Abstract
Introduction.
Sikh Asian Indians are an underserved, minority group demonstrating high rates of diabetes. Community health workers (CHWs) are effective in addressing health disparities by reaching socially and linguistically isolated populations. There are no culturally-adapted programs for diabetes prevention among Sikh Asian Indians, thus, this study tests the efficacy of a culturally-tailored CHW intervention to improve diabetes prevention-related outcomes among Sikh Asian Indians at-risk for diabetes.
Methods.
A quasi-experimental two-arm intervention among Sikh Asian Indian adults at-risk for diabetes and living in New York City (n=160) was conducted in 2013–2014. The treatment group received six monthly CHW group education sessions and ten follow-up phone calls; the control group received the first session. Main outcomes included weight, body mass index (BMI), blood pressure (BP), physical activity (PA), diet, and health self-efficacy.
Results.
Positive and significant changes in weight, BMI, and diabetes prevention-related indicators were seen among both study groups. However, only treatment group participants showed significant changes over time for weight, BMI, PA self-efficacy, and health-related self-efficacy. Significant between-group differences were seen in adjusted analyses for weight, BMI, systolic BP, total weekly PA, PA self-efficacy, PA social interaction, portion control, barriers to healthy eating, and health self-efficacy. At 6-months, treatment participants were more likely to lose ≥5% and ≥7% of their weight compared to control participants (p=0.071, and p=0.015, respectively).
Conclusions.
Findings demonstrate that a culturally-adapted CHW diabetes prevention program in the Sikh community is efficacious, adding to the growing literature on CHWs’ capacity to address health inequity among underserved populations.
South Asians make up one-fifth of the world’s total population (Misra, Ramchandran, Jayawardena, Shrivastava, & Snehalatha, 2014) and include a diverse group representing individuals from India, Pakistan, Bangladesh and other parts of the South Asian subcontinent. Often misconstrued as a homogenous ethnic group, South Asians comprise a variety of subgroups with unique sociocultural characteristics that may differentially influence diabetes prevention behaviors and outcomes. The New York metropolitan area (consisting of cities in New York, New Jersey, and Connecticut) is home to the largest Asian Indian population in the US, with greater than 700,000 (South Asian Americans Leading Together and the Asian American Federation, 2012; U.S. Census Bureau).
Sikhism is a monotheistic religion founded during the 16th century in the Punjab district of what is now India. The Sikh population in New York City (NYC) is estimated around 50,000, and many Sikh Asian Indians live in the Richmond Hill and South Ozone Park areas in the borough of Queens (Haller, January 11, 2013; Lasky, October 10, 2018; Mann, Numrich, & Williams, 2007; The Sikh Coalation, 2008). Sikh Asian Indians in the US and in NYC face significant social vulnerabilities to adverse health. Approximately 19.7% of Asian Indians in the US and 24.3% of Asian Indians in NYC speak English less than very well (U.S. Census Bureau); Similar limited English proficiency rates have been shown among NYC Sikh Asian Indians (The Sikh Coalation, 2008), which may affect their ability to access quality care. In NYC, Asian Indian men are concentrated in service sector jobs such as taxi driving and construction work or are small business owners, and similar job categories are shown for Sikh Asian Indians (Cao, Ahmed, & Islam, 2007; The Sikh Coalation, 2008). Taxi driving, in particular, is associated with a sedentary life-style, high stress, and unhealthy diet, leading to poorer health among taxi drivers (Burgel, Gillen, & White, 2012; Gany, Gill, Ahmed, Acharya, & Leng, 2013). Since the terrorist attacks of September 11, 2001, Sikh Asian Indians have often been the target of discrimination and harassment due to misconceptions about turbans, the traditional head covering for Sikhs’ uncut hair (The Sikh Coalation, 2008, 2014). Increased discrimination among Sikh Asian Indians, in turn, have been linked to poorer self-reported mental and physical health (Nadimpalli et al., 2016).
South Asians, including Sikh Asian Indians, bear a high burden of Type 2 diabetes. Type 2 diabetes can lead to serious complications and is characterized by insulin resistance and relative lack of insulin. The diabetes prevalence among South Asians in the US and NYC is higher than that of other Asian subgroups (Barnes, Adams, & Powell-Griner, 2008; Centers for Disease Control and Prevention, 2017; Gupta, Wu, Young, & Perlman, 2011; N. S. Islam, Wyatt, Kapadia, et al., 2013; King & Deng, March 2018; Rajpathak et al., 2010). The most recent national estimates with Asian subgroups, using 2013–2015 (Burgel et al., 2012; Gany et al., 2013) National Health Interview Survey data, report a diabetes prevalence of 11.2% among Asian Indians, compared to 4.3% among Chinese, 8.9% among Filipinos, and 8.0% among overall non-Hispanic Asians. Prevalence rates among Asian Indians are also approaching those of non-Hispanic Blacks (12.7%) and Hispanics (12.1%) (Centers for Disease Control and Prevention, 2017). However, recent estimates of diabetes in NYC show a prevalence of approximately 20% among South Asians and 20.9% among Asian Indians (N. S. Islam, Wyatt, Kapadia, et al., 2013; King & Deng, March 2018). In comparison, national diabetes prevalence is estimated at 9.3% and NYC diabetes prevalence is estimated between 11.0–16.0% (Centers for Disease Control and Prevention, 2017; King & Deng, March 2018; New York City Department of Health and Mental Hygiene, 2016; Thorpe et al., 2018). While diabetes prevalence data within the Sikh Asian Indian subgroup is limited, a random sample of Sikh Asian Indians residing in urban India indicated a diabetes prevalence of 23.2% (Singh, Shenoy, & Singh Sandhu, 2016); additionally, a study in the UK found that migrants from the Punjab region had a less favorable coronary risk profile when compared with sibling who had not migrated from India, and a study on South Asians from California (which included a sub-sample collected in Punjabi) found a self-reported diabetes prevalence of 10.2% (Bhatnagar et al., 1995; Ivey et al., 2004).
The Diabetes Prevention Program (DPP) is an evidence-based program to prevent type 2 diabetes (Diabetes Prevention Program (DPP) Research Group, 2002) and has been shown to be more effective than metformin (Knowler et al., 2002). The DPP addresses diabetes prevention through dietary changes and weight loss, increased physical activity (PA), and other lifestyle changes. The program includes a weight loss goal to lose 7% of initial body weight (or between 5–10% of initial body weight) (Diabetes Prevention Program (DPP) Research Group, 2002; National Institute of Diabetes and Digestive and Kidney Diseases, 2018). Given the evidence-base supporting DPP effectiveness, there are efforts to widely scale the DPP across the U.S, including recent efforts to reimburse DPP programs through Medicare, consistent with population health approaches that aim to reach wide populations yielding broad improvements in net outcomes (Kindig & Stoddart, 2003).
There is increasing recognition that population-based approaches alone, however, are not sufficient in reducing health disparities faced by minority groups – and in fact, focusing on a single approach may actually widen the health disparity gap as large segments of communities and subpopulations are not reached and are being left behind by these population-wide efforts. In contrast to population health approaches, health equity approaches aim to achieve the highest attainment of health for all populations (Srinivasan & Williams, 2014). Population health equity approaches, then, encompass both targeted interventions for socially disadvantaged and medically underserved communities and population-wide interventions using a health equity lens to maximize health impact (Trinh-Shevrin, Islam, Nadkarni, Park, & Kwon, 2015; Trinh-Shevrin, Kwon, Park, Nadkarni, & Islam, 2015). In the context of diabetes prevention, this entails culturally adapting the DPP intervention to ensure the program is relevant to the unique contextual milieu of minority populations. DPP studies have been culturally adapted for various racial/ethnic groups throughout the US (N. S. Islam, Zanowiak, et al., 2013; Jaber et al., 2011; Mau et al., 2010; Merriam et al., 2009; Ockene et al., 2012) as well as abroad (Ramachandran & Snehalatha, 2011). In the US, culturally adapted DPP initiatives have been implemented in a select and limited number of Asian American communities (N. S. Islam, Zanowiak, et al., 2013; N. S. Islam et al., 2014; U.S. National Library of Medicine). However, outside of a previous pilot study conducted by the current investigators (N. S. Islam et al., 2014), no study has evaluated a DPP adaptation in the South Asian community.
