Abstract
Six γ-oxa-ε-lactones, 4-phenyl-3,4-dihydro-2H-1,5-benzodioxepin-2-one (5a) and its five derivatives with methoxy groups in different positions of A and B rings (5b–f), were synthesized from corresponding flavanones. Three of the obtained lactones (5b,c,f) have not been previously described in the literature. Structures of all synthesized compounds were confirmed by complete spectroscopic analysis with the assignments of signals on 1H and 13C-NMR spectra to the corresponding atoms. In most cases, lactones 5a–f exerted an inhibitory effect on the growth of selected pathogenic bacteria (Escherichia coli, Bacillus subtilis, and Staphylococcus aureus), filamentous fungi (Fusarium graminearum, Aspergillus niger, and Alternaria sp.), and yeast (Candida albicans). The broadest spectrum of activity was observed for unsubstituted lactone 5a, which was particularly active against filamentous fungi and yeast. Lactones with methoxy groups in the 3′ (5c) and 4′ (5d) position of B ring were more active towards bacteria whereas lactone substituted in the 7 position of the A ring (5e) exhibited higher antifungal activity. In most cases, the introduction of lactone function increased the activity of the compound compared to its flavonoid precursors, chalcones 3a–e, and flavanones 4a–f.
Keywords: ε-lactones, chalcones, flavanones, antibacterial activity, anifungal activity, lag-phase, optical density
1. Introduction
Antibiotic resistance is a serious problem on a global scale, becoming the biggest challenge for global health, food security, and development today. Statistics show that 70% of two million bacterial infections in United States hospitals are caused by the strains resistant to at least one drug [1]. In Great Britain, 50% of Staphylococcus aureus strains are resistant to methicillin. A growing number of diseases, such as tuberculosis, pneumonia, or salmonellosis, are becoming difficult to treat as antibiotics become less effective, which leads to longer hospital stays, increasing medical costs and mortality. Another serious problem are fungal diseases, which affect over a billion people. For example, 3,000,000 cases of chronic pulmonary aspergillosis, about 700,000 cases of invasive candidiasis, over 10,000,000 cases of fungal asthma, and about 1,000,000 cases of fungal keratitis have been noticed annually. Early diagnosis allows the application of proper therapy; incorrect treatment may cause complications, including serious chronic illness or even death [2].
Therefore, compounds inhibiting the growth of microorganisms are still isolated and synthesized. One of the extensively studied compounds in this field are those with lactone ring. Many groups of natural or synthetic sesquiterpenes with an α- methylene-γ-lactone ring inhibit the growth of fungi [3,4], bacteria [5], or viruses [6]. Antimicrobial activity has been proven for bicyclic γ-lactones with an alkylsubstituted cyclohexane or cyclohexene ring, including hydroxylactones and epoxylactones obtained by biotransformation [7,8,9], synthetic δ-halo-γ-lactones [10], or products of their reductive dehalogenation [11]. Simple alkylsubstituted lactones found in food as flavor substances, namely γ-decalactone, δ-decalactone, and whisky lactone, limited the growth of pathogenic strains of Candida albicans and Trichophyton rubrum [12].
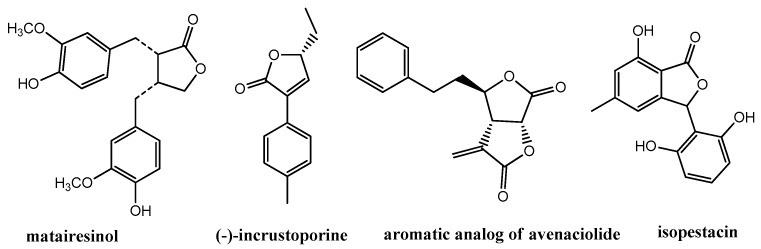
Antimicrobial activity is also a characteristic property of natural lactones and their synthetic analogues containing aromatic rings (Figure 1). Some lignane lactones, matairesinol and its oxidized derivatives, showed activity against some gram-negative bacteria, i.e., Bacillus subtilis and Staphylococcus aureus [13]. Antifungal activity, mainly against strains of Candida, was found for series of 3-(substituted phenyl)-5-alkyl-2,5-dihydrofuran-2-ones related to a natural product, (−)-incrustoporine [14,15,16]. Aromatic bis-γ-lactones analogous to avenaciolide displayed activity against Colletotrichum gloeosporioides [17] whereas 3,4-diphenyl-α-methylene-γ-butyrolactone turned out to be a lead scaffold for discovering compounds with high activity against Colletotrichum lagenarium [18]. β-Aryl-γ-lactones derived from substituted benzaldehydes inhibited the growth of some Fusarium strains [19] or selected bacteria [20]. An interesting example is also the strong inhibition of plant pathogenic fungi by isopestacin, the compound with the γ-lactone ring obtained from the endophytic fungus Pestalotiopsis microspora [21].
Figure 1.
Lactones with aromatic rings exhibiting antimicrobial activity.
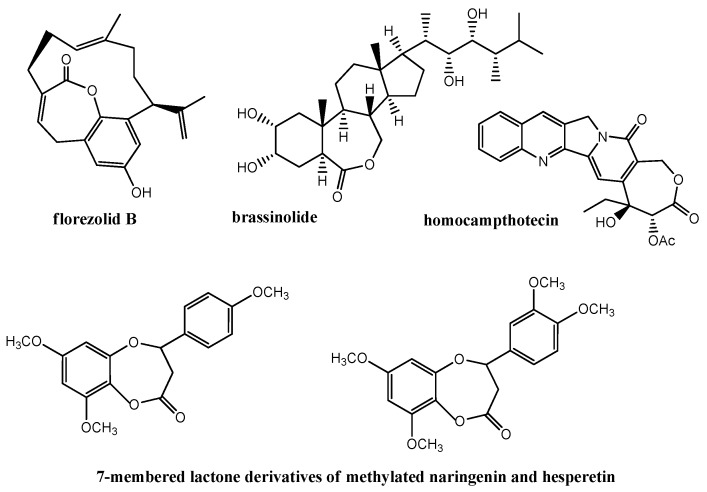
Among the biologically active lactones, only a few examples of those with a seven-membered ring are reported (Figure 2). Cytotoxic activity against KB tumor cells was proven for florezolid B isolated from an extract of an ascidian of genus Aplidium [22]. Numerous groups of compounds containing ε-lactone rings are brassinosteroids (e.g., brassinolide), which are essential for normal plant growth by promoting cell elongation [23]. Expanding the δ-lactone ring of natural alkaloid, camphtotecin [24], to a seven-membered lactone ring significantly increased the stability of the molecule. The obtained analogue, homocampthotecin and its derivatives, retained antiproliferative activity against different cancer cell lines [25,26].
Figure 2.
Examples of biologically active ε-lactones.
Due to our interest in the synthesis of biologically active lactones with aromatic rings, we also paid attention to flavanone-derived ε-lactones. Some of them have been obtained earlier by the oxidation of flavanones [27,28]. Two compounds from this group, shown in Figure 2 above, were obtained from methylated naringenin and hesperetin and evaluated for apoptic activity against thee E2 human lymphoma cell line. They were found to be more active than the corresponding flavanone precursors [28].
The antimicrobial activity of chalcone 3a and flavanone 4a as well as their methoxy-substituted derivatives 3a–e and 4a–f was studied previously. Chalcones 3a, 3b, 3d, and 3e and flavanones 4a and 4d have been tested against methicillin-resistant Staphylococcus aureus [29,30], chalcone 3e against Candida albicans [31], and chalcones 3e and 3b against Mycobacterium tuberculosis [32]. Flavanones 4a, 4d, and 4f were tested against the following bacterial strains: Staphylococcus aureus, Shiegella sonnei, Shiegella dysenteriae, Salmonella typhimurium, Escherichia coli, Vibrio cholerae, Vibrio parahemolyticus, and Enterobacter sakazakii [33,34]; whereas flavanone 4e was tested against the following fungal strains: Trichoderma koningii, Fusarium oxysporum, and Cladosporium cladosporioides [35]. In this work, we would like to present the results of the antimicrobial activity of a series of flavanone-derived γ-oxa-ε-lactones 5a–f and their flavonoid precursors, the corresponding chalcones 3a–e and flavanones 4a–f, against selected pathogenic bacteria, filamentous fungi, and yeast. To the best of our knowledge, this kind of biological activity has not been evaluated so far for flavanone-derived ε-lactones. The main goal of this research was to determine the effect of the introduction of a lactone moiety into the flavanoid skeleton on the activity of the studied compounds.
