In a phase I clinical trial of Venetoclax in patients with t(11;14) relapsed/refractory MM, 40% of the patients treated achieved an objective response(1). While this response rate with a single agent in MM is impressive, it demonstrates that t(11;14) is not an optimal biomarker for response to venetoclax. Functional profiling of BCL2 family members has been shown to predict responses to therapy in myeloma and other diseases(2). Therefore, we set out to determine how ex vivo sensitivity to venetoclax corresponds to clinical response and resistance.
We performed ex vivo apoptosis assays on a cohort of 76 samples collected from 65 patients (Supplemental Table 1). Consistent with previous preclinical results we found that samples from patients with t(11;14) myeloma were significantly more sensitive than samples from t(11;14)-negative patients (Median IC50 70 vs. 910 nM, P=0.0176, Figure 1A)(3). However, a range of sensitivity was observed in both populations.
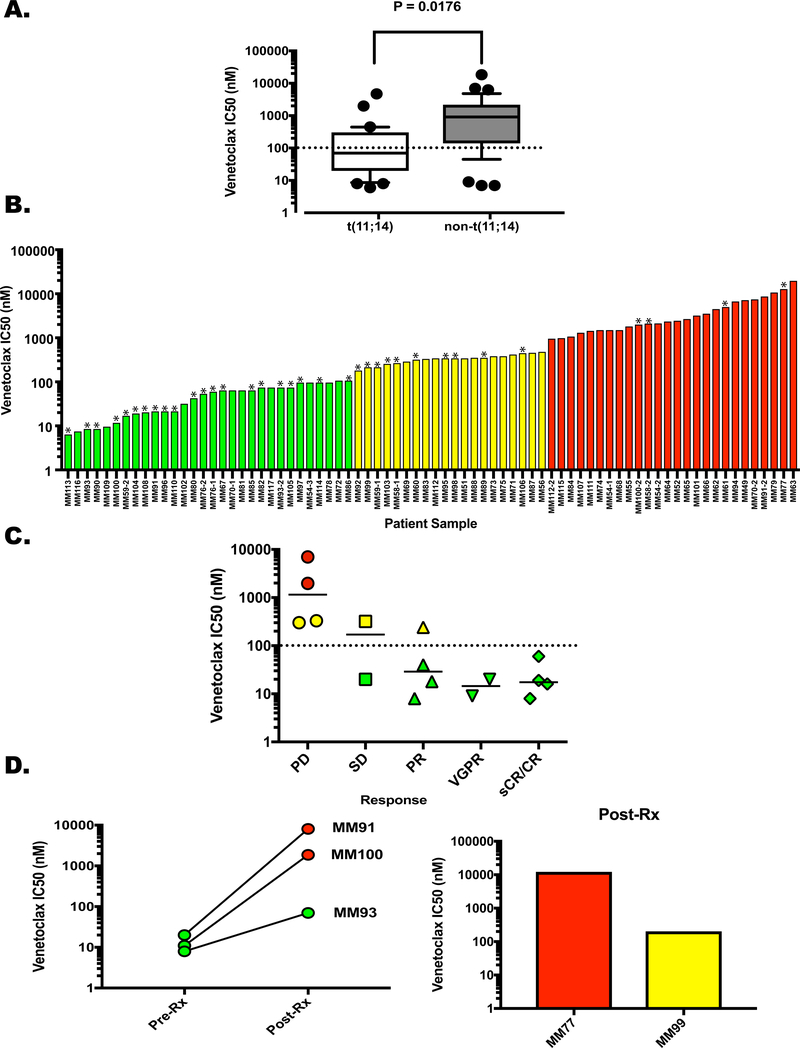
Figure 1. Ex Vivo Response of Patient-Derived Plasma Cells to Venetoclax.
