Abstract
Ocimum sanctum (OS) is tropical herbal plant which is easy to find and widely used as a vegetable food in Indonesia. In last decade, lung adenocarcinoma was in top position as male cancer disease in Indonesia. Recently, emerging data showing the extracts of different species of Ocimum exhibiting the anti-tumor properties. Further studies on lung lewis carcinoma demonstrated pro-apoptosis effects after the treatment with Ocimum extracts. However, the effect of OS of Indonesian origin in human alveolar pulmonary adenocarcinoma A549 cells remain unclear. Therefore, we aimed to investigate effects of ethanolic extract OS (EEOS) in A549 cell culture systems. Cell adhesion and viability assays revealed that EEOS significantly decreased the attachment into extracellular matrix of A549 cells. Morphological examination AO/EB and DAPI staining indicated that EEOS induced the cells shrinkage, DNA fragmentation and condensation of A549 cells. Further, EEOS treatment induced the apoptosis rate followed by up-regulation of reactive oxygen species (ROS), caspase-3 expression and decreased anti-apoptotic protein Bcl-2. This condition also suppressed the expression of SOD2 as well as the GPx. In conclusion, our findings indicate that EEOS suppressed the viability of A549 cells, which may result from the activation of ROS promoting the apoptosis signaling via mitochondrial intrinsic pathway. Taken together, EEOS might be a good therapeutic potential to further understand its properties in the treatment of lung carcinoma.
Keywords: Food science, Cell biology, A549, ethanolic extract Ocimum sanctum, Detachment, Apoptosis
Food science; Cell biology; A549; ethanolic extract Ocimum sanctum; Detachment; Apoptosis.
1. Introduction
Lung alveolar pulmonary adenocarcinoma is one of the primary cause of lung cancer in related to high mortality not only in developing countries but also worldwide [1, 2]. In Indonesia, lung cancer is on the first position cause of death in cancer patients. The high prevalence of lung adenocarcinoma in Indonesia is mainly due to smoking habits and also industrial waste, such as fly ash, bottom ash, and coal ash [3]. Recently, clinical therapies including surgery, chemotherapy, allopathy medicine and radiotherapies are successful as concurrent therapeutic approaches against lung cancer, however, several marked chances of undesirable and adverse side effects caused by such therapies need to be managed [4]. Moreover, especially in developing countries, the therapeutic system is extremely expensive. Therefore, there is huge demand for alternative medicine in Indonesia.
The use of medicinal plants in chemoprevention is need to be consideration as an ideal treatment with good efficacy and few side effects compared with allopathic medicine [5]. Recently, evidences have shown that the prescription of new treatment methods that are derived from plants or herbal medicine could reduce cancer mortalities until 25%, thus it proves that plants or herbal medicine plays an important role in curing cancer symptoms and treatments [6, 7]. The use of herbal remedies as complementary and alternative medicines (CAMs) has also been widely used on United Kingdom (UK) and the rest of Europe such as France and Germany, as well as in North America and Australia [8]. In addition, dietary intake of phytochemicals as a chemoprevention agents may reduce the risk of cancer, detoxification of highly reactive molecules and has antitumor potential against lung cancer [9].
Basically, chemoprevention agents are compounds that prevent development of cancer [10]. Their preventive effects are attributed to intervening in interaction of the carcinogen with cellular DNA, altering intracellular signaling pathways as results of stopping progression of an initiated cell through pre-neoplastic changes into a malignant cell, inhibiting angiogenesis, inducing cell cycle arrest, and triggering apoptosis [11, 12, 13]. It is believed that the apoptosis induced by chemoprevention agents may not only inhibit the carcinogenesis induced by carcinogens, but also may suppress the growth of tumor and enhance the cytotoxic effects of antitumor drug on tumor, which plays an important role in the antitumor therapies [14].
The genus Ocimum, belonging to the family Labiatae or Laminaceae, is widely found in tropical and subtropical countries [15]. In Indonesia, Ocimum sanctum is very easy to find and use commonly as vegetable food. It is well-known that Ocimum sanctum acts as a chemopreventive, anti-carcinogenic, free radical scavenger and also used as a medication for neurodegenerative diseases [15, 16, 17, 18].
