Abstract
Introduction
Patients with IgA nephropathy (IgAN) have elevated serum levels of galactose-deficient IgA1 (Gd-IgA1) that are bound by Gd-IgA1–specific autoantibodies in pathogenic immune complexes. Renal biopsy histopathologic features of IgA vasculitis (IgAV) with nephritis (IgAV-N) are similar to those of IgAN. Mucosal infections often are associated with clinical onset and exacerbation in both diseases. We investigated whether patients with IgAV-N share pathogenic characteristics of IgAN.
Methods
We generated IgA1- and IgG-secreting cell lines from Epstein-Barr virus (EBV)–immortalized cells of patients with IgAV without nephritis (IgAV-woN), IgAV-N, and IgAN and from healthy individuals. Sera and cell-culture supernatants were used for analysis of Gd-IgA1 and Gd-IgA1–specific IgG autoantibodies.
Results
IgA1-producing cells from patients with IgAV-N, like cells from patients with IgAN, secreted more Gd-IgA1 than did cells from patients with IgAV-woN or healthy control subjects, in agreement with elevated serum Gd-IgA1 levels in patients with IgAV-N and IgAN. IgA1-producing cells from patients with IgAV-N had altered expression of genes involved in O-glycan biosynthesis: decreased for core 1 synthase (glycoprotein-N-acetylgalactosamine 3-β-galactosyltransferase 1; C1GALT1) and C1GALT1 Specific Chaperone 1 (C1GALTC1; COSMC) and elevated for N-acetylgalactosaminide α-2,6-sialyltransferase 2 (ST6GALNAC2). Levels of Gd-IgA1–specific IgG in sera and supernatants of IgG-producing cells were similar for patients with IgAV-N and IgAN and higher than those for IgAV-woN patients or healthy control subjects. Moreover, patients with IgAV-N who had active disease, manifested by hematuria and substantial proteinuria, had higher serum levels of Gd-IgA1–specific IgG autoantibodies than did patients with IgAV-N who had inactive disease.
Conclusion
Serum levels and cellular production of Gd-IgA1 and Gd-IgA1–specific IgG autoantibodies were elevated in patients with IgAV-N, supporting the hypothesis that IgAV-N and IgAN share pathogenic features.
Keywords: autoantibody, galactose-deficient IgA1, glycosyltransferase, IgA nephropathy, IgA vasculitis, immune complex
See Commentary on Page 1661
IgAV is a systemic vasculitis characterized by vascular wall immunodeposits of predominantly IgA, typically involving small vessels in the skin and gut, often associated with purpura and colitis. IgAV-N occurs in 30% to 50% of patients with IgAV, usually within 4 to 6 weeks after the onset of the typical purpuric rash, with IgA glomerular deposits upon examination of renal biopsy specimens.1, 2 Whereas other organ manifestations of IgAV are mostly benign and self-limiting, IgAV-N may lead to end-stage kidney disease.2 Renal involvement is the principal cause of morbidity and mortality in children with IgAV-N.3, 4 Thus it is important to clarify the onset mechanism(s) of IgAV-N and identify the most appropriate treatment. The renal biopsy findings of IgAV-N are indistinguishable from those of IgAN. Of note, Gd-IgA1 specifically deposits in the kidneys of patients with IgAN and IgAV-N.5 In addition, some persons with typical IgAV-N later exhibit the clinical phenotype of IgAN with one or more episodes of macroscopic hematuria in the absence of the vasculitic rash or abdominal or joint symptoms. Apart from the IgAV-N–specific extrarenal clinical signs, a demographic feature distinguishes IgAV-N from IgAN: peak age at onset ranges between 15 and 30 years for IgAN, whereas IgAV-N manifests mainly in childhood.
It has been long postulated that IgAN and IgAV-N share pathogenetic features.6 IgA1-containing immune complexes from sera of patients with IgAN and IgAV-N stimulate proliferation of cultured human mesangial cells.7 Of note, galactose deficiency of O-linked glycans in the hinge region of IgA1 has been reported in patients with IgAV-N but not in patients with IgAV-woN.7, 8, 9 Moreover, production of Gd-IgA1 is genetically regulated in IgAN and IgAV-N.10, 11, 12, 13 Thus we hypothesized that development of Gd-IgA1–specific autoantibodies and the subsequent formation of Gd-IgA1–containing immune complexes may play a key role in the pathogenesis of IgAV-N as in IgAN. To test our hypothesis, we measured the amounts of Gd-IgA1–specific IgG autoantibodies and Gd-IgA1 (autoantigen) secreted by the IgG- and IgA-producing cell lines, respectively, derived from the EBV-immortalized peripheral blood mononuclear cells of patients with IgAV-N, IgAV-woN, and IgAN and healthy control subjects. We also determined serum levels of Gd-IgA1–specific IgG autoantibodies and Gd-IgA1.
