors (MG and SB) independently evaluated the risk of bias within each included study using the criteria recommended in the Cochrane Handbook for Systematic Reviews of Interventions and set out in Appendix 2 (Higgins 2011a; Deeks 2011). For each included study, both review authors independently assigned ratings of high, low or unclear risk of bias for each of the following domains: sequence generation; allocation concealment; blinding of parents and health professionals; blinding of outcome assessment; incomplete outcome data; selective outcome reporting; and other potential threats to validity. Both review authors then compared their judgments, and discussed and resolved any inconsistencies in the assessments. We completed a 'Risk of bias' table for each included study and present a summary of the risk of bias (Figure 2).
2.
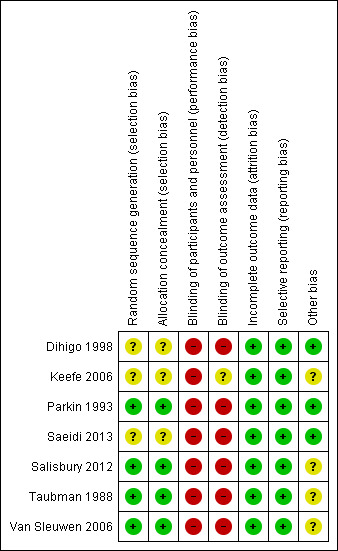
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
Dichotomous data
We present dichotomous data as risk ratios (RR), since the effects of the RR are readily understood (Walter 2000). We report all dichotomous data with their associated 95% CI and probabilities of control and intervention groups (where possible).
Continuous data
If all studies used the same measurement scale, we calculated mean differences (MD) for change scores. Where studies used different scales, we calculated the SMD using Hedges' (adjusted) g. If necessary, we calculated effect estimates from P values, t statistics, analysis of variance (ANOVA) tables or other statistics, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
For this analysis we used, according to the need of the outcome being considered, either change scores or final values, without combining them. We did not combine these two different indices in a meta‐analysis and only meta‐analysed homogeneous data sets.
If both continuous and dichotomous data were available for an outcome, we included only the continuous outcome in the primary analysis. If some studies reported an outcome as a dichotomous measure, and others used a continuous measure of the same construct, we converted the results for the former from the dichotomous measure to an SMD, provided that we could assume the underlying continuous measure had approximately a normal or logistic distribution (otherwise we carried out two separate analyses).
Unit of analysis issues
Cluster‐randomised trials
For each included study, we determined whether the unit of analysis was appropriate for the unit of randomisation and the design of that study (that is, whether the number of observations matched the number of 'units' that were randomised (Deeks 2011)). We did not find cluster‐randomised trials because this design is uncommon in this field. See Table 2 for our strategy of analysis should we find cluster‐randomised trials in future updates of this review.
1. Unused methods.
| Type of study | Unit of analysis |
| Assessment of risk of bias in included studies | A third review author (JG) will resolve any persisting disagreements. |
| Unit of analysis issues |
Cluster‐randomised trials Should we find cluster‐randomised trials in future updates of this review, we will use the intra‐class correlation coefficient (ICC) to convert trials to their effective sample size before incorporating them into the meta‐analysis, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). Where the ICC cannot be used, we will use values available in the published literature as an external source, as well as contacting the author to supply more data to allow an estimate of the ICC to be calculated (Campbell 2000). |
|
Cross‐over trials In randomised cross‐over studies, individuals receive each intervention sequentially, in a random order. Cross‐over studies usually contain a washout period, which is a stage after the first intervention but before the second intervention, where time is given for the active effects of the first intervention to wear off before the new intervention begins in order to reduce the carry‐over effect (when the first intervention affects the second). Carry‐over effects are of particular concern in this review, given the educational nature of the interventions being assessed. Should we include cross‐over trials in future updates of this review, we will not include any data in cross‐over studies after the first intervention period. | |
|
Qualitative analysis For qualitative educational content data, we will avoid making a priori hypotheses and conclusions, in keeping with a grounded theory approach. Following data collection and processing, two review authors (MG and SB) will code the data using Nvivo 2015. They will develop an initial thematic index, with the addition of emerging thematic categories according to interpretation of the content of the data. The analysis will proceed through three stages consisting of open, axial and selective coding, with constant comparison taking place throughout each phase. Each stage will provide categories that could be used to explore the themes of the data and build an interpretation that could address the overarching research questions. | |
| Dealing with missing data | We will explore the impact of including studies with high levels of missing data in the overall assessment of intervention effect by conducting sensitivity analyses (see Sensitivity analysis). |
| Data synthesis | While there may be heterogeneity in the interventions, as well as the comparisons, we considered that the consensus on definitions of symptoms for eligibility manages the risk of 'blurring' the results. However, if we perceive a risk upon evaluation of our findings, we will conduct a sensitivity analysis by removing such studies to provide more definite findings. |
| Subgroup analysis and investigation of heterogeneity | Where data permit, we will conduct the following subgroup analyses:
These analyses will be exploratory as they will involve non‐experimental (cross‐study) comparisons on primary outcomes only. We will treat any conclusions with caution. |
| Sensitivity analysis | Where data permit, we will conduct sensitivity analyses to determine whether findings are sensitive to:
|
Studies with multiple arms
In the primary analysis, we combined results across all eligible intervention arms (parent training programmes, i.e. courses or written materials), and compared them with the combined results across all eligible control arms, making single, pairwise comparisons. Where such a strategy prevented investigation of potential sources of heterogeneity, we analysed each programme type separately (against a common control group – placebo), but divided the sample size for common comparator arms proportionately across each comparison (Higgins 2011c). This simple approach allowed the use of standard software (including Review Manager 2014), and prevented the inappropriate double‐counting of individuals.
Cross‐over trials
We did not include cross‐over trials in this review. See Table 2 for our strategy of analysis should we include cross‐over trials in future updates of this review.
Qualitative analysis
In order to gain a clear understanding of the content of the parent training interventions we planned to complete a qualitative assessment of the interventions using the details provided in the reports of the included RCTs. Such data were not available and so this could not be done. Details of the planned methods are given in Table 2. Instead, we provide a simple descriptive analysis of the content of the interventions.
To clarify, as per the section on Types of outcome measures above we did not include studies that were qualitative reports of parent training. Rather, we extracted and synthesised qualitative data on the intervention itself.
Dealing with missing data
Where data were missing, we contacted the corresponding authors of included studies, requesting them to supply any unreported data. For all outcomes in all studies, we carried out analyses as far as possible on an intention‐to‐treat basis; that is, we attempted to include all participants randomised to each group in the analyses, and we analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. For missing statistics we estimated, for example, missing SDs from other available data, such as standard errors, or we imputed them using the methods suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). We made no assumptions about loss to follow‐up for continuous data, and we based analyses on those participants completing the trial. If there was a discrepancy between the number randomised and the number analysed in each intervention group, we calculated and reported the percentage lost to follow‐up in each group. Where it was not possible to obtain missing data, we recorded this on the data collection form, reported it in the ‘Risk of bias’ tables, and discussed the extent to which the missing data could alter the results and hence the conclusions of the review. For included studies, we noted levels of attrition.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by comparing the distribution of important participant (e.g. age) and trial characteristics (e.g. randomisation, concealment, blinding of outcome assessment, losses to follow‐up, intervention type, co‐interventions) between studies. We assessed statistical heterogeneity by examining the I² statistic (Deeks 2011), a quantity that describes the proportion of variation in point estimates that is due to variability across studies rather than sampling error. We interpreted the I² statistic as suggested in Deeks 2011:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; or
75% to 100%: suggests considerable heterogeneity.
We employed a Chi² test of homogeneity, with a 10% level of significance, to determine the strength of evidence that heterogeneity was genuine. We also reported Tau².
Once they had extracted data, the review team judged clinical and methodological heterogeneity by discussion.
Assessment of reporting biases
In order to minimize publication bias, we attempted to obtain the results of any unpublished studies in order to compare the results extracted from published reports with the results obtained from other sources (including correspondences).
In addition, for comparisons that included 10 or more studies we planned to evaluate whether reporting biases were present by using funnel plots to investigate any relationship between effect estimates and study size or precision, or both, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011).
Data synthesis
Where interventions were similar in type of parental training programme and type of outcome assessed, we grouped the studies and synthesized their results in a meta‐analysis. We presented results for each combination of parental training programme, and assessed outcome and colic definition, with the exception of those studies for which we observed no data. For instance, if two or more studies assessed the effects of a parental training programme for parents of otherwise healthy children with colic and both measured the daily crying, we performed a meta‐analysis of the results. Because we assumed that clinical heterogeneity was very likely to impact on our results, given the wide breadth and types of interventions included, we combined the studies using a random‐effects model regardless of evidence of statistical heterogeneity. We calculated all overall effects using inverse variance methods. We carried out statistical analysis using RevMan 5 (Review Manager 2014). Where data were insufficient to allow meta‐analysis or qualitative analysis, we provided a narrative synthesis and descriptive summary of the study outcomes.
Subgroup analysis and investigation of heterogeneity
Large numbers of subgroup analyses may result in misleading conclusions (Oxman 1992; Yusuf 1991). We planned to carry out subgroup analysis as per our published protocol (Thomas 2016), but could not complete these due to a lack of data. See Table 2 for planned analyses.
Sensitivity analysis
We conducted a sensitivity analysis to test the robustness of the results to the choice of model used, by comparing results from the random‐effects model with those from the fixed‐effects model.
We were unable to conduct our other preplanned sensitivity analyses due to a lack of data (Thomas 2016). See Table 2.
Presentation of main results
We assessed the overall certainty of the evidence using the GRADE approach (Guyatt 2008). The GRADE approach appraises the certainty of a body of evidence based on the extent to which one can be confident that an estimate of effect, or association, reflects the item being assessed. RCTs start as high‐quality evidence but may be downgraded due to: risk of bias (methodological quality); indirectness of evidence; unexplained heterogeneity; imprecision (sparse data); and publication bias. Intention‐to‐treat data would be of better quality than per protocol results. Two review authors (SB and MG) independently assessed and agreed the overall certainty of the evidence for each outcome after considering each of these factors, and graded them as:
high certainty: further research is very unlikely to change confidence in the estimate of effect;
moderate certainty: further research is likely to have an important impact on confidence in the estimate of effect, and may change the estimate;
low certainty: further research is very likely to have an important impact on confidence in the estimate of effect, and is likely to change the estimate; or
very low certainty: any estimate of effect is very uncertain.
We planned to assess the certainty of the evidence for the following outcomes.
Change in duration of crying (post‐intervention versus baseline).
Crying time at completion.
Adverse effects, including choking and apparent life‐threatening events.
We present the results of our assessment of the primary outcomes in Table 1 for the main comparison, which we created using GRADEpro GDT software (GRADEpro GDT). The comparisons used are those outlined in the studies. Our table includes information on the type of participants, the interventions and comparisons used in each case, and the outcomes and their measurements for each study, as well as the setting and the length of follow‐up. We include any rationale for downgrading the certainty of the evidence in the footnotes of the table.
Results
Description of studies
Results of the search
The literature searches, which we conducted in September 2016, February 2018 and June 2019, identified a total of 6064 records. After duplicates were removed, a total of 3530 records remained. Two review authors independently screened the titles and abstracts of these records and selected 20 for full‐text review. Of these 20, we excluded nine (see Excluded studies) and included 10 reports of seven studies (see Included studies). One study is awaiting classification. See Figure 1.
Included studies
This review includes 10 reports of 7 studies with 1187 participants (Dihigo 1998; Keefe 2006; Parkin 1993; Saeidi 2013; Salisbury 2012; Taubman 1988; Van Sleuwen 2006). Below we summarise the key characteristics of the studies. For further detail, please see the Characteristics of included studies tables.
Study design
All seven studies included in this review were RCTs (Dihigo 1998; Keefe 2006; Parkin 1993; Saeidi 2013; Salisbury 2012; Taubman 1988; Van Sleuwen 2006).
Location/setting
Four of the included studies were conducted in the USA (Dihigo 1998; Keefe 2006; Salisbury 2012; Taubman 1988), one in Canada (Parkin 1993), one in the Netherlands (Van Sleuwen 2006), and one in Iran (Saeidi 2013).
Participants
Participants in all studies were infants under four months of age who were already suffering from infantile colic. The infants' ages at which the intervention commenced ranged between two weeks (Keefe 2006) up to three months (Saeidi 2013; Salisbury 2012; Taubman 1988; Van Sleuwen 2006).
Four studies defined colic using the Wessel Criteria (Parkin 1993; Saeidi 2013; Salisbury 2012; Van Sleuwen 2006), with remaining studies defining colic as:
diagnosed by a paediatrician (Dihigo 1998);
meeting the Rome III criteria (Keefe 2006; Mostafa 2008); and
crying every day for more than two hours (Taubman 1988).
Symptoms of colic in all participants commenced before three months of age. Six studies included both breast‐fed and formula‐fed infants, whilst one study included breast‐fed infants only (Saeidi 2013).
Interventions (including comparators)
The duration of the interventions varied between six days and three months (Dihigo 1998 and Van Sleuwen 2006 respectively).
The content of the parent training programmes was generally presented in limited detail in the study reports (see Table 3); we gathered no further information after contacting the study authors. As limited information was provided, we were unable to complete the planned qualitative analysis (see Table 2 for planned methods). Instead, we have completed descriptive analyses. The content of the parental training programmes varied and included soothing techniques for crying infants (Dihigo 1998; Keefe 2006; Parkin 1993; Saeidi 2013; Salisbury 2012; Taubman 1988); understanding of babies' needs (Dihigo 1998; Taubman 1988); general care advice, including sleep (Keefe 2006; Salisbury 2012; Taubman 1988; Van Sleuwen 2006); feeding advice (Dihigo 1998; Salisbury 2012); feeding management and normalisation methods (Keefe 2006); stress reduction and empathic counselling for parents (Keefe 2006; Salisbury 2012); and positive play interaction advice (Van Sleuwen 2006). One study taught 'kangaroo care', a specific form of skin‐to‐skin cuddling (Saeidi 2013). Studies used a variety of methods to deliver the parental training programmes, including printed materials (Dihigo 1998; Keefe 2006), face‐to‐face courses (Parkin 1993), home visits (Keefe 2006; Parkin 1993), coaching or counselling (Dihigo 1998; Saeidi 2013; Taubman 1988), remote support (Dihigo 1998), remote counselling (Parkin 1993), and written instructions (Salisbury 2012; Taubman 1988; Van Sleuwen 2006).
