Abstract
Background
A substantial proportion of people living with HIV (PLHIV) present for care with advanced HIV disease (AHD), which may result in difficulty reaching the “90–90–90” target to end AIDS in 2030. We assessed the risk of AHD for different transmission routes to summarize the evidence for priority prevention strategies for key populations.
Methods
Observational studies published before September 10th, 2019 in the PubMed, EMBASE, Web of Science and Chinese electronic databases were analysed. The outcomes of interest were the number of PLHIV and AHD patients and their associated transmission routes. We assessed the risk of AHD among the different transmission routes using the multi-armed network meta-analysis based on the Bayesian method. The associations between AHD and regional policies for sex work and compulsory drug treatment were estimated using ecological linear regression.
Findings
One hundred and one articles were included, covering 129,780 PLHIV with 478,830 patients who developed AHD. The network analysis revealed that among PLHIV, heterosexual contact was associated with the highest risk of AHD, followed by injection drug use (odds ratio [OR]=0•56, 95% credible interval [CrI] 0•47–0•68), and men who have sex with men (OR=0•54, 95% CrI 0•46–0•63). Regions that criminalized sex work and compulsory drug treatment had higher risks for AHD than those that did not.
Interpretation
Our findings suggest HC is at a higher risk of AHD compared to IDU and MSM. This justifies the need to expand prevention campaigns and maintain efforts to increase HIV testing in the heterosexual population.
Introduction
According to the Joint United Nations Programme on HIV and AIDS (UNAIDS), despite the tremendous progress on the prevention of HIV, there are still 36.9 million people living with HIV (PLHIV).1 A significant portion of PLHIV are not aware of their infection status, even when it develops into advanced HIV disease (AHD), due to prolonged asymptomatic phases after HIV infection.2
In some countries, PLHIV are discriminated against by their communities, generating stigma1 that has a negative impact on seeking medical advice, which results in a higher risk of developing AHD.3,4 Furthermore, the effectiveness of antiretroviral therapy (ART) declines significantly after the patients develop AHD, causing not only greater health burden for PLHIV, but also greater financial burden for the patients and government.5 This may result in difficulty reaching the “90–90–90” target to end AIDS in 2030;1 therefore, it is vital to identify groups at high risk for AHD.
In most countries, the HIV epidemic is sustained through the following transmission routes: men who have sex with men (MSM), heterosexual contact (HC), and injection drug user (IDU), with about one in three PLHIV developing AHD.1,6,7 Despite enhanced prevention efforts, many PLHIV in the modern era still have AHD at the time of diagnosis. One study suggested that CD4 cell counts among PLHIV at their first presentation to medical care did not increase significantly from 1992 to 2011 in developed countries,8 and another found that median CD4+ T cell counts at the time of initiation of ART generally remained below 350/μL in 2015.9 Whether different transmission routes predispose to different risks for AHD is largely unknown; although several studies suggest that the risk of AHD differs among PLHIV depending on their HIV transmission routes, findings have been inconsistent.10,11
In addition, several structural factors shape HIV acquisition and transition risk.12 Some studies suggested that criminalization of sex work, compulsory drug treatment, and homosexuality could lower the odds of access to HIV care among key populations.[13], [14], [15]
It is essential to understand the relationship between AHD and HIV transmission routes to prioritize prevention strategies and achieve early diagnosis, early linkage to care and early initiation of ART for key populations. Additionally, no systematic reviews have summarized the evidence regarding HIV transmission routes and AHD; therefore, this systematic review and network meta-analysis was conducted to summarize the pooled evidence.
Methods
Search strategy and selection criteria
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Network Meta-Analyses (PRISMA-NMA) statement.16 We searched PubMed, EMBASE, and Web of Science for studies published before September 10th 2019, as well as Chinese electronic databases including Wanfang, China national knowledge infrastructure (CNKI), and China Science and Technology Journal Database (CQVIP). The search terms were as follows: transmission mode/route, men who have sex with men/women, heterosexual, injection, intravenous drug users, transgender, HIV testing, delayed/late diagnosis/presentation/testers, advanced HIV disease, CD4 (cell count) less than/below/< 200, AIDS-defining/related disease/illness. The details of the search strategies are provided in appendix A2 (pp 2–3).
We specified no language restriction in the literature search and the following inclusion criteria were established: ①The study was an observational study, including a cohort, case-control, cross-sectional, and surveillance study. ②The article provided explicit definition of AHD or other related terms that followed the European Late Presenter Consensus Working Group's proposed common definition: persons presenting for care with a CD4 count below 200 cells/μL or presenting with an AIDS-defining event, regardless of the CD4 cell count.17 ③The article provided the exact number of individuals with AHD and PLHIV via the following transmission routes: MSM, IDU, and HC. The following exclusion criteria were adopted: ①The article did not study on the topic of AHD. ②The outcomes did not follow the consensus definition proposed by the European Presenter Consensus Working Group. ④The article did not present the relationship between selected transmission routes and AHD. ④The article was a review, case series, or conference abstract. ⑤The article contained a distinct data error.
