Abstract
Leptin signaling pathways, stemming primarily from the hypothalamus, are necessary for maintaining normal energy homeostasis and body weight. In both rodents and humans, dysregulation of leptin signaling leads to morbid obesity and diabetes. Since leptin resistance is considered a primary factor underlying obesity, understanding the regulation of leptin signaling could lead to therapeutic tools and provide insights into the causality of obesity. While leptin actions in some hypothalamic regions such as the arcuate nuclei have been characterized, less is known about leptin activity in the hypothalamic ventromedial nuclei (VMN). Recently, pituitary adenylate cyclase activating-polypeptide (PACAP) has been shown to reduce feeding behavior and alter metabolism when administered into the VMN in a pattern similar to that of leptin. In the current studies, we examined whether leptin and PACAP actions in the VMN share overlapping pathways in the regulation of energy balance. Interestingly, PACAP administration into the VMN increased STAT3 phosphorylation and SOCS3 mRNA expression both of which are hallmarks of leptin receptor activation. In addition, BDNF mRNA expression in the VMN was also increased by both leptin and PACAP administration. Moreover, antagonizing PACAP receptors fully reversed the behavioral and cellular effects of leptin injections into the VMN. Electrophysiological studies further illustrated that leptin-induced effects on VMN neurons were blocked by antagonizing PACAP receptors. We conclude that leptin dependency on PACAP signaling in the VMN suggests a potential common signaling cascade allowing a tonically and systemically secreted neuropeptide to be more precisely regulated by central neuropeptides.
Keywords: food intake, metabolism, thermoregulation, BDNF, STAT3
INTRODUCTION
With nearly forty-percent of Americans suffering from excessive weight gain coupled with co-morbidities, there is an urgent need to address the growing national obesity epidemic [1]. Since its clinical potential was established over twenty years ago, leptin has been explored as a possible target for therapeutic solutions addressing obesity but with limited success. Released peripherally from adipose cells, leptin is a peptide hormone that acts both peripherally and centrally to suppress feeding behavior and increase metabolism to manage long-term energy stores [2–4]. As leptin production and release increases in over weight individuals, [5, 6] the chronically-elevated circulating leptin levels result in peripheral and central leptin resistance [7] leading to diminished metabolism, increased feeding behavior and ultimately obesity [8]. Signaling endpoints implicated in leptin resistance include trafficking of cell-surface leptin receptors and the upregulation or downregulation of positive and negative regulators of the leptin signaling cascade. Therefore, detailing the mechanisms modulating central leptin signaling and the leptin insensitivity that leads to excess weight gain is an important step towards identifying effective therapeutics to treat obesity.
However, revealing the biological basis of leptin resistance has been limited by the extent to which we fully understand leptin signaling mechanisms. Currently, the canonical signaling cascade has been repeatedly confirmed in which leptin binding activates the Janus kinase-2 (JAK2) tyrosine kinase, promoting tyrosine phosphorylation of the leptin receptor at several sites including Tyr1138. Phosphorylation of the leptin receptor recruits transcription factors of the signal transducer and activator of transcription family such as STAT3 and promotes their binding, phosphorylation and transcription activation [9, 10]. Moreover, the hypothalamus has been implicated as a primary target of leptin signaling [11, 12] especially in hypothalamic sub-groups such as the arcuate, dorsomedial, and ventromedial nuclei (VMN) [13–15]. While the nature of leptin signaling in the arcuate is better characterized [16–19], less is known about the precise mechanisms leptin uses in the VMN. Leptin receptors in the VMN are not only expressed in neurons expressing the nuclear receptor steroidogenic factor 1 (SF-1) but that knocking down leptin receptors in SF-1 neurons produces an obese phenotype in rodents [14, 20, 21]. Interestingly, SF-1 neurons also express receptors for the neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) [21], which invites the possibility for leptin and PACAP receptor interactions. Administration of the PACAP receptor (PAC1R) antagonist, PACAP6–38, into the third ventricle of mice attenuates leptin-induced suppression of food intake, body weight, and changes in thermogenesis [21], further suggesting that leptin and PACAP functionally interact. PACAP microinjections into the VMN induce hypophagia, body weight reduction, and changes in core body temperature [22–24], in a manner nearly identical to that of leptin. The current findings demonstrate that administration of PACAP6–38 just prior to leptin in the VMN inhibits leptin-induced behavior and cell signaling, indicating that leptin is dependent on PAC1R activation in the VMN.
Given the similarity in the cellular and behavioral actions of leptin, PACAP, and other peptide transmitters such as melanocortins [25, 26] and brain-derived neurotrophic factor [27, 28] there is an obvious need to take a more integrative perspective to determine whether there is a common signaling cascade that is accessed by multiple peptide transmitters. This would broaden our understanding of the network that interfaces with leptin and promote opportunities to develop therapeutic approaches to reverse leptin insensitivity.
MATERIALS & METHODS
Animals and Experimental Design
Male Sprague-Dawley rats (Envigo; Madison, WI) weighing 275–300 grams were housed individually in either standard tub cages or BioDAQ feeding cages (Research Diets; New Brunswick, NJ) and acclimated for one week to a climate-controlled colony room under a 12:12 light-dark cycle. Animals were ad lib-fed standard chow (Teklad rodent diet #8604; 32% protein, 54% carbohydrate, 14% fat; 3.0 kcal/g; Madison, Wisconsin) and body weights were measured daily. Following the acclimation period, all animals were stereotactically implanted with double-guide cannula targeting the hypothalamic ventromedial nuclei (VMN) and then allowed approximately a week to recover. Afterwards, animals were assigned to one of the following microinjection treatment groups: vehicle, leptin, PACAP6–38, and leptin+PACAP6–38. A subset of studies also included PACAP and PACAP+ PACAP6–38 groups. All animal procedures were approved by Marquette University Institutional Animal Care and Use Committee.
