Abstract
For approximately the first 2 billion years of Earth history, atmospheric oxygen levels were extremely low. It wasn’t until at least half a billion years after the evolution of oxygenic photosynthesis, perhaps as early as 3 billion years ago, that oxygen rose to appreciable levels during the Great Oxidation event. Shortly after, marine carbonates experienced a large positive spike in carbon isotope ratios known as the Lomagundi event. The mechanisms responsible for the Great Oxidation and Lomagundi events remain debated. Using a carbon-oxygen box model which tracks surface and interior C fluxes and reservoirs while also tracking C isotopes and atmospheric oxygen levels we demonstrate that about 2.5 billion years ago a tectonic transition resulting in increased volcanic CO2 emissions could have led to increased deposition of both carbonates and organic carbon via enhanced weathering and nutrient delivery to oceans. Increased burial of carbonates and organic carbon would have allowed accumulation of atmospheric oxygen while also increasing delivery of carbon to subduction zones. Coupled with preferential release of carbonates at arc volcanoes and deep recycling of organic C to ocean island volcanoes we find such a tectonic transition can simultaneously explain the Great Oxidation and Lomagundi events without any change in the fraction of carbon buried as organic carbon relative to carbonate, which is often invoked to explain carbon isotope excursions.
Carbon dioxide and oxygen, two gases critical for Earth’s habitability, are linked via photosynthesis:
| (1) |
The link between CO2 and O2 is evident in the geologic record from the association of the most dramatic events in the histories of both gases. The Great Oxidation Event (GOE), during which Earth’s atmospheric oxygen levels increased by several orders of magnitude, and the Lomagundi Event (LE), during which carbon isotope ratios of marine carbonates increased by up to ~10‰, both occurred around 2.3–2.0 Ga1–3 (Fig. 1). The temporal association of the two events suggests both may be attributed to a single mechanism, such as enhanced organic carbon burial4. This is because increasing the fraction of carbon buried as organic carbon (forg) relative to carbonate will increase the amount of oxygen produced and allowed to accumulate via equation (1). Increased forg also increases δ13C of carbonates because organic matter preferentially incorporates 12C, leaving the carbonate-forming C reservoir enriched in 13C (ref. 4).
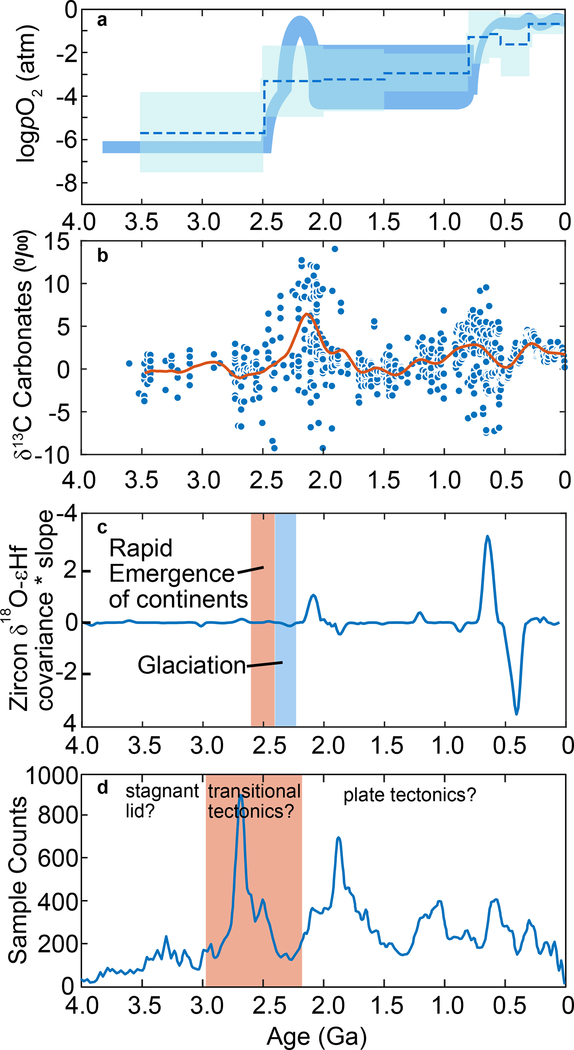
Figure 1. Variations in atmospheric O2, δ13C of carbonates, and tracers of tectonic processes through geologic time.
a, Qualitative model of atmospheric O2 (solid blue curve)1 and estimation of atmospheric O2 from Zn/Fe ratios in carbonates (dashed blue line with shaded region for uncertainties)46. b, Carbonate δ13C through time43. c, Zircon δ18O-εHf covariance*slope from Keller, et al.24, where positive values indicate increases in crustal contribution to magmatism. Orange band is proposed timing for rapid emergence of subaerial continents inferred from triple-oxygen isotopes15, blue band is timing large-scale glaciations24.d, U/Pb age distribution of detrital and orogenic zircons47, with orange band showing the proposed timing of transitional tectonics between stagnant lid and plate tectonics12.
Despite the apparent temporal association of the GOE and LE and the conceptual consistency of explaining both events via increased forg, its role as the driver of the GOE and LE has been questioned. There is evidence that oxygenic photosynthesis evolved at least half a billion years before the GOE5, so why would atmospheric oxygen have remained low for so long?6. In addition, other studies have suggested that the GOE may have preceded the LE by ~100 million years2,3,7,8. If the onset of the GOE and LE were indeed temporally disparate, it becomes difficult to explain both events via a single mechanism. This has led researchers to propose mechanisms invoking decreased sinks of atmospheric oxygen as the main driver of the GOE9–11. While decreased oxygen sinks would allow atmospheric O2 to accumulate, it is unclear how they would drive the associated positive C isotope excursion.
Here, we propose a single mechanism for the GOE and LE. Our modeling shows that the proposed mechanism can simultaneously explain increased oxygen production as well as a positive C isotope excursion in marine carbonates. In addition, we show that the proposed mechanism is consistent with a delay between the evolution of photosynthesis and increased atmospheric oxygen levels, as well as a delay between the build-up of oxygen in the atmosphere and the positive C isotope excursion observed in marine carbonates.
