Abstract
Background
Current World Health Organization (WHO) guidelines recommend annual mass drug administration using praziquantel in areas with high schistosome endemicity. Yet little is known about incidence and reinfection rates after treatment in women with frequent exposure to schistosomes. We sought to quantify response to anti-schistosome treatment and incident S. mansoni infections in a cohort of rural women living in a schistosome-endemic area of northwest Tanzania.
Methods and principal findings
We enrolled women with and without S. mansoni infection into a 12-month longitudinal cohort. Every 3 months, women were tested for schistosome infection using microscopic examinations for ova on filtered urine, Kato Katz slides, and serum Circulating Anodic Antigen (CAA). Those with schistosome infection received treatment with praziquantel 40 mg/kg according to the standard of care.
We studied 35 women who were S. mansoni positive by stool microscopy and 46 women without schistosome infection who returned for at least one follow-up. Of the women who were initially infected, 14 (40%) were schistosome-positive at a follow-up visit. Four women developed incident infections, for a cumulative incidence of 8.7% and incidence rate of 0.99 per 100 person-months throughout the year among initially uninfected women. Only 3 women were egg-positive at any follow-up.
Women with persistent, recurrent, or incident infection during the study period were significantly younger (p = 0.032) and had fewer children than women who remained uninfected or those who cleared the infection and did not experience recurrence (p = 0.003). Having fewer children remained significant after controlling for age (p = 0.023). There was no difference in initial intensity of infection by CAA or stool egg count, HIV status, or socioeconomic status. Although most water contact behaviors were comparable between the two groups, women with recurrent or incident schistosome infections were significantly more likely to have recently swum in the lake (p = 0.023).
Conclusions
Our data suggests that annual praziquantel treatment reduces intensity of schistosome infections but is insufficient in providing stable parasite eradication in over a third of women in endemic communities. Furthermore, microscopy lacks adequate sensitivity to evaluate efficacy of treatment in this population. Our work demonstrates that further investigation into treatment efficacy and reinfection rates is warranted and suggests that increased frequency of praziquantel treatment is needed to improve cure rates in high-risk populations.
Author summary
Schistosomiasis is a parasitic infection transmitted through contaminated water that primarily affects the gastrointestinal and urogenital tracts. Previous studies in Tanzania have shown that adult women infected with schistosomes also have a higher risk of contracting HIV. Although it is recommended that people living in areas where they are exposed to schistosomes be treated with praziquantel once a year, the rate of new infections or reinfection after treatment in adult women is not known. We followed a group of schistosome-infected women and an uninfected control group for 12 months. They were tested for schistosomes every 3 months, and treated with praziquantel if they were infected. Over 40% of the women tested positive for schistosome infection at some point during the follow-up period, and the majority of them were from the group that was infected at the beginning of the study. These women may not have fully cleared the infection after one treatment, or they may be more susceptible to reinfection due to variations in their immune systems. Further studies are recommended to investigate whether a higher frequency of treatment is needed to control schistosome infection in adult women, especially given that reducing schistosome infection may help to reduce HIV risk in populations similar to ours.
Introduction
Schistosomiasis is a water-borne helminthic infection, most prominently affecting the urogenital and gastrointestinal tracts, that causes disease in over 200 million people worldwide [1]. Schistosomes have a complex life cycle that includes freshwater snails as intermediate hosts, from which infective cercariae are released into fresh water and can penetrate unbroken skin. The disease is highly prevalent in tropical climates where the parasite is common and access to clean water is limited. Over 90% of people with schistosome infections live in Africa [1], and the Lake Victoria region in Tanzania has one of the highest prevalences of schistosome infections in the world [2,3].
World Health Organization guidelines recommend annual mass treatment with praziquantel 40 mg/kg in schistosome-endemic regions [4]. Although recent studies have suggested higher cure rates with repeated dosing or single doses of 60 mg/kg, a single dose of 40 mg/kg remains the mainstay of most schistosome control programmes [5] and is the standard of care in Tanzania. Moreover, these recommendations are largely based on studies in school-age or younger children, and little is known about incidence and reinfection rates after treatment in adults. In rural communities where women’s responsibilities to collect water, cook and wash cause them to contact contaminated water on a daily basis, more than half of women have schistosome infections [3,6,7]. Furthermore, recent studies in Tanzania and Zambia have shown that infection with Schistosoma mansoni increases susceptibility to HIV infection in women, but not in men, and raises HIV-1 RNA viral load set point in those who become infected [8,9]. If incidence and reinfection rates among women are high, then recommendations for yearly anti-schistosome treatment may need to be increased. This could serve not only to decrease the morbidity and mortality related to schistosome infection itself and to improve population-level control of schistosomiasis, but additionally to decrease the HIV risk that accompanies schistosome infection in women.