2. Results and Discussion
2.1. Synthesis
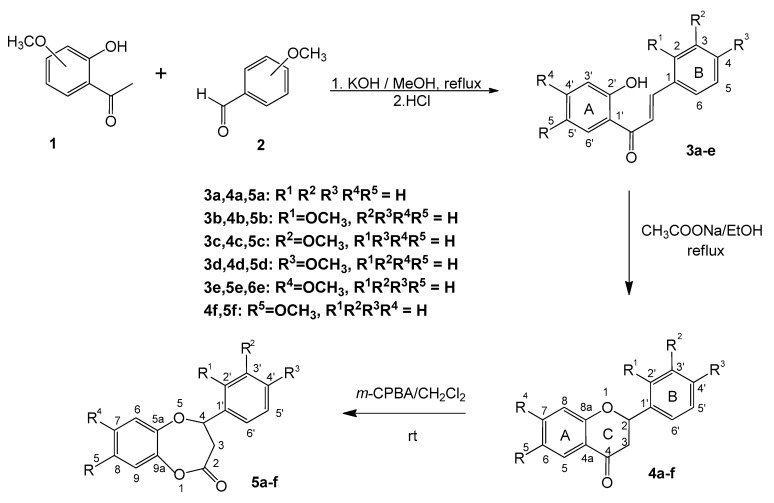
The γ-oxa-ε-lactones were obtained in a three-step synthesis (Scheme 1). The first step of the synthetic route was Claisen–Schmidt condensation between corresponding 2′-hydroxyacetophenones and benzaldehydes under alkaline conditions using a standard procedure [36] to afford 2′-hydroxychalcones 3a–e in a 69% to 94% yield. Their physical and spectroscopic data were in accordance with detailed literature data [37,38]. Cyclization of 2′-hydroxychalcones 3a–e in the presence of sodium acetate [39] yielded flavanones 4a–e in a 61% to 79% yield. Synthesized flavanones 4a–e and commercially available 6-methoxyflavanone 4f, all of them characterized spectrally in the literature [37,40,41,42], were regioselectively oxidized with m-CPBA to corresponding γ-oxa-ε-lactones 5a–f in which oxygen was introduced into the C ring between the carbonyl atom C-4 and atom C-4a.
Scheme 1.
Synthesis of ε-lactones 5a–f.
2.2. Structural Analysis of ε-Lactones 5a–f
The structures of synthesized γ-oxa-ε-lactones 5a–f were confirmed by spectroscopic methods (IR, NMR). Three of the synthesized lactones (5b,c,f) were new compounds that have not been described previously. Apart from 1H-NMR and 13C-NMR spectroscopy, we also used two-dimensional spectroscopic techniques, namely COSY, HSQC, and HMBC, to assign as many signals as possible on the NMR spectra to the corresponding protons and carbons, which in the case of spectroscopic characterization of known lactones 5a,d,e was not reported earlier [27,43]. Full spectroscopic description of three known (5a,d,e) as well as three newly synthesized (5b,c,f) lactones was helpful for the determination of the effect of the methoxy group on the chemical shifts of corresponding atoms.
The presence of the lactone moiety was confirmed in the IR spectra by absorption bands corresponding to C=O and C-O bonds in the region of 1766 to 1773 cm−1 and 1205 to 1236 cm−1, respectively. One can see a significant difference between the absorption of carbonyl moiety in these seven-membered rings in comparison with flavanones 4a–f, in which the carbonyl group absorbs in the region of 1670 to 1690 cm−1 [44]. On the 1H-NMR spectra, the protons of the CH2-3 methylene group are non-equivalent (diastereotopic) due to the presence of a chiral center and they couple to each other and the adjacent methine C-4 proton. As a result, their signals are observed as two doublets of doublets, with the higher (J = 13.2 Hz) and lower (in the range 4.8–7.3 Hz) coupling constant. These signals are located in the region of 3.00 to 3.20 ppm and the difference between their chemical shift (Δδ) is significantly lower when compared to the flavanone precursors 4a–f, in which one of the protons from the methylene CH2-3 group is shifted upfield to the range 2.88–2.90 ppm [41]. Methine proton H-4 in lactones 5a–f resonates in the region of 5.70 to 6.00 ppm and is slightly shifted upfield compared to the flavanones 4a–f. Relatively small differences between vicinal coupling constants of H-4 with both methylene protons at C-3 suggest that, in these lactones, methine proton H-4 is oriented gauche to these protons, which implies that the phenyl substituent at C-4 is in the pseudoequatorial position. These assignments were in accordance with previous studies on flavanone-derived γ-oxa-ε-lactones [43].
Generally, in the case of lactones with unsubstituted benzene rings, most of the aromatic protons are represented by overlapping multiplets at 6.90 to 7.20 (protons from the A-ring) and 7.36 to 7.42 ppm (protons from the B-ring). However, in the spectra of two lactones with an unsubstituted A ring (5a and 5c), a doublet from H-9 (J = 7.7 and 7.6 Hz, respectively) can be easily recognized because of the shielding effect of the ester group at the ortho position. The result of this effect is the location of H-9 signals at the higher field (7.04 and 7.07 ppm, respectively) in comparison with other aromatic protons.
Comparative analysis of spectroscopic data for methoxy-substituted derivatives 5b–f showed the effect of this group on the chemical shift of the aromatic protons and carbons. As a strong electron-donating substituent, the methoxy group increases the electronic density of the benzene ring, particularly on the ortho and para positions. As a result, the protons on these positions become more shielded than the meta-situated protons. The observed electronic effect of the methoxy group facilitated the assignment of most of the proton signals in the NMR spectra of lactones 5b–f. In all lactones 5a–f, three-proton singlets from these groups are observed at 3.75 to 3.84 ppm. The location of the -OCH3 group at C-7 in lactone 5e determines the chemical shifts of H-6, H-8, and H-9 protons. The signal from H-6, which is coupled only with H-8 (J = 2.8 Hz), is located at the higher field (6.58 ppm) because of the shielding effect of the ortho-situated methoxy group at C-7 and oxygen atom O-5. The signal from H-8 appears at 6.71 ppm as a doublet of doublets (J = 9.0 and 2.8 Hz). The doublet of H-9 is located at thee significantly lower field (7.10 ppm) as a result of the deshielding effect of the meta-situated methoxy group on this proton.
Substitution of the A ring with the methoxy group at C-8 (lactone 5f) influences the chemical shifts of H-6 and H-9. The signals of these protons are visible as doublets; H-6 (J = 9.0 Hz) at the lower field (6.93 ppm) and H-9 (J = 3.0 Hz) at the higher field (6.74 ppm), which is the effect of the shielding effect of the methoxy group on the ortho proton H-9 and the deshielding effect of this group on the meta proton H-6.
The location of the methoxy group at the B ring has a significant effect on the location of signals from aromatic protons H-2′-H-5′. Taking into account the deshielding effect of this group on the protons at meta positions, in the spectrum of lactone 5b, signals shifted towards the lower field, namely the triplet of doublets (J = 7.8 and 1.8 Hz) at 7.34 ppm and doublet of doublets (J = 7.8 and 1.8 Hz) at 7.61 ppm, belonging to H-4′ and H-6′, respectively. A reverse effect can be observed for protons at the ortho (H-3′) and para (H-5′) position, which are represented by a doublet of doublets (J = 7.8 and 0.6 Hz) at 6.93 ppm and triplet of doublets (J = 7.8 and 0.6 Hz) at 7.01 ppm, respectively.
In the case of lactone 5c, the deshielding effect of the methoxy group at C-3′ applies only to proton H-5’ at the meta position and its signal is clearly shifted downfield (7.31 ppm), whereas other protons located at ortho or para positions are shielded by this substituent. A lack of neighboring protons allows the assignment of the singlet at 6.95 ppm to proton H-2′. Assignments of the two remaining signals to corresponding protons are possible using HMBC spectrum in which only the multiplet at 6.91 ppm correlates with signal from C-3′ which clearly shows that this multiplet comes from proton H-4′ whereas the multiplet at 6.94 ppm must be assigned to proton H-6′.
The substitution pattern of the B ring in ε-lactone 5d generates the symmetry of this aromatic fragment of the molecule. Thus, the multiplet of protons located at the ortho position towards the methoxy group (H-3′, H-5′) is observed at the higher field (6.90–6.92 ppm), whereas the multiplet of protons located at meta positions (H-2′, H-6′) is observed at the lower field (7.28–7.29 ppm).
Many similarities can be observed in the 13C-NMR spectra of ε-lactones 5a–f. Signals from C-2, observed at about 168 ppm, are characteristic for carbonyl atoms in seven-membered lactone rings. Signals from C-3 are located at about 38 ppm, whereas signals from C-4 are generally shifted downfield to approximately 80 ppm by the neighboring oxygen O5. In comparison with corresponding flavanones [41], in which the signal from C-4a was observed in the range 121–122 ppm, the signal of this carbon in lactones 5a–f (C-9a) was significantly shifted downfield, to the range 139–146 ppm, which unequivocally confirmed the introduction of oxygen in the Baeyer–Villiger reaction between the benzene ring and the carbonyl group. Analysis of the correlations on the HSQC and HMBC spectra enabled the assignment of most of the signals at the 13C-NMR spectra to the corresponding carbon atoms.