Enhanced volcanic CO2 driven by tectonic transitions
We propose that around 2.5 billion years ago a major tectonic transition increased volcanic CO2 emissions, driving increased rates carbonate and organic C burial via enhanced weathering and nutrient delivery to oceans. A transition from stagnant/sluggish lid to plate tectonics has been proposed around 2.5 Ga12, which would have caused increased volcanic CO2 emissions13,14. Also proposed around 2.5 billion years ago are the rapid emergence of subaerial continents15 and major glaciations16. These events would have increased the supply of hydrated sediments to trenches, lubricating the subduction interface and enhancing plate tectonic activity, which would have led to enhanced volcanic CO2 emissions17. Moussallam et al.18 propose that mantle cooling resulted in lower eruptive temperatures for volcanic gases, which caused the gases to become more oxidized, resulting in CO2 emissions to increase relative to emissions of more reduced C gases.
A GOE resulting from tectonically-driven increases in volcanic CO2 emissions is consistent with oxygenic photosynthesis evolving prior to the GOE5 because the production rate of oxygen is controlled by the flux of organic C burial:
| (2) |
where Forg is the flux of organic carbon burial, and Ftot is the flux of total carbon burial (carbonate + organic C). Therefore, Forg, and the oxygen production flux, can increase by increasing Ftot, instead of forg. At the onset of oxygenic photosynthesis, oxygen production could have been low because Earth’s tectonic mode promoted low weathering fluxes (i.e., stagnant-lid, un-emerged continents), resulting in low Ftot and thus low Forg. Then a tectonic transition could have increased Ftot and thus Forg, resulting in a delay between the evolution of photosynthesis and the GOE5,19. This could have all been achieved without any significant changes to forg.
Carbonate release at arcs drive positive spike in δ13C
Positive δ13C excursions in carbonates are commonly attributed to increased forg:
| (3) |
where δ13Cin is δ13C of atmospheric inputs of CO2 and δ13Corg is δ13C of organic C. If fractionation of C isotopes between carbonates and organic C (Δ13Ccarb-org ≡ δ13Ccarb- δ13Corg ≈ 25‰), and δ13Cin remain constant, increased forg increases δ13Ccarb. δ13Ccarb also increases if δ13Cin increases at constant forg and Δ13Ccarb-org. We propose that the increased δ13Ccarb of the LE could have been caused by increased δ13Cin rather than increased forg.
δ13Cin depends on the balance of CO2 being emitted at different volcanic settings:
| (4) |
where Fi is CO2 flux at volcanic setting i and δ13Ci is δ13C of CO2 emitted at volcanic setting i. δ13CMORB ≈ −5‰20,21, CO2 flux-weighted δ13Carc ≈ −3‰22, and sparse studies on δ13COIB suggest it could be similar to MORB or more negative21,23. Therefore, increasing increases δ13Cin by shifting the balance of δ13Cin towards δ13Carc.
The tectonic transitions discussed above can increase carbonate and organic C burial leading to increased subduction of C. Upon reaching subarc depths the increased flux of subducted C would be released from the slab at an increased rate resulting in increased , shifting the balance of δ13Cin towards δ13Carc. This mechanism is consistent with oxygenation preceding increased δ13Ccarb8 because oxygen accumulates immediately after burial of organic C. Contrastingly, δ13Ccarb increases after the increased C flux is buried, subducted, released from the slab, emitted at arcs as CO2, and incorporated into marine carbonates. Evidence for increased delivery of subducted sediments to arc volcanic sources is provided by the record of covariance of δ18O and εHf in zircons - both of which quantify the contribution of subducted weathering products to magma generation24. Fig 1c shows that the peak in δ13Ccarb closely correlates with the peak in δ18O-εHfzircon covariance, supporting the idea that δ13Ccarb may be controlled by the balance of δ13C of CO2 emitted at different volcanic centers.
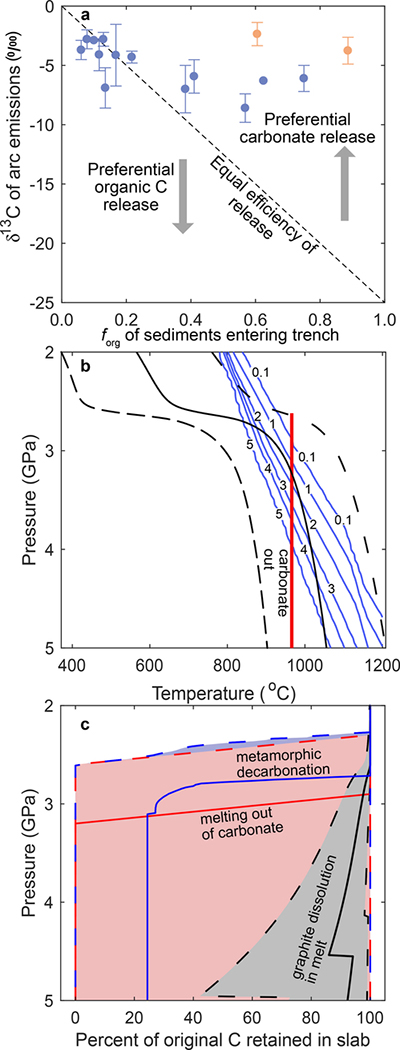
Increasing δ13Cin by increasing is possible if δ13Carc is high, which is most easily explained by a heavy contribution of carbonates to arc CO2 emissions. A recent study determined that the flux-weighted, average δ13Carc≈−3‰22. Whether high δ13Carc is due to contribution of carbonates from the subducted slab or upper crust remains debated22,25. Figure 2a shows δ13Carc versus forg of the sediments entering the respective trench. As forg of subducted C becomes greater, δ13Carc becomes more negative, implying that forg of subducted sediments is the dominant controller of δ13Carc26. The dashed line in Fig. 2a shows what δ13Carc would be if carbonates and organic C were released with equal efficiency. Data in Fig. 2a suggest preferential carbonate release, supporting the idea that δ13Carc is high due to preferential release of carbonates relative to organic C from the subducted slab. Below, we test whether ancient arcs may have also had high δ13C.
