Abstract
Paraquat is one of the most commonly used lethal herbicide. Even the small amount of paraquat is hazardous to human beings. Poisoning by paraquat is most commonly happening in agricultural based economical country. This poison is a threat to public health and its high mortality rate is responsible for a significant number of death. We hereby report a case of 38-year-old male with a history of accidental paraquat ingestion. He works as a gardener which contributes to the ease in availability of the poison. He had the post accidental paraquat consumption complaints of lip sores, swallowing difficulty and hypersalivation in more amount. The patient was admitted in the Casualty and underwent the supportive treatment. Laboratory investigations were found to be normal except WBC count which was elevated. Early diagnosis and proper management can reduce the mortality rate and even small amount of paraquat can lead to some major fatal outcomes. At present there is no specific antidote available so there is a need to focus more on the prevention and management of paraquat poisoning.
Keywords: Poison, Casualty, Paraquat, Fatal, Accidental, Herbicide
Introduction
Herbicide has been increasing in our agriculture. Now a day’s Ooty (Nilgiris) is more prominent for agriculture. Paraquat is nitrogen herbicide which is highly toxic to humans and animals [1]. Paraquat is used to kill unwanted weeds and other vegetation before planting crops. Paraquat belongs to bipyridyl group, chemically 1, 1 dimethyl 1, 4, 4 bipyridylium dichloride. It was discovered in 1950 and it began to use as herbicide from 1960. It is available in granular and liquid form [2]. Paraquat is highly toxic herbicide for which fatality rate of paraquat ranges from 60 to 80% [3]. In India paraquat is available in 10–20% solution, hence 10 ml of 20% solution contains about 2 g of paraquat [4].
Paraquat distributes into all organs. It can be absorbed, inhaled or ingested where its minimal amount can be hazardous. Rapidly it absorbs via ingestion and it tends to accumulate in organs such as lungs and kidneys. Paraquat’s volume of distribution is large [5]. Concentrated solutions of paraquat corrodes the Gastrointestinal mucosa. In case of ingestion difficulty in swallowing, sore throat and mouth ulcers can occur. Here in our study patient developed all these symptoms.
Reason for report
The main reason for reporting the case study is that in the particular region The Nilgiris, it is rare and unusual. It can create further knowledge among who will come across this type of poison cases.
Case presentation
A 38 years old male patient was admitted in casualty, at Secondary care hospital, Ooty for the paraquat accidental ingestion after two days of ingestion. Patient was opening the container of paraquat with his mouth and accidentally it got ingested. And post ingestion he developed the delayed symptoms of hyper salivation, lip sores and difficulty in swallowing. On examination the patient was conscious, oriented, afebrile and no history of breathing difficulty. His vital reports were found to be BP (Blood pressure): 160/110 mmHg, PR (Pulse rate): 80 beats/min, RR (Respiratory rate): 14 per minute, P/A (Per abdomen): soft. His laboratory investigations found to be normal and appropriate except the WBC (White blood count): 14.7/mm3. Other laboratory values which were observed on day 1 are presented in Table 1. From casualty department he was transferred to ICU (Intensive care unit) and on day 1 he was treated with Ringer lactate, Normal saline and Dextrose normal saline each of 500 ml, Inj. Ranitidine 50 mg IV BD, Inj. Cefotaxime 1 g IV BD, Inj. Ondansetron 4 mg IV SOS, Inj. Diclofenac 25 mg/2 ml IM STAT.
Table 1.
Laboratory investigations at the day of admission in secondary care hospital, Ooty
| Investigation | Patient observed value | Normal value |
|---|---|---|
| Haemoglobin (Hb) | 17.9 g/dL | 14-18 g/dL |
| Total count (Tc) | 14.7 × 103cells/mm3 | 3.2–.9.8 × 103cells/mm3 |
| Platelet count(Pt) | 388 × 103/mm3 | 130–400 × 103/mm3 |
| Red blood cell(RBC’s) | 5.76 × 106/mm3 | 4.3–59 × 106/mm3 |
| Hematrocrit(Hct) | 51.3% | 39–49% |
| Mean cell volume (MCV) | 89.1m3 | 76-100 m3 |
| Mean cell hemoglobin (MCH) | 31.1 pg/cell | 27-33 pg/cell |
| Mean cell hemoglobin concentration (MCHC) | 34.9 g/dL | 33.37 g/dL |
| Random blood sugar (RBS) | 94 mg/dL | <200 mg/dL |
| Blood urea | 35 mg/dL | 20-40 mg/dL |
| Serum creatinine (Sr.cr) | 1.1 mg/dL | 0.6–1.2 mg/dL |
| AST-Asparate aminotransferase (SGOT) | 26 U/L | 0-35 U/L |
| ALT-Alanie aminotransferase (SGPT) | 18 U/L | 0-35 U/L |
| Alkaline phosphate (ALP) | NA | 30-120 U/L |
| Bilirubin: Total | 1.3 mg/dL | 0.1-1 mg/dL |
| Direct | 0.8 mg/dL | 0.-0.2 mg/dL |
| Indirect | 0.5 mg/dL | 0.1–0.8 mg/dL |
| Pus cell (pc) | 4–5/hpf | 1–2/hpf |
| Epithelial pus cell (epc) | 2–4/hpf | 1–2/hpf |
On day 2, at examination, patient was conscious, oriented and afebrile. BP: 130/80 mmHg, SPO2: 94%. Patient was given with RL and NS 1 pint and DNS 2 pint of IVF and each followed with Inj. Ranitidine 50 mg IV BD, Inj. Vit K 1 ampule 10 mg IM BD, Inj. dexamethasone 8 mg IV BD, and Inj. cefotaxime 1 g IV BD.