We hypothesized that the overall incidence of schistosome infections in reproductive-aged women would be at least 6% at 6 months and 12% over the course of the year, including women who were schistosome-infected at baseline and those who were not. We based these hypotheses on 10 years of clinical experience in this region. We also predicted that at least 20% of women treated for schistosomiasis would show evidence of incomplete clearance with standard of care single-dose praziquantel [10,11]. Secondary hypotheses were that incident schistosome infections would be associated with age, water security, and the intensity of initial schistosome infection. Our overall objective was to determine the clearance, recurrence, and incidence of S. mansoni infection in a population of women of childbearing age living in rural northwest Tanzania in the year after treatment with praziquantel.
Materials and methods
Ethics
Women provided written informed consent, obtained by the nurse in a private setting in Kiswahili, for study participation. All participants were adults. All treatment was provided free of charge. Any woman who tested positive for HIV on an initial point-of-care test (SD Bioline, Standard Diagnostics, Inc., Korea) underwent confirmatory testing using a second point-of-care test (Uni-gold, Trinity Biotech, Wicklow, Ireland) in accordance with Tanzanian national guidelines. HIV test results were provided on the day of screening, and any woman with a new diagnosis of HIV was counselled and provided with a referral letter to obtain free treatment at a nearby HIV care and treatment center.
Permission for the conduct of this study was obtained from the joint research ethics committee of Bugando Medical Centre/the Catholic University for Health and Allied Sciences in Mwanza, Tanzania (CREC/171/2017), from the National Institute for Medical Research in Dar es Salaam (NIMR/HQ/R.8a/Vol.IX/2446), and from Weill Cornell Medicine in New York (1612017800).
Study population and design
We conducted a longitudinal study of women with and without S. mansoni infection in villages in rural Tanzania in which the prevalence of S. mansoni infection in adults is known to be approximately 40% and the prevalence of S. haematobium infection is < 2% [8]. Enrollment was conducted between June and November 2017 in the schistosome-endemic villages of Kisesa, Lumeji, Welamasonga, and Kayenze. Women of reproductive age living in these villages were invited to receive free screening for schistosomiasis via urine, stool, and blood tests. Women who had schistosome infections were treated with praziquantel free of charge, and all women were asked to return after 3, 6, 9, and 12 months for repeat testing and examinations.
This analysis was conducted as part of a larger cohort study to characterize alterations induced by S. mansoni infection in the female genital tract and to determine the effect of anti-schistosome praziquantel treatment on these alterations. In order to study the dynamics of S. mansoni infection and praziquantel treatment, we restricted our analysis to women who returned for at least one of the four follow-up visits.
Sample collection and laboratory procedures
At screening, women provided urine, stool, and blood to be tested for schistosome infection. Five Kato Katz slides were prepared from each stool sample for examination for S. mansoni ova, a technique that maximizes sensitivity without the need for stool examinations on multiple days [12]. 10 mL of urine was filtered from each sample and examined microscopically in the field for S. haematobium ova by trained parasitologists.
A three milliliter sample of blood was also collected for quantification of serum CAA, which was performed using up-converting phosphor technology at the Tanzanian National Institute for Medical Research laboratory in Mwanza as previously described [13]. We defined a negative CAA value as < 30 pg/mL [13].
Women were given cards with a follow-up date within 1–2 weeks to receive their urine, stool, and serum CAA results verbally and in writing, and to receive directly observed treatment with praziquantel if any test was positive. At that time, we invited those who were positive for S. mansoni infection to participate in our cohort. Although we expected Kato Katz sensitivity to be lower than CAA testing in these women [14], we required stool eggs in order to confirm the species of schistosome. For every positive woman invited to participate, we also invited one uninfected woman to participate who presented for care in the same village on the same day. Women who consented to participate completed a structured interview involving demographic and clinical information, which was administered by the study nurse, and underwent additional testing for the larger study.
Each study participant provided 1–2 phone numbers, which were used by our study nurses to maintain contact with them for the duration of the study. Participants were asked to return for follow-up appointments at 3-month intervals for one year. At each follow-up, women again provided urine, stool, and blood samples for testing following the procedures described above. At the 6-month follow-up, women completed the survey administered at enrollment for a second time. At the 12-month follow-up, women completed the enrollment survey and also answered more detailed questions about water sources and exposure to lake water. All women who tested positive for schistosomiasis by urine, stool or serum CAA at any follow-up appointment were contacted by a nurse to return to clinic in order to receive directly observed treatment with praziquantel.
Statistical analysis
Data was analyzed using Stata Version 13 (College Station, Texas). Demographic and clinical characteristics were quantified as number (percent) or median [interquartile range, or IQR] and compared using Wilcoxon rank-sum test for continuous variables, and chi-squared test for categorical variables. Fisher’s Exact test was used for categorical variables for which any category had less than 5 people. For factors that were significantly associated with recurrent or persistent schistosome infection using these initial tests, a multivariable logistic regression analysis was performed to determine which factors remained significantly associated with recurrent or persistent schistosome infection. Population at risk was defined as women non-infected with schistosomes. Cumulative incidence was calculated as the number of new cases divided by the total population at risk, and incidence rate in person-months was calculated as the number of new cases divided by the total months at-risk.
Results
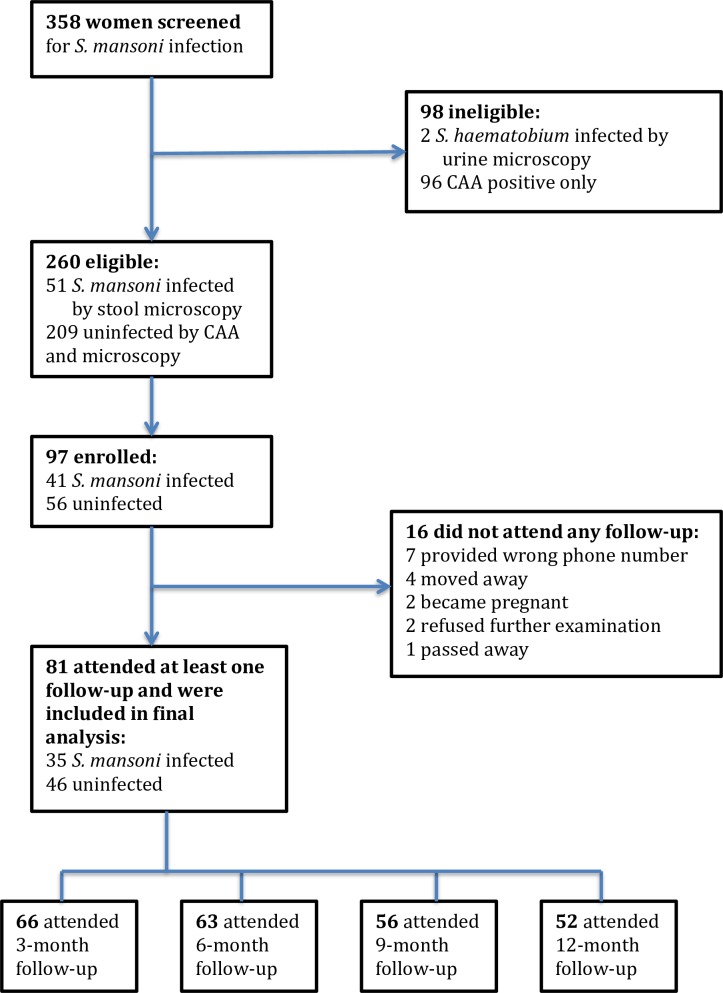
We screened a total of 358 women for schistosome infections by urine and stool microscopy and serum CAA testing. Of these, 51 women (14.2%) had S. mansoni eggs detected in stool and 2 women (0.5%) had S. haematobium eggs detected in urine (Fig 1). Serum CAA was positive in 141 women (39.4%).
Fig 1. Flow chart of study screening and enrollment.
41 of the 51 women who had S. mansoni eggs detected in stool returned to receive results and provided written informed consent to participate in the cohort study, along with an additional 56 women who came to receive their results on the same day and were completely negative for schistosome infection. Of these 97 women, 16 were not able to return for their first follow-up visit, as described in Fig 1. Ultimately, we analyzed data from 81 women who remained eligible for the larger study and returned for at least one follow-up visit: 35 with confirmed S. mansoni infection and 46 confirmed to be negative for schistosome infection.
Among the 35 women with S. mansoni infection, 31 were both CAA and stool microscopy positive and 4 were stool microscopy positive alone. The median stool eggs per gram was 24 [9.6–57.6] and the median CAA value was 1527.1 [216.2–10,000] pg/mL. At baseline, women with S. mansoni infection were younger at first pregnancy compared to uninfected women (p = 0.002). 3 women (8.6%) in the S. mansoni infected group, and 7 women (15.2%) in the uninfected group, were HIV-infected (p = 0.502) as expected due to decreased sensitivity of egg excretion in women with HIV [14]. There was also a trend towards more women with S. mansoni infection missing meals due to food insufficiency (66% versus 46%, p = 0.086). No other significant demographic or clinical differences were found between the two groups (Table 1).
