Abstract
Methamphetamine (METH) increases metabolic neuronal activity in the mesolimbic dopamine (DA) system and mediates the reinforcing effect. To explore the underlying mechanism of acupuncture intervention in reducing METH-induced behaviors, we investigated the effect of acupuncture on locomotor activity, ultrasonic vocalizations, extracellular DA release in the nucleus accumbens (NAcs) using fast-scan cyclic voltammetry and alterations of brain temperature (an indicator of local brain metabolic activity) produced by METH administration. When acupuncture was applied to HT7, but not TE4, both locomotor activity and 50-kHz ultrasonic vocalizations were suppressed in METH-treated rats. Acupuncture at HT7 attenuated the enhancement of electrically stimulated DA release in the NAc of METH-treated rats. Systemic injection of METH produced a sustained increase in NAc temperature, which was reversed by the DA D1 receptor antagonist SCH 23390 or acupuncture at HT7. Acupuncture inhibition of METH-induced NAc temperature was prevented by pre-treatment with a group II metabotropic glutamate receptors (mGluR2/3) antagonist EGLU into the NAc or mimicked by injection of an mGluR2/3 agonist DCG-IV into the NAc. These results suggest that acupuncture reduces extracellular DA release and metabolic neuronal activity in the NAc through activation of mGluR2/3 and suppresses METH-induced affective states and locomotor behavior.
Keywords: acupuncture, brain temperature, dopamine, locomotor activity, mGluR2/3, ultrasonic vocalizations
INTRODUCTION
Methamphetamine (METH) is a powerful psychostimulant with potent addictive and neurotoxic properties. While METH use is a serious public health problem presenting a growing drug problem in the world (UNODC 2011), there are no approved pharmacological treatments. METH enhances dopaminergic transmission in the mesolimbic system, projecting from the ventral tegmental area (VTA) to the nucleus accumbens (NAcs) (Fleckenstein et al. 2007). Acute or chronic exposure to METH increases extracellular dopamine (DA) levels in the dorsal and ventral striatum (Clausing & Bowyer 1999; Jang et al. 2016) via direct blockade of DA uptake at the vesicular monoamine transporter-2 and reversal of the plasmalemma DA transporter (Fleckenstein et al. 2007; Guillot & Miller 2009). These contribute to the rewarding effects (Vollm et al. 2004), enhanced locomotor activity (Jang et al. 2016) and behavioral sensitization (Yang et al. 2006) of METH.
Drug addiction is characterized by drug-induced positive affective states, followed by negative affective states associated with withdrawal. The affective states provide crucial sources of motivation that trigger drug craving and use (Koob 1996). METH is reported to influence both positive and negative affective states in humans (Parrott et al. 2011). Ultrasonic vocalizations (USVs) in rodents have been employed for assessment of affective states in preclinical models of drug abuse. Rats emit USVs of two distinct classes: low-frequency USVs (called 22-kHz USVs) and high-frequency USVs (called 50-kHz USVs), reflecting negative and positive affective states, respectively. The 22-kHz USVs are emitted under conditions of aversive stimuli (e.g. electrical footshock and predators), chronic pain and drug withdrawal, while the 50-kHz USVs are elicited by rewarding stimuli including exposure to euphorigenic drugs of abuse, food intake and sexual behaviors (Portfors 2007; Barker, Simmons & West 2015). In a previous study, increased numbers of 50-kHz USVs were observed during METH self-administration and cue-induced reinstatement in METH-administering rats (Mahler et al. 2013). The 50-kHz USVs have also been shown to be positively correlated with extracellular DA levels in the mesolimbic system. In support of this, the number of 50-kHz USVs is increased by DA agonists (i.e. amphetamine) or electrical stimulation of the mesolimbic DA system and lowered by treatment of D1 or D2 receptor antagonist (Thompson, Leonard & Brudzynski 2006; Burgdorf et al. 2007).
Brain temperature has been used frequently to monitor metabolic activity in specific brain regions in response to pharmacological or physiological stimuli (Kiyatkin 2010). Previous studies have suggested that an increase of local brain temperature is associated with increased neural metabolism. METH induces hyperthermia in the brain, especially NAc, of rodents (Brown, Wise & Kiyatkin 2003; Kiyatkin 2013), which is closely correlated with enhanced dopamiergic activity and drug-seeking behaviors (Kiyatkin 2005).
While acupuncture has been used to treat a variety of diseases and symptoms for thousands of years, the use of acupuncture for drug addiction began with an incidental discovery in 1972 by Wen and Cheung, who reported that opium addicts receiving electroacupuncture as postsurgical analgesia experienced relief from withdrawal symptoms (Wen & Cheung 1973). Over the last four decades, public and scientific interest is increasing in acupuncture as an approach for the treatment of drug addiction around the world (Cui, Wu & Li 2013). For example, in public health, an acupuncture protocol known as NADA (National Acupuncture Detoxification Association) was originally developed to treat drug withdrawal and is increasingly recognized as an effective intervention in the treatment of patients with addictions (Carter & Olshan-Perlmutter 2014). We and others have shown that acupuncture at HT7 (Shenmen) points suppresses addictive behaviors associated with abused drugs. Acupuncture at HT7 significantly attenuated cocaine-induced, alcohol-induced or morphine-induced DA release in the NAc (Yoon et al. 2004; Kim et al. 2005; Jin et al. 2017), cocaine-induced locomotor activity (Kim et al. 2013), morphine-induced behavioral sensitization and c-Fos expression in the dorsal and ventral striatum (Lee et al. 2010), alcohol-taking (Yang et al. 2010; Kang et al. 2017) or morphine-taking behaviors (Yoon et al. 2010; Lee et al. 2014) and cocaine-seeking behavior (Jin et al. 2017). The HT7 effects are blocked by severing ulnar nerve or by local anesthesia and mediated by activation of peripheral sensory afferents, such as Pacinian and Meissener corpuscles. The peripheral signals from HT7 are conveyed via large A fibers within ulnar nerve and spinal dorsal column somatosensory pathway in order to inhibit the reinforcing effects of drugs of abuse in the mesolimbic dopaminergic system (Kim et al. 2013; Chang et al. 2017). We also proved that the HT7 effects on drug-induced behaviors are point specific, since needling at control acupoints such as TE4 (Yangji) or LI5 (Yangxi), located 3–5 mm apart from HT7 acupoints, is ineffective in reducing drug-induced behaviors (Lee et al. 2002; Kim et al. 2013; Chang et al. 2017). On the other hand, group II metabotropic glutamate receptors (mGluR2/3), mostly located on presynaptic axon terminals, have been implicated as a therapeutic target for addiction, because activation of mGluR2/3 decreases drug-seeking behaviors by reducing DA release in the NAc (Karasawa, Yoshimizu & Chaki 2006; Crawford, Roberts & Beveridge 2013). In previous studies, acupuncture suppresses drug-induced DA release in the NAc (Jin et al. 2017) or an intrastriatal injection of an mGluR2/3 antagonist blocks acupuncture effects on the symptoms of Parkinson’s disease in rats (Jia et al. 2017). Thus, we speculate that mGluR2/3 may be involved in acupuncture inhibition of METH-induced behaviors.