2. Content of parent training programmes.
| Study | Content |
| Dihigo 1998 | Provided a protocol for parent‐infant interaction consisting of counselling and flow charts. Counselling focused on feeding patterns based upon the results of a Nursing Child Assessment Feeding Scale and diaries. The flow charts enabled parents to understand their infants needs when they were crying (to be fed, to sleep, to suck, to be held, to be stimulated). The control group received empathy and support from the researcher. |
| Keefe 2006 | Used the REST Routine for Infant Irritability (REST ROUTINE) programme, which consisted of two parts. In the first part, which involved the infant, parents were informed to regulate the infant's behaviour and protect them from overstimulation and exhaustion. They were also told to synchronise the infant's sleep‐wake cycle and various infant holds and positions. Repetition of these methods was recommended. In the second part of the programme, which focused on the parent, a nurse provided reassurance, empathy, support and suggested caregivers' take a break. The control group received routine care, which was not described in the study. |
| Parkin 1993 | Taught mothers different management methods to try to reduce crying, including showing an early response to the baby's crying, soothing the baby, avoiding overstimulation, using a pacifier, holding and carrying the infant, using an infant carrier, and maintaining a day‐night orientation. Written summaries were provided. The second intervention group used a sleep‐tight car ride simulation device when the infant's crying was lasting up to 1 hour. The control group received general information and reassurance. |
| Saeidi 2013 | Taught mothers 'kangaroo care', which involves skin‐to‐skin contact between the mother and infant, during which breastfeeding can also occur. A blanket was provided to cover both infant and mother. Mothers were instructed to do this for a minimum of two hours a day. The control group were asked to rock their infants in the crib. |
| Salisbury 2012 | A paediatrician and mental health clinician delivered family‐centred treatment consisting of strategies to manage the infant's distress and the caregiver's stress. Strategies focused on sleep, feeding, routine and the family's mental health, and included soothing strategies, analysis of day and night behaviour to increase sleep, and encouraging daytime naps. Routine standard care which often includes a brief 30‐minute office visit. |
| Taubman 1988 | Provided parents with written instructions on how to meet the needs of their crying infant. The instructions detailed five needs the infant is possibly signalling (food, sleep, stimulation, suck, or to be held). Parents were instructed to identify the need and respond to it appropriately. The control group received a hydrolysed casein formula and mothers were informed to exclude milk from their infants' diets. After nine days, parents received counselling and were informed to return to the original diets and original formulas. |
| Van Sleuwen 2006 | Healthcare nurses provided parent training, which consisted of a sequence of sleeping, feeding the infant after s/he woke, interacting positively with the baby and allowing the baby to play alone in a playpen. Parents were instructed to watch for signs of tiredness in the baby while in the playpen. When tired, the baby was put to bed and tucked in tightly with sheet and blankets, after which a new cycle started. Essential is the repetitiveness of the elements, feeding the infant directly after waking (with an assumption that a well‐rested baby is able to drink effectively) and not feeding to stop crying, and after playing alone putting the child to bed sleepy but awake. The comparator group received the same parent training course, with the addition of swaddling for sleeping periods, where an infant was wrapped in two cloths. Parents were trained to swaddle ‒ shoulders and arms are wrapped tightly; from the hip down the wrapping was less tight, allowing for leg flexion and abduction. |
In general, the studies conducted the following comparisons.
Parent training versus control (Dihigo 1998; Keefe 2006; Parkin 1993; Saeidi 2013; Salisbury 2012)
Parent training versus specialised baby seat (Parkin 1993)
Parent training versus a milk‐exclusion diet (Taubman 1988)
Parent training versus the same parent training plus swaddling (Van Sleuwen 2006)
Outcomes
The seven included studies reported on two of the three primary outcomes ('Crying time', 'Adverse effects') and two of the three secondary outcomes ('Parental or family quality of life', 'Infant sleep duration'). We describe these below. None of the studies reported on the primary outcome 'Change in duration of crying', or the secondary outcome 'Number of responders in each group post‐intervention: change in frequency of crying episodes per 24 hours'.
Primary outcomes
Crying time at completion of study period
Four studies measured crying time at completion of study period (Dihigo 1998; Keefe 2006; Parkin 1993; Salisbury 2012).
Adverse effects
None of the studies explicitly stated results for this outcome.
Secondary outcomes
Parental or family quality of life
Two studies (183 participants) measured parental stress (Keefe 2006; Salisbury 2012). Keefe 2006 measured parental stress using the Parental Stress Index‒Short Form (PSI‐SF) at baseline, four weeks and eight weeks. Salisbury 2012 measured parental stress using the Parental Stress Index (PSI) at baseline and at 10 weeks. The PSI‐SF is a 36‐item questionnaire, which is completed by parents and measures parental stress. A rating scale between one (strongly agree) and five (strongly disagree) is used for each item.
One study, Parkin 1993, measured maternal anxiety at pre‐ and post‐intervention using the Spielberger's State‐Trait Anxiety Inventory (STAI).
One study (62 participants) reported on depression, measured with the Beck Depression Inventory (BDI), at baseline and at 10 weeks (Salisbury 2012). The BDI measures if depression symptoms and signs are present using a 21‐item scale with possible scores ranging from 0 to 63. A high score is suggested to be correlated with depression. A score above 10 suggests a mood disturbance is present, whilst scores of 21 and above are suggestive of clinical depression.
Infant sleep duration
Three studies (124 participants) measured infant sleep duration (Dihigo 1998; Saeidi 2013; Salisbury 2012).
Funding sources
Four studies stated the funding sources for their studies (Keefe 2006; Salisbury 2012; Taubman 1988; Van Sleuwen 2006). These included national research institutes (Keefe 2006), foundations (Salisbury 2012), and nutritional companies (Taubman 1988). The rest did not state any sources of income.
Excluded studies
We excluded nine studies for various reasons. Two studies were not RCTs (Taubman 1984; Wolke 1994); one did not use a definition of colic that met our inclusion criteria (Van den Boom 1994); three included participants without pre‐existing colic (Cook 2012; McRury 2010; St James‐Roberts 2001); and one included shaken baby syndrome patients (Barr 2009). We were unable to obtain the full‐text reports of two studies, but from screening their abstracts we determined that they were ineligible for inclusion as they did not describe an appropriate intervention (Lim 2013; Smith 2004). See the Characteristics of excluded studies tables.
Studies awaiting classification
One report, Cook 2015, is a protocol for an in‐progress study and is described in further detail in the Characteristics of studies awaiting classification table.
Risk of bias in included studies
Below we present the results of our 'Risk of bias' assessment. Further details can be found in the 'Risk of bias' tables (beneath Characteristics of included studies tables), while a graphical summary of the information can be seen in Figure 2.
Allocation
We rated four studies at low risk of selection bias as the method of random allocation of participants to intervention groups and allocation concealment was described and we judged it to be adequate (Parkin 1993; Salisbury 2012; Taubman 1988; Van Sleuwen 2006).
We rated the three remaining studies at unclear risk of selection bias and allocation concealment (Dihigo 1998; Keefe 2006; Saeidi 2013). Although allocation was described as random, the studies did not describe the method of randomisation.
Blinding
None of the seven studies blinded participants, personnel or outcome assessors, so we rated all seven at high risk of performance bias (Dihigo 1998; Keefe 2006; Parkin 1993; Saeidi 2013; Salisbury 2012; Taubman 1988; Van Sleuwen 2006). However, we only rated six studies at high risk of detection bias; Keefe 2006 stated that the data collection team were unaware of the group assignments or the intervention content, so we rated it at unclear risk of detection bias.
Incomplete outcome data
We considered all seven studies to be at low risk of attrition bias due to clear patient flow and description of attrition, with balanced rates between groups (Dihigo 1998; Keefe 2006; Parkin 1993; Saeidi 2013; Salisbury 2012; Taubman 1988; Van Sleuwen 2006)
Selective reporting
We judged all seven studies to be at low risk of reporting bias, since they each reported on all of their prespecified outcomes (Dihigo 1998; Keefe 2006; Parkin 1993; Saeidi 2013; Salisbury 2012; Taubman 1988; Van Sleuwen 2006). Whilst adverse effects were not specifically reported, we judged no impact on reporting bias to have occurred, as all studies considered non‐medicinal interventions and specified appropriate proxy outcomes of adverse events.
Other potential sources of bias
We rated four studies at unclear risk of other bias (Keefe 2006; Salisbury 2012; Taubman 1988; Van Sleuwen 2006). Three of these studies were funded through external commercial sources and provided no details as to the level of involvement — we could not obtain this information, even after contacting the authors (Keefe 2006; Salisbury 2012; Taubman 1988). One study was led by the developer of the intervention (Keefe 2006). One study was funded by a public national body, but we also received no response from the authors to our request for further information, so we rated it as unclear (Van Sleuwen 2006). We considered the three remaining studies to be at low risk of other bias (Dihigo 1998; Parkin 1993; Saeidi 2013).
Effects of interventions
See: Table 1
There was limited scope for meta‐analysis due to inconsistent reporting of the primary and secondary outcomes.
Parent training versus control
Five studies (283 participants) compared a parent training intervention (Table 3) with a control (two provided 'no intervention', two stated they provided reassurance, and one asked the parents to rock their babies in their crib) (Dihigo 1998; Keefe 2006; Parkin 1993; Saeidi 2013; Salisbury 2012). One of these studies had three trial arms, two of which compared parent training and a control (Parkin 1993).
We were unable to include two studies in a meta‐analysis of 'Crying time at completion of study period'. We could not include Saeidi 2013 (48 participants) because the study did not provide standard deviation (SD) values, which we could not calculate. We could not include Salisbury 2012 (71 participants) because the study did not report figures at completion (10 weeks), only at six weeks in the middle of the study, and did not provide SD values. We could not calculate or generate these figures despite contacting the study authors.
Primary outcomes
Crying time at completion of study period
We conducted a random‐effects meta‐analysis of three studies (157 participants) for the primary outcome of 'Crying time at completion of study period' (Dihigo 1998; Keefe 2006; Parkin 1993). These three studies all employed a training programme with parents: this programme consisted of counselling to cover how to be responsive to an infant, understand the cues and needs of the baby, and appropriate responses.
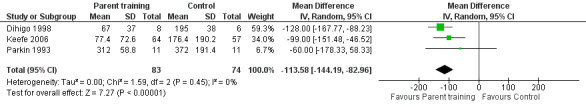
We found that parent training was more effective than a control (MD −113.58 minutes per day, 95% CI −144.19 to −82.96; Analysis 1.1; Figure 3). Using the GRADE approach, we rated the certainty of this evidence as low, due to concerns about risk of bias and imprecision from low participant numbers (see Table 1). A sensitivity analysis using the fixed‐effect model also found a result favouring parent training (MD −113.58 minutes per day, 95% CI −144.19 to −82.96; Analysis 1.2; Figure 4). There was no statistical heterogeneity in either analysis (random‐effects model ‒ Tau² = 0.00; Chi² = 1.59, degrees of freedom (df) = 2 (P = 0.45); I² = 0%; fixed‐effect model ‒ Chi² = 1.59, df = 2 (P = 0.45), I² = 0%).
1.1. Analysis.
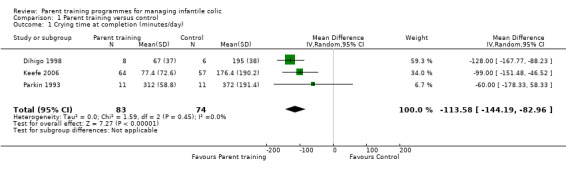
Comparison 1 Parent training versus control, Outcome 1 Crying time at completion (minutes/day).
3.
Forest plot of comparison: 1 Parent training versus control, outcome: 1.1 Crying time at completion (minutes/day).
1.2. Analysis.
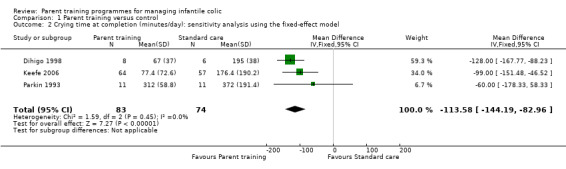
Comparison 1 Parent training versus control, Outcome 2 Crying time at completion (minutes/day): sensitivity analysis using the fixed‐effect model.
4.
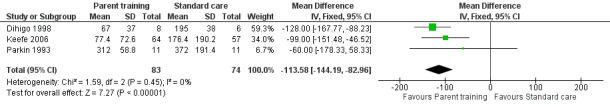
Forest plot of comparison: 1 Parent training versus control, outcome: 1.2 Crying time at completion (minutes/day): sensitivity analysis using the fixed‐effect model.
Single study results
Saeidi 2013 (48 participants) compared kangaroo care versus a control (rock their infants in the crib, Table 3), and found a reduction in crying time of 1.8 hours per day from baseline in the group given kangaroo care, compared to a reduction of 0.3 hours per day in the group instructed to rock their infants in the crib. This difference between the two groups was significant.
Salisbury 2012 (71 participants) compared family‐centred treatment versus a brief office visit, and found a reduction in crying time of 3.10 hours per day (64% reduction, 95% CI 60 to 69) from baseline in the family‐centred treatment group, compared to a reduction of 0.97 hours (27% reduction, 95% CI 24 to 30) from baseline in the control group.
Secondary outcomes
Parental or family quality of life
Two studies measured parental stress (183 participants) (Keefe 2006; Salisbury 2012). Keefe 2006 measured parental stress using the PSI‐SF at baseline, four weeks and eight weeks. Keefe 2006 found no significant difference between the treatment and control groups at baseline on total PSI‐SF scores; although for the difficult child subscale (demonstrating the most difficult infants), the baseline score in the control group was significantly higher than the treatment group (P = 0.009). At baseline the total mean PSI‐SF score was 87.0 for the parent training group and 92.5 for the control group. There was a reduction in total mean PSI‐SF scores for both parent training and control groups from baseline, and at four and eight weeks. At four weeks the total mean PSI‐SF score was 76.6 for the parent training group and 78.8 for the control group. At eight weeks the total mean PSI‐SF score was 71.0 for the parent training group and 77.2 for the control group. The study also found reduced parental stress on the subscale for parental‐child dysfunctional interaction in the parent group compared to the control group (P = 0.04). Salisbury 2012 measured parental stress using the PSI at baseline and at 10 weeks. The study reported no group differences between the parent training and control groups.
