Abstract
Programmed cell death ligand 1 (PD-L1) is a key suppressor of the cytotoxic immune response. In colorectal carcinoma (CRC), PD-L1 expression results in immune escape and poor prognosis. Extensive researches suggested that metformin had a potential efficacy of enhancing anti-tumor immune response in different types of cancer. However, the detail mechanisms underlying the efficacy in CRC are unclear. Here, we showed that metformin decreases PD-L1 and YAP1 expression in vitro and in vivo. After silencing or inhibiting YAP1 expression by Verteporfin (VP), the inhibitor of YAP1, the expression of PD-L1 were decreased in protein level in CRC cells. Furthermore, metformin directly phosphorylated YAP1 and restricted YAP1 to entry in the nucleus, so that PD-L1 was reduced via western blot and immunofluorescence assays in SW480 and HCT116 cells. Finally, subcutaneous xenotransplanted tumor models of HCT116 cells were established in BALB/c nude mice. Compared with the control group, PD-L1 and YAP1 expressions in tumor tissues, detected by immunohistochemistry, were reduced in the group of metformin treatment. These findings illuminate a new regulatory mechanism, metformin activates Hippo signaling pathway to regulate PD-L1 expression and suggests that metformin has the possibility to increase the efficacy of immunotherapy in human CRC.
Keywords: Metformin, PD-L1, Hippo signaling pathway, colorectal carcinoma
Introduction
Colorectal carcinoma (CRC) is a lethal disease with a large demand for treatment. At present, chemotherapy is the only option for most patients with metastatic CRC [1]. A clinical trial study reported that the incidence and number of metachronous colorectal adenomas and polyps after polypectomy were reduced by low-dose metformin in a comparative randomised trial [2]. Metformin has been a frontline therapy for type 2 diabetes (T2D) for many years for its safety, effective and well tolerated [3]. In addition, a great number of studies have shown metformin has the anti-tumor effect on clinical trials between diabetic cancer patients or non-diabetic cancer patients [4-7]. Besides, metformin also enhances cytotoxic T lymphocyte (CTL) activity to eliminate cancer cells in tumor tissues [8]. Those findings indicate that metformin may be involved in anti-tumor effects by immune response.
Literatures has investigated that cancer cells smartly escape immune surveillance by manipulating immune checkpoint molecules, which are expressed to maintain the balance of the immune system, such as circumstances of tolerance to self-antigens and chronic infections, as well as in inflammatory settings [9]. One of the important immune checkpoint molecular is programmed cell death ligand 1 (PD-L1; also known as CD274 or B7-H1) in the surface of tumor cells, which is the ligand of programmed cell death 1 (PD-l) [10]. PD-L1 positivity tumors were connected with poorly differentiated. Lots of colorectal cancer with ‘stem-like’ immunophenotype expressed PD-L1 [11]. Increasing evidences have revealed that many signaling pathways and transcriptional factors played a critical role in controlling the expression of PD-L1 to regulate the immune surveillance. The Janus kinase-signal transducer and activator of transcription (JAK-STAT)/mitogen-activated protein kinase (MAPK)/phosphatidylinositol 3-kinase (PI3K)/Hippo/epidermal growth factor receptor (EGFR)/nuclear factor kappa beta (NF-κB) signaling pathways are all mediated the expression and function of PD-L1 in various tumors [12-17].
The Hippo signaling pathway was discovered nearly two decades ago emerged as an important player in tissue regeneration, stem cell renewal and tumorigenesis [18-20]. Yes-associated protein 1 (YAP1) is a key oncogenic mediator in the Hippo signaling pathway and negatively regulated by the Hippo pathway. The role of YAP1 in the cancer immunology has started to be researched [21]. Recent studies showed that YAP1 was involved in the regulation of the immune checkpoints PD-L1 and tumor-associated immune cells, such asmyeloid-derived suppressor cells, tumor-associated macrophages and regulatory T cells in BRAFV600 inhibitor (BRAFi)-resistant melanoma [22-26]. More interesting, metformin suppressed YAP1 by interfering with interferon regulatory factor 1 (IRF-1) binding to the YAP1 promoter in non-small cell lung cancer (NSCLC) [27].
The exact molecular mechanism of metformin-mediated antitumor immunity is still unclear. Here, we demonstrated that metformin downregulated PD-L1 expression via Hippo signaling pathway in human colorectal cancer cells. In detail, metformin accelerated phosphorylation of YAP1, promoted YAP1 degradation via ubiquitination, and decreased the total protein of YAP1. Our study indicated that metformin might lay the foundation for future development of metformin-based immunotherapy for colorectal cancer.
Materials and methods
Cell lines and culture
SW480 and HCT116 cell lines were purchased from the Culture of Collection of the Chinese Academy of sciences (Shanghai, China). SW480 cells were cultured in DMEM medium containing 10% fetal bovine serum. HCT116 cells were cultured in RPMI1640 medium containin 10% fetal bovine serum. They both were incubated with a humidified 5% CO2 atmosphere at 37°C.
RNA isolation and quantitative real-time PCR
Total RNA was extracted from cells after treatment using Trizol (TaKaRa, Japan). PrimeScript RT Reagent Kit (TaKaRa, Japan) was used to reverse-transcribe cDNA from total RNA. Quantification of target and reference (GAPDH) genes was performed on an iQ5 real-time PCR detection system (Bio-Rad, USA). After being normalized to GAPDH gene, the expression level of target genes were calculated by using 2-ΔΔct methods. Changes in mRNA expression were expressed as fold change relative to control.
