Abstract
Mucosal wound repair is coordinated by dynamic crosstalk between endogenous and exogenous mediators and specific receptors on epithelial cells and infiltrating immune cells. One class of such receptor-ligand pairs involves formyl peptide receptors (FPRs) that have been shown to influence inflammatory response and repair. Here we explored the role of murine Fpr2/3, an ortholog of human FPR2/receptor for lipoxin A4 (ALX), in orchestrating intestinal mucosal repair. Compared with wild-type (WT) mice, Fpr2/3−/− mice exhibited delayed recovery from acute experimental colitis and perturbed repair after biopsy-induced colonic mucosal injury. Decreased numbers of infiltrating monocytes were observed in healing wounds from Fpr2/3−/− mice compared with WT animals. Bone marrow transplant experiments revealed that Fpr2/3−/− monocytes showed a competitive disadvantage when infiltrating colonic wounds. Moreover, Fpr2/3−/− monocytes were defective in chemotactic responses to the chemokine CC chemokine ligand (CCL)20, which is up-regulated during early phases of inflammation. Analysis of Fpr2/3−/− monocytes revealed altered expression of the CCL20 receptor CC chemokine receptor (CCR)6, suggesting that Fpr2/3 regulates CCL20-CCR6–mediated monocyte chemotaxis to sites of mucosal injury in the gut. These findings demonstrate an important contribution of Fpr2/3 in facilitating monocyte recruitment to sites of mucosal injury to influence wound repair.—Birkl, D., O’Leary, M. N., Quiros, M., Azcutia, V., Schaller, M., Reed, M., Nishio, H., Keeney, J., Neish, A. S., Lukacs, N. W., Parkos, C. A., Nusrat, A. Formyl peptide receptor 2 regulates monocyte recruitment to promote intestinal mucosal wound repair.
Keywords: epithelium, GPCRs, FPR2, inflammation, inflammatory bowel disease
Intestinal epithelial cells are at the interface between the gut lumen and underlying tissue compartments, creating a dynamic, selectively permeable barrier that not only plays an important role in host defense but in mediating crosstalk between the mucosal immune system and luminal antigens and microbes. Pathologic states that occur during chronic inflammation, ischemia, and mechanical injury are associated with disruption of the epithelial barrier and mucosal ulceration. Efficient repair of the epithelium is critical in restoring the barrier and in regaining mucosal homeostasis (1–4).
Mucosal repair is a dynamic process orchestrated by a spatiotemporal network of mediators that include secreted factors that crosstalk with epithelial and immune cells (2–7). Chemoattractants, such as chemokines and cytokines, are secreted by immune cells and injured epithelial cells and signal through receptors to recruit other immune cells, thus orchestrating the inflammatory response. After an initial recruitment of neutrophils, there is an influx of monocytes to sites of mucosal injury in response to locally produced chemoattractants, such as CC chemokine ligand (CCL)2 and CCL20. In later phases of the acute inflammatory response, cells in healing wounds secrete factors that mediate active resolution of inflammation and repair (8, 9). For example, in response to bacterial LPS stimulation, IL-1β or TNF-α signaling via NFκB activation induces CCL20 expression in intestinal epithelial cells (10–13). CCL20-mediated signaling through the receptor CC chemokine receptor (CCR)6 (14) induces chemotaxis of CCR6-expressing monocytes, dendritic cells, and macrophages to sites of infection that influence resolution and repair (15, 16).
In addition to expressing CCR6, monocytes express a wide variety of GPCRs, including formyl peptide receptors (FPRs) and CCRs (17, 18). FPRs serve as important receptors during wound repair because they regulate recruitment of immune cells to sites of inflammation and promote migration of epithelial cells (19, 20). Although 3 human FPRs (FPR1–3) have been identified, mice express at least 8 Fpr genes. Human FPR2, also referred to as the receptor for lipoxin A4 (ALX) gene, has been proposed to function as 2 mouse genes referred to as Fpr2 and Fpr3, which share their first 2 exons (21). FPRs are expressed on epithelial (22–27) and immune cells (neutrophils, monocytes, macrophages, and dendritic cells) (20, 28, 29). Fpr2-deficient mice have shortened colonic crypts, reduced epithelial proliferation, and delayed recovery from acute colitis compared with wild-type (WT) mice (25). Previous work has suggested that Fpr2/3 plays a role in monocyte recruitment during inflammation induced by polymicrobial sepsis (30) and chronic airway inflammation in a manner dependent on CCR2 (6, 31).
CCR signaling has been shown to cooperate with activation of FPRs to play an important role in coordinating immune cell recruitment to sites of injury and inflammation to initiate resolution. Specifically, the chemokine CCL3 and the FPR1/2 ligand fMLF have been shown to synergistically activate chemotaxis of human monocytes (32). The mechanisms by which these receptors cooperate to recruit immune cells and facilitate intestinal mucosal repair are not well understood. Here, we used Fpr2/3−/− mice to identify the role of Fpr2/3 in facilitating recruitment of monocytes to sites of mucosal injury to promote wound repair. We found that Fpr2/3 expression in epithelial and immune cells was required for colonic mucosal wound repair. Deletion of Fpr2/3 resulted in reduced monocyte recruitment to sites of mucosal injury due to an alternate CCR6-CCL20 signaling axis, demonstrating that FPR2/3 regulates monocyte migration to influence colonic mucosal repair.
MATERIALS AND METHODS
Mice
Experiments were performed on mice 8–12 wk of age. Male C57BL/6J (Fpr2/3+/+, WT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Fpr2/3−/− mice on a C57BL/6 background were generated as previously described by Dufton et al. (19). Ccr6−/− mice (Ccr6tm(EGFP)lrw; 013061, The Jackson Laboratory) were generated as previously described by Kucharzik et al. (33). Animals were housed under a standard day-night cycle with free access to food and water. Animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Michigan and were done in accord with accepted national guidelines.
Dextran sodium sulfate–induced colitis
Mice were provided with 2.5% (w/v) dextran sodium sulfate (DSS) dissolved in drinking water for 3 d followed by 5 d of recovery on water. To assess response to chronic DSS-induced colitis, the treatment was repeated twice. Daily clinical assessment of DSS-treated animals was monitored by a Clinical Disease Activity Index (DAI) ranging from 0 to 4 (34), which was calculated using stool consistency, presence or absence of fecal blood (Hemoccult; Thermo Fisher Scientific, Waltham, MA, USA), and weight loss. Mice were euthanized after 2 cycles of treatment, and colons were isolated for histology and analysis of pathobiology.
In vivo wounding of colonic mucosa
Mice were anesthetized by intraperitoneal injection of a ketamine (100 mg/kg)/xylazine (10 mg/kg) solution. A high-resolution, miniaturized colonoscope system equipped with biopsy forceps (Coloview Veterinary Endoscope; Karl Storz, Tuttlingen, Germany) was used to injure the colonic mucosa at 5–10 sites along the dorsal artery, and healing was quantified on d 1 and 3 postinjury. Endoscopic procedures were viewed with high-resolution (1024 × 768 pixels) images on a flat-panel color monitor. Wound size averaged ∼1 mm2, which is equivalent to removal of ∼250–300 crypts. For each analysis, 20–25 lesions from 5 mice/group were examined. Mucosal wounds and intact mucosa were harvested for quantitative PCR (qPCR) analysis of mRNA expression or flow cytometry. Wound area was quantified using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Bone marrow transplantation
For total bone marrow (BM) transplant experiments, donor BM cells were harvested from WT (C57BL/6 CD45.1; 002014, The Jackson Laboratory) and Fpr2/3−/− (CD45.2) mice. Recipient mice were sublethally irradiated using 2 times 5 Gy 4 h apart. 1 × 106 donor BM cells were transplanted by tail vein injection into recipient mice. For competitive BM transplant experiments, a 1:1 ratio of CD45.1 (WT) and CD45.2 (Fpr2/3−/−) at 5 × 105 was used for each genotype for a total of 1 × 106 BM donor cells/recipient. Blood samples were collected from the recipients 5 wk after BM transplantation to confirm engraftment. Experiments using the recipients were conducted 8–10 wk after BM transplantation and cardiac puncture was used to collect blood for engraftment and complete blood cell (CBC) analysis.
