Abstract
Objective:
We studied 8 mental health conditions diagnosed before bilateral oophorectomy performed for nonmalignant indications.
Methods:
We identified 1,653 premenopausal women who underwent bilateral oophorectomy for a nonmalignant indication in Olmsted County, Minnesota during the 20-year period 1988-2007. Each woman was matched by age (±1 year) to 1 population-based control who had not undergone bilateral oophorectomy before the index date (age range: 21-49 years). Both cases and controls were identified using the records-linkage system of the Rochester Epidemiology Project (REP http://www.rochesterproject.org). For 8 mental health conditions we calculated odds ratios (OR) and their 95% confidence intervals (95% CI) adjusted for race, education, and income using conditional logistic regression.
Results:
Preexisting mood disorders, anxiety disorders, and somatoform disorders were associated with increased risk of bilateral oophorectomy in overall analyses. These associations were also significant in women ≤45 years of age at index date. Personality disorders were associated with increased risk only in overall analyses, and adjustment disorders only in women 46-49 years of age. Some of the associations were significantly different across strata by age at index date and by indication. There was also a linear trend of increasing adjusted ORs from 1.55 (95% CI, 1.31-1.83) for one mental health condition to 2.19 (95% CI, 1.40-3.41) for three or more conditions (trend P<0.001).
Conclusions:
We identified several mental health conditions that were associated with bilateral oophorectomy for nonmalignant indications. Awareness of these associations may guide women and physicians in future decision-making and limit unindicated bilateral oophorectomies.
Keywords: Bilateral oophorectomy, mood disorders, anxiety disorders, somatoform disorders, personality disorders, mental health
Several studies have investigated the effects of hysterectomy with or without concurrent bilateral oophorectomy on mental health outcomes over short-term and long-term follow-up; however, the results are mixed.1–7 Some of these studies reported that some women had mental health conditions preceding surgery, and these women were, in general, excluded from analyses for mental health conditions after the surgery (de novo conditions). 4–7 However, a formal study of psychiatric conditions before bilateral oophorectomy has not been conducted.
In 2016, we reported results from the Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (MOA-2) showing a higher frequency of depression, anxiety, and substance abuse disorders diagnoses occurring before the date of bilateral oophorectomy compared to controls.8 In 2017, we reported an association between adverse childhood or adult experiences and the risk of bilateral oophorectomy in a case-control study conducted in a subset of the MOA-2 sample. However, in that case-control study, the effect of the adverse experiences appeared not to be mediated by psychiatric symptoms or diagnoses such as substance abuse disorders, eating disorders, suicidal attempts or ideation, and more specifically, of depression or anxiety before the index date.9
The overall group of women in the MOA-2 study may have included two or more subgroups of women with different conditions associated with bilateral oophorectomy. In one subgroup of women, depression and anxiety symptoms developed before the index date and may have contributed to the circumstances that led the women and their physicians to opt for a gynecological surgery, including a bilateral oophorectomy. In another subgroup of women, adverse childhood or adult experiences may have been involved. Even though psychiatric symptoms or adverse experiences are not an indication for bilateral oophorectomy, these conditions may have directly or indirectly influenced the decision.8–10 To further investigate the psychiatric conditions that may be diagnosed before bilateral oophorectomy, we conducted a case-control study based on data collected via the manual review of the medical records in a population-based records-linkage system.
METHODS
Study population
Several previous publications have reported the overall methodology of this study and the clinical characteristics of the women in the MOA-2 study.8, 11–13 Briefly, MOA-2 included all of the premenopausal women who underwent bilateral oophorectomy for a nonmalignant indication in Olmsted County, Minnesota (USA) during the 20-year period 1988 to 2007. This cohort of women was compared to a cohort of age-matched referent women (1:1 matching for age ± 1 year). Both cohorts were representative of a geographically-defined population, and all data were collected using the records-linkage system of the Rochester Epidemiology Project (REP). The REP has been described in several publications.14–17 The women in the MOA-2 study were originally sampled to serve as exposed and referent women for a set of cohort analyses; however, they were considered cases and controls in the case-control analyses conducted in this study. The date of bilateral oophorectomy was used as the index date for each matched pair. The same case-control design was used in a previous study focusing on familial, personal, and reproductive characteristics associated with bilateral oophorectomy.13 All study procedures and ethical aspects received approval from the institutional review boards of both Mayo Clinic and Olmsted Medical Center. Because the data were abstracted electronically or manually from the medical records, women were not contacted for the study and did not need to provide a study-specific informed consent. However, we included only women who had signed a general consent to use their medical records for research, as mandated by the State of Minnesota.15, 16
Data collection
As described more extensively elsewhere, a physician (LGR) and a trained nurse abstractor reviewed the complete medical records of women with oophorectomy (cases) and their age-matched controls to collect demographic, social, and reproductive history data, and information about adult life characteristics and family history of cancer.13 Data were abstracted and recorded using an electronic data entry application. To increase the agreement between the two abstractors, we developed a manual of instructions that included definitions and examples for the characteristics to be abstracted. Specific to this study, all psychiatric diagnoses recorded in the medical record by any physician at any time before the index date were manually abstracted, regardless of the diagnostic coding for billing purposes. The diagnoses were grouped into 8 broad categories using terminology consistent with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).18 A full listing and description of the specific diagnoses is provided in the DSM-IV.18 In particular, we considered mood disorders, bipolar disorders, anxiety disorders, schizophrenia, somatoform disorders, personality disorders, dissociative disorders, and adjustment disorders.18 The physician who abstracted the records (LGR) was trained in psychiatry and the nurse abstractor consulted with her when terminology was uncertain; however, the two abstractors did not apply the formal diagnostic criteria of the DSM-IV.18 The abstractors also recorded the date of first diagnosis for each specific condition. Women who did not receive a diagnosis for a mental health condition were considered not to have that condition.
Reliability and validity
We measured the agreement between the data obtained through manual review of the medical records (current study) and the data reported previously using electronic extraction of diagnostic codes from the REP indexes.8 The diagnostic codes from the International Classification of Diseases, Ninth Revision (ICD-9) were grouped into several broad psychiatric conditions as suggested by the Department of Health and Human Services (DHHS).19, 20 Because the DHHS grouping for depression includes also bipolar disorders, we grouped mood disorders and bipolar disorders obtained from manual review for this agreement study. When considering the two data abstractors together, the percent agreement was 87.6 for depression (kappa 0.66; 95% confidence interval [CI], 0.63-0.69), 88.2 for anxiety (kappa 0.45; 95% CI, 0.41-0.50), and 99.7 for schizophrenia (kappa 0.78; 95% CI, 0.65-0.91).
To study the inter-rater agreement between the two data abstractors, we also compared separately the data obtained by each abstractor with the corresponding DHHS coding. The psychiatrist (LGR) abstracted 2,116 medical records and the nurse abstractor 1,190 records. The percent agreements and the kappa values were similar across the abstractors. For depression (including both mood disorders and bipolar disorders), the percent agreement was 87.2 (kappa 0.66, 95% CI, 0.62-0.69) for the psychiatrist and 88.2 (kappa 0.66; 95% CI, 0.61-0.71) for the nurse abstractor. For anxiety, the percent agreement was 88.0 (kappa 0.43, 95% CI, 0.37-0.49) for the psychiatrist and 88.6 (kappa 0.49, 95% CI, 0.41-0.56) for the nurse abstractor. For schizophrenia, the percent agreement was 99.6 (kappa 0.69, 95% CI, 0.49-0.89) for the psychiatrist and 99.8 (kappa 0.90, 95% CI, 0.76-1.00) for the nurse abstractor.
Statistical analysis
We used conditional logistic regression models for matched pairs to compare cases and controls and to calculate odds ratios (ORs) and 95% CIs. Analyses were adjusted for education (≤12; 13-16; >16 years), race (white; nonwhite), and household income (quartiles: <$42,000; $42,000-56,999; $57,000-71,999; ≥$72,000) because these characteristics were considered potential confounders.11 Household income was derived from the 2000 United States Census, at the census block level, as described elsewhere.13 We considered each psychiatric diagnosis individually, and also compared the number of psychiatric conditions occurring in the same woman (dose-effect analyses; Cochrane-Armitage test for linear trend in the ORs using conditional logistic regression).
As in our previous case-control analyses in the MOA-2 study, we conducted a set of analyses including the complete sample, and three sets of analyses stratified by age at the time of oophorectomy or at the index date (≤45 years and 46-49 years), by indication for the oophorectomy (benign ovarian condition and no ovarian indication), and by calendar year of the oophorectomy or index year (1988-1997 and 1998-2007 decades).13
We also conducted a set of sensitivity analyses for the overall sample after excluding 165 case-control pairs for which the control had undergone hysterectomy (with conservation of at least one ovary) before the index date and 24 pairs for which the case had not undergone hysterectomy (the woman underwent bilateral oophorectomy with conservation of the uterus) as of the index date. Because 3 pairs were excluded for both reasons, we excluded a total of 186 pairs. Finally, we compared the median lag time between the first diagnosis of each of the psychiatric conditions and the index date. All analyses were conducted using SAS v.9.4 (SAS Institute Inc., Cary, NC), and tests of statistical significance were conducted at the two-tailed alpha level of 0.05.
RESULTS
Table 1 shows the demographic and social characteristics of our case-control sample (1,653 cases and 1,653 matched controls). Fewer cases were of nonwhite race than controls; however, the numbers for nonwhite women were small. Cases also reported fewer years of education than controls. Table 1 also provides a distribution of the indications for the bilateral oophorectomy. Approximately 60% of the women did not have any ovarian condition mentioned in their medical record, and most of them had undergone bilateral oophorectomy for a generic prophylaxis of ovarian and breast cancer, at the time of a hysterectomy. Case-control analyses for race, education, and income conducted in the overall sample and in strata by age at oophorectomy and by indication for oophorectomy were reported and discussed elsewhere.13
TABLE 1.
Demographic and social characteristics for women who underwent bilateral oophorectomy and their matched controls as measured at the index date
| Bilateral oophorectomy (N=1,653) |
Control women (N=1,653) |
||||
|---|---|---|---|---|---|
| Characteristica | N | % | N | % | Pb |
| Age at oophorectomy | -- | ||||
| ≤35 | 161 | 9.7 | 161 | 9.7 | |
| 36-39 | 183 | 11.1 | 183 | 11.1 | |
| 40-45 | 687 | 41.6 | 687 | 41.6 | |
| 46-49 | 622 | 37.6 | 622 | 37.6 | |
| Calendar year of oophorectomy | -- | ||||
| 1988-1992 | 317 | 19.2 | 317 | 19.2 | |
| 1993-1997 | 406 | 24.6 | 406 | 24.6 | |
| 1998-2002 | 553 | 33.5 | 553 | 33.5 | |
| 2003-2007 | 377 | 22.8 | 377 | 22.8 | |
| Race | <0.001 | ||||
| White | 1,611 | 97.5 | 1,570 | 95.0 | |
| Black | 18 | 1.1 | 29 | 1.8 | |
| Asian | 18 | 1.1 | 49 | 3.0 | |
| Other | 6 | 0.4 | 5 | 0.3 | |
| Hispanic ethnicity | 20 | 1.2 | 23 | 1.4 | 0.65 |
| Years of education | <0.001 | ||||
| <9 | 8 | 0.5 | 31 | 1.9 | |
| 9-12 | 518 | 31.4 | 447 | 27.6 | |
| 13-16 | 895 | 54.2 | 861 | 53.2 | |
| >16 | 229 | 13.9 | 279 | 17.2 | |
| Household incomec | 0.28 | ||||
| <$42,000 | 414 | 25.1 | 406 | 24.6 | |
| $42,000-56,999 | 440 | 26.7 | 412 | 25.0 | |
| $57,000-71,999 | 419 | 25.4 | 412 | 25.0 | |
| ≥$72,000 | 374 | 22.7 | 421 | 25.5 | |
| Indication for oophorectomyd | -- | ||||
| Benign ovarian condition | 675 | 40.8 | -- | -- | |
| Benign tumor | 382 | 23.1 | -- | -- | |
| Endometriosis | 225 | 13.6 | -- | -- | |
| Cyst | 60 | 3.6 | -- | -- | |
| Othere | 8 | 0.5 | -- | -- | |
| No ovarian indicationf | 978 | 59.2 | -- | -- | |
Women with missing or unknown data were not included in the respective analysis for the following characteristics: 38 women for years of education (3 cases and 35 controls) and 8 women for household income (6 cases and 2 controls).
