Abstract
Reading is a complex process involving recruitment and coordination of a distributed network of brain regions. The present study sought to establish a methodologically sound evidentiary base relating specific reading and phonological skills to neuro-metabolic concentration. Single voxel proton magnetic resonance spectroscopy was performed to measure metabolite concentration in a left hemisphere region around the angular gyrus for 31 young adults with a range of reading and phonological abilities. Correlation data demonstrated a significant negative association between phonological decoding and normalized choline concentration and as well as a trend toward a significant negative association between sight word reading and normalized choline concentration, indicating that lower scores on these measures are associated with higher concentrations of choline. Regression analyses indicated that choline concentration accounted for a unique proportion of variance in the phonological decoding measure after accounting for age, cognitive ability and sight word reading skill. This pattern of results suggests some specificity for the negative relationship between choline concentration and phonological decoding. To our knowledge, this is the first study to provide evidence that choline concentration in the angular region may be related to phonological skills independently of other reading skills, general cognitive ability, and age. These results may have important implications for the study and treatment of reading disability, a disorder which has been related to deficits in phonological decoding and abnormalities in the angular gyrus.
1. Introduction
Reading is a complex process involving recruitment and coordination of a distributed network of brain regions. Researchers have been exploring the relationship between reading and neurobiology since as early as 1891 when Dejerine described left hemisphere posterior brain lesions as causal to acquired alexia, or, inability to read (Dejerine, 1891). Research has converged on several left hemisphere regions, often collectively termed the reading network, that are critical to the reading process including inferior frontal gyrus, temporo-parietal regions (angular and supramarginal gyri), temporal lobe and occipito-temporal junction (Price, 2000). Despite this long history of research the exact relationship between neurobiological processes and reading skills is not well understood, especially the relationship between neurochemical composition and reading skills. The goal of the present study was to examine the relationship between neurochemical composition and reading processes, especially phonological processing.
Phonological processing is a crucial component of the reading process because it allows the reader to detect and discriminate differences in phonemes found in words. Phonological processing is necessary in the decoding of unfamiliar words and, therefore, highly involved in learning to read. Individuals who have deficits in phonological processing have an extremely difficult time learning to read and are often diagnosed with developmental dyslexia or reading disability. Indeed, phonological processing deficits are thought to be the core deficit associated with developmental dyslexia (Brady, 1997; Snowling, 2000; Vellutino et al., 2004).
Various neuroimaging techniques have been employed to examine the structural and/or functional brain correlates of reading and phonological processing. Variations in structure and function throughout the reading network, identified with functional and/or structural magnetic resonance imaging studies, have been associated with variations in reading and phonological skills (Eckert et al., 2003; Shaywitz et al., 2003). When combined with cognitive profiles, functional activation and morphological patterns can be used to predict phonological abilities (Hoeft et al., 2007).
Magnetic resonance spectroscopy (MRS) may be a particularly useful technique because it is able to detect concentrations of specific neuro-metabolites in vivo, providing a measure of the quality of brain tissues. It may be a more sensitive measure than traditional structural MRI, which is only able to measure the quantity of brain tissues. Furthermore, MRS can be employed to measure steady state chemical concentrations in the absence of explicit task performance thus eliminating confounds such as effort, accuracy, reaction time and/or cognitive strategy differences which occur with task dependent functional MRI.
Concentrations of metabolites such as choline, Myoinositol, and N-acetyl aspartate (NAA) have been linked to specific neurobiological disorders and to cognitive functioning in a general sense. Choline and myoinositol are involved in the synthesis and breakdown of phospholipid cell membranes and are considered markers of membrane synthesis, degeneration and/or inflammation (Brand et al., 1993; Ross and Sachdev, 2004). Increases in choline concentrations have been reported for individuals suffering from traumatic brain injury (Padma, 2005) and multiple sclerosis (Tartaglia et al., 2002). Increased myoinositol has been reported in patients with mild cognitive impairment and Alzheimer’s disease (Catani et al., 2001). Although the exact role of NAA is unclear it is generally considered a marker of intact neuronal cell functions (Moffett et al., 2007) and research suggests it varies with general cognitive skill (Jung et al., 2009a; Jung et al., 2005), creativity (Jung et al., 2009b), and personality (Ryman et al., 2011).
In a previous MRS study with reading impaired individuals, Rae et al., (Rae et al., 1998) found a lower choline/NAA ratio in the left temporo-parietal region of dyslexic adult males, which they interpreted as evidence of abnormal development of cells, intercellular connections or both. This interpretation is in line with structural studies that indicate abnormal structure in gray (Silani et al., 2005) and white matter (Niogi and McCandliss, 2006) of the temporo-parietal regions in the left hemisphere in individuals with dyslexia. However, the specific finding of decreased choline in dyslexic individuals does not fit with previous reports that have associated various pathologies with increases in choline (Padma, 2005). Furthermore, the use of NAA as a baseline may add ambiguity to the results because of the relationship between NAA and general cognitive skill (Jung et al., 2005). The choice of a baseline is crucial in MRS research to account for individual variance in metabolic concentration. The abundant metabolite creatine is most commonly utilized as a baseline because its concentration remains stable regardless of changes in energy metabolism or disease progression (Yiannoutsos et al., 2008).