2.3. Antimicrobial Activity Assay
Compounds were tested for their antimicrobial activity against selected strains of pathogenic bacteria (Escherichia coli, Bacillus subtilis, Staphylococcus aureus), filamentous fungi (Fusarium graminearum, Aspergillus niger, Alternaria sp.), and yeast (Candida albicans). The results presented in Table 1 and Table 2 show the duration of the lag phase and changes in the optical density (ΔOD), with both values determined on the basis of the resultant microbial growth curves. Two main aspects were taken into consideration analyzing the results: The effect of the location of the methoxy substituent on the activity of lactones 5a–f and the comparison of the activity of lactones 5a–f with their precursors: 2′-hydroxychalcones 3a–e and flavanones 4a–f.
Table 1.
Effect of chalcones 3a–e, flavanones 4a–f, and ε-lactones 5a–f on the growth of selected bacterial strains.
| Compound | Escherichia coli | Bacillus subtilis | Staphylococcus aureus | ||||
|---|---|---|---|---|---|---|---|
| Lag-Phase [h] | ΔOD 1 | Lag-Phase [h] | ΔOD | Lag-Phase [h] | ΔOD | ||
| chalcones | 3a | n.a.3 | 0 ± 0.01 2 | 13.5 | 0.15 ± 0.02 | n.a. | 0 ± 0.01 |
| 3b | 29 | 0.34 ± 0.09 | n.a. | 0 ± 0.01 | 4.5 | 0.36 ± 0.04 | |
| 3c | n.a. | 0 ± 0.03 | n.a. | 0 ± 0.01 | n.a. | 0 ± 0.03 | |
| 3d | 10 | 0.31 ± 0.08 | 15 | 0.79 ± 0.20 | 17.5 | 0.32 ± 0.06 | |
| 3e | 14.5 | 0.52 ± 0.09 | n.a. | 0 ± 0.03 | 21 | 0.31 ± 0.07 | |
| flavanones | 4a | 4.5 | 0.42 ± 0.05 | 5.5 | 0.76 ± 0.09 | 11 | 0.86 ± 0.03 |
| 4b | 22.5 | 0.26 ± 0.06 | 31.5 | 0.35 ± 0.08 | 33.5 | 0.31 ± 0.06 | |
| 4c | 21 | 0.44 ± 0.01 | 31.5 | 0.60 ± 0.04 | 30.5 | 0.63 ± 0.03 | |
| 4d | 28 | 0.27 ± 0.01 | 15 | 0.78 ± 0.09 | 29.5 | 0.26 ± 0.06 | |
| 4e | 27 | 0.26 ± 0.06 | n.a. | 0 ± 0.07 | 5 | 0.50 ± 0.09 | |
| 4f | 30 | 0.30 ± 0.07 | 13 | 0.57 ± 0.09 | 22.5 | 0.27 ± 0.02 | |
| ε-lactones | 5a | 21 | 0.43 ± 0.05 | 7 | 1.04 ± 0.03 | n.a. | 0 ± 0.01 |
| 5b | 12 | 0.72 ± 0.08 | 21 | 0.44 ± 0.04 | 21 | 0.38 ± 0.06 | |
| 5c | n.a. | 0 ± 0.07 | n.a. | 0 ± 0.02 | n.a. | 0 ± 0.05 | |
| 5d | n.a. | 0 ± 0.09 | 17.5 | 0.31 ± 0.09 | n.a. | 0 ± 0.04 | |
| 5e | 22 | 0.33 ± 0.08 | 7.5 | 0.77 ± 0.09 | 28 | 0.25 ± 0.06 | |
| 5f | 13.5 | 0.58 ± 0.05 | n.a. | 0 ± 0.04 | n.a | 0 ± 0.03 | |
| Control cultures 4 | 2 | 1.77 ± 0.09 | 3 | 1.27 ± 0.10 | 4 | 1.58 ± 0.11 | |
1 Results presented as means ± standard deviation (SD). 2 Underlined number means the average significantly different from control. 3 n.a.—not applicable. 4 Control cultures were cultivated in the medium supplemented with DMSO without tested compounds
Table 2.
Effect of chalcones 3a–e, flavanones 4a–f, and ε-lactones 5a–f on the growth of selected filamentous fungi and yeast.
| Compound | Fusarium graminearum | Aspergillus niger | Alternaria sp. | Candida albicans | |||||
|---|---|---|---|---|---|---|---|---|---|
| Lag-Phase [h] | ΔOD 1 | Lag-Phase [h] | ΔOD | Lag-Phase [h] | ΔOD | Lag-Phase [h] | ΔOD | ||
| chalcones | 3a | 26 | 1.45 ±0.01 2 | 15 | 1.69 ± 0.01 | 42 | 0.28 ± 0.06 | 39 | 0.26 ± 0.09 |
| 3b | n.a.3 | 0 ± 0.04 | 15 | 1.36 ± 0.12 | 48 | 0.21 ± 0.07 | n.a. | 0 ± 0.03 | |
| 3c | 46 | 0.51 ± 0.09 | 24.5 | 0.61 ± 0.07 | n.a. | 0 ± 0.01 | 14 | 1.12 ± 01 | |
| 3d | 58.5 | 0.23 ± 0.06 | 17.5 | 1.07 ± 0.09 | 57 | 0.12 ± 0.03 | 20.5 | 0.88 ± 0.10 | |
| 3e | n.a. | 0 ± 0.07 | 14 | 0.68 ± 0.06 | n.a. | 0 ± 0.01 | n.a. | 0 ± 0.04 | |
| flavanones | 4a | 22 | 0.36 ± 0.09 | n.a. | 0 ± 0.06 | n.a. | 0 ± 0.07 | n.a. | 0 ± 0.05 |
| 4b | 42 | 0.12 ± 0.02 | 17.5 | 0.67 ± 0.09 | n.a. | 0 ± 0.06 | 18 | 0.33 ± 0.06 | |
| 4c | 28 | 0.16 ± 0.08 | 31.5 | 0.35 ± 0.08 | n.a. | 0 ± 0.01 | 16 | 0.53 ± 0.08 | |
| 4d | n.a. | 0 ± 0.01 | 27 | 0.38 ± 0.04 | n.a. | 0 ± 0.01 | 23 | 0.49 ± 0.07 | |
| 4e | 53 | 0.18 ± 0.06 | 13 | 1.51 ± 0.09 | 9.5 | 0.32 ± 0.01 | 13 | 1.66 ± 0.06 | |
| 4f | 27.5 | 0.60 ± 0.02 | 36 | 1.36 ± 0.10 | n.a. | 0 ± 0.01 | 15.5 | 0.52 ± 0.09 | |
| ε-lactones | 5a | n.a. | 0 ± 0.01 | n.a. | 0 ± 0.01 | n.a. | 0 ± 0.01 | n.a. | 0 ± 0.04 |
| 5b | n.a. | 0 ± 0.01 | 15 | 1.48 ± 0.02 | n.a. | 0 ± 0.06 | 16.5 | 1.16 ± 0.07 | |
| 5c | 35 | 0.17 ± 0.01 | 20 | 0.85 ± 0.02 | 46 | 0.58 ± 0.07 | 20 | 1.22 ± 0.08 | |
| 5d | 46.5 | 0.43 ± 0.08 | 13 | 2.13 ± 0.10 | n.a. | 0 ± 0.01 | 10 | 0.22 ± 0.06 | |
| 5e | n.a. | 0 ± 0.03 | 43 | 0.20 ± 0.02 | n.a. | 0 ± 0.01 | n.a. | 0 ± 0.04 | |
| 5f | n.a. | 0 ± 0.00 | 15 | 1.34 ± 0.02 | 28 | 0.70 ± 0.05 | n.a. | 0 ± 0.05 | |
| Control cultures 4 | 12.5 | 0.81 ± 0.01 | 12.5 | 1.19 ± 0.17 | 9 | 1.04 ± 0.09 | 8 | 1.03 ± 0.01 | |
1 Results presented as means ± Standard Deviation (SD). 2 Underlined number means the average significantly different from control. 3 n.a.—not applicable. 4 Control cultures were cultivated in the medium supplemented with DMSO without tested compounds
2.3.1. The Effect of the Methoxy Group on the Activity of Lactones 5a–f
In most experiments tested, ε-lactones exerted an inhibitory effect on the growth of the tested strains by the prolongation of the lag phase and lowering the ΔOD of the microbial cultures in comparison to the controls. The broadest spectrum of activity was observed for unsubstituted compound 5a, which completely inhibited the growth of S. aureus (Table 1) and all tested fungi and yeast (Table 2). The presence of the methoxy group at C-7 (lactone 5e) did not significantly affect the mentioned activity but increased the growth inhibition of E. coli and B. subtilis (Table 1). Compared to lactone 5a, the presence of the methoxy group in the C-8 position of the A ring (lactone 5f) resulted in the total inhibition of B. subtilis (Table 1) whereas the growth of A. niger was not inhibited at all by this compound (Table 2). In the case of E. coli (Table 1) and Alternaria sp. (Table 2), the inhibitory effect of lactone 5f was lower in comparison with lactone 5a. Similar to unsubstituted ε-lactone 5a, its derivatives with methoxy groups in the A ring (5e,f) turned out to be total growth inhibitors of the yeast C. albicans (Table 2). In contrast, among the three lactones with different substitution patterns of ring B, only 4′-methoxyderivative 5d exhibited some activity against this strain. Lactones 5b–d were not active against A. niger but inhibited strongly (5c) or very strongly (5b,d) the growth of Alternaria sp. (Table 2). In the case of F. graminearum (Table 2), the location of the methoxy group in the B ring affected the degree of inhibition: The most active was 2′-methoxylactone 5b and the least active was 4′-methoxyderivative 5d. Considering antibacterial activity (Table 1), the positive impact of the methoxy groups in the B ring was observed particularly at the 3′ and 4′ position. Complete growth inhibition of S. aureus and E. coli was found for lactones 5c and 5d. For lactone 5c, a similar effect was also noticed in the case of B. subtilis.
