Abstract
Background
Disulfiram (DSF) has a long history of being used as a first-line promising therapy for treatment of alcoholism in human. Besides its prominence in the treatment of alcoholism, extensive investigations have been carried out to explore other biomedical and pharmacological effects of DSF. Amongst other biomedical implications, plenty researches have shown evidence of promising anticancer efficacy of this agent for treatment of wide range of cancers such as breast cancer, liver cancer and lung carcinoma.
Methods
Electronic databases, including Google scholar, PubMed and Web of science were searched with the keywords disulfiram, nanoparticles, cancer, drug delivery systems.
Result
Despite its excellent anticancer efficacy, the pharmaceutical significance and clinical applicability of DSF are hampered due to poor stability, low solubility, short plasma half-life, rapid metabolism, and early clearance from systemic circulation. Various attempts have been made to eradicate these issues. Nanotechnology based interventions have gained remarkable recognition in improving pharmacokinetic and pharmacodynamic profile of DSF by improving its stability and avoiding its degradation.
Conclusion
The aim of the present review is to critically analyse all recent developments in designing various nanotechnology-based delivery systems, to ponder their relevance in improving stability, pharmacokinetic and pharmacodynamic profile, and achieving target-specific delivery of this agent to cancer cells to effectively eradicate cancer and abolish its metastasis. Nanotechnology is a novel approach for overcoming such obstacles faced presently, the results obtained so far using different novel drug delivery systems seem to be very promising to increase the stability and half-life of DSF.
Graphical abstract.
Nanocrrier mediated drug delivery systems for disulfiram
Keywords: Disulfiram, Polymeric nanoparticles, Cancer, Nanotechnology, Drug delivery systems
Introduction
Anti-cancer drug discovery has demonstrated to be an expensive and laborious procedure, such as structured based drug designing, computational chemistry and personal genomics, etc. [1–3]. In this framework, the exploration of new features of existing drugs could be the shortcut for the drug development application. Among those medications, the disulfiram (DSF) is an old drug that has been shown to play new tricks on its anti-cancer property. Disulfiram which is also known as bis (diethyl thiocarbamoyl) disulphide has been used for treatment of alcoholism since the 1930s [4].
The exploration of novel anti-cancer drugs still remains a challenge in selective targeted killing of cancer cells without impairing the normal cell. In the past, disulfiram is the first medicine approved by FDA U.S. for the remedial of alcoholism. The mechanism behind the treatment of alcoholism is when DSF blocks the activity of aldehyde dehydrogenase (ALDH), an enzyme converting the acetaldehyde into acetate [5, 6].
Anti-cancer activity of Disulfiram
Years after the discovery of DSF, more scientific based evidence of DSF having anti-cancer properties have been reported. The scientific studies show that DSF has been used against various type of cancers, such as pancreatic [7, 8], brain [9], skin [10], breast [6, 11], colorectal [12, 13], non-small cell lung [14], prostate cancer [15] and bladder cancer cells [16]. The anti- neoplastic property of disulfiram has been confirmed in ex-vivo (cell lines) and in-vivo (animal models) [14, 17–21].
The anti- neoplastic activity of DSF is more prominent when combined with copper (Cu) before given to the patient [22]. The cytotoxicity effect of DSF along with Cu appears through inhibition of proteasome pathway leading to the induction of apoptosis activity in human cancer cell line [6]. The Cu (II), ions are abundantly present in human body and are easily found in the stomach. These ions can combine with DSF at its thiol group and main metabolite, diethyldithiocarbamate (DDC), to form much more stable compound known as copper-DDC (Cu DDC) [5]. Cu DDC is a hydrophobic metabolite of disulfiram and is quickly absorbed in the gut. Because of its stability problem, Cu DDC rapidly degrades into the DDC and Cu in the bloodstream [5].
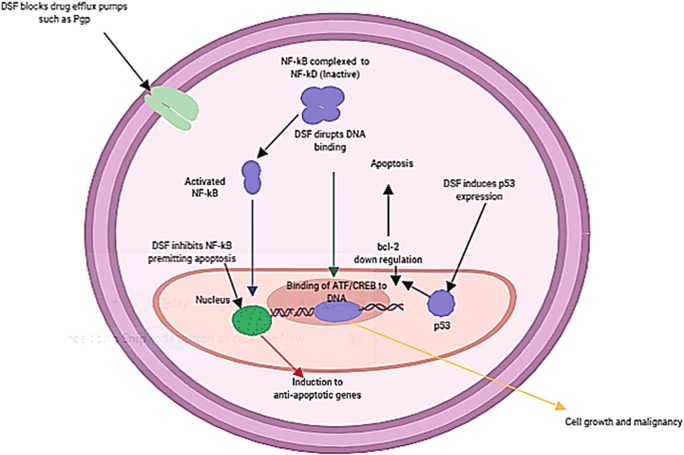
Numerous anti-cancer mechanisms of disulfiram have been reported, including triggering oxidative stress by the generation of (ROS) reactive oxygen species, inhibition of the superoxide dismutase activity and activation of the mitogen-activated protein kinase pathway. Moreover, DSF can reverse the resistance to anti-cancer drugs by inhibiting the P-glycoprotein multidrug efflux pump and suppressing the activation of NF-kB, both of which play a vital role in the progress of drug resistance [23] (Fig. 1).
Fig. 1.
A schematic illustration showing cellular mechanisms of DSF anti-cancer activity
The therapeutic potential of DSF as an emerging anti-neoplastic agent has led to several clinical investigation to further evaluating the anti-cancer potential of repurposed DSF against different types of carcinomas (ClinicalTrials.gov Identifiers: NCT03323346, NCT01118741, NCT02963051, NCT00742911, NCT00312819, NCT01907165, NCT00256230, NCT03034135, and NCT03151772) [24].
DSF limitations
Disulfiram is highly unstable in the acidic environment and blood circulation, the half-life of DSF is only 4 min in-vivo [9, 25, 26]. Hence, when disulfiram is uncovered to blood circulation, it shows only anti-alcoholism potential, but the anti-cancer property is lost. As a result, the DSF instability remains as a challenge to retain its anti-cancer activity against various cancers. Besides, it is necessary to design and develop novel nano drug delivery systems for DSF to maintain its stability in the blood stream and improve cellular uptake in tumour tissue. It also belongs to the less water-soluble class drug with a solubility of 0.2 mg/mL [27, 28]. The encapsulation of DSF into the nanoparticles (NPs) can prolong its half-life and stability from the rapid deprivation in blood stream. [9]. The novel drug delivery of disulfiram can improve the drug accumulation in tumour cells [15, 29, 30]. Meanwhile, nanotechnology-based smart nanomaterials have various advantages as a drug cargo. These nanocarriers can increase the obstacles associated with drugs, by increasing the aqueous solubility and protecting the rapid degradation in the blood circulation, increasing the pharmacokinetics property of the drugs, target delivery of drug moiety in a tissue or cell and enhancing the therapeutic efficacy of encapsulated molecules for therapy [31–33].
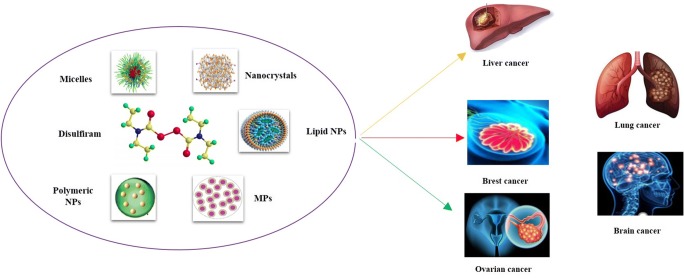
Various nanotechnology-based therapeutic drug delivery systems such as micelles, polymeric NPs, nanosuspension, lipid-based NPs have been designed to potentiate the stability of DSF towards anti-cancer therapy (Fig. 2). Here in this review, we will highlight the various nanotechnology approaches designed for the delivery of DSF towards anti-cancer therapy. Besides, this article also covers the brief anti-cancer activity of DSF.
Fig. 2.
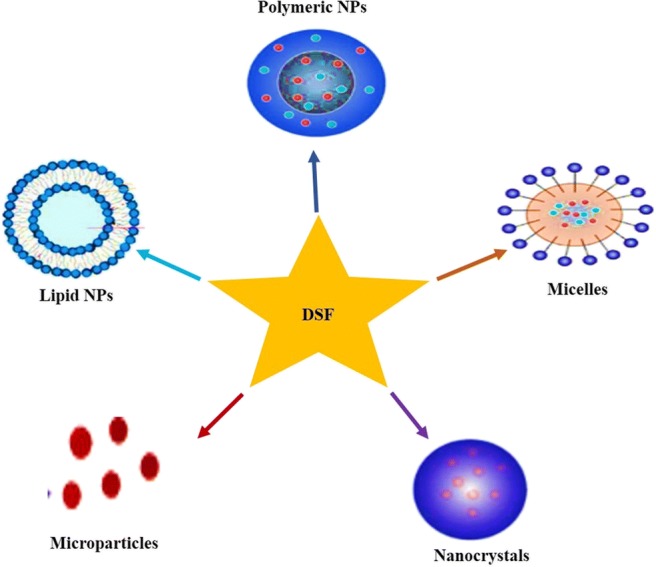
Illustration depicting the nanotechnology-based approaches for effective delivery of DSF in cancer
Method
Electronic databases, including Google scholar, PubMed and Web of science were searched with the keywords disulfiram, nanoparticles, cancer, drug delivery systems. Data was included in this study until June 2019 with focus only on English language articles.
Nanotechnology based approaches for DSF
Polymeric nanoparticles
In current eras, polymeric NPs have accomplished significant consideration from researchers. The noteworthy benefits of these NPs for novel drug delivery systems (DDs) are their outstanding bio-compatibility, efficiency, bioavailability, furthermost, the simple expansion, designing and extensive structural diversity. Besides, polymeric nanoparticles might be model entrant for tumour remedy [34]. One of the main recompenses of polymeric nanoparticles is their nano-size, which permits them to enter and infuse the cell membrane, vessels and walls, while taking the protracted passage in a different partition of the body until they reach to their target sites [35]. The deatil of disulfiram-loaded polymeric based nano-carriers is shown in Table 1.
Table 1.
Nanoparticles studied as nano-drug carriers in designing of DSF
| Fabrication technique | Components | Particle Size(nm) | Z.P⃰ (mV) | %EE⃰, %D. L⃰ | Indication | Route of Administration | Cell line/Animal | Major outcome | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Polymeric NPs | ||||||||||
| High-Pressure Homogenization Method | mPEG-PLA/PLA or mPEG-PCL/PCL,DSF | 100.5 ± 10.8 | – | 5.21 | Liver Cancer | i.v. | H22/ Male Sprague-Dawley (SD) rats |
-Improved the pharmacokinetic of DSF -Enhanced the tumour inhibition growth -Better drug loading and stability |
[36] | |
| Emulsion-Solvent Evaporation Method | PLGA, Polysorbate 80, DSF | 120 | – | 24 | Liver Cancer | – | Hep3B |
-Achieved sustained drug release patterns -Reduced dosage regimens -Improved anti-cancer efficacy of DSF-NPs |
[37] | |
| Emulsion-Solvent Evaporation Method | PLGA, PVA, DSF | 145.9 ± 5.0 | +36.2 ± 4.6 | 94 | Non-small cell lung Cancer | – | A549 |
-Improved tumour inhibition growth -Better loading capacity -Improved drug stability |
[38] | |
| Synthesized | LBA-PDA-PEG-DSF | 30.05 ± 0.42 | – | – | Metastatic ovarian Cancer | i.p. | SKOV-3/ Mice |
- stronger inhibitory effect as compared blanked NPs - Reduced toxicity |
[39] | |
| Nanoprecipitation Method | PGA-CisPt, DSF | 15.7 ± 1.3 | −9.4 ± 2.3 | 19.5 | Lung Cancer | i.v. | A549/ Balb/C nude mice |
-Improved the therapeutic efficacy of cisplatin -Reduced toxicity associated with cisplatin -Overcome drug induced resistance |
[40] | |
| Nanoprecipitation Method | PEG-PLGA/PCL, DSF | 91.8 ± 25.3 | – | 2.0 | Breast Cancer | i.v. | MCF-7/ Balb/C mice |
-High drug loading -Improved stability - preventing from rapid degradation |
[41] | |
| Nanoprecipitation Method | PLGA, PEG, PVA, DSF | 204 | – | – | Breast Cancer | i.v., i.p. | MCF-7 Female BALB/c mice |
-Extended the drug half-life -More potent effects as compared with raw drug |
[42] | |
| Emulsion Solvent Evaporation Technique | PLGA, mPEG-COOH, DSF | 81.74 ± 8.7 | −8.27 | 18.47, 92.1 | Brain Cancer | i.v. | T98G and DAOY/female athymic nude |
-Increased plasma half-life of DSF -Efficient penetration toward tumour inhibition growth |
[43] | |