Data extraction
An extraction information table was projected, and three researchers (Chen, Zeng, and Lyu) extracted data using the prepared table independently. Before the statistical analyses, four researchers (Jiang, Chen, Zeng, and Lyu) conferred about the details about the extraction and scrutinized the extraction table.
The following information was extracted: publication year, study period, region, continent, definition of AHD, median/mean age with an interquartile range (IQR)/standard error, study method, time lag (the time between the initial diagnosis of HIV and a CD4 count below 200 cells/μL or the diagnosis of AIDS), number of PLHIV/AHD for each transmission routes, and the number of male/female.
Methodological quality assessment
Two researchers (Chen and Zeng) evaluated the methodological quality of included cohort studies using the Newcastle-Ottawa scale (NOS) independently.18 The included cross-sectional and surveillance studies were assessed by the adaptive NOS updated by Herzog and colleagues.19 Any disagreement was discussed with Jiang to reach a consensus.
Data analysis
A multi-armed Bayesian network meta-analysis20 was conducted to assess the risk of AHD associated with the MSM, IDU, HC transmission routes using crude data to estimate the odds ratio (OR). A random/fixed consistency model was selected according to the deviance information criterion (DIC). Additionally, a node-splitting analysis21 was conducted to evaluate potential inconsistencies, along with subgroup analysis to explore potential heterogeneity. A meta-analysis of single proportion was conducted to assess the prevalence of AHD internationally, and a trend chart was created to evaluate the tendency of the prevalence of AHD, based on locally weighted scatterplot smoothing (LOESS) regression.22 A map was plotted to illustrate the relationship between the prevalence of AHD and the level of economic development. Furthermore, the ecological linear regressions were conducted to explore the associations between the prevalence of AHD among PLHIV through different transmission routes and the potential factors including the level of economic development (gross domestic product per capita [PPP] adjusted by international dollars), time lag, regional policies regarding sex work, and compulsory drug treatment policies. Sensitivity analyses were conducted to test the robustness of the network meta-analysis and ecological linear regression. Sample size was weighted in all the above analyses.
The data analyses were conducted using R software (x64 3.5.1). The packages ‘dplyr’ and ‘reshape’ were used for data wrangling. Network meta-analysis, meta-analysis of single proportion, Egger/Begg-Mazumdar/Thompson-Sharp test and conventional/contour-enhanced funnel plots for publication bias,23 and the ecological linear regression were conducted based on ‘gemtc’, ‘meta’, ‘netmeta’ and ‘ecoreg’ respectively. Additionally, data visualization was based on software R (x64 3.5.1) and software Graphpad Prism v.8.0.2.263. The R packages ‘ggplot2’, ‘RColorBrewer’, ‘maps’ and ‘ggpubr’ contributed to trend chart and maps. The surface under the cumulative ranking curve (SUCRA) was generated using software Graphpad Prism v.8.0.2.263.
Result
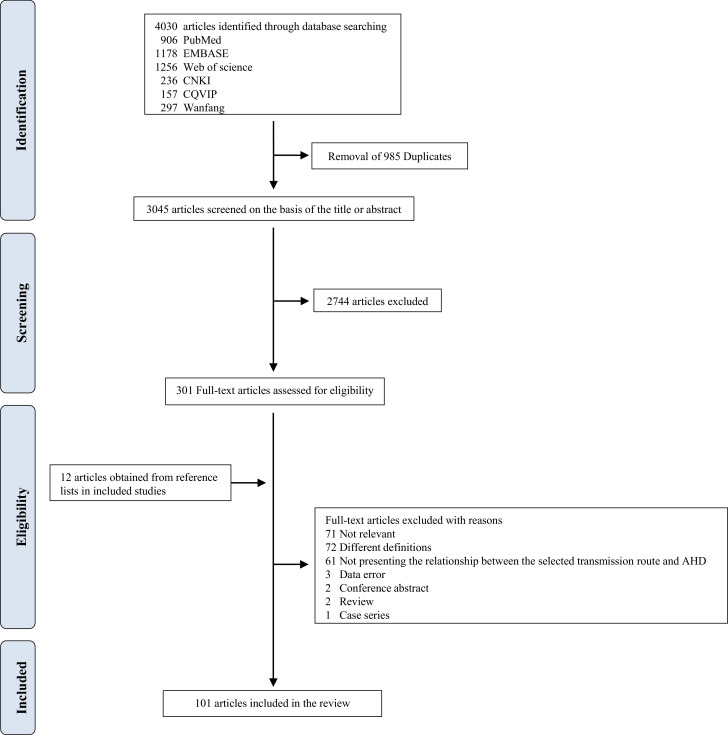
After the removal of duplicates, we identified 3045 unique articles in the first stage. A preliminary screening of abstracts and titles was conducted, and 301 articles were screened for eligibility. Subsequently, 212 articles were excluded (71 articles did not study on AHD, 72 contained different definitions, three contained data errors, two were reviews, two were conference abstracts, one was a case series, 61 did not present the relationship between the selected transmission routes and AHD). We also retrieved an additional 12 articles from reference lists, and 101 articles were included in the final analysis (Fig. 1).
