Abstract
B cell–mediated autoimmunity may contribute to poor neurological outcomes after spinal cord injury (SCI). B cell–activating factor (BAFF) is a key cytokine involved in B cell development, proliferation, activation, and survival whose expression is elevated in men with chronic SCI. The aim of this study was to assess factors associated with circulating BAFF in non-ambulatory males with chronic SCI. We assessed the association between clinical and demographic factors, health habits, and circulating BAFF levels in a convenience sample of 43 non-ambulatory men with chronic spinal cord injury (≥ 1 year post-injury). Serum BAFF and total testosterone levels were quantified by enzyme-linked immunosorbent assay. Body composition was determined by whole body dual-energy X-ray absorptiometry. In multivariable models, active smokers had significantly greater BAFF levels than former/nonsmokers (871 pg/mL vs. 665 pg/ml, p = 0.002). BAFF decreased 36 ± 11.1 pg/mL for every 1 ng/mL increase in total testosterone (p = 0.002). This model explained 41% of the variation in circulating BAFF levels (model p < 0.0001). Our findings suggest that modifiable health habits may be associated with elevated BAFF levels in men with non-ambulatory chronic SCI. Further, the significant and independent negative association between testosterone levels and BAFF would suggest a link between androgen deficiency and autoimmunity observed in SCI via modulation of BAFF and B cell numbers. This points toward BAFF as a potential biomarker of injury severity and a target of therapies designed to reduce neuroinflammation and improve neurological outcomes after SCI.
Keywords: B cell–activating factor (BAFF), biomarker, rehabilitation medicine, spinal cord injury, testosterone
Introduction
Neurotrauma, including spinal cord injury, disrupts the blood–spinal cord barrier. This causes exposure of central nervous system (CNS) antigens to the immune system.1 Activation of lymphocytes with receptors specific to these antigens and production of auto-antibodies ensues which results in an autoimmune response.1,2 For instance, auto-antibodies specific to CNS antigens can be detected following injury in a mouse model of SCI. These antibodies worsen neurological outcomes and are thought to prevent neurological recovery.1,2 Antibodies against myelin basic protein are elevated in subjects with chronic SCI and levels are greatest in subjects with motor complete SCI.3 Consistent with these findings, there is abundant evidence (in rodents as well as in humans) that B cells mediate a harmful autoimmune response following SCI. Ankeny and colleagues2 have shown that B cell–deficient mice experience less severe motor dysfunction after SCI than wild-type (WT) mice. In addition, injection of antibodies purified from SCI mice into uninjured spinal cord caused paralysis.2 Elevated levels of autoantibodies were observed in SCI subjects. This was associated with several complications of autoimmunity including kidney disease, infertility, neuropathic pain, and pressure ulcers.4 Similarly, B and T cell–deficient mice with spinal cord injury experienced better neurological outcomes compared with WT mice.5
The mechanisms mediating the autoimmune response after SCI have not been clearly defined. A prior study demonstrated elevated B cell–activating factor (BAFF) levels in men with chronic SCI compared with men without SCI.6 BAFF is a key cytokine involved in B cell survival, proliferation, and differentiation into both antibody-secreting plasma cells and long-lived memory B cells.7 Elevated circulating levels of BAFF also have been reported in patients with autoimmune disorders including systemic lupus erythematosus, Sjogren's syndrome,8 and multiple sclerosis.9 There is emerging evidence supporting circulating BAFF as a biomarker of disease severity and progression in autoimmune disorders. However, there is limited information on BAFF after SCI. In this study, we sought to assess clinical and demographic factors associated with circulating BAFF levels in non-ambulatory men with chronic SCI.