| Nanoprecipitation Method | PLGA, PVA, DSF | 165 | – | 5.35 ± 0.03%, 58.85% | Breast Cancer | – | MCF-7 | - Provide better stability to drug molecules | [44] | |
| Emulsion-Solvent 1Evaporation Method | PLGA, PVA, DSF | 136.2 ± 6.2 | −21.7 ± 0.96 | 27.67 ± 3.47, 78.92 ± 2.16 | Liver Cancer | i.v. | HCC/nude mice |
-Better EE, DLC -In -vitro controlled release and improved half-life. |
[45] | |
| Dialysis Method | PCL, mPEG. DSF, DOX | 143.9 ± 2.1 | – | 1.02, 21.42 | Breast Cancer | i.v. | MCF-7 and MDA-MB-231/ female BALB/c mice | -Exhibited increased cellular uptake by tumour cells, an improved drug synergistic effect. | [46] | |
| Synthesized | Cu (DCC), DSF. mPEG PEC | 200 | −25 | 4.55% | i.v. | A549 |
-Excellent stability -Improved anti-cancer efficacy |
[47] | ||
| Micelles | ||||||||||
| Emulsification-Solvent Diffusion Method | mPEG5k-b-PLGA2k/PCL3.4 k/MCT, DSF | 86.4 ± 13.2 | −24.5 ± 1.5 | 5.90 | Liver Cancer | i.v. | H22/ Kunming mice |
-Improved plasma stability of DSF -More potent as compared with free drug |
[48] | |
| Ring-Opening Polymerization | PEG-b-PLL, PTX, DSF | 138 ± 8.5 | −12.4 ± 1.3 | 2.15 ± 0.5 | 89.3 ± 1 | Breast Cancer | – | MCF-7 |
-Reverse MDR to breast cancer -Achieved synergistic effect |
[49] |
| Synthesized | Poly (styrenecomaleic anhydride) (SMA), DSF | 80 | −21.9 | 7.5,75.4 | Breast Cancer | i.v. | 4 T1/female BABL/c mice |
-Improve the targeted intracellular delivery of DSF -Highly accumulation in tumour site |
[29] | |
| Synthesized | SMA, DSF, PTX |
88.58± 4.12 |
−22.8 | 5 | Breast Cancer | i.v. | MCF-7/female BALB/c nude |
-High drug loading -Improved cellular interlization |
[30] | |
| Nanocrystals | ||||||||||
| Anti-solvent Precipitation Method | Beta lactoglobulin, DSF, PTX | 160 | −24 | 36.23 ± 0.9, 96.6 ± 0.24 | Lung Cancer | – | A549 |
-Efficient MDR Reversal -Enhanced Apoptosis |
[50] | |
| Anti-solvent Precipitation-Ultrasonication Method | Beta lactoglobulin, DSF, PTX | 160 | −24 | 36, 7 | Lung Cancer | i.v. | A549 cells/ female BALB/c nude mice | -PTX-DSF Ns improve the MDR-reversal | [51] | |
| Microparticles | ||||||||||
| Emulsion Solvent Evaporation Method | PLGA, PVA, DSF | 47.83 ± 13.21 μm | −14.9 ± 4.7 | 4.09 ± 0.11, 81.84 ± 2.3 | Non-small-cell lung Cancer | – | A549, HCC827 cells |
-Efficient anti-proliferation -Improved anti-tumour efficiency |
[52] | |
| Lipid based Nanocarriers | ||||||||||
| Phase-Inversion Method | HS-PEG1k-TATp, DSF | 93.7 | −40.13 ± 2.83 | 3.59 ± 0.36 | Liver Cancer | i.v. | Hep G2 cells/BALB/cA nude mice |
-Better stability -Better tumour internalization and increased cytotoxicity |
[53] | |
| Emulsification Ultrasonication Method | TPGS. lecithin, PC, 98%), tween80, Labrafac, DSF | 188.6 ± 1.5 | −27.91 ± 2.5 | 80.7 ± 2.68 | Breast Cancer | i.v. | MCF-7/female BALB/c mic |
-Improve the drug loading, release, stability, -Improve the overall anti-cancer effect of DSF both in-vitro and in vivo. |
[54] | |
In addition, these polymeric nanoparticles can be modified with a various functional group including polyethylene glycol (PEG) and target mediator for improving the therapeutic or gene delivery to the tumor sites [55]. These NPs are composed of various type of synthetic constituents, e.g., poly-lactic-co-glycolic acid (PLGA), PEG, poly-L-lysine), micelle, and polymer gained from the natural sources, e.g., chitosan [56]. These polymeric nanoparticles also improved the bioavailability and reduced toxicity as of their biodegradable and biocompatible property [57]. Xuezhi Zhuo et al. 2018 formulated the novel mixed nanoparticles for improving the stability and drug loading of disulfiram through a high-pressure homogenization technique. The loading efficiency of prepared NPs improved from 3.35% to 5.50% by reducing the crystallinity of PCL NPs core. The crystallinity of these nanoparticles also reduced from 51.13% to 25.15% after the addition of medium chain tri-glyceride. The drug loading capacity was improved because of expanded amorphous regions in the NPs core and the better stability of NPs might be associated to the curved tunnels [36]. In this experiment, the PLGA nanoparticles were synthesized for encapsulation of DSF against Hep3B cell lines. The in-vitro cellular investigation demonstrated that the inhibitory effects of prepared nanoparticles were much better as compared to the free drug and also achieved sustained release that reduced the dosage regimen [37]. Similarly, this study also stated fabrication of PLGA nanoparticles for the encapsulation of DSF against xenograft mouse lung cancer model. The resultant nanoparticles have obtained size around 145 nm with excellent encapsulation efficiency of 94%. They have improved the stability of nanoparticles in serum and enhanced the cytotoxicity in A549 lung cancer cell lines [38]. He et al. have explored the anti-cancer potential of disulfiram against metastatic ovarian cancer. They have designed a redox sensitive diethyldithiocarbamate (DDTC) polymeric nanoparticles for the target drug delivery of DSF. These nanoparticles can enter efficiently into cancer cells through receptor-mediated endocytosis. The 3D cell culture investigation reveals that, this NPs has stronger inhibitory effects as compared to the blank nanoparticles [39].
