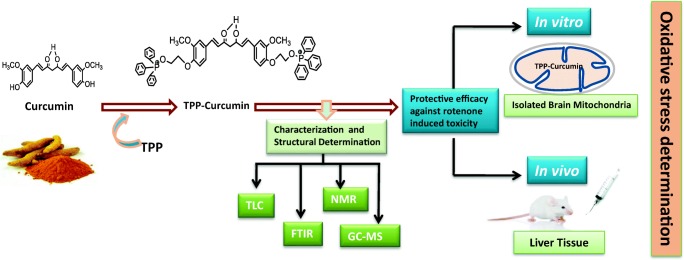
Abstract
Background
Mitochondrial impairments due to free radicals are implicated in a wide range of neurotoxicological alterations. Curcumin, an active ingredient of turmeric has shown protective efficacy against oxidative damage due to its strong antioxidant potential, but its efficiency is restricted due to low bioavailability in the mitochondria. In view of this, we have synthesized mitochondria-targeted curcumin (MTC) with an aim to investigate its efficacy against rotenone-induced oxidative damage in mice and isolated mitochondria.
Methods
MTC was synthesized by attaching the triphenylphosphonium cation (TPP) as a cationic carrier to the curcumin to assess its protective efficacy in rotenone-induced in-vitro and in-vivo toxicity in mice.
Results
In-vitro treatment of rotenone in isolated mitochondria caused a significant increase in lipid peroxidation (2.74 fold, 3.62 fold), protein carbonyl contents (2.62 fold, 1.81 fold), and decrease in levels of reduced glutathione (2.02 fold, 1.70 fold) as compared to control. Pre-treatment of curcumin and MTC along with rotenone in the isolated mitochondria significantly reduce the oxidative stress as compared to those treated with rotenone alone. Rotenone treatment in mice significantly increased lipid peroxidation (2.02 fold) and decreased the levels of reduced glutathione (2.99 fold), superoxide dismutase (2.09 fold) and catalase (3.60 fold) in the liver as compared to controls. Co-treatment of curcumin and MTC along with rotenone significantly reduced lipid peroxidation (1.26 fold, 1.76 fold) and increased the levels of reduced glutathione (1.60 fold, 2.43 fold), superoxide dismutase (1.45 fold, 1.99 fold) and catalase (2.32 fold, 2.90 fold) as compared to those treated with rotenone alone.
Conclusion
The results of the present study indicate that the protective efficacy of MTC against rotenone-induced oxidative damage was more promising than curcumin in both in-vitro and in-vivo system which indicates the enhanced bioavailability of MTC.
Graphical abstract.
Effect of mitochondrial targeted delivery of TPP-curcumin in rotenone-induced toxicity.
Electronic supplementary material
The online version of this article (10.1007/s40199-019-00283-2) contains supplementary material, which is available to authorized users.
Keywords: TPP-curcumin, Curcumin, Rotenone, Mice, Mitochondria, Oxidative stress
Introduction
A number of neurotoxicants are present in the environment which may affect human health and cause neurotoxicity and behavioral abnormalities. The primary target of these neurotoxicants is mitochondria to generate free radical species and enhanced oxidative stress. These free radicals further trigger the apoptotic cascade and also involved in the process of neurodegeneration. Mitochondria play a significant role in cellular bioenergetics, amino acid biosynthesis, fatty acid oxidation, urea cycle, lipid metabolism and homeostasis of cellular calcium ions [1–3]. Rotenone, an insecticide compound can easily cross biological membranes and work as a potential inhibitor of complex I of the electron transport chain [4, 5]. It has been shown to generate nigrostriatal dopaminergic neurodegeneration and behavioral alterations when infused in rodents [6, 7]. During electron transfer in the respiratory chain, electrons continually leak to form reactive oxygen species (ROS) and reactive nitrogen species (RNS) which cause damaging effects in the mitochondrial membranes through lipid peroxidation and protein oxidation [8, 9]. This oxidative damage induces mitochondrial permeability transition and disrupts calcium homeostasis leading to apoptosis and other pathological conditions, like ischemia-reperfusion injury, aging and neurodegenerative diseases. Rotenone-induced neuronal death has been found to be associated with the increased levels of pro-caspases – 3, 9 and 12, suggesting the activation and involvement of caspases in both mitochondrial and endoplasmic reticulum [10, 11]. Further, rotenone-induced dopaminergic neuronal death linked to the activation of p38 and JNK pathways have also been reported [12]. Incomplete electron transfer to oxygen due to rotenone increases hydrogen peroxide (H2O2) concentration that inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways leading to apoptosis in neuronal cells [13, 14].
Studies have been carried out to decipher the protective efficacy of natural and pharmacological agents, including vitamin E, vitamin C, curcumin, gallic acid, quercetin and resveratrol. Curcumin was reported several times more potent than other antioxidants as a free radical scavenger and effective against ROS and RNS [15–17]. Pre-clinical and clinical trials of curcumin have been reported in a wide variety of therapeutic effects, ranging from anti-inflammatory, antioxidant, chemopreventive, chemotherapeutic and anti-cancer activity [18]. All these antioxidants have been found to reduce oxidative damage in various disease conditions, but their efficiency is restricted due to low bioavailability within mitochondria. Mitochondria are the cytoplasmic organelles that maintain strong membrane potential (ΔΨ) across the inner mitochondrial membrane with highly negatively charged mitochondrial matrix [19]. Lipophilic cations such as triphenylphosphonium cation (TPP) accumulate into the mitochondrial matrix in response to membrane potential [19]. The antioxidant compounds conjugated with mitochondria targeted positively charged cations can be used to target mitochondria for exploring the potential of antioxidant [20, 21]. Mitochondria-targeted antioxidant transport through non-mediate transport, membrane potential driven and accumulate within the mitochondrial matrix and preserve mitochondrial functions against oxidative damage. In various studies, lipophilic TPP is used as a carrier for facilitating antioxidant to mitochondrial matrix [19, 22]. To date, researchers have developed various mitochondria-targeted antioxidants including curcumin, but the synthesis procedure of mitochondria-targeted curcumin (MTC) and its characterization is limited. In the present study, a three steps simple procedure of synthesis and characterization of MTC was developed by attaching TPP as a cationic carrier with an aim to evaluate its protective efficacy against rotenone-induced oxidative damage in isolated mitochondria and mice model.
Materials and methods
Chemicals and reagents
Potassium carbonate (K2CO3), sodium iodide (NaI), 1-bromo-4-chlorobutane, dimethyl formamide, ethyl acetate, and TPP were purchased from Himedia, India. Hydrochloric acid (HCl), dichloromethane (CH2Cl2), magnesium sulfate (MgSO4), curcumin, acetone, and toluene were purchased from CDH, India. n-hexane, diethyl ether, and pre-coated silica gel GF aluminum plate were purchased from Merck, India. Superoxide dismutase assay kit (Abcam, ab65354) and catalase assay kit (Abcam, ab83464) were purchased from Abcam, UK.