The primers used for the qPCR are listed as follows: PD-L1, forward: 5’-GGCATTTGCTGAACGCAT-3’; PD-L1, reverse: 5’-CAATTAGTGCAGCCAGGT-3’; YAP1, forward: 5’-TAGCCCTGCGTAGCCAGTTA-3’; YAP1, reverse: 5’-TCATGCTTAGTCCACTGTCTGT-3’; GAPDH, forward: 5’-TGTGTCCGTCGTGGATCTGA-3’; GAPDH, reverse: 5’-TTGCTGTTGAAGTCGCAGGAG-3’.
Immunofluorescence
To detection the expressions of YAP1 in cytoplasm and cell nucleus by immune-fluorescence staining, cells were fixed with 4% paraformaldehyde for 30 min and cell membranes were perforated by 0.3% triston-100 for 15 min. Then, the cells were blocked with ready-to-use normal goat serum for 45 min at room temperature, stained with anti-YAP1 antibody (GeneTex, USA) at 4°C overnight. After that, cells were washed with PBS for three times and incubated with FITC-conjugated secondary antibodies (Bioworld, USA) for 2 h at room temperature, then, stained with 4’,6-diamidino-2-phenylindole (DAPI) for 4 min. Finally, representative images were taken using an FV3000 laser scanning confocal microscope (Olympus, Japan) with a 60 × objective for analyzing YAP1 nuclear translocation.
Flow cytometry
SW480 and HCT116 cell lines were seeded on 6-well plates (2.0 × 105 cells/well) overnight, incubated with IFN-γ (50 ng/mL) for 24 h, and dealt with 4 mM metformin for 24 h. First, collecting these cells and washing them for two times with PBS, next, staining them with anti-PD-L1 primary antibodies (GeneTex, USA) at 4°C overnight, then, washing them two times again with PBS, staining them with FITC-conjugated secondary antibodies for 2 h at room temperature in the dark. Flow cytometry was performed on a flow cytometer (Millipore, USA) followed by analysis with FlowJo Version 10.0.7 software, after gating on live singlet cells.
Western blot analysis
The cells were washed two times with cold PBS and then lysed in 1 × loading buffer. Proteins (40 μg) were loaded per well and separated on 10% SDS-polyacrylamide gels, electrophoretically transferred onto 0.22 μm PVDF membranes. Following sealing with 5% defatted milk at room temperature for 2 h, the membranes were incubated with primary antibodies at 4°C overnight, washing them three times with PBS and then incubated with HRP-conjugated secondary antibodies (Cell Signaling, USA) for 2 h at room temperature. Blots were analyzed using Chemiluminescent reagent (TANON, China). All the normalized protein expressions were analyzed by Image J.
siRNA transfection
The cells were seeded in 6-well-plates (2.0 × 105 cells/well) overnight. When the cell density reached from 60% to 70%, 50 nM siRNA (RiboBio, China) mixed with Lipofectamine 3000 (Invitrogen, USA) were transfected into these cells for 24 h in complete culture medium. Then, 4 mM metformin were added into them for another 24 h. The sequence of siYAP1# 1 is GCGTAGCCAGTTACCAACA, siYAP1# 2 is CAGTGGCACCTATCACTCT and siYAP1# 3 is GGTGATACTATCAACCAAA.
Xenograft models
BALB/c female nude mice were purchased from Gempharmatech Co., Ltd (Nanjing, China). When the experiments were begun, the mice were between 4 and 5 weeks of age, between 14 g and 16 g of weight. The animal use protocol was in line with institutional guidelines and approved by Institutional Animal Care and Use Committee of Sun Yat-Sen University (Guangzhou, China).
Immunohistochemistry staining and evalution
Tissue paraffin sections from allograft tumors were dewaxed by xylol and were treated with 3% hydrogen peroxide solution for 20 min to reduce endogenous peroxidase activity. Then, antigens were repaired by boiling in 0.01 M citrate buffer (pH=6.0) for 10 min. After 2 h pre-incubation with ready-to-use normal goat serum for 45 min at 37°C to prevent non-specific staining, sections were incubated with the anti-YAP1 primary antibodies and anti-PD-L1 primary antibodies at 4°C overnight, washing them three times again with PBS and then incubated with HRP-conjugated secondary antibodies for 2 h. Finally, DAB was applied as substrate and hematoxylin was used as a counterstain. Each stained slice was observed by three independent scorers (Jiawang Zhou, Ziqian Li and Junjie Zhang). They were graded by assessing (m) the proportion of positive cells in the whole cells (0, < 5%; 1, 6-25%; 2, 26-50%; 3, 51-75%; 4, 76-100%) and (n) the intensity of staining (0, negative; 1, weak staining; 2, medium staining; 3, strong staining). The score was calculated by m × n [28]. The percentage of PD-L1+ and YAP1+ were calculated by Image J.
Statistical analyses
Results are expressed as mean ± S.D. of three independent experiments unless otherwise specified. Statistical significance was analyzed with two-tailed unpaired Student’s t-test (2 groups) and one-way ANOVA (> 2 groups). GraphPad Prism Software Version 5.0 (GraphPad Software Inc., La Jolla, CA, USA) was used to calculated P values. *P < 0.05.
