Abstract
Pharmacokinetic modelling on dynamic positron emission tomography (PET) data is a quantitative technique. However, the long acquisition time is prohibitive for routine clinical use. Instead, the semi-quantitative standardised uptake value ratio (SUVR) from a shorter static acquisition is used, despite its sensitivity to blood flow confounding longitudinal analysis. A method has been proposed to reduce the dynamic acquisition time for quantification by incorporating cerebral blood flow (CBF) information from arterial spin labelling (ASL) magnetic resonance imaging (MRI) into the pharmacokinetic modelling. In this work, we optimise and validate this framework for a study of ageing and preclinical Alzheimer's disease. This methodology adapts the simplified reference tissue model (SRTM) for a reduced acquisition time (RT-SRTM) and is applied to [18F]-florbetapir PET data for amyloid-β quantification. Evaluation shows that the optimised RT-SRTM can achieve amyloid burden estimation from a 30-min PET/MR acquisition which is comparable with the gold standard SRTM applied to 60 min of PET data. Conversely, SUVR showed a significantly higher error and bias, and a statistically significant correlation with tracer delivery due to the influence of blood flow. The optimised RT-SRTM produced amyloid burden estimates which were uncorrelated with tracer delivery indicating its suitability for longitudinal studies.
Keywords: Positron emission tomography, arterial spin labelling, pharmacokinetic modelling, reduced acquisition time, cerebral blood flow
Introduction
Positron emission tomography (PET) facilitates the quantification of a range of important biomarkers through the injection and detection of targeted radiotracers. To interpret the measured signal and derive the biological parameters of interest, data are collected dynamically from injection, covering radiotracer delivery to tissue, interaction with the target, and tracer washout. This provides a map of the spatio-temporal concentration of the tracer in vivo. A pharmacokinetic model which describes these processes may then be fitted to these dynamic data to estimate the biological parameters such as radiotracer target density.
Depending on the radiotracer administered, the dynamic data acquisition time required to fit the model may be 60 min or more. This is prohibitive in a clinical context due to patient discomfort, restrictions on scanner time availability, and the increased chance of subject motion which corrupts the data. Consequently, a simplified technique is commonly employed.
The standardised uptake value ratio (SUVR) is a measure of relative tracer uptake which can be calculated from a static scan lasting approximately 10 min. SUVR is calculated by dividing the tracer concentration within the tissue of interest by the concentration in a reference region. The reference region consists of tissue considered to be free of the radiotracer target and represents the non-displaceable () tracer concentration (i.e. tracer in the tissue which is not bound to the intended target). When the ratio of the tracer concentration within the target tissue and the reference tissue has reached a steady-state, SUVR approximates the distribution volume ratio (DVR). DVR can be estimated from pharmacokinetic modelling and is related to target density.
However, as SUVR is calculated from a single static scan, the tracer concentration present during the acquisition will depend on the delivery of the tracer, as well as target density. Tracer delivery is intrinsically linked to blood flow, and since blood flow can change during the progression of disease,1 and indeed fluctuate over the course of a day,2 SUVR estimates may be confounded.
The influence of cerebral blood flow (CBF) changes on SUVR estimates has been highlighted in longitudinal Alzheimer's disease studies in which the target of interest was the protein amyloid-β. Amyloid-β is an early indicator of disease onset and a therapeutic target, hence accurately quantifying amyloid-β density is of paramount importance. Here, variation in blood flow has been shown to cause spurious changes in SUVR which are unrelated to target density.3,4 Conversely, target density estimates derived from pharmacokinetic modelling of dynamic data starting from radiotracer injection can account for blood flow, as tracer delivery is parameterised within the model.
Dynamic PET data can be divided into two phases: the early phase, in which the signal is dominated by tracer delivery to tissue,5 and the late phase, which contains information related to tracer binding and washout, and is where SUVR is estimated. The intrinsic correlation between tracer delivery and CBF has been demonstrated for an amyloid-β tracer.6 Therefore, if CBF can be measured independently from the PET acquisition, then the data acquisition time may be reduced such that only the late phase data are acquired to estimate the remaining parameters.
Arterial spin labelling (ASL) magnetic resonance imaging (MRI) is a non-invasive imaging technique which applies a magnetic ‘tag’ to arterial blood, such that it can be used as an endogenous contrast agent. ASL can be used to estimate CBF, as validated by comparison with the gold standard radiolabelled water PET.7 While the accuracy of the technique is dependent on the implementation, high quantitative accuracy has been achieved when ASL data are normalised to a reference region.8
The introduction of combined PET/MRI scanners, which facilitate simultaneous acquisition, means that ASL and late phase PET data can be acquired concurrently. By incorporating CBF information from ASL into the PET pharmacokinetic modelling to provide early phase delivery information, the total acquisition time can be significantly reduced, increasing patient comfort and throughput, without sacrificing quantitative accuracy.
In this paper, we build on the framework which combines ASL-derived CBF data with PET kinetic modelling to reduce the acquisition time from 60 to 30 min.9 This is referred to here as the reduced acquisition time simplified reference tissue model (RT-SRTM). In the present work, the relationship between PET radiotracer delivery and ASL measured CBF is investigated with a larger group of subjects. An improved methodology for the estimation of the early phase data is proposed and compared to that used previously.9 Finally, the timing of the late phase acquisition is optimised before applying the improved methodology to a different set of subjects. This was applied to [18F]-florbetapir amyloid PET data from a study of ageing and preclinical Alzheimer's disease.
Material and methods
PET kinetic modelling
The simplified reference tissue model (SRTM)10 was used as the gold standard for PET pharmacokinetic modelling, as it is commonly applied in amyloid studies5,6,11,12 and has been validated against pharmacokinetic modelling with arterial sampling for [18F]-florbetapir.13 The SRTM employs a reference region, which is considered to be devoid of the imaging target, to replace the plasma input function. Cerebellar grey matter was used as it is assumed to be devoid of amyloid-β.14
The operational equation between the tracer concentration in the target tissue and the reference region is formulated as shown in equation (1). Here t denotes time with tracer injection at , and ⊗ represents the convolution operator
| (1) |
The SRTM contains three parameters: R1, which is the rate constant of tracer delivery to the target tissue relative to the reference tissue; k2, which is the rate constant from target tissue to blood; and the ND binding potential , which is proportional to target density, i.e. the density of amyloid-β, see Supplementary Materials.
An in-house implementation of the SRTM using the basis function method15 was fitted to the PET data to derive regional gold standard parameter estimates, denoted by an asterisk, of , and from dynamic PET data conventionally acquired for min, denoted here as min. For more details see Supplementary Materials.