Lamina propria isolation
Punch biopsies (2 mm) of wounded or intact colon tissue from each experimental condition were placed in 10 ml of Rosewell Park Memorial Institute (RPMI) 1640 medium containing 150 μl Liberase stock (2.5 mg/ml) and 150 μl DNase I stock (2 × 104 Kuntz U/ml) (both from MilliporeSigma, Burlington, MA, USA). Biopsied tissue samples were digested at 37°C for 30 min, passed several times through an 18-gauge needle plus a 3-cc syringe, and then filtered through a 70-μm cell strainer into a clean 50-ml tube on ice. Samples were centrifuged to pellet the immune cells then resuspended in PBS containing 2% fetal bovine serum (FBS)/1 mM EDTA.
Flow cytometry and cell sorting
Isolated lamina propria cells from colonic wounds were resuspended in flow buffer (PBS containing 2% FBS/1 mM EDTA), filtered through 70-μm nylon mesh, and then incubated for 30 min at 4°C with a Live/Dead dye (eBioscience Fixable Viability Dye eFluor 780; Thermo Fisher Scientific). After being washed thoroughly, cells were stained with a labeled primary Ab cocktail in the presence of Fc block for 30 min at 4°C. Cells were then washed and fixed in 4% paraformaldehyde for 10 min at room temperature in the dark. Flow cytometric analysis was performed on a NovoCyte Flow Cytometer (Acea Biosciences, San Diego, CA, USA). The results were plotted and analyzed using FlowJo software (Becton Dickinson, Franklin Lakes, NJ, USA). For blood, 200 μl was collected into 1 ml PBS/2 mM EDTA and kept on ice. Blood was centrifuged, and RBC were lysed in 400 μl of ACK lysis buffer for 2–3 min on ice. After being washed in flow buffer, cells were stained and analyzed as previously described.
Monocyte isolation
Monocytes were isolated from spleen by antibody-based negative selection. Briefly, spleens were collected in PBS supplemented with 1% FBS and 1 mM EDTA, passed through a 70-μm Falcon strainer (Corning, Corning, NY, USA), and collected in a 50-ml conical tube. Cell suspension was centrifuged at 400 g for 5 min and red blood cells were lysed with 1 ml of distilled water for 30 s. Lysing reaction was stopped by adding 1 ml of 1.8% NaCl. Subsequently, monocytes were isolated from the cell suspension according to EasySep Mouse Monocyte Isolation protocol (Stemcell Technologies, Vancouver, BC, Canada).
Monocyte migration assay
Transcollagen migration experiments were performed using collagen-coated (10 μg/ml rat tail collagen type I), permeable, 0.33-cm2 polycarbonate Transwells (5 μm pore size; Costar, Cambridge, MA, USA), in the presence of a chemotactic gradient of 100 ng/ml CCL9 or 100 ng/ml CCL20 (PeproTech, Rocky Hill, NJ, USA). 2 × 105 of splenic monocytes were added to the upper chambers of Transwell, and migration to the lower chamber was assayed for 2 h at 37°C. Migrated monocytes were fixed and stained with crystal violet. Representative images from 5 fields were acquired and migrated cells were quantified. The rate of migration is represented as the percentage of the total monocytes added to the upper chamber of the Transwell that have migrated into the bottom.
CBC analysis
CBC analysis was performed by the University of Michigan Animal Diagnostic Laboratory. Whole blood samples (50 µl) were collected by cardiac puncture into EDTA microtainers (Becton Dickinson) and stored at 4°C until analysis.
Reagents
The following antibodies were used for flow cytometry analysis: eFluor 450–conjugated Ly-6C (48-5932-82), phycoerythrin (PE)-Cyanine7–conjugated CD19 (25-0193-82), allophycocyanin (APC)-conjugated CD45.1 (17-0453-82), FITC-conjugated CD45.2 (11-0454-85), APC-eFluor 780–conjugated CD4 (47-0041-82), and PE-conjugated F4/80 (MF48004) antibodies from Thermo Fisher Scientific; BV510-conjugated CD11b (562950), PE-conjugated CD3e (553063), PerCP-conjugated CD8a (553036), APC-Cy7–conjugated sialic acid-binding immunoglobulin-like lectin (Siglec)-F (565527), and BD Fc Block CD16/CD32 (553142) antibodies from BD Biosciences (San Jose, CA, USA); and BV608-conjugated Ly-6G (127639), PE/Cy7-conjugated CD64 (139314), APC-conjugated CCR1 (152504), and BV785-conjugated CCR6 (129823) antibodies from BioLegend (San Diego, CA, USA). Antibodies were used at 1:200 for flow cytometry.
qPCR
Total RNA was isolated from colonic wounds or isolated monocytes using the RNeasy Kit (Qiagen, Germantown, MD, USA) with DNAse I treatment following the manufacturer’s protocol. Equal amounts of total RNA (500 ng–1 μg) were transcribed into cDNA using the iScript Reverse Transcription Supermix (Bio-Rad, Hercules, CA, USA). Individual gene expression was determined by qPCR using SsoAdvanced Universal SYBR Green (Bio-Rad) with a Bio-Rad CTX Cycler measuring SYBR green incorporation for product detection. Reactions were performed in triplicate with at least 3 biologic replicates. The relative expression of the gene of interest was calculated by 2−ΔΔCt and normalized to the housekeeping gene TATA-box-binding protein (TBP). The primer sequences are shown in Table 1.
TABLE 1.
Primers
| Gene | Primer name | Primer sequence, 5′–3′ |
|
|---|---|---|---|
| Forward | Reverse | ||
| Ccr1 | Mm Ccr1 | AGGAACTGGTCAGGAATAATAGC | CAAAGGCCCAGAAACAAAGTC |
| Ccr2 | Mm Ccr2 | ACTGAGGTAACATATTATTGTCTTCCA | GAGCCATACCTGTAAATGCCA |
| Ccr6 | Mm Ccr6 | GTCACTGTCATGCTTACTTGAATG | CTTAGGACTGGAAGCCTGGATA |
| FPR2 | Mm Fpr2 | AAGGAGACCTCAGCTGGTTGTG | TCCACAGAACTCTGGAGATGGTAG |
| TBP | Mm Tbp | GGAATTGTACCGCAGCTTCAAA | GATGACTGCAGCAAATCGCTT |
Luminex assay
Intestinal tissue CCL-20 protein concentration was determined using Bio-Plex Pro Mouse Chemokine Assay (12009159) purchased from Bio-Rad following the manufacturer’s instructions.
Statistical analysis
Statistical comparisons were performed by 1- or 2-way ANOVA with Bonferroni’s multiple comparison, or unpaired 2-tailed Student’s t test, as appropriate. A value of P < 0.05 was considered significant.
RESULTS
Fpr2/3−/− mice display delayed colonic mucosal wound repair
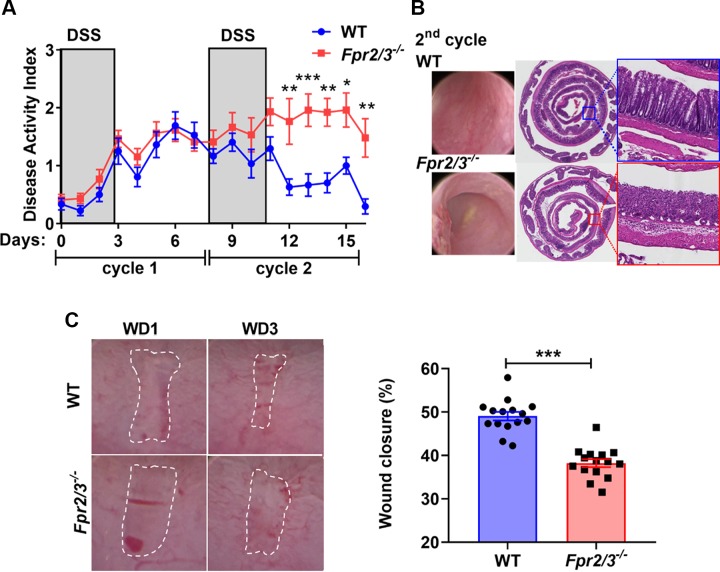
Intestinal epithelial expression of FPR2 has been reported to regulate epithelial homeostasis and response to mucosal inflammation (25). Inflammatory bowel disease is commonly associated with repeated bouts of active inflammation followed by reparative events. To model the process of repetitive bouts of mucosal inflammation and repair, we subjected Fpr2/3−/− and WT mice to cycles of DSS administration in drinking water followed by recovery on water alone. Mice were administered 2 cycles of 2.5% (w/v) DSS for 3 d in the drinking water with a recovery period of 5 d/cycle. The concentration of DSS and duration of administration were based on close monitoring of disease activity. We determined that administration of 2.5% DSS resulted in optimal conditions that allowed for evaluation of resolution of inflammation and repair in FPR2/3 mice. Higher DSS concentration or longer exposure resulted in severe disease activity that necessitated euthanasia during the repair phase in FPR2/3 mice. The DAI was monitored by evaluating body weight, stool consistency, and fecal blood. Mice lacking Fpr2/3 (Fpr2/3−/−) displayed similar DAI to that observed with WT mice during the first cycle of DSS treatment. However, increased DAI was noted in Fpr2/3−/− mice during the second DSS cycle, as evidenced by a marked delay in recovery from colitis (d 11–16) (Fig. 1A). Endoscopic images of the colonic mucosa showed increased hyperemia and ulceration in Fpr2/3−/− mice compared with WT controls (C57BL/6J) at the termination of the experiments (Fig. 1B). Histologic evaluation showed increased mucosal ulceration and delayed mucosal healing in Fpr2/3−/− mice compared with WT mice (Fig. 1B). Colonic mucosa from Fpr2/3−/− mice displayed loss of epithelial architecture, increased immune cell infiltration, and decreased epithelial repair (Fig. 1B). These results are consistent with delayed healing responses in Fpr2/3−/− mice. To further evaluate the contribution of Fpr2/3 in controlling colonic mucosal wound repair, we analyzed mucosal wound healing using a colon biopsy-induced injury model. As shown in Fig. 1C, Fpr2/3−/− mice demonstrated delayed mucosal wound healing compared with control mice (38.2 ± 3.5, Fpr2/3−/−; 49.0 ± 3.8, WT; P < 0.001). Taken together, these results support an important role of Fpr2/3−/− in colonic mucosal wound repair.
Figure 1.
Fpr2−/− mice show decreased acute and chronic wound healing. A) Disease activity index comparing C57BL/6J (WT) with Fpr2/3−/− mice after 2 cycles of DSS treatment consisting of 3 d of 2.5% DSS followed by 5 d of recovery with water consumption. B) Representative images of endoscopic videos and hematoxylin and eosin–stained histologic sections of WT or Fpr2/3−/− colonic mucosa after the second cycle of DSS treatment. Boxed areas are magnified in insets equidistant from the rectum. The total magnification of the photomicrographs is original magnification, ×2, and the inset is original magnification, ×40. C) Endoscopic images of colonic mucosal wounds 1 and 3 d after biopsy-induced injury in Fpr2/3−/− (n = 15) or WT control (n = 15) mice. Wound area was quantified using ImageJ. WD, wound day. Graph shows quantification of wound closure. Statistical comparisons were performed using an unpaired 2-tailed Student’s t test with Welch’s correction. ***P < 0.001 (means ± sem).
Fpr2/3 expressed in epithelial and immune cells regulates colonic mucosal wound repair
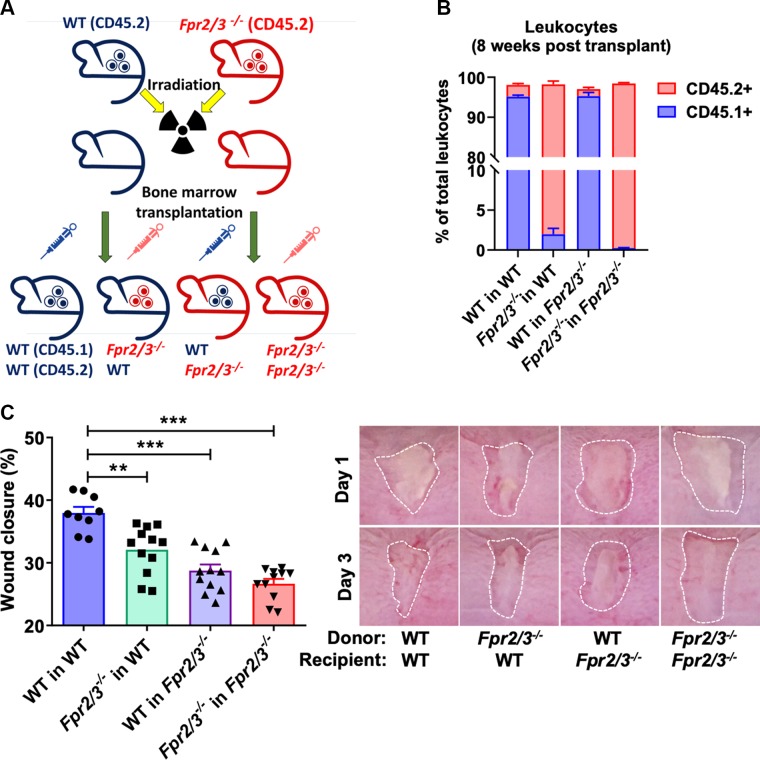
Mucosal repair is orchestrated by the epithelium as well as infiltrating immune cells at sites of injury. Because epithelial cells and leukocytes express FPR2 (28, 35, 36), we evaluated the relative contributions of epithelial and immune cell expression of Fpr2/3 in regulating mucosal repair. Mice were subjected to BM transplantation followed by biopsy-induced mucosal wound healing experiments. Irradiated WT or Fpr2/3−/− recipient mice were reconstituted with BM from either WT or Fpr2/3−/− donor mice (Fig. 2A). Engraftment was assessed by flow cytometry to evaluate allelic markers 8 wk posttransfer (Fig. 2B), and reconstitution of hematopoietic cells was determined by CBC analyses at the end of the experiment (Supplemental Fig. S1). Analysis of healing wounds at d 3 postinjury revealed delayed wound repair in Fpr2/3−/− mice reconstituted with WT BM (WT in Fpr2/3−/−) (Fig. 2C), supporting a role of epithelial Fpr2/3 in regulation of wound healing. Additionally, WT mice that received a BM transplant from Fpr2/3−/− mice (Fpr2/3−/− in WT) had delayed wound repair (Fig. 2C), suggesting that immune cell–expressed Fpr2/3 also plays a role in regulating mucosal repair.
Figure 2.
Expression of Fpr2/3 on epithelial and immune cells is necessary for colonic mucosal wound repair. A) Illustration of BM transplant experiment. B) Engraftment verification after BM transplantation by flow cytometry analysis of leukocytes in irradiated WT or Fpr2/3−/− host mice that were reconstituted with BM from either WT (CD45.1+) or Fpr2/3−/− (CD45.2+) donor mice (WT→WT; Fpr2/3−/− →WT; WT→ Fpr2/3−/−; Fpr2/3−/− → Fpr2/3−/−; n = 6; means ± sem). C) Quantification and endoscopic images of colonic mucosal wound repair after biopsy-induced injury, comparing irradiated WT or Fpr2/3−/− host mice that were reconstituted with BM from either WT or Fpr2/3−/− donor mice (WT→WT, n = 9; Fpr2/3−/− →WT, n = 12; WT→ Fpr2/3−/−, n = 12; Fpr2/3−/− → Fpr2/3−/−, n = 11).
Fpr2/3 influences monocyte recruitment into healing mucosal wounds
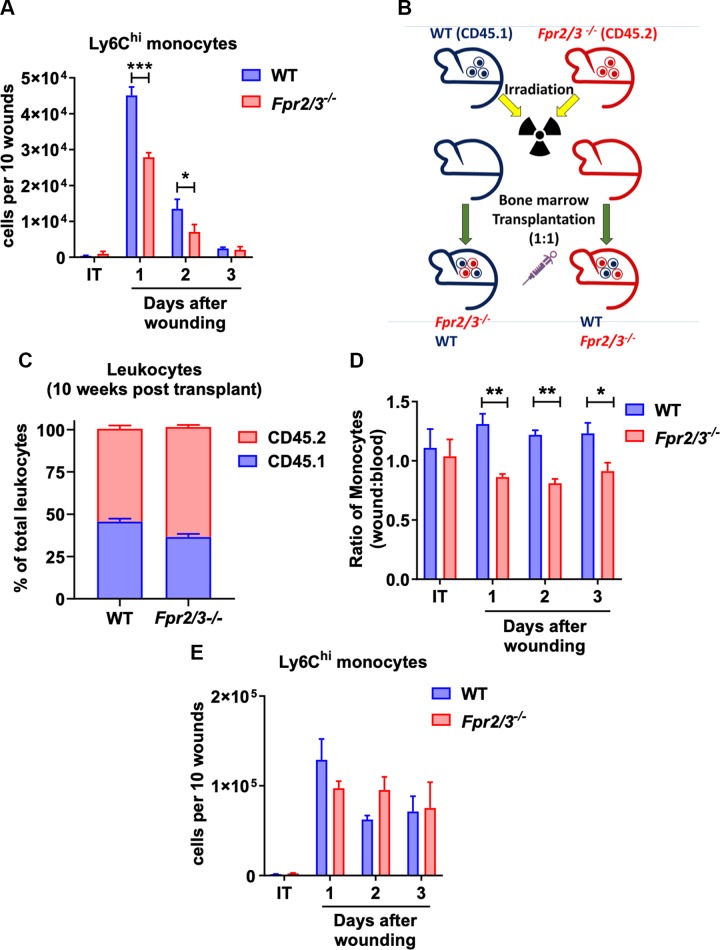
Given that previous reports supported a role of epithelial-expressed FPR2 in regulating mucosal wound repair (23–25), we investigated the contribution of FPR2 in regulating immune cell recruitment to sites of injury and mucosal repair in vivo. We first examined wound-associated immune cells in the colonic mucosa 1–3 d after biopsy-induced injury of WT and Fpr2/3−/− mice using flow cytometric approaches. Immune cells were flow sorted using the following gating strategy (Supplemental Fig. S3) to analyze immune cells’ infiltration into the wounds: neutrophils (CD45+ Ly6G+ Siglec-F−), eosinophils (CD45+ Ly6G− Siglec-F+), and monocytes/macrophages (CD45+ CD11b+ Ly6G− Siglec-F−). Monocyte and macrophage populations were further sorted into 3 populations: macrophages (CD45+ CD11b+ Ly6G− Siglec-F− F4/80+), Ly6Clo monocytes (CD45+ CD11b+ Ly6G− Siglec-F− F4/80lo Ly6Clo), and Ly6Chi monocytes (CD45+ CD11b+ Ly6G− Siglec-F− F4/80lo Ly6Chi). CBC analyses revealed no differences between immune cells in blood from Fpr2/3−/− mice compared with WT (Supplemental Fig. S2). As shown in Fig. 3A, on d 1 and 2 after wounding, mice lacking Fpr2/3 had reduced numbers of inflammatory monocytes (defined as viable, CD45+ CD11b+ Ly6G− Siglec-F− F4/80lo Ly6Chi). Although changes in other immune cell populations were also noted (Supplemental Fig. S3), monocytes have been strongly linked to playing important roles in regulating wound repair in a number of organ systems (37). We therefore focused on defining the role of FPR2/3 in regulating monocyte migration and infiltration to sites of intestinal mucosal injury.
Figure 3.
FPR2 expression is required for competitive advantage of monocytes to migrate to sites of mucosal injury. A) Analysis of monocytes from intact (IT) or wounded lamina propria tissue on different postinjury days isolated from WT or Fpr2/3−/− (n = 6). B) Illustration of competitive BM transplant experiment. C) Engraftment verification after competitive BM transplantation by flow cytometry analysis of leukocytes in irradiated WT or Fpr2/3−/− host mice that were reconstituted with a 1:1 mixture of BM from WT (CD45.1) and Fpr2/3−/− (CD45.2) donor mice (n = 19–20, means ± sem). WT, C57BL/6J. D, E) Analysis of monocytes isolated from IT or wounded lamina propria tissue on different postinjury days isolated from irradiated WT or Fpr2/3−/− host mice were reconstituted with a 1:1 mixture of BM from WT (CD45.1) and Fpr2/3−/− (CD45.2) donor mice. D) Graphs represent the ratio of WT (CD45.1) or Fpr2/3−/− (CD45.2) Ly6Chi cells in the wound vs. in the blood (n = 10) of combined WT and Fpr2/3−/− mice submitted to a competitive BM transplant. E) Numbers of Ly6Chi monocytes on different postinjury days and in IT lamina propria tissue (n = 5). IT, intact tissue; WT, C57BL/6J. Statistical comparisons performed using 2-way ANOVA with Bonferroni’s multiple comparison. *P < 0.5, **P < 0.01, ***P < 0.001 (means ± sem).
Based on the above results, we hypothesized reduced recruitment of Ly6Chi monocytes into healing colonic mucosal wounds of Fpr2/3−/− mice. To explore the role of Fpr2/3 in monocyte recruitment, competitive BM chimera experiments were performed. Irradiated WT or Fpr2/3−/− host mice were reconstituted with a 1:1 mixture of BM from WT (CD45.1) and Fpr2/3−/− (CD45.2) donor mice (Fig. 3). Engraftment was assessed by allelic markers at 10-wk posttransfer, and reconstitution of hematopoietic cells was determined by CBC analyses at the end of experiments (Supplemental Fig. S4). Colonic mucosal wounds were induced by biopsy, and infiltrating immune cell populations were evaluated after injury. The ratio of WT (CD45.1) to Fpr2/3−/− (CD45.2) Ly6Chi monocytes in normal colonic mucosa and after injury was compared with the ratio of CD45.1:CD45.2 cells in blood. Results of such experiments demonstrated that WT CD45.1 cells have a competitive advantage in recruitment from blood to wounded mucosa compared with cells from Fpr2/3-deficient CD45.2 mice that is independent of the genetic background (Fig. 3D). There was no difference in recruitment of Ly6Chi monocytes into wound beds of Fpr2/3−/− mice reconstituted with 1:1 WT: Fpr2/3−/− BM compared with WT recipients (Fig. 3E), suggesting that the presence of WT cells may compensate for reduced monocyte recruitment in Fpr2/3−/− mice. Together, these data suggest that Fpr2/3 expression influences inflammatory monocyte recruitment into healing mucosal wounds.
Fpr2/3 impacts intestinal wound cytokine and chemokine expression profile
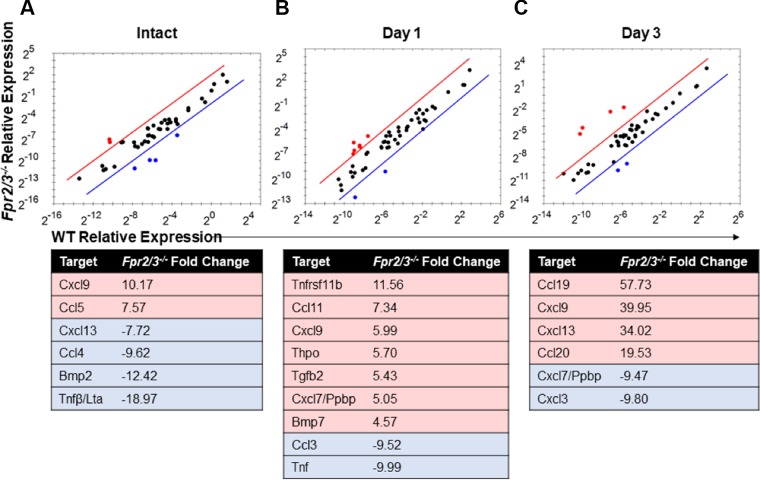
To determine the basis of delayed Fpr2/3−/− monocyte recruitment into healing wounds, we analyzed expression of cytokines and chemokines using a PCR array. RNA was isolated from intact colonic mucosa and healing biopsy-induced wounds 1 and 3 d after biopsy-induced injury. Pooled samples from multiple mice were reverse transcribed and analyzed. In intact mucosa, expression of the leukocyte chemoattractants Ccl5 and chemokine (C-X-C motif) ligand (Cxcl)9Cxcl9 was up-regulated, whereas expression of Bmp2, Ccl4, Cxcl13, and Tnf-β was down-regulated relative to WT (Fig. 4A). Analysis of colonic mucosa 1 d after injury revealed up-regulation of targets linked to regulation of immune cell recruitment (Ccl11, Cxcl7, and Cxcl9) (40, 41) (Fig. 4B). Additionally, in Fpr2/3−/− wounded mucosa isolated on d 1 after injury, Tnf-α expression was down-regulated relative to WT along with Ccl3, a ligand for CCR1, CCR4, and CCR5 (Fig. 4B). In wounded mucosa isolated on d 3 after injury, up-regulation of T-cell and B-cell chemoattractants (Ccl19, Cxcl9, and Cxcl13) and down-regulation of chemoattractants involved in neutrophil recruitment (Cxcl7 and Cxcl3) were found in Fpr2/3−/− wounded mucosa relative to WT (Fig. 4C). The chemokine Ccl20 was found to be highly up-regulated in wounds 3 d after injury in Fpr2/3−/− mice when compared to WT (Fig. 4C). CCL20 has been described to have an important role in migration of Ly6Chi monocytes in inflamed tissues through its interaction with the CCR6 receptor (15, 38, 39). Furthermore, CCL20 protein in healing wounds was increased 3 d postinjury in Fpr2/3−/− mice compared with WT mice (Supplemental Fig. S5). Overall, these data suggest there is a different chemokine and cytokine milieu in Fpr2/3−/− mice compared with WT animals. Because monocytes play an important role in repair, our study was focused on identifying contribution of Fpr2/3−/− in monocyte recruitment to healing wounds.
Figure 4.
Cytokine and chemokine expression analysis in intact and injured tissue. Scatter plots comparing gene expression changes determined by PCR array performed on RNA isolated from 3-mm punch biopsies of WT or Fpr2/3−/− intact tissue (A) or resealing colonic wounds on d 1 (B) and d 3 (C) postinjury isolated from mice. Tables detail the fold change in expression observed in Fpr2/3−/− samples relative to WT (C57BL/6J).
Fpr2/3 promotes monocyte tissue migration cooperatively with CCR6 signaling
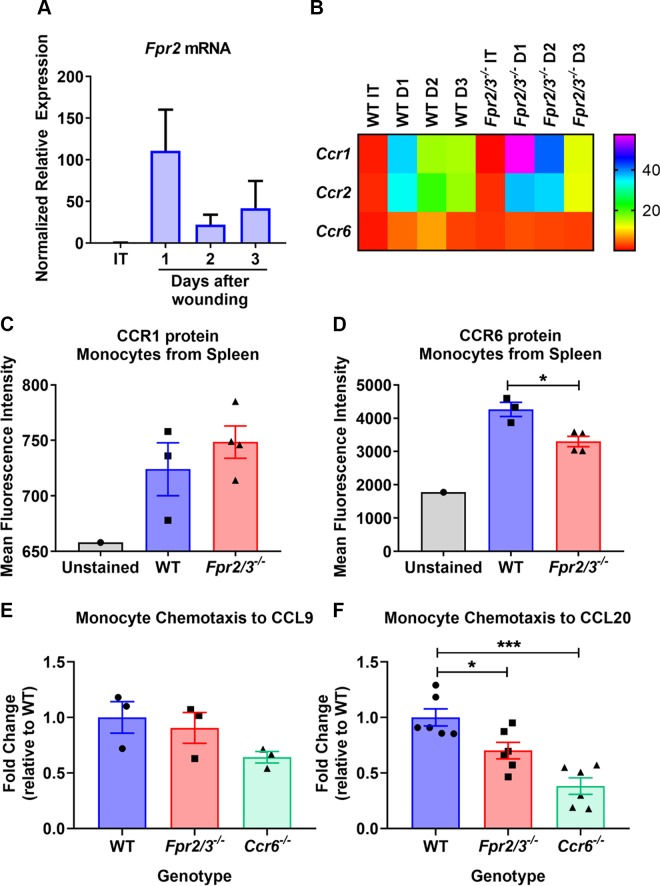
Given the delayed recruitment of inflammatory monocytes in healing wounds of Fpr2/3−/− mice, we evaluated whether elevated Ccl20 mRNA levels might be secondary to an altered CCL20-CCR6 signaling axis, offering a possible explanation for the defect in monocyte recruitment observed. Expression of monocyte chemokine receptors previously shown to influence Ly6C+ monocyte migration from blood to inflamed tissues was evaluated (42, 43). To specifically evaluate the expression of chemokine receptors on wound-associated monocytes, intact mucosa or wound beds 1–3 d after biopsy-induced injury were obtained from WT or Fpr2/3−/− mice. Monocytes were isolated from the lamina and qPCR was performed. Notably, increased Fpr2 mRNA expression was detected in WT monocytes isolated from colonic wounds on d 1 after biopsy-induced injury relative to monocytes in intact tissue (Fig. 5A). Interestingly, Ccr6 mRNA was up-regulated on d 1 postinjury in WT controls but not Fpr2/3−/− monocytes (Fig. 5B). These data suggest that in the absence of Fpr2/3−/−, monocyte recruitment to healing wounds may be impaired secondary to altered expression of Ccr6.
Figure 5.
Monocytes lacking FPR2 expression have a deficit in their chemotactic response. A) Fpr2 mRNA levels were determined by qPCR of RNA isolated from monocytes collected from the lamina propria of WT intact tissue or resealing colonic wounds isolated from mice (n = 3–4). B) Heat map representation of expression levels of Ccr1, Ccr2, and Ccr6 mRNA in monocytes was determined by qPCR analysis of RNA (n = 3–4). C, D) Flow cytometric analysis of CCR1 (C) and CCR6 (D) expression on monocytes isolated from spleens of WT or Fpr2/3−/− mice. E, F) In vitro migration of spleen monocytes isolated from WT or Fpr2/3−/− mice toward CCL9 (n = 3) (E) or CCL20 (n = 6) (F). The rate of migration is represented as the fold change relative to WT based on the ratio of total monocytes added to the upper chamber of the Transwell to the number of cells migrated into the bottom. MFI, mean fluorescence intensity. Statistical comparisons performed using 1-way ANOVA with Bonferroni’s multiple comparison or paired Student’s t test. *P < 0.5, ***P < 0.001 (means ± sem).
To determine if monocytes lacking Fpr2/3 have reduced chemokine receptor expression, we harvested splenic monocytes from Fpr2/3−/− or WT mice and determined expression of CCR1 and CCR6 receptors by flow cytometry. Although there was no change in CCR1, CCR6 expression was indeed reduced on Fpr2/3−/− splenic monocytes (Fig. 5C, D and Supplemental Fig. S6). Because CCR6 receptor expression was reduced in Fpr2/3−/− monocytes, we compared WT or Fpr2/3−/− monocyte chemotaxis toward the CCR6 ligand CCL20 using Transwell migration assays. Although we did not detect a significant change in chemotaxis of Fpr2/3−/− or Ccr6−/− monocytes toward the CCR1 ligand CCL9 (Fig. 5E), there was decreased Fpr2/3−/− chemotaxis toward CCL20 compared with WT monocytes (Fig. 5F). As an additional control, Ccr6−/− monocytes displayed significantly reduced chemotaxis toward CCL20 compared with WT monocytes (Fig. 5F). These data are consistent with Fpr2/3 indirectly regulating monocyte chemotaxis through the CCL20-CCR6 signaling axis to influence mucosal repair.
DISCUSSION
Gastrointestinal mucosal injury associated with wounds is observed in a number of pathologic states, including inflammatory bowel disease. The murine ortholog of FPR2/ALX, Fpr2/3, has been implicated in resolution of inflammation in models of polymicrobial sepsis, mesenteric ischemia–reperfusion injury, and carrageenan-induced paw edema (19, 30). In this study, we report that Fpr2/3−/− mice have 2 distinct functions that extend our knowledge of mucosal repair: 1) FPR2/3 has a central role in monocyte recruitment into colonic mucosal wounds to promote healing responses, and 2) FPR2/3 further promotes recruitment and positioning of prorepair myeloid cells through regulation of other chemokine receptors (i.e., CCR6). These observations are consistent with other studies using Fpr2/3−/− mice that demonstrate impaired recruitment of monocytes to apoptotic murine neutrophils (44) and reduced peritoneal recruitment of monocytes in a murine sepsis model (30). In the skin, immune cell Fpr1/2 depletion has been shown to impair repair (45), whereas stimulation with the FPR2-specific peptide agonist WKYMVm increases macrophage infiltration into wounds and promotes repair (46). Liu et al. (45) reported decreased wound repair associated with defects in neutrophil migration to sites of dermal injury that was attributed to lack of FPR1 and FPR2 receptors. In our study, we also observed decreased neutrophil recruitment in healing intestinal mucosal wounds of mice lacking FPR2/3 receptors (Supplemental Fig. S3). Given the current appreciation of mononuclear cells in regulating tissue repair and our previous findings showing the importance of inflammatory monocytes in orchestrating colonic mucosal wound repair (47), we performed studies to investigate the contribution FPR2/3 in monocyte-mediated intestinal mucosal wound repair.
Infiltrating monocytes/macrophages accumulate in close proximity to wound-associated healing epithelial cells (2, 48). In the current study, we observed delayed recovery from DSS-induced colitis and biopsy-induced mucosal wound repair in Fpr2/3−/− compared with WT mice associated with decreased recruitment of Ly6Chi inflammatory monocytes into healing mucosal wounds normally observed within a day following biopsy-induced injury. BM transplant experiments revealed contribution of epithelial and immune cell expression of Fpr2/3 in orchestrating mucosal repair. A previous study reported shortened colonic crypts with reduced epithelial proliferation, decreased acute inflammatory response, and delayed recovery from acute DSS colitis in Fpr2-deficient mice compared with WT mice (25). Additionally, mice with myeloid-specific Fpr2 deletion (Fpr2f/f;LysMcre) displayed a moderate reduction in disease associated with decreased myeloid cell recruitment into the colonic mucosa without a change in mucosal recovery (25). The differences in our findings compared with those reported (25) might be related to the reparative experimental models, mouse strains (19), and institutional animal facilities.
The importance of FPRs on immune cells in the context of host defense during inflammatory diseases has been previously described (49–52). Monocyte recruitment to specific tissues is mediated through activation of specific surface receptors by various ligands, including chemokines. Chemokine receptor signaling is critical for chemotactic movement and cell activation, and FPR signaling has been implicated in desensitization and internalization of chemotactic receptors, such as CXCR1, PAFR, C5aR, CCR1, CCR5, and CXCR4 (53–58).
We observed selective up-regulation of Ccr6 in monocytes isolated from wounds on d 1 of WT mice, which was not detected in wounds from Fpr2/3−/− mice, suggesting a functional relationship between these 2 receptors. Furthermore, we observed reduced CCR6 surface receptor expression on Fpr2/3−/− splenic monocytes compared with WT cells, suggesting that the expression of CCR6 is linked to Fpr2/3. Although CCL20 expression is tightly regulated under normal physiologic processes, aberrant expression of CCR6 and CCL20 has also been linked to pathologic inflammation, as reviewed in ref. 59. Epithelial expression of CCL20 has been shown to induce chemotaxis of inflammatory monocytes and monocyte-derived dendritic cells in response to inflammation (15, 38, 39). Inflammatory mediators, such as TNF-α, have been shown to up-regulate Ccl20 mRNA and protein expression in colonic epithelial cells (13) as well as play an important role in intestinal wound repair in humans and mice (60, 61). Indeed, we found elevated Ccl20 mRNA and protein levels in harvested mucosal wounds. In humans, CCL20 levels are elevated in the injured/inflamed colonic mucosa of individuals with active ulcerative colitis (UC) (62).
In summary, we found that epithelial and immune cell expression of FPR2/3 is required for colonic mucosal repair by regulating monocyte chemotaxis through the CCL20-CCR6 signaling axis. The reduced monocyte influx into the wound bed might cause a lack of neutrophil efferocytosis, altered production of SPMs, and reduced macrophage differentiation, thus interrupting the process of resolution and resulting in chronic inflammation and impaired wound healing. Our results show that FPR2 promotes resolution of inflammation through the recruitment of inflammatory monocytes into the sites of injury, therefore transducing proinflammatory responses. Under physiologic conditions, this acute inflammatory response sets the stage for a complex process leading to resolution. In chronic diseases, such as UC, or conditions associated with vascular compromise, including ischemia or atherosclerosis, the shift from inflammation to resolution is impaired, leading to a nonresolving state (chronic inflammation) and persistent tissue damage (2–5). Under such conditions, there is persistent high expression of FPR2, as observed in biopsies of patients with UC as well as in atherosclerotic lesions (63). Due to altered resolution signals, down-regulation of FPR2 expression does not occur and serves to perpetuate a chronic inflammatory response. An example is highlighted by the increased macrophage infiltration observed in Fpr2+/+ compared with Fpr2−/− mice suffering from atherosclerosis (63). It is possible that during long-term disease processes, proinflammatory and anti-inflammatory responses resolve, leading to UC remission or plaque stabilization even with persistent elevation of FPR2 receptors. Our results highlight the importance of Fpr2 in mediating resolution of inflammation by influencing monocyte cell trafficking and could have clinical implications that include the use of FPR2 agonists to promote monocyte migration and neutrophil clearance and enhance intestinal mucosal wound repair in chronic intestinal inflammatory disorders, such as inflammatory bowel disease.
ACKNOWLEDGMENTS
The authors thank Prof. Mauro Perretti (Queen Mary University of London London, United Kingdom) for providing the Fpr2/3−/− mice, and Madeline R. Barron and Darius Feier (University of Michigan) for experimental help during their experimental rotation in the researchers’ laboratory. This work was supported by U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grants DK055679, DK089763, and DK059888 (to A.N.) and DK072564, DK079392, and DK061379 (to C.A.P.); German Research Foundation (DFG) Research Fellowship SI 2282/1-1 (to D.B.); and Crohn’s and Colitis Foundation Career Development Award 544599 (to M.Q.). The authors declare no conflicts of interest.