Community health workers (CHWs) are frontline health workers who often serve vulnerable minority populations (N. S. Islam et al., 2015). CHWs are respected members of their community who are well-suited to provide linguistically and culturally tailored services (Anthony, Gowler, Hirsch, & Wilkinson, 2009). Indeed, studies have demonstrated that CHWs are effective in improving a wide range of health outcomes among minority populations, including among Asian Americans. Specifically, studies have shown that CHWs can improve diabetes prevention and management among South Asians and other Asian subgroups (N. S. Islam et al., 2018; N. S. Islam, Zanowiak, et al., 2013; Sinclair et al., 2013). Accordingly, the purpose of this study was to test the efficacy of a culturally tailored CHW intervention designed to improve health behaviors and health outcomes related to diabetes prevention among Sikh Asian Indians identified as at-risk for diabetes who are living in NYC.
Methods
Study Population
Project RICE (Reaching Immigrants through Community Empowerment), conducted between November 2013 and September 2014, was a quasi-experimental two-arm CHW intervention. The project utilized the principles of community-based participatory research (CBPR) to guide the development, implementation, and evaluation of the study. The New York University (NYU) School of Medicine worked in partnership with UNITED SIKHS, a community-based organization serving the Sikh Asian Indian population; UNITED SIKHS is the largest Sikh Asian Indian social service agency in the United States. The current study builds upon the previously published pilot data (N. S. Islam et al., 2014) by including a second cohort of randomized study participants and excluding the initial round (which included only treatment group individuals). This allowed us to adequately power the study for the planned analyses, including assessing the impact of the intervention on achieving desired weight loss (5–10% of initial weight) based on DPP standards. Inclusion criteria was set as the following: (1) self-identification as Sikh South Asian; (2) between 18 and 75 years of age; and (3) at-risk for diabetes as determined by an interviewer-administered diabetes risk assessment tool adapted from the American Diabetes Association (at-risk scores based on family history of diabetes, body mass index [BMI], and other factors). Individuals were ineligible to participate if they had self-reported diabetes from a health professional, had serious health problems (e.g. terminal illness or recent history of an acute medical problem), or had previously participated in a cardiovascular disease (CVD) study. Human subjects approval was obtained in 2011, and the trial was registered at ClinicalTrials.gov (NCT03530579).
Screening and randomization
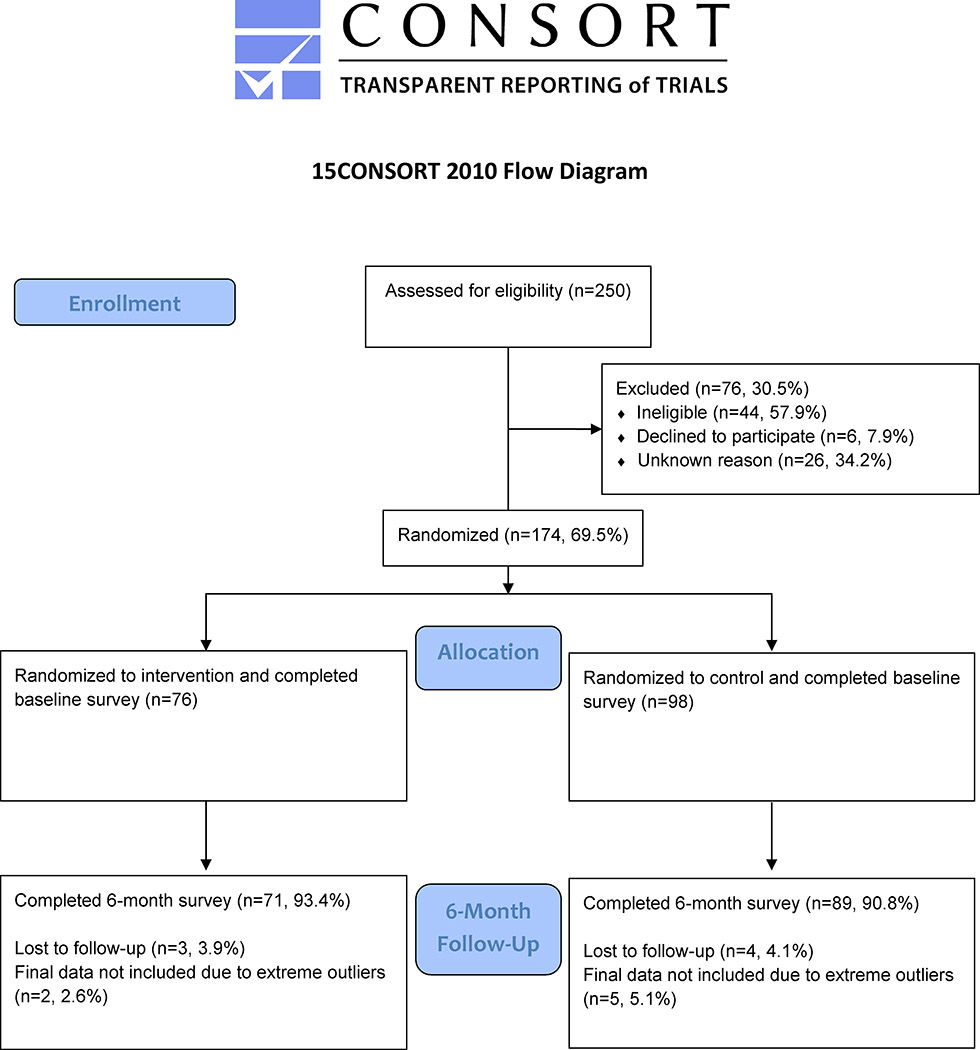
In order to alleviate concerns regarding contamination due to the close-knit nature of the Sikh community and the concentration of Sikh Asian Indians by neighborhood location, randomization occurred at the site (neighborhood) level rather than at the individual level. The two neighborhoods (Richmond Hill and South Ozone Park, located in the Southwestern portion of the borough of Queens) were selected due to their high concentration of South Asian Sikhs (Department of City Planning - City of New York, 2013). The neighborhoods were demographically similar, with similar educational attainment, foreign-born population, Asian ancestry, and limited English proficiency (U.S. Census Bureau, 2012–2016). Two recruitment rounds were completed at two gurdwaras (Sikh religious institutions), one within each neighborhood. Health fairs were held at the two gurdwaras in order to recruit individuals, and CHWs also set up tables during additional times at the gurdwaras in order to meet the recruitment numbers. The randomization assignments switched between the first and second rounds. A total of 250 individuals were screened for eligibility at the two neighborhood gurdwaras, and 174 (69.6%) were eligible and enrolled, consenting to participate in the study and completing the baseline assessment (see Figure 1). In the first round, Richmond Hill was allocated to the treatment group (n=50) and South Ozone Park was allocated to the control group (n=50). In the second round, South Ozone Park was allocated to the treatment group (n=26) and Richmond Hill was allocated to the control group (n=48). Among treatment group participants, three (3.9%) were lost to follow-up and two (2.6%) were excluded from analysis due to extreme outliers, whereas among control group participants four (4.1%) were lost to follow-up and five (5.1%) were excluded from analysis due to extreme outliers in the BMI measure. The majority of individuals completed all sessions and all follow-up phone calls with the exception of two individuals with missing attendance data.
Figure 1.
