Abstract
Not all genetic loci follow Mendel's rules, and the evolutionary consequences of this are not yet fully known. Genomic conflict involving multiple loci is a likely outcome, as restoration of Mendelian inheritance patterns will be selected for, and sexual conflict may also arise when sexes are differentially affected. Here, we investigate effects of the t haplotype, an autosomal male meiotic driver in house mice, on genome-wide gene expression patterns in males and females. We analysed gonads, liver and brain in adult same-sex sibling pairs differing in genotype, allowing us to identify t-associated differences in gene regulation. In testes, only 40% of differentially expressed genes mapped to the approximately 708 annotated genes comprising the t haplotype. Thus, much of the activity of the t haplotype occurs in trans, and as upregulation. Sperm maturation functions were enriched among both cis and trans acting t haplotype genes. Within the t haplotype, we observed more downregulation and differential exon usage. In ovaries, liver and brain, the majority of expression differences mapped to the t haplotype, and were largely independent of the differences seen in the testis. Overall, we found widespread transcriptional effects of this male meiotic driver in the house mouse genome.
Keywords: gene drive, meiotic drive, house mice, gene expression, t complex
1. Introduction
As a rule, nuclear genes in diploids have an equal 50% chance of being transmitted from the germline into gametes. This has important implications, as the route to increasing fitness lies in cooperating with other genes to increase organismal relative fitness [1]. Some loci, called meiotic drivers, adopt a different, selfish strategy, by distorting segregation in their favour in heterozygotes, so that the genomic region bearing the driver is over-represented among gametes. As a consequence, meiotic drivers increase in frequency through segregation distortion if they have positive, neutral or even detrimental fitness effects on the organism, as long as the fitness costs are not too high. Negative fitness effects of drivers slow fixation or prevent it altogether, and select for counter-adaptations of the genome against the driver in the form of drive resistance loci. This in turn selects for evolution in the driver to overcome such defences in the genome, potentially leading to an arms race between the driver and the genome that affects multiple genes.
Detailed studies of several systems where drive occurs in males, including the SD drive system in Drosophila melanogaster, SR in D. simulans, D. neotestacea, the stalk-eyed fly Teleopsis dalmanni and the t haplotype in house mice, have shown that these systems have in common that one or more driver loci act in trans on a target locus on the homologous autosome or on the other sex chromosome, which causes the developmental failure of wild-type sperm [2–6]. Loci in linkage disequilibrium with a driver, such as within an inversion, may also be divergent from wild-type variants and increase in frequency by hitchhiking. With reduced recombination, Muller's ratchet predicts an accumulation of deleterious mutations [7]. Selection could favour the evolution of linked enhancers of a driver, to improve drive, or linked loci that otherwise improve the fitness of drive carriers. Sexually antagonistic selection could also play a role, as meiotic drive favours transmission either in males or in females. In male meiotic drive, for example, drive loci are transmitted more often through males than females, so trait expression of driver-linked genes could be pulled away from the average optimum for both sexes to one closer to the optimum for males, the sex in which the driver is active [8,9]. This could potentially explain shared patterns of expression of genes in ovaries and testes of driving X chromosome carriers in stalk-eyed flies, T. dalmanni [10]. Drive also selects for the evolution of unlinked suppressor loci. Thus, meiotic drive might lead not only to differences in expression of genes associated with the drive phenotype, but also in linked genes, sexually antagonistic genes and unlinked loci throughout the genome.
Here, we use the t haplotype in house mice to investigate genome-wide patterns of gene expression related to a male meiotic driver. We already have a relatively rich understanding of specific effects of the t haplotype. It is transmitted to about 90% of offspring when inherited from the father. Females transmit the t gametes in the usual 50% ratio [11,12]. The mechanism of segregation distortion is like a poison-antidote system. Males heterozygous for the t haplotype (+/t) produce equal proportions of + and t sperm [13], but maturing t sperm produce gene products from the transmission distortion loci (Tagap, Fgd2, Nme3 and Tiam2) that ultimately hyperactivate a target gene (Sperm motility kinase Smok) in + sperm [14–19], leaving + sperm impaired. t sperm themselves have a hypoactive version of the target Smok gene (SmokTcr) to compensate [17]. The balance of these effects is that t sperm are successful when competing against damaged + sperm within the ejaculate, but are very poor in competition with + sperm from other males [20,21] when females mate multiply. Sperm from +/t males have been found to be slower, to hyperactivate more quickly and to be less able to enter the oocyte [22–26].
The distorter loci are situated within four large adjacent inversions on chromosome 17 that greatly reduce recombination and make it more likely that the complex is inherited as one intact entity [27]. A recent study has shown a more heterogeneous picture—some regions of the t haplotype show signs of recombination, while others do not [28]. However, as predicted under the mutation accumulation hypothesis, the t haplotype often carries a recessive lethal allele, so that t homozygotes die prenatally [21,29]. Litter sizes are reduced by 40% in crossings of +/t individuals [11]. There are, however, several different t variants, each with different recessive lethal effects, or that do not have lethal effects, but male-sterile effects when homozygous [12]. Detailed studies of a natural population of t-bearing house mice have led to the discovery of other traits associated with heterozygosity at the t haplotype: increased longevity in females [30], decreased activity levels in females [31], an increased propensity to emigrate [32] and decreased fitness in males [20,21,33].
In a previous study, Kelemen & Vicoso [28] made use of the finding of +/t individuals among a set of wild-caught house mice from a genome sequencing and gene expression study [34]. They analysed the pooled transcriptomes of 4 male +/t mice with 12 male +/+ mice from two different populations in France and Germany [34]. Their main findings were that gene regulation divergence was highly variable across the t haplotype, with increased divergence in regions in which no recombination events with the wild-type chromosome could be detected, parallel with an increase in the ratio of non-synonymous to synonymous SNPs in this region. They also found very few genes that were differentially expressed (DE) that were not within the t haplotype.
In this study, we compare the transcriptome of pairs of full sibling male and female adult house mice reared under standardized conditions in which one same-sex sibling is heterozygous for the t haplotype, while the other carries two wild-type alleles. The same laboratory population has been used in numerous studies of the t haplotype [11,21,25,31,35–37] and descends from a well-studied field population [30,32]. We analyse gene expression divergence in gonads, liver and brain attributable to the t haplotype in both males and females, making this the first study of effects of a male meiotic driver on female gene expression. By incorporating analysis of differential exon usage (DEU), we also advance on previous studies of gene regulation associated with meiotic drivers.