Results
Metformin downregulates PD-L1 expression in SW480 and HCT116 cells
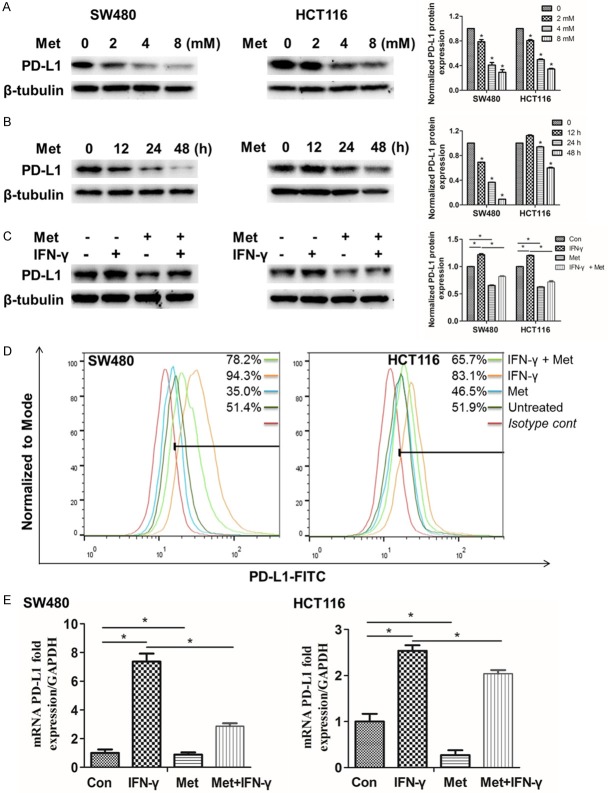
To investigate how metformin impacted on PD-L1 in SW480 and HCT116, cells were incubated with metformin at different concentrations and different times. Surprisingly, we found that when the concentration of metformin was 4 mM and the treated time was 48 h, the protein expressions of PD-L1 were significantly decrease (Figure 1A, 1B). Generally, IFN-γ effectively increased PD-L1 expression via JAK-STAT signaling pathway in various tumor cells [29]. Subsequently, we detected PD-L1 expression by western blot, flow cytometery and qRT-PCR. We discovered that metformin still inhibited the expression of PD-L1 while the cells were stimulated with IFN-γ (Figure 1C-E). The relatively original western blots images were provided (Figure S1). Collectively, the results of this study indicated that metformin lessened the expression of PD-L1 in protein and mRNA levels in SW480 and HCT116 cells.
Figure 1.
Metformin reduces PD-L1 expression in SW480 and HCT116 cells. A. SW480 and HCT116 cells were respectively treated with 0, 2, 4 and 8 mM metformin for 24 h, the proteins were collected for western blot. B. SW480 and HCT116 cells were respectively treated with 4 mM metformin for 0, 12, 24, and 48 h, the proteins were collected for western blot. C. The cells were incubated with IFN-γ (50 ng/mL) for 24 h, and then treated with 4 mM metformin for 24 h, the proteins were collected for western blot. D. The cells were collected for flow cytometry analysis, the gate was based on nearly half untreated SW480 and HCT116 cells. E. The mRNA was collected for qRT-PCR analysis. Data were presented as means ± S.D. (n=3), *P < 0.05.
Metformin activates Hippo signaling pathway by inducing phosphorylation level of YAP1
Recently, increasing studies confirmed that PD-L1 was associated with JAK-STAT, MAPK and PI3K signaling pathways. IRF1 was activated by the JAK-STAT signaling, which directly bonded to the PD-L1 promoter to induce the transcription of PD-L1 [12]. The MAPK/ERK pathway increased PD-L1 expression by activating the transcription factor c-Jun. PI3K pathway induced PD-L1 expression through activating transcription factor STAT3 [13]. In our results, metformin almost did influence on not MAPK and PI3K signaling pathways but JAK-STAT and Hippo signaling pathways. However, metformin validly upregulated the phosphorylation level of YAP1 regardless of in a short time or a long time (Figures 2A, 2B and S2). That meant Hippo signaling pathway was activated by metformin.
Figure 2.
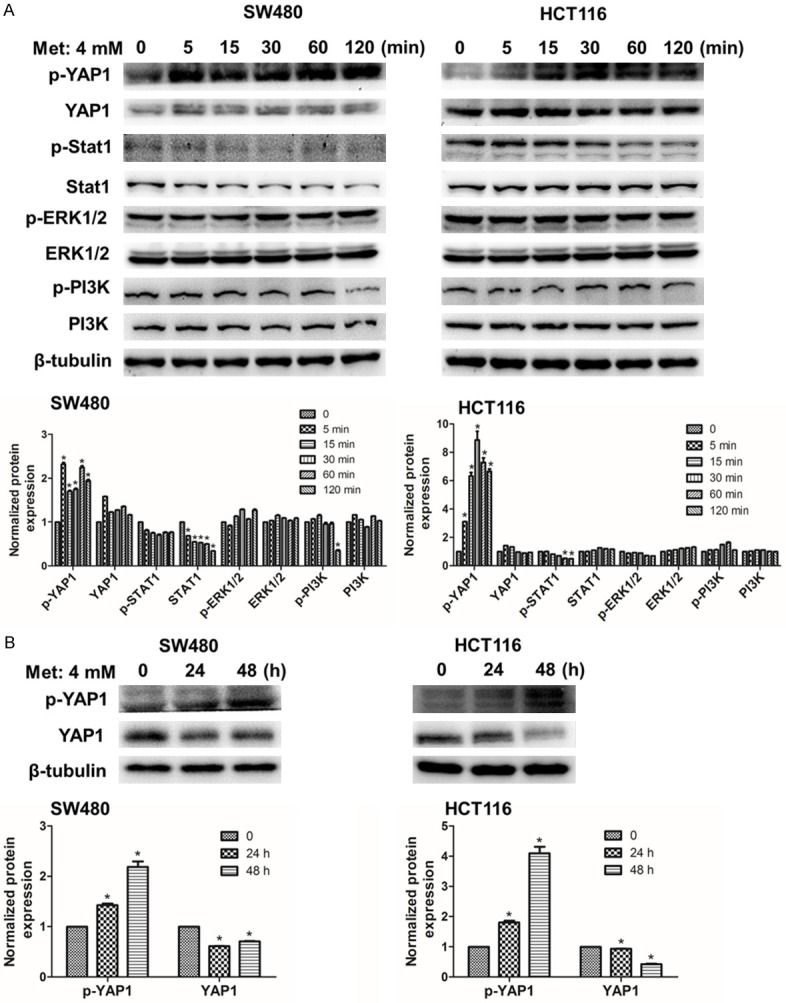
Metformin upregulates phosphorylation level of YAP1. A. SW480 and HCT116 cells were respectively incubated with 4 mM metformin for a short time: 0, 5, 15, 30, 60 and 120 min. B. The two kinds of cells were incubated for a long time: 0, 24 and 48 h. Data were presented as means ± S.D. (n=3), *P < 0.05.