CONSORT Diagram of Study Sample
Intervention
The intervention consisted of six CHW-facilitated group sessions of approximately two hours in length, with the following six topics that were culturally-adapted to the South Asian Sikh community: 1) An overview of diabetes prevention, including a concept of prevention tied to Sikh core values and the increased risk of diabetes among Asians and Asian Indians; 2) Nutrition, including healthy elements in traditional Indian cooking, following the plate method with traditional Punjabi foods, and working with women participants to improve nutrition in the household; 3) Physical activity, including encouragement to practice physical activity in a similar manner as in prayer; 4) Diabetes complications and other cardiovascular diseases, including discussion of the prevention and inter-connectedness of chronic diseases; 5) Stress and family support, including discussions around stigma associated with mental health and Naam Simran, a meditation practice used in Sikhism; and 6) Access to healthcare, including communicating with doctors and health access resources and providers in Punjabi. All sessions included the incorporation of culturally appropriate images and language, and the cultural adaptations are further described in our pilot paper (N. S. Islam et al., 2014). A total of six sessions were held monthly in a convenient community setting, either at the neighborhood gurdwara or a nearby education center in close proximity to both neighborhoods. The project curriculum was adapted from materials previously validated in minority communities: the National Heart, Lung, and Blood Institute’s Healthy Heart, Healthy Family; the DPP; the National Diabetes Education Program’s Power to Prevent and Road to Health curricula; and a diabetes management curriculum used in the NYC Bangladeshi population (Centers for Disease Control and Prevention, 2008; Diabetes Prevention Program (DPP) Research Group, 2002; N. S. Islam, Wyatt, Patel, et al., 2013; N. S. Islam et al., 2018). In addition to the cultural tailoring, there were other differences between our intervention and the DPP intervention. Differences include: 1) the number of sessions (DPP’s 16 sessions over a six-month period compared to our six sessions over the same time period); 2) the length of each session (DPP sessions were 30 minutes to one hour long compared to our approximately two-hour long sessions); 3) the mode of delivery (DPP’s individual sessions compared to our group sessions); 4) the program delivery (DPP used case managers or “Lifestyle Coaches” while we used trained CHWS); 5) follow-up (DPP had biweekly calls and six follow-up sessions while our intervention had weekly phone call follow-ups; and 6) provision of supplemental sessions (DPP’s optional group exercise sessions and regular group/individual sessions after the six month curriculum compared to no supplemental sessions for our study) (Diabetes Prevention Program (DPP) Research Group, 2002).
Treatment group participants received a total of ten follow-up phone calls from CHWs, during which individualized challenges, strategies, and goal-setting for improving diet and PA and stress reduction were discussed. Calls were made bi-monthly, between sessions 1 and 6. Individuals in the control group attended only the first health education session, which was identical to the first session received by treatment group participants, while treatment group participants received all sessions. The intervention was led by two trained, bilingual, Sikh Asian Indian female CHWs and one bilingual Asian Indian CHW female supervisor at the partnering community-based organization. The CHW supervisor participated in a core competency and curriculum based training, which focused on comprehensive skills for CHWs. The CHWs were trained by the supervisor and study staff on the study protocol, delivery, and curriculum, and all study staff attended approximately 30 hours of additional trainings on motivational interviewing, basic action planning, mental health, and other topics.
Measures
A baseline survey was completed by treatment and control group participants after consenting to take part in the study, and a follow-up assessment was conducted at 6-month. All survey questions were developed in English and administered in Punjabi by the CHWs. Consents were obtained in Punjabi or English. Clinical measures were also obtained at these timepoints by the CHWs.
Height was collected at baseline using a tape measure taped to the wall. Guidance was provided on how to measure for men who wore turbans. Weight was obtained at baseline and 6-month follow-up using a scale, and BMI was calculated using weight at each timepoint and height taken at baseline. BP was collected at baseline and 6-month follow-up by CHWs using an OMRON automatic BP monitor, and by health professionals at the gurdwara (at screenings) using manual BP machines. Participants were in a seated position, and three resting BP measurements approximately two to three minutes apart were collected; the average of the second and third readings were used for analysis. Two-hour fasting glucose and total cholesterol measurements were collected via a finger stick test. CHWs were trained to collect all measures.
A series of questions assessing self-reported moderate and vigorous physical activity was asked of participants. For both moderate and vigorous activities, total days per week and total minutes per day were reported; a weekly measure of both moderate and vigorous physical activity was then calculated using the following equation: days x minutes. Based on 2008 PA guidelines, it is recommended that adults perform at least 150 minutes a week of moderate-intensity PA or at least 75 minutes a week of vigorous-intensity PA (U.S. Department of Health and Human Services). Thus, recommended weekly PA was calculated as follows: total minutes of weekly moderate PA + (total minutes of weekly vigorous PA x 2). Individuals engaging in at least 150 minutes per week of PA using the new calculated variable met weekly recommended PA (Nothwehr, Dennis, & Wu, 2007).
PA self-efficacy was adapted from the Bandura Self-Efficacy Scale (Bandura, 2006). The mean of two questions was calculated for a score of one to four, with four representing the highest self-efficacy. PA barriers were adapted from the Exercise Benefits and Barriers Scale (Sechrist, Walker, & Pender, 1987). Participants were asked if they agreed or disagreed with each, and the responses were totaled for a scale of zero to seven, where seven represents the greatest barriers to exercise. PA social interaction were adapted from a previous intervention (Nothwehr et al., 2007). The mean of four questions was calculated for a scale of one to four, where four represents the highest PA social interaction.
Portion control questions were adapted from measurement of the behavioral objectives of a weight management intervention (Nothwehr et al., 2007). The mean of five questions was taken for a scale of one to four, where four represents the highest portion control. Barriers to eating healthy were adapted from the previous interventions (N. S. Islam et al., 2018; N. S. Islam, Zanowiak, et al., 2013; Ursua et al., 2018). Participants were asked if they agreed or disagreed with each barrier question; the responses of eight questions were totaled for a scale of zero to eight, where eight represents the greatest barriers to healthy eating. Nutrition self-efficacy was adapted from the Bandura Self-Efficacy Scale (Bandura, 2006); participants were asked about confidence for eight questions; the response of each question was totaled for a scale of zero to eight, where eight represents the highest self-efficacy.
Questions on diabetes knowledge were adapted from the Diabetes Knowledge Test and risk assessment questions from the American Diabetes Association (American Diabetes Association, 2018; Fitzgerald et al., 2016; Fitzgerald et al., 1998). Two scales were included, and each scale included seven questions. True responses were totaled for a score of zero to seven, where seven represents highest knowledge.
Health self-efficacy was adapted from the Bandura Self-Efficacy Scale (Bandura, 2006); the mean of four questions was calculated for a score of one to four, where four represents the highest self-efficacy.
Exact questions used for scale variables can be found in Table 1. Additional variables for analysis include gender, age, country of birth, years lived in the US, marital status, employment, education, English spoken frequency, and family history of diabetes.
Table 1.