Table 1. Demographic and clinical characteristics of women with and without S. mansoni infection at enrollment.
| Characteristic (Median [IQR] or Number (Percent)) |
S. mansoni -infected (n = 35) |
Schistosome-uninfected (n = 46) | p-value |
|---|---|---|---|
| Age in years | 29 [23–38] | 34 [27–42] | 0.13 |
| Years of schooling | 7 [2–7] | 7 [4–7] | 0.43 |
| Number of children | 3 [1–5] | 4 [2–6] | 0.16 |
| Age at first pregnancy | 18 [16–19] | 19 [18–20] | 0.007 |
| Admits missing meals due to insufficient food in household in past month | 23 (66) | 21 (46) | 0.089 |
| HIV infected | 3 (8.6) | 7 (15) | 0.50 |
| Reports ever being treated for schistosomiasis prior to study enrollment | 6 (17) | 15 (33) | 0.12 |
| Serum schistosome circulating anodic antigen (pg/ml)* | 1527.1 [216.2–10,000] | 3.45 [0.0–8.5] | N/A** |
| S. mansoni ova per 1 gram stool | 24 [9.6–57.6] | 0 [0–0] | N/A** |
* CAA < 30 pg/mL is negative.
** P-value is not applicable since these were criteria used to define the study groups.
Fig 2 shows the number of women from each group (S. mansoni-infected or uninfected at enrollment) who were seen at each follow-up time point and the proportion who were schistosome-infected at that visit. 14 of the 81 women included in analysis were lost to follow-up at some point during the year. Of these, 4 did not want additional gynecologic examinations, 5 lost their phones and were no longer reachable, and 2 had family members who prohibited them from returning.
Fig 2. Number of women from each group who were seen at each follow-up time point, and their infection status.
Blue bars represent women who were S. mansoni-infected at enrollment. Yellow bars represent women who were uninfected at enrollment. Darker color indicates the proportion of women who were S. mansoni-infected at the time point shown.
In total, 14 of the 35 women who had S. mansoni infections at baseline (40%) had infection again at some point during the 12-month follow-up period. The other 21 women (60%) cleared the infection after one treatment and did not become reinfected. Of the women who were S. mansoni-infected at baseline, 3 cleared the infection and then developed recurrent infection. 3 cleared the S. mansoni after multiple treatments, while 8 remained infected throughout the study period. Four women who were uninfected at baseline had developed incident S. mansoni infection by the 12-month follow-up, for a cumulative incidence of 8.7% and an incidence rate of 0.985 per 100 person-months among initially uninfected women throughout the entire study period.
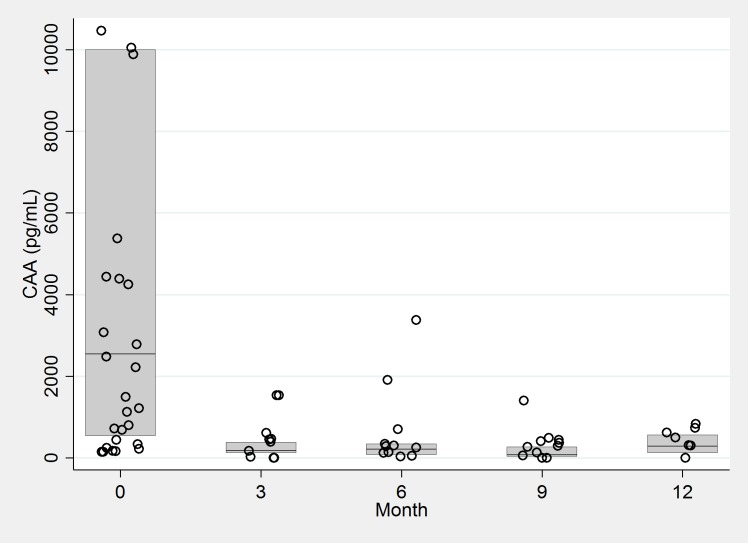
The majority of infections throughout the follow-up period were identified by elevated CAA levels only (CAA ≥ 30 pg/ml), with only 3 women found to be excreting eggs at any follow-up time point and an egg reduction rate of 98% from Month 0 to Month 3 [15]. The median CAA values at follow-up among those who were still CAA positive were significantly lower than the baseline CAA values at each subsequent time point (p = 0.001, Fig 3). No women were found to be excreting S. haematobium eggs in urine at any follow-up time point.
Fig 3. CAA values in women with Schistosoma mansoni infection at each time point.
6 data points with values > 12,000, all occurring at Month 0, were included in the derivation of this box plot but are not shown.
When compared with women who were schistosome-negative throughout all follow-ups, women with recurrent or incident infection during the study period were significantly younger (p = 0.032). They also had fewer children than women who remained uninfected (p = 0.003). Number of children remained significantly lower in women with schistosomiasis when controlling for age (p = 0.023). 8 out of 65 of the women without recurrent or incident infection (12.3%), and 2 out of 16 of those with recurrent or incident infection (12.5%) were HIV-infected (p = 1.00). No other significant demographic or clinical differences were found (Table 2).