One study (62 participants) reported on depression, measured with the BDI (possible scores range from 0 to 63), at baseline and at 10 weeks (Salisbury 2012). The study found that the parent training group scored higher than the group who undertook a brief office visit at baseline. At 10 weeks, the parent training group had 25 mothers (83.3%) with a minimum of a 3‐point reduction in BDI score, whereas the group who undertook a brief office visit had 12 mothers (40%) with a minimum of a 3‐point reduction in BDI scores. The difference in scores was not significant when adjusted for baseline scores.
Infant sleep duration
Three studies (124 participants) measured sleep duration (Dihigo 1998; Saeidi 2013; Salisbury 2012).
Dihigo 1998 (14 participants) reported qualitative and sporadic comments only from diary entries completed by parents, and suggested those in the parent training group found their infants were sleeping more.
Saeidi 2013 (48 participants) found that sleep duration increased significantly more in the group given kangaroo care, from 8.3 hours per day (mean baseline sleep duration) to 12 hours per day, than in the other group where parents were asked to rock their infants in the crib (from eight hours per day to nine hours per day) over seven days of treatment (P = 0.02).
Salisbury 2012 (62 participants) found that the group that received family‐centred treatment were sleeping more than the group asked to attend for a brief office visit at two weeks (P = 0.032). The study also reported the mean hours per day on a graph at baseline, two weeks, six weeks and 10 weeks. However, due to the graphical representation of the data, we were unable to elicit accurate values from the graphs with any certainty and so this was not done.
None of the studies included in this comparison reported data on our other primary ('Change in duration of crying' and 'Adverse effects') or secondary outcomes ('Number of responders in each group post‐intervention: change in frequency of crying episodes per 24 hours').
Parent training versus specialised baby seat
One study (38 participants) compared parent training with a specialised baby seat (Parkin 1993).
Primary outcome
Crying time at completion of study period
The values of mean hours of crying per day in each group were not reported and only displayed on a graph. The study stated there were no significant differences between the three groups but did not report a P value in support of this statement.
Secondary outcome
Parental or family quality of life
Parkin 1993 measured maternal anxiety at pre‐ and post‐intervention using Spielberger's State‐Trait Anxiety Inventory (STAI). This study used a component of this tool which was the 'state' component. This component involved 20 questions measuring an adult's anxiety levels (possible scores range from 20 to 80).
The study does not present STAI data in a meaningful way for us to use in our study.
The study did not report data on our other primary ('Change in duration of crying' and 'Adverse effects') or secondary outcomes ('Number of responders in each group post‐intervention: change in frequency of crying episodes per 24 hours' and 'Infant sleep duration').
Parent training versus a milk‐exclusion/soy milk formula diet
One study (20 participants) compared parent training versus a milk‐exclusion/soy milk formula diet (Taubman 1988).
Primary outcome
Crying time at completion of study period
In Taubman 1988, the mean crying time of the parent training group (10 participants) at baseline was 3.21 hours per day (SD = 1.10). After nine days this decreased to 1.08 hours per day (SD = 0.70) (P = 0.01). The mean crying time of the milk‐exclusion diet group (10 participants) was 3.19 hours (SD = 0.69). After nine days this decreased to 2.03 hours per day (SD = 1.03) (P = 0.01). The study reports that the amount and rate at which crying decreased in the parent training group versus the milk‐exclusion diet group was statistically significant (P < 0.02).
The second phase of this study involved measuring only the crying times in the milk‐exclusion diet group who thereafter received the same counselling as the parent training group.
The study did not report data on our other primary outcomes ('Change in duration of crying' and 'Adverse effects'), or any of our secondary outcomes ('Number of responders in each group post‐intervention: change in frequency of crying episodes per 24 hours'; 'Parental or family quality of life'; and 'Infant sleep duration').
Parent training versus the same parent training plus swaddling training
One study (398 participants) compared a parent training intervention with the same parent training plus swaddling training (Van Sleuwen 2006).
Primary outcome
Crying time at completion of study period
Van Sleuwen 2006 reported crying times during the first week. The study found that the amount of crying in the parent training group increased by 20 to 25 minutes on the first day before decreasing over the next few days. Crying time in the parent training group plus swaddling decreased by "30 to 40 minutes" (quote); the study does not, however, state the baseline mean crying time in each individual group. The study states that there was no significant difference between the groups. We were unable to extract actual figures for crying times in each group at the end of the first week as they were presented on a graph only.
At four weeks, two parents in the 'parent training plus swaddling' group stopped the swaddling technique, and 16 parents in the 'parent training only' group commenced swaddling.
The study did not report data on our other primary outcomes ('Change in duration of crying' and 'Adverse effects'), or any of our secondary outcomes ('Number of responders in each group post‐intervention: frequency of crying episodes per 24 hours'; 'Parental or family quality of life'; and 'Infant sleep duration').
Discussion
Summary of main results
Seven studies (1187 participants) met our inclusion criteria (Dihigo 1998; Keefe 2006; Parkin 1993; Saeidi 2013; Salisbury 2012; Taubman 1988; Van Sleuwen 2006). Of these, five studies compared parent training to a control; one study compared parent training to a milk‐exclusion diet; one study contained three arms, comparing parent training to a specialised baby seat and parents receiving general information and reassurance; and one study compared parent training to the same parent training plus swaddling.
A meta‐analysis of three studies (157 participants) of parent training versus control found a larger reduction in the primary outcome of 'Crying time at completion of study period' in the parent training group compared to the control group (Dihigo 1998; Keefe 2006; Parkin 1993). This is low‐certainty evidence, downgraded by two levels (see Quality of the evidence).
Narrative results of single studies
Three studies (221 participants) reported on the secondary outcome of 'Parental or family quality of life' (Keefe 2006; Parkin 1993; Salisbury 2012). Salisbury 2012 (62 participants) measured parental stress at baseline and 10 weeks, and found no differences between the parent‐training group and the control group that received 'standard care' possibly including a 30‐minute office visit. Keefe 2006 measured parental stress at baseline, and at four and eight weeks, and found a reduction in parental stress levels in both the parent training group and the control group that received what the authors termed "routine care". Keefe 2006 also found reduced parental stress for the subscale of parent‒child dysfunctional interaction in the parent training group compared to the control group.
Salisbury 2012 (62 participants) also measured depression using the BDI and found no difference in scores between the parent training group and control group.
Three studies measured the secondary outcome of infant sleep duration (124 participants). Two studies (110 participants) reported increased sleeping duration of infants in the parent training group in comparison to a control: Saeidi 2013 found that sleep duration increased significantly more in the group given kangaroo care; Salisbury 2012 found that the group that received family‐centred treatment were sleeping more than the group asked to attend for a brief office visit at two weeks. One study (14 participants) stated increased sleeping of infants in the parent training group based on qualitative data from diary entries (Dihigo 1998).
One study (38 participants) comparing parent training with a specialised baby seat reported no differences between the groups for crying time at completion, displayed on a graph and without a P value (Parkin 1993).
One study (20 participants) comparing parent training with a milk‐exclusion/soy milk formula diet found a reduction in crying time at completion in the parent training group compared to the milk‐exclusion/soy milk formula group (Taubman 1988).
One study (398 participants) comparing parent training with the same parent training plus swaddling reported that there was no significant difference between the groups in crying time at completion (Van Sleuwen 2006).
None of the studies reported change in duration of crying or adverse events.
Overall completeness and applicability of evidence
There are a few issues that limit the completeness and applicability of the evidence. The studies used multiple, different interventions and controls, resulting in a heterogenous evidence base. Given the small number of studies, this limited the current scope for analysis and certainty results, thereby impacting the applicability of evidence. A key area for this is in the length of study, which ranged from just six days to three months.
Studies were generally poor at reporting what the intervention was, which reduces the ability of other researchers to replicate the work, and again leads to a heterogenous evidence base. What is key is the poor reporting of the actual training programme, impacting dissemination.
No data were provided regarding adverse effects and a paucity of trials measured our secondary outcomes, with only three studies reporting on 'Parental and family quality of life' (Keefe 2006; Parkin 1993; Salisbury 2012), and three studies reporting 'Infant sleep duration' per 24 hours (Dihigo 1998; Saeidi 2013; Salisbury 2012).
Quality of the evidence
The studies were open‐label and thus susceptible to the placebo effect. They were also at high risk of performance bias, as participants were not blinded to the interventions they received (although this is not possible for parent training programmes). In addition, the majority of studies had unclear or absent methodology regarding random sequence and allocation concealment, leading to concerns of selection bias. Due to the serious risks of bias from lack of blinding and unclear allocation concealment in the majority of studies, and because the studies involved low participant numbers leading to concerns of imprecision, we downgraded the certainty of the evidence for the primary outcome of 'Crying time at completion of study period' to low in our GRADE analysis.
Potential biases in the review process
We encountered difficulty when having to decide what constituted parent training versus specific, baby‐focused interventions using parents. For example, one study used kangaroo care and we had to consider whether the study focused on the actual care itself or on parental training on how to deliver this as a behavioural intervention (Saeidi 2013). It is possible that such judgements could be considered by readers as inappropriate; they have, however, been reported transparently to support readers.
Agreements and disagreements with other studies or reviews
No other systematic reviews have investigated the effectiveness and safety of parent training programmes for managing colic in infants under four months of age. One review, however, has assessed the effectiveness and safety of dietary interventions for reducing or preventing infantile colic (Gordon 2018). This review found little evidence regarding the effectiveness of dietary interventions for reducing colic, but some evidence that they may prevent its onset.
Authors' conclusions
Implications for practice.
This review yielded limited evidence on the effectiveness and safety of parent training programmes for managing infantile colic. A single meta‐analysis found that parent training programmes may reduce crying times for infantile colic compared to no intervention. However, the certainty of this evidence is low due to a number of methodological issues (e.g. small number of studies, low participant numbers, and high risks of bias). No other analyses were possible. Limited data were presented in primary reports to allow any descriptive analysis of content, so we can arrive at no firm conclusions to guide future production of such interventions.
Implications for research.
Despite a number of studies in the area, many clinical, methodological and writing issues pervade the publications and must be addressed in future research.
Key for new studies is to define and appropriately deploy the intervention in question. Given the educational nature of these parent training strategies, it is vital to consider the pedagogy, conceptual underpinning, resources needed and specific outcomes for learning. This will allow useful dissemination and allow other researchers and clinicians to replicate work and form a more applicable and less heterogenous evidence base.
Furthermore, outcome measures of interest detailed in this review should be utilised by future studies. It is not appropriate for studies investigating the potential to reduce the onset of new infantile colic to not report such outcomes and instead focus on proxies such as crying time. For example, set outcomes at one to two weeks and two to four weeks after commencing the intervention would be of interest to parents.
One final area of improvement for future study designs is to reduce the risk of bias. In particular, ensuring appropriate allocation concealment and measures to account for the difficulty in blinding participants, personnel and outcomes assessors due to the nature of such interventions, is key.