PET kinetic modelling with reduced acquisition time
Early PET signal is dominated by the delivery of the tracer to the tissue and hence is important for the estimation of R1. The later part of the signal contains information about the binding of the tracer to the target and its subsequent washout, which is essential for the estimation of k2 and .
Consequently, if the blood flow information can be estimated independently from the PET data, then the acquisition time can be reduced, by recording only the late signal to allow the estimation of k2 and . This requires two modifications to the model: (i) the estimation of R1 from another source, and (ii) extrapolation of the reference region curve, CR to , as the model contains a convolution term which requires the full time series from injection to compute. Here, we refer to this modified model as the reduced acquisition time SRTM (RT-SRTM).
Derivation of PET-R1 from ASL–CBF
R1 is defined as where K1 is the transfer rate constant from blood to target tissue and is the transfer rate constant from blood to reference tissue. According to the Renkin–Crone16,17 capillary model, the relationship between tracer delivery, K1, and blood flow, F, can be described as
| (2) |
The Renkin–Crone model includes a term for the net extraction of the tracer from the capillaries, E, which is dependent on the vessel permeability surface area product, . Under common physiological flow conditions, where is high (), the relationship between K1 and flow F is approximately linear. If we assume that is sufficiently high, the relationship between K1 and F, and in turn the relationship between R1 and F, can be approximated as a linear function,6 as expressed in equation (3)
| (3) |
ASL can be used to measure the CBF, and may be converted into an R1 estimate to use in the RT-SRTM, using the relationship from equation (3), where indicates the CBF measured in the reference region.
In this study, linear regression between R1 and CBF was performed in a group of subjects to determine whether this approximation is valid. The slope and intercept of the linear regression, and , can account for systematic differences between the modalities and an extraction fraction of . The derived and can then be applied to a different group of subjects to convert ASL-CBF to a derived R1 value.
Due to systematic errors in ASL-CBF estimates in certain regions of the brain, particularly for single inversion time ASL data,7 multi-linear analysis was also performed, to determine whether and may be region dependent. Multi-linear regression has the capacity to model interaction terms between ASL-CBF and the region and can be described as in equation (4)
| (4) |
Here is equal to 1 when is being considered, and 0 otherwise. Two further multi-linear analyses were also performed (i) using the subject as a covariate, and (ii) using both subject and regions as covariates to determine their relative influence on R1 estimation.
Extrapolation of PET reference region,
To compute the convolution term in equation (1), CR must be known from injection, at , to the end of the scan, , where min for the gold standard. However, when reducing the acquisition time, CR is only measured between ts and where , and therefore a strategy is required to extrapolate the missing data. Here we define as a vector containing the reference region tracer concentration over time, as a matrix containing the reference region concentration for a population of subjects, and as a vector containing the mean population tracer concentration. For clarity, the acquisition time, t, is expressed as a discrete variable, as the dynamic data are binned into frames.
In this work, two different approaches are evaluated to estimate the whole reference input vector for an unseen subject's . Both techniques make use of a population of subjects for which the full was measured.
The first method, referred to as the scaled mean CR method and proposed by Scott et al.,9 scales the population average reference region curve, , as
| (5) |
where α is a subject specific scaling factor determined through a least squares fit of to . This results in an individual estimate of to be used in the RT-SRTM.
The second method employs statistical shape modelling to build a model of the variation in CR within the population of subjects.18 This requires principal component analysis (PCA) of a set of subjects to determine the M components, where , and is therefore referred to as the PCA CR method.
The of each subject in the set can be expressed as the mean population reference curve, , plus a linear combination of the weighted principal components, where the weight of the mode, , is wi. A subset, L where , of the components which describe the majority of the variation are selected. An unseen reference region curve can then be fitted by adjusting the weights, as per equation (6). The same weights and modes can then be used with to generate . See Supplementary Materials for more details
| (6) |
Fitting the SRTM with CBF-derived R1 and extrapolated CR
To apply the modifications to the SRTM with reduced acquisition time, the operational equation in (1) is re-written as in equation (7). This groups the measured parameter , with the derived and derived R1 into a dummy variable, , as they are determined prior to fitting
where
| (7) |
To solve equation (7) for a reduced acquisition time where , the basis function approach15 is used to pre-calculate the convolution term using the extrapolated with a range of biologically plausible values for θ. A least squares fit to the pre-determined data, , is performed for each θ to estimate φ, and the instance of θ which yields the lowest sum of squares difference is selected. and k2 are then derived from φ, θ and the CBF-derived R1. For more details, including the pseudo-code, see Supplementary Materials.
Data
Imaging data were collected from 60 cognitively normal subjects participating in Insight 46, a neuroimaging sub-study of the Medical Research Council National Survey of Health and Development,19 and 4 subjects from a study of young onset Alzheimer's Disease (YOAD) with an intermediate or high certainty diagnosis.20 These studies were conducted in line with the principles of the Declaration of Helsinki. For Insight 46, ethical approval was obtained from the National Research Ethics Service (ref 14/LO/1173), and for YOAD, approval was obtained from the London Queen Square Ethics Committee (ref 15/LO/1412). Written informed consent was obtained from all participants.
All subjects underwent 60 min of simultaneous amyloid PET and multi-modal MR imaging on a Siemens Biograph mMR PET/MR scanner. Of the 64 subjects used for analysis, mean age 69.6 years (range 61.7–70.5 years), 45 had both PET and ASL data, and for 19 the ASL data were missing, either due to repetition of other scans (4) or imaging artefacts (15).
The subjects were divided into 2 sets; an optimisation set containing 39 subjects, and a testing set containing the remaining 25 subjects, see Figure 1. The clinically diagnosed YOAD subjects were evenly split between the two sets, as were amyloid positive () subjects from Insight 46. Amyloid positivity was defined using mean cortical grey matter SUVR with a whole cerebellum reference region, see Supplementary Materials for details.
Figure 1.
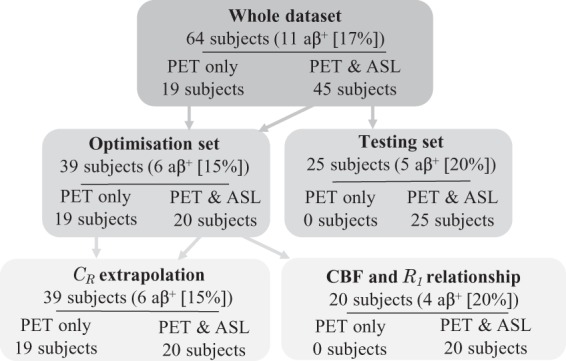
Flow chart describing data division between optimisation and testing sets, with the number of subjects defined as amyloid positive () using SUVR with a whole cerebellum reference region.
Within the optimisation set, the 20 subjects with PET and ASL data were used to derive the relationship between PET-R1 and ASL-CBF. The whole optimisation set was used to optimise the extrapolation of CR and the acquisition timing window using leave-one-out cross validation. Finally, the relationship between PET-R1 and ASL-CBF and the optimised CR method from the optimisation set was used to apply the RT-SRTM to the 25 testing set subjects, see Supplementary Materials.
CBF estimation from ASL MRI
CBF was estimated from a pseudo-continuous ASL (pCASL) acquisition with the following parameters: 3D GRASE readout21 with 36 partitions and a reconstructed voxel size of mm, TE/TR=20.3/4000 ms, 4-shot with turbo-factor/EPI-factor=14/28, bandwidth 2298 Hz/pixel; 10 control-label pairs were acquired with a pulse duration (τ) and post labelling delay (PLD) both equal to 1800 ms. Acquisition time was 5 min 20 s (). CBF maps were computed with equation (8) 22
| (8) |
with 0.9 ml/g for the plasma/tissue partition coefficient (λ), a blood T1 of 1650 ms (), and a labelling efficiency of 0.85 (α) as recommended in the ASL consensus paper.23 is the signal difference between the control and label images, S0 maps were estimated by fitting saturation recovery images acquired with the same sequence at three different saturation times (1,2,4 s) using NiftyFit.24
Dynamic PET acquisition and reconstruction
List mode PET data were acquired for 60 min following intravenous injection of [18F]-florbetapir, which targets amyloid-β. For PET image reconstruction, simultaneously acquired structural T1- and T2-weighted MR images were used to synthesise CT data and calculate the attenuation map (μ-map),25 as validated in Ladefoged et al.26 The μ-map was propagated into PET space by registering the T1-weighted images to a full 60-min non-attenuation-corrected reconstructed PET image.
Dynamic PET data were binned into 31 time frames (15 s ×4, 30 s × 8, 60 s ×9, 180 s ×2, 300 s ×8), and reconstructed into 2×2×2 mm voxels using the open source NiftyPET package.27 An ordered subset expectation maximisation (OSEM) algorithm was used with 4 iterations, 14 subsets, and a 2 mm Gaussian filter, accounting for dead-time, attenuation, scatter, randoms and normalisation.
Regional analysis
T1-weighted MR images were parcellated into 17 regions: accumbens, amygdala, brainstem, caudate, cerebellum (white and grey separately), hippocampus, cerebral white matter, pallidum, putamen, thalamus and six cortical grey matter regions, with left and right hemispheres combined;28 16 regions were used for analysis, excluding the reference region.
Analysis was performed in native space such that the T1-weighted MR image was rigidly registered to both ASL (saturation recovery image target) and PET (µ-map transformation) space, and the transformation was propagated to the parcellation.29
Statistical analysis
To compare different techniques with the gold standard, mean square error ( and mean error were used, where n is the number of estimates, is the gold standard estimate or measured value and Yi is the estimate being evaluated. To compare different techniques, statistical significance was tested using paired, two-tailed Wilcoxon signed-rank test for MSE (as the data are not normally distributed), and paired two-tailed t-tests for ME.
Results
Relationship between ASL–CBF and PET-R1
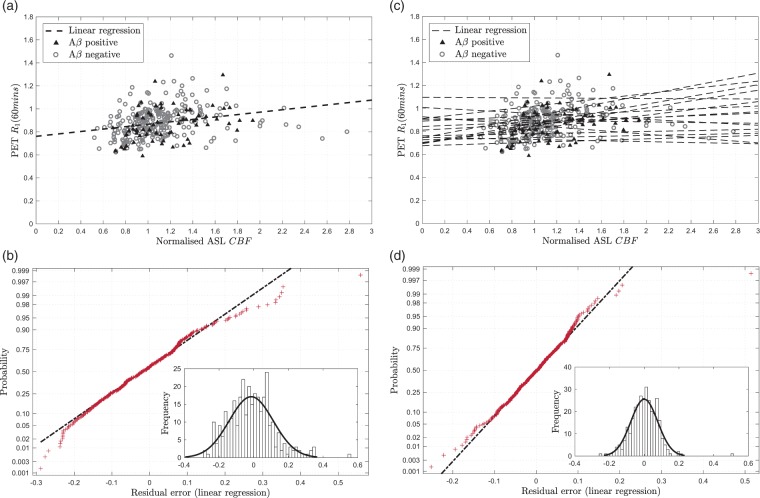
Figure 2(a) shows the relationship between PET-R1 and ASL-CBF across the 16 regions for 20 optimisation set subjects. Linear regression shows a statistically significant correlation between the two parameters (ρ = 0.349, 0.001); however, there is some variability which is not explained by this linear model. Noise and artefacts in the ASL data are considered to be the main causes of the variability; however, violations of the model assumptions may also contribute.
Figure 2.
Correlation of PET-R1 with ASL-CBF for 20 optimisation set subjects where the regression was calculated and applied to the ASL-CBF data to show the residual error in the fit. (a) PET-R1 against ASL-CBF with single linear regression (black dashed line), (b) Residual normality plot for single linear regression R1 estimation, inset: histogram of residuals, (c) PET-R1 against ASL-CBF with multi-linear regression (black dashed lines), (d) Residual normality plot for multi-linear regression R1 estimation, inset: histogram of residuals.
Residual analysis was performed to determine whether a non-linear model could be fitted to the data, as suggested by equation (2). The normality plot for the residual error in Figure 2(a) is shown in Figure 2(b). This demonstrates that the residuals follow an approximately normal distribution, which supports the use of a linear model. However, there is some deviation from normality at the extremes, which is illustrated by the histogram inset in Figure 2(b). This shows that there are some outlying positive residuals which skew the distribution.
To quantify the regional and subject effects, multi-linear analysis was also performed. Multi-linear regression using the region name as a covariate was found to explain much of the variation seen in the single linear regression and gives an R2 value of 0.650 (adjusted R2 = 0.613). Figure 2(d) shows that the residual error using multi-linear regression is lower and more normally distributed than single linear regression.
Figure 2(c) shows the multi-linear regression by region which demonstrates the variability in slope and intercept between regions. These differences can be attributed to two main causes: regional differences in bolus transit times meaning that the ASL label image is acquired before the bolus reaches the tissue, and regional differences in tracer extraction. Acquiring ASL data with multiple post-labelling delay times can be used to reduce some of this variability and would be particularly helpful when transit time changes are caused by pathology which cannot be modelled. However, this multi-linear regression using region as a covariate provides a good model for the data used here.