Understanding the effects of the t haplotype may be useful in contexts other than evolutionary biology, as it has been proposed as a conservation tool to control house mouse populations on islands [38]. The t haplotype could be transformed into a sex ratio driver by genetically engineering the incorporation of the male Sry gene, a Y-linked gene that initiates male sexual development, onto the t [38,39]. Offspring inheriting the modified t would develop a male phenotype regardless of their sex chromosomes, thereby reducing the proportion of fertile females to all offspring produced. Strongly biased sex ratios can lead to population crashes [40–42]. An improved understanding of the effects of the t haplotype on gene expression may be useful in better predicting the fate of a t-Sry construct.
2. Methods
(a). Animal breeding
We maintain a laboratory population of wild house mice descended from animals wild-caught from an intensively studied free-living population in Illnau, Switzerland (see [11,37,43] for details of the source population, and the t haplotype). All mice were maintained at standard conditions (22–25°C, 40–50% humidity, at a 14 h : 10 h light : dark cycle starting at 5.30 CET) in the same room. Further details are available in the electronic supplementary material.
We created 10 breeding pairs from unrelated +/t females and +/+ males. Each pair contributed their first litter to the study, over 21 days. These 10 litters generated 7 male and 8 female sibling pairs, with each pair consisting of one +/+ and one +/t. Five litters provided both male and female sibling pairs. At 23 days of age, offspring were moved to single sex cages and an ear punch taken for t haplotype diagnosis by PCR [44]. At five weeks of age, males were placed into single cages (Macrolon type II), and at eight weeks of age were euthanized with CO2. Sister pairs were put into two single cages connected by a tube, to equalize per capita space available to males and females. For females, we prioritized standardization between paired siblings of the female oestrus cycle over age. From eight weeks of age, we examined females visually for oestrus [45], checking for colour and swelling of the external genitalia, backed up by a vaginal smear [46] when in doubt. Females in a sibling pair were euthanized as soon as both were not in oestrus and appeared to be in dioestrus. Mice within pairs were processed sequentially during daylight hours, with one researcher extracting the brain and another gonads and liver. The organs were put into an RNA stabilization buffer [47] at 10× volume of the sample, and stored at 4°C. After 4 h, the RNA buffer was replaced. After 24 h, the buffer was removed and samples were frozen at −80°C. All experimental procedures were conducted blind with respect to genotype.
(b). RNA isolation, sequencing and processing
Samples were shipped on dry ice to the MPI for Evolutionary Biology in Germany for RNA isolation and sequencing. RNA from testis, ovaries and liver was extracted using the Ambion PureLink RNA Mini Kit with Trizol and a DNase step, while RNA from whole brain was isolated using a phenol–chloroform protocol. RNA libraries were prepared using Set A and B of the TruSeq Stranded mRNA Library Kit (Illumina). All samples, in total 90, were measured with the Fluoreszenz NanoDrop (NanoDrop 3300 Fluorospectrometer) and pooled at equimolar concentrations. The final pools were measured with the Agilent 2100 Bioanalyzer using the Agilent DNA 7500 Kit. The library was clustered to a density of approximately 180 K mm−2. Sequencing was performed on an Illumina NextSeq 500 using five HighOutput 300 cycle kits, corresponding to five lanes. PhiX Control library (Illumina) was combined with the library at 1%.
The RNA-seq data processing consisted of the following steps. Adapter trimming of the reads was performed with Trimmomatic [48]. We filtered reads with an average quality below 20. Poor quality reads led to the omission of one testis, one male brain and one female liver sample. Read-alignment was performed with STAR 2.5.3a [49]. As reference, we used the Ensembl genome build GRCm38, with the gene annotations downloaded on 31 May 2017. Gene expression values were computed with the function featureCounts from Rsubread [50]. The electronic supplementary material details the STAR alignment and featureCounts options. Genes were considered as expressed in a sample if they had at least 10 reads assigned. All figures were generated using more general R/Bioconductor functionality and UpsetR [51].
(c). Differential gene expression and differential exon usage
For the tissues, we computed differential expression as gene-level differences and DEU. In the gene-level differences, we analysed the total number of reads assigned to a gene locus, using the generalized linear model implemented in the Bioconductor package DESeq2 [52]. Specifically, the design parameter we used as input for DESeq2 was Genotype + Sex + Sibling. These factors are available as sample descriptions in GEO under the accession GSE138839. We computed the contrast between +/t and +/+. The model used for DEXSeq [53] was extended to include exons and the genotype–exon interaction. DEU will be significant if there is alternative splicing, or usage of alternative transcript start sites. If DEU is detected for a gene, the increase or decrease in the gene's total read counts must be interpreted with caution. For those genes, it cannot be assumed that an increase in the gene counts is associated with an overexpression. Thus, we report genes only as DEU if they show next to the DEU also an increased or decreased global read count. The thresholds we used were the following: upregulation: log2 ratio > 0.1, fdr < 0.1; downregulation: log2 ratio<−0.1, fdr < 0.1; DEU: splicing q-value < 0.1.
We used the software VLAD [54] via the Mouse Genomic Informatics website (http://proto.informatics.jax.org/prototypes/vlad, accessed 25 July 2019) to test for functional themes among DE gene sets. To test for enrichment of ontology terms within the t haplotype, we had to define the set of genes associated with the t haplotype. The precise boundaries of the t haplotype have not been identified [55]. Kelemen & Vicoso [28] gave approximate beginning and endpoints of the t haplotype as 5–40 Mb, based on increased SNP heterozygosity in a heterogeneous sample of +/+ and +/t mice. However, a recently discovered t haplotype distorter, Tiam2 [18], is located at 3.2 Mb, suggesting that this window is too small. We therefore expanded the window by 2 Mb in each direction (including 20 additional genes), at the risk of considering genes beyond the start and endpoints of the t haplotype as within it. We set q < 0.01 to limit results to the top terms.
3. Results
We found in all tissues a total of 434 genes with a differential expression status between +/t and +/+ siblings; 198 of these genes mapped to chromosome 17, with 195 falling within our defined limit of the t haplotype (3–42 Mb, making up 1.4% of the mouse genome [56] and containing 708 annotated genes). These 195 genes were located from 5.9 to 40.9 Mb, the remaining three being at 43.9, 80.5 and 86.9 Mb, and the latter two clearly outside of the t haplotype.
We found that the t haplotype region is highly enriched for antigen processing and presentation genes, as the t contains 78% of all MHC protein complex genes. The t haplotype also includes 82% of all pheromone activity genes (via exocrine gland-secreted peptide genes), and is enriched for plasma membrane part genes, and response to pheromone and G-protein coupled receptor activity genes, due to numerous vomeronasal receptor genes. It does not show depletion of any biological or molecular process, but in terms of cellular processes, it is deficient in intracellular parts.