Scheme for synthesis
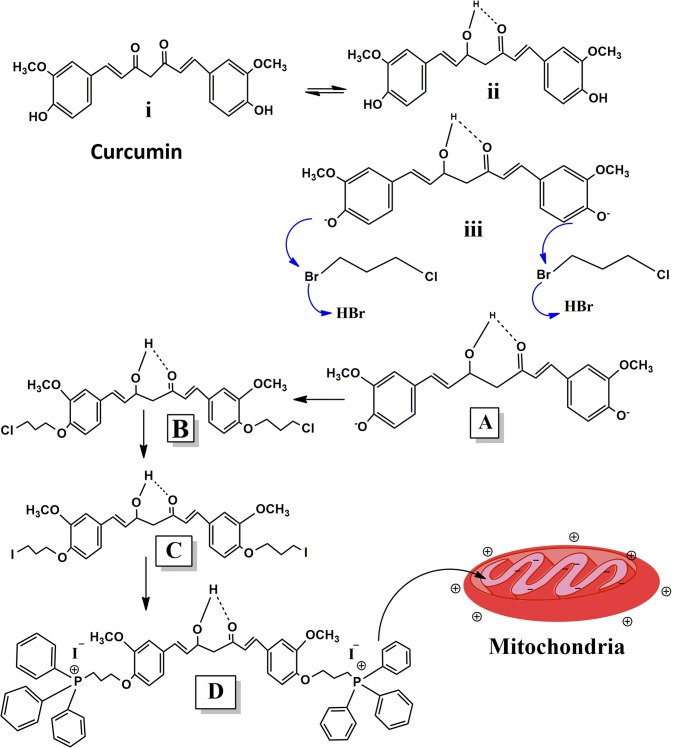
The reaction mechanism involved in the synthesis of MTC (TPP-Curcumin) is shown in Scheme 1 (Figure: A-D). In this scheme for the synthesis of desired compound ‘D’ (MTC) was synthesized in three steps reactions starting from the compound curcumin (Scheme 1,i). Due to quasi-aromaticity of curcumin, it is the more stable form is enol form (Scheme 1,ii) those going to the conversion of phenolic OH group into phenoxide ion (Scheme 1,iii) in the presence of K2CO3 which act as a base. In the first reaction, the addition of 1-bromo-4-chlorobutane in a reaction mixture led to SN2 type reaction and formed compound “B”. In this reaction, the phenoxide ion of curcumin acts as a nucleophilic attack on the 1-bromo-4-chlorobutane (Scheme 1, iii). I− is better leaving the group as well as good nucleophilic atoms, when NaI is adding in the reaction medium in the second reaction, I− atom of NaI compound replace the chloride and get converted into iodide derived compound ‘C’. In the reaction of the third step, when TPP is added in the reaction medium, compound ‘D’ was formed. In this process, TPP gets attached to compound ‘C’ by replacing iodide atom (Scheme 1d).
Scheme 1.
A: (i) Keto form of curcumin (IUPAC Name- (1E,6E)-1, 7-bis (4-hydroxy-3methoxyphenyl) hepta-1,6-diene-3,5-dione). A(ii) Enol form of curcumin. A (iii) Ionic form of curcumin. a: 4,4′-((1E,6E)-3-hydroxy-5-oxohepta-1,6-diene-1,7diyl) bis (2methoxyphenolate) b: ((1E,6E)-1,7- bis (4-(3-chloropropoxy)-3-methoxyphenyl)-5-hydroxyhepta-1,6-dien-3-one c: (1E,6E)-5-hydroxy-1,7-bis(4-(3-idopropoxy)-3-methoxyphenyl) hepta-1,6-dien-3-one d: Mitochondria-targeted derived compound 1,7-bis{3-methoxy-4-[3-(triphenylphosphonium) propoxy]-phenyl} hepta-1,6-diene-3,5-dionedi-iodide (TPP-curcumin)
Synthesis procedure
Step I
The first step was for the synthesis of compound ‘B’ i.e. (1E,6E)-1,7-bis(4-(3-chloropropoxy)-3-methoxyphenyl)-5-hydroxyhepta-1,6-dien-3-one. All the reactions were carried out under nitrogen atmosphere. Curcumin (4,4′-{(1E,6E)-3-hydroxy-5-oxohepta-1,6-diene 1,7diyl} bis(2methoxyphenolate) (3 g, 8.1 mmol) was dissolved in 10 mL dimethyl formamide. K2CO3 (0.750 g, 5.4 mmol) was added immediately to covert phenolic -OH into phenolic -O (Scheme 1 Figure- A) and then 1-bromo-4-chlorobutane (1.52 g, 8.86 mmol) was mixed into the reaction medium. The solution was kept stirring at 37 °C for 24 h. Progress of every reaction was monitored by thin-layer chromatography (TLC). After stirring, the solution was diluted with 100 mL ethyl acetate and washed with 1 N HCl three times (3x50mL) using the separating funnel. During the washing process, the two layers were formed, the upper layer (organic layer) was collected in a separate conical flask and dried with MgSO4 for two to three hours and filtered with the help of Whatman filter paper. The solvent was evaporated under reduced pressure in rotary vacuum evaporator (Jain Scientific Glass Works, India) and residue was purified by flash chromatography and eluted with ethyl acetate/petroleum ether (1:3) and 1E,6E)-1,7-bis(4-(3-chloropropoxy)-3-methoxyphenyl)-5-hydroxyhepta-1,6- dien-3-one (chromo curcumin) ‘B’ was collected.
Step II
It is for the synthesis of compound ‘C’ i.e. (1E,6E)-5-hydroxy-1,7-bis(4-(3-idopropoxy)-3-methoxyphenyl)hepta-1,6-dien-3-one. Synthesized product ‘B’ was added in a saturated solution of NaI in dry acetone (10 mL), and the solution was kept at room temperature and subsequently refluxed at 100 °C for two days. Progress of reaction and product formation was monitored by TLC. After cooling, the resultant mixture was diluted with ethyl acetate (100 mL), filtered and washed three times with water. The upper organic layer was taken into a separate conical flask and dried over MgSO4 for two hours and then filtered. The solvent was evaporated under reduced pressure in a rotary vacuum evaporator, and the residue was purified by flash chromatography.
Step III
This step is for the synthesis of compound ‘D’ i.e. 1,7-bis{3-methoxy-4-[3-(triphenylphosphonium) propoxy]-phenyl} hepta-1,6-diene-3,5-dionedi-iodide (TPP-curcumin). Compound ‘C’ [(1E,6E)-5-hydroxy-1,7-bis (4-(3-idopropoxy)-3-methoxyphenyl)hepta-1,6-dien-3-on] obtained from step II was dissolved in toluene (15 mL), and the appropriate amount of triphenylphosphine was added to it. The solution was refluxed at 100 °C until completion of the reaction (white precipitate was observed). The reaction mixture was allowed to cool at room temperature, the solvent was eliminated at reduced pressure, and the yielded colorless crystalline powder was dissolved in the minimum volume of acetone and precipitated with diethyl ether (100 mL). The precipitate was filtered and then dried in an oven at 55 °C. The yield was 73%, and the melting point of the final product (MTC) was 108–120 °C. The molecular weight of curcumin was 368 g/mol, and molecular weight of the synthesized product (TPP-curcumin) is 1044 g/mol.
Solubility, characterization and structural determination of synthesized product
The solubility of both curcumin and MTC has been checked and observed that MTC (10 mg/ml) was soluble in acetone, chloroform, dimethyl sulfoxide (DMSO), moderate soluble in methanol, ethanol and insoluble in water. In case of pure curcumin (10 mg/ml), best solubility found in acetone, DMSO, moderate solubility in methanol, ethanol, chloroform and poorly soluble in water. Both the compound showed similar solubility pattern.
Characterization and structural determination of the synthesized product was carried out using analytical techniques such as TLC, Fourier transform infra-red spectroscopy (FTIR), Nuclear magnetic resonance (NMR) and Gas chromatography-mass spectrometry (GC-MS).
Thin layer chromatography
It is a simple, easy to use the analytical method without any requirement of sophisticated instrumentation. During reaction in every step, the progress of every reaction was monitored by TLC method on pre-coated silica-gel sheets under a flow of mobile phase, i.e. chloroform: methanol (9:1 ratio). TLC plates were dried and visualized under the saturated iodine fuming chamber.
Fourier-transform infrared spectroscopy
FTIR spectra of curcumin and MTC were recorded with a spectrophotometer using KBr pellets, the scanning range was used from 400 to 4000 cm−1 with the resolution set at 2 cm−1. A change in the peak intensity of the wave number of products (TPP-Curcumin) was compared to the already reported FTIR spectrum (curcumin) [23] which indicated that TPP has got attached to native curcumin.
Gas chromatography - mass spectrometry
GC-MS was performed under electro-spray ionization (ESI) conditions at a resolution of 61,800 using a JEOL AccuTOF4GCV mass spectrometer (MS;4.02;17.81;/El+), Agilent 7890AGC (GC; A.01.15). Elemental analysis was done on a JEOL AccuTOF4GCV FLASH-2000 CHN analyzer, and NIST MS search 2.0 library and Mass centre V2.6.6b version was used [24].