On day 3, patient was conscious, afebrile. Urea level was found to be 45 mg/dL and creatinine level was 3.2 mg/dL. Patient was treated with same medications. Patient’s prognosis was poor and for this he was referred to Primary health care Centre for further management.
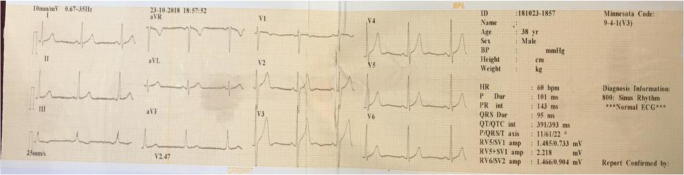
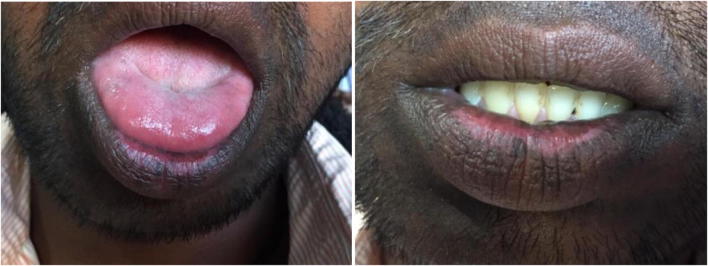
Follow-up was done for the patient at Primary health care Centre. At the time of admission, he was treated with Inj. Methylprednisolone 500 mg, Inj. Ceftriaxone 1 g, Inj. Ciprofloxacin 100 mg, Inj. Pantoprazole 40 mg and he undergone there for hemodialysis. The laboratory investigation at the day of admission in primary health care hospital is presented in Table 2. His ECG report is presented in Fig. 1. He was admitted there for further 15 days and he was given the treatment of Inj. Ceftriaxone, Inj Pantoprazole, Inj. Ciprofloxacin, Tab. Mecosan, Tab. Folvite, Tab. Shelcal, and Cap. Becosules. His creatinine and urea levels are presented in Table 3. The creatinine and urea level was normal on 14th day. And he was discharged with Tab. Liv 52, Practin syrup, Tab. Hepa merz and Oral fast ointment for oral infections. His prognosis was better and lip sores got treated and it is presented in Fig. 2.
Table 2.
Laboratory investigations at the day of admission in Primary health care Centre
| Investigation | Patient observed value | Normal value |
|---|---|---|
| Haemoglobin (Hb) | 15.6 g/dl | 13.5–18 g/dl |
| Total count (Tc) | 7600 cells/cmm | 4000–1000 cells/cmm |
| Platelet count (Pt) | 2,75,000 cells/cmm | 1,50,000–4,00,000 cells/cmm |
| Red blood cell (RBC’s) | 5.09 million/cmm | 4.2–5.4 million/cmm |
| Hematrocrit (Hct) | 46.8% | 42–52% |
| Mean cell volume (MCV) | 96.0cubic/μm | 80–95cubic/μm |
| Mean cell hemoglobin (MCH) | 30.7 pg | 27–31 pg |
| Mean cell hemoglobin concentration (MCHC) | 32.0% | 28–36% |
| Random Blood sugar | 111.1 mg/dl | 80–140 mg/dl |
| Cholinesterase | 5180 U/L | 4620–11,500 U/L |
| Electrolyte sodium | 142.0 mEq/l | 133–145 mEq/l |
| Potassium | 4.7 mEq/l | 3.5–5.5 mEq/l |
| Chloride | 102.3 mEq/l | 94–107 mEq/l |
| Bicarbonate | 24.6 mmol/l | 22–30 mmol/l |
| Calcium | 9.5 mEq/l | 8–11 mEq/l |
| AST-Asparate aminotransferase (SGOT) | 48.2 IU/L | 17–50 IU/L |
| ALT-Alanine aminotransferase (SGPT) | 29 IU/L | 10–55 IU/L |
| Alkaline phosphate (ALP) | 168.3 IU/L | 37–147 IU/L |
| Bilirubin: Total | 1.16 mg/dl | 0.2–1.0 mg/dl |
| Direct | 0.35 mg/dl | 0.0–0.3 mg/dl |
| Indirect | 0.81 mg/dl | 0.2–0.8 mg/dl |
| Albumin | 4.6 g/dl | 3.5–5.0 g/dl |
| Globulin | 2.9 g/dl | |
| A/G Ratio | 1.59 | |
| Protein | 7.5 g/dl | 6.0–8.2 g/dl |
| Total count: Polymorph | 85.9% | 50–70% |
| Lymphocyte | 10.7% | 25–40% |
| Eosinophils | 01% | 1–5% |
| Basophils | 0% | 0.5–1.0% |
| monocytes | 2.4% | 0–2% |
Fig. 1.
ECG reveals normal values
Table 3.
Urea and creatinine value at primary health care centre
| Date | Urea (mg/dL) | Creatinine (mg/dL) |
|---|---|---|
| 23/10/2018 | 127.7 | 6.24 |
| 24/10/2018 | 120.8 | 5.72 |
| 25/10/2018 | 129.3 | 4.59 |
| 26/10/2018 | 135.0 | 4.06 |
| 28/10/2018 | 124.0 | 2.53 |
| 29/10/2018 | 123.5 | 2.84 |
| 31/10/2018 | 77.1 | 2.03 |
| 01/11/2018 | 62.3 | 2.02 |
| 02/11/2018 | 53.1 | 1.68 |
| 03/11/2018 | 41.5 | 1.50 |
| 05/11/2018 | 30.8 | 1.20 |
| 12/11/2018 | 22.0 | 0.98 |
Fig. 2.
Lip sores and ulcers got treated but marks are left behind (Patient consent was taken for publishing his information and pictures)
Discussion
Accidental poisoning can be hazardous and can lead to fatal outcomes. In India paraquat has been trade under gramoxone name. It is a fast acting herbicide [6]. There is no specific antidote available so primary supportive treatment can be given [7]. In case of accidental ingestion, the victim should approach the Health care professional immediately for the better treatment. India is known as an agricultural country where most of the farmers uses herbicides in day to day life but lack of knowledge is present about the products that how far it can be dangerous, so for that some workshops or Government running programs should be conducted. In our case patient accidentally ingested the small amount of paraquat which led him to fatal outcome of poison. The treatment was given according to the protocol but patient visited hospital after two days of ingestion to which supportive treatment was given. He was counselled about the various toxic and adverse effects of paraquat. The consent of patient was taken for publishing this data.
Outcome
The outcome of this presentation is every individual who is in the usage of pesticides and herbicides should be aware of the fatal outcomes of the substance. A small awareness can save a life and prevent it for future damage.
Conclusion
Paraquat poison is highly toxic in nature, early diagnosis and appropriate treatment can reduce the morbidity and mortality rate among the victims. This report is to view about the importance of early treatment of small ingestion that can lead to some fatal outcomes. Hyper salivation condition occurred due to the severity of the toxins. Condition of the patient was treated according to the symptoms of toxins and this patient recovered at the end of the treatment. Hence there is a crucial need to focus more on the poison management.
Acknowledgements
The authors acknowledge the support and cooperation provided by the staff of Government Headquarters Hospital Ooty. I would like to acknowledge our HOD Dr. S. Ponnusankar for his immense support and guidance.
Compliance with ethical standards
Conflict of interest
The authors do not report any Conflict of Interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180(1):65–77. doi: 10.1016/S0300-483X(02)00382-7. [DOI] [PubMed] [Google Scholar]
- 2.Arts J, Schuit G, Schipper A, Kleij B. A case report of paraquat poisoning. Eur J Hosp Pharm. 2006;12:22–24. [Google Scholar]
- 3.Weng CH, Hu CC, Lin JL, Lin-Tan DT, Huang WH, Hsu CW, Yen TH. Sequential organ failure assessment score can predict mortality in patients with paraquat intoxication. PLoS One. 2012;7(12):e51743. doi: 10.1371/journal.pone.0051743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal P, Wali JP. Diagnosis and management of acute poisoning. Delhi: Oxford University Press; 1997. pp. 1–38. [Google Scholar]
- 5.Bismuth C, Scherrman JM, Garnier R, et al. Elimination of paraquat. Hum Toxicol. 1987;6:63–67. doi: 10.1177/096032718700600110. [DOI] [PubMed] [Google Scholar]
- 6.https://www.syngenta.co.in/herbicides. © 2019 Syngenta. Accessed 2019.
- 7.Khayati M, Sreeja R, Anju R, Sivasankaran P. Paraquat induced acute renal failure: A case report. Int Res J Pharm. 2018;9(6):125–127. doi: 10.7897/2230-8407.096102. [DOI] [Google Scholar]