Methods
Subjects
We studied a convenience sample of participants with SCI who were enrolled in an exercise-based clinical trial to improve bone health.10 Subjects were recruited for the parent study from individuals who receive care at our outpatient rehabilitation clinic or Veterans Affairs Medical Center. Participants were eligible for the parent study if they were 18 years or older, had a C4 or lower SCI (American Spinal Injury Association classification A, B, or C) with 3/5 greater biceps strength, and were non-ambulatory due to their injury. Subjects were excluded if they were actively being treated for epilepsy, actively using medications potentially affecting bone metabolism, including parathyroid hormone (PTH) and PTH analogs, bisphosphonates, androgenic steroids, estrogenic steroids, anti-epileptics, lithium, or oral glucocorticoid (use for more than 3 months); if they had a history of peripheral nerve compression or rotator cuff injury that limited the ability to exercise; uncontrolled diabetes; active renal disease; implanted defibrillator or pacemaker; an active grade 2 or greater pressure ulcer in a location that could be worsened with exercise; had an active bone fracture or lower extremity contractures; or were pregnant or lactating. For this BAFF substudy, we excluded 16 participants because biomarker results were not available, six participants with acute SCI (< 1 year post-injury), and five women, as there were too few to make meaningful comparisons based on sex. The final consisted of 43 men with chronic SCI who completed baseline testing between June 2011 and June 2013. The Institutional Review Boards approved all protocols prior to initiation of the study, and all participants gave their written informed consent to participate.
Motor score
Motor level and completeness of injury were confirmed by physical exam at study entry by the study physician according to the American Spinal Injury Association Impairment Scale (AIS) as previously described.6,11 Participants were classified as AIS A (sensory and motor complete, no sensory or motor function below the neurological level of injury), AIS B (motor complete, preservation of sensory but no motor function below the neurological level of injury), or AIS C (motor incomplete, sensory and motor function preserved below the neurological level, and more than half the key muscles below the neurological level are not strong enough to overcome gravity).
Dual x-ray absorptiometry for bone mineral density
We used a 5th generation GE Healthcare iDXA dual x-ray absorptiometry (DXA) scanner with enCore configuration version 12.3 to assess body composition. Total fat mass (kg) and total lean mass (kg) were calculated by the system software from whole body scans, as previously described.
Biochemical analyses
Plasma samples were drawn into an ethylenediaminetetraacetic acid tube and immediately delivered to the core blood research laboratory at our facility. The samples were centrifuged for 15 min at 2600 rpm (1459 × g) at 40°C and stored at −800°C until batch analysis. All biochemical analyses were performed at the Clinical and Epidemiologic Research Laboratory, Department of Laboratory Medicine at Children's Hospital in Boston, MA. Assays were performed in duplicate and any duplicate with >10% coefficient of variation (CV) was repeated. BAFF was measured by enzyme-linked immunosorbent assay from R&D Systems (Minneapolis, MN). The assay employs the quantitative sandwich enzyme immunoassay technique with a sensitivity of 2.7 pg/mL and run-to-run imprecision at BAFF concentrations of 474, 1118, and 2111 pg/mL are 9.9, 10.0 and 11.6%, respectively. Total testosterone was measured by a competitive electrochemiluminescence immunoassay (Roche Diagnostics, Indianapolis, IN). This assay is approved by the U.S. Food and Drug Administration (FDA) for clinical use. The lowest detection limit of this assay is 0.02 ng/mL and the day-to-day imprecision values at concentrations of 0.24, 2.75, and 7.01 ng/mL are 7.4, 2.2 and 1.7%, respectively. 25OH vitamin D was measured by high performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) using an API-5000 (AB Sciex, Foster City, CA) with a detection limit of 1.0 ng/mL.
Variable definition
Information regarding SCI, medical history, health habits, and medication use was obtained by a questionnaire at the time of enrollment. Participants completed a health questionnaire based on the American Thoracic Society adult respiratory disease questionnaire.12 Smokers were defined as smoking 20 or more packs of cigarettes or using 336 g (12 oz) of tobacco or more in a lifetime or smoking 1 or more cigarettes a day for at least 1 year. Current smokers reported cigarette use within 1 month of testing. Smoking status was considered dichotomously (current smoker vs. never/past smoker). Age and body mass index (BMI), were considered as continuous variables. 25OH vitamin D level was considered as a continuous variable and categorized as sufficient (≥ 30 ng/mL) or deficient (< 30 ng/mL). Lifetime alcohol consumption and alcohol consumption after SCI were calculated based on report of average daily, weekly, or monthly quantity and frequency of alcohol consumption and duration of alcohol use before and after injury. Each glass of wine (4 oz = 10.8 g), beer (12 oz = 13.2 g), and shot of liquor (1.5 ounce = 15.1 g) was converted to grams of alcohol.13,14 Participants were asked about a history of urinary infections or a pressure injury in the week prior to testing.
