Introduction
In its seminal report, “Relieving Pain in America: A Blueprint for Transforming Pain Prevention, Care, Education, and Research,” the Institute of Medicine (IOM; now the National Academy of Medicine) asserted that pain is a significant public health problem and estimated that as many as 100 million Americans experience persistent pain, at a cost of as much as $635 billion in treatment costs and lost productivity [1]. Following the recommendations of this report, the Department of Health and Human Services published a National Pain Strategy in March 2016 that offered a comprehensive framework and specific recommendations for transforming pain care in America [2]. Key findings and recommendations for this transformation encouraged integrated, multimodal, and interdisciplinary pain care tailored to each person’s experience of pain and preferences for treatment. Growing concern about harms associated with long-term opioid therapy, other pain medications, and invasive procedures encouraged consideration of alternative approaches. In fact, numerous evidence-based nonpharmacological approaches were identified that may reduce risk of harms and hold special appeal to persons with pain, delivered either in conjunction with or alone as alternatives to traditional medical and rehabilitation approaches. In addition to these National Pain Strategy recommendations, other health professional organizations have similarly promulgated guidelines that recommend nonpharmacological approaches as firstline treatment for chronic pain [3]. Unfortunately, as the National Pain Strategy acknowledged, evidence suggests that these recommendations are rarely enacted due to myriad organizational, clinician-, and patient-level barriers [4].
Military service members and veterans have been identified as particularly vulnerable to the high prevalence and complexity of chronic pain related to their military experiences, including their high risk of musculoskeletal injuries and combat-related trauma. Estimates suggest that approximately 45% of active duty military service members experience pain [5], and low back pain, in particular, is one of the most common reasons service members seek medical care and one of the most likely conditions to interrupt combat duty [6]. As many as 50% of male veterans receiving care in Department of Veterans Affairs (VA) primary care settings report the presence of pain [7], and the prevalence may be as high as 75% among female veterans [8]. In a recent Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention, compared with nonveterans, veterans were significantly more likely to report chronic pain [9]. Pain is among the most frequent presenting complaints for veterans, particularly among those with polytrauma [10]. Data document that the prevalence of pain among veterans is growing steadily with each passing year [11,12]. The presence of pain among veterans receiving primary care in VA facilities is associated with poorer self-rated health, greater utilization of health care resources, greater prevalence of health risk behaviors and factors such as tobacco use, excessive alcohol use, diet/weight concerns, decreased social and physical activity, lower social support, and greater ratings of affective distress [7]. Presence of pain among veterans and military service members is highly comorbid with depressive and anxiety disorders [13], post-traumatic stress disorder [14], and substance use disorders [15]. Among women veterans, pain is associated with high rates of military and nonmilitary sexual harassment and trauma [16]. Finally, pain is also among the most costly disorders treated in VA settings [17].
The National Pain Strategy highlighted a critical gap between the science and practice of pain management, and both the Department of Defense (DoD) and the VA have developed comprehensive strategic initiatives to improve pain care that encourage research to address these gaps, including large-scale, pragmatic effectiveness studies and strategies to implement effective strategies in clinical practice [18,19]. Similarly, in 2015, the National Institutes of Health (NIH)/National Center for Complementary and Integrative Health (NCCIH) Council Working Group Report on “Strengthening Collaborations with DoD and VA” was published and called for an investment in the conduct of pragmatic clinical trials for nonpharmacological approaches to pain and comorbidities in DoD and VA health systems [20].
Pragmatic clinical trials (PCTs) test interventions in real-world settings with fewer restrictions than are commonly employed in explanatory efficacy studies. PCTs distinguish themselves from explanatory trials by 1) having broader eligibility criteria to promote the external validity and generalizability of findings to the larger population for which interventions may be applied; 2) engaging health care providers who may have little research training or experience delivering interventions; 3) delivering interventions in a manner that mimics usual care and attempts to minimally disrupt normal workflow in the clinical setting; and 4) collecting data, when possible, in the context of routine clinical care, often extracting data from electronic health records. The DoD and VA health systems, as large national integrated learning health systems with robust electronic health records, are ideal sites for robust PCTs [21].
