Abstract
Cognitive dysfunction, including mild cognitive impairment and dementia, is increasingly recognised as an important comorbidity and complication of diabetes that affects an individual’s well-being and diabetes management, and is associated with diabetes treatment-related complications. Recent guidelines therefore recommend screening for cognitive impairment in older individuals with diabetes. In addition, these guidelines suggest that glucose-lowering treatment should be tailored in those diagnosed with cognitive impairment, to reduce the risk of hypoglycaemia and improve treatment adherence. This review gives an overview of cognitive dysfunction in people with diabetes, briefly describing the clinical features of different stages of cognitive dysfunction and their epidemiology. In particular, it addresses essential additional steps that need to be taken to fully implement the emerging guidelines on screening and management of cognitive dysfunction in diabetes into daily practice.
Keywords: Alzheimer’s disease, Cognitive dysfunction, Dementia, Guidelines, Implementation, Mild cognitive impairment, Review, Screening, Type 1 diabetes, Type 2 diabetes
Introduction
Cognitive dysfunction is an important comorbidity of diabetes. This may reflect brain changes as a consequence of diabetes [1] but the co-occurrence of diabetes and cognitive dysfunction clearly also reflects shared risk factors, the most obvious of which is age. Currently, the worldwide prevalence of diabetes in people older than 65 years is 18.8% and the 2017 estimate of the number of people aged 65–99 years living with diabetes around the world was 122.8 million [2]. This is expected to double over the next three decades, primarily due to the increase in the number of older people [2]. For dementia, population trends are similar. The worldwide prevalence of dementia in people older than 60 years is 6–7%, with limited variation between different regions in the world [3]. According to 2015 estimates, there were at that time 46.8 million people living with dementia around the world, with an expected doubling in the next two decades [3]. Data from a large veteran’s registry in the USA showed that, among people with diabetes, the prevalence of dementia and cognitive impairment combined was 13.1% for those aged 65–74 years and 24.2% for those aged 75 years and older [4].
Although both individuals with diabetes and their physicians are increasingly aware of cognitive dysfunction in relation to diabetes, this awareness still lags behind that of other diabetes complications [5]. Patients report that their healthcare providers sometimes have difficulty addressing cognitive dysfunction in relation to diabetes [6]. Professional diabetes societies do recognise this important knowledge gap and increasingly include information on cognitive dysfunction in educational activities. Moreover, because it is clear that particularly the more severe stages of cognitive dysfunction affect many aspects of life, including diabetes management, professional guidelines on medical care in diabetes also increasingly address cognition [7–11]. This is an important development but (as we will argue) additional steps need to be taken before these guidelines can be fully put into practice. In this review, we summarise the different manifestations of cognitive dysfunction in adults with diabetes, both in terms of clinical features and epidemiology. We also address current evidence on the impact of cognitive dysfunction on diabetes management. Finally, we discuss the emerging guidelines and address knowledge gaps and further actions that should be taken for full implementation.
Manifestations of cognitive dysfunction in adults with diabetes
In this review, the term ‘cognitive dysfunction’ in relation to diabetes refers to any deviation of cognitive functioning compared with people without diabetes. Of note, cognitive dysfunction in people with diabetes is not a unitary construct. It is important to distinguish between ‘cognitive impairment’, which refers to dysfunction that is severe enough to be classified as ‘abnormal’ at an individual patient level based on normative cognitive test values, and more subtle forms of dysfunction wherein the mean performance of people with diabetes as a group is lower than that of people without diabetes but does not meet formal criteria for abnormal test scores.
Dementia
Dementia is the most severe of the different stages of cognitive dysfunction, with objective impairment of multiple cognitive domains, by definition affecting activities of daily life. Most of the epidemiological work to date has focused on type 2 diabetes and dementia, with estimates in risk increase ranging from 50% to 100% [12]. A study in 2016 analysed pooled data from 14 studies comprised of over 2.3 million people [13]. This study analysed over 100,000 occurrences of dementia and found a 60% increased risk for all-cause dementia; when limiting the outcome to ‘non-vascular dementia’, mostly defined as clinically diagnosed Alzheimer’s disease, the risk increase was 50%. Interestingly, the magnitude of risk increase for diabetes on vascular dementia was 18% greater in women than in men [13] but it is unclear whether this reflects a true sex difference in the impact of diabetes on dementia risk or whether this is an artefact of selective survival and increased longevity in women.