Methods
Participants
Peripheral blood was collected from 20 patients with IgAV-N (mean age, 10.7 ± 5.7 years; serum creatinine, 0.7 ± 0.3 mg/dl; urinary protein/creatinine ratio, 2.2 ± 2.6 g/g; Table 1) and from 10 patients with IgAV-woN (mean age, 7.3 ± 2.0 years). The extrarenal manifestations (skin, joints, and gastrointestinal tract) of patients with IgAV-N are specified in Table 1. Sixteen of the 20 subjects with IgAV-N had a renal biopsy showing glomerular IgA deposition; the other 4 subjects had clinical signs and symptoms resulting in diagnosis of IgAV-N. The patients with IgAV-N included 8 white males, 8 white females, 1 African American male, and 3 African American females (Table 1). Active IgAV-N at the time of study (n = 14) was defined as hematuria by dipstick urinalysis (Supplementary Table S1). Inactive IgAV-N (n = 6) was defined by the absence of hematuria (Supplementary Table S1). We further divided active IgAV-N into 2 subgroups based on duration between diagnosis and study: acute active (patients 3, 5–12, and 17) and chronic active (patients 1, 2, 4, and 16) (Supplementary Table S2). Estimated glomerular filtration rate was calculated using the bedside Schwartz equation for subjects <18 years old and the Modification of Diet in Renal Disease Study formula for those ≥18 years old.
Table 1.
Clinical features of patients with IgAV-N
| Patient no. | Age at study, yr | Age at diagnosis, yr | Sex | Race | Serum creatinine, mg/dla | eGFR, ml/min per 1.73 m2 | Hematuriaa,b | UP/Cr, g/ga | Extrarenal manifestationsc |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 15.9 | 5.8 | M | W | 0.7 | 101 | 4+ | 6.7 | Joint |
| 2 | 18.5 | 9.6 | F | W | 1.0 | 72 | 3+ | 1.3 | Joint, abdominal |
| 3 | 12.2 | 12.1 | F | W | 0.5 | 135 | 4+ | 3.0 | Abdominal |
| 4 | 19.3 | 17.7 | F | B | 0.9 | 75 | 3+ | 2.6 | |
| 5 | 3.0 | 3.0 | F | B | 0.4 | 95 | 4+ | 2.9 | Joint, abdominal |
| 6 | 9.2 | 9.1 | M | W | 0.6 | 84 | 3+ | 1.1 | Joint, abdominal |
| 7 | 3.5 | 3.3 | M | W | 0.4 | 131 | 2+ | 9.8 | Joint, abdominal |
| 8 | 3.5 | 3.5 | M | W | 0.6 | 72 | 3+ | 2.1 | Abdominal |
| 9 | 7.8 | 7.7 | F | W | 0.7 | 77 | 3+ | 2.5 | Joint |
| 10 | 2.4 | 2.4 | M | W | 0.4 | 88 | 3+ | 6.6 | Abdominal |
| 11 | 8.6 | 8.4 | F | W | 0.5 | 99 | 3+ | 1.4 | Abdominal |
| 12 | 7.5 | 6.6 | M | W | 0.7 | 74 | 1+ | 0.1 | Joint, abdominal |
| 13 | 10.5 | 7.5 | F | B | 0.6 | 94 | N | 0.3 | Joint, abdominal |
| 14 | 17.8 | 5.2 | M | W | 1.5 | 50 | N | 0.9 | Abdominal |
| 15 | 11.6 | 9.9 | M | W | 0.6 | 107 | N | 0.5 | Joint |
| 16 | 9.0 | 7.4 | F | W | 0.4 | 135 | 3+ | 0.2 | Joint, abdominal |
| 17 | 6.5 | 6.2 | M | B | 0.5 | 99 | 3+ | 0.6 | Joint |
| 18 | 12.9 | 7.5 | F | W | 0.6 | 113 | N | 0.6 | Joint, abdominal |
| 19 | 12.1 | 8.2 | F | W | 0.8 | 75 | N | 0.5 | Joint, abdominal |
| 20 | 21.4 | 14.6 | F | W | 0.9 | 91 | N | 0.0 |
B, black; eGFR, estimated glomerular filtration rate; IgAV-N, IgA vasculitis with nephritis; F, female; M, male; N, negative for hematuria by dipstick; UP/Cr, urinary protein/creatinine ratio; W, white.
Serum creatinine, hematuria, and proteinuria were measured at the time of study.
Hematuria by urinalysis dipstick (0–4+).
All subjects had typical rash.
The Institutional Review Boards of the University of Alabama at Birmingham and the University of Tennessee Health Sciences Center approved this study. Informed written consent was obtained from all adults and from a parent or legally authorized representative for all children; children age 8 years or older provided signed assent.
