Hart characterizes a novel interaction between genetics and environmental stress on experience-dependent neuron remodeling and plasticity in Caenorhabditis elegans, defining an interplay between different forms of adolescent stress and the autism-associated gene neurexin...
Keywords: Caenorhabditis elegans, neurexin, plasticity, stress, neuron, neuroscience
Abstract
Neurexins are neuronal adhesion molecules important for synapse maturation, function, and plasticity. Neurexins have been genetically associated with neurodevelopmental disorders, including autism spectrum disorders (ASDs) and schizophrenia, but can have variable penetrance and phenotypic severity. Heritability studies indicate that a significant percentage of risk for ASD and schizophrenia includes environmental factors, highlighting a poorly understood interplay between genetic and environmental factors. The singular Caenorhabditis elegans ortholog of human neurexins, nrx-1, controls experience-dependent morphologic remodeling of a GABAergic neuron in adult males. Here, I show remodeling of this neuron’s morphology in response to each of three environmental stressors (nutritional, heat, or genotoxic stress) when applied specifically during sexual maturation. Increased outgrowth of axon-like neurites following adolescent stress is the result of an altered morphologic plasticity in adulthood. Despite remodeling being induced by each of the three stressors, only nutritional stress affects downstream behavior and is dependent on neurexin/nrx-1. Heat or genotoxic stress in adolescence does not alter behavior despite inducing GABAergic neuron remodeling, in a neurexin/nrx-1 independent fashion. Starvation-induced remodeling is also dependent on neuroligin/nlg-1, the canonical binding partner for neurexin/nrx-1, and the transcription factors FOXO/daf-16 and HSF1/hsf-1. hsf-1 and daf-16, in addition, each have unique roles in remodeling induced by heat and UV stress. The differential molecular mechanisms underlying GABAergic neuron remodeling in response to different stressors, and the disparate effects of stressors on downstream behavior, are a paradigm for understanding how genetics, environmental exposures, and plasticity may contribute to brain dysfunction in ASDs and schizophrenia.
AUTISM and schizophrenia are neurodevelopmental disorders characterized by social and cognitive phenotypes that can be extremely heterogeneous in makeup and severity. Their complex etiology is a consequence of pathogenic roles of both genetic and environmental risk factors (Hallmayer et al. 2011; Chaste and Leboyer 2012; Sandin et al. 2014; Schmitt et al. 2014; Tick et al. 2016; Cattane et al. 2018; Hertz-Picciotto et al. 2018). Exposure to stress is a specific environmental risk factor for autism and schizophrenia, and may influence the course of these disorders (Schmitt et al. 2014; Bishop-Fitzpatrick et al. 2015; Fuld 2018). Recent work on the genetics of autism spectrum disorders (ASDs) and schizophrenia has implicated genes involved in overlapping pathways to be associated with both disorders, including many neuronal/synaptic genes (Waltereit et al. 2014; Autism Spectrum Disorders Working Group of The Psychiatric Genomics Consortium 2017). However, the potentially important interplay between variants in these genes and environmental exposures, including stress, is still not clearly defined at the cellular and molecular level.
Stress exposure affects the brain in many ways that might explain the connection between stress and neurodevelopmental disorders. For example, changes in neuron morphology (especially of the dendrites and axons), which are known to be induced by stress, can mediate changes in neuron and circuit function by dictating connectivity potential. More than a decade of work has characterized the effect of stress on neuron morphology in the vertebrate brain, specifically with regard to atrophy and/or hypertrophy of excitatory dendrites in the hippocampus, amygdala, and cortex in response to stress (Vyas et al. 2002; Cook and Wellman 2004; McEwen et al. 2016). Recent work has also identified stress-induced dendritic remodeling in GABAergic inhibitory interneurons in the same brain regions, in some cases mirroring the direction of dendrite remodeling of excitatory neurons (Gilabert-Juan et al. 2011, 2013, 2017).
However, much less is known about remodeling of neuronal axons in response to stress, in large part due to the technical difficulty of performing such studies in the vertebrate brain. Stress has been found to alter the presynaptic dynamics of inhibitory neurons, indicating that inhibitory axons can be affected by stress (McKlveen et al. 2016; Czéh et al. 2018). Studies have attempted to connect stress-induced changes in neuron morphology with changes in the functional output of neurons, namely relevant behavioral changes. While stress-induced dendritic hypertrophy of neurons in the rodent hippocampus have been shown to correlate with changes in spatial learning tasks, there is also evidence that behavioral changes following stress can be experimentally separated from the neuron remodeling events (Conrad et al. 1999, 2017; Vyas et al. 2002; Conrad 2006). Thus, a direct connection between stress-induced remodeling and behavior has been difficult to fully define. The majority of vertebrate studies also lack the temporal resolution to analyze the dynamics of neuron morphologic changes following stress, and while the mechanisms controlling excitatory dendrite remodeling in response to stress have been studied (McEwen et al. 2016), those controlling remodeling in inhibitory neurons are still relatively unknown.
Caenorhabditis elegans has been utilized as a powerful and simple model for neuronal response to stress, including various environmental, internal/developmental, and aging stressors (Kagias et al. 2012). We recently described experience-dependent morphologic plasticity in the nematode C. elegans, where the GABAergic DVB neuron undergoes progressive experience-dependent outgrowth of axon-like neurites in adult males. This plasticity alters circuit connectivity to cause changes in male mating and defecation behaviors (Hart and Hobert 2018). We identified the singular C. elegans ortholog of neurexins, nrx-1, to be required in the DVB neuron for this neurite outgrowth. Neurexins are synaptic adhesion molecules with diverse functions in synaptic formation, maturation, maintenance, function, and plasticity (Südhof 2017), and have been genetically associated with both ASDs and schizophrenia (Kirov et al. 2009; Gauthier et al. 2011; Reichelt et al. 2012). Therefore, neurexins are strong candidates for mediating changes in neuron morphology and behavior in response to environmental stress.
This C. elegans model of GABAergic morphologic plasticity provides a platform to investigate the effect of stress on GABAergic axonal morphology, including the temporal dynamics of remodeling. The results presented here demonstrate that stress, specifically during sexual maturation of the male nervous system (fourth larval stage, “adolescence;” Snoek et al. 2014), alters the morphology of the DVB GABAergic neuron, with outgrowth of axon-like neurites becoming more elaborate in early adulthood. Each of three distinct stressors—nutritional (4- or 18-hr fasting/starvation), heat (30 min at 37°), or genotoxic (254 nm light set to 200 × 100 μJ/cm2)—affect GABAergic neuron morphology, resulting in lasting morphologic changes. Despite this, each stressor differentially affects downstream functional output and behavior with disparate dependence on nrx-1, its canonical binding partner neuroligin/nlg-1 (also an ASD-associated gene), and two conserved stress-responsive transcription factors (FOXO/daf-16 and HSF1/hsf-1). These findings demonstrate that different stressors alter axonal morphology and morphologic dynamics in GABAergic neurons with disparate functional effect, and provide an example of how underlying genetic defects (nrx-1 or nlg-1 mutation) can interact with environmental stressors to affect distinct neuronal and behavioral responses. These results provide a platform for understanding the combinatorial effect of genetics and environment on risk for pathogenesis of neuropsychiatric disorders.
Materials and Methods
C. elegans strains
Wild-type strains were C. elegans variety Bristol, strain N2. Worms were grown at 23° on nematode growth media (NGM) plates seeded with bacteria (Escherichia coli OP50) as a food source. All males contained either him-8(e1489) IV or him-5(e1490) V as indicated by strain. Mutant alleles used in this study were as follows: him-8(e1489) IV, him-5(e1490) V, unc-119(ed3) III; nrx-1(wy778[unc-119(+)]) V, nrx-1(gk246237) V, nlg-1(ok259) X, daf-16(mu86) I, hsf-1(sy441) I.
All transgenic strains used in this study are listed in Table 1, ordered by figures. All plasmids were injected at 25 ng µl−1 with co-injection marker myo-3::gfp at 25 ng µl−1 to generate extrachromosomal arrays.
Table 1. Strain table.
| Figure | Strain | Mutant background | Array | DNA on array |
|---|---|---|---|---|
| 1 | OH15098 | him-8(e1489) | otIs525 | lim-6int4::gfp |
| 4 | MPH1 | him-5(e1490) | otIs541 | lim-6int4::wCherry |
| hpmEx1 | lim-6int4::cla-1::gfp; myo-3::gfp | |||
| 5 | OH15099 | him-5(e1490) | otIs541 | see above |
| otIs659 | lim-6int4::gfp::rab-3; ttx-3::gfp | |||
| 6 | OH15111 | unc-119(ed3); nrx-1(wy778[unc-119(+)]); | otIs525 | see above |
| him-8(e1489) | ||||
| 6 | OH15116 | nrx-1(gk246237); him-8(e1489) | otIs525 | see above |
| 8 | OH15112 | unc-119(ed3); nrx-1(wy778[unc-119(+)]); | otIs525 | see above |
| him-8(e1489) | otEx7013 | lim-6int4::birA::nrx-1 | ||
| 8 | MPH2 | unc-119(ed3); nrx-1(wy778[unc-119(+)]); | otIs525 | see above |
| him-8(e1489) | hpmEx2 | ric-19prom6::birA::nrx-1 | ||
| 9 | MPH3 | nlg-1(ok259); him-8(e1489) | otIs525 | see above |
| 9 | OH15114 | unc-119(ed3); nrx-1(wy778[unc-119(+)]); | otIs525 | see above |
| nlg-1(ok259); him-8(e1489) | ||||
| 10 | MPH4 | daf-16(mu86); him-8(e1489) | otIs525 | see above |
| 10 | MPH5 | hsf-1(sy441); him-8(e1489) | otIs525 | see above |
Cloning and constructs
To generate lim-6int4::cla-1::gfp (pMPH21), a 291 bp fragment of the lim-6 fourth intron was amplified with primers (forward: GATTACGCCAAGCTTGCATGCGGATCCTTAGCCAGTTGCATAA; reverse: GAGGCGCGCCAATCCCGGGGATCCCCTGAAAATGTTCTATG) and cloned into PK068 (a gift from Peri Kurshan; Kurshan et al. 2018) to replace the unc-17 promoter, using restriction-free cloning.
To generate ric-19prom6::BirA::nrx-1 (pMPH24), a 147 bp fragment of the ric-19 promoter was PCR amplified from genomic DNA using primers (forward:GAAATGAAATAAAGCTTGCATGCATTAAAGAGTGTGCTCCAC; reverse: CTTTGGGTCCTTTGGCCAATCCCGGGTTCAAAGTGAAGAGCTCTCTCGAC), and cloned into pMH27 (lim-6int4::BirA::nrx-1) to replace the lim-6int4 promoter, using restriction-free cloning.
Stress induction
Male worms were picked at early L4, mid-L4, or following L4 molt (adults), onto plates described below, as indicated in figures. Nonstressed males were placed onto NGM plates with OP50 as a food source at 23°. Starved and fasted males were placed onto NGM plates lacking peptone and any food source at times indicated and for duration indicated (18 or 4 hr), then analyzed or returned to NGM plates with OP50 as a food source. Heat stress was performed by placing males onto NGM plates with OP50 as a food source, setting the plate in a 37° incubator for 30 min, then returning plate to 23°. For genotoxic stress, or ultraviolet (UV) exposure, males were placed on NGM plates with a thin layer of OP50 as a food source to minimize blocking UV rays, which was set uncovered in a Spectrolinker XL-1500 (Spectroline) and irradiated with 254 nm light using the energy input function that varies the exposure time (DeBardeleben et al. 2017), set to 200 × 100 μJ/cm2. In all nonstress and stress conditions, males were subjected to image analysis or behavioral assays at times indicated in figures.
Microscopy
Worms were anesthetized using 5 μl of 100 mM of sodium azide on a pad of 5% agarose on glass slides, and covered with a glass coverslip. Worms were analyzed by fluorescence microscopy, using an upright stand Leica TCS SP8 laser-scanning confocal microscope operated by LAS X software. Confocal laser and photomultiplier tube settings were set at identical levels for image acquisition of all experimental conditions. Confocal z-stacks were reconstructed as maximum intensity projections in FIJI. Figures were prepared using Adobe Photoshop CS6 and Adobe Illustrator CS6.
Neurite tracing
Neurite tracing was performed as previously described (Hart and Hobert 2018). Briefly, confocal z-stacks were opened in FIJI, and analyzed using the Simple Neurite Tracer plugin (Longair et al. 2011). The primary neurite of DVB was traced from the center of the cell soma to where the axon projects ventrally and turns anteriorly into the ventral nerve cord. Neurite branches were added by tracing off the primary neurite, including all neurites emanating posterior of the last branch point. The simple neurite tracer plugin was used to analyze the skeletons for neurite length, which were summed to calculate total neurite length, and the number of neurite junctions (a proxy for the number of neurite branches).
Aldicarb spicule protraction assay
Aldicarb solution was added on the top of NGM agar plates and allowed to dry for 1 hr with a plate lid on. For a concentration of 0.8 mM aldicarb, 80 μl of a 100 mM in 70% ethanol stock solution was added onto 10 ml NGM plates and spread evenly onto the surface (Locke et al. 2008). Ten male worms were placed onto the aldicarb plates and observed for spicule protraction longer than 3 s, when the time was recorded for each worm. Aldicarb assay was performed on two technical replicates and at least three independent replicates. Investigator was blinded to experimental conditions before aldicarb assay was performed.
Quantification of CLA-1::GFP puncta and particle analysis of GFP::RAB-3 synaptic puncta
Confocal z-stacks were opened in FIJI and CLA-1::GFP puncta overlapping with DVB neurites (labeled with mCherry) were counted by scanning through the stack for distinct puncta. Puncta were included for quantification on any DVB neurites, minus the DVB cell body, up to the last branch point before the DVB projection turns anteriorly along ventral nerve cord, the same as the cutoff for neurite tracing. Confocal z-stacks of GFP::RAB-3 micrographs were opened and turned into maximum projections in FIJI. A region of interest was drawn around all of the neurites of DVB, defined the same way as for neurite tracing. Particles, representing synaptic puncta, were defined by manual thresholding of projections (top slider 20, bottom slider 255) and subsequent particle analysis to outline particles. Without applying threshold, these regions were then analyzed using Analyze Particle tool. Particle number, particle area, and mean particle intensity were determined for each particle and used to determine total particle number, mean particle area, and mean particle intensity for each worm.
Statistics and reproducibility
One-way ANOVA with post hoc Tukey’s test tests were performed using RStudio, with P-values shown on each graph. Beeswarm and boxplot graphs were created in RStudio.
Data availability
All data that supports the findings of this study are represented in the article, tables, and figures. All plasmids and strains are available from the corresponding author upon request.
Results
Adolescent stress alters GABAergic neuron morphology by altering morphologic plasticity in adulthood
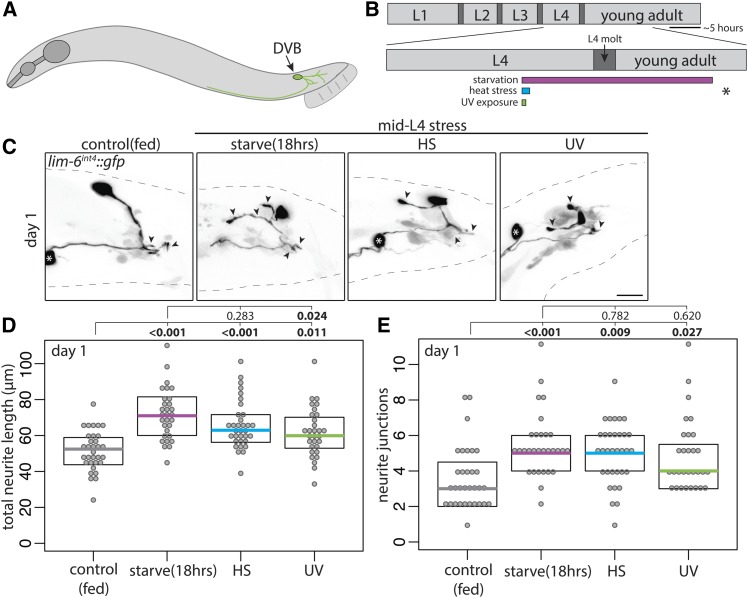
The GABAergic DVB neuron is present in both male and hermaphrodite C. elegans, but undergoes experience-dependent morphologic plasticity only in males (Hart and Hobert 2018). In studying morphologic plasticity of the GABAergic DVB neuron in adult male C. elegans (Figure 1A), I noted that dietary history (fed vs. starved) appeared to affect DVB neurite outgrowth. To confirm this observation, I compared DVB neurite outgrowth between well-fed males and males cultivated without food for 18 hr beginning in the mid-L4 stage (Figure 1B). DVB in males “starved” for 18 hr had increased total neurite length and neurite junctions (a proxy for the number of neurite branches) compared to fed males (Figure 1, C–E). To determine if this effect was starvation-specific or a more general neuronal response to stress, fed males were subjected to heat or genotoxic stress (UV light exposure) at mid-L4 (Figure 1B). At 18 hr after stress induction, both heat- and UV-stressed males had increases in DVB neurite outgrowth compared to unstressed animals (Figure 1, C–E). Thus, three different stressors applied during the mid-L4 stage affected DVB neuron morphology in a similar manner.
Figure 1.
Multiple stressors during adolescence induce neurite outgrowth of the DVB neuron in adult male C. elegans. (A) Illustration of male C. elegans with DVB neuron and neurites depicted. (B) Timeline of postembryonic development of male C. elegans with inset of L4 to adult period. Boxes below timeline indicate timing of different stressors labeled for each panel; gray indicates control/fed, magenta indicates starvation/fasting, teal indicates heat stress, and green indicates UV exposure. Asterisk indicates time of confocal imaging and DVB neurite quantification. (C) Confocal z-stack maximum projections of DVB neuron in control and mid-L4 stressed males at day 1 of adulthood (arrowheads indicate DVB neurites, white asterisk indicates soma of PVT neuron). Bar, 10 μm. Quantification of (D) total neurite length and (E) neurite junctions for DVB in control and mid-L4 stressed males at day 1. Each dot represents one worm; colored bar indicates the median, boxes indicate quartiles. Comparison using one-way ANOVA and post hoc Tukey’s test, P values shown above plots with bold showing significance (P < 0.05). True for all subsequent figures.
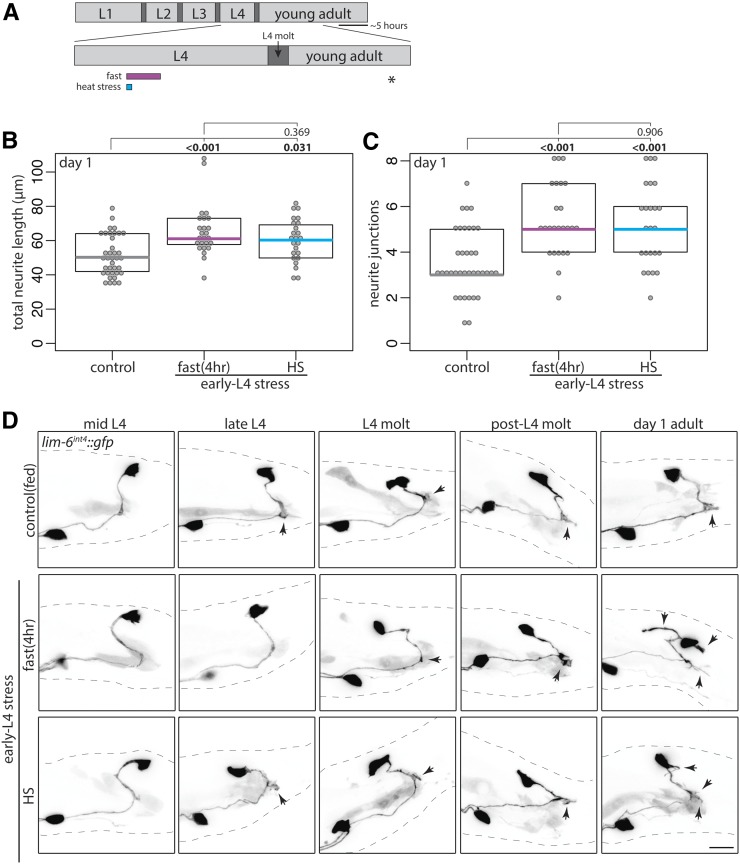
The L4 stage, which is analogous to vertebrate adolescence, is when sexual maturation of the nervous system and other sexual tissues occurs, followed by the L4 molt, a period of behavioral quiescence before adulthood. Previous work demonstrates that transient fasting of a few hours has similar effect to 18–20 hr of starvation on male muscle and neuron excitability (LeBoeuf et al. 2011). To test the effect of timing during L4/L4 molt and the duration of starvation stress on DVB neuron neurite outgrowth, males were stressed early in the L4 stage (Figure 2A). Early L4 heat stress or 4-hr fast each resulted in increases in DVB neurite outgrowth in day 1 adult males (Figure 2, B–D, last panel). UV exposure at early L4 resulted in the majority of males arresting before or during the L4 molt, precluding analysis of this stressor at the early L4 time point.