Fig. 1.
Flow diagram of search strategy and study selection.
The final 105 studies from 101 articles (15 cross-sectional studies from 15 articles and 25 cohort studies from 25 articles, respectively, 65 surveillance studies from 61 articles) included 129,780 PLHIV with 478,830 patients who developed AHD, and estimated the risk between AHD and the following transmission routes: MSM, IDU, and HC. The detailed characteristics of the included articles provided in the appendix A3 (pp 4–8).
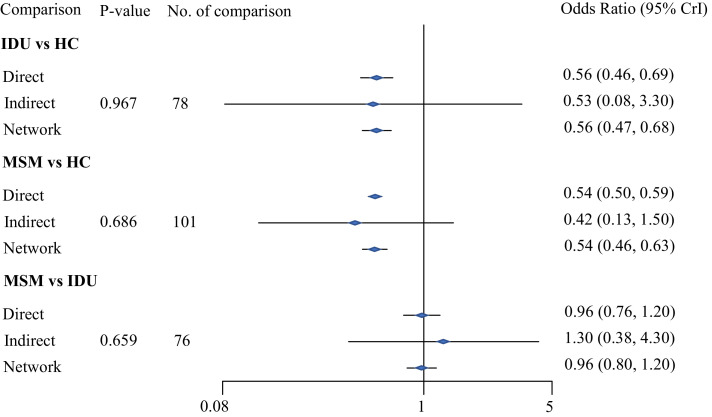
A consistent random-effects network model for AHD and the three specific transmission routes was constructed for analysis. Fig. 2 illustrates the results of the network analysis with direct, indirect and network comparison estimates using raw data. Fig. 2 reveals that the PLHIV associated with HC had the highest risk of AHD, followed by those associated with IDU, with an OR of 0•56 (95% credible interval [CrI] 0•47–0•68), and MSM (OR=0•54, 95% CrI 0•46–0•63). On the basis of the node-splitting analysis, the P value of consistency among the three transmission routes was greater than 0•05, which suggested that the model was consistent and suitable to provide useful estimates. Also, we conducted a sensitivity analysis including high-quality studies assessed by NOS, which showed the consistent result that PLHIV infected via HC had the highest risk of AHD compared with those infected via IDU and MSM (see appendix A5 pp19).
Fig. 2.
Direct, indirect and network odds ratio for advanced HIV disease among people living with HIV through heterosexual contact (HC), injection drug users (IDU), and men who have sex with men (MSM)
A vs B: the latter (B) is the reference. P-value >0.05 indicates that accepting the null hypothesis of consistency between direct and indirect results.
Two conventional and contour-enhanced funnel plots showed that there was little risk of publication bias in the meta-analysis. Additionally, the results of Egger/Begg-Mazumdar/Thompson-Sharp tests were in accordance with the funnel plots, which suggested accepting the null-hypothesis of no publication bias due to non-statistical significance consistently (p>0•10) (see appendix A6 pp 20–24).
Since there were a relatively large number of studies from five continents, the heterogeneity of the estimates was predictably high; and the details are illustrated in the appendix A (pp 18). Subgroup analyses of the following potential factors were conducted: time lag, the level of economic development of regions, continent, prevalence of AHD, and study method. Generally, the ranking of the risk of AHD associated with the three transmission routes remained stable and HC had the highest risk of AHD compared with IDU and MSM (see appendix A8 pp 25–39).