Surgery & microinjections
Cannulation surgery
Animals were anesthetized with a ketamine/xylazine/acepromazine (77:1.5:1.5 mg/ml/kg; i.p.) cocktail and placed in a stereotaxic apparatus. 26-gauge bilateral guide cannulae (Plastics One; Roanoke VA) were placed 3 mm dorsal to VMN of the hypothalamus and secured to the surface of the skull with acrylic resin. Stereotaxic coordinates for VMN injections were 1) anterior/posterior, −2.5 mm from bregma; 2) medial/lateral, ± 0.6 mm from midline; 3) dorsal/ventral, −6.2 mm from surface of the skull based on The Rat Brain [29]. Following the conclusion of each study, animals were sacrificed and brains collected by rapid decapitation then sectioned and Nissl stained to determine cannula placement. Only those with correct placements were included in the studies.
Telemetry probe
Prior to VMN cannulation, some animals (n=15) were implanted intraperitoneally with a telemetry probe (Mini-Mitter, Sunriver, OR). Animals were allowed one week to recover from surgery before baseline body temperatures were monitored for one week prior to VMN microinjections.
Microinjections
PACAP (50 pmol/0.25μl per side; California Peptide Research, Napa, CA), PACAP6–38 (500 pmol/0.25μl per side; Anaspec, Fremont, CA), leptin (0.025 μg/0.25μl per side; R&D Systems, Minneapolis, MN) or vehicle (saline) were microinjected into the VMN over a two-minute period in gently-restrained awake animals. Groups included vehicle (n=4–11), leptin (n=6–10), PACAP (n=4–10), PACAP6–38 + PACAP (n=4–10) or PACAP6–38 + leptin (n=6–12) at the onset of the dark cycle. All compounds were dissolved in saline. After injection, an additional minute was allotted before removing injectors to prevent backflow.
In situ Hybridization
Rat brains were sectioned coronally at 12μm and then post-fixed in 4% paraformaldehyde, rinsed in 0.1 M PBS (pH 7.4), equilibrated in 0.1 M triethanolamine (pH 8.0), and acetylated in triethanolamine containing 0.25% acetic anhydride. Standard in vitro transcription methods were used to generate both sense and antisense riboprobes recognizing PAC1R, PACAP, long-form of the leptin receptor (LepRb) and BDNF transcripts (Choi, Milwaukee, WI), which were subsequently diluted in hybridization cocktail (Amresco, Solon, OH) with tRNA. Sections were hybridized overnight at 60°C with either digoxigenin (DIG) or fluorescein (FITC)-labeled riboprobes. After hybridization, slides were treated with RNase A and stringently washed in 0.3X SSC at 65°C (all riboprobes) for 30 min. Slides were incubated with an antibody against DIG or FITC conjugated to horseradish peroxidase (HRP; Roche) overnight at 4°C. Riboprobe signal was amplified using the TSA-Plus fluorophore system with either fluorescein or Cy3 (PerkinElmer; Waltham, MA). Image capture was performed using fluorescent microscopy at 10X and magnified 20X (Axioskop-2; Zeiss, Thornwood, NY) with Axiovision image analysis software (Zeiss, Thornwood, NY) or 60X (Nikon-confocal; Nyquist sampling) with Nikon NIS Elements software (Nikon, Melville, NY). ImageJ [30] was used to subtract the background with a rolling ball radius of 300 pixels and adjusted for optimal contrast equally across all non-confocal images.
Quantitative polymerase chain reaction
Tissue punches for both mRNA and protein analysis were collected following rapid desanguination. The ventral hypothalamus was demarcated in a stainless steel brain matrix and divided into five 1mm divisions. The 1mm slice containing the VMN was isolated and briefly placed on dry ice in order to bilaterally punch the VMN using a 2mm tissue puncher. Care was taken to avoid nearby hypothalamic cell groups such as the arcuate nuclei. Dissected VMN tissue was frozen in liquid nitrogen and stored at −80 °C. Total RNA was isolated from hypothalamic VMN punches by TRIzol extraction (Invitrogen-ThermoFisher). cDNA was constructed using the Reverse Transcription System (Promega, Madison, WI). Quantitative PCR was performed using a StepOne Real-Time PCR System (Applied Biosystems-ThermoFisher), and PerfeCTa SYBR Green FastMix with ROX (QuantaBio, Beverly, MA). Quantification of mRNA expression was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primers GAPDH: 5’-CTCCCATTCTTCCACCTTTGA-3’ and 5’- ATGTAGGCCATGAGGTCCAC-3’, SOCS3: 5’ CCC CGCTTTGACTGTGTACT 3’and 5’ AAAGGAAGGTTCCGTCGGTG 3’, BDNF: 5’ AAAACCATAAGGACG CGGACTT 3’ and 5’ AAAGAGCAGAGGAGGCTCCAA 3’.
Western blotting
VMN tissue (see above) was collected 30 min after microinjections and flash frozen in liquid nitrogen and then homogenized by hand (10 strokes) in ice-cold homogenization buffer (300 mM sucrose, 10 mM Tris-HCl, pH 7.4, 10 mM EDTA, 10 mM EGTA) containing Halt protease and phosphatase inhibitor cocktail (Pierce; Rockford, IL), followed by 3–4 seconds of sonication. Homogenates were centrifuged at 1000 × g for 10 min at 4 °C to obtain the nuclear fractionation. Protein levels were measured using a bicinchoninic (BCA) assay (Pierce). Nuclear protein (25 μg) was run on an 8% gel by SDS-PAGE and transferred to a polyvinylidine fluoride (PVDF) membrane. Membranes were blocked in odyssey blocking buffer (PBS; LI-COR Biosciences; Lincoln, NE) with 0.1% Tween-20 and then probed with rabbit anti-phospho-STAT3 (Tyr705) antibody (Cell Signaling; Beverly, MA) overnight at 4°C, and then with an IRDye 680 CW goat anti-rabbit secondary antibody (LI-COR Biosciences; Lincoln, NE) at room temperature for 1 hour. Band intensities for Tyr705 were visualized using the Odyssey Fc Dual Mode Imaging System. Blots were stripped and re-probed for total endogenous STAT3 expression using mouse anti-STAT3 (124H6) (Cell Signaling; Beverly, MA) and IRDye 800CW donkey anti-mouse (LI-COR Biosciences; Lincoln, NE) antibodies.