Figure 2.
Adolescent stress alters DVB neurite outgrowth dynamics in adulthood. (A) Diagram of L4 to adult period with boxes depicting stressors applied in early L4. Quantification of (B) total neurite length and (C) neurite junctions for DVB in control and early L4 stressed males at day 1 of adulthood corresponding confocal maximum projections are rightmost column panels of D. (D) Confocal z-stack maximum projections of DVB neuron in control and early L4 stressed males at different times throughout the L4 stage into early adulthood (ten males observed for each time point and condition). Bar, 10 μm. Arrowheads indicate single neurites before, during, and after L4 molt.
Two explanations could account for these stress-induced morphologic changes in DVB neurite outgrowth. One possibility is that neurite outgrowth begins earlier in response to stress, such that by the first day of adulthood males have more DVB neurites. Alternatively, DVB neurite outgrowth may start at the same time as in unstressed males, but occur with different dynamics, resulting in adult males with increased neurites. To distinguish between these possibilities, I monitored DVB morphology in unstressed and in early L4 stressed males at different time points from mid L4 into day 1 of adulthood (Figure 2, A and D). Strikingly, despite stress being applied during early L4, DVB morphology was not different between nonstressed and stressed males until after the L4 molt and entry into adulthood (Figure 2D). At late-L4 and L4 molt, males had either no neurites or a single short neurite, regardless of stress exposure. Only after L4 molt did differences in neurite outgrowth appear (Figure 2D), with stressed males having increased DVB neurites. This result demonstrates that adolescent stress does not change the timing of DVB neurite outgrowth, but rather changes the degree of neurite outgrowth occurring in adult males. Therefore, adolescent stress exposure changes the dynamics of DVB morphologic plasticity in adulthood, and demonstrate that even a 4-hr fast during adolescence can alter adult GABAergic neuron morphology.
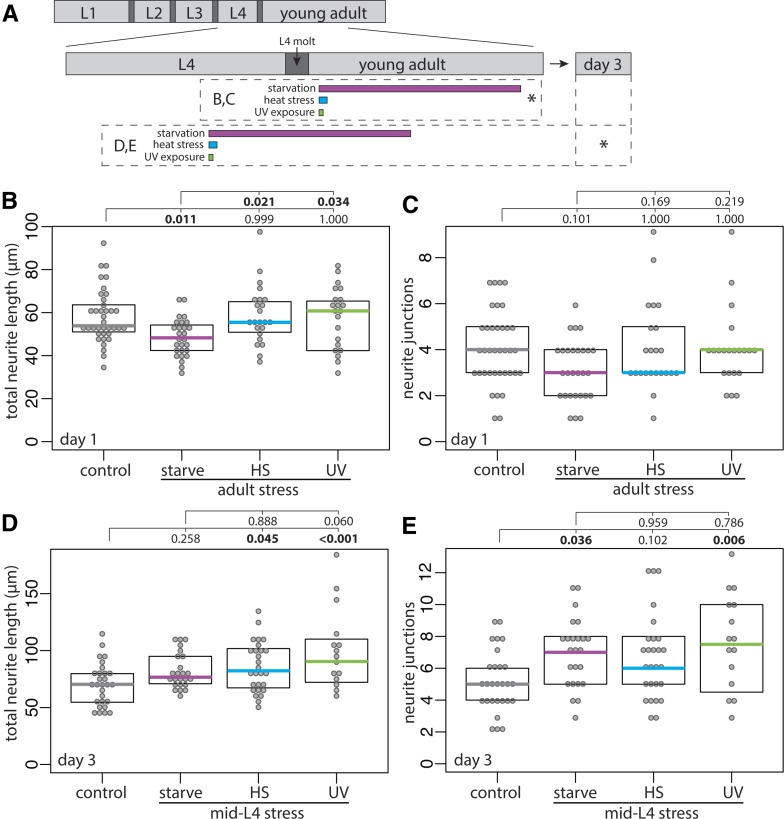
To test if stress-induced DVB remodeling were specific to the adolescent stage, I exposed males to stress shortly after entry into adulthood (Figure 3A). Surprisingly, stress in early adulthood did not increase DVB neurite outgrowth (Figure 3, A–C). In contrast to the effects of starvation during adolescence, 18-hr starvation during adulthood resulted in a small, but significant, decrease in DVB total neurite length (Figure 3, A–C). These results suggest that the increase in DVB neurite outgrowth in response to stress is specific to the L4 adolescent stage. Are stress-induced DVB morphologic changes long-lasting or a temporary and reversible stress response? DVB morphology was analyzed at day 3 of adulthood following mid-L4 stress (Figure 3A). Day 3 of adulthood corresponds to the end of peak male C. elegans reproductive capability (Guo et al. 2012). Remarkably, all three stressors resulted in increases in at least one parameter of DVB neurite outgrowth >60 hr after stress (Figure 3, D and E). In UV-exposed males both neurite length and junctions were increased, while in heat-stressed males only neurite length was increased, and in starved males only neurite junctions were increased (Figure 3, D and E). These results demonstrate that although all stressors increase neurite outgrowth at day 1, different stresses have unique long-term effects on DVB morphology (Figure 3, D and E). Thus, adult DVB morphology is altered by adolescent stress as a consequence of enhanced morphologic plasticity in adulthood, producing long-lasting changes to GABAergic neuron morphology.
Figure 3.
Early adulthood stress does not alter DVB morphology, while adolescent stress results in sustained changes to DVB morphology. (A) Diagram of L4 to adult period, including inset indicating day 3 of adulthood. Boxes below timeline indicate timing of stressors, asterisk indicates time of confocal imaging and quantification. Quantification of (B) total neurite length and (C) neurite junctions for DVB in control and post-L4 molt stressed males at day 1. Quantification of (D) total neurite length and (E) neurite junctions for DVB in control and mid-L4 stressed males at day 3.
Adolescent stressors differentially affect DVB function in male spicule protraction behavior and DVB presynaptic number and morphology
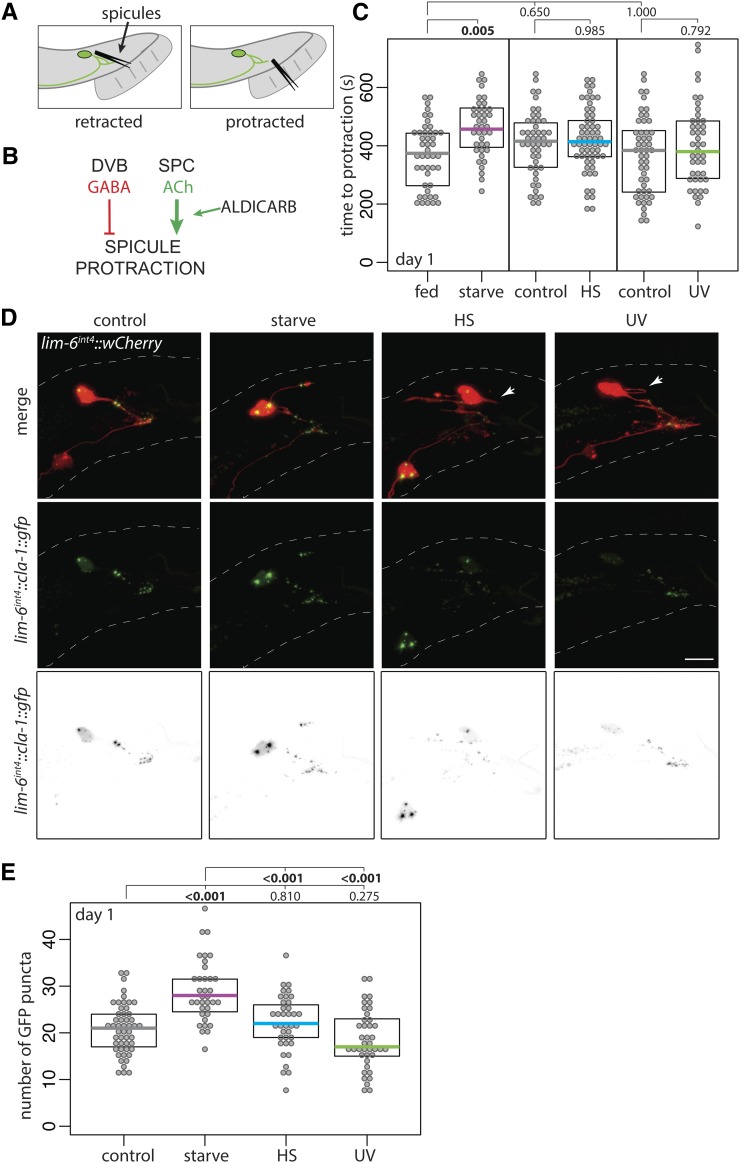
The functional effect of DVB morphologic plasticity in adulthood was previously characterized on a number of behaviors, including male spicule protraction behavior (Hart and Hobert 2018). The spicules (Figure 4A) protract out of the male tail to penetrate the hermaphrodite vulva during copulation, and are therefore required for successful sperm transfer (Garcia et al. 2001). Males exposed to aldicarb, an acetylcholinesterase inhibitor, protract their spicules due to acetylcholine buildup at the neuromuscular synapses between the cholinergic SPC neuron and the spicule protraction muscles (Figure 4B) (Garcia et al. 2001). The GABAergic DVB neuron synapses onto the SPC neuron as well as onto the spicule protractor muscles, to inhibit spicule protraction (Figure 4B). Therefore, DVB neurite elaboration, which adds synapses specifically onto these targets (Hart and Hobert 2018), results in increased SPC and spicule protractor inhibition, and a delay in the time to aldicarb-induced spicule protraction. This increased time to spicule protraction serves as a readout for the amount of DVB GABAergic input (Hart and Hobert 2018).
Figure 4.