Nuclear magnetic resonance
1H NMR spectra of compounds (in CDCl3) were recorded on a JEOL, ECX-500 at 500 MHz. Singlet, s; doublet, d; triplet, t; multiplet, m; double doublet, dd; were designed as peak multiplicities with a singlepulse.ex2. Chemical shifts were reported as δ (5.0 ppm) [24].
Morphological analysis of curcumin and MTC
Scanning Electron microscopy (SEM)
SEM is a qualitative technique for the measurement and analysis of the surface roughness and to get images of the surface texture. The morphology of the curcumin and MTC surface was examined through a scanning electron microscope (NOVA NANO SEM-450) operating at 15–18 kV. Before imaging, the samples were coated with gold using a sputter-coater (Denton Desk V) in an inert argon atmosphere.
Biological evaluation of synthesized product on mice model
Animals
Healthy male Swiss albino adult mice were procured from the Institute of Veterinary Science and Animal Husbandry, Mhow, India. The animals were housed in polypropylene cages under standard conditions (25 ± 1 °C with 12 h light / dark cycle) and were provided with standard food and water ad libitum. All procedures were done in accordance with CPCSEA and study was approved by the Institutional Animal Ethical Committee (IAEC No. AIPS/2015/2459/IAEC-04) of Adina College of Pharmacy, Sagar (MP).
In-vitro evaluation of synthesized product on isolated brain mitochondria
Mitochondria preparation
The healthy animals were sacrificed by decapitation. The brain was rapidly excised and placed into ice cold isolation medium (0.25 M sucrose). The brain was weighed finely, minced and homogenized (10% w/v) in an isolating medium using Teflon homogenizer (Remi Stirrer, 125S/U, Bombay India). Mitochondria were isolated from the mice brain by conventional differential centrifugation method as described [25]. The isolation buffer contains sucrose (0.25 M), ethylene diamine tetra acetic acid (EDTA, 1 mM) and Tris- HC1 (10 mM, pH 7.4). The experiments were performed immediately after purification of the mitochondria.
Mitochondria treatment
Mitochondria were incubated in an incubation medium containing potassium chloride (KCl) (120 mM), phosphoric acid (2 mM) and Tris-HCl buffer (15 mM). Isolated mitochondria were divided into four groups. The first group served as control and the second group was treated with rotenone 2 μM for 30 min. The third group was treated with curcumin 1 mM for 15 min and then incubated with rotenone 2 μM for 30 min. The fourth group was treated with MTC 1 mM for 15 min and then incubated with rotenone 2 μM for 30 min.
Sample preparation for assays
After incubation, different groups of treated mitochondria were assayed using suitable buffers. Phosphate buffer (0.05 M) was used for lipid peroxidation assay, phosphate buffer (100 mM, pH 7.4) containing 0.1% digitonin was used for protein carbonyl assay and 0.1 M cold metaphosphoric acid containing 0.02% EDTA and 0.1 mM5,5-dithio-bis-2-nitrobenzoic acid (DTNB) was used for reduced glutathione (GSH) assay.
In-vivo evaluation of synthesized product on liver
Animals and treatment
Mice were randomly divided into four groups having four mice in each group. In group I, mice were treated with corn oil as vehicle control. In group II, mice were treated with rotenone (suspended in corn oil, 3 mg/kg body weight, p.o., daily for 60 days). Mice in group III were simultaneously treated with rotenone and curcumin (suspended in corn oil, 30 mg/kg body weight, p.o., daily for 60 days). Mice in group IV were simultaneously treated with rotenone and MTC (suspended in corn oil, 30 mg/kg body weight, p.o., for 60 days). On the last day of treatment, mice from all the groups were sacrificed by cervical dislocation and liver was removed rapidly and washed with chilled saline solution. Tissue was minced into small pieces with scissors and homogenized in 10% homogenate buffer using Teflon homogenizer (Remi Stirrer, 125S/U, Bombay India). Homogenate was centrifuged (1000 rpm for 10 min at 4 °C) to remove the debris and the supernatant was taken out. The supernatant was again centrifuged (12,500 rpm for 15 min at 4 °C), and the pellet was discarded. The clear supernatant was taken for analysis of lipid peroxidation, GSH, catalase and SOD assays.
Biochemical assays
Lipid peroxidation
Lipid peroxidation was determined by measuring thiobarbituric acid reactive substance (TBARS) in terms of malonaldehyde (MDA) yielding a pink colored compound trimethine complex using the method of Ohkawa et al., [26]. The product was determined by measuring absorbance at 548 nm. The result was expressed as nmol MDA/mg protein.
Protein carbonyl content
Protein carbonyl content was measured according to the method of Levine et al., [27] using 2,4-dinitrophenylhydrazine (DNPH) as substrate. Briefly, in a 0.5 ml of mitochondrial sample, streptomycin sulfate solution (10% w/v) was added to a final concentration of 1% to precipitate DNA. The mixed solution was left to stand for 15 min at room temperature, and then it was centrifuged at room temperature. The supernatant was removed and divided equally between two test tubes. Now DNPH was added to one tube and 2 M HCl to the other tube. The tubes were then incubated for 1 h at room temperature, and then the protein was precipitated by adding an equal volume of trichloroacetic acid (TCA) and leaving them for 15 min. The protein was centrifuged at room temperature, and the pellet was washed with ethyl acetate: ethanol mixture (1:1, v/v) to remove excess DNPH. The final protein pellet was dissolved in guanidine hydrochloride, and the absorbance of both DNPH and HCl solution was measured at 370 nm in a UV spectrophotometer. The results of carbonyl contents were expressed in terms of nmol carbonyl group/mg protein.
Assay of reduced glutathione
The GSH content was quantified as described [28], involving the spectrophotometric assessment of the formation of 5-thio-2-nitrobenzoate from DTNB in the presence of NADPH and glutathione reductase. In parallel, a calibration curve was prepared using glutathione (2-10 μg) as standard. The values were expressed as μmol GSH/mg protein.
Assay of superoxide dismutase activity
Activity of SOD was assayed in liver tissue by colorimetric assay kit (Abcam, ab65354). The assay utilizes a tetrazolium salt WST-1 that produces a water-soluble formazan dye upon reduction with superoxide anion that can be detected at 450 nm. The rate of WST-1 reduction is linearly related to the inhibition activity of xanthine oxidase (XO) by SOD. SOD catalyzes the dismutation of the superoxide anion into hydrogen peroxide and molecular oxygen, resulting in a decrease of WST-1 reduction. The activity of SOD was expressed in percent inhibition, which in turn reflects the percent inhibition of the superoxide anions.
Assay of catalase activity
Activity of catalase was assayed in liver tissue by colorimetric assay kit (Abcam, ab83464). In this assay, H2O2 reacts with catalase present in the sample and produce water and oxygen. The unconverted H2O2 reacts with a probe to produce a product that can be measured colorimetrically at OD 570 nm.
Protein estimation
Mitochondrial protein concentration was determined by Folin-phenol reaction assay as described by Lowry et al. [29]. The peptide bonds in protein react with copper sulfate to give a blue colored complex. Briefly, mitochondrial sample and water were taken in the appropriate amount and then protein reagent was added to all tubes, vortex and allowed to stand for 5 min at room temperature. This was followed by the addition of Folins-Ciocalteau reagent to all the tubes and incubated for 30 min at room temperature. The absorbance was read at 660 nm. A standard curve was prepared using BSA for calculation of protein concentration, and the value was expressed as mg protein/ml of sample.
Statistical analysis
All the statistical analysis was performed using statistical software Microsoft office excel 2007 version and Sigma plot 12.0. Values were expressed as the mean ± standard error of the mean (SEM). The comparison of groups was performed by one-way analysis of variance (ANOVA), using the Tukey test method. The value of p < 0.05 was considered as significant.
Results
Characterization and structural determination of compound
Thin layer chromatography
Changes in retardation factor (Rf) during progress in every reaction by using method thin-layer chromatography, which confirmed that compound would be formed (figures are in supplementary copy).