Glossary
- ALX
receptor for lipoxin A4
- APC
allophycocyanin
- BM
bone marrow
- CBC
complete blood cell
- CCL
CC chemokine ligand
- CCR
CC chemokine receptor
- CXCL
chemokine (C-X-C motif) ligand
- DAI
Disease Activity Index
- DSS
dextran sodium sulfate
- FBS
fetal bovine serum
- FPR
formyl peptide receptor
- PE
phycoerythrin
- qPCR
quantitative PCR
- Siglec
sialic acid-binding immunoglobulin-like lectin
- TBP
TATA-box-binding protein
- UC
ulcerative colitis
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. Birkl, M. N. O’Leary, M. Quiros, N. W. Lukacs, C. A. Parkos, and A. Nusrat designed research and analyzed the data; D. Birkl, M. N. O’Leary, M. Quiros, V. Azcutia, M. Schaller, M. Reed, H. Nishio, and J. Keeney performed experiments; D. Birkl, M. N. O’Leary, M. Quiros, A. S. Neish, N. W. Lukacs, C. A. Parkos, and A. Nusrat wrote, reviewed, and edited the manuscript; and A. S. Neish and A. Nusrat acquired funding.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Kagnoff M. F. (2014) The intestinal epithelium is an integral component of a communications network. J. Clin. Invest. 124, 2841–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leoni G., Neumann P. A., Kamaly N., Quiros M., Nishio H., Jones H. R., Sumagin R., Hilgarth R. S., Alam A., Fredman G., Argyris I., Rijcken E., Kusters D., Reutelingsperger C., Perretti M., Parkos C. A., Farokhzad O. C., Neish A. S., Nusrat A. (2015) Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J. Clin. Invest. 125, 1215–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan C. (2002) Points of control in inflammation. Nature 420, 846–852 [DOI] [PubMed] [Google Scholar]
- 4.Serhan C. N., Savill J. (2005) Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197 [DOI] [PubMed] [Google Scholar]
- 5.Fullerton J. N., Gilroy D. W. (2016) Resolution of inflammation: a new therapeutic frontier. Nat. Rev. Drug Discov. 15, 551–567 [DOI] [PubMed] [Google Scholar]
- 6.Chen K., Le Y., Liu Y., Gong W., Ying G., Huang J., Yoshimura T., Tessarollo L., Wang J. M. (2010) A critical role for the g protein-coupled receptor mFPR2 in airway inflammation and immune responses. J. Immunol. 184, 3331–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luissint A. C., Parkos C. A., Nusrat A. (2016) Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 151, 616–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy P. M., Baggiolini M., Charo I. F., Hébert C. A., Horuk R., Matsushima K., Miller L. H., Oppenheim J. J., Power C. A. (2000) International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 52, 145–176 [PubMed] [Google Scholar]
- 9.Hughes C. E., Nibbs R. J. B. (2018) A guide to chemokines and their receptors. FEBS J. 285, 2944–2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harant H., Eldershaw S. A., Lindley I. J. (2001) Human macrophage inflammatory protein-3alpha/CCL20/LARC/Exodus/SCYA20 is transcriptionally upregulated by tumor necrosis factor-alpha via a non-standard NF-kappaB site. FEBS Lett. 509, 439–445 [DOI] [PubMed] [Google Scholar]
- 11.Starner T. D., Barker C. K., Jia H. P., Kang Y., McCray P. B., Jr (2003) CCL20 is an inducible product of human airway epithelia with innate immune properties. Am. J. Respir. Cell Mol. Biol. 29, 627–633 [DOI] [PubMed] [Google Scholar]
- 12.Fujiie S., Hieshima K., Izawa D., Nakayama T., Fujisawa R., Ohyanagi H., Yoshie O. (2001) Proinflammatory cytokines induce liver and activation-regulated chemokine/macrophage inflammatory protein-3alpha/CCL20 in mucosal epithelial cells through NF-kappaB [correction of NK-kappaB]. Int. Immunol. 13, 1255–1263; erratum: 1443 [DOI] [PubMed] [Google Scholar]
- 13.Kwon J. H., Keates S., Bassani L., Mayer L. F., Keates A. C. (2002) Colonic epithelial cells are a major site of macrophage inflammatory protein 3alpha (MIP-3alpha) production in normal colon and inflammatory bowel disease. Gut 51, 818–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baba M., Imai T., Nishimura M., Kakizaki M., Takagi S., Hieshima K., Nomiyama H., Yoshie O. (1997) Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J. Biol. Chem. 272, 14893–14898 [DOI] [PubMed] [Google Scholar]
- 15.Dieu-Nosjean M. C., Massacrier C., Homey B., Vanbervliet B., Pin J. J., Vicari A., Lebecque S., Dezutter-Dambuyant C., Schmitt D., Zlotnik A., Caux C. (2000) Macrophage inflammatory protein 3alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J. Exp. Med. 192, 705–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Power C. A., Church D. J., Meyer A., Alouani S., Proudfoot A. E., Clark-Lewis I., Sozzani S., Mantovani A., Wells T. N. (1997) Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3alpha from lung dendritic cells. J. Exp. Med. 186, 825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He H. Q., Ye R. D. (2017) The formyl peptide receptors: diversity of ligands and mechanism for recognition. Molecules 22, E455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckley C. D., Gilroy D. W., Serhan C. N. (2014) Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40, 315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dufton N., Hannon R., Brancaleone V., Dalli J., Patel H. B., Gray M., D’Acquisto F., Buckingham J. C., Perretti M., Flower R. J. (2010) Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J. Immunol. 184, 2611–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen K., Bao Z., Gong W., Tang P., Yoshimura T., Wang J. M. (2017) Regulation of inflammation by members of the formyl-peptide receptor family. J. Autoimmun. 85, 64–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye R. D., Boulay F., Wang J. M., Dahlgren C., Gerard C., Parmentier M., Serhan C. N., Murphy P. M. (2009) International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61, 119–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babbin B. A., Jesaitis A. J., Ivanov A. I., Kelly D., Laukoetter M., Nava P., Parkos C. A., Nusrat A. (2007) Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J. Immunol. 179, 8112–8121 [DOI] [PubMed] [Google Scholar]
- 23.Babbin B. A., Laukoetter M. G., Nava P., Koch S., Lee W. Y., Capaldo C. T., Peatman E., Severson E. A., Flower R. J., Perretti M., Parkos C. A., Nusrat A. (2008) Annexin A1 regulates intestinal mucosal injury, inflammation, and repair. J. Immunol. 181, 5035–5044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babbin B. A., Lee W. Y., Parkos C. A., Winfree L. M., Akyildiz A., Perretti M., Nusrat A. (2006) Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J. Biol. Chem. 281, 19588–19599 [DOI] [PubMed] [Google Scholar]
- 25.Chen K., Liu M., Liu Y., Yoshimura T., Shen W., Le Y., Durum S., Gong W., Wang C., Gao J. L., Murphy P. M., Wang J. M. (2013) Formylpeptide receptor-2 contributes to colonic epithelial homeostasis, inflammation, and tumorigenesis. J. Clin. Invest. 123, 1694–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leoni G., Alam A., Neumann P. A., Lambeth J. D., Cheng G., McCoy J., Hilgarth R. S., Kundu K., Murthy N., Kusters D., Reutelingsperger C., Perretti M., Parkos C. A., Neish A. S., Nusrat A. (2013) Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J. Clin. Invest. 123, 443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alam A., Leoni G., Wentworth C. C., Kwal J. M., Wu H., Ardita C. S., Swanson P. A., Lambeth J. D., Jones R. M., Nusrat A., Neish A. S. (2014) Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 7, 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Migeotte I., Communi D., Parmentier M. (2006) Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 17, 501–519 [DOI] [PubMed] [Google Scholar]
- 29.Muto Y., Guindon S., Umemura T., Kőhidai L., Ueda H. (2015) Adaptive evolution of formyl peptide receptors in mammals. J. Mol. Evol. 80, 130–141 [DOI] [PubMed] [Google Scholar]
- 30.Gobbetti T., Coldewey S. M., Chen J., McArthur S., le Faouder P., Cenac N., Flower R. J., Thiemermann C., Perretti M. (2014) Nonredundant protective properties of FPR2/ALX in polymicrobial murine sepsis. Proc. Natl. Acad. Sci. USA 111, 18685–18690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen K., Liu M., Liu Y., Wang C., Yoshimura T., Gong W., Le Y., Tessarollo L., Wang J. M. (2013) Signal relay by CC chemokine receptor 2 (CCR2) and formylpeptide receptor 2 (Fpr2) in the recruitment of monocyte-derived dendritic cells in allergic airway inflammation. J. Biol. Chem. 288, 16262–16273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gouwy M., Struyf S., Leutenez L., Pörtner N., Sozzani S., Van Damme J. (2014) Chemokines and other GPCR ligands synergize in receptor-mediated migration of monocyte-derived immature and mature dendritic cells. Immunobiology 219, 218–229 [DOI] [PubMed] [Google Scholar]