Generation of IgA- and IgG-Secreting Cell Lines
Peripheral blood mononuclear cells from patients with IgAV-woN and IgAV-N were isolated and immortalized with EBV.14 Next we subcloned IgA1- and IgG-secreting cells using limiting dilutions and maintained the cells in Roswell Park Memorial Institute 1640 medium supplemented with L-glutamine, 20% fetal calf serum, penicillin, and streptomycin. After several rounds of cloning and screening, IgA1-producing cell lines from 5 patients with IgAV-N and 4 patients with IgAV-woN were generated. In addition, IgG-producing cell lines from 6 patients with IgAV-N and 5 patients with IgAV-woN were generated. The data from previous experiments using cell lines originating from 10 patients with IgAN (mean age, 39.2 ± 18.6 years) and 10 healthy control subjects (mean age, 42.6 ± 16.6 years) were used as the reference.14
Determination of Gd-IgA1
Concentrations of total IgA in serum specimens and cell-culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA), as previously described.10, 11, 12, 13, 14 F(ab’)2 fragment of goat anti-human IgA (2.5 μg/ml; Jackson ImmunoResearch Laboratories, West Grove, PA) was used to coat MaxiSorp ELISA plates (Nunc; Thermo Fisher Scientific, Waltham, MA). Serially diluted samples were applied on the plates and the captured IgA was treated with 10 mU/ml neuraminidase (Roche Diagnostics Corp., Indianapolis, IN) to remove sialic acid. After washing, the samples were probed with biotin-labeled lectin from Helix aspersa (Sigma-Aldrich, St. Louis, MO) that is specific for N-acetylgalactosamine, followed by avidin–horseradish peroxidase conjugate and peroxidase substrate o-phenylenediamine-H2O2 (Sigma-Aldrich). Absorbance was measured at 490 nm. The Helix aspersa reactivity of IgA1 in each sample was then calculated as units of Gd-IgA1 per 100 ng of total IgA. A galactose-deficient IgA1 myeloma protein (Ale) purified from plasma of a patient with IgA1 myeloma was used as standard.14, 15, 16 Optical density at 490 nm for 50 ng neuraminidase-treated IgA1 (Ale) was defined as 100 U of Gd-IgA1.
Assay for Gd-IgA1–Specific IgG Antibodies
Concentrations of total IgG in serum specimens and cell-culture supernatants were determined by ELISA, as previously described.17 To determine amounts of Gd-IgA1–specific IgG autoantibodies in serum or secreted by IgG-producing cells, we performed an ELISA that measures the binding of IgG using an Fab fragment of Gd-IgA1 containing part of the hinge region with O-glycans (Fab-IgA1) as antigen.15, 16, 17 MaxiSorp ELISA plates (Nunc) were coated with 1 μg/ml Fab-IgA1. Serum or cell culture supernatant samples diluted in phosphate-buffered saline solution were then added to each well. The starting dilution of all samples was normalized according to total amount of IgG determined previously by ELISA. The captured IgG was detected with biotin-labeled F(ab’)2 fragment of goat anti-human IgG antibody (BioSource; Invitrogen, Carlsbad, CA). Avidin–horseradish peroxidase conjugate (ExtrAvidin; Sigma-Aldrich) followed by peroxidase substrate were added and absorbance was measured at 490 nm.15, 16, 17
RNA Isolation and Real-Time Reverse Transcription Polymerase Chain Reaction
Total RNA was isolated from 1 × 106 cells treated with RNA Stat-60 (Thermo Fisher Scientific). Following deoxyribonuclease treatment and conversion to cDNA, real-time polymerase chain reaction was performed using LightCycler FastStart DNA Master SYBR Green I (Roche Diagnostics Corp.). Gene-specific primers were used for detection of transcripts of C1GALT1, COSMC, and ST6GALNAC2.14, 18 Primers for beta-actin amplification were purchased from R&D Systems (Minneapolis, MN) and used for quantitation of the housekeeping gene to normalize gene-expression data. Real-time polymerase chain reaction was performed for 42 cycles of denaturation at 95 °C, annealing at 60 °C, and extension at 72 °C.
Statistics
Correlations between the different parameters were analyzed by the Student t test, 2-tailed, or by the Mann-Whitney test. Analysis of variance was used to determine differences in the characteristics among multiple groups. Data were expressed as mean ± SD or median values. P values < 0.05 were considered significant. P values ≥ 0.05 are not included in the text. All statistical analyses were performed with StatView 5.0 software (Abacus Concept Inc., Cary, NC).
Results
Serum IgA and IgG Levels in Patients With IgAV-N and IgAV-woN
Serum levels of total IgA in patients with IgAV-N and IgAV-woN were 400.2 ± 187.1 and 257.5 ± 107.9 mg/dl, respectively (P < 0.05). In patients with active and inactive IgAV-N, serum levels of IgA were 395.0 ± 208.2 and 373.7 ± 156.3 mg/dl, respectively.
Serum levels of total IgG in patients with IgAV-N and IgAV-woN were 1332.3 ± 467.8 and 1278.1 ± 446.1 mg/dl, respectively. In patients with active and inactive IgAV-N, serum levels of IgG were 1134.2 ± 404.4 and 1728.7 ± 318.4 mg/dl, respectively.