Fourier-transform infrared spectroscopy
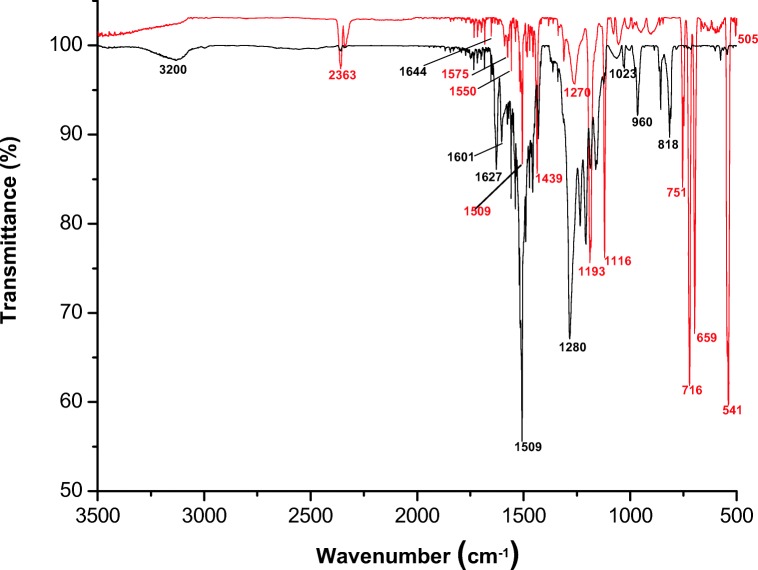
FTIR spectroscopy has been performed to determine the attachment of TPP with curcumin. In Fig. 1, black line shows the FTIR spectrum of curcumin, the characteristic broad peak of O-H at 3200 cm−1. The sharp peak at 1621 cm−1 and 1510 cm−1 were assigned to C=C, and C=O aromatic ring stretching and peak at 1282 cm−1 shows C-O in enol form of curcumin. The 1023 cm−1, 965 cm−1 peak shows C-O-C, and trans -CH vibration respectively and 818 cm−1 shows cis CH vibration of aromatic rings.
Fig. 1.
FTIR spectra mitochondria targeted curcumin (red line) compared with standard curcumin (black line)
In the product (TPP-Curcumin) the stretching vibration of P=O is observed at a lower frequency of 1109 cm−1 which indicated the presence of bonds between TPP and carbon of curcumin. Moreover, the absence of -OH frequency of curcumin at 3200 cm−1 indicated that -OH group involved in bond formation with TPP. The shift in the wave number value of the carbonyl absorption peak from 2363 cm−1 to 1734 cm−1 was observed in the FTIR spectrum, which also confirmed the TPP attached with curcumin. Another conformation proved that the presence of N = C and ring N–C–S vibrations at 1654 cm−1 and 1299 cm−1 corroborated the formation of a thiazolidinone ring (Fig. 1).
Gas chromatography-mass spectroscopy
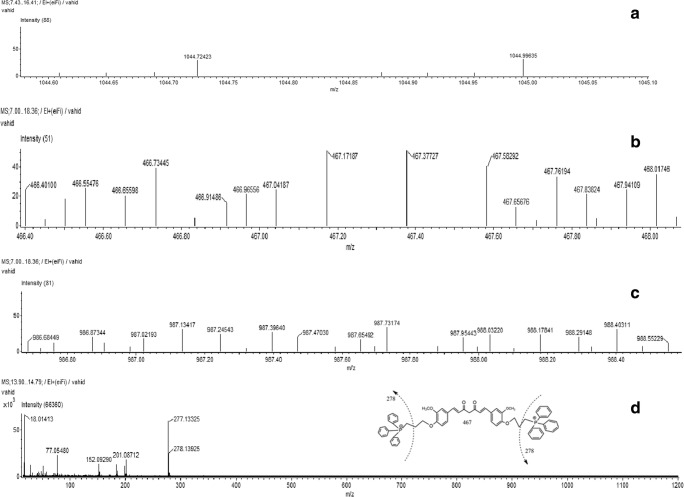
The mass spectra exhibited that the molecular ion produced was in agreement with the molecular weight (Fig. 2). In GC/MS analysis, the synthesized compound peaks show molecular ions at m/z 1044 and 987. The major fragment ions of peaks m/z 467 and 278 were the same as those of TPP-curcumin. The structure of the synthesized product was confirmed by 1H-NMR analysis.
Fig. 2.
a-d Gas Chromatography–Mass Spectrometry (GC–MS) analysis of synthesized product (Mitochondria targeted curcumin and its major fragments)
Nuclear magnetic resonance
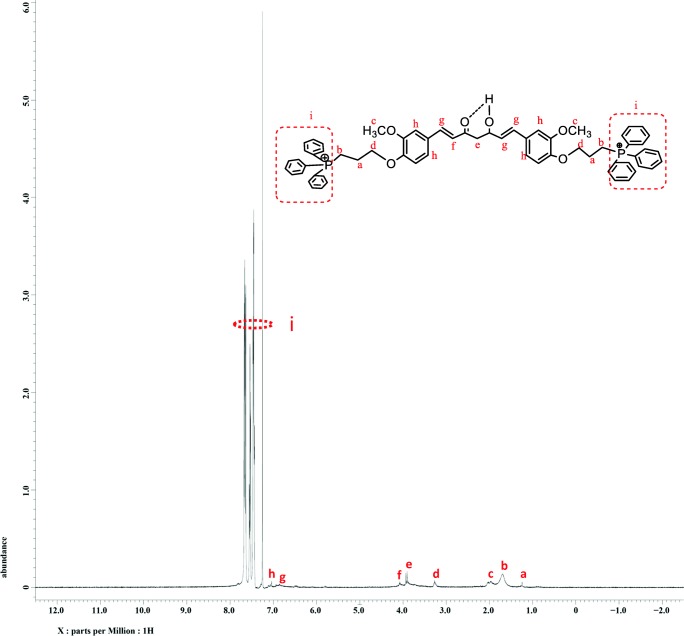
The reaction product (TPP-curcumin) was purified, isolated and analyzed for structural determination through 1H-NMR and represented in Fig. 3. The operating condition were 1H-NMR (500 MHZ, CDCl3, TMS, δppm): 1.2(m,4H,CH2), 1.7(t, 4H, CH2PPh2), 3.2 (t, 3H, OCH3), 3.9 (t, 4H, OCH2), 4.1 (m,2H, CH2CO) 6.6(S,1H, CH), 7.1 (s, 1H, CH), 7.3–8(m, 36H, PPh3). Peak integral assigned to the TPP protons at 7.3–7.8 ppm, and curcumin represent the synthesis of TPP-curcumin (Fig. 3).
Fig. 3.
1H-NMR spectra of mitochondria targeted curcumin and its chemical structures, provided for the interpretation of the 1H-NMR spectra
Morphological analysis of curcumin and MTC
Scanning Electron microscopy (SEM)
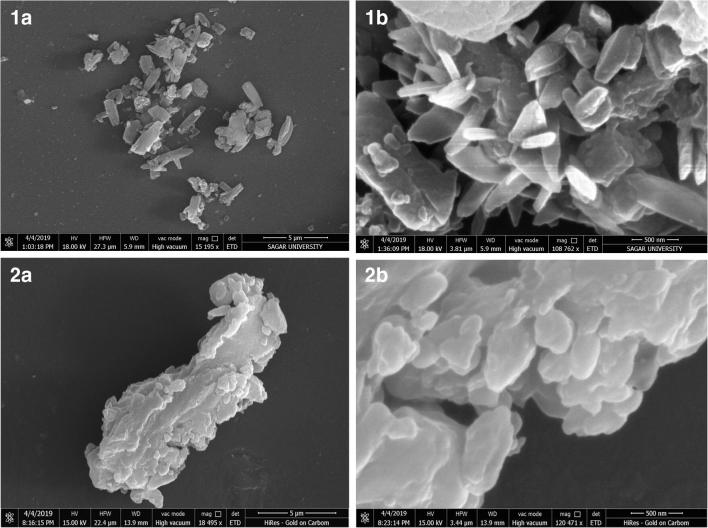
Ultra structural imaging of curcumin and MTC showed that the curcumin particles are irregular and flat needle-shaped with sharp edges while the MTC particles have spherical shaped with smooth edges as shown in the Fig. 4.
Fig. 4.
Scanning electron microscopy images of curcumin (1A and 1B) and TPP-curcumin (2A and 2B)
Effect on oxidative stress
In-vitro study
Isolated brain mitochondria were used for the evaluation of the protective effect of curcumin and MTC against well known complex I inhibitor, rotenone. Pre-treatment of curcumin and MTC were given to the isolated mitochondria to enrich them with the protective agent to investigate the protective role against rotenone-induced toxicity.
Effect on lipid peroxidation
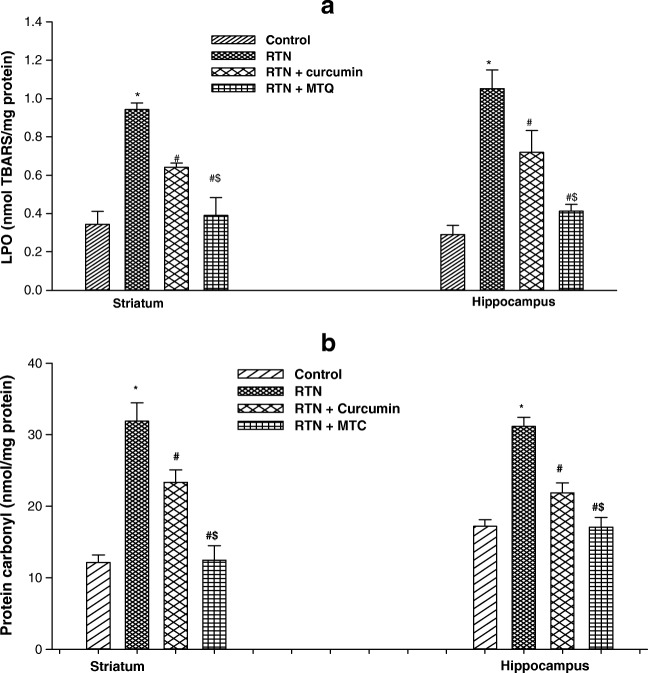
A significant increase in the level of lipid peroxidation in isolated mitochondria of the striatum (2.74 fold) and hippocampus (3.62 fold) was observed in the rotenone-treated mice as compared to controls (Fig. 4a). Pre-treatment of curcumin and MTC reduced the levels of lipid peroxidation in the striatum (1.47 fold, 2.41 fold) and hippocampus (1.46 fold, 2.54 fold) respectively in isolated mitochondria as compared to those treated with rotenone alone. Pre-treatment of mitochondria with MTC was more effective in reducing the level of lipid peroxidation in the striatum (1.64 fold) and hippocampus (1.74 fold) against rotenone-induced toxicity as compared to curcumin (Fig. 5a).
Fig. 5.
a Effect on lipid peroxidation b protein carbonylation in isolated mitochondria from brain regions of mice following exposure of RTN, co-treatment of curcumin and MTC with RTN . Values are mean ± SE of four animals in each group. RTN — Rotenone, CUR — Curcumin and MTC-mitochondria targeted curcumin. *Significantly differs from control group, #Significantly differs from rotenone treated group, $Significantly differs from curcumin group. Significantly differs (p < 0.05)
Effect on protein carbonyl contents
The protective effect of curcumin and MTC was also assessed against rotenone-induced increased protein carbonyl contents in the striatum and hippocampal mitochondria. Figure 5b shows a significant increase in the level of protein carbonyl contents in isolated mitochondria of the striatum (2.62 fold) and hippocampus (1.81 fold) treated with rotenone in comparison to control. Pre-treatment of curcumin and MTC reduced protein carbonyl contents in the striatum (1.36 fold, 2.55 fold) and hippocampus (1.42 fold, 1.82 fold) respectively in isolated mitochondria as compared to those treated with rotenone alone. Pre-treatment of mitochondria with MTC was more effective in reducing the level of protein carbonyl contents in the striatum (1.87 fold) and hippocampus (1.27 fold) against rotenone-induced toxicity as compared to curcumin.
Effect on reduced glutathione levels
Mitochondrial GSH serves as major antioxidants and detoxifying enzymes and maintains the appropriate mitochondrial redox environment to avoid or repair oxidative modifications leading to mitochondrial dysfunctions and cell death. As shown in Fig. 6, the rotenone-treated isolated mitochondria caused a significant lowering of GSH levels in the striatum (2.02 fold) and hippocampus (1.70 fold) as compared to control. Pre-incubating mitochondria with curcumin and MTC along with rotenone, maintain the GSH level compared with rotenone one. The GSH level maintained in curcumin and MTC treated striatum (1.29 fold, 1.30 fold) and hippocampal (1.19 fold, 1.42 fold) respectively in isolated mitochondria as compared to those treated with rotenone alone. The MTC was found more effective in preserving the GSH level in the striatum (1.30 fold) and hippocampus (1.19 fold) in rotenone-induced toxicity as compared to curcumin.
Fig. 6.
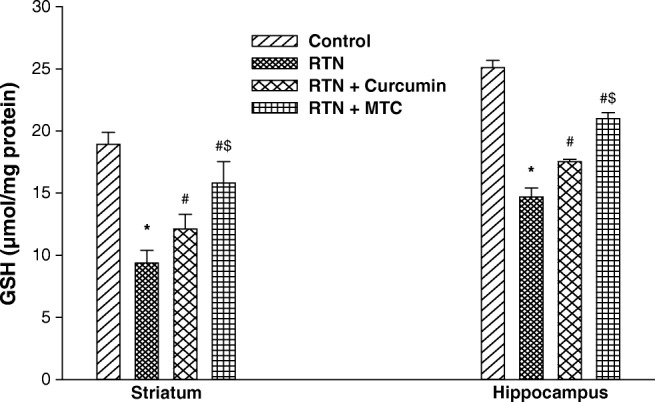
Effect on reduced glutathione level in isolated mitochondria from brain regions of mice following exposure of RTN, co-treatment of curcumin and MTC with RTN . Values are mean ± SE of four animals in each group. RTN — Rotenone, CUR — Curcumin and MTC-mitochondria targeted curcumin. *Significantly differs from control group, #Significantly differs from rotenone treated group, $Significantly differs from curcumin group. Significantly differs (p < 0.05)
In-vivo study
The protective effect of curcumin and MTC against well-known complex I inhibitor, rotenone was also assessed in in-vivo studies on mice model. The effect of rotenone and co-treatment of curcumin and MTC in mice was measured in the liver. Being an inhibitor of complex I, rotenone-induced oxidative stress results in liver damage that causes liver dysfunctions.
Effect on lipid peroxidation
In-vivo evaluation of the protective effect of curcumin and MTC against well known complex I inhibitor rotenone. The result showed that the administration of rotenone significantly elevated the MDA level (2.02 fold) in the liver of mice when compared to control group. Co-treatment with rotenone along with curcumin and MTC significantly decrease the MDA level (1.26 fold, 1.76 fold) respectively as compared to those treated with rotenone alone. MTC supplements more effectively (1.39 fold) diminish the levels of MDA than curcumin in the liver (Fig. 7a).
Fig. 7.
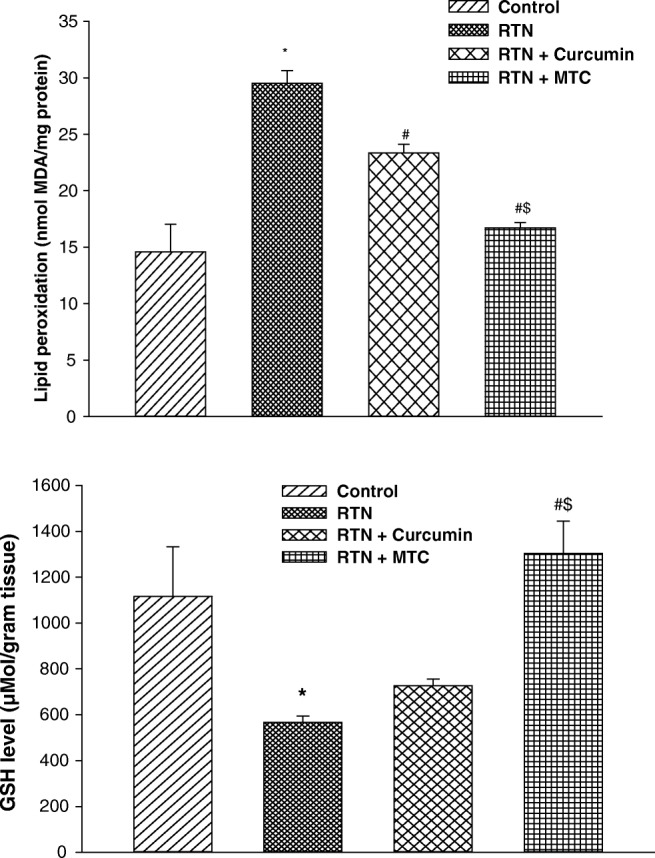
a Effect on lipid peroxidation b reduced glutathione levels in liver of mice exposed to RTN, cotreatment of curcumin and MTC with RTN. Values are mean ± SE of four animals in each group. RTN —Rotenone, CUR — Curcumin and MTC-mitochondria targeted curcumin. *Significantly differs from control group, #Significantly differs from rotenone treated group, $Significantly differs from curcumin group. Significantly differs (p < 0.05)
Effect on reduced glutathione levels
Treatment of rotenone significantly depleted the GSH levels in the liver (2.99 fold) as compared to the control group. Curcumin and MTC supplements normalized the GSH levels (1.60 fold, 2.43 fold) respectively as compared to those treated with rotenone alone. MTC treated group more effectively normalized (1.52 fold) the GSH level as compared to curcumin-treated group (Fig. 7b).
Effect on superoxide dismutase activity
The activity of SOD was significantly lower in the rotenone-treated group (2.09 fold) as compared to the control group. Treatment of MTC and curcumin, along with rotenone significantly enhanced the SOD activity (1.45 fold, 1.99 fold) respectively as compared to those treated with rotenone alone. MTC treated group was more potentially enhanced SOD activity (1.37 fold) than curcumin (Fig. 8a).
Fig. 8.
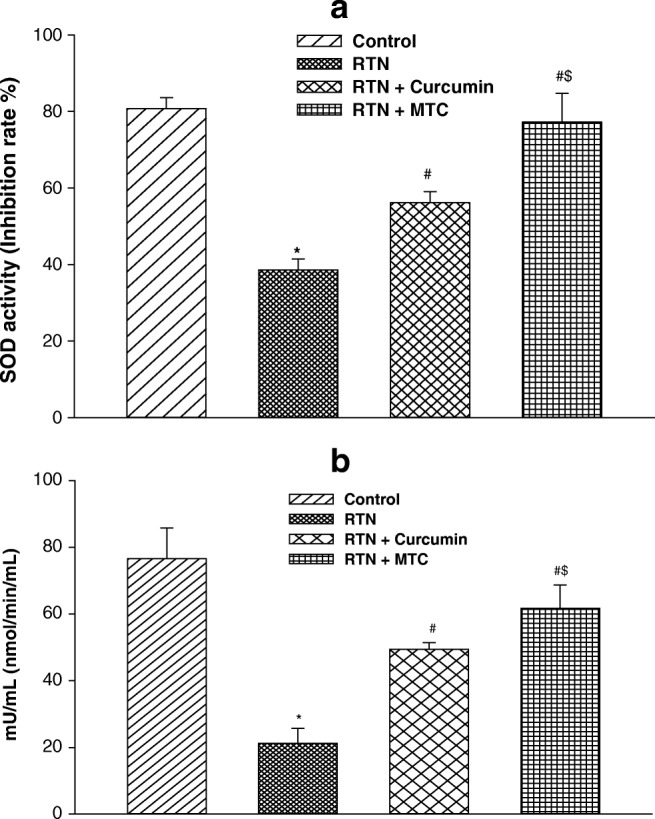
a Effect on superoxide dismutase activity b catalase activity in liver of mice exposed to RTN, cotreatment of curcumin and MTC with RTN. Values are mean ± SE of four animals in each group. RTN —Rotenone, CUR — Curcumin and MTC-mitochondria targeted curcumin. *Significantly differs from control group, #Significantly differs from rotenone treated group, $Significantly differs from curcumin group. Significantly differs (p < 0.05)
Effect on catalase activity
The activity of catalase was significantly diminished in the rotenone-treated group (3.60 fold) as compared to control. Treatment of MTC and curcumin, along with rotenone significantly increased the catalase activity (2.32 fold, 2.90 fold) respectively as compared to those treated with rotenone alone. Catalase activity prevented more in MTC treated group (1.24 fold) against rotenone as compared to curcumin-treated group (Fig. 8b).
Discussion
The curcumin possesses significant antioxidant and free radical scavenging activities due to the presence of both phenolic and β-diketone functional groups in its structure [30]. In addition, the supplementation of curcumin also enhances the activities of antioxidant enzymes such as SOD, catalase and glutathione peroxidase in the cellular system [31]. Curcumin has been shown to reduce ROS and prevent oxidative damage in the mitochondria and also exhibits anti-inflammatory and anti-cancer properties. The protective efficacy of curcumin against oxidative damage is enhanced many folds when it enters into the mitochondria than the curcumin present outside the mitochondria. The TPP has a positive charge, and lipophilic cationic properties enable it to cross lipid bilayer easily and accumulate several hundred folds within the mitochondrial matrix, because of the significant negative environment (−150 to -170 mV) [32] inside mitochondria. It also crosses the plasma membrane due to the negative environment inside the cell (−30 to −60 mg) in comparison to the extracellular environment [32–34]. The protective effects of TPP attached bioactive compound including MitoQ [35], MitoVitE [36], MitoCP [37], MitoSNO [38], etc. have been investigated in isolated mitochondria as well as following oral, intra-peritoneal and intra-venous administration. In view of this, the present study contributed to developing strategies for delivery of curcumin into the mitochondrial matrix by covalently coupled with the TPP. A novel procedure for the attachment of TPP with curcumin by the chemical reaction was developed, and delivers it into mitochondria, isolated from brain and liver of mice to check the bioavailability. The action mechanism of MTC is similar to the curcumin, indicating that covalently attached TPP does not interfere with the action of curcumin.
Rotenone exposure on isolated mitochondria from striatum has been found to cause oxidative damage to the biological system by enhancing the generation of free radical species which leads to increase lipid peroxidation, protein carbonylation and decrease GSH levels [39]. Rotenone administration in rodents induced the oxidative damage in different organs such as brain, liver, kidney, etc. [40–42]. The formation of protein carbonylation is a presumptive marker for oxidative damage. Therefore, in the present study, the effect of rotenone in isolated brain mitochondria and liver of mice was examined. Mitochondria incubated with rotenone alone showed increased levels of lipid peroxide and protein carbonyl contents. The result of the present study shows that rotenone treatment induced oxidative stress by increasing lipid peroxidation, protein carbonylation and reduces the GSH level in isolated mitochondria from mice brain. In the present in-vivo study, rotenone also caused oxidative damage as evident by increased levels of lipid peroxidation and reduced levels of antioxidant enzymes, including GSH, catalase, and SOD in the liver of mice. The result shows that rotenone significantly blocks this antioxidant defense system of isolated brain mitochondria and also in the liver of treated mice.
Various herbal agents such as curcumin, quercetin, resveratrol and gallic acid [43–45] have been used to protect against oxidative damage by reducing lipid peroxidation, protein carbonylation and increasing the GSH level, SOD and catalase activity. Curcumin has been shown to have potent inhibitor of oxidative stress, inhibits lipid peroxidation in isolated mitochondria as well as in mice liver, kidney and brain and retain the activity of antioxidant enzymes [46]. Earlier studies have indicated that curcumin may diminish oxidative stress conditions and delay the age-related increase in protein carbonyl content in brain cells [47]. Curcumin also found to preserve mitochondrial redox and reduce mitochondrial protein carbonylation after 4-HNE intoxicant [48]. Some study has been reported to amplify the protective efficacy of herbal agents by directly sending into mitochondria such as VitE, MitoQ, MitR, etc. In an in-vitro study, the curcumin was found to reinstate the GSH when cells are intoxicated with sodium fluoride [49].
Similarly, potassium dichromate-induced decrease of GSH was potentially protected by curcumin pretreatment in rat hepatic mitochondria [50]. Curcumin also increases levels of GSH in mitochondria purified from cortical neurons [30]. The decrease GSH level under a stressed condition can increase mitochondrial damage and cause a defect in mitochondrial energy conservation [51]. In the present study, increased lipid peroxidation, protein carbonylation and reduced GSH level in isolated mitochondria from mice brain regions significantly protected by the pretreatment of curcumin and MTC suggesting the free radical scavenging activity of curcumin and MTC. The protective effect of MTC was more prominent than curcumin further indicating its enhanced bioavailability in the cellular system.
Oxidative stress induces when there is an imbalance between pro-oxidants and antioxidants, excess production of ROS, and lowering in cellular antioxidant defenses such as GSH, catalase, and SOD levels. GSH is a tripeptide, and its level is necessary for maintaining cellular redox potential, and it protects cells against oxidative stress [52]. Catalase and SOD antioxidant enzyme response against oxidative stress, SOD converts superoxide into H2O2 and catalase neutralized H2O2 into water and oxygen. The impairment in SOD activity may lead to an increased flux of superoxide in cellular compartments which may be the cause for the enhanced lipid peroxidative as observed in the present study. Catalase serves as a protective antioxidant and plays an important role in the protection against oxidative stress. Catalase and SOD depletion under a stressed condition can increase mitochondrial damage. The results of the present study revealed that rotenone-induced free radical generation associated with increased levels of lipid peroxidation and reduced levels of antioxidant enzymes including GSH, catalase and SOD significantly protected in mice liver co-treated with curcumin and MTC for 60 days. The efficacy of MTC against rotenone-induced oxidative damage was more promising than curcumin clearly indicates that the bioavailability of MTC was also increased in the in-vivo system. The findings of the present study were consistent with the earlier studies demonstrated the efficacy of MTC in defending the mitochondria from the harmful effects of oxidative stress by increasing its bioavailability in the mitochondria.
In conclusion, the present investigation contributes to a novel approach towards the synthesis of MTC through the three simple and easy steps. The result shows a significant increase in the lipid peroxide level and protein carbonyl in the liver of mice treated with rotenone. Incubation of mitochondria with curcumin and MTC prior to rotenone treatment showed a significant decrease in the level of lipid peroxide and protein carbonyls. Co-treatment with curcumin and MTC along with rotenone for 60 days reduced the oxidative stress in the liver of mice. The MTC was more effective than curcumin in preventing rotenone-induced lipid peroxidation and protein carbonylation. The results of present in-vitro study of brain and in-vivo study in the liver of mice showed that MTC was more effective in obstructing oxidative burden and mitochondrial impairment than curcumin. Thus, targeting mitochondria with these synthesized antioxidants may enhance the bioavailability and could be the preferred approach to diminish the oxidative burden for making suitable therapeutic strategies.
Electronic supplementary material
(DOCX 96 kb)
Acknowledgements
The authors are thankful to the CIL, Dr. Harisingh Gour Vishwavidyalaya (A Central University), Sagar (MP), India for providing instrumentation facilities. Financial support from UGC New-Delhi, India is also acknowledged.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wanders RJ, Ruiter JP, IJLst L, Waterham HR, Houten SM. The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. J Inherit Metab. 2010;33:479–494. doi: 10.1007/s10545-010-9104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: the calcium connection. Biochim Biophys Acta. 2010;1797:607–618. doi: 10.1016/j.bbabio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Ahn CS, Metallo CM. Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab. 2015;3(1):1. [DOI] [PMC free article] [PubMed]
- 4.Jimenez JJ, Bernal JL, Nozal MJ. Determination of rotenone residues in raw honey by solid-phase extraction and high-performance liquid chromatography. J Chromatogr. A. 2000;A871:67–73. doi: 10.1016/s0021-9673(99)01063-8. [DOI] [PubMed] [Google Scholar]
- 5.Talpade Deepa J., Greene James G., Higgins Donald S., Greenamyre J. Timothy. In Vivo Labeling of Mitochondrial Complex I (NADH:UbiquinoneOxidoreductase) in Rat Brain Using [3H]Dihydrorotenone. Journal of Neurochemistry. 2008;75(6):2611–2621. doi: 10.1046/j.1471-4159.2000.0752611.x. [DOI] [PubMed] [Google Scholar]
- 6.Yong R, Liu RW, Jiang H, Jiang Q, Feng J. Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J Biol Chem. 2005;280:34105–34112. doi: 10.1074/jbc.M503483200. [DOI] [PubMed] [Google Scholar]
- 7.Nehru B, Verma R, Khanna P, Sharma SK. Behavioral alterations in rotenone model of Parkinson’s disease: attenuation by co-treatment of centrophenoxine. Brain Res. 2008;1201:122–127. doi: 10.1016/j.brainres.2008.01.074. [DOI] [PubMed] [Google Scholar]
- 8.Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. doi: 10.1042/bse0470053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondria electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 10.Im AR, Kim YH, Uddin MR, Chae S, Lee HW, Kim YH, Kim YS, Lee MY. Betaine protects against rotenone-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol. 2013;33(5):625–635. doi: 10.1007/s10571-013-9921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samantaray S, Knaryan VH, Guyton MK, Matzelle DD, Ray SK, Banik NL. The parkinsonian neurotoxin rotenone activates calpain and caspase-3 leading to motoneuron degeneration in spinal cord of Lewis rats. Neuroscience. 2007;146(2):741–755. doi: 10.1016/j.neuroscience.2007.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newhouse K, Hsuan SL, Chang SH, Cai B, Wang Y, Xia Z. Rotenone-induced apoptosis is mediated by p38 and jnk map kinases in human dopaminergic SH-SY5Y cells. Toxicol Sci. 2004;79:137–146. doi: 10.1093/toxsci/kfh089. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Liu C, Chen S, Ye Y, Guo M, Ren Q, Liu L, Zhang H, Xu C, Zhou Q, Huang S, Chen L. Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in-vitro models of Parkinson's disease. Cell Signal. 2014;26(8):1680–1689. doi: 10.1016/j.cellsig.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Q, Liu C, Liu W, Zhang H, Zhang R, Liu J, Zhang J, Xu C, Liu L, Huang S, Chen L. Rotenone induction of hydrogen peroxide inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, leading to neuronal apoptosis. Toxicol Sci. 2015;143(1):81–96. doi: 10.1093/toxsci/kfu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav RS, Sankhwar ML, Shukla RK, Chandra R, Pant AB, Islam F, Khanna VK. Attenuation of arsenic neurotoxicity by curcumin in rats. Toxicol Appl Pharmacol. 2009;240:367–376. doi: 10.1016/j.taap.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Yadav RS, Chandravanshi LP, Shukla RK, Sankhwar ML, Ansari RW, Shukla PK, Pant AB, Khanna VK. Neuroprotective efficacy of curcumin in arsenic induced cholinergic dysfunctions in rats. Neurotoxicology. 2011;32:760–768. doi: 10.1016/j.neuro.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava P, Yadav RS, Chandravanshi LP, Shukla RK, Dhuriya YK, Chauhan LK, Dwivedi HN, Pant AB, Khanna VK. Unraveling the mechanism of neuroprotection of curcumin in arsenic induced cholinergic dysfunctions in rats. Toxicol Appl Pharmacol. 2014;15:428–440. doi: 10.1016/j.taap.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Lin J, Shih CA. Inhibitory effect of curcumin on xanthine dehydrogenase/oxidase induced by phorbol-12-myristate-13-acetate in NIH3T3 cells. Carcinogenesis. 1994;15:1717–1721. doi: 10.1093/carcin/15.8.1717. [DOI] [PubMed] [Google Scholar]