Study Scale Questions and Response Choices
| Weekly Physical Activity | |
| Including what you do at your job, home, gym, or elsewhere, do you do any sustained physical activity for 10 minutes or more? | Yes; No |
| During the last 7 days, on how many days did you do moderate physical activities? | [Days per week] |
| How much time did you usually spend doing these moderate types of physical activities on a normal day that you do activity? | [Minutes per day] |
| During the last 7 days, on how many days did you do activities that required large amounts of physical exertion or effort to make your heart rate and breathing much faster? | [Days per week] |
| On one of those days, how much time did you usually spend doing these hard types of physical activities? | [Minutes per day] |
| Physical Activity Self-efficacy | |
| How sure you do feel that you will be able to know what exercises are healthy for you? | Not at all sure; Not very sure; Somewhat sure; Very sure |
| How sure do you feel that you will be able to exercise for at least 30 minutes five times each week in the future? | |
| Physical Activity Barriers | |
| I don’t have time to exercise | Agree; Disagree |
| I am not motivated to exercise | |
| I don’t have a safe place to exercise | |
| Health problems prevent me from exercising | |
| I don’t like to exercise | |
| I need someone to exercise with but don’t have one | |
| I don’t know what exercises to perform | |
| Physical Activity Social Interaction | |
| How often do you suggest doing something active when you get together with family members or friends? | Never or almost never; Sometimes; Often; Always or almost always |
| How often do you set aside a special time to do physical activity? | |
| How often do you ask a friend or relative to do some physical activity with you? | |
| How often do you talk to others about the benefits of physical activity? | |
| Portion Control | |
| How often do you stop eating when full? | Almost never or never; Sometimes; Often; Almost always or always |
| How often do you refuse offers of food when you are not hungry? | |
| How often do you try to limit the number of food servings you ate? | |
| How often do you try to limit the size of food servings you ate? | |
| How often do you try to find something else to do instead of snacking? | |
| Barriers to Healthy Eating | |
| It is difficult for me to choose a healthy snack | Agree; Disagree |
| I cannot afford to buy healthy foods | |
| I do not have time to prepare healthy foods | |
| There is no store for me to buy healthy foods | |
| It is difficult for me to eat healthy food on holidays or special occasions | |
| It is uncomfortable for me to refuse unhealthy foods when they are offered to me at social events or get-togethers | |
| I do not like how healthier foods tastes | |
| I do not cook healthy foods because my family does not like them | |
| Nutrition Self-efficacy | |
| Are you confident that you can stay on a healthy diet? | Yes; No |
| Are you confident that you can cook a healthy diet? | |
| Are you confident that you can decrease the amount of sugar and sweets you eat? | |
| Are you confident that you can decrease the amount of fat and cholesterol in the foods you eat? | |
| Are you confident that you can increase the amount of fiber and vegetables you eat? | |
| Are you confident that you know what foods you should eat on a healthy diet? | |
| Are you confident that you can stay on a healthy diet when eating outside your home? | |
| Are you confident that you can stay on a healthy diet when you are busy? | |
| Diabetes Knowledge Scale 1 | |
| How much does each of the following affect a person’s risk for getting diabetes? | Increases or raises the risk; Has no effect; Decreases or lowers the risk |
| Being South Asian | |
| Eating a healthy diet | |
| Having had diabetes during pregnancy | |
| Having a blood relative with diabetes | |
| Being 65 years of age or older | |
| Exercising regularly | |
| Controlling weight gain | |
| Diabetes Knowledge Scale 2 | |
| Can a person get diabetes if he or she has a normal body weight? | Yes; No |
| Which of the following is highest in carbohydrate? | Baked chicken; Rice; Cheese; Peanut butter |
| Eating foods lower in fat decreases your risk for… | Nerve disease; Kidney disease; Heart disease; Eye disease |
Statistical Analyses
Descriptive statistics summarize and compare the baseline characteristics of all participants randomized to treatment and control groups; Pearson Chi-square tests assess group differences for categorical variables and Student’s t-tests assess group differences for continuous variables. To test within-group differences between baseline and follow-up, we used paired t-tests and McNemar tests for each outcome measure. To assess change between groups for each continuous outcome, we ran generalized estimated equations (GEE) models for repeated measures over time using the PROC GENMOD procedure in SAS, while adjusting for study arm, time point, and the interaction between study arm and time point (the intervention effect). Adjusted models were also run to include age, gender, education, years lived in the US and insurance status. The interaction variable tests the intervention effect and indicates whether there are significant differences in changes in the outcome between intervention and control groups. Missing data was excluded from analysis unless greater than 5%. Analyses were performed between May 2016 and August 2018 using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Sociodemographic characteristics and baseline outcomes and clinical information are presented for the 160 individuals with baseline and follow-up data (Table 2). There were no statistically significant differences in PA, diet, diabetes knowledge and health-related characteristics between the groups. However, treatment group participants were significantly more likely than control group participants to be female (80.3% vs. 60.7%, respectively; p-value=0.008), and treatment group participants were also significantly less likely than control group participants to have insurance (50.8% vs 73.3%, respectively; p-value=0.005). In addition, treatment group participants had significantly higher mean glucose levels at baseline when compared to control group participants (114.5 mg/dL vs. 96.8 mg/dL, p<0.001). When comparing the first and second rounds of the intervention, there was a significantly smaller percentage of males in round 1 compared to round 2; it is likely that this was because the CHWs were female, making it more difficult to enroll males into the intervention. During round 2, the CHWs focused on enrolling a greater number of males, while working with male gurdwara leadership.
Table 2.
Baseline characteristics of Project RICE participants (N=160)
| Treatment (n=71) | Control (n=89) | ||
|---|---|---|---|
| n (%) | n (%) | p-value | |
| Socio-demographics | |||
| Female, n (%) | 57 (80.3) | 54 (60.7) | 0.008 |
| Years of age, mean (95% CI) | 45.4 (42.9–47.9) | 47.3 (45.2–49.4) | 0.242 |
| Country of birth | 1.000 | ||
| India | 71 (100.0) | 88 (98.9) | |
| Bangladesh | 0 (0.0) | 1 (1.1) | |
| Years lived in the US, mean (95% CI) | 13.3 (11.3–15.2) | 13.9 (12.2–15.6) | 0.634 |
| Marital status | 1.000 | ||
| Married | 68 (97.1) | 83 (96.5) | |
| Unmarried | 2 (2.9) | 3 (3.5) | |
| Employed | 0.493 | ||
| Employed | 19 (27.1) | 28 (32.2) | |
| Unemployed | 51 (72.9) | 59 (67.8) | |
| Education | 0.641 | ||
| < High school | 11 (15.7) | 10 (11.8) | |
| High school/Some college | 48 (68.6) | 64 (75.3) | |
| College graduate | 11 (15.7) | 11 (12.9) | |
| Speaks English at home | 37 (54.4) | 35 (39.3) | 0.060 |
| How well do you speak English? | 0.777 | ||
| Very well/Well | 38 (55.1) | 47 (52.8) | |
| Not well/Not at all | 31 (44.9) | 42 (47.2) | |
| Family history of diabetes | 52 (74.3) | 57 (64.0) | 0.167 |
| Physiological measures | |||
| BMI (kg/m2), mean (95% CI) | 28.2 (27.3–29.0) | 27.7 (27.1–28.4) | 0.420 |
| Weight (lbs.), mean (95% CI) | 166.7 (161.1–172.4) | 169.2 (164.2–174.1) | 0.521 |
| Systolic BP (mmHG), mean (95% CI) | 130.6 (127.4–133.7) | 126.6 (123.6–129.5) | 0.064 |
| Diastolic BP (mmHG), mean (95% CI) | 83.6 (81.8–85.5) | 83.5 (81.5–85.5) | 0.958 |
| Cholesterol (mg/dL), mean (95% CI) | 154.1 (142.5–165.6) | 155.8 (148.4–163.2) | 0.797 |
| Glucose (mg/dL), mean (95% CI) | 114.5 (106.3–122.7) | 96.8 (92.0–101.6) | <0.001 |
| Physical activity (PA) | |||
| Met weekly recommended PA | 7 (10.3) | 7 (9.5) | 0.868 |
| Weekly PA, minutes, mean (95% CI) | 47.7 (16.0–79.5) | 23.8 (7.9–39.6) | 0.172 |
| Self-efficacy, 1–4, 4=highest, mean (95% CI) | 3.2 (3.0–3.4) | 3.2 (3.1–3.4) | 0.734 |
| Social Interaction, 1–4, 4=highest, mean (95% CI) | 1.5 (1.4–1.7) | 1.5 (1.4–1.7) | 0.902 |
| Barriers, 0–7, 0=highest, mean (95% CI) | 3.0 (2.6–3.5) | 3.3 (2.9–3.7) | 0.409 |
| Diet | |||
| Portion control, 1–4, 4=highest, mean (95% CI) | 1.9 (1.8–2.1) | 2.2 (1.9–2.4) | 0.151 |
| Barriers, 0–8, 8=greatest barriers, mean (95% CI) | 4.8 (4.3–5.4) | 5.0 (4.5–5.4) | 0.746 |
| Self-efficacy, 0–8, 8=highest, mean (95% CI) | 7.8 (7.6–8.0) | 7.6 (7.4–7.9) | 0.286 |
| Diabetes knowledge | |||
| Knowledge scale 1, 0–7, 7=highest | 3.8 (3.3–4.3) | 3.4 (2.9–3.8) | 0.245 |
| Knowledge scale 2, 0–7, 7=highest | 1.7 (1.4–2.0) | 1.7 (1.4–1.9) | 0.944 |
| Health | |||
| Insured | 33 (50.8) | 63 (73.3) | 0.005 |
| Has a doctor in the US | 56 (90.3) | 76 (92.7) | 0.612 |
| Self-efficacy, 1–5, 5=highest, mean (95% CI) | 3.9 (3.8–4.0) | 3.8 (3.7–3.9) | 0.245 |
Table 3 presents changes in physiological measurements and diabetes prevention-related indicators (PA, diet, knowledge, self-efficacy) from baseline to 6-month follow-up by study group. At 6 months, mean BMI decreased by 0.7 kg/m2 (p=<0.001) and mean weight decreased by 3.9 pounds in the treatment group, whereas no change was seen in the control group; the adjusted intervention effect was significant for BMI (p=0.010) and for weight (p=0.018). At 6-months, mean systolic BP decreased by 12.3 mm/Hg (p<0.001) and mean diastolic BP decreased by 3.3 mm/Hg (p=0.003) in the treatment group; while mean systolic BP decreased by 9.2 mm/Hg (p<0.001) and mean diastolic BP decreased by 3.1 mm/Hg (p<0.010) in the control group. The adjusted intervention effect for systolic BP was significant (p<0.001); a significant intervention effect was not shown for diastolic BP. At 6-months, mean glucose decreased by −19.6 mg/dL in the treatment group, whereas no change was seen in the control group; the adjusted intervention effect was significant for glucose (p<0.001).