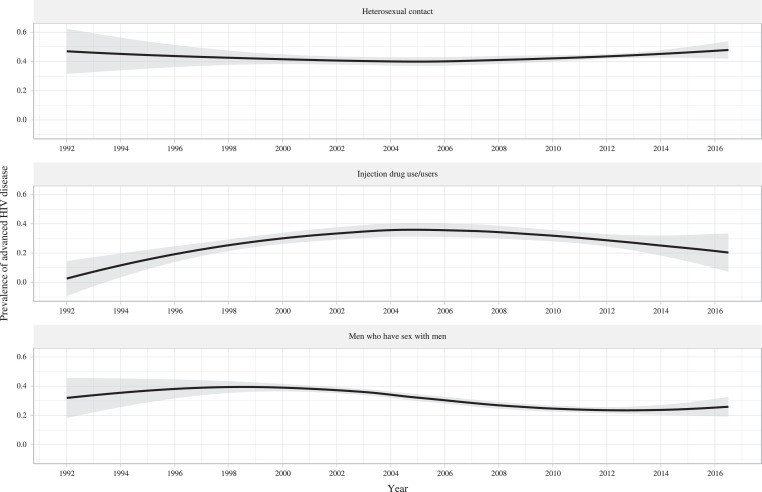
Fig. 3 shows the temporal changes in the prevalence of AHD among the three specific transmission routes from 1992 to 2016. Compared to the prevalence of AHD associated with MSM and IDU, that associated with HC remained the highest during the period. Generally, the prevalence of AHD associated with HC did not change materially, while that associated with IDU and MSM witnessed a downward tendency, respectively. Recently, the prevalence of AHD associated with HC showed a slightly upward tendency, while that associated with MSM and IDU experienced a plateau and a decrease, respectively.
Fig. 3.
Temporal tendencies for the prevalence of advanced HIV disease among people living with HIV through heterosexual contact, injection drug use, and men who have sex with men.
Results from locally weighted scatterplot smoothing (LOESS) regression are based on all the included studies. 95% confidence intervals are illustrated as grey shaded areas. Years are estimated by the median year of study period.
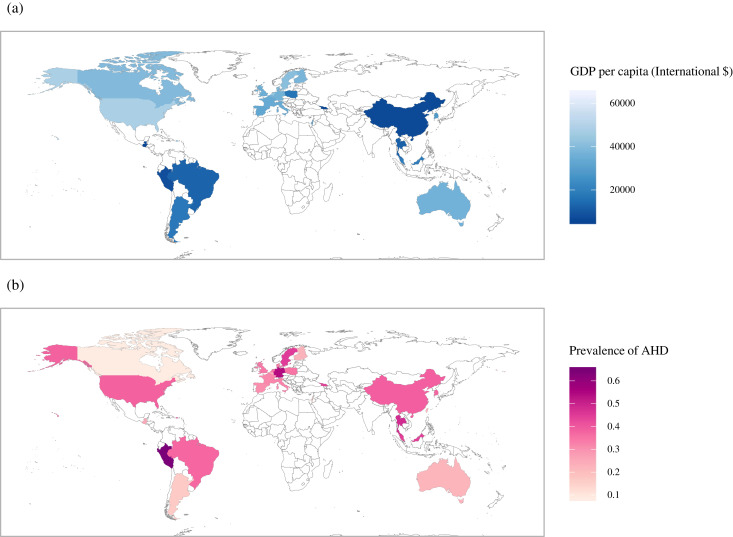
Fig. 4 consists of two different maps; one is an epidemic map of the prevalence of AHD, and the other displays the level of economic development of regions in the included studies. The pooled prevalence of AHD among PLHIV in all of the included studies was 34•87% (95% confidence interval [CI] 33•30%−36•45%). There was a much higher prevalence of AHD (37•45%, 95% CI 35•35%−39•58%) in regions with lower PPP than in the regions with higher PPP (32•82%, 95% CI 30•38%−35•30%). PLHIV associated with other transmission routes, including but not limited to transfusion and unknown, were included to show the pooled prevalence of AHD in this figure.
Fig. 4.
Two world maps regarding gross domestic product per capita (PPP) 2009 adjusted by international $ (a) and the prevalence of advanced HIV disease (b)
PPP 2009 was adopted because the median year of study period for all the included studies is 2009. Due to data unavailable of PPP 2009 in China (Taiwan) from the World Bank, the area of China (Taiwan) filled with dark grey in the map (a).
Table 1 and Table 2 demonstrate the relationship between legislation and prevalence of AHD among PLHIV through different transmission routes. According to Table 1, there was a higher risk of AHD (crude OR [cOR]=1•30, 95% CI 1•27–1•33) in regions where sex work was criminalized than in those regions where sex work was legalized. Considering the potential bias due to the level of economic development and time lag, they were evaluated separately as well, revealing a lower risk of AHD in regions with higher PPP (cOR=0•91, 95% CI 0•89–0•93) and a higher risk of AHD in studies with time lag ≥ three months (cOR=1•90, 95% CI 1•85–1•96). After adjusting for the level of economic development and time lag, consistent results showed that there was a higher risk of AHD in criminalized sex work regions (adjusted OR [aOR]=1•21, 95% CI 1•18–1•25) than in legalized sex work regions. Similarly, after taking the level of economic development and time lag into consideration, those regions that criminalized compulsory commitment to care of substance misusers (CCC) had a higher risk of AHD (aOR=4•38, 95%CI 4•10–4•67) than those that did not criminalize CCC (Table 2). Results of sensitivity analyses indicated that the relation between criminal justice to sex work, CCC and AHD did not significantly change when the PPP in different year was adjusted in the multivariate model; and when studies with small sample size were excluded (see appendix A9-10 pp 40–43). The relationship between the criminalization of homosexuality and the prevalence of AHD among MSM was not analysed because homosexuality was criminalized in only two countries (Singapore and Malaysia) included in the study.