Acknowledgements
We would like to acknowledge the editor and statistician for their helpful comments on this review, and the support received from the Cochrane Developmental, Psychosocial and Learning Problems Editorial Team. We would also like to thank Dr Bruce Taubman, Dr Rupert Hinds and Rachel Marshall, who commented on an earlier version of this review.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane LIbrary
Searched 2 September 2016 (612 records); 5 February 2018 (108 new records), 4 June 2019 (148 new records)
#1 [mh Colic] #2 colic* #3 ((stomach or abdominal or abdomen*) near/3 (spasm* or pain* or cramp*)) #4 ((gastric or gastro*) near/3 (spasm* or pain* or cramp*)) #5 [mh Crying] #6 (cry or crying or cries) #7 {or #1‐#6} #8 [mh infant] #9 (baby or babies or infant* or child* or newborn* or neonat*) #10{or #8‐#9} #11 #7 and #10 #12 [mh Parents] #13 [mh Parenting] #14 [mh "Parent‐Child Relations"] #15 [mh ^"family relations"] #16 [mh "maternal behavior"] #17 [mh "paternal behavior"] #18 [mh "maternal deprivation"] #19 [mh "paternal deprivation"] #20(parent* or mother* or father* or maternal* or paternal* or famil*) #21{or #12‐#20} #22 [mh "Health Education"] #23 [mh Education] #24 [mh teaching] #25 (class* or coach* or counsel* or curricul* or educat* or group* or intervention* or learn* or package* or program* or support* or teach* or train*) 26 {or #22‐#25} #27 #21 and #26 #28 [mh Parents/ED] #29 ((home* or nurse* or famil* or mother* or parent* or father*) near/2 visit*) #30 {or #27‐#29} #31 #11 and #30
MEDLINE Ovid
Searched 1 September 2016 (803 records); 5 February 2018 (55 new records), 4 June 2019 (53 new records)
Lines 32 to 42 are the sensitivity maximizing version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE Ovid (Lefebvre 2008),
1 colic/ 2 colic$.tw. 3 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).tw. 4 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).tw. 5 crying/ 6 (cry or crying or cries).tw. 7 or/1‐6 8 exp Infant/ 9 (baby or babies or infant$ or child$ or newborn$ or neonat$).tw. 10 or/8‐9 11 7 and 10 12 exp Parents/ 13 Parenting/ 14 exp Parent‐Child Relations/ 15 family relations/ 16 exp maternal behavior/ 17 maternal deprivation/ 18 paternal behavior/ 19 paternal deprivation/ 20 (parent$ or mother$ or father$ or maternal$ or paternal$ or famil$).tw. 21 or/12‐20 22 Health Education/ 23 education/ 24 teaching/ 25 (class$ or coach$ or counsel$ or curricul$ or educat$ or group$ or intervention$ or learn$ or package$ or program$ or support$ or teach$ or train$).tw. 26 or/22‐25 27 21 and 26 28 exp Parents/ed [Education] 29 ((home$ or nurse$ or famil$ or mother$ or parent$ or father$) adj2 visit$).tw. 30 or/27‐29 31 11 and 30 32 randomized controlled trial.pt. 33 controlled clinical trial.pt. 34 randomi#ed.ab. 35 placebo$.ab. 36 drug therapy.fs. 37 randomly.ab. 38 trial.ab. 39 groups.ab. 40 or/32‐39 41 exp animals/ not humans.sh. 42 40 not 41 43 31 and 42
MEDLINE(R) In‐Process & Other Non‐Indexed Citations Ovid
Searched 1 September 2016 (170 records); 5 February 2018 (114 new records), 4 June 2019 (55 new records)
1 colic$.tw,kw. 2 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).tw,kw. 3 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).tw,kw. 4 (cry or crying or cries).tw,kw. 5 or/1‐4 6 (baby or babies or infant$ or child$ or newborn$ or neonat$).tw,kw. 7 5 and 6 8 (parent$ or mother$ or father$ or maternal$ or paternal$ or famil$).tw,kw. 9 (class$ or coach$ or counsel$ or curricul$ or educat$ or group$ or intervention$ or learn$ or package$ or program$ or support$ or teach$ or train$).tw,kw. 10 8 and 9 11 ((home$ or nurse$ or famil$ or mother$ or parent$ or father$) adj2 visit$).tw,kw. 12 10 or 11 13 7 and 12
MEDLINE(R) Epub Ahead of Print Ovid
Searched 1 September 2016 (38 records); 5 February 2018 (44 new records), 4 June 2019 (48 new records)
1 colic$.tw,kw. 2 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).tw,kw. 3 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).tw,kw. 4 (cry or crying or cries).tw,kw. 5 or/1‐4 6 (baby or babies or infant$ or child$ or newborn$ or neonat$).tw,kw. 7 5 and 6 8 (parent$ or mother$ or father$ or maternal$ or paternal$ or famil$).tw,kw. 9 (class$ or coach$ or counsel$ or curricul$ or educat$ or group$ or intervention$ or learn$ or package$ or program$ or support$ or teach$ or train$).tw,kw. 10 8 and 9 11 ((home$ or nurse$ or famil$ or mother$ or parent$ or father$) adj2 visit$).tw,kw. 12 10 or 11 13 7 and 12
Embase Ovid
Searched 1 September 2016 (881 records); 5 February 2018 (130 new records), 4 June 2019 (126 new records)
1 colic/ 2 crying/ 3 colic$.tw. 4 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).tw. 5 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).tw. 6 (cry or crying or cries).tw. 7 or/1‐6 8 exp infant/ 9 (baby or babies or infant$ or child$ or newborn$ or neonat$).tw. 10 8 or 9 11 7 and 10 12 Infantile colic/ 13 11 or 12 14 exp Parent/ 15 exp child parent relation/ 16 family relation/ 17 parental deprivation/ 18 (parent$ or mother$ or father$ or maternal$ or paternal$ or famil$).tw. 19 or/14‐18 20 health education/ 21 education/ 22 teaching/ 23 (class$ or coach$ or counsel$ or curricul$ or educat$ or group$ or intervention$ or learn$ or package$ or program$ or support$ or teach$ or train$).tw. 24 or/20‐23 25 19 and 24 26 parenting education/ 27 ((home$ or nurse$ or famil$ or mother$ or parent$ or father$) adj2 visit$).tw. 28 25 or 26 or 27 29 13 and 28 30 Randomized controlled trial/ 31 controlled clinical trial/ 32 Single blind procedure/ 33 Double blind procedure/ 34 triple blind procedure/ 35 Crossover procedure/ 36 (crossover or cross‐over).tw. 37 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 38 Placebo/ 39 placebo.tw. 40 prospective.tw. 41 factorial$.tw. 42 random$.tw. 43 assign$.ab. 44 allocat$.tw. 45 volunteer$.ab. 46 or/30‐45 47 29 and 46
CINAHL Plus EBSCOhost
Searched 2 September 2016 (270 records); 5 February 2018 (81 new records), 4 June 2019 (63 new records)
S1 (MH "Colic") S2 colic* S3 TI((stomach or abdominal or abdomen*) N3 (spasm* or pain* or cramp*)) or AB((stomach or abdominal or abdomen*) N3 (spasm* or pain* or cramp*)) S4 TI((gastric or gastro*) N3 (spasm* or pain* or cramp*)) or AB((gastric or gastro*) N3 (spasm* or pain* or cramp*)) S5 (MH "Crying") S6 TI(cry or crying or cries) or AB(cry or crying or cries) S7 S1 or S2 or S3 or S4 or S5 or S6 S8 (MH "Infant+") S9 TI(baby or babies or infant* or child* or newborn* or neonat*) or AB(baby or babies or infant* or child* or newborn* or neonat*) S10 S8 or S9 S11 S7 and S10 S12 (MH "Infant Colic") S13 S11 or S12 S14 (MH "Parents+") S15 (MH "Parent‐Child Relations+") S16 (MH "Family Relations") S17 TI (parent* or mother* or father* or maternal* or paternal* or famil*) or AB (parent* or mother* or father* or maternal* or paternal* or famil*) S18 S14 or S15 or S16 or S17 S19 (MH "Health Education") S20 (MH "Education") S21 (MH "Teaching") S22 TI(class* or coach* or counsel* or curricul* or educat* or group* or intervention* or learn* or package* or program* or support* or teach* or train*) or AB (class* or coach* or counsel* or curricul* or educat* or group* or intervention* or learn* or package* or program* or support* or teach* or train*) S23 S19 or S20 or S21 or S22 S24 S18 and S23 S25 (MH "Parents/ED") S26 (MH "Home Visits") S27 TI ((home* or nurse* or famil* or mother* or parent* or father*) N2 visit*) or AB((home* or nurse* or famil* or mother* or parent* or father*) N2 visit*) S28 S24 or S25 or S26 or S27 S29 S13 and S28 S30 (MH "Clinical Trials+") S31 MH random assignment S32 (MH "Meta Analysis") S33 (MH "Crossover Design") S34 (MH "Quantitative Studies") S35 PT randomized controlled trial S36 PT Clinical trial S37 (clinical trial*) or (control* N2 trial*) S38 ("follow‐up study" or "follow‐up research") S39 (prospectiv* study or prospectiv* research) S40 (evaluat* N2 study or evaluat* N2 research) S41 (MH "Program Evaluation") S42 (MH "Treatment Outcomes") S43 TI(single N2 mask* or single N2 blind*) or AB(single N2 mask* or single N2 blind*) S44 TI((doubl* N2 mask*) or (doubl* N2 blind*)) or AB((doubl* N2 mask*) or (doubl* N2 blind*)) S45 TI ((tripl* N2 mask*) or (tripl* N2 blind*)) or ((trebl* N2 mask*) or (trebl* N2 blind*)) or AB((tripl* N2 mask*) or (tripl* N2 blind*)) or ((trebl* N2 mask*) or (trebl* N2 blind*) S46 random* S47 S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 S48 S29 and S47
PsycINFO Ovid
Searched 1 September 2016 (543 records); 5 February 2018 (41 new records), 4 June 2019 (32 new records)
1 Crying/ 2 colic$.tw. 3 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).tw. 4 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).tw. 5 (cry or crying or cries).tw. 6 or/1‐5 7 (infancy 2 23 mo or neonatal birth 1 mo).ag. 8 (baby or babies or infan$ or child$ or neonat$ or newborn$).tw. 9 7 or 8 10 6 and 9 11 exp parents/ 12 exp parenting/ 13 parenting skills/ 14 family relations/ 15 (parent$ or mother$ or father$ or maternal$ or paternal$ or famil$).tw. 16 or/11‐15 17 education/ 18 Educational Programs/ 19 teaching/ 20 health education/ 21 (class$ or coach$ or counsel$ or curricul$ or educat$ or group$ or intervention$ or learn$ or package$ or program$ or support$ or teach$ or train$).tw. 22 or/17‐21 23 16 and 22 24 parent training/ 25 23 or 24 26 10 and 25 27 clinical trials/ 28 longitudinal studies/ 29 exp program evaluation/ 30 exp Treatment Effectiveness Evaluation/ 31 random$.tw. 32 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).tw. 33 (crossover$ or "cross over$").tw. 34 trial$.tw. 35 group$.ab. 36 treatment effectiveness evaluation/ 37 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 38 prospective.tw. 39 factorial$.tw. 40 random$.tw. 41 (assign$ or allocat$).ab. 42 control.ab. 43 treatment as usual.ab. 44 placebo.ab. 45 (crossover or cross‐over).tw. 46 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. 47 or/27‐46 48 26 and 47
Science Citation Index and Social Sciences Citation Index Web of Science
Searched 5 September 2016 (1272 records); 5 February 2018 (39 new records), 4 June 2019 (157 new records)
# 20 #16 AND #15 Indexes=SCI‐EXPANDED, SSCI Timespan=2018‐2019 # 19 #16 AND #15 Indexes=SCI‐EXPANDED, SSCI Timespan=2016‐2018 # 18 #16 AND #15 Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 17 #16 AND #15 Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 16 TS=(random* or trial* or control* or placebo* or group* or blind* or longitudinal* or prospectiv*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 15 #14 AND #9 Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 14 #13 OR #12 Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 13 TS= ("home visit*") Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 12 #11 AND #10 Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 11 TS=(class* or coach* or counsel* or curricul* or educat* or group* or intervention* or learn* or package* or program* or support* or teach* or train*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 10 TS=(parent* or mother* or father* or maternal* or paternal* or famil*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 9 #8 AND #7 Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 8 TS=(baby or babies or infant* or child* or newborn* or neonat*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 7 #6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 6 TS= (abdomen* Near/3 spasm* or abdomen* near/3 pain* or abdomen* near/3 cramp*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 5 TS= (abdominal Near/3 spasm* or abdominal near/3 pain* or abdominal near/3 cramp*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 4 TS= (stomach Near/3 spasm* or stomach near/3 pain* or stomach near/3 cramp*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 3 TS= (gastro* Near/3 spasm* or gastro* near/3 pain* or gastro* near/3 cramp*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 2 TS= (gastric Near/3 spasm* or gastric near/3 pain* or gastric near/3 cramp*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 1 TS=(colic* or cry or cries or crying) Indexes=SCI‐EXPANDED, SSCI Timespan=All years
Conference Proceedings Citation Index ‐ Science and Conference Proceedings Citation Index Social Science and Humanities Web of Science
Searched 5 September 2016 (57 records); 5 February 2018 (3 new records), 4 June 2019 (0 new records)
#19 #16 AND #15 Indexes=CPCI‐S, CPCI‐SSH Timespan=2018‐2019 # 18 #16 AND #15 Indexes=CPCI‐S, CPCI‐SSH Timespan=2016‐2018 # 17 #16 AND #15 Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 16 TS=(random* or trial* or control* or placebo* or group* or blind* or longitudinal* or prospectiv*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 15 #14 AND #9 Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 14 #13 OR #12 Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 13 TS= ("home visit*") Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 12#11 AND #10 Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 11 TS=(class* or coach* or counsel* or curricul* or educat* or group* or intervention* or learn* or package* or program* or support* or teach* or train*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 10 TS=(parent* or mother* or father* or maternal* or paternal* or famil*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 9 #8 AND #7 Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 8 TS=(baby or babies or infant* or child* or newborn* or neonat*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years #7 #6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 6 TS= (abdomen* Near/3 spasm* or abdomen* near/3 pain* or abdomen* near/3 cramp*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 5 TS= (abdominal Near/3 spasm* or abdominal near/3 pain* or abdominal near/3 cramp*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 4 TS= (stomach Near/3 spasm* or stomach near/3 pain* or stomach near/3 cramp*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 3TS= (gastro* Near/3 spasm* or gastro* near/3 pain* or gastro* near/3 cramp*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 2TS= (gastric Near/3 spasm* or gastric near/3 pain* or gastric near/3 cramp*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 1 TS=(colic* or cry or cries or crying) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years
LILACS http://lilacs.bvsalud.org/en/
Searched 2 September 2016 (6 records); 5 February 2018 (no new records), 5 June 2019 (0 new records)
(tw:(baby OR babies OR infant* OR neonat* OR newborn*)) AND (tw:(colic* OR cries OR crying OR cry)) AND (tw:(parent* OR mother* OR father* OR famil* OR maternal* OR paternal*)) AND (tw:((class* OR coach* OR counsel* OR curricul* OR educat* OR group* OR intervention* OR learn* OR package* OR program* OR support* OR teach* OR train* OR visit* ) )) AND (instance:"regional") AND ( type_of_study:("clinical_trials"))
Cochrane Database of Systematic Reviews (CDSR) part of the Cochrane Library
Searched 2 September 2016 (15 records); 5 February 2018 (8 new records), 4 June 2019 (4 new records)
#1 colic*:ti,ab,kw #2 ((bout* or episod* or excess* or time* or hours or period*) near (cry or crying or cries)):ti,ab,kw #3 {or #1‐#2} #4 (baby or babies or infant* or child* or newborn* or neonat*):ti,ab,kw #5 #3 and #4 in Cochrane Reviews, Cochrane Protocols
Database of Abstracts of Reviews of Effects (DARE) part of the Cochrane Library
Searched 2 September 2016 (9 records).
#1 colic*:ti,ab,kw #2 ((bout* or episod* or excess* or time* or hours or period*) near (cry or crying or cries)):ti,ab,kw #3 {or #1‐#2} #4 (baby or babies or infant* or child* or newborn* or neonat*):ti,ab,kw #5 #3 and #4 in Other Reviews
Epistemonikos
Searched 2 September 2016 (8 records); 5 February 2018 (3 new records), 5 June 2019 (6 new records)
Full query:title:(colic* OR cry OR crying OR cries) AND title:(infant* OR child* OR baby OR babies OR neonat* OR newborn*) AND (title:(parent* OR father* OR mother* OR famil*) OR abstract:(parent* OR father* OR mother* OR famil*)) AND (title:(class* OR coach* OR counsel* OR curricul* OR educat* OR group* OR intervention* OR learn* OR package* OR program* OR support* OR teach* OR train*) OR abstract:(class* OR coach* OR counsel* OR curricul* OR educat* OR group* OR intervention* OR learn* OR package* OR program* OR support* OR teach* OR train*)) Limited to systematic reviews
WorldCat https://www.worldcat.org/
Searched 2 September 2016 (8 records); 5 February 2018 (1 new record), 5 June 2019 (0 new records)
kw:(colic* OR crying OR cries OR cry) AND (baby OR babies OR infant* OR neonat* OR newborn*) AND kw:(PARENT* OR MOTHER* OR FATHER* OR FAMIL*) AND (kw:class* OR coach* OR counsel* OR curricul* OR educat* OR group* OR intervention* OR learn* OR package* OR program* OR support* OR teach* OR train* OR visit*) Limited to Theses
ClinicalTrials.gov
2 September 2016 (10 records) (parents OR mothers OR fathers OR carers OR caregivers ) AND ( education OR training OR support OR program or class or group ) | Interventional Studies | colic OR crying
6 February 2018 (4 records after duplicates removed) Search string 1 Interventional Studies | colic OR crying | education OR training OR support OR program or class or group | First posted from 09/01/2016 to 02/06/2018 (2 records)
Search string 2 Interventional Studies | colic OR crying | parents OR mothers OR fathers OR carers OR caregivers | First posted from 09/01/2016 to 02/06/2018 (4 records)
5 June 2019 (5 records after duplicates removed)
Search string 1 Interventional Studies | colic OR crying | education OR training OR support OR program or class or group | First posted from 02/06/2018 to 06/05/2019 (2 records)
Search string 2 Interventional Studies | colic OR crying | parents OR mothers OR fathers OR carers OR caregivers | First posted from 02/06/2018 to 06/05/2019 [4 records]
WHO ICTRP http://apps.who.int/trialsearch
Searched 2 September 2016 (25 records); 6 February 2018 (3 new records), 5 June 2019 (10 new records)
Advanced search: TITLE| baby OR babies OR child* OR neonat* OR newborn* OR infant* AND Condition|(colic* OR cries OR Cry OR crying) AND INTERVENTION| parent* OR mother* OR father* OR famil* OR maternal* OR paternal*
Appendix 2. Criteria for assigning 'Risk of bias' judgements
Sequence generation
We included only RCTs or quasi‐RCTs in this review. We assessed randomisation as being at low risk of selection bias if the procedure of sequence generation was explicitly described to confirm it was random; examples included computer‐generated random numbers, a random numbers table or coin‐tossing. If no description was given, we contacted the study authors for further information and if we failed to receive a response, we assigned a judgment of unclear risk of selection bias. We considered studies that use non‐randomised procedures to be at high risk of selection bias.
