Abstract
Inhibiting transmission of Plasmodium is an essential strategy in malaria eradication, and the biological process of gamete fusion during fertilization is a proven target for this approach. Lack of knowledge of the mechanisms underlying fertilization have been a hindrance in the development of transmission-blocking interventions. Here we describe a protein disulphide isomerase essential for malarial transmission (PDI-Trans/PBANKA_0820300) to the mosquito. We show that PDI-Trans activity is male-specific, surface-expressed, essential for fertilization/transmission, and exhibits disulphide isomerase activity which is up-regulated post-gamete activation. We demonstrate that PDI-Trans is a viable anti-malarial drug and vaccine target blocking malarial transmission with the use of PDI inhibitor bacitracin (98.21%/92.48% reduction in intensity/prevalence), and anti-PDI-Trans antibodies (66.22%/33.16% reduction in intensity/prevalence). To our knowledge, these results provide the first evidence that PDI function is essential for malarial transmission, and emphasize the potential of anti-PDI agents to act as anti-malarials, facilitating the future development of novel transmission-blocking interventions.
Subject terms: Mechanisms of disease, Protein folding, Parasite biology, Parasite physiology
Introduction
Malaria remains a significant widespread health challenge with an estimated 216 million cases and 435,000 deaths globally in 20171. Recent approaches have significantly reduced global burden but recent progress has stalled1, and it is widely accepted that a range of new tools will be required to attain malaria elimination2. The causative agent of malaria, the protozoan parasite Plasmodium, is transmitted almost exclusively by anopheline mosquitoes. Transmission of Plasmodium from vertebrate to mosquito hosts is entirely dependent on the circulation of sexually viable gametocytes within circulating blood, which differentiate into micro- (male) and macro- (female) gametes upon ingestion by the mosquito within a blood meal. The essential process of fertilization is a two stage process, initiated by gamete adhesion, followed by membrane fusion3,4. A small number of proteins have previously been implicated in plasmodial fertilization; the 6-Cys protein family members P48/45, P47 and P230 have demonstrable roles in the mutual recognition and adhesion of micro- and macro-gametes5–7, whereas the conserved male-specific Class II fusion protein HAP2/GCS1 has been shown to be the key driver of membrane fusion by mediating merger of lipid bilayers3,4. Following successful fertilization, resulting zygotes develop into ookinetes, which migrate to and invade the mosquito midgut, establishing infection in the insect. Despite the key importance of parasitic transmission and its undoubted potential as a point to disrupt the plasmodial lifecycle with various therapeutic classes8, our knowledge of the mechanisms underlying fertilization and subsequent zygote formation in Plasmodium is surprisingly incomplete.
It is recognized that to achieve malarial control or eradication, it is vital to use interventions that inhibit transmission from humans to mosquitoes2. A potential mechanism to achieve this is to target Plasmodium using transmission-blocking interventions (TBIs); i.e. transmission blocking vaccines (TBVs), or transmission blocking drugs (TBDs) against parasitic sexual stages9–11. Antibodies targeting three of the five currently proven, potent TBV targets (P48/45, P230, HAP2) have demonstrable localization to proteins found on the plasma membrane of the gametes12–22, indicating the potential value of targeting this lifecycle stage21. Additionally, multiple anti-malarial compounds have been demonstrated to have activity against this parasitic stage23–27. In summary, the comparatively short life span, fragility, and availability of proteins on the surface of the Plasmodium male gamete make targeting this stage of the lifecycle a potential method of impeding transmission11,27. Similarly, potent TBIs targeting the parasitic ookinete post-fertilization are well characterized in multiple vaccine and drug studies10,17,18,28–30.
Protein Disulphide Isomerase (PDI) (EC: 5.3.4.1) is a multifunctional member of the thioredoxin superfamily of redox proteins, characterized by the presence of the βαβαβαββα fold31. PDIs typically have three catalytic activities; disulphide isomerase, thiol-disulphide oxidoreductase, and redox-dependent chaperone. PDI homologues have been identified in multiple species, where they are “classically” located in the endoplasmic reticulum (ER) and facilitate the folding and assembly of secretory and membrane proteins within the lumen32. In Plasmodium, a small number of proteins have been putatively identified (by sequence homology) as PDI-like molecules in Plasmodium falciparum, vivax, knowlesi, berghei and yoelii33–35. Conclusive demonstration of PDI activity has currently only been demonstrated with PF3D7_0827900/PDI-833, with transcription and translation demonstrated in asexual blood schizonts, gametocytes and sporozoites. Knowledge regarding the process of disulphide bond-dependent protein folding in Plasmodium is scarce.
Similarly, an increased understanding of transmission and mechanisms of fertilization within Plasmodium is vital, and offers prospective opportunities for the development of novel TBIs. Here, we describe the identification, characterization and role of a protein disulphide isomerase (PDI-Trans/PBANKA_0820300) essential for malarial transmission to the mosquito host in P. berghei. We demonstrate that PDI-Trans is transcribed and translated across the entire parasitic lifecycle, and exhibits activity at the sexual stages of the lifecycle, when fertilization of gametes occurs. We show that PDI-Trans function is male specific after microgamete release, and essential for successful fertilization/transmission, and exhibits disulphide isomerase function which is up-regulated post-gamete activation. Furthermore, we show that PDI-Trans is a viable anti-malarial drug and vaccine target, expressed on the surface of the sexual stages of Plasmodium, by blocking malarial transmission with the use of repurposed compounds that target PDI activity, and anti-PDI-Trans peptide antibodies. These results demonstrate that protein disulphide isomerase function is essential for malarial transmission; emphasize the potential of anti-PDI agents to act as anti-malarials, and demonstrate the potential utility of rationally-selected targets to facilitate the development of novel anti-malarial transmission-blocking interventions.
Results
PDI-Trans is located on the surface on the transmission stages of P. berghei
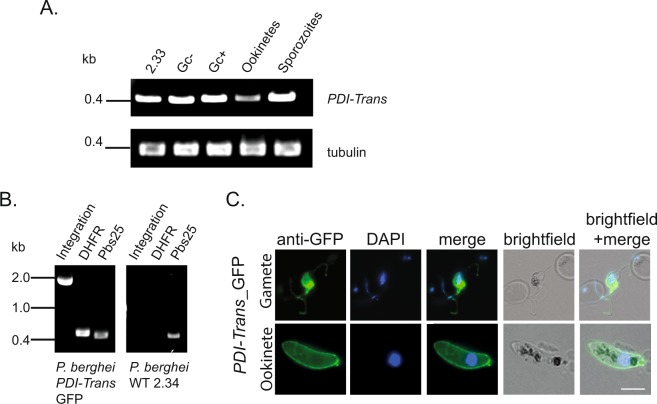
Previous proteomic analysis of a P. berghei male gamete proteome generated in36–38 followed by advanced bioinformatics analysis encompassing a suite of functional and localization-based algorithms36 identified the expression of PDI-Trans (PBANKA_0820300) in the male gamete, and suggested that the resulting transmembrane protein was potentially located on the surface of the plasma membrane of male gametes. A brief analysis of PDI-Trans is described within39, where following a BarSeq Screen for asexual growth on an extensive library of non-clonal P. berghei KO parasites, posited that the gene is dispensable for the progression of blood-stage parasitemia. Our subsequent analysis of transcription levels by RT-PCR support this, demonstrating that PDI-Trans transcripts were present in wild-type asexual erythrocytic stages of the gametocyte the deficient strain 2.33, in addition to non-activated (Gc−) and activated (Gc+) gametocytes, ookinetes and sporozoites of the parental line 2.34 (Fig. 1A). To investigate the cellular localization of PDI-Trans across the parasitic lifecycle targeted-single homologous recombination was utilized to generate a transgenic P. berghei parasite expressing the endogenous PDI-Trans protein with a C-terminal EGFP fusion tag. Successful integration following drug selection was confirmed by PCR (Fig. 1B). The presence of the EGFP tag caused no observable impact on blood or sexual stages, and did not impact transmission through An. stephensi mosquitoes. Immunofluorescence microscopy on non-permeablized parasites confirmed PDI-Trans-GFP expression at the surface of activated male gametes and ookinetes (Fig. 1C). Live microscopy of mixed blood stages, female gametes and fixed immunofluorescence of sporozoites demonstrated that PDI-Trans-GFP is expressed across the entire parasitic lifecycle (Figure S1).
Figure 1.
Constitutive expression of Plasmodium berghei PDI-Trans, and localization on the surface of gametocytes and ookinetes. (A) RT-PCR analysis of PDI-Trans in asexual blood stages using the non-gametocyte producing strain 2.33; non-activated (Gc-) and activated (Gc+) gametocytes; purified in vitro ookinetes and day 21 salivary gland dissected sporozoites. The analysis was complemented with alpha-tubulin loading controls (B). PCR confirmation of integration of egfp into the PDI-Trans locus. Oligonucleotides 35 and 14 were used to detect integration. Oligonucleotide 91 and 92 were used to detect DHFR presence, pbs25 oligonucleotides were used as positive controls. P. berghei WT 2.34 gDNA was used as a negative control for integration. (C) IFA of fixed, non-permeablised PDI-Trans-GFP parasites probed with anti-GFP; exflagellating male gametocytes (top) and ookinetes (bottom). Each panel shows an overlay of GFP fluorescence (green) and DNA labelled with DAPI (blue). White scale bar = 5 μm.