The present study was designed to further explore the neurochemical correlates of reading and phonological skills in individuals with a range of reading skill, several of whom would meet criteria for developmental dyslexia (i.e., reading in the bottom 15% on standardized measures). Specifically, we sought to relate individual differences in phonological and reading skills to the integrity and/or composition of brain tissues as measured by MRS. The single voxel MRS protocol we employed dictates ROI specification a priori yet neuroimaging studies of reading skill and disability have indicated that multiple brain regions are involved in the reading process including inferior frontal, parietal and temporal regions of the left hemisphere (Eckert et al., 2003). We chose to examine a region around the left hemisphere angular gyrus, within the parietal system, for several reasons. First, the left angular gyrus is a critical region for reading ability due to its proposed role in acquired dyslexia (Dejerine, 1891) and current theories indicate that it may be a kind of convergence zone for neurophysiological processes involved in reading (Pugh et al., 2001). The angular gyrus is implicated in the integration of visual input (from extrastriate visual areas) with phonological codes processed in the superior temporal gyrus (Damasio and Damasio, 1983; Geschwind, 1965). Neuroimaging research suggests that the angular gyrus, along with the supramarginal gyrus, may be part of a dorsal phonological system (Church et al., 2008; Pugh et al., 2001) and/or a semantic processing system alongside the supramarginal phonological system (Binder et al., 2005; Lee et al., 2007; Price, 2000). Abnormal neural activity patterns within angular gyrus and between angular gyrus and other regions have been reported for individuals with reading disability or developmental dyslexia (Hampson et al., 2006; Pugh et al., 2000; Schulz et al., 2008) and it has been demonstrated that illiterates who gain reading experience later in life display altered angular gyrus anatomy (Carreiras et al., 2009).
2. Materials and Methods
2.1. Participants
Thirty-one university students, recent university graduates, or graduate students (age range 18–30, 17 females, 14 males) participated in the present study. Ten individuals reported receiving a diagnosis of reading disability at some point in their lifetimes. Participants were monolingual native English speakers who were strongly right-handed as assessed by the Edinburgh Handedness Questionnaire (Oldfield, 1971). Participants presented with normal or corrected to normal vision and hearing as well as negative histories for neurological, serious emotional, and mood disorders. We sought to recruit individuals of varying reading skill, with and without a history of reading problems, however we wanted to ensure that individuals were equated in terms of general cognitive abilities including verbal and spatial abilities. To that end participants were required to score in the average range or above (standard score ≥ 85) on an estimate of general cognitive ability (the Woodcock-Johnson III Tests of Cognitive Abilities –Spatial Relations and Verbal Comprehension subtests [mean of test scores was used], (Woodcock et al., 2001).
2.2. Reading/phonological testing
In addition to the Woodcock subtests, participants were administered several standardized reading and phonological tests comprising the following domains 1) sight word reading efficiency, 2) phonological decoding and 3) reading comprehension. For the domain of sight word efficiency the Word Identification subtest of the Woodcock Johnson III Tests of Cognitive Achievement (Woodcock et al., 2001) and the Test of Word Reading Efficiency Sight Word Efficiency (TOWRE-SWE) subtest (Torgesen et al., 1999) were administered. The WID measures single word reading ability in an untimed setting and the SWE subtest measures single word reading ability in a time-pressured setting in which both accuracy and efficiency are stressed. For the domain of phonological decoding, the Word Attack (WAT) subtest of the Woodcock Johnson III tests and the Phonemic Decoding Efficiency (TOWRE-PDE) subtest of the TOWRE were included. Both the WAT and the TOWRE-PDE measure the ability to pronounce pseudowords (e.g. ‘blort’) and therefore are considered measures of phonological decoding because the pseudowords must be decoded in order for one to read them. The WAT is untimed whereas the PDE subtest is timed and stresses accuracy and speed. The Nelson-Denny Test (Brown et al., 1993) was employed as a standardized measure of reading comprehension. Summary statistics for the reading/phonological testing scores are presented in Table 1. Also included in the table are the number of individuals who scored less than or equal to the 25th percentile on each reading/phonological measure (criteria commonly used as diagnosis for reading disability). Z scores are reported for both the TOWRE-PDE and TOWRE-SWE subtests because those tests are normalized on a sample of children and young adults (≤ age 24) and standard scores were not available for our entire sample. The relatively narrow age range for the present sample justified using z-score conversions of raw scores. We created composite measures of 1) sight word reading and 2) phonological decoding by computing the mean of the two sight word subtest Z scores and the two phonological decoding subtest Z scores respectively. Composite measures were used for subsequent analyses.
Table 1.
Descriptive statistics: Cognitive tests and metabolite concentrations
| Mean | Standard Deviation | Minimum | Maximum | N <25% | |
|---|---|---|---|---|---|
| Age (years) | 22.14 | 3.15 | 18.50 | 30.00 | |
| Education (years) | 15.62 | 1.36 | 13.60 | 18.00 | |
| WID | 102.32 | 12.14 | 76 | 125 | 6 |
| TOWRE – SWE | 91.68 | 12.32 | 62 | 104 | 12 |
| WAT | 95.90 | 13.22 | 65 | 119 | 10 |
| TOWRE – PD | 45.76 | 13.97 | 13 | 61 | 13 |
| ND Comprehension | 15.71 | 3.81 | 4.10 | 18.90 | 7 |
| General cognitive ability | 108.85 | 8.18 | 92 | 123 | 0 |
| Cho/Cr | 0.75 | 0.10 | 0.60 | 1.01 | |
| NAA/Cr | 2.01 | 0.31 | 1.31 | 2.65 | |
| mIns/Cr | 0.55 | 0.19 | 0.31 | 1.17 | |
| Grey Matter/White Matter | 0.75 | 0.28 | 0.32 | 1.39 | |
N < 25% = number of indiviudals scoring less than or equal to the 25th percentile on the measure. ND Comprehension = Grade equivalency score on the Nelson-Denny Test of Reading Comprehension [For the Nelson Denny test percentile scores were not available therefore the number in the N< 25% column is the number of individuals whose grade equivalency score was 3 or more uyears below their level of education], TOWRE – PDE = Test of word reading efficiency – phonemic decoding efficiency subtest; TOWRE – SWE = Test of word reading efficiency – sight efficiency subtest. For both versions of the TOWRE raw scores are reported because standardized scores were not available for our entire sample. WID (Word Identification), WAT (Word Attack), and cognitive ability estimate are from the WJ-III and standard scores are reported. Cho/cr = ratio of choling/creatine; mIns/Cr = ratio of myoinositol/creatine; Naa/Cr = ratio of N-acetyl aspertate/creatine concentrations.
2.3. Scanning Procedures
All scanning was performed on a Siemens 3T TIM TRIO (Siemens Medical Solutions, Malvern, PA) using a Siemens Matrix head coil. Earplugs and sound dampening headphones were employed to shield the participants from acoustic noise. Foam padding was used to minimize head movement.
High-resolution anatomical images were acquired via a T1-weighted MPRAGE sequence (FoV 256 mm; TI 900 ms; TR 2300 ms; TE 2.98 ms; 160 saggital slices). 3D volumes were compiled and used for placement of the 20mm3 ROI (See Figure 1a) on an individual basis using neuro-anatomical landmarks. All voxel placement was performed by the first author. The boundaries were prescribed such that the voxel would encompass the maximum amount of grey and white matter surrounding the angular gyrus (Brodmann’s area 39). Due to the voxel’s cubic shape it was not possible to include only grey or white matter, nor was it possible to include only the angular gyrus. Parts of the supramarginal gyrus (Brodmann’s area 40) and posterior superior temporal gyrus (Brodmann’s area 22) were also included. The inferior boundary of the voxel was established at the level of the superior temporal sulcus at the most lateral surface of the cortex. The posterior boundary was the occipito-temporal sulcus, which was aligned with the center (in the anterior posterior direction) of the voxel. Finally, the voxel was shifted laterally to the most lateral surface of cortex such that no portion of the voxel contained non-brain material.
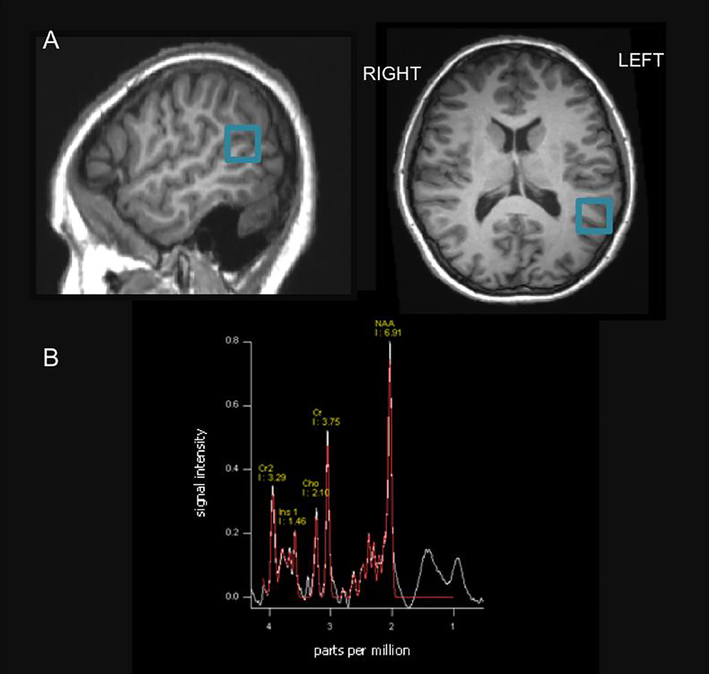
Figure1.
Voxel Placement and Spectrograph for one example participant
a) Placement of voxel in one example participant illustrated on T2 image.
b) Spectrograph for one example participant. NAA, N-acetyl aspertate; Cho, choline; Cr, creatine; Ins, myoinositol; Cr2, second resononance of creatine (with methelyne group, not used in analyses). Signal intensity is in arbitrary units.
Single voxel spectroscopy was performed utilizing spin echo pulse sequence (SVS-SE, TR 2000 ms, TE 30 ms, acquisition time 4:17, number of acquisitions or averages 128). Spectrograph processing was performed by the Siemens Spectroscopy Toolbox. The metabolite concentrations estimated by the toolbox were choline, myoinsositol, NAA and creatine. A spectrograph for one participant is shown in Figure 1b. Peak integrals indicating concentrations of each metabolite relative to internal water content (Christiansen et al., 1993) were extracted and compiled in SPSS for further analysis. Normalized concentration of each metabolite of interest (choline, myoinositol and NAA) was computed relative to creatine.
Following the MRI scanning session, a 20mm3 voxel was placed on each participant’s T1 anatomical image by the first author using the same landmarks as in the scanning session. The FMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl) brain extraction and segmentation tools were then used to segment each T1 image and calculate the percentage of each tissue type (grey matter, white matter and cerebrospinal fluid) within each MRS voxel.
3. Results
3.1. Correlations
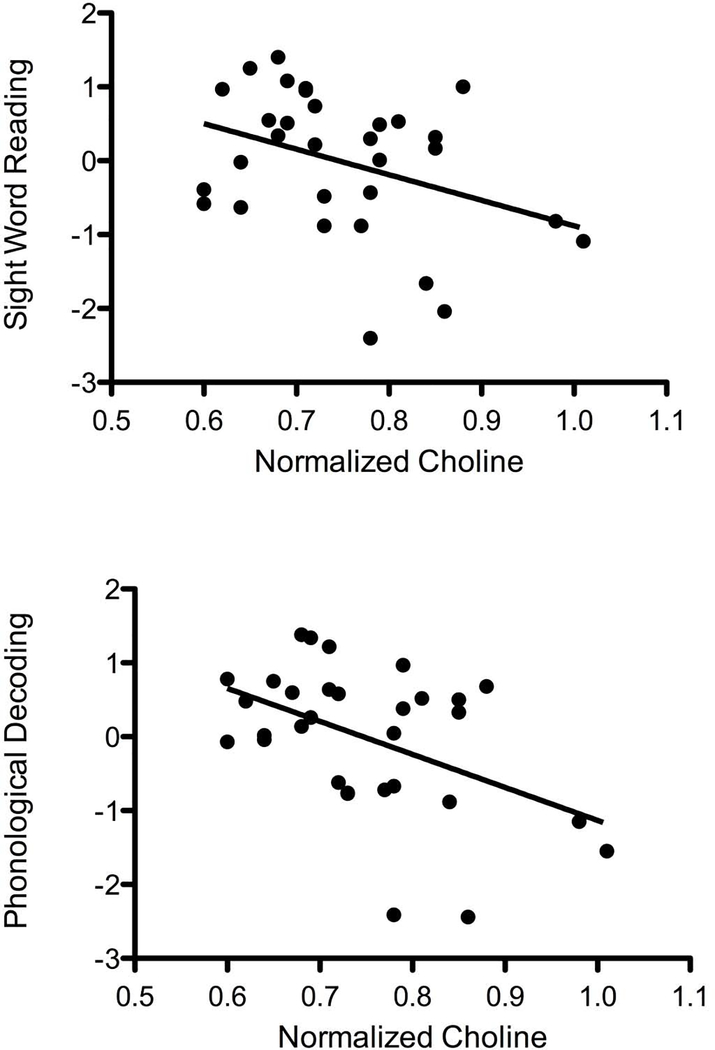
The relationship between normalized choline concentration and phonological decoding composite was significant (rho = –0.383, p=0.034), and the relationship between choline concentration and sight word reading composite trended toward significance (rho = –0.344, p=0.058). Figure 2 presents scatter plots illustrating the aforementioned relationships. Normalized choline did not correlate significantly with ND reading comprehension score (p>0.10). Levels of normalized myoinositol and NAA did not correlate significantly with any of the reading/phonological measures (all p>0.10). Age, grade level and cognitive ability estimate did not correlate with any measure of metabolite concentration (all p>0.10). Sex was significantly correlated with NAA/Cr (rpb = 0.356, p=0.049, higher NAA/Cr concentration for males), and with general cognitive ability (rpb = 0.490, p=0.010, higher cognitive ability scores for males), but sex did not correlate significantly with any other metabolite of interest or any of the reading/phonological measures (all p <0.10). Voxel tissue concentration was not significantly related to any of the metabolite concentrations (including the ratios Cho/Cr, NAA/Cr, mIns/Cr and absolute creatine all p <0.10), therefore tissue concentrations were not included in any subsequent analyses.
Figure 2.
Scatter plots of relationships between normalized choline concentration and sight word reading composite (top), and between normalized choline concentration and phonological decoding composite (bottom). Normalized choline concentration is in arbitrary usits, sight word composite and phonological decoding composite scores have been standardized.
3.2. Regressions
Due to the significant inter-correlation among reading component variables and the correlations between age, education cognitive ability and reading component variables, hierarchical multiple regression was used to statistically test the hypothesis that choline concentration contributed unique variance to the sight word or phonological decoding composite scores after controlling for the other reading components and for age and general cognitive ability. Reading comprehension was not included in a regression analysis as an independent variable of interest due to the lack of a significant correlation or a trend towards a significant correlation with choline concentration. Given that age and education level were highly correlated (rho = 0.89, p < 0.001) we included only age in the regressions.
Two multiple regression analysis were computed, each with three blocks of independent variables and either sight word or phonological decoding composite as the dependant variable. The first block contained age and cognitive ability as variables to be controlled for. The second block contained the reading component measure that was not the independant variable and the third block contained the normalized choline measure. Regression results are presented in Table 3. Results indicate that normalized choline concentration does not account for a significant unique proportion of variance in sight word reading after accounting for age, cognitive ability and phonological decoding (p>0.10). Normalized choline concentration accounts for a significant unique proportion of variance in phonological decoding after accounting for age, cognitive ability and sight word reading (ΔR2 = 0.027, p =0.029).
Table 3a.
Multiple regression analysis using three blocks to address the specificity of sight word reading to choline/creatine concentration, controlling for age and general cognitive ability, and phonological decoding.
| Model | Change Statistics |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| R | R2 | Adjusted R2 | SE of the Estimate | R2 Change | F Change | df1 | df2 | Sig. F Change | |
| 1 | .522a | .273 | .221 | .851 | .273 | 5.245 | 2 | 28 | .012 |
| 2 | .925b | .856 | .840 | .385 | .584 | 109.609 | 1 | 27 | .000 |
| 3 | .931c | .866 | .845 | .379 | .010 | 1.906 | 1 | 26 | .179 |
Predictors: (Constant), age, cognitive ability
Predictors: (Constant), age, cognitive ability, phonological decoding composite
Predictors: (Constant), age, cognitive ability, phonological decoding composite, normalized choline
4. Discussion
The present study sought to establish a methodologically sound evidentiary base relating neuro-metabolite concentration to specific reading and phonological skills. Single voxel proton MRS was performed on one ROI around the left hemisphere angular gyrus, of 31 young adults with a range of reading and phonological abilities. Correlation data demonstrated a significant negative relationship between normalized choline concentration and phonological decoding as well as a trend toward a significant correlation between normalized choline and sight word composite, indicating that lower scores on these measures are associated with higher levels of choline. No significant relationships were demonstrated between normalized myoinositol or NAA concentration and reading/phonological variables. To our knowledge, this is the first study to provide evidence that choline concentration in the angular region is related to phonological decoding independently of age, general cognitive ability and sight word reading.
Our pattern of results tentatively indicates some specificity for the negative relationship between choline concentration and phonological decoding because choline concentration remained a significant predictor of phonological decoding after accounting for age, general cognitive ability and sight word reading skill. Conversely, choline concentration did not remain a significant predictor of sight word reading skill after accounting for age, general cognitive ability and phonological decoding. However, we note that phonological decoding and sight word reading measures were moderately correlated with one another and more specific measures of each skill may be required to adequately tease apart these relationships. Furthermore, it is possible that metabolite concentration in other parts of the reading network may be more specifically related to sight word reading. Functional specialization of the occipitotemporal region (close to what has been termed the visual word form area) correlates with sight word reading efficiency (Ben-Shachar et al., 2011) suggesting the potential for stronger metabolite-skill relationships there.
Phonological decoding has been proposed as the core deficit of developmental dyslexia and the distal cause for impaired word reading (Vellutino et al., 2004). Abnormalities in the structure and function of the angular gyrus have been implicated in individuals with reading disability (Hampson et al., 2006; Pugh et al., 2000; Schulz et al., 2008) and illiterates who gain reading experience later in life (Carreiras et al., 2009). Therefore, the relationship revealed between metabolite concentration and phonological decoding in the angular region may have important implications for the study and treatment of reading disability. In the present study an inverse relationship was revealed between choline concentration and phonological decoding for individuals ranging in reading ability, some whom scored well below average on tests of reading/phonological skills indicating they may have a reading disability.
Inverse relationships between cognitive measures and choline concentration have been reported for both healthy and clinical samples (Ross and Sachdev, 2004). Elevated levels of choline may indicate inflammation or demyelination, as is the case in several CNS disorders (Ross and Sachdev, 2004). In healthy samples less dramatically elevated choline levels may reflect more active myelin turnover (Jung et al., 2005; Ryman et al., 2011) which could result in less efficient white matter functioning and, therefore, reduced connectivity within the specified region and between it and other regions. This interpretation may explain findings of less anisotropic white matter microstructure in dyslexic individuals in a nearby region of the tempero-parietal lobe (Beaulieu et al., 2005; Klingberg et al., 2000; Niogi and McCandliss, 2006).
It is not possible within the scope of this study to test the precise reasons why metabolite concentration was related to reading or phonological abilities. White matter systems are dynamic and white matter development occurs into adulthood (Qiu et al., 2008). One report demonstrated increases in white matter microstructure integrity for poor readers following intensive remediation (Keller and Just, 2009). Therefore, future research examining metabolite concentrations at various ages and before/after remediation would be useful to provide evidence 1) whether the relationship between choline concentration and reading/phonological skills is present throughout development and 2) of how metabolite concentration and the relationship between metabolite concentrations and reading/phonological skills may change depending on development or improvement of skills.
We did not find a relationship between choline concentration and reading comprehension scores. Reading comprehension is a high-level process involving reasoning, memory and, particularly, executive function (Sesma et al., 2009) in addition to basic reading component skills. Therefore, reading comprehension may be more closely related to neurobiological structure or function of regions outside the angular region or the relationship between reading comprehension and neurobiology may be distributed across multiple regions. Furthermore, we only included one measure of reading comprehension, the Nelson-Denny Test, because standardized measures of reading comprehension for college-age individuals are very limited. It is possible that the Nelson-Denny Test was not sensitive enough to reveal underlying relationships with metabolite concentration.