Table 3.
Changes in Clinical and Behavioral Measurements of Study Participants in the RICE Project from Baseline to Follow-up
| Intervention group (n=71) | Control Group (n=89) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Baseline Mean (SD) | 6-Month Mean (SD) | Change in Mean (95% CI) | N | Baseline Mean (SD) | 6-Month Mean (SD) | Change in Mean (95% CI) | Intervention Effect: Unadjusted | Intervention Effect: Adjusteda | |
| Physiological Measures | ||||||||||
| Weight (lbs.), mean (95% CI) | 70 | 166.2 (23.8) | 162.3 (22.6) | −3.9 (−5.8, −2.2)† | 89 | 169.2 (23.5) | 168.8 (21.8) | −0.4 (−1.9, 1.1) | −3.7 (−6.0, −1.4)** | −3.0 (−5.4, −0.5)* |
| BMI (kg/m2), mean (95% CI) | 70 | 28.1 (3.5) | 27.4 (3.3) | −0.7 (−1.0, −0.4)† | 89 | 27.7 (3.1) | 27.7 (3.0) | 0.0 (−0.3, 0.2) | −0.7 (−1.0, −0.3)† | −0.5 (−1.0, −0.1)* |
| Systolic BP (mmHG), mean (95% CI) | 68 | 131.0 (13.2) | 118.7 (8.9) | −12.3 (−15.4, −9.2)† | 88 | 126.6 (13.7) | 117.4 (11.1) | −9.2 (−12.9, −5.5)† | −2.4 (−7.1, 2.2) | −2.5 (−11.4, −4.6)† |
| Diastolic BP (mmHG), mean (95% CI) | 68 | 83.9 (7.6) | 80.6 (7.8) | −3.3 (−5.3, −1.2)** | 88 | 83.5 (9.5) | 80.4 (5.0) | −3.1 (−5.2, −1.1)** | −0.4 (−3.2, 2.5) | −1.2 (−4.5, 2.0) |
| Cholesterol | 66 | 154.1 (47.0) | 167.5 (28.8) | 13.4 (1.5, 25.3)* | 79 | 155.8 (33.0) | 157.2 (32.7) | 1.4 (−5.5, 8.3) | 12.0 (−1.2, 25.3) | 10.4 (−4.2, 24.9) |
| Glucose | 67 | 114.5 (33.6) | 94.9 (16.2) | −19.6 (−28.0, −11.1)† | 82 | 96.8 (21.7) | 97.8 (18.6) | 1.0 (−2.6, 4.6) | −22.0 (−30.9, −13.2)† | −20.1 (−29.6, −10.7)† |
| Physical activity (PA) | ||||||||||
| Weekly PA, minutes | 67 | 48.4 (132.1) | 299.3 (214.3) | 250.9 (194.7, 307.1)† | 69 | 25.1 (69.7) | 128.7 (132.9) | 103.5 (74.4, 132.6)† | 135.3 (74.1, 196.4)† | 136.0 (70.6, 201.4)† |
| Self-efficacy (1–4, 4=highest) | 43 | 3.2 (0.6) | 3.9 (0.3) | 0.7 (0.5, 0.9)† | 77 | 3.2 (0.7) | 3.3 (0.5) | 0.1 (−0.2, 0.2) | 0.7 (0.5, 0.9)† | 0.8 (0.5, 1.0)† |
| Social interaction (1–4, 4=highest) | 69 | 1.5 (0.6) | 3.2 (0.7) | 1.7 (1.4, 2.0)† | 84 | 1.5 (0.6) | 2.0 (0.6) | 0.5 (0.4, 0.7)† | 1.2 (0.9, 1.5)† | 1.1 (0.7, 1.4)† |
| Barriers to exercise (0–7, 0=fewest) | 71 | 3.0 (1.8) | 1.1 (1.1) | −1.9 (−2.3, −1.5)† | 85 | 3.3 (1.8) | 2.2 (1.6) | −1.1 (−1.6, −0.6)† | −0.8 (−1.4, 0.2)* | −0.7 (−1.4, 0.0) |
| Diet | ||||||||||
| Portion control (1–4, 4=highest) | 64 | 2.0 (0.8) | 3.5 (0.6) | 1.5 (1.2, 1.8)† | 87 | 2.2 (1.1) | 2.6 (0.6) | 0.4 (0.2, 0.6)† | 1.2 (0.8, 1.5)*** | 1.1 (0.7, 1.4)*** |
| Barriers (0–8, 0=fewest) | 67 | 4.8 (2.2) | 1.0 (1.3) | −3.8 (−4.4, −3.3)† | 93 | 5.0 (2.2) | 3.6 (2.8) | −1.4 (−2.0, −0.8)† | −2.4 (−3.1, −1.6)† | −2.1 (−3.0, −1.2)† |
| Self-efficacy (0–8, 8=highest) | 67 | 7.8 (0.7) | 7.6 (0.8) | −0.2 (−0.4, 0.0) | 86 | 7.7 (1.2) | 7.2 (1.1) | −0.4 (−0.8, −0.1)* | 0.2 (−0.2, 0.6) | 0.2 (−0.2, 0.7) |
| Diabetes knowledge | ||||||||||
| Knowledge scale 1 (0–7, 7=highest) | 67 | 3.8 (2.0) | 6.5 (0.7) | 2.7 (2.3, 3.3)† | 85 | 3.4 (2.1) | 5.5 (1.3) | 2.1 (1.5, 2.6)† | 0.5 (−0.2, 1.2) | 0.4 (−0.4, 1.2) |
| Knowledge scale 2 (0–7, 7=highest) | 65 | 1.6 (1.3) | 4.0 (0.8) | 2.3 (2.1, 2.6)† | 86 | 1.7 (1.3) | 3.5 (1.1) | 1.8 (1.4, 2.2)† | 0.4 (0.0, 0.8) | 0.4 (−0.1, 0.9) |
| Health | ||||||||||
| Self-efficacy (1–5, 5=highest) | 65 | 3.9 (0.5) | 4.1 (0.5) | 0.2 (0.1, 0.4)** | 87 | 3.8 (0.6) | 3.7 (0.6) | −0.1 (−0.2, 0.1) | 0.3 (0.1, 0.5)** | 0.4 (0.2, 0.6)** |
Boldface indicates statistical significance
p<0.05
p<0.01
p<0.001
Adjusted for age, sex, education, years in US; time-varying: insurance
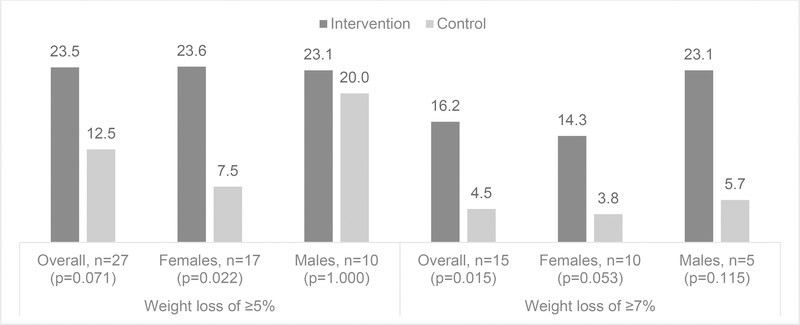
Among the treatment group, 73.5% lost weight over the intervention period, and among the control group, 42.1% lost weight; this group difference was significant (p<0.001). Figure 2 presents weight loss of ≥5% and ≥7% among both the treatment and control groups while stratifying by gender. Individuals in the treatment group were more likely to lose ≥5% of their initial body weight during the intervention period compared to the control group (23.5% vs. 12.5%); although this difference was not statistically significant (p=0.071), the magnitude of change between treatment and control groups was large. When stratified by gender, females in the treatment group were significantly more likely than females in the control group to lose ≥5% of their body weight (23.6% vs. 7.5%, p=0.022); no difference was shown among males. Individuals in the treatment group were also significantly more likely to lose ≥7% of their initial body weight compared to the control group (16.2% vs. 4.5%, p=0.015); a similar trend was shown when stratifying by gender, but the differences were not significant.