Slice Electrophysiology
Coronal slices (250μm) containing the VMN of the hypothalamus were obtained from adult male rats and prepared using a vibratome (Leica VT1000S). Slices were prepared in ice-cold solution containing 229mM sucrose, 1.9mM KCl, 1.2mM NaH2PO4, 33mM NaHCO3, 10mM glucose, 0.4mM ascorbic acid, 6mM MgCl2, and 0.5mM CaCl2 oxygenated using 95% O2 5% CO2. Slices were then incubated at 31°C for ten minutes in a solution containing 119mM NaCl, 2.5mM KCl, 1mM NaH2PO4, 26.2mM NaHCO3, 11mM glucose, 0.4mM ascorbic acid, 4mM MgCl2, and 1mM CaCl2 and further incubated a minimum of 35 minutes at room temperature. Whole-cell recordings were performed in slices continuously perfused with aerated aCSF (125mM NaCl, 2.5mM KCl, 25mM NaHCO3, 10mM glucose, 0.4mM ascorbic acid, 1.3mM MgCl2, and 2mM CaCl2) at a temperature of 29°- 33°C using a gravity-fed perfusion system with a flow rate of ~2 ml/min oxygenated. Borosilicate (2.5–4.5MΩ) glass pipettes were filled with 140mM K-Gluconate, 5.0mM HEPES, 1.1mM EGTA, 2.0mM MgCl2, 2.0mM Na2-ATP, 0.3mM Na-GTP, and 5.0mM phosphocreatine (pH 7.3, 290mOsm). Action potential (AP) firing was filtered at 1kHz, sampled at 5kHz, and measured using a Sutter Integrated Patch Amplifier (IPA) with Igor Pro (Wave Metrics, Inc.) data acquisition software. Current-clamp recordings were performed in spontaneously-firing neurons in the VMN with adequate whole-cell access (Ra<20 MΩ) and capacitance verified at the beginning of recording. Picrotoxin (100μM) and NBQX (10μM) were added to the recording aCSF to better isolate postsynaptic effects of PACAP and leptin. Following completion of 2–5 minute baseline with <20% variation in spike firing, either PACAP (100nM), leptin (100nM), or PACAP6–38 (100nM) was added to the recording solution. For recordings measuring spike firing after application of PACAP6–38, leptin or PACAP were subsequently added to the following acquisition of a stable (<20% variation) baseline in the presence of PACAP6–38.
Statistical Analysis
Data are presented as means ± standard errors of the mean and were analyzed statistically (Sigma Plot 11; Systat Software Inc.; San Jose, CA) by analysis of variance (with repeated measures when appropriate), or Student’s t-test. Fischer LSD, Tukey’s HSD, and Cohen’s d analyses were used when appropriate for post-hoc group comparisons. P values less than 0.05 were considered statistically significant. Electrophysiology data are presented in figure legends with n= number of cells and N= number of rats per group.
RESULTS
Antagonizing VMN-PAC1R blocks leptin-induced changes in energy expenditure.
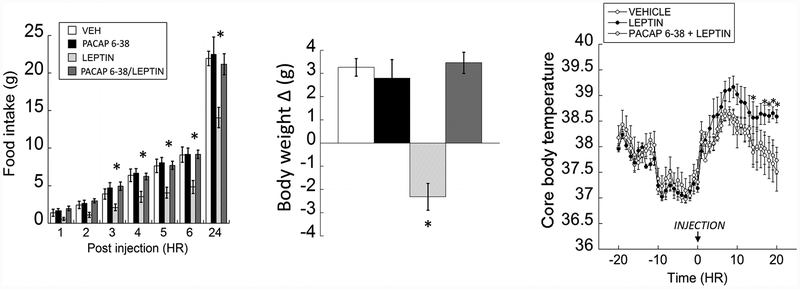
Feeding behavior in VMN-cannulated animals receiving a microinjection of either vehicle, leptin, PACAP6–38 or PACAP6–38 + leptin at the onset of the dark cycle showed significant changes in food intake (Fig 1; left panel). Repeated measures two-way ANOVA revealed significant main effects of treatment (F(3,293)= 11.319, p =0.001) and treatment × time interaction (F(18, 293)= 3.555, p=0.001). Animals injected with leptin into the VMN consumed significantly less standard chow compared to vehicle-injected animals (vehicle vs. leptin; p= 0.001) after 3 hours post-injection. Interestingly, VMN microinjections of PACAP6–38 prior to leptin administration blocked behavioral responses to the leptin and produced nearly identical feeding behavior to controls (vehicle vs. PACAP6–38 + leptin; p= 0.792). Administering PACAP6–38 alone in the VMN produced no significant effect on feeding behavior (vehicle vs. PACAP6–38; p= 0.708) or body weight (vehicle vs. PACAP6–38 + leptin; p= 0.792). Post-microinjection analysis of 24-hour body weight change using a two-way ANOVA showed a significant interaction of leptin and PACAP6–38 (Fig 1; middle panel; F(1,41)=28.952; p< 0.001). Leptin-injected animals lost a significant amount of weight compared to controls whereas pretreatment with PACAP6–38 prior to leptin administration prevented leptin-induced weight loss.
Figure 1.
In the VMN, antagonizing PAC1 receptors blocks leptin-induced hypophagia, weight loss and thermogenesis. (Left) Bilateral VMN-leptin (0.025μg/0.25μl/side) significantly suppressed feeding behavior, which was blocked with pretreatment with PACAP6–38 (500 pmol/0.25μl/side). (Middle) A similar pattern of data was observed in body weight change 24-hours post microinjection. (Right) Leptin bilaterally microinjected into the VMN significantly stimulated thermogenesis for up to 20 hours compared to vehicle and PACAP6–38 pretreated animals. Data are presented ± SEM. * = P < 0.05
Using a two-way repeated measures ANOVA, we found that leptin produced a significant effect of treatment over time (F(38,279)=1.572, p= 0.025) on core body temperature (CBT) compared to vehicle or PACAP6–38 pretreated animals (Fig 1; right panel). Post-hoc analysis revealed animals injected with leptin alone displayed a significant increase in CBT that remained elevated 20 hours post-injection compared to controls (vehicle vs. leptin, p=0.002). Interestingly, antagonizing PAC1R with PACAP6–38 prevented leptin-induced increases in CBT and were nearly identical to vehicle-injected animals (PACAP6–38 + leptin vs. leptin, p= 0.385). The same results were reported previously demonstrating that PACAP administration not only significantly elevated core body temperature in the VMN but pre-treatment with PACAP6–38 completely reversed PACAP-induced core body temperature changes [24]. Differences in body temperatures subsided by 30 hours post injection. Overall, leptin-induced increases in core body temperature, and decreases in feeding behavior and body weight were fully reversed after pre-treating with a PACAP receptor antagonist.