Adolescent stress differentially affects adult spicule protraction behavior and number of DVB presynaptic active zone sites. (A) Cartoon of C. elegans male tail with DVB and spicules depicted in retracted and protracted positions. (B) Logic diagram of the male C. elegans spicule protraction circuit involving DVB GABAergic and SPC cholinergic signaling, showing location of action of the acetylcholinesterase inhibitor aldicarb. (C) Time to protraction of spicules in control and mid-L4 stressed males on aldicarb plates at day 1 of adulthood. (D) Confocal z-stack maximum projections of DVB neuron and DVB presynaptic puncta (lim-6int4::cla-1::gfp) in control and mid-L4 stressed males at day 1 of adulthood (arrowheads indicate DVB neurites with no GFP signal). Bar, 10 μm. (E) Number of GFP puncta in DVB neurites in control and mid-L4 stressed males at day 1.
To test if the outgrowth of DVB neurites induced by adolescent stress affects the function of DVB and the downstream spicule protraction behavior, unstressed and mid-L4 stressed males (from this point, “stress” exposure is at mid-L4, with scoring done on day 1 of adulthood) were exposed to aldicarb immediately following starvation stress (Figure 4C). Starved males had increased time to spicule protraction on aldicarb compared to fed males (Figure 4C), suggesting that the elaborated DVB neurites result in functionally enhanced GABAergic tone. In contrast, males exposed to either heat stress or UV had no change in time to spicule protraction in comparison to unstressed males (Figure 4C). These results suggest that although all of the stressors result in increased outgrowth of DVB neurites, the functional effect of these neurites is different between stressors.
The above data demonstrate that elaborated DVB neurites induced by stress may not have the same effect on circuit connectivity, potentially explaining the disparate behavioral phenotypes. To explore this possibility, I compared DVB presynaptic puncta in unstressed and stressed males. GFP-tagged Clarinet1 (CLA-1), a piccolo/bassoon-like protein involved in synaptic vesicle release, localizes to presynaptic active zones (Xuan et al. 2017; Kurshan et al. 2018). GFP-tagged RAB-3, a presynaptic GTPase that marks vesicle clusters, shows overlapping localization with CLA-1 in the synaptic bouton (Kurshan et al. 2018). DVB-specific expression of CLA-1::GFP localized to bright distinct puncta on DVB processes, allowing visualization and quantification of presynaptic sites by counting puncta (Figure 4D). Males exposed to starvation stress had more CLA-1::GFP puncta on DVB neurites compared with nonstressed controls and heat- or UV-stressed males (Figure 4, D and E). Localization of puncta within neurites did not appear different between conditions, except with some heat- and UV-stressed males having neurites without any discernible GFP signal, which never occurred in nonstressed or starvation-stressed males (Figure 4D).
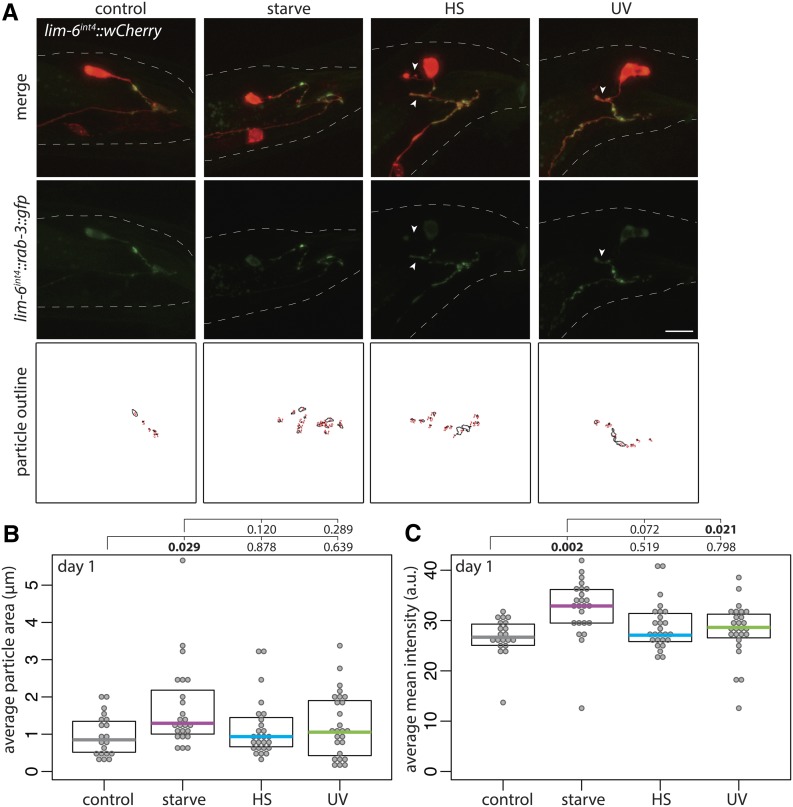
GFP:RAB-3 expression in DVB was more diffuse, as expected, with fewer distinct puncta. Overall, the distribution mimicked that of CLA-1::GFP, with starved males having brighter puncta on all DVB neurites, compared with dimmer and more diffuse puncta in nonstressed males. This could indicate more mature synaptic structures following starvation that correspond to the increased number of CLA-1::GFP puncta (Figure 5A). Heat- or UV-stressed males had dimmer and more diffuse puncta, with some neurites lacking GFP signal (arrowheads, Figure 5A). To quantify the potential differences, I outlined “puncta” by applying consistent thresholding on confocal z-stack maximum projections using the particle analysis feature in FIJI. I drew a region of interest around DVB neurites, and analyzed particles (puncta). While there was no difference in the number of puncta between unstressed and any of the stressed males (data not shown), the average area of puncta in starved males was significantly increased compared to controls, while heat-stressed or UV-exposed males showed no difference from controls (Figure 5B). Additionally, the mean intensity of puncta was also increased in starved males compared to controls, but not males exposed to heat or UV (Figure 5C). Considering the results of both presynaptic markers, starved males have more presynaptic active zones with larger and brighter vesicle clusters than controls, a difference not observed in males exposed to heat or UV stress. These differences in presynaptic morphology may begin to explain the functional differences observed in spicule protraction behavior between the stressors.
Figure 5.
Adolescent stress differentially affects DVB presynaptic vesicle cluster morphology. (A) Confocal z-stack maximum projections of DVB neuron and DVB presynaptic puncta (lim-6int4::gfp::rab-3) in fed control and mid-L4 stressed males at day 1 of adulthood (arrowheads indicate DVB neurites with little/no gfp or diffuse gfp). Bar, 10 μm. Identically thresholded z-stack maximum projections of the gfp::rab-3 channel created particle outlines of synaptic puncta on DVB neurites for analysis of intensity and other measures. Quantification of lim-6int4::gfp::rab-3 (B) average particle area and (C) average mean intensity in control and mid-L4 stressed males at day 1.
The C. elegans neurexin ortholog nrx-1 is required for starvation-induced DVB neurite outgrowth
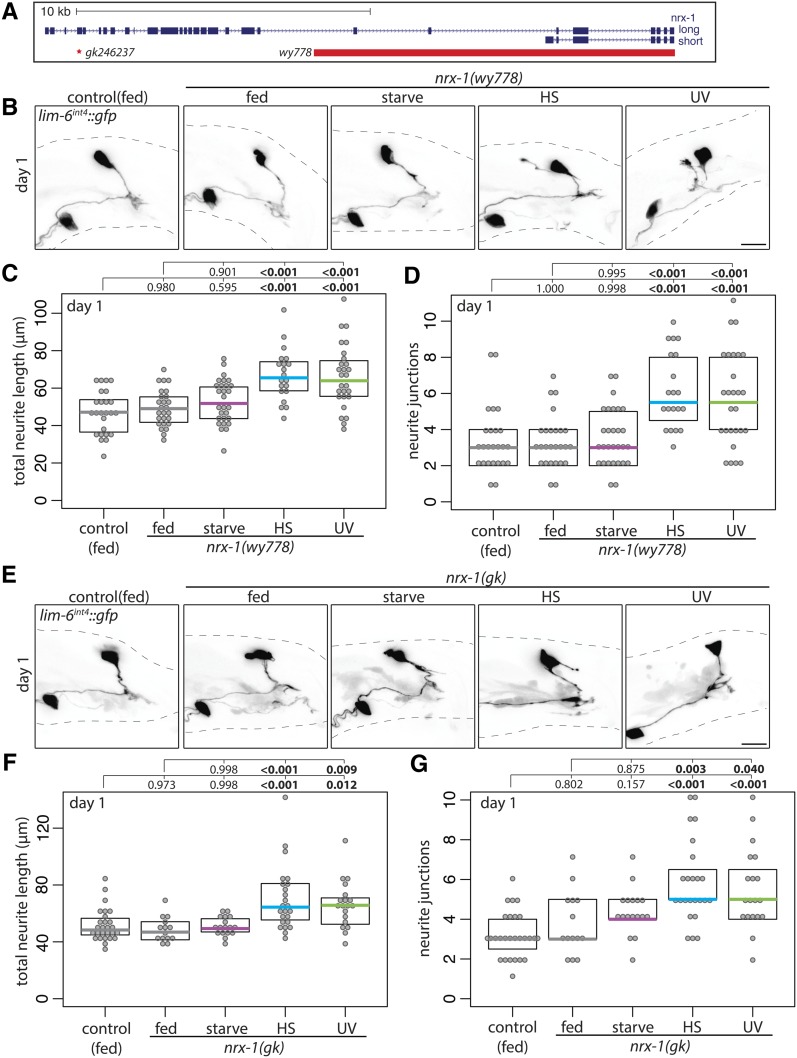
DVB neurite outgrowth in early adulthood is dependent on the ortholog of the synaptic adhesion molecule neurexin, nrx-1 (Hart and Hobert 2018). To determine if stress-induced DVB neurite outgrowth is also dependent on nrx-1, I analyzed neurite outgrowth in nrx-1 mutant males. I used two nrx-1 alleles that strongly reduce gene function but differ in their effects. Allele wy778 harbors a large deletion that removes the entire short isoform as well as the transmembrane and intracellular portion of the long isoform (which was recently described to have phenotypes indistinguishable from those of worms with a deletion covering the entire nrx-1 locus; Kurshan et al. 2018). Allele gk246237 contains a point mutation resulting in a premature stop codon affecting only the long isoform (Figure 6A). As previously observed, loss of nrx-1 did not affect DVB neurite outgrowth under fed conditions at day 1 of adulthood (Figure 6, B–G) (Hart and Hobert 2018). However, the increase in DVB neurite outgrowth observed in wild-type males following starvation did not occur in males with either nrx-1 loss-of-function allele; DVB neurites were similar in starved nrx-1 mutant males and fed wild-type males (Figure 6, B–G). Remarkably, following either heat or UV stress, animals harboring either nrx-1 loss-of-function allele showed increases in DVB neurite outgrowth compared to wild-type nonstressed males, similar to levels of outgrowth seen in heat-stressed or UV-exposed wild-type animals (Figure 6, B–G). These results imply that while DVB neurite outgrowth and morphologic plasticity in response to starvation is dependent on nrx-1, heat- and UV-induced DVB neurite outgrowth is not. Despite all three stressors resulting in increased DVB neurite outgrowth, a role for nrx-1 is uniquely required for DVB response to starvation.
Figure 6.
Neurexin/nrx-1 is required for DVB neurite outgrowth following adolescent starvation stress. (A) Genomic locus of nrx-1 shown 5′ to 3′ with long and short isoforms included; red bar indicates region deleted in nrx-1(wy778) and red asterisk indicates point mutation of early stop in nrx-1(gk246237), referred to in figures as nrx-1(gk). (B) Confocal z-stack maximum projections of DVB neuron in fed control and fed and mid-L4 stressed nrx-1(wy778) males at day 1 of adulthood. Bar, 10 μm. Quantification of (C) total neurite length and (D) neurite junctions for DVB in fed control and fed and mid-L4 stressed nrx-1(wy778) males at day 1. (E) Confocal z-stack maximum projections of DVB neuron in fed control and fed and mid-L4 stressed nrx-1(gk246237) males at day 1 of adulthood. Bar, 10 μm. Quantification of (F) total neurite length and (G) neurite junctions for DVB in fed control and fed and mid-L4 stressed nrx-1(gk246237) males at day 1.
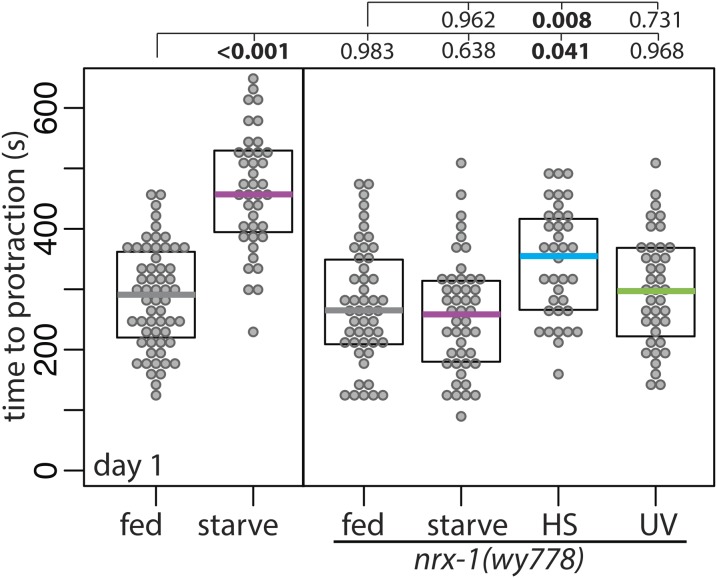
To test if nrx-1 also has an effect on the functional output of DVB neurite outgrowth following adolescent stress, I exposed males with nrx-1 deletion to stress and then performed the aldicarb spicule protraction assay. Nonstressed nrx-1 mutants were not different from nonstressed wild-type animals in time to protraction on aldicarb (Figure 7). Starved wild-type animals were slower to protract spicules on aldicarb than fed wild-type animals; however, there was no difference in time to spicule protraction between fed and starved nrx-1 mutants (Figure 7), thus implicating nrx-1 in both the underlying mechanism responsible for DVB neurite outgrowth and the downstream behavioral response to starvation. nrx-1 males exposed to UV stress had no difference in the time to protraction compared to nonstressed nrx-1 males (Figure 7), similar to UV stress not affecting time to protraction in wild-type animals. Unexpectedly, nrx-1 males exposed to heat stress had an increase in the time to protraction on aldicarb compared to nonstressed nrx-1 males (Figure 7), a difference not observed in wild-type males exposed to heat stress (Figure 4C). This suggests that even though nrx-1 plays no role in heat stress-induced DVB morphologic changes, it may play a role in modifying the functional output of DVB or the spicule protraction circuit in response to heat stress, although how and where this occurs will require further investigation. Together, these results show that both the morphologic and functional plasticity of DVB in response to starvation are dependent on nrx-1, and demonstrate that different types of stress can have unique behavioral outcomes and depend on different underlying molecular mechanisms.
Figure 7.
Neurexin/nrx-1 is required for adolescent starvation-induced changes in adult spicule protraction behavior. Time to protraction of spicules in fed control, starved control (repeated data from Figure 3C, for comparison), and mid-L4 control and stressed nrx-1(wy778) males on aldicarb plates at day 1 of adulthood.
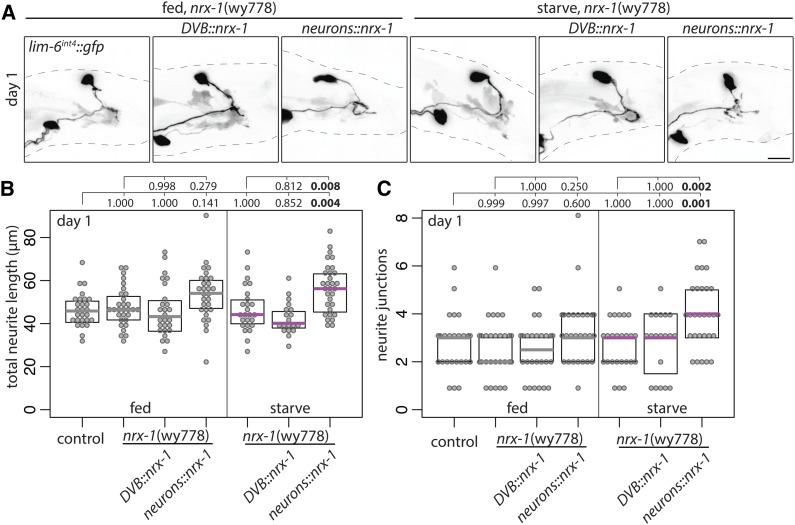
I previously found that neurexin controls experience-dependent DVB neurite outgrowth in adult males in a cell-autonomous manner (Hart and Hobert 2018). Surprisingly, NRX-1 expression in DVB was unable to restore starvation-induced neurite outgrowth in nrx-1 mutants following starvation (also observed for a GFP-tagged NRX-1, data not shown) (Figure 8, A–C). NRX-1 is expressed in all neurons and some muscles in C. elegans and recent work has described cell-nonautonomous functions of NRX-1 (Tong et al. 2017; Philbrook et al. 2018). NRX-1 expression in all neurons using a pan-neuronal promoter (ric-19p; Stefanakis et al. 2015) did not significantly alter DVB neurite outgrowth in nonstressed males, but restored starvation-induced neurite outgrowth in nrx-1 mutants (Figure 8, A–C). Thus, NRX-1 expression in DVB alone cannot restore starvation-induced neurite outgrowth, while NRX-1 expression in additional neurons is sufficient to promote neurite outgrowth following starvation.
Figure 8.
Neurexin/nrx-1 expression in all neurons, but not in DVB alone, rescues starvation-induced DVB neurites in nrx-1 mutants. (A) Confocal z-stack maximum projections of DVB neuron in fed and mid-L4 starved nrx-1(wy778) males with control, DVB::nrx-1 (lim-6int4::birA::nrx-1), or neurons::nrx-1 (ric-19prom6::birA::nrx-1) transgenes at day 1 of adulthood. Bar, 10 μm) Quantification of (B) total neurite length and (C) neurite junctions for DVB in fed control and fed and mid-L4 starved nrx-1(wy778) males with control, DVB::nrx-1 (lim-6int4::birA::nrx-1), or neurons::nrx-1 (ric-19prom6::birA::nrx-1) transgenes at day 1 of adulthood.
Neuroligin/nlg-1 promotes starvation-induced DVB neurite outgrowth
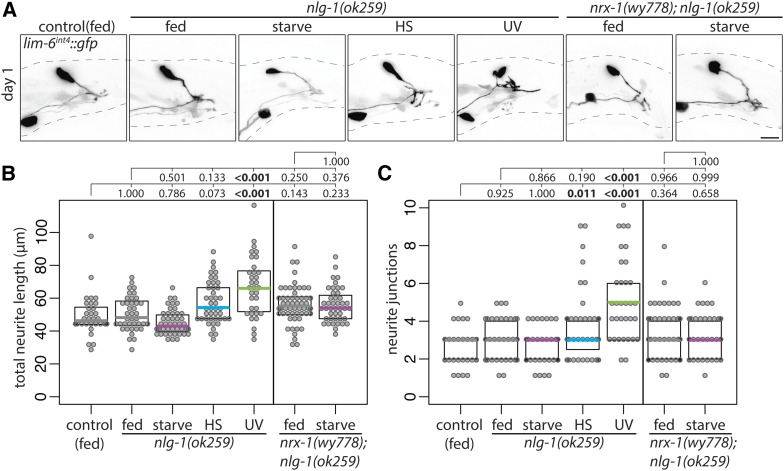
Neuroligins are synaptic adhesion molecules that interact with neurexins at synapses, commonly serving as the postsynaptic ligands for neurexins (Maro et al. 2015; Tong et al. 2015; Tu et al. 2015). DVB experience-dependent neurite outgrowth in adult males requires NRX-1 in DVB to promote neurite outgrowth, while the C. elegans singular ortholog of neuroligins, NLG-1, restricts DVB neurite outgrowth from postsynaptic targets (Hart and Hobert 2018). Does NLG-1 play a role in adolescent stress-induced DVB neurite outgrowth? DVB neurite outgrowth was not increased in nlg-1 mutant males following starvation or heat stress, but did increase after UV exposure (Figure 9, A–C), suggesting that NLG-1 is required for DVB neurite outgrowth induced by starvation and heat stress. In males mutant for both nrx-1 and nlg-1, starvation did not induce DVB neurite outgrowth (Figure 9, A–C). The similar double and single mutant phenotypes suggest that, in contrast to their later antagonistic roles (Hart and Hobert 2018), NRX-1 and NLG-1 function together to promote adolescent starvation-induced DVB neurite outgrowth.