Statistical analysis
All analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC). T-tests or χ2 tests were used to compare subject characteristics as appropriate. BAFF was found to be normally distributed and therefore was not logged transformed. General linear models (PROC GLM) were applied to assess associations between clinical/demographic factors and BAFF. Factors with a p value of <0.10 in the univariate models were included in the multivariable models. Factors with a p value of <0.05 were considered statistically significant.
Results
Subject characteristics
Subject characteristics are presented in Table 1. Participants were age 40.8 ± 11.2 (standard deviation; SD) years (range 21.1–63.5) years and were 13.0 ± 10.9 (1.3–37.5) years post-injury. All participants used a wheelchair as their primary mode of mobility and most were paraplegic (74.4%). The mean BMI was 26.2 ± 5.4 (13.8–38.3), mean total mass was 84.6 ± 19.2 kg, and mean total lean mass was 52.4 ± 9.1 kg. Fifty-eight percent of participants were vitamin D deficient (< 30 ng/mL). 11% of participants were active smokers at the time of testing and the mean cigarette exposure was 12 ± 12 (SD) pack-years. The mean lifetime alcohol consumption was 352 19.2 kg-years. 19% of participants reported either a urinary tract infection (UTI; n = 5) or active skin ulcer (n = 3) at the time of testing.
Table 1.
Baseline Participant Characteristics
| Variable | Total cohort (n = 43) |
|---|---|
| Demographics | |
| Age (years) mean ± SD | 40.8 ± 11.2 |
| White, n (%) | 33 (76.7) |
| Years post injury mean ± SD | 13.0 ± 10.9 |
| Injury level | |
| Tetraplegia n (%) | 11 (25.6) |
| Paraplegia n (%) | 32 (74.4) |
| Injury completeness | |
| Motor complete: | |
| • A/B n (%) | 35 (81.4) |
| Motor incomplete: | |
| • C n (%) | 8 (18.6) |
| Body composition | |
| BMI (kg/m2) mean ± SD | 26.6 ± 5.9 |
| Total fat mass (%) mean ± SD | 34.6 ± 8.8** |
| Total lean mass (kg) mean ± SD | 52.4 ± 9.1** |
| Health habits | |
| Current smoker, n (%) | 5 (11.6) |
| Cigarette exposure (pack-years) mean ± SD | 11.79 ± 11.63 |
| Lifetime alcohol consumption (kg-years) mean ± SD | 323.07 ± 736.05 |
| Post-SCI alcohol consumption (kg-years) mean ± SD | 115.08 ± 174.85 |
| Current skin ulcers, n (%) | 3 (7.0) |
| Current UTI, n (%) | 5 (11.6) |
| Biomarkers | |
| BAFF (pg/mL) mean ± SD | 688.5 ± 159.5 |
| Total testosterone (ng/mL) mean ± SD | 4.3 ± 1.7 |
| 25OH Vitamin D (ng/mL) mean ± SD | 28.6 ± 10.5* |
| Vitamin D level | |
| • Normal (≥ 30 ng/mL), n (%) | 17 (39.5)* |
| • Deficient (< 30 ng/mL), n (%) | 25 (58.2) |
n = 42; **n = 41
SD, standard deviation; BMI, body mass index; SCI, spinal cord injury; UTI, urinary tract infection,; BAFF, B-cell–activating factor.