Despite growing evidence of the efficacy of nonpharmacological approaches for the management of chronic pain, including studies that address pain and comorbidities in VA and DoD settings, few large-scale PCTs that can inform clinical practice have been conducted [22]. Therefore, efforts to conduct relevant clinical research studies within these settings will require considerable collaboration within these systems, as well as infrastructure support. Ultimately, many unanswered questions remain about how to successfully implement policy-driven models of care such as the VA and DoD Stepped Care Model of Pain Management [23] and the VA Whole Health Initiative [24], how best to address important medical and mental health comorbidities to promote improved outcomes, and how to optimally incorporate nonpharmacological approaches into routine care.
Several factors encourage optimism that the time is right to begin to address these scientific knowledge and practice gaps within the DoD and VA health systems. Important advances include methods for optimal use of comprehensive electronic health records; innovation in the design and methods for pragmatic clinical trials; availability of resources and infrastructure to conduct multisite trials such as the existing VA Cooperative Studies Program; dissemination of effective approaches for partnered research conducted by integrated teams of scientists, practitioners, patients, and other stakeholders such as publications describing a formative evaluation and implementation study of the VA’s Stepped Care Model of Pain Management [25]; and advances in quality improvement and dissemination and implementation science methods that address ethical and regulatory challenges and other barriers to the conduct of high-impact PCTs.
Structure and Function of the Collaboratory
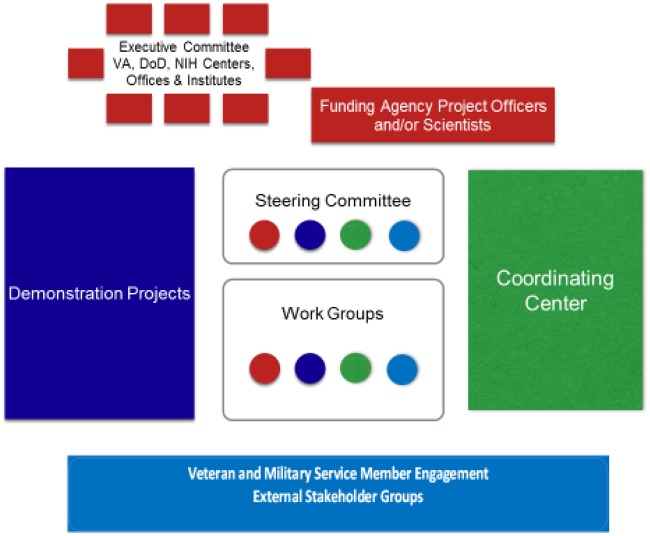
In September 2017, a significant and innovative intergovernment agency partnership involving the NIH, DoD, and VA was announced to support a multicomponent research initiative focusing on nonpharmacological approaches for pain management addressing the needs of military service members and veterans. The NIH-DoD-VA Pain Management Collaboratory (PMC) represents an investment of approximately $81 million over six years, focused on developing, implementing, and testing cost-effective, large-scale, real-world research on nonpharmacological approaches for management of pain and comorbid medical and mental health conditions in DoD and VA health care delivery organizations. The NIH/NCCIH serves as the lead funding agency or sponsor in partnership with the VA’s Health Services Research and Development Service and the DoD’s Clinical Rehabilitation Medicine Research Program, Military Operational Medicine Research Program. Numerous additional NIH institutes, centers, and offices are supporting the Collaboratory’s projects. The PMC represents a major advance in the field of pain and pain management by developing the first-ever comprehensive guidance for the conduct of PCTs of nonpharmacological approaches for the management of pain in DoD and VA health care settings. Products will take several forms, including technical policy guidelines and summaries of best practices and lessons learned based on the experiences of highly qualified teams of investigators who will conduct the PCTs, complemented by the expertise of a Pain Management Collaboratory Coordinating Center (PMC3). The products of the PMC3 will serve a foundational role for future similar efforts in the field of pain management and related fields and for PCTs in other integrated health care settings. A schematic of the PMC components is presented in Figure 1.