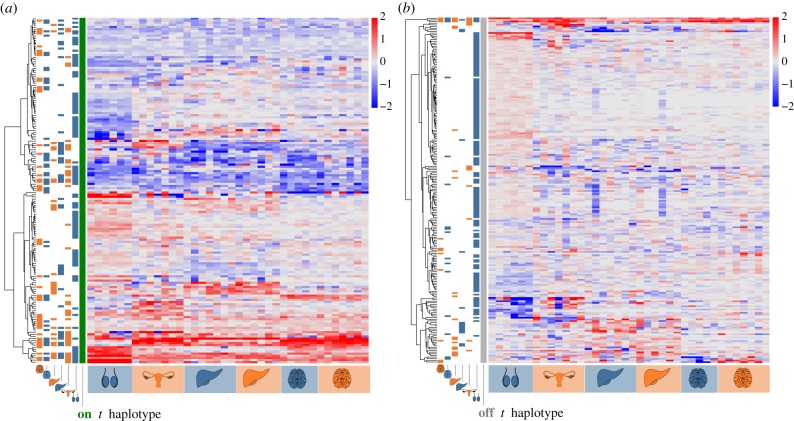
We visualized gene regulation differences between +/t and +/+ siblings as heatmaps (figure 1; see also electronic supplementary material, figure S1). Expression differences were observed in all tissues. In testes, expression differences, measured as absolute log2 ratios, were greater in the t haplotype region (mean ± s.d. of 0.83 ± 1.09) than in the rest of the genome (0.35 ± 0.65; Wilcoxon test, Z = 2883, p < 0.00001; electronic supplementary material, figure S2). Remarkably, brain tissue also showed a strong upregulation of a few t haplotype genes not DE in testes. This contrasts with non-t haplotype genes in the brain, which showed only very small changes.
Figure 1.
Heatmaps indicating expression differences between +/t and +/+ males and females for all genes DE in at least one tissue, with red indicating increased expression and blue decreased expression in +/t. Each column indicates the comparison between a +/t mouse and its +/+ sibling, for gonads, liver and brain for males (blue) and females (orange). On the left of each panel, blue and orange bars indicate in which comparison a specific gene showed a significant gene-level expression difference: (a) with the green bar shows the genes located on the t haplotype, while (b) with the grey bar shows genes located in other genomic regions.
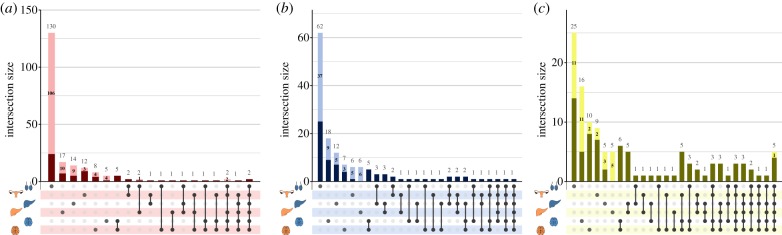
We also observed DEU in all tissues, and mostly in genes mapping to the t haplotype (figure 2). Examples include the t haplotype distorter genes Tagap (but notably not Tiam2), the responder SmokTcr locus element Rps6ka2, and spermatogenesis genes such as Dynlt1c, Dynlt1f, Tcp1, Tcp10a, Tcp10b, Tcp11, Tcte2, Tcte3, as well as the embryonic lethal gene Vps52. As an example of DEU, we show in electronic supplementary material, figure S3 differences in the distribution of reads across exons between +/t and +/+ individuals in the chromosome 5 gene Ppp1cb, identified as showing the strongest differential expression between +/t and +/+ in Kelemen & Vicoso [28]. Thus, our final dataset scores three categories: significant DEU, or significant up- or downregulation. Electronic supplementary material, table S2 provides the differential expression status of all genes, and electronic supplementary material, figure S4 illustrates differential expression status of genes along chromosome 17.
Figure 2.
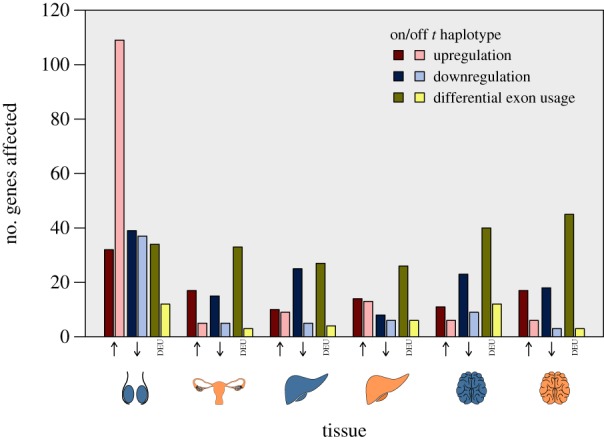
The number of significantly DE genes per tissue and sex (with blue representing male tissue, and orange female tissue), grouped by up- or downregulation (indicated by up or down facing arrows) or DEU. Darker bars represent genes in the t haplotype region; lighter bars represent genes elsewhere in the genome.
The testis dominated other tissues regarding the number of DE genes (figure 2), but only 40% (105/263) of all DE testis genes mapped to the t haplotype. This was significantly less than in other tissues: in ovaries 83% (65/78, χ2 = 43.6, p > 0.001), in male and female liver combined 64% (74/116, χ2 = 17.5, p < 0.001) and in male and female brain combined, 77.0% (99/135, χ2 = 38.5, p < 0.001) of DE genes were within the t haplotype. The t haplotype also significantly influenced the pattern of gene expression differences. In the testis, upregulation was observed in 30.5% (32/105) of DE t genes, compared with 69.0% (108/158) of non-t haplotype genes (χ2 = 34.8, p < 0.001).
We next asked whether the direction or type of expression difference in the ovary, liver and brain mirrored those of the testis. Usually they did not (figure 3; see also electronic supplementary material, figure S4). Many genes were up- or downregulated only in the testis, but in every tissue (male and female gonad, liver and brain), we found genes with tissue-specific changes. We also identified other genes with similar expression changes in multiple tissues. Remarkably, those genes were nearly always located on the t haplotype (figure 3). Males and females showed independent, but also some shared regulation differences within tissues. A small number of genes showed upregulation in all tissues (Rsph3a, Zrnd1), downregulation in all tissues (Jmjd8) and DEU in all tissues (H2-D1, Ppp1r11, Rnpepl1, Tcp1, Tmem181), with all these genes but Rnpepl1 located within the t haplotype.
Figure 3.