Metformin reduces protein and mRNA level of YAP1 in SW480 and HCT116 cells
Above experimental results prompted us to investigate YAP1 expression in SW480 and HCT116 cells after incubated with metformin. As expected, we found that when concentrations of metformin were increased and cells incubation time was prolonged, protein level of YAP1 were obviously decreased (Figure 3A, 3B). Then, the mRNA level of YAP1 in SW480 and HCT116 was significantly reduced after incubated with 4 mM metformin for 48 h (Figure 3C). Besides, the downstream of Hippo signaling pathway, such as ANKRD1, EDN1 and CTGF, the mRNA level also were decreased in SW480 and HCT116 cells (Figure S4). To further explore the reason why YAP1 was considerably reduced by treating with metformin in SW480 and HCT116 cells, MG132, a kind of proteasome inhibitors, was used. By contrast, the protein level of YAP1 was increased after the cells were treated with MG132 in our results (Figure 3D) All the original western blots images were offered (Figure S3). These findings, while preliminary, suggested that metformin regulated YAP1 by promoting phosphorylation level of YAP1 and may accelerate YAP1 degradation via ubiquitination.
Figure 3.
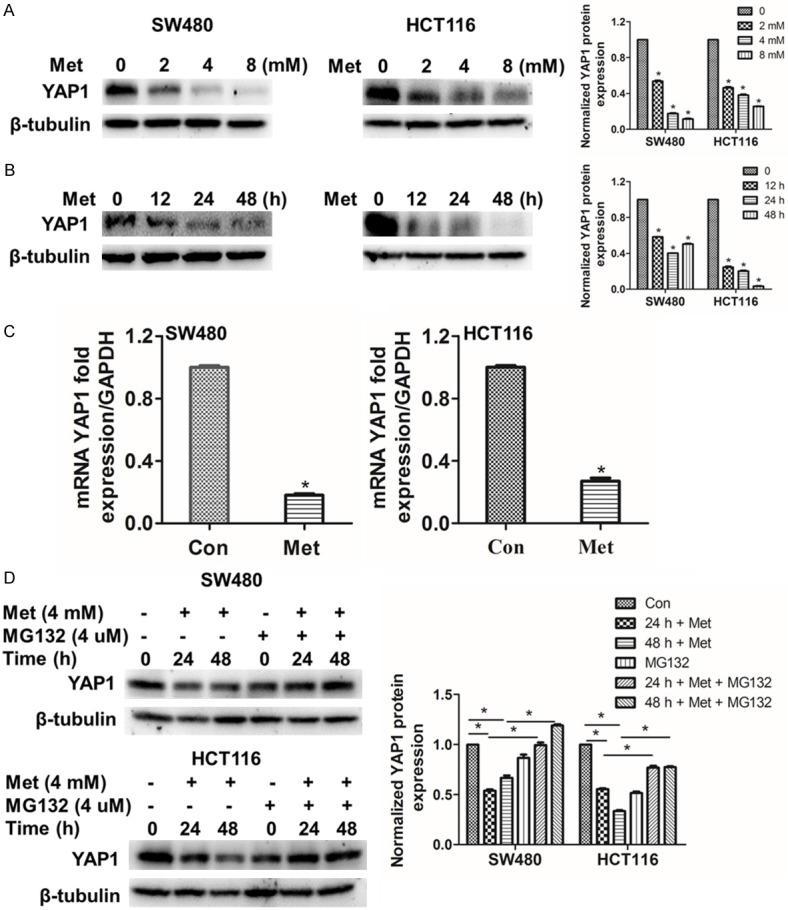
Metformin-induced down-regulation of YAP1 is significantly inhibited by MG132. A. SW480 and HCT116 cells were respectively treated with 0, 2, 4 and 8 mM metformin for 24 h. B. SW480 and HCT116 cells were respectively treated with 4 mM metformin for 0, 12, 24 and 48 h. C. The cells were treated with 4 mM metformin for 48 h, the mRNA were collected for qRT-PCR analysis. D. SW480 and HCT116 cells were treated with 4 mM metformin for 0, 24 and 48 h, the proteasome inhibitor MG132 (4 μM) or DMSO were added for 2 h after metformin stimulation. Western blot was used to detect the proteins. Data were presented as means ± S.D. (n=3), *P < 0.05.