Figure 1.
Schematic depiction of the components of the Pain Management Collaboratory.
The PMC3 supports 11 PCTs selected based on peer review that evaluated the significance of the scientific questions, innovation and quality of the approach, and potential to address impediments to the research with health care delivery organizations. The trials span those focused on examining questions related to specific nonpharmacological approaches (e.g., “dose” of chiropractic care necessary to achieve optimal, sustained benefit; comparative effectiveness of two approaches to delivering mindfulness-based meditation) and/or models of pain care (e.g., comparative effectiveness of a sequenced, integrated multimodal care pathway vs care management via a patient resource group; comparative effectiveness of integrated health care professional teams delivering personalized care planning, health coaching and targeted complementary and integrative health strategies vs traditional primary care–based group education). One project examines acute pain management in the perioperative setting, and the others focus on chronic pain management in outpatient settings. The trials are supported by a phased award mechanism, with a two-year planning phase followed by a four-year implementation phase. All projects are milestone-driven (e.g., regulatory approvals are obtained, feasible recruitment plans are finalized), and moving to the implementation phase will be dependent upon the successful progress made during the planning phase. Table 1 provides a list of each of these projects with principal investigator(s) and sponsors.
Table 1.
Pragmatic clinical trials and principal investigators
| PI Name | Affiliation | PCT Name |
|---|---|---|
| A. Heapy | VA Connecticut Healthcare System & Yale School of Medicine | Cooperative Pain Education and Self-Management: Expanding Treatment for Real-world Access (Copes ExTRA) |
|
VA Connecticut Healthcare System & Yale School of Medicine | Engaging Veterans Seeking Service Connection Payments in Pain Treatment |
|
|
Chiropractic Care for Veterans, a Pragmatic Randomized Trial Addressing Dose Effects for cLBP |
|
|
Improving Veteran Access to Integrated Management of Chronic Back Pain (AIM-Back) |
|
|
SMART Stepped Care Management for Low Back Pain in the Military Health System |
|
|
Whole Health Team vs. Primary Care Group Education to Promote Non-Pharmacological Strategies to Improve Pain, Functioning, and Quality of Life in Veterans |
|
|
APPROACH: Assessing Pain, Patient Reported Outcomes and Complementary and Integrative Health: A National Dissemination Project |
| D. Burgess | Minneapolis VA & University of Minnesota Medical School | Testing Two, Scalable, Veteran-Centric Mindfulness Based Interventions for Chronic Musculoskeletal Pain: A Pragmatics Multisite Trial |
|
|
Targeting Chronic Pain in Primary Care Settings Using Internal Behavioral Health Consultants |
| B. M. Ilfeld | University of California, San Diego | Ultrasound-Guided Percutaneous Peripheral Nerve Stimulation: A Non-Pharmacologic Alternative for the Treatment of Postoperative Pain |
|
|
Resolving the Burden of Low Back Pain in Military Service Members and Veterans: A Multi-Site Pragmatic Clinical Trial (RESOLVE Trial) |
PCT = pragmatic clinical trial.
Following on the heels of a successful Health Care Systems Research Collaboratory, also sponsored by the NIH (https://rethinkingclinicaltrials.org/), the PMC3 was established to facilitate collective learning across the PCTs and to optimize the impact of the PMC as an integrated whole. The PMC3 is based at the Yale School of Medicine and its affiliate, the VA Connecticut Healthcare System. The PMC3 draws on four existing centers, including the Yale Center for Analytical Sciences, the Yale Center for Medical Informatics, the VA Connecticut Pain Research, Informatics, Multimorbidities and Education Center of Innovation, and the VA Cooperative Studies Program Coordinating Center. An additional partnership was established with the Uniformed Services University of the Health Science Center for Rehabilitation Sciences Research to extend the reach of the PMC3 to the DoD research community. The PMC3 provides leadership and serves as a resource for development and refinement of innovative tools, best practices, and other resources in the conduct of PCTs. The leadership of the PMC3 includes three co-directors with complementary expertise in pain management (RDK), health informatics (CAB), and biostatistics/clinical trials (PP). An administrative/operations and communications core ensures a high level of productivity overall and that key milestones are met.