Shared expression patterns according to whether genes are located within the t haplotype or in the rest of the genome, for (a) upregulated genes, (b) downregulated genes or (c) genes with differential exon usage. The y-axis indicates the number of genes of each category, with the darker part of each bar representing genes in the t haplotype region, and the lighter part representing genes elsewhere in the genome. The x-axis indicates categories of shared expression. Single dots indicate expression differences within a category of sex and tissue, and vertical lines connect dots whenever the same gene is DE in more than one category. Blue and orange organs represent male and female tissues, respectively.
We also compared females and males more generally, asking how often a gene is significantly DE in females but not in males between +/t and +/+. In the gonads, 51 genes were DE in ovaries but not in testes, 26 genes in female compared to male brains, and 36 genes in female versus male livers. One gene Zfp994 showed upregulation in all female, but not in any male tissue, and one gene, Tagap, showed differential expression in all male but not in any female tissues.
We then tested if DE genes from the testis showed enrichment for particular functions (electronic supplementary material, figure S5 and GO_analysis). Spermatogenesis genes were significantly enriched, with 11% of all DE genes having this function, representing 4% of all known spermatogenesis genes. Some functions were upregulated, such as syncytium formation, with 15% of syncytium formation genes affected. Downregulated functions included regulation of protein catabolic process (5% of DE genes, 3% of genes of this function) and chylomicron remnant clearance (1% of DE genes, 50% of genes attached to this function). DE genes were involved in endocytic recycling (2% of DE genes, 10% of endocytic recycling genes). The top category of enrichment of cellular process was upregulation of genes contributing to the sperm flagellum (7% of DE genes, 14% of all sperm flagellum genes), followed by DEU of dynein complex genes (4% of DE genes, 14% of genes of this function) and protein phosphatase type 1 complex genes, including the gene Ppp1cb (1.5% of DE genes, 23% of genes of this function). No molecular functions were significantly enriched.
We next asked whether the DE genes from the t haplotype were significantly associated with biological functions, also using a Gene Ontology enrichment analysis. In t genes of the testis, we found significant enrichment and upregulation of the cytoplasmic dynein complex and enrichment of sperm flagellum genes (electronic supplementary material, table S1 and GO_analysis). With the analogous analysis for non-t genes, we found enrichment of sperm flagellum genes, spermatogenesis, but also syncytium formation, GTP biosynthetic process and alcohol and steroid metabolism.
Some functions carried over to other tissues. In the ovary, there was enrichment of gene function only within the t region. The dynein complex was enriched but downregulated. Sperm flagellum genes and the function of membrane-bound organelles were also enriched. Unlike in testis, there was enrichment of the MHC protein complex. In male liver, no functions were enriched, but in females, some metabolic processes were enriched, in the non-t part of the genome. In male brain, differential expression of cytoplasmic dynein genes was enriched, but mainly due to DEU, unlike in the ovary. In both male and female brain intracellular organelle was enriched.
4. Discussion
In this study of gene expression associated with the t haplotype, including analysis of DEU, we show that most expression differences are associated with the testis, where the loci causing drive of the t haplotype are known to be active. Expression effects were larger within the t haplotype than in chromosomal regions outside of the t haplotype, in line with previous studies comparing the magnitude of cis and trans expression [57]. We found that 60% of genes showing expression differences in testes map outside of the t haplotype, suggesting a high degree of activity in trans of t haplotype genes. In ovaries, liver and brain, most expression changes were associated with genes of the t haplotype itself. Both cis and trans changes are important in adaptive evolution, but it appears that trans changes feature more prominently in adaptation within species, whereas cis changes play a larger role in divergence between species [58–60]. This is interesting in the context of the t haplotype because it is thought to have evolved 1–3 Ma [61,62] and then recently introgressed into Mus musculus populations, either from an isolated M. musculus lineage [12] or from another species [63]. This suggests that the gene expression differences outside of the testis may simply reflect a neutral evolution of cis-acting elements. The trans effects in the testis, on the other hand, indicate the direct interaction of genes from the t haplotype with other parts of the genome to regulate sperm maturation.
Activity by transcription factors in trans may explain why the vast majority of expression differences off the t haplotype were in activation or repression of transcription, rather than in alternative splicing, which also requires cis-elements [64]. Testis genes affected in trans included those conferring functions that seem beneficial to the driving t haplotype genes. For example, t distorter products encounter developing + sperm in the syncytium, thus upregulation of syncytium formation genes may enhance dissemination of t distorter proteins, potentially increasing drive. The t haplotype influences flagellar function, decreasing sperm motility and altering movement patterns [24,25], and it is thought that + sperm of the +/t male are primarily affected, with the t sperm retaining normal function [17,65]. As dynein genes affect flagellar function, and spermatogenesis genes can affect many aspects of sperm function, activating such genes off the t haplotype could enhance drive. Protein phosphatase type 1 complex genes were enriched in the testis. These function in signalling, in an opposite way to protein kinases, such as Smok, and thus could conceivably also play a role in drive. DE genes involved in protein catabolism, endocytic recycling and chylomicron (small lipoprotein particle) clearance may contribute to general maturation pathways of the sperm that respond to the signalling changes induced by the t haplotype genes.
Expression of t genes with function in the testis was generally tissue-specific, according with organ-specific gene expression in mammals [66]. Some t genes were DE in the testis and also in other tissues, but most of these are probably due to the differences in cis-elements that have accumulated on the t haplotype. One unusual example is that of dynein complex genes, which showed DEU in testis and male brain, but were downregulated in ovaries, were they are unlikely to be useful and were not DE in female brain. Sex- and tissue-specific expression of many t genes suggests that selection has finely tuned expression patterns of most t-beneficial genes, and that sexually antagonistic selection for male beneficial gene regulation has not generally shaped expression profiles in females. Differential expression of t genes outside of the testis is not associated with an enrichment of gene ontology functions that link to phenotypic changes that have been observed in female +/t, including increased viability [30], decreased activity in the home cage [31] or altered mate choice [67], although we measured gene expression in dioestrus, rather than oestrus, when it is more relevant for mate choice [68]. Carboxylic acid metabolism in the liver has been linked to sexual dimorphism in mice [69] and this function was enriched outside of the t haplotype in +/t female liver. In all +/t female tissues, there was upregulation of one gene, a zinc finger protein gene with KRAB domain, associated with retrotransposon defence [70].