Another team of researchers designed a novel strategy for lung cancer treatment by combining disulfiram with cisplatin to overcome the resistance in solid tumours. The DSF significantly reduced the severe toxicity, cisplatin-resistant index from 46.2 to 14.5 and improved the synergistic effect and therapeutic efficiency of cisplatin. That was likely caused by disulfiram reducing the glutathione level, inhibiting NFκB activation and modulating the apoptosis related proteins in the A549 cell lines [40]. Wantong et al. 2015 constructed mPEG-PLGA/PCL nanoparticles for DSF entrapment by nanoprecipitation method. This nanoparticle improved the loading capacity up to 7.8%, had better stability and prevented its degradation in blood circulation while maintaining its anti-tumour efficacy. In-vivo studies have shown enhanced concentration of DSF after administration via tail vein in tumour induced mice [41]. Another interesting study reported novel folate receptor-targeted PEG-PLGA NPs for targeted based delivery of DSF into breast cancer by passive and active targeting. The encapsulated disulfiram was found more potent at lower concentration in comparison to the drug alone. The developed nanoparticles prolonged the half-life of loaded drug and increased its target delivery to breast cancer. The cytotoxicity mechanisms of DSF may be the catalyst of ROS production against cancer cells [42]. Another team engineered a novel polymeric nanoparticle for brain cancer to overawed the in-stability and low therapeutic efficiency of DSF. The confocal microscopic observations show a quick internalization of the NPs in the cancer cell and cytotoxicity assay reveals that this NPs induced a potent killing of many brain tumour cells [43]. This strategy was developed for the protection of the DSF from rapid degradation in the gastric environment, blood circulation and attaining its sustained effect in tumour cells [44]. Wang et al. constructed PLGA nanoparticles through emulsion-solvent evaporation method for the entrapment of disulfiram as a model drug. They obtained a size around 136 nm with excellent entrapment efficiency and better drug loading. The encapsulation of DSF in PLGA nanoparticles not only extended the half-life from 2 min to 7 h in serum but also improved its toxicity in liver cancer mouse model [45]. Another interesting study was designed based on folate-receptor targeted PLGA-PEG nanoparticles for entrapment and target delivery of DSF into the breast carcinoma. The developed formulation was accumulated into cancer cells and also prevented fast degradation of disulfiram in blood stream [58].
Recently Xiaoguang Tao 2018 utilized a dialysis method for designing core-shell-corona structured NPs for loading of hydrophilic doxorubicin (DOX) and hydrophobic DSF for improving cellular intake and synergistic effect against breast cancer. In-vivo imaging study reveals that, this nanoparticle can easily accumulate in tumour cells [46].
Xinyu Peng and his team fabricated coordinated polymeric nanoparticles loaded with DSF for combinational cancer therapy. These nanoparticles exhibited the excellent stability in both neutral and acidic solutions. The in-vivo anti-tumour results demonstrated that the better efficacy of DSF loaded nanoparticles [47].
Micelles
Micelles have unique features for delivering the numerous therapeutic moieties including the cancer drugs. Micelles are designed for DDs mostly composed of di-block, tri-block co-polymers or graft co-polymers through hydrophilic or hydrophobic segments. The micelles have exclusive properties, such as lower molecular weight, better entrapment of loaded agent, improve stability, lower critical micelle concentration, and enhanced drug accretion at the tumor sites for targeting [59]. Polymeric micelles are round shape made of amphiphilic di-block or tri-block copolymers and have hydrophilic and hydrophobic blocks in an aqueous medium. It can infiltrate simply into the tumor tissues due to their nanosize as compared with other nanoparticles [60, 61].
Lanolin Miao et al. 2018 designed a polymeric micelle for entrapment of disulfiram by using the solvent diffusion method. The medium chain triglyceride was fused with developed micelle for increasing the loading capacity by reducing the core crystallinity. The micelles efficiently improved its stability in plasma while maintaining its effects with 58% remaining after 48 h as compared to free drug that is degraded 90% after the same period [48]. Qiang Huo et al. 2018 designed a novel nano-drug delivery system to reduce the multi drug resistance (MDR) and improve effectiveness by encapsulating DSF and PTX into the core of poly (ethylene glycol)-block-poly(l-lysine) (PEG-b-PLL) block copolymer micelles. The developed micelles have negative surface charge that is helpful for prolongation in blood circulation, and in a weak acid environment of tumour tissue (pH 6.5–6.8). The in-vitro studies show that, the DSF and paclitaxel micelles exhibited more cellular uptake and enhanced its cytotoxicity against MCF-7 cell lines which may be because of inhibitory effects of disulfiram on the efflux function of P-gp [49].
Xiaopin Duan and his team developed a redox sensitive shell crosslinked micelle for loading of DSF to achieve intracellular targeted delivery and prevent metastasis. The resulted formulation significantly improved the stability and released disulfiram under a reductive environment. In addition, these micelles repressed cell proliferation, induced cell apoptosis and suppressed cell invasion in breast cancer cell line. The findings suggested that, it can be novel strategy for inhibiting the metastasis of breast cancer [29]. In this study a novel drug delivery system was developed to overcome multidrug resistance in breast cancer. A smart pH-sensitive micelle encapsulated with DSF and DOX was designed for simultaneous delivery to tumour site at the optimum ratio. The loaded disulfiram is released faster to inhabited the activity of P-gp and restore cell apoptotic signalling pathway, while doxorubicin was released in a sustained and pH-dependent manner and accrued into the drug resistant cells to exert therapeutic outcome [30].