Clinical factors associated with BAFF levels
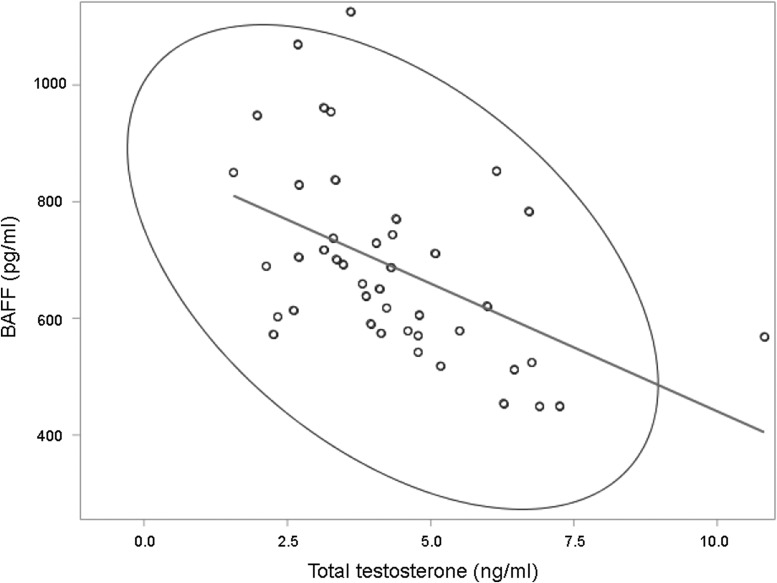
BAFF levels did not vary significantly based on time since last meal or snack (p = 0.34). In univariate analyses (Table 2), BAFF levels were positively associated with BMI (p = 0.03), and lifetime alcohol consumption (p = 0.02) and were significantly greater in active smokers compared with former/never smokers (p = 0.0006). BAFF levels were negatively associated with total testosterone levels (Fig. 1; p = 0.0009, Pearson Correlation r = -0.49). We found no association between BAFF levels and current UTI (p = 0.96) or skin ulcer (p = 0.65). In multi-variable models (Table 3), BMI and lifetime alcohol consumption were no longer significantly associated with BAFF (Model A, p = 0.91 and p = 0.11, respectively). Active smokers had significantly greater BAFF levels than former/nonsmokers (Model B, 871 pg/mL vs. 664 pg/mL; p = 0.002). BAFF decreased 36.31 ± 11.13 pg/mL for every 1 ng/mL increase in total testosterone (Model B, p = 0.002). This model explained 41% of the variation in circulating BAFF levels in non-ambulatory men with chronic SCI (model B; p < 0.0001).
Table 2.
Univariate Factors Associated with BAFF in Non-Ambulatory Men with Chronic SCI
| Variable | β ± SE | p |
|---|---|---|
| Age (years) | 0.66 ± 2.22 | 0.77 |
| Injury duration (years) | -3.29 ± 2.21 | 0.14 |
| BMI (kg/m2) | 7.83 ± 4.06 | 0.06 |
| Weight (kg) | 1.95 ± 1.23 | 0.12 |
| Total fat mass (%) | 4.32 ± 2.84 | 0.14** |
| Total fat (kg) | 3.08 ± 2.02 | 0.14** |
| Total lean mass (kg) | 1.61 ± 2.81 | 0.57** |
| Total testosterone (ng/mL) | -43.78 ± 12.21 | 0.0009 |
| 25OH vitamin D (ng/mL) | -1.41 ± 2.39 | 0.56* |
| Post-SCI alcohol consumption | -0.16 ± 0.14 | 0.28^ |
| Lifetime alcohol consumption | 0.07 ± 0.03 | 0.03^ |
| Means ± SE | p | |
| Race | ||
| • White | 700.39 ± 164.15 | 0.38 |
| • Others | 649.50 ± 144.12 | |
| Smoking status | ||
| • Active smokers | 906.04 ± 232.71 | 0.0006 |
| • Former/never smokers | 659.94 ± 125.73 | |
| Current skin ulcers | ||
| • Yes | 647.93 ± 55.25 | 0.65** |
| • No | 692.14 ± 167.36 | |
| Current UTI | ||
| • Yes | 690.85 ± 221.21 | 0.96*** |
| • No | 695.05 ± 161.25 |
n = 42; **n = 41; ***n = 38; ^n = 40.
SCI, spinal cord injury; SE, standard error; BMI, body mass index; SCI, spinal cord injury; UTI, urinary tract infection.
FIG. 1.