Our success in identifying expression differences in nearly all genes in which we expected to find differences validates our experimental and analytical approach. In testis, many t haplotype loci previously investigated in the search for genes directly involved in drive showed expression differences in this study. Nearly half of all t haplotype genes that showed DEU are known to be associated with differences between +/t and +/+: Tagap [14], Rps6ka2 [17], Tcte2 [71], Tcte3 [72], Tcp1 [73], Tcp10a and Tcp10b [74,75], Tcp11 [76], Dynlt1c and Dynlt1f [77], Vps52 [78], Fgfr1op [17], Ppp1r11 [79], Synj2 [80] and Tulp4 [81]. Nineteen genes showing alternative transcripts are left which have as yet no known function in the t haplotype, but are now new candidates: the protein kinase Map3k4, sperm genes Ift140, Hmga1, Slc26a8, as well as a mixed collection: Tmem181, Ube2i, Rsph3b, 1700010I14Rik, Rab11b, Rab11fip3, Fkbpl, Afdn, Znrd1as, Gm3448, Tbp, Rps2 and H2-D1. Copy number variation in the t haplotype [28] could contribute to the prevalence of alternative splicing among DE genes. The driving gene Tagap, for example, is present in four copies [14]. We found, however, that DEU was largely tissue-specific, and not across tissues. Downregulation was seen in five well-studied t haplotype genes: Dynlt1b [82], Tagap1 [17], Fgd2 [15] and DNAh8 [83,84]. Upregulation of known t haplotype active genes was rare, with only two cases: the maternally imprinted gene Slc22a3 [85,86] and the sperm glycolysis gene Pgk2 [87,88]. We found no expression differences in the t loci Tiam2 [18], Nme3 [16], Tcp10c [89], Slc22a2 [85], Acat2 and Acat3 [90], Serac1 [80], and Prdm9 [91], or the classic markers T, qk, Itpr3 (previously tf) [55]. The lack of detecting differences in Tiam2 was unexpected, given previously reported DEU [18]. Expression differences might be due to differences between wild-type strains or between t variants, as shown for Fgd2 [15], possibly indicating variation in which transmission distortion loci function in different t variants or different genetic backgrounds. Nme3, by contrast, was not expected to show expression differences [16]. These genes are not typical of the content of the t haplotype region, as it is enriched for immune function and pheromone activity and pheromone response, yet none of these functions were enriched in DE genes of the testis, liver or brain.
Our finding that most DE genes in the testis were not within the t haplotype region differs from stalk-eyed flies T. dalmanni and Drosophila neotestacea, and from a previous study of the t haplotype, in which most expression differences in testis were linked to the driving chromosome [5,10,28]. The differences with the latter study are likely to have been influenced by sampling scheme, as distant populations were pooled for that analysis [28]. Strikingly, functional themes among enriched transcripts in T. dalmanni and D. neotestacea show little overlap with each other or with this study, even though all three systems damage + sperm development. This highlights the varied evolutionary routes leading to male meiotic drive, but more work is needed to better understand the differences and commonalities between systems.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Patricia Lopes helped collect brain tissue and Jari Garbely performed +/t genetic diagnosis.
Ethics
This research was approved by the Veterinaeramt of Kanton Zurich, under permit 110/2013.
Data accessibility
Sequence reads can be accessed at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE138839. Other data supporting this study are available in the electronic supplementary material.
Authors' contributions
A.L. and D.T. designed the study. A.L. set up the live animal study and A.L. and A.S. collected samples. S.K. performed RNA isolation and RNA sequencing. H.R. analysed the RNA data. A.L. wrote the manuscript with input from H.R., A.S. and D.T. All authors contributed to editing the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by the SNF grant no. 310030M_133156.
References
- 1.Frank SA. 2003. Repression of competition and the evolution of cooperation. Evolution 57, 693–705. ( 10.1111/j.0014-3820.2003.tb00283.x) [DOI] [PubMed] [Google Scholar]
- 2.Larracuente AM, Presgraves DC. 2012. The selfish Segregation Distorter gene complex of Drosophila melanogaster. Genetics 192, 33–53. ( 10.1534/genetics.112.141390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao Y, Araripe L, Kingan SB, Ke Y, Xiao H, Hartl DL. 2007. A sex-ratio meiotic drive system in Drosophila simulans. II: an X-linked distorter. PLoS Biol. 5, e293 ( 10.1371/journal.pbio.0050293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrmann BG, Bauer H. 2012. The mouse t-haplotype: a selfish chromosome—genetics, molecular mechanism, and evolution. In Evolution of the house mouse (eds Macholán M, Baird SJE, Munclinger P, Piálek J), pp. 297–314. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Pieper KE, Unckless RL, Dyer KA. 2018. A fast-evolving X-linked duplicate of importin-α2 is overexpressed in sex-ratio drive in Drosophila neotestacea. Mol. Ecol. 27, 5165–5179. ( 10.1111/mec.14928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burt A, Trivers R. 2006. Genes in conflict: the biology of selfish genetic elements. Cambridge, MA: Harvard University Press. [Google Scholar]
- 7.Muller HJ. 1964. The relation of recombination to mutational advance. Mutat. Res. 1, 2–9. ( 10.1016/0027-5107(64)90047-8) [DOI] [PubMed] [Google Scholar]
- 8.Manser A, Lindholm AK, König B, Bagheri HC. 2012. The effect of polyandry on a distorter system with differential viabilities in the sexes. Commun. Integr. Biol. 5, 550–552. ( 10.4161/cib.21955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patten MM. 2014. Meiotic drive influences the outcome of sexually antagonistic selection at a linked locus. J. Evol. Biol. 27, 2360–2370. ( 10.1111/jeb.12493) [DOI] [PubMed] [Google Scholar]