Figure 9.
Neuroligin/nlg-1 is required for DVB neurite outgrowth following adolescent starvation and heat stress. (A) Confocal z-stack maximum projections of DVB neuron in fed control, fed and mid-L4 stressed nlg-1(ok259) males, and fed and mid-L4 starved nrx-1(wy778); nlg-1(ok259) males at day 1 of adulthood. Bar, 10 μm. Quantification of (B) total neurite length and (C) neurite junctions for DVB in fed control, fed and mid-L4 stressed nlg-1(ok259) males, and fed and mid-L4 starved nrx-1(wy778); nlg-1(ok259) males at day 1 of adulthood.
Stress-responsive transcription factors daf-16 and hsf-1 are required for starvation-induced DVB neurite outgrowth
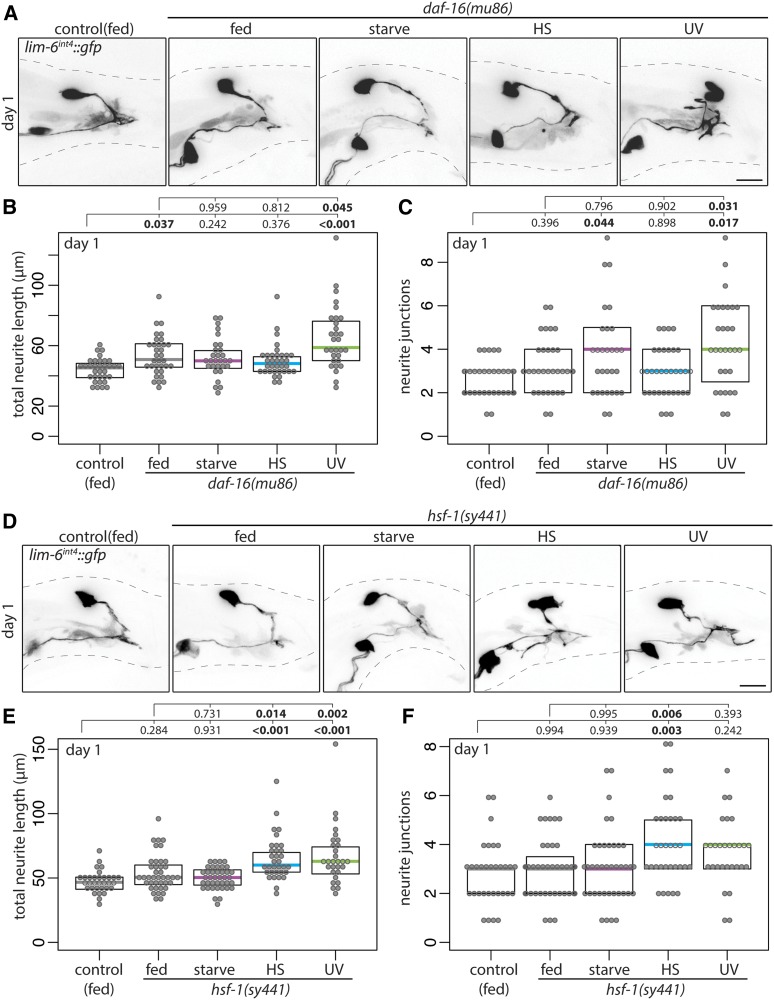
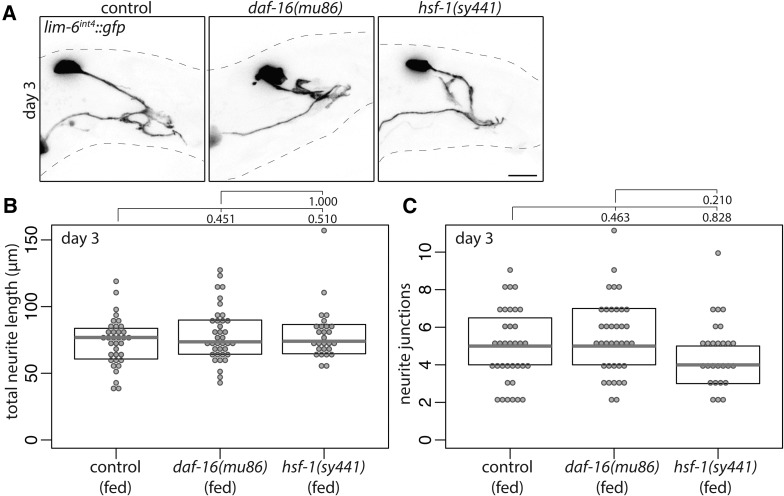
How is adolescent stress signaled to the DVB neuron to induce neurite outgrowth at day 1 of adulthood? In C. elegans, stress resistance, stress response, and the interplay between longevity and stress are signaled through two conserved transcription factors, FOXO/daf-16 and HSF1/hsf-1, both with roles in conserved insulin signaling pathways (Hsu et al. 2003; Baumeister et al. 2006). To test if these factors play a role in signaling stress to DVB, males mutant for daf-16 or hsf-1 were exposed to stress. Starvation stress partially induced DVB neurite outgrowth in daf-16 mutant males, but did not in hsf-1 mutant males (Figure 10, A–F). Conversely, heat stress did not induce DVB neurite outgrowth in daf-16 mutant males, but did in hsf-1 mutant males (Figure 10, A–F). Finally, DVB neurite outgrowth was induced by UV exposure in daf-16 mutant males, but not in hsf-1 mutant males (Figure 10, A–F). Thus, daf-16 and hsf-1 are differentially involved in stress signaling and DVB neurite outgrowth, further highlighting the complexity of molecular mechanisms underlying neuronal remodeling in response to different stressors. The role of daf-16 and hsf-1 in DVB neurite outgrowth are unique to stress response, as in the absence of stress, neither affected experience-dependent DVB neurite outgrowth analyzed at day 3 of adulthood (Figure 11, A–C).
Figure 10.
Stress-responsive transcription factors daf-16 and hsf-1 are required for DVB neurite outgrowth following adolescent starvation. (A) Confocal z-stack maximum projections of DVB neuron in fed control and fed and mid-L4 stressed daf-16(mu86) males at day 1 of adulthood. Bar, 10 μm. Quantification of (B) total neurite length and (C) neurite junctions for DVB in fed control and fed and mid-L4 stressed daf-16(mu86) males at day 1. (D) Confocal z-stack maximum projections of DVB neuron in fed control and fed and mid-L4 stressed hsf-1(sy441) males at day 1 of adulthood. Bar, 10 μm. Quantification of (E) total neurite length and (F) neurite junctions for DVB in fed and fed and mid-L4 stressed hsf-1(sy441) males at day 1.
Figure 11.
Stress-responsive transcription factors daf-16 and hsf-1 are not required for experience-dependent DVB neurite outgrowth in adulthood. (A) Confocal z-stack maximum projections of DVB neuron in fed control, fed daf-16(mu86), and fed hsf-1(sy441) males at day 3 of adulthood. Bar, 10 μm. Quantification of (B) total neurite length and (C) neurite junctions for DVB in fed control, fed daf-16(mu86), and fed hsf-1(sy441) males at day 3.
Discussion
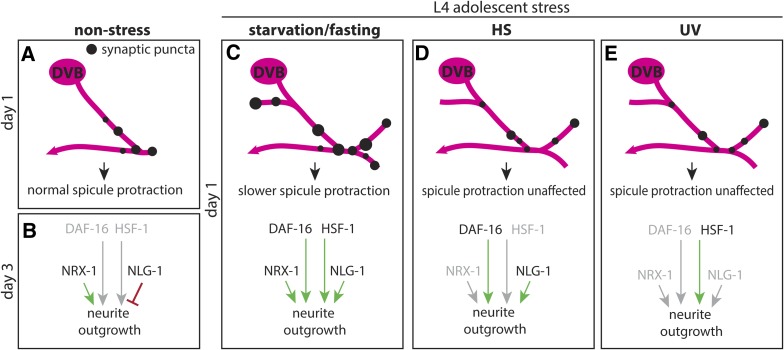
Neurons can respond to stress by altering the morphology of their dendritic trees, altering their connectivity with other neurons within circuits, and thus contributing to alterations in behavioral output (McEwen et al. 2016). Utilizing a recently-discovered form of experience-dependent morphologic plasticity in adult male C. elegans, I report that stress induces morphologic remodeling of axon-like neurites of a GABAergic neuron and directly affects behavior. In this study, I find that three stressors during adolescence result in altered morphologic plasticity of a GABAergic neuron upon entry into adulthood. Remarkably, although each stressor increased neurite outgrowth, their effects on a specific behavior (i.e., spicule protraction) were different, which was further reflected in slight differences between stressors on long-term morphologic changes. Interestingly, it is only stressors with a functional effect on behavior that were found to be dependent on nrx-1 (Figure 12); only nutritional stress resulted in both morphologic and functional changes dependent on neurexin. In contrast, genotoxic stress resulted in morphologic changes without any effect on behavior and was not dependent on nrx-1 (Figure 12), while heat stress also resulted in morphologic changes not dependent on nrx-1 and without any effect on behavior. Further, when analyzing the role of neuroligin/nlg-1 and stress-responsive transcription factors FOXO/daf-16 and HSF1/hsf-1, it appears that in response to each stressor, a unique combination of molecular mechanisms controls remodeling (Figure 12). Together, these findings provide evidence that neuronal morphologic changes in response to stress are dynamic, and that different types of stress can have different functional effects and underlying molecular mechanisms. Furthermore, neuronal stress response at the functional and behavioral level is more complex than a readout of morphologic changes, even in a simple nervous system.
Figure 12.
Model of DVB morphologic, synaptic, and behavioral response to adolescent stressors with differential underlying molecular mechanisms. (A) DVB in nonstressed males at day 1 has few neurites and few presynaptic sites. (B) After day 1, neurites will grow out in response to experience/activity, increasing the number of presynaptic puncta; outgrowth is dependent on NRX-1 in DVB, antagonized by NLG-1 in postsynaptic tissues. (C) Adolescent starvation increases DVB neurite outgrowth at day 1 of adulthood with corresponding increases in presynaptic sites and slower spicule protraction. Starvation-induced neurites are dependent on NRX-1 (not in a DVB-autonomous manner) and NLG-1, as well as the transcription factors DAF-16 and HSF-1. (D) Adolescent exposure to heat stress increases DVB neurite outgrowth at day 1, dependent on NLG-1 and partly on DAF-16, but not NRX-1. This outgrowth does not increase presynaptic sites or alter spicule protraction behavior. (E) Adolescent exposure to UV increases DVB neurite outgrowth at day 1 without increasing presynaptic sites or altering spicule protraction behavior. UV-induced DVB neurite outgrowth is not dependent on NRX-1, NLG-1, but is partly dependent on HSF-1.
Further work will be required to identify the specific neurons/tissues in which this network of genes is required to signal stress and induce DVB neurite outgrowth. Given the cell-autonomous function of NRX-1 in DVB neurite outgrowth in adulthood (Hart and Hobert 2018), NRX-1 may be required in both DVB and other neurons. However, NRX-1 function in starvation-induced DVB neurite outgrowth may be completely cell-nonautonomous. NRX-1 and NLG-1 may be required in neurons upstream of DVB to signal starvation stress, contribute to normal synaptic transmission required for activity-dependent signaling/outgrowth, or a combination of both. It is also possible that NRX-1/NLG-1 have cell-nonautonomous functions, although previous examples of cell-nonautonomous functions of NRX-1 were found to be independent of NLG-1 (Philbrook et al. 2018). The requirement for NRX-1 and NLG-1 in promoting DVB neurite outgrowth is in striking contrast to their antagonistic relationship during later adulthood (Hart and Hobert 2018). Perhaps at day 1, NRX-1 and NLG-1 provide adhesive connection required for early outgrowth and/or synapse stability, emphasized by stress, which later becomes antagonistic to balance outgrowth and synaptic strength. Stress signaling by daf-16 and hsf-1 involves many genes in many tissues, and downstream gene expression for each factor is extremely complex, including distinct and overlapping target genes regulated in a tissue-specific manner (Hsu et al. 2003; Baumeister et al. 2006; Volovik et al. 2014). One intriguing possibility is that nrx-1/nlg-1 are regulated by daf-16 and hsf-1 in neurons in response to stress, and there is some limited evidence insulin signaling pathways regulate nrx-1 and nlg-1 under certain circumstances (Shen et al. 2007; Staab et al. 2014). Future work will include testing the mechanisms of this stress signaling, the location of action of each gene, and the interplay between these genes to regulate DVB stress-induced remodeling.
It was recently reported that past life experience, specifically starvation stress, affects the development of male-specific nervous system wiring in C. elegans through monoamine signaling (Bayer and Hobert 2018). Starvation during development before (L1–L3), but not during adolescence (L4), blocks synaptic pruning events that are required for male-specific circuit wiring and behavior (Oren-Suissa et al. 2016; Bayer and Hobert 2018). Preadolescent starvation stress produced a “memory” of starvation in the form of sex-specific connections not being pruned. Interestingly, the results reported here demonstrate that starvation stress during adolescence produces changes in male-specific connectivity through a different mechanism, as a result of altering GABAergic morphologic plasticity. In combination, these results show that early life and adolescent stress both influence the connectivity and behavioral output of sexually dimorphic circuits;, the former by affecting the development of sex-specific neuronal connectivity, and the latter by altering the dynamics of an adult form of morphologic plasticity.
Early life and adolescence are important periods in brain development and maturation marked by reorganization of neurons and circuits in the brain, thereby making these periods sensitive to stress disruption. In adolescence, stress has been shown to affect dendritic morphology of neurons in a number of brain regions, in some cases having a unique effect on morphology and behavior compared to the same stress during adulthood (Tsai et al. 2014; Tzanoulinou et al. 2014), with stresses during critical periods in brain development linked to neuropsychiatric disorders. A two-hit model has been proposed where stress inflicted on a genetically predisposed individual during critical brain development windows (early brain development and adolescent maturation) plays a role in the emergence of symptoms. Interestingly, there is evidence that neuron morphology and relevant behavioral phenotypes are affected differently in animals stressed during both early life and adolescent critical periods of brain development compared to stress during one critical period or only in adulthood (Eiland and McEwen 2012). The results reported here demonstrate that even in the nematode C. elegans, adolescence and sexual maturation of the nervous system are a period of particular sensitivity to stress.
It has been proposed that to develop models of severe psychiatric disorders, studies should combine genetic variants in susceptibility genes with several stressors during critical periods (Schmitt et al. 2014). Recent work has identified many genetic risk factors for autism and schizophrenia that converge on shared pathways, some of which could potentially enhance sensitivity of the nervous system to stress. Neurexin and neuroligin have been associated genetically with ASDs and schizophrenia, but their relationships to neuronal stress response have not been studied. The results reported here indicate that neurexin/neuroligin play a role in neuronal stress response. Additional adhesion molecules have roles in stress-induced neuronal remodeling, suggesting that cellular adhesion molecules and partners may be important in this process (Gilabert-Juan et al. 2011; McCall et al. 2013; Yang et al. 2019). The pathogenic role of NRXN1 in ASDs and schizophrenia has been complicated by the presence of deletions/mutations in unaffected parents and siblings, suggesting variable penetrance or involvement in these disorders (Todarello et al. 2014; Lowther et al. 2017; Woodbury-Smith et al. 2017). The results reported here in C. elegans describe the effect of alteration in a single gene on the morphologic and behavioral consequences of different stress exposures on a single neuron, and suggest that some phenotypic heterogeneity may potentially be explained by the requirement for a genetic risk to be uncovered by a specifically timed stress. In terms of neuropsychiatric disease, perhaps multiple genetic risk factors, in combination with different environmental exposures, act together to affect the complex human brain, but with varying phenotypic consequences depending on the specific genetic/environmental combination. In fact, a recent study suggests that variation in genetic background in combination with defects in developmental susceptibility genes could explain phenotypic variability (Pizzo et al. 2019), and perhaps stress could also be added to this model. Underlying genetic differences, including defects in susceptibility genes, may only affect some neuronal responses to stress during certain critical periods, which may differ for each stress/genetic risk, adding further complexity and variability to relevant phenotypes.
Acknowledgments
I thank Meera Sundaram, David Raizen, Oliver Hobert, Emily Bayer, Laura Pereira, and Theodore G. Drivas for discussions and comments on the included experiments and manuscript; and Meera Sundaram and members of the Sundaram lab for technical support. I would also like to thank the members and anonymous donor of the Autism Spectrum Program of Excellence at the Perelman School of Medicine for collaborative discussions on neurexin and financial support. Some strains were provided by the Caenorhabditis Genetics Center, funded by National Institutes of Health Office of Research Infrastructure Programs (grant P40 OD010440). The author declares no conflicts of interest.
Footnotes
Communicating editor: H. Bülow
Literature Cited
- Autism Spectrum Disorders Working Group of The Psychiatric Genomics Consortium , 2017. Meta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol. Autism 8: 21 10.1186/s13229-017-0137-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister R., Schaffitzel E., and Hertweck M., 2006. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J. Endocrinol. 190: 191–202. 10.1677/joe.1.06856 [DOI] [PubMed] [Google Scholar]
- Bayer E. A., and Hobert O., 2018. Past experience shapes sexually dimorphic neuronal wiring through monoaminergic signalling. Nature 561: 117–121. 10.1038/s41586-018-0452-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop-Fitzpatrick L., Mazefsky C. A., Minshew N. J., and Eack S. M., 2015. The relationship between stress and social functioning in adults with autism spectrum disorder and without intellectual disability. Autism Res. 8: 164–173. 10.1002/aur.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattane N., Richetto J., and Cattaneo A., 2018. Prenatal exposure to environmental insults and enhanced risk of developing schizophrenia and autism spectrum disorder: focus on biological pathways and epigenetic mechanisms. Neurosci. Biobehav. Rev., pp. S0149-S7634 30972-7 10.1016/j.neubiorev.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Chaste P., and Leboyer M., 2012. Autism risk factors: genes, environment, and gene-environment interactions. Dialogues Clin. Neurosci. 14: 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C. D., 2006. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav. Cogn. Neurosci. Rev. 5: 41–60. 10.1177/1534582306289043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C. D., LeDoux J. E., Magarinos A. M., and McEwen B. S., 1999. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci. 113: 902–913. 10.1037/0735-7044.113.5.902 [DOI] [PubMed] [Google Scholar]
- Conrad C. D., Ortiz J. B., and Judd J. M., 2017. Chronic stress and hippocampal dendritic complexity: methodological and functional considerations. Physiol. Behav. 178: 66–81. 10.1016/j.physbeh.2016.11.017 [DOI] [PubMed] [Google Scholar]
- Cook S. C., and Wellman C. L., 2004. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 60: 236–248. 10.1002/neu.20025 [DOI] [PubMed] [Google Scholar]
- Czéh B., Vardya I., Varga Z., Febbraro F., Csabai D. et al. , 2018. Long-term stress disrupts the structural and functional integrity of GABAergic neuronal networks in the medial prefrontal cortex of rats. Front. Cell. Neurosci. 12: 148 10.3389/fncel.2018.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBardeleben H. K., Lopes L. E., Nessel M. P., and Raizen D. M., 2017. Stress-induced sleep after exposure to ultraviolet light is promoted by p53 in Caenorhabditis elegans. Genetics 207: 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L., and McEwen B. S., 2012. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus 22: 82–91. 10.1002/hipo.20862 [DOI] [PubMed] [Google Scholar]
- Fuld S., 2018. Autism spectrum disorder: the impact of stressful and traumatic life events and implications for clinical practice. Clin. Soc. Work J. 46: 210–219. 10.1007/s10615-018-0649-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L. R., Mehta P., and Sternberg P. W., 2001. Regulation of distinct muscle behaviors controls the C. elegans male’s copulatory spicules during mating. Cell 107: 777–788. 10.1016/S0092-8674(01)00600-6 [DOI] [PubMed] [Google Scholar]