The results of the present study do not seem to correspond with the findings of Rae et al. (Rae et al., 1998) who reported decreased choline in the temporo-parietal region for reading impaired individuals. In fact, we report a relationship in the opposite direction – choline concentrations are increased in individuals who scored lower on reading or phonological measures. This may be due to several methodological differences between the two studies including metabolite ratio calculations, localization and sampling frame. Rae et al., used NAA as a baseline and reported levels of choline/NAA. It is possible that their reported decrease in choline was actually an increase in NAA for the dyslexic group. This result may seem odd given the various reports indicating structural abnormalities in this region, but it could reflect an overall metabolic difference present in the dyslexic group or an interaction between NAA and choline. NAA is known to vary with general cognitive skill (Jung et al., 2005), and creatine is reported to be a more stable baseline (Yiannoutsos et al., 2008). Furthermore, Rae et al’s sample was limited to male participants, and the reading impaired individuals were identified based on earlier diagnosis. It is not clear whether all individuals were still experiencing reading and/or phonological difficulties (although the authors do report that the group mean on a phonological decoding test was lower for the reading impaired group). To facilitate comparison of our results with the report of Rae et al. we conducted a further analysis examining correlations with choline/NAA and found no significant relationships (all p>0.10). Creatine seems to be the preferred metabolite to use for normalization, but it is possible that the present results indicate that a decrease in creatine or an interaction between creatine and other metabolites was driving the negative correlations we report. This seems unlikely in light of previous reports indicating stable levels of creatine with disease progression (Yiannoutsos et al., 2008). Moreover, absolute levels of creatine did not correlate significantly with any of the cognitive variables reported here (all p>0.10, results not shown). Normalization of metabolites to creatine is limits our interpretations and future studies measuring absolute metabolite concentrations relative to water signal would be valuable.
Our sample size (17 females and 14 males) did not provide adequate power to examine sex-based subgroups. Instead we report correlations of metabolite concentration and cognitive variables with gender. NAA concentration and general cognitive ability were significantly correlated with gender, suggesting higher NAA concentration and higher cognitive ability for males. Given our small sample these differences may be due to sampling bias. Gender was not significantly correlated with choline concentration or reading/phonological measures thus it was not included in the regression analyses.
The significant negative relationships between reading measures (sight word reading and reading comprehension), general intelligence and age/education level were unexpected. It is possible that the younger individuals, who were still attending college level classes, had an advantage on our tests of reading and general intelligence because they were more accustom to taking regular tests/exams when compared to the older individuals who had already graduated from college. In addition, these results may be due to sampling bias. We did note that more of the older individuals reported current/past reading problems and/or a diagnosis of reading disorder.
Limitations of available technology and time constraints prevented us from examining regions outside the left hemisphere angular region, but future studies examining multiple brain regions, utilizing chemical shift imaging, would be ideal. It is possible that relationships between metabolite concentration and reading components including phonological decoding and sight word reading are present in multiple regions of the brain and the reading components that most strongly relate to metabolite concentration are likely to vary according to region. Additionally, hemispheric differences in metabolite concentration may exist and they may vary with reading and/or phonological skills. Due to the cubic nature of the ROI it likely included parts of the supramarginal gyrus and posterior superior temporal gyri, thus we acknowledge that our results may reflect contributions of these neighboring structures, which are also related to reading/phonological skill (Church et al., 2008; Graves et al., 2008; Lee et al., 2007). The size of the ROI was rather large (20 mm3, also limited by technology) and therefore included both gray and white matter, but this relatively wide scope of tissue types does not preclude specificity of interpretation. Specific neurometabolites are able to describe integrity or functionality (i.e. viability) of gray as opposed to white matter. For example, NAA is specifically related to intact neuronal functionality and choline is specifically related to membrane synthesis and breakdown. Combining MRS with white matter diffusion tensor studies as well as structural MRI studies measuring cortical thickness would be necessary to fully describe the altered brain structure that underlies skilled and disabled reading.
5. Conclusions
This study presents the first evidence that choline concentration in the left hemisphere angular region may be related to phonological skills independently of other reading skills, general cognitive ability, and age. The study population included adults with a range of skill levels, including some individuals with reading disability. MRS techniques have great potential in the study of reading disability and the field of cognitive neuroscience in general. Our basic research findings provide a theoretical mechanism, which may be useful for applied investigations of reading/phonological disorders or aid in the design and assessment of treatments for such disorders.
Table 2.