- 10.Reinhardt JA, Brand CL, Paczolt KA, Johns PM, Baker RH, Wilkinson GS. 2014. Meiotic drive impacts expression and evolution of X-linked genes in stalk-eyed flies. PLoS Genet. 10, e1004362 ( 10.1371/journal.pgen.1004362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindholm AK, Musolf K, Weidt A, König B. 2013. Mate choice for genetic compatibility in the house mouse. Ecol. Evol. 3, 1231–1247. ( 10.1002/ece3.534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver LM. 1993. The peculiar journey of a selfish chromosome: mouse t haplotypes and meiotic drive. Trends Genet. 9, 250–254. ( 10.1016/0168-9525(93)90090-5) [DOI] [PubMed] [Google Scholar]
- 13.Hammerberg C, Klein JAN. 1975. Evidence for postmeiotic effect of t factors causing segregation distortion in mouse. Nature 253, 137–138. ( 10.1038/253137a0) [DOI] [PubMed] [Google Scholar]
- 14.Bauer H, Willert J, Koschorz B, Herrmann BG. 2005. The t-complex-encoded GTPase-activating protein Tagap1 acts as a transmission ratio distorter in mice. Nat. Genet. 37, 969–973. ( 10.1038/ng1617) [DOI] [PubMed] [Google Scholar]
- 15.Bauer H, Véron N, Willert J, Herrmann BG. 2007. The t-complex-encoded guanine nucleotide exchange factor Fgd2 reveals that two opposing signaling pathways promote transmission ratio distortion in the mouse. Genes Dev. 21, 143–147. ( 10.1101/gad.414807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer H, Schindler S, Charron Y, Willert J, Kusecek B, Herrmann BG. 2012. The nucleoside diphosphate kinase gene Nme3 acts as quantitative trait locus promoting non-Mendelian inheritance. PLoS Genet. 8, e1002567 ( 10.1371/journal.pgen.1002567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrmann BG, Koschorz B, Wertz K, McLaughlin J, Kispert A. 1999. A protein kinase encoded by the t complex responder gene causes non-mendelian inheritance. Nature 402, 141–146. ( 10.1038/45970) [DOI] [PubMed] [Google Scholar]
- 18.Charron Y, Willert J, Lipkowitz B, Kusecek B, Herrmann BG, Bauer H. 2019. Two isoforms of the RAC-specific guanine nucleotide exchange factor TIAM2 act oppositely on transmission ratio distortion by the mouse t-haplotype. PLoS Genet. 15, e1007964 ( 10.1371/journal.pgen.1007964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veron N, Bauer H, Weisse AY, Luder G, Werber M, Herrmann BG. 2009. Retention of gene products in syncytial spermatids promotes non-Mendelian inheritance as revealed by the t complex responder. Genes Dev. 23, 2705–2710. ( 10.1101/gad.553009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manser A, Lindholm AK, Simmons LW, Firman RC. 2017. Sperm competition suppresses gene drive among experimentally evolving populations of house mice. Mol. Ecol. 26, 5784–5792. ( 10.1111/mec.14215) [DOI] [PubMed] [Google Scholar]
- 21.Sutter A, Lindholm AK. 2015. Detrimental effects of an autosomal selfish genetic element on sperm competitiveness in house mice. Proc. R. Soc. B 282, 20150974 ( 10.1098/rspb.2015.0974) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson LR, Pilder SH, Bailey JL, Olds-Clarke P. 1995. Sperm from mice carrying one or two t haplotypes are deficient in investment and oocyte penetration. Dev. Biol. 168, 138–149. ( 10.1006/dbio.1995.1067) [DOI] [PubMed] [Google Scholar]
- 23.Olds-Clarke P. 1989. Sperm from tw32/+ mice: capacitation is normal, but hyperactivation is premature and nonhyperactivated sperm are slow. Dev. Biol. 131, 475–482. ( 10.1016/S0012-1606(89)80018-1) [DOI] [PubMed] [Google Scholar]
- 24.Olds-Clarke P, Johnson LR. 1993. t haplotypes in the mouse compromise sperm flagellar function. Dev. Biol. 155, 14–25. ( 10.1006/dbio.1993.1002) [DOI] [PubMed] [Google Scholar]
- 25.Sutter A, Lindholm AK. 2016. Meiotic drive changes sperm precedence patterns in house mice: potential for male alternative mating tactics? BMC Evol. Biol. 16, 133 ( 10.1186/s12862-016-0710-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redkar AA, Si Y, Twine SN, Pilder SH, Olds-Clarke P. 2000. Genes in the first and fourth inversions of the mouse t complex synergistically mediate sperm capacitation and interactions with the oocyte. Dev. Biol. 226, 267–280. ( 10.1006/dbio.2000.9870) [DOI] [PubMed] [Google Scholar]
- 27.Artzt K, McCormick P, Bennett D. 1982. Gene mapping within the T/t complex of the mouse. I: t-lethal genes are nonallelic. Cell 28, 463–470. ( 10.1016/0092-8674(82)90200-8) [DOI] [PubMed] [Google Scholar]
- 28.Kelemen RK, Vicoso B. 2018. Complex history and differentiation patterns of the t-haplotype, a mouse meiotic driver. Genetics 208, 365–375. ( 10.1534/genetics.117.300513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Safronova LD. 2009. Embryonal effects of t-haplotypes in mice. Russ. J. Dev. Biol. 40, 23–30. ( 10.1134/S1062360409010032) [DOI] [PubMed] [Google Scholar]
- 30.Manser A, Lindholm AK, König B, Bagheri HC. 2011. Polyandry and the decrease of a selfish genetic element in a wild house mouse population. Evolution 65, 2435–2447. ( 10.1111/j.1558-5646.2011.01336.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auclair Y, König B, Lindholm AK. 2013. A selfish genetic element Influencing longevity correlates with reactive behavioural traits in female house mice (Mus domesticus). PLoS ONE 8, e67130 ( 10.1371/journal.pone.0067130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Runge J-N, Lindholm AK. 2018. Carrying a selfish genetic element predicts increased migration propensity in free-living wild house mice. Proc. R. Soc. B 285, 20181333 ( 10.1098/rspb.2018.1333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carroll LS, Meagher S, Morrison L, Penn DJ, Potts WK. 2004. Fitness effects of a selfish gene (the Mus t complex) are revealed in an ecological context. Evolution 58, 1318–1328. ( 10.1111/j.0014-3820.2004.tb01710.x) [DOI] [PubMed] [Google Scholar]
- 34.Harr B, et al. 2016. Genomic resources for wild populations of the house mouse, Mus musculus and its close relative Mus spretus. Sci. Data 3, 160075 ( 10.1038/sdata.2016.75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manser A, König B, Lindholm AK. 2015. Female house mice avoid fertilization by t haplotype incompatible males in a mate choice experiment. J. Evol. Biol. 28, 54–64. ( 10.1111/jeb.12525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutter A, Lindholm AK. 2016. No evidence for female discrimination against male house mice carrying a selfish genetic element. Curr. Zool. 62, 675–686. ( 10.1093/cz/zow063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrari M, Lindholm AK, König B. 2014. A genetic tool to manipulate litter size. Front. Zool. 11, 18 ( 10.1186/1742-9994-11-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leitschuh CM, Kanavy D, Backus GA, Valdez RX, Serr M, Pitts EA, Threadgill D, Godwin J. 2018. Developing gene drive technologies to eradicate invasive rodents from islands. J. Responsible Innov. 5, S121–S138. ( 10.1080/23299460.2017.1365232) [DOI] [Google Scholar]