IgA1 Secreted by Cell Lines and Serum IgA1 From IgAV-N Patients Exhibited O-Glycan Galactose Deficiency
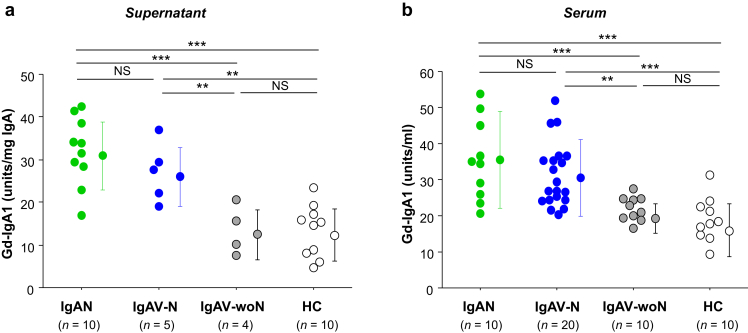
IgA1 secreted by cell lines derived from EBV-immortalized peripheral blood mononuclear cells of patients with IgAV-N was galactose-deficient, to a similar degree as IgA1 secreted by IgA1-producing cells from patients with IgAN. In contrast, IgA1 secreted by cell lines of patients with IgAV-woN exhibited normal galactosylation (Figure 1a). The amount of Gd-IgA1 produced by IgA1-secreting cells was higher for patients with IgAV-N than that by cells from patients with IgAV-woN (P < 0.01). In addition, serum levels of Gd-IgA1 were higher in patients with IgAV-N than in patients with IgAV-woN (Figure 1b, P < 0.01). Serum levels of Gd-IgA1 in patients with IgAV-N were similar to those in patients with IgAN.
Figure 1.
Galactose-deficient IgA1 (Gd-IgA1) in serum and secreted by IgA1-producing cells derived from patients with IgA vasculitis with nephritis (IgAV-N) and IgA vasculitis without nephritis (IgAV-woN) compared with those derived from patients with IgA nephropathy (IgAN) and healthy control subjects (HC). (a) IgA1-secreting cells from patients with IgAV-N or IgAN produced more Gd-IgA1 than did those from patients with IgAV-woN or HC. Gd-IgA1 data were normalized to total IgA1 and are expressed in U/mg IgA1. (b) Serum levels of Gd-IgA1 were higher in patients with IgAV-N or IgAN than those in patients with IgAV-woN or HC. Gd-IgA1 data are expressed in U/ml. NS, not significant. **P < 0.01; ***P < 0.001.
Expressions of Key Glycosyltransferases in IgA1-Producing Cell Lines From Patients With IgAV-woN, IgAV-N, and IgAN and From Healthy Control Subjects
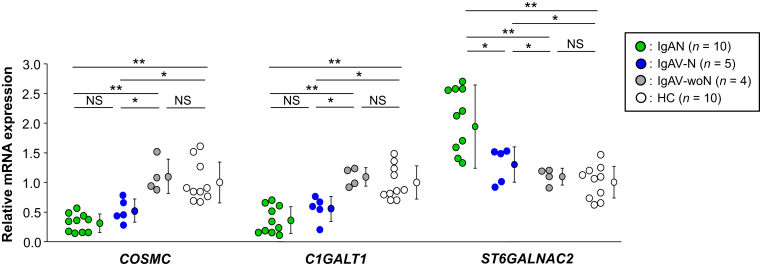
Quantitative analysis (real-time reverse transcription polymerase chain reaction) of gene expression of specific glycosyltransferases in IgA1-producing cells revealed lower levels for C1GALT1 and COSMC in cells from IgAV-N patients than in those from patients with IgAV-woN (Figure 2, P < 0.01). The expression of ST6GALNAC2 gene in cells from patients with IgAV-N was higher than that in cells from patients with IgAV-woN patients (P < 0.01). Expression of C1GALT1 and COSMC in cells from patients with IgAV-N was similar to that in cells from patients with IgAN. In contrast, expression levels of the 3 genes in IgA1-producing cells from patients with IgAV-woN were comparable with those in cells from healthy control subjects (Figure 2).
Figure 2.
Expressions of specific glycosyltransferase-encoding genes in IgA1-producing cell lines from patients with IgA vasculitis with nephritis (IgAV-N) and IgA vasculitis without nephritis (IgAV-woN) compared with those derived from patients with IgA nephropathy (IgAN) and healthy control subjects (HC). Transcription of the genes encoding core 1 synthase (glycoprotein-N-acetylgalactosamine 3-β-galactosyltransferase 1; C1GALT1) and its molecular chaperone COSMC (C1GALT1 specific chaperone 1; C1GALTC1) was lower in IgA1-secreting cells from patients with IgAV-N than in those from patients with IgAV-woN (P < 0.01). Transcription of the gene encoding N-acetylgalactosaminide α-2,6-sialyltransferase 2 (ST6GALNAC2) was higher in cells from patients with IgAV-N than in those from patients with IgAV-woN (P < 0.01). Expression of C1GALT1 and COSMC in cells from patients with IgAV-N was comparable with that in cells from patients with IgAN. Gene expression data for the 3 genes were similar in the cells from patients with IgAV-woN and HC. NS, not significant. *P < 0.01; **P < 0.001.