B cell–activating factor (BAFF) levels based on total testosterone levels. There is an inverse relationship between total testosterone and BAFF in non-ambulatory men with chronic SCI (β ± standard error = -39.38 ± 13.31, r = -0.49; p = 0.006). The ellipse approximates a region that contains 95% of population.
Table 3.
Multivariable model of Factors Associated with BAFF in Non-Ambulatory Men with Chronic SCI
| Model A, <0.0001, r2 = 0.50, n = 40 | Model B, <0.0001, r2 = 0.41, n = 43 | |||||
|---|---|---|---|---|---|---|
| Variable | β ± SE | lsmeans | p | β ± SE | lsmeans | p |
| BMI (kg/m2) | -0.47 ± 3.94 | . | 0.91 | . | . | . |
| Lifetime alcohol consumption | 0.05 ± 0.03 | . | 0.11 | . | . | . |
| Total testosterone (ng/mL) | -39.76 ± 13.22 | . | 0.005 | -36.31 ± 11.13 | . | 0.002 |
| Smoking status | ||||||
| • Smokers | 180.58 ± 61.41 | 840.27 | 0.006 | 206.55 ± 61.05 | 871.08 | `0.002 |
| • Non smokers | reference | 659.69 | reference | 664.54 | ||
BAFF, B-cell–activating factor; SCI, spinal cord injury; lsmeans, least squares means; BMI, body mass index.
Discussion
We examined circulating BAFF and total testosterone levels in 43 healthy, community dwelling non-ambulatory men with chronic SCI enrolled in an exercise-based clinical trial. We found that BAFF levels were negatively associated with total testosterone and were significantly greater in active smokers compared with former or never smokers. BAFF is an emerging biomarker of disease severity in rheumatoid arthritis, Grave's disease, and systemic lupus erythematosus (SLE). Based on our findings, BAFF may also be a valid biomarker of injury severity and/or spontaneous recovery after SCI. Moreover, treatment with a BAFF-neutralizing antibody (belimumab or Benlysta) reduces symptoms and slows disease progression in patients with SLE, rheumatoid arthritis, and multiple sclerosis.15–17 Belimumab is an FDA-approved drug that is routinely used in autoimmune disorders that is well tolerated and has a favorable safety profile. It is plausible that belimumab treatment may reduce the SCI-induced autoimmune response thereby reducing tissue damage and neurotoxicity. Additional pre-clinical work and subsequent randomized control clinical trials are needed to test this.
There are currently no validated biomarkers of injury severity, neurological recovery, or response to therapy. This void is considered a major limitation to both clinical care and to the development of adequately designed and powered clinical trials in SCI. Identification of a biomarker that is easy to obtain and measure (i.e., circulates in blood) would represent a major advancement in the field. While injury severity is routinely determined by neurological exam, there are instances where complete neurological testing cannot be performed (coma, intubated, or nonverbal patient, presence of cast or other physical barrier). Further, the variability in spontaneous neurologic recovery at 1-year post-injury is great and neurological examination does not differentiate between those who will experience recovery from those who will not. This variability in spontaneous recovery necessitates very large numbers for clinical trials testing pharmacologic interventions. Identification of a novel biomarker of spontaneous recovery would make clinical trial recruitment more economical and efficient.
Our findings suggest that smoking, a modifiable risk factor, may elevate BAFF levels, thereby potentially exacerbating SCI-induced autoimmunity. Similarly, testosterone may mitigate SCI-induced autoimmunity via BAFF modulation. Little is known about the impact of health habits, comorbid conditions, or clinical factors on circulating BAFF levels in the general population, and there is no information regarding these associations after SCI. Elevated BAFF levels in non-ambulatory men with chronic SCI who smoke suggests that factors promoting systemic inflammation also impact BAFF expression, production, and or release into the circulation. It was recently reported that exposure to cigarette smoke in mice induced increased BAFF expression by alveolar macrophages and that circulating BAFF levels were elevated in smokers compared with non-smokers.18,19 Circulating B cells produce excess BAFF levels after SCI.6 Adipose tissue is a known source of BAFF and SCI is associated with increased central fat mass and greater rates of obesity.20 Therefore, it is possible that multiple tissues contribute to BAFF detected in circulation.