Co-expression patterns of PAC1R, PACAP, BDNF and the leptin receptor mRNA in the VMN.
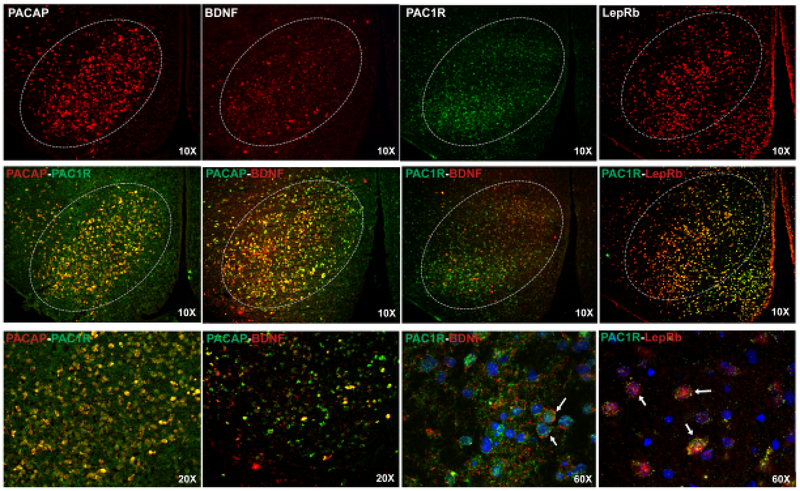
Figure 2 shows double fluorescent in situ hybridization targeting mRNA transcripts for PACAP and PAC1R are co-localized together in the VMN. PACAP mRNA is also co-localized with BDNF mRNA expression. In addition, PAC1R mRNA in the VMN co-expresses with brain derived neurotrophic factor (BDNF) and the leptin receptor (LepRb).
Figure 2.
Fluorescent photomicrographs of mRNA expression in the VMN (Bregma = 2.5–2.7mm) for PACAP, BDNF, PAC1R, and LepRb (top row) at 10X magnification. Second row: 10X images illustrate co-expression of PACAP/PAC1R, PACAP/BDNF, PAC1R/BDNF, and PAC1R/LepRb in cells of the VMN. Label colors correlate with similarly colored fluorophores. Third row: Higher magnification images of PACAP/PAC1R, PACAP/BDNF (20X) and PAC1R/BDNF, PAC1R/LepRb (60x). The 60X images show co-expression PAC1R, BDNF, and LepRb in DAPI delineated cells. Arrows identify examples of DAPI-labeled cells showing co-expression. 3V=third ventricle
PACAP-leptin molecular interactions in the VMN.
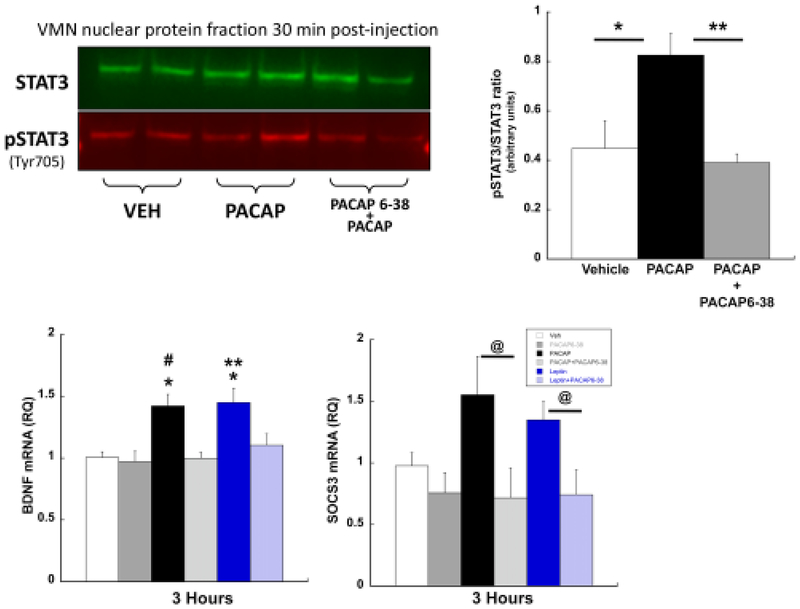
Previous studies have demonstrated that activation of the leptin receptor drives the JAK2-STAT3 intracellular signaling cascade by increasing the immunoreactivity of phosphorylated STAT3 in the VMN [9]. PACAP administered into the VMN similarly induced phosphorylation of STAT3 specifically through the PAC1R since pretreatment with the PAC1R antagonist blocked STAT3 phosphorylation (Fig 3; treatment F(2,9)=7.733, p=0.011; Tukey’s HSD: vehicle vs. PACAP p=0.029; PACAP6–38 + PACAP vs. PACAP p= 0.014; vehicle vs. PACAP6–38 + PACAP p=0.884). Given that the group sizes in this study were small, we conducted a Levene’s test for homogeneity of variance and this assumption was not violated (F=3.6; p>0.05). Even so, out of an abundance of caution, we also conducted a non-parametric Kruskal-Wallis test and the results are consistent with the original analysis (H(2)=7.42, p=0.0245). The robust effect, despite the small n, is likely due to the very large effect size in relation to the overall variance as is revealed by the large Cohen’s d values (Veh vs. PACAP: Cohen’s d = 1.68; PACAP vs PACAP6–38 + PACAP: Cohen’s d=2.61).
Figure 3.