Serum IgG and IgG Secreted by Immortalized Cells From Patients With IgAV-N Contained Elevated Amounts of Autoantibodies Specific for Gd-IgA1
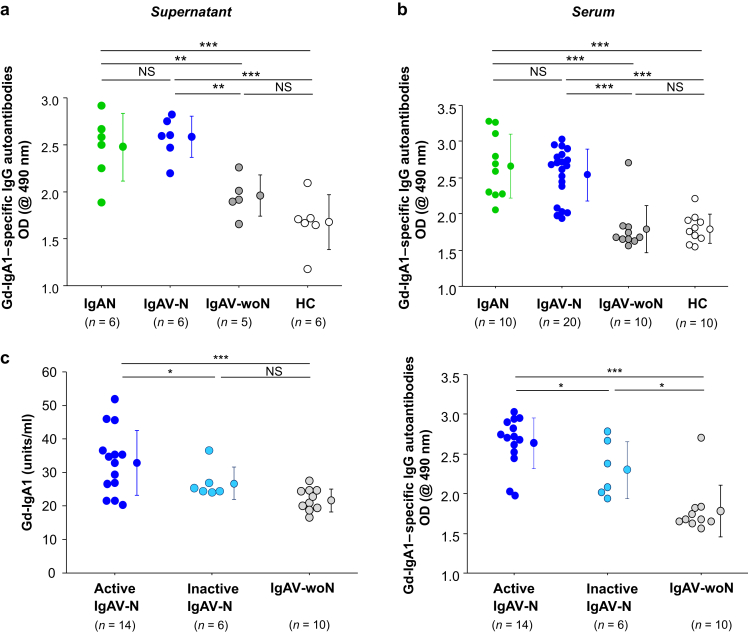
IgG secreted by cell lines from patients with IgAV-N contained higher amounts of IgG autoantibodies specific for Gd-IgA1 than did the supernatants from cells derived from patients with IgAV-woN (Figure 3a, P < 0.01). Amounts of IgG autoantibody produced by cells from patients with IgAV-N were comparable with those produced by cells from patients with IgAN. Serum levels of IgG autoantibody specific for Gd-IgA1 in patients with IgAV-N were similar to those in patients with IgAN but higher compared with those in patients with IgAV-woN or healthy control subjects (P < 0.001 for each of the 4 comparisons; Figure 3b). The levels of serum IgG autoantibody in patients with IgAV-woN were similar to those in healthy control subjects (Figure 3b).
Figure 3.
Serum levels of IgG autoantibody specific for galactose-deficient IgA1 (Gd-IgA1) and the autoantibody amounts secreted by cell lines derived from patients with IgA vasculitis with nephritis (IgAV-N), IgA vasculitis without nephritis (IgAV-woN), IgA nephropathy (IgAN), and healthy control subjects (HC). (a) Amounts of IgG autoantibody produced by cells derived from patients with IgAV-N were comparable with those produced by cells from patients with IgAN. IgG-producing cells from patients with IgAV-N or IgAN secreted higher amounts of IgG autoantibodies specific for Gd-IgA1 than did those derived from patients with IgAV-woN or HC. (b) Serum levels of IgG autoantibody specific for Gd-IgA1 in patients with IgAV-N were similar to those in patients with IgAN. Serum levels of IgG autoantibodies specific for Gd-IgA1 were higher in patients with IgAV-N or IgAN compared with those in patients with IgAV-woN or HC. (c) Serum levels of Gd-IgA1 and Gd-IgA1–specific IgG autoantibodies in patients with active IgAV-N, inactive IgAV-N, and IgAV-woN. Serum levels of Gd-IgA1 and IgG autoantibodies were elevated in patients with IgAV-N with active disease compared with those in patients with inactive disease (P < 0.05). The serum levels of Gd-IgA1–specific IgG autoantibodies in patients with IgAV-woN were lower than those of patients with inactive IgAV-N (P < 0.05). NS, not significant. *P < 0.05; **P < 0.01; ***P < 0.001.
Moreover, we observed a weak correlation between serum levels of Gd-IgA1 and Gd-IgA1–specific IgG autoantibodies (P < 0.01, R2 = 0.24) when all subjects were included (Supplementary Figure S1). However, this correlation did not reach statistical significance for any single group, probably because of the small numbers of subjects.
Serum Levels of Gd-IgA1 and Gd-IgA1–Specific IgG Were High in Patients With Active IgAV-N
Next we analyzed a possible association between the clinical activity of renal disease and serum levels of Gd-IgA1 and Gd-IgA1–specific IgG. Serum levels of Gd-IgA1 and IgG autoantibodies were higher in patients with IgAV-N who had active disease compared with patients who had inactive IgAV-N (Figure 3c, P < 0.05 for both comparisons). Furthermore, the levels of Gd-IgA1–specific IgG autoantibodies in sera of patients with IgAV-woN were lower than those of patients with inactive IgAV-N (Figure 3c, P < 0.05).