The prevalence of nonalcoholic fatty liver disease is high after SCI and is associated with androgen deficiency.21 Moreover, upregulation of BAFF and B2-lymphocyte activation is an early finding in nonalcoholic fatty liver disease and contributes to disease progression implicating a role for SCI-induced autoimmunity in the pathophysiology.22 Therefore, our findings are consistent with these reports and suggest that an independent negative association between total testosterone levels and BAFF may contribute to the increased prevalence of nonalcoholic fatty liver disease after SCI. Our findings also are consistent with a recent report that testosterone is an endogenous regulator of BAFF.23 BAFF levels are inversely associated with testosterone levels in healthy men, in agreement with our current findings. Moreover, androgen receptor–deficient mice have greater numbers of B cells and higher circulating BAFF levels that wild-type counterparts. The authors speculate that testosterone is protective against autoimmunity via modulation of BAFF.
Studies of men with spinal cord injury are in agreement that testosterone levels decline after spinal cord injury and are substantially lower in men with spinal cord injury than in age-matched healthy controls.24–26 Forty to sixty percent of men with spinal cord injury have serum testosterone levels that are well below the lower limit of normal. The prevalence of testosterone deficiency is significantly greater in participants with motor complete (AIS A and B) injuries compared with those with motor incomplete (AIS C, D, and E). Low testosterone levels are associated with time since injury and lower hemoglobin levels. A number of factors, including the use of opiate analgesics, clustering of chronic illnesses, 25OH vitamin D deficiency,27–31 and neurologic injury, may all contribute to low testosterone levels. While an association between vitamin D and testosterone has been reported in SCI,27 we report that the negative association between BAFF and total testosterone is independent of 25OH vitamin D levels. Given that testosterone is a negative regulator of BAFF, this raises the intriguing possibility that testosterone deficiency contributes to BAFF over-expression and subsequent autoimmunity after SCI. Moreover, testosterone may be an effective neuroprotective agent after SCI,32 potentially via modulation of BAFF.
There are several limitations of the current study to consider. First, this is a small sample of non-ambulatory men with chronic SCI and who are relatively homogeneous in respect to injury severity, age, and medical comorbidities. Larger studies including women and across the spectrum of age and neurological impairment are required to identify other factors associated with circulating BAFF. This convenience sample did not include individuals with less severe injury or uninjured controls. Sample collection in the parent study did not include B cell isolation. Therefore, the current study cannot address downstream effectors of BAFF, such as NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) activation, or B cell subtype profiling. While we previously reported no significant increase in NF-κB complex gene expression in circulating peripheral blood mononuclear cells derived from men with chronic SCI compared with uninjured controls,6 additional work is needed to examine the downstream targets of BAFF and to perform B cell subtype profiling. These studies would add important information on the mechanisms of B cell activation in SCI and will be the focus of future work. Additionally, this is a cross-sectional analysis. Longitudinal studies are required to assess the relationship between circulating BAFF and spontaneous recovery. Finally, we only assayed total testosterone and therefore cannot assess associations between BAFF and free testosterone. Despite these limitations, this analysis provides important insight into factors that may regulate BAFF levels, and therefore autoimmunity, after SCI.