- 19.Lenaz G, Fato R, Genova ML, Bergamini C, Bianchi C, Biondi A, Mitochondrial Complex I. Structural and functional aspects. Biochimica. Et. Biophysica.Acta. 2006;1757:1406–1420. doi: 10.1016/j.bbabio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Trnka Jan, Elkalaf Moustafa, Anděl Michal. Lipophilic Triphenylphosphonium Cations Inhibit Mitochondrial Electron Transport Chain and Induce Mitochondrial Proton Leak. PLOS ONE. 2015;10(4):e0121837. doi: 10.1371/journal.pone.0121837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 22.Murphy MP. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta. 2008;1777:1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Krishna Mohan PR, Sreelakshmi G, Muraleedharan CV, Joseph R. Water soluble complexes of curcumin with cyclodextrins: characterization by FT-Raman spectroscopy. Vib Spectrosc. 2012;62:77–84. [Google Scholar]
- 24.Reddy CA, Somepalli V, Golakoti T, Kanugula AK, Karnewar S, Rajendiran K, Vasagiri N, Prabhakar S, Kuppusamy P, Kotamraju S, Kutala VK. Mitochondrial-targeted curcuminoids: a strategy to enhance bioavailability and anticancer efficacy of curcumin. PLoS One. 2014;9(3):e89351. doi: 10.1371/journal.pone.0089351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jat D, Parihar P, Kothari SC, Parihar MS. Curcumin reduces oxidative damage by increasing reduced glutathione and preventing membrane permeability transition in isolated brain mitochondria. Cell Mol Biol. 2013;59:OL1899–OL1905. [PubMed] [Google Scholar]
- 26.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 27.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 28.Hasan M, Haider SS. Acetyl homocysteinthiolactone protect against some neurotoxic effects of thallium. Neurotoxicology. 1989;10:257–262. [PubMed] [Google Scholar]
- 29.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 30.Reddy AC, Lokesh BR. Effect of dietary turmeric (Curcuma longa) on iron-induced lipid peroxidation in the rat liver. Food Chem Toxicol. 1994;32:279–283. doi: 10.1016/0278-6915(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 31.Zhu YG, Chen XC, Chen ZZ, Zeng YQ, Sh GB, Su YH, Peng X. Curcumin protects mitochondria from oxidative damage and attenuates apoptosis in cortical neurons. Acta Pharmacol Sin. 2004;25:1606–1612. [PubMed] [Google Scholar]
- 32.Smith R, Porteous CM, Gane AM, Michael MP. Delivery of bioactive molecules to mitochondria in-vivo. Proc Natl Acad Sci U S A. 2003;100(9):5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azzone GF, Pietrobon D, Zoratti M. Determination of the proton electrochemical gradient across biological membranes. Curr Top Bioenerg. 1984;13:1–77. [Google Scholar]
- 34.Brand MD, Brown GC, Cooper CE. Measurement of mitochondrial proton motive force. IRL Press. Oxford. UK . 1995; 4: 39–62.
- 35.Hu Q, Ren J, Li G, Wu J, Wu X, Wang G, Gu G, Ren H, Hong Z, Li J. The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis. 2018;9(3):403. doi: 10.1038/s41419-018-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes G, Murphy MP, Ledgerwood EC. Mitochondrial reactive oxygen species regulate the temporal activation of nuclear factor NF-kappaB to modulate tumour necrosis factor-induced apoptosis: evidence from mitochondria-targeted antioxidants. Biochem J. 2005;389:83–89. doi: 10.1042/BJ20050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng G, Zielonka J, McAllister D, Hardy M, Ouari O, Joseph J, Dwinell MB, Kalyanaraman B. Anti proliferative effects of mitochondria-targeted cationic antioxidants and analogs: role of mitochondrial bioenergetics and energy-sensing mechanism. Cancer Lett. 2015;365(1):96–106. doi: 10.1016/j.canlet.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Methner C, Chouchani ET, Buonincontri G, Pell VR, Sawiak SJ, Murphy MP, Krieg T. Mitochondria selective S-nitrosation by mitochondria-targeted S-nitrosothiol protects against post-infarct heart failure in mouse hearts. Eur J Heart Fail. 2014;16:712–717. doi: 10.1002/ejhf.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chinta SJ, Kumar M, Hsu M, Rajagopalan S, Kaur D, Rane A. Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J Neurosci. 2007;27:13997–14006. doi: 10.1523/JNEUROSCI.3885-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terzia A, Irazb M, Sahinc S, Ilhand A, Idize N, Fadilliogluf E. Protective effects of erdosteine on rotenone-induced oxidant injury in liver tissue. Toxicol Ind Health. 2004;20:141–147. doi: 10.1191/0748233704th208oa. [DOI] [PubMed] [Google Scholar]
- 41.Khurana N, Gajbhiye A. Ameliorative effect of Sida cordifolia in rotenone induced oxidative stress model of Parkinson’s disease. NeuroToxicology. 2013;39:57–64. doi: 10.1016/j.neuro.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Jiang XW, Qiao L, Feng XX, Liu L, Wei QW, Wang XW, Yu WH. Rotenone induces nephrotoxicity in rats: oxidative damage and apoptosis. Toxicol Mech Methods. 2017;27(7):528–536. doi: 10.1080/15376516.2017.1333553. [DOI] [PubMed] [Google Scholar]
- 43.Yadav RS, Shukla RK, Sankhwar ML, Patel DK, Ansari RW, Pant AB, Islam F, Khanna VK. Neuroprotective effect of curcumin in arsenic-induced neurotoxicity in rats. NeuroToxicology. 2010;31:533–539. doi: 10.1016/j.neuro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Parihar P, Jat D, Ghafourifar P, Parihar MS. Effciency of mitochondrially targeted gallic acid in reducing brain mitochondrial oxidative damage. Cell Mol Biol. 2014;60(2):35–41. [PubMed] [Google Scholar]
- 45.Kale A, Pişkin Ö, Baş Y, Aydın BG, Can M, Elmas Ö, Büyükuysal Ç. Neuroprotective effects of quercetin on radiation-induced brain injury in rats. J Radiat Res. 2018;59(4):404–410. doi: 10.1093/jrr/rry032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulkarni S, Dhir A, Akula KK. Potentials of curcumin as an antidepressant. Sci World J. 2009;9:1233–1241. doi: 10.1100/tsw.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dkhar P, Sharma R. Effect of dimethylsulphoxide and curcumin on protein carbonyls and reactive oxygen species of cerebral hemispheres of mice as a function of age. Int J Dev Neurosci. 2010;28:351–357. doi: 10.1016/j.ijdevneu.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Raza H, John A, Brown EM, Benedict S, Kambal A. Alterations in mitochondrial respiratory functions, redox metabolism and apoptosis by oxidant 4-hydroxynonenal and antioxidants curcumin and melatonin in PC12 cells. Toxicol Appl Pharmacol. 2008;226:161–168. doi: 10.1016/j.taap.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Nabavi SF, Moghaddam AH, Eslami S, Nabavi SM. Protective effects of curcumin against sodium fluoride-induced toxicity in rat kidneys. Biol Trace Elem Res. 2012;145:369–374. doi: 10.1007/s12011-011-9194-7. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Nino WR, Tapia E, Zazueta C, Zatarain-Barron ZL, Hernandez-Pando R, Vega-Garcia CC, Pedraza-Chaverri J. Curcumin pretreatment prevents potassium dichromate-induced hepatotoxicity, oxidative stress, decreased respiratory complex I activity and membrane permeability transition pore opening. Evid Based Complement Alternat Med. 2013;2013:42469243. doi: 10.1155/2013/424692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049–2063. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 96 kb)