To quantify the subject specific component of the relationship between normalised ASL-CBF and PET-R1 which cannot be modelled in a new set of subjects, multi-linear regression with the subject as a covariate was performed. This gave an R2 value of 0.436 (adjusted R2 = 0.358) indicating that there is some variation between subjects, but that this accounts for less of the variation than the variation between regions. Finally, multi-linear regression using both region and subject as covariates yielded an R2 value of 0.862 (adjusted R2 = 0.824), showing that most of the variation can be explained by these two parameters.
There is insufficient evidence that the data used here could support a more complex non-linear model, which supports the linear Renkin–Crone model in equation (2) under the assumption that . Since the multi-linear regression using region as a covariate is better able to describe the variation in the data, this model was selected for use when applying the RT-SRTM in the subsequent sections.
Extrapolation of PET reference region, CR
Optimisation of PCA CR method
Table 1 shows the percentage of the variation described as the number of components are increased for the population of 39 subjects, . This demonstrates that six principal components are required to describe 99.9% of the variation within the data; therefore, a maximum of six components are used in the optimisation ().
Table 1.
The percentage of the variation explained using increasing number of principal components following PCA on the of 39 optimisation set subjects.
| Number of components (L) | Variation described (%) |
|---|---|
| 1 | 76.4 |
| 2 | 95.0 |
| 3 | 97.8 |
| 4 | 99.1 |
| 5 | 99.6 |
| 6 | 99.9 |
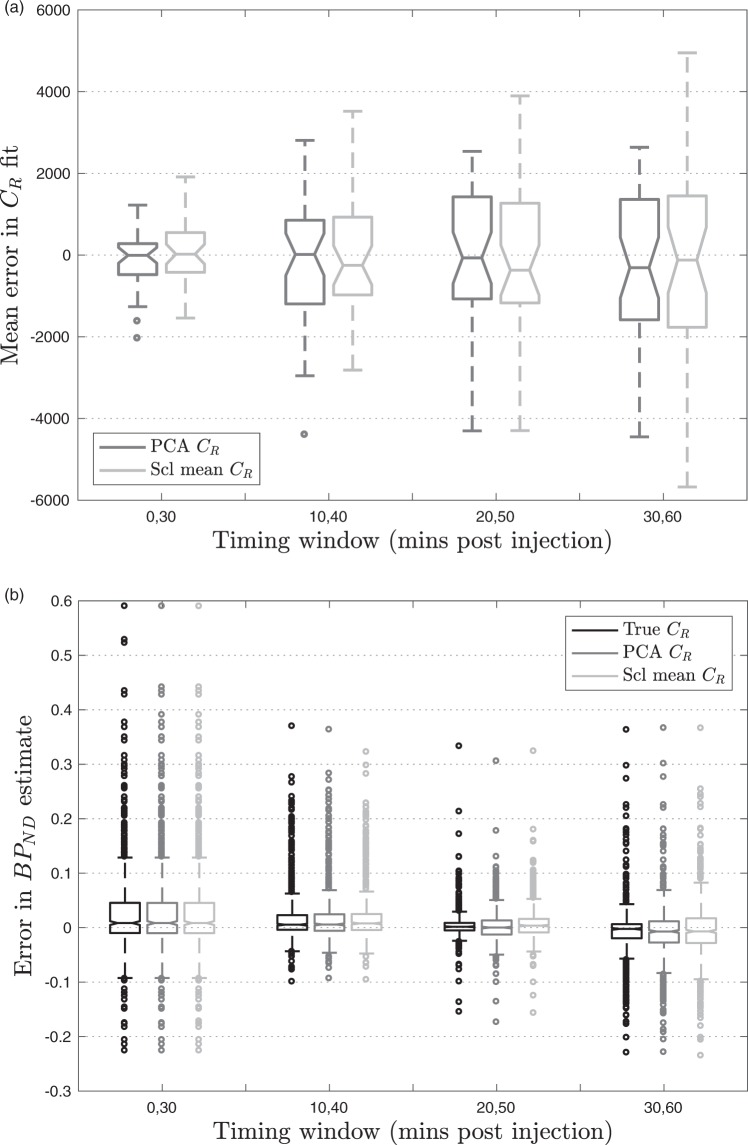
Figure 3 shows the MSE in the fit of using leave-one-out cross validation and averaged across subjects. This demonstrates that constraining the upper and lower bounds of the weights reduces the error when fitting to data with missing timepoints. The number of components used (L) has less of an influence on the error, and overall the combinations of components and weight bounds with the lowest error are with either ± 0.5 or 1 times the standard deviation for the bounds, or L = 3 with ± 1 times the standard deviation for the bounds. These three combinations give a similar MSE for ; however, with 1 times the standard deviation for the bounds was found to perform consistently better across different timing windows, and therefore this was used for comparison with the scaled mean CR method in the following sections.
Figure 3.
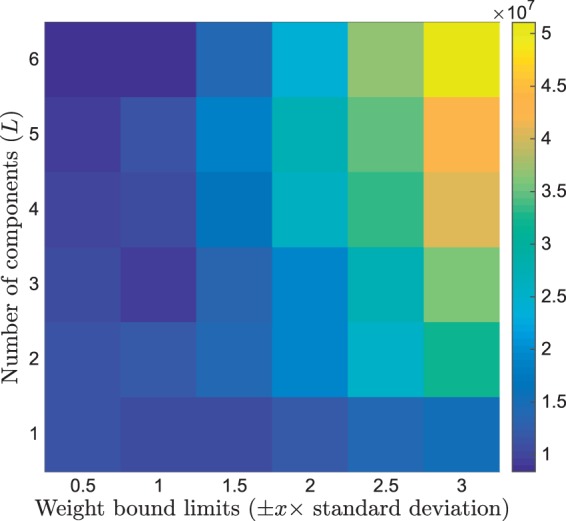
MSE in the fit of using the PCA CR method when optimising the number of components used and the upper and lower bounds for the weights using leave-one-out analysis on 39 optimisation set subjects.
Comparison of PCA CR and scaled mean CR methods
The boxplot in Figure 4(a), which summarises across all subjects, shows that the PCA CR method performs better for and ; however, the difference in MSE did not reach statistical significance at any timepoint (). The influence of this error on the estimation of at different acquisition windows is assessed in the next section.
Figure 4.
Box-plots calculated using leave-one-out cross validation in 39 optimisation set subjects. (a) ME in the PCA CR method and scaled mean CR method compared to measured CR, (b) Error in estimates across different timing windows using different estimates of CR.