Allocation concealment
We assessed concealment of treatment allocation as being at low risk of selection bias if the procedure was explicitly described and adequate efforts were made to ensure that intervention allocations could not have been foreseen in advance of, or during, enrolment; examples included centralized randomisation, numbered or coded containers, or sealed envelopes. Procedures considered to have a high risk of selection bias included alternation or reference to case record numbers or dates of birth. We also assigned a high risk of selection bias if allocation concealment did not occur, as intervention allocations could have been foreseen and thus introduced potential bias. If no description was given, we contacted the study authors, and if we did not receive a response, we assigned a judgment of unclear risk of selection bias.
Blinding of parents and health professionals
In this context, the intervention is administered by parents and so, in effect, we considered them to be the target of the blinding procedures. Indeed, as the participants were less than four months of age by the defined inclusion criteria, we deemed that this item was not applicable to them. Furthermore, parents often act as outcome assessors. We primarily assessed the risk of bias associated with the blinding of participants based on the likelihood that such blinding was sufficient to ensure that parents had no knowledge as to which intervention the infant received. We assessed blinding at low risk of bias where it was explicitly described how the parents could have no knowledge of which intervention the infant was receiving, and at high risk of bias where it was clear that parents were aware of which intervention the infant was receiving. We assessed blinding at unclear risk of bias where we did not have enough information to make an assessment of either high or low risk of bias, based on the information provided in the papers, or after contacting the study authors.
Blinding of outcome assessment
We described, for each included study, the methods used, if any, to blind the outcome assessors from knowledge of which intervention a participant received. We judged studies to be at low risk of detection bias if they were blinded or if we considered that the lack of blinding could not have affected the results. If blinding was not done, or was not possible due to the nature of intervention, we judged the study to be at high risk of detection bias because it was possible that the lack of blinding influenced the results. If no description was given, we contacted the study authors for more information, and if we did not receive a response, we assigned a judgment of unclear risk of detection bias. We noted the blinding of health professionals, if reported.
Incomplete outcome data
Incomplete outcome data essentially included attrition, exclusions, and missing data.
We assigned a judgment of low risk of attrition bias if:
participants included in the analysis were exactly those who were randomised into the trial, if missing outcome data were balanced in terms of numbers across intervention groups with similar reasons for missing data across groups, or if there were no missing outcome data;
for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was not sufficient to have a clinically relevant impact on the intervention effect estimate;
for continuous outcome data, the plausible effect size (standardized mean differences (SMD)) among missing outcomes was not sufficient to have a clinically relevant impact on observed effect size; or
missing data were imputed using appropriate methods.
We assigned a judgment of high risk of attrition bias when:
reasons for missing outcome data were likely to be related to the true outcome, with either an imbalance in numbers or reasons for missing data across intervention groups;
for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was sufficient to induce clinically relevant bias in the intervention effect estimate;
for continuous outcome data, the plausible effect size (SMD) among missing outcomes was sufficient to induce clinically relevant bias in the observed effect size;
an 'as‐treated’ analysis was carried out in cases where there was substantial departure of the intervention received from that assigned at randomisation;
there was a potentially inappropriate application of simple imputation; or
the dropout rate was reported as higher than 20%, since there is evidence that dropout rates higher than 20% are likely to increase bias in treatment estimates (Unnebrink 2001; Wright 2003).
We assigned a judgment of unclear risk of attrition bias when:
there was insufficient reporting of attrition or exclusions, or both, to permit a judgment of low or high risk of bias;
the study reported incomplete outcome data; or
the numbers randomised to intervention and control groups were not clearly reported.
Selective outcome reporting
We assessed the reporting of outcomes as being at low risk of reporting bias if all study outcomes declared in the Methods section were reported in the Results section. We also evaluated whether different reports of the study were available, including protocols, and examined them to ensure there was no suggestion of selective outcome reporting. If no report was given of all outcomes declared in the Methods section, we contacted the study authors for more information, and if we did not receive a response we assigned a judgment of unclear risk of reporting bias. If there was evidence of selective reporting (such as a significant departure from the protocol or key outcomes that were due to be investigated were not reported), we assigned a judgment of high risk of reporting bias.
Other potential threats to validity
If the study was at risk of other sources of bias, we assessed it as being at high risk of other bias. For instance, if it was stopped early due to a data‐dependent process, having a baseline imbalance between the groups, or sources of sponsorship or funding. We assessed the study as being at low risk of other bias if it appeared to be free from such threats to validity. When the risk of bias was unclear from published information, we attempted to contact the study authors for clarification. If this was not forthcoming, we assessed these studies as being at unclear risk of other bias.
Data and analyses
Comparison 1. Parent training versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Crying time at completion (minutes/day) | 3 | 157 | Mean Difference (IV, Random, 95% CI) | ‐113.58 [‐144.19, ‐82.96] |
| 2 Crying time at completion (minutes/day): sensitivity analysis using the fixed‐effect model | 3 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐113.58 [‐144.19, ‐82.96] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dihigo 1998.
| Methods |
Study design: randomised controlled trial of parental counselling to modify the parent‒infant interaction vs empathy and support only Location: USA Setting: recruitment occurred in paediatrician offices in Dallas/Fort Worth Metroplex |
|
| Participants |
Sample size: 14 infants
Number of dropouts/withdrawals: 0 infants
Mean age: intervention group = 6.31 weeks (SD = 2.36), control group = 6.25 weeks (SD = 3.37) Inclusion criteria:
Exclusion criteria: none stated |
|
| Interventions | Intervention group (n = 8): received individualised parental counselling, flow charts, and handouts to teach the parent of the colicky infant how to respond quickly and appropriately to their infant's cues Control group (n = 6): received empathy and support from the researcher Duration of intervention: 6 days | |
| Outcomes |
Primary outcome: crying time. Data were collected in parental diaries.
Secondary outcome: infant sleep, measured by qualitative data recorded in parental diaries. Parents in the intervention group suggested their infants were sleeping more than before. |
|
| Notes |
Study start date: not stated
Study end date: not stated
Declared DOI: none stated
Perceived DOI: none perceived
Funding source: none stated Other comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: study states participants randomly assigned but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: method not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and researcher both aware of interventions received |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: parents of infants recorded crying times in diaries and were aware of intervention received |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no withdrawals or losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: study appears free of selective reporting |
| Other bias | Low risk | Comment: none noted |
Keefe 2006.
| Methods | Study design: randomised clinical trial of REST routine and video vs control (routine care) Location: USA Setting: interventions carried out in the patients' homes in Charleston (SC), Denver (CO) | |
| Participants |
Sample size: 137 infants
Number of dropouts/withdrawals: 16 infants Mean age: 5.1 weeks Inclusion criteria:
Exclusion criteria: none stated |
|
| Interventions |
Intervention group (n = 64): REST programme, which is a home‐based intervention where a nurse provides support for the family. 4 home visits were conducted at weekly intervals and lasted 1 hour. The REST programme consisted of informing the parents to protect infants from exhaustion and overstimulation. To achieve this, parents aimed to synchronize the infant's sleep‐wake cycle and use various holds and positions repetitively. Educational material with videos and worksheets was also provided. Nurses aimed to provide support and reassurance to the parents. Control group (n = 57): received standard routine care, which was not described in the study Duration of intervention: 8 weeks |
|
| Outcomes |
Primary outcome: crying time, measured using the Fussiness Rating Scale, which was completed by parents.
Secondary outcome: parental or family quality of life: parental stress, measured using the Parental Stress Index Short Form (PSI‐SF). There was a reduction in total mean PSI‐SF scores for both the intervention and control groups.
The study also found reduced parental stress on the subscale for parental‒child dysfunctional interaction in the parent group compared to the control (P = 0.04). |
|
| Notes |
Study start date: not stated
Study end date: not stated
Declared DOI: none stated
Perceived DOI: study led by developer of REST technique
Funding source: National Institute of Nursing Research Other comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: authors state only participants randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Comment: not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants aware of interventions received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: participants and personnel aware of interventions received. The data collection team were unaware of the group assignments or the intervention content. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: data from 16 participants lost to follow‐up not included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | Unclear risk | Comment: study led by developer of REST technique |
Parkin 1993.
| Methods | Study design: unblinded, randomised controlled trial of 3 interventions for management of persistent crying in infancy vs general information and reassurance Location: Canada Setting: urban community bordering Toronto | |
| Participants |
Sample size: 45 infants
Number of dropouts/withdrawals: 7 infants Mean age: intervention group 1 = 5.9 weeks (SD = 3.7), intervention group 2 = 7.8 weeks (SD = 2.6), control group = 6.3 weeks (SD = 2.6) Inclusion criteria: infants who met modified Wessel Criteria Exclusion criteria:
|
|
| Interventions |
Intervention group 1* (n = 11): taught management techniques, early response to crying, soothing motion, avoidance of overstimulation, use of pacifier, prophylactic holding, using infant carrier and maintenance of day‒night orientation Intervention group 2* (n = 16): used a sleep‐tight car‐ride simulation device when infant's crying lasted up to 1 hour Control group* (n = 11): parents were provided with general information and reassurance *All 3 groups were provided with general information and offered a home visit from a nurse. Telephone calls and visits after this were also offered. Duration of intervention: 2 weeks |
|
| Outcomes |
Primary outcome: crying time. Mean daily hours of crying decreased in the total study population (n = 38) during a 2‐week period, from 5.7 hours crying per day (SD = 2.4) before the intervention, to an average of 4.3 hours crying per day (SD = 3.7 hours) in the second week of the study (P > 0.01). There was no significant difference between the groups.
Secondary outcome: parental or family quality of life: maternal anxiety, measured using the Speilberg State‐Trait Anxiety Inventory (STAI) at baseline and after study. In all 3 groups the mean pre‐intervention STAI score was 45 (SD = 13) and the mean post‐intervention STAI score was 37 (SD = 11), which represents an 18% improvement overall in maternal anxiety in all groups (P < 0.001).
The study stated there was no significant difference between the 3 groups but did not report a figure with this statement. |
|
| Notes |
Study start date: not stated
Study end date: not stated
Declared DOI: none stated
Perceived DOI: none apparent
Funding source: none stated Other comments: figures for baseline crying times not provided except on graph |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: used a random numbers table |
| Allocation concealment (selection bias) | Low risk | Comment: used sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants aware of intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: researchers aware of intervention received by participants. Participants aware of intervention and recorded crying time in diaries |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 7 infants (5 withdrawn and 2 loss to follow‐up) excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | Low risk | Comment: none |
Saeidi 2013.
| Methods | Study design: randomised controlled trial of kangaroo care vs conventional care Location: Iran Setting: recruitment at Sheikh Children Hospital Clinic but intervention applied at home | |
| Participants |
Sample size: 70 infants
Number of dropouts/withdrawals: 22 infants
Mean age: 7.2 weeks (SD = 2.3)
Inclusion criteria:
Exclusion criteria Infant:
Mother:
|
|
| Interventions | Intervention group (n = 25): parents instructed to provide kangaroo care for 2 hours a day Control group (n = 23): parents asked to "shaking the baby in the crib" (quote). No further clarification was given on this, although the authors do clearly elicit in their discussion that this standard care was in no way comparable to other forms of shaking that the word may infer. So as not to confuse readers, we use the word 'rocking' (or 'rock') in the text. Duration of intervention: 9 days | |
| Outcomes |
Primary outcome: crying time
The difference was statistically significant (P < 0.05). Secondary outcome: infant sleep, measured by parental diaries.
The difference in sleep duration when comparing the kangaroo group to the control group over 7 days of treatment was significant (P = 0.02). No standard deviation values were provided. |
|
| Notes |
Study start date: May 2008
Study end date: May 2009
Declared DOI: none stated
Perceived DOI: none perceived
Funding source: none stated Other comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: states participants were randomised but does not describe how this was done and no response from request from author |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: researcher aware of personnel in each study arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: parents recorded crying times in diaries |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 22 (out of 70) infants dropped out and were excluded from analysis. Reasons for dropout included mothers being unwell, being too busy or "difficulty in regularly completing the diaries" (quote) |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | Low risk | Comment: none apparent |
Salisbury 2012.
| Methods | Study design: randomised controlled trial of a family‐based intervention for infantile colic vs control (standard paediatric care) Location: USA Setting: intervention applied at clinic appointment | |
| Participants |
Sample size: 71 infants
Number of dropouts/withdrawals: 1 infant
Mean age: intervention group = 38.88 weeks (SD = 1.51), control group = 39.16 (SD = 1.32)
Inclusion criteria: Infant:
Parent:
Mother:
Exclusion criteria: not explicitly stated |
|
| Interventions |
Intervention group (n = 30): psycho‐social family treatment, which consisted of 2 clinicians (behavioural paediatrician and mental health clinician) assessing together the causes of and responses to the infant's crying. The mental health clinician focused on reducing stress and self‐criticism. They provided information on normative sleeping, feeding and crying patterns. Methods to manage their stress and the distress of the infant were also provided. This culminated in an individualised family treatment plan provided as written recommendations. Control group (n = 31): standard paediatric care, which involved a brief office visit. Did not provide written recommendations or mental health management Duration of interventions: 6 weeks |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Study start date: not stated
Study end date: not stated
Declared DOI: none stated
Perceived DOI: none perceived
Funding source: supported by Gerber foundation Other comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: parents selected their group assignment from a container in the presence of a research assistant |
| Allocation concealment (selection bias) | Low risk | Comment: parents selected their group assignment from a container |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participant aware of intervention received |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: researcher aware of personnel in study arms |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1 infant dropped out and was not included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | Unclear risk | Comment: supported by the Gerber Foundation |
Taubman 1988.
| Methods | Study design: randomised controlled trial of parent counselling vs elimination of cows' milk or soy milk protein for infantile colic Location: USA Setting: outpatient paediatric appointments | |
| Participants |
Sample size: 21 infants
Number of dropouts/withdrawals: 1 infant
Mean age: The mean age of intervention group infants was 5.4 ± 2.2 weeks, and the mean age of control group infants was 6.5 ± 1.8 weeks.