Buffy coat cells were treated with Venetoclax for 24 h and apoptosis of the plasma cells was determined as described in the Supplemental Methods section. Dose curves using concentrations of 0, 10, 100 and 1000 nM venetoclax were used to calculate the IC50 for each patient sample. A. The samples were clustered based on the presence (N=31) or absence (N=36) of the t(11;14) translocation and graphed according to IC50. Whiskers extend from 10–90%, P=0.0176. Post-venetoclax treatment samples were removed from this analysis due to the effects of acquired resistance. B. Samples separated into 3 categories according to venetoclax sensitivity; Green – highly sensitive, IC50 up to 100 nM, Yellow – intermediate sensitivity, IC50 170 nM – 450 nM, Red – resistant, IC50 ≥ 900 nM. Asterisks denote samples from t(11;14)-positive patients C. Clinical response of 16 patients graphed versus IC50. Progressive disease (PD) N = 4, Single agent venetoclax; stable disease (SD) N = 2, single agent venetoclax. Partial response (PR) N = 4, venetoclax monotherapy; very good partial response (VGPR) N = 2, 1 single agent venetoclax, 1 venetoclax plus daratumamab and dexamethasone; stringent complete response sCR N = 4, 2 venetoclax monotherapy, 2 venetoclax plus dexamethasone. Dotted line represents the cutoff for the most sensitive cohort (100 nM), solid lines represent the median IC50 for each response. Symbol colors represent sensitivity as shown in Figure 1B. D. (Left) Ven IC50 for MM91, MM100, and MM93 before treatment and upon progression. (Right) Post-Ven IC50 for MM77 and MM99. Pre-treatment samples were not obtained.
The samples appeared to cluster into three groups based on sensitivity. Thirty of the 76 total samples (39.5%) had an IC50 of 100 nM or less (Figure 1B, green bars), consisting primarily from t(11;14)-positive samples (73.3%). A second cohort of 20 samples (26.3%) had an IC50 from 170 to 450 nM (Figure 1B, yellow bars). In the remaining 26 samples (34.2%) the IC50 was 900 nM or greater (range 900–18400 nM, Figure 1B, red bars). The presence of t(11;14) was the only significant difference between the sensitive group and the others (Supplemental Table 2). Twenty patients received venetoclax as a single agent (N=10) or in combination with dexamethasone (N=9) or daratumamab/dexamethasone (N=1) and 18 had ex vivo sensitivity tested immediately prior to venetoclax treatment (Supplemental Tables 1 and 3). A patient with non-secretory disease and another that withdrew consent prior to completing one cycle were not included in this analysis. Ten of the 16 remaining patients had a clinical response (PR or better), 9 of which were in the most sensitive cohort. Only 1 of the 6 non-responding patients was in the most sensitive group (Figure 1C). Thus, the sensitivity of the assay is 0.9 while the specificity is 0.83 (Fisher’s exact test, P=0.0076). Examples of ex vivo concentration-response curves and clinical responses are provided in Supplemental Figure 1. Together these data suggest that ex vivo pre-treatment sensitivity can predict patient responses to venetoclax.
Of the patients who responded to venetoclax, 3 had both a pre-treatment and post-relapse sample that was analyzed (Figure 1D). MM93 had an initial IC50 of 8 nM and achieved a PR for 8 months (Supplemental Figure 2). In the post-treatment sample the IC50 increased to 70 nM (Figure 1D, left panel). While this would still be considered a sensitive sample in the ex vivo analysis, it does represent a nearly 9-fold increase in IC50 post-treatment. MM100 had non-secretory disease however while on venetoclax the patient demonstrated a hematologic recovery. This “response” was transient and the patient became cytopenic after 3 months (Supplemental Figure 2). Consistent with development of venetoclax resistance, ex vivo testing indicated that the IC50 had increased from 4 nM to 1870 nM (Figure 1D). MM91 had an initial IC50 of 20 nM when the combination of daratumumab, venetoclax and dexamethasone was initiated. The patient had a VGPR that lasted for over 11 months before progression. The post-treatment IC50 increased over 400-fold to 8100 nM (Figure 1D). Finally, for two patients who were treated, no pre-treatment sample was available however a post-relapse sample was tested for ex vivo sensitivity. In both cases the sample tested as moderately or highly resistant to venetoclax (Figure 1D, right panel). MM77 achieved a sCR that was maintained for 15 months. The post-relapse sample was highly resistant to ex vivo venetoclax treatment (IC50, 12000 nM). MM99 had stable disease for nearly 6 months before treatment was stopped. The post-treatment sample displayed sensitivity consistent with our pre-treatment non-responder population (IC50, 200 nM). Thus, post-treatment ex vivo testing reflects development of venetoclax resistance.