The effect of acupuncture on METH-induced behaviors and the underlying mechanisms are unclear. The present study examined the effect of acupuncture on locomotor activity, 50-kHz USVs, extracellular DA release and brain temperature in the NAc in acute METH-treated rats. We also explored the involvement of mGluR2/3 in the acupuncture effects on brain temperature.
MATERIAL AND METHODS
Animals and ethic statement
Male Sprague–Dawley rats (Daehan Animal, Seoul, Korea) weighing 270–300 g were housed in groups of 2–3 animals per cage on a 12-hour light and dark cycle and received ad libitum food and water. Experimental procedures were approved by the Institutional Animal Care and Use Committee at the Daegu Haany University (ethics approval references number: DHU2014–072) and conducted in accordance with National Institutes of Health guidelines for the care and use of laboratory animals.
Chemicals
Methamphetamine hydrochloride (0.5 mg/kg; Sigma-Aldrich, St. Louis, MO, USA); SCH 23390 (a DA D1 receptor antagonist, 0.2 mg/kg; Sigma-Aldrich); (2S,1’R, 2’R,3’R)-2-(2,3-dicarboxycyclopropyl)glycine [DCG-IV; a group II metabotropic glutamate receptors (mGluR2/3) agonist, 1 nmol/μl per side; Tocris Bioscience, Avonmouth, Bristol, UK]; (s)-alpha-ethylglutamic acid (EGLU; an mGluR2/3 antagonist, 5 nmol/μl per side; Tocris Bioscience); radioimmunoprecipitation assay buffer (Cell Signaling Technology, Beverly, MA, USA); horseradish peroxide-conjugated secondary antibody (#sc-2004, Santa Cruz Biotechnology, Inc, Dallas, TX, USA); and anti-mGluR2/3 antibody (#G9790, Sigma-Aldrich). METH was dissolved in saline immediately before injection, and all other chemicals were dissolved in saline and kept at −80°C until use.
Acupuncture treatment
The effects of acupuncture on locomotor activity, USVs in freely moving rats and brain temperature and DA release in the NAc in anesthetized rats were evaluated by applying manual acupuncture, as described previously (Jin et al. 2017). In brief, stainless-steel needles (0.10 mm diameter and 7 mm length; Dongbang Medical Co., Boryung-si, Chungcheongnam-do Korea) were inserted vertically to a depth of 3 mm from surface of skin over HT7 or TE4 and remained intact for 1 minute. The needle was manually rotated during insertion and withdrawal at a frequency of 2 Hz for 2 seconds. The acupuncture treatment was performed immediately after intravenous (i.v.) or intraperitoneal (i.p.) injection of METH (0.5 mg/kg). In locomotor activity experiments, acupuncture was applied bilaterally at acupoints on wrists for 1 minute immediately after METH injection while an assistant lightly restrained the rat without general anesthesia, as performed in our previous experiments (Chang et al. 2017; Jin et al. 2017). HT7 acupoint, located on the transverse crease of the wrist of the forepaw, radial to the tendon of flexor carpi ulnaris muscle in rats, has been used to treat mental and psychiatric disorders in oriental medicine (Stux & Pomeranz 1987) and was used as the main point for the present study, based on our pervious findings (Kim et al. 2013; Jin et al. 2017). To control the possibility of locomotor disturbance by motor impairment in the wrist by acupuncture at HT7, a nearby point at wrist TE4, about 5 mm apart from HT7, was chosen as the corresponding control point to HT7, located at the midpoint of the dorsal crease of wrist (Fig. 1a).
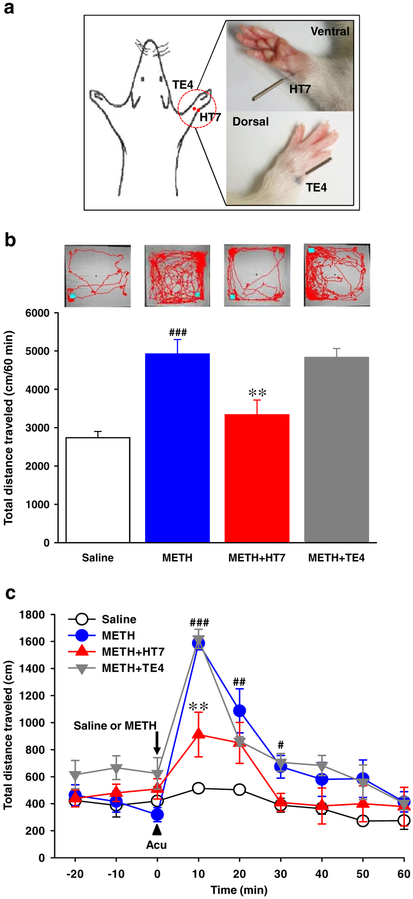
Figure 1.
Effect of acupuncture on methamphetamine (METH)-induced locomotor activity. (a) Location of HT7 or TE4 acupoint in rat. (b) Total distance traveled (expressed in cm) and representative moving trace in open-field box for 1 hour. (c) Time course of distance traveled during every 10 minutes after systemic injection of saline or METH. Data are presented as mean ± standard error of the mean. #P < 0.05, ##P < 0.01, ###P < 0.001 versus saline; **P < 0.01 versus METH. N value is 5–6 per group. Acu = acupuncture; METH = METH-treated rats; METH + HT7 = acupuncture at HT7 in METH-treated rats; METH + TE4 = acupuncture at TE4 in METH-treated rats; saline = injected with saline instead of METH
Locomotor activity
As described previously (Jang et al. 2016; Jin et al. 2017), locomotor activity was measured with a video-tracking system (Ethovision, Nodus Information Technology BV, Wageningen, The Netherlands) that provided automatic measures of traveled distance (cm). In brief, each rat was placed in a square open-field box made of black acrylic (40 × 40 × 45 cm) in a dimly lit room. On test day, the animals were habituated for 1 hour in the open field. After measuring basal activity for 30 minutes, rats received METH (0.5 mg/kg, i.p.) injection and were then immediately given acupuncture treatment for 1 minute, and the locomotor activity was recorded for an additional 1 hour data are expressed as total distance traveled (cm) for 1 hour after METH or during every 10-minute period for 90 minutes.
Recordings of ultrasonic vocalizations
Ultrasonic vocalizations were recorded in customized sound-attenuating chambers that consisted of two boxes to minimize exterior noise (Fig. 2a; inside box: 60 × 42 × 42 cm, outside box: 68 × 50 × 51 cm). A condenser ultrasound microphone (Ultramic250K, Dodotronic, Italy) was positioned at the center of top of inside chambers and suspended ~2.5 cm from the top. The microphone signals were recorded using an Avisoft-RECORDER USGH software (Avisoft Bioacoustics, Glienicke, Germany) with a sampling rate 250 kHz and a 16-bit resolution. For 50-kHz USVs, the signals were band filtered between 38 and 60 kHz and analyzed using Avisoft SASLab Pro (version 4.2, Avisoft Bioacoustics). Spectrograms were generated with a fast Fourier transform length of 512 points and an overlap of 75 percent (FlatTop window, 100 percent frame size). The spectrograms had a frequency resolution of 490 Hz and a time resolution of 0.5 milliseconds. Animals were habituated for at least 30 minutes in the chambers prior to experiments. The USVs were recorded for 20 minutes as baseline and then recorded for 40 minutes after METH (0.5 mg/kg, i.p.) administration. Data are expressed as number of 50-kHz USVs for 40 minutes after METH or during every 10-minute period for 60 minutes.