Table 1.
Relation between different sex work policies and prevalence of advanced HIV disease among people living with HIV through heterosexual contact.
| Variable (Number of studies) | Unadjusted model | Adjusted model |
|---|---|---|
| OR a (95%CIb) | OR (95%CI) | |
| PPP | ||
| ≤ World PPP 2005c (9) | Ref. | Ref. |
| > World PPP 2005 (46) | 0•91 (0•89–0•93) | 0•85 (0•82–0•87) |
| Sex work Policy | ||
| Legal (38) | Ref. | Ref. |
| Illegal (17) | 1•30 (1•27–1•33) | 1•21 (1•18–1•25) |
| Time Lag | ||
| ≤ 3 months (36) | Ref. | Ref. |
| > 3 months (19) | 1•90 (1•85–1•96) | 2.05 (1•99–2.12) |
OR: odds ratio.
CI: confidence interval.
PPP: gross domestic product per capita (current international $). The median year of study period for the included studies in this ecological linear model is 2005. The median time lag (months) for the included studies in this ecological linear model is 3. The regression model is weighed by sample sizes. The adjusted model incorporates PPP 2005 of the regions, time lag and sex work legislation. The studies which do not report explicit time lag are excluded.
Table 2.
Relationship between criminal justice legislation to the compulsory commitment to care of substance misusers and the prevalence of advanced HIV disease among people living with HIV through injection drug use.
| Variable (Number of studies) | Unadjusted model | Adjusted model |
|---|---|---|
| ORa (95%CIb) | OR (95%CI) | |
| PPP | ||
| ≤ World PPP 2004 c (6) | Ref. | Ref. |
| > World PPP 2004 (36) | 0•51 (0•46–0•55) | 0•45 (0•41–0•48) |
| CCCd | ||
| Non-Criminalized CCC (8) | Ref. | Ref. |
| Criminalized CCC (34) | 1•95 (1•85–2•05) | 4•38 (4•10–4•67) |
| Time Lag | ||
| ≤ 3 months (28) | Ref. | Ref. |
| > 3 months (14) | 1•44 (1•39–1•50) | 2•72 (2•57–2•87) |
: OR: odds ratio.
: CI: confidence interval.
: PPP: gross domestic product per capita (current international $).
: CCC: compulsory commitment to care of substance misusers. The median year of study period for the included studies in this ecological linear model is 2004. The median time lag (months) for the included studies in this ecological linear model is 3. The regression model is weighed by sample sizes. The adjusted model incorporates PPP 2004 of the regions, time lag and legislation to CCC. The studies which do not report explicit time lag are excluded.
Discussion
To our knowledge, this network meta-analysis is the first and most comprehensive synthesis of data examining the risk of AHD associated with different transmission routes. This is also the first study to explore the association between criminalization and the risk of AHD at the country/region level. Our findings indicated that PLHIV who acquired HIV through heterosexual contact had the highest risk of AHD, followed by those through IDU, and MSM. The prevalence of AHD decreased among the MSM and IDU transmission routes generally, but the trend among the HC transmission route seemed to slightly increase recently. Regions with criminalized sex work and CCC polices presented a higher risk of AHD than those regions without. These results reinforced the challenges in achieving the ambitious “90–90–90” target in 2020 and the end of AIDS in 2030: The prevalence of AHD remained high, especially among PLHIV who acquired HIV through heterosexual contact. More efforts should be made to facilitate early diagnosis among key populations.
There are several implications and considerations related to these findings. Our results revealed that the prevalence of AHD was high (34•87%) among PLHIV, which was consistent with that (one-third) estimated by UNAIDS. More than two out of every three PLHIV did not know their HIV status in 2016,1 resulting in late HIV diagnosis. Male gender, older age, low risk perception, lack of awareness about HIV and decreased HIV testing coverage were risk factors for a late HIV diagnosis.5,10,11,24 A previous study showed that even after adjusting for gender and age, late presentation for care was more common among individuals with low socioeconomic status than among those with high socioeconomic status,25 which aligns the fact that a higher prevalence of AHD was observed in regions with a lower level of economic development than in regions with a higher level of economic development in our study.