In addition to testing serial samples from patients who were treated with venetoclax we were able to test samples from 6 patients who were not treated with venetoclax to determine if clonal changes associated with other therapy could influence ex vivo sensitivity. We observed a decrease in ex vivo IC50 with 2 patients, an increase in ex vivo IC50 in 3 patients and 1 patient who had no change (Supplementary Figures 3 and 4).
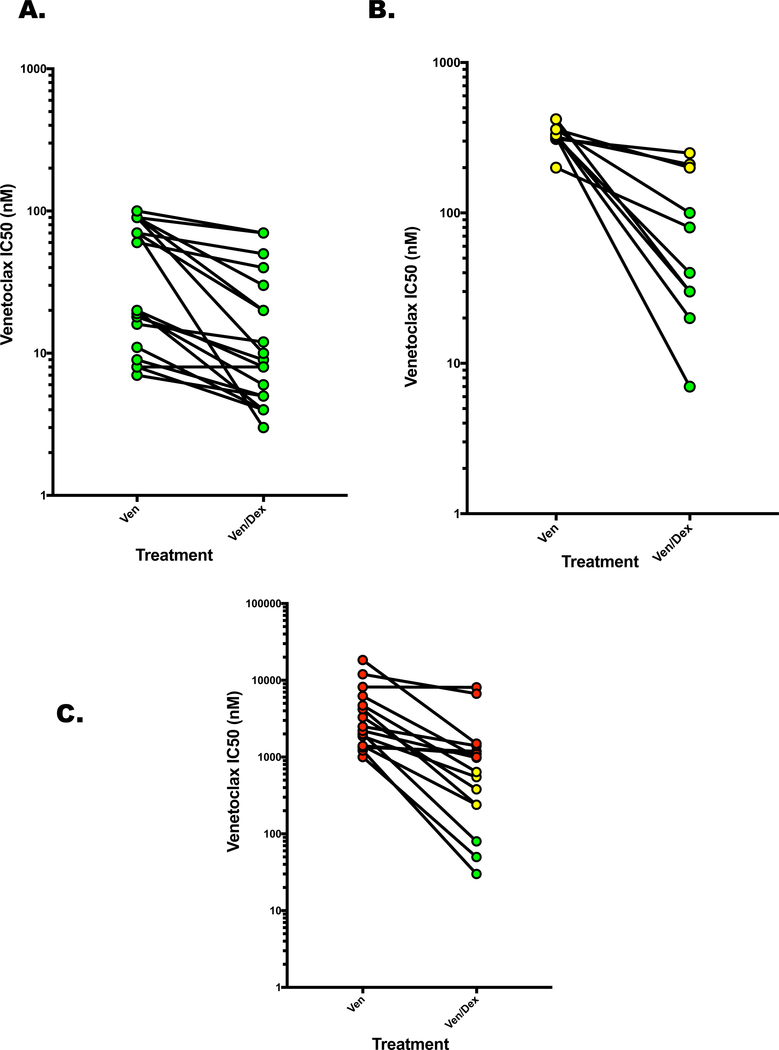
We previously demonstrated that dexamethasone could synergize with venetoclax by increasing the expression of the pro-apoptotic BH3-only protein BIM and subsequent priming of BCL2(4). This combination resulted in an increase in the response rate of t(11;14)-positive patients from 40% in the initial study to 65%(5). We analyzed the ven/dex combination in 46 samples, including our original cohort of 9. Only 11 samples demonstrated ex vivo sensitivity to dexamethasone alone as defined by induction of at least 25% cell death. Therefore, the majority of responses observed are due to sensitization while in the 11 that showed dexamethasone sensitivity we cannot rule out additive or synergistic effects if the cells were also sensitive to venetoclax. The decrease in IC50 with dexamethasone was significant in all three sensitivity cohorts. In the most sensitive cohort, the median IC50 decreased from 40 nM to 9.5 nM with dexamethasone (P≤0.0001). However, 5/20 samples were sensitive to dexamethasone alone suggesting that this change could be in part due to an additive response to two active drugs (Figure 2A). Dexamethasone had a more profound effect on the intermediate population where 7/10 samples had an IC50 shift to the most sensitive cohort (Figure 2B, median IC50 325 nM vs. 60 nM with dexamethasone, P=0.0020). While 5/10 samples were sensitive to dexamethasone alone, they are not considered sensitive to venetoclax alone so this is unlikely to be an additive response, more likely reflecting synergy or sensitization. Similar results were observed in the most resistant population where 8/15 samples shifted to an IC50 consistent with a more sensitive cohort (median IC50 3250 nM vs. 810 nM for the combination, P≤0.0001). Only 1 of these samples was sensitive to dexamethasone alone. Amazingly 3 of these samples were now in the most sensitive cohort demonstrating a greater than one log shift in the venetoclax IC50 (Figure 2C). Of the 10 samples that were sensitized by dexamethasone to an IC50 consistent with the sensitive cohort only half were t(11;14) suggesting this may be an effective way to increase the response rate of venetoclax in both t(11;14)-positive and -negative myeloma.
Figure 2. Combining Dexamethasone with Venetoclax Decreases IC50 and Shifts Sample Classification.
Buffy coat cells were isolated and treated with Ven as before, +/− 0.5 μM Dex. A. IC50 changes in samples sensitive to Ven alone. B. Shifts in IC50 of samples displaying intermediate sensitivity to Ven C. IC50 shifts in samples resistant to Ven alone. Symbol colors represent sensitivity as shown in Figure 1B.