Figure 2.
Proportion of study participants who decreased weight by ≥5% and ≥7% at 6-month follow-up, overall and stratified by gender
Significant intervention effects were shown for diabetes prevention-related indicators. Compared to control group participants, treatment group participants were more likely to increase total PA (p<0.001), more likely to have increased PA self-efficacy (p<0.001), and more likely to have increased social interaction p<0.001). Treatment group participants were also more likely to have decreased barriers to exercise but the adjusted intervention effect was not significant (p=0.054). For diet, treatment group participants showed improved portion control (p<0.001) and decreased barriers to healthy eating (p<0.001). At 6 months, nutrition self-efficacy decreased in both groups and this difference across groups was not statistically significant (p=0.281). Diabetes knowledge (scales 1 and 2) increased in both groups equally and the intervention effect was not statistically significant (p=0.363 and p=0.148, respectively). Lastly at 6 months, health self-efficacy increased in the treatment group and decreased in the control group; the adjusted intervention effect was statistically significant (p=0.001).
Discussion
In this culturally adapted CHW-led intervention of Sikh Asian Indians to prevent type 2 diabetes, the decrease in total weight and BMI was significantly greater for the treatment group compared to the control group. Treatment group participants were more likely than control group participants to lose at least 5% and 7% of their weight (p=0.071, and p=0.015, respectively). Positive changes in other diabetes prevention-related indicators such as PA, diet, and health self-efficacy were also seen among treatment group participants.
There are some limitations to this study that should be noted. There were significant sociodemographic differences between the treatment and control group participants. Specifically, treatment group participants were more likely to be female and less likely to have insurance compared to the control group. Due to the convenience sampling used, our study findings may not be generalizable to the wider Sikh population in NYC, especially given that much of the recruitment took place at health fairs within the gurdwaras. However, the study had very low loss to follow-up and high attendance rates. It must be noted that the NYC Indian Sikh Community, especially those recruited from the implementing community partner, form a geographically and socially tight knit community. For these reasons, similar interventions conducted in other Sikh communities in the US may not produce comparable results. Further, baseline BMI measurements for both treatment and control group participants were relatively high and far above the American Diabetes Association recommended BMI cutoff for Asians of ≥23 kg/m2 (Yi, Islam, & Trinh-Shevrin, 2015), which may have motivated participants to attend and complete all sessions. Some of the data should be interpreted with caution; two-hour fasting could not be verified for glucose and total cholesterol, although it was protocol that individuals should have been fasting for two hours. Additionally, much of the data was collected by self-report, including self-reported diabetes diagnosis by a health professional (an ineligibility criteria). Receiving a diabetes diagnosis requires that an individual recalls the test and understands the information provided. Participants were not screened for diabetes after enrollment, and undiagnosed diabetes could be a potential confounder. Lastly, because group allocation occurred at the neighborhood-level, there may have been some contamination as shown by increased PA and social interaction among control participants at six months. However, these changes were significantly greater in the treatment group than in the control group.
Despite these limitations, our study demonstrated positive results in diabetes prevention indicators using a culturally adapted DPP curriculum. Results of the current study expand the knowledge base on the efficacy of culturally adapted diabetes prevention programs as well as on the role of CHWs in addressing health disparities and improving health equity. Having two trusted CHWs who are linguistically and culturally congruent with the participants likely enhanced the acceptability of the intervention as seen in earlier pilot data (N. S. Islam et al., 2014). CHWs were also trained in motivational interviewing and other techniques to help empower participants, which may have contributed to the statistically significant increase in self-efficacy among treatment participants and positively influenced main study outcomes. In addition, our past work has demonstrated that CHWs inter-personal attributes, their ability to serve as a bridge between health and non-health resources, and their ability to extend accessibility beyond health providers are important aspects of CHWs pathways of action in effectively engaging immigrant communities (N. Islam et al., 2017). Asian Americans are comprised of diverse subgroups, and within South Asians there are similarly distinct ethnic subgroups. While healthcare systems continue other population-wide approaches, these types may be insufficient in addressing the health needs of diverse communities. Our findings demonstrate that culturally and linguistically concordant CHWs that delivery culturally relevant interventions have the potential to improve health outcomes for specific populations and positively impact health disparities. While the current study did not have a sample size large enough to investigate the specific mechanisms by which CHWs impact health outcomes, this is an important area of future research.
Further, our culturally adapted curriculum condensed DPP material into six sessions coupled with follow-up phone calls, compared to the more intensive DPP 22 session curriculum. This modification, made with feedback from community partners, was implemented due to concerns that the Sikh population would be unable to attend 22 sessions due to family and work commitments. While our adapted intervention saw smaller changes in weight loss compared to the DPP (≥7% weight loss: 16.2% versus 50%, respectively) (Knowler et al., 2002), these changes were statistically significant. Mean weight loss from our study was also comparable to other CHW intervention studies; a systematic review of CHW interventions that have been adapted from DPP showed that the mean weight loss among intervention participants was 4.3% (Ali, Echouffo-Tcheugui, & Williamson, 2012) compared to 2.3% in our study. (Ali et al., 2012)
Under the framework of community-based participatory research, the study team partnered with community-based organizations to implement the intervention, adding to the literature on successful community-academic partnerships that build capacity of local communities (Trinh-Shevrin, Islam, et al., 2015; Trinh-Shevrin, Kwon, et al., 2015). Our positive results also underscore the valuable role that community partners can play in making adaptation to programs that ensure their success (as evidenced by our high retention rate) and produce positive and meaningful results in terms of improved health outcomes.
Implications for Health Behavior Research and Dissemination
The promising findings of our study point to several future areas of research that can further advance the field of improving healthy equity through health behaviors. A particular innovation of our study is the incorporation of religious and cultural principles into an adapted diabetes prevention curriculum. Though our study did not have a large enough sample size to assess the moderating influence of discrimination on health outcomes, this may be an important area of future research given that Sikh Asian Indians are particularly vulnerable to discrimination based on physical manifestations of their faith and such discrimination is linked to adverse health outcomes (Nadimpalli et al., 2016). Given the positive findings that we demonstrated in terms of the impact of the intervention on enhancing self-efficacy, future studies should also examine how such intermediary mechanisms may lead to improved prevention behaviors. Similar to the DPP, implementation of studies with a longer follow-up period assessing incidence of diabetes or sustained weight loss would also be scientifically valuable (Diabetes Prevention Program Research et al., 2009). Additionally, delivery of future interventions among Sikh Asian Indians may be improved through adoption of innovative and multi-level strategies such as healthy food policies for communal meals at places of worship (Kwon et al., 2017) and integration of CHWs into clinical practices to improve chronic disease outcomes (Lopez et al., 2017). While at this time, Medicare has only agreed to reimburse unadapted versions of the DPP, including English and Spanish language versions only, New York State has recently agreed to reimburse linguistically translated versions of the DPP program; however, these programs are not culturally adapted and consist only of verbatim language translations of materials, which may be rendered meaningless in some contexts (e.g. certain food items that are not culturally-appropriate or consumed by certain racial/ethnic groups may not have direct translations for particular racial/ethnic minority groups). For this reason, it is necessary to continue to build the evidence base for the effectiveness of culturally adapted DPPs and advocate for changes to current Medicare reimbursement policy, aligning with a health equity approach.
Although the value of our study intervention relies precisely on its cultural specificity, we believe that study components such as incorporation of religious elements and family values can be generalizable to other minority communities in which religion holds a prominent place in their daily lives. The study’s curriculum as well as the protocol (particularly on engagement of community members and CHW training) have wide potential for dissemination and adaption by researchers seeking to implement CHW-driven CBPR interventions.