The varied risks of AHD among different transmission routes might depend on the current or past testing rate and the epidemic patterns.10 More and earlier testing among MSM might contribute to a decreased rate of late presentation.10,26 A previous study showed that MSM population had the highest possibility of undergoing a past HIV test in the last year, followed by individuals engaging in IDU and HC.26 The higher proportion of MSM among the newly diagnosed PLHIV might decrease the risk of late presentation as well.10 Another recent study indicated that gay/bisexual men were more likely to attend health services and underwent testing than women and heterosexual men because of the greater perceived risk, which could explain the lower risk of AHD among MSM.4 Apart from variations in access to health services, the perception of risk of HIV among different groups or the establishment of programmes for HIV diagnosis for specific risk groups could result in different risks of AHD.4,27 Moreover, PLHIV with IDU were more likely to have less access to healthcare and lower socioeconomic status, which was associated with late presentation.3
The decreasing trend in prevalence of AHD might be attributed to the global response, including the substantial rise in HIV testing in many countries, and early HIV treatment as prevention to reduce the HIV transmission.[28], [29], [30] However, this might not have been the case for sex workers and drug users. Limited ART coverage and retention might be due to the criminalization of sex workers, and called for a continuing investment in community and structural interventions so that this population could profit from the preventions and treatments that other key populations received.30 The results from a national observational cohort in the Netherlands revealed that although the prevalence of late presentation decreased over time from 62% in 1996 to 42% in 2013, it did not decline significantly among individuals associated with heterosexual transmission;31 these results were in line with the trend of prevalence of AHD among PLHIV associated with HC in our study. The trend among PLHIV associated with IDU in our study was consistent with the results of the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study from 2010 to 2013.7
Stigma, discrimination, and criminalization remain obstacles for early diagnosis in key populations.1 A previous review showed that the criminalization of sex work increased the risk of sexual/physical violence, HIV/sexually transmitted infections (STIs), and unprotected sex among sex workers.32 Moreover, an ecological linear regression analysis suggested that the legalization of some aspects of sex work might help reduce the HIV prevalence in sex workers.13 The criminalization of sex work can force sex workers to work in isolation, disrupt peer support networks, increase their marginalization, and reduce their access to HIV services due to a fear of prosecution and moral judgement,13,32 ultimately increasing the risk of AHD. Therefore, more efforts should be made to improve the health of sex workers and access to HIV services. Previous studies revealed that the compulsory treatment for drug dependence was not related to improved outcomes as a whole, with some studies revealing potential harms.14,33 In the context of criminal justice to CCC, drug users might fail to seek care due to the threat of detainment within compulsory drug detention centres and the fear of being targeted by police,14 potentially contributing to a late HIV diagnosis. Another systematic review showed that criminalization of drug use including forced detention as addiction treatment, had a negative effect on HIV prevention and treatment such as HIV testing and counselling programs in 85 of the 106 eligible studies, which could result in AHD.34 Accordingly, UNAIDS has been advocating for the global adoption of public health alternatives to the criminalization and imprisonment to IDU.35
The sensitivity analysis and subgroup analyses showed the stable rankings among the three transmission routes, which suggested the robustness of the network results. No publication bias was observed, which revealed the validity of our results. The sensitivity analyses also highlighted the stability of ecological linear regression when the analyses were conducted using PPP of different years and were restricted to studies with larger sample size.
There were several limitations in the current study. First, we used the crude number of individuals with AHD and the number of PLHIV from observational studies instead of randomized controlled trials, which were usually used for network meta-analysis. Confounding factors could impact the results of the network meta-analysis, as the adjusted risk estimates were not used for the pooled data.36 Second, no study conducted in Africa was included in the current study, which should be considered when interpreting our results. Third, the median year of study period37 was used to present the temporal trend of AHD prevalence, which could result in some biases. Fourth, ecological analyses of country/region-level data to explore the relationships between criminalization and AHD among key populations could result in ecological fallacies.13 In addition, sex workers were not equivalent to PLHIV associated with HC, although we examined the relationships between the criminalization of sex work and AHD among PLHIV associated with HC. The exclusion of studies without known legislation policies and specified time lag in the ecological linear regression and the change in sex work and CCC criminal justice over time could lead to biases and restrict the extrapolation of our results. Nevertheless, the effects of criminalizing sex work and CCC criminal justice on the prevalence of AHD remain an important topic of future research.
In conclusion, our findings indicated that the prevalence of AHD among PLHIV was high, and PLHIV associated with HC had the highest risk of AHD, followed by individuals who engaged in IDU, and MSM. The temporal trend of the prevalence of AHD justified the need to expand prevention campaigns and to maintain efforts to increase HIV testing in the heterosexual population. The positive association between criminalization and AHD suggest that more efforts should be made to improve access to HIV services, and attention should be focused on reducing structural inequalities, stigma, discrimination, and the exclusion on sex workers and drug users.
Authors' contributions
HJ had the idea for the study, designed the protocol. HJ, QC, DZ, and YL did the study selection and data extraction. QC, DZ, and HJ conducted the methodological quality assessment. QC, DZ and HJ contributed to the statistical analysis. QC and DZ performed the data visualization. HJ and QC wrote the draft report. HJ, YS, XG, MJF, and YY critically revised the report. All authors approved the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
National Natural Science Foundation of China (81703282).
Declaration of Competing Interest
We declare no competing interests.
Acknowledgements
This work was funded by National Natural Science Foundation of China (81703282). The funding source of the study had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit the paper for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2019.10.003.
Contributor Information
Yi Yang, Email: yangyigz@163.com.
Hongbo Jiang, Email: hongbojiang3@163.com.
Appendix. Supplementary materials
References
- 1.UNAIDS. Ending AIDS: Progress towards the 90-90-90 targets, July 20, 2017. https://www.unaids.org/sites/default/files/media_asset/Global_AIDS_update_2017_en.pdf(accessed March 23, 2019).</bib
- 2.van Sighem A., Pharris A., Quinten C. Reduction in undiagnosed hiv infection in the european union/european economic area, 2012 to 2016. Euro surveill. 2017;22(48) doi: 10.2807/1560-7917.ES.2017.22.48.17-00771. pii=17-00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Socio-economic Inequalities and HIV Writing Group for COHERE in EuroCoord. Lodi S., Dray-Spira R. Delayed hiv diagnosis and initiation of antiretroviral therapy: inequalities by educational level, cohere in eurocoord. AIDS. 2014;28(15):2297–2306. doi: 10.1097/QAD.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 4.Fakoya I., Alvarez-Del Arco D., Monge S. HIV testing history and access to treatment among migrants living with hiv in europe. J Int AIDS Soc. 2018;21(Suppl 4):e25123. doi: 10.1002/jia2.25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang H., Yin J., Fan Y. Gender difference in advanced hiv disease and late presentation according to european consensus definitions. Sci Rep. 2015;5:14543. doi: 10.1038/srep14543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Althoff K.N., Gange S.J., Klein M.B. Late presentation for human immunodeficiency virus care in the united states and canada. Clin Infect Dis. 2010;50(11):1512–1520. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Late presenters working group in COHERE in EuroCoord Late presentation for hiv care across europe: update from the collaboration of observational hiv epidemiological research europe (COHERE) study, 2010 to 2013. Euro surveill. 2015;20(47) doi: 10.2807/1560-7917.ES.2015.20.47.30070. pii=30070. [DOI] [PubMed] [Google Scholar]
- 8.Lesko C.R., Cole S.R., Zinski A., Poole C., Mugavero M.J. A systematic review and meta-regression of temporal trends in adult CD4(+) cell count at presentation to hiv care, 1992-2011. Clin Infect Dis. 2013;57(7):1027–1037. doi: 10.1093/cid/cit421. [DOI] [PubMed] [Google Scholar]
- 9.IeDEA and COHERE Cohort Collaborations Global trends in CD4 cell count at the start of antiretroviral therapy: collaborative study of treatment programs. Clin Infect Dis. 2018;66(6):893–903. doi: 10.1093/cid/cix915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickson N., McAllister S., Sharples K., Paul C. Late presentation of hiv infection among adults in new zealand: 2005-2010. HIV Med. 2012;13(3):182–189. doi: 10.1111/j.1468-1293.2011.00959.x. [DOI] [PubMed] [Google Scholar]
- 11.Hachfeld A., Ledergerber B., Darling K. Reasons for late presentation to hiv care in switzerland. J Int AIDS Soc. 2015;18:20317. doi: 10.7448/IAS.18.1.20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shannon K., Strathdee S.A., Goldenberg S.M. Global epidemiology of hiv among female sex workers: influence of structural determinants. Lancet. 2015;385(9962):55–71. doi: 10.1016/S0140-6736(14)60931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeves A., Steele S., Stuckler D., McKee M., Amato-Gauci A., Semenza J.C. National sex work policy and hiv prevalence among sex workers: an ecological regression analysis of 27 european countries. Lancet HIV. 2017;4(3) doi: 10.1016/S2352-3018(16)30217-X. e134-e40. [DOI] [PubMed] [Google Scholar]
- 14.Werb D., Kamarulzaman A., Meacham M.C. The effectiveness of compulsory drug treatment: a systematic review. Int J Drug Policy. 2016;28:1–9. doi: 10.1016/j.drugpo.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arreola S., Santos G.M., Beck J. Sexual stigma, criminalization, investment, and access to hiv services among men who have sex with men worldwide. AIDS Behav. 2015;19(2):227–234. doi: 10.1007/s10461-014-0869-x. [DOI] [PubMed] [Google Scholar]
- 16.Hutton B., Salanti G., Caldwell D.M. The prisma extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 17.Antinori A., Coenen T., Costagiola D. Late presentation of hiv infection: a consensus definition. HIV Med. 2011;12(1) doi: 10.1111/j.1468-1293.2010.00857.x. 61-4. [DOI] [PubMed] [Google Scholar]
- 18.Wells G, Shea B, O'Connell D, et al. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2018. http://www.ohri.ca/programs/clinicalepidemiology/oxford.asp(accessed September 20, 2019).
