Abstract
We evaluated functional measures of neuromuscular integrity and bone’s resistance to fracture as a combined tool in discriminating osteoporosis patients with and without fractures. Functional aspects of neuromuscular integrity were quantified with a noninvasive measure of static and dynamic functional postural stability (FPS), and fracture resistance was obtained with bone shock absorption in patients with osteoporosis aged 65–85 and compared our measures with dual-energy X-ray absorptiometry and Fracture Risk Assessment Tool (FRAX [World Health Organization Collaborating Center for Metabolic Bone Diseases, Sheffield, UK]) in women with osteoporosis, some with and some without vertebral fractures. Patients with vertebral fracture showed larger static FPS (postural sway excursion) in the mediolateral and anterior-posterior directions, suggesting poorer balance. Most of the variables of dynamic FPS showed significant differences between fracture and no-fracture groups (e.g., the fracture group took significantly longer during turning, implying poorer dynamic balance control). Also, compared with healthy control subjects, all 4 dynamic FPS responses for osteoporosis patients with and without fracture were significantly poorer, suggesting potential risk for falls. In summary, patients with osteoporosis who have vertebral fractures (compared with patients with similarly low bone mineral density and other nonfracture risk fractures) have not only lower bone shock absorption damping (ζ) but also increased postural imbalance.
Keywords: Bone shock absorption, dynamic bone quality, fracture, functional postural balance, osteoporosis
Introduction
Improved measures of fracture discrimination are needed as bone mineral density (BMD) alone is a crude predictor of fracture risk (1–9). The Fracture Risk Assessment Tool (FRAX [World Health Organization Collaborating Center for Metabolic Bone Diseases, Sheffield, UK]) (including clinical risk factors) is better than BMD alone but does not include falling, a major contributing factor to fracture (1,10–17). Bone shock absorption (BSA) and static and dynamic functional postural balance/stability (FPS) (13,18–21) encompass measures of structural bone health and neuromuscular integrity, which are both compromised in osteoporosis (1,16,22–26). The purpose of this study was to evaluate functional measures of bone’s resistance to fracture and neuromuscular integrity in discriminating osteoporosis patients with and without fractures.
The musculoskeletal system consists of natural shock absorbers (soft tissue, joints with cartilage and synovial fluids, and trabecular bone, including mineralized collagen fibrils, nonfibrillar organic matrix, and noncollagenous proteins in bone), which absorb external loads of activities of daily life, minimizing the potential for fractures (27–33). Although each natural shock absorber provides different degrees of absorption, ranging from 11% to 70% based on loading frequency, the collective absorption capacities help minimize the potential for fracture (34). We developed BSA, a quantitative measure of combined bone and musculature (CBAM) system’s resistance to fracture when exposed to dynamic loads such as heel strike (35). Damping, the BSA metric, as a measure of fracture resistance in the musculoskeletal system, is supported by classical structural engineering studies quantifying composite materials’ and structures’ ability to absorb/dissipate external loads (36–38). Bone, as a composite material, has mineralized collagen fibrils and nonfibrillar organic matrix, which can dissipate external loads to decrease fracture risk (39–42). Resistance to fracture depends on the musculoskeletal systems’ ability to absorb and/or dissipate externally applied loads but not necessarily the maximum strength of the structure (42–44). In osteoporosis, both bone integrity and the neuromuscular system are compromised, as evidenced by higher prevalence of fractures and falls (1,16,22–26). Although our previous publications showed that BSA discriminates between fracture and no-fracture groups, we have not looked at its association with postural instability, another risk factor for fracture (35). One of our goals was to test the hypothesis that osteoporosis patients with decreased bone damping capacity (i.e., stiffer, more brittle bone) have increased postural sway as measured by FPS outcomes.
The objective of this study was to determine the fracture discriminating abilities of BSA and FPS in patients with osteoporosis, aged 65–85. Secondary objectives were to investigate contributions of static postural balance (postural sway), dynamic FPS outcomes, damping and age in modifying the FRAX scores and contributions of damping, BMD and age in modifying static postural balance outcomes and dynamic FPS outcomes.
Materials and Methods
Subjects
The University Institutional Review Board approved the protocol, and all subjects gave informed consent. We recruited postmenopausal women with osteoporosis from a convenience sample of patients who already had dual-energy X-ray absorptiometry and vertebral fracture assessment or lateral spine radiographs. One investigator (NBW) was aware of the subjects’ fracture status but was blinded to the BSA test results; the other investigators were aware of the BSA test results but blinded about the fracture status.
Bone Densitometry, FRAX Scores, and Fracture Assessment
BMD results for spine, femoral neck (FN), and total hip (TH) were obtained using Hologic equipment performed within 12 mo of the study entry. FRAX scores were calculated using version 3.8 without BMD and with lowest ever FN (BMD_FN). Vertebral fractures were assessed from lateral spine images acquired using dual-energy X-ray absorptiometry or lateral spine X-rays; reduction in anterior, middle, and/or posterior vertebral height by ≥20% constituted a fracture (45). Seven of the subjects had 1 or more vertebral fractures, whereas 22 had no vertebral fractures.
Dynamic Bone Quality Measure
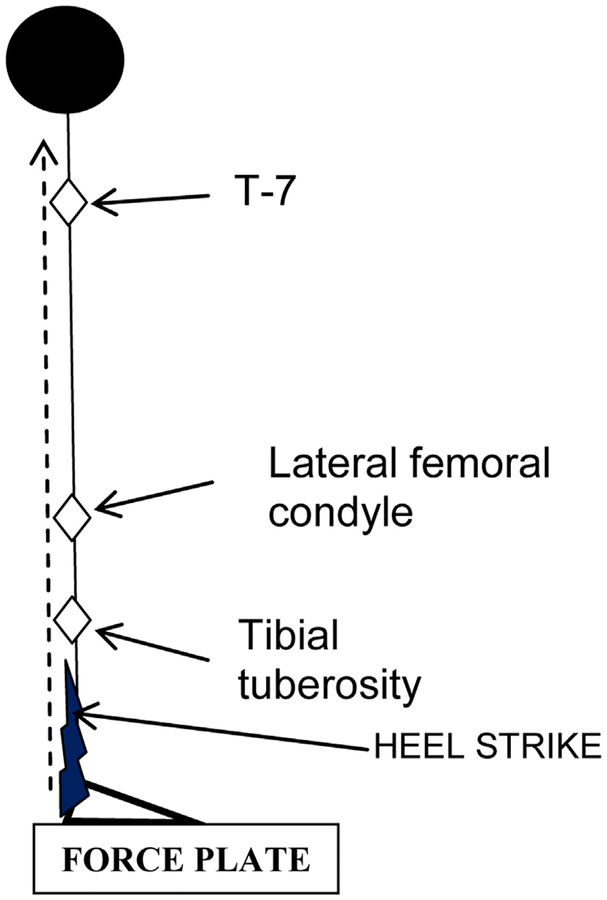
CBAM capacity outcome, damping (ζ), was quantified from acceleration measurements at 3 anatomical sites (3,35) (Fig. 1): (1) below the knee at the tibial tuberosity (ζBELOWKNEE-R and ζBELOWKNEE-L; R for right heel strike and L for left heel strike), (2) above knee at the lateral femoral condyle (ζABOVEKNEE-R and ζABOVEKNEE-L) for each leg, and (3) upper back (T-7) (ζUPPERBACK-R and ζUPPERBACK-L). Low mass skin-mounted accelerometers were attached to bony prominences, and signals obtained as per our previous publication (35). Each subject performed 5 stationary tasks, lifting the bare foot and placing it down with the heel striking the force platform with a force equivalent (or slightly higher) than used for during natural walking. Our custom BSA software (BSA software 2009–15; University of Cincinnati, Cincinnati, OH) was used to collect data and calculate shock absorption response variables. An average of 5 heel strikes was used for statistical analysis. Reproducibility of BSA test trials was demonstrated in our previous study (46). CBAM damping (ζ) and resonance/dominant frequencies at anatomical sites were calculated as described previously (3,35,46).
Fig. 1.
Schematic of accelerometer-placement sites for bone shock absorption test.
Measures of Static and Dynamic FPS
Each subject underwent 2 tests: (1) static postural balance standing on a force platform system (this quantifies movement of center of pressure [CP] as a measure of static postural balance) (47) and (2) dynamic FPS test with instrumented timed up and go (iTUG) protocol as described herein:
Static postural balance test: Each subject performed tests addressing vision, proprioception, and the vestibular system in maintaining upright balance using our published protocol (47). Tests were performed with subjects standing on a force platform (1) with eyes open (EO) and eyes closed (EC) standing directly on the platform and (2) with eyes open (FO) and eyes closed (FC) on a 4 inch thick foam pad placed on the platform. Outcome variables of postural balance tests are sway area (SA; cm2), sway length (SL; cm), excursion in mediolateral direction (Excur-ML; cm), and excursion in anterior-posterior direction (Excur-AP; cm), which are significant discriminators of faller status (26). The SA is encompassed by movement patterns of X-Y coordinates of CP movements during postural balance tests. SL is the total distance traveled by the subjects’ CP during postural balance tests. The Excur-ML and Excur-AP are maximum displacements of subject’s CP in the ML and AP directions, respectively, during postural balance tests.
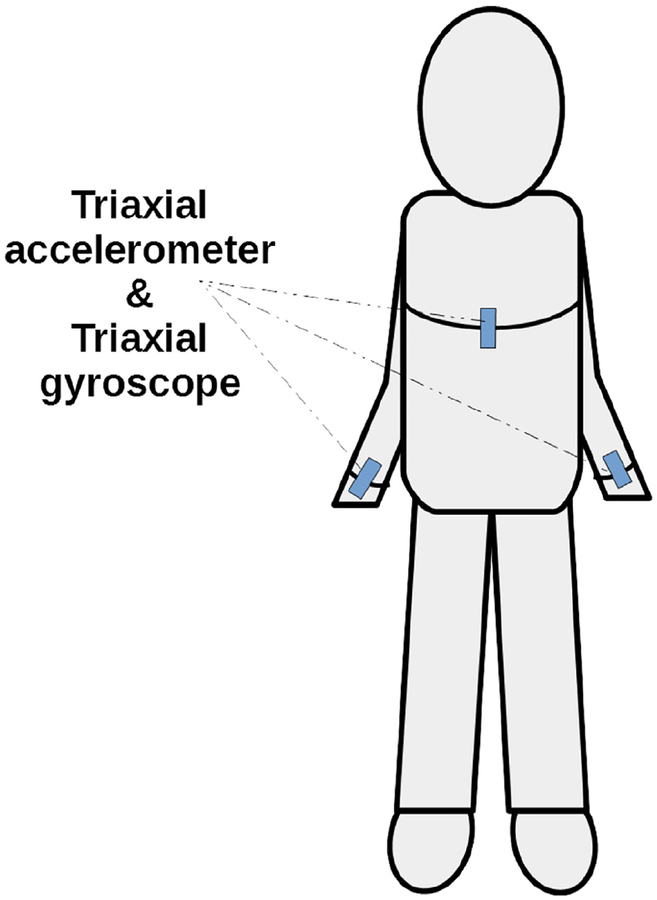
Dynamic FPS iTUG test: he FPS iTUG test is performed using an inertial link 6D sensor system (3-dimensional accelerometers and 3-dimensional gyroscopes) for quantifying dynamic FPS (48,49). The wireless sensor is attached to the chest and 2 wrists, as per the protocol of Zampieri et al and others (48,49) (Fig. 2). The TUG test is designed to assess balance control status during the dynamic task of getting up from a chair, walking, and turning (50–54). The outcomes of TUG test are (1) peak turn velocity (PTV, deg/s) of torso, (2) peak swing velocity of right arm (PSVr, deg/s), (3) peak swing velocity of left arm (PSVl, deg/s), (4) turn duration (TD, s), (5) average turning velocity of torso (degrees/s), and (6) range of motion (ROM left and right arms) about the shoulder joint (in the sagittal plane), spanned by each arm swing during walking (degrees). The receiver operating characteristic (ROC) analysis of data from TUG test revealed that these variables have high discrimination for postural mobility between healthy and diseased patients’ values with area under the curve (AUC) ranging between 0.76 and 0.87 (55–58).
Fig. 2.
Schematic of triaxial accelerometers and gyroscopes—placement sites for the functional postural stability instrumented timed up and go test.
Data Analysis
Data are described using mean, standard deviation, or standard error. Because of the small sample size, outcomes were compared between fracture and no-fracture groups using Wilcoxon rank sum test. As a pilot study with expected directions of all outcomes’ responses, a 1-sided p < 0.10 was used for statistical significance. The ROC curve analysis was carried out for damping, static postural balance outcomes (postural sway variables), dynamic FPS (or balance [FPS]; iTUG outcomes), and BMD measures for differentiating facture from no-fracture groups. The results of ROC analysis are summarized with AUC. Spearman rank correlations (r) were obtained among static and dynamic FPS outcomes with damping outcomes, BMD outcomes, and FRAX outcomes. Similarly, Spearman rank correlations were also computed between outcomes for damping BMD and FRAX. Because of smaller sample size for the fracture group (n = 7), correlations among outcome variables were carried out for the no-fracture group (N = 22) only. In addition, exploratory multivariable regression analysis was also done. Two regression models were developed: for model 1, the dependent variable was FRAX score (based on lowest ever BMD) and independent variables were static postural balance outcomes for FO and FC, dynamic FPS, damping, and age. We first assessed the univariate association of cofactors using ordinary linear regression; significant cofactors at 20% level of significance in the univariate regression analyses with known expected directions were included in the multivariable linear regression analysis. Backward elimination was used, starting from the full model, including all independent effects, then deleting effects 1 by 1 until a stopping condition (p = 0.10; 1 sided) was satisfied. Similarly, for model 2, individual regression models were developed for each of the dependent variables of static postural balance and dynamic FPS outcomes. The results of regression model are reported using regression coefficient, standard error of regression coefficient, 1-tailed p value along with model-adjusted coefficient of determination (R2). Statistical software used was SAS, version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Table 1 provides demographic data, fracture status, BMD scores, and FRAX scores for the study groups. There were no significant differences in body weight, height, BMI, and BMD between groups except that on the average the fracture group was 6 yr older than the no-fracture group and FRAX scores were higher for the fracture group (Table 1). Except for fractures, which increase the FRAX scores, the 2 groups had similar risk based on other risk factors.
Table 1.
Demographics and Patient Characteristics of Fracture and No-Fracture Groups
| Variables | Fracture (N = 7) | No fracture (N = 22) | p (1 tailed) | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (yr) | 76.20 | 5.94 | 70.6 | 3.97 | 0.0233a |
| Height (cm) | 158.7 | 10.15 | 161.3 | 6.34 | 0.6463a |
| Weight (kg) | 62.9 | 5.96 | 61.5 | 10.57 | 0.6464a |
| BMI (kg/m2) | 25 | 2.29 | 23.6 | 3.97 | 0.1768a |
| BMD-femoral neck (g/cm2) | 0.586 | 0.087 | 0.597 | 0.052 | 0.3606 |
| BMD-total hip (g/cm2) | 0.699 | 0.074 | 0.724 | 0.062 | 0.2457 |
| FRAX-lowest BMD | 26.71 | 9.76 | 17.33 | 6.68 | 0.0053 |
| FRAX-lowest hip fracture | 9.91 | 7.82 | 5.98 | 5.09 | 0.0487 |
Abbr: BMD, bone mineral density; BMI, body mass index; FRAX, Fracture Risk Assessment Tool; SD, standard deviation.
Two tailed p value obtained from Wilcoxon rank sum test.
Comparison of Static and Dynamic FPS Responses Between Fracture and No-Fracture Groups
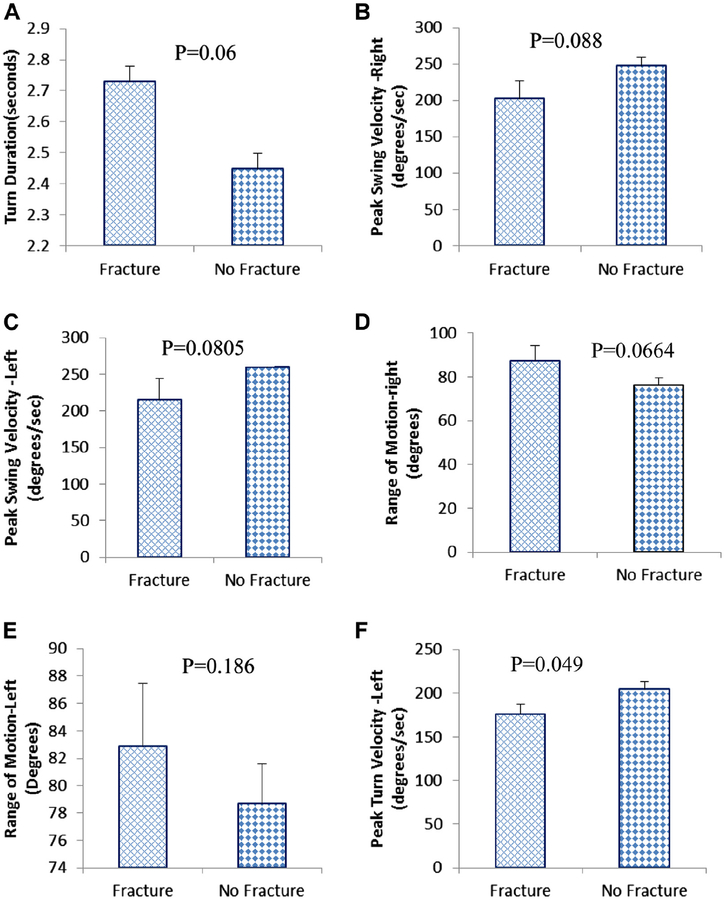
Five of 6 variables of dynamic FPS showed statistically significant differences (p values ranged between 0.0488 and 0.088) between fracture and no-fracture groups (Fig. 3A–F). For example, the fracture group took significantly longer (+12%) than the no-fracture group during turning (TD). ROM responses were higher for the fracture group, an unexpected finding. Static postural balance responses to all 4 test conditions were not statistically different between the groups.
Fig. 3.
Dynamic functional postural stability responses to timed up and go test. (A) Turn duration. (B) Peak swing velocity—right arm. (C) Peak swing velocity—left arm, (D) Range of motion—right arm. (E) Range of motion—left arm. (F) Peak turn velocity.
We also tested the diagnostic performance of static postural balance and dynamic FPS responses in differentiating fracture and no-fracture groups. Maximum AUC was for PTV (0.71, 95% confidence interval [CI]: 0.51–0.92, p = 0.045) followed by TD (0.70, 95% CI: 0.47–0.94, p = 0.057), left PSV (0.68, 95% CI: 0.41–0.95, p = 0.077), and right PSV (0.68, 95% CI: 0.42–0.93, p = 0.085). In the static postural balance responses, the AUC was statistically significant for Excur-ML (0.66, 95% CI: 0.41–0.91, p = 0.10) in FC test and SL (0.71, 95% CI: 0.53–0.89, p = 0.05) in EC test.
Comparison of Damping and BMD Values Between Fracture and No-Fracture Groups
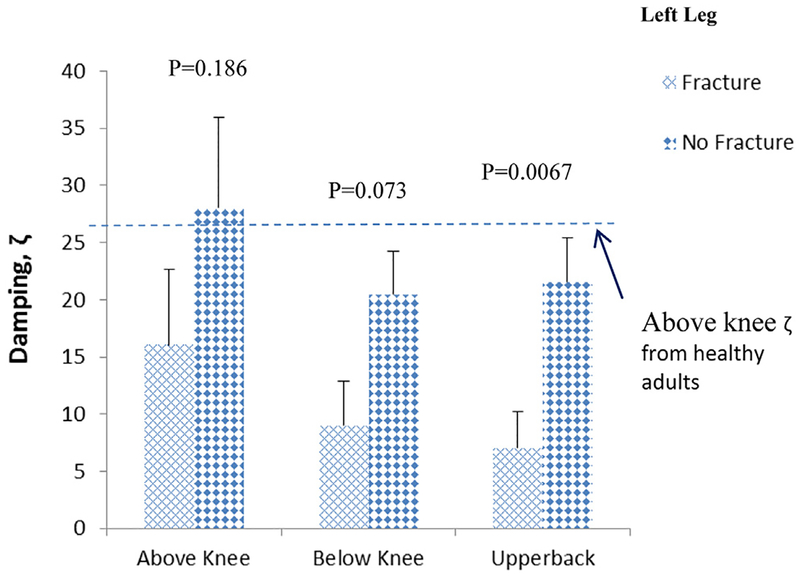
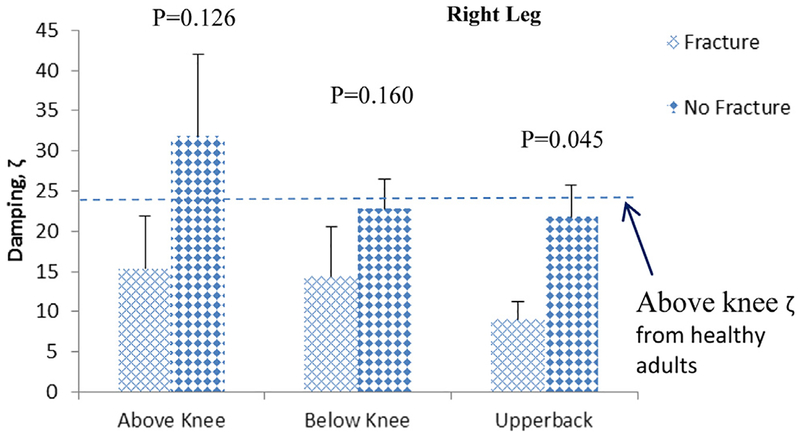
Regarding BSA outcomes, the left heel strike-associated mean damping (ζ) values for the fracture group were 55%, 42%, and 71% lower than for the no-fracture group for sites above knee, below knee, and upper back, respectively (Fig. 4). The right heel strike mean damping values for the fracture group were 50%, 38%, and 61% lower than values for no-fracture group for sites above knee, below knee, and upper back, respectively (Fig. 5). The AUC for damping upper back (ζUPPERBACK) was 0.72 (95% CI: 0.52–0.92, p = 0.043), followed by above knee (ζABOVEKNEE [0.65, 95% CI: 0.42–0.87]) and below knee (ζBELOWKNEE [0.62, 95% CI: 0.39–0.85]). Although the mean damping values for the fracture group were lower than the no-fracture group for all sites, the AUC of ζUPPERBACK was the only site reaching significance, possibly because of small sample size. AUCs of BMD measures were between 0.50 and 0.58. None of the BMD measures had significant AUC for differentiating fracture from no-fracture groups.
Fig. 4.
Mean ± standard error of the mean damping values at various anatomical sites of patients with osteoporosis for left leg.
Fig. 5.
Mean ± standard error of the mean damping values at various anatomical sites of patients with osteoporosis for right leg.
Associations Between Static Postural Balance and Damping
Three of 4 postural balance outcomes (SA, SL, and Excur-ML) for the FC test showed significant negative association with damping of the upper back or torso. Damping upper back (ζUPPERBACK) was correlated with SA (r = −0.046, p = 0.018), SL (r = −0.39, p = 0.039), and Excur-ML (r = −0.52, p = 0.007). On the other hand, although the Excur-AP was in the expected direction (i.e., negatively associated with damping), the differences between groups was not significant (Excur-AP [r = −0.27, p = 0.12]). Two of 4 postural sway outcomes (SL and Excur-ML) for the EC test showed significant negative association with ζUPPERBACK (r: −0.30 to −0.31; p: 0.085–0.09).
Associations Between Dynamic FPS and Damping
There were no consistent association among dynamic FPS outcomes and damping variables.
Associations Between Static Postural Balance and BMD
Higher BMD_TH was significantly associated with decrease in postural sway outcomes in all conditions, suggesting better postural balance. For 3 of 4 test conditions, EO, EC, and FO, all 4 postural sway outcomes (SA, SL, Excur-ML, and Excur-AP) were significantly negatively correlated with BMD_TH. The correlation coefficient ranged from −0.28 to −0.48. For the FC test condition, only 1 of 4 sway outcomes (Excur-AP, r = −0.31, p = 0.08) was significantly negatively associated with BMD_TH. BMD_FN showed significantly negative correlations with 3 of 4 postural sway outcomes (SA, SL, and Excur-ML) for the EC test condition. The correlation coefficient ranged from −0.36 to −0.52.
Associations Between Dynamic FPS and BMD
Higher BMD_TH (r = 0.57, p = 0.003) was significantly associated with an increase in PSVr, an increase in PTV (r = 0.32, p = 0.075), and a decrease in TD (r = −0.28; p = 0.10).
Associations Between Dynamic FPS and FRAX Scores
The FPS outcomes correlated negatively with FRAX scores—patients with higher FRAX scores had reduced dynamic functional balance, poorer balance, and increased potential of falling during walking. Of 6 FPS outcomes, 4 (mean PTV, mean PSV right arm, mean ROM right arm, and mean ROM left arm) showed significant negative associations with FRAX scores for major fracture risk (based on lowest BMD) (r = −0.53 to −0.40; p = 0.006–0.031) and hip fracture risk (r = −0.56 to −0.40; p = 0.0036–0.018). Similar correlations with FRAX scores without BMD were found.
Associations Between Static Postural Balance and FRAX Scores
Static postural sway was positively correlated with FRAX scores—patients with higher FRAX scores demonstrated higher postural sway, poorer balance, and increased potential of falling, even during static conditions. For the FO test, all sway outcomes demonstrated significant positive associations with FRAX scores (r = 0.55–0.28; p = 0.0041–0.11). For the EO test, except for SL (not significant), Excur-ML, Excur-AP, and SA were significantly associated with higher FRAX scores (r = 0.50–0.34; p = 0.009–0.062). For the FC test, only Excur-AP and SA were significantly associated with FRAX scores (r = 0.36–0.30; p = 0.048–0.085). For the EC test, only Excur-ML showed significant association with FRAX scores (r = 0.30; p = 0.089).
Associations Between Damping and FRAX Scores
There were no correlations between FRAX scores and damping values, but each was an independent discriminator of fracture groups (Figs. 4 and 5; Table 1).
Regression Models Relating Static and Dynamic FPS Outcomes to FRAX Scores
After covariate adjustment within the regression analysis for static postural sway outcomes, only Excur-ML sway for the FO test reached significance with FRAX score (based on lowest BMD) for major hip fracture risk (Table 2). Age was the only covariate significant with both FRAX scores. After covariate adjustment within the regression analysis for dynamic FPS outcomes, none of the variables reached significance with FRAX scores.
Table 2.
Regression Models Relating FRAX Scores to Static Postural Balance outcomes
| Dependent variable | Independent variable | Regression coefficient | Standard error | p (1 tailed) | Model R2 (adjusted) |
|---|---|---|---|---|---|
| FRAX based on lowest | Intercept | −43.17 | 18.63 | 0.014 | 0.42 |
| BMD major fracture risk | Age | 0.75 | 0.29 | 0.008 | |
| Excur-ML (FO) | 3.31 | 1.82 | 0.040 | ||
| FRAX based on lowest | Intercept | −32.28 | 14.58 | 0.018 | 0.30 |
| BMD hip fracture risk | Age | 0.47 | 0.23 | 0.025 | |
| Excur-ML (FO) | 1.96 | 1.42 | 0.090 |
Abbr: BMD, bone mineral density; Excur-ML, excursion in mediolateral direction; FO, eyes open standing on a 4 inch thick foam pad placed on the platform; FRAX, Fracture Risk Assessment Tool.
Regression Models Relating Damping to Dynamic FPS Outcomes
After covariate adjustment within the regression analysis, only ζABOVEKNEE reached significance with only 2 of the dynamic FPS variables, TD and PTV, respectively (Table 3). Table 3 provides 1-tailed p values. BMD_TH was the only covariate that was significant with both TD and PTV. Age was significant in the PTV regression model only.
Table 3.
Regression Models Relating Damping to Dynamic Functional Postural Balance/Stability Outcomes
| Dependent variable | Independent variable | Regression coefficient | Standard error | p (1 tailed) | Model R2 (adjusted) |
|---|---|---|---|---|---|
| TD | Intercept | 4.60 | 0.94 | <.0001 | 0.14 |
| ζABOVEKNEE-R | 0.00 | 0.00 | 0.018 | ||
| BMD_TH | −2.72 | 1.26 | 0.021 | ||
| PTV | Intercept | 156.62 | 166.13 | 0.178 | 0.26 |
| Age | −2.06 | 1.45 | 0.083 | ||
| ζABOVEKNEE-R | 0.31 | 0.19 | 0.052 | ||
| BMD TH | 250.09 | 122.38 | 0.026 |
Abbr: ζABOVEKNEE-R, above knee at the lateral femoral condyle; BMD_TH, total hip bone mineral density; PTV, peak turn velocity; R for right heel strike; TD, turn duration.
Regression Models Relating Damping to Static Postural Balance Outcomes
The regression models were developed only for FC and FO tests as they showed more consistent bivariate associations for multiple postural balance outcomes with damping. After covariate adjustment within the regression analysis, ζUPPERBACK-R reached significance with static postural balance variables SL and Excur-ML for FO and FC tests and with SA and Excur-AP for FC test. Table 4 provides 1-tailed p values. In addition, ζABOVEKNEE-R also reached significance with Excur-ML for FO and FC tests, whereas ζABOVEKNEE-L was significantly associated with Excur-AP for the FC test only. Only 1 of 4 damping outcomes, ζBELOWKNEE-R, showed an unexpected positive relationship with postural balance outcomes; this may be because of chance and/or small sample size. Covariate of age, as expected, showed significant positive relationships with all postural balance outcomes for all test conditions, consistent with previous studies (55–58).
Table 4.
Regression Models Relating Damping to Static Postural Balance Outcomes
| Test | Dependent variable | Independent variable | Regression coefficient | Standard error | p (1 tailed) | Model R2 (adjusted) |
|---|---|---|---|---|---|---|
| FO | SA | Intercept | −0.46 | 6.47 | 0.472 | 0.50 |
| Age | 0.23 | 0.06 | 0.001 | |||
| BMD_TH | −15.38 | 4.94 | 0.002 | |||
| FO | SL | Intercept | 44.54 | 78.21 | 0.287 | 0.10 |
| Age | 1.10 | 0.78 | 0.085 | |||
| BMD_TH | −82.06 | 59.71 | 0.091 | |||
| FO | Excur-ML | Intercept | 4.79 | 2.59 | 0.039 | 0.60 |
| Age | 0.05 | 0.02 | 0.013 | |||
| ζABOVEKNEE-R | 0.00 | 0.00 | 0.079 | |||
| ζUPPERBACK-R | −0.01 | 0.01 | 0.041 | |||
| FO | Excur-AP | BMD_TH | −7.71 | 1.94 | 0.000 | 0.38 |
| Intercept | 2.35 | 2.64 | 0.191 | |||
| Age | 0.07 | 0.03 | 0.009 | |||
| BMD_TH | −5.57 | 2.02 | 0.005 | |||
| FC | SA | Intercept | −13.87 | 13.17 | 0.151 | 0.44 |
| Age | 0.48 | 0.13 | 0.001 | |||
| ζUPPERBACK-R | −0.07 | 0.04 | 0.045 | |||
| BMD_TH | −15.38 | 10.07 | 0.070 | |||
| FC | SL | Intercept | −53.66 | 128.14 | 0.340 | 0.08 |
| Age | 2.40 | 1.76 | 0.092 | |||
| ζUPPERBACK-R | −0.77 | 0.52 | 0.078 | |||
| FC | Excur-ML | Intercept | 2.05 | 4.31 | 0.319 | 0.39 |
| Age | 0.08 | 0.04 | 0.019 | |||
| ζABOVEKNEE-R | −0.01 | 0.00 | 0.086 | |||
| ζUPPERBACK-R | −0.02 | 0.01 | 0.045 | |||
| BMD_TH | −5.33 | 3.22 | 0.056 | |||
| FC | Excur-AP | Intercept | 3.93 | 4.00 | 0.168 | 0.29 |
| Age | 0.08 | 0.04 | 0.022 | |||
| ζABOVEKNEE-L | −0.01 | 0.01 | 0.086 | |||
| BMD_Troch | −7.37 | 3.09 | 0.013 |
Abbr: ζABOVEKNEE-R, above knee at the lateral femoral condyle; BMD_TH, total hip bone mineral density; BMD_Troch, Trochanter bone mineral density; Excur-AP, excursion in anterior-posterior direction; Excur-ML, excursion in mediolateral direction; FC, eyes open standing on a 4 inch thick foam pad placed on the platform; FO, eyes open standing on a 4 inch thick foam pad placed on the platform; L, left heel strike; R, right heel strike; SA, sway area; SL, sway length; ζUPPERBACK-R, upper back (T-7).
Discussion
We noninvasively quantitated static and dynamic FPS as well as dynamic bone quality (damping [ζ]) among patients with osteoporosis with and without vertebral fractures. As before, damping values were significantly lower in the fracture group compared with patients without fracture (Figs. 4 and 5) and normal healthy younger groups (3,35,46). Although area under the ROC curve analysis (0.72) for ζUPPERBACK showed significant fracture discrimination, none of the BMD measures had significant AUC (0.50–0.58) for differentiating fracture status. This further supports the literature that BMD alone is not sufficient to discriminate between fracture and no-fracture groups.
In addition to BMD, bone quality, postural balance, and age-related decreases in muscle strength contribute independently to fracture risk (1,5,13,19,22,25,59–61). Our dual approach of combining impaired bone quality with poor postural balance risk factors or fracture is consistent with the concept of sarco-osteoporosis (18). With aging, muscle mass decreases and muscle strength is reduced even more, and, with the additional effects of osteoporosis, detrimentally impacts FPS, thereby increasing the risk of falls/fracture (62,63).
Older age and certain diseases (e.g., osteoporosis, Parkinson disease) bring about gradual changes in posture, spinal flexibility, mobility, and decreased sensory capacity, which collectively affect postural balance and contribute to fall-related injuries (1,13,16,22,23,64–66). Greig et al (22) reported that vertebral fracture interferes with the vertical alignment of skeleton, shifting the body’s center of gravity, thereby impairing postural balance. Recent studies (1,12) show that falls are stronger predictor of fractures than BMD.
It is hypothesized that structural integrity of CBAM system will be an equally important fall/fracture risk factors in osteoporosis. Postural control associated with upright balance depends on interactions between neural and musculoskeletal systems (i.e., CBAM system). Age-associated declines in both musculoskeletal system and neural system play a significant role in jeopardizing postural balance. In particular, age-associated decreasing capacity of somatosensory systems has serious consequences in perceiving the degree of slipperiness of a wet and/or uneven surface, which will impact the ability to negotiate a threatening environment and may increase susceptibility to falling (67,68).
Our area under the ROC analyses results show that dynamic FPS responses (i.e., PTV, TD, and left PSV) during performance of tasks of daily living were able to discriminate fracture from no-fracture groups (Fig. 3). In comparison to the no-fracture group, osteoporosis patients with fracture had lower PTV and PSV and took longer to make the turn during the TUG test. In addition, static postural balance outcomes for FC and EC test conditions allowed discrimination between fracture groups for Excur-ML and Excur-SL, respectively. In comparison to the no-fracture group, osteoporosis patients with fracture demonstrated larger postural sway excursion in the ML direction and increased movement of body’s CP (SL outcome) in both ML and AP directions, suggesting poorer balance. Our findings suggest that both static and dynamic FPS outcomes can discriminate between fracture and no-fracture groups. Interestingly, the mean TD value of the osteoporosis patients with fracture (standard error of the mean [SEM]: 2.73 [0.15 s]) was similar to the values (SEM: 2.67 [0.13 s]) obtained in Parkinson disease patients with history of falls in another study in our laboratory (69), suggesting potential high risk for falling in our osteoporosis patients. Also, in comparison to healthy control subjects (mean age [SEM]: 64.2 [2.86]; n = 10) in another of our studies, all 4 dynamic FPS responses for osteoporosis patients with and without fracture were significantly poorer, suggesting potential risk for falls (69).
FRAX scores were not associated with dynamic FPS outcomes, and only 1 static postural sway outcome (Excur-ML) showed significant association with FRAX (Table 2). Therefore, FRAX does not quantify fall-related fracture risk associated with reduced dynamic postural balance as determined by the TUG test. Although FRAX and BSA were not correlated, individually each was an independent discriminator of fracture status as shown in Figs. 4 and 5 and Table 1. Although both BMD and FRAX provide a measure of fracture risk because of skeletal fragility, they do not assess risks associated with falling, a common outcome in osteoporosis (1,16,22,23). On the other hand, our findings provide encouraging results supporting the abilities of BSA-FPS for discriminating fracture groups with measures of dynamic bone quality (damping, ζ) and dynamic FPS outcomes.
We hypothesized that reduced damping capacity of CBAM is detrimental to the static postural sway or balance and the dynamic FPS in osteoporosis patients, increasing the risk of falling and fracturing. To evaluate potential interplay between damping and static postural balance outcomes and dynamic FPS outcomes, we used regression modeling (Tables 3 and 4). Within the regression analysis between damping and dynamic FPS outcomes, the negative relationship between TD and ζABOVEKNEE-R suggests that a higher damping is associated with shorter TD (i.e., the subject is turning quicker during the TUG test), an indication of better dynamic motor control. Similarly, a positive relationship between PTV and ζABOVEKNEE-R suggests that increased damping is associated with increased PTV during TUG, also an indication of better dynamic motor control. Regression models relating damping to static postural balance outcomes were also carried out (Table 4). A negative relationship between postural sway outcomes and ζUPPERBACK-R suggests that higher damping is associated with lower SL and Excur-ML for FO and FC tests and lower SA and Excur-AP for FC test, implying better static balance/stability. Both ζABOVEKNEE-R and ζABOVEKNEE-L were negatively associated with Excur-ML and Excur-AP, respectively—higher damping was associated with better balance in ML and AP directions, less postural sway, implying better balance. This is consistent with the hypothesis that higher damping capacity of CBAM would effectively absorb the perturbing energy associated with movement of body segments trying to maintain upright balance and thereby would reduce postural sway or movement of CP. It is reasonable that both dynamic and static balances are influenced by the damping capacity of the CBAM system. Although static postural balance is primarily impacted by neuromuscular properties of large body mass segments such as torso, dynamic functional balance as measured by TUG has multiple contributing factors provided by all moving body segments, such as torso, head, legs, and swinging arms. Under dynamic conditions, such as getting up from chair and walking, interactions among damping properties of the various body segments of the musculoskeletal system affecting dynamic FPS outcomes would be complex. Further study with larger numbers and mechanistic biomechanical modeling will be needed to better understand intrinsic mechanisms of interplay between damping capacity of the CBAM system and postural balance influencing fall-related fracture risk.
In summary, this study provides an approach for identification of patients at risk of fracture using the dual approach noninvasive BSA-FPS tool to quantify the status of structural integrity of subjects’ CBAM system and their FPS. Poor structural integrity is characterized by reduced damping capacity of the CBAM system tested under realistic in vivo loading of simple heel strike (35). Reduced damping capacity of CBAM is likely detrimental to the FPS, increasing the risk of falling and fracturing. Further larger studies are warranted to confirm these findings, but results from this study provide support for potential new diagnostic approach, which could have positive impact (lower cost, fewer patients exposed to radiation) compared with testing with BMD alone and FRAX (70,71) and also to better target therapy to those in need. Our study is based on a small sample size; therefore, findings should be interpreted with caution.
Acknowledgments
The authors thank the patients and their families for their participation and cooperation. Thanks are due Cyndy Cox, Kelley James, Ramya Raghavan, for bone vibration, postural balance, and iTUG data collection and analyses. Thanks are also due Zhouyang Weng for her assistance in SAS programming and analysis. This study was supported by a grant from University of Cincinnati Provost Pilot Research Project Program, College of Medicine’s Discovery Acceleration Initiative. The BSA technology used in this study is a patented device under the names of the first two authors, A. Bhattacharya and Nelson B Watts (Patent approved 2014-Serial No. 12/402,586).
References
- 1.Abreu DC, Trevisan DC, Costa GC, et al. 2010. The association between osteoporosis and static balance in elderly women. Osteoporos Int 21(9):1487–1491. [DOI] [PubMed] [Google Scholar]
- 2.Heaney R, Avioli L, Chestnut C, et al. 1988. Is bone loss the cause of osteoporotic fracture or its consequence? J Bone Miner Res 3:88. [Google Scholar]
- 3.Huang S, Bhattacharya A. 1993. The effects of osteoarthritis on the biomechanical properties of the tibia. Chin J Med Biol Eng 13(3):255–264. [Google Scholar]
- 4.Kimmel D, Recker R, Gallagher J, et al. 1990. A comparison of iliac bone histomorphometric data in postmenopausal osteoporotic and normal subjects. Bone Miner 11:217–235. [DOI] [PubMed] [Google Scholar]
- 5.Lang T, Cauley JA, Tylavsky F, et al. 2010. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res 25: 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuit SC, van der KM, Weel AE, et al. 2004. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 34:195–202. Erratum in: Bone. 2006 Apr;38(4):603. PMID: 14751578. [DOI] [PubMed] [Google Scholar]
- 7.Siris ES, Chen YT, Abbott TA, et al. 2004. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 164:1108–1112. [DOI] [PubMed] [Google Scholar]
- 8.Stone KL, Seeley DG, Lui LY, et al. 2003. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 18:1947–1954. [DOI] [PubMed] [Google Scholar]
- 9.Watts N. 2002. Bone quality: getting closer to a definition. J Bone Miner Res 17:1148–1150. [DOI] [PubMed] [Google Scholar]
- 10.FRAX: WHO fracture risk assessment tool. Available at: http://www.shef.ac.uk/FRAX/. Accessed April 20, 2015.
- 11.Kanis JA, Oden A, Johnell O, et al. 2007. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int 18:1033–1046. [DOI] [PubMed] [Google Scholar]
- 12.Kaptoge S, Benevolenskaya LI, Bhalla AK, et al. 2005. Low BMD is less predictive than reported falls for future limb fractures in women across Europe: results from the European Prospective Osteoporosis Study. Bone 36:387–398. [DOI] [PubMed] [Google Scholar]
- 13.Liu-Ambrose T, Eng JJ, Khan KM, et al. 2003. Older women with osteoporosis have increased postural sway and weaker quadriceps strength than counterparts with normal bone mass: overlooked determinants of fracture risk? J Gerontol A Biol Sci Med Sci 58:M862–M866. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook PN, Cameron ID, Chen JS, et al. 2007. Influence of fall related factors and bone strength on fracture risk in the frail elderly. Osteoporos Int 18:603–610. [DOI] [PubMed] [Google Scholar]
- 15.Seeman E, Delmas PD. 2006. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med 354:2250–2261. [DOI] [PubMed] [Google Scholar]
- 16.Waters DL, Hale L, Grant AM, et al. 2010. Osteoporosis and gait and balance disturbances in older sarcopenic obese New Zealanders. Osteoporos Int 21:351–357. [DOI] [PubMed] [Google Scholar]
- 17.Wren TA, Gilsanz V. 2009. Evolving role of imaging in the evaluation of bone structure. J Bone Miner Res 24:1943–1945. [DOI] [PubMed] [Google Scholar]
- 18.Binkley N, Buehring B. 2009. Beyond FRAX: it’s time to consider “sarco-osteopenia”. J Clin Densitom 12:413–416. [DOI] [PubMed] [Google Scholar]
- 19.Leeming DJ, Henriksen K, Byrjalsen I, et al. 2009. Is bone quality associated with collagen age? Osteoporos Int 20(9):1461–1470. [DOI] [PubMed] [Google Scholar]
- 20.Shuster S. 2005. Osteoporosis, a unitary hypothesis of collagen loss in skin and bone. Med Hypotheses 65:426–432. [DOI] [PubMed] [Google Scholar]
- 21.Trento LK, Pietropolli A, Ticconi C, et al. 2009. Role of type I collagen C telopeptide, bone-specific alkaline phosphatase and osteocalcin in the assessment of bone status in postmenopausal women. J Obstet Gynaecol Res 35:152–159. [DOI] [PubMed] [Google Scholar]
- 22.Greig AM, Bennell KL, Briggs AM, et al. 2007. Balance impairment is related to vertebral fracture rather than thoracic kyphosis in individuals with osteoporosis. Osteoporos Int 18: 543–551. [DOI] [PubMed] [Google Scholar]
- 23.Lynn SG, Sinaki M, Westerlind KC. 1997. Balance characteristics of persons with osteoporosis. Arch Phys Med Rehabil 78: 273–277. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen ND, Eisman JA, Center JR, Nguyen TV. 2007. Risk factors for fracture in nonosteoporotic men and women. J Clin Endocrinol Metab 92:955–962. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen T, Sambrook P, Kelly P, et al. 1993. Prediction of osteoporotic fractures by postural instability and bone density. BMJ 307:1111–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeifer M, Begerow B, Minne HW, et al. 2001. Vitamin D status, trunk muscle strength, body sway, falls, and fractures among 237 postmenopausal women with osteoporosis. Exp Clin Endocrinol Diabetes 109:87–92. [DOI] [PubMed] [Google Scholar]
- 27.Day JS, Van Der Linden JC, Bank RA, et al. 2004. Adaptation of subchondral bone in osteoarthritis. Biorheology 41: 359–368. [PubMed] [Google Scholar]
- 28.Hoshino A, Wallace WA. 1987. Impact-absorbing properties of the human knee. Joint Surg Br 69:807–811. [DOI] [PubMed] [Google Scholar]
- 29.Paul JL, Munro MB, Abernethy P. 1978. Musculoskeletal shock absorption: relative contribution of bone and soft tissues at various frequencies. J Biomech 11:237–239. [DOI] [PubMed] [Google Scholar]
- 30.Radin EL, Burr DB, Caterson B, et al. 1991. Mechanical determinants of osteoarthrosis. Semin Arthritis Rheum 21:12–21. [DOI] [PubMed] [Google Scholar]
- 31.Radin EL, Paul IL. 1970. Does cartilage compliance reduce skeletal impact loads? The relative force-attenuating properties of articular cartilage, synovial fluid, periarticular soft tissues and bone. Arthritis Rheum 13:139–144. [DOI] [PubMed] [Google Scholar]
- 32.Wei HW, Sun SS, Jao SH, et al. 2005. The influence of mechanical properties of subchondral plate, femoral head and neck on dynamic stress distribution of the articular cartilage. Med Eng Phys 27:295–304. [DOI] [PubMed] [Google Scholar]
- 33.Zioupos P. 2001. Accumulation of in-vivo fatigue microdamage and its relation to biomechanical properties in ageing human cortical bone. J Microsc 201:270–278. [PubMed] [Google Scholar]
- 34.Dodge T, Wanis M, Ayoub R, et al. 2012. Mechanical loading, damping, and load-driven bone formation in mouse tibiae. Bone 51:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhattacharya A, Watts NB, Davis K, et al. 2010. Dynamic bone quality—a non-invasive measure of bone’s biomechanical property in osteoporosis. J Clin Densitom 13(2):228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng CF, Wang DZ, Zang XX, et al. 2007. Damping characteristics of carbon nanotube reinforced aluminum composite. Mater Lett 61:3229–3231. [Google Scholar]