- 39.Backus G, Gross K. 2016. Genetic engineering to eradicate invasive mice on islands: modeling the efficiency and ecological impacts. Ecosphere 7, e01589 ( 10.1002/ecs2.1589) [DOI] [Google Scholar]
- 40.Hamilton WD. 1967. Extraordinary sex ratios. Science 156, 477–488. ( 10.1126/science.156.3774.477) [DOI] [PubMed] [Google Scholar]
- 41.Lyttle TW. 1977. Experimental population genetics of meiotic drive systems. 1. Pseudo-Y chromosomal drive as a means of eliminating cage populations of Drosophila melanogaster. Genetics 86, 413–445. [PMC free article] [PubMed] [Google Scholar]
- 42.Kyrou K, Hammond AM, Galizi R, Kranjc N, Burt A, Beaghton AK, Nolan T, Crisanti A. 2018. A CRISPR–Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat. Biotechnol. 36, 1062 ( 10.1038/nbt.4245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.König B, Lindholm AK. 2012. The complex social environment of female house mice (Mus domesticus). In Evolution of the house mouse (eds Macholán M, Baird SJE, Munclinger P, Piálek J), pp. 114–134. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 44.Schimenti J, Hammer M. 1990. Rapid identification of mouse t haplotypes by PCR polymorphism (PCRP). Mouse Genome 87, 108. [Google Scholar]
- 45.Champlin AK, Dorr DL, Gates AH. 1973. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol. Reprod. 8, 491–494. ( 10.1093/biolreprod/8.4.491) [DOI] [PubMed] [Google Scholar]
- 46.Byers SL, Wiles MV, Dunn SL, Taft RA. 2012. Mouse estrous cycle identification tool and images. PLoS ONE 7, e35538 ( 10.1371/journal.pone.0035538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Wit P, Pespeni MH, Ladner JT, Barshis DJ, Seneca F, Jaris H, Therkildsen NO, Morikawa M, Palumbi SR.. 2012. The simple fool's guide to population genomics via RNA-Seq: an introduction to high-throughput sequencing data analysis. Mol. Ecol. Resour. 12, 1058–1067. ( 10.1111/1755-0998.12003) [DOI] [PubMed] [Google Scholar]
- 48.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. ( 10.1093/bioinformatics/btu170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. ( 10.1093/bioinformatics/bts635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao Y, Smyth GK, Shi W. 2013. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 41, e108 ( 10.1093/nar/gkt214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conway JR, Lex A, Gehlenborg N. 2019. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics 33, 2938–2940. ( 10.1093/bioinformatics/btx364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 ( 10.1186/s13059-014-0550-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anders S, Reyes A, Huber W. 2012. Detecting differential usage of exons from RNA-seq data. Genome Res. 22, 2008 ( 10.1101/gr.133744.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson JE, Bult CJ. 2015. Visual annotation display (VLAD): a tool for finding functional themes in lists of genes. Mamm. Genome 26, 567–573. ( 10.1007/s00335-015-9570-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugimoto M. 2014. Developmental genetics of the mouse t complex. Genes Genet. Syst. 89, 109–120. ( 10.1266/ggs.89.109) [DOI] [PubMed] [Google Scholar]
- 56.EMBL-EBI. 2019. Mouse assembly and gene annotation. See https://www.ensembl.org/Mus_musculus/Info/Annotation
- 57.Meiklejohn CD, Coolon JD, Hartl DL, Wittkopp PJ. 2014. The roles of cis- and trans-regulation in the evolution of regulatory incompatibilities and sexually dimorphic gene expression. Genome Res. 24, 84–95. ( 10.1101/gr.156414.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Metzger BPH, Duveau F, Yuan DC, Tryban S, Yang B, Wittkopp PJ. 2016. Contrasting frequencies and effects of cis- and trans-regulatory mutations affecting gene expression. Mol. Biol. Evol. 33, 1131–1146. ( 10.1093/molbev/msw011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhoné B., Mariac C, Couderc M, Berthouly-Salazar C, Ousseini IS, Vigouroux Y. 2017. No excess of cis-regulatory variation associated with intraspecific selection in wild pearl millet (Cenchrus americanus). Genome Biol. Evol. 9, 388–397. ( 10.1093/gbe/evx004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Signor SA, Nuzhdin SV. 2018. The evolution of gene expression in cis and trans. Trends Genet. 34, 532–544. ( 10.1016/j.tig.2018.03.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morita T, et al. 1992. Evolution of the mouse t haplotype: recent and worldwide introgression to Mus musculus. Proc. Natl Acad. Sci. USA 89, 6851–6855. ( 10.1073/pnas.89.15.6851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hammer MF, Silver LM. 1993. Phylogenetic analysis of the alpha-globin pseudogene-4 (Hba-ps4) locus in the house mouse-species complex reveals a stepwise evolution of t-haplotypes. Mol. Biol. Evol. 10, 971–1001. ( 10.1093/oxfordjournals.molbev.a040051) [DOI] [PubMed] [Google Scholar]
- 63.Silver LM. 1982. Genomic analysis of the H-2-complex region associated with mouse t-haplotypes. Cell 29, 961–968. ( 10.1016/0092-8674(82)90459-7) [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, et al. 2015. Mechanism of alternative splicing and its regulation. Biomed. Rep. 3, 152–158. ( 10.3892/br.2014.407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olds-Clarke P, Peitz B. 1985. Fertility of sperm from t/+ mice: evidence that + -bearing sperm are dysfunctional. Genet. Res. 47, 49–52. ( 10.1017/S0016672300024502) [DOI] [PubMed] [Google Scholar]
- 66.Brawand D, et al. 2011. The evolution of gene expression levels in mammalian organs. Nature 478, 343 ( 10.1038/nature10532) [DOI] [PubMed] [Google Scholar]
- 67.Lenington S, Egid K. 1985. Female discrimination of male odors correlated with male genotype at the T locus: a response to T-locus or H-2-locus variability? Behav. Genet. 15, 53–67. ( 10.1007/BF01071932) [DOI] [PubMed] [Google Scholar]