Table 2. Demographic and clinical characteristics of women with and without recurrent or incident S. mansoni infection at any follow-up.
| Characteristic (Median [IQR] or Number (Percent)) |
Recurrent* or incident infection at any follow-up (n = 16) | Schistosome-uninfected at all follow-ups (n = 65) |
p-value |
|---|---|---|---|
| Age in years | 27 [22–33] | 33 [27–41] | 0.032 |
| Years of schooling | 7 [1.5–7] | 7 [4–7] | 0.37 |
| Number of children | 1.5 [1–3.5] | 4 [2–6] | 0.003 |
| Age at first pregnancy | 17 [17–19] | 18 [18–20] | 0.13 |
| Admits missing meals due to insufficient food in household in past month | 10 (62) | 34 (53) | 0.51 |
| HIV-infected | 2 (12.5) | 8 (12.3) | 1.00 |
| Reports ever being treated for schistosomiasis prior to study enrollment | 4 (25.0) | 17 (26.0) | 0.92 |
*Recurrent infection was defined as the detection of eggs or serum CAA ≥ 30 pg/ml in a person who was previously schistosome-positive but most recently had been negative by both serum CAA and microscopy.
There was no significant difference in initial CAA level between women who were S. mansoni positive and successfully treated (1308 [161–10,000] pg/mL) compared to those with recurrent or persistent infection (1809 [586–7840], p = 0.587). Similarly, no difference was seen in the initial number of S. mansoni eggs per gram of stool in women who were successfully treated (24 [5–58]), compared to those with recurrent or persistent infection (34 [19–81], p = 0.423).
At enrollment, women with S. mansoni infection were significantly more likely to report the lake as their primary water source (20.0% versus 0.0%, p = 0.002), while women without schistosome infection were more likely to report piped water as their primary water source (17.1% versus 37.0%, p = 0.046). However, no difference in primary water source was seen when comparing women with recurrent or incident schistosome infection during the study to women who remained schistosome-negative throughout follow-up; lake water was used by 12.5% of women in the infected group and 7.7% of the schistosome-negative group (p = 0.620). Furthermore, no differences were noted between the groups in distance to primary water source (median of 30 [10–45] minutes walking in both groups) or number of water collection trips per week (median of 7 [7–14]).
A detailed survey about water contact was administered to women who were seen at the 12-month follow-up (n = 45). Women with recurrent or incident schistosomiasis were more likely to obtain drinking water from a well, while women who remained schistosome-negative were more likely to obtain drinking water from a pipe (p = 0.028). No difference was found in water sources used for bathing, cooking, washing clothes, or farming. Women were also asked whether they ever used lake water for any of these activities, and no significant difference was seen between the two groups in response to these questions (S1 Table).
Under 10% of women in each group reported fishing in the lake, and some also reported going into the lake for travel or fetching water in addition to the activities mentioned above. Women with recurrent or incident schistosomiasis were more likely to have gone swimming in the past 3 months (p = 0.023). Women in both groups lived a median of a 30 minute walking distance from the lake. Participants were also asked whether they were aware of the symptoms of schistosomiasis. 76% of responses correctly included “urinating blood”, “painful urination” and/or “pain in the stomach”, while 21% responded that they did not know. When asked whether they knew how to prevent schistosomiasis, 58% of respondents mentioned avoidance of drinking and bathing in dirty or standing water, wearing shoes or boots to enter the water, and boiling water before use. An incorrect response was given by 13%, and 29% responded that they did not know. There was no difference in correct knowledge of prevention strategies between women in either initial group (p = 0.406) or between women with recurrent or incident infection versus those who remained uninfected (p = 0.499).
Discussion
Annual mass treatment of school-aged children with praziquantel is the current mainstay of schistosomiasis control. It has been hypothesized that protective immunity against schistosomes may develop over time, leading to a decreased prevalence and intensity of infection in adulthood despite continued exposure [16,17]. However, estimated rates of recurrence or persistence of infection after praziquantel treatment vary widely by region and population. By following up women with S. mansoni at 3-month intervals post-treatment, our study shows that an annual single-dose treatment with praziquantel is insufficient to clear the infection for as many as 40% of infected adult women living in this heavily endemic area.
In our cohort, the majority of women who were found to be schistosome-positive at follow-ups were women who were treated multiple times and were repeatedly CAA-positive. In these women the intensity of infection decreased after treatment, as evidenced by elimination of egg excretion and significant decreases in serum CAA, but CAA level remained above the threshold of positivity. This could have had several causes. First, praziquantel has decreased activity against juvenile worms [18] and therefore could lead to incomplete cure in recently infected people who harbored juvenile worms at the time of praziquantel treatment. Second, given that CAA becomes detectable 3–4 weeks post-infection [19], we were unable to determine whether women cleared infection but became re-infected, or whether the infection never cleared during the 3-month period between successive CAA measurements. Third, these findings could reflect technical or administrative imperfections or the presence of single-sex or non-egg producing infections [20].