Epidemiological data on type 1 diabetes and dementia is relatively sparse. This is because type 1 diabetes is much less common than type 2 diabetes and individuals with type 1 diabetes have only recently been living to old age [14]. Hence, late-life cognitive dysfunction and dementia risk is a more recent consideration for those with type 1 diabetes. The largest study to date in type 1 diabetes is a retrospective cohort study of individuals hospitalised for type 1 or type 2 diabetes and risk of dementia [15]. This study examined risk of dementia in over 300,000 people with type 1 diabetes, 1.8 million people with type 2 diabetes and a reference cohort. Those with type 1 diabetes had a 65% increased risk of dementia and those with type 2 diabetes had a 37% increased risk, suggesting that the risk increase is somewhat larger for those with type 1 diabetes. Thus far, no studies have delineated type 1 diabetes and risk of vascular vs non-vascular dementia. Clearly, additional studies are needed but evidence to date suggests that, on a population level, those with either type 1 or type 2 diabetes have a 40–60% increased risk of all-cause dementia.
Mild cognitive impairment
Mild cognitive impairment (MCI) is defined as acquired cognitive complaints with objective abnormal test results in one or more domains on formal cognitive testing. The primary distinction from dementia is that, by definition, in MCI cognitive deficits should not (or only minimally) interfere with instrumental activities of daily living [16]. MCI can be further categorised into memory-impaired (amnestic) MCI vs non-memory-impaired MCI. When compared with individuals without MCI, those with MCI have an increased risk of dementia (meta-analysis: RR 3.3 [16]), although not everyone with MCI will get dementia and, in some people, cognition may even revert back to normal.
Fewer population-based studies have explored the association between type 2 diabetes and increased risk of MCI. A meta-analysis identified two studies including 393 individuals with type 2 diabetes showing a 20% pooled increased risk of MCI [17]. Research also indicates that type 2 diabetes further increases the rate of conversion from MCI to dementia, possibly accelerating the process, though there is heterogeneity in these findings for amnestic MCI vs non-amnestic MCI [18]. To date, no such studies have been carried out in individuals with type 1 diabetes. Given the preliminary data on type 1 diabetes and dementia, one can also expect that those with type 1 diabetes are also at increased risk of MCI. More research is needed on the continuum of cognitive ageing, including progression to MCI, among individuals with type 1 diabetes. Given the decreased life expectancy of individuals with type 1 vs type 2 diabetes, the earlier age of diabetes onset and the higher exposure to acute hypoglycaemia and microvascular complications, it is plausible that the continuum of MCI to dementia may vary according to type of diabetes.
Diabetes-specific cognitive decrements
Type 1 and type 2 diabetes are both associated with subtle so-called cognitive decrements [19]. These decrements are defined as a deviation from normal cognitive functioning but, unlike MCI, this deviation is not severe enough to be classified formally as cognitive impairment [19]. In type 2 diabetes, these decrements usually manifest themselves in a cognitive test performance result on average a one-third to one-half SD lower than in those without diabetes [20]. These subtle decrements may impact all cognitive domains in type 2 diabetes, including memory, processing speed and executive function, and appear to be present at all ages [21], although clearly in most individuals type 2 diabetes has a mid- to late-life onset.
In type 1 diabetes, the subtle cognitive decrements are slightly larger and impact some domains more than others. Systematic reviews posit a one-third to three-quarter SD reduction overall, with effects most pronounced in mental flexibility, general intelligence and psychomotor speed [22]. An earlier age of onset and longer duration are risk factors for worse cognitive performance in the type 1 diabetic population [23]. While type 1 diabetes is mainly diagnosed in childhood or adolescence, it can also develop in mid and late life. It is not known whether individuals with older age of onset of type 1 diabetes exhibit the same pattern of impairment seen in the typical childhood-onset population of type 1 diabetes.