P values were obtained from chi-squared tests or Fisher’s exact tests.
Household income was stratified using quartiles calculated from the overall sample (case-control groups pooled).
The indication was recorded in the medical record by the gynecologist at the time of oophorectomy. For women with different indications in the two ovaries, we reported the most severe indication (only one indication for each woman, in the order shown).
Torsion, tubo-ovarian abscess, pelvic congestion, pelvic inflammatory disease, chronic abdominal pain, post-hysterectomy vaginal bleeding, and other rare indications.
Women without a known or suspected malignant or benign ovarian condition. Historically, the terms “prophylactic”, “elective”, or “incidental” oophorectomy were used; however, we avoided these terms.
Table 2 shows separate case-control analyses for 8 psychiatric conditions diagnosed before the index date in the overall sample, and Table 3 shows case-control analyses stratified by age. Figure 1 shows the effect of age in modifying the associations. Mood disorders (OR=1.77; 95% CI, 1.51-2.09), anxiety disorders (OR=1.29; 95% CI, 1.04-1.59), and somatoform disorders (OR=3.65; 95% CI, 1.75-7.64) were associated with increased risk of bilateral oophorectomy in overall analyses and in the age stratum ≤45 years. The association for somatoform disorders was particularly strong in women ≤45 years of age (OR=5.09; 95% CI, 1.75-14.86). Personality disorders were associated with increased risk of bilateral oophorectomy in overall analyses. Adjustment disorders were associated with increased risk of bilateral oophorectomy in the age stratum 46-49 years. The ORs for mood disorders and anxiety disorders were significantly greater in the younger age stratum than in the older age stratum, whereas the OR for adjustment disorders were significantly greater in the older age stratum (significant interaction by age). There was also a linear trend of increasing ORs with increasing numbers of mental health conditions before the index date.
TABLE 2.
Overall case-control analyses for psychiatric conditions before the index date.
| Bilateral oophorectomy (N=1,653) |
Control women (N=1,653) |
Unadjusted modelsb |
Adjusted modelsc |
|||||
|---|---|---|---|---|---|---|---|---|
| Conditiona | N | % | N | % | Odds ratio (95% CI) | P | Odds ratio (95% CI) | P |
| Mood disorders | 529 | 32.0 | 346 | 20.9 | 1.79 (1.53-2.11) | <0.001 | 1.77 (1.51-2.09) | <0.001 |
| Bipolar disorders | 31 | 1.9 | 18 | 1.1 | 1.76 (0.97-3.20) | 0.06 | 1.71 (0.94-3.12) | 0.08 |
| Anxiety disorders | 236 | 14.3 | 189 | 11.4 | 1.30 (1.06-1.61) | 0.01 | 1.29 (1.04-1.59) | 0.02 |
| Schizophrenia | 7 | 0.4 | 13 | 0.8 | 0.54 (0.21-1.35) | 0.19 | 0.57 (0.23-1.46) | 0.24 |
| Somatoform disorders | 34 | 2.1 | 9 | 0.5 | 3.78 (1.81-7.88) | <0.001 | 3.65 (1.75-7.64) | <0.001 |
| Personality disorders | 37 | 2.2 | 20 | 1.2 | 1.94 (1.10-3.43) | 0.02 | 1.90 (1.07-3.38) | 0.03 |
| Dissociative disorders | 12 | 0.7 | 8 | 0.5 | 1.50 (0.61-3.67) | 0.37 | 1.42 (0.57-3.53) | 0.45 |
| Adjustment disorders | 123 | 7.4 | 112 | 6.8 | 1.11 (0.85-1.45) | 0.45 | 1.13 (0.86-1.48) | 0.38 |
| Number of conditionsd | ||||||||
| 0 | 980 | 59.3 | 1,158 | 70.1 | 1.00 (reference)e | 1.00 (reference)e | ||
| 1 | 427 | 25.8 | 323 | 19.5 | 1.55 (1.31-1.83) | <0.001 | 1.55 (1.31-1.83) | <0.001 |
| 2 | 185 | 11.2 | 138 | 8.3 | 1.62 (1.27-2.07) | <0.001 | 1.61 (1.26-2.06) | <0.001 |
| ≥3 | 61 | 3.7 | 34 | 2.1 | 2.22 (1.43-3.45) | <0.001 | 2.19 (1.40-3.41) | <0.001 |
Psychiatric conditions before the index date were determined from abstraction of the medical records.
Odds ratios calculated using conditional logistic regression models (matched pairs).
Odds ratios calculated using conditional logistic regression models (matched pairs), adjusted for race (white vs nonwhite), years of education (≤12, 13-16, >16), and quartiles of household income (<$42,000, $42,000-56,999, $57,000-71,999, ≥$72,000). Women missing years of education (3 cases, 35 controls) were assigned to the ≤12 years group and women missing household income (6 cases, 2 controls) were assigned to the second quartile.
Total number of psychiatric conditions before the index date for each woman.
Cochrane-Armitage test for linear trend in the odds ratios, P<0.001 for the unadjusted models and P<0.001 for the adjusted models.
TABLE 3.
Case-control analyses for psychiatric conditions before the index date, stratified by age at index date.