Finally, it is possible that our findings could reflect incomplete clearance of infections, whether due to some level of resistance to praziquantel in schistosomes, or to varying pharmacokinetics of praziquantel or its synergy with host immunity in different persons [18]. Patterns of reduced treatment efficacy against S. mansoni have been observed in areas with heavy use of praziquantel. In Senegal, cure rates as low as 18–38% were seen with single-dose praziquantel, while treatment with oxamniquine yielded normal cure rates, leading to concern for developing drug resistance [21]. Reduced egg reduction rates have also been observed in Ugandan populations following multiple rounds of mass treatment [22]. In Egypt, S. mansoni isolated from patients who repeatedly failed treatment showed 3- to 5-fold lower sensitivity to praziquantel [23]. Although parasite resistance to praziquantel has not been demonstrated in Tanzania, we cannot rule this out as a contributing factor to our results.
Alternatively, the women who were repeatedly schistosome-positive may be more susceptible to reinfection than others for reasons related to individual immune response [24]. The women in our cohort who were persistently infected or reinfected were younger than those who were successfully treated. While this could be because younger women had more frequent or intense contact with contaminated water that was not captured by our survey, younger women also may have a less developed immune response due to less lifetime exposure to the parasite. Repeated treatment with praziquantel may further contribute to the development of immunity by increasing exposure to antigens from dying adult schistosome worms [25,26]. In our cohort, a similar proportion of women in each group reported previous treatment for schistosomiasis before the study, but we did not inquire how many times they had been treated. In contrast to some other reports, we did not find that intensity of initial infection by CAA or number of S. mansoni ova per gram, socioeconomic factors, or HIV infection were associated with treatment failure or increased risk of re-infection [27–29].
Only two women were found to be egg-positive after treatment at any follow-up. This could support the idea that the infections detected by CAA only were new infections rather than the result of unsuccessful treatment, since eggs would only be seen with an infection established for longer than about 6 weeks [30]. However, treatment with praziquantel is known to reduce schistosome egg production even in cases where it does not lead to cure [31]. Our results suggest that relying on microscopic examination for ova in this setting may lead us to overestimate the efficacy of praziquantel treatment in women, particularly given the documented sex differences in egg-to-CAA ratios between men and women [14].
In terms of parasite exposure during the study period, the majority of water-related risk factors were the same between the two groups, with the exception of swimming. This is usually considered an uncommon activity for adult women, but one-third of the infected women reported swimming in the lake in the past 3 months, so it may be more significant than often believed. Finally, our qualitative questions about how schistosomiasis can be prevented showed that many women are aware of precautions they should take against contaminated water. The women we surveyed are not necessarily representative of the rest of the community, as they have been educated about schistosomiasis multiple times through participating in the study. However, their responses indicate the difficulty of following water contact precautions in such a setting, even when education is provided.
Limitations of our study include a small sample size, which makes it difficult to control for confounders such as duration of infection, previous praziquantel treatments, or other medication use. Our conclusions are also limited by lack of information about other parasitic infections and immunologic data about the participants. The lack of post-treatment testing within 4–6 weeks after praziquantel to prove cure caused us to be unable to distinguish reinfection from persistent infection in some cases.
Our study identified an incidence of 8.7% among uninfected women over the course of one year, and recurrent or incompletely cleared infection affecting 40% of infected women. A single praziquantel treatment reduced intensity of schistosome infection, but was insufficient in providing stable parasite eradication in over a third of women. Further investigation into the efficacy of single-dose praziquantel in adults is warranted, especially as the interaction of schistosomiasis with HIV indicates that schistosomiasis control may have effects beyond control of the parasitic infection itself. Further analysis of data from this cohort will examine differences in cervical gene expression, immune environment, and microbiota in women with and without schistosomiasis, and the effects of treatment with praziquantel on these factors. Future studies may address effective treatment of schistosomiasis to reduce HIV risk in adult women in endemic areas.
Supporting information
(PDF)
(PDF)
Acknowledgments
We thank the women for their willing participation in this study. We also thank Dr. Humphrey Mazigo for his helpful comments and suggestions for this manuscript.
Data Availability
Data cannot be shared publicly because of the small sample size and the risk of a breach of confidentiality given the detailed nature of demographic and clinical questions in small rural villages. Data are available from the National Institute for Medical Research (contact: Mark Urassa, urassamark@yahoo.co.uk) for researchers who meet the criteria for access to confidential data.