PDI-Trans is essential for parasite transmission, is male specific and demonstrates classical PDI activity
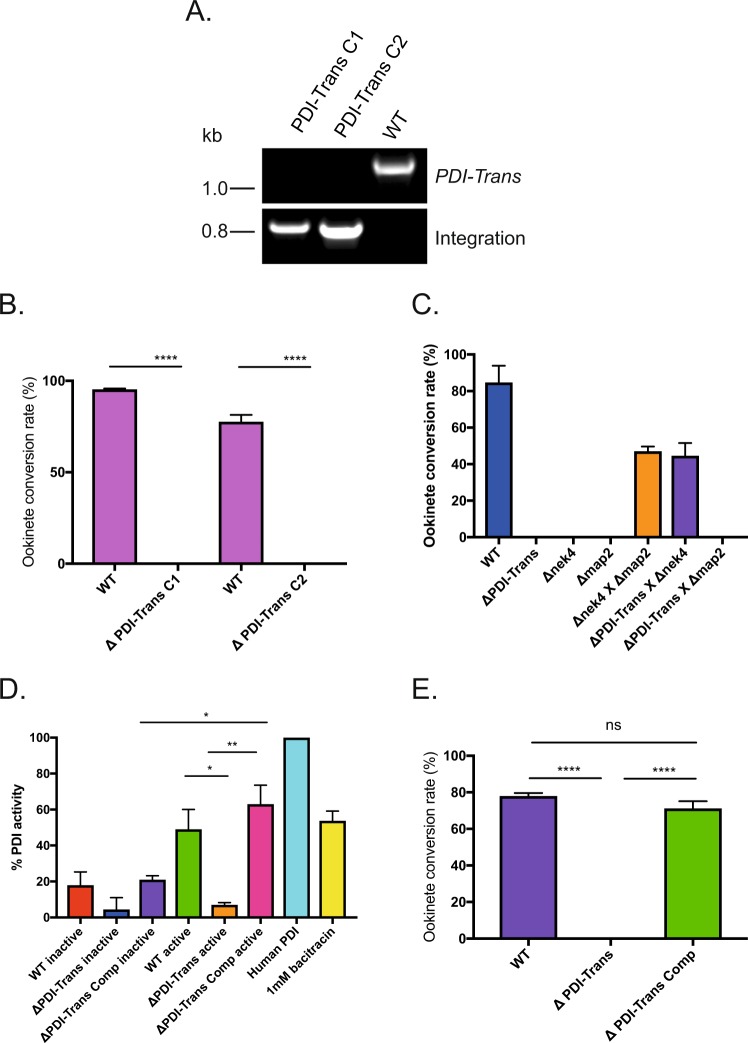
To investigate the function of PDI-Trans targeted gene disruption was used to replace the entire PDI-Trans coding sequence. This was performed by double homologous recombination as described in39,40, with constructs designed and manufactured by PlasmoGem (Sanger Institute, UK). Following dilution cloning of drug-resistant parasites, genotyping by PCR of two independently produced clones (Fig. 2A) indicates that the replacement construct had integrated at the targeted site, disrupting the endogenous locus. Consistent with previous predictions40,41, examination of mice infected with ΔPDI-Trans clones showed that the parasites underwent normal asexual development in erythrocytes (Figure S2). Rates of gametocytogenesis and sex ratio were unaffected, and gametocytes were able to emerge from their host cells and differentiate into gametes when exposed to standard gamete activation conditions (i.e. drop in pH or temperature, presence of xanthaurenic acid). To examine non-inactivated gametes, incubation in coelenterazine loading buffer followed by Nycodenz purification was performed42. To examine for a specific role during fertilization we specifically examined in vitro ookinete formation in blood collected from mice infected with ΔPDI-Trans parasites. Blood cultures from mice infected with ΔPDI-Trans parasites failed to produce ookinetes, a finding confirmed by triplicate experiments on two independent ΔPDI-Trans clones (Fig. 2B). To further explore this phenotype in vivo, An. stephensi mosquitoes were fed on mice infected with ΔPDI-Trans parasites in triplicate studies, and 12 days later microscopy was used to examine the presence of oocysts (Figure S3). Triplicate experiments of each clone showed a mean reduction of 94.38% inhibition in intensity and 63.68% inhibition in oocyst prevalence with in ΔPDI-Trans clone 1, and a 96.43%/65.62% inhibition in intensity/prevalence with ΔPDI-Trans clone 2 when compared to wild type P. berghei (Table 1). The phenomenon of total blockade in transmission in vitro, while still observing low levels in vivo, is often observed with fertilization-null phenotypes following gene deletion (e.g. following KOs of HAP2 and P48/453,6), and is often attributed to the fragile nature of the midgut epithelium post-blood meal. The resulting oocysts are morphologically normal and produce sporozoites that are capable of successfully establishing a blood stage infection. These results suggest that Trans-PDI plays a key role in the successful of Plasmodium.
Figure 2.
Deletion of PDI-Trans strongly inhibits transmission and is male specific. (A) Diagnostic PCR with genomic DNA templates and primers 69 and 70 to test for the presence of PDI-Trans, and primers 72 and 9 to detect a unique 930 bp product across the integrations site. (B) The bar chart shows ookinete conversion rates for wild type and both ΔPDI-Trans clones. Conversion rate is expressed as a percentage of P28-positive parasites that had progressed to the ookinete stage (error bar indicates SEM; n = 3). Asterisks indicate P value < 0.05 Paired t test (C). In vitro ookinete conversion analysis demonstrates that PDI-Trans mutant shows production cross-fertilisation with the Δnek4 sterility mutant, which produces functional males only, and not with Δmap2 mutant, which produces functional females only. (error bar indicates SEM; n = 3). (D) PDI activity of purified whole parasite active and non-activated gametocytes from wild type, ΔPDI-Trans and ΔPDI-Trans Comp parasite lines. PDI activity is expressed as a percent relative to the positive control (human recombinant PDI). Bacitracin at 1 mM was used on human recombinant PDI as a control for a 50% reduction of activity as outlined by kit protocol. Experiments were performed in triplicate (error bar indicates SEM; n = 3). Asterisks indicate P value < 0.05 Paired t test. (E) Bar chart of ookinete conversion rates for wild type ΔPDI-Trans and ΔPDI-Trans Comp parasite lines. Conversion rate is expressed as a percentage of P28-positive parasites that had progressed to the ookinete stage (error bar indicates SEM; n = 3). Asterisks indicate P value < 0.05 Paired t test.
Table 1.
Mean in vivo evaluation of deleting PDI-Trans on transmission by direct feeding.
| Wild type | ΔPDI-Trans Clone 1 | Wild type | ΔPDI-Trans Clone 2 | |
|---|---|---|---|---|
| Mean intensity (n = 3) | 59.81 | 3.19 | 60.17 | 2.23 |
| Mean prevalence (n = 3) | 92.67 | 34 | 93.86 | 32.67 |
| Inhibition in intensity (%) | — | 94.38a | — | 96.43a |
| Inhibition in prevalence (%) | — | 63.68b | — | 65.62b |
The mean (from three replicates) inhibition in intensity (mean number of oocysts per midgut) and prevalence of two independent PDI-Trans knockout clones were calculated with respect to wild type controls. aP < 0.05, Mann-Whitney U test bP < 0.05, Fisher’s exact test.
Cross-fertilization studies, with pre-characterized sex-specific mutants, such as the male-deficient map2 or female-defective nek4 mutant43,44, make it possible to identify gender-specific sterility phenotypes in P. berghei. As demonstrated in Fig. 2C, neither Δmap2 nor Δnek4 strains produce ookinetes when cultured alone, but when cultures containing both KO lines were mixed, Δnek4 male gametes are able to fertilize map2 female gametes, restoring fertility and the capacity to produce ookinetes (Fig. 2C). Reduced conversion rates (compared with wild type parasites) are expected3,42,43, due to the persistence of Δnek4 female and Δmap2 gametes which are unable to fertilize. In ΔPDI-Trans /Δnek4 crosses the PDI-Trans female gametes were fertilized by Δnek4 micro-gametes, but Δmap2 females remained unable to differentiate into ookinetes in ΔPDI-Trans/Δmap2 crosses (Fig. 2C), indicating that ΔPDI-Trans males are sterile. Correspondingly, these results demonstrate that during plasmodial fertilization, PDI-Trans is essential for microgamete (male) fertility.
PDI enzymes typically catalyze the rearrangement of disulphide bonds between cysteine residues within proteins. To determine whether PDI-Trans exhibits classical PDI activity we utilized a fluorescent PDI insulin-reduction assay to determine reductase activity. Recombinant human PDI was used as a positive control for PDI activity, and the well-characterized PDI inhibitor bacitracin was used as a negative control. Gametocytes from ΔPDI-Trans, ΔPDI-Trans Comp (See below) and wild type lines were purified on a density gradient and used in the assay in either a non-activated or activated form. PDI activity was expressed as a percentage relative to the positive control (Fig. 2D). In wild type parasites, PDI activity is increased post-gametocyte activation, implicating broad PDI activity throughout gamete activation/fertilization. The activated gametes of the ΔPDI-Trans line had significantly reduced PDI activity with respect to wild type gametes, specifically indicating that PDI-Trans exhibits true PDI reductase function during fertilization. PDI activity was significantly increased when ΔPDI-Trans was complemented with the endogenous gene (ΔPDI-Trans Comp). To investigate whether complementation restored not only PDI activity, but the ability of these parasites to successfully fertilize we performed ookinete conversion assays. Wild type parasites had a mean conversion rate of 77.98%. Ookinete conversion was not observed in the ΔPDI-Trans parasite line. Conversely, ΔPDI-Trans Comp parasites exhibited a mean ookinete conversion rate of 72.25% indicating that complementation of the PDI-Trans restored the ability to of male gametes to fertilize. Assays were performed in triplicate (Fig. 2E).