Together our data represent an alternative approach to precision medicine that utilizes functional testing rather than targeting of acquired mutations or structural variations(6,7). The latter have had limited success due to the heterogeneity of these events within a tumor or availability of targeted therapy (8) (9). Targeting tumor dependence on BCL2 for survival, which is typically not based on mutations or translocations, is a promising new approach and has been used effectively in CLL (10) (11). Our results build on the pre-clinical BH3 profiling and ex vivo testing with venetoclax first used to demonstrate BCL2 dependence in t(11;14) myeloma (3, 12, 13).
We applied functional profiling of BCL2 dependence to a large series of myeloma patient samples and consistent with previous findings demonstrated that t(11;14) was the only commonly tested marker that was associated with ex vivo venetoclax sensitivity(3). However consistent with previous studies of cell lines, patients samples, and the phase I venetoclax monotherapy trial, t(11;14) samples can be resistant to venetoclax and non-t(11;14) samples can be sensitive(1). Therefore, we focused on the functional responses of the samples as opposed to genomic alterations to define BCL2 dependence. Since these cohorts included patients treated with venetoclax (both pre-and post-treatment) we were able to determine what level of ex vivo drug sensitivity associated with clinical response. There was a strong correlation with ex vivo sensitivity as 90% of the responding patients fell in the most sensitive cohort and 83% of the non-responding patients were in the other 2 cohorts. This compares favorably to the use of expression ratios, which worked best when the ratio was high (1). Moreover, all post-treatment samples showed a significant increase in their IC50. While the total number of treated patients evaluated in this study is small it is the first example of a patient cohort where clinical and ex vivo responses were both measured.
Our findings also demonstrate the dynamic nature of BCL2 dependence and the importance of when sensitivity is tested relative to initiation of treatment. We observed significant changes in sensitivity of samples taken from patients at different points of treatment. This could reflect clonal heterogeneity of myeloma and the effects of previous treatments on the makeup of the disease at any given time(14, 15). Alternatively, it could be due to changes in mitochondrial priming associated with therapy(16).
Finally, ex vivo testing demonstrates the ability of dexamethasone to convert some venetoclax-insensitive or even -resistant cells to clinically sensitive samples. This combination has already been shown to have significant activity in t(11;14) patients compared to single agent venetoclax(5). Our data point to the possibility of using this combination in a broader spectrum of myeloma patients beyond the 15–20% that are t(11;14)-positive.