Figure 2. Natural data and petrologic calculations suggesting preferential release of carbonate over organic carbon in present-day and ancient subduction zones.
a, δ13Carc22 versus forg of sediments at modern subduction zones48. Orange symbols represent volcanoes erupting through carbonates, increasing likelihood of crustal carbonate dominating δ13Carc49. Dashed line shows equal efficiency of release for carbonates and organic C. Error bars are 1σ. b, Ancient slab P-T paths27 (Dashed black lines are cold and hot P-T paths, solid black line is average of the two) compared to metamorphic decarbonation (blue lines labeled with wt% CO2 retained in slab), and melting out of carbonates29 (red line). c, Percentage of C retained in slab versus depth. Blue lines show how much C remains in slab (solid and dashed lines calculated along respective P-T paths of b) during metamorphic decarbonation. Red lines calculated for carbonates released by carbonated melting (red line in b). Black lines for organic C release by graphite dissolution in silicate melts.
Ancient subduction zones were likely hotter than present day due to secular mantle cooling27. Figure 2b,c demonstrates that along P-T paths of subducting slabs adjusted for secular mantle cooling27, carbonate will be removed from the slab more efficiently than organic C, which metamorphoses to graphite during subduction28. In a carbonated pelitic sediment, metamorphic decarbonation and partial melting29 can release up to 100% of subducted carbonates (Fig. 2c). Graphite removal via CO2 dissolution in pelitic sediment partial melts can only extract up to ~50% of the subducted organic C. This demonstrates that like present-day arcs and perhaps even more so, ancient δ13Carc was more strongly influenced by δ13Ccarb than δ13Corg.
Considering the results provided above, we propose enhanced subduction of C can increase δ13Ccarb by increasing δ13Cin due to preferential release of carbonates relative to organic C at arcs. This explains increased δ13Ccarb observed in the LE, however it doesn’t explain why δ13Ccarb returned to around 0‰ (Fig. 1). We propose that the return to “normal” δ13Ccarb (~ 0‰) can be explained by delayed release of organic C at intraplate ocean island volcanoes.
Return to normal δ13Ccarb via deep recycling of organic C
After releasing carbonates, the slab becomes enriched in organic C, which remains in the slab as refractory graphite/diamond27,30. Subducted slabs have been proposed to descend to the core-mantle boundary and reside in “slab graveyards” until being entrained in upwelling mantle plumes, fueling volcanism at ocean islands31,32. Upon ascent through the mantle, reduced organic C oxidizes and initiates redox melting33, allowing organic C to be emitted at ocean islands as CO2. The large reservoir of Fe3+ relative to organic C in the mantle can effectively oxidize organic C without experiencing major changes over geologic time. Unlike arc δ13Carc, δ13COIB has received relatively little attention. However, several studies report δ13COIB, which may support the hypothesis of ocean islands being enriched in organic C.
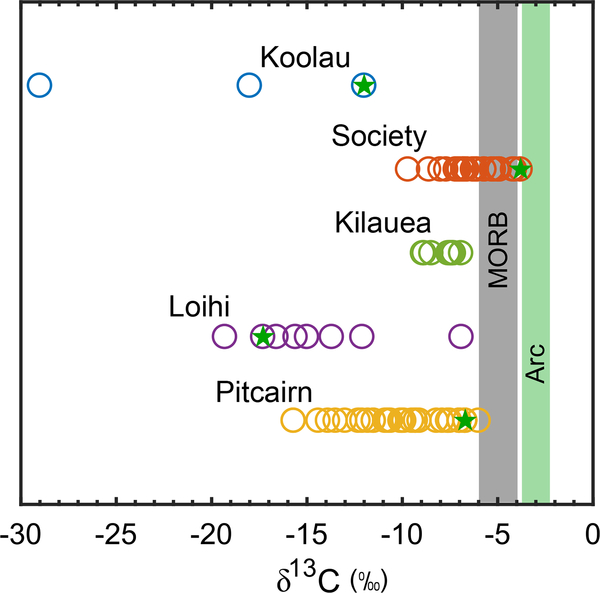
Figure 3 shows a compilation of δ13COIB. δ13COIB measurements at Kilauea were made on high-temperature volcanic CO234, making them the most appropriate to compare with the arc dataset, which are largely fumarolic measurements. Figure 3 shows that δ13C at Kilauea is lower than δ13Carc. Measurements made at Koolau35, Society23, Loihi36, and Pitcairn21 were made on CO2 from vesicles, glass, or melt inclusions and are subject to isotope fractionation during degassing37. For this reason, we identify the least degassed sample from each dataset. Except for Society, which has been shown to have similar noble gas ratios to MORB23, the least degassed samples from ocean islands have lower δ13C than MORBs and arcs. These measurements support the possibility of organic C enrichment in ocean island basalts. We propose that delayed release of organic C at ocean islands can explain the return to “normal” δ13Ccarb (about 0‰) at the tail-end of the LE. This mechanism requires that subducted organic C retains its low δ13C, which is supported by the observation of light δ13C values in eclogitic diamonds38. Additionally, organic C hosted in subducted oceanic crust may remain physically and chemically isolated from C hosted in ambient mantle precluding any alteration of δ13C values. Other elements, such as nitrogen, have also been argued to show an enrichment in subducted organic components at ocean island volcanoes39. A schematic diagram showing preferential release of organic C at ocean islands and carbonates at arcs shown in Fig. 4.
Figure 3. Comparison of carbon isotope compositions of CO2 emissions from different volcanic settings.