Optimisation of data acquisition window
The MSE and ME in the estimation of using different data acquisition windows are shown in Table 2. Extrapolation of CR is not strictly necessary for t = 0,30 as it starts from injection; thus, the basis functions can be generated using the measured data alone. However, this leads to a higher MSE of 0.1960 due to higher errors in the computation of the convolution, and therefore the results given always extrapolate CR for t = 0,30.
Table 2.
MSE and ME between gold standard and at different 30-min acquisition windows averaged across 16 regions and 39 optimisation set subjects.
| Time window (t= ts, te) |
True CR |
PCA CR |
Mean CR |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0,10 | 10,20 | 20,30 | 30,40 | 40,50 | 50,60 | MSE | ME | MSE | ME | MSE | ME |
| • | • | • | ◯ | ◯ | ◯ | 0.0089 | 0.0303 | 0.0085 | 0.0299 | 0.0085 | 0.0299 |
| ◯ | • | • | • | ◯ | ◯ | 0.0035 | 0.0202 | 0.0036 | 0.0202 | 0.0035 | 0.0211 |
| ◯ | ◯ | • | • | • | ◯ | 0.0008 | 0.0050 | 0.0010 | 0.0041 | 0.0012 | 0.0088 |
| ◯ | ◯ | ◯ | • | • | • | 0.0030 | –0.0084 | 0.0032 | –0.0090 | 0.0034 | –0.0050 |
Note: True CR uses the true reference region curve, PCA CR and mean CR extrapolate the reference region curve using the PCA CRand scaled mean CR methods, respectively. All methods used the gold standard .
In Table 2, the true CR column uses the full measured , therefore errors are introduced purely due to the limited number of datapoints available. When the later frames are omitted and only t = 0,30 min of data are acquired, large errors are introduced as there is little information about the late phase which contains the signal relating to target binding. As the time window is shifted later, the MSE and ME are reduced.
However, the results in Table 2 also show that there is little to be gained by including data acquired more than 50 min post injection as the error increases. This is because the signal has plateaued by this point so, for a fixed 30-minute window, a better fit can be obtained by including some of the earlier data where the tracer concentration changes more rapidly over time. This is further illustrated in Figure 4(b), which shows the smallest distribution of errors in at t = 20,50.
For the two techniques which extrapolate CR from the available time window, additional variability is introduced into the estimates due to errors in the extrapolation, which is reflected by the higher MSE in Table 2. For both the PCA CR and the scaled mean CR method, the early phase of the data becomes more important, as much of the variation between the CR of subjects is contained in these frames. This can be seen in Figure 4(a), as for t = 0,30 the error in CR estimation is at a minimum and increases as the acquisition window shifts later. When the early frames are included, the error in is very similar to using the true CR, whereas when only the late frames are used, the error in for the extrapolation methods increases relative to the true CR, Figure 4(b). Table 2 shows that the optimal timing window for this technique is t = 20,50 min post injection as it yields the lowest MSE and ME.
The PCA CR method produces a consistently lower MSE which was statistically significantly lower than that obtained using the scaled mean CR method for t = 20,50 (p = 0.002). This is due to the increased flexibility in this method which allows it to better describe the variation in unseen CR shapes. The PCA CR method also produces estimates with lower bias at t = 20,50, as demonstrated by the median lines in Figure 4(b) and the ME in Table 2 (p < 0.001).
Due to the lower MSE and ME, the PCA CR method with the t = 20,50 min timing window was selected for the full implementation of the RT-SRTM in subsequent sections.
Comparison of proposed RT-SRTM with gold standard
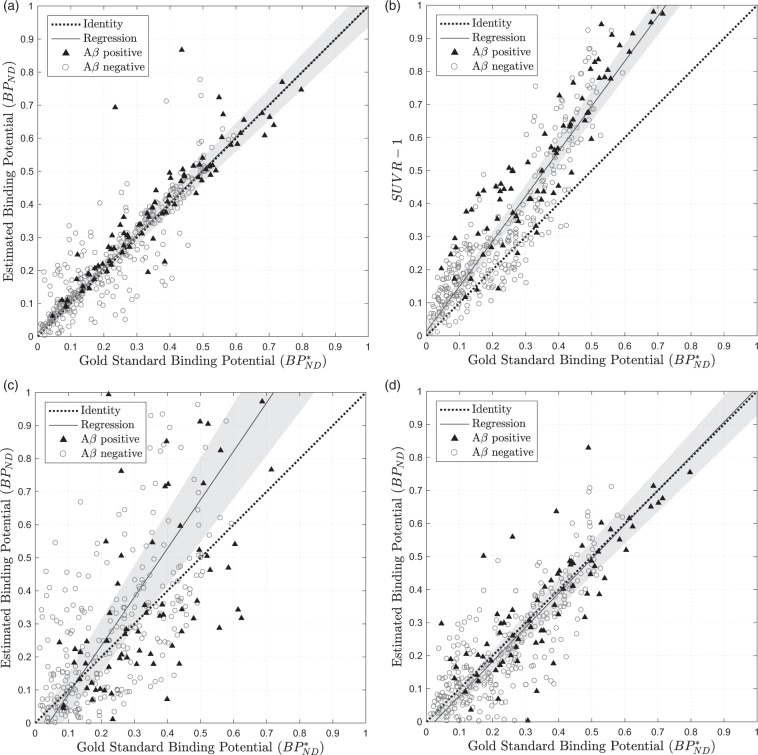
Figure 5(a) shows , estimated using the RT-SRTM with t = 20,50 min of data plotted against the gold standard . Linear regression of the data shows that the RT-SRTM method offers a good approximation of the gold standard as it closely follows the line of identity (dashed), which is within the 95% confidence interval (CI) of the regression (shaded). The linear correlation between the two estimates was tested using the Pearson correlation coefficient which demonstrated a high, statistically significant result (ρ = 0.896, p < 0.001, 95% CI [0.875, 0.914]).
Figure 5.
Estimated amyloid burden against the gold standard value calculated using full PET time series for 25 testing set subjects using 4 different methods: (a) RT-SRTM where t = 20,50 min (ASL-derived R1), (b) where t = 50,60 min, (c) SRTM where t = 0,30 min (PET data only, no CR extrapolation), (d) RT-SRTM where t = 20,50 min (PET data only, extrapolated CR). The grey-shaded region covers the 95% confidence interval in the regression.
To determine the influence of CBF derived R1 errors on , sensitivity analysis was performed as described in the supplementary materials. Briefly, PET time activity curves were simulated using the realistic parameters with a range of noise values30 and fitted using the optimised RT-SRTM with a fixed R1 error. It was found that, for regional analysis where noise < 3.4%, the mean absolute error was smaller than the blood flow component found in SUVR in Cselenyi et al.4 However, if the methodology were to be extended to voxel-wise analysis, a lower R1 error would be required due to the increased noise in the PET data which increases the uncertainty in the model fit.
Comparison of proposed RT-SRTM with
To ensure a fair comparison between RT-SRTM and SUVR, was calculated at five 10-minute acquisition windows, starting at t = 30, to cover the recommended time range and compared to the gold standard , see supplementary materials. The optimal timing window with the lowest bias and error was found to be t = 50,60 minutes post injection, which is in concordance with the literature,4 hence t = 50,60 was used for comparison.
Figure 5(b) shows the amyloid burden estimates generated for (t = 50,60 minutes). Whilst the correlation between and is evident, a positive bias is shown as overestimates the binding potential at higher values. This is due to the fact that the target and reference tissue concentrations reach equilibrium with blood plasma at different points depending on tracer binding, as has been explored in detail in the literature.31 The ME quantifies the bias between the estimates and the gold standard which is 0.1038 for , indicative of the systematic overestimation, compared to 0.0079 for the RT-SRTM method (p < 0.001). The RT-SRTM method also has a lower MSE (0.0066 compared to 0.0235 for , p < 0.001), showing that overall this technique is more accurate at estimating than the simplified technique.
This overestimation in is likely to be a combination of a linear systematic error in the estimation, as well as the influence of blood flow. Systematic error could result from estimating when not at steady-state, and could potentially be accounted for using a population correction factor.
To determine the influence of blood flow on the estimation of target density, the correlation between estimated target density and tracer delivery was calculated, as in Cselényi and Farde4 Spearman's correlation (ρ) between and the estimates was calculated, as the relationship is in theory non-linear. For the gold standard and using RT-SRTM, there was no significant correlation with (p = 0.336 and 0.106 respectively). However, for , there was a significant negative correlation (ρ = −0.226, p < 0.001), which suggests that the proposed RT-SRTM method may be more robust to changes in blood flow than .
Comparison of the proposed RT-SRTM with short acquisition time PET
Pharmacokinetic modelling can be applied to reduced acquisition time PET data without incorporating ASL data in several ways, e.g.: (i) fitting the first 30 min of PET data only using the standard SRTM in equation (1), (ii) extrapolating CR as in RT-SRTM, but R1 is estimated from the PET data.
For the first method, (i), Figure 5(c) compares the estimation of using the PET data for t = 0,30 min to the gold standard. It is evident that the absence of late-phase data leads to a high error in the estimate, which is significantly higher than using the RT-SRTM (MSE = 0.0813, p < 0.001; ME = 0.0569, p < 0.001).
Using the second method, the estimation of using t = 20,50 min of PET data and extrapolating using the PCA CR method, Figure 5(d) shows a significantly lower MSE compared to using the first 30 min (MSE = 0.0121, p < 0.001). The timing window t = 20,50 was selected by calculating the MSE for all the timing windows used in Table 2 and t = 20,50 yielded the lowest value. This method also outperforms with a lower MSE (p < 0.001) and ME (p < 0.001); however, it is significantly correlated with the gold standard (p = 0.004).
Comparison of the PET only method with the proposed RT-SRTM including ASL derived R1 estimates shows that the additional CBF information improves the estimate of , yielding a significantly lower MSE (p = 0.028), ME (p < 0.001) and variance (two-tailed F-test p < 0.001). This can be seen in Figure 5(a) where the points are more tightly clustered around the line of identity when using the proposed RT-SRTM compared to Figure 5(d) using PET data only.
Discussion
In this paper, we have presented an improved framework for quantitative PET analysis with significantly reduced acquisition time, exploiting blood flow information from simultaneously acquired ASL MRI data.
We have demonstrated that the relationship between the blood flow and tracer delivery for [18F]-florbetapir, which is described by the Renkin–Crone model, may be approximated as regionally linear and used to convert ASL relative CBF values into pseudo-R1 estimates. We have also evaluated a new technique for extrapolating the reference region time activity curve, CR, using PCA which introduces a lower error than the method used by Scott et al.,9 where the mean population is scaled. The timing of the PET acquisition was then optimised, and found to be t = 20,50 min post injection.
When the RT-SRTM estimates of using t = 20,50 min of PET data and ASL derived R1 were compared to the gold standard using the full 60 min of PET data, a strong linear correlation was found. This demonstrates that the RT-SRTM with a 30-min acquisition could potentially be used as a proxy for the full 60-min acquisition for this tracer and subject group.
By comparison, the simplified measure, , using 10 min of data showed a strong positive bias in the target density estimation, and the results were correlated with the delivery of the tracer as determined by the gold standard R1 estimates. This implies that, in addition to systematic error within the estimates, there is also a bias introduced due to local differences in blood flow. This may confound longitudinal studies, as blood flow may change over time, over the progression of disease, or due to disease modifying interventions. Conversely, the RT-SRTM estimates of target density were not correlated with R1, suggesting that this technique may be robust to changes in blood flow and could be a suitable alternative for longitudinal studies. However, this needs to be validated in a longitudinal dataset.
estimation at different timing windows showed that t = 50,60 min gave the best estimation of . Since appears to have plateaued by this point, it is unlikely that the estimation can be improved by acquiring data at a later timepoint.
This paper focused on the optimisation of a 30-min PET/MR acquisition. Simultaneous acquisition ensures that the CBF measured by ASL represents the flow at tracer injection, avoiding errors introduced by physiological flow changes throughout the day.2 This assumes negligible change in blood flow between the tracer injection and the end of the scan. This can be controlled through measures used for routine clinical PET scans, such as keeping the patient lying down in an uptake room from injection to scan start. The influence of auditory stimulation, which would be present during the ASL scan but not during tracer injection, on CBF should also be considered, and if necessary the conditions in the scanner should be emulated in the uptake bay. If conditions could be adequately controlled, the technique could be extended to separate PET and MRI acquisitions. However, this would be heavily dependent on the scheduling of the scans and the acquisition time saved is reduced compared to the simultaneous PET/MRI method.
A 30-min acquisition was selected to accommodate a typical MRI neuroimaging session, while still greatly increasing patient throughput and comfort. This could be further reduced depending on the MRI data acquired, where the minimum time is determined by the acquisition of the ASL data and the images required for attenuation correction of the PET data. In this case, using the PCA CR method with fewer principal components should be considered to avoid an under-determined problem where there are more parameters to fit than datapoints available.
The ASL data used in this study were acquired for just 5.5 min over 50 min into the scan with no motion correction and no patient restraint. For this reason, approximately 30% of the ASL-CBF maps failed quality control checks, largely due to motion-induced artefacts. This represents a challenging dataset which could be significantly improved by increasing the number of acquisitions and motion correction. However, the fact that the RT-SRTM worked so well on this dataset indicates that it could be a clinically useful tool. It is worth noting that the data acquired from the four clinically diagnosed YOAD subjects all passed the quality control checks.
The limited time available within the protocol for the ASL acquisition also meant that a single delay time between blood tagging and image acquisition was used. This yields errors in CBF estimation due to different bolus transit times for different brain regions, either due to normal physiology or pathological changes. A multi-delay time ASL acquisition would make the methodology more robust as the transit time is parameterised within the model and this will be evaluated in future work.
The RT-SRTM was here applied to an amyloid-β tracer; however, the methodology could potentially be used for any tracer which can be described by the SRTM, and which has a sufficiently high extraction fraction, such as [18F]-flutametamol, another amyloid-β tracer, or tau (τ) tracers.32 The kinetics of these tracers are slower than those of [18F]-florbetapir, and as such require longer dynamic acquisitions. Here, the RT-SRTM could potentially offer a greater reduction in acquisition time, and in future work, we intend a comparison with the dual time-window protocol, another acquisition time reduction method used on such tracers, where early and late PET data are acquired with a break in-between.33 Furthermore, our approach could be broadened to other kinetic models which have a tracer delivery parameter that can be approximated using CBF from ASL. Reference region curve extrapolation could also be used in reference Logan analysis,34 as an alternative to a previously proposed reduced acquisition time method which cannot account for blood flow changes.35
The main limitation of this study is that the optimisation of the RT-SRTM has been performed on cross-sectional dynamic scans of mostly healthy volunteers. The introduction of subjects with disease may increase the variability in CR between subjects. However, some variability already exists in the dataset used as the healthy subjects undergo normal ageing and we include four clinically diagnosed subjects, which the PCA CR method can handle. Provided that the dataset used to build the model for the PCA CR method includes diseased subjects, this variability can also be accounted for.
In future work, this technique will be validated on longitudinal data including symptomatic patients, to further investigate its robustness to disease progression and local changes in blood flow. The linear relationship found between R1 and relative CBF will require verification on such datasets to ensure that the assumption of still holds in symptomatic subjects.
Another limitation within the proposed technique is that tracer delivery is estimated directly from ASL-CBF using a region-dependent linear relationship. This works well for regional data as artefacts can be averaged out; however, this is not possible for voxel-wise analysis where errors will propagate through to the R1 estimate. A more robust methodology which propagates database R1 values into the subject space based on local image similarity has been proposed36 and will be investigated in the future. This would facilitate voxel-wise analysis for quantitative parametric imaging within a clinically feasible time frame.
Supplemental Material
Supplemental material for Reduced acquisition time PET pharmacokinetic modelling using simultaneous ASL–MRI: proof of concept by Catherine J Scott, Jieqing Jiao, Andrew Melbourne, Ninon Burgos, David M Cash, Enrico De Vita, Pawel J Markiewicz, Antoinette O'Connor, David L Thomas, Philip SJ Weston, Jonathan M Schott, Brian F Hutton and Sébastien Ourselin in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The 3D GRASE pCASL sequence used in this work was kindly provided by Dr Federico von Samson-Himmelstjerna and Professor Matthias Günther (Fraunhofer MEVIS, Bremen, Germany). We acknowledge Roger Gunn for providing help with the simplified reference tissue model and Kjell Erlandsson for the useful comments and discussion of the manuscript. We are grateful to the Insight 46 and YOAD participants for their involvement in this study.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the EPSRC UCL Centre for Doctoral Training in Medical Imaging (EP/L016478/1), UCL Leonard Wolfson Experimental Neurology Centre (PR/ylr/18575), EPSRC (EP/H046410/1, EP/J020990/1, EP/K005278), MRC (MR/J01107X/1), NIHR UCLH Biomedical Research Centre (Inc. High Impact Initiative, BW.mn.BRC10269), and the Alzheimer's Society (AS-PG-15-025). The Tesla K20 used for the PET reconstruction was donated by the NVIDIA Corporation. Insight 1946 receives funding from Alzheimer's Research UK (ARUKPG2014-1946, ARUKPG2014-1946), MRC Dementia Platform UK (CSUB19166), The Wolfson Foundation, and Brain Research Trust. The Florbetapir tracer was kindly supplied by Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly. This work was partly supported by the Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z].
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
CJS performed the analysis, data interpretation, and wrote and revised the manuscript. JJ, PJM and NB advised on PET data analysis, and AM, EDV and DLT advised on ASL analysis. JJ, AM, EDV and DLT contributed to manuscript structure. JJ, AM, EDV, DLT, AOC, PSJW, PJM, NB, DMC, JMS, BFH and SO contributed to study design, data acquisition and critically revised and approved the manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Benedictus MR, Leeuwis AE, Binnewijzend MAA, et al. Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer’s disease. Eur Radiol 2017; 27: 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkes LM, Rashid W, Chard DT, et al. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med 2004; 51: 736–743. [DOI] [PubMed] [Google Scholar]
- 3.van Berckel BNM, Ossenkoppele R, Tolboom N, et al. Longitudinal amyloid imaging using 11C-PiB: methodologic considerations. J Nucl Med 2013; 54: 1570–1576. [DOI] [PubMed] [Google Scholar]
- 4.Cselényi Z, Farde L. Quantification of blood flow-dependent component in estimates of beta-amyloid load obtained using quasi-steady-state standardized uptake value ratio. J Cereb Blood Flow Metab 2015; 35: 1485–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsiao IT, Huang CC, Hsieh CJ, et al. Correlation of early-phase 18F-florbetapir (AV-45/Amyvid) PET images to FDG images: preliminary studies. Eur J Nucl Med Mol Imag 2012; 39: 613–620. [DOI] [PubMed] [Google Scholar]
- 6.Chen YJ, Rosario BL, Mowrey W, et al. PiB relative delivery (R1) as a proxy of relative cerebral blood flow: quantitative evaluation using single session 15O-water and 11C-PiB PET. J Nucl Med 2015; 56: 1199–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan AP, Jahanian H, Holdsworth SJ, et al. Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: a systematic review. J Cereb Blood Flow Metab 2016; 36: 842–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goetti R, Warnock G, Kuhn FP, et al. Quantitative cerebral perfusion imaging in children and young adults with moyamoya disease: comparison of arterial spin-labeling–MRI andH2[15O]-PET. AJNR Am J Neuroradiol 2014; 35: 1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott CJ, Jiao J, Melbourne A, et al. ASL- incorporated pharmacokinetic modelling of PET data with reduced acquisition time: application to amyloid imaging. In: Ourselin S, Joskowicz L, Sabuncu MR, et al. (eds) MICCAI 2016. LNCS, vol. 9902, 2016, pp. 406–413. Springer, Cham. DOI: 10.1007/978-3-319-46726-9 47.