| Bilateral oophorectomy (N=1,031 age ≤45, N=622 age 46-49) |
Control women (N=1,031 age ≤45, N=622 age 46-49) |
Unadjusted modelsb |
Adjusted modelsc |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Conditiona | N | % | N | % | Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | Interaction P |
| Mood disorders | |||||||||
| Age ≤45 years | 363 | 35.2 | 205 | 19.9 | 2.22 (1.81-2.74) | <0.001 | 2.16 (1.75-2.66) | <0.001 | 0.003 |
| Age 46-49 years | 166 | 26.7 | 141 | 22.7 | 1.25 (0.96-1.62) | 0.10 | 1.29 (0.99-1.68) | 0.06 | |
| Bipolar disorders | |||||||||
| Age ≤45 years | 24 | 2.3 | 13 | 1.3 | 1.92 (0.95-3.85) | 0.07 | 1.71 (0.84-3.50) | 0.14 | 0.72 |
| Age 46-49 years | 7 | 1.1 | 5 | 0.8 | 1.40 (0.44-4.41) | 0.57 | 1.34 (0.42-4.26) | 0.63 | |
| Anxiety disorders | |||||||||
| Age ≤45 years | 150 | 14.5 | 101 | 9.8 | 1.57 (1.20-2.06) | 0.001 | 1.56 (1.19-2.06) | 0.002 | 0.04 |
| Age 46-49 years | 86 | 13.8 | 88 | 14.1 | 0.97 (0.69-1.36) | 0.86 | 0.97 (0.69-1.37) | 0.88 | |
| Schizophrenia | |||||||||
| Age ≤45 years | 3 | 0.3 | 9 | 0.9 | 0.33 (0.09-1.23) | 0.10 | 0.31 (0.08-1.16) | 0.08 | 0.19 |
| Age 46-49 years | 4 | 0.6 | 4 | 0.6 | 1.00 (0.25-4.00) | 1.00 | 1.13 (0.28-4.55) | 0.87 | |
| Somatoform disorders | |||||||||
| Age ≤45 years | 23 | 2.2 | 4 | 0.4 | 5.75 (1.99-16.63) | 0.001 | 5.09 (1.75-14.86) | 0.003 | 0.30 |
| Age 46-49 years | 11 | 1.8 | 5 | 0.8 | 2.20 (0.76-6.33) | 0.14 | 2.31 (0.80-6.68) | 0.12 | |
| Personality disorders | |||||||||
| Age ≤45 years | 26 | 2.5 | 15 | 1.5 | 1.85 (0.94-3.63) | 0.08 | 1.74 (0.88-3.45) | 0.11 | 0.63 |
| Age 46-49 years | 11 | 1.8 | 5 | 0.8 | 2.20 (0.76-6.33) | 0.14 | 2.36 (0.81-6.90) | 0.12 | |
| Dissociative disorders | |||||||||
| Age ≤45 years | 8 | 0.8 | 7 | 0.7 | 1.14 (0.41-3.15) | 0.80 | 0.98 (0.35-2.75) | 0.96 | 0.22 |
| Age 46-49 years | 4 | 0.6 | 1 | 0.2 | 4.00 (0.45-35.79) | 0.22 | 4.49 (0.48-41.75) | 0.19 | |
| Adjustment disorders | |||||||||
| Age ≤45 years | 60 | 5.8 | 75 | 7.3 | 0.79 (0.55-1.12) | 0.18 | 0.80 (0.56-1.15) | 0.23 | 0.004 |
| Age 46-49 years | 63 | 10.1 | 37 | 5.9 | 1.84 (1.19-2.85) | 0.006 | 1.85 (1.19-2.88) | 0.006 | |
Psychiatric conditions before the index date were determined from abstraction of the medical records.
Odds ratios calculated using conditional logistic regression models (matched pairs).
Odds ratios calculated using conditional logistic regression models (matched pairs), adjusted for race (white vs nonwhite), years of education (≤12, 13-16, >16), and quartiles of household income (<$42,000, $42,000-56,999, $57,000-71,999, ≥$72,000). The interaction p-values were calculated using adjusted conditional logistic regression models (matched pairs) which also included a variable for the multiplicative interaction between each psychiatric condition and age (≤45 years vs 46-49 years). Women missing years of education (age ≤45 years: 2 cases, 23 controls; age 46-49 years: 1 case, 12 controls) were assigned to the ≤12 years group and women missing household income (age ≤45 years: 5 cases, 2 controls; age 46-49 years: 1 case, 0 controls) were assigned to the second quartile.
Fig 1.
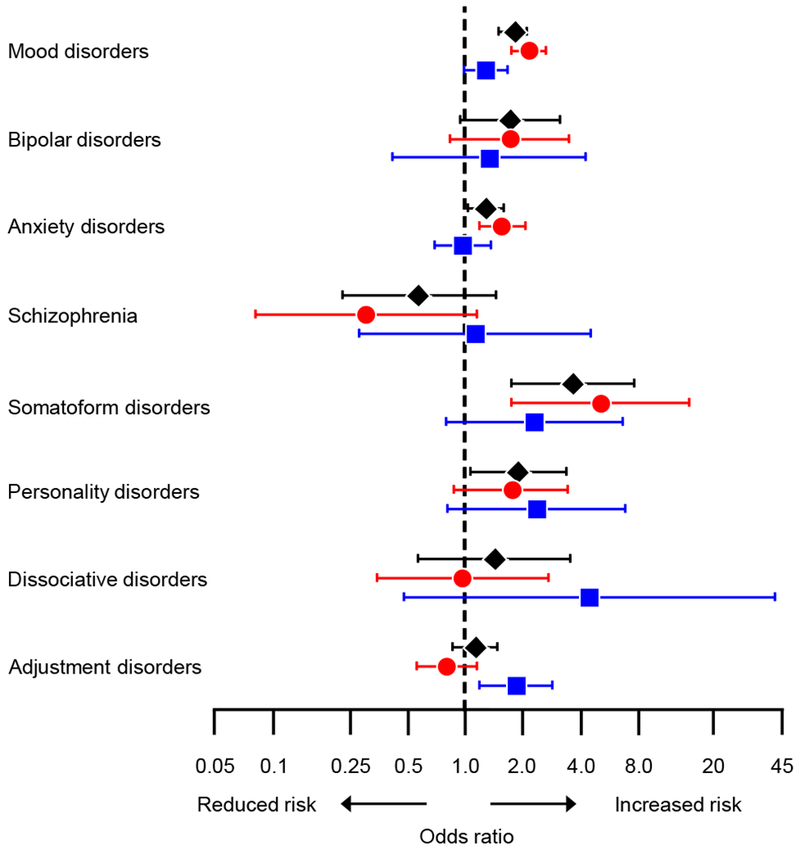
Odds ratios and 95% confidence intervals for psychiatric conditions before the index date, overall (black diamonds), and in the age strata ≤45 years (red circles) and 46-49 years (blue squares). The analyses were adjusted for race (white vs nonwhite), years of education (≤12, 13-16, >16), and household income (four quartiles).
Table 4 shows case-control analyses stratified by indication and Figure 2 shows the effect of the indication for the bilateral oophorectomy in modifying the associations. Mood disorders were associated with increased risk of bilateral oophorectomy both in women with an ovarian indication (OR=1.69; 95% CI, 1.31-2.18) and without an ovarian indication (OR=1.86; 95% CI, 1.50-2.30), with similar magnitude of the ORs. Anxiety disorders and adjustment disorders were associated with increased risk only in the group without an ovarian indication. Bipolar disorders and somatoform disorders were associated only in the group with an ovarian indication. The OR for bipolar disorders was significantly greater in women with an ovarian indication, and the OR for adjustment disorders was significantly greater in women without an ovarian indication (significant interaction by indication).