In recent years, there is a tremendous research work going on OS to understand its additional pharmacological properties. For instance ethanolic extract of Ocimum sanctum induced apoptosis of lewis lung carcinoma [19], and aqueous extract of Ocimum gratissimum avoided the breast cancer proliferation through inhibition of matrix metalloproteases [20]. Further, Ocimum sanctum essential oil demonstrated in promoting cytotoxic and apoptotic activity in human colorectal adenocarcinoma cells [21]. It has been used in a variety of forms for consumption, the aqueous leaf extract and seed oil are reported to show chemopreventive and antiproliferative activity in Hela cells [22]. Ethanolic extract of Ocimum sanctum (EEOS) leaf also has been shown to have a significant influence on carcinogen metabolizing enzymes including cytochrome P450, cytochrome b5, and aryl hydrocarbon hydroxylase [23, 24]. Additionally, Ocimum sanctum prepared in the form of fresh leaf paste, aqueous, and ethanolic extract has been reported to reduce the incidence of papillomas and squamous cell carcinoma in carcinogen-treated hamsters [25]. Nevertheless, how the mechanisms of ethanolic extract of Ocimum sanctum (EEOS) underlying anticancer property remains unclear. Therefore, in the present study, the anticancer effects of EEOS were investigated using human lung carcinoma A549 cells.
2. Materials and methods
2.1. Preparation of Ocimum sanctum linn. ethanolic extract
O. sanctum leafs were derived from Center for Research and Development of Medicinal Plants and Traditional Medicines, Ministry of Health in Tawangmangu, Central Java, Indonesia. Crude extracts and ethanolic extracts of O. sanctum were prepared as previously described [15]. Then, the ethanolic extracts were diluted with phosphate buffer saline (PBS) pH 7,4 to prepare there different concentrations (50 μg/ml, 70 μg/ml, 100 μg/ml) (Gibco, Waltham, MA, USA).
2.2. Cell culture of A549 cells
Lung adenocarcinoma cell A549 were maintained and cultured in DMEM supplemented with FBS containing 100 g/ml penicillin/streptomycin at 37 °C in a humidified atmosphere. Cells were seeded in T-75 culture flask and grown to approximately confluence. EEOS treatments were performed by incubating cells with several concentrations (50, 70, 100 ug/ml) of EEOS (w/v) in serum-free DMEM for 24 h. After the EEOS treatments, the cells were washed with PBS pH 7,4 and collected for following analyses.
2.3. Cell viability assay
Cell viability was determined by MTT assay as previously describe [17] in the absence or presence of 50, 70, 100 or 200 ug/ml EEOS. After 48 h treatments, culture medium was aspirated and cells were incubated with MTT (0,5 mg/ml) at 37 °C for 4 h. The viable cell number was directly proportional to the production of formazan, which was dissolved in isopropanol and determined by measuring the absorbance at 570 nm using a microplate reader (SpectraMAX 360 pc, Molecular Devices, Sunnyvale, CA).
2.4. Adhesion assay
The adhesion assay are performed like previously describe [26, 27, 28]. Microtiter wells (Greiner Bio-one, Frickenhausen, Germany) were coated with vitronectin (Athens Research and Technology, Athens, GA, USA) or BSA (Serva, Heidelberg, Germany) in HBS buffer (119 mM NaCl, 4 mM KCl, 11 mM Glucose in 20 mM Hepes buffer) overnight at 4 °C. After washings, wells were then blocked with 100 μl 3% BSA for 1 h at 4 °C. Aliquots of washed A549 cells (1-4 x106) were added together with EEOS (final concentration 50 μg/ml, 70 μg/ml, and 100 μg/ml) for 1 h at 37 °C. cRGD was run as positive control detachment. Wells were washed once, and adherent cells were stained with crystal violet (Sigma, Steinheim, Germany) and measured in a microtiter reader at 592 nm (Sunrise™, Tecan, Männedorf, Germany).
2.5. Apoptosis assay
The apoptosis assay are performed like previously describe [26, 27, 28]. Cell apoptosis was measured by the Caspase-Glo 3/7 assay (Promega, Madison, WI, USA). 100 μl vitronectin (2 μg/mL; Athens Research & Technology, Athens, GA, USA) were coated on 96 white well plates (Corning Incorporated, Corning, NY, USA) for 8 h. Aliquots of 450 μl A549 suspensions (1-2x106 cells in DMEM medium) were incubated with EEOS (final concentration 100 μg/ml), and cRGD (final concentration 20 μg/ml), then seeded onto vitronectin coated wells for 16 h at 37 °C, 5% CO2. 100 μl of Caspase-Glo 3/7 reagents were then added at room temperature and luminescence was measured using a microtiter reader (FLX800, Biotek Instrument Winooski, VT, USA).
2.6. DAPI staining
DAPI staining was performed to assess morphological changes in the chromatin structure of A549 cells undergoing apoptosis as previously described [28]. Aliquots of 105 A549 were seