Abstract
Sperm retrieval combined with intracytoplasmic sperm injection (ICSI) is the treatment of choice for couples with untreatable azoospermia-related infertility. However, an increasing body of evidence has been mounting, suggesting that ICSI with testicular sperm instead of ejaculated sperm (when both are available) increases pregnancy outcomes in some specific scenarios. This has led to the exploration of extended indications for sperm retrieval. This review summarizes the current literature concerning sperm retrieval and ICSI for non-azoospermic men with elevated sperm DNA fragmentation, oligozoospermia, and cryptozoospermia.
Keywords: sperm DNA fragmentation, sperm chromatin damage, sperm retrieval, testicular sperm, ejaculated sperm, assisted reproductive technology, in vitro fertilization, intracytoplasmic sperm injection, oligozoospermia, cryptozoospermia, pregnancy, offspring health, male infertility
Introduction
Intracytoplasmic sperm injection (ICSI) was an extraordinary achievement in the field of assisted reproduction technology (ART). Introduced in 1992 as a modification of conventional in vitro fertilization (IVF), ICSI enables men with low sperm quantity and quality to father a child 1, 2. Nowadays, ICSI has become not only the most commonly used method of fertilization in ART but also the method of choice for overcoming untreatable severe male factor infertility 3.
ICSI is typically carried out with ejaculated sperm, which are generally regarded as having the highest fertilization potential since they have completed their transit through the male reproductive tract. By contrast, sperm retrieval methods—developed a few years after the introduction of ICSI—have been used to harvest sperm from the epididymides and testes of men with azoospermia-related infertility 4, 5. After retrieval of epididymal or testicular sperm, ICSI is mandatory as the retrieved gametes are unable to fertilize the oocytes by conventional IVF.
However, as experience accumulated, reports of an association between semen quality and ICSI outcomes increased steadily 6– 8. Concerns of a possible role of the paternal gamete on ICSI outcomes led Greco et al., in 2005, to investigate the utility of sperm retrieval in a group of 18 non-azoospermic patients with elevated sperm DNA fragmentation (SDF) on neat semen 9. On the day of oocyte retrieval, the male partners underwent sperm retrieval using percutaneous or open methods to harvest sperm from the seminiferous tubules. In this series, ICSI with testicular sperm (Testi-ICSI) resulted in eight clinical pregnancies (44.5%) whereas only one pregnancy (5.6%) that ended in miscarriage had been obtained in previous ICSI cycles with the use of ejaculated sperm.
Given this information, the utility of sperm retrieval in indications other than azoospermia has been investigated. Here, the current support for these indications, including elevated SDF, severe oligozoospermia, and cryptozoospermia—denoted by very few spermatozoa (or none) in the fresh ejaculate but observed after microscopic examination of centrifuged pellet—will be summarized.
Extended sperm retrieval indications: biological plausibility
It is well established that sperm chromatin integrity is vital for the birth of healthy infants 10. Fertilization of oocytes by sperm with DNA fragmentation might increase the risk of fertilization failure, embryo development arrest, implantation failure, miscarriage, congenital malformations, and perinatal and postnatal morbidity 11– 13. Notably, infertile men often have elevated SDF rates in neat semen 14, 15. Varicocele, systemic diseases, male accessory gland infections, advanced paternal age, obesity, lifestyle and environmental factors, radiation, and heat exposure are some of the conditions associated with SDF 16, 17. These stressors have in common the trait of oxidative stress, which represents a significant cause of SDF 18. The mechanisms involve reactive oxygen species (ROS) attack on sperm membranes and nuclear and mitochondrial DNA, mostly during sperm transit through the male reproductive tract 19– 21.
Interestingly, data from human studies assessing paired testicular and ejaculated specimens of non-azoospermic men indicate that SDF is two to three times lower in testicular sperm than in ejaculated sperm 9, 22– 25. A 2017 systematic review—followed by a meta-analysis—compiled the results of five studies including 143 patients and showed that the mean difference (MD) in SDF rates was −24.6% (95% confidence interval [CI] −32.5 to −16.6%, I 2 = 92%, P <0.001) in favor of testicular sperm 26. In that report, SDF was measured by using the terminal deoxyribonucleotide transferase–mediated dUTP nick-end labeling (TUNEL) assay (four studies, pooled MD: −19.8%, 95% CI −22.3 to −17.2%, I 2 = 15%, P <0.001) or the sperm chromatin dispersion (SCD) assay (one study, MD: −32.4%, 95% CI −34.85 to −29.95%, P <0.001).
Elevated sperm DNA fragmentation
After the report by Greco et al. 9, several authors investigated the utility of sperm retrieval in non-azoospermic men with elevated SDF in neat semen ( Table 1) 19, 24, 25, 27– 32. In a 2017 systematic review, we aggregated the evidence of five studies including 507 ICSI cycles 26. In total, 3,840 oocytes were injected with either ejaculated sperm or testicular sperm. Using meta-analysis, we showed higher clinical pregnancy rates (odds ratio [OR] 2.42, 95% CI 1.57 to 3.73, I 2 = 34%, P <0.0001) and live birth rates (OR 2.58, 95% CI 1.54 to 4.35, I 2 = 0%, P = 0.0003), and lower miscarriage rates (OR 0.28, 95% CI 0.11 to 0.68, I 2 = 11%, P = 0.005) when comparing Testi-ICSI with ejaculated ICSI.
Table 1. Studies reporting ICSI outcomes with testicular versus ejaculated sperm in non-azoospermic men with high sperm DNA fragmentation in the neat semen.
| Study characteristics | Indication | Sperm retrieval method | Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Author (year) | Design | Subjects and cohort size (N) | Test used for sperm
chromatin damage assessment and cutoff values (%) |
Paired SDF
results in testicular and ejaculated sperm (%) |
Sperm retrieval
method |
Sperm
retrieval success and complication rates (%) |
Fertilization
rate (%) |
Clinical
pregnancy rate (%) |
Ongoing
pregnancy rate or live birth rate a (%) |
| Greco
et al.
9
(2005) |
Case series | Predominantly normozoospermic
infertile men (18); couples with history of ICSI failure performed with ejaculated sperm |
TUNEL (15) | 23.6 ± 5.1 (E)
and 4.8 ± 3.6 (T) ( P <0.001) |
TESE and TESA | 100.0 and NR | 74.9 b | 44.4 c | NR |
| Sakkas and
Alvarez 19 (2010) |
Case series | Couples with history of IVF/ICSI
failure (68) with ejaculated sperm |
TUNEL (20) | NR | TESA | NR | 58.0; range:
20.0–100.0 |
40.0 | NR |
| Esteves
et al. 24 (2015) |
Prospective
cohort |
Oligozoospermic (sperm
concentration 5–15 million/mL) infertile men (172); couples with no history of ICSI failure (Testi-ICSI, n = 81 and Ejac-ICSI, n = 91) |
SCD (30) | 40.9 ± 10.2 (E)
and 8.3 ± 5.3 (T) ( P <0.001) |
TESE and TESA | 100.0 and 6.2 | 69.4 (E) vs.
56.1 (T) ( P = 0.0001) |
40.2 (E) vs. 51.9
(T) (NS) |
LBR: 26.4 (E)
vs. 46.7 (T) ( P = 0.007) |
| Mehta
et al.
25
(2015) |
Case series | Oligozoospermic (sperm
concentration <5 million/mL) infertile men (24); couples with one or more failed IVF or ICSI cycles using ejaculated sperm |
TUNEL (7) | 24.0 (95% CI
19–34) (E) and 5.0 (95% CI 3–7) (T) ( P = 0.001) |
Micro-TESE | 100.0 and NR | 54.0 | 50.0 | 50.0 |
| Bradley
et al. 27 (2016) |
Retrospective
cohort |
Predominantly oligozoospermic
infertile men; Testi-ICSI (n = 148) d, Ejac-ICSI (n = 80) d |
SCIT (29) | NR | TESE and TESA | NR | 66.0 (E) vs.
57.0 (T) ( P <0.001) |
27.5 (E) vs. 49.5
(T) ( P <0.01) |
LBR: 24.2 (E)
vs. 49.8 (T) ( P <0.05) |
| Pabuccu
et al. 28 (2016) |
Retrospective
cohort |
Normozoospermic infertile men (71);
couples with history of ICSI failure using ejaculated sperm (Testi-ICSI, n = 31; Ejac-ICSI, n = 40) |
TUNEL (30) | 41.7 ± 8.2 (E) | TESA | 100.0 and NR | 74.1 ± 20.7
(T) vs. 71.1 ± 26.9 (E) (NS) |
41.9 (T) vs. 20.0
(E) ( P = 0.04) |
OPR: 38.7 (T)
vs. 15.0 (E) ( P = 0.02) |
| Arafa
et al.
29
(2018) |
Prospective
cohort; interventions applied in the same patients |
Oligozoospermic and
normozoospermic infertile men (36); couples with history of ICSI failure performed with ejaculated sperm |
SCD (30) | 56.3 ± 15.3 (E) | TESA | 100.0 and
NR |
46.4 (T) vs.
47.8 (E) (NS) |
38.9 (T) vs. 13.8
(E) ( P <0.0001) |
LBR: 38.9 (T)
vs. 8.0 (E) ( P <0.0001) |
| Zhang
et al.
30
(2018) |
Prospective
cohort e |
Oligozoospermic and
normozoospermic infertile men (102); couples with no history of ICSI failure (Testi-ICSI, n = 61; Ejac-ICSI, n = 41) |
SCSA (30) | NR | TESA | 100.0 and
NR |
70.4 (T) vs.
75.0 (E) (NS) |
36.0 (T) vs. 14.6
(E) ( P = 0.01) |
LBR: 36.0 (T)
vs. 9.8 (E) ( P = 0.001) |
| Herrero
et al. 31 (2019) |
Retrospective
cohort |
Couples with no previous live births
and a history of at least two previous failed ICSI cycles with ejaculated sperm (Testi-ICSI, n = 77; Ejac-ICSI, n = 68) |
SCSA (25); TUNEL
(36%) |
NR | TESE | NR | SCSA: 66.3
(T); 62.9 (E) (NS) TUNEL: 61.2 (T); 57.6 (E) (NS) |
SCSA: 18.2
(T); 9.1% (E) ( P <0.02) TUNEL: 23.1 (T); 0.0 (E) ( P <0.02) |
fSCSA: 21.7
(T); 9.1 (E) ( P <0.01) TUNEL: 20.0 (T); 0.0 (E) ( P <0.02) |
| Alharbi
et al. 32 (2019) |
Retrospective
cohort |
Couples with one or more failed ICSI
cycles with ejaculated sperm Testi- ICSI, n = 52; Ejac-ICSI, n = 48) |
SCSA (15);
subgroup analysis using SCSA thresholds of 30% |
NR | TESA | 100.0 and
NR |
58.0 ± 27.0
(T) vs. 70.0 ± 23.0 ( P = 0.03) |
DFI >15%: 48.6
(T) vs. 38.7 (E); DFI >30%: 48.0% vs. 25.0% ( P = 0.25) |
gDFI >15%:
36.4 (T) vs. 30.0 (E); DFI >30%: 29.2 vs. 25.0 (NS) |
aHerrero et al. 31 reported cumulative live birth rates.
b2PN fertilization rate with use of testicular sperm; data from previous cycles with use of ejaculated sperm not provided.
cThe authors reported only one pregnancy with ejaculated sperm which miscarried.
dNumber of intracytoplasmic sperm injection (ICSI) cycles.
eInferred from the study’s reported data.
fCumulative live birth rates.
gAlharbi et al. 32 reported pregnancy rates per embryo transfer; live birth data were incomplete as a number of patients achieving clinical pregnancy were lost in follow-up. E, ejaculated sperm group; Ejac-ICSI, ICSI with ejaculated sperm; LBR, live birth rate; micro-TESE, microdissection testicular sperm extraction; NR, not reported; NS, not significantly different; OPR, ongoing pregnancy rate; SCD, sperm chromatin dispersion; SCIT, sperm chromatin integrity test, a variation of sperm chromatin structure assay (SCSA); SDF, sperm DNA fragmentation; T, testicular sperm group; TESA, testicular sperm aspiration; TESE, Testicular sperm extraction, Testi-ICSI, ICSI with testicular sperm; TUNEL, terminal deoxyribonucleotide transferase–mediated dUTP nick-end labeling assay.
Recent studies providing live birth data corroborate the effectiveness of testicular sperm for ICSI in men with high SDF 29– 31. Thus, despite the limited evidence and lack of randomized controlled trials, data from seven retrospective studies and three prospective studies, including a total of 830 patients and 902 ICSI cycles, suggest that Testi-ICSI is superior to ICSI with ejaculated sperm to overcome infertility among non-azoospermic men with elevated SDF in semen. Testi-ICSI has been postulated to bypass post-testicular sperm chromatin damage caused by oxidative stress during sperm transit through the epididymis 33. As a result, the chances of oocyte fertilization by genomically intact spermatozoa and formation of a normal embryonic genome are increased, thus positively impacting the likelihood of achieving a live birth. Notably, a single study 32 including 110 couples with sperm DNA damage data failed to corroborate the latter findings; however, in that study, SDF thresholds of 15% (by sperm chromatin structure assay, or SCSA) were used to select couples eligible for Testi-ICSI; those thresholds are not fully consistent with the 30% SCSA thresholds reported to be associated with adverse pregnancy outcomes in ART 34. Thus, the inclusion of ~30% of men with SDF values between 15% and 30% in the above study might have diluted the positive effect of Testi-ICSI.
Severe oligozoospermia and cryptozoospermia
Weissman et al., in 2008, reported the first series of Testi-ICSI in patients with severe oligozoospermia (<5 million sperm/mL) 35. The authors performed testicular sperm injections in four couples with a history of multiple failed IVF/ICSI cycles after the use of poor-quality ejaculated sperm. The male partners had sperm counts ranging from 0.2 million/mL to 2.0 million/mL. On the day of oocyte retrieval, sperm retrieval was performed, and in all cases, motile spermatozoa were retrieved from the testis. All couples achieved embryo implantation and delivery of healthy offspring after embryo transfers.
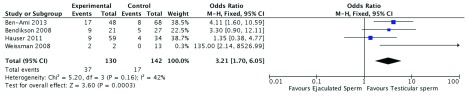
Given the success reported by Weissman et al. 35, many authors sought to investigate the utility of sperm retrieval for ICSI in non-azoospermic patients with severe oligozoospermia or cryptozoospermia ( Table 2) 36– 39. These studies report an overall better pregnancy outcome with the use of testicular than ejaculated sperm. But surprisingly, in 2016, a systematic review and meta-analysis aggregating the data of the above studies concluded that sperm retrieval should not be recommended in men with severe oligozoospermia or cryptozoospermia 40. In that report, the relative risk (RR) of achieving pregnancy (272 cycles, RR = 0.53, 95% CI 0.19 to 1.42) with the use of testicular or ejaculated sperm for ICSI was not different. However, we performed a careful examination of the authors’ data and discovered that they inadvertently inverted the number of pregnancies reported in the study by Bendikson et al. 36 concerning the group of patients undergoing ICSI with testicular and ejaculated sperm. This critical mistake inflated the total number of pregnancies in the ejaculate sperm group, thus leading to an erroneous RR calculation. We reassessed the pregnancy results of the meta-analysis by Abhyankar et al. 40—after correcting the incongruency mentioned above—and found a significantly higher pregnancy rate with the use of testicular sperm than with ejaculated sperm in men with cryptozoospermia and severe oligozoospermia (272 cycles, RR = 3.21, 95% CI 1.70 to 6.05, I 2 = 42%, P = 0.0003) ( Figure 1; unpublished data).
Table 2. Characteristics and main outcome measures of studies reporting ICSI outcomes with testicular versus ejaculated sperm in non-azoospermic men with severe oligozoospermia/cryptozoospermia.
| Study characteristics | Indication | Sperm retrieval method | Outcomes | |||||
|---|---|---|---|---|---|---|---|---|
| Author (year) | Design | Subjects and cohort size (N) | SDF
assessment |
Sperm
retrieval method |
Sperm retrieval
success and complication rates (%) |
Fertilization rate (%) | Clinical
pregnancy rate (%) |
Live birth rate (%) |
| Weissman
et al. 35 (2008) |
Case series | Severe oligozoospermic (<5 million/mL)
infertile men (4) undergoing Testi-ICSI; couples with a history of multiple failed ICSI cycles with ejaculated sperm; in total, five TESA-ICSI cycles were carried out in the cohort of four patients |
No | TESA | 100.0 and NR | 67.6 | 75.0 | 75.0 |
| Bendikson
et al. 36 (2008) |
Case series | Cryptozoospermic infertile men (16);
couples with history of IVF/ICSI failure (16) with ejaculated sperm; in total, 21 TESA-ICSI cycles were carried out in the cohort of 16 patients |
No | Micro-TESE | 100.0 and NR | 51.7 (T) vs. 59.9 (E)
(NS) |
20.8 (E) vs. 47.4
(T) (NS) |
20.8 (E) vs. 42.1
(T) (NS) |
| Hauser
et al.
37
(2011) |
Prospective
cohort |
Cryptozoospermic infertile men (13); in
total, 93 ICSI cycles (ICSI with ejaculated sperm, n = 34; ICSI with fresh testicular sperm, n = 9; ICSI with frozen-thawed testicular sperm, n = 50) were carried out in the cohort of 13 patients |
No | TESE | 100.0 and NR | 38.2 (E) vs. 50.0
(T, fresh) vs. 46.7 (T, frozen-thawed) a ( P <0.05, pairwise comparisons between T and E sperm) |
14.3 (E) vs. 42.9
(T, fresh) vs. 12.8 (T, frozen- thawed) (NS) |
14.3 (E) vs. 42.9
(T, fresh) vs. 12.8 (T, frozen-thawed) (NS) |
| Ben-Ami
et al. 39 (2013) |
Case series | Cryptozoospermic (17) infertile men;
couples with multiple failed ICSI cycles using ejaculated sperm; in total, 116 ICSI cycles (Testi-ICSI, n = 48; Ejac-ICSI, n = 68) were carried out in the cohort of 16 patients |
No | TESE | 100.0 and NR | 38.0 (E) vs. 46.7 (T)
(NS) |
15.1 (E) vs. 42.5
(T) ( P = 0.004) |
9.4 (E) vs. 27.5 (T)
( P = 0.028) |
| Ketabchi
41 (2016) |
Prospective
cohort |
Cryptozoospermic (<10
3 sperm/mL)
infertile men (73) undergoing ICSI with sperm retrieved from the epididymis or testis (18) |
No | PESA and
TESE |
100.0 and NR | 55.3 (E) vs. 85.7.
(T+E) ( P <0.001) |
31.6 (E) vs. 57.1
(T) ( P <0.001) |
NR |
| Cui
et al.
42
(2017) |
Retrospective
cohort |
Cryptozoospermic infertile men
undergoing Testi-ICSI; couples (285) undergoing ICSI with ejaculated sperm (214) or testicular sperm (71) |
No | TESA and
TESE |
97.9 and NR | 59.6 (E) vs. 60.6 (T)
(NS) |
33.3 (E) vs. 53.6
(T) ( P <0.01) |
27.1 (E) vs. 44.0
(T) ( P = 0.03) |
| Yu
et al.
45 (2019) |
Retrospective
cohort |
Cryptozoospermic infertile men (35)
undergoing Testi-ICSI; in total, 19 cycles (18 patients) were performed with ejaculated sperm and 19 cycles (17 patients) with testicular sperm |
No | TESA and
micro-TESE |
100.0 and NR | 74.7 (E) and 62.4 (T)
in men <35 years old ( P = 0.01); 60.9 (E) and 56.6 (T) in men ≥35 years old (NS) |
74.7 (E) and
62.4 (T) in men <35 years old ( P = 0.01); 60.9 (E) and 56.6 (T) in men ≥35 years old (NS) |
44.4 (E) and 52.9
(T) in men <35 years old (NS); 0.0 (E) and 42.9 (T) in men ≥35 years old |
a2PN fertilization using motile sperm. E, ejaculated sperm group; Ejac-ICSI, intracytoplasmic sperm injection with ejaculated sperm; LBR, live birth rate; micro-TESE, microdissection testicular sperm extraction; NR, not reported; NS, not significantly different; OPR, ongoing pregnancy rate; SDF, sperm DNA fragmentation; T, testicular sperm group; TESA, testicular sperm aspiration; TESE, Testicular sperm extraction, Testi-ICSI, intracytoplasmic sperm injection with testicular sperm.
Figure 1. Pregnancy rates according to sperm source in non-azoospermic men with cryptozoospermia or severe oligozoospermia.
Forest plot showing odds ratio for pregnancy with use of ejaculated sperm or testicular sperm for intracytoplasmic sperm injection in men with cryptozoospermia/severe oligozoospermia. CI, confidence interval; M-H, Mantel–Haenszel analysis.
Recently, additional reports and systematic reviews on the matter concerned were published 41– 45. In a 2018 systematic review and meta-analysis, Kang et al. pooled the data of six studies including a total of 578 patients and 761 ICSI cycles 43. The authors showed that sperm retrieval and Testi-ICSI improved the likelihood of achieving good-quality embryos (RR = 1.17, 95% CI 1.05 to 1.30, P = 0.005), implantation (RR = 1.52, 95% CI 1.02 to 2.26, P = 0.04), and pregnancy (RR = 1.74, 95% CI 1.20 to 2.52, P = 0.004). These results were corroborated by Ku et al., who pooled the evidence of studies that provided miscarriage and live birth data 44. The authors included a total of 331 patients and 479 ICSI cycles. In that report, miscarriage rates were not affected by the use of testicular or ejaculated sperm for ICSI (RR = 1.06, 95% CI 0.48 to 2.35), but live birth rates per initiated cycle were increased among couples that had undergone Testi-ICSI (RR = 1.77, 95% CI 1.28 to 2.44, P = 0.0005).
Collectively, evidence from seven retrospective studies and one prospective study, including a total of 613 patients and 799 ICSI cycles, suggests that Testi-ICSI is superior to ICSI with ejaculated sperm to overcome infertility among non-azoospermic men with severe oligozoospermia or cryptozoospermia ( Table 2). Likewise, Testi-ICSI has been postulated to bypass post-testicular sperm damage during sperm transit through the genital tract. However, no randomized controlled study has been published yet to support the routine use of sperm retrieval and testicular sperm for ICSI to non-azoospermic men with low sperm count undergoing ICSI.
Confounding factors
The relatively low testicular sperm positivity for DNA damage might explain the better reproductive outcomes with the use of testicular sperm rather than ejaculated sperm for ICSI. Nevertheless, it is important to acknowledge that the evidence concerning the superiority of Testi-ICSI relies overwhelmingly on cohort studies with few patients, in which confounding factors, such as maternal and paternal age, etiology of male factor infertility, use of medication with possible gonadotoxic effect, and lifestyle factors, to cite a few, were not properly controlled. For instance, it has been suggested that the adverse effect of sperm DNA damage on reproductive outcomes is modulated by female age because of the intrinsic (albeit limited) capacity of oocytes from young women to repair the DNA damage 46– 48. On the other hand, women of advanced reproductive age have significantly fewer euploid embryos available for transfer, which will reduce ART success irrespective of the type of sperm used 49. Since not all sperm DNA damage is repairable, it seems sound to suggest that surgically retrieved sperm should not be used as a last resort after years of treatment with ejaculated sperm because the oocyte apparatus to repair sperm DNA damage is less efficient as both ovarian reserve and maternal age increase 48. These observations highlight the importance of controlling for confounders in future studies evaluating the clinical utility of testicular sperm in non-azoospermic men.
Technical aspects
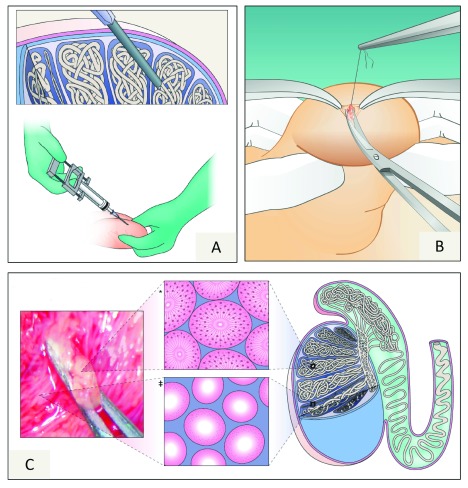
Both percutaneous and open sperm retrieval procedures can be used to harvest sperm from the seminiferous tubules in non-azoospermic men ( Figure 2) 50– 52. The testicle rather than the epididymis is the target organ because of the reported lower SDF rates in the former 53– 55. In such patients, the reported sperm retrieval success rates are close to 100% with the use of testicular sperm aspiration (TESA), testicular sperm extraction (TESE), or microdissection TESE (micro-TESE) ( Table 1 and Table 2). Our choices are TESA for men with elevated SDF and TESE or micro-TESE for cryptozoospermic patients 24, 56. In our hands, these methods are carried out on an outpatient basis on the same day of oocyte retrieval 50– 52, 56– 58. The reason relates to the fact that prolonged sperm incubation—in particular, at 37°C—and sperm freezing might negatively affect sperm chromatin integrity 21, 59, 60.