YAP1 is crucial for PD-L1 expression in SW480 and HCT116 cells
In order to confirm the relationship between PD-L1 and YAP1, we silenced YAP1 and we found that the expression of PD-L1 was reduced (Figure 4A). The YAP1 inhibitor Verteporfin (VP), which inhibited the interaction of YAP1 and TEAD family transcription factors, mitigated the function to activate the downstream gene [30]. Further, the PD-L1 expression decreased as the concentration of VP increased (Figure 4B). All the original western blots images were provided (Figure S5). Those findings corroborated the idea of Min Hwan Kim, who suggested that YAP1 bound to the PD-L1 enhancer region and promoted PD-L1 transcription, an intact YAP1-TEAD interaction was essential for the upregulation of PD-L1 transcription [25].
Figure 4.
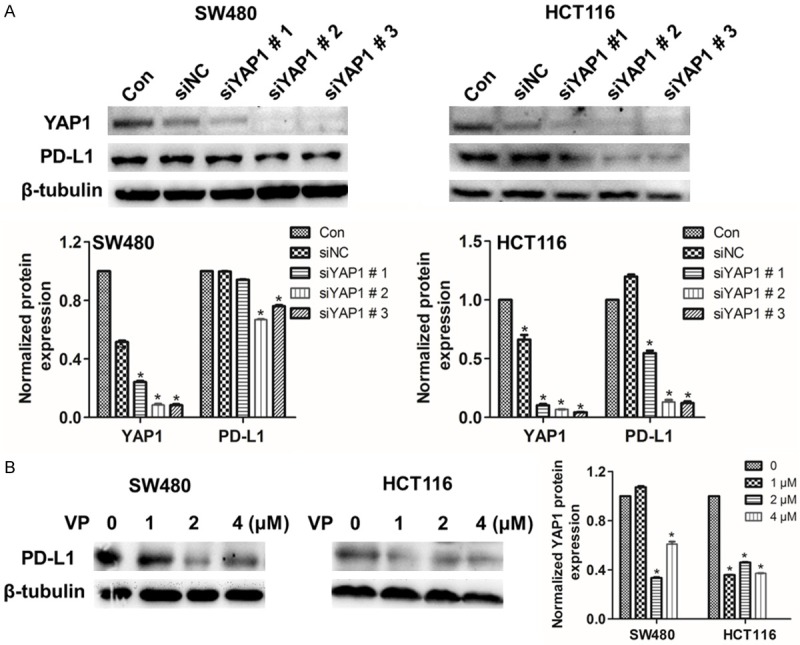
Silence or inhibition of YAP1 lessenes PD-L1 expression in SW480 and HCT116 cells. A. SW480 and HCT116 cells were exposed to siNC or YAP1 siRNA for 48 h, the protein expression of PD-L1 was detected by western blot. B. SW480 and HCT116 cells were respectively treated with 0, 1, 2 and 4 mM VP for 48 h, protein analysis of PD-L1 by western blot. Data were presented as means ± S.D. (n=3), *P < 0.05.
Metformin suppresses YAP1 travels to the cell nucleus
YAP1 and PDZ-binding motif (TAZ) are well known for transcriptional coactivator of target genes in Hippo signaling cascade and mechanical cues [31]. To further explore how metformin restricted the function of YAP1, we performed the following experiments. One was nuclear/cytoplasmic fractionation assays, the other was immunofluorescence staining. The protein of YAP1 in SW480 and HCT116 cells was obvious reduction in the nucleus, while it changed a little in the cytoplasm after incubated with metformin (Figures 5A and S6). Meanwhile, immunofluorescence staining showed the same results. The location of YAP1 was both in the nucleus and cytoplasm. Moreover, the intensity of green fluorescence in the nuclear had distinct weakening (Figure 5B). The above results illuminated that YAP1 could be restricted to enter into the nucleus after incubated with metformin.
Figure 5.