Risk factors for cognitive dysfunction in diabetes
For both type 1 and type 2 diabetes, poor glycaemic control (including glycaemic variability), hypoglycaemic and hyperglycaemic events, age, depression and vascular complications are associated with increased risk of dementia (in type 2 diabetes) and worse cognitive performance (type 1 and type 2 diabetes) [19, 23–26]. Thus, there is heterogeneity in individual risk increase for significant cognitive impairment in the population with diabetes. Of note, there is currently no evidence that intensified glycaemic control has benefit (or harm) for preserving cognitive functioning in people with type 1 [27] or type 2 diabetes [26, 28]. Observational data suggest that some glucose-lowering agents may be associated with lower dementia risk than others, but this needs to be regarded with caution as confounding by indication may be an important issue [29].
Impact of cognitive dysfunction in diabetes
Cognitive dysfunction, most evidently for MCI, and even more so for dementia in more advanced cognitive impairment stages, has a major impact on people’s life. This is clearly not specific to diabetes. However, some aspects of cognitive dysfunction, particularly in relation to an individual’s self-management of disease, are relevant for those with diabetes. Glucose monitoring, having to follow often-complicated medication regimens and keeping those aligned with one’s diet and exercise require planning, oversight and sometimes complex decision making. It is not surprising that people with diabetes and cognitive impairment are more likely to perform these rather demanding tasks less well [30, 31]. Cognitive impairment also predisposes people with diabetes to treatment-related complications, such as acute severe hypo- or hyperglycaemic episodes [31, 32]. Of note, occurrence of severe hypo- or hyperglycaemic episodes also predicts future development of cognitive impairment [24, 33], indicating that the relationship between cognitive impairment and these acute metabolic emergencies is bidirectional. This is one of the reasons why guidelines advise tailoring diabetes therapy to prevent further hypoglycaemic episodes in individuals over the age of 60–65 years [9, 10]. In addition, it has been established that, compared with people with diabetes and intact cognition, those with cognitive impairment are at increased risk of major cardiovascular events and death [32].
Emerging guidelines
In recent years, several societies have put forward guidelines on the management of older individuals with diabetes and cognitive impairment (see Text box ‘Summy of recommendations on cognitive dysfunction in diabetes from recent guidelines’ [7–11, 31]). The recommendations have two main general components: (1) cognitive impairment in individuals with diabetes should be actively sought for, because unrecognised cognitive impairment is associated with adverse health outcomes, and (2) findings should lead to an individualised diabetes management regimen, compatible with the individual’s capabilities, generally with more lenient treatment targets and simplified treatment regimens to improve treatment compliance and reduce treatment-related risks.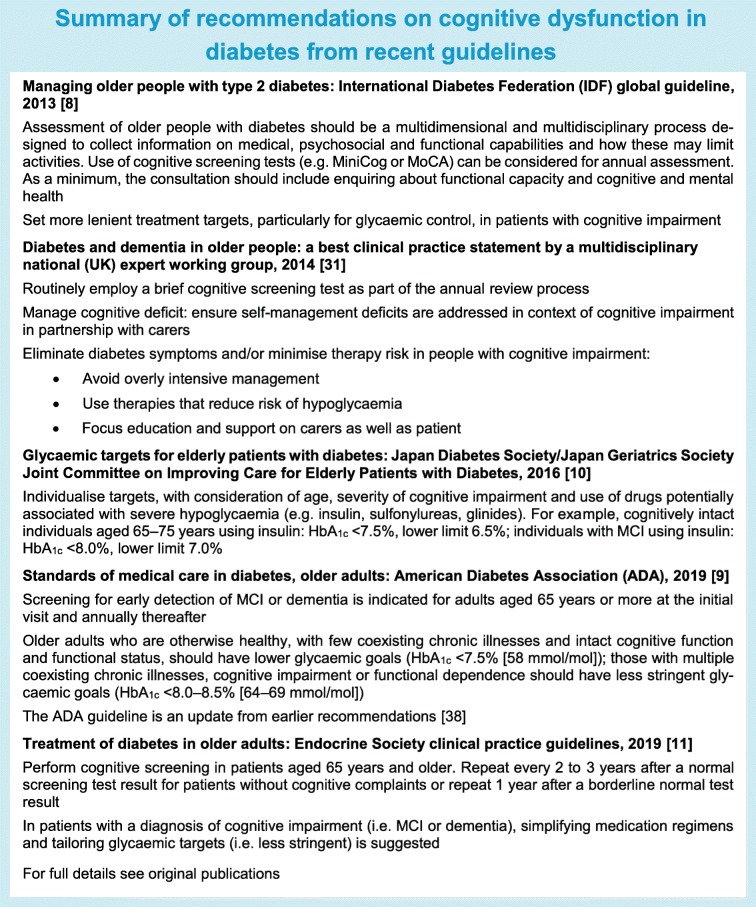
The emergence of this guidance is clearly important, as cognitive dysfunction was not really considered in diabetes management guidelines until up to a decade ago. Yet, from a practical perspective, additional steps will need to be taken to fully integrate these recommendations into daily routine.