PACAP mimics molecular actions of leptin in the VMN. (Top) Western blot image (left) and its graphical representation (right) from VMN punches 30 minutes post PACAP treatment (50pmol/0.25μl/side) show significant PACAP-induced stimulation of phosphorylated STAT3 (pSTAT3) compared to vehicle. Pretreatment with PACAP6–38 (500pmol/0.25μl/side) reversed PACAP-induced STAT3 phosphorylation. In VMN punches, (bottom, left) BDNF and (bottom, right) SOCS3 mRNA were elevated following microinjection of PACAP or leptin. These increases were blocked with pretreatment with PACAP6–38. Data are presented ± SEM. * = P < 0.05
Both leptin and PACAP microinjected into the VMN increased mRNA levels of BDNF and suppressor of cytokine signaling-3 (SOCS3) to a similar degree (lower panels, respectively). Moreover, both PACAP- and leptin-induced increases in BDNF and SOCS3 mRNA levels and both were equally blocked by antagonizing PAC1R. Leptin- and PACAP-induced increases in BDNF mRNA were similar in magnitude (F(5, 46)= 6.800, p< 0.001; vehicle vs. PACAP p<0.001; vehicle vs. leptin p<0.001) and were equally inhibited by PACAP6–38 (vehicle vs. PACAP6–38 + PACAP, p=0.950; vehicle vs. PACAP6–38 + leptin, p=0.405). Like BDNF, a one-way ANOVA of SOCS3 mRNA expression revealed a significant effect of treatment (F(5,45)= 2.901, p=0.025). PACAP or leptin microinjections into the VMN did not reach statistical significance for changes in SOCS3 mRNA, although there is a clear trend towards increased expression (vehicle vs. PACAP, p=0.055; vehicle vs. leptin p=0.103). However, PACAP and leptin SOCS3 mRNA levels were significantly higher than mRNA levels measured in animals treated with PACAP6–38 prior to leptin or PACAP administration (PACAP vs. PACAP6–38 + PACAP, p=0.006; leptin vs. PACAP6–38 + leptin, p=0.050). Thus, PACAP6–38 reversed the intracellular effects of both PACAP and leptin in VMN neurons.
PACAP receptor antagonism on PACAP and leptin-induced changes in action potential frequency in VMN cells
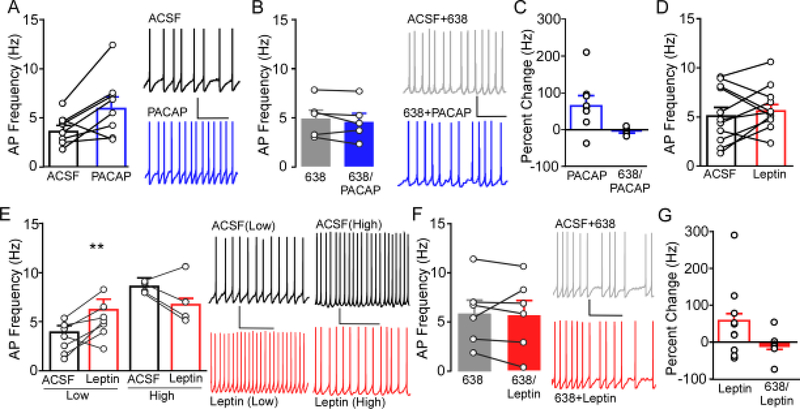
Similar to our previous reports [31], bath application of PACAP (100nM) increased action potential (AP) firing of neurons in the VMN (Fig 4A; t(7)= −2.783, p= 0.027). Repeated measures ANOVA assessing within cell changes in AP frequency revealed no significant difference between firing frequency following application of PACAP6–38 alone compared to in the presence of PACAP6–38 + PACAP (Fig 4B; F(4,9)=1.64, p=0.27). Examination of percent change showed that PACAP produced a +70% increase in firing frequency compared to ACSF baseline frequency (n=9), but a −6.96% reduction when applied in the presence of PACAP6–38 (Fig4C).
Figure 4.
Ex vivo slice electrophysiology was used to determine the role of PAC1R signaling on action potential (AP) frequency in the VMN. A) Application of 100nM PACAP (blue) in slice increases AP firing of neurons in the VMN (n= 8, N= 5) compared to baseline firing in ACSF (black). Representative trace of VMN AP firing (right) during bath application of ACSF (top) and following bath application of PACAP (bottom). * P<0.05 PACAP compared to ACSF. B) Prior and concomitant application of PACAP6–38 (6–38, gray filled) blocked the effects on AP firing induced by PACAP (6–38/PACAP, blue filled) (n=6, N=3). Representative traces of AP firing in the presence of PACAP6–38 (ACSF+6–38, top) and PACAP6–38 along with PACAP (6–38+PACAP, bottom). C) Percent change of firing produced by PACAP in the presence of ACSF (data from panel A) and in the presence of PACAP6–38 (data from panel B). D) Application of 100nM leptin (red) did not alter overall AP firing compared to baseline ACSF (n= 11, N= 5). D) Cells exhibiting a baseline AP frequency below the overall mean (Low) showed a significant increase in firing (left), whereas VMN cells showing a baseline AP frequency above the mean (High) showed a trend towards reduced firing (right). Representative traces (right) from Low and High cells in ACSF (top, black) and leptin (bottom, red). ** P<0.01 leptin compared to ACSF. F) Bath application of PACAP6–38 blocked the effects on AP firing induced by leptin (n= 6, N= 4). Representative traces of AP firing in PACAP6–38 + ACSF (top, gray filled) and PACAP6–38 + leptin (bottom, red filled). G) Percent change of firing produced by leptin in the presence of ACSF (data from panel D) and in the presence of PACAP6–38 (data from panel F).
In contrast to PACAP, application of leptin (100nM) did not alter mean frequency of AP firing (Fig 4D; t(10)= −0.86, p=0.41). Subsequent examination of within-cell effects of leptin compared to baseline revealed that 7 of 11 cells exhibited an increase in firing frequency, while 4 showed a reduction in firing rate. This suggests that leptin may exert bimodal effects in certain cell populations in the VMN. To gain further insight into what might influence the response of individual cells to Leptin, post-hoc analysis was performed based on the mean basal firing frequency (in ACSF), with cells exhibiting a frequency above the mean and below the mean grouped together. Repeated measures ANOVA showed that cells exhibiting a baseline firing rate lower than the mean (Low, n=7) exhibited a significant increase in AP frequency in response to leptin (F(6,13)=8.43, p=0.017), whereas cells with baseline firing rates below the mean (High, n=4) showed a trend towards reduced firing (Fig4E; F(3,7)=2.08, p=0.105). Repeated measures ANOVA revealed no significant difference between firing frequency following application of PACAP6–38 compared to PACAP6–38 + leptin (Fig 4F; F(4,9)=1.64, p=0.27). Examination of percent change in all cells regardless of response, show that leptin produced a mean +45% increase in firing frequency, whereas mean percent change was −10.5% when leptin was applied in the presence of PACAP6–38 (Fig4G). Notably, low frequency cells showed a mean +82% increase and High frequency cells showed a mean −28% decrease in frequency compared to ACSF baseline. Notably, within cell comparison of all cells receiving initial application of PACAP6–38 showed no significant impact on firing frequency compared to ACSF baseline (F(10,21)=3.63, p=0.096; data not shown). Taken together, these data indicate that PACAPs excitatory effects on neuronal firing are blocked by PACAP6–38 and that PACAP6–38 blunts leptins ability to increase and decrease firing.
DISCUSSION
A novel perspective towards understanding the central mechanisms underlying feeding is to consider whether there are common intracellular signaling cascades that are accessed by multiple hormones and neurotransmitters. This is evidenced by the numerous neurotransmitters and hormones that can produce hypophagia within specific hypothalamic regions, such as the VMN. Hence, an important question for understanding obesity is whether specific VMN neurons have a common molecular sequence that produces the activation pattern needed to produce hypophagia, and whether this pathway is stimulated and utilized by a variety of signals. If there is a molecular convergence onto a master signaling sequence, then the concept of leptin resistance can be broadened to include additional signaling molecules or hormones and could then be examined as a source for insensitivity or a potential therapeutic strategy to normalize activity. In our studies, PACAP acts in the VMN to produce hypophagia and metabolic changes similar to that of leptin. To the extent that PACAP and leptin display obligatory convergence on a shared signaling pathway, alterations to PACAP signaling could either present as leptin insensitivity or the solution to ameliorate leptin insensitivity.
Exogenous administration of PACAP and leptin in the hypothalamic VMN produce similar behavioral and cellular outcomes whereas, anatomically, receptor mRNA for both peptides are co-expressed in VMN cells as further evidence to suggest that PACAP and leptin signaling could intersect. Leptin’s canonical signaling pathway has been repeatedly shown in the hypothalamus to involve the JAK2-STAT3 cascade [9, 10]. Remarkably, PACAP administration into the VMN mimic canonical leptin activation markers including the phosphorylation of STAT3 and increased transcription of SOCS3 and BDNF mRNA, while also suppressing food intake, body weight, and increasing core body temperature. However, direct manipulation of PACAP and leptin signaling revealed that leptin’s behavioral and cellular actions can be reversed in rats pre-treated with a PACAP receptor antagonist demonstrating that these two peptides functionally intersect [24]. Thus, the molecular, cellular, and behavioral data not only show the close relationship between PACAP and leptin functionality in the VMN, but that leptin-induced actions in the VMN require active PACAP signaling.
To further demonstrate leptin’s dependency on PACAP signaling in cells of the VMN, electrophysiological characterization demonstrate that VMN cells are responsive to both PACAP and leptin. In contrast to the behavioral and cellular/molecular responses, PACAP and leptin produced identical electrophysiological responses, but only in a sub-set of VMN neurons. Bath application of PACAP produced excitatory responses in VMN cells, which we have observed previously [22] and is similar to the excitatory actions of PACAP observed in other regions of the brain [32–34]. By contrast, leptin application produced both excitatory and inhibitory responses in the VMN. Similar bidirectional responses have been previously observed in neuronal subpopulations of other hypothalamic subgroups such as the arcuate nuclei [35], where leptin depolarized and increase firing rates of POMC/CART neurons, while hyperpolarizing and decreasing firing rates of AgRP/NPY neurons [18, 36]. Leptin has also been shown to depolarize and increase firing of VMN steroidogenic factor-1 (SF-1) cells [20, 35]. Notably, this effect was not observed in all cells, however it was unclear whether cells not showing enhanced firing exhibited no response to leptin or a reduction in firing, thus we provide the first evidence that leptin can bidirectionally alter VMN neuron firing rates. The mechanisms underlying this bidirectionality remain unclear, but likely reflect distinctions in molecular (e.g., ion channels, receptors), intrinsic neurophysiology, synaptic innervation, and perhaps anatomical connectivity that endow specific subpopulations of specific SF-1 cells with unique properties to integrate and communicate information. In support, VMN neurons exhibiting reduced firing showed a trend towards higher basal firing rates compared to those that increase firing, however this was not true in all cells, suggesting that firing rates are not absolute predictors of responsivity. Regardless, important future studies will involve a more rigorous characterization of what defines these potential subpopulations.
Our study demonstrates that PAC1R mRNA expression overlaps with that of PACAP mRNA in the VMN and so a likely conclusion may be that SF-1 cells express the PACAP receptor, PAC1R. Consistent with this, we found that excitatory effects of PACAP on neuronal firing were blocked by prior and concomitant application of the PAC1R antagonist, PACAP6–38. Although cell-specific markers have not been fully characterized for leptin-sensitive VMN cells, we and others have shown that leptin can exert an excitatory effect on SF-1 cells [20], which have been shown to co-express PACAP mRNA [21]. Although no significant effects of leptin were observed overall, as well as in the presence of PACAP6–38, comparison of percent change shows that leptin increases firing by 45% when combining all cells, whereas in the presence of PACAP6–38, there is a −10% percent change, indicating an overall blunting of leptin effects. Further, because all cells showed a change in firing in response to leptin alone (+82% in High frequency cells, −28% in Low frequency cells), antagonism of PAC1R appears to inhibit leptin effects regardless of response, and suggests that divergent effects of leptin do not likely reflect the presence or absence of PAC1R. As leptin and PACAP receptors are not known to localize presynaptically, and there is little evidence of collateral communication between VMN neurons, observed effects of Leptin and PACAP alone as well as antagonism of these effects with PACAP6–38, are likely due to postsynaptic activation of receptors on cells being recording from. Thus, despite the differential in vitro actions of PACAP and leptin, the electrophysiological studies still suggest there is an interaction between leptin and PACAP signaling.