- 37.Gibson RF, Chen Y, Zhao H. 2001. Improvement of vibration damping capacity and fracture toughness in composite laminates by the use of polymeric interleaves. J Eng Mater Technol Trans ASME 123:309–314. [Google Scholar]
- 38.Kireitseu M, Hui D, Tomlinson G. 2008. Advanced shock-resistant and vibration damping of nanoparticle-reinforced composite material. Composites 39:128–138. [Google Scholar]
- 39.Fantner GE, Adams J, Turner P, et al. 2007. Nanoscale ion mediated networks in bone: osteopontin can repeatedly dissipate large amounts of energy. Nano Lett 7:2491–2498. [DOI] [PubMed] [Google Scholar]
- 40.Fantner GE, Oroudjev E, Schitter G, et al. 2006. Sacrificial bonds and hidden length: unraveling molecular mesostructures in tough materials. Biophys J 90:1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansma PK, Fantner GE, Kindt JH, et al. 2005. Sacrificial bonds in the interfibrillar matrix of bone. J Musculoskelet Neuronal Interact 5:313–315. [PubMed] [Google Scholar]
- 42.Wang W, Elbanna A. 2014. Crack propagation in bone on the scale of mineralized collagen fibrils: role of polymers with sacrificial bonds and hidden length. Bone 68:20–31. [DOI] [PubMed] [Google Scholar]
- 43.Buehring B, Krueger D, Binkley N. 2010. Jumping mechanography: a potential tool for sarcopenia evaluation in older individuals. J Clin Densitom 13:283–291. [DOI] [PubMed] [Google Scholar]
- 44.Burr DB. 2011. Why bones bend but don’t break. J Musculoskelet Neuronal Interact 11:270–285. [PubMed] [Google Scholar]
- 45.Genant HK, Delmas PD, Chen P, et al. 2007. Severity of vertebral fracture reflects deterioration of bone microarchitecture. Osteoporos Int 18:69–76. [DOI] [PubMed] [Google Scholar]
- 46.Bhattacharya A, Watts N, Gordon J, et al. 2007. Bone quantity and quality of youths working in farm—a pilot study. J Agro-medicine 12:27–38. [DOI] [PubMed] [Google Scholar]
- 47.Bagchee A, Bhattacharya A, Succop PA, et al. 1998. Postural stability assessment during task performance. Occup Ergon 1: 41–53. [Google Scholar]
- 48.Zampieri C, Salarian A, Carlson-Kuhta P, et al. 2010. The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson’s disease. J Neurol Neurosurg Psychiatr 81:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zampieri C, Salarian A, Carlson-Kuhta P, et al. 2011. Assessing mobility at home in people with early Parkinson’s disease using an instrumented Timed Up and Go test. Parkinsonism Relat Disord 17:277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Creel GL, Light KE, Thigpen MT. 2001. Concurrent and construct validity of scores on the Timed Movement Battery. Phys Ther 81:789–798. [DOI] [PubMed] [Google Scholar]
- 51.Katzman WB, Vittinghoff E, Ensrud K, et al. 2011. Increasing kyphosis predicts worsening mobility in older community-dwelling women: a prospective cohort study. J Am Geriatr Soc 59:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Podsiadlo D, Richardson S. 1991. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39:142–148. [DOI] [PubMed] [Google Scholar]
- 53.Shumway-Cook A, Baldwin M, Polissar NL, Gruber W. 1997. Predicting the probability for falls in community-dwelling older adults. Phys Ther 77:812–819. [DOI] [PubMed] [Google Scholar]
- 54.Shumway-Cook A, Brauer S, Woollacott M. 2000. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther 80: 896–903. [PubMed] [Google Scholar]
- 55.El-Sobkey SB. 2011. Balance performance of community-dwelling older people. Saudi Med J 32:283–287. [PubMed] [Google Scholar]
- 56.Haibach PS, Slobounov SM, Slobounova ES, et al. 2007. Aging and time-to-postural stability following a visual perturbation. Aging Clin Exp Res 19:438–443. [DOI] [PubMed] [Google Scholar]
- 57.Kangas M, Konttila A, Winblad I, Jämsä T. 2007. Determination of simple thresholds for accelerometry-based parameters for fall detection. Conf Proc IEEE Eng Med Biol Soc 2007: 1367–1370. [DOI] [PubMed] [Google Scholar]
- 58.Kangas M, Vikman I, Wiklander J, et al. 2009. Sensitivity and specificity of fall detection in people aged 40 years and over. Gait Posture 29:571–574. [DOI] [PubMed] [Google Scholar]
- 59.Ishikawa Y, Miyakoshi N, Kasukawa Y, et al. 2009. Spinal curvature and postural balance in patients with osteoporosis. Osteoporos Int 20(12):2049–2053. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen ND, Pongchaiyakul C, Center JR, et al. 2005. Identification of high-risk individuals for hip fracture: a 14-year prospective study. J Bone Miner Res 20:1921–1928. [DOI] [PubMed] [Google Scholar]
- 61.Sinaki M, Lynn SG. 2002. Reducing the risk of falls through proprioceptive dynamic posture training in osteoporotic women with kyphotic posturing: a randomized pilot study. Am J Phys Med Rehabil 81:241–246. [DOI] [PubMed] [Google Scholar]
- 62.Janssen I, Heymsfield SB, Ross R. 2002. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50:889–896. [DOI] [PubMed] [Google Scholar]
- 63.Morley JE. 2008. Sarcopenia: diagnosis and treatment. J Nutr Health Aging 12:452–456. [DOI] [PubMed] [Google Scholar]
- 64.Hasselkus BR. 1974. Aging and the human nervous system. Am J Occup Ther 28:16–21. [PubMed] [Google Scholar]
- 65.Kerr GK, Worringham CJ, Cole MH, et al. 2010. Predictors of future falls in Parkinson disease. Neurology 75:116–124. [DOI] [PubMed] [Google Scholar]
- 66.Sheldon JH. 1960. On the natural history of falls in old age. BMJ 2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhattacharya A, Succop P, Modawal A, et al. 2007. Impact of mismatch between actual and perceived risks on slip/fall while negotiating a ramp Proceedings of International Conference on Slips, Trips and Falls—From Research to Practice, Liberty Mutual Research Institute for Safety, Hopkinton, MA, August 23–24, 2007. [Google Scholar]
- 68.Bhattacharya A, Succop P, Lu ML, et al. Workers’ postural balance response on dry surface can predict their balance performance on slippery surface American Industrial Hygiene Conference and Exposition, Chicago, IL, May 13–16, 2006. [Google Scholar]
- 69.Mani A, Dunning K, Larsh T, et al. Dynamic fall-risk predictors in Parkinson’s disease Presented at American Academy of Neurology 66th Annual Meeting, at the Pennsylvania Convention Center, Philadelphia, PA, April 26–May 3, 2014. [Google Scholar]
- 70.Kraemer DF, Nelson HD, Bauer DC, Helfand M. 2006. Economic comparison of diagnostic approaches for evaluating osteoporosis in older women. Osteoporos Int 17:68–76. [DOI] [PubMed] [Google Scholar]
- 71.Schousboe JT. 2008. Cost effectiveness of screen-and-treat strategies for low bone mineral density: how do we screen, who do we screen and who do we treat? Appl Health Econ Health Policy 6:1–18. [DOI] [PubMed] [Google Scholar]