Malarial fertilization is inhibited reversibly by the PDI inhibitor Bacitracin in vitro
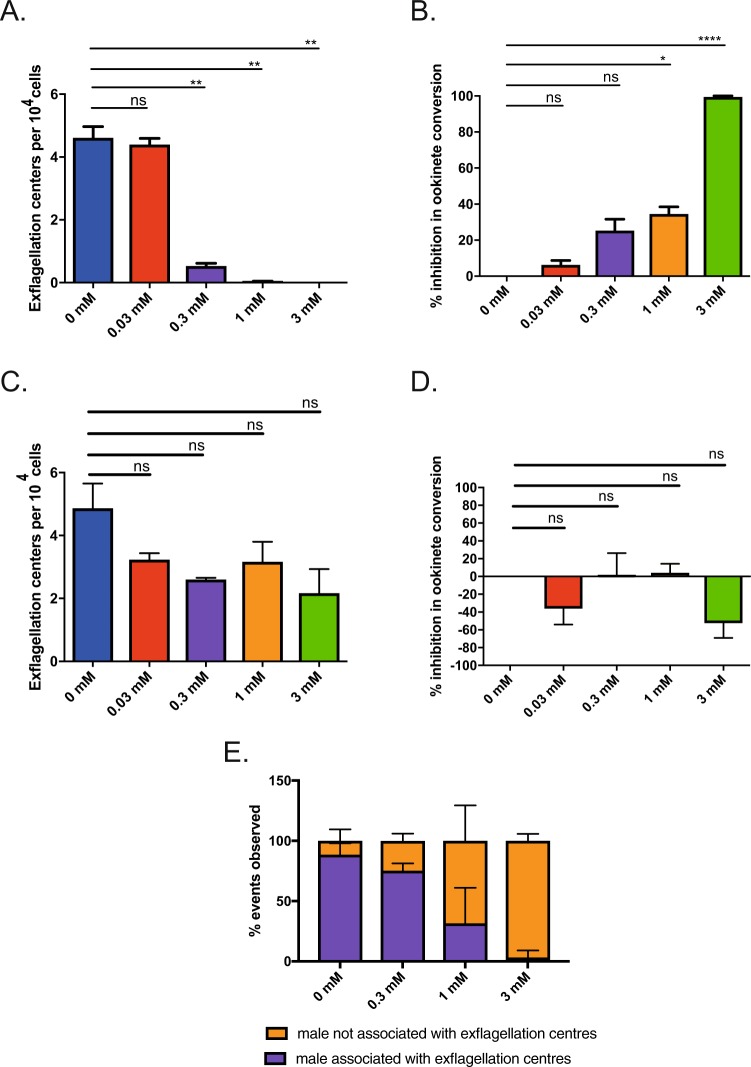
To further explore PDI-Trans activity, and to examine the ability of specific PDI inhibitors to block malarial transmission, we utilized the classical PDI inhibitor bacitracin44. The peptide antibiotic bacitracin was first reported to be an inhibitor of PDI in 198145, and has subsequently been used in a wide range of studies to demonstrate the role of PDI in cellular processes, including glioma cell invasion46, melanoma cell death47, viral entry48,49, and platelet function50. Subsequent experimentation has demonstrated direct interaction of PDI with bacitracin, resulting in disulfide bond formation between an open thiol of the bacitracin thiazoline ring, and corresponding cysteines within the substrate‐binding domain of PDI51. Bacitracin has proven low membrane permeability51, giving it utility as a tool to assess PDI-dependent cell-surface reductase activity52. In vitro addition of bacitracin pre-fertilization resulted in a dose-dependent reduction of exflagellation centres (defined as motile flagellate male gametes bound to at least three additional cells), with a complete inhibition in formation of exflagellation centers at 3 mM (Fig. 3A). This subsequently inhibited the ability of gametocytes to form ookinetes, with complete inhibition of ookinete conversion at 3 mM bacitracin (Fig. 3B). In order to test whether bacitracin has a broad and non-specific toxic effect on parasites, potentially unrelated to PDI-Trans function, gametocytes were pre-incubated in bacitracin at a range of concentrations for 30 minutes, washed to remove the PDI inhibitor, then assayed for formation of exflagellation centers/ookinetes respectively (post-wash). Results show that parasites pre-incubated with bacitracin, then washed, resulted in no detectable reduction (Paired t test) in the number of exflagellation centres compared to the untreated control across all concentrations examined (Fig. 3C,D).
Figure 3.
The PDI inhibitor bacitracin reversibly inhibits Plasmodium berghei fertilization. (A) Exflagellation centers in the presence of Bacitracin at 0, 0.03, 0.3, 1 mM. Asterisks indicate P value < 0.05 Paired t test. (B) In vitro ookinete development assay supplemented with Bacitracin at 0, 0.03, 0.3, 1 and 3 mM. Results are shown as percent inhibition in ookinete conversion. Asterisks indicate P value < 0.05 Paired t test, ns indicate P value not significant. (C) Exflagellation centers after 30 minutes in the presence of Bacitracin at 0, 0.03, 0.3, 1. P value < 0.05 Paired t test indicates P value not significant at any concentration. (D) In vitro ookinete development assay supplemented with Bacitracin at 0, 0.03, 0.3, 1 and 3 mM for 30 min prior to removal of Bacitracin. Results are shown as percent inhibition in ookinete conversion. P value < 0.05 Paired t test indicates P value not significant at any concentration. (E) Triplicate counts of free floating male gametes and male gametes in exflagellation centers. Represented as a percentage of total events observed in the presence of 0, 0.3, 1 and 3 mM Bacitracin.
In an attempt to further examine the activity of PDI-Trans and the mechanism of PDI-inhibitor based blockade of transmission, following bacitracin treatment we examined the number of visible free floating male gametes present, compared with the number of exflagellation centers present (rosettes comprising the adherence of newly emerged microgametes adhering to neighboring erythrocytes, defined as a motile male gamete adhered to three cells). As bacitracin concentrations increased. the number of visible exflagellation centres decreased, conversely, the number of free floating observed microgametes was unaffected (Fig. 3E), suggesting that PDI activity is essential for the association of male gametes to additional cells to form exflagellation centers, but not for the ability of gametogenesis/activation to form microgametes.
Malarial transmission is inhibited by Bacitracin in P. berghei and P. falciparum
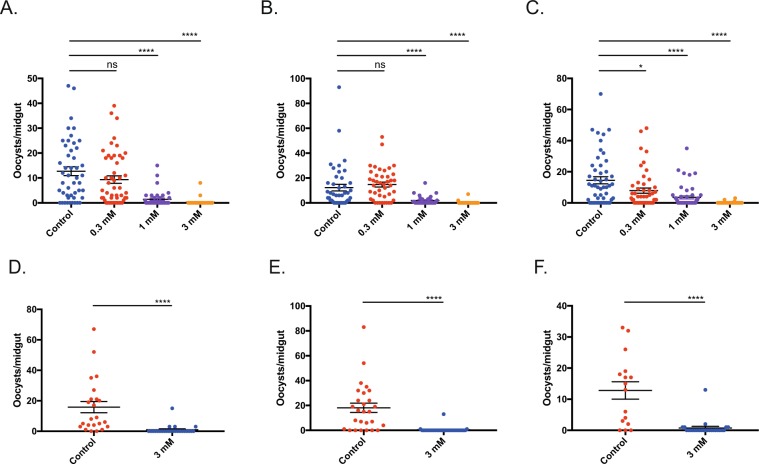
To test the ability of bacitracin to block the transmission of P. berghei ex vivo we performed standard membrane feeding assays (SMFA) in triplicate with 0.3, 1 and 3 mM doses of bacitracin (Fig. 4A–C). Bacitracin inhibited transmission at all concentrations in a dose-dependent manner with a maximum inhibition in oocyst intensity of 98.21% and infection prevalence of 92.48% with 3 mM bacitracin (Table 2).
Figure 4.
The PDI inhibitor bacitracin inhibits transmission in Plasmodium berghei and Plasmodium falciparum. (A–C) Triplicate P. berghei standard membrane feeding assays with Bacitracin compared to control at concentrations of 0.3, 1 and 3 mM. Individual data points represent the number of oocysts found in individual mosquitoes 12-days post feed. Horizontal bars indicate mean intensity of infection, whilst error bars indicate S.E.M within individual samples. Asterisks indicate P value < 0.05 Mann-Whitney U test, ns indicate P value not significant. (D–F) Triplicate P. falciparum standard membrane feeding assays with Bacitracin compared to control at a concentration of 3 mM. Individual data points represent the number of oocysts found in individual mosquitoes 8-days post feed. Horizontal bars indicate mean intensity of infection, whilst error bars indicate S.E.M within individual samples. Asterisks indicate P value < 0.05 Mann-Whitney U test.
Table 2.