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group (n = 10): parents received counselling with written instructions to seek why the infant was crying and a request to respond to this. Control group (n = 10): infants received a hydrolysed casein formula and mothers were informed to exclude milk from their infants' diets. After 9 days, parents received counselling and were informed to return to the original diets and original formulas. Duration of intervention: 9 days |
|
| Outcomes |
Primary outcome: crying time
|
|
| Notes |
Study start date: not reported
Study end date: not reported
Declared DOI: not reported
Perceived DOI: none perceived
Funding source: Mead Johnson Nutrition group Other comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: infants assigned by random numbers generator |
| Allocation concealment (selection bias) | Low risk | Comment: states participants assigned by a gastroenterology office co‐ordinator |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: investigator and participants aware of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: parents of participants recorded crying times |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1 infant dropped out and was not included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | Unclear risk | Comment: funded by Mead Johnson Nutrition group |
Van Sleuwen 2006.
| Methods | Study design: randomised controlled trial of behavioural modification (regularity and stimuli reduction) vs behavioural modification and swaddling Location: the Netherlands Setting: intervention carried out at home | |
| Participants |
Sample size: 398 infants
Number of dropouts/withdrawals: 16 infants
Mean age: intervention group 1 = 8 weeks (SD = 2.53), intervention group 2 = 7.9 weeks (SD = 2.52)
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group 1 (n = 204): intervention consisted of parents applying a sequence of sleeping, feeding, positive interactions with their infant, and awake time of the infant in the playpen alone. Parents were informed to look out for signs of tiredness and, if present, to place the infant into bed. Provided with written instructions of this intervention. Intervention group 2 (n = 194): received the same intervention as intervention group 1 and were also taught a swaddling technique and provided with written instructions on this. Duration of intervention: 3 months |
|
| Outcomes |
Primary outcome: crying time. The study reported mainly crying times for the total group.
The study did not measure any of the other primary or secondary outcomes defined by this review. After 4 weeks, a total of 18 pairs (group 1 = 2, group 2 = 16) swapped intervention, so we could not use the data from after 4 weeks. |
|
| Notes |
Study start date: February 2001
Study end date: recruitment to March 2003
Declared DOI: none stated
Perceived DOI: none apparent
Funding source: funded by The Netherlands Organization for Health Research and Development. Frisocare provided hypoallergenic formulas, Weleda Nederland NV provided swaddling cloths Other comments: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: states randomly allocated by telephone through independent computerised centre |
| Allocation concealment (selection bias) | Low risk | Comment: states randomly allocated by telephone through independent computerised centre |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and researchers aware of intervention allocated and received |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: investigators aware of the intervention allocated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: data from 16 withdrawn participants excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | Unclear risk | Comment: funded by The Netherlands Organization for Health Research and Development. Frisocare provided hypoallergenic formula, and Weleda Nederland NV provided the swaddling cloths |
APGAR: appearance, pulse, grimace, activity and respiration. DOI: declaration(s) of interest. REST: regulation, entrainment, structure and touch. SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barr 2009 | Study did not investigate the management of existing colic |
| Cook 2012 | Participants did not have pre‐existing infantile colic before entry into study |
| Lim 2013 | No access to full paper, but excluded on basis of abstract screening: highly unlikely to meet inclusion criteria as not the correct intervention |
| McRury 2010 | Participants did not have pre‐existing infantile colic before entry into study |
| Smith 2004 | No access to full paper, but excluded on basis of abstract screening: highly unlikely to meet inclusion criteria as not the correct intervention |
| St James‐Roberts 2001 | Participants did not have pre‐existing infantile colic before entry into study |
| Taubman 1984 | Not a randomised controlled trial. Control group did not have infantile colic |
| Van den Boom 1994 | Included participants do not satisfy definition of infantile colic |
| Wolke 1994 | Not a randomised controlled trial |
Characteristics of studies awaiting assessment [ordered by study ID]
Cook 2015.
| Methods | Study design: randomised, parallel group, superiority trial Location: Australia Setting: not clear |
| Participants |
Target sample size: 152 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention group: received access to the online 'Cry Baby' program. The online 'Cry Baby' program covers evidence‐based information and strategies on parent self‐care (e.g. taking care of your body, parent sleep, postnatal depression), infant crying (e.g. why crying is normal and healthy, why babies cry, how to cope with crying) and infant sleep (e.g. infant sleep cycles and cues, settling your baby, safe sleep conditions). All content is delivered within the online platform. The program is brief, taking no longer than an hour to complete. The program has inbuilt activities: for example, parents can move objects to make a cot a safe place for a baby to sleep, they can also ‘burst’ bubbles (using the mouse pointer) to bust common myths about baby care. There is a video showing how to wrap (swaddle) a baby for sleep, links to a coping plan for parents who are feeling overwhelmed by persistent infant crying, as well as links to local parenting support services. Control group: received access to the online 'Cry Baby' program and e‐mail prompts. |
| Outcomes |
Primary outcome:
Secondary outcome:
|
| Notes | Other comments: none |
Differences between protocol and review
-
Authors
Jesal Gohil replaced Megan Thomas on the review team and is lead author of the review.
-
As with a number of protocols published at the same time as this one by our group (i.e. Probiotics to prevent infantile colic (Banks 2016), Dietary modifications for infantile colic (Savino 2014b)), it became apparent very quickly that our primary outcome did not match the published literature. Consequently, the team made the decision that the primary reported outcome was an appropriate clinical parameter as so few studies reported reduction in cases of colic as a dichotomous outcome, so this was replaced by 'Crying time at completion of study period'.
We reworded the outcomes of 'Reduction in the duration of crying' and 'Reduction in frequency of crying episodes per 24 hours' to the more neutral definitions of 'Change in duration of crying' and 'Frequency of crying episodes per 24 hours', to reflect the fact that we are assessing the variable rather than a deterioration in the variable.
-
We planned to translate studies published in languages other than English, but this did not occur as we did not identify any such studies.
-
'Summary of findings' tables (beneath Data synthesis)
We updated the GRADE criteria in line with current guidance, to refer to 'certainty', instead of 'quality' (e.g. high certainty, moderate certainty, low certainty, very low certainty).
We planned to include all outcomes in our 'Summary of findings' table but decided to present only primary outcomes as these are the most clinically relevant.
Contributions of authors
Jesal Gohil extracted data from trials, carried out and interpreted the analysis and led the write‐up of the results. Morris Gordon co‐wrote the review and supported data extraction and analysis and is guarantor of the review. Shel Banks completed searches, data extraction and analysis.
Sources of support
Internal sources
-
Blackpool Teaching Hospitals NHS Foundation Trust, UK.
Blackpool Teaching Hospitals is the employer of two of the review authors: Morris Gordon, Families division, and Shel SC Banks, Project Manager in infant feeding in the families division who had a 12‐month sabbatical as a Research Assistant to complete this review.
The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of Blackpool Teaching Hospitals NHS Foundation Trust or Department of Health.
-
NIHR, UK.
NIHR Cochrane Programme grant was obtained by MG in October 2017 and, whilst not primarily supporting this review, this grant gives some funded time for Cochrane works for MG
External sources
None, Other.
Declarations of interest
MG has received unrestricted travel grants in the last three years from Ferring Pharmaceuticals, Tillots Pharma AG, BioGaia and Synergy Pharma to attend meetings to present results of previous works. They have had no input or involvement in any aspect of the review process during this or previous systematic reviews carried out by MG. MG declares that whilst Ferring and BioGaia produce products that can be used for colic, they were not involved in any of the studies in the scope of this review. MG been involved as an author on other Cochrane Reviews, including 'Bowel preparation for paediatric colonoscopy' (Gordon 2012) and 'Probiotics for maintenance of remission in ulcerative colitis' (Naidoo 2011). MG is a paediatrician with an interest in gastroenterology. This involves seeing patients referred with infantile colic and managing them in line with current accepted evidence‐based practice. MG is also an NIHR Cochrane programme grant holder in the UK.
Shel Banks (SB) is being paid as a Research Assistant for this review from Blackpool Teaching Hospitals NHS Foundation Trust. SB is Chair of the Local Infant Feeding Information Board (LIFIB) — providing syntheses of evidence‐based information on infant feeding topics for health professionals. SB works as a Consultant for LIFIB and the Sudden Unexpected Death of a Child Prevention Team, and until March 2016, this post was funded by money from the local authority via Breastfeeding Network and SB was paid by the hour. SB also works as an Internationally Board Certified Lactation Consultant in private practice. SB declares that neither she personally nor any of the entities she represents take funding of any kind from any commercial interests in infant feeding or early years and that she works completely within the professional code of ethics as an Internationally Board Certified Lactation Consultant.
Jesal Gohil (JG) has no declarations of interest. He is currently a core medical trainee.
New
References
References to studies included in this review
Dihigo 1998 {published data only}
- Dihigo SK. New strategies for the treatment of colic: modifying the parent/infant interaction. Journal of Pediatric Health Care 1998;12(5):256‐62. [PUBMED: 9987256] [DOI] [PubMed] [Google Scholar]
Keefe 2006 {published data only}
- Keefe MR, Barbosa GA, Froese‐Fretz A, Kotzer AM, Lobo M. An intervention program for families with irritable infants. MCN. The American Journal of Maternal Child Nursing 2005;30(4):230‐6. [PUBMED: 16000966] [DOI] [PubMed] [Google Scholar]
- Keefe MR, Kajrlsen KA, Lobo ML, Kotzer AM, Dudley WN. Reducing parenting stress in families with irritable infants. Nursing Research 2006;55(3):198‐205. [PUBMED: 16708044] [DOI] [PubMed] [Google Scholar]
- Keefe MR, Lobo ML, Froese‐Fretz A, Kotzer AM, Barbosa GA, Dudley WN. Effectiveness of an intervention for colic. Clinical Pediatrics 2006;45(2):123‐33. [DOI: 10.1177/000992280604500203; PUBMED: 16528432] [DOI] [PubMed] [Google Scholar]
- Keefe MR, Lobo ML, Kotzer AM, Froese‐Fretz A, Barbosa G, Smith SL. Overview: family focused interventions for irritable infants: issues and outcomes. Communicating Nursing Research 2004;37:24. [Google Scholar]
Parkin 1993 {published data only}
- Parkin PC, Schwartz CJ, Manuel BA. Randomized controlled trial of three interventions in the management of persistent crying of infancy. Pediatrics 1993;92(2):197‐201. [PUBMED: 8337017] [PubMed] [Google Scholar]
Saeidi 2013 {published data only}
- Saeidi R, Abadi MZL, Saeidi A, Robatsangi MG. The effectiveness of mother infant interaction on infantile colic. Iranian Journal of Neonatology 2014;4(4):34‐8. [DOI: 10.22038/IJN.2013.2090] [DOI] [Google Scholar]
Salisbury 2012 {published data only}
- Salisbury AL, High P, Twomey JE, Dickstein S, Chapman H, Liu J, et al. A randomized control trial of integrated care for families managing infant colic. Infant Mental Health Journal 2012;33(2):110‐22. [DOI: 10.1002/imhj.20340; PUBMED: 28520096] [DOI] [PubMed] [Google Scholar]
Taubman 1988 {published data only}
- Taubman B. Parental counseling compared with elimination of cow's milk or soy milk protein for the treatment of infant colic syndrome: a randomized trial. Pediatrics 1988;81(6):756‐61. [PUBMED: 3285312] [PubMed] [Google Scholar]
Van Sleuwen 2006 {published data only}
- Sleuwen BE, L'hoir MP, Engelberts AC, Busschers WB, Westers P, Blom MA, et al. Comparison of behavior modification with and without swaddling as interventions for excessive crying. Journal of Pediatrics 2006;149(4):512‐7. [DOI: 10.1016/j.jpeds.2006.06.068; PUBMED: 17011324] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barr 2009 {published data only}
- Barr RG, Barr M, Fujiwara T, Conway J, Catherine N, Brant R. Do educational materials change knowledge and behaviour about crying and shaken baby syndrome? A randomized controlled trial. CMAJ : Canadian Medical Association Journal 2009;180(7):727‐33. [DOI: 10.1503/cmaj.081419; PMC2659818; PUBMED: 19255065] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cook 2012 {published data only}
- Cook F, Bayer J, Le HN, Mensah F, Cann W, Hiscock H. Baby Business: a randomised controlled trial of a universal parenting program that aims to prevent early infant sleep and cry problems and associated parental depression. BMC Pediatrics 2012;12:13. [DOI: 10.1186/1471-2431-12-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2013 {published data only}
- Lim SK. A Behavioral Intervention for Mothers of Colicky Infants [PhD thesis]. Houston (TX): The University of Health Science Center at Houston, 2013. [search.proquest.com/docview/1456034744] [Google Scholar]
McRury 2010 {published data only}
- McRury JM, Zolotor AJ. A randomized, controlled trial of a behavioral intervention to reduce crying among infants. Journal of the American Board of Family Medicine 2010;23(3):315‐22. [DOI: 10.3122/jabfm.2010.03.090142; PUBMED: 20453177] [DOI] [PubMed] [Google Scholar]
Smith 2004 {published data only}
- Smith SL. Family focused interventions for irritable infants: issues and outcomes. The impact of an intervention on sleep states in irritable infants. Communicating Nursing Research 2004;31:44. [Google Scholar]
St James‐Roberts 2001 {published data only}
- James‐Roberts I, Sleep J, Morris S, Owen C, Gillham P. Use of a behavioural programme in the first 3 months to prevent infant crying and sleeping problems. Journal of Paediatrics and Child Health 2001;37(3):289‐97. [DOI: 10.1046/j.1440-1754.2001.00699.x; PUBMED: 11468047] [DOI] [PubMed] [Google Scholar]
Taubman 1984 {published data only}
- Taubman B. Clinical trial of the treatment of colic by modification of parent‐infant interaction. Pediatrics 1984;74(6):998‐1003. [PUBMED: 6390331] [PubMed] [Google Scholar]