Overall, our data demonstrates an obligatory convergence relationship between leptin and PACAP in the VMN. Leptin receptors are a type I cytokine receptor in the IL-6 receptor family [40] and following ligand binding activates the associated Janus kinase-2 (JAK2) tyrosine kinase. JAK2 activation promotes tyrosine phosphorylation of the leptin receptor leading to the creation of docking sites for signal transducer and activator of transcription (STAT) factors, which ultimately leads to induction of gene expression [10, 41]. Known inhibitors of leptin signaling include SOCS3 binding to the Tyr985 residue of the leptin receptor [42] and dephosphorylation of JAK2 by protein tyrosine phosphatase [43]. While cytokine signaling pathways have been at the core of a number of human disorders, there is still a poor understanding of their mechanistic circuitry. Even though the regulation of leptin-induced STAT3 activation may be well understood, cytoplasmic STAT proteins have been shown to be modulated by other signals such as proteins, cytokines, and growth factors [44–46]. Such cross-talk may be physiologically important for STAT activity and normal homeostatic control. One possibility of this type of cross-talk may include PACAP-induced STAT3 phosphorylation subsequently leading to SOCS3 mRNA expression and ultimately the ability to regulate leptin actions through PACAP signaling. In fact, STAT3 phosphorylation has been shown to occur independently of the JAK2 pathway via Src family kinases [9]. The possibility for interaction grows as PACAP receptors are a class B GPCR known to use Src- and PKA-dependent signaling [47]. Src family kinases including Blk, Brk, Fgr, Frk, Fyn, Hck, Lck, Lyn, Src, Srm and Yes are not only widely expressed in multiple tissues [48] but are the means by which PACAP has been shown to facilitate the activity of NMDA receptors in the hippocampus and hypothalamic VMN through Src-mediated phosphorylation [33, 49]. Although such evidence presents potential opportunities for PACAP modulation of leptin signaling, there has been no direct evidence yet of their interaction. Interestingly, leptin receptors have been shown to have allosteric interactions with GPCR’s such as orexin/hypocretin and ghrelin receptors suggesting a possibility that leptin and PACAP receptors could interact through similar receptor interactions [50].
Developing therapeutics to address the growing obesity epidemic requires a better understanding of the cellular mechanisms that underlie energy regulation. While leptin resistance is recognized as a significant contributor to obesity, revealing the mechanisms leading to leptin insensitivity has been limited. We show that leptin receptor actions in the VMN show functional dependency on PACAP signaling to regulate feeding behavior, metabolism, and gene transcription. This suggests that intersecting signaling cascades in VMN neurons for regulating energy homeostasis could allow tonic signals such as leptin to be potentially regulated by discretely released neuropeptides such as PACAP. Interconnecting pathways activated by multiple ligands could broaden our understanding of leptin resistance as well as allow for future therapeutic strategies. Further studies will be needed to reveal possible intersectional activity between PAC1 and leptin receptors that could permit differential behavioral and metabolic variability informed by concomitant indicators of energy resources.
Funding Sources:
This work was supported by the US National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; DK074734).
Footnotes
Statement of Ethics: Animal experiments conform to internationally accepted standards and have been approved by Marquette University’s IACUC committee.
Disclosure Statement: The authors have no conflicts of interest to declare.
References
- 1.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS: Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA : the journal of the American Medical Association 2003;289:76–79. [DOI] [PubMed] [Google Scholar]
- 2.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F: Effects of the obese gene product on body weight regulation in ob/obmice. Science 1995;269:540–543. [DOI] [PubMed] [Google Scholar]
- 3.Pelleymounter MA, Cullen MJ, Wellman CL: Characteristics of BDNF-induced weight loss. Exp Neurol 1995;131:229–238. [DOI] [PubMed] [Google Scholar]
- 4.Zhange Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM: Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–432. [DOI] [PubMed] [Google Scholar]
- 5.Considine RV, Considine EL, Williams CJ, Nyce MR, Magosin SA, Bauer TL, Rosato EL, Colberg J, Caro JF: Evidence against either a premature stop codon or the absence of obese gene mRNA in human obesity. The Journal of clinical investigation 1995;95:2986–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funahashi T, Shimomura I, Hiraoka H, Arai T, Takahashi M, Nakamura T, Nozaki S, Yamashita S, Takemura K, Tokunaga K, et al. : Enhanced expression of rat obese (ob) gene in adipose tissues of ventromedial hypothalamus (VMH)-lesioned rats. Biochemical and biophysical research communications 1995;211:469–475. [DOI] [PubMed] [Google Scholar]
- 7.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS: Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nature medicine 1995;1:1311–1314. [DOI] [PubMed] [Google Scholar]
- 8.Berthoud HR, Lenard NR, Shin AC: Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol 2011;300:R1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang L, Li Z, Rui L: Leptin stimulates both JAK2-dependent and JAK2-independent signaling pathways. The Journal of biological chemistry 2008;283:28066–28073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banks AS, Davis SM, Bates SH, Myers MG Jr.: Activation of downstream signals by the long form of the leptin receptor. The Journal of biological chemistry 2000;275:14563–14572. [DOI] [PubMed] [Google Scholar]
- 11.Maniscalco JW, Rinaman L: Systemic leptin dose-dependently increases STAT3 phosphorylation within hypothalamic and hindbrain nuclei. Am J Physiol Regul Integr Comp Physiol 2014;306:R576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB: Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology 1997;138:839–842. [DOI] [PubMed] [Google Scholar]
- 13.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC Jr., Elmquist JK, Lowell BB: Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 2004;42:983–991. [DOI] [PubMed] [Google Scholar]
- 14.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL: Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 2008;149:2138–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson CM, Leshan RL, Jones JC, Myers MG Jr.: Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res 2011;1378:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baver SB, Hope K, Guyot S, Bjorbaek C, Kaczorowski C, O’Connell KM: Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. The Journal of neuroscience: the official journal of the Society for Neuroscience 2014;34:5486–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG: Central nervous system control of food intake. Nature 2000;404:661–671. [DOI] [PubMed] [Google Scholar]