Consistent with the successful model enacted for the Health Care Systems Research Collaboratory, shared learning, harmonization across projects, and problem solving related to the PCTs largely occur through participation in seven work groups spanning the domains of 1) biostatistics and study design, 2) phenotypes and outcomes, 3) electronic health records, 4) ethics and regulatory issues, 5) stakeholder engagement, 6) data sharing, and 7) implementation science. Each work group is led by two co-chairs with expertise related to the work group domain, along with representatives from each of the PCT teams, and supported by an experienced project manager. Representatives from the NIH, DoD, and VA, as well as investigators with additional relevant expertise, participate in work group discussions. Work groups usually meet virtually at least monthly; one-on-one sessions are scheduled as necessary with individual PCT teams to address specific issues. Work groups discuss topics of mutual interest, share best practices, and build consensus. The objectives of the work groups are to promote harmonization and synergy among the trials, to optimize the performance and success of the individual trials and the PMC, and to support dissemination of new knowledge. Table 2 presents a summary of work group objectives.
Table 2.
Pain Management Collaboratory work group objectives
| Work Group Name | Work Group Objectives |
|---|---|
| Biostatistics and Study Design |
|
| Data Sharing |
|
| Electronic Health Record (EHR) |
|
| Ethics and Regulatory |
|
| Implementation Science |
|
| Phenotypes and Outcomes |
|
| Stakeholder Engagement |
|
DoD = Department of Defense; PCT = pragmatic clinical trial; PI = principal investigator; VA = Department of Veterans Affairs.
Multiple innovations of the PMC3 promise to address known barriers to the successful conduct and implementation of PCTs. A few examples of key barriers include obtaining all necessary regulatory approvals from multiple sites where the trials will be conducted; successfully obtaining clinician and administrator support for the study, including engaging clinicians as study therapists and other staff in recruitment efforts; and modifying electronic health records to incorporate collection of key study measures within the context of clinical care, among many others. Innovations highlight the opportunity afforded by this important initiative to support advances in the design (e.g., cluster randomized designs [26]) and successful enactment of PCTs and to address numerous acknowledged scientific knowledge and practice gaps related to the role of nonpharmacological approaches to the management of pain and comorbidities. Table 3 presents a summary of these innovations.
Table 3.
PMC innovations
| Development and enactment of a comprehensive, evidence-based, and stakeholder-informed approach to address previously identified organizational, clinician-, and patient-level barriers to access, engagement, and participation in PCTs of nonpharmacological approaches for the management of chronic pain and comorbidities. |
| Development and dissemination of a first-of-its-kind comprehensive toolkit comprised of VA and DoD technical guides, policies, and regulations relevant to the ethical conduct of pragmatic clinical trials consistent with good clinical practice and standards for the protection of human subjects’ research participants. The toolkit will include state-of-the-art risk-based monitoring methods to ensure adequate protection of the rights, welfare, and safety of human subjects and the quality and integrity of the resulting data to yield high-quality approaches for monitoring clinical trials. |
| Development and dissemination of methodological standards for the design and implementation of PCTs of nonpharmacological approaches for chronic pain management in VA and DoD health care systems. |
| Application of innovative biomedical informatics tools (e.g., machine learning, natural language processing, ontologies) to facilitate optimal use of existing electronic health record structured and unstructured data and to optimize integration of complementary patient-reported outcomes to support the successful conduct of pragmatic clinical trials in DoD and VA settings. |
| Harmonization of the measurement approaches, especially measurement of key outcomes proposed by pragmatic trial investigators, when feasible. |
| Facilitation of data sharing to explore development of integrated patient-level de-identified database(s) combining data from participants in the pragmatic trials in these settings through the application of state-of-the-science tools for data sharing and consistent with existing ethical policies and standards and regulations governing such activities. The availability of an integrated database could serve as the foundation for application of innovative approaches to trial participant phenotyping and evaluating and comparing trial outcomes and effect modifiers. |
| Development and enactment of a comprehensive communication approach that supports timely and reliable sharing of information, activities, and best practices among collaborating teams of scientists and internal DoD and VA stakeholders, as well as a similar approach that fosters optimal external communication and dissemination of lessons learned, white papers, online text books, and webinars. |
| Implementation of new informatics approaches to augment and supplement the informed consent process such as the addition of multimedia materials to enhance participant comprehension and reduce provider burden for obtaining patient consent. |
DoD = Department of Defense; PCT = pragmatic clinical trial; VA = Department of Veterans Affairs.
The PMC is supported by a Steering Committee comprised of the leadership of the PMC3 (including work group co-chairs and program managers), the PCT PIs, and sponsors/funders. The Steering Committee aligns with the mission of the PMC, spans all PCTs, provides input into the policies and processes of the PMC, addresses immediate and long-term cross-cutting issues that impact the PCTs, ensures the quality, performance, management, and regulatory compliance of the PCTs, promotes an integrated and collaborative culture among all PCTs, assists in the dissemination of policies and processes that enable research in partnership with the DoD and VA health systems, their patients, and practitioners, and advances knowledge and education related to nonpharmacological approaches to pain management. The Steering Committee meets virtually monthly and in person at least once annually. Meetings are structured to provide updates on PCTs and to discuss key cross-cutting or priority topics that have emerged from work group discussions. As just a few examples, the Steering Committee has discussed priority issues including refinement of PCT designs, harmonization of primary and secondary outcome measures across trials, best practices for ensuring timely approval of human subjects’ research protocols, shared recruitment challenges, and regulatory issues related to data sharing.
It is important to emphasize that all PMC trials are informed by substantial evidence of the efficacy, even effectiveness, of specific nonpharmacological approaches and models of care. For the most part, although these trials will likely contribute to the body of evidence about effectiveness, they are designed to address important additional scientific knowledge and practice gaps related to the successful implementation of these approaches and models of care in real-world clinical settings, particularly in the VA and DoD. As such, an important implication of the PMC initiative is that clinicians, policy-makers, and administrators can have confidence that the approaches and models that are the targets of the PMC trials are all already considered to be appropriate for implementation in routine clinical practice.
The PMC will employ a multipronged communication plan to disseminate information relevant to the Collaboratory, as well as new knowledge and products. Even in advance of the conduct of the PCTs that comprise the PMC, the PMC3 has constructed a publicly accessible website (www.painmanagementcollaboratory.org) that is intended to be an important resource for investigators, clinicians, policy-makers, and advocates invested in addressing important scientific knowledge and practice gaps and in promoting the equitable and timely availability of high-quality pain management services. Already available is a growing array of resources including timely updates of evidence relevant to those involved in delivering pain care, upcoming education and training opportunities, and information about best practices, guidance, and policy statements in support of this objective. For example, “white papers” will be published on the website, such as summaries of innovative processes or methods that have been employed to address key issues. Although the results of trials will not likely be available for six years, the results of pilot work are already starting to be reported. As early results and products emerge from the PMC and the individual trials, these updates will be highlighted on the website. Other outlets for dissemination include publication in scientific and professional journals, presentations at scientific meetings, and social media outlets such as Twitter (@painmc3).
Conclusions
The PMC has already begun to show evidence of the potential to fulfill its promise as a significant and innovative approach to addressing key scientific knowledge and clinical practice gaps in the delivery of high-quality pain care in DoD and VA health systems and supporting improved patient outcomes. A major difference between the ongoing Health Care Systems Research Collaboratory and the PMC is that, although the existing Collaboratory includes trials from multiple domains of health care (e.g., cardiovascular disease, renal disease, pain management), the PMC includes a specific focus on nonpharmacological approaches to management of pain and comorbid conditions in DoD and VA health systems. The conduct of these trials in large integrated health systems has potential advantages relative to the Health Care Systems Research Collaboratory, which is primarily conducted in civilian fee-for-service settings. The focus on military service members and veterans is significant due to the higher burden of pain relative to the civilian population. As such, the PMC represents a significant investment and offers a unique opportunity to rapidly advance the science and practice of pain management in these settings.