What is needed to bring the new guidelines into practice?
In the general population, screening for cognitive impairment is generally not recommended [34], based on the argument that no disease-modifying therapy is currently available to stop or slow down the processes that lead to dementia. Hence, early identification in people without evident complaints has even been suggested to be unethical, because an early diagnosis might be stressful while there is little to offer those that screen positive. The guidelines for people with diabetes now take a different stance, with the argument that early detection may help to avoid diabetes treatment-related risks and improve diabetes management.
Although the recommendation to screen people with diabetes for cognitive impairment seems straightforward, there are still many loose ends (summarised in the Text box ‘Suggested steps for optimal implementation of guidelines’). First, we should determine who to screen. Even brief tests generally take over 10 min to complete [35]. Implementing this for all people with diabetes is clearly labour intensive. The guidelines therefore advise primarily assessing ‘older’ people (i.e. over the age of 60–65 years) [8, 9], because the prior likelihood of detecting unrecognised cognitive impairment clearly increases with age. Yet, more individualised strategies for identifying who to screen, based on risk factor profiles beyond age, might even be more effective.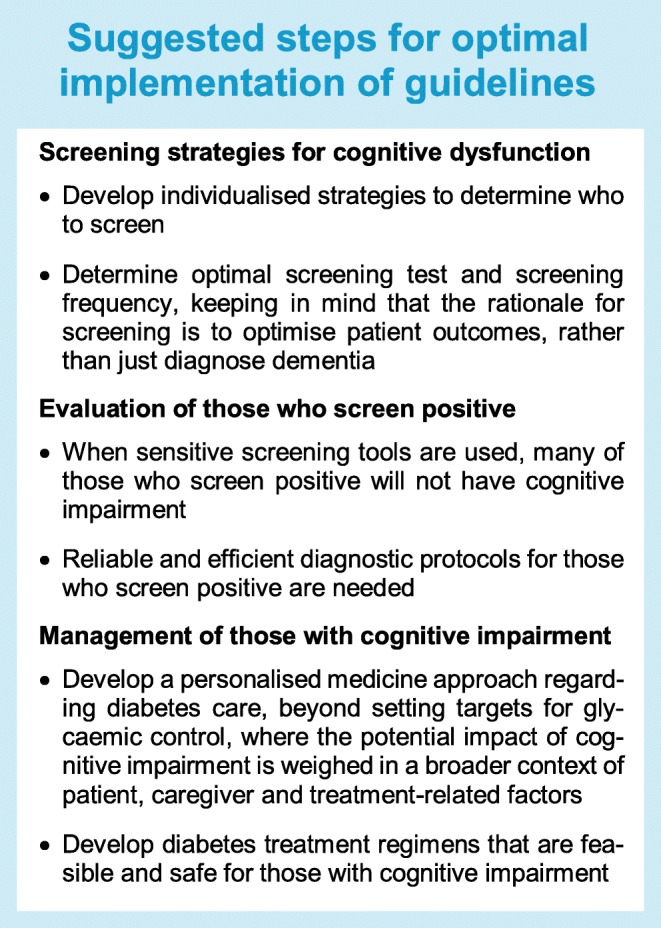
A further issue is the question of which test to use and with which cut-off. The guidelines suggest use of validated tests such as the mini-mental state examination (MMSE) or the Montreal cognitive assessment (MoCA) [8, 9]. Self-administered screening tests could be an efficient alternative [11, 36]. What is not explicitly addressed by the guidelines is whether screening should primarily try to ‘rule in’ or rather ‘rule out’ cognitive impairment. In general, most healthcare screening strategies have a multistep approach where the first step is intended to rule out the condition sought for, often by using a test with a high sensitivity, generally at the cost of specificity (i.e. lower positive predictive value). After a positive screening test, additional tests are used to establish the diagnosis (i.e. rule in). The point is that, while screening tests such as the MMSE (also depending on the cut-off used) have a reasonable sensitivity for dementia, for MCI this is much worse [35]. In a study that validated screening instruments in a population-based sample of individuals with type 2 diabetes, in which most cases identified indeed proved to have MCI, the self-administered ‘test your memory (TYM)’ and ‘self-administered gerocognitive examination (SAGE)’ questionnaires clearly outperformed the MMSE in terms of sensitivity [36]. The choice of screening tests and appropriate cut-offs for widespread and repeated use in older people with type 2 diabetes clearly warrants further evaluation. Of note, because screening aims to avoid poor outcomes of diabetes treatment in relation to cognitive impairment, sensitivity to predict these outcomes should be an essential feature of an optimal screening test.