Measurements made on samples from Koolau35, Society23, Loihi36, and Pitcairn21 were made on CO2 from vesicles, glass, or melt inclusions and are therefore subject to isotope fractionation during degassing37. Least degassed samples are marked for each location with a green star. Measurements at Kilauea34 are made on high-T volcanic gas emissions and are therefore the most directly applicable for comparison to the global flux-weighted average of arc CO2 emissions22. Available data suggests that ocean islands may emit CO2 with lower δ13C than MORB and arcs.
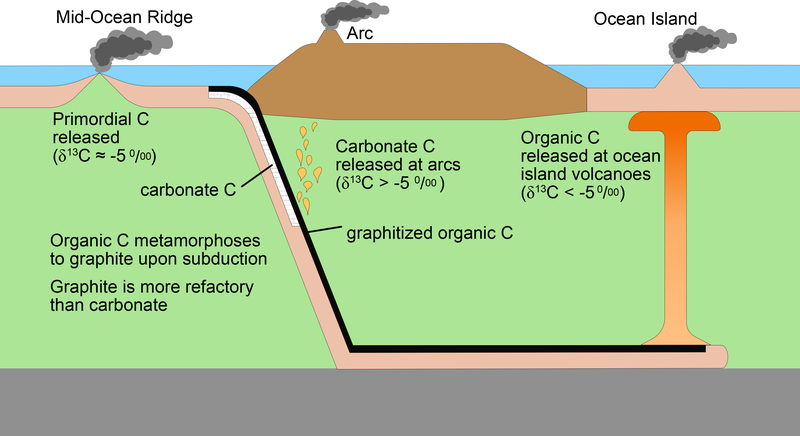
Figure 4. Schematic diagram showing preferential release of carbonate C at arc volcanoes and organic C at ocean island volcanoes.
Both carbonates and organic carbon are deposited on the seafloor and subducted into Earth’s interior. Upon subduction, organic C metamorphoses to graphite, which is more refractory than carbonate. At subarc conditions carbonates are preferentially released from the slab (Fig. 2) relative to graphitized organic C, increasing arc volcano δ13C to values greater than −5‰. Graphitized organic C remains in slab and is transported deep into the mantle, where it may become entrained in upwelling mantle plumes feeding ocean island volcanoes. Upwelling, reduced organic C likely undergoes redox melting, decreasing ocean island volcano δ13C to <−5‰.
The proposed mechanism combined with the observation that δ13Ccarb has always returned to ~0‰ after large excursions (Fig. 1), leads us to infer that C fluxes into and out of the mantle are at near steady-state with response times to perturbations on the time-scale of mantle transit times, which are on the order of hundreds of million years40. If C fluxes into and out of the mantle were out of balance, then due to the more refractory nature of graphitized organic C, we would expect δ13Ccarb to increase with time due to the preferential sequestration of light organic C in the mantle. However, δ13Ccarb is not observed to increase with time, rather it experiences excursions then returns to ~0‰ (Fig. 1).
Modeling oxygen levels and δ13Ccarb
Here, we present a carbon-oxygen box model to test whether increased volcanic CO2 emissions can drive oxygenation and a positive δ13Ccarb excursion. The key model assumptions are: 1) Atmospheric oxygen levels are proportional to the mass of organic C in the system. 2) The mass of organic C buried depends on the flux of carbon deposition, which is proportional to the concentration of atmospheric CO2:
| (5) |
where Fw is the flux of CO2 drawdown from the atmosphere, k is the strength of the weathering feedback, and [CO2,atm] is the concentration of atmospheric CO2. 3) δ13Carc is strongly influenced by δ13Ccarb and δ13COIB by δ13Corg. 4) Farc responds to surficial changes in CO2 fluxes on the order of 10 Myr, while FOIB responds on the order of 100 Myr, corresponding to one mantle transit time for subducted crust32,40. These timescales are equivalent to residence times of subducted carbonates and organic C in their respective mantle reservoirs. These residence times appear short compared to those estimated in previous studies (e.g.41). However, the > 1 billion years residence time of carbon in the mantle is calculated assuming the mantle as a single reservoir for C, which is an oversimplification of the natural C cycle because the mantle contains both recycled and primordial carbon. We assume the residence time of subducted carbonates in the ancient mantle is on the order of 10 million years because they are rapidly released to arc source mantle during subduction42. We relate the residence time of organic C to the timescales of mantle convection because organic C is hosted as refractory/graphite diamond in subducted oceanic crust and is unlikely to efficiently mix with surrounding mantle. Therefore, as soon as the subducted oceanic crust completes a convective cycle and undergoes partial melting in the oxidized upper mantle, organic C will be efficiently degassed as CO2.
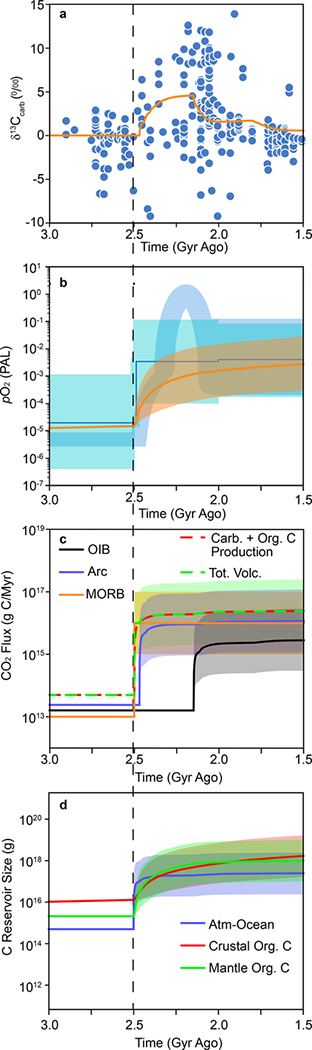
Figure 5 was generated by increasing mid-ocean ridge CO2 emissions by 1000-fold instantaneously at 2.5 Ga. Figure 5 demonstrates that in response to increased CO2 emissions, the production flux of carbonates and organic C increases, causing atmospheric oxygen to increase immediately due to the increased reservoir of buried organic C. Shortly after oxygen increases, δ13Ccarb increases as increases, with the delay being dictated by the time required for carbonate to be deposited, subducted, and released at arcs as CO2 (Fig. 5). After a longer delay, dictated by the time required for organic C to be buried and later released as CO2 at ocean islands, δ13Ccarb decreases in response to increased . Note that model results were generated keeping forg constant at 0.20 throughout the model run.