Overall evaluation of transmission blocking effect of PDI inhibitor bacitracin in P. berghei by SMFA.
| Control | 0.3 mM | 1 mM | 3 mM | |
|---|---|---|---|---|
| Mean intensity (n = 3) | 13.17 | 10.68 | 2.32 | 0.23 |
| Mean prevalence (n = 3) | 77.37 | 72.32 | 38.29 | 5.81 |
| Inhibition in intensity (%) | — | 17.20a | 82.77a | 98.21a |
| Inhibition in prevalence (%) | — | 6.72b | 50.23b | 92.48b |
Mean (from three replicates) reductions in intensity (mean number of oocysts per midgut) and prevalence with bacitracin at 0.3, 1 and 3 mM were calculated with respect to control feeds. aP < 0.05, Mann-Whitney U test bP < 0.05, Fisher’s exact test.
To investigate if PDI function is implicated in fertilization in additional Plasmodium species, and to extend our observations in P. berghei to human malaria parasites, we performed SMFAs with P. falciparum gametocyte cultures in the presence of bacitracin. Given the results observed with P. berghei we chose to perform the P. falciparum feeds at the highest concentration of bacitracin (3 mM) in triplicate to detect maximal effect (Fig. 4D–F). The addition of bacitracin significantly inhibited transmission, with a mean inhibition in oocyst intensity of 95.05% and an inhibition of oocyst prevalence of 81.71% observed (Table 3).
Table 3.
Mean ex vivo evaluation of transmission blocking effect of PDI inhibitor bacitracin in P. falciparum.
| Control Feed 1 | Bacitracin Feed 1 | Control Feed 2 | Bacitracin Feed 2 | Control Feed 3 | Bacitracin Feed 3 | |
|---|---|---|---|---|---|---|
| Mean intensity | 15.83 | 0.96 | 18.15 | 0.52 | 12.81 | 0.76 |
| Mean prevalence | 91.30 | 16 | 78.57 | 10 | 81.25 | 20 |
| Inhibition in intensity (%) | — | 93.93 | — | 97.14 | — | 94.07 |
| Inhibition in prevalence (%) | — | 82.48 | — | 87.27 | — | 75.38 |
The mean (from three replicates) changes in intensity (mean number of oocysts per midgut) and prevalence with Bacitracin at 3 mM were calculated with respect to control feeds. aP < 0.05, Mann-Whitney U test bP < 0.05, Fisher’s exact test.
PDI-Trans can be targeted specifically with antibodies to block transmission in vitro and ex vivo
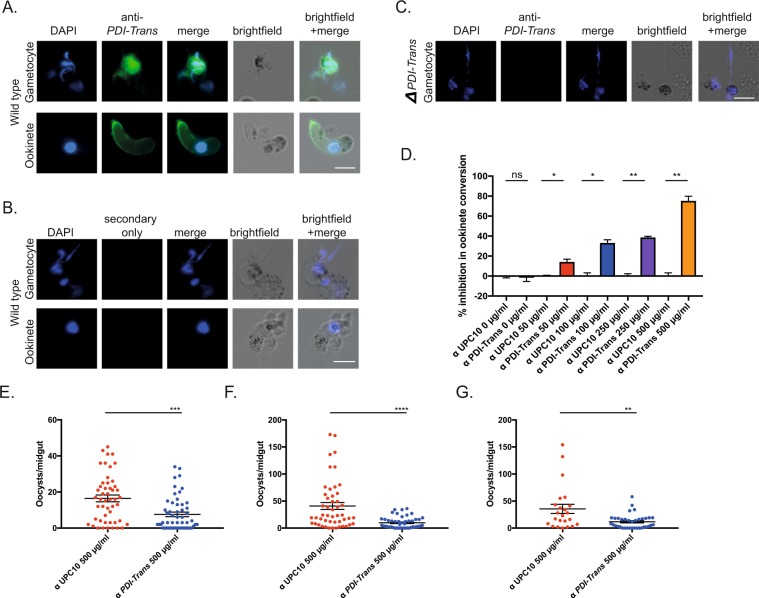
Activity of the membrane impermeant53 drug Bacitracin and localization strongly suggest PDI-Trans is located on the surface of the microgamete and ookinete, therefore the ability of anti-PDI-Trans antibodies to initiate a specific anti-parasitic transmission blocking response was additionally examined. A polyclonal anti-peptide antibody was raised against residues bioinformatically predicted to be within the extracellular domain of PDI-Trans (amino acids 30–43). Antibodies were raised in rabbits, IgG purified, and examined for their ability to inhibit transmission in vitro and ex vivo. Anti-PDI-Trans IgG recognized both non-permeabilized gametocytes and ookinetes by IFA (Fig. 5A). Staining was absent in secondary only controls Fig. 5B), and on activated ΔPDI-Trans male gametes (Fig. 5C) indicating the ability of these antibodies to specifically recognize natively folded PDI-Trans on the gamete and ookinete surface.
Figure 5.
Anti-PDI-Trans antibodies inhibit fertilization and transmission in Plasmodium berghei. IFA of wildtype P. berghei ANKA male gametes and ookinetes with (A). anti PDI-Trans and (B). Secondary-only control antibodies (green) DAPI (blue). IFA of male gametes and ookinetes with anti PDI-Trans shows broad surface staining. White scale bars = 5 μm (C). IFA of ΔPDI-Trans gametes with anti PDI-Trans. Absence of staining illustrates anti PDI-Trans recognises native protein. White scale bar = 10 μm (D). Inhibition in ookinete conversion in in vitro ookinete development assay with anti PDI-Trans compared to negative control antibody UPC10 at concentrations of 0, 50, 100, 250 and 500 µg/ml. Asterisks indicate P value < 0.05 Paired t test, ns indicate P value not significant. (E–G) Triplicate standard membrane feeding assays with anti PDI-Trans compared with negative control antibody UPC10 at a concentration of 500 µg/ml. Individual data points represent the number of oocysts found in individual mosquitoes 12-days post feed. Horizontal bars indicate mean intensity of infection, whilst error bars indicate S.E.M within individual samples. Asterisks indicate P value < 0.05 Mann-Whitney U test, ns indicate P value not significant.
To examine the ability of PDI-Trans to act a transmission blocking antigen, in vitro ookinete conversion assays were perfumed in the presence of anti-PDI-Trans. Anti-PDI-Trans inhibited ookinete conversion in a dose-dependent manner, further suggesting specificity. At antibody concentrations of 50, 100, 250 and 500 μg/ml, ookinete formation was inhibited by 14.3%, 33.2%, 38.7% and 75.4% respectively. In contrast, as previously demonstrated4, the presence of the isotypic IgG UPC10 (negative control) had no discernable effect on ookinete conversion (Fig. 5D).
The transmission blocking activity of anti-PDI-Trans antibodies was additionally assessed by triplicate SMFA (Fig. 5E–G). Given the in vitro results observed previously (Fig. 5D), we assessed in vivo transmission blocking ability of anti-PDI-Trans antibodies only at the highest concentration where an in vitro effect was demonstrated. Anti-PDI-Trans antibodies significantly inhibited P. berghei transmission in all experiments. At a concentration of 500 μg/ml anti-PDI-Trans antibodies inhibited oocyst intensity by a mean of 66.22% and reduced prevalence of infection by 33.16% (Table 4).
Table 4.
Mean ex vivo evaluation of transmission blocking effect of anti-PDI-Trans antibodies.
| α UPC10 500 μg/ml | α PDI-Trans 500 μg/ml | |
|---|---|---|
| Mean intensity (n = 3) | 31.37 | 9.82 |
| Mean prevalence (n = 3) | 93.28 | 62.18 |
| Inhibition in intensity (%) | — | 66.22a |
| Inhibition in prevalence (%) | — | 33.16b |
The mean (from three replicates) change in intensity (mean number of oocysts per midgut) and prevalence with anti PDI-Trans antibody at 500 µg/ml were calculated with respect to appropriate negative control antibody UPC10 at the same concentration. aP < 0.05, Mann-Whitney U test bP < 0.05, Fisher’s exact test.
Discussion
Fertilization is a key process in the Plasmodium lifecycle, encompassing the active fusion of activated male (micro) and female (macro) gametes to form a zygote within the mosquito bloodmeal. Despite its essential nature to the success of the parasitic lifecycle, the cellular and molecular mechanisms that underlie fertilization remain largely opaque. Merely three proteins have been discovered that have a perceptible role in the mutual recognition of Plasmodium gametes; the 6-Cys family members, P48/45, P47 and P2305. The specific mechanism of action of these proteins are currently unknown. Following gamete recognition, the plasma membranes of the male and female gametes come into intimate contact and fuse, resulting in cytoplasmic continuity. The conserved class II fusion protein HAP2 is essential for gamete fusion during fertilization, and initiates merger of lipid bilayers post gamete adhesion. Following a (currently unidentified) trigger, it is postulated that a short-conserved region within the Plasmodium HAP2 ectodomain becomes exposed on the microgamete membrane surface, leading to the subsequent parallel trimerization of protein subunits4,54,55. Polar residues within this “fusion loop” are subsequently inserted into the macrogamete membrane, followed by a conformational change in HAP2 domain III which distorts the target membrane, causing hemifusion followed by fusion/cytoplasmic continuity. Further related upstream and downstream effector molecules that specifically mediate the process of fertilization are at present unclear.
Here, we demonstrate that protein disulphide isomerase function, specifically encoded by a single plasmodial gene (PDI-Trans/PBANKA_0820300) is essential for malarial transmission. We demonstrate that PDI-Trans is constitutively expressed throughout the parasitic lifecycle, in both the blood and mosquito stages, but is only essential in the male gamete. This is consistent with transcriptomic data described in55, where transcripts of PBANKA_082030 have demonstrated upregulation in male gametes. Absence of PDI-Trans only confers a detectable effect post-gamete activation, prior to gamete adhesion, and is null for male fertility, and consequently, zygote/ookinete formation, and transmission to the mosquito host. Complementation of the disrupted locus restores fertility. Furthermore, we demonstrate reductase activity, indicative of classical PDI activity. Inhibition of PDI-Trans using the widely available (topical) antibiotic and PDI inhibitor, bacitracin, reversibly blocks plasmodial transmission in vivo and ex vivo. Specifically, the process of gamete activation remains unaffected by bacitracin, whereas the ability of treated gametes to adhere to other cells (i.e. form exflagellation centers) appears to be compromised. Bacitracin-derived transmission blockade is observed in both P. berghei and P. falciparum. Finally, we show that antibodies specifically raised against the extracellular region of PDI-Trans can recognize the surface of the sexual stages of the parasite by immunofluorescence, and can initiate transmission-blocking activity both in vitro and ex vivo.
In all living cells, the appropriate formation and cleavage of disulphide bonds between cysteine residues in secreted and membrane anchored proteins is essential for native conformation, and therefore, function. PDIs are traditionally known to be versatile enzymes with key roles in the mediation of disulfide bond formation, isomeration and reduction in the endoplasmic reticulum33. PDI function is also associated with varied chaperone activity32. Little is known regarding the expression and function of PDI-like proteins in Plasmodium. A previous study34 has bioinformatically identified nine PDI-like molecules across five species of malaria parasites (four in falciparum, one in vivax, berghei, knowlesi and yoelii), indicated by the presence of classical thioredoxin domains. A more detailed analysis of one of these PDI candidates in P. falciparum; PfPDI-8 (PF3D7_0827900), demonstrated expression within the endoplasmic reticulum of asexual blood schizonts, gametocytes and sporozoites, with biochemical analysis indicating a function in the disulfide-dependent conformational folding of a recombinant form of the erythrocyte-binding protein (and putative bloodstage vaccine target) EBA-175. As further evidence of the chaperone function of PDI enzymes, studies utilizing the overexpression of PfPDI-8 resulted in the enhanced expression and folding of the transmission-blocking vaccine candidate, Pfs25, in a Picha pastoris expression system56. Broadly, PDI function is bioinformatically predicted to be conferred by multiple ORF throughout the parasitic genome, with recent bioinformatics predictions (PlasmoDB) suggesting the presence of six PDI-related ORFs in P. berghei (with homologues in P. falciparum). Expression, localization and function of these proteins are still largely undefined. Future study to further dissect the function of PDIs (and associated mechanisms of protein folding) in Plasmodium may be advantageous.
Although classically considered to be key mediators of protein folding in the endoplasmic reticulum, key evidence showing localization and function of PDIs in other cell compartments does exist. In some organisms, PDIs have been demonstrated to on occasion escape the ER, and exhibit cytoplasmic and cell surface localization, where their predominant function appears to be the reduction of disulphide bonds33,57. In terms of fertilization, PDI activity on the sperm head has previously proved to be essential for sperm-egg cell fusion in multiple vertebrates32,58–60, and implicated in male fertility in mice61,62. PDI function has previously been implicated in the progression of multiple infectious diseases, with a specific role in mediating pathogen entry. In viruses, overexpression of PDI enhances the fusion of vial membranes, leading to increased internalization of HIV-163. Cell surface PDI has been shown to facilitate the infection of HeLa cells by mouse polyoma virus64, and in endothelial cells a surface localized (lipid-raft associated) PDI reduces β1 and β2 integrins, allowing for the entry of dengue virus52,65. Its function is also essential for release of cholera toxin active chain A from the ER to the cytosol of the infected cell66. In protozoan pathogens, previous experimentation has demonstrated that increased levels of PDI enhance phagocytosis of the L. chagasi promastigote (but not the amastigote)67. It has previously been hypothesized that T. gondii and L. donovani PDI could be putative targets for vaccine development68,69. The specific function of PDI-Trans in the parasite is still unknown, however, it is clear that successful fertilization in Plasmodium requires the presence and function of a range of proteins with conserved disulphide bonds between cysteine residues on the gamete surface. The 6-Cys family members P48/45, P47, P230 are all definitively evidenced to mediate gamete adhesion, whereas HAP2 requires the correct formation of multiple crucial disulphide bridges to enable membrane fusion. It cannot be discounted that PDI-Trans may in some way catalyse disulphide bond rearrangement in one of these transmission-essential proteins, exposing key residues critical for fertility-based function.
The results described here clearly indicate that PDI-Trans is a potential target for anti-malarial strategies to successfully inhibit malarial transmission. The generation of novel TBIs to reduce disease burden is a key component of the current anti-malarial strategy, and it is accepted that to achieve eradication, it will be hugely advantageous to use interventions that inhibit transmission of parasites from humans to mosquitoes2. We demonstrate that bacitracin reversibly inhibits malarial transmission with high efficacy, and additionally, that antibodies targeting PDI-Trans mediate significant transmission reducing immunity. It should be noted that bacitracin is already an FDA approved compound, traditionally used clinically against gram-positive bacteria. This example illustrates the potential value of re-purposing drugs with observed efficacy against non-malarial species. Previous studies examining the anti-malarial efficacy of bacitracin only examined impact on asexual growth, where no effect was demonstrated70. The data here provides further evidence that anti-malarial transmission blocking efficacy can be achieved by targeting the male/micro gamete. Previously described compounds effective against the process of fertilization (methylene blue and atovaquone) are effective in blocking transmission, as are antibodies against multiple male gamete-surface proteins22–24. A deeper understanding of transmission and the mechanisms of fertilization within Plasmodium will be advantageous to develop novel interventions in the future. More broadly, PDI-like proteins are expressed across multiple species and taxa, including in a range of pathogens of veterinary and clinical importance30,31,48. Given the ability of both anti-PDI-Trans compounds and antibodies to inhibit malarial transmission described here, and the proven role of PDI function in the regulation of infection across multiple species, it is logical to suggest that future studies may want to examine the possibility of targeting PDI proteins/functions using specifically designed novel anti-malarial drugs or vaccines.
Materials and Methods
General parasite maintenance
Parasite maintenance was carried out as described in71. P. berghei was maintained in ~6 week-old female TO mice (Harlan)ƒ. If necessary, hyper-reticulosis was induced three days pre-infection by treating intraperitoneally (i.p) with 200 μl phenylhydrazinium chloride (PH; 6 mg/ml; ProLabo). Infections were monitored via Giemsa-stained thin smears as described previously43.
Purification of gametocytes
Purification of gametocytes was achieved using a protocol modified from7. Mice were treated i.p with 200 μl phenylhydrazinium chloride (PH; 6 mg/ml in PBS; ProLabo UK). Day four post infection (p.i.) mice were treated with sulfadiazine (Sigma) at 20 mg/L in their drinking water for two days to eliminate asexual blood stage parasites. On day six p.i. mice were bled by cardiac puncture into heparin and gametocytes separated from uninfected erythrocytes on a 48% NycoDenz gradient (27.6% w/v NycoDenz in 5 mM Tris-HCl, pH 7.20, 3 mM KCl, 0.3 mM EDTA) in coelenterazine loading buffer (CLB), containing PBS, 20 mM HEPES, 20 mM Glucose, 4 mM sodium bicarbonate, 1 mM EGTA, 0.1% w/v bovine serum albumin, pH 7.25. Gametocytes were harvested from the interface and washed twice in RPMI 1640 ready for activation of gamete formation.
Generation and analysis of transgenic parasite lines
PDI-Trans-GFP
To examine the expression and localization of PDI-Trans, the PDI-Trans-GFP transgenic line was created, introducing a C-terminal GFP tag to the native protein by single homologous recombination. The targeting construct pPDI-Trans-GFP was produced using the backbone of the EGFP-tagging vector p27772. The terminal 1527 bp of the PDI gene (PBANKA_082030) was synthesized to remove an internal ApaI site and introduce unique AvrII site within the gene and flanking KpnI and ApaI sites to the amplicon. This block was cloned in frame into ApaI/KpnI sites of p277, resulting in pPDI-Trans -GFP. For transfection, this construct was linearized at a unique AvrII site within the PDI sequence.
Parasites were transfected using the Nucleofector device (Amaxa Biosystems) as described previously65. Integration of the DNA constructs into the chromosome was confirmed by PCR flanking a region upstream from the 5′ integration site into the EGFP sequence (oligo 35; 5′-GCATGTGCGATTGTATTGGG-3; oligo 14; 5′-ACGCTGAACTTGTGGCCG-3′) and the presence of the DHFR selection cassette (oligo 91 5′- TTCGCTAAACTGCATCGT -3′; oligo 92 5′-GTACTTAATGCCTTTCTCCT-3′). Oligos against the Pbs25 gene (PBANKA_0051500) were used as positive control (oligos F1: 5′-CAACTTAGCATAAATATAATAATGCGAAAGTTACCGTGG-3′; F2 5′-CCATCTTTACAATCACATTTATAAATTCCATC-3′). GFP expression in transfected, drug resistant parasites were confirmed by fluorescence microscopy. Two independent clones were obtained from two independent transfections, demonstrating identical phenotypes and GFP expression.