- 68.Williams JR, Lenington S. 1993. Factors modulating preferences of female house mice for males differing in t-complex genotype: role of t-complex genotype, genetic background, and estrous condition of females. Behav. Genet. 23, 51–58. ( 10.1007/BF01067553) [DOI] [PubMed] [Google Scholar]
- 69.Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ. 2006. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 16, 995–1004. ( 10.1101/gr.5217506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jacobs FMJ, Greenberg D, Nguyen N, Haeussler M, Ewing AD, Katzman S, Paten B, Salama SR, Haussler D. 2014. An evolutionary arms race between KRAB zinc-finger genes ZNF91/93 and SVA/L1 retrotransposons. Nature 516, 242 ( 10.1038/nature13760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braidotti G, Barlow DP. 1997. Identification of a male meiosis-specific gene, Tcte2, which is differentially spliced in species that form sterile hybrids with laboratory mice and deleted in t chromosomes showing meiotic drive. Dev. Biol. 186, 85–99. ( 10.1006/dbio.1997.8574) [DOI] [PubMed] [Google Scholar]
- 72.Huw LY, Goldsborough AS, Willison K, Artzt K. 1995. TCTEX2-a sperm tail surface protein mapping to the t-complex. Dev. Biol. 170, 183–194. ( 10.1006/dbio.1995.1206) [DOI] [PubMed] [Google Scholar]
- 73.Silver LM, Kleene KC, Distel RJ, Hecht NB. 1987. Synthesis of mouse t complex proteins during haploid stages of spermatogenesis. Dev. Biol. 119, 605–608. ( 10.1016/0012-1606(87)90063-7) [DOI] [PubMed] [Google Scholar]
- 74.Schimenti J, Cebra-Thomas JA, Decker CL, Islam SD, Pilder SH, Silver LM. 1988. A candidate gene family for the mouse t complex responder (Tcr) locus responsible for haploid effects on sperm function. Cell 55, 71–78. ( 10.1016/0092-8674(88)90010-4) [DOI] [PubMed] [Google Scholar]
- 75.Cebrathomas JA, Decker CL, Snyder LC, Pilder SH, Silver LM. 1991. Allele-specific and haploid-specific product generated by alternative splicing from a mouse t-complex-responder locus candidate. Nature 349, 239–241. ( 10.1038/349239a0) [DOI] [PubMed] [Google Scholar]
- 76.Fraser LR, Hosseini R, Hanyalogou A, Talmor A, Dudley RK. 1997. TCP-11, the product of a mouse t-complex gene, plays a role in stimulation of capacitation and inhibition of the spontaneous acrosome reaction. Mol. Reprod. Dev. 48, 375–382. () [DOI] [PubMed] [Google Scholar]
- 77.Ha H, Howard CA, Yeom YI, Abe K, Uehara H, Artzt K, Bennett D. 1991. Several testis-expressed genes in the mouse t-complex have expression differences between wild-type and t-mutant mice. Dev. Genet. 12, 318–332. ( 10.1002/dvg.1020120409) [DOI] [PubMed] [Google Scholar]
- 78.Sugimoto M, et al. 2012. Molecular identification of tw5: Vps52 promotes pluripotential cell differentiation through cell–cell interactions. Cell Rep. 2, 1363–1374. ( 10.1016/j.celrep.2012.10.004) [DOI] [PubMed] [Google Scholar]
- 79.Han YB, Feng HL, Cheung CK, Lam PM, Wang CC, Haines CJ. 2007. Expression of a novel T-complex testis expressed 5 (Tctex5) in mouse testis, epididymis, and spermatozoa. Mol. Reprod. Dev. 74, 1132–1140. ( 10.1002/mrd.20631) [DOI] [PubMed] [Google Scholar]
- 80.Schimenti JC, Reynolds JL, Planchart A. 2005. Mutations in Serac1 or Synj2 cause proximal t haplotype-mediated male mouse sterility but not transmission ratio distortion. Proc. Natl Acad. Sci. USA 102, 3342–3347. ( 10.1073/pnas.0407970102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chao HHJ, Mentzer SE, Schimenti JC, You Y. 2003. Overlapping deletions define novel embryonic lethal loci in the mouse t complex. Genesis 35, 133–142. ( 10.1002/gene.10174) [DOI] [PubMed] [Google Scholar]
- 82.Duguay D, Bélanger-Nelson E, Mongrain V, Beben A, Khatchadourian A, Cermakian N. 2011. Dynein light chain Tctex-type 1 modulates orexin signaling through its interaction with orexin 1 receptor. PLoS ONE 6, e26430 ( 10.1371/journal.pone.0026430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fossella J, et al. 2000. An axonemal dynein at the Hybrid Sterility 6 locus: implications for t haplotype-specific male sterility and the evolution of species barriers. Mamm. Genome 11, 8–15. ( 10.1007/s003350010003) [DOI] [PubMed] [Google Scholar]
- 84.Samant SA, Ogunkua OO, Hui L, Lu J, Han Y, Orth JM, Pilder SH. 2005. The mouse t complex distorter/sterility candidate, Dnahc8, expresses a γ-type axonemal dynein heavy chain isoform confined to the principal piece of the sperm tail. Dev. Biol. 285, 57–69. ( 10.1016/j.ydbio.2005.06.002) [DOI] [PubMed] [Google Scholar]
- 85.Zwart R, Verhaagh S, de Jong J, Lyon M, Barlow DP.. 2001. Genetic analysis of the organic cation transporter genes Orct2/Slc22a2 and Orct3/Slc22a3 reduces the critical region for the t haplotype mutant tw73 to 200kb. Mamm. Genome 12, 734–740. ( 10.1007/s00335-001-3016-8) [DOI] [PubMed] [Google Scholar]
- 86.Zwart R, Sleutels F, Wutz A, Schinkel AH, Barlow DP. 2001. Bidirectional action of the Igf2r imprint control element on upstream and downstream imprinted genes. Genes Dev. 15, 2361–2366. ( 10.1101/gad.206201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fraser LR, Dudley K. 1999. New insights into the t-complex and control of sperm function. Bioessays 21, 304–312. () [DOI] [PubMed] [Google Scholar]
- 88.Danshina PV, Geyer CB, Dai Q, Goulding EH, Willis WD, Kitto GB, McCarrey JR, Eddy EM, O'Brien DA. 2010. Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biol. Reprod. 82, 136–145. ( 10.1095/biolreprod.109.079699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bullard DC, Schimenti JC. 1991. Molecular structure of Tcp-10 genes from the t complex responder locus. Mamm. Genome 1, 228–234. ( 10.1007/BF00352329) [DOI] [PubMed] [Google Scholar]
- 90.Ashworth A. 1993. Two acetyl-CoA acetyltransferase genes located in the t-complex region of mouse chromosome 17 partially overlap the Tcp-1 and Tcp-1x genes. Genomics 18, 195–198. ( 10.1006/geno.1993.1454) [DOI] [PubMed] [Google Scholar]
- 91.Kono H, Tamura M, Osada N, Suzuki H, Abe K, Moriwaki K, Ohta K, Shiroishi T. 2014. Prdm9 polymorphism unveils mouse evolutionary tracks. DNA Res. 21, 315–326. ( 10.1093/dnares/dst059) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence reads can be accessed at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE138839. Other data supporting this study are available in the electronic supplementary material.