2.3.2. Effect of the Introduction of the Lactone Moiety into the Flavanoid Skeleton on the Antimicrobial Activity
Apart from ε-lactones, we also tested the antimicrobial activity of their corresponding precursors, 2′-hydroxychalcones 3a–e and flavanones 4a–f, to assess the effect of the lactone ring on the activity of flavanone-derived lactones. In many cases, ε-lactones were more active than both their precursors. Considering antibacterial activity (Table 1), the most distinct effect of the lactone ring on the inhibitory effect of the studied compounds was observed in the case of lactone 6d, which completely inhibited the growth of E. coli and S. aureus and was a strong inhibitor of B. subtilis. Inhibition of these microorganisms by chalcone 3d and flavanone 4d was significantly lower. A positive effect of lactone function on the activity was also observed in the group of compounds with a methoxy group at position 7 of the A ring. A significantly higher inhibitory effect was noticed for 8-methoxylactone 5f compared to its parent flavanone 4f but only towards B. subtilis and S. aureus, whereas E. coli was more sensitive to flavanone 4f than lactone 3f. In turn, 7-methoxylactone 5e was more active towards S. aureus than both chalcone 3e and flavanone 4e. In the case of compounds with a methoxy group at position 3′ of the B ring, both lactone 5c and chalcone 3c were strong inhibitors against three bacterial strains, whereas flavanone 4c exhibited lower activity. The same dependence was observed for unsubstituted compounds 3a,4a,5a in the tests with S. aureus. In some cases, ε-lactone was more active than the corresponding flavanone but less active than chalcone; in others, the following order of compounds in terms of increasing activity can be observed: Chalcone < ε-lactone < flavanone. The first case was observed for compounds with unsubstituted benzene rings 3a,4a,5a in the tests carried out with E. coli; the latter case was found for the activity of compounds with the 2′-methoxy-substituted B ring (3b,4b,5b) towards S. aureus and those with a 7-methoxy-substituted A ring (3e,4e,5e) towards E. coli. Only in the case of the activity of compounds 3b,4b,5b towards E. coli and B. subtilis and compounds 3a,4a,5a towards B. subtilis did the introduction of the lactone ring not support the inhibitory effect of the compound in comparison with both chalcone and flavanone.
The most noticeable increase of antifungal activity (Table 2) after the introduction of the lactone moiety into the flavanoid molecule was observed in the case of unsubstituted compounds 4a,5a,6a. Lactone 5a was a significantly stronger inhibitor towards F. graminearum than both chalcone 3a and flavanone 4a; in the case of A. niger, Alternaria sp., and C. albicans, this activity was as strong as the activity of flavanone 4a but still considerably higher compared with chalcone 3a. Substitution of A ring at position 7 resulted in a significant increase of activity of lactone 5e compared to its flavanone precursor 4e towards filamentous fungi A. niger, F. graminearum, and Alternaria sp. as well as yeast C. albicans. In the cases of the last three strains, a comparably strong inhibition was also observed for starting chalcone 3e. Lactone with the methoxy group at the 8 position of the A ring (5f) was a significantly stronger inhibitor of F. graminearum and C. albicans than flavanone 4f, whereas a reverse relationship was found in the case of Alternaria sp.
The substitution pattern of the B ring also exhibited an impact on the antifungal and anti-yeast activity of lactones in relation to the corresponding flavanoid precursors. A negative effect on the activity towards filamentous fungi was found for lactones with the 3′-methoxy or 4′-methoxy substituent (5c,5d), which in most cases were weaker inhibitors than the corresponding chalcones 3c,3d and flavanones 4c,4d. An exception in this regard was a strong inhibition of Alternaria sp. by lactone 6d, comparable with the activity of flavanone 4d and stronger than chalcone 3d. On the contrary, the lactone moiety of the compound with the 2′-methoxyphenyl substituent (5b) increased the activity towards F. graminearum and Alternaria sp. in comparison with flavanone 4b or chalcone 3b, respectively. Comparing the activity of compounds substituted at the B ring against C. albicans, one can see that 2′-methoxy-substituted lactone 5b and 3′-methoxy-substituted lactone 5c were not growth inhibitors in contrast to chalcone 3b and flavanones 4b,c respectively, whereas 4′-methoxy-substituted lactone 5d decreased ΔOD to a higher extent than chalcone 3d and flavanone 4d.
3. Materials and Methods
3.1. Chemicals
Benzaldehyde (purity >99%) was purchased from Chempur (Piekary Śląskie, Poland). Methoxy-substituted benzaldehydes (purity 97–98%), 2′-hydroxyacetophenones (purity 97–98%), and m-chloroperbenzoic acid (m-CPBA, ≤77%) were purchased from Sigma-Aldrich (St. Louis, MO, USA), and 6-methoxyflavanone 4f (purity 98%) was from Alfa Aesar (Kandel, Germany). Other chemicals purchased from Chempur were of analytical grade. Silica gel used for column chromatography (Kieselgel 60, 230–400 mesh) was purchased from (Merck, Darmstadt, Germany).
Hexane was purified by distillation before use for column chromatography. m-Chloroperbenzoic acid (m-CPBA) was dried immediately before use over anhydrous magnesium sulphate(VI) in the solution of methylene chloride (20 mL), then the dried solution of m-CPBA was filtered and concentrated under nitrogen.
3.2. Analysis
Analytical thin layer chromatography was carried out on silica gel-coated aluminum plates (DC-Alufolien Kieselgel 60 F254, Merck, Darmstadt, Germany). Spots were visualized using a solution of 1% Ce(SO4)2 and 2% H3[P(Mo3O10)4] × H2O in 10% H2SO4.
Gas chromatography (GC) analysis was performed on an Agilent Technologies 6890N instrument equipped with an autosampler, split injection (5:1), and Flame Ionization Detector (FID) detector using a DB-5HT column (30 m × 0.32 mm × 0.10 µm) with hydrogen as the carrier gas. For the analysis of compounds, the following temperature program was applied: Injector 200 °C, detector (FID) 280 °C, column temperature: 140 °C, 140–340 °C (rate 30 °C /min), and 340 °C (hold 1 min).
Nuclear magnetic resonance (NMR) spectra were recorded in CDCl3 solutions on a Bruker Avance II 600 MHz Bruker spectrometer (Bruker, Rheinstetten, Germany) with signals of residual solvent (δH = 7.26, δC = 77.0) as references for chemical shifts. Infrared spectroscopy (IR) spectra were determined using a Mattson IR 300 Thermo Nicolet spectrophotometer using KBr pellets or as neat. High-resolution mass spectra (HRMS) were recorded on a spectrometer Waters ESI-Q-TOF Premier XE (Waters Corp., Millford, MA, USA) by the electron spray ionization (ESI) technique.
The melting points (uncorrected) were determined on a Boetius apparatus.
3.3. General Procedure for the Synthesis of 2′-Hydroxychalcones 3a–e
A mixture of 2′-hydroxyacetophenone (1 equiv) and the corresponding benzaldehyde (1 equiv) was heated at reflux in 50 mL of 2% KOH methanolic solution and a drop of water. When the substrates reacted completely (TLC, 24–48 h), the reaction mixture was transferred to the flask containing 50 mL of water, 80 mL of 1M HCl, and 50 g of crushed ice. The resulting solution was gently mixed with a glass rod until the precipitate was formed (2–3 min). The precipitate was then filtered under reduced pressure and washed with 30 to 40 mL of water and recrystallized from ethanol. The yields of the reaction and physical and spectral data of the obtained 2′-hydroxychalcones 3a–e are given below:
2′-Hydroxychalcone (3a): Obtained from 2′-hydroxyacetophenone (10.31 g, 0.075 mol) and benzaldehyde (8.03 g, 0.075 mol); yield 14.6 g (86%); yellow crystals; mp 77 °C (lit. 75–78 °C [45]) spectroscopic data in accordance with the literature [38].
2′-Hydroxy-2-methoxychalcone (3b): Obtained from 2′-hydroxyacetophenone (5.01 g, 0.037 mol) and 2-methoxybenzaldehyde (5 g, 0.037 mol); yield 7.64 g (82%); yellow crystals, mp 92–93 °C (lit. 111–112 °C [46]) spectroscopic data in accordance with the literature [38].
2′-Hydroxy-3-methoxychalcone (3c): Obtained from 2′-hydroxyacetophenone (4.02 g, 0.029 mol) and 3-methoxybenzaldehyde (4 g, 0.029 mol); yield 5.15 g (69%); yellow crystals, mp 73–75 °C (lit. 90–92 °C [46]); spectroscopic data in accordance with the literature [37].
2′-Hydroxy-4-methoxychalcone (3d): Obtained from 2′-hydroxyacetophenone (5.04 g, 0.037 mol) and 4-methoxybenzaldehyde (5 g, 0.037 mol); yield 7.83 g (84%); yellow crystals, mp 70–71 °C (lit. 70–75 °C [47]), spectroscopic data in accordance with the literature [38].
2′-Hydroxy-4′-methoxychalcone (3e): Obtained from 2′-hydroxy-4′-methoxyacetophenone (5.03 g, 0.03 mol) and benzaldehyde (3.2 g, 0.03 mol); yield 7.01 g (92%); yellow crystals, mp 93–94 °C (lit. 96–99 °C [45]); spectroscopic data in accordance with the literature [37].
3.4. General Procedure for the Preparation of Flavanones 4a–e
A mixture of 2′-hydroxychalcone 3a–e (1 equiv), sodium acetate (10 equiv), and a drop of water was heated at reflux in 200 mL of ethanol for 48 h. Then, the mixture was diluted with water and the products were extracted with methylene chloride (3 × 40 mL). The extracts were pooled, dried with anhydrous magnesium sulphate, and concentrated in vacuo. Products were crystallized from ethanol to afford pure flavanones 4a–e with the following data:
Flavanone (4a): Obtained from 2′-hydroxychalcone 3a (14.6 g, 0.065 mol); yield 11.68 g (84%); white crystals, mp 68 °C (lit. 69–72 °C [48]), spectroscopic data in accordance with the literature [40].
2′-Methoxyflavanone (4b): Obtained from 2′-hydroxy-2-methoxychalcone 3b (7.64 g, 0.03 mol); yield 4.66 g (61%); dense oil; spectroscopic data in accordance with the literature [41].
3′-Methoxyflavanone (4c): Obtained from 2′-hydroxy-3-methoxychalcone 3c (5.15 g, 0.02 mol); yield 3.76 g (73%); white crystals, mp 67–68 °C (lit. 78–79 °C [49]); spectroscopic data in accordance with the literature [41].
4′-Methoxyflavanone (4d): Obtained from 2′-hydroxy-4-methoxychalcone 3d (7.83 g, 0.031 mol); yield 5.01 g (64%); white crystals, mp 74–75 °C (lit. 75–77 °C [50]), spectroscopic data in accordance with the literature [41].
7-Methoxyflavanone (4e): Obtained from 2′-hydroxy-4′-methoxychalcone 3e (7.01 g, 0.028 mol); yield 5.47 g (78%); white crystals, mp 77–78 °C (lit. 62–63 °C [51]); spectroscopic data in accordance with the literature [37].
3.5. General Procedure for Preparation of γ-Oxa-ε-Lactones 5a–f
Flavanone 4a–f (1 equiv) and freshly dried m-CPBA (3 equiv) were dissolved in methylene chloride (200 mL). The mixture was stirred at room temperature until the substrate was totally consumed (48 h). The reaction was monitored by gas chromatography and TLC (hexane/acetone, 3:1). The reaction mixture was then diluted with diethyl ether, and successively washed with saturated solutions of sodium sulphite and sodium carbonate. Ethereal layer was dried over anhydrous magnesium sulphate and the solvent was evaporated in vacuo. Crystallization from hexane afforded pure lactones 5a–f. Their physical and spectral data are given below:
4-Phenyl-3,4-dihydro-2H-1,5-benzodioxepin-2-one (5a): Obtained from flavanone 4a (11.68 g, 0.05 mol); yield 9.72 g (89%); white crystals, mp 81–83 °C (lit. 85–86 °C [27]); 1H-NMR (600 MHz, CDCl3) δ: 3.11 (dd, J = 13.2 and 7.2 Hz, 1H, one of CH2-3), 3.16 (dd, J = 13.2 and 6.0 Hz, 1H, one of CH2-3), 5.73 (dd, 1H, J = 7.2 and 6.0 Hz, 1H, H-4), 7.04 (d, J = 7.7 Hz, 1H, H-9), 7.14–7.20 (m, 3H, H-6, H-7, H-8), 7.36–7.42 (m, 5H, -C6H5); 13C-NMR (150 MHz, CDCl3) δ: 38.61 (C-3), 83.59 (C-4), 120.56 (C-6), 124.39 (C-9), 125.82 (C-8), 126.28 (C-2′ and C-6′), 126.67 (C-7), 128.93 (C-3′ and C-5′), 129.17 (C-4′), 138.69 (C-1′), 145.28 (C-5a), 145.77 (C-9a), 167.59 (C-2); IR (KBr, cm−1): 1770, 1485, 1451, 1250, 1225, 1040, 972, 765, 711.
4-(2′-Methoxyphenyl)-3,4-dihydro-2H-1,5-benzodioxepin-2-one (5b): Obtained from 2′-methoxyflavanone 4b (4.66 g, 0.018 mol); yield 4.37 g (90%); white crystals, mp 141–142 °C; 1H-NMR (600 MHz, CDCl3) δ: 3.01 (dd, J = 13.2 and 4.8 Hz, 1H, one of CH2-3), 3.20 (dd, J = 13.2 and 6.0 Hz, 1H, one of CH2-3), 3.84 (s, 3H, -OCH3), 6.02 (dd, 1H, J = 6.0 and 4.8 Hz, H-4), 6.93 (dd, J = 7.8 and 0.6 Hz, 1H, H-3′), 7.01 (td, J = 7.8 and 0.6 Hz, 1H, H-5′), 7.13–7.19 (m, 4H, H-6, H-7, H-8, H-9), 7.34 (td, J = 7.8 and 1.8 Hz, 1H, H-4′), 7.61 (dd, J = 7.8 and 1.8 Hz, 1H, H-6′); 13C-NMR (150 MHz, CDCl3) δ: 37.89 (C-3), 55.47 (-OCH3), 78.52 (C-4), 110.58 (C-3′), 120.55, 123.98, 125.58 and 126.60 (C-6, C-7, C-8, C-9), 120.84 (C-5′), 126.29 (C-6′), 127.19 (C-1′), 129.87 (C-4′), 145.76 (C-9a), 146.27 (C-5a), 155.72 (C-2′), 167.89 (C-2); IR (KBr, cm−1): 1772, 1493, 1438, 1272, 1205, 1037, 764. HRMS: calcd for C16H14O4[M + Na]+: 293.0790, found: 293.0792.
4-(3′-Methoxyphenyl)-3,4-dihydro-2H-1,5-benzodioxepin-2-one (5c): Obtained from 3′-methoxyflavanone 4c (3.76 g, 0.015 mol); yield 2.68 g (67%); dense oil; 1H-NMR (600 MHz, CDCl3) δ: 3.09 (dd, J = 13.2 and 7.3 Hz, 1H, one of CH2-3), 3.15 (dd, J = 13.2 and 5.3 Hz, 1H, one of CH2-3), 3.82 (s, 3H, OCH3), 5.69 (dd, J = 7.3 and 5.3 Hz, 1H, H-4), 6.91 (m, 1H, H-4′), 6.94 (m, 1H, H-6′), 6.95 (s, 1H, H-2′), 7.07 (d, J = 7.6 Hz, 1H, H-9), 7.15–7.19 (m, 3H, H-6, H7, H-8), 7.31 (m, 1H, H-5′); 13C-NMR (150 MHz, CDCl3) δ: 38.66 (C-3), 55.45 (OCH3), 83.45 (C-4), 112.00 (C-2′), 114.55 (C-4′), 118.41 (C-6′), 120.57 and 125.84 (C-7 and C-8), 124.38 (C-9), 126.68 (C-6), 130.01 (C-5′), 140.28 (C-1′), 145.30 (C-5a), 145.73 (C-9a), 159.99 (C-3′), 167.56 (C-2); IR (KBr, cm−1): 1773, 1490, 1437, 1268, 1211, 1035, 765. HRMS: calcd for C16H14O4[M + Na]+: 293.0790, found: 293.0794.
4-(4′-Methoxyphenyl)-3,4-dihydro-2H-1,5-benzodioxepin-2-one (5d): Obtained from 4′-methoxyflavanone 4d (5.01 g, 0.02 mol); yield 4.20 g (79%); white crystals; mp 108–109 °C (lit. 121–122 °C, [27]); 1H-NMR (600 MHz, CDCl3) δ: 3.09 (dd, J = 13.2 and 6.6 Hz, 1H, one of CH2-3), 3.12 (dd, J = 13.2 and 7.2 Hz, 1H, one of CH2-3), 3.82 (s, 3H, -OCH3), 5.69 (dd, J = 7.2 and 6.6 Hz, 1H, H-4), 6.90–6.92 (m, 2H, H-3′ and H-5′), 6.90–7.18 (m, 4H, H-6, H-7, H-8, H-9), 7.28–7.29 (m, 2H, H-2′ and H-6′); 13C-NMR (150 MHz, CDCl3) δ: 38.54 (C-3), 55.46 (-OCH3), 83.39 (C-4), 114.22 (C-3′ and C-5′), 120.46, 124.52, 125.77 and 126.60 (C-6, C-7, C-8, C-9), 127.77 (C-2′ and C-6′), 130.73 (C-1′), 145.13 (C-5a), 145.87 (C-9a), 160.21 (C-4′), 167.78 (C-2); IR (KBr, cm−1): 1770, 1488, 1437, 1258, 1210, 1030, 766.
7-Methoxy-4-phenyl-3,4-dihydro-2H-1,5-benzodioxepin-2-one (5e); Obtained from 7-methoxyflavanone 4e (5.47 g, 0.022 mol); yield 4.01 g (69%); white crystals, mp 71–73 °C (lit. 72–74 °C [27]); 1H-NMR (600 MHz, CDCl3) δ: 3.09–3.15 (two m, 2H, CH2-3), 3.75 (s, 3H, -OCH3), 5.72 (m, 1H, H-4), 6.58 (d, J = 2.8 Hz, 1H, H-6), 6.71 (dd, J = 9.0 and 2.8 Hz, 1H, H-8), 7.10 (d, J = 9.0 Hz, 1H, H-9), 7.37–7.42 (m, 5H, H-2′, H-3′, H-4′, H-5′, H-6′); 13C-NMR (150 MHz, CDCl3) δ: 38.64 (C-3), 55.89 (-OCH3), 83.50 (C-4), 109.63 (C-6), 110.79 (C-8), 120.87 (C-9), 126.27 (C-2′ and C-6′), 128.96 (C-3′ and C-5′), 129.19 (C-4′), 138.82 (C-1′), 139.25 (C-9a), 145.92 (C-5a), 157.90 (C-7), 168.07 (C-2); IR (KBr, cm−1): 1768, 1500, 1470, 1260, 1211, 1027, 703.
8-Methoxy-4-phenylo-3,4-dihydro-2H-1,5-benzodioxepin-2-one (5f): Obtained from 6-methoxyflavanone 4f (3.0 g, 0.012 mol); yield 2.36 g (74%); white crystals; mp 122–123 °C; 1H-NMR (600 MHz, CDCl3) δ: 3.09 (dd, J = 13.2 and 7.2 Hz, 1H, one of CH2-3), 3.15 (dd, J = 13.2 and 6.0 Hz, 1H, one of CH2-3), 3.80 (s, 3H, -OCH3), 5.67 (dd, J = 7.2 and 6.0 Hz, 1H, H-4), 6.67 (dd, J = 9.0 and 3.0 Hz, 1H, H-7), 6.74 (d, J = 3.0 Hz, 1H, H-9), 6.93 (d, J = 9.0 Hz, 1H, H-6), 7.37–7.39 (m, 5H, H-2′, H-3′, H-4′, H-5′, H-6′); 13C-NMR (150 MHz, CDCl3) δ: 38.60 (C-3), 55.94 (-OCH3), 83.70 (C-4), 105.98 (C-9), 111.63 (C-7), 124.58 (C-6), 126.36 (C-2′ and C-6′), 128.90 (C-3′ and C-5′), 129.13 (C-4′), 138.64 and 138.71 (C-1′ and C-5a), 146.27 (C-9a), 157.32 (C-8), 167.65 (C-2); IR (KBr, cm−1): 1766, 1502, 1468, 1445, 1260, 1236, 1028, 764, 704. HRMS: calcd for C16H14O4[M + Na]+: 293.0790, found: 293.0790.
3.6. Antimicrobial Activity Assay
Antimicrobial tests were carried out using the bacteria strains: Escherichia coli PCM 2560, Bacillus subtilis B5, Staphylococcus aureus D1; filamentous fungi: Fusarium graminearum 109, Aspergillus niger XP, Alternaria sp., and yeast Candida albicans KL-1. All strains originated from the collection of the Department of Biotechnology and Food Microbiology, Wroclaw University of Environmental and Life Sciences.
The tests were performed on the automated Bioscreen C system (Automated Growth Curve Analysis System, Lab Systems, Finland). The working volume in the wells of the Bioscreen plate was adjusted to 200 μL, containing 180 μL of culture medium and 10 μL of cell or spore solution. The final density of the cultures was 1 × 106 cells mL−1. The bacteria were cultivated for 48 h in a liquid medium composed of nutrient broth (Biocorp) 15 g · L−1 and glucose 10 g · L−1. Yeast and fungi were cultured in YPG medium containing 10 g · L−1 of yeast extract, 10 g · L−1 of bacteriological peptone, and 10 g · L−1 of glucose for 48 and 96 h, respectively. Tested compounds were added to the cultures as 0.1% solutions in 10 μL of dimethyl sulfoxide (DMSO) (w/v). The cultures were carried out for 2 to 3 days at 30 °C (bacteria, yeasts) or 25 °C (filamentous fungi), under constant agitation. The optical density of the cell suspensions were measured automatically at the 560-nm wavelength at 30-min intervals. Each culture was performed in three replicates. The results (Table 1 and Table 2) were analyzed using spreadsheet software (Excel 97) and the means for the triplicates of each culture type were calculated. Statistics on a completely randomized design were determined using the one-way analysis of variance (ANOVA) procedure at a level of significance set at p < 0.050. A comparison of average growth OD microorganisms relative to the control was carried out using Dunnett’s test.
4. Conclusions
It was shown that γ-oxa-ε-lactones 5a–f containing methoxy groups with different substitution patterns in the A and B ring inhibited the growth of selected bacteria, filamentous fungi, and yeast. The most sensitive strains were S. aureus, F. graminearum, and Alternaria sp. whereas the most resistant one turned out to be A. niger. The broadest spectrum of activity was observed for unsubstituted lactone 5a, which was a particularly strong inhibitor of fungi and yeast. The activity and selectivity of the methoxy-substituted lactones depended on the substitution pattern of the A and B rings. A-ring substitution with a methoxy group in position 7 improved the activity and selectivity against fungal strains and yeast whereas substitution at position 8 increased the antibacterial activity. Lactones possessing methoxy groups in the 3′ and 4′ position of the B ring were highly active against bacteria whereas the compound with the methoxy group at the 2′ position was a strong inhibitor of F. graminearum and Alternaria sp. In most cases, the activity of the compounds was improved after introduction of the lactone function into the molecule compared to their flavonoid precursors, chalcones 3a–e and/or flavanones 4a–f. The most spectacular example was 4′-methoxy-substituted lactone 5d, which exhibited significantly higher activity against all bacterial strains than both its flavonoid precursors. Unsubstituted lactone 5a was a much stronger inhibitor of filamentous fungi and yeast than the starting chalcone 3a whereas the activity of 7-methoxy lactone 5e was significantly higher than flavanone 4e towards these microorganisms. The γ-Oxa-ε-lactone scaffold derived from the flavanone skeleton is a good candidate for the screening of compounds with high antimicrobial activity. Our results indicate that future studies on the antifungal flavanone-derived lactones should focus on compounds substituted in the A ring whereas a candidate for an antibacterial drug will probably be a derivative substituted in the B ring.
Author Contributions
Conceptualization, M.S. and C.W.; Investigation, M.S., T.J., E.K.-S., J.P., M.M., B.Ż., W.Ł. and G.M.; Validation, W.G. and C.W.; Writing – original draft, W.G.
Funding
This research was funded by the statutory activities of the Department of Chemistry, Wrocław University of Environmental and Life Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 5a, 5b, 5d, 5f are available from the authors.
References
- 1.Spellberg B., Guidos R., Gilbert D., Bradley J., Boucher H.W., Michael Scheld W., Bartlett J.G., Edwards J.J. The epidemic of antibiotic-resistance infections: A call to action for the medical community from the infectious diseases society of America. Clin. Infect. Dis. 2008;46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 2.Bongomin F., Gago S., Oladele R.O., Denning D.W. Global and multi-national prevalence of fungal diseases—estimate precision. J. Fungi. 2017;3:57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wedge D.E., Galindo J.C.G., Macías F.A. Fungicidal activity of natural and synthetic sesquiterpene lactone analogs. Phytochemistry. 2000;53:747–757. doi: 10.1016/S0031-9422(00)00008-X. [DOI] [PubMed] [Google Scholar]
- 4.Barrero A.F., Oltra J.E., Álvarez M., Raslan D.S., Saúde D.A., Akssira M. New sources and antifungal activity of sesquiterpene lactones. Fitoterapia. 2000;71:60–64. doi: 10.1016/S0367-326X(99)00122-7. [DOI] [PubMed] [Google Scholar]
- 5.Fortuna A.M., Juárez Z.N., Bach H., Nematallah A., Av-Gay Y., Sánchez-Arreola E., Catalán C.A.N., Turbay S., Hernández L.R. Antimicrobial activities of sesquiterpene lactones and inositol derivatives from Hymenoxys robusta. Phytochem. 2011;72:2413–2418. doi: 10.1016/j.phytochem.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Özçelik B., Gürbüz I., Karaoglu T., Yeşilada E. Antiviral and antimicrobial activities of three sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis. Microbiol. Res. 2009;164:545–552. doi: 10.1016/j.micres.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Grabarczyk M., Mączka W., Wińska K., Żarowska B., Anioł M. Antimicrobial activity of hydroxylactone obtained by biotransformation of bromo- and iodolactone with gem-dimethylcyclohexane ring. J. Braz. Chem. Soc. 2013;24:1913–1919. doi: 10.5935/0103-5053.20130238. [DOI] [Google Scholar]
- 8.Wińska K., Grabarczyk M., Mączka W., Żarowska B., Maciejewska G., Anioł M. Influence of structure of lactones with the methylcyclohexene and dimethylcyclohexene ring on their biotransformation and antimicrobial activity. Zeitschrift Fur Naturforsch. Sect. C J. Biosci. 2017;72:209–217. doi: 10.1515/znc-2016-0188. [DOI] [PubMed] [Google Scholar]
- 9.Wińska K., Grabarczyk M., Mączka W., Żarowska B., Maciejewska G., Anioł M. Antimicrobial activity of new bicyclic lactones with three or four methyl groups obtained both synthetically and biosynthetically. J. Saudi Chem. Soc. 2018;22:363–371. doi: 10.1016/j.jscs.2016.04.004. [DOI] [Google Scholar]
- 10.Grabarczyk M., Wińska K., Mączka W., Żołnierczyk A.K., Żarowska B., Anioł M. Lactones with methylcyclohexane systems obtained by chemical and microbiological methods and their antimicrobial activity. Molecules. 2015;20:3335–3353. doi: 10.3390/molecules20023335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazur M., Gładkowski W., Podkowik M., Bania J., Nawrot J., Białońska A., Wawrzeńczyk C. Lactones 43. New biologically active lactones: β-cyclocitral derivatives. Pest. Manag. Sci. 2014;70:286–294. doi: 10.1002/ps.3557. [DOI] [PubMed] [Google Scholar]
- 12.Kalinowska K., Boratyński F., Baran E., Wawrzeńczyk C., Hryncewicz-Gwóźdź A. Antifungal in vitro activity of lactones, ketones and diols. Mikol. Lek. 2010;17:217–220. [Google Scholar]
- 13.Akiyama K., Maruyama M., Yamauchi S., Nakashima Y., Nakato T., Tago R., Sugahara T., Kishida T., Koba Y. Antimicrobiological activity of lignan: Effect of benzylic oxygen and stereochemistry of 2,3-dibenzyl-4-butanolide and 3,4-dibenzyltetrahydrofuran lignans on activity. Biosci. Biotechnol. Biochem. 2007;71:1745–1751. doi: 10.1271/bbb.70168. [DOI] [PubMed] [Google Scholar]
- 14.Pour M., Špulák M., Balšánek V., Kuneš J., Buchta V., Waisser K. 3-Phenyl-5-methyl-2H,5H-furan-2-ones: Tuning antifungal activity by varying substituents on the phenyl ring. Bioorganic Med. Chem. Lett. 2000;10:1893–1895. doi: 10.1016/S0960-894X(00)00376-0. [DOI] [PubMed] [Google Scholar]
- 15.Pour M., Špulák M., Balšánek V., Kuneš J., Kubanová P., Buchta V. Synthesis and structure-antifungal activity relationships of 3-aryl-5-alkyl-2,5-dihydrofuran-2-ones and their carbanalogues: Further refinement of tentative pharmacophore group. Bioorganic Med. Chem. 2003;11:2843–2866. doi: 10.1016/S0968-0896(03)00220-7. [DOI] [PubMed] [Google Scholar]
- 16.Šenel P., Tichotová L., Votruba I., Buchta V., Špulák M., Kuneš J., Nobilis M., Krenk O., Pour M. Antifungal 3,5-disubstituted furanones: From 5-acyloxymethyl to 5-alkylidene derivatives. Bioorganic Med. Chem. 2010;18:1988–2000. doi: 10.1016/j.bmc.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Castelo-Branco P.A., Rubinger M.M.M., Alves Lde C., de Barros P.M., Pereira S.G., de Melo V.J., Pilo-Veloso D., Zambolim L. Synthesis and antifungal activity of aromatic bis-γ-lactones analogous to avenaciolide. Chem. Biodivers. 2007;4:2745–2754. doi: 10.1002/cbdv.200790223. [DOI] [PubMed] [Google Scholar]
- 18.Jun-Tao F., De-Long W., Yong-Ling W., He Y., Xing Z. New antifungal scaffold derived from a natural pharmacophore: Synthesis of α-methylene-γ-butyrolactone derivatives and their antifungal activity against Colletotrichum Lagenarium. Bioorganic Med. Chem. Lett. 2013;23:4393–4397. doi: 10.1016/j.bmcl.2013.05.073. [DOI] [PubMed] [Google Scholar]
- 19.Skrobiszewski A., Gładkowski W., Walczak P., Gliszczyńska A., Maciejewska G., Klejdysz T., Nawrot J., Wawrzeńczyk C. Synthesis of β-aryl-γ-lactones and relationship: Structure—Antifeedant and antifungal activity. J. Chem. Sci. 2015;127:687–699. doi: 10.1007/s12039-015-0823-0. [DOI] [Google Scholar]
- 20.Mazur M., Skrobiszewski A., Gladkowski W., Podkowik M., Bania J., Nawrot J., Klejdysz T., Wawrzeńczyk C. Lactones 46. Synthesis, antifeedant and antibacterial activity of γ-lactones with a p-methoxyphenyl substituent. Pest. Manag. Sci. 2016;72:489–496. doi: 10.1002/ps.4012. [DOI] [PubMed] [Google Scholar]
- 21.Strobel G., Ford E., Worapong J., Harper J.K., Arif A.M., Grant D.M., Fung P.C.W., Ming Wah Chau R. Isopestacin, an isobenzofuranone from Pestalotiopsis microspora, possessing antifungal and antioxidant activities. Phytochemistry. 2002;60:179–183. doi: 10.1016/S0031-9422(02)00062-6. [DOI] [PubMed] [Google Scholar]
- 22.Nicolaou K.C., Xu H. Total synthesis of floresolide B and Δ6,7-Z-floresolide B. Chem. Commun. 2006;6:600–602. doi: 10.1039/b517385j. [DOI] [PubMed] [Google Scholar]
- 23.Yakota T. The structure, biosynthesis and function of brassinosteroids. Trends Plant. Sci. 1997;2:137–143. doi: 10.1016/S1360-1385(97)01017-0. [DOI] [Google Scholar]
- 24.Pizao P.E., Smitskamp-Wilms E., Van Ark-Otte J., Beijnen J.H., Peters G.J., Pinedo H.M., Giaccone G. Antiproliferative activity of the topoisomerase I inhibitors topotecan and camptothecin, on sub- and postconfluent tumor cell cultures. Biochem. Pharmacol. 1994;48:1145–1154. doi: 10.1016/0006-2952(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 25.Luo Y., Yu S., Tong L., Huang Q., Lu W., Chen Y. Synthesis and biological evaluation of new homocamptothecin analogs. Eur. J. Med. Chem. 2012;54:281–286. doi: 10.1016/j.ejmech.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Wang L., Xie S., Ma L., Chen Y., Lu W. Design, synthesis and biological evaluation of novel homocamptothecin analogues as potent antitumor agents. Bioorganic Med. Chem. 2015;23:1950–1962. doi: 10.1016/j.bmc.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Gelebe A.C., Kaye P.T., Liddell J.R. Synthesis and Structure of 3,4-dihydro-4-phenyl-1,5-benzodioxepin-2-ones. Synth. Commun. 1991;21:2263–2268. doi: 10.1080/00397919108021584. [DOI] [Google Scholar]
- 28.Bernini R., Mincione E., Cortese M., Saladino R., Gualandi G., Belfiore M.C. Conversion of naringenin and hesperetin by heterogeneous catalytic Baeyer-Villiger reaction into lactones exhibiting apoptotic activity. Tetrahedron Lett. 2003;44:4823–4825. doi: 10.1016/S0040-4039(03)01139-0. [DOI] [Google Scholar]
- 29.Tran T.D., Do T.H., Tran N.C., Ngo T.D., Huynh T.N.P., Tran C.D., Thai K.M. Synthesis and anti Methicillin resistant Staphylococcus aureus activity of substituted chalcones alone and in combination with non-beta-lactam antibiotics. Bioorganic Med. Chem. Lett. 2012;22:4555–4560. doi: 10.1016/j.bmcl.2012.05.112. [DOI] [PubMed] [Google Scholar]
- 30.Alcaráz L.E., Blanco S.E., Puig O.N., Tomás F., Ferretti F.H. Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains. J. Theor. Biol. 2000;205:231–240. doi: 10.1006/jtbi.2000.2062. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y.H., Dong H.H., Zhao F., Wang J., Yan F., Jiang Y.Y., Jin Y.S. The synthesis and synergistic antifungal effects of chalcones against drug resistant Candida albicans. Bioorganic Med. Chem. Lett. 2016;26:3098–3102. doi: 10.1016/j.bmcl.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y.M., Zhou Y., Flavin M.T., Zhou L.M., Nie W., Chen F.C. Chalcones and flavonoids as anti-tuberculosis agents. Bioorganic Med. Chem. 2002;10:2795–2802. doi: 10.1016/S0968-0896(02)00094-9. [DOI] [PubMed] [Google Scholar]
- 33.Moorthy N.S.H.N., Singh R.J., Singh H.P., Gupta S.D. Synthesis, biological evaluation and in silico metabolic and toxicity prediction of some flavanone derivatives. Chem. Pharm. Bull. 2006;54:1384–1390. doi: 10.1248/cpb.54.1384. [DOI] [PubMed] [Google Scholar]
- 34.Xie Y., Chen J., Xiao A., Liu L. Antibacterial activity of polyphenols: Structure-activity relationship and influence of hyperglycemic condition. Molecules. 2017;22:1913. doi: 10.3390/molecules22111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernini R., Pasqualetti M., Provenzano G., Tempesta S. Ecofriendly synthesis of halogenated flavonoids and evaluation of their antifungal activity. New J. Chem. 2015;39:2980–2987. doi: 10.1039/C5NJ00258C. [DOI] [Google Scholar]
- 36.Janeczko T., Dymarska M., Siepka M., Gniłka R., Leśniak A., Popłoński J., Kostrzewa-Susłow E. Enantioselective reduction of flavanone and oxidation of cis- and trans-flavan-4-ol by selected yeast cultures. J. Mol. Catal. B Enzym. 2014;109:47–52. doi: 10.1016/j.molcatb.2014.08.006. [DOI] [Google Scholar]
- 37.Kostrzewa-Susow E., Dymarska M., Guzik U., Wojcieszynska D., Janeczko T. Stenotrophomonas maltophilia: A gram-negative bacterium useful for transformations of flavanone and chalcone. Molecules. 2017;22:1830. doi: 10.3390/molecules22111830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yong Y., Ahn S., Hwang D., Yoon H., Jo G., Kim Y.H., Kim S.H., Koh D., Lim Y. 1H and 13C NMR spectral assignments of 2′-hydroxychalcones. Magn. Reson. Chem. 2013;51:364–370. doi: 10.1002/mrc.3949. [DOI] [PubMed] [Google Scholar]
- 39.Murti Y., Mishra P. Synthesis and evaluation of flavanones as anticancer agents. Indian J. Pharm. Sci. 2014;76:163–166. [PMC free article] [PubMed] [Google Scholar]
- 40.Kostrzewa-Susłow E., Dmochowska-Gładysz J., Białońska A., Ciunik Z., Rymowicz W. Microbial transformations of flavanone and 6-hydroxyflavanone by Aspergillus niger strains. J. Mol. Catal. B Enzym. 2006;39:18–23. doi: 10.1016/j.molcatb.2006.01.020. [DOI] [Google Scholar]
- 41.Dymarska M., Janeczko T., Kostrzewa-Susłow E. Glycosylation of methoxylated flavonoids in the cultures of Isaria fumosorosea KCH J2. Molecules. 2018;23:2578. doi: 10.3390/molecules23102578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kostrzewa-Susłow E., Dymarska M., Białońska A., Janeczko T. Enantioselective conversion of certain derivatives of 6-hydroxyflavanone. J. Mol. Catal. B Enzym. 2014;102:59–65. doi: 10.1016/j.molcatb.2014.01.013. [DOI] [Google Scholar]
- 43.English R.B., Gelebe A.C., Kaye P.T., Sewry J.D. Benzodiazepine analogues. Part 22. Conformational analysis of benzodioxepine and benzoxathiepine derivatives. J. Chem. Res. 2006;15:512–514. doi: 10.3184/030823406778256315. [DOI] [Google Scholar]
- 44.Kondhare D.D., Gyananath G., Tamboli Y., Kumbhar S.S., Choudhari P.B., Bhatia M.S., Zubaidha P.K. An efficient synthesis of flavanones and their docking studies with aldose reductase. Med. Chem. Res. 2017;26:987–998. doi: 10.1007/s00044-017-1813-1. [DOI] [Google Scholar]
- 45.Jeon J.H., Yang D.M., Jun J.G. Selective synthesis of 3,4-dihydrocoumarins and chalcones from substituted aryl cinnamic esters. Bull. Korean Chem. Soc. 2011;32:65–70. doi: 10.5012/bkcs.2011.32.1.65. [DOI] [Google Scholar]
- 46.Detsi A., Majdalani M., Kontogiorgis C.A., Hadjipavlou-Litina D., Kefalas P. Natural and synthetic 2′-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorganic Med. Chem. 2009;17:8073–8085. doi: 10.1016/j.bmc.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Sarojini B.K., Yathirajan H.S., Mustafa K., Sarfraz H., Bolte M. (2E)-1-(2-Hydroxyphenyl)-3-(4-methoxy-phenyl)prop-2-en-1-one. Acta Crystallogr. Sect. E Struct. Rep. Online. 2007;63 doi: 10.1107/S1600536807052737. [DOI] [Google Scholar]
- 48.Dubrovskiy A.V., Larock R.C. Intermolecular C-O addition of carboxylic acids to arynes. Org. Lett. 2010;12:3117–3119. doi: 10.1021/ol101017z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mondal R., Gupta A., Das, Mallik A.K. Synthesis of flavanones by use of anhydrous potassium carbonate as an inexpensive, safe, and efficient basic catalyst. Tetrahedron Lett. 2011;52:5020–5024. doi: 10.1016/j.tetlet.2011.07.072. [DOI] [Google Scholar]
- 50.Chimenti F., Fioravanti R., Bolasco A., Chimenti P., Secci D., Rossi F., Yáñez M., Orallo F., Ortuso F., Alcaro S., et al. A new series of flavones, thioflavones, and flavanones as selective monoamine oxidase-B inhibitors. Bioorganic Med. Chem. 2010;18:1273–1279. doi: 10.1016/j.bmc.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 51.Ganguly A.K., Mahata P.K., Biswas D. Synthesis of oxygen heterocycles. Tetrahedron Lett. 2006;47:1347–1349. doi: 10.1016/j.tetlet.2005.12.062. [DOI] [Google Scholar]