ΔPDI-Trans
To examine the function of PBANKA_082030 the ΔPDI-Trans transgenic line was generated. The plasmid was designed and constructed by PlasmoGEM (PlasmoGem ID PbGEM-239637) using recombinase-mediated engineering followed by a Gateway® mediated exchange41,73. Prior to transfection the construct was digested by NotI to release the P. berghei insert from its vector backbone. Parasites were transfected using the Nucleofector device (Amaxa Biosystems) as described previously72,73. Integration of the DNA constructs into the chromosome was confirmed by PCR region flanking 5′ of the modified target locus and 3′ DHFR selection cassette (oligo 72; 5′- ACGTGCATGTGCGATTGTATTGGGT -3; oligo 9; 5′- CTTTGGTGACAGATACTAC -3′) and the absence of the wildtype locus (oligo 69 5′- ATGGGAAACTATACTTATATATATATTTTTTTCA -3′; oligo 70 5′- TTATAAATCAGAATTTTCTTCTCCTTC -3′). Two independent clones were obtained from two independent transfections, demonstrating statistically indistinguishable phenotypes.
ΔPDI-Trans-Comp
For the complementation construct the clonal knockout line was injected into mice and mice were treated with 5-Fluorocytosine (5FC) nucleoside analog (Sigma) drinking water, 1.5 mg/ml to recycle the Hdhfr-yfcu marker. The subsequent marker free line was subjected to dilution cloning to achieve a pure population of marker free parasites. Following this the full length endogenous PBANKA_082030 gene was transfected on top using the artificial chromosome library clone mapping to PBANKA_082030 from PlasmoGEM (clone ID PbAC02-74d11) as described previously (Figure S4)39,73.
RT-PCR
P. berghei RNA was isolated from gametocyte deficient strain 2.33, activated or inactivated gametocytes, ookinetes and sporozoites from wild type P. berghei 2.34 strain using Trizol reagent (Invitrogen). cDNA synthesis was performed using Prime script kit from (Clonetech). PCR reactions were set up to amplify sections of PDI-Trans ORF (Forward 5′-ATGGGAAACTATACTTATATATATATTTTTTTCA-3′; and reverse 5′- CTACATATTTATCGACATCTCCAA-3′). The expected RT amplicon was 481 bp. The ubiquitously expressed α-tubulin gene PBANKA_0522700 was amplified for each sample to ensure amplifiability of cDNA from respective RNA samples (Forward, 5′-CCAGATGGTCAAATGCCC-3′; Reverse, 5′-CTGTGGTGATGGCCATGAAC-3′). The expected products were 435 bp (cDNA). Thirty RT–PCR cycles were carried out with denaturation for 1 min at 94 °C, annealing for 45 secs at 50 °C, and extension for 1.5 min at 68 °C, and products were visualized on a 0.8% agarose gel.
Direct feeding assay (DFA)
Routine maintenance of P. berghei was carried out as described above. Prior to challenge, mice were PH treated, and 3 days later infected i.p. with 106 P. berghei ANKA 2.34 or ΔPDI-Trans parasites. Three-days post-infection, animals were anesthetized, and >50 female Anopheles stephensi mosquitoes allowed to blood feed on each mouse. Twenty-four hours later, unfed mosquitoes were removed. Mosquitoes were maintained on 8% (w/v) fructose, 0.05% (w/v) p-aminobenzoic acid at 19–22 °C and 50–80% relative humidity. Day 14 post-feeding, mosquito midguts were dissected and oocyst intensity and prevalence observed by standard phase microscopy and recorded. Reduction in oocyst intensity and prevalence in knockout mice were calculated with respect to wild type controls.
In Vitro ookinete conversion assay (IVOA)
PH-treated mice were injected with 5 × 107 parasites i.p. On day 3 or 4 of infection, parasitaemia was counted on a Giemsa- stained tail blood smear and exflagellation of male gametocytes was checked by addition of a drop of exflagellation medium to a drop of tail blood. Hosts observed to have exflagellating parasites were exsanguinated by cardiac puncture and each 20 μl of blood taken up in 450 μl ookinete medium. Individual cultures were then added to pre-prepared 24 well plates (Nunc) and incubated for 24 h at 19 °C. Cultures were harvested after 24 h by centrifugation (500 × g, 5 min), washed once in 100 μl ookinete medium, and the pellet taken up in 50 μl ookinete medium containing Cy3-conjugated Pbs28 mAb clone 13.1 (1:500). Ookinetes and macrogametocytes were then immediately counted by fluorescence microscopy. Ookinete conversion rates were calculated as described previously18. In bacitracin experiments harvested parasites were added to ookinete medium containing a range of Bacitracin (Sigma Aldrich: #B0125) concentrations and either left in or washed and put into fresh medium 30 min after drug treatment. In antibody experiments harvested parasites were added to ookinete medium containing anti-PDI-Trans rabbit sera or anti-UPC10 (negative control). In each set of experiments results were collated from three separate experiments and inhibition expressed as the percentage reduction in ookinete conversion with respect to wild type parasites, samples with no bacitracin or the anti-UPC10 control.
Crosses
At day 3 post infection of phenylhydrazine treated mice, infected with parasites with either ΔPDI-Trans, Δnek4, Δmap2 and wt were harvested by heart puncture and mixed at a 1:1 ratio in ookinete medium. After 24 h, ookinete conversion assays were performed by incubating samples with 13.1 antibody (antibody against Pb28 conjugated with Cy3). The proportion of ookinetes to all 13.1-positive cells (unfertilised macrogametes and ookinetes) was established, counting fields at 60 × magnification. Experiments were performed in biological triplicate42,43.
PDI activity assay
PDI activity was measured in a microplate PDI inhibitor screening assay kit from Abcam (ab139480). Briefly, ΔPDI-Trans ΔPDI-Trans Comp and wild-type gametocytes were purified42. Non-activated gametocytes were Nycodenz purified in coelentrazine loading buffer and liberated from the red blood cell with lysis buffer before use. Both activated and non-activated gametocytes of each parasite line were used in the assay and the PDI-catalyzed reduction of insulin in the presence of Dithiothreitol resulting in the formation of insulin aggregates which bind avidly to the red-emitting fluorgenic PDI detection reagent were measured on Tecan, Infinite M200 Pro. The background media signal for each sample was subtracted and PDI activity was calculated as a percent relative to the positive control (human recombinant PDI). Bacitracin at 1 mM was used on human recombinant PDI as a control for a 50% reduction of activity as outlined by kit protocol. Experiments were performed in triplicate.
Standard membrane feeding assay (SMFA)
P. berghei
Female An. stephensi (SDA 500 strain) were starved for twenty-four hours and then fed on heparinized P. berghei infected blood using standard membrane feeding methods [6460]. For each feed, 350 µl of P. berghei ANKA 2.34 infected blood containing asexual and sexual stages of the parasite was mixed with 150 µl of PBS containing either antibody to yield final concentration of 500 μg/ml or drug at 0.3, 1 and 3 mM. Mosquitoes were handled, maintained and analyzed as described above. Reductions in oocyst intensity and prevalence was calculated with respect to control feeds as described in4.
P. falciparum
Mature gametocytes of P. falciparum (NF54) were produced in vitro as described previously74 with slight modifications. Briefly, mature gametocyte cultures (0.5 to 2% final gametocytaemia) were fed for 15–20 min at room temperature to An. gambiae mosquitoes through an artificial membrane kept at 37 °C. For each feed 300 µl of mature P. falciparum gametocytes were mixed with bacitracin at a concentration of 3 mM. Engorged mosquitoes were housed in pots at 26 °C and 60–80% relative humidity. On days 7–9, midguts were dissected and the results analyzed as outlined in the above P. berghei section.
Antibody production
Synthetic peptide to PDI-Trans (VSDDFAKKVNHLTHC) was produced, conjugated to KLH and used to raise polyclonal rabbit antisera (Genscript, USA). Resulting sera was IgG purified and validated by Genscript via ELISA.
Microscopy
Immunofluorescence assay (IFA)
PDI-Trans-GFP parasites were assessed by IFA for the presence of GFP tag with anti-GFP, Roche at a dilution of 1:500. Signal was detected by Alexa Fluor 488-labelled goat, anti-mouse IgG (Molecular Probes) at 1:500. Rabbit antibodies to PDI-Trans were assessed by IFA on wild-type P. berghei ANKA 2.34 gametocytes and ookinetes at a dilution of 1:500. Signal was detected by Alexa Fluor 488-labelled goat, anti-rabbit IgG (Molecular Probes) at 1:500. Parasites were cultured and IFAs were performed as described previously4. Slides were visualized under x60 objective magnification using a fluorescence microscope (EVOSFL Cell Imaging System, Life Technologies).
Live imaging
PDI-Trans-GFP parasites were examined for GFP signal by live microscopy. Parasites were cultured and allowed to settle on glass slides before microscopy. Slides were visualized under X40 objective magnification using a fluorescence microscope (Leica DMR).
Statistical analysis
Statistical analysis was performed using Graphpad Prism. For DFA, SMFA and DMFA, significance was assessed using Mann–Whitney U (to examine differences in intensity) and Fisher’s exact probability tests (to examine differences in prevalence). Parametric ELISA tests were assessed using t-test. P values < 0.05 were considered statistically significant (***< 0.0001, ***0.001, **0.001–0.01, *0.01–0.05).