Acknowledgments
Correspondence to: Robert D. Kerns, VA Connecticut Healthcare System, 950 Campbell Avenue, West Haven, Connecticut, 06516, USA. Tel: 203-937-3841; E-mail: robert.kerns@yale.edu.
Funding sources: The NIH-DoD-VA Pain Management Collaboratory is supported by multiple US Government agencies and entities, including the National Institutes of Health (NIH) National Center for Complementary and Integrative Health (NCCIH), National Institute of Neurological Disorders and Stroke (NINDS), National Institute of Drug Abuse (NIDA), National Institute of Alcohol Abuse and Alcoholism (NIAAA), National Institute for Child Health and Human Development (NICHD), National Center for Medical Rehabilitation Research (NCMRR), Office of Research on Women’s Health (ORWH), and National Institute of Nursing Research (NINR); the Department of Defense (DoD) Clinical and Rehabilitative Medicine Research Program (CRMRP) and Military Operational Medicine Research Program (MOMRP); and the Department of Veterans Affairs (VA) Health Services Research & Development (HSR&D) Service of the Office of Research and Development.
Conflict of interest: R.D.K is a member of the Senior Board of Advisors, Rialto, Inc and a member of the Board of Directors of A Place to Nourish your Health (APNH), and has received honoraria as Senior Editor of Pain Medicine, speaker for the International Massage Therapy Association and speaker for the American Case Management Association. S. P. C. has in the past 12 months, received money as a consultant from Scilex, Sorrento, Avanos, SPR Therapeutics, AcelRx. B. I.'s institution, the University of California, has received funding and product for other research projects from cryoneurolysis device manufacturers Myoscience and Epimed; infusion pump manufacturer Infutronics; perineural catheter manufacturer Ferrosan Medical; a manufacturer of a peripheral nerve stimulation device, SPR Therapeutics; and a manufacturer of long-acting local anesthetics, Heron Pharmaceuticals. All other authors report no conflicts of interest.
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily reflect the official policy of the Department of Health and Human Services, National Institutes of Health, Department of Defense, Department of Veterans Affairs, or US Government.
Appendix
Table A1.
Authors and Affiliations
| Name | Affiliation |
|---|---|
| Joe Ali, JD | Johns Hopkins Berman Institute of Bioethics |
| Margaret Antonelli | VA Connecticut Healthcare System & Yale School of Medicine |
| Lori Bastian, MD, MPH | VA Connecticut Healthcare System & Yale School of Medicine |
| William Becker, MD | VA Connecticut Healthcare System & Yale School of Medicine |
| Cynthia Brandt, MD, MPH | VA Connecticut Healthcare System & Yale School of Medicine |
| Diana Burgess, PhD | Minneapolis VA Medical Center & University of Minnesota Medical School |
| Amy Burns, JD, OTR/L | VA Connecticut Healthcare System & Yale School of Medicine |
| Steven P. Cohen, MD | Johns Hopkins Medical Institutions |
| Christopher Dearth, PhD | DoD-VA Extremity Trauma and Amputation Center of Excellence (EACE), Walter Reed National Military Medical Center & Uniformed Services University of the Health Sciences |