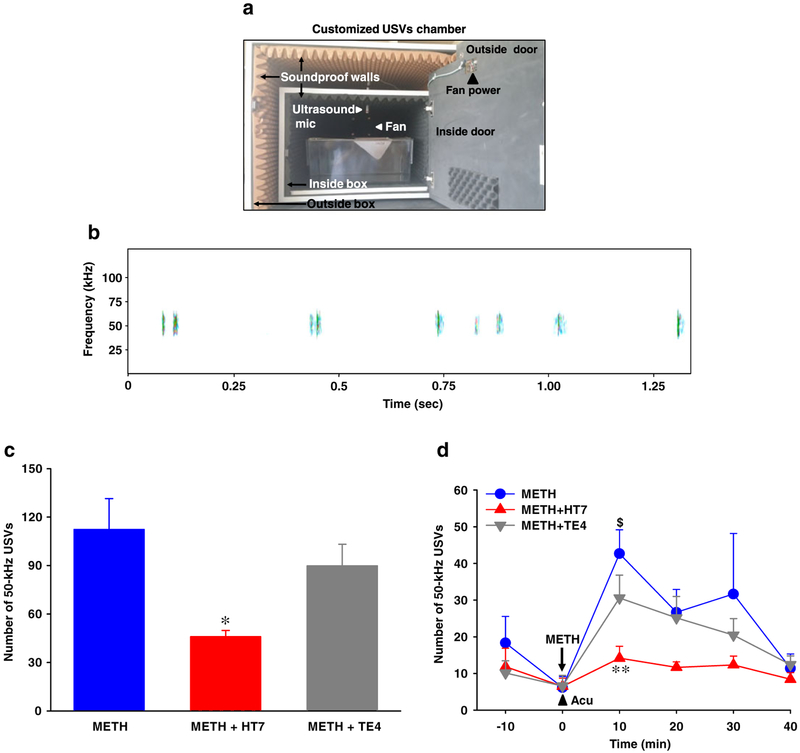
Figure 2.
Effect of acupuncture on production of 50-kHz ultrasonic vocalizations (USVs) in methamphetamine (METH)-administered rats. (a) A customized experimental chamber to measure USVs in freely moving rats. (b) Exemplary spectrograms of 50-kHz USVs. (c) Total number of 50-kHz USVs for 40 minutes and (d) time course of 50-kHz USVs during every 10 minutes after systemic injection of METH. Data are presented as mean ± standard error of the mean. $P < 0.05 versus baseline of METH. *P < 0.05, **P < 0.01 versus METH. N value is 6–7 per group. Acu = acupuncture; METH = METH-treated rats; METH + HT7 = acupuncture at HT7 in METH-treated rats; METH + TE4 = acupuncture at TE4 in METH-treated rats
Measurement of dopamine release using fast-scan cyclic voltammetry
Electrically stimulated DA release in the NAc was measured by fast-scan cyclic voltammetry (FSCV) under urethane anesthesia, as described previously (Addy et al. 2010; Jang et al. 2015; Jang et al. 2016). Briefly, a 7.0-μm diameter carbon fiber was inserted into borosilicate glass capillary tubing (1.2 mm o.d., A-M Systems, Sequim, WA, USA) under negative pressure and subsequently pulled on a pipette puller (Sutter Instrument, Model P-97, Nocato, CA, USA). The carbon fiber electrode (CFE) was then cut under microscopic control with 150–200 μm of bare fiber protruding from the end of the glass micropipette. The tip of the CFE was dipped in cyanoacrylate, allowed to dry and backfilled with 3 M KCl. The electrode potential was linearly scanned with a triangular waveform from −0.4 to +1.3 V and back to −0.4 V versus Ag/AgCl using a scan rate of 400 V/second Cyclic voltammograms were recorded at the CFE every 100 milliseconds (10 Hz) by means of a ChemClamp voltage clamp amplifier (Dagan Corporation, Minneapolis, MN, USA). Voltammetry recordings were performed and analyzed using LabVIEW-based (National Instruments, Austin, TX, USA) customized software (Demon Voltammetry) (Yorgason, Espana & Jones 2011). For in vivo voltammetry recordings of DA signals in the NAc, rats were anesthetized with urethane (1.5 g/kg, i.p.) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). Bipolar, coated stainless steel electrodes and a capillary glass-based CFE were stereotaxically positioned into the medial forebrain bundle (MFB; AP −2.5, ML +1.9 and DV −8.0 to −8.3 from the skull) and the NAc (AP +1.6, ML +0.8~1.9 and DV −6.5 to −8.0 from the skull), respectively, according to a rat brain atlas (Paxinos & Watson 2007). The MFB was stimulated with 60 monophasic pulses (60 Hz, 4-millisecond pulse width) at 2-minute intervals. DA levels in the NAc were monitored for a stabilization period typically lasting about 20–60 minutes. Once the stimulated DA response was stable and did not vary by more than 10 percent for three or four successive collections, baseline measurements were taken for control and drug treatment. After a stable baseline was established for at least 6–8 minutes rats were given an injection of METH (0.5 mg/kg, i.v.) followed by acupuncture treatment. DA signals were recorded in 2-minute intervals up to 60 minutes after METH administration.
Measurement of nucleus accumben temperature
Temperature changes in the NAc were monitored under pentobarbital anesthesia, as performed previously (Jang et al. 2015). Briefly, rats were placed in a stereotaxic apparatus under sodium pentobarbital anesthesia (50 mg/kg, i.p.). The body temperature was warmed at 36.5°C with a thermostatically controlled heating blanket with a rectal probe. To measure the change of local brain temperature in the NAc, the thermocouple needle microprobe (NJ-7013, WPI, Sarasota, LF, USA) was inserted into the NAc (AP +1.7, ML +0.8 and DV −7.0 from the skull). Body temperature was also monitored with a regular thermocouple probe inserted into the rectum. After stable basal temperature in brain was recorded for 20–30 minutes, the animals were given either saline, METH (0.5 mg/kg, i.v.), SCH 23390 (0.2 mg/kg, i.p.) or SCH 23390 together with METH (Fig. 4). In another set of animals, acupuncture was applied immediately after METH injection (Fig. 5). The temperatures were then recorded for a further 30 minutes.