TABLE 4.
Case-control analyses for psychiatric conditions before the index date, stratified by oophorectomy indication.
| Bilateral oophorectomy (N=675 indication, N=978 no indication) |
Control women (N=675 indication, N=978 no indication) |
Unadjusted modelsb |
Adjusted modelsc |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Conditiona | N | % | N | % | Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | Interaction P |
| Mood disorders | |||||||||
| Benign indication | 209 | 31.0 | 138 | 20.4 | 1.73 (1.35-2.22) | <0.001 | 1.69 (1.31-2.18) | <0.001 | 0.58 |
| No ovarian indication | 320 | 32.7 | 208 | 21.3 | 1.84 (1.49-2.27) | <0.001 | 1.86 (1.50-2.30) | <0.001 | |
| Bipolar disorders | |||||||||
| Benign indication | 16 | 2.4 | 3 | 0.4 | 5.33 (1.55-18.30) | 0.008 | 5.58 (1.55-20.08) | 0.008 | 0.02 |
| No ovarian indication | 15 | 1.5 | 15 | 1.5 | 1.00 (0.48-2.10) | 1.00 | 0.96 (0.46-2.02) | 0.92 | |
| Anxiety disorders | |||||||||
| Benign indication | 78 | 11.6 | 73 | 10.8 | 1.08 (0.77-1.50) | 0.67 | 1.04 (0.74-1.47) | 0.82 | 0.12 |
| No ovarian indication | 158 | 16.2 | 116 | 11.9 | 1.47 (1.12-1.93) | 0.005 | 1.48 (1.12-1.94) | 0.005 | |
| Schizophrenia | |||||||||
| Benign indication | 3 | 0.4 | 2 | 0.3 | 1.50 (0.25-8.98) | 0.66 | 1.70 (0.28-10.35) | 0.57 | 0.18 |
| No ovarian indication | 4 | 0.4 | 11 | 1.1 | 0.36 (0.12-1.14) | 0.08 | 0.39 (0.12-1.25) | 0.11 | |
| Somatoform disorders | |||||||||
| Benign indication | 20 | 3.0 | 4 | 0.6 | 5.00 (1.71-14.63) | 0.003 | 4.67 (1.57-13.88) | 0.006 | 0.47 |
| No ovarian indication | 14 | 1.4 | 5 | 0.5 | 2.80 (1.01-7.77) | 0.048 | 2.69 (0.96-7.54) | 0.06 | |
| Personality disorders | |||||||||
| Benign indication | 13 | 1.9 | 5 | 0.7 | 3.00 (0.97-9.30) | 0.06 | 2.80 (0.88-8.86) | 0.08 | 0.43 |
| No ovarian indication | 24 | 2.5 | 15 | 1.5 | 1.64 (0.85-3.19) | 0.14 | 1.63 (0.83-3.18) | 0.16 | |
| Dissociative disorders | |||||||||
| Benign indication | 4 | 0.6 | 3 | 0.4 | 1.33 (0.30-5.96) | 0.71 | 0.97 (0.20-4.64) | 0.97 | 0.61 |
| No ovarian indication | 8 | 0.8 | 5 | 0.5 | 1.60 (0.52-4.89) | 0.41 | 1.61 (0.52-5.01) | 0.41 | |
| Adjustment disorders | |||||||||
| Benign indication | 37 | 5.5 | 53 | 7.9 | 0.67 (0.43-1.04) | 0.08 | 0.70 (0.44-1.11) | 0.13 | 0.007 |
| No ovarian indication | 86 | 8.8 | 59 | 6.0 | 1.51 (1.07-2.14) | 0.02 | 1.53 (1.08-2.18) | 0.02 | |
Psychiatric conditions before the index date were determined from abstraction of the medical records.
Odds ratios calculated using conditional logistic regression models (matched pairs).
Odds ratios calculated using conditional logistic regression models (matched pairs), adjusted for race (white vs nonwhite), years of education (≤12, 13-16, >16), and quartiles of household income (<$42,000, $42,000-56,999, $57,000-71,999, ≥$72,000). The interaction p-values were calculated using adjusted conditional logistic regression models (matched pairs) which also included a variable for the multiplicative interaction between each psychiatric condition and surgical indication (benign indication vs no ovarian indication). Women missing years of education (benign indication: 3 cases, 15 controls; no ovarian indication: 0 cases, 20 controls) were assigned to the ≤12 years group and women missing household income (benign indication: 3 cases, 2 controls; no ovarian indication: 3 cases, 0 controls) were assigned to the second quartile.
Fig. 2.
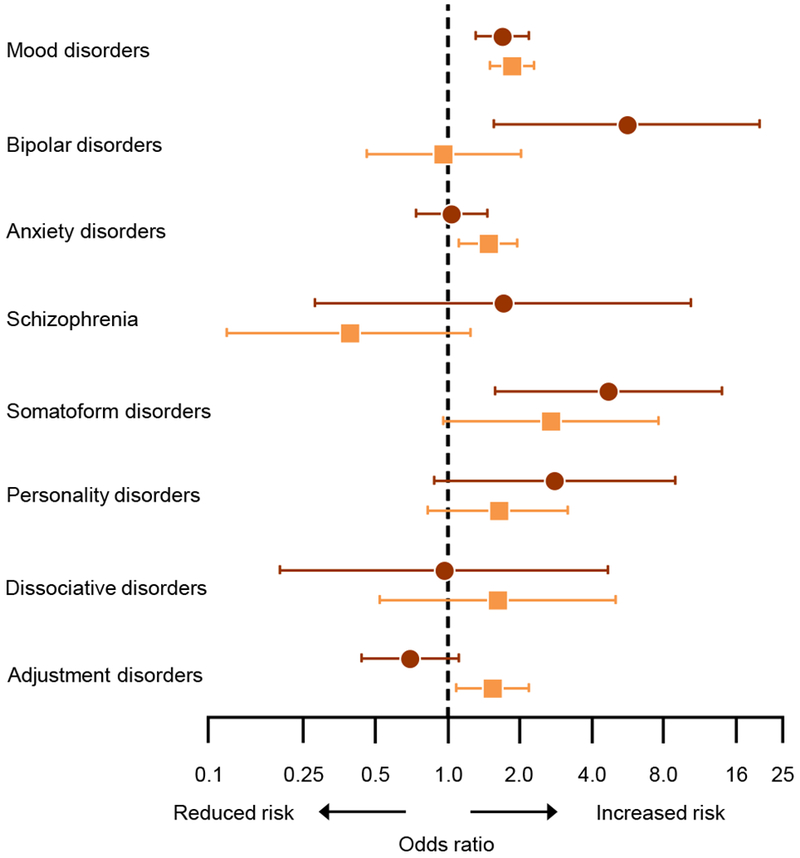
Odds ratios and 95% confidence intervals for psychiatric conditions before the index date in strata with an ovarian indication (brown circles) and without an ovarian indication (orange squares). The analyses were adjusted for race (white vs nonwhite), years of education (≤12, 13-16, >16), and household income (four quartiles).