Spearman’s Rho Correlation coefficients of metabolite concentration to reading component scores
| Cho/Cr | NAA/Cr | mIns/Cr | NAA/Cho | General Cog | Sight Word | Phono Decoding | ND Comp | Sex | Age | |
|---|---|---|---|---|---|---|---|---|---|---|
| NAA/Cr | −0.19 | |||||||||
| mIns/Cr | 0.43* | −0.26 | ||||||||
| NAA/Cho | 0.67** | −0.83** | 0.42* | |||||||
| General Cog | −0.11 | 0.24 | −0.11 | −0.18 | ||||||
| Sight Word | −0.34 | 0.04 | −0.12 | −0.17 | 0.54** | |||||
| Phono Decoding | −0.38* | 0.03 | −0.07 | −0.19 | 0.39* | 0.87** | ||||
| ND Comp | −0.27 | −0.09 | −0.21 | −0.03 | 0.47** | 0.78** | 0.54** | |||
| Sex | 0.19 | 0.33 | −0.23 | −0.12 | 0.49** | −0.01 | −0.09 | −0.21 | ||
| Age | 0.16 | −0.11 | 0.27 | 0.13 | −0.48** | −0.52** | −0.30 | −0.79** | 0.11 | |
| Education | 0.19 | −0.12 | 0.24 | 0.15 | −0.43* | −0.47** | −0.24 | −0.69** | 0.07 | 0.89** |
Cho/cr = ratio of choling/creatine; mIns/Cr = ratio of myoinositol/creatine; Naa/Cr = ratio of N-acetyl aspertate/creatine Naa/Cho = ratio of N-acetyl aspartate/creatine. General cog = general cognitive ability estimate, Sight word = sight word reading composite, Phono Decoding = phonological decoding composite, ND Comp= Nelson-Denny Test of Reading Comprehension.
p < 0.05, two tailed
p < 0.01, two tailed
p =.058, two tailed and p<.05, two tailed Spearman correlation.
Table 3b.
Multiple regression analysis using three blocks to address the specificity of phonological decoding to choline/creatine concentration, controlling for age, cognitive ability estimate, and sight word reading.
| Model | Change Statistics |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| R | R2 | Adjusted R2 | SE of the Estimate | R2 Change | F Change | df1 | df2 | Sig. F Change | |
| 1 | .455a | .207 | .151 | .901 | .207 | 3.663 | 2 | 28 | .039 |
| 2 | .918b | .843 | .826 | .408 | .636 | 109.609 | 1 | 27 | .000 |
| 3 | .933c | .870 | .850 | .379 | .027 | 5.320 | 1 | 26 | .029 |
Predictors: (Constant), age, cognitive ability
Predictors: (Constant), age, cognitive ability, sight word composite
Predictors: (Constant), age, cognitive ability, sight word composite, normalized choline
Highlights.
Primary evidence relating specific reading processes to neuro-metabolites.
Magnetic resonance spectroscopy measured neuro-metabolites in angular gyrs.
Negative relationship between phonological decoding and choline was revealed.
Relationship between phonological decoding and choline concentration unique.
Results may have implications for study/tretment of reading disability.
Acknowledgments
This research was supported by grant number HD 29891 from the National Institute of Child Health and Human Development to FM. The authors express gratitude to the individuals who participated in this research and to Allison Zumberge-Orechwa, JC Zhuang and Jason Goldman for their assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, Phillips L, 2005. Imaging brain connectivity in children with diverse reading ability. Neuroimage 25, 1266–1271. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Deutsch GK, Wandell BA, 2011. The development of cortical sensitivity to visual word forms. Journal of Cognitive Neuroscience 23, 2387–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E, 2005. Some neurophysiological constraints on models of word naming. Neuroimage 27, 677–693. [DOI] [PubMed] [Google Scholar]
- Brady SA (Ed.), 1997. Ability to encode phonological representations: An underlying difficulty of poor readers. Lawrence Erlbaum Associates, Hillsdale. [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D, 1993. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Developmental Neuroscience 15, 289–298. [DOI] [PubMed] [Google Scholar]
- Brown JI, Fishco VV, Hanna G, 1993. Nelson-Denny Reading Test forms G and H. Riverside Publishing, Itasca, Ill. [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estevez A, Lozano A, Devlin JT, Price CJ, 2009. An anatomical signature for literacy. Nature 461, 983–986. [DOI] [PubMed] [Google Scholar]
- Catani M, Cherubini A, Howard R, Tarducci R, Pelliccioli GP, Piccirilli M, Gobbi G, Senin U, Mecocci P, 2001. (1)H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport 12, 2315–2317. [DOI] [PubMed] [Google Scholar]
- Christiansen P, Henriksen O, Stubgaard M, Gideon P, Larsson HB, 1993. In vivo quantification of brain metabolites by 1H-MRS using water as an internal reference. Magnetic Resonance Imaging 11, 107–118. [DOI] [PubMed] [Google Scholar]
- Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL, 2008. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cerebral Cortex 18, 2054–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Damasio H, 1983. The anatomic basis of pure alexia. Neurology 33, 1573–1583. [DOI] [PubMed] [Google Scholar]
- Dejerine, 1891. Sur un cas de cectie verbale avec agraphie, suivi d’autopsie. CR Societe du Biologie 43, 197–201. [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, J, 2003. Anatomical correlates of dyslexia: frontal and cerebellar findings. Brain 126, 482–494. [DOI] [PubMed] [Google Scholar]
- Geschwind N, 1965. Disconnexion syndromes in animals and man. Brain 88, 237–294. [DOI] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, Gupta P, 2008. The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. Journal of Cognitive Neuroscience 20, 1698–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT, 2006. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca’s area varies with reading ability. Neuroimage 31, 513–519. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Ueno T, Reiss AL, Meyler A, Whitfield-Gabrieli S, Glover GH, Keller TA, Kobayashi N, Mazaika P, Jo B, Just MA, Gabrieli JD, 2007. Prediction of children’s reading skills using behavioral, functional, and structural neuroimaging measures. Behavioral Neuroscience 121, 602–613. [DOI] [PubMed] [Google Scholar]