Van den Boom 1994 {published data only}
- Boom DC. The influence of temperament and mothering on attachment and exploration: an experimental manipulation of sensitive responsiveness among lower‐class mothers with irritable infants. Child Development 1994;65(5):1457‐77. [DOI: 10.2307/1131511] [DOI] [PubMed] [Google Scholar]
Wolke 1994 {published data only}
- Wolke D, Gray P, Meyer R. Excessive infant crying: a controlled study of mothers helping mothers. Pediatrics 1994;94(3):322‐32. [PUBMED: 8065857] [PubMed] [Google Scholar]
References to studies awaiting assessment
Cook 2015 {published data only}
- Cook F, Seymour M, Giallo R, Cann W, Nicholson JM, Green J, et al. Comparison of methods for recruiting and engaging parents in online interventions: study protocol for the Cry Baby infant sleep and settling program. BMC Pediatrics 2015;15:174. [DOI: 10.1186/s12887-015-0502-9; 26556032; ACTRN12613001098729; PMC4640160] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ali 2012
- Ali AM. Helicobacter pylori and infantile colic. Archives of Pediatrics & Adolescent Medicine 2012;166(7):648‐50. [DOI: 10.1001/archpediatrics.2011.1241; PUBMED: 22751879] [DOI] [PubMed] [Google Scholar]
Arslanoglu 2012
- Arslanoglu S, Moro GE, Boehm G, Weinz F, Stahl B, Bertino E. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. Journal of Biological Regulators and Homeostatic Agents 2012;26(3 Suppl):49‐59. [PUBMED: 23158515] [PubMed] [Google Scholar]
Axelsson 1986
- Axelsson I, Jakobsson I, Lindberg T, Benediktsson B. Bovine beta‐lactoglobulin in human milk. A longitudinal study during the whole lactation period. Acta Paediatrica 1986;75(5):702‐7. [PUBMED: 3564937] [DOI] [PubMed] [Google Scholar]
Banks 2016
- Banks SSC, Thomas MR, Gordon M, Wallace C, Akobeng AK. Probiotics to prevent infantile colic. Cochrane Database of Systematic Reviews 2016, Issue 12. [DOI: 10.1002/14651858.CD012473] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barr 1991
- Barr RG, McMullan SJ, Spiess H, Leduc DG, Yaremko J, Barfield R, et al. Carrying as colic "therapy": a randomized controlled trial. Pediatrics 1991;87(5):623‐30. [PUBMED: 2020506] [PubMed] [Google Scholar]
Bryanton 2013
- Bryanton J, Beck CT, Montelpare W. Postnatal parental education for optimizing infant general health and parent‐infant relationships. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD004068.pub4; PUBMED: 24284872] [DOI] [PubMed] [Google Scholar]
Campbell 1989
- Campbell JP. Dietary treatment of infant colic: a double‐blind study. British Journal of General Practice 1989;39(318):11‐4. [PMC1711543; PUBMED: 2553940] [PMC free article] [PubMed] [Google Scholar]
Campbell 2000
- Campbell M, Grimshaw J, Steen N. Sample size calculations for cluster randomised trials. Changing professional practice in Europe Group (EU BIOMED II Concerted Action). Journal of Health Services Research & Policy 2000;5(1):12‐6. [PUBMED: 10787581] [DOI] [PubMed] [Google Scholar]
Chandran 2014
- Chandran A, Zhu J, Paul IM, Kjerulff KH. Infant colic in relation to maternal social support, partner involvement in caring for newborn, and happiness of the mother partner relationship. Journal of Pediatric Gastroenterology and Nutrition 2014;59(2 Suppl):S51. [journals.lww.com/jpgn/Documents/NASPGHAN%202014%20abstracts.pdf] [Google Scholar]
Clifford 2002
- Clifford TJ, Campbell MK, Speechley KN, Gorodzinsky F. Infant colic: empirical evidence of the absence of an association with source of early infant nutrition. Archives of Pediatrics & Adolescent Medicine 2002;156(11):1123‐8. [PUBMED: 12413341] [DOI] [PubMed] [Google Scholar]
De Weerth 2013
- Weerth C, Fuentes S, Puylaert P, Vos WM. Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics 2013;131(2):e550‐8. [DOI: 10.1542/peds.2012-1449; PUBMED: 23319531] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP,Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dobson 2012
- Dobson D, Lucassen PL, Miller JJ, Vlieger AM, Prescott P, Lewith G. Manipulative therapies for infantile colic. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD004796.pub2; PUBMED: 23235617] [DOI] [PubMed] [Google Scholar]
Dupont 2010
- Dupont C, Rivero M, Grillon C, Belaroussi N, Kalindjian A, Marin V. Alpha‐lactalbumin‐enriched and probiotic‐supplemented infant formula in infants with colic: growth and gastrointestinal tolerance. European Journal of Clinical Nutrition 2010;64(7):765‐7. [DOI: 10.1038/ejcn.2010.81; PUBMED: 20517331] [DOI] [PubMed] [Google Scholar]
Estep 2000
- Estep DC, Kulczycki A Jr. Treatment of infant colic with amino acid‐based infant formula: a preliminary study. Acta Paediatrica 2000;89(1):22‐7. [PUBMED: 10677052] [DOI] [PubMed] [Google Scholar]
Freedman 2009
- Freedman SB, Al‐Harthy N, Thull‐Freedman J. The crying infant: diagnostic testing and frequency of serious underlying disease. Pediatrics 2009;123(3):841‐8. [DOI: 10.1542/peds.2008-0113; PUBMED: 19255012] [DOI] [PubMed] [Google Scholar]
Furlong 2012
- Furlong M, McGilloway S, Bywater T, Hutchings J, Smith SM, Donnelly M. Behavioural and cognitive‐behavioural group‐based parenting programmes for early‐onset conduct problems in children aged 3 to 12 years. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008225.pub2; PUBMED: 22336837] [DOI] [PubMed] [Google Scholar]
Garrison 2000
- Garrison MM, Christakis DA. A systematic review of treatments for infant colic. Pediatrics 2000;106(1 Pt 2):184‐90. [PUBMED: 10888690] [PubMed] [Google Scholar]
Gordon 2011
- Gordon M, Findley R. Educational interventions to improve handover in health care: a systematic review. Medical Education 2011;45(11):1081‐9. [DOI: 10.1111/j.1365-2923.2011.04049.x; PUBMED: 21933243] [DOI] [PubMed] [Google Scholar]
Gordon 2012
- Gordon M, Harper V, Thomas AG, Akobeng AK. Bowel Preparation for Paediatric Colonoscopy. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009976] [DOI] [Google Scholar]
Gordon 2013
- Gordon M. Non‐technical skills training to enhance patient safety. Clinical Teacher 2013;10(3):170‐5. [DOI: 10.1111/j.1743-498X.2012.00640.x; PUBMED: 23656679] [DOI] [PubMed] [Google Scholar]
Gordon 2018
- Gordon M, Biagioli E, Sorrenti M, Lingua C, Moja L, Banks SS, et al. Dietary modifications for infantile colic. Cochrane Database of Systematic Reviews 2018, Issue 10. [DOI: 10.1002/14651858.CD011029.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 30 December 2015. Hamilton (ON): McMaster University (developed by Evidence Prime).
Gupta 2007
- Gupta SK. Update on infantile colic and management options. Current Opinion in Investigational Drugs (London, England : 2000) 2007;8(11):921‐6. [PUBMED: 17979025] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendation. BMJ 2008;336(7650):924‐6. [DOI: 10.1136/bmj.39489.470347.AD; PMC2335261; PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haji 2013
- Haji F, Morin MP, Parker K. Rethinking programme evaluation in health professions education: beyond 'did it work?'. Medical Education 2013;47(4):342‐51. [DOI: 10.1111/medu.12091; PUBMED: 23488754] [DOI] [PubMed] [Google Scholar]
Hall 2012
- Hall B, Chesters J, Robinson A. Infantile colic: a systematic review of medical and conventional therapies. Journal of Paediatrics and Child Health 2012;48(2):128‐37. [DOI: 10.1111/j.1440-1754.2011.02061.x; PUBMED: 21470331] [DOI] [PubMed] [Google Scholar]
Harb 2015
Heine 2013
- Heine RG. Cow's‐milk allergy and lactose malabsorption in infants with colic. Journal of Pediatric Gastroenterology and Nutrition 2013;57(Supplement 1):25‐7. [DOI: 10.1097/01.mpg.0000441930.13307.9b] [DOI] [Google Scholar]
Heine 2014
- Heine RG, Hill DJ. Infantile colic and food allergy. In: Metcalfe DD, Sampson HA, Simon RA, Lack G editor(s). Food Allergy: Adverse Reactions to Foods and Food Additives. 5th Edition. Chichester (UK): John Wiley & Sons Ltd, 2014:171‐81. [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hill 1995
- Hill DJ, Hudson IL, Sheffield LJ, Shelton MJ, Menahem S, Hosking CS. A low allergen diet is a significant intervention in infantile colic: results of a community‐based study. Journal of Allergy and Clinical Immunology 1995;96(6 Pt 1):886‐92. [PUBMED: 8543745] [DOI] [PubMed] [Google Scholar]
Hill 2000
- Hill DJ, Hosking CS. Infantile colic and food hypersensitivity. Journal of Pediatric Gastroenterology and Nutrition 2000;30(1 Suppl):67‐76. [DOI: 10.1097/00005176-200001001-00011; PUBMED: 10634302] [DOI] [PubMed] [Google Scholar]
Hill 2005
- Hill DJ, Roy N, Heine RG, Hosking CS, Francis DE, Brown J, et al. Effect of a low‐allergen maternal diet on colic among breastfed infants: a randomized, controlled trial. Pediatrics 2005;116(5):e709‐15. [DOI: 10.1542/peds.2005-0147; PUBMED: 16263986] [DOI] [PubMed] [Google Scholar]
Hiscock 2014
- Hiscock H, Cook F, Bayer J, Le HN, Mensah F, Cann W, et al. Preventing early infant sleep and crying problems and postnatal depression: a randomized trial. Pediatrics 2014;133(2):e346‐54. [DOI: 10.1542/peds.2013-1886; PUBMED: 24394682] [DOI] [PubMed] [Google Scholar]
Huhtala 2000
- Huhtala V, Lehtonen L, Heinonen R, Korvenranta H. Infant massage compared with crib vibrator in the treatment of colicky infants. Peditatrics 2000;105(6):e84. [PUBMED: 10835097] [DOI] [PubMed] [Google Scholar]
Hyams 1989
- Hyams JS, Geertsma MA, Etienne NL, Treem WR. Colonic hydrogen production in infants with colic. Journal of Pediatrics 1989;115(4):592‐4. [PUBMED: 2795353] [DOI] [PubMed] [Google Scholar]
Hyman 2006
- Hyman PE, Milla PJ, Benninga MA, Davidson GP, Fleisher DF, Taminiau J. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology 2006;130(5):1519‐26. [DOI: 10.1053/j.gastro.2005.11.065; PUBMED: 16678565] [DOI] [PubMed] [Google Scholar]
Iacono 1991
- Iacono G, Carroccio A, Montalto G, Cavataio F, Bragion E, Lorello D, et al. Severe infantile colic and food intolerance: a long‐term prospective study. Journal of Pediatric Gastroenterology and Nutrition 1991;12(3):332‐5. [PUBMED: 2072224] [DOI] [PubMed] [Google Scholar]
Iacono 2005
- Iacono G, Merolla R, D'Amico D, Bonci E, Cavataio F, Prima L, et al. Gastrointestinal symptoms in infancy: a population‐based prospective study. Digestive and Liver Disease 2005;37(6):432‐8. [DOI: 10.1016/j.dld.2005.01.009; PUBMED: 15893282] [DOI] [PubMed] [Google Scholar]
Iacovou 2012
- Iacovou M, Ralston RA, Muir J, Walker KZ, Truby H. Dietary management of infantile colic: a systematic review. Maternal and Child Health Journal 2012;16(6):1319‐31. [DOI: 10.1007/s10995-011-0842-5; PUBMED: 21710185] [DOI] [PubMed] [Google Scholar]
Indiro 2014
- Indiro F, Mauro A, Riezzo G, Civardi E, Intini C, Corvaglia L, et al. Prophylactic use of a probiotic in the prevention of colic, regurgitation, and functional constipation: a randomized clinical trial. JAMA Pediatrics 2014;168(3):228‐33. [DOI: 10.1001/jamapediatrics.2013.4367; PUBMED: 24424513] [DOI] [PubMed] [Google Scholar]
Infante 2011
- Infante D, Segarra O, Luyer BL. Dietary treatment of colic caused by excess gas in infants: biochemical evidence. World Journal of Gastroenterology 2011;17(16):2104‐8. [DOI: 10.3748/wjg.v17.i16.2104; PMC3084395; PUBMED: 21547129] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakobsson 1983
- Jakobsson I, Lindberg T. Cow’s milk proteins cause infantile colic in breast‐fed infants: a double‐blind crossover study. Pediatrics 1983;71(2):268‐71. [PUBMED: 6823433] [PubMed] [Google Scholar]
Jakobsson 2000
- Jakobsson I, Lothe L, Ley D, Borschel MW. Effectiveness of casein hydrolysate feedings in infants with colic. Acta Paediatrica 2000;89(1):18‐21. [PUBMED: 10677051] [DOI] [PubMed] [Google Scholar]
Karp 2003
- Karp H. The Happiest Baby on the Block. New York: Bantam Dell, 2003. [Google Scholar]
Landgren 2010a
- Landgren K, Hallström I. Parents' experience of living with a baby with infantile colic ‐ a phenomenological hermeneutic study. Scandinavian Journal of Caring Sciences 2011;25(2):317‐24. [DOI: 10.1111/j.1471-6712.2010.00829.x; PUBMED: 20723153] [DOI] [PubMed] [Google Scholar]
Landgren 2010b
- Landgren K, Kvorning N, Hallström I. Acupuncture reduces crying in infants with infantile colic: a randomised, controlled, blind clinical study. Acupuncture in Medicine 2010;28(4):174‐9. [DOI: 10.1136/aim.2010.002394; PMC3002757; PUBMED: 20959312] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Levy 2015
- Levy RL, Tilburg MA, Langer S, Romano J, Mancl LA, Feld SI. Parent‐only intervention reduces symptoms and disability in abdominal pain patients. Gastroenterology 2016;150(4 Suppl 1):S100. [DOI: 10.1016/S0016-5085(16)30444-9] [DOI] [Google Scholar]
Lindberg 1999
- Lindberg T. Infantile colic and small intestinal function: a nutritional problem?. Acta Paediatrica. Supplement 1999;88(430):58‐60. [PUBMED: 10569224] [DOI] [PubMed] [Google Scholar]
Lothe 1982
- Lothe L, Lindberg T, Jakobsson I. Cow's milk formula as a cause of infantile colic: a double‐blind study. Pediatrics 1982;70(1):7‐10. [PUBMED: 7088636] [PubMed] [Google Scholar]
Lothe 1989
- Lothe L, Lindberg T. Cow's milk whey protein elicits symptoms of infantile colic in colicky formula‐fed infants: a double‐blind crossover study. Pediatrics 1989;83(2):262‐6. [PUBMED: 2913556] [PubMed] [Google Scholar]
Lucas 1998
- Lucas A, James‐Roberts I. Crying, fussing and colic behaviour in breast‐ and bottle‐fed infants. Early Human Development 1998;53(1):9‐18. [PUBMED: 10193923] [DOI] [PubMed] [Google Scholar]
Lucassen 2000
- Lucassen PL, Assendelft WJ, Gubbels JW, Eijk JT, Douwes AC. Infantile colic: crying time reduction with a whey hydrolysate: a double‐blind, randomized, placebo‐controlled trial. Pediatrics 2000;106(6):1349‐54. [PUBMED: 11099588] [DOI] [PubMed] [Google Scholar]
Markestad 1997
- Markestad T. Use of sucrose as a treatment for infant colic. Archives of Disease in Childhood 1997;76(4):356‐8. [DOI: 10.1136/adc.76.4.356] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenzie 1991
- McKenzie S. Troublesome crying in infants: effect of advice to reduce stimulation. Archives of Disease in Childhood 1991;66(12):1416‐20. [ PMC1793390; PUBMED: 1776889] [DOI] [PMC free article] [PubMed] [Google Scholar]
Merras‐Salmio 2013
- Merras‐Salmio L, Pelkonen AS, Kolho KL, Kuitunen M, Mäkelä MJ. Cows' milk‐associated gastrointestinal symptoms evaluated using the double‐blind, placebo‐controlled food challenge. Journal of Pediatric Gastroenterology and Nutrition 2013;57(3):281‐6. [DOI: 10.1097/MPG.0b013e3182993fe0; PUBMED: 23974059] [DOI] [PubMed] [Google Scholar]
Metcalf 1994
- Metcalf TJ, Irons TG, Sher LD, Young PC. Simethicone in the treatment of infant colic: a randomized, placebo‐controlled, multicenter trial. Pediatrics 1994;94(1):29‐34. [PUBMED: 8008533] [PubMed] [Google Scholar]
Miller 1990
- Miller JJ, Brand JC, McVeagh P. Breath hydrogen excretion in infants with colic. Archives of Disease in Childhood 1990;65(2):248. [PMC1792219; PUBMED: 2317079] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264‐9. [PUBMED: 19622511] [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore DJ, Robb TA, Davidson GP. Breath hydrogen response to milk containing lactose in colicky and noncolicky infants. Journal of Pediatrics 1988;113(6):979‐84. [PUBMED: 3193321] [DOI] [PubMed] [Google Scholar]
Mostafa 2008
- Mostafa R. Rome III: the functional gastrointestinal disorders, third edition, 2006. World Journal of Gastroenterology 2008;14(13):2124‐5. [DOI: 10.3748/wjg.14.2124; PMC2701540] [DOI] [Google Scholar]
Naidoo 2011
- Naidoo K, Gordon M, Fagbemi AO, Thomas AG, Akobeng AK. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD007443.pub2] [DOI] [PubMed] [Google Scholar]
NICE CKS 2014
- NICE Clinical Knowledge Summary. Colic ‐ infantile. cks.nice.org.uk/colic‐infantile (accessed 30 December 2015).