Figure 5. Model results showing how tectonically-driven increased CO2 emissions and deep recycling of organic C can drive GOE and LE.
a. δ13Ccarb versus time. Orange curve is model result, blue symbols are natural data. When increases to MOR CO2 emissions are large, magnitude of C isotope excursion becomes insensitive to magnitude of changes to MOR CO2 emissions (See SI). b. Atmospheric O2 versus time. Orange curve shows results for 1000-fold increase in MOR CO2 emissions, while bands show results when increases range from 100-fold to 10,000-fold. Blue curves from Fig. 1. Vertical dashed line emphasizes that oxygen increases before C isotope excursion. c. CO2 fluxes versus time. Shaded regions as in b. d. C reservoir size versus time. Shaded regions as in b.
A notable result of the model is that simply increasing volcanic CO2 emissions can match the magnitude of oxygen increase while nearly matching the magnitude of δ13C excursions observed for the GOE and LE, which can’t be achieved by solely changing forg43,44. Another notable result of the model is that it closely reproduces the topology of the Lomagundi event, which displays a gradual increase in δ13C and terminates abruptly with δ13C rapidly decreasing to ~0‰. Supplementary Fig. 2 shows how varying different model parameters affects the shape and magnitude of the C isotope excursion. Furthermore, the present model shows that a permanent transition to increased volcanic CO2 emissions produces a permanent transition to higher atmospheric O2 but causes a transient excursion in δ13C as observed in natural data. We note that our model is consistent with any event that increases CO2 emissions. Therefore, the proposed mechanism is not only applicable to the Great Oxidation and Lomagundi event (see Supp. Fig. 3), it can also explain other large oxidation and C isotope excursions such as the events observed at the end of the Proterozoic1, which have also been proposed to result from large emissions of CO245. This is in contrast to the model of Duncan and Dasgupta27, which relies on initiation of plate tectonics to coincide with the GOE.
In summary, we propose that the GOE and LE can be explained by a single mechanism – increased carbonate and organic C deposition driven by enhanced volcanic CO2 degassing, possibly driven by a transition from stagnant/sluggish lid to plate tectonics12. Alternatively, the rapid emergence of subaerial continents15 or global glaciation17,24, proposed to have occurred ~2.5–2.3 Ga, have been argued to facilitate enhanced volcanic CO2 emissions by supplying sediments to convergent margins thereby facilitating more efficient subduction and related volcanism17. The presented hypothesis is consistent with natural observations such as contemporaneous increases in continental weathering24, heavy δ13Carc22, and possible light δ13COIB21. We present a carbon-oxygen model which demonstrates increased volcanic CO2 emissions coupled with preferential release of carbonate-derived CO2 at arc volcanoes and the delayed release of organic C at ocean island volcanoes can drive atmospheric oxygenation and positive C isotope excursions without requiring any changes to forg
The present study raises several important implications. 1) Large-scale, long-lived positive δ13C isotope excursions such as the LE do not require changes in forg, they may be the result of fractionation of carbonates and organic C in Earth’s interior. 2) Oxygen and biomass production may be controlled by tectonic conditions dictating volcanic CO2 emissions. 3) Carbon fluxes into and out of the mantle may be close to steady state, with response times to perturbations on the order of mantle transit times. These conclusions provide an alternate interpretation of the evolution of CO2 and O2 on Earth and can inform our thinking on how Earth, the only known inhabited planet in our Solar System and beyond, became and remained oxygenated, allowing for the proliferation of complex life on the surface.
Methods
Quantifying C release from the subducting slab
To quantify carbon release from the subducting slab via metamorphic decarbonation, we performed thermodynamic calculations with the software package Perple_X 50. We calculate the stable mineral assemblage of a model carbonated pelite as a function of pressure and temperature, with a bulk composition from Tsuno et al.29. We use the calculated bulk composition of the residual solid lithology to determine the amount of CO2 that can be retained in the solid lithology in the form of carbonates. The CO2 wt.% of the bulk rock decreases as P and T increase (Fig. 2a). We then calculate how much of the original carbon is retained in the solid lithology along subduction zone P-T paths relevant to ancient subduction zones. Following the arguments of Duncan and Dasgupta27 we assume that ancient subduction zones were hotter than those in the present day by about 70 °C/Gyr. Therefore, to investigate conditions relevant to the GOE and LE, we increased the temperatures of slab top P-T paths from Syracuse et al.51 by 175 °C. Assuming a starting CO2 of 5 wt.% as mineral carbonates, the amount of original subducted CO2 retained in the slab as carbonate was calculated along P-T paths according using the following equation:
| (6) |
Where 5 is the initial amount of CO2 in the bulk rock composition in wt%, and ‘CO2 wt% of bulk rock’ is the CO2 content of the bulk calculated as a function of pressure and temperature using Perple_X.
We note that the extent of metamorphic decarbonation calculated in this fashion provides an underestimate if extraneous fluid source is available. Incorporating open system, fluid infiltration induced metamorphic decarbonation more efficiently breaks down carbonates in the slab52. Similarly, the availability of extraneous fluid allows carbonate dissolution to be an important process of carbonate removal42. Furthermore, for warm subduction zones of ancient past, complete carbonate removal from the downgoing slab likely took place via carbonated melting53. Hence there are several mechanisms that could have led to complete exhaustion of carbonates from the slab in ancient subduction zones.