Ethical statement
All procedures were performed in accordance with the UK Animals (Scientific Procedures) Act (PPL 70/8788) and approved by the Imperial College AWERB. The Office of Laboratory Animal Welfare Assurance for Imperial College covers all Public Health Service supported activities involving live vertebrates in the US (no. A5634-01).
Supplementary information
Acknowledgements
This work was funded by the MRC (New Investigator Research Grant; award number MR/N00227X/1). A.M.B thanks PATH-MVI for funding. Funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. We gratefully acknowledge Mark Tunnicliff for mosquito production and Tibebu Habtewold for advice regarding P. falciparum infections.
Author contributions
A.M.B. conceived the study. A.M.B. and G.K.C. acquired funding. Investigation performed by F.A., K.A.S., S.T., A.M.B.. Data analysis by A.M.B. and F.A. Writing – original draft by A.M.B. & F.A. Writing – review and editing by F.A., K.A.S., S.T., G.K.C., A.M.B.
Data availability
All datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-54613-0.
References
- 1.World Health Organization W. World Malaria Report (2017).
- 2.Rabinovich RN, et al. malERA: An updated research agenda for malaria elimination and eradication. PLoS Med. 2017;14(11):e1002456. doi: 10.1371/journal.pmed.1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, et al. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes and Development. 2008;22:1051–1068. doi: 10.1101/gad.1656508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angrisano F, et al. Targeting the Conserved Fusion Loop of HAP2 Inhibits the Transmission of Plasmodium berghei and falciparum. Cell Reports. 2017;21(10):2868–2878. doi: 10.1016/j.celrep.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dijk MR, et al. Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog. 2010;6:e1000853. doi: 10.1371/journal.ppat.1000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Dijk MR, et al. A central role for P48/45 in malaria parasite male gamete fertility. Cell. 2001;104(1):153–164. doi: 10.1016/S0092-8674(01)00199-4. [DOI] [PubMed] [Google Scholar]
- 7.van Schaijk BCL, et al. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Molecular and Biochemical Parasitology. 2006;149(2):216–222. doi: 10.1016/j.molbiopara.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Sinden RE. Developing transmission-blocking strategies for malaria control. PLoS Pathogens. 2017;13:e1006336. doi: 10.1371/journal.ppat.1006336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sauerwein RW, Bousema T. Transmission blocking malaria vaccines: Assays and candidates in clinical development. Vaccine. 2015;33(52):7476–82. doi: 10.1016/j.vaccine.2015.08.073. [DOI] [PubMed] [Google Scholar]
- 10.Fowler RE, Sinden RE, Pudney M. Inhibitory activity of the anti-malarial atovaquone (566C80) against ookinetes, oocysts, and sporozoites of Plasmodium berghei. J. Parasitol. 1995;81:452–458. doi: 10.2307/3283831. [DOI] [PubMed] [Google Scholar]
- 11.Gwadz RW. Malaria: successful immunization against the sexual stages of Plasmodium gallinaceum. Science. 1976;193:1150–1151. doi: 10.1126/science.959832. [DOI] [PubMed] [Google Scholar]
- 12.Singh SK, et al. Plasmodium falciparum 48/45 single epitope R0.6C subunit protein elicits high levels of transmission blocking antibodies. Vaccine. 2015;33(16):1981–6. doi: 10.1016/j.vaccine.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 13.Singh SK, et al. Improving the malaria transmission-blocking activity of a Plasmodium falciparum 48/45 based vaccine antigen by SpyTag/SpyCatcher mediated virus-like display. Vaccine. 2017;35(30):3726–3732. doi: 10.1016/j.vaccine.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 14.Outchkourov NS, et al. Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc Natl Acad Sci USA. 2008;105(11):4301–5. doi: 10.1073/pnas.0800459105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Read D, et al. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite Immunol. 1994;10:511–9. doi: 10.1111/j.1365-3024.1994.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 16.Miura K, et al. Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay. Infect. Immun. 2013;81:4377–4382. doi: 10.1128/IAI.01056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapulu MC, et al. Comparative assessment of transmission-blocking vaccine candidates against Plasmodium falciparum. Sci Rep. 2015;11(5):11193. doi: 10.1038/srep11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bompard A, et al. Evaluation of two lead malaria transmission blocking vaccine candidate antibodies in natural parasite-vector combinations. Scientific Reports. 2017;7:2045–2322. doi: 10.1038/s41598-017-06130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theisen M, Jore MM, Sauerwein R. Towards clinical development of a Pfs48/45-based transmission blocking malaria vaccine. Expert Rev Vaccines. 2017;16(4):329–336. doi: 10.1080/14760584.2017.1276833. [DOI] [PubMed] [Google Scholar]
- 20.Farrance CE, et al. A Plant-Produced Pfs230 Vaccine Candidate Blocks Transmission of Plasmodium falciparum. Clin Vaccine Immunol. 2011;18(8):1351–7. doi: 10.1128/CVI.05105-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blagborough AM, Sinden RE. Plasmodium berghei HAP2 induces strong malaria transmission-blocking immunity in vivo and in vitro. Vaccine. 2009;27(38):5187–94. doi: 10.1016/j.vaccine.2009.06.069. [DOI] [PubMed] [Google Scholar]
- 22.Canepa GE, et al. Antibody targeting of a specific region of Pfs47 blocks Plasmodium falciparum malaria transmission. NPJ Vaccines. 2018;10(3):26. doi: 10.1038/s41541-018-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miguel-Blanco C, et al. Imaging-Based High-Throughput Screening Assay To Identify New Molecules with Transmission-Blocking Potential against Plasmodium falciparum Female Gamete Formation. Antimicrob. Agents Chemother. 2015;59:3298–3305. doi: 10.1128/AAC.04684-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burrows JN, et al. New developments in anti-malarial target candidate and product profiles. Malar. J. 2017;16:26. doi: 10.1186/s12936-016-1675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dicko A, et al. Efficacy and safety of primaquine and methylene blue for prevention of Plasmodium falciparum transmission in Mali: a phase 2, single-blind, randomised controlled trial. Lancet Infect. Dis. 2018;18(6):627–639. doi: 10.1016/S1473-3099(18)30044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchholz K, et al. Interactions of Methylene Blue with Human Disulfide Reductases and Their Orthologues from Plasmodium falciparum. Antimicrob. Agents Chemother. 2008;52:183–191. doi: 10.1128/AAC.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter R, Chen DH. Malaria transmission blocked by immunisation with gametes of the malaria parasite. Nature. 1976;263:57–60. doi: 10.1038/263057a0. [DOI] [PubMed] [Google Scholar]
- 28.Goodman AL, et al. A viral vectored prime-boost immunization regime targeting the malaria Pfs25 antigen induces transmission-blocking activity. PLoS One. 2011;6(12):e29428. doi: 10.1371/journal.pone.0029428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones RM, et al. A novel plant-produced Pfs25 fusion subunit vaccine induces long-lasting transmission blocking antibody responses. Hum Vaccin Immunother. 2015;11(1):124–32. doi: 10.4161/hv.34366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sala KA, et al. Immunization with Transgenic Rodent Malaria Parasites Expressing Pfs25 Induces Potent Transmission-Blocking Activity. Scientific Reports. 2018;8(1):1573. doi: 10.1038/s41598-017-18831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan A, Mutus B. Protein disulfide isomerase a multifunctional protein with multiple physiological roles. Front Chem. 2014;26(2):70. doi: 10.3389/fchem.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noiva R. Protein disulfide isomerase: the multifunctional redox chaperone of the endoplasmic reticulum. Semin Cell Dev Biol. 1999;10(5):481–93. doi: 10.1006/scdb.1999.0319. [DOI] [PubMed] [Google Scholar]