Funding Statement
This project was supported by the Doris Duke Charitable Foundation (Grant Number 2017067 to J.A.D., www.ddcf.org), by the Gilead Sciences Research Scholars Program in HIV from Gilead Sciences (to J.A.D., http://researchscholars.gilead.com/en/hiv_portal), and by the National Institutes of Health (K23 AI 110238 to J.A.D., www.nih.gov). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Schistosomiasis. In: WHO Fact Sheet [Internet]. 2018 [cited 24 Feb 2019]. Available: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis
- 2.Ruganuza DM, Mazigo HD, Waihenya R, Morona D, Mkoji GM. Schistosoma mansoni among pre-school children in Musozi village, Ukerewe Island, North-Western-Tanzania: prevalence and associated risk factors. Parasit Vectors. 2015;8: 377 10.1186/s13071-015-0997-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Downs JA, de Dood CJ, Dee HE, McGeehan M, Khan H, Marenga A, et al. Schistosomiasis and Human Immunodeficiency Virus in Men in Tanzania. Am J Trop Med Hyg. 2017;96: 856–62. 10.4269/ajtmh.16-0897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crompton DWT (David WT, World Health Organization. Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. World Health Organization; 2006. [Google Scholar]
- 5.Kabuyaya M, Chimbari MJ, Mukaratirwa S. Efficacy of praziquantel treatment regimens in pre-school and school aged children infected with schistosomiasis in sub- Saharan Africa: a systematic review. Infectious Diseases of Poverty; 2018; 1–7. 10.1186/s40249-017-0384-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N, Gwanzura L, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20: 593–600. 10.1097/01.aids.0000210614.45212.0a [DOI] [PubMed] [Google Scholar]
- 7.Yegorov S, Galiwango RM, Good S V., Mpendo J, Tannich E, Boggild AK, et al. Schistosoma mansoni infection and socio-behavioural predictors of HIV risk: a cross-sectional study in women from Uganda. BMC Infect Dis. 2018;18: 586 10.1186/s12879-018-3481-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downs JA, Dupnik KM, van Dam GJ, Urassa M, Lutonja P, Kornelis D, et al. Effects of schistosomiasis on susceptibility to HIV-1 infection and HIV-1 viral load at HIV-1 seroconversion: A nested case-control study. Hsieh MH, editor. PLoS Negl Trop Dis. Public Library of Science; 2017;11: e0005968 10.1371/journal.pntd.0005968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wall KM, Kilembe W, Vwalika B, Dinh C, Livingston P, Lee Y-M, et al. Schistosomiasis is associated with incident HIV transmission and death in Zambia. Bustinduy AL, editor. PLoS Negl Trop Dis. 2018;12: e0006902 10.1371/journal.pntd.0006902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Expert Committee on the Control of Schistosomiasis. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. World Heal Organ; 2002; [PubMed] [Google Scholar]
- 11.Munisi DZ, Buza J, Mpolya EA, Angelo T, Kinung’hi SM. The Efficacy of Single-Dose versus Double-Dose Praziquantel Treatments on Schistosoma mansoni Infections: Its Implication on Undernutrition and Anaemia among Primary Schoolchildren in Two On-Shore Communities, Northwestern Tanzania. Biomed Res Int. Hindawi Limited; 2017;2017: 7035025 10.1155/2017/7035025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berhe N, Medhin G, Erko B, Smith T, Gedamu S, Bereded D, et al. Variations in helminth faecal egg counts in Kato–Katz thick smears and their implications in assessing infection status with Schistosoma mansoni. Acta Trop. 2004;92: 205–212. 10.1016/j.actatropica.2004.06.011 [DOI] [PubMed] [Google Scholar]
- 13.Corstjens PLAM, De Dood CJ, Kornelis D, Fat EMTK, Wilson RA, Kariuki TM, et al. Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology. 2014;141: 1841–55. 10.1017/S0031182014000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombe S, Lee MH, Masikini PJ, van Lieshout L, de Dood CJ, Hoekstra PT, et al. Decreased Sensitivity of Schistosoma sp. Egg Microscopy in Women and HIV-Infected Individuals. Am J Trop Med Hyg. 2018;98: 1159–1164. 10.4269/ajtmh.17-0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vercruysse J, Albonico M, Behnke JM, Kotze AC, Prichard RK, McCarthy JS, et al. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int J Parasitol Drugs drug Resist. Elsevier; 2011;1: 14–27. 10.1016/j.ijpddr.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabatereine NB, Vennervald BJ, Ouma JH, Kemijumbi J, Butterworth AE, Dunne DW, et al. Adult resistance to schistosomiasis mansoni: age-dependence of reinfection remains constant in communities with diverse exposure patterns. Parasitology. 1999;118 (Pt 1): 101–5. [DOI] [PubMed] [Google Scholar]