To quantify graphitized organic carbon release from subducting slabs we followed the methods of Duncan and Dasgupta27, in which the authors predict dissolved CO2 concentrations in hydrous rhyolitic melts in equilibrium with graphite/diamond as a function of P, T, and fO2. In our calculations of graphite/diamond dissolution in silicate melts we use the same P-T paths described above and fO2 was calculated along the CCO buffer54. Organic C removal efficiency would be even lower if more reduced slab conditions are used, i.e., the fO2 conditions used give the highest CO2 dissolution capacity and graphite/diamond saturation. The results shown in Fig. 2 were generated assuming 1 wt% graphite in the initial bulk composition. See Duncan and Dasgupta27 for more details on calculations.
Modeling atmospheric oxygen levels and δ13C of carbonates
We developed a new coupled carbon-oxygen cycle model which tracks δ13C. The model tracks how a set of carbon reservoirs (Ci) respond to changes in carbon fluxes (Fi) between these reservoirs. The surface reservoirs are the atmosphere-ocean (Catm), solid carbonates (Ccarb), and organic carbon (Corg), which are generated from the atmospheric reservoir (in this model we do not treat any fractionations between the ocean-atmosphere reservoirs). The transfer of carbon from the atmosphere-ocean reservoir into the carbonate and organic carbon reservoirs is controlled by a weathering flux (Fw, which is divided into Fcarb and Forg) which is proportional to the weathering constant (k) and the concentration of CO2 in the atmosphere (Catm). Carbon that is fluxed out of the atmosphere can be deposited as either carbonates or organic carbon. In Fig. 5 we prescribe that 40% of carbonates and organic C fluxed out of the atmosphere are added to a crustal reservoir (Ccarb and Corg), while the other 60% are subducted (χ =0.60). To demonstrate that a carbon isotope excursion is possible without changing the fraction of organic C buried relative to carbonate, we hold forg constant at 0.20 throughout the model.
While treating carbon in the Earth’s interior, we divide the mantle into three different carbon reservoirs. The first reservoir holds primitive carbon Cprm, which we treat as having existed in the mantle since its formation, additionally it receives no contribution from subducted components throughout the model evolution. The other two mantle carbon reservoirs consist of carbon hosted in subducted carbonate (Cmcarb), and carbon hosted in organic carbon (Cmorg), which have influxes from surfaces reservoirs controlled by the flux of subducted carbonate (Fsubc) and organic carbon (Fsubo) respectively. To relate oxygen levels to the carbon cycle we assume the atmospheric oxygen levels are directly proportional to the mass of organic carbon in the surface and mantle reservoirs.
We prescribe that subducted carbon can leave the mantle reservoirs via arc volcanoes (Farc) and ocean island volcanoes (FOIB). In order to keep track of how differential release of carbonates versus organics at different volcanic settings affects C isotopes of CO2 emissions through time, we treat volcanic CO2 fluxes at arc volcanoes and ocean island volcanoes as the sum of CO2 fluxes derived from carbonates (Farcc & FOIBc), organic carbon (Farco & FOIBo), and primitive mantle carbon (Farcp & FOIBp), each having their own distinct δ13C. To account for the differential release of carbonates and organics at arcs and OIBs we prescribe that a certain fraction of subducted carbonates (αcarb) and organics (αorg) are released at arcs, with the remaining fraction released at OIBs (1- αcarb, and 1- αorg). In accordance with our calculations in the main text (Fig. 2), we treat carbonates as being completely released at arcs (αcarb = 1), and organics as being completed released at OIBs (αorg = 0) when generating Fig. 5. Supplementary Figs. 2 and 3 show how probable variations in αcarb and αorg would influence the modeled evolution of δ13Ccarb.
Subducted carbon cycled through the mantle and released at arc volcanoes travel on the order of hundreds of kilometers, while carbon cycled through ocean island volcanoes may travel to the core-mantle boundary, traveling distances on the order of thousands of kilometers. Assuming mantle convection occurs on the order of 1 cm/yr the residence time of mantle carbon released at arcs (τarc) is on the order of 10 Myr, while the residence time of mantle carbon released at ocean island volcanoes (τOIB) is on the order of 100 Myr. Therefore, perturbations to subduction carbon fluxes will manifest themselves in arc volcanoes before ocean island volcanoes. The total volcanic CO2 outgassing flux (Fout) is a sum of the fluxes at arcs, OIBs, and MORB.
The system of equations representing this coupled surface and interior carbon cycle are:
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
| (15) |
| (16) |
| (17) |
| (18) |
| (19) |
| (20) |
| (21) |
| (22) |
| (23) |
| (24) |
| (25) |
| (26) |
| (27) |
A main focus of the model is to demonstrate how δ13Ccarb evolves in response to perturbations to carbon fluxes. Therefore, we track the δ13C evolution of different carbon reservoirs and fluxes with the following set of equations:
| (28) |
| (29) |
| (30) |
| (31) |
| (32) |
where is the value for carbon reservoir or flux i at time t, with atm = atmospheric reservoir, carb = carbonate reservoir, org = organic carbon reservoir, prm = primitive mantle reservoir, arc = arc flux, and OIB = ocean island flux, and MORB = mid-ocean ridge flux. We set to a constant value of −5‰ for the duration of the model, as we have assumed it has no influx of carbon from the surface reservoirs. The equations for and are offset from by +5 and −20 respectively because we assume that Δ13Ccrb-org = 25‰ and forg = 0.20.
The model run to generate Fig. 5 was designed to simulate increased CO2 emissions. To simulate this scenario, the model was run with the initial conditions given in supplementary Table 1. After evolving with no perturbations for 1 billion years, we prescribed that MORB emissions increase from 1013 to 1016 g C/Myr. In order to check whether the model is an acceptable representation of the global C cycle model we increased MORB emissions a second time to 1019 g C/Myr (similar to present day estimates41), and model-dependent reservoir sizes and fluxes are in line with present day estimates (Supp. Fig. 3). The increased CO2 emissions cause increased deposition of carbonates and organic C, which drives increased subduction fluxes of both carbonates and organic C. The increased subduction flux of C coupled with the differential release of carbonates and organics at different volcanic settings described above results in increased oxygen levels and the positive C isotope excursion shown in Fig. 5.