- 19.Herzog R., Álvarezpasquin M.J., Díaz C., Barrio J.L.D., Estrada J.M., Á Gil. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health. 2013;13:1–17. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Valkenhoef G., Lu G., de Brock B., Hillege H., Ades A.E., Welton N.J. Automating network meta-analysis. Res Synth Methods. 2012;3(4):285–299. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 21.van Valkenhoef G., Dias S., Ades A.E., Welton N.J. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. 2016;7(1):80–93. doi: 10.1002/jrsm.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleveland W.S., Devlin S.J. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83(403):596–610. [Google Scholar]
- 23.Peters J.L., Sutton A.J., Jones D.R., Abrams K.R., Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Ransome Y., Terzian A., Addison D. Expanded hiv testing coverage is associated with decreases in late hiv diagnoses. AIDS. 2015;29(11):1369–1378. doi: 10.1097/QAD.0000000000000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gueler A., Schoeni-Affolter F., Moser A. Neighbourhood socio-economic position, late presentation and outcomes in people living with hiv in switzerland. AIDS. 2015;29(2):231–238. doi: 10.1097/QAD.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 26.Dailey A.F., Hoots B.E., Hall H.I. Vital signs: human immunodeficiency virus testing and diagnosis delays - United States. MMWR Morb Mortal Wkly Rep. 2017;66(47):1300–1306. doi: 10.15585/mmwr.mm6647e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suarez-Garcia I., Sobrino-Vegas P., Dalmau D. Clinical outcomes of patients infected with hiv through use of injected drugs compared to patients infected through sexual transmission: late presentation, delayed anti-retroviral treatment and higher mortality. Addiction. 2016;111(7):1235–1245. doi: 10.1111/add.13348. [DOI] [PubMed] [Google Scholar]
- 28.Cohen M.S., Chen Y.Q., McCauley M. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beyrer C., Baral S.D., Collins C. The global response to hiv in men who have sex with men. Lancet. 2016;388(10040):198–206. doi: 10.1016/S0140-6736(16)30781-4. [DOI] [PubMed] [Google Scholar]
- 30.Shannon K., Crago A.L., Baral S.D. The global response and unmet actions for hiv and sex workers. Lancet. 2018;392(10148):698–710. doi: 10.1016/S0140-6736(18)31439-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Op de Coul E.L., van Sighem A., Brinkman K. Factors associated with presenting late or with advanced hiv disease in the netherlands, 1996–2014: results from a national observational cohort. BMJ Open. 2016;6(1) doi: 10.1136/bmjopen-2015-009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Platt L., Grenfell P., Meiksin R. Associations between sex work laws and sex workers' health: a systematic review and meta-analysis of quantitative and qualitative studies. PLoS Med. 2018;15(12) doi: 10.1371/journal.pmed.1002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lunze K., Idrisov B., Golichenko M., Kamarulzaman A. Mandatory addiction treatment for people who use drugs: global health and human rights analysis. BMJ. 2016;353:i2943. doi: 10.1136/bmj.i2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeBeck K., Cheng T., Montaner J.S. HIV and the criminalisation of drug use among people who inject drugs: a systematic review. Lancet HIV. 2017;4(8) doi: 10.1016/S2352-3018(17)30073-5. e357-e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.UNAIDS. Harm reduction saves lives, June 26, 2017. https://www.unaids.org/sites/default/files/media_asset/harm-reduction-saves-lives_en.pdf(accessed March 23, 2019).
- 36.Stegeman B.H., de Bastos M., Rosendaal F.R. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298. doi: 10.1136/bmj.f5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siedner M.J., Ng C.K., Bassett I.V., Katz I.T., Bangsberg D.R., Tsai A.C. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan africa, 2002-2013: a meta-analysis. Clin Infect Dis. 2015;60(7):1120–1127. doi: 10.1093/cid/ciu1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.