| Jim Dziura, MPH, PhD | Yale School of Medicine |
| Joe Erdos, MD | VA Connecticut Healthcare System & Yale School of Medicine |
| Shawn Farrokhi, MS, DPT, PhD | DoD-VA Extremity Trauma and Amputation Center of Excellence (EACE) & Naval Medical Center San Diego |
| Julie Fritz, PhD, PT | University of Utah |
| Mary Geda, RN, BSN, MSN | Yale School of Medicine |
| Steven Z. George, PT, PhD | Durham VA Health Care System & Duke University |
| Christine Goertz, DC, PhD | Spine Institute for Quality |
| Jeffrey Goodie, PhD. | Uniformed Services University of the Health Sciences |
| Susan Hastings, MHS., MD | Durham VA Medical Center & Duke University |
| Alicia Heapy, PhD | VA Connecticut Healthcare System & Yale School of Medicine |
| Jorielle Houston, PhD | Defense Health Agency |
| Brian Ilfeld, MD, MS | University of California, San Diego |
| Gary Johnson, MS | VA Connecticut Healthcare System |
| Robert Kerns, PhD | Yale University & VA Connecticut Healthcare System |
| Tassos Kyriakides, PhD | West Haven Cooperative Studies Program Coordinating Center, VA Connecticut Healthcare System & Yale School of Public Health |
| Allison Lee | Yale School of Medicine & VA Connecticut Healthcare System |
| Cyndy Long, PhD | Palmer Center for Chiropractic Research |
| Steve Martino, PhD. | VA Connecticut Healthcare System & Yale School of Medicine |
| Michael E. Matheny, MD, MS, MPH | Vanderbilt University Medical Center |
| Don McGeary, PhD, ABPP | University of Texas Health Science Center – San Antonio |
| Amanda Midboe, PhD | VA Palo Alto Health Care System |
| Paul Pasquina, MD | Uniformed Services University of the Health Sciences & Walter Reed National Military Medical Center |
| Peter Peduzzi, PhD | Yale School of Medicine |
| Michael Raffanello | VA Connecticut Healthcare System & Yale School of Medicine |
| Daniel Rhon, MPT, DPT, DSc | Brooke Army Medical Center |
| Will Roddy | Uniformed Services University of the Health Sciences |
| Marc Rosen, MD | VA Connecticut Healthcare System & Yale School of Medicine |
| Elizabeth Russell Esposito, PhD | DoD-VA Extremity Trauma and Amputation Center of Excellence (EACE), VA Puget Sound Health Care System, University of Washington & Uniformed Services University of the Health Sciences |
| Dylan Scarton, MS | Uniformed Services University of the Health Sciences |
| Karen Seal, MD, MPH | San Francisco VA Health Care System & University of California-San Francisco |
| Norman Silliker | Yale School of Medicine |
| Jerika Taylor, MA | Uniformed Services University of the Health Sciences |
| Sakasha Taylor, MS | VA Connecticut Healthcare System |
| Stephanie Taylor, PhD, MPH | VA Greater Los Angeles Healthcare System & UCLA Department of Health Policy and Management |
| Fred S. Wright, MD | VA Connecticut Healthcare System & Yale School of Medicine |
| Steven Zeliadt, PhD, MPH | VA Puget Sound Health Care System & University of Washington, School of Public Health |
References
- 1.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: Institute of Medicine; 2011.
- 2.Department of Health and Human Services. National Pain Strategy: A Comprehensive Population Health-Level Strategy for Pain. Washington, DC: Department of Health and Human Services; 2016.
- 3. Qaseem A, Wilt TJ, McLean RM, Forceia MA.. Noninvasive treatments for acute, subacute, and chronic low back pain: A clinical practice guideline from the American College of Physicians. Ann Intern Med 2017;1667:514–30. [DOI] [PubMed] [Google Scholar]
- 4. Heyward J, Jones CM, Compton WM, et al. Coverage of nonpharmacological treatments for low back pain by US public and private insurers. JAMA Open Netw 2018;16:e183044.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toblin RL, Quartana PJ, Riviere LA, Walper K, Hoge CW.. Chronic pain and opioid use in us soldiers after combat deployment. JAMA Int Med 2014;1748:1400–1. [DOI] [PubMed] [Google Scholar]
- 6. Cohen SP, Gallagher RM, DaVIS s, Griffith SR, Carragee EJ.. Spine-area pain in military personnel: A review of epidemiology, etiology, diagnosis and treatment. Spine J 2012;129:833–42. [DOI] [PubMed] [Google Scholar]
- 7. Kerns RD, Otis J, Rosenberg R, Reid MC.. Veterans' reports of pain and associations with ratings of health, health-risk behaviors, affective distress, and use of the healthcare system. J Rehabil Res Dev 2003;405:371–80. [DOI] [PubMed] [Google Scholar]
- 8. Haskell SG, Heapy A, Reid MC, Papas RK, Kerns RD.. The prevalence and age-related characteristics of pain in a sample of women veterans receiving primary care. J Womens Health (Larchmt) 2006;157:864–71. [DOI] [PubMed] [Google Scholar]
- 9. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep 2018;6736:1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX.. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: Polytrauma clinical triad. J Rehabil Res Dev 2009;466:697–702. [DOI] [PubMed] [Google Scholar]
- 11. Haskell SG, Ning Y, Krebs E.. Prevalence of painful musculoskeletal conditions in female and male veterans in 7 years after return from deployment in Operation Enduring Freedom/Operation Iraqi Freedom. Clin J Pain 2012;282:163–7. [DOI] [PubMed] [Google Scholar]
- 12. Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: Examining pain and pain care among veterans. Pain 2016;1578:1696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K.. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med 2008;708:890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shipherd JC, Keyes M, Jovanovic T, et al. Veterans seeking treatment for posttraumatic stress disorder: What about comorbid chronic pain? J Rehabil Res Dev 2007;442:153–66. [DOI] [PubMed] [Google Scholar]
- 15. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA 2012;3079:940–7. [DOI] [PubMed] [Google Scholar]
- 16. Haskell SG, Papas RK, Heapy A, Reid MC, Kerns RD.. The association of sexual trauma with persistent pain in a sample of women veterans receiving primary care. Pain Med 2008;96:710–7. [DOI] [PubMed] [Google Scholar]
- 17. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev 2003;60(3 Suppl):146S–67S. [DOI] [PubMed] [Google Scholar]
- 18.Department of Veterans Affairs. VHA National Pain Management Strategy. Washington, D.C.: Department of Veterans Affairs; 1998.
- 19.Office of the Army Surgeon General. Pain Management Task Force: Providing a Standardized DoD and VHA Vision and Approach to Pain Management to Optimize the Care for Warriors and their Families. Washington, D.C.: Office of the Army Surgeon General; 2010.
- 20. National Center for Complementary and Integrative Health. NCCIH Council Working Group Report on Strengthening Collaborations with the U.S. Department of Defense and U.S. Department of Veterans Affairs: Effectiveness Researches on Mind Body Interventions. Bethesda, MD: National Center for Complementary and Integrative Health; 2015.
- 21. Veterans Health Administration. VHA Operations Activities That May Constitute Research. VA Handbook 1058.05. Washington, DC: Veterans Health Administration; 2011.
- 22. Goertz CM, Long CR, Vining RD, Pohlman KA, Walter J, Coulter I.. Effect of usual medical care plus chiropractic care vs usual medical care alone on pain and disability among US service members with low back pain. JAMA Netw Open 2018;11:e180105.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosenberger PH, Philip E, Lee A, Kerns RD VHA.. National pain management strategy: Implementation of stepped pain management. Fed Pract 2011;28:39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krejci LP, Carter K, Gaudet T.. Whole health: The vision and implementation of personalized, proactive, patient-driven healthcare for veterans. Med Care 2014;52:S5–S8. [DOI] [PubMed] [Google Scholar]
- 25. Dorflinger L, Moore B, Goulet J, et al. A partnered approach to opioid management, guideline concordant care and the stepped care model of pain management. JGIM 2014;29(S4):870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cook AJ, Delong E, Murray DM, Vollmer WM, Heagerty PJ.. Statistical lessons learned for designing cluster randomized pragmatic clinical trials from the NIH Health Care Systems Collaboratory Biostatistics and Design Core. Clin Trials 2016;135:504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]