Supplementary Material
Acknowledgements
The authors thank Jeremy Caves and an anonymous reviewer for their constructive reviews. R.D acknowledges support from an NSF grant OCE-1338842, NASA grant 80NSSC18K0828, and the Deep Carbon Observatory. J.E. acknowledges support from a NASA Postdoctoral Program fellowship with the NASA Astrobiology Institute.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
Data Availability
Data used in the generation of Fig. 1 were taken directly without any alteration from the references given in the figure caption and can be accessed in the original publications cited in the figure caption. Additionally the original author has made the data compilation of C isotopes available on his personal website: http://www.krisstott.com/publications.html. Data used in Fig. 2a is reported in Supplementary Table 2 and can be accessed from the original publications cited in the table. Additionally, the data in Supplementary Table 2 has been made publicly available at earthchem.org (DOI:10.1594/IEDA/111406). Compiled data used in Fig. 3 is reported in Supplementary Table 3 and can be accessed from the original publications cited in the table. Additionally, the data in Supplementary Table 3 has been made publicly available at earthchem.org (DOI:10.1594/IEDA/111406).
Code Availability
All equations required by the model are presented in the methods section. The python code for the model is included in the online supplementary material, and is available online at https://github.com/jameseguchi.
Additional information
Supplementary information is available in the online version of the paper. Reprints and permissions information is available online at www.nature.com/reprints.
References
- 1.Lyons TW, Reinhard CT & Planavsky NJ The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506, 307–315 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Luo G et al. Rapid oxygenation of Earth’s atmosphere 2.33 billion years ago. Sci. Adv 2, e1600134 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekker A, Karhu JA & Kaufman AJ Carbon isotope record for the onset of the Lomagundi carbon isotope excursion in the Great Lakes area, North America. Precambrian Res. 148, 145–180 (2006). [Google Scholar]
- 4.Karhu JA & Holland HD Carbon isotopes and the rise of atmospheric oxygen. Geology 24, 867–870 (1996). [Google Scholar]
- 5.Planavsky NJ et al. Evidence for oxygenic photosynthesis half a billion years before the Great Oxidation Event. Nat. Geosci 7, 283–286 (2014). [Google Scholar]
- 6.Kasting JF What caused the rise of atmospheric O2? Chem. Geol 362, 13–25 (2013). [Google Scholar]
- 7.Bekker A et al. Dating the rise of atmospheric oxygen. Nature 427, 117–120 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Bekker A et al. Fractionation between inorganic and organic carbon during the Lomagundi (2.22–2.1 Ga) carbon isotope excursion. Earth Planet. Sci. Lett 271, 278–291 (2008). [Google Scholar]
- 9.Kump LR & Barley ME Increased subaerial volcanism and the rise of atmospheric oxygen 2.5 billion years ago. Nature 448, 1033–1036 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Lee C-TA et al. Two-step rise of atmospheric oxygen linked to the growth of continents. Nat. Geosci 9, 417–424 (2016). [Google Scholar]
- 11.Holland HD Why the atmosphere became oxygenated: A proposal. Geochim. Cosmochim. Acta 73, 5241–5255 (2009). [Google Scholar]
- 12.Condie KC, Aster RC & Van Hunen J A great thermal divergence in the mantle beginning 2.5 Ga: Geochemical constraints from greenstone basalts and komatiites. Geosci. Front 7, 543–553 (2016). [Google Scholar]
- 13.Korenaga J Initiation and evolution of plate tectonics on Earth: theories and observations. Annu. Rev. Earth Planet. Sci 41, 117–151 (2013). [Google Scholar]
- 14.Fuentes JJ, Crowley JW, Dasgupta R & Mitrovica JX The influence of plate tectonic style on melt production and CO2 outgassing flux at mid-ocean ridges. Earth Planet. Sci. Lett 511, 154–163 (2019). [Google Scholar]
- 15.Bindeman IN et al. Rapid emergence of subaerial landmasses and onset of a modern hydrologic cycle 2.5 billion years ago. Nature 557, 545–548 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Gumsley AP et al. Timing and tempo of the Great Oxidation Event. Proc. Natl. Acad. Sci 114, 1811–1816 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobolev SV & Brown M Surface erosion events controlled the evolution of plate tectonics on Earth. Nature 570, 52–57 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Moussallam Y, Oppenheimer C & Scaillet B On the relationship between oxidation state and temperature of volcanic gas emissions. Earth Planet. Sci. Lett 520, 260–267 (2019). [Google Scholar]
- 19.Campbell IH & Allen CM Formation of supercontinents linked to increases in atmospheric oxygen. Nat. Geosci 1, 554–558 (2008). [Google Scholar]
- 20.Marty B & Zimmermann L Volatiles (He, C, N, Ar) in mid-ocean ridge basalts: assesment of shallow-level fractionation and characterization of source composition. Geochim. Cosmochim. Acta 63, 3619–3633 (1999). [Google Scholar]
- 21.Aubaud C, Pineau F, Hékinian R & Javoy M Carbon and hydrogen isotope constraints on degassing of CO2 and H2O in submarine lavas from the Pitcairn hotspot (South Pacific). Geophys. Res. Lett. 33, L02308 (2006). [Google Scholar]