Another key issue is how to proceed after screening. Where a screen result is negative, a repeat examination is warranted. The most recent version of the ‘Standards of medical care in diabetes’ from the ADA recommends annual assessment [9]. Yet, annual testing has the drawbacks of increased effort and costs. Moreover, most screening tests have not been developed for repeated administration and practice effects may reduce their validity. The question is therefore what screening frequency is best. Because the incidence of dementia in people with diabetes over the age of 60 years varies widely, from below 0.5% per year to over 7%, depending on age and risk factor profile [24], it might be an option to adapt the screening interval according to the patient’s individualised dementia risk.
In those who screen positive, appropriate further diagnostic evaluation is indicated. In our own study in individuals with type 2 diabetes we observed that the predictive value of a positive screening test for a diagnosis of cognitive impairment (formally established at a memory clinic) is modest, below 50% [36]. Many diabetes outpatient clinics may currently not have the expertise to make an initial distinction between true- and false-positive test results. Training will be required to avoid inappropriate referral of large numbers of patients to memory clinics.
Finally, and importantly, recommendations for the management of people with diabetes who have an established diagnosis of cognitive impairment are now predominantly based on expert opinion (see Text box ‘Summary of recommendations on cognitive dysfunction in diabetes from recent guidelines’). Although there is clear logic to these recommendations, there is evidently a need for further studies into optimal management of these individuals. This includes, but is clearly not limited to, determining optimal and safe targets for glycaemic control. With regard to the latter, the question is whether we can expect formal randomised controlled trials on glycaemic targets for people with cognitive impairment, as there may be important ethical and practical barriers to such studies. As an alternative, leveraging of large healthcare databases with longitudinal measures of glycaemic control and exposures to severe hypo- and hyperglycaemic events can be a valuable resource. Examples are to use such data to assess cumulative exposure over time to low or high HbA1c and risk of diagnosed dementia [37] or include causal modelling of HbA1c and vascular complications on dementia risk. Better insight into these factors can support a personalised medicine approach, where risk factors and patients’ abilities and preferences are assessed in an integral fashion to support optimal treatment.
Evidently, support and care for people with diabetes and cognitive impairment extends well beyond medical treatment. There is increasing awareness of the multifaceted impact of cognitive impairment and dementia on those affected. There are important calls for action (e.g. from the WHO) to improve care and support for people with dementia and their carers to live a life with meaning and dignity [39]. This includes efforts to make societies more dementia friendly and also, and this is clearly also relevant to the management of diabetes, to actively engage patients and their carers in policy making and the development of treatment and care approaches that are person-centred, cost-effective, sustainable and affordable and take public health principles and cultural aspects into account [39].
Abbreviations
- MCI
Mild cognitive impairment
- MMSE
Mini-mental state examination
- MoCA
Montreal cognitive assessment
Contribution statement
Both authors were responsible for drafting the article and revising it critically for important intellectual content. Both authors approved the version to be published.
Funding
The research of GJB is supported by Vici Grant 918.16.616 from the Netherlands Organisation for Scientific Research (NWO). He consults for and receives research support from Boehringer Ingelheim. All financial compensation for these services is transferred to his employer, the UMCU Utrecht. The research of RAW is supported by a US grant from the National Institute on Aging, National Institute of Health (NIH), 5R01AG047500-04.
Duality of interest
GJB consults for and receives research support from Boehringer Ingelheim. RAW declares that there is no duality of interest associated with her contribution to this manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14(10):591–604. doi: 10.1038/s41574-018-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation (IDF) (2017) IDF diabetes atlas, 8th ed, 2017. Available from www.diabetesatlas.org/resources/2017-atlas.html. Accessed 15 March 2019
- 3.Alzheimer’s Disease International (2015) World Alzheimer report 2015: the global impact of dementia. Available from https://www.alz.co.uk/research/world-report-2015. Accessed 15 March 2019
- 4.Feil DG, Rajan M, Soroka O, Tseng CL, Miller DR, Pogach LM. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc. 2011;59(12):2263–2272. doi: 10.1111/j.1532-5415.2011.03726.x. [DOI] [PubMed] [Google Scholar]
- 5.Dolan C, Glynn R, Griffin S, et al. Brain complications of diabetes mellitus: a cross-sectional study of awareness among individuals with diabetes and the general population in Ireland. Diabet Med. 2018;35(7):871–879. doi: 10.1111/dme.13639. [DOI] [PubMed] [Google Scholar]
- 6.Cuevas HE, Stuifbergen AK, Brown SA, Rock JL. Thinking about cognitive function: perceptions of cognitive changes in people with type 2 diabetes. Diabetes Educ. 2017;43(5):486–494. doi: 10.1177/0145721717729806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown AF, Mangione CM, Saliba D, Sarkisian CA. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51(5 Suppl Guidelines):S265–S280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 8.International Diabetes Federation (IDF) (2013) Global guideline for managing older people with type 2 diabetes. Available from www.idf.org/e-library/guidelines/78-global-guideline-for-managing-older-people-with-type-2-diabetes.html, accessed 15 March 2019
- 9.American Diabetes Association Standards of medical care in diabetes-2019: section 12. Older adults. Diabetes Care. 2019;42(Supplement 1):S139–S147. doi: 10.2337/dc19-S012. [DOI] [PubMed] [Google Scholar]
- 10.Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes Committee report: glycemic targets for elderly patients with diabetes. J Diabetes Investig. 2017;8:126–128. doi: 10.1111/jdi.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of diabetes in older adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1520–1574. doi: 10.1210/jc.2019-00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee S, Peters SA, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300–307. doi: 10.2337/dc15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrie D, Lung TW, Rawshani A, et al. Recent trends in life expectancy for people with type 1 diabetes in Sweden. Diabetologia. 2016;59(6):1167–1176. doi: 10.1007/s00125-016-3914-7. [DOI] [PubMed] [Google Scholar]
- 15.Smolina K, Wotton CJ, Goldacre MJ. Risk of dementia in patients hospitalised with type 1 and type 2 diabetes in England, 1998-2011: a retrospective national record linkage cohort study. Diabetologia. 2015;58(5):942–950. doi: 10.1007/s00125-015-3515-x. [DOI] [PubMed] [Google Scholar]
- 16.Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90:126–135. doi: 10.1212/WNL.0000000000004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42(5):484–491. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 18.Pal K, Mukadam N, Petersen I, Cooper C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2018;53(11):1149–1160. doi: 10.1007/s00127-018-1581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GE, Biessels GJ. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol. 2015;14(3):329–340. doi: 10.1016/S1474-4422(14)70249-2. [DOI] [PubMed] [Google Scholar]