Nanocrystals
Nanocrystals are colloidal dispersions of pure drug particles (amorphous, crystalline) stabilized by surfactants [62]. In Comparison to the other drug delivery systems e.g., liposome, micelle, and inorganic nano-carrier, nanocrystal has a better dais for accomplishing the improved drug loading because they contain of pure drug nanoparticles [63].
The dual drug loaded beta lactoglobulin coated nanosuspension was prepared by the antisolvent precipitation method. These nanocrystals were designed for reversing multi drug resistance in the lung carcinoma. Meanwhile this protein coated nanoparticles increased 14-fold cellular uptakes and a 5-fold increase in apoptosis, respectively [50]. Similarly, another novel smart NCs was developed for co-delivery of PTX and DSF for reversing the MDR in lung carcinoma. The rod-shaped nanoparticles around 160 nm size was obtained with excellent drug loading up to 43%. These novel nanoparticles improved their circulation in blood stream over time, improved their accumulation in cancer cells and reduced the tumour size by 12-fold in tumour bearing BALB/c nude mice [60].
Microparticles
In past decade, the drug delivery systems based on microparticles composed of biopolymers have been utilized as a carrier for controlled release [64].
Chenhui Wang and his team developed a PLGA porous microparticles for disulfiram through emulsion-solvent evaporation method. The resultant microparticles obtained highly porous surface and sustained release profile with favourable drug loading (4.09% ± 0.11%). The flow cytometry and western blotting analysis elucidate the mechanisms beyond the enhanced induction of cell apoptosis and cell cycle arrest at S phase [52].
Lipid based nanoparticles
Lipid-based NPs were initially stated in 1991 as an alternate of other nano-carriers, such as polymeric nanoparticles, emulsions and microparticle. Lipid carriers have attracted researchers because of its properties like maximum drug loading, sustained release of the therapeutic moiety, selective targeting of drugs, and most importantly, the enhancement of bioavailability of therapeutic agents. The lipid-based NPs are colloidal nano-carriers that have a size rang between 50 to 1000 nm. Lipids and surfactants are important constituents for designing the lipid NPs. These lipids are must be bio-degradable and bio-compatible [65, 66].
Ling Zhang, 2015 formulated novel pH-triggered PEG lipid nano capsules for encapsulation of DSF with Cu in cancer treatment. The lipid nano capsules exhibited good stability, sustained release, improved bioavailability and prolonged half-life. The developed nanoparticles have shown better cellular uptake as compared to free drug [53]. Parikshit Banerjee, 2019 prepared nanostructured lipid carriers for encapsulation of disulfiram altered with vitamin E-TPGS. Meanwhile, the addition of TPGS in DSF enhanced the stability of prepared nanoparticles. The histopathological studies also confirmed that anti-tumour activity of resultant formulation as compared to free drug. The lipid in nanostructured lipid carrier can be an auspicious vehicle for delivery of disulfiram [54].
Conclusion and future perspectives
Disulfiram has confirmed its anti-tumor potential in the several types of solid cancers such as brain, skin, prostate, colorectal, breast, non-small cell lung and prostate cancer. Current review highlights the polymeric nano formulations, micelles, microparticles, nanocrystals and lipid-based novel advance drug delivery systems designed for encapsulation of DSF. However, the therapeutic application of DSF is imperfect due to its poor stability, solubility and rapid degradation in blood stream within 4 min. Our understanding from previous literature confirms that the nanotechnology-based DSF nanomedicine is a sensible approach for cancer therapy by targeting cancer cells. Effectiveness of various nano formulations of disulfiram in cellular study highlights their potential for in-vivo assessment in animal models. Nanotechnology is a novel approach for overcoming such obstacles faced presently, the results obtained so far using different novel drug delivery systems seem to be very promising to increase the stability and half-life of DSF.
We have confidence in that targeted novel drug delivery systems based nanocarriers might be desigend for effective delivery of DSF. Besides, the co-delivery of disulfiram with other chemo toxic agents has also improved the therapeutic potential, safety and efficiency. Forthcoming nanomedicine-based DDs still looks auspicious, where it can deliver a healthier outcome in cancer management. Also, additional novel efforts must be planned for targeted based delivery to lessen the toxicities which is the main problematic for cancer therapeutics. The surface modifications of these nano-formulations may overlay a new technique to achieve targeted drug delivery. Further studies must be conducted to investigate the efficacy and safety of DSF in clinical trials.
Author’s contributions
Muhammad Asim Farooq designed the review and wrote the manuscript, Md Aquib and Daulat Haleem Khan contributed to searching and data collection, Zahid Hussain critical revision of the manuscript, Anam Ahsan checked the grammar and proofread, Mirza Muhammad Faran Ashraf Baig and Dickson Pius Wande revised the manuscript based on reviewers comments, Muhammad Masood Ahmad and Hafiz Muhammad Ahsan edited the manuscript, Jiang jiajie designed the figures, Bo wang supervised the project. All the authors read and approved the final manuscript.