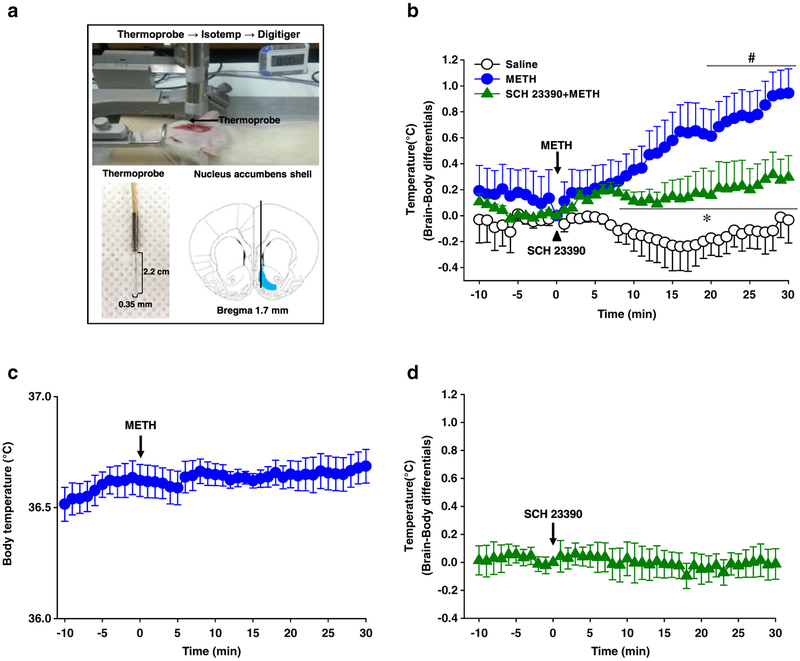
Figure 4.
Blockade of nucleus accumbens (NAc) temperature by D1 receptor antagonist SCH 23390 in methamphetamine (METH)-treated rats. (a) Measurement of NAc temperature using a thermocouple needle microprobe. (b) Effect of pretreatment with SCH 23390 (D1 receptor antagonist, 0.2 mg/kg, i.p.) on METH-induced changes of NAc–body temperature differentials. (c) Effect of METH alone on body temeprature. (d) Effect of SCH 23390 alone on NAc–body temperature differentials. Data are presented as mean ± standard error of the mean. #P < 0.05 versus saline and *P < 0.05 versus METH. N value is 4–6 per group. METH = METH-treated rats; saline = injection of saline, instead of METH; SCH 23390 = SCH 23390 only in normal rats; SCH 23390 + METH = rats were treated with SCH 23390 prior to METH injection
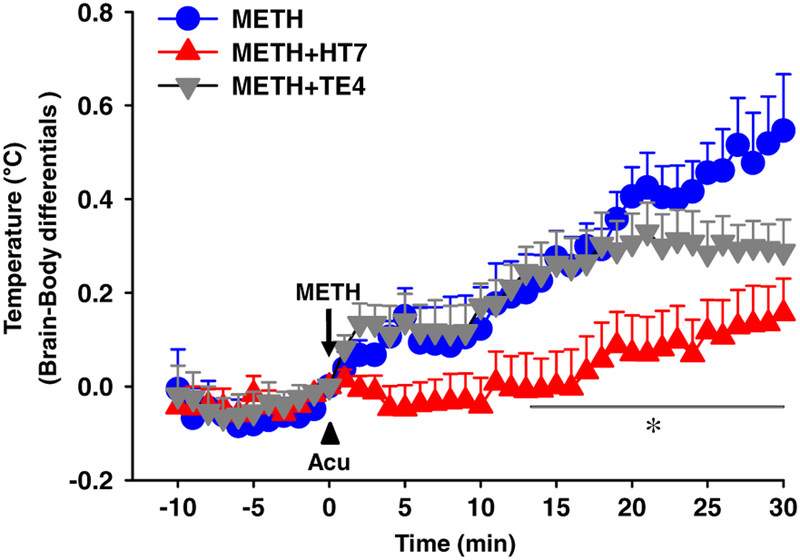
Figure 5.
Effect of acupuncture on methamphetamine (METH)-induced brain temperature in the nucleus accumbens (NAc). Effect of acupuncture on NAc–body temperature differentials in METH-treated rats. Data are presented as mean ± standard error of the mean. *P < 0.05 versus METH. N value is 5–10 per group. Acu = acupuncture; METH = METH-treated rats; METH + HT7 = acupuncture at HT7 in METH-treated rats; METH + TE4 = acupuncture at TE4 in METH-treated rats
Microinjection of an metabotropic glutamate receptor agonist, DCG-IV, or antagonist, EGLU, into the nucleus accumben
Rats were placed in a stereotaxic apparatus, and a Hamilton syringe (Hamilton Company, Reno, NV, USA) was oriented into the NAc (AP +1.7, ML ± 0.8 and DV −7.0 from the skull). DCG-IV (1 nmol/μl per side) or EGLU (5 nmol/μl per side) was slowly infused into the bilateral NAc for 1 minute. For facilitating diffusion of the drug, the injector was left for 30 seconds after completion of microinjection. Basal temperatures were measured for 10 minutes after injection of saline, EGLU or DCG-IV. Temperature changes were recorded for another 30 minutes following METH with or without HT7 acupuncture (Fig. 6).
Figure 6.
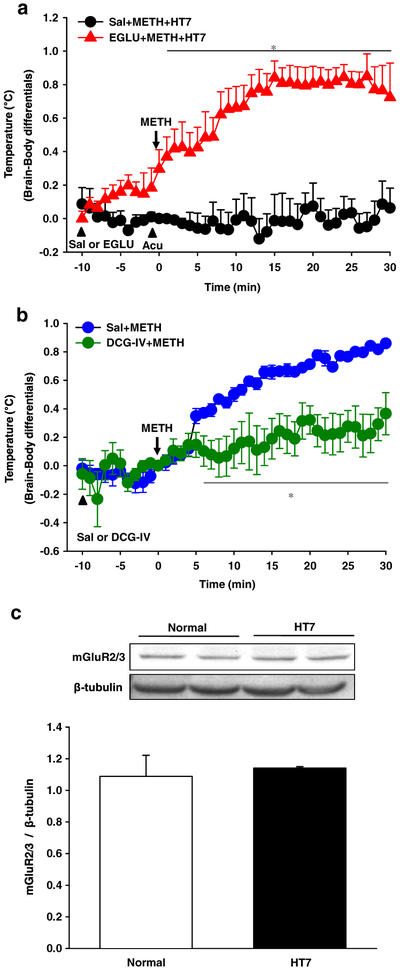
Mediation of mGluR2/3 in inhibitory effect of acupuncture on methamphetamine (METH)-induced nucleus accumbens (NAc)–body temperature differentials. (a) Effect of an mGluR2/3 antagonist EGLU on acupuncture inhibition of NAc–body temperature differentials in METH-treated rats. *P < 0.05 versus Sal + METH + HT7. (b) Effect of an mGluR2/3 agonist DCG-IV on NAc–body temperature differentials in METH-treated rats. *P < 0.05 versus Sal + METH. Sal + METH + HT7, microinjection of saline (Sal) prior to acupuncture at HT7 in METH-treated rats; EGLU + METH + HT7, microinjection of EGLU prior to acupuncture at HT7 in METH-treated rats; Sal + METH, microinjection of Sal in METH-treated rats; DCG-IV + METH, microinjection of DCG-IV in METH-treated rats. (c) Western blot results for expression of mGluR2/3 in the NAc of normal rats or rats given acupuncture at HT7 (HT7). Data are presented as mean ± standard error of the mean. N value is 5–7 per group for brain temperature and 4 per group for Western blot. Acu = acupuncture; DCG4 IV + METH = microinjection of DCG-IV in METH-treated rats; EGLU + METH + HT7 = microinjection of EGLU prior to acupuncture at HT7 in METH-treated rats; Sal + METH = microinjection of Sal in METH-treated rats; Sal + METH + HT7 = microinjection of Sal prior to acupuncture at HT7 in METH-treated rats
Western blotting
Twenty minutes after acupuncture treatment, the rats were sacrificed, and brains were quickly removed and homogenized in a lysis buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1 percent Triton X-100, 2 mM EDTA, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride and a protease inhibitor cocktail. Homogenates were kept on ice for 20 minutes and then centrifuged for 15 minutes at 12 000 rpm at 4°C. Total proteins were fractionated by 10 percent gel electrophoresis and electrophoretically transferred to a polyvinylidene fluoride membrane. The membranes were incubated with primary anti-mGluR2/3 antibody (1:500) overnight at 4°C and with horseradish peroxidase-conjugated secondary antibody (1:1000) and developed using enhanced chemiluminescent detection methods (ECL kit; Amersham Pharmacia Biotech, Buckinghamshire, UK). Densities were measured using ATTO DENSITOGRAPH Software (ATTO Corporation, Tokyo, Japan) and quantified as the ratio to β-tubulin.
Statistical analysis
All data were presented as mean ± standard error of the mean. All data were analyzed using SPSS statistical software (version 16.0; Somers, NY, USA) and compared by one-way or two-way repeated measures analysis of variance (ANOVA) followed by post hoc Tukey test or, when appropriate, t-test. Statistical significance was established at P < 0.05.
RESULTS
Effect of acupuncture on methamphetamine-induced locomotor activity and 50-kHz ultrasonic vocalizations
Systemic injection of METH significantly increased locomotor activity (expressed as total distance traveled in cm) compared with saline injection (Fig. 1b), which lasted up to about 30 minutes with a peak at 10 minutes (Fig. 1c). Manual stimulation of needles inserted into HT7, but not TE4, inhibited the increase of locomotion produced by METH (Fig. 1b & c: F(2, 14)= 13.38, P = 0.001).
To see if acupuncture at HT7 reduces positive affective states following acute injection of METH, the effect of acupuncture on 50-kHz USVs was assessed in METH-treated rats. Acute injection of METH significantly increased the number of calls of 50-kHz USVs at 10 minutes (P < 0.05), compared with baseline, and the levels returned to baseline levels over 40 minutes after injection (Fig. 2c & d), similar to what was seen in the locomotor activity experiment. The METH-enhanced 50-kHz USVs were suppressed, when acupuncture was applied to HT7, but not TE4, immediately after METH administration (Fig. 2c & d; F(2, 16) = 5.776, P = 0.013).
Effect of acupuncture on electrically stimulated dopamine release in the nucleus accumben
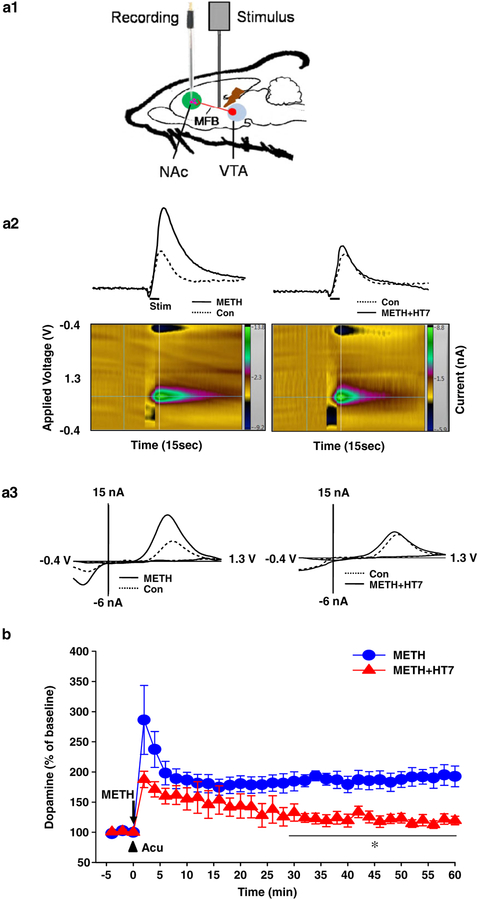
To explore whether the inhibitory effects of acupuncture at HT7 on METH-induced locomotion and USVs were due to inhibition of DA release in the NAc, we evaluated the effect of acupuncture at HT7 on electrically stimulated DA release in the NAc following METH using in vivo FSCV (Fig. 3a1). Intravenous METH administration increased the peak amplitude of electrically stimulated DA release in the NAc by about average 286.29 ± 57.18 percent over baseline (Con) at 2 minutes after METH administration and remained elevated for up to 60 minutes after METH injection (Fig. 3a2 & a3 and Fig. 3b). The METH enhancement of DA release was lowered by about 187.59 ± 13.55 percent in the peak value at 2 minutes after METH injection when acupuncture was applied to HT7 (Fig. 3b; F(1, 9) = 8.807, P = 0.016).
Figure 3.
Effect of acupuncture (Acu) on electrically stimulated dopamine (DA) release in the nucleus accumbens (NAc). (a1) Schematic of fast-scan cyclic voltammetry for recording of NAc DA release. (a2) Representative trace (upper) and pseudo-color plots (lower) triggered by electrical stimulation of the NAc before [control (Con)] and after METH injection (METH) in normal (left) or acupuncture-treated rat (right). (a3) Insets show cyclic volt-ammograms from peaks of representative traces. (b) Effect of acupuncture at HT7 on electrically stimulated DA release in METH-treated rats. Data are presented as mean ± standard error of the mean. METH, METH-treated rats; METH + HT7, acupuncture at HT7 in METH-treated rats. *P < 0.05 versus METH. N value is 5–6 per group. MFB = medial forebrain bundle; Stim = stimulation; VTA = ventral tegmental area
Inhibitory effects of acupuncture on methamphetamine-induced nucleus accumben hyperthermia
Most drugs of abuse increase metabolic neuronal activity accompanied by enhanced brain temperature, especially in mesolimbic dopaminergic system (Kiyatkin 2014). Initially, to examine whether metabolic activity in the NAc is enhanced by METH and the effect is mediated via NAc DA receptors, we monitored the changes of local brain temperature following METH administration and tested the effects of the D1 receptor antagonist SCH 23390 to explore the role of DA in mediating METH-induced NAc hyperthermia. Acute METH (0.5 mg/kg, i.v.) administration showed a sustained increase in NAc–body temperature differentials compared with saline (Fig. 4b: P < 0.05 versus saline), while there was no significant change in body temperature (Fig. 4c: F(8, 287) = 0.216, P = 1.0). An increase of NAc–body temperature differentials by METH was blocked by D1 receptor antagonist SCH 23390 (0.2 mg/kg, i.p.) (Fig. 4b; F(2, 14) = 7.175, P = 0.007; P < 0.05 versus METH). However, SCH 23390 itself did not affect NAc–body temperature differentials compared with baseline (Fig. 4d; F(5, 185) = 0.989, P = 0.989). To test whether the METH-induced hyperthermia in the NAc is reversed by acupuncture, acupuncture was applied to either HT7 or TE4 immediately after METH injection and the NAc temperature was monitored over 30 minutes after injection. Acupuncture at HT7, but not TE4, significantly attenuated an elevation of NAc–body temperature differentials following METH (Fig. 5; F(2, 20) = 5.156, P = 0.01). To measure the involvement of group II metabotropic glutamatergic transmission in the inhibitory effects of acupuncture on METH-induced NAc hyperthermia, we injected an mGluR2/3 antagonist EGLU into the NAc 10 minutes prior to acupuncture at HT7. Pretreatment with mGluR2/3 antagonist EGLU completely blocked the acupuncture effects on METH-induced NAc hyperthermia (Fig. 6a: F(1, 8) = 10.251, P = 0.013), which was mimicked by NAc injection of an mGluR2/3 agonist DCG-IV (Fig. 6b: F(1,9) = 26.320 , P = 0.001). Finally, we further explored whether acupuncture at HT7 causes substantial changes in the expression of mGluR2/3 in the NAc. However, no difference in total mGluR2/3 levels between normal and HT7 acupuncture groups was seen (Fig. 6c: F(1, 7) = 0.21, P = 0.66).
DISCUSSION
The present study showed that acupuncture at HT7 significantly (1) attenuated locomotor activity, (2) reduced the number of 50-kHz USVs in METH-treated rats, (3) decreased electrically stimulated DA release in the NAc of METH-treated rats and (4) attenuated NAc temperature enhanced by METH. In addition, the acupuncture effect on NAc temperature following METH administration was reversed by antagonism of mGlu2/3 activity. Our findings suggest that acupuncture reduces the positive affective states, mesolimbic DA release and metabolic activity in the NAc, in order to generate inhibitory effects on METH-enhanced locomotion, and the effects are mediated, in part, by activation of mGluR2/3 in the NAc.
Consistent with previous studies (Mahler et al. 2013; Jang et al. 2016), acute exposure to METH increased locomotor activity and calls of 50-kHz USVs. Moreover, the patterns of 50-kHz USV responses to METH were largely similar to those seen with locomotor activity. It has been reported that increases of both locomotion and 50-kHz USVs are closely linked with extracellular DA level in the NAc (Knutson, Burgdorf & Panksepp 1999; Jang et al. 2016) or activation of mesolimbic dopaminergic neuronal pathways (Mahler et al. 2013). In support of this, DA depleting lesions in the MFB, in which DA neuronal projections end axons rostrally before reaching the striatum, NAc and prefrontal cortex, abolish emission of 50-kHz USVs in rats (Ciucci et al. 2009). Furthermore, playback of 50-kHz USVs elicits phasic DA release, using FSCV, in the NAc of freely moving rats (Willuhn et al. 2014). In addition, intra-NAc amphetamine injection produces a significant increase in 50-kHz USVs (Thompson et al. 2006). While acupuncture can be effective in treating substance abuse including cocaine, morphine, heroine and alcohol (Lorini et al. 1979; Yoon et al. 2004; Kim et al. 2005; Jin et al. 2017), there are few experimental studies regarding acupuncture treatment of METH addiction. One recent study reported that electroacupuncture significantly reduced METH-induced behavioral sensitization and conditioned place preference by regulating DA release and synthesis in the NAc of chronic METH-exposed mice (Ho et al. 2017). Similar to this study, we showed that acupuncture suppressed METH-induced both locomotor activity and 50-kHz USVs and attenuated DA receptor-related NAc temperature in METH-treated rats. These findings were further supported by our voltammetry experiment for recordings of phasic DA release in the NAc. By using FSCV, we found that METH administration increased stimulated DA release in the NAc of rats and acupuncture at HT7 significantly reduced electrically stimulated DA release in the NAc. This is consistent with our previous studies involving cocaine, morphine, ethanol (Kim et al. 2005; Zhao et al. 2006; Jin et al. 2017) and a recent METH study (Ho et al. 2017) measuring drug-induced DA release in the NAc. Taken together, our findings suggest that acupuncture at HT7 attenuated METH-induced locomotor activity and 50-kHz USVs by reducing NAc DA release.
Psychoactive drugs such as psychostimulants (cocaine or METH) increase hyperthermia, as a typical symptom of acute intoxication, which may underlie the physiological effect of these drugs. In addition, drug-induced changes of brain temperature are important parameters reflecting metabolic brain activity as well as affecting neuronal activity and functioning. These changes enhance the mesolimbic DA system (Lee, Mora & Myers 1985; Kiyatkin 2005). In support of this, previous studies have shown that intravenous injection of cocaine increases NAc temperature and locomotor activity in freely moving rats (Kiyatkin 2014). Moreover, administration of amphetamine increases the extracellular DA levels and simultaneously elevates brain temperature in the caudate/putamen of the same rats (Clausing & Bowyer 1999). In addition, increased brain temperature by cocaine is blocked by DA receptor antagonists (Kiyatkin & Brown 2006). Similarly, in our present study, acute METH administration increased NAc temperature, which was blocked by D1 receptor antagonist SCH23390. Furthermore, acupuncture at HT7 suppressed the METH-induced NAc hyperthermia, indicating HT7 acupuncture can attenuate metabolic activity, by, in part, decreasing dopaminergic activity in the NAc and thus reducing METH-induced behaviors such as locomotor activity and production of 50-kHz USVs.
Dopaminergic neuronal activity can be regulated by glutamatergic neurotransmission, projecting from the prefrontal cortex to the VTA or NAc, via activation of ionotropic and metabotropic glutamate receptors (Tye, Miller & Blaha 2013; Johnson, Mateo & Lovinger 2017). It has been shown that intra-VTA infusion of AMPA enhanced basal NAc DA release (Tye et al. 2013), and pretreatment with AMPA antagonist CNQX blocked flutriafol-induced DA release in the striatum (Faro et al. 2012). In our preliminary experiment, either microinjection of an AMPA receptor antagonist CNQX into the VTA or electrolytic lesion of infralimbic cortex failed to block inhibitory effect of HT7 acupuncture on METH-induced NAc temperature, suggesting a lack of involvement of AMPA receptors (data not shown). On the other hand, group II mGluRs (mGluR2/3), located primarily on presynaptic terminals, modulate the release of neurotransmitters as autoreceptors (Xi et al. 2002; Pehrson & Moghaddam 2010). For example, activation of mGluR2/3 by infusion of agonist LY379268 into the NAc attenuates DA release in the NAc (Karasawa et al. 2006), heroin seeking (Bossert et al. 2006) and cocaine seeking (Peters & Kalivas 2006). The present study also showed that the inhibitory effect of acupuncture on the NAc temperature by METH was blocked by infusion of mGluR2/3 antagonist EGLU into the NAc. In addition, an mGluR2/3 agonist DCG-IV decreased METH-induced temperature in the NAc. Based on these results, acupuncture at HT7 might activate mGluR2/3 in the terminal of the NAc to attenuate the rewarding effect of METH. However, in our Western blot data for mGluR2/3 level in the NAc, no significant change in total mGluR2/3 levels was observed between normal and acupuncture groups, although inhibitory acupuncture effect on NAc temperature was blocked via mGluR2/3 antagonist. We speculate that acupuncture may inhibit METH-induced behaviors via transient activation of mGluR2/3, but not structural changes in the expression level of mGluR2/3. It is a limitation of the present study, and we need to explore the timeline of change in mGluR2/3 expression after stimulation of acupuncture perhaps via phosphorylated mGluR2/3 expression tests in future studies.
In conclusion, acupuncture at HT7 significantly reduced extracellular DA release and metabolic activity in the NAc through activation of mGluR2/3 and thus suppressed METH-induced affective states and locomotor behaviors. These findings provide the mechanisms underlying acupuncture treatment of METH addiction.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1B03935206) to E.Y.J., NRF 2014R1A2A1A11053104 and 2017R1E1A2A01079599 to H.Y.K. and the Korea Institute of Oriental Medicine (KIOM) K18181 to Y.H.R.
Footnotes
Disclosure/Conflict of Interest
The authors declare that they have no competing interests.
References
- Addy NA, Daberkow DP, Ford JN, Garris PA, Wightman RM (2010) Sensitization of rapid dopamine signaling in the nucleus accumbens core and shell after repeated cocaine in rats. Journal of Neurophysiology 104:922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Simmons SJ, West MO (2015) Ultrasonic vocalizations as a measure of affect in preclinical models of drug abuse: a review of current findings. Current Neuropharmacology 13:193–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y (2006) Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology 31:2197–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Wise RA, Kiyatkin EA (2003) Brain hyperthermia is induced by methamphetamine and exacerbated by social interaction. The Journal of Neuroscience 23:3924–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J (2007) Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behavioural Brain Research 182:274–283. [DOI] [PubMed] [Google Scholar]
- Carter K, Olshan-Perlmutter M (2014) NADA protocol: integrative acupuncture in addictions. Journal of Addictions Nursing 25:182–187. quiz 188–189. [DOI] [PubMed] [Google Scholar]
- Chang S, Ryu Y, Gwak YS, Kim NJ, Kim JM, Lee JY, Kim SA, Lee BH, Steffensen SC, Jang EY, Yang CH, Kim HY (2017) Spinal pathways involved in somatosensory inhibition of the psychomotor actions of cocaine. Scientific Reports 7: 5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Ahrens AM, Ma ST, Kane JR, Windham EB, Woodlee MT, Schallert T (2009) Reduction of dopamine synaptic activity: degradation of 50-kHz ultrasonic vocalization in rats. Behavioral Neuroscience 123:328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausing P, Bowyer JF (1999) Time course of brain temperature and caudate/putamen microdialysate levels of amphetamine and dopamine in rats after multiple doses of d-amphetamine. Annals of the New York Academy of Sciences 890:495–504. [DOI] [PubMed] [Google Scholar]
- Crawford JT, Roberts DC, Beveridge TJ (2013) The group II metabotropic glutamate receptor agonist, LY379268, decreases methamphetamine self-administration in rats. Drug and Alcohol Dependence 132:414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui CL, Wu LZ, Li YJ (2013) Acupuncture for the treatment of drug addiction. International Review of Neurobiology 111:235–256. [DOI] [PubMed] [Google Scholar]
- Faro LR, Alfonso M, Maues LA, Duran R (2012) Role of ionotropic glutamatergic receptors and nitric oxide in the effects of flutriafol, a triazole fungicide, on the in vivo striatal dopamine release. The Journal of Toxicological Sciences 37:1135–1142. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR (2007) New insights into the mechanism of action of amphetamines. Annual Review of Pharmacology and Toxicology 47: 681–698. [DOI] [PubMed] [Google Scholar]
- Guillot TS, Miller GW (2009) Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Molecular Neurobiology 39:149–170. [DOI] [PubMed] [Google Scholar]
- Halpin LE, Collins SA, Yamamoto BK (2014) Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sciences 97:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TJ, Lee CW, Lu ZY, Lane HY, Tsai MH, Ho IK, Huang CL, Chiang YC (2017) Effects of electroacupuncture on methamphetamine-induced behavioral changes in mice. Evidence-based Complementary and Alternative Medicine: eCAM 2017:5642708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang EY, Ryu YH, Lee BH, Chang SC, Yeo MJ, Kim SH, Folsom RJ, Schilaty ND, Kim KJ, Yang CH, Steffensen SC, Kim HY (2015) Involvement of reactive oxygen species in cocaine-taking behaviors in rats. Addiction Biology 20:663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang EY, Yang CH, Hedges DM, Kim SP, Lee JY, Ekins TG, Garcia BT, Kim HY, Nelson AC, Kim NJ, Steffensen SC (2016) The role of reactive oxygen species in methamphetamine self-administration and dopamine release in the nucleus accumbens. Addiction Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia YJ, Deng JH, Zhang WZ, Sun ZL, Yang J, Yu Y, Gong XL, Jia J, Wang XM (2017) The role of group II metabotropic glutamate receptors in the striatum in electroacupuncture treatment of Parkinsonian rats. CNS Neuroscience & Therapeutics 23:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Kim MS, Jang EY, Lee JY, Lee JG, Kim HY, Yoon SS, Lee BH, Chang S, Kim JH, Choi KH, Koo H, Gwak YS, Steffensen SC, Ryu YH, Yang CH (2017) Acupuncture reduces relapse to cocaine-seeking behavior via activation of GABA neurons in the ventral tegmental area. Addiction Biology. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Mateo Y, Lovinger DM (2017) Metabotropic glutamate receptor 2 inhibits thalamically-driven glutamate and dopamine release in the dorsal striatum. Neuropharmacology 117:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SY, Kwon OS, Moon JY, Cho SJ, Choi KH, Kim J, Ahn SH, Ryu Y (2017) Mechanical stimulation of the HT7 acupuncture point to reduce ethanol self-administration in rats. Evidence-based Complementary and Alternative Medicine eCAM 2017:6578621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa J, Yoshimizu T, Chaki S (2006) A metabotropic glutamate 2/3 receptor antagonist, MGS0039, increases extracellular dopamine levels in the nucleus accumbens shell. Neuroscience Letters 393:127–130. [DOI] [PubMed] [Google Scholar]
- Kim MR, Kim SJ, Lyu YS, Kim SH, Lee Y, Kim TH, Shim I, Zhao R, Golden GT, Yang CH (2005) Effect of acupuncture on behavioral hyperactivity and dopamine release in the nucleus accumbens in rats sensitized to morphine. Neuroscience Letters 387:17–21. [DOI] [PubMed] [Google Scholar]