- 10.Lammertsma A, Hume S. Simplified reference tissue model for PET receptor studies. Neuroimage 1996; 158: 153–158. [DOI] [PubMed] [Google Scholar]
- 11.Wong DF, Rosenberg PB, Zhou Y, et al. In vivo imaging of amyloid deposition in Alzheimer Disease using the radioligand 18F-AV-45 (Flobetapir F 18). J Nucl Med 2010; 51: 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sojkova J, Goh J, Bilgel M, et al. Voxelwise relationships between distribution volume ratio and cerebral blood flow: implications for analysis of beta-amyloid images. J Nucl Med 2015; 56: 1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golla SSV, Verfaillie SCJ, Boellaard R, et al. Quantification of [18 F] florbetapir: a test – retest tracer kinetic modelling study. J Cereb Blood Flow Metab Epub ahead of print 13 June 2018. DOI: 10.1177/0271678X18783628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol 2004; 55: 306–319. [DOI] [PubMed] [Google Scholar]
- 15.Gunn RN, Lammertsma AA, Hume SP, et al. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 1997; 6: 279–287. [DOI] [PubMed] [Google Scholar]
- 16.Renkin E. Transport of potassium-42 from blood to tissue in isolated mammalian skeletal muscles. Am J Physiol 1959; 197: 1205–1210. [DOI] [PubMed] [Google Scholar]
- 17.Crone C. The permeability of capillaries in various organs as determined by use of the indicator diffusion method. Acta Physiol Scand 1963; 58: 292–305. [DOI] [PubMed] [Google Scholar]
- 18.Cootes TF, Taylor CJ, Cooper DH, et al. Active shape models: their training and application. Comp Vis Image Underst 1995; 61: 38–59. [Google Scholar]
- 19.Lane CA, Parker TD, Cash DM, et al. Study protocol: Insight 46 – a neuroscience sub-study of the MRC National Survey of Health and Development. BMC Neurol 2017; 17: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Günther M, Oshio K, Feinberg DA. Single-shot 3D imaging techniques improve arterial spin labeling perfusion measurements. Magn Reson Med 2005; 54: 491–498. [DOI] [PubMed] [Google Scholar]
- 22.Buxton RB, Frank LR, Wong EC, et al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 1998; 40: 383–396. [DOI] [PubMed] [Google Scholar]
- 23.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled Perfusion mri for clinical applications: a consensus of the ISMRM Perfusion Study group and the European consortium for ASL in dementia. Magn Reson Med 2015; 73: 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melbourne A, Toussaint N, Owen D, et al. niftyfit: a software package for multi-parametric model-fitting of 4D magnetic resonance imaging data. Neuroinformatics 2016; 14: 319–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgos N, Cardoso MJ, Thielemans K, et al. Multi-contrast attenuation map synthesis for PET/MR scanners: assessment on FDG and Florbetapir PET tracers. Eur J Nucl Med Mol Imag 2015; 42: 1447–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladefoged CN, Law I, Anazodo U, et al. A multi-centre evaluation of eleven clinically feasible brain PET/MRI attenuation correction techniques using a large cohort of patients. Neuroimage 2017; 147: 346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markiewicz PJ, Ehrhardt MJ, Erlandsson K, et al. NiftyPET: a high-throughput software platform for high quantitative accuracy and precision PET imaging and analysis. Neuroinformatics 2018; 16: 95–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardoso MJ, Modat M, Wolz R, et al. Geodesic information flows: spatially-variant graphs and their application to segmentation and fusion. IEEE Trans Med Imag 2015; 34: 1976–1988. [DOI] [PubMed] [Google Scholar]
- 29.Modat M, Cash DM, Daga P, et al. Global image registration using a symmetric block-matching approach. J Med Imag 2014; 1: 024003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ichise M, Liow J-S, Lu J-Q, et al. Linearized reference tissue parametric imaging methods: application to 11C.DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab 2003; 23: 1096–1112. [DOI] [PubMed] [Google Scholar]
- 31.Slifstein M. Revisiting an old issue: the discrepancy between tissue ratio-derived binding parameters and kinetic modeling-derived parameters after a bolus of the serotonin transporter radioligand 123I-ADAM. J Nucl Med 2008; 49: 176–178. [DOI] [PubMed] [Google Scholar]
- 32.Baker SL, Lockhart SN, Price JC, et al. Reference tissue-based kinetic evaluation of 18F-AV-1451 in aging and dementia. J Nucl Med 2016; 5279: 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bullich S, Barthel H, Koglin N, et al. Validation of noninvasive tracer kinetic analysis of 18 F-florbetaben PET using a dual–time-window acquisition protocol. J Nucl Med 2018; 57: 1104–1110. [DOI] [PubMed] [Google Scholar]
- 34.Logan J, Fowler JS, Volkow ND, et al. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 1996; 16: 834–840. [DOI] [PubMed] [Google Scholar]
- 35.Tantawy MN, Jones CK, Baldwin RM, et al. 18F.Fallypride dopamine D2 receptor studies using delayed microPET scans and a modified Logan plot. Nucl Med Biol 2009; 36: 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott CJ, Jiao J, Cardoso MJ, et al. Short acquisition time PET quantification using MRI-based pharmacokinetic parameter synthesis. In: Descoteaux M, Maier-Hein L, Franz A, et al. (eds) MICCAI 2017. LNCS, vol. 10434, 2017, pp. 737–744. Springer, Cham. doi:https://doi.org/10.1007/978-3-319-66185-8_83.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Reduced acquisition time PET pharmacokinetic modelling using simultaneous ASL–MRI: proof of concept by Catherine J Scott, Jieqing Jiao, Andrew Melbourne, Ninon Burgos, David M Cash, Enrico De Vita, Pawel J Markiewicz, Antoinette O'Connor, David L Thomas, Philip SJ Weston, Jonathan M Schott, Brian F Hutton and Sébastien Ourselin in Journal of Cerebral Blood Flow & Metabolism