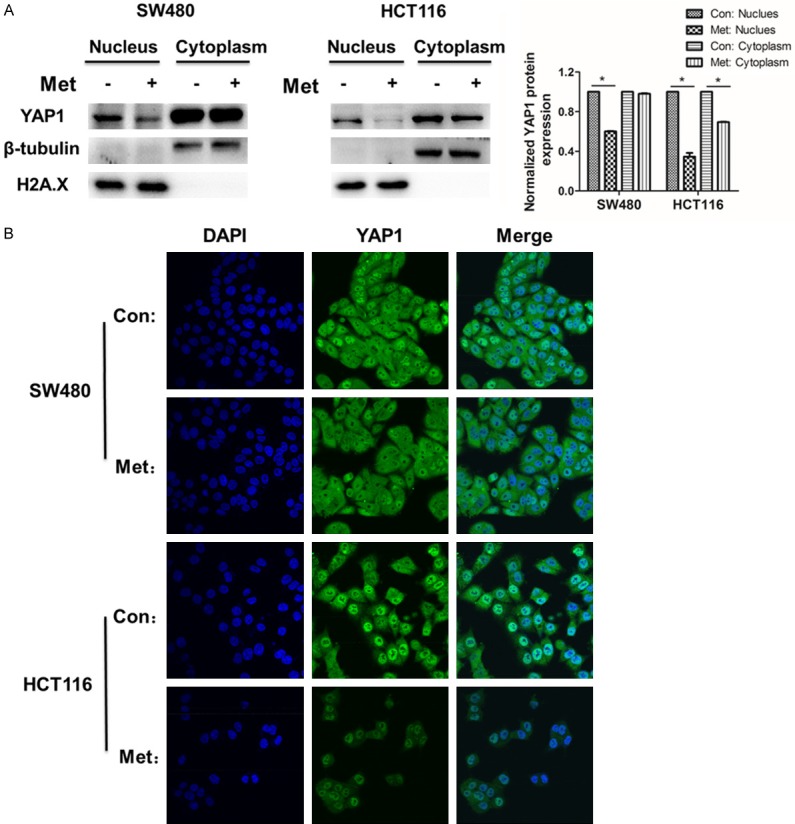
Metformin inhibits YAP1 entry into the nucleus. A. The YAP1 protein expression level was tested in the nuclear and cytoplasmic fractions in SW480 and HCT116 cells treated with metformin for 48 h. The protein expression levels were normalized to H2A.X and β-tubulin in the nuclear and cytosolic fractions, respectively. B. Immunofluorescence staining was performed to observe the location of YAP1 and the changes of YAP1 protein expression level in SW480 and HCT116 cells treated with metformin for 24 h. Each bar represents Mean ± S.D. (n=3), *P < 0.05.
Metformin inhibits PD-L1 and YAP1 expression in xenograft tumors
Finally, we wanted to explore whether metformin reduced PD-L1 and YAP1 in vivo, so we constructed subcutaneous transplantation tumor model in BALB/c female nude mice (Figure 6A). The tumor volume was monitored and the primary tumors were collected after termination of the experiments for immunohistochemistry analysis. There was no significant difference in tumor volume and weight (Figure 6B). After immunohistochemistry staining, PD-L1 and YAP1 expression score showed that stronger positive expressions happened in control group but weaker expressions in the metformin group (Figure 6C).
Figure 6.
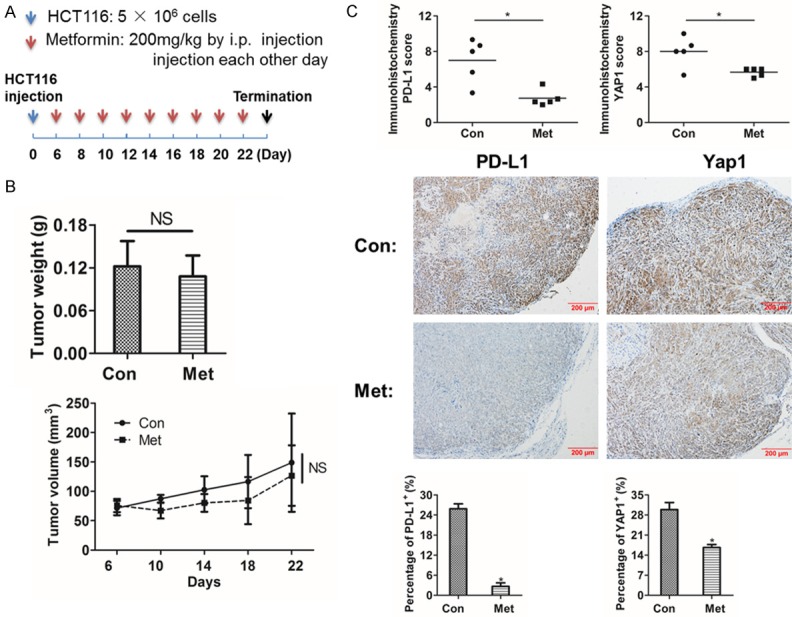
Metformin decreases PD-L1 and YAP1 expression in xenografted tumors. A. BALB/c female nude mice were subcutaneous injection with 5 × 106 HCT116 cells. After 6 days, the mice were i.p. injection 200 mg/kg metformin or PBS each other day for 3 weeks. B. Values represent the median tumor volume and the weight of xenografted tumors. C. Immunohistochemistry was performed to detect and evaluated the protein expressions of PD-L1 and YAP1 in tumor tissues. Data were presented as means ± S.D. (n=3), no statistical significance (NS) and *P < 0.05.
Discussion
The major objective of this study was to explore the molecular mechanisms about PD-L1 being inhibited by metformin in human colorectal cancer cells. Priorly studies have reported that metformin has been shown to directly induce antitumor effects by inhibiting the mechanistic target of rapamycin (mTOR) and MAPK signaling pathways in breast cancer cells, which were critical for cancer cell growth and proliferation [32-34]. Besides, more recent studies have shown that AMP-activated protein kinase (AMPK) affected T cell effector responses [35]. Metformin activated AMPK via inhibiting oxidative phosphorylation, which seemed to provide basis for effects on immune function. Indeed, metformin has been used as a tool to upregulate AMPK to demonstrate the contacts between energy metabolism and CD8 T cell memory [36]. These imply that the relevance of metformin and anti-tumor immune response is supported by the current findings. Here, our results prove a new regulatory mechanism of PD-L1 expression to further confirm this possibility.