Next we divided active IgAV-N into 2 subgroups, acute active and chronic active. When we compared patients who had chronic active IgAV-N with patients who had inactive IgAV-N, the serum levels of Gd-IgA1 were higher (P < 0.05), whereas levels of Gd-IgA1–specific IgG autoantibodies were similar (Supplementary Figure S2).
Discussion
The pathogenic mechanisms of IgAV-N have not been fully elucidated but are thought to include perturbations in the immune system, resulting in increased serum levels of IgA1 and IgA1-containing circulating immune complexes.19 Notably, patients with IgAV have IgA1-containing circulating immune complexes of relatively small molecular mass, whereas patients with IgAV-N have additional IgA1-IgG containing circulating complexes of large molecular mass.20 It has been postulated that increased synthesis and/or reduced clearance of IgA may be involved in the pathogenesis of various diseases characterized by glomerular deposition of IgA1-containing circulating immune complexes. The clinical onset of IgAV frequently follows after an upper respiratory tract infection; many different viral and bacterial pathogens have been implicated as triggers. Increased production of polymeric IgA by the mucosal immune system in response to locally presented antigens has been hypothesized as a potential disease mechanism of IgAV.21, 22 Decreased galactosylation of serum IgA1 O-glycans has been documented for patients with IgAV-N and IgAN.23 Based on these studies, Gd-IgA1 is likely to play a pivotal role in the pathogenesis of IgAV-N.7, 8, 22, 23
In this study, we confirmed that the degree of galactose deficiency of the IgA1 hinge region O-linked glycans differentiated patients with IgAV who did and did not have clinical nephritis. Serum levels of Gd-IgA1 were higher in patients with IgAV-N than in patients with IgAV-woN; serum Gd-IgA1 levels in patients with IgAV-woN were similar to those in healthy control subjects. Moreover, IgA1 secreted by EBV-immortalized cells from patients with IgAV-N was galactose deficient to a degree similar to that for IgA1 secreted by cells from patients with IgAN. In contrast, IgA1 secreted by EBV-immortalized cells derived from peripheral blood cells from patients with IgAV-woN was normally galactosylated. Quantitative analysis of expression of genes encoding specific glycosyltransferases in IgA1-producing cells from patients with IgAV showed a pattern favoring greater synthesis of galactose-deficient hinge region O-glycans in patients with nephritis than in those without nephritis: less expression for C1GALT1 and COSMC and higher expression for ST6GALNAC2. These observations are consistent with prior reports of glycosylation defects of serum IgA1 in patients with IgAV-N8, 23 and with our recent genome-wide association study that associated serum Gd-IgA1 levels with variants in the C1GALT1 and COSMC genes.24, 25
Circulating levels of Gd-IgA1 are heritable in children with IgAN and IgAV-N,10 but many asymptomatic blood relatives also have high serum Gd-IgA1 levels. Therefore, a high serum Gd-IgA1 level alone is not sufficient for the development of clinical symptoms, and it is likely that the second hit, that is, autoantibody against Gd-IgA1, is required to produce overt disease.26 We found that the serum level of IgG directed against galactose-deficient O-linked glycans of IgA1 was higher in patients with IgAV who had nephritis compared with patients who did not have nephritis. IgG secreted by immortalized cells from patients with IgAV-N and IgAN contained higher content of IgG autoantibodies against Gd-IgA1. In contrast, the amounts of these IgG autoantibodies produced by cells from patients with IgAV-woN and healthy control subjects were similar. Moreover, serum levels of Gd-IgA1–specific IgG were higher in patients with IgAV-N who had active disease (characterized by hematuria and substantial proteinuria) compared with those in patients with IgAV-N who had inactive disease. Importantly, serum levels of Gd-IgA1 were elevated in patients with chronic active IgAV-N. This finding suggests that continuous production of Gd-IgA1 contributes to persistent glomerular injury.
Based on the findings of prior publications5 and our current study, we speculate that IgAN and IgAV-N share pathogenetic features. Naturally occurring IgA1-containing immune complexes from sera of patients with IgAV-N stimulate proliferation of cultured human mesangial cells, as do such complexes of patients with IgAN.7 The mechanisms of renal injury by the Gd-IgA1–containing immune complexes in IgAV-N may include these sequential steps: (i) circulating Gd-IgA1 is bound by Gd-IgA1–specific antibody to form immune complexes that can activate complement;27, 28 (ii) some of these complexes deposit in the mesangium and (iii) activate mesangial cells, subsequently inducing cellular proliferation and overproduction of extracellular matrix components and cytokines/chemokines.