- Gauthier J., Siddiqui T. J., Huashan P., Yokomaku D., Hamdan F. F. et al. , 2011. Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia. Hum. Genet. 130: 563–573. 10.1007/s00439-011-0975-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert-Juan J., Castillo-Gomez E., Perez-Rando M., Molto M. D., and Nacher J., 2011. Chronic stress induces changes in the structure of interneurons and in the expression of molecules related to neuronal structural plasticity and inhibitory neurotransmission in the amygdala of adult mice. Exp. Neurol. 232: 33–40. 10.1016/j.expneurol.2011.07.009 [DOI] [PubMed] [Google Scholar]
- Gilabert-Juan J., Castillo-Gomez E., Guirado R., Molto M. D., and Nacher J., 2013. Chronic stress alters inhibitory networks in the medial prefrontal cortex of adult mice. Brain Struct. Funct. 218: 1591–1605. 10.1007/s00429-012-0479-1 [DOI] [PubMed] [Google Scholar]
- Gilabert-Juan J., Bueno-Fernandez C., Castillo-Gomez E., and Nacher J., 2017. Reduced interneuronal dendritic arborization in CA1 but not in CA3 region of mice subjected to chronic mild stress. Brain Behav. 7: e00534 10.1002/brb3.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Navetta A., Gualberto D. G. and Garcia L. R., 2012. Behavioral decay in aging male C. elegans correlates with increased cell excitability. Neurobiol. Aging 33: 1483.e5–1483.e23. 10.1016/j.neurobiolaging.2011.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J., Cleveland S., Torres A., Phillips J., Cohen B. et al. , 2011. Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry 68: 1095–1102. 10.1001/archgenpsychiatry.2011.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M. P., and Hobert O., 2018. Neurexin controls plasticity of a mature, sexually dimorphic neuron. Nature 553: 165–170. 10.1038/nature25192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I., Schmidt R. J., and Krakowiak P., 2018. Understanding environmental contributions to autism: causal concepts and the state of science. Autism Res. 11: 554–586. 10.1002/aur.1938 [DOI] [PubMed] [Google Scholar]
- Hsu A. L., Murphy C. T., and Kenyon C., 2003. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300: 1142–1145. 10.1126/science.1083701 [DOI] [PubMed] [Google Scholar]
- Kagias K., Nehammer C., and Pocock R., 2012. Neuronal responses to physiological stress. Front. Genet. 3: 222 10.3389/fgene.2012.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G., Rujescu D., Ingason A., Collier D. A., O’Donovan M. C. et al. , 2009. Neurexin 1 (NRXN1) deletions in schizophrenia. Schizophr. Bull. 35: 851–854. 10.1093/schbul/sbp079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurshan P. T., Merrill S. A., Dong Y., Ding C., Hammarlund M. et al. , 2018. γ-neurexin and frizzled mediate parallel synapse assembly pathways antagonized by receptor endocytosis. Neuron 100: 150–166.e4. 10.1016/j.neuron.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBoeuf B., Guo X., and Garcia L. R., 2011. The effects of transient starvation persist through direct interactions between CaMKII and ether-a-go-go K+ channels in C. elegans males. Neuroscience 175: 1–17. 10.1016/j.neuroscience.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke C., Berry K., Kautu B., Lee K., Caldwell K. et al. , 2008. Paradigms for pharmacological characterization of C. elegans synaptic transmission mutants. J. Vis. Exp. 18: 837. 10.3791/837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longair M. H., Baker D. A., and Armstrong J. D., 2011. Simple Neurite Tracer: open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics 27: 2453–2454. 10.1093/bioinformatics/btr390 [DOI] [PubMed] [Google Scholar]
- Lowther C., Speevak M., Armour C. M., Goh E. S., Graham G. E. et al. , 2017. Molecular characterization of NRXN1 deletions from 19,263 clinical microarray cases identifies exons important for neurodevelopmental disease expression. Genet. Med. 19: 53–61. 10.1038/gim.2016.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro G. S., Gao S., Olechwier A. M., Hung W. L., Liu M. et al. , 2015. MADD-4/Punctin and neurexin organize C. elegans GABAergic postsynapses through neuroligin. Neuron 86: 1420–1432. 10.1016/j.neuron.2015.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall T., Weil Z. M., Nacher J., Bloss E. B., El Maarouf A. et al. , 2013. Depletion of polysialic acid from neural cell adhesion molecule (PSA-NCAM) increases CA3 dendritic arborization and increases vulnerability to excitotoxicity. Exp. Neurol. 241: 5–12. 10.1016/j.expneurol.2012.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S., Nasca C., and Gray J. D., 2016. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41: 3–23. 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen J. M., Morano R. L., Fitzgerald M., Zoubovsky S., Cassella S. N. et al. , 2016. Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biol. Psychiatry 80: 754–764. 10.1016/j.biopsych.2016.03.2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren-Suissa M., Bayer E. A., and Hobert O., 2016. Sex-specific pruning of neuronal synapses in Caenorhabditis elegans. Nature 533: 206–211. 10.1038/nature17977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philbrook A., Ramachandran S., Lambert C. M., Oliver D., Florman J. et al. , 2018. Neurexin directs partner-specific synaptic connectivity in C. elegans. eLife 7: e35692. 10.7554/eLife.35692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo L., Jensen M., Polyak A., Rosenfeld J. A., Mannik K. et al. , 2019. Rare variants in the genetic background modulate cognitive and developmental phenotypes in individuals carrying disease-associated variants. Genet. Med. 21: 816–825. 10.1038/s41436-018-0266-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt A. C., Rodgers R. J., and Clapcote S. J., 2012. The role of neurexins in schizophrenia and autistic spectrum disorder. Neuropharmacology 62: 1519–1526. 10.1016/j.neuropharm.2011.01.024 [DOI] [PubMed] [Google Scholar]
- Sandin S., Lichtenstein P., Kuja-Halkola R., Larsson H., Hultman C. M. et al. , 2014. The familial risk of autism. JAMA 311: 1770–1777. 10.1001/jama.2014.4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A., Malchow B., Hasan A., and Falkai P., 2014. The impact of environmental factors in severe psychiatric disorders. Front. Neurosci. 8: 19 10.3389/fnins.2014.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. L., Wang Y., and Wang D. Y., 2007. Involvement of genes required for synaptic function in aging control in C. elegans. Neurosci. Bull. 23: 21–29. 10.1007/s12264-007-0003-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek L. B., Sterken M. G., Volkers R. J., Klatter M., Bosman K. J. et al. , 2014. A rapid and massive gene expression shift marking adolescent transition in C. elegans. Sci. Rep. 4: 3912 10.1038/srep03912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab T. A., Evgrafov O., Knowles J. A., and Sieburth D., 2014. Regulation of synaptic nlg-1/neuroligin abundance by the skn-1/Nrf stress response pathway protects against oxidative stress. PLoS Genet. 10: e1004100 (erratum: PLoS Genet. 10: e1004361). 10.1371/journal.pgen.1004100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanakis N., Carrera I., and Hobert O., 2015. Regulatory logic of pan-neuronal gene expression in C. elegans. Neuron 87: 733–750. 10.1016/j.neuron.2015.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C., 2017. Synaptic neurexin complexes: a molecular code for the logic of neural circuits. Cell 171: 745–769. 10.1016/j.cell.2017.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tick B., Bolton P., Happe F., Rutter M., and Rijsdijk F., 2016. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J. Child Psychol. Psychiatry 57: 585–595. 10.1111/jcpp.12499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todarello G., Feng N., Kolachana B. S., Li C., Vakkalanka R. et al. , 2014. Incomplete penetrance of NRXN1 deletions in families with schizophrenia. Schizophr. Res. 155: 1–7. 10.1016/j.schres.2014.02.023 [DOI] [PubMed] [Google Scholar]
- Tong X. J., Hu Z., Liu Y., Anderson D., and Kaplan J. M., 2015. A network of autism linked genes stabilizes two pools of synaptic GABA(A) receptors. eLife 4: e09648 10.7554/eLife.09648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X. J., López-Soto E. J., Li L., Liu H., Nedelcu D. et al. , 2017. Retrograde synaptic inhibition is mediated by α-neurexin binding to the α2δ subunits of N-type calcium channels. Neuron 95: 326–340.e5. 10.1016/j.neuron.2017.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Huang T. Y., Chang C. Y., Hsu Y. C., Chen S. J. et al. , 2014. Social instability stress differentially affects amygdalar neuron adaptations and memory performance in adolescent and adult rats. Front. Behav. Neurosci. 8: 27 10.3389/fnbeh.2014.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H., Pinan-Lucarre B., Ji T., Jospin M., and Bessereau J. L., 2015. C. elegans punctin clusters GABA(A) receptors via neuroligin binding and UNC-40/DCC recruitment. Neuron 86: 1407–1419. 10.1016/j.neuron.2015.05.013 [DOI] [PubMed] [Google Scholar]
- Tzanoulinou S., Garcia-Mompo C., Castillo-Gomez E., Veenit V., Nacher J. et al. , 2014. Long-term behavioral programming induced by peripuberty stress in rats is accompanied by GABAergic-related alterations in the Amygdala. PLoS One 9: e94666 10.1371/journal.pone.0094666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volovik Y., Moll L., Marques F. C., Maman M., Bejerano-Sagie M. et al. , 2014. Differential regulation of the heat shock factor 1 and DAF-16 by neuronal nhl-1 in the nematode C. elegans. Cell Rep. 9: 2192–2205. 10.1016/j.celrep.2014.11.028 [DOI] [PubMed] [Google Scholar]
- Vyas A., Mitra R., Shankaranarayana Rao B. S., and Chattarji S., 2002. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 22: 6810–6818. 10.1523/JNEUROSCI.22-15-06810.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R., Banaschewski T., Meyer-Lindenberg A., and Poustka L., 2014. Interaction of neurodevelopmental pathways and synaptic plasticity in mental retardation, autism spectrum disorder and schizophrenia: implications for psychiatry. World J. Biol. Psychiatry 15: 507–516. 10.3109/15622975.2013.838641 [DOI] [PubMed] [Google Scholar]
- Woodbury-Smith M., Nicolson R., Zarrei M., Yuen R. K. C., Walker S. et al. , 2017. Variable phenotype expression in a family segregating microdeletions of the NRXN1 and MBD5 autism spectrum disorder susceptibility genes. NPJ Genom. Med. 2: 17. 10.1038/s41525-017-0020-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan Z., Manning L., Nelson J., Richmond J. E., Colon-Ramos D. A. et al. , 2017. Clarinet (CLA-1), a novel active zone protein required for synaptic vesicle clustering and release. eLife 6: e29276. 10.7554/eLife.29276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Walder-Christensen K. K., Kim N., Wu D., Lorenzo D. N. et al. , 2019. ANK2 autism mutation targeting giant ankyrin-B promotes axon branching and ectopic connectivity. Proc. Natl. Acad. Sci. USA 116: 15262–15271. 10.1073/pnas.1904348116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that supports the findings of this study are represented in the article, tables, and figures. All plasmids and strains are available from the corresponding author upon request.