Compliance with ethical standards
Declaration of interest
The authors state no conflict of interest and have received no funding in preparation of this manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3(8):673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 2.Cvek B. Nonprofit drugs as the salvation of the world's healthcare systems: the case of Antabuse (disulfiram) Drug Discov Today. 2012;17(9–10):409–412. doi: 10.1016/j.drudis.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Suggitt M, Bibby MC. 50 years of preclinical anticancer drug screening: empirical to target-driven approaches. Clin Cancer Res. 2005;11(3):971–981. [PubMed] [Google Scholar]
- 4.Suh JJ, Pettinati HM, Kampman KM, O'Brien CP. The status of disulfiram: a half of a century later. J Clin Psychopharmacol. 2006;26(3):290–302. doi: 10.1097/01.jcp.0000222512.25649.08. [DOI] [PubMed] [Google Scholar]
- 5.Johansson B. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. ActaPsychiatr. Scand. 1992;86(S369):15–26. doi: 10.1111/j.1600-0447.1992.tb03310.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen D, Cui QC, Yang H, Dou QP. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66(21):10425–10433. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 7.Dalla Pozza E, Donadelli M, Costanzo C, Zaniboni T, Dando I, Franchini M, Arpicco S, Scarpa A, Palmieri M. Gemcitabine response in pancreatic adenocarcinoma cells is synergistically enhanced by dithiocarbamate derivatives. Free Radic Biol Med. 2011;50(8):926–933. doi: 10.1016/j.freeradbiomed.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Kim SK, Kim H, Lee DH, Kim TS, Kim T, Chung C, Koh GY, Kim H, Lim DS. Reversing the intractable nature of pancreatic cancer by selectively targeting ALDH-high, therapy-resistant cancer cells. PLoS One. 2013;8(10):e78130. doi: 10.1371/journal.pone.0078130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu P, Brown S, Goktug T, Channathodiyil P, Kannappan V, Hugnot JP, Guichet PO, Bian X, Armesilla AL, Darling JL, Wang W. Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br J Cancer. 2012;107(9):1488–1497. doi: 10.1038/bjc.2012.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cen D, Brayton D, Shahandeh B, Meyskens FL, Farmer PJ. Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J Med Chem. 2004;47(27):6914–6920. doi: 10.1021/jm049568z. [DOI] [PubMed] [Google Scholar]
- 11.Liu P, Kumar IS, Brown S, Kannappan V, Tawari PE, Tang JZ, Jiang W, Armesilla AL, Darling JL, Wang W. Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells. Br J Cancer. 2013;109(7):1876–1885. doi: 10.1038/bjc.2013.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Serie MM, El-Rashidy FH. In vitro collapsing colon cancer cells by selectivity of disulfiram-loaded charge switchable nanoparticles against cancer stem cells. Recent Pat Anticancer Drug Discov. 2017;12(3):260–271. doi: 10.2174/1574892812666170424144925. [DOI] [PubMed] [Google Scholar]
- 13.You SY, Rui W, Chen ST, Chen HC, Liu XW, Huang J, Chen HY. Process of immunogenic cell death caused by disulfiram as the anti-colorectal cancer candidate. Biochem Biophys Res Commun. 2019;513(4):891–897. doi: 10.1016/j.bbrc.2019.03.192. [DOI] [PubMed] [Google Scholar]
- 14.Duan L, Shen H, Zhao G, Yang R, Cai X, Zhang L, Jin C, Huang Y. Inhibitory effect of Disulfiram/copper complex on non-small cell lung cancer cells. Biochem Biophys Res Commun. 2014;446(4):1010–1016. doi: 10.1016/j.bbrc.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 15.Sharma V, Verma V, Lal N, Yadav SK, Sarkar S, Mandalapu D, Porwal K, Rawat T, Maikhuri JP, Rajender S, Sharma VL. Disulfiram and its novel derivative sensitize prostate cancer cells to the growth regulatory mechanisms of the cell by re-expressing the epigenetically repressed tumor suppressor—estrogen receptor β. Mol Carcinog. 2016;55(11):1843–1857. doi: 10.1002/mc.22433. [DOI] [PubMed] [Google Scholar]
- 16.Spillier Q, Vertommen D, Ravez S, Marteau R, Thémans Q, Corbet C, Feron O, Wouters J, Frédérick R. Anti-alcohol abuse drug disulfiram inhibits human PHGDH via disruption of its active tetrameric form through a specific cysteine oxidation. Sci Rep. 2019;9(1):4737. doi: 10.1038/s41598-019-41187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iljin K, Ketola K, Vainio P, Halonen P, Kohonen P, Fey V, Grafström RC, Perälä M, Kallioniemi O. High-throughput cell-based screening of 4910 known drugs and drug-like small molecules identifies disulfiram as an inhibitor of prostate cancer cell growth. Clin Cancer Res. 2009;15(19):6070–6078. doi: 10.1158/1078-0432.CCR-09-1035. [DOI] [PubMed] [Google Scholar]
- 18.Lin J, Haffner MC, Zhang Y, Lee BH, Brennen WN, Britton J, Kachhap SK, Shim JS, Liu JO, Nelson WG, Yegnasubramanian S. Disulfiram is a DNA demethylating agent and inhibits prostate cancer cell growth. Prostate. 2011;71(4):333–343. doi: 10.1002/pros.21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison BW, Doudican NA, Patel KR, Orlow SJ. Disulfiram induces copper-dependent stimulation of reactive oxygen species and activation of the extrinsic apoptotic pathway in melanoma. Melanoma Res. 2010;20(1):11–20. doi: 10.1097/CMR.0b013e328334131d. [DOI] [PubMed] [Google Scholar]
- 20.Rae C, Tesson M, Babich JW, Boyd M, Sorensen A, Mairs RJ. The role of copper in disulfiram-induced toxicity and radiosensitization of cancer cells. J Nucl Med. 2013;54(6):953–960. doi: 10.2967/jnumed.112.113324. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Deng Q, Feng X, Sun J. Use of the disulfiram/copper complex for breast cancer chemoprevention in MMTV-erbB2 transgenic mice. Mol Med Rep. 2015;12(1):746–752. doi: 10.3892/mmr.2015.3426. [DOI] [PubMed] [Google Scholar]