YAP/TAZ has been recognized as a contextspecific oncogene [37]. Suppression of YAP decreased epithelial-mesenchymal transition (EMT) marker expressions and impeded tumor migration and invasion [38]. Another study was linked energy stress to YAP1 activity because AMPK directly phosphorylated YAP1 [39]. That may be an explanation for our research. In our study, metformin phosphorylated and deactivated YAP1, inhibited the translocation of YAP1 from the cytoplasm to the nucleus, which abolished its transcriptional activity. What’s more, dephosphorylation of YAP1 at serine 127 disrupted the association with 14-3-3 proteins, leading to its nuclear accumulation and transcriptional activity [40].
We identified YAP1 as a key point in human colorectal cancer cells under metformin treatment and determined it was the upstream of PD-L1 expression at the transcriptional level. First, we screened various signaling pathways and chose the Hippo signaling pathway, which was the most significantly changed in phosphorylation levels. Then, to explore the biological function of YAP1 in human colorectal cancer cells, siRNAs and inhibitor VP were used to inhibit the endogenous YAP1 and then downregulated PD-L1 protein level in SW480 and HCT116 cells. It was reported that YAP1 decreased PD-L1 expression in BRAFi resistant melanoma, NSCLS, malignant pleural mesothelioma [15,25,41]. However, YAP1 regulating PD-L1 in human colorectal cancer cells has not been reported previously.
In conclusion, the results of this study indicated that metformin decreased the total protein of YAP1, induced phosphorylation of YAP1 and blocked YAP1 entry into the nucleus to regulate PD-L1 expression in human colorectal cancer cells. At present, few people pay attention to the research on how metformin inhibits PD-L1 expression in human colorectal cancer cells. But, our research just based on SW480 and HCT116 cells, which only represent some characteristics of the human colorectal cancer. We have done a preliminary study and there has been still a mountain of intensive work to do. Further investigation should focus on whether metformin regulates PD-L1 expression via suppressing YAP1 expression in other types of tumor or the other potential mechanisms are urgent. In the future, metformin may be a good choice targeting on PD-L1 in tumor immunotherapy and drug combination.
Acknowledgements
This work was supported by National Natural Science Foundation of China (NO. 41030429).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Kather JN, Halama N, Jaeger D. Genomics and emerging biomarkers for immunotherapy of colorectal cancer. Semin Cancer Biol. 2018;52:189–197. doi: 10.1016/j.semcancer.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E, Uchiyama T, Taniguchi L, Hata Y, Uchiyama S, Hattori A, Nagase H, Kessoku T, Arimoto J, Matsuhashi N, Inayama Y, Yamanaka S, Taguri M, Nakajima A. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475–483. doi: 10.1016/S1470-2045(15)00565-3. [DOI] [PubMed] [Google Scholar]
- 3.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Zingales V, Distefano A, Raffaele M, Zanghi A, Barbagallo I, Vanella L. Metformin: a bridge between diabetes and prostate cancer. Front Oncol. 2017;7:243. doi: 10.3389/fonc.2017.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang ZJ, Bi Y, Li S, Zhang Q, Zhao G, Guo Y, Song Q. Reduced risk of lung cancer with metformin therapy in diabetic patients: a systematic review and meta-analysis. Am J Epidemiol. 2014;180:11–14. doi: 10.1093/aje/kwu124. [DOI] [PubMed] [Google Scholar]
- 6.Hadad SM, Coates P, Jordan LB, Dowling RJ, Chang MC, Done SJ, Purdie CA, Goodwin PJ, Stambolic V, Moulder-Thompson S, Thompson AM. Evidence for biological effects of metformin in operable breast cancer: biomarker analysis in a pre-operative window of opportunity randomized trial. Breast Cancer Res Treat. 2015;150:149–155. doi: 10.1007/s10549-015-3307-5. [DOI] [PubMed] [Google Scholar]
- 7.Hosono K, Endo H, Takahashi H, Sugiyama M, Sakai E, Uchiyama T, Suzuki K, Iida H, Sakamoto Y, Yoneda K, Koide T, Tokoro C, Abe Y, Inamori M, Nakagama H, Nakajima A. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila) 2010;3:1077–1083. doi: 10.1158/1940-6207.CAPR-10-0186. [DOI] [PubMed] [Google Scholar]
- 8.Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A. 2015;112:1809–1814. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inaguma S, Lasota J, Wang Z, Felisiak-Golabek A, Ikeda H, Miettinen M. Clinicopathologic profile, immunophenotype, and genotype of CD274 (PD-L1)-positive colorectal carcinomas. Mod Pathol. 2017;30:278–285. doi: 10.1038/modpathol.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mimura K, Teh JL, Okayama H, Shiraishi K, Kua LF, Koh V, Smoot DT, Ashktorab H, Oike T, Suzuki Y, Fazreen Z, Asuncion BR, Shabbir A, Yong WP, So J, Soong R, Kono K. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018;109:43–53. doi: 10.1111/cas.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 2013;19:598–609. doi: 10.1158/1078-0432.CCR-12-2731. [DOI] [PubMed] [Google Scholar]