- 22.Mason E, Edmonds M & Turchyn AV Remobilization of crustal carbon may dominate volcanic arc emissions. Science 357, 290–294 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Aubaud C, Pineau F, Hékinian R & Javoy M Degassing of CO2 and H2O in submarine lavas from the Society hotspot. Earth Planet. Sci. Lett 235, 511–527 (2005). [Google Scholar]
- 24.Keller CB et al. Neoproterozoic glacial origin of the Great Unconformity. Proc. Natl. Acad. Sci 116, 1136–1145 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avanzinelli R, Casalini M, Elliott T & Conticelli S Carbon fluxes from subducted carbonates revealed by uranium excess at Mount Vesuvius, Italy. Geology 46, 259–262 (2018). [Google Scholar]
- 26.Sano Y & Marty B Origin of carbon in fumarolic gas from island arcs. Chem. Geol 119, 265–274 (1995). [Google Scholar]
- 27.Duncan MS & Dasgupta R Rise of Earth’s atmospheric oxygen controlled by efficient subduction of organic carbon. Nat. Geosci 10, 387–392 (2017). [Google Scholar]
- 28.Buseck PR & Beyssac O From organic matter to graphite: graphitization. Elements 10, 421–426 (2014). [Google Scholar]
- 29.Tsuno K, Dasgupta R, Danielson L & Righter K Flux of carbonate melt from deeply subducted pelitic sediments: Geophysical and geochemical implications for the source of Central American volcanic arc. Geophys. Res. Lett 39, L16307 (2012). [Google Scholar]
- 30.Galvez ME et al. Graphite formation by carbonate reduction during subduction. Nat. Geosci 6, 473–477 (2013). [Google Scholar]
- 31.Hofmann AW & White WM Mantle plumes from ancient oceanic crust. Earth Planet. Sci. Lett 57, 421–436 (1982). [Google Scholar]
- 32.Li M & McNamara AK The difficulty for subducted oceanic crust to accumulate at the Earth’s core-mantle boundary. J. Geophys. Res. Solid Earth 118, 1807–1816 (2013). [Google Scholar]
- 33.Stagno V, Ojwang DO, McCammon CA & Frost DJ The oxidation state of the mantle and the extraction of carbon from Earth’s interior. Nature 493, 84–88 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Gerlach TM & Taylor BE Carbon isotope constraints on degassing of carbon dioxide from Kilauea Volcano. Geochim. Cosmochim. Acta 54, 2051–2058 (1990). [Google Scholar]
- 35.Hauri E SIMS analysis of volatiles in silicate glasses, 2: isotopes and abundances in Hawaiian melt inclusions. Chem. Geol 183, 115–141 (2002). [Google Scholar]
- 36.Exley RA, Mattey DP, Clague DA & Pillinger CT Carbon isotope systematics of a mantle ‘hotspot’: a comparison of Loihi Seamount and MORB glasses. Earth Planet. Sci. Lett 78, 189–199 (1986). [Google Scholar]
- 37.Holloway J & Blank J Application of experimental results to C-O-H species in natural melts. Reviews in Mineralogy and Geochemistry 30, 187–230 (1994). [Google Scholar]
- 38.Shirey SB et al. Diamonds and the Geology of Mantle Carbon. Rev. Mineral. Geochemistry 75, 355–421 (2013). [Google Scholar]
- 39.Marty B & Dauphas N The nitrogen record of crust–mantle interaction and mantle convection from Archean to Present. Earth Planet. Sci. Lett 206, 397–410 (2003). [Google Scholar]
- 40.Christensen UR & Hofmann AW Segregation of subducted oceanic crust in the convecting mantle. J. Geophys. Res. Solid Earth 99, 19867–19884 (1994). [Google Scholar]
- 41.Dasgupta R & Hirschmann MM The deep carbon cycle and melting in Earth’s interior. Earth Planet. Sci. Lett 298, 1–13 (2010). [Google Scholar]
- 42.Kelemen PB & Manning CE Reevaluating carbon fluxes in subduction zones, what goes down, mostly comes up. Proc. Natl. Acad. Sci. 112, E3997–E4006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krissansen-Totton J, Buick R & Catling DC A statistical analysis of the carbon isotope record from the Archean to phanerozoic and implications for the rise of oxygen. Am. J. Sci 315, 275–316 (2015). [Google Scholar]
- 44.Olson SL et al. Volcanically modulated pyrite burial and ocean–atmosphere oxidation. Earth Planet. Sci. Lett 506, 417–427 (2019). [Google Scholar]
- 45.Williams JJ, Mills BJW & Lenton TM A tectonically driven Ediacaran oxygenation event. Nat. Commun 10, 2690 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X-M et al. Tracing Earth’s O2 evolution using Zn/Fe ratios in marine carbonates. Geochemical Perspect. Lett 2, 24–34 (2016). [Google Scholar]
- 47.Condie KC & Aster RC Episodic zircon age spectra of orogenic granitoids: The supercontinent connection and continental growth. Precambrian Res. 180, 227–236 (2010). [Google Scholar]
- 48.Clift PD A revised budget for Cenozoic sedimentary carbon subduction. Rev. Geophys 55, 97–125 (2017). [Google Scholar]
- 49.Carter LB & Dasgupta R Decarbonation in the Ca-Mg-Fe carbonate system at mid-crustal pressure as a function of temperature and assimilation with arc magmas – Implications for long-term climate. Chem. Geol 492, 30–48 (2018). [Google Scholar]
References
- 50.Connolly JAD The geodynamic equation of state: What and how. Geochemistry, Geophys. Geosystems 10, Q10014 (2009). [Google Scholar]
- 51.Syracuse EM et al. The global range of subduction zone thermal models. Phys. Earth Planet. Inter 183, 73–90 (2010). [Google Scholar]
- 52.Gorman PJ, Kerrick DM & Connolly JAD Modeling open system metamorphic decarbonation of subducting slabs. Geochemistry, Geophys. Geosystems 7, Q04007 (2006). [Google Scholar]
- 53.Dasgupta R Ingassing, storage, and outgassing of terrestrial carbon through geologic time. Rev. Mineral. Geochemistry 75, 183–229 (2013). [Google Scholar]
- 54.Frost DJ & Wood BJ Experimental measurements of the fugacity of CO2 and graphite/diamond stability from 35 to 77 kbar at 925 to 1650°C. Geochim. Cosmochim. Acta 61, 1565–1574 (1997). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.