- 22.Cvek B. Targeting malignancies with disulfiram (Antabuse): multidrug resistance, angiogenesis, and proteasome. Curr Cancer Drug Tar. 2011;11(3):332–337. doi: 10.2174/156800911794519806. [DOI] [PubMed] [Google Scholar]
- 23.Ekinci E, Rohondia S, Khan R, Dou QP. Repurposing Disulfiram as an anti-Cancer agent: updated review on literature and patents. Recent Pat Anticancer Drug Discov. 2019. [DOI] [PubMed]
- 24.Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:S131–S155. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 25.Hu P, Jin L, Baillie TA. Studies on the metabolic activation of Disulfiram in rat. Evidence for electrophilic S-oxygenated metabolites as inhibitors of aldehyde dehydrogenase and precursors of UrinaryN-Acetylcysteine conjugates. J Pharmacol Exp Ther. 1997;281(2):611–617. [PubMed] [Google Scholar]
- 26.Wang W, Darling JL. How could a drug used to treat alcoholism also be effective against glioblastoma? Rev Anticancer Ther. 2013;13(3):239–241. doi: 10.1586/era.12.169. [DOI] [PubMed] [Google Scholar]
- 27.Liu P, Wang Z, Brown S, Kannappan V, Tawari PE, Jiang W, Irache JM, Tang JZ, Britland S, Armesilla AL, Darling JL. Liposome encapsulated Disulfiram inhibits NFκB pathway and targets breast cancer stem cells in vitro and in vivo. Oncotarget. 2014;5(17):7471. doi: 10.18632/oncotarget.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Löbler Marian, Rohm H. W., Schmitz K.-P., Johnston A. H., Newman T. A., Ranjan S., Sood R., Kinnunen P. K. J. IFMBE Proceedings. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009. Drug delivery by nanoparticles — facing the obstacles; pp. 2335–2338. [Google Scholar]
- 29.Duan X, Xiao J, Yin Q, Zhang Z, Yu H, Mao S, Li Y. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS Nano. 2013;7(7):5858–5869. doi: 10.1021/nn4010796. [DOI] [PubMed] [Google Scholar]
- 30.Duan X, Xiao J, Yin Q, Zhang Z, Yu H, Mao S, Li Y. Multi-targeted inhibition of tumor growth and lung metastasis by redox-sensitive shell crosslinked micelles loading disulfiram. Nanotechnology. 2014;25(12):125102. doi: 10.1088/0957-4484/25/12/125102. [DOI] [PubMed] [Google Scholar]
- 31.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41(7):2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgess P, Hutt PB, Farokhzad OC, Langer R, Minick S, Zale S. On firm ground: IP protection of therapeutic nanoparticles. Nat Biotechnol. 2010;28(12):1267–1270. doi: 10.1038/nbt.1725. [DOI] [PubMed] [Google Scholar]
- 33.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 34.Khalid M, El-Sawy HS. Polymeric nanoparticles: promising platform for drug delivery. Int J Pharm. 2017;528(1–2):675–691. doi: 10.1016/j.ijpharm.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 35.Lai P, Daear W, Löbenberg R, Prenner EJ. Overview of the preparation of organic polymeric nanoparticles for drug delivery based on gelatine, chitosan, poly (d, l-lactide-co-glycolic acid) and polyalkylcyanoacrylate. Colloids Surf B Biointerfaces. 2014;118:154–163. doi: 10.1016/j.colsurfb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Zhuo X, Lei T, Miao L, Chu W, Li X, Luo L, Gou J, Zhang Y, Yin T, He H, Tang X. Disulfiram-loaded mixed nanoparticles with high drug-loading and plasma stability by reducing the core crystallinity for intravenous delivery. J Colloid Interface Sci. 2018;529:34–43. doi: 10.1016/j.jcis.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 37.Hoda M, Pajaniradje S, Shakya G, Mohankumar K, Rajagopalan R. Anti-proliferative and apoptosis-triggering potential of disulfiram and disulfiram-loaded polysorbate 80-stabilized PLGA nanoparticles on hepatocellular carcinoma Hep3B cell line. Nanomed-Nanotechnol. 2016;12(6):1641–1650. doi: 10.1016/j.nano.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Najlah M, Ahmed Z, Iqbal M, Wang Z, Tawari P, Wang W, McConville C. Development and characterisation of disulfiram-loaded PLGA nanoparticles for the treatment of non-small cell lung cancer. Eur J Pharm Biopharm. 2017;112:224–233. doi: 10.1016/j.ejpb.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 39.He H, Markoutsa E, Li J, Xu P. Repurposing disulfiram for cancer therapy via targeted nanotechnology through enhanced tumor mass penetration and disassembly. Acta Biomater. 2018;68:113–124. doi: 10.1016/j.actbio.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song W, Tang Z, Shen N, Yu H, Jia Y, Zhang D, Jiang J, He C, Tian H, Chen X. Combining disulfiram and poly (l-glutamic acid)-cisplatin conjugates for combating cisplatin resistance. J Control Release. 2016;231:94–102. doi: 10.1016/j.jconrel.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 41.Song Wantong, Tang Zhaohui, Lei Tian, Wen Xue, Wang Guanyi, Zhang Dawei, Deng Mingxiao, Tang Xing, Chen Xuesi. Stable loading and delivery of disulfiram with mPEG-PLGA/PCL mixed nanoparticles for tumor therapy. Nanomedicine: Nanotechnology, Biology and Medicine. 2016;12(2):377–386. doi: 10.1016/j.nano.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 42.Fasehee H, Zarrinrad G, Tavangar SM, Ghaffari SH, Faghihi S. The inhibitory effect of disulfiram encapsulated PLGA NPs on tumor growth: different administration routes. Mat Sci Eng C-Mater. 2016;63:587–595. doi: 10.1016/j.msec.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 43.Hanumantha Rao Madala SR, Ali-Osman F, Zhang R, Srivenugopal KS. Brain-and brain tumor-penetrating disulfiram nanoparticles: Sequence of cytotoxic events and efficacy in human glioma cell lines and intracranial xenografts. Oncotarget. 2018;9(3):3459. doi: 10.18632/oncotarget.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fasehee H, Ghavamzadeh A, Alimoghaddam K, Ghaffari SH, Faghihi S. A comparative cytotoxic evaluation of disulfiram encapsulated PLGA nanoparticles on MCF-7 cells. Int J Hematol Oncol Stem Cell Res. 11(2):102. [PMC free article] [PubMed]