Figure 2. Sperm retrieval methods.
( A) Testicular sperm aspiration. The illustration depicts a 13G needle—connected to a 20-mL syringe and fitted to the Cameco holder—being percutaneously inserted into the testis. Negative pressure is created, and the tip of the needle is moved within the testis to disrupt the seminiferous tubules and sample different areas. ( B) Testicular sperm extraction (TESE). Single or multiple incisions are made on the tunica albuginea, and one or several testicular biopsies are taken. ( C) Microsurgical TESE (micro-TESE). With aid of an operating microscope, the dilated seminiferous tubules are identified and removed with microforceps. The illustration in the middle of the figure depicts histopathology cross-sections of dilated seminiferous tubules with active spermatogenesis* and a thin tubules with germ cell aplasia ‡. Adapted by permission from Macmillan Publishers Ltd 3.
In the context of non-azoospermic men, sperm retrieval is associated with few complications (less than 5%) as minimal tissue extraction yields sufficient numbers of sperm for ICSI 26, 33, 51, 52. Nevertheless, given the potential risk for complications and adverse effects on testicular function, sperm retrieval should be performed by well-trained urologists.
Offspring health
The use of sperm retrieval in non-azoospermic men has raised concerns about the health of resulting offspring because of the reports of increased sperm aneuploidy rates in testicular sperm (versus ejaculated sperm) 23, 61– 64. On the one hand, ICSI has been associated with possible increased risks of congenital malformations, epigenetic disorders, chromosomal abnormalities, infertility, cancer, delayed psychological and neurological development, and impaired cardiometabolic profile compared with naturally conceived children and this is probably due to the influence of parental subfertility 3. On the other hand, data concerning risks and sequelae to offspring health with the use of surgically retrieved gametes from azoospermic men are overall reassuring albeit limited 3, 65– 70. However, no study has yet examined whether ICSI with testicular instead of ejaculated sperm (when both are available) affects the risk of malformations and long-term health of offspring.
Nevertheless, new data generated by whole-exome sequencing molecular karyotype suggest that sperm aneuploidy in testicular specimens is not a major concern 71. In this series, paired assessments in ejaculated and surgically retrieved testicular samples of non-azoospermic patients with elevated SDF in semen showed that the rates of aneuploidy (1.3% versus 8.4%, respectively, P = 0.02) were lower in testicular sperm than in ejaculated sperm. Along these lines, Weng et al. showed that the origin of sperm used for ICSI had no marked influence on embryo aneuploidy rates 72. Moreover, a 2019 ICSI study from our group—using 24-chromosome genetic testing—revealed that euploid blastocyst rate per metaphase II oocyte was not differently affected whether ejaculated or testicular sperm retrieved from men with elevated SDF was used for ICSI (18.7% versus 18.2%, respectively) 73. These observations corroborate the safe utilization of sperm retrieval in non-azoospermic men, but owing to limited data concerning the health of resulting offspring, continuous monitoring is warranted.
Conclusions
A growing body of evidence supports sperm retrieval for ICSI in non-azoospermic men with elevated SDF, severe oligozoospermia, and cryptozoospermia. In these scenarios, Testi-ICSI instead of ICSI with ejaculated sperm seems to be associated with improvements in pregnancy outcomes. Percutaneous aspiration and open TESE (with and without the aid of microsurgery) are the methods that have been applied, with high success rates and few complications, to harvest sperm from the seminiferous tubules of non-azoospermic men. However, it is essential to acknowledge the limitations of existing evidence. First, most of the data summarized derive from small observational studies in which confounder factors were not properly controlled. Thus, level 1 evidence in support of Testi-ICSI is still lacking. Second, sperm retrieval is an invasive procedure with potential complications. Thus, identification and treatment of the male factor associated with high SDF, oligozoospermia, and cryptozoospermia are essential to potentially avoid the use of surgical retrieval. We recommend a holistic approach to improve paternal health—whenever there is an opportunity—to patients embarking on any type of ART treatment. Lastly, there are limited data concerning the health of resulting offspring with the use of sperm retrieval and ICSI in cases where both ejaculated and testicular sperm are available. Keeping these limitations in mind, by summarizing the current literature, this article might guide health-care providers in presenting available evidence to patients to help them make informed decisions.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Sheena Lewis, Examen Ltd, Belfast, UK; Queen's University, Belfast, UK
Ricardo P. Bertolla, Department of Surgery, Division of Urology, Universidade Federal de São Paulo, São Paulo, Brazil; Hospital São Paulo, São Paulo, Brazil
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 2 approved]
References
- 1. Palermo G, Joris H, Devroey P, et al. : Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–8. 10.1016/0140-6736(92)92425-f [DOI] [PubMed] [Google Scholar]
- 2. Esteves SC: Novel concepts in male factor infertility: clinical and laboratory perspectives. J Assist Reprod Genet. 2016;33(10):1319–1335. 10.1007/s10815-016-0763-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Esteves SC, Roque M, Bedoschi G, et al. : Intracytoplasmic sperm injection for male infertility and consequences for offspring. Nat Rev Urol. 2018;15(9):535–62. 10.1038/s41585-018-0051-8 [DOI] [PubMed] [Google Scholar]
- 4. Esteves SC, Miyaoka R, Orosz JE, et al. : An update on sperm retrieval techniques for azoospermic males. Clinics (Sao Paulo). 2013;68 Suppl 1:99–110. 10.6061/clinics/2013(sup01)11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flannigan RK, Schlegel PN: Microdissection testicular sperm extraction: preoperative patient optimization, surgical technique, and tissue processing. Fertil Steril. 2019;111(3):420–426. 10.1016/j.fertnstert.2019.01.003 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Strassburger D, Friedler S, Raziel A, et al. : Very low sperm count affects the result of intracytoplasmic sperm injection. J Assist Reprod Genet. 2000;17(8):431–6. 10.1023/a:1009413201849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchell V, Rives N, Albert M, et al. : Outcome of ICSI with ejaculated spermatozoa in a series of men with distinct ultrastructural flagellar abnormalities. Hum Reprod. 2006;21(8):2065–74. 10.1093/humrep/del130 [DOI] [PubMed] [Google Scholar]
- 8. Verza S, Esteves SC: Sperm defect severity rather than sperm Source is associated with lower fertilization rates after intracytoplasmic sperm injection. Int Braz J Urol. 2008;34(1):49–56. 10.1590/s1677-55382008000100008 [DOI] [PubMed] [Google Scholar]
- 9. Greco E, Scarselli F, Iacobelli M, et al. : Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod. 2005;20(1):226–30. 10.1093/humrep/deh590 [DOI] [PubMed] [Google Scholar]
- 10. Krawetz SA: Paternal contribution: new insights and future challenges. Nat Rev Genet. 2005;6(8):633–42. 10.1038/nrg1654 [DOI] [PubMed] [Google Scholar]
- 11. Agarwal A, Majzoub A, Esteves SC, et al. : Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol. 2016;5(6):935–50. 10.21037/tau.2016.10.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aitken RJ: DNA damage in human spermatozoa; important contributor to mutagenesis in the offspring. Transl Androl Urol. 2017;6(Suppl 4):S761–S764. 10.21037/tau.2017.09.13 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Rima D, Shiv BK, Bhavna Ch, et al. : Oxidative Stress Induced Damage to Paternal Genome and Impact of Meditation and Yoga - Can it Reduce Incidence of Childhood Cancer? Asian Pac J Cancer Prev. 2016;17(9):4517–25. [PubMed] [Google Scholar]
- 14. Santi D, Spaggiari G, Simoni M: Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management - meta-analyses. Reprod Biomed Online. 2018;37(3):315–26. 10.1016/j.rbmo.2018.06.023 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Esteves SC, Agarwal A, Cho CL, et al. : A Strengths-Weaknesses-Opportunities-Threats (SWOT) analysis on the clinical utility of sperm DNA fragmentation testing in specific male infertility scenarios. Transl Androl Urol. 2017;6(Suppl 4):S734–S760. 10.21037/tau.2017.08.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Esteves SC: Interventions to Prevent Sperm DNA Damage Effects on Reproduction. Adv Exp Med Biol. 2019;1166:119–48. 10.1007/978-3-030-21664-1_8 [DOI] [PubMed] [Google Scholar]
- 17. Roque M, Esteves SC: Effect of varicocele repair on sperm DNA fragmentation: a review. Int Urol Nephrol. 2018;50(4):583–603. 10.1007/s11255-018-1839-4 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Agarwal A, Parekh N, Panner Selvam MK, et al. : Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J Mens Health. 2019;37(3):296–312. 10.5534/wjmh.190055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sakkas D, Alvarez JG: Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93(4):1027–36. 10.1016/j.fertnstert.2009.10.046 [DOI] [PubMed] [Google Scholar]
- 20. Muratori M, Tamburrino L, Marchiani S, et al. : Investigation on the Origin of Sperm DNA Fragmentation: Role of Apoptosis, Immaturity and Oxidative Stress. Mol Med. 2015;21:109–22. 10.2119/molmed.2014.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Gosálvez J, López-Fernández C, Fernández JL, et al. : Unpacking the mysteries of sperm DNA fragmentation: ten frequently asked questions. J Reprod Biotechnol Fertil. 2015;4:205891581559445 10.1177/2058915815594454 [DOI] [Google Scholar]
- 22. Moskovtsev SI, Jarvi K, Mullen JBM, et al. : Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil Steril. 2010;93(4):1142–6. 10.1016/j.fertnstert.2008.11.005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Moskovtsev SI, Alladin N, Lo KC, et al. : A comparison of ejaculated and testicular spermatozoa aneuploidy rates in patients with high sperm DNA damage. Syst Biol Reprod Med. 2012;58(3):142–8. 10.3109/19396368.2012.667504 [DOI] [PubMed] [Google Scholar]
- 24. Esteves SC, Sánchez-Martín F, Sánchez-Martín P, et al. : Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril. 2015;104(6):1398–405. 10.1016/j.fertnstert.2015.08.028 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Mehta A, Bolyakov A, Schlegel PN, et al. : Higher pregnancy rates using testicular sperm in men with severe oligospermia. Fertil Steril. 2015;104(6):1382–7. 10.1016/j.fertnstert.2015.08.008 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Esteves SC, Roque M, Bradley CK, et al. : Reproductive outcomes of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with high levels of DNA fragmentation in semen: systematic review and meta-analysis. Fertil Steril. 2017;108(3):456–467.e1. 10.1016/j.fertnstert.2017.06.018 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Bradley CK, McArthur SJ, Gee AJ, et al. : Intervention improves assisted conception intracytoplasmic sperm injection outcomes for patients with high levels of sperm DNA fragmentation: a retrospective analysis. Andrology. 2016;4(5):903–10. 10.1111/andr.12215 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Pabuccu EG, Caglar GS, Tangal S, et al. : Testicular versus ejaculated spermatozoa in ICSI cycles of normozoospermic men with high sperm DNA fragmentation and previous ART failures. Andrologia. 2017;49(2). 10.1111/and.12609 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Arafa M, AlMalki A, AlBadr M, et al. : ICSI outcome in patients with high DNA fragmentation: Testicular versus ejaculated spermatozoa. Andrologia. 2018;50(1). 10.1111/and.12835 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Zhang J, Xue H, Qiu F, et al. : Testicular spermatozoon is superior to ejaculated spermatozoon for intracytoplasmic sperm injection to achieve pregnancy in infertile males with high sperm DNA damage. Andrologia. 2019;51(2):e13175. 10.1111/and.13175 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Herrero MB, Lusignan MF, Son WY, et al. : ICSI outcomes using testicular spermatozoa in non-azoospermic couples with recurrent ICSI failure and no previous live births. Andrology. 2019;7(3):281–287. 10.1111/andr.12591 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Alharbi M, Hamouche F, Phillips S, et al. : Use of testicular sperm in couples with SCSA-defined high sperm DNA fragmentation and failed intracytoplasmic sperm injection using ejaculated sperm. Asian J Androl. 2019. 10.4103/aja.aja_99_19 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Esteves SC, Roque M, Garrido N: Use of testicular sperm for intracytoplasmic sperm injection in men with high sperm DNA fragmentation: a SWOT analysis. Asian J Androl. 2018;20(1):1–8. 10.4103/aja.aja_7_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Evenson DP: Evaluation of sperm chromatin structure and DNA strand breaks is an important part of clinical male fertility assessment. Transl Androl Urol. 2017;6(Suppl 4):S495–S500. 10.21037/tau.2017.07.20 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Weissman A, Horowitz E, Ravhon A, et al. : Pregnancies and live births following ICSI with testicular spermatozoa after repeated implantation failure using ejaculated spermatozoa. Reprod Biomed Online. 2008;17(5):605–9. 10.1016/s1472-6483(10)60306-9 [DOI] [PubMed] [Google Scholar]
- 36. Bendikson KA, Neri QV, Takeuchi T, et al. : The outcome of intracytoplasmic sperm injection using occasional spermatozoa in the ejaculate of men with spermatogenic failure. J Urol. 2008;180(3):1060–4. 10.1016/j.juro.2008.05.025 [DOI] [PubMed] [Google Scholar]
- 37. Hauser R, Bibi G, Yogev L, et al. : Virtual azoospermia and cryptozoospermia--fresh/frozen testicular or ejaculate sperm for better IVF outcome? J Androl. 2011;32(5):484–90. 10.2164/jandrol.110.011353 [DOI] [PubMed] [Google Scholar]
- 38. Amirjannati N, Heidari-Vala H, Akhondi MA, et al. : Comparison of intracytoplasmic sperm injection outcomes between spermatozoa retrieved from testicular biopsy and from ejaculation in cryptozoospermic men. Andrologia. 2012;44 Suppl 1:704–9. 10.1111/j.1439-0272.2011.01253.x [DOI] [PubMed] [Google Scholar]
- 39. Ben-Ami I, Raziel A, Strassburger D, et al. : Intracytoplasmic sperm injection outcome of ejaculated versus extracted testicular spermatozoa in cryptozoospermic men. Fertil Steril. 2013;99(7):1867–71. 10.1016/j.fertnstert.2013.02.025 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Abhyankar N, Kathrins M, Niederberger C: Use of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with cryptozoospermia: a meta-analysis. Fertil Steril. 2016;105(6):1469–1475.e1. 10.1016/j.fertnstert.2016.02.013 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Ketabchi AA: Intracytoplasmic Sperm Injection Outcomes with Freshly Ejaculated Sperms and Testicular or Epididymal Sperm Extraction in Patients with Idiopathic Cryptozoospermia. Nephrourol Mon. 2016;8(6):e41375. 10.5812/numonthly.41375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cui X, Ding P, Gao G, et al. : Comparison of the Clinical Outcomes of Intracytoplasmic Sperm Injection Between Spermatozoa Retrieved From Testicular Biopsy and From Ejaculate in Cryptozoospermia Patients. Urology. 2017;102:106–10. 10.1016/j.urology.2016.08.071 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Kang YN, Hsiao YW, Chen CY, et al. : Testicular sperm is superior to ejaculated sperm for ICSI in cryptozoospermia: An update systematic review and meta-analysis. Sci Rep. 2018;8(1):7874. 10.1038/s41598-018-26280-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Ku FY, Wu CC, Hsiao YW, et al. : Association of sperm source with miscarriage and take-home baby after ICSI in cryptozoospermia: A meta-analysis of testicular and ejaculated sperm. Andrology. 2018;6(6):882–9. 10.1111/andr.12546 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Yu Y, Wang R, Xi Q, et al. : Effect of paternal age on intracytoplasmic sperm injection outcomes in cryptozoospermic men: Ejaculated or testicular sperm? Medicine (Baltimore). 2019;98(26):e16209. 10.1097/MD.0000000000016209 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Lewis SEM: The place of sperm DNA fragmentation testing in current day fertility management. Middle East Fertility Society Journal. 2013;18(2):78–82. 10.1016/j.mefs.2013.01.010 [DOI] [Google Scholar]
- 47. Meseguer M, Santiso R, Garrido N, et al. : Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril. 2011;95(1):124–8. 10.1016/j.fertnstert.2010.05.055 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Jin J, Pan C, Fei Q, et al. : Effect of sperm DNA fragmentation on the clinical outcomes for in vitro fertilization and intracytoplasmic sperm injection in women with different ovarian reserves. Fertil Steril. 2015;103(4):910–6. 10.1016/j.fertnstert.2015.01.014 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Esteves SC, Carvalho JF, Bento FC, et al. : A Novel Predictive Model to Estimate the Number of Mature Oocytes Required for Obtaining at Least One Euploid Blastocyst for Transfer in Couples Undergoing in vitro Fertilization/Intracytoplasmic Sperm Injection: The ART Calculator. Front Endocrinol (Lausanne). 2019;10:99. 10.3389/fendo.2019.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miyaoka R, Orosz JE, Achermann AP, et al. : Methods of surgical sperm extraction and implications for assisted reproductive technology success. Panminerva Med. 2019;61(2):164–77. 10.23736/S0031-0808.18.03508-5 [DOI] [PubMed] [Google Scholar]
- 51. Lopes LS, Esteves SC: Testicular sperm for intracytoplasmic sperm injection in non-azoospermic men: a paradigm shift. Panminerva Med. 2019;61(2):178–186. 10.23736/S0031-0808.18.03534-6 [DOI] [PubMed] [Google Scholar]