The Supplemental Digital Content 1 shows case-control analyses stratified by calendar year of the index date. The results were similar in the two decades. The Supplemental Digital Content 2 shows sensitivity analyses after excluding a total of 186 matched pairs in which the control underwent hysterectomy before the index date or the case did not undergo hysterectomy on or before the index date (uterine conservation). The results of these sensitivity analyses were similar to the results of the primary analyses. We also explored the lag time between the first diagnosis of a psychiatric condition and the index date. The median lag time was longer in cases than in controls for anxiety disorders, somatoform disorders, dissociative disorders, and adjustment disorders; however, none of the differences were statistically significant (data not shown).
DISCUSSION
Principal findings
Our study identified several psychiatric conditions that may have influenced the decision of women to request a surgery resulting in bilateral oophorectomy for a nonmalignant indication, or the decision of primary care providers to endorse or suggest the surgery, and/or of gynecologists to perform the surgery. Several associations varied significantly for bilateral oophorectomies performed at ages ≤45 years compared to 46-49 years, and for women with or without a benign ovarian indication. These associations have not been previously investigated in large epidemiologic studies. Some of our findings can be considered independent replications of previous findings because the associations with depression and anxiety have been reported before.8 However, some of the findings for less common psychiatric conditions are novel and may be important for some subgroups of women. Thirty-four women who underwent bilateral oophorectomy had somatoform disorders and 37 had personality disorders, for a combined frequency of 3.6% (accounting for 12 women with overlap of both conditions). Although these conditions were less common than mood disorders (32.0%) or anxiety disorders (14.3%), their associations were stronger (e.g., OR=3.65 for somatoform disorders). As expected from general psychiatric practice, these conditions often co-occurred with anxiety or depression, or both. For example, of the 37 women with somatoform disorders who underwent bilateral oophorectomy, 8 also had a diagnosis of depression, 2 of anxiety, and 17 of both depression and anxiety. These conditions or combinations of conditions should be considered in a psychiatric evaluation of women who are candidates for a bilateral oophorectomy. Unfortunately, somatoform disorders and personality disorders are often overlooked even in routine psychiatric practice.
Comparison with previous studies
Figure 3 shows a comparison of the ORs obtained using manual abstraction of diagnoses from the medical records in the present study with the ORs obtained in a previous study in the same sample using diagnostic codes extracted from the electronic indexes of the REP.8 Figure 3 also compares the results obtained in the present study for bilateral oophorectomy with the results from a previous study of women who underwent hysterectomy with ovarian conservation in the same Olmsted County population (including 2,094 women with hysterectomy and 2,094 women without hysterectomy).21 In both previous studies, depression, anxiety, and schizophrenia were defined using ICD-9 diagnostic codes (as suggested by the DHHS). The results for bilateral oophorectomy using manually abstracted data and data extracted electronically were similar for mood disorders and bipolar disorders combined, for anxiety disorders, and for schizophrenia.8 Interestingly, the ORs for bilateral oophorectomy were also similar to the results from a previous study of hysterectomy with ovarian conservation.21 Therefore, women undergoing hysterectomy with or without concurrent bilateral oophorectomy have a similar profile of psychiatric conditions diagnosed before the surgery. These psychiatric conditions are associated with an increased risk of performing a surgery of the reproductive organs; however, the extent of the surgery may be influenced by other factors (e.g., family history of ovarian cancer or preference of the gynecologist).
Fig. 3.
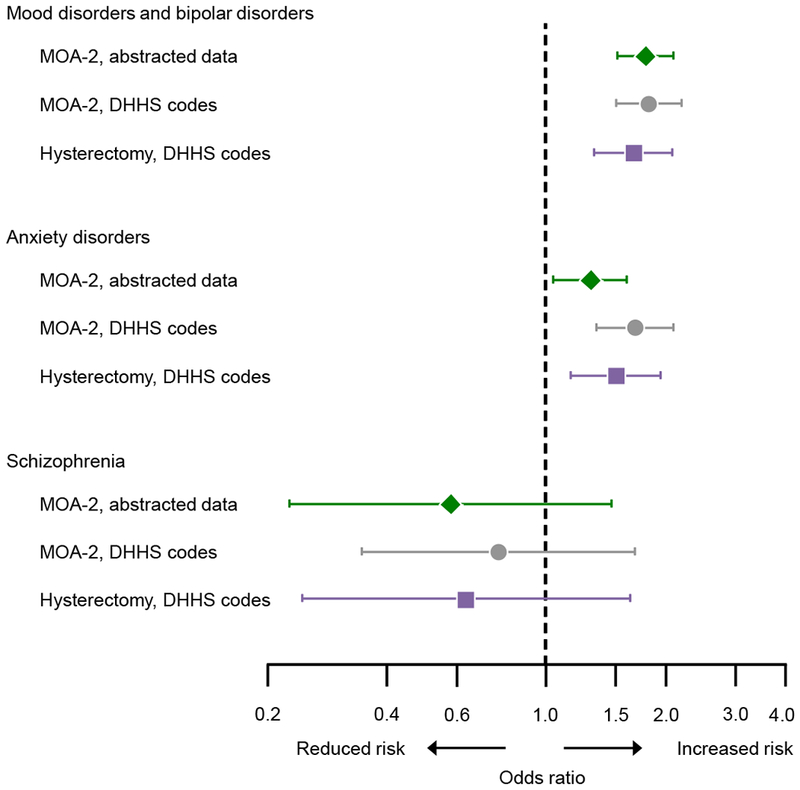
Comparison of the results from the current study using medical record abstraction (green diamonds; Mayo Clinic Cohort Study of Oophorectomy and Aging-2 [MOA-2]) with results from a previous study of bilateral oophorectomy using diagnostic codes extracted electronically (grey circles; MOA-2),8 and with results of a previous study of hysterectomy with ovarian conservation (purple squares).21 Because the Department of Health and Human Services (DHHS) codes for depression include also bipolar disorders, we pooled mood disorders and bipolar disorders as obtained through medical records abstraction in the current study to increase comparability. The analyses were adjusted for race (white vs nonwhite), years of education (≤12, 13-16, >16), and household income (four quartiles) in the two MOA-2 studies, and only for race and education in the hysterectomy study.