There is a need for reliable noninvasive biomarkers for monitoring response to treatment of IgAV-N. We have demonstrated in this study that the serum levels of Gd-IgA1 and Gd-IgA1–specific IgG autoantibodies were significantly higher in patients with IgAN and IgAV-N compared with patients who had IgAV-woN and healthy control subjects. These findings indicate that serum levels of Gd-IgA1 and the corresponding IgG autoantibodies may have diagnostic and/or prognostic potential in newly manifested IgAV. Follow-up studies are needed to elucidate the precise pathophysiological mechanisms related to the onset and progression of IgAV-N.
A significant limitation of this study is the lack of data for another immune-mediated glomerular disease and pediatric healthy-control subjects. Serum levels of total IgA1 are lower in children compared with those in adults.9 Therefore, we may have underestimated the levels of Gd-IgA1 in serum of the pediatric patients.
Despite the noted limitations, we found that the composition of circulating IgA1-containing immune complexes is a major factor that determines whether patients with IgAV exhibit nephritis. Immortalized cells from the patients with IgAV-N and IgAN secreted Gd-IgA1 and Gd-IgA1–specific IgG in amounts that exceeded that from cells from patients with IgAV-woN. Circulating levels of Gd-IgA1 and Gd-IgA1–specific autoantibodies were elevated in patients with IgAV-N but not in patients with IgAV-woN, supporting the hypothesis that IgAV-N and IgAN share pathogenetic components. These findings open new possibilities for management of patients with IgAV using assessment of serum Gd-IgA1 and Gd-IgA1–specific IgG autoantibodies to potentially predict which patients with IgAV are likely to experience development of nephritis and to monitor their clinical course.29, 30
Disclosure
BAJ and JN report that they are 2 of the co-founders of Reliant Glycosciences, LLC, in which they retain equity; they have current research support from Alexion and Retrophin; and they previously had sponsored-research agreements with Pfizer and Anthera and consulted for Visterra, Inc. RJW reports consultation agreements with Omeros Corporation, Apellis Pharmaceuticals, Aduro Biotech, and Catabasis Pharmaceuticals that represent no conflicts of interest for the present study. All the other authors declared no competing interests.
Acknowledgments
This study was supported in part by National Institutes of Health grants DK078244 and DK082753, a gift from the IGA Nephropathy Foundation of America, and JSPS KAKENHI grant No. 18K08252. We thank Catherine V. Barker, Sandy Grimes, and Sue Y. Woodford for assistance with the collection of blood samples and the management of clinical data.
Footnotes
Figure S1. Association between serum levels of galactose-deficient IgA1 (Gd-IgA1) and Gd-IgA1–specific IgG autoantibodies. There was a weak correlation between serum levels of Gd-IgA1 and Gd-IgA1–specific IgG autoantibodies in all subjects (P < 0.01, R2 = 0.24). However, this correlation did not reach statistical significance for any single group, probably because of small numbers of subjects in these subgroups.
Figure S2. Serum levels of galactose-deficient IgA1 (Gd-IgA1) were elevated in patients with chronic active IgA vasculitis with nephritis (IgAV-N). We divided active IgAV-N into 2 subgroups, acute active and chronic active. Serum levels of Gd-IgA1 were elevated in patients with chronic active IgAV-N compared with patients who had inactive IgAV-N. Serum levels of Gd-IgA1–specific IgG autoantibodies were not significantly different among the 3 groups.
Table S1. Clinical data of patients with active and inactive IgA vasculitis with nephritis (IgAV-N).
Table S2. Clinical data of patients with acute and chronic active IgA vasculitis with nephritis (IgAV-N).
Supplementary Material
References
- 1.Sanders J.T., Wyatt R.J. IgA nephropathy and Henoch-Schönlein purpura nephritis. Curr Opin Pediatr. 2008;20:163–170. doi: 10.1097/MOP.0b013e3282f4308b. [DOI] [PubMed] [Google Scholar]
- 2.Davin J.C., Coppo R. Henoch-Schönlein purpura nephritis in children. Nat Rev Nephrol. 2014;10:563–573. doi: 10.1038/nrneph.2014.126. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein A.R., White R.H., Akuse R. Long-term follow-up of childhood Henoch-Schönlein nephritis. Lancet. 1992;339:280–282. doi: 10.1016/0140-6736(92)91341-5. [DOI] [PubMed] [Google Scholar]
- 4.Schärer K., Krmar R., Querfeld U. Clinical outcome of Schönlein-Henoch purpura nephritis in children. Pediatr Nephrol. 1999;13:816–823. doi: 10.1007/s004670050707. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H., Yasutake J., Makita Y. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1 oriented pathogenesis. Kidney Int. 2018;93:700–705. doi: 10.1016/j.kint.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Waldo F.B. Is Henoch-Schönlein purpura the systemic form of IgA nephropathy? Am J Kidney Dis. 1988;12:373–377. doi: 10.1016/s0272-6386(88)80028-3. [DOI] [PubMed] [Google Scholar]