Acknowledgments
This study received support from: the Department of Defense [W81XWH-10-1-1043], The National Institute of Arthritis and Musculoskeletal and Skin Diseases [R01AR064793 and R01AR064793], and the Salah Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Ankeny D.P. and Popovich P.G. (2010). B cells and autoantibodies: complex roles in CNS injury. Trends Immunol. 31, 332–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ankeny D.P., Guan Z., and Popovich P.G. (2009). B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Invest. 119, 2990–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zajarias-Fainsod D., Carrillo-Ruiz J., Mestre H., Grijalva I., Madrazo I., and Ibarra A. (2012). Autoreactivity against myelin basic protein in patients with chronic paraplegia. Eur. Spine J. 21, 964–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davies A.L., Hayes K.C., and Dekaban G.A. (2007). Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 88, 1384–1393 [DOI] [PubMed] [Google Scholar]
- 5. Wu B., Matic D., Djogo N., Szpotowicz E., Schachner M., and Jakovcevski I. (2012). Improved regeneration after spinal cord injury in mice lacking functional T- and B-lymphocytes. Exp. Neurol. 237, 274–285 [DOI] [PubMed] [Google Scholar]
- 6. Saltzman J.W., Battaglino R.A., Salles L., Jha P., Sudhakar S., Garshick E., Stott H.L., Zafonte R., and Morse L.R. (2013). B-cell maturation antigen, a proliferation-inducing ligand, and B-cell activating factor are candidate mediators of spinal cord injury-induced autoimmunity. J. Neurotrauma 30, 434–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dekaban G.A. and Thawer S. (2009). Pathogenic antibodies are active participants in spinal cord injury. J. Clin. Invest. 119, 2881–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vadacca M., Margiotta D., Sambataro D., Buzzulini F., Lo Vullo M., Rigon A., and Afeltra A. (2010). [BAFF/APRIL pathway in Sjogren syndrome and systemic lupus erythematosus: relationship with chronic inflammation and disease activity]. Reumatismo 62, 259–265 [DOI] [PubMed] [Google Scholar]
- 9. Mackay F. and Schneider P. (2009). Cracking the BAFF code. Nat. Rev. Immunol. 9, 491–502 [DOI] [PubMed] [Google Scholar]
- 10. Morse L.R., Troy K.L., Fang Y., Nguyen N., Battaglino R., Goldstein R.F., Gupta R., and Taylor J.A. (2019). Combination therapy with zoledronic acid and FES-row training mitigates bone loss in paralyzed legs: results of a randomized comparative clinical trial. JBMR Plus 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morse L.R., Sudhakar S., Danilack V., Tun C., Lazzari A., Gagnon D.R., Garshick E., and Battaglino R.A. (2012). Association between sclerostin and bone density in chronic spinal cord injury. J. Bone Miner. Res. 27, 352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferris B.G. (1978). Epidemiology Standardization Project (American Thoracic Society). Am. Rev. Respir. Dis. 118, 1–120 [PubMed] [Google Scholar]
- 13. Dawson D.A. (2003). Methodological issues in measuring alcohol use. Alcohol Res. Health 27, 18–29 [PMC free article] [PubMed] [Google Scholar]
- 14. Garshick E., Segal M.R., Worobec T.G., Salekin C.M., and Miller M.J. (1989). Alcohol consumption and chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 140, 373–378 [DOI] [PubMed] [Google Scholar]
- 15. Jin X. and Ding C. (2013). Belimumab—an anti-BLyS human monoclonal antibody for rheumatoid arthritis. Expert. Opin. Biol. Ther. 13, 315–322 [DOI] [PubMed] [Google Scholar]
- 16. Blair H.A. and Duggan S.T. (2018). Belimumab: a review in systemic lupus erythematosus. Drugs 78, 355–366 [DOI] [PubMed] [Google Scholar]
- 17. Hawker K. (2008). B-cell-targeted treatment for multiple sclerosis: mechanism of action and clinical data. Curr. Opin. Neurol. 21 Suppl 1, S19–S25 [DOI] [PubMed] [Google Scholar]