Possible interpretations of findings
Among 1,653 women in our study, a total of 675 women (40.8%) had a benign ovarian condition listed in their medical records as the indication for the bilateral oophorectomy (benign tumor, cyst, endometriosis, or other benign ovarian condition).13 It can be argued that the removal of both ovaries was not needed to control the benign ovarian conditions for many of these women. In addition, we observed that 24.9% of these women had normal ovaries at pathology. Only for the 225 women (13.6%) with suspected ovarian endometriosis, bilateral oophorectomy had a strong indication. However, of these 225 women, less than half (44.4%) had pathologically confirmed ovarian endometriosis. The remaining 978 women (59.2%) who underwent bilateral oophorectomy in our study did not have any specified ovarian indication.13 Women without a benign ovarian condition were historically considered to have “prophylactic”, “elective”, or “incidental” bilateral oophorectomy for a generic prophylaxis of ovarian or breast cancer. Many of these women underwent a hysterectomy for another gynecological indication (most commonly, excessive bleeding, pelvic pain, fibroids, or prolapse), and the presumed healthy ovaries were removed during the surgery. Unfortunately, for some women, it is not possible to reconstruct with certainty the indication from the medical record or from the pathology report.
As shown in our previous study,9, 10 the first subgroup of women who experienced adverse childhood experiences or adverse experiences later in life had undergone prior abdominal surgeries outside of the reproductive system (e.g., appendectomy) or involving the reproductive system (e.g., Cesarean section or tubal removal or ligation). These women requested the oophorectomy because they were convinced that it might definitively eliminate pain or other distress. These women were probably unaware that remote psychological or emotional traumas could be related to their pain and distress. A second subgroup of women may have developed psychiatric symptoms long before the index date, and these symptoms may have influenced their preference for a surgical intervention. For example, in women with a family history of ovarian or breast cancer, some of the psychiatric symptoms may have been prompted by the fear of developing cancer. In other women, the gynecological symptoms such as excessive bleeding or abdominal pain, may have prompted or exacerbated the symptoms of anxiety or depression.
In both subgroups of women, a problem of miscommunication may have arisen between the women and the care providers. Some women may have reported to primary care providers with gynecological symptoms that may have been in part related to anxiety, depression, somatoform disorders, or personality disorders. Some of these women may have interpreted their pain and distress as somatic symptoms, and requested treatment for a somatic condition. If the pharmacological treatments failed to control the symptoms (e.g., treatment with oral contraceptives or pain medications), some women requested a gynecological surgery as a more definitive solution to the problem. The primary care providers may have endorsed the request or even suggested a gynecological surgery.
At this point, women may have been referred by their primary care providers to a gynecologist with a specific recommendation. Unfortunately, the gynecologists may also not have been trained to consider the psychological or emotional origin of the pain and distress suffered by the woman. In the past, the gynecologists have been more willing to perform a hysterectomy with or without a bilateral oophorectomy because they were unaware of the possible long-term harmful consequences of these surgeries.9, 10, 21 In summary, psychiatric conditions may have played a role in the decision to perform a hysterectomy with or without a concurrent bilateral oophorectomy, and a better recognition of psychiatric problems may change the practice in the future and avoid unnecessary surgeries. In particular, some of these women may benefit from a multidisciplinary team able to offer a psychiatric evaluation and possibly a psychiatric therapy program (e.g., cognitive behavioral therapy) before proceeding to bilateral oophorectomy without a clear pathologic indication.22
Strengths
Our case-control study has several strengths. First, we abstracted details about bilateral oophorectomy, prior psychiatric diagnoses, and the medical conditions present at the index date from the medical records included in a records-linkage system. Thus, we did not need to interview or examine the women included in the study (recall bias was minimized). The recall of psychiatric symptoms or diagnoses may be particularly problematic. Second, because the data were abstracted from medical records, women did not need to provide a study-specific informed consent, but only a general state-mandated research authorization.15, 16 Thus, we minimized the risk of non-participation.
Third, we used controls who were representative of the general population. This is contrary to the design of other studies in which women who underwent bilateral oophorectomy were compared to women who underwent hysterectomy with ovarian conservation.23, 24 We elected to use general population controls because hysterectomy itself is associated with increased risk of mental health conditions, as shown in a previous study (Figure 3).21, 25 Finally, using the REP medical records-linkage system, we were able to include virtually the entire population without restrictions by socioeconomic status, insurance status, and the setting in which health care was received.16
Limitations
First, because the psychiatric diagnoses were abstracted from medical records in a records-linkage system, absence of a diagnosis was considered evidence that the condition was absent. However, the misdiagnoses of mental health conditions or the under-ascertainment of diagnoses, if any, should be similar for women with and without bilateral oophorectomy (non-differential misclassification). In either case, any resulting bias would be toward the null hypothesis. Second, surgical practices have changed over the 20 year period of the study (from 1988 through 2007). To address these possible effects, we conducted a set of analyses considering the decades of the surgery separately (1988-1997 vs. 1998-2007), and the association did not change. Third, we derived income level using census data at the census block group level and for only one point in time. Therefore, we may have experienced some misclassification of income. On the other hand, we used the year 2000 that was approximately the central year of the study period. In addition, we used the same methods to derive income for women with and without bilateral oophorectomy, and any misclassification should be non-differential.
Fourth, some of the analyses stratified by age and by ovarian indication were based on small numbers and should be interpreted with caution. In particular, the study was underpowered for schizophrenia and dissociative disorders. Finally, we were able to study only one geographically defined US population. Therefore, our findings may differ from the findings in other populations. A previous study showed that the demographic and socioeconomic characteristics of our population are similar to those of the population living in the upper Midwest and of a large segment of the entire US population.15, 17 Nevertheless, only the replication of this study in other populations in the United States and worldwide can guide the interpretation of our findings.