Supplementary Material
Acknowledgments
The authors thank the patients for providing the samples used in this study. They also acknowledge the hard work and dedication of the Winship Myeloma Research Team for consenting patients and collecting samples. Funding provided by R01 CA192844, P30 CA138292 and the Emory Myeloma Working Group.
Footnotes
Conflict of Interest
NJB funding and honorarium from Celgene and Janssen
PM and JDL employment, Abbvie
LTH, research funding, Abbvie
SL consultancy Takeda, Celgene, Novartis, Abbvie, Amgen, Janssen, Bristol-Myers Squibb
AKN advisory board or consultancy, Amgen, Adaptive Technologies, Janssen, Celgene, Spectrum Pharmaceuticals, Bristol-Myers Squibb, GlaxoSmithKline, Takeda
JLK Consultancy, Abbvie, Janssen, Bristol-Myers Squibb, Takeda, Data Monitoring Committee, Karyopharm, Pharmacyclics
LHB, consultancy, Abbvie, honorarium, AstraZeneca.
References
- 1.Kumar S, Kaufman JL, Gasparetto C, Mikhael J, Vij R, Pegourie B, et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130(22):2401–9. [DOI] [PubMed] [Google Scholar]
- 2.Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334(6059):1129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Touzeau C, Dousset C, Le Gouill S, Sampath D, Leverson JD, Souers AJ, et al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia. 2014;28(1):210–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matulis SM, Gupta VA, Nooka AK, Hollen HV, Kaufman JL, Lonial S, et al. Dexamethasone treatment promotes Bcl-2 dependence in multiple myeloma resulting in sensitivity to venetoclax. Leukemia. 2016;30(5):1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufman JL, Gasparetto CJ, Mikhael J, Moreau P, Touzeau C, Vij R, et al. Phase 1 Study of Venetoclax in Combination with Dexamethasone As Targeted Therapy for t(11;14) Relapsed/Refractory Multiple Myeloma. Blood. 2017;130(Suppl 1):3131-. [Google Scholar]
- 6.Friedman AA, Letai A, Fisher DE, Flaherty KT. Precision medicine for cancer with next-generation functional diagnostics. Nature reviews Cancer. 2015;15(12):747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letai A. Functional precision cancer medicine-moving beyond pure genomics. Nature medicine. 2017;23(9):1028–35. [DOI] [PubMed] [Google Scholar]
- 8.Cheng ML, Solit DB. Opportunities and Challenges in Genomic Sequencing for Precision Cancer Care. Ann Intern Med. 2018;168(3):221–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moscow JA, Fojo T, Schilsky RL. The evidence framework for precision cancer medicine. Nat Rev Clin Oncol 2018;15(3):183–92. [DOI] [PubMed] [Google Scholar]
- 10.Leverson JD. Chemical parsing: Dissecting cell dependencies with a toolkit of selective BCL-2 family inhibitors. Mol Cell Oncol 2016;3(1):e1050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. The Journal of clinical investigation. 2007;117(1):112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touzeau C, Ryan J, Guerriero J, Moreau P, Chonghaile TN, Le Gouill S, et al. BH3 profiling identifies heterogeneous dependency on Bcl-2 family members in multiple myeloma and predicts sensitivity to BH3 mimetics. Leukemia. 2016;30(3):761–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodet L, Gomez-Bougie P, Touzeau C, Dousset C, Descamps G, Maiga S, et al. ABT-737 is highly effective against molecular subgroups of multiple myeloma. Blood. 2011;118(14):3901–10. [DOI] [PubMed] [Google Scholar]
- 14.Lohr JG, Stojanov P, Carter SL, Cruz-Gordillo P, Lawrence MS, Auclair D, et al. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer cell. 2014;25(1):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker BA, Wardell CP, Melchor L, Brioli A, Johnson DC, Kaiser MF, et al. Intraclonal heterogeneity is a critical early event in the development of myeloma and precedes the development of clinical symptoms. Leukemia. 2014;28(2):384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montero J, Sarosiek KA, DeAngelo JD, Maertens O, Ryan J, Ercan D, et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell. 2015;160(5):977–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.