Conclusions
Our study is the first to document the efficacy of a diabetes prevention intervention among Sikh Asian Indians, filling an important gap in the literature on developing culturally adapted interventions for underserved, high-risk, racial ethnic minority communities. Although there is a strong evidence base for the effectiveness of diabetes prevention programs like DPP, diabetes rates among Asian Indians are rising worldwide and there exists tremendous diversity within this population, underscoring the need for population health equity approaches like the one described here. Continued development, implementation, and evaluation of effective and culturally adapted interventions are needed to lessen the diabetes burden in this population.
Discussion Questions.
How can evidenced-based strategies for diabetes prevention enhance health equity?
What is the role of community health workers in advancing health equity?
What further evidence is needed to enhance the science of health equity?
Acknowledgments
This study was supported by grant U58DP005621, U48DP005008, and 1U48DP001904-01 from the Centers of Disease Control and Prevention, grants P60MD000538 and P60MD001786 from the National Institutes of Health, grant U54MD000538 from the National Institutes of Health National Institute on Minority Health and Health Disparities, Grant R01DK110048 from the National Institutes of Health National Institute of Diabetes and Digestive Kidney Diseases, and grant UL1TR000038 from the National Center for Advancing Translational Sciences, National Institute of Health.
The findings and conclusions are those of the authors and do not necessarily represent the official position of the funding organizations.
The authors would also like to thank Surinder Kaur and Satinder Kaur for their service and dedication on the project.
Financial Disclosure: The authors have no conflicts of interest to report, financial or otherwise.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
The article contents have not been previously published elsewhere.
Contributor Information
Sahnah Lim, NYU School of Medicine.
Laura C. Wyatt, NYU School of Medicine.
Harmanpreet Chauhan, California State University Fullerton.
Jennifer M. Zanowiak, NYU School of Medicine.
Rucha Kavathe, UNITED SIKHS.
Hardayal Singh, UNITED SIKHS.
Simona C. Kwon, NYU School of Medicine.
Chau Trinh-Shevrin, NYU School of Medicine.
Nadia S. Islam, NYU School of Medicine.
References
- Ali MK, Echouffo-Tcheugui J, & Williamson DF (2012). How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood), 31(1), 67–75. doi: 10.1377/hlthaff.2011.1009 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. (2018). Type 2 Diabetes Risk Test. Retrieved from http://www.diabetes.org/are-you-at-risk/diabetes-risk-test/
- Anthony S, Gowler R, Hirsch G, & Wilkinson G (2009). Community Health Workers in Massachusetts: Improving Health Care and Public Health. Retrieved from Boston, MA: [Google Scholar]
- Bandura A (2006). A Guide for Constructing Self-Efficacy Scales In Self-Efficacy Beliefs of Adolscents: Information Age Publishing. [Google Scholar]
- Barnes PM, Adams PF, & Powell-Griner E (2008). Health characteristics of the Asian adult population: United States, 2004–2006. Adv Data(394), 1–22. [PubMed] [Google Scholar]
- Bhatnagar D, Anand IS, Durrington PN, Patel DJ, Wander GS, Mackness MI, … et al. (1995). Coronary risk factors in people from the Indian subcontinent living in west London and their siblings in India. Lancet, 345(8947), 405–409. [DOI] [PubMed] [Google Scholar]
- Burgel BJ, Gillen M, & White MC (2012). Health and safety strategies of urban taxi drivers. J Urban Health, 89(4), 717–722. doi: 10.1007/s11524-012-9685-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao A, Ahmed T, & Islam N (2007). Community Health Needs & Resource Assessment: An Exploratory Study of South Asians in NYC. Retrieved from https://med.nyu.edu/sites/default/files/asian-health2/chnra_southasian_0.pdf
- Centers for Disease Control and Prevention. (2008). Road to Health Resource Guide. Retrieved from Atlanta, GA: [Google Scholar]
- Centers for Disease Control and Prevention. (2017). National Diabetes Statistics Report, 2017. Retrieved from Atlanta, GA: [Google Scholar]
- Department of City Planning - City of New York. (2013). The Newest New Yorkers: Characteristics of the City’s Foreign-born Population - 2013 Edition. Retrieved from https://www1.nyc.gov/assets/planning/download/pdf/data-maps/nyc-population/nny2013/nny_2013.pdf
- Diabetes Prevention Program (DPP) Research Group. (2002). The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care, 25(12), 2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research, G., Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, … Nathan DM (2009). 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet, 374(9702), 1677–1686. doi: 10.1016/S0140-6736(09)61457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JT, Funnell MM, Anderson RM, Nwankwo R, Stansfield RB, & Piatt GA (2016). Validation of the Revised Brief Diabetes Knowledge Test (DKT2). Diabetes Educ, 42(2), 178–187. doi: 10.1177/0145721715624968 [DOI] [PubMed] [Google Scholar]
- Fitzgerald JT, Funnell MM, Hess GE, Barr PA, Anderson RM, Hiss RG, & Davis WK (1998). The reliability and validity of a brief diabetes knowledge test. Diabetes Care, 21(5), 706–710. [DOI] [PubMed] [Google Scholar]
- Gany FM, Gill PP, Ahmed A, Acharya S, & Leng J (2013). “Every disease...man can get can start in this cab”: focus groups to identify south Asian taxi drivers’ knowledge, attitudes and beliefs about cardiovascular disease and its risks. J Immigr Minor Health, 15(5), 986–992. doi: 10.1007/s10903-012-9682-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta LS, Wu CC, Young S, & Perlman SE (2011). Prevalence of diabetes in New York City, 2002–2008: comparing foreign-born South Asians and other Asians with U.S.-born whites, blacks, and Hispanics. Diabetes Care, 34(8), 1791–1793. doi: 10.2337/dc11-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller V (January 11, 2013). I ndo-Caribbean Content, Victorian Style. The New York Times. [Google Scholar]
- Islam N, Shapiro E, Wyatt L, Riley L, Zanowiak J, Ursua R, & Trinh-Shevrin C (2017). Evaluating community health workers’ attributes, roles, and pathways of action in immigrant communities. Prev Med, 103, 1–7. doi: 10.1016/j.ypmed.2017.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam NS, Wyatt LC, Kapadia SB, Rey MJ, Trinh-Shevrin C, & Kwon SC (2013). Diabetes and associated risk factors among Asian American subgroups in New York City. Diabetes Care, 36(1), e5. doi: 10.2337/dc12-1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam NS, Wyatt LC, Patel SD, Shapiro E, Tandon SD, Mukherji BR, … Trinh-Shevrin C (2013). Evaluation of a community health worker pilot intervention to improve diabetes management in Bangladeshi immigrants with type 2 diabetes in New York City. Diabetes Educ, 39(4), 478–493. doi: 10.1177/0145721713491438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam NS, Wyatt LC, Taher MD, Riley L, Tandon SD, Tanner M, … Trinh-Shevrin C (2018). A Culturally Tailored Community Health Worker Intervention Leads to Improvement in Patient-Centered Outcomes for Immigrant Patients With Type 2 Diabetes. Clin Diabetes, 36(2), 100–111. doi: 10.2337/cd17-0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam NS, Zanowiak JM, Riley L, Nadkarni SK, Kwon SC, & Trinh-Shevrin C (2015). Characteristics of Asian American, Native Hawaiian, and Pacific Islander community health worker programs: a systematic review. J Health Care Poor Underserved, 26(2 Suppl), 238–268. doi: 10.1353/hpu.2015.0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam NS, Zanowiak JM, Wyatt LC, Chun K, Lee L, Kwon SC, & Trinh-Shevrin C (2013). A randomized-controlled, pilot intervention on diabetes prevention and healthy lifestyles in the New York City Korean community. J Community Health, 38(6), 1030–1041. doi: 10.1007/s10900-013-9711-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam NS, Zanowiak JM, Wyatt LC, Kavathe R, Singh H, Kwon SC, & Trinh-Shevrin C (2014). Diabetes prevention in the New York City Sikh Asian Indian community: a pilot study. Int J Environ Res Public Health, 11(5), 5462–5486. doi: 10.3390/ijerph110505462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey SL, Patel S, Kalra P, Greenlund K, Srinivasan S, & Grewal D (2004). Cardiovascular health among Asian Indians (CHAI): a community research project. J Interprof Care, 18(4), 391–402. [DOI] [PubMed] [Google Scholar]