- 18. Morissette M.C., Gao Y., Shen P., Thayaparan D., Berube J.C., Pare P.D., Brandsma C.A., Hao K., Bosse Y., Ettinger R., Herbst R., Humbles A.A., Kolbeck R., Zhong N., Chen R., and Stampfli M.R. (2016). Role of BAFF in pulmonary autoantibody responses induced by chronic cigarette smoke exposure in mice. Physiol. Rep. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seys L.J., Verhamme F.M., Schinwald A., Hammad H., Cunoosamy D.M., Bantsimba-Malanda C., Sabirsh A., McCall E., Flavell L., Herbst R., Provoost S., Lambrecht B.N., Joos G.F., Brusselle G.G., and Bracke K.R. (2015). Role of B cell-activating factor in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 192, 706–718 [DOI] [PubMed] [Google Scholar]
- 20. Kim M.Y., Kim D.H., and Do M.S. (2013). B-cell-activating factor is a regulator of adipokines and a possible mediator between adipocytes and macrophages. Exp. Mol. Med. 45, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barbonetti A., Caterina Vassallo M.R., Cotugno M., Felzani G., Francavilla S., and Francavilla F. (2016). Low testosterone and non-alcoholic fatty liver disease: evidence for their independent association in men with chronic spinal cord injury. J. Spinal Cord Med. 39, 443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bruzzi S., Sutti S., Giudici G., Burlone M.E., Ramavath N.N., Toscani A., Bozzola C., Schneider P., Morello E., Parola M., Pirisi M., and Albano E. (2018). B2-Lymphocyte responses to oxidative stress-derived antigens contribute to the evolution of nonalcoholic fatty liver disease (NAFLD). Free Radical Biol. Med. 124, 249–259 [DOI] [PubMed] [Google Scholar]
- 23. Wilhelmson A.S., Lantero Rodriguez M., Stubelius A., Fogelstrand P., Johansson I., Buechler M.B., Lianoglou S., Kapoor V.N., Johansson M.E., Fagman J.B., Duhlin A., Tripathi P., Camponeschi A., Porse B.T., Rolink A.G., Nissbrandt H., Turley S.J., Carlsten H., Martensson I.L., Karlsson M.C.I., and Tivesten A. (2018). Testosterone is an endogenous regulator of BAFF and splenic B cell number. Nat. Commun. 9, 2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barbonetti A., Vassallo M.R., Pacca F., Cavallo F., Costanzo M., Felzani G., Francavilla S., and Francavilla F. (2014). Correlates of low testosterone in men with chronic spinal cord injury. Andrology 2, 721–728 [DOI] [PubMed] [Google Scholar]
- 25. Rosety-Rodriguez M., Rosety I., Fornieles G., Rosety J.M., Elosegui S., Rosety M.A., and Ordonez F.J. (2014). A short-term arm-crank exercise program improved testosterone deficiency in adults with chronic spinal cord injury. Int. Braz. J. Urol. 40, 367–372 [DOI] [PubMed] [Google Scholar]
- 26. Durga A., Sepahpanah F., Regozzi M., Hastings J., and Crane D.A. (2011). Prevalence of testosterone deficiency after spinal cord injury. PM R 3, 929–932 [DOI] [PubMed] [Google Scholar]
- 27. Barbonetti A., Vassallo M.R., Felzani G., Francavilla S., and Francavilla F. (2016). Association between 25(OH)-vitamin D and testosterone levels: evidence from men with chronic spinal cord injury. J. Spinal Cord Med. 39, 246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bauman W.A., Zhong Y.G., and Schwartz E. (1995). Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism 44, 1612–1616 [DOI] [PubMed] [Google Scholar]
- 29. Barbonetti A., Sperandio A., Micillo A., D'Andrea S., Pacca F., Felzani G., Francavilla S., and Francavilla F. (2016). Independent association of vitamin D with physical gunction in people with chronic spinal cord injury. Arch. Phys. Med. Rehabil. 97, 726–732 [DOI] [PubMed] [Google Scholar]
- 30. Barbonetti A., Cavallo F., D'Andrea S., Muselli M., Felzani G., Francavilla S., and Francavilla F. (2017). Lower vitamin D levels are associated with depression in people with chronic spinal cord injury. Arch. Phys. Med. Rehabil. 98, 940–946 [DOI] [PubMed] [Google Scholar]
- 31. Barbonetti A., D'Andrea S., Martorella A., Felzani G., Francavilla S., and Francavilla F. (2018). Low vitamin D levels are independent predictors of 1-year worsening in physical function in people with chronic spinal cord injury: a longitudinal study. Spinal Cord 56, 494–501 [DOI] [PubMed] [Google Scholar]
- 32. Gurer B., Kertmen H., Kasim E., Yilmaz E.R., Kanat B.H., Sargon M.F., Arikok A.T., Erguder B.I., and Sekerci Z. (2015). Neuroprotective effects of testosterone on ischemia/reperfusion injury of the rabbit spinal cord. Injury 46, 240–248 [DOI] [PubMed] [Google Scholar]