- 20.Palta P, Schneider AL, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc. 2014;20(3):278–291. doi: 10.1017/S1355617713001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2(3):246–255. doi: 10.1016/S2213-8587(13)70088-3. [DOI] [PubMed] [Google Scholar]
- 22.Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28(3):726–735. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Huang E, Gao S. Type 1 diabetes mellitus and cognitive impairments: a systematic review. J Alzheimers Dis. 2017;57(1):29–36. doi: 10.3233/JAD-161250. [DOI] [PubMed] [Google Scholar]
- 24.Exalto LG, Biessels GJ, Karter AJ, et al. Risk score for prediction of 10 year dementia risk in individuals with type 2 diabetes: a cohort study. Lancet Diabetes Endocrinol. 2013;1(3):183–190. doi: 10.1016/S2213-8587(13)70048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feinkohl I, Price JF, Strachan MW, Frier BM. The impact of diabetes on cognitive decline: potential vascular, metabolic, and psychosocial risk factors. Alzheimers Res Ther. 2015;7(1):46. doi: 10.1186/s13195-015-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015;3(1):75–89. doi: 10.1016/S2213-8587(14)70148-2. [DOI] [PubMed] [Google Scholar]
- 27.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Areosa Sastre A, Vernooij RW, Gonzalez-Colaco Harmand M, Martinez G (2017) Effect of the treatment of type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst Rev (6):CD003804 [DOI] [PMC free article] [PubMed]
- 29.McMillan JM, Mele BS, Hogan DB, Leung AA. Impact of pharmacological treatment of diabetes mellitus on dementia risk: systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2018;6(1):e000563. doi: 10.1136/bmjdrc-2018-000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munshi MN. Cognitive dysfunction in older adults with diabetes: what a clinician needs to know. Diabetes Care. 2017;40(4):461–467. doi: 10.2337/dc16-1229. [DOI] [PubMed] [Google Scholar]
- 31.Sinclair A. J., Hillson R., Bayer A. J. Diabetes and dementia in older people: a Best Clinical Practice Statement by a multidisciplinary National Expert Working Group. Diabetic Medicine. 2014;31(9):1024–1031. doi: 10.1111/dme.12467. [DOI] [PubMed] [Google Scholar]
- 32.de Galan BE, Zoungas S, Chalmers J, et al. Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia. 2009;52(11):2328–2336. doi: 10.1007/s00125-009-1484-7. [DOI] [PubMed] [Google Scholar]
- 33.Yaffe K, Falvey CM, Hamilton N, et al. Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med. 2013;173(14):1300–1306. doi: 10.1001/jamainternmed.2013.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brayne C, Fox C, Boustani M. Dementia screening in primary care: is it time? JAMA. 2007;298(20):2409–2411. doi: 10.1001/jama.298.20.2409. [DOI] [PubMed] [Google Scholar]
- 35.Lin JS, O’Connor, E, Rossom RC et al (2013) Screening for cognitive impairment in older adults: an evidence update for the U.S preventive services task force. Agency for Healthcare Research and Quality (US): report no. 14-05198-EF-1. Available from www.ncbi.nlm.nih.gov/books/NBK174643/pdf/Bookshelf_NBK174643.pdf. Accessed 1 March 2019 [PubMed]
- 36.Koekkoek PS, Janssen J, Kooistra M, et al. Case-finding for cognitive impairment among people with type 2 diabetes in primary care using the test your memory and self-administered gerocognitive examination questionnaires: the Cog-ID study. Diabet Med. 2016;33(6):812–819. doi: 10.1111/dme.12874. [DOI] [PubMed] [Google Scholar]
- 37.Lacy ME, Gilsanz P, Karter AJ, Quesenberry CP, Pletcher MJ, Whitmer RA. Long-term glycemic control and dementia risk in type 1 diabetes. Diabetes Care. 2018;41(11):2339–2345. doi: 10.2337/dc18-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization (2017) Global action plan on the public health response to dementia 2017–2025 Available from www.who.int/mental_health/neurology/dementia/action_plan_2017_2025/en/. Accessed 5 June 2019