- Jaber LA, Pinelli NR, Brown MB, Funnell MM, Anderson R, Hammad A, & Herman WH (2011). Feasibility of group lifestyle intervention for diabetes prevention in Arab Americans. Diabetes Res Clin Pract, 91(3), 307–315. doi: 10.1016/j.diabres.2010.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindig D, & Stoddart G (2003). What is population health? Am J Public Health, 93(3), 380–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L, & Deng WQ (March 2018). Health Disparities among Asian New Yorkers. Retrieved from https://www1.nyc.gov/assets/doh/downloads/pdf/epi/databrief100.pdf
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, … Diabetes Prevention Program Research, G. (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med, 346(6), 393–403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SC, Patel S, Choy C, Zanowiak J, Rideout C, Yi S, … Islam N (2017). Implementing Health Promotion Activities Using Community-engaged Approaches in Asian American Faith-Based Organizations in New York City and New Jersey. Translational Behavioral Medicine, 7(3), 444–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky J (October 10, 2018). Ozone Park, Queens: A Congenial Area Welcomes a New Wave of Residents. The New York Times. [Google Scholar]
- Lopez PM, Zanowiak J, Goldfeld K, Wyka K, Masoud A, Beane S, … Islam N (2017). Protocol for project IMPACT (improving millions hearts for provider and community transformation): a quasi-experimental evaluation of an integrated electronic health record and community health worker intervention study to improve hypertension management among South Asian patients. BMC Health Serv Res, 17(1), 810. doi: 10.1186/s12913-017-2767-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann GS, Numrich P, & Williams R (2007). Buddhists, Hindus and Sikhs in America: A Short History. New York, NY: Oxford University Press. [Google Scholar]
- Mau MK, Keawe’aimoku Kaholokula J, West MR, Leake A, Efird JT, Rose C, … Gomes H (2010). Translating diabetes prevention into native Hawaiian and Pacific Islander communities: the PILI ‘Ohana Pilot project. Prog Community Health Partnersh, 4(1), 7–16. doi: 10.1353/cpr.0.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam PA, Tellez TL, Rosal MC, Olendzki BC, Ma Y, Pagoto SL, & Ockene IS (2009). Methodology of a diabetes prevention translational research project utilizing a community-academic partnership for implementation in an underserved Latino community. BMC Med Res Methodol, 9, 20. doi: 10.1186/1471-2288-9-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra A, Ramchandran A, Jayawardena R, Shrivastava U, & Snehalatha C (2014). Diabetes in South Asians. Diabet Med, 31(10), 1153–1162. doi: 10.1111/dme.12540 [DOI] [PubMed] [Google Scholar]
- Nadimpalli SB, Cleland CM, Hutchinson MK, Islam N, Barnes LL, & Van Devanter N (2016). The association between discrimination and the health of Sikh Asian Indians. Health Psychol, 35(4), 351–355. doi: 10.1037/hea0000268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. (2018). Your Game Plan to Prevent Type 2 Diabetes. Retrieved from https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-type-2-diabetes/game-plan
- New York City Department of Health and Mental Hygiene. (2016). EpiQuery. Retrieved from https://a816-healthpsi.nyc.gov/epiquery/CHS/CHSXIndex.html
- Nothwehr F, Dennis L, & Wu H (2007). Measurement of behavioral objectives for weight management. Health Educ Behav, 34(5), 793–809. doi: 10.1177/1090198106288559 [DOI] [PubMed] [Google Scholar]
- Ockene IS, Tellez TL, Rosal MC, Reed GW, Mordes J, Merriam PA, … Ma Y (2012). Outcomes of a Latino community-based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. Am J Public Health, 102(2), 336–342. doi: 10.2105/AJPH.2011.300357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpathak SN, Gupta LS, Waddell EN, Upadhyay UD, Wildman RP, Kaplan R, … Wylie-Rosett J (2010). Elevated risk of type 2 diabetes and metabolic syndrome among Asians and south Asians: results from the 2004 New York City HANES. Ethn Dis, 20(3), 225–230. [PubMed] [Google Scholar]
- Ramachandran A, & Snehalatha C (2011). Diabetes prevention programs. Med Clin North Am, 95(2), 353–372, viii. doi: 10.1016/j.mcna.2010.11.006 [DOI] [PubMed] [Google Scholar]
- Sechrist KR, Walker SN, & Pender NJ (1987). Development and psychometric evaluation of the exercise benefits/barriers scale. Res Nurs Health, 10(6), 357–365. [DOI] [PubMed] [Google Scholar]
- Sinclair KA, Makahi EK, Shea-Solatorio C, Yoshimura SR, Townsend CK, & Kaholokula JK (2013). Outcomes from a diabetes self-management intervention for Native Hawaiians and Pacific People: Partners in Care. Ann Behav Med, 45(1), 24–32. doi: 10.1007/s12160-012-9422-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Shenoy S, & Singh Sandhu J (2016). Prevalence of Type 2 Diabetes Mellitus among Urban Sikh Popultion of Amritsar. Indian Journal of Community Medicine, 41(4), 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South Asian Americans Leading Together and the Asian American Federation. (2012). A Demographic Snapshot of South Asians in the United States. Retrieved from http://saalt.org/wp-content/uploads/2012/09/Demographic-Snapshot-Asian-American-Foundation-2012.pdf
- Srinivasan S, & Williams SD (2014). Transitioning from health disparities to a health equity research agenda: the time is now. Public Health Rep, 129 Suppl 2, 71–76. doi: 10.1177/00333549141291S213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Sikh Coalation. (2008). Making Our Voices Heard: A Civil Rights Agenda for New York City’s Sikhs. Retrieved from http://www.sikhcoalition.org/documents/pdf/RaisingOurVoicesReport.pdf
- The Sikh Coalation. (2014). Fact Sheet on Post-9/11 Discrimination and Violence Against Sikh Americans. Retrieved from http://www.sikhcoalition.org/images/documents/fact%20sheet%20on%20hate%20against%20sikhs%20in%20america%20post%209-11%201.pdf
- Thorpe LE, Kanchi R, Chamany S, Rodriguez-Lopez JS, Chernov C, Freeman A, & Perlman SE (2018). Change in Diabetes Prevalence and Control among New York City Adults: NYC Health and Nutrition Examination Surveys 2004–2014. J Urban Health. doi: 10.1007/s11524-018-0285-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh-Shevrin C, Islam NS, Nadkarni S, Park R, & Kwon SC (2015). Defining an integrative approach for health promotion and disease prevention: a population health equity framework. J Health Care Poor Underserved, 26(2 Suppl), 146–163. doi: 10.1353/hpu.2015.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh-Shevrin C, Kwon SC, Park R, Nadkarni SK, & Islam NS (2015). Moving the dial to advance population health equity in New York City Asian American populations. Am J Public Health, 105 Suppl 3, e16–25. doi: 10.2105/AJPH.2015.302626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. American Community Survey 1-year Estimates 2016.
- U.S. Census Bureau. (2012–2016). Selected Social Characteristics in the United States, American Community Survey 5-Year Estimates. Retrieved from https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_16_5YR_DP02&prodType=table
- U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Retrieved from https://health.gov/paguidelines/2008/pdf/paguide.pdf
- U.S. National Library of Medicine. Chinese Diabetes Prevention Program (Chinese DPP). Retrieved from https://clinicaltrials.gov/ct2/show/NCT02277509
- Ursua RA, Aguilar DE, Wyatt LC, Trinh-Shevrin C, Gamboa L, Valdellon P, … Islam NS (2018). A community health worker intervention to improve blood pressure among Filipino Americans with hypertension: A randomized controlled trial. Prev Med Rep, 11, 42–48. doi: 10.1016/j.pmedr.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SS, Islam N, & Trinh-Shevrin C (2015). Comment on Hsu et al. BMI cut points to identify at-risk asian americans for type 2 diabetes screening. Diabetes Care 2015;38:150–158. Diabetes Care, 38(6), e90. doi: 10.2337/dc15-0470 [DOI] [PMC free article] [PubMed] [Google Scholar]