- 18.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ: Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001;411:480–484. [DOI] [PubMed] [Google Scholar]
- 19.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB: Distributions of leptin receptor mRNA isoforms in the rat brain. Journal of Comparative Neurology 1998;395:535–547. [PubMed] [Google Scholar]
- 20.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S Jr., Elmquist JK, Lowell BB: Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 2006;49:191–203. [DOI] [PubMed] [Google Scholar]
- 21.Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM: PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. The Journal of neuroscience: the official journal of the Society for Neuroscience 2009;29:14828–14835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurley MM, Maunze B, Block ME, Frenkel MM, Reilly MJ, Kim E, Chen Y, Li Y, Baker DA, Liu QS, Choi S: Pituitary Adenylate-Cyclase Activating Polypeptide Regulates Hunger- and Palatability-Induced Binge Eating. Front Neurosci 2016;10:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Resch JM, Boisvert JP, Hourigan AE, Mueller CR, Yi SS, Choi S: Stimulation of the hypothalamic ventromedial nuclei by pituitary adenylate cyclase-activating polypeptide induces hypophagia and thermogenesis. Am J Physiol Regul Integr Comp Physiol 2011;301:R1625–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Resch JM, Maunze B, Gerhardt AK, Magnuson SK, Phillips KA, Choi S: Intrahypothalamic pituitary adenylate cyclase-activating polypeptide regulates energy balance via site-specific actions on feeding and metabolism. American journal of physiology Endocrinology and metabolism 2013;305:E1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF: Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nature neuroscience 2003;6:736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu LY, van den Pol AN: Agouti-related peptide and MC3/4 receptor agonists both inhibit excitatory hypothalamic ventromedial nucleus neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience 2008;28:5433–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Bomberg E, Billington CJ, Levine AS, Kotz CM: Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure. Brain Res 2010;1336:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Bomberg E, Levine A, Billington C, Kotz CM: Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol 2007;293:R1037–1045. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C: The rat brain in stereotaxic coordinates, ed 6th. Amsterdam; Boston, Elsevier Academic Press, 2007. [Google Scholar]
- 30.Rasband WS: ImageJ. U.S. National Institutes of Health, Bethesda, Maryland, USA, 1997–2018, [Google Scholar]
- 31.Hurley MM, Resch JM, Maunze B, Frenkel MM, Baker DA, Choi S: N-acetylcysteine decreases binge eating in a rodent model. Int J Obes (Lond) 2016;40:1183–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michel S, Itri J, Han JH, Gniotczynski K, Colwell CS: Regulation of glutamatergic signalling by PACAP in the mammalian suprachiasmatic nucleus. BMC Neurosci 2006;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macdonald DS, Weerapura M, Beazely MA, Martin L, Czerwinski W, Roder JC, Orser BA, MacDonald JF: Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires G alpha q, protein kinase C, and activation of Src. The Journal of neuroscience: the official journal of the Society for Neuroscience 2005;25:11374–11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Mauro M, Cavallaro S, Ciranna L: Pituitary adenylate cyclase-activating polypeptide modifies the electrical activity of CA1 hippocampal neurons in the rat. Neuroscience letters 2003;337:97–100. [DOI] [PubMed] [Google Scholar]
- 35.Irani BG, Le Foll C, Dunn-Meynell A, Levin BE: Effects of leptin on rat ventromedial hypothalamic neurons. Endocrinology 2008;149:5146–5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D: Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nature neuroscience 2004;7:493–494. [DOI] [PubMed] [Google Scholar]
- 37.Ikeda Y, Luo XR, Abbud R, Nilson JH, Parker KL: The Nuclear Receptor Steroidogenic Factor-1 Is Essential for the Formation of the Ventromedial Hypothalamic Nucleus. Molecular Endocrinology 1995;9:478–486. [DOI] [PubMed] [Google Scholar]
- 38.Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL: Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology 2002;143:607–614. [DOI] [PubMed] [Google Scholar]
- 39.Kim KW, Zhao L, Donato J Jr., Kohno D, Xu Y, Elias CF, Lee C, Parker KL, Elmquist JK: Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proceedings of the National Academy of Sciences of the United States of America 2011;108:10673–10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA: The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proceedings of the National Academy of Sciences of the United States of America 1996;93:8374–8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heim MH: The Jak-STAT pathway: cytokine signalling from the receptor to the nucleus.J Recept Signal Transduct Res 1999;19:75–120. [DOI] [PubMed] [Google Scholar]
- 42.Dunn SL, Bjornholm M, Bates SH, Chen Z, Seifert M, Myers MG Jr.: Feedback inhibition of leptin receptor/Jak2 signaling via Tyr1138 of the leptin receptor and suppressor of cytokine signaling 3. Mol Endocrinol 2005;19:925–938. [DOI] [PubMed] [Google Scholar]
- 43.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB: Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol 2000;20:5479–5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong LH, Sim H, Chatterjee-Kishore M, Hatzinisiriou I, Devenish RJ, Stark G, Ralph SJ: Isolation and characterization of a human STAT1 gene regulatory element. Inducibility by interferon (IFN) types I and II and role of IFN regulatory factor-1. The Journal of biological chemistry 2002;277:19408–19417. [DOI] [PubMed] [Google Scholar]
- 45.Krebs DL, Hilton DJ: SOCS proteins: negative regulators of cytokine signaling. Stem Cells 2001;19:378–387. [DOI] [PubMed] [Google Scholar]
- 46.David M, Petricoin E 3rd, Larner AC: Activation of protein kinase A inhibits interferon induction of the Jak/Stat pathway in U266 cells. The Journal of biological chemistry 1996;271:4585–4588. [DOI] [PubMed] [Google Scholar]
- 47.Delcourt N, Thouvenot E, Chanrion B, Galeotti N, Jouin P, Bockaert J, Marin P: PACAP type I receptor transactivation is essential for IGF-1 receptor signalling and antiapoptotic activity in neurons. EMBO J 2007;26:1542–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roskoski R Jr.: Src protein-tyrosine kinase structure and regulation. Biochemical and biophysical research communications 2004;324:1155–1164. [DOI] [PubMed] [Google Scholar]
- 49.Resch JM, Maunze B, Phillips KA, Choi S: Inhibition of food intake by PACAP in the hypothalamic ventromedial nuclei is mediated by NMDA receptors. Physiology & behavior 2014;133:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medrano M, Aguinaga D, Reyes-Resina I, Canela EI, Mallol J, Navarro G, Franco R: Orexin A/Hypocretin Modulates Leptin Receptor-Mediated Signaling by Allosteric Modulations Mediated by the Ghrelin GHS-R1A Receptor in Hypothalamic Neurons. Mol Neurobiol 2018;55:4718–4730. [DOI] [PubMed] [Google Scholar]