CONCLUSIONS
We identified several psychiatric conditions that were associated with an increased risk of undergoing bilateral oophorectomy over a 20-year period. Some of these conditions, alone or in combination, may have influenced the decision of women to request a gynecological surgery for a nonmalignant indication, the decision of primary care providers to endorse or suggest the surgery, and/or the decision of gynecologists to perform the surgery. Understanding the psychiatric conditions that may have influenced the practice of hysterectomy with or without concurrent bilateral oophorectomy in the past is important for developing more conservative strategies in the future. For example, future practice could involve multidisciplinary teams able to help women address sensitive and emotional issues and to offer alternative treatment plans. In addition, our finding may help to explain the complex relationship between mental health and gynecological health.
Supplementary Material
Supplemental Digital Content 1. Table showing case-control analyses for psychiatric conditions before the index date, stratified by index calendar year.
Supplemental Digital Content 2. Table showing overall case-control analyses for a subset of women, excluding 186 matched pairs in which the control underwent hysterectomy before the index date, or in which the case did not undergo hysterectomy on or before the index date.
Acknowledgement:
We thank Ms. Deborah C. Olson for her abstraction of information from medical records and Ms. Kristi Klinger for her assistance in typing and formatting the manuscript.
Sources of funding: The MOA-2 study used the resources of the Rochester Epidemiology Project, which was supported by the National Institute on Aging of the National Institutes of Health (grants R01 AG034676 and R01 AG052425). However, the content of this article is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. This study was also supported by funds from the Mayo Clinic Research Committee (to WAR). WAR was partly supported by the National Institutes of Health (P50 AG044170, U01 AG006786, and P01 AG004875). Dr. Bobo’s research has been funded by the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Mayo Foundation for Medical Education and Research.
Conflicts of interest/financial disclosures: Over the past 36 months, Dr. Stewart received funding from AbbVie, Allergan, Bayer, Med Learning Group, Myovant and Welltwigs, and received royalties from UpToDate for articles on uterine diseases. None of these activities were related to this manuscript. Dr. Laughlin-Tommaso consults for Allergan, has a research grant from Bayer, is on the DSMB for the ULTRA trial (Halt Medical), and receives royalties from UpToDate for fibroid articles. Dr. Bobo receives royalties from UpToDate for bipolar disorder articles. For the remaining authors, no disclosures were declared.
REFERENCES
- 1.Farquhar CM, Harvey SA, Yu Y, Sadler L, Stewart AW. A prospective study of 3 years of outcomes after hysterectomy with and without oophorectomy. Am J Obstet Gynecol. 2006;194:711–7. [DOI] [PubMed] [Google Scholar]
- 2.Gibson CJ, Joffe H, Bromberger JT, et al. Mood symptoms after natural menopause and hysterectomy with and without bilateral oophorectomy among women in midlife. Obstet Gynecol. 2012;119:935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darwish M, Atlantis E, Mohamed-Taysir T. Psychological outcomes after hysterectomy for benign conditions: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;174:5–19. [DOI] [PubMed] [Google Scholar]
- 4.Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186–91. [DOI] [PubMed] [Google Scholar]
- 5.Hickey M, Schoenaker DA, Joffe H, Mishra GD. Depressive symptoms across the menopause transition: findings from a large population-based cohort study. Menopause. 2016;23:1287–93. [DOI] [PubMed] [Google Scholar]
- 6.Wilson L, Pandeya N, Byles J, Mishra G. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women’s Health. Epidemiol Psychiatr Sci. 2018;27:381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocca WA, Grossardt BR, Geda YE, et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15:1050–9. [DOI] [PubMed] [Google Scholar]
- 8.Rocca WA, Gazzuola-Rocca L, Smith CY, et al. Accelerated accumulation of multimorbidity after bilateral oophorectomy: a population-based cohort study. Mayo Clin Proc. 2016;91:1577–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazzuola Rocca L, Smith CY, Grossardt BR, et al. Adverse childhood or adult experiences and risk of bilateral oophorectomy: a population-based case-control study. BMJ Open. 2017;7:e016045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gazzuola Rocca L, Smith CY, Stewart EA, Rocca WA. Adverse childhood experiences and adult abuse are predictors of hysterectomy and oophorectomy. Maturitas. 2017;106:95–6. [DOI] [PubMed] [Google Scholar]
- 11.Rocca WA, Gazzuola Rocca L, Smith CY, et al. Cohort profile: the Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (MOA-2) in Olmsted County, Minnesota (USA). BMJ Open. 2017;7:e018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocca WA, Gazzuola Rocca L, Smith CY, et al. Bilateral oophorectomy and accelerated aging: cause or effect? J Gerontol A Biol Sci Med Sci. 2017;72:1213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocca WA, Gazzuola Rocca L, Smith CY, et al. Personal, reproductive, and familial characteristics associated with bilateral oophorectomy in premenopausal women: a population-based case-control study. Maturitas. 2018;117:64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 19.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen JW, Cohen SB, Banthin JS. The Medical Expenditure Panel Survey: a national information resource to support healthcare cost research and inform policy and practice. Med Care. 2009;47:S44–50. [DOI] [PubMed] [Google Scholar]
- 21.Laughlin-Tommaso SK, Khan Z, Weaver AL, Smith CY, Rocca WA, Stewart EA. Cardiovascular and metabolic morbidity after hysterectomy with ovarian conservation: a cohort study. Menopause. 2018;25:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: The process and practice of mindful change. 2nd ed. New York / London: Guilford Press; 2012. [Google Scholar]
- 23.Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:1027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses’ Health Study. Obstet Gynecol. 2013;121:709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laughlin-Tommaso SK, Khan Z, Weaver AL, Schleck CD, Rocca WA, Stewart EA. Cardiovascular risk factors and diseases in women undergoing hysterectomy with ovarian conservation. Menopause. 2016;23:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Table showing case-control analyses for psychiatric conditions before the index date, stratified by index calendar year.
Supplemental Digital Content 2. Table showing overall case-control analyses for a subset of women, excluding 186 matched pairs in which the control underwent hysterectomy before the index date, or in which the case did not undergo hysterectomy on or before the index date.