- 17.Woolhouse MEJ, Taylor P, Matanhire D, Chandiwana SK. Acquired immunity and epidemiology of Schistosoma haematobium. Nature. 1991;351: 757–759. 10.1038/351757a0 [DOI] [PubMed] [Google Scholar]
- 18.Wu W, Wang W, Huang Y-X. New insight into praziquantel against various developmental stages of schistosomes. Parasitol Res. 2011;109: 1501–7. 10.1007/s00436-011-2670-3 [DOI] [PubMed] [Google Scholar]
- 19.van Dam GJ, Bogitsh BJ, van Zeyl RJ, Rotmans JP, Deelder AM. Schistosoma mansoni: in vitro and in vivo excretion of CAA and CCA by developing schistosomula and adult worms. J Parasitol. 1996;82: 557–64. [PubMed] [Google Scholar]
- 20.Secor WE. Toward Mass Drug Administration Stopping Criteria for Schistosoma mansoni Control Programs. Am J Trop Med Hyg. 2019; 1–2. 10.4269/ajtmh.19-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danso-Appiah A, De Vlas SJ. Interpreting low praziquantel cure rates of Schistosoma mansoni infections in Senegal. Trends Parasitol. 2002;18: 125–9. 10.1016/s1471-4922(01)02209-7 [DOI] [PubMed] [Google Scholar]
- 22.Crellen T, Walker M, Lamberton PHL, Kabatereine NB, Tukahebwa EM, Cotton JA, et al. Reduced Efficacy of Praziquantel Against Schistosoma mansoni Is Associated With Multiple Rounds of Mass Drug Administration. Clin Infect Dis. 2016;63: ciw506 10.1093/cid/ciw506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ismail M, Metwally A, Farghaly A, Bruce J, Tao L, Bennett AL. CHARACTERIZATION OF ISOLATES OF SCHISTOSOMA MANSONI FROM EGYPTIAN VILLAGERS THAT TOLERATE HIGH DOSES OF PRAZIQUANTEL. 1996;55: 214–218. [DOI] [PubMed] [Google Scholar]
- 24.Walter K, Fulford AJC, McBeath R, Joseph S, Jones FM, Kariuki HC, et al. Increased human IgE induced by killing Schistosoma mansoni in vivo is associated with pretreatment Th2 cytokine responsiveness to worm antigens. J Immunol. 2006;177: 5490–8. 10.4049/jimmunol.177.8.5490 [DOI] [PubMed] [Google Scholar]
- 25.Woolhouse MEJ, Hagan P. Seeking the ghost of worms past. Nat Med. 1999;5: 1225–1227. 10.1038/15169 [DOI] [PubMed] [Google Scholar]
- 26.Black CL, Mwinzi PNM, Muok EMO, Abudho B, Fitzsimmons CM, Dunne DW, et al. Influence of exposure history on the immunology and development of resistance to human Schistosomiasis mansoni. PLoS Negl Trop Dis. Public Library of Science; 2010;4: e637 10.1371/journal.pntd.0000637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gazzinelli A, Oliveira-Prado R, Matoso LF, Veloso BM, Andrade G, Kloos H, et al. Schistosoma mansoni reinfection: Analysis of risk factors by classification and regression tree (CART) modeling. PLoS One. Public Library of Science; 2017;12: e0182197 10.1371/journal.pone.0182197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gryseels B, Mbaye A, De Vlas SJ, Stelma FF, Guissé F, Van Lieshout L, et al. Are poor responses to praziquantel for the treatment of Schistosoma mansoni infections in Senegal due to resistance? An overview of the evidence. Trop Med Int Health. 2001;6: 864–73. 10.1046/j.1365-3156.2001.00811.x [DOI] [PubMed] [Google Scholar]
- 29.Karanja DM, Hightower AW, Colley DG, Mwinzi PN, Galil K, Andove J, et al. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. Lancet. 2002;360: 592–596. 10.1016/S0140-6736(02)09781-7 [DOI] [PubMed] [Google Scholar]
- 30.Schwartz C, Fallon PG. Schistosoma “Eggs-Iting” the Host: Granuloma Formation and Egg Excretion. Front Immunol. Frontiers; 2018;9: 2492 10.3389/fimmu.2018.02492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamberton PHL, Faust CL, Webster JP. Praziquantel decreases fecundity in Schistosoma mansoni adult worms that survive treatment: evidence from a laboratory life-history trade-offs selection study. Infect Dis Poverty. 2017;6: 110 10.1186/s40249-017-0324-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
Data cannot be shared publicly because of the small sample size and the risk of a breach of confidentiality given the detailed nature of demographic and clinical questions in small rural villages. Data are available from the National Institute for Medical Research (contact: Mark Urassa, urassamark@yahoo.co.uk) for researchers who meet the criteria for access to confidential data.