- 33.Mahajan B, et al. Protein disulfide isomerase assisted protein folding in malaria parasites. Int J Parasitol. 2006;36(9):1037–48. doi: 10.1016/j.ijpara.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Mouray E, et al. Biochemical properties and cellular localization of Plasmodium falciparum protein disulfide isomerase. Biochimie. 2007;89(3):337–46. doi: 10.1016/j.biochi.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Novo C, Martins TM, Prata S. Lopes, Armada A. Gene sequencing, modelling and immunolocalization of the protein disulfide isomerase from Plasmodium chabaudi. Macromolecules. 2009;45(4):339–406. doi: 10.1016/j.ijbiomac.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Talman AM, et al. Proteomic analysis of the Plasmodium male gamete reveals the key role for glycolysis in flagellar motility. Malar J. 2014;13(13):315. doi: 10.1186/1475-2875-13-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wass MN, et al. Proteomic analysis of Plasmodium in the mosquito: progress and pitfalls. Parasitology. 2012;139(9):1131–45. doi: 10.1017/S0031182012000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan SM, et al. Proteome Analysis of Separated Male and Female Gametocytes Reveals Novel Sex-Specific Plasmodium Biology. Cell. 2005;121(5):675–687. doi: 10.1016/j.cell.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 39.Bushell E, et al. Functional Profiling of a Plasmodium Genome Reveals an Abundance of Essential Genes. Cell. 2017;170(2):260–272. doi: 10.1016/j.cell.2017.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwach F, et al. PlasmoGEM, a database supporting a community resource for large-scale experimental genetics in malaria parasites. Nucleic Acids Res. 2015;43:1176–82. doi: 10.1093/nar/gku1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomes AR, et al. A genome-scale vector resource enables high-throughput reverse genetic screening in a malaria parasite. Cell Host Microbe. 2015;11(17(3)):404–413. doi: 10.1016/j.chom.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beetsma AL, Van de Wiel TJJM, Sauerwein RW, Eling WMC. Plasmodium berghei ANKA: Purification of Large Numbers of Infectious Gametocytes. Exp Parasitol. 1998;88(1):69–72. doi: 10.1006/expr.1998.4203. [DOI] [PubMed] [Google Scholar]
- 43.Tewari R, Dorin D, Moon R, Doerig C, Billker O. An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Mol Microbiol. 2005;58(5):1253–63. doi: 10.1111/j.1365-2958.2005.04793.x. [DOI] [PubMed] [Google Scholar]
- 44.Reininger Luc, Tewari Rita, Fennell Clare, Holland Zoe, Goldring Dean, Ranford-Cartwright Lisa, Billker Oliver, Doerig Christian. An Essential Role for the Plasmodium Nek-2 Nima-related Protein Kinase in the Sexual Development of Malaria Parasites. Journal of Biological Chemistry. 2009;284(31):20858–20868. doi: 10.1074/jbc.M109.017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roth RA. Bacitracin: an inhibitor of the insulin degrading activity of glutathione‐insulin transhydrogenase. Biochem Biophys Res Commun. 1981;98:431–438. doi: 10.1016/0006-291X(81)90858-5. [DOI] [PubMed] [Google Scholar]
- 46.Goplen D, et al. Protein disulfide isomerase expression is related to the invasive properties of malignant glioma. Cancer Res. 2006;66:9895–9902. doi: 10.1158/0008-5472.CAN-05-4589. [DOI] [PubMed] [Google Scholar]
- 47.Lovat PE, et al. Increasing melanoma cell death using inhibitors of protein disulfide isomerases to abrogate survival responses to endoplasmic reticulum stress. Cancer Res. 2008;68:5363–5369. doi: 10.1158/0008-5472.CAN-08-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Markovic I, et al. Thiol/disulfide exchange is a prerequisite for CXCR4‐tropic HIV‐1 envelope‐mediated T‐cell fusion during viral entry. Blood. 2004;103:1586–1594. doi: 10.1182/blood-2003-05-1390. [DOI] [PubMed] [Google Scholar]
- 49.Ryser HJ, Levy EM, Mandel R, DiSciullo GJ. Inhibition of human immunodeficiency virus infection by agents that interfere with thiol–disulfide interchange upon virus–receptor interaction. Proc Natl Acad Sci USA. 1994;91:4559–4563. doi: 10.1073/pnas.91.10.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Essex DW, Li M, Miller A, Feinman RD. Protein disulfide isomerase and sulfhydryl‐dependent pathways in platelet activation. Biochemistry. 2001;40:6070–6075. doi: 10.1021/bi002454e. [DOI] [PubMed] [Google Scholar]
- 51.Dickerhof N, Kleffmann T, Jack R, McCormick S. Bacitracin inhibits the reductive activity of protein disulfide isomerase by disulfide bond formation with free cysteines in the substrate-binding domain. FEBS J. 2011;278(12):2034–43. doi: 10.1111/j.1742-4658.2011.08119.x. [DOI] [PubMed] [Google Scholar]
- 52.Wan SW, et al. Endothelial cell surface expression of protein disulfide isomerase activates β1 and β3 integrins and facilitates dengue virus infection. J Cell Biochem. 2012;113(5):1681–91. doi: 10.1002/jcb.24037. [DOI] [PubMed] [Google Scholar]
- 53.Bell SE, Shah CM, Gordge MP. Protein disulphide-isomerase mediates delivery of nitric oxide redox derivatives into platelets. Biochem. J. 2007;15(403):283–288. doi: 10.1042/BJ20061146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fédry J, et al. The Ancient Gamete Fusogen HAP2 Is a Eukaryotic Class II Fusion Protein. Cell. 2017;168:904–915. doi: 10.1016/j.cell.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinello JF, et al. Structure-Function Studies Link Class II Viral Fusogens with the Ancestral Gamete Fusion Protein HAP2. Current Biology. 2017;27(5):651–660. doi: 10.1016/j.cub.2017.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai CW, Duggan PF, Shimp RL, Jr, Miller LH, Narum DL. Overproduction of Pichia pastoris or Plasmodium falciparum protein disulfide isomerase affects expression, folding and O-linked glycosylation of a malaria vaccine candidate expressed in P. pastoris. J Biotechnol. 2006;24(121(4)):458–70. doi: 10.1016/j.jbiotec.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 57.Kozlov G, Määttänen P, Thomas DY, Gehring K. A structural overview of the PDI family of proteins. FEBS J. 2010;277(19):3924–36. doi: 10.1111/j.1742-4658.2010.07793.x. [DOI] [PubMed] [Google Scholar]
- 58.Benham AM. The protein disulfide isomerase family: key players in health and disease. Antioxid Redox Signal. 2012;16(8):781–9. doi: 10.1089/ars.2011.4439. [DOI] [PubMed] [Google Scholar]
- 59.van Lith M, Hartigan N, Hatch J, Benham AM. PDILT, a divergent testis-specific protein disulfide isomerase with a non-classical SXXC motif that engages in disulfide-dependent interactions in the endoplasmic reticulum. J Biol Chem. 2005;280(2):1376–83. doi: 10.1074/jbc.M408651200. [DOI] [PubMed] [Google Scholar]
- 60.Turano C, Coppari S, Altieri F, Ferraro A. Proteins of the PDI family: unpredicted non-ER locations and functions. J Cell Physiol. 2002;193(2):154–63. doi: 10.1002/jcp.10172. [DOI] [PubMed] [Google Scholar]
- 61.Ellerman DA, Myles DG, Primakoff PA. role for sperm surface protein disulfide isomerase activity in gamete fusion: evidence for the participation of ERp57. Dev Cell. 2006;10(6):831–7. doi: 10.1016/j.devcel.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Primakoff P, Myles DG. Cell-cell membrane fusion during mammalian fertilization. FEBS Lett. 2007;22(581(11)):2174–80. doi: 10.1016/j.febslet.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 63.Reiser K, et al. Thioredoxin-1 and protein disulfide isomerase catalyze the reduction of similar disulfides in HIV gp120. Int J Biochem Cell Biol. 2012;44(3):556–62. doi: 10.1016/j.biocel.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 64.Gilbert J, Ou W, Silver J, Benjamin T. Downregulation of protein disulfide isomerase inhibits infection by the mouse polyomavirus. J Virol. 2006;80(21):10868–70. doi: 10.1128/JVI.01117-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diwaker D, Mishra KP, Ganju L, Singh SB. Protein disulfide isomerase mediates dengue virus entry in association with lipid rafts. Viral Immunol. 2015;28(3):153–60. doi: 10.1089/vim.2014.0095. [DOI] [PubMed] [Google Scholar]
- 66.Taylor M, et al. Substrate-induced unfolding of protein disulfide isomerase displaces the cholera toxin A1 subunit from its holotoxin. PLoS Pathog. 2014;10(2):e1003925. doi: 10.1371/journal.ppat.1003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santos CX, et al. Protein disulfide isomerase (PDI) associates with NADPH oxidase and is required for phagocytosis of Leishmania chagasi promastigotes by macrophages. J Leukoc Biol. 2009;86(4):989–98. doi: 10.1189/jlb.0608354. [DOI] [PubMed] [Google Scholar]
- 68.Wang Hai-Long, Li Ya-Qing, Yin Li-Tian, Meng Xiao-Li, Guo Min, Zhang Jian-Hong, Liu Hong-Li, Liu Juan-Juan, Yin Guo-Rong. Toxoplasma gondii Protein Disulfide Isomerase (TgPDI) Is a Novel Vaccine Candidate against Toxoplasmosis. PLoS ONE. 2013;8(8):e70884. doi: 10.1371/journal.pone.0070884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kushawaha PK, Gupta R, Tripathi CD, Sundar S, Dube A. Evaluation of Leishmania donovani protein disulfide isomerase as a potential immunogenic protein/vaccine candidate against visceral Leishmaniasis. PLoS One. 2012;7(4):e35670. doi: 10.1371/journal.pone.0035670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shookhoff HB. The Present Status of Antibiotics in the Treatment of Protozoan Diseases. Bull N Y Acad Med. 1951;27(7):439–451. [PMC free article] [PubMed] [Google Scholar]
- 71.Ramakrishnan C, et al. Laboratory maintenance of rodent malaria parasites. Methods Mol. Biol. 2013;923:51–72. doi: 10.1007/978-1-62703-026-7_5. [DOI] [PubMed] [Google Scholar]
- 72.Sebastian S, et al. A Plasmodium calcium-dependent protein kinase controls zygote development and transmission by translationally activating repressed mRNAs. Cell Host Microbe. 2012;12:9–19. doi: 10.1016/j.chom.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfander C, et al. A scalable pipeline for highly effective genetic modification of a malaria parasite. Nat. Methods. 2011;8:1078–1082. doi: 10.1038/nmeth.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Habtewold T, Povelones M, Blagborough AM, Christophides GK. Transmission blocking immunity in the malaria non-vector mosquito Anopheles quadriannulatus species A. PLoS Pathog. 2008;23(4(5)):e1000070. doi: 10.1371/journal.ppat.1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.