- 7.Novak J., Moldoveanu Z., Renfrow M.B. IgA nephropathy and Henoch-Schoenlein purpura nephritis: aberrant glycosylation of IgA1, formation of IgA1-containing immune complexes, and activation of mesangial cells. Contrib Nephrol. 2007;157:134–138. doi: 10.1159/000102455. [DOI] [PubMed] [Google Scholar]
- 8.Lau K.K., Suzuki H., Novak J. Pathogenesis of Henoch-Schönlein purpura nephritis. Pediatr Nephrol. 2010;25:19–26. doi: 10.1007/s00467-009-1230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau K.K., Wyatt R.J., Moldoveanu Z. Serum levels of galactose-deficient IgA in children with IgA nephropathy and Henoch-Schönlein purpura. Pediatr Nephrol. 2007;22:2067–2072. doi: 10.1007/s00467-007-0623-y. [DOI] [PubMed] [Google Scholar]
- 10.Kiryluk K., Moldoveanu Z., Sanders J.T. Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schönlein purpura nephritis. Kidney Int. 2011;80:79–87. doi: 10.1038/ki.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gharavi A.G., Moldoveanu Z., Wyatt R.J. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol. 2008;19:1008–1014. doi: 10.1681/ASN.2007091052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiryluk K., Julian B.A., Wyatt R.J. Genetic studies of IgA nephropathy: past, present, and future. Pediatr Nephrol. 2010;25:2257–2268. doi: 10.1007/s00467-010-1500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hastings M.C., Moldoveanu Z., Julian B.A. Galactose-deficient IgA1 in African Americans with IgA nephropathy: serum levels and heritability. Clin J Am Soc Nephrol. 2010;5:2069–2074. doi: 10.2215/CJN.03270410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki H., Moldoveanu Z., Hall S. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest. 2008;118:629–639. doi: 10.1172/JCI33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanagawa H., Suzuki H., Suzuki Y. A panel of serum biomarkers differentiates IgA nephropathy from other renal diseases. PLoS One. 2014;23 doi: 10.1371/journal.pone.0098081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Placzek W.J., Yanagawa H., Makita Y. Serum galactose-deficient-IgA1 and IgG autoantibodies correlate in patients with IgA nephropathy. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki H., Fan R., Zhang Z. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009;119:1668–1677. doi: 10.1172/JCI38468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki H., Raska M., Yamada K. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem. 2014;289:5330–5339. doi: 10.1074/jbc.M113.512277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppo R., Mazzucco G., Cagnoli L. Long-term prognosis of Henoch-Schönlein nephritis in adults and children. Nephrol Dial Transplant. 1997;12:2277–2283. doi: 10.1093/ndt/12.11.2277. [DOI] [PubMed] [Google Scholar]
- 20.Levinsky R.J., Barratt T.M. IgA immune complexes in Henoch-Schönlein purpura. Lancet. 1979;24:1100–1103. doi: 10.1016/s0140-6736(79)92505-4. [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki Y. The pathogenesis and treatment of pediatric Henoch-Schönlein purpura nephritis. Clin Exp Nephrol. 2011;15:648–657. doi: 10.1007/s10157-011-0478-1. [DOI] [PubMed] [Google Scholar]
- 22.Pohl M. Henoch-Schönlein purpura nephritis. Pediatr Nephrol. 2015;30:245–252. doi: 10.1007/s00467-014-2815-6. [DOI] [PubMed] [Google Scholar]
- 23.Allen A.C., Willis F.R., Beattie T.J. Abnormal IgA glycosylation in Henoch-Schönlein purpura restricted to patients with clinical nephritis. Nephrol Dial Transplant. 1998;13:930–934. doi: 10.1093/ndt/13.4.930. [DOI] [PubMed] [Google Scholar]
- 24.Kiryluk K., Li Y., Moldoveanu Z. GWAS for serum galactose-deficient IgA1 implicates critical genes of the O-glycosylation pathway. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gale D.P., Molyneux K., Wimbury D. Galactosylation of IgA1 is associated with common variation in C1GALT1. J Am Soc Nephrol. 2017;28:2158–2166. doi: 10.1681/ASN.2016091043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki H., Kiryluk K., Novak J. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795–1803. doi: 10.1681/ASN.2011050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maillard N., Wyatt R.J., Julian B.A. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol. 2015;26:1503–1512. doi: 10.1681/ASN.2014101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyatt R.J. The complement system in IgA nephropathy and Henoch-Schönlein purpura: functional and genetic aspects. Contrib Nephrol. 1993;104:82–91. doi: 10.1159/000422400. [DOI] [PubMed] [Google Scholar]
- 29.Berthelot L., Robert T., Vuiblet V. Recurrent IgA nephropathy is predicted by altered glycosylated IgA, autoantibodies and soluble CD89 complexes. Kidney Int. 2015;88:815–822. doi: 10.1038/ki.2015.158. [DOI] [PubMed] [Google Scholar]
- 30.Pillebout E., Jamin A., Ayari H. Biomarkers of IgA vasculitis nephritis in children. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.