- 33.Kucharzik T., Hudson J. T., III, Waikel R. L., Martin W. D., Williams I. R. (2002) CCR6 expression distinguishes mouse myeloid and lymphoid dendritic cell subsets: demonstration using a CCR6 EGFP knock-in mouse. Eur. J. Immunol. 32, 104–112 [DOI] [PubMed] [Google Scholar]
- 34.Laukoetter M. G., Nava P., Lee W. Y., Severson E. A., Capaldo C. T., Babbin B. A., Williams I. R., Koval M., Peatman E., Campbell J. A., Dermody T. S., Nusrat A., Parkos C. A. (2007) JAM-A regulates permeability and inflammation in the intestine in vivo. J. Exp. Med. 204, 3067–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Y., Murphy P. M., Wang J. M. (2002) Formyl-peptide receptors revisited. Trends Immunol. 23, 541–548 [DOI] [PubMed] [Google Scholar]
- 36.Le Y., Oppenheim J. J., Wang J. M. (2001) Pleiotropic roles of formyl peptide receptors. Cytokine Growth Factor Rev. 12, 91–105 [DOI] [PubMed] [Google Scholar]
- 37.Wynn T. A., Vannella K. M. (2016) Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook D. N., Prosser D. M., Forster R., Zhang J., Kuklin N. A., Abbondanzo S. J., Niu X. D., Chen S. C., Manfra D. J., Wiekowski M. T., Sullivan L. M., Smith S. R., Greenberg H. B., Narula S. K., Lipp M., Lira S. A. (2000) CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12, 495–503 [DOI] [PubMed] [Google Scholar]
- 39.Le Borgne M., Etchart N., Goubier A., Lira S. A., Sirard J. C., van Rooijen N., Caux C., Aït-Yahia S., Vicari A., Kaiserlian D., Dubois B. (2006) Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity 24, 191–201 [DOI] [PubMed] [Google Scholar]
- 40.Brown A. J., Sepuru K. M., Sawant K. V., Rajarathnam K. (2017) Platelet-derived chemokine CXCL7 dimer preferentially exists in the glycosaminoglycan-bound form: implications for neutrophil-platelet crosstalk. Front. Immunol. 8, 1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menzies-Gow A., Ying S., Sabroe I., Stubbs V. L., Soler D., Williams T. J., Kay A. B. (2002) Eotaxin (CCL11) and eotaxin-2 (CCL24) induce recruitment of eosinophils, basophils, neutrophils, and macrophages as well as features of early- and late-phase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J. Immunol. 169, 2712–2718 [DOI] [PubMed] [Google Scholar]
- 42.Provoost S., Maes T., Joos G. F., Tournoy K. G. (2012) Monocyte-derived dendritic cell recruitment and allergic T(H)2 responses after exposure to diesel particles are CCR2 dependent. J. Allergy Clin. Immunol. 129, 483–491 [DOI] [PubMed] [Google Scholar]
- 43.Domínguez P. M., Ardavín C. (2010) Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol. Rev. 234, 90–104 [DOI] [PubMed] [Google Scholar]
- 44.McArthur S., Gobbetti T., Kusters D. H., Reutelingsperger C. P., Flower R. J., Perretti M. (2015) Definition of a novel pathway centered on lysophosphatidic acid to recruit monocytes during the resolution phase of tissue inflammation. J. Immunol. 195, 1139–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu M., Chen K., Yoshimura T., Liu Y., Gong W., Le Y., Gao J. L., Zhao J., Wang J. M., Wang A. (2014) Formylpeptide receptors mediate rapid neutrophil mobilization to accelerate wound healing. PLoS One 9, e90613; erratum: e99541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwon Y. W., Heo S. C., Jang I. H., Jeong G. O., Yoon J. W., Mun J. H., Kim J. H. (2015) Stimulation of cutaneous wound healing by an FPR2-specific peptide agonist WKYMVm. Wound Repair Regen. 23, 575–582 [DOI] [PubMed] [Google Scholar]
- 47.Quiros M., Nishio H., Neumann P. A., Siuda D., Brazil J. C., Azcutia V., Hilgarth R., O’Leary M. N., Garcia-Hernandez V., Leoni G., Feng M., Bernal G., Williams H., Dedhia P. H., Gerner-Smidt C., Spence J., Parkos C. A., Denning T. L., Nusrat A. (2017) Macrophage-derived IL-10 mediates mucosal repair by epithelial WISP-1 signaling. J. Clin. Invest. 127, 3510–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pull S. L., Doherty J. M., Mills J. C., Gordon J. I., Stappenbeck T. S. (2005) Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. USA 102, 99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Southgate E. L., He R. L., Gao J. L., Murphy P. M., Nanamori M., Ye R. D. (2008) Identification of formyl peptides from Listeria monocytogenes and Staphylococcus aureus as potent chemoattractants for mouse neutrophils. J. Immunol. 181, 1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu M., Chen K., Yoshimura T., Liu Y., Gong W., Wang A., Gao J. L., Murphy P. M., Wang J. M. (2012) Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci. Rep. 2, 786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao J. L., Lee E. J., Murphy P. M. (1999) Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J. Exp. Med. 189, 657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giebeler A., Streetz K. L., Soehnlein O., Neumann U., Wang J. M., Brandenburg L. O. (2014) Deficiency of formyl peptide receptor 1 and 2 is associated with increased inflammation and enhanced liver injury after LPS-stimulation. PLoS One 9, e100522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blackwood R. A., Hartiala K. T., Kwoh E. E., Transue A. T., Brower R. C. (1996) Unidirectional heterologous receptor desensitization between both the fMLP and C5a receptor and the IL-8 receptor. J. Leukoc. Biol. 60, 88–93 [DOI] [PubMed] [Google Scholar]
- 54.Ali H., Richardson R. M., Haribabu B., Snyderman R. (1999) Chemoattractant receptor cross-desensitization. J. Biol. Chem. 274, 6027–6030 [DOI] [PubMed] [Google Scholar]
- 55.Tomhave E. D., Richardson R. M., Didsbury J. R., Menard L., Snyderman R., Ali H. (1994) Cross-desensitization of receptors for peptide chemoattractants. Characterization of a new form of leukocyte regulation. J. Immunol. 153, 3267–3275 [PubMed] [Google Scholar]
- 56.Li B. Q., Wetzel M. A., Mikovits J. A., Henderson E. E., Rogers T. J., Gong W., Le Y., Ruscetti F. W., Wang J. M. (2001) The synthetic peptide WKYMVm attenuates the function of the chemokine receptors CCR5 and CXCR4 through activation of formyl peptide receptor-like 1. Blood 97, 2941–2947 [DOI] [PubMed] [Google Scholar]
- 57.Shen W., Li B., Wetzel M. A., Rogers T. J., Henderson E. E., Su S. B., Gong W., Le Y., Sargeant R., Dimitrov D. S., Oppenheim J. J., Wang J. M. (2000) Down-regulation of the chemokine receptor CCR5 by activation of chemotactic formyl peptide receptor in human monocytes. Blood 96, 2887–2894 [PubMed] [Google Scholar]
- 58.Bednar F., Song C., Bardi G., Cornwell W., Rogers T. J. (2014) Cross-desensitization of CCR1, but not CCR2, following activation of the formyl peptide receptor FPR1. J. Immunol. 192, 5305–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schutyser E., Struyf S., Van Damme J. (2003) The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 14, 409–426 [DOI] [PubMed] [Google Scholar]
- 60.Bradford E. M., Ryu S. H., Singh A. P., Lee G., Goretsky T., Sinh P., Williams D. B., Cloud A. L., Gounaris E., Patel V., Lamping O. F., Lynch E. B., Moyer M. P., De Plaen I. G., Shealy D. J., Yang G. Y., Barrett T. A. (2017) Epithelial TNF receptor signaling promotes mucosal repair in inflammatory bowel disease. J. Immunol. 199, 1886–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Birkl D., Quiros M., Garcia-Hernandez V., Zhou D. W., Brazil J. C., Hilgarth R., Keeney J., Yulis M., Bruewer M., Garcia A. J., O Leary M. N., Parkos C. A., Nusrat A. (2019) TNFα promotes mucosal wound repair through enhanced platelet activating factor receptor signaling in the epithelium. Mucosal Immunol. 12, 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trivedi P. J., Adams D. H. (2018) Chemokines and chemokine receptors as therapeutic targets in inflammatory bowel disease; pitfalls and promise. J. Crohn’s Colitis 12, 1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petri M. H., Laguna-Fernández A., Gonzalez-Diez M., Paulsson-Berne G., Hansson G. K., Bäck M. (2015) The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc. Res. 105, 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.