- 45.Wang Z, Tan J, McConville C, Kannappan V, Tawari PE, Brown J, Ding J, Armesilla AL, Irache JM, Mei QB, Tan Y. Poly lactic-co-glycolic acid-controlled delivery of disulfiram to target liver cancer stem-like cells. Nanomedicine. 2017;13(2):641–657. doi: 10.1016/j.nano.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tao X, Gou J, Zhang Q, Tan X, Ren T, Yao Q, Tian B, Kou L, Zhang L, Tang X. Synergistic breast tumor cell killing achieved by intracellular co-delivery of doxorubicin and disulfiram via core–shell–corona nanoparticles. Biomater Sci. 2018;6(7):1869–1881. doi: 10.1039/c8bm00271a. [DOI] [PubMed] [Google Scholar]
- 47.Peng X, Pan Q, Zhang B, Wan S, Li S, Luo K, Pu Y, He B. Highly stable, coordinated polymeric nanoparticles loading copper (II) diethyldithiocarbamate for combinational chemo/chemodynamic therapy of cancer. Biomacromolecules. 2019;6:2372–2383. doi: 10.1021/acs.biomac.9b00367. [DOI] [PubMed] [Google Scholar]
- 48.Miao L, Su J, Zhuo X, Luo L, Kong Y, Gou J, Yin T, Zhang Y, He H, Tang X. mPEG5k-b-PLGA2k/PCL3. 4k/MCT mixed micelles as carriers of Disulfiram for improving plasma stability and antitumor effect in vivo. Mol. Pharm. 2018;15(4):1556–1564. doi: 10.1021/acs.molpharmaceut.7b01094. [DOI] [PubMed] [Google Scholar]
- 49.Huo Q, Zhu J, Niu Y, Shi H, Gong Y, Li Y, Song H, Liu Y. pH-triggered surface charge-switchable polymer micelles for the co-delivery of paclitaxel/disulfiram and overcoming multidrug resistance in cancer. Int J Nanomedicine. 2017;12:8631. doi: 10.2147/IJN.S144452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohammad IS, He W, Yin L. A smart paclitaxel-disulfiram nanococrystals for efficient MDR reversal and enhanced apoptosis. Pharm. Res. 2018;35(4):77. doi: 10.1007/s11095-018-2370-0. [DOI] [PubMed] [Google Scholar]
- 51.Mohammad IS, Teng C, Chaurasiya B, Yin L, Wu C, He W. Drug-delivering-drug approach-based codelivery of paclitaxel and disulfiram for treating multidrug-resistant cancer. Int J Pharm. 2019;557:304–313. doi: 10.1016/j.ijpharm.2018.12.067. [DOI] [PubMed] [Google Scholar]
- 52.Wang C, Yang J, Han H, Chen J, Wang Y, Li Q, Wang Y. Disulfiram-loaded porous PLGA microparticle for inhibiting the proliferation and migration of non-small-cell lung cancer. Int J Nanomedicine. 2017;12:827. doi: 10.2147/IJN.S121948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Tian B, Li Y, Lei T, Meng J, Yang L, Zhang Y, Chen F, Zhang H, Xu H, Zhang Y. A copper-mediated disulfiram-loaded pH-triggered PEG-shedding TAT peptide-modified lipid nanocapsules for use in tumor therapy. ACS Appl Mater Interfaces. 2015;7(45):25147–25161. doi: 10.1021/acsami.5b06488. [DOI] [PubMed] [Google Scholar]
- 54.Banerjee P, Geng T, Mahanty A, Li T, Zong L, Wang B. Integrating the drug, disulfiram into the vitamin E-TPGS-modified PEGylated nanostructured lipid carriers to synergize its repurposing for anti-cancer therapy of solid tumors. Int J Pharm. 2019;557:374–389. doi: 10.1016/j.ijpharm.2018.12.051. [DOI] [PubMed] [Google Scholar]
- 55.Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov Today. 2003;8(24):1112–1120. doi: 10.1016/s1359-6446(03)02903-9. [DOI] [PubMed] [Google Scholar]
- 56.Hu CM, Aryal S, Zhang L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther Deliv. 2010;1(2):323–334. doi: 10.4155/tde.10.13. [DOI] [PubMed] [Google Scholar]
- 57.Sung JC, Pulliam BL, Edwards DA. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007;25(12):563–570. doi: 10.1016/j.tibtech.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Fasehee H, Dinarvand R, Ghavamzadeh A, Esfandyari-Manesh M, Moradian H, Faghihi S, Ghaffari SH. Delivery of disulfiram into breast cancer cells using folate-receptor-targeted PLGA-PEG nanoparticles: in vitro and in vivo investigations. J Nanobiotechnol. 2016;14(1):32. doi: 10.1186/s12951-016-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deshmukh AS, Chauhan PN, Noolvi MN, Chaturvedi K, Ganguly K, Shukla SS, Nadagouda MN, Aminabhavi TM. Polymeric micelles: basic research to clinical practice. Int J Pharm. 2017;532(1):249–268. doi: 10.1016/j.ijpharm.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 60.Xu W, Ling P, Zhang T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv. 2013;13:340–415. doi: 10.1155/2013/340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yokoyama M. Clinical applications of polymeric micelle carrier systems in chemotherapy and image diagnosis of solid tumors. Med Sci Monit. 2011;3(4):151–158. [Google Scholar]
- 62.Farooq MA, Aquib M, Ghayas S, Bushra R, Haleem Khan D, Parveen A, Wang B. Whey protein: a functional and promising material for drug delivery systems recent developments and future prospects POLYM ADVAN TECHNOL. 2019. [Google Scholar]
- 63.Farooq MA, Aquib M, Khan DH, Ghayas S, Ahsan A, Ijaz M, et al. Nanocarrier-mediated co-delivery systems for lung cancer therapy: recent developments and prospects. Environ Chem Lett. 2019:1–9.
- 64.Wong CY, Al-Salami H, Dass CR. Microparticles, microcapsules and microspheres: a review of recent developments and prospects for oral delivery of insulin. Int J Pharm. 2018;537(1–2):223–244. doi: 10.1016/j.ijpharm.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 65.Kesharwani R, Sachan A, Singh S, Patel D. Formulation and evaluation of solid lipid nanoparticle (SLN) based topical gel of etoricoxib. J appl pharm sci. 2016;10:124–131. [Google Scholar]
- 66.Amol MP, Rohit RT, Dipak SG, Mohite SK, Magdum CS. A review on solid lipid nanoparticle. Res J of Pharm and Tech. 2016;8(3):218. [Google Scholar]