- Jung RE, Gasparovic C, Chavez RS, Caprihan A, Barrow R, Yeo RA, 2009a. Imaging intelligence with proton magnetic resonance spectroscopy. Intelligence 37, 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Gasparovic C, Chavez RS, Flores RA, Smith SM, Caprihan A, Yeo RA, 2009b. Biochemical support for the “threshold” theory of creativity: a magnetic resonance spectroscopy study. The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 5319–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ, Yeo RA, Rowland LM, Petropoulos H, Levine AS, Sibbitt WL, Brooks WM, 2005. Sex differences in N-acetylaspartate correlates of general intelligence: an 1H-MRS study of normal human brain. Neuroimage 26, 965–972. [DOI] [PubMed] [Google Scholar]
- Keller TA, Just MA, 2009. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron 64, 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA, 2000. Microstructure of Temporo-Parietal White Matter as a Basis for Reading Ability. Neuron 25, 493–500. [DOI] [PubMed] [Google Scholar]
- Lee H, Devlin JT, Shakeshaft C, Stewart LH, Brennan A, Glensman J, Pitcher K, Crinion J, Mechelli A, Frackowiak RS, Green DW, Price CJ, 2007. Anatomical traces of vocabulary acquisition in the adolescent brain. The Journal of neuroscience 27, 1184–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett J, Ross B, Arun P, Madhavarao C, Namboodiri M, 2007. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Progress in Neurobiology 81, 89–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi SN, McCandliss BD, 2006. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia 44, 2178–2188. [DOI] [PubMed] [Google Scholar]
- Oldfield RC, 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Padma M, Adineh M, Pugar K, Mukherjee J, Satter M, Shi B, Dunigan K, Bidwell K, Ezzeddine B, Mantil J, 2005. Functional imaging of a large demyelinating lesion. Journal of Clinical Neuroscience 12, 176–178. [DOI] [PubMed] [Google Scholar]
- Price CJ, 2000. The anatomy of language: contributions from functional neuroimaging. Journal of Anatomy 197, 335–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA, 2001. Neurobiological studies of reading and reading disability. Journal of Communication Disorders 34, 479–492. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Shaywitz BA, Shaywitz SE, Fulbright RK, Constable RT, Skudlarski P, Marchione KE, Jenner AR, Fletcher JM, Liberman AM, Shankweiler DP, Katz L, Lacadie C, Gore JC, 2000. The angular gyrus in developmental dyslexia: task-specific differences in functional connectivity within posterior cortex. Psychological Science 11, 51–56. [DOI] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Zhou K, Khong PL, 2008. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage 41, 223–232. [DOI] [PubMed] [Google Scholar]
- Rae C, Lee MA, Dixon RM, Blamire AM, Thompson CH, Styles P, Talcott J, Richardson AJ, Stein JF, 1998. Metabolic abnormalities in developmental dyslexia detected by 1H magnetic resonance spectroscopy. Lancet 351, 1849–1852. [DOI] [PubMed] [Google Scholar]
- Ross AJ, Sachdev PS, 2004. Magnetic resonance spectroscopy in cognitive research. Brain Research Reviews 44, 83–102. [DOI] [PubMed] [Google Scholar]
- Ryman SG, Gasparovic C, Bedrick EJ, Flores RA, Marshall AN, Jung RE, 2011. Brain biochemistry and personality: a magnetic resonance spectroscopy study. PloS one 6, e26758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz E, Maurer U, van der Mark S, Bucher K, Brem S, Martin E, Brandeis D, 2008. Impaired semantic processing during sentence reading in children with dyslexia: combined fMRI and ERP evidence. Neuroimage 41, 153–168. [DOI] [PubMed] [Google Scholar]
- Sesma HW, Mahone EM, Levine T, Eason SH, Cutting LE, 2009. The contribution of executive skills to reading comprehension. Child Neuropsychology 15, 232–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Pugh KR, Holahan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC, 2003. Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biological Psychiatry 54, 25–33. [DOI] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet JF, Fazio F, Perani D, Price C, Frith CD, Paulesu E, 2005. Brain abnormalities underlying altered activation in dyslexia: a voxel based morphometry study. Brain 128, 2453–2461. [DOI] [PubMed] [Google Scholar]
- Snowling MJ, 2000. Dyslexia. Blackwell, Malden. [Google Scholar]
- Tartaglia MC, Narayanan S, De Stefano N, Arnaoutelis R, Antel SB, Francis SJ, Santos AC, Lapierre Y, Arnold DL, 2002. Choline is increased in pre-lesional normal appearing white matter in multiple sclerosis. Journal of Neurology 249, 1382–1390. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA, 1999. TOWRE: Test of Word Reading Efficiency. Pro-Ed, Austin. [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM, 2004. Specific reading disability (dyslexia): what have we learned in the past four decades? Journal of Child Psychology and Psychiatry, and Allied Disciplines 45, 2–40. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N, 2001. Woodcock-Johnson Revised Tests of Cognitive Ability - III. Riverside Publishing, Chicago. [Google Scholar]
- Yiannoutsos CT, Nakas CT, Navia BA, 2008. Assessing multiple-group diagnostic problems with multi-dimensional receiver operating characteristic surfaces: application to proton MR Spectroscopy (MRS) in HIV-related neurological injury. Neuroimage 40, 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]