- 52. Esteves SC: Should a Couple with Failed In Vitro Fertilization or Intracytoplasmic Sperm Injection and Elevated Sperm DNA Fragmentation Use Testicular Sperm for the Next Cycle? Eur Urol Focus. 2018;4(3):296–298. 10.1016/j.euf.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 53. Steele EK, McClure N, Maxwell RJ, et al. : A comparison of DNA damage in testicular and proximal epididymal spermatozoa in obstructive azoospermia. Mol Hum Reprod. 1999;5(9):831–5. 10.1093/molehr/5.9.831 [DOI] [PubMed] [Google Scholar]
- 54. O'Connell M, McClure N, Lewis SE: Mitochondrial DNA deletions and nuclear DNA fragmentation in testicular and epididymal human sperm. Hum Reprod. 2002;17(6):1565–70. 10.1093/humrep/17.6.1565 [DOI] [PubMed] [Google Scholar]
- 55. Hammoud I, Bailly M, Bergere M, et al. : Testicular Spermatozoa Are of Better Quality Than Epididymal Spermatozoa in Patients With Obstructive Azoospermia. Urology. 2017;103:106–111. 10.1016/j.urology.2016.11.019 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Esteves SC: Clinical management of infertile men with nonobstructive azoospermia. Asian J Androl. 2015;17(3):459–70. 10.4103/1008-682X.148719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Esteves SC, Miyaoka R, Agarwal A: Surgical treatment of male infertility in the era of intracytoplasmic sperm injection - new insights. Clinics (Sao Paulo). 2011;66(8):1463–78. 10.1590/s1807-59322011000800026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Esteves SC: Microdissection testicular sperm extraction (micro-TESE) as a sperm acquisition method for men with nonobstructive azoospermia seeking fertility: operative and laboratory aspects. Int Braz J Urol. 2013;39(3):440; discussion 441. 10.1590/S1677-5538.IBJU.2013.03.21 [DOI] [PubMed] [Google Scholar]
- 59. Paoli D, Pelloni M, Lenzi A, et al. : Cryopreservation of Sperm: Effects on Chromatin and Strategies to Prevent Them. Adv Exp Med Biol. 2019;1166:149–167. 10.1007/978-3-030-21664-1_9 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Nabi A, Khalili MA, Halvaei I, et al. : Prolonged incubation of processed human spermatozoa will increase DNA fragmentation. Andrologia. 2014;46(4):374–9. 10.1111/and.12088 [DOI] [PubMed] [Google Scholar]
- 61. Levron J, Aviram-Goldring A, Madgar I, et al. : Sperm chromosome abnormalities in men with severe male factor infertility who are undergoing in vitro fertilization with intracytoplasmic sperm injection. Fertil Steril. 2001;76(3):479–84. 10.1016/s0015-0282(01)01957-4 [DOI] [PubMed] [Google Scholar]
- 62. Palermo GD, Colombero LT, Hariprashad JJ, et al. : Chromosome analysis of epididymal and testicular sperm in azoospermic patients undergoing ICSI. Hum Reprod. 2002;17(3):570–5. 10.1093/humrep/17.3.570 [DOI] [PubMed] [Google Scholar]
- 63. Rodrigo L, Rubio C, Peinado V, et al. : Testicular sperm from patients with obstructive and nonobstructive azoospermia: aneuploidy risk and reproductive prognosis using testicular sperm from fertile donors as control samples. Fertil Steril. 2011;95(3):1005–12. 10.1016/j.fertnstert.2010.10.022 [DOI] [PubMed] [Google Scholar]
- 64. Vozdova M, Heracek J, Sobotka V, et al. : Testicular sperm aneuploidy in non-obstructive azoospermic patients. Hum Reprod. 2012;27(7):2233–9. 10.1093/humrep/des115 [DOI] [PubMed] [Google Scholar]
- 65. Belva F, de Schrijver F, Tournaye H, et al. : Neonatal outcome of 724 children born after ICSI using non-ejaculated sperm. Hum Reprod. 2011;26(7):1752–8. 10.1093/humrep/der121 [DOI] [PubMed] [Google Scholar]
- 66. Bonduelle M, van Assche E, Joris H, et al. : Prenatal testing in ICSI pregnancies: Incidence of chromosomal anomalies in 1586 karyotypes and relation to sperm parameters. Hum Reprod. 2002;17(10):2600–14. 10.1093/humrep/17.10.2600 [DOI] [PubMed] [Google Scholar]
- 67. Meijerink AM, Ramos L, Janssen AJ: Behavioral, cognitive, and motor performance and physical development of five-year-old children who were born after intracytoplasmic sperm injection with the use of testicular sperm. Fertil Steril. 2016;106(7):1673–1682.e5. 10.1016/j.fertnstert.2016.09.011 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Tsai CC, Huang FJ, Wang LJ, et al. : Clinical outcomes and development of children born after intracytoplasmic sperm injection (ICSI) using extracted testicular sperm or ejaculated extreme severe oligo-astheno-teratozoospermia sperm: A comparative study. Fertil Steril. 2011;96(3):567–71. 10.1016/j.fertnstert.2011.06.080 [DOI] [PubMed] [Google Scholar]
- 69. Esteves SC, Agarwal A: Reproductive outcomes, including neonatal data, following sperm injection in men with obstructive and nonobstructive azoospermia: Case series and systematic review. Clinics (Sao Paulo). 2013;68 Suppl 1:141–50. 10.6061/clinics/2013(sup01)16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Esteves SC, Prudencio C, Seol B, et al. : Comparison of sperm retrieval and reproductive outcome in azoospermic men with testicular failure and obstructive azoospermia treated for infertility. Asian J Androl. 2014;16(4):602–6. 10.4103/1008-682X.126015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cheung S, Schlegel PN, Rosenwaks Z, et al. : Revisiting aneuploidy profile of surgically retrieved spermatozoa by whole exome sequencing molecular karyotype. PLoS One. 2019;14(1):e0210079. 10.1371/journal.pone.0210079 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Weng SP, Surrey MW, Danzer HC, et al. : Chromosome abnormalities in embryos derived from microsurgical epididymal sperm aspiration and testicular sperm extraction. Taiwan J Obstet Gynecol. 2014;53(2):202–5. 10.1016/j.tjog.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 73. Figueira R, Carvalho JF, Bento FC, et al. : ICSI using surgically retrieved testicular sperm of non-azoospermic men with high sperm DNA fragmentation index and blastocyst ploidy: a safe approach. Abstracts of the 35th Annual Meeting of the European Society of Human Reproduction and Embryology. Hum Reprod. 2019;34(Supp 1):i1–i543. [Google Scholar]