- Kim SA, Lee BH, Bae JH, Kim KJ, Steffensen SC, Ryu YH, Leem JW, Yang CH, Kim HY (2013) Peripheral afferent mechanisms underlying acupuncture inhibition of cocaine behavioral effects in rats. PLoS One 8:e81018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA (2005) Brain hyperthermia as physiological and pathological phenomena. Brain Research. Brain Research Reviews 50:27–56. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA (2010) Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front Biosci (Landmark Ed) 15:73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA (2013) The hidden side of drug action: brain temperature changes induced by neuroactive drugs. Psychopharmacology 225:765–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA (2014) State-dependent and environmental modulation of brain hyperthermic effects of psychoactive drugs of abuse. Temperature (Austin) 1:201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL (2006) The role of peripheral and central sodium channels in mediating brain temperature fluctuations induced by intravenous cocaine. Brain Research 1117:38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J (1999) High-frequency ultrasonic vocalizations index conditioned pharmacological reward in rats. Physiology & Behavior 66:639–643. [DOI] [PubMed] [Google Scholar]
- Koob GF (1996) Drug addiction: the yin and yang of hedonic homeostasis. Neuron 16:893–896. [DOI] [PubMed] [Google Scholar]
- Lee B, Shim I, Lee H, Yin CS, Park HK, Yang JS, Hahm DH (2010) Morphine-induced locomotor response and Fos expression in rats are inhibited by acupuncture. Neurological Research 32:107–110. [DOI] [PubMed] [Google Scholar]
- Lee BB, Shim IS, Yang CH, Lee HI, Hahm DH, Lee HJ (2002) Effect of acupuncture (HT7) on acute cocaine-induced locomotor activity and Fos-like immunoreactivity in the brain of the rats. Korean Journal Acupuncture 19:25–33. [Google Scholar]
- Lee BH, Ku JY, Zhao RJ, Kim HY, Yang CH, Gwak YS, Chang SC, Kim NJ, Kim JS, Lee YK, Lee HJ, Lim SC (2014) Acupuncture at HT7 suppresses morphine self-administration at high dose through GABA system. Neuroscience Letters 576:34–39. [DOI] [PubMed] [Google Scholar]
- Lee TF, Mora F, Myers RD (1985) Dopamine and thermoregulation: an evaluation with special reference to dopaminergic pathways. Neuroscience and Biobehavioral Reviews 9:589–598. [DOI] [PubMed] [Google Scholar]
- Lorini G, Fazio L, Cocchi R, Fusari A, Roccia L (1979) Acupuncture as a part of a program of detoxification and weaning from opiates: 25 cases. Minerva Medica 70:3831–3836. [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Feltenstein MW, Cox BM, Ogburn KB, Bachar M, McGonigal JT, Ghee SM, See RE (2013) A rodent “self-report” measure of methamphetamine craving? Rat ultrasonic vocalizations during methamphetamine self-administration, extinction, and reinstatement. Behavioural Brain Research 236:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC, Gibbs A, Scholey AB, King R, Owens K, Swann P, Ogden E, Stough C (2011) MDMA and methamphetamine: some paradoxical negative and positive mood changes in an acute dose laboratory study. Psychopharmacology 215:527–536. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Elsevier: Amsterdam; Boston. [Google Scholar]
- Pehrson AL, Moghaddam B (2010) Impact of metabotropic glutamate 2/3 receptor stimulation on activated dopamine release and locomotion. Psychopharmacology 211:443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW (2006) The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology 186:143–149. [DOI] [PubMed] [Google Scholar]
- Portfors CV (2007) Types and functions of ultrasonic vocalizations in laboratory rats and mice. Journal of the American Association for Laboratory Animal Science: JAALAS 46:28–34. [PubMed] [Google Scholar]
- Stux G, Pomeranz B (1987) Acupuncture. Spinger: Berlin. [Google Scholar]
- Thompson B, Leonard KC, Brudzynski SM (2006) Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behavioural Brain Research 168:64–73. [DOI] [PubMed] [Google Scholar]
- Tye SJ, Miller AD, Blaha CD (2013) Ventral tegmental ionotropic glutamate receptor stimulation of nucleus accumbens tonic dopamine efflux blunts hindbrain-evoked phasic neurotransmission: implications for dopamine dysregulation disorders. Neuroscience 252:337–345. [DOI] [PubMed] [Google Scholar]
- UNODC (2011) World Drug. Report 2011. United Nations Office on Drugs and Crime; pSales No. E.11.XI.10. [Google Scholar]
- Vollm BA, de Araujo IE, Cowen PJ, Rolls ET, Kringelbach ML, Smith KA, Jezzard P, Heal RJ, Matthews PM (2004) Methamphetamine activates reward circuitry in drug naive human subjects. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology 29:1715–1722. [DOI] [PubMed] [Google Scholar]
- Wen HL, Cheung SYC (1973) Treatment of drug addiction by acupuncture and electrical stimulation. American Journal of Acupuncture 1:71–75. [Google Scholar]
- Willuhn I, Tose A, Wanat MJ, Hart AS, Hollon NG, Phillips PE, Schwarting RK, Wohr M (2014) Phasic dopamine release in the nucleus accumbens in response to pro-social 50 kHz ultrasonic vocalizations in rats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 34:10616–10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW (2002) Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. The Journal of Pharmacology and Experimental Therapeutics 300:162–171. [DOI] [PubMed] [Google Scholar]
- Yang CH, Yoon SS, Hansen DM, Wilcox JD, Blumell BR, Park JJ, Steffensen SC (2010) Acupuncture inhibits GABA neuron activity in the ventral tegmental area and reduces ethanol self-administration. Alcoholism, Clinical and Experimental Research 34:2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PP, Huang EY, Yeh GC, Tao PL (2006) Co-administration of dextromethorphan with methamphetamine attenuates methamphetamine-induced rewarding and behavioral sensitization. Journal of Biomedical Science 13:695–702. [DOI] [PubMed] [Google Scholar]
- Yoon SS, Kim H, Choi KH, Lee BH, Lee YK, Lim SC, Choi SH, Hwang M, Kim KJ, Yang CH (2010) Acupuncture suppresses morphine self-administration through the GABA receptors. Brain Research Bulletin 81:625–630. [DOI] [PubMed] [Google Scholar]
- Yoon SS, Kwon YK, Kim MR, Shim I, Kim KJ, Lee MH, Lee YS, Golden GT, Yang CH (2004) Acupuncture-mediated inhibition of ethanol-induced dopamine release in the rat nucleus accumbens through the GABAB receptor. Neuroscience Letters 369:234–238. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Jones SR (2011) Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. Journal of Neuroscience Methods 202:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao RJ, Yoon SS, Lee BH, Kwon YK, Kim KJ, Shim I, Choi KH, Kim MR, Golden GT, Yang CH (2006) Acupuncture normalizes the release of accumbal dopamine during the withdrawal period and after the ethanol challenge in chronic ethanol-treated rats. Neuroscience Letters 395:28–32. [DOI] [PubMed] [Google Scholar]