Norcini 2011
- Norcini J, Anderson B, Bollela V, Burch V, Costa MJ, Duvivier R, et al. Criteria for good assessment: consensus statement and recommendations from the Ottawa 2010 Conference. Medical Teacher 2011;33(3):206‐14. [DOI: 10.3109/0142159X.2011.551559; PUBMED: 21345060] [DOI] [PubMed] [Google Scholar]
Nvivo 2015 [Computer program]
- QSR International Pty Ltd. Nvivo 11 for Windows. Version 11. Doncaster (AU): QSR International Pty Ltd, 2015.
Olafdottir 2001
- Olafdottir E, Forshei S, Fluge G, Markestad T. Randomised controlled trial of infantile colic treated with chiropractic spinal manipulation. Archives of Disease in Childhood 2001;84(2):138‐41. [PMC1718650; PUBMED: 11159288] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oozeer 2013
- Oozeer R, Limpt K, Ludwig T, Amor KB, Martin R, Wind RD, et al. Intestinal microbiology in early life: specific prebiotics can have similar functionalities as human‐milk oligosaccharides. American Journal of Clinical Nutrition 2013;98(2):561S‐71S. [DOI: 10.3945/ajcn.112.038893; PUBMED: 23824728] [DOI] [PubMed] [Google Scholar]
Oshikoya 2009
- Oshikoya KA, Senbanjo IO, Njokanma OF. Self‐medication for infants with colic in Lagos, Nigeria. BMC Pediatrics 2009;9(9):1‐8. [DOI: 10.1186/1471-2431-9-9; PMC2645392; PUBMED: 19193235] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78‐84. [PUBMED: 1530753] [DOI] [PubMed] [Google Scholar]
Perry 2011
- Perry R, Hunt K, Ernst E. Nutritional supplements and other complementary medicines for infantile colic: a systematic review. Pediatrics 2011;127(4):720‐33. [DOI: 10.1542/peds.2010-2098; PUBMED: 21444591] [DOI] [PubMed] [Google Scholar]
Pitkin 1999
- Pitkin RM, Branagan MA, Burmeister LF. Accuracy of data in abstracts of published research articles. JAMA 1999;281(12):1110‐1. [PUBMED: 10188662] [DOI] [PubMed] [Google Scholar]
Reijneveld 2001
- Reijneveld SA, Brugman E, Hirasing RA. Excessive infant crying: the impact of varying definitions. Pediatrics 2001;108(4):893‐7. [PUBMED: 11581441] [DOI] [PubMed] [Google Scholar]
Reinthal 2008
- Reinthal M, Andersson S, Gustafsson M, Plos K, Lund I, Lundeberg T, et al. Effects of minimal acupuncture in children with infantile colic ‐ a prospective, quasi‐randomised single blind controlled trial. Acupuncture in Medicine 2008;26(3):171‐82. [PUBMED: 18818563] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romanello 2013
- Romanello S, Spiri D, Marcuzzi E, Zanin A, Boizeau P, Riviere S, et al. Association between childhood migraine and history of infantile colic. JAMA 2013;309(15):1607‐12. [DOI: 10.1001/jama.2013.747; PUBMED: 23592105] [DOI] [PubMed] [Google Scholar]
Rosen 2007
- Rosen LD, Bukutu C, Le C, Shamseer L, Vohra S. Complementary, holistic, and integrative medicine. Pediatrics in Review 2007;28(10):381‐5. [DOI: 10.1542/pir.28-10-381; PUBMED: 17908860] [DOI] [PubMed] [Google Scholar]
Saps 2011
- Saps M, Lu P, Bonilla S. Cow's‐milk allergy is a risk factor for the development of FGIDs in children. Journal of Pediatric Gastroenterology and Nutrition 2011;52(2):166‐9. [DOI: 10.1097/MPG.0b013e3181e85b55; PUBMED: 20975580] [DOI] [PubMed] [Google Scholar]
Savino 2001
- Savino F, Cresi F, Silvestro L, Oggero R. Use of an amino acid formula in the treatment of colicky breastfed infants. Acta Paediatrica 2001;90(3):359‐60. [PUBMED: 11332183] [PubMed] [Google Scholar]
Savino 2002
- Savino F, Brondello C, Cresi F, Oggero R, Silvestro L. Cimetropium bromide in the treatment of crisis in infantile colic. Journal of Pediatric Gastroenterology and Nutrition 2002;34(4):417‐9. [PUBMED: 11930101] [DOI] [PubMed] [Google Scholar]
Savino 2005
- Savino F, Castagno E, Bretto R, Brondello C, Palumeri E, Oggero R. A prospective 10‐year study on children who had severe infantile colic. Acta Paediatrica. Supplement 2005;94(449):129‐32. [DOI: 10.1080/08035320510043691; PUBMED: 16214780] [DOI] [PubMed] [Google Scholar]
Savino 2006
- Savino F, Palumeri E, Castagno E, Cresi F, Dalmasso P, Cavallo F, et al. Reduction of crying episodes owing to infantile colic: a randomized controlled study on the efficacy of a new infant formula. European Journal of Clinical Nutrition 2006;60(11):1304‐10. [DOI: 10.1038/sj.ejcn.1602457; PUBMED: 16736065] [DOI] [PubMed] [Google Scholar]
Savino 2007
- Savino F. Focus on infantile colic. Acta Paediatrica 2007;96(9):1259‐64. [DOI: 10.1111/j.1651-2227.2007.00428.x; PUBMED: 17718777] [DOI] [PubMed] [Google Scholar]
Savino 2010
- Savino F, Tarasco V. New treatments for infant colic. Current Opinion in Pediatrics 2010;22(6):791‐7. [DOI: 10.1097/MOP.0b013e32833fac24; PUBMED: 20859207] [DOI] [PubMed] [Google Scholar]
Savino 2012
- Savino F, Tarasco V, Lingua C, Moja L, Ricceri F. Pain‐relieving agents for infant colic. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009999] [DOI] [PMC free article] [PubMed] [Google Scholar]
Savino 2014a
- Savino F, Tarasco V, Sorrenti M, Lingua C, Moja L, Gordon M, et al. Dietary modifications for infantile colic. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD011029] [DOI] [PMC free article] [PubMed] [Google Scholar]
Savino 2014b
- Savino F, Tarasco V, Sorrenti M, Lingua C, Moja L, Gordon M, et al. Dietary modifications for infantile colic. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD011029] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schach 2002
- Schach B, Haight M. Colic and food allergy in the breastfed infant: is it possible for an exclusively breastfed infant to suffer from food allergy?. Journal of Human Lactation 2002;18(1):50‐2. [DOI: 10.1177/089033440201800108; PUBMED: 11845737] [DOI] [PubMed] [Google Scholar]
Schmitt 1987
- Schmitt BD. Seven deadly sins of childhood: advising parents about difficult developmental phases. Child Abuse & Neglect 1987;11(3):421‐32. [PUBMED: 3479226] [DOI] [PubMed] [Google Scholar]
Shannon 1921
- Shannon WR. Colic in breast‐fed infants as a result of sensitization to foods in the mother's diet. Archives Paediatrica 1921;38:756‐61. [Google Scholar]
Skjeie 2013
- Skjeie H, Skonnord T, Fetveit A, Brekke M. Acupuncture for infantile colic: a blinding‐validated, randomized controlled multicentre trial in general practice. Scandinavian Journal of Primary Health Care 2013;31(4):190‐6. [DOI: 10.3109/02813432.2013.862915; DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
St James‐Roberts 1991
- James‐Roberts I, Halil T. Infant crying patterns in the first year: normal community and clinical findings. Journal of Child Psychology and Psychiatry, and Allied Disciplines 1991;32(6):951‐68. [PUBMED: 1744198] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Thomas 2010
- Thomas DW, Greer FR, American Academy of Pediatrics Committee on Nutrition, American Academy of Pediatrics Section on Gastroenterology, Hepatology, Nutrition. Probiotics and prebiotics in pediatrics. Pediatrics 2010;126(6):1217‐31. [DOI: 10.1542/peds.2010-2548; PUBMED: 21115585] [DOI] [PubMed] [Google Scholar]
Unnebrink 2001
- Unnebrink K, Windeler J. Intention‐to‐treat: methods for dealing with missing values in clinical trials of progressively deteriorating diseases. Statistics in Medicine 2001;20(24):3931‐46. [PUBMED: 11782044] [DOI] [PubMed] [Google Scholar]
UpToDate 2016
- Turner TL, Palamountain S. Infantile colic: management and outcome. www.uptodate.com/contents/infantile‐colic‐management‐and‐outcome (accessed 30 December 2015).
UpToDate Patient Information Tips
- UpToDate. Tips for reducing colic. www.uptodate.com/contents/image?imageKey=PI/69458&topicKey=PI%2F15897&utdPopup=true (accessed 30 December 2015).
Walter 2000
- Walter SD. Choice of effect measure for epidemiological data. Journal of Clinical Epidemiology 2000;53(9):931‐9. [PUBMED: 11004419] [DOI] [PubMed] [Google Scholar]
Wessel 1954
- Wessel MA, Cobb JC, Jackson EB, Harris GS Jr, Detwiler AC. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics 1954;14(5):421‐35. [PUBMED: 13214956] [PubMed] [Google Scholar]
Wolfe 1994
- Wolfe D, Gray P, Meyer R. Excessive infant crying: a controlled study of mothers helping mothers. Pediatrics 1994;94(3):322‐32. [PUBMED: 8065857] [PubMed] [Google Scholar]
Wright 2003
- Wright CC, Sim J. Intention‐to‐treat approach to data from randomized controlled trials: a sensitivity analysis. Journal of Clinical Epidemiology 2003;56(9):833‐42. [PUBMED: 14505767] [DOI] [PubMed] [Google Scholar]
Yusuf 1991
- Yusuf S, Wittes J, Probstfield K, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991;266(1):93‐8. [PUBMED: 2046134] [PubMed] [Google Scholar]
Zwi 2011
- Zwi M, Jones, H, Thorgaard C, York A, Dennis JA. Parent training interventions for Attention Deficit Hyperactivity Disorder (ADHD) in children aged 5 to 18 years. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD003018.pub3; PUBMED: 22161373] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Thomas 2016
- Thomas MR, Gordon M, Banks SS, Wallace C. Parent training programmes for managing infantile colic. Cochrane Database of Systematic Reviews 2016, Issue 12. [DOI: 10.1002/14651858.CD012459] [DOI] [PMC free article] [PubMed] [Google Scholar]