- 14.Okkenhaug K, Graupera M, Vanhaesebroeck B. Targeting PI3K in cancer: impact on tumor cells, their protective stroma, angiogenesis, and immunotherapy. Cancer Discov. 2016;6:1090–1105. doi: 10.1158/2159-8290.CD-16-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu PC, Miao J, Wang YC, Zhang WQ, Yang YL, Wang CW, Yang CT, Huang Z, You J, Xu Z, Jablons DM, You L. Inhibition of yes-associated protein down-regulates PD-L1 (CD274) expression in human malignant pleural mesothelioma. J Cell Mol Med. 2018;22:3139–3148. doi: 10.1111/jcmm.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, Wilkerson MD, Fecci PE, Butaney M, Reibel JB, Soucheray M, Cohoon TJ, Janne PA, Meyerson M, Hayes DN, Shapiro GI, Shimamura T, Sholl LM, Rodig SJ, Freeman GJ, Hammerman PS, Dranoff G, Wong KK. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bi XW, Wang H, Zhang WW, Wang JH, Liu WJ, Xia ZJ, Huang HQ, Jiang WQ, Zhang YJ, Wang L. PD-L1 is upregulated by EBV-driven LMP1 through NF-kappaB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J Hematol Oncol. 2016;9:109. doi: 10.1186/s13045-016-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulford A, Tapon N, Ribeiro PS. Upstairs, downstairs: spatial regulation of Hippo signalling. Curr Opin Cell Biol. 2018;51:22–32. doi: 10.1016/j.ceb.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebhardt T, Harvey KF. Hippo wades into cancer immunology. Dev Cell. 2016;39:635–637. doi: 10.1016/j.devcel.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, Fang Z, Zhao K, Konaparthi R, Hua S, Zhang J, Li-Ning-Tapia EM, Kapoor A, Wu CJ, Patel NB, Guo Z, Ramamoorthy V, Tieu TN, Heffernan T, Zhao D, Shang X, Khadka S, Hou P, Hu B, Jin EJ, Yao W, Pan X, Ding Z, Shi Y, Li L, Chang Q, Troncoso P, Logothetis CJ, McArthur MJ, Chin L, Wang YA, DePinho RA. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov. 2016;6:80–95. doi: 10.1158/2159-8290.CD-15-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami S, Shahbazian D, Surana R, Zhang W, Chen H, Graham GT, White SM, Weiner LM, Yi C. Yes-associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma. Oncogene. 2017;36:1232–1244. doi: 10.1038/onc.2016.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni X, Tao J, Barbi J, Chen Q, Park BV, Li Z, Zhang N, Lebid A, Ramaswamy A, Wei P, Zheng Y, Zhang X, Wu X, Vignali P, Yang CP, Li H, Pardoll D, Lu L, Pan D, Pan F. YAP is essential for treg-mediated suppression of antitumor immunity. Cancer Discov. 2018;8:1026–1043. doi: 10.1158/2159-8290.CD-17-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MH, Kim CG, Kim SK, Shin SJ, Choe EA, Park SH, Shin EC, Kim J. YAP-induced PD-L1 expression drives immune evasion in brafi-resistant melanoma. Cancer Immunol Res. 2018;6:255–266. doi: 10.1158/2326-6066.CIR-17-0320. [DOI] [PubMed] [Google Scholar]
- 26.Taha Z, Janse van Rensburg HJ, Yang X. The hippo pathway: immunity and cancer. Cancers (Basel) 2018;10 doi: 10.3390/cancers10040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin D, Guo J, Wang D, Wu Y, Wang X, Gao Y, Shao C, Xu X, Tan S. The antineoplastic drug metformin downregulates YAP by interfering with IRF-1 binding to the YAP promoter in NSCLC. EBioMedicine. 2018;37:188–204. doi: 10.1016/j.ebiom.2018.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Li Z, Zhou J, Zhang J, Li S, Wang H, Du J. Cancer-associated fibroblasts promote PD-L1 expression in mice cancer cells via secreting CXCL5. Int J Cancer. 2019;145:1946–1957. doi: 10.1002/ijc.32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501–1509. doi: 10.1038/bjc.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen CG, Moroishi T, Guan KL. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 2015;25:499–513. doi: 10.1016/j.tcb.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alimova IN, Liu B, Fan Z, Edgerton SM, Dillon T, Lind SE, Thor AD. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–915. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 34.Goodwin PJ, Ligibel JA, Stambolic V. Metformin in breast cancer: time for action. J. Clin. Oncol. 2009;27:3271–3273. doi: 10.1200/JCO.2009.22.1630. [DOI] [PubMed] [Google Scholar]
- 35.Blagih J, Coulombe F, Vincent EE, Dupuy F, Galicia-Vazquez G, Yurchenko E, Raissi TC, van der Windt GJ, Viollet B, Pearce EL, Pelletier J, Piccirillo CA, Krawczyk CM, Divangahi M, Jones RG. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity. 2015;42:41–54. doi: 10.1016/j.immuni.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 36.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ling HH, Kuo CC, Lin BX, Huang YH, Lin CW. Elevation of YAP promotes the epithelial-mesenchymal transition and tumor aggressiveness in colorectal cancer. Exp Cell Res. 2017;350:218–225. doi: 10.1016/j.yexcr.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 39.DeRan M, Yang J, Shen CH, Peters EC, Fitamant J, Chan P, Hsieh M, Zhu S, Asara JM, Zheng B, Bardeesy N, Liu J, Wu X. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 2014;9:495–503. doi: 10.1016/j.celrep.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang P, Bai Y, Song B, Wang Y, Liu D, Lai Y, Bi X, Yuan Z. PP1A-mediated dephosphorylation positively regulates YAP2 activity. PLoS One. 2011;6:e24288. doi: 10.1371/journal.pone.0024288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miao J, Hsu PC, Yang YL, Xu Z, Dai Y, Wang Y, Chan G, Huang Z, Hu B, Li H, Jablons DM, You L. YAP regulates PD-L1 expression in human NSCLC cells. Oncotarget. 2017;8:114576–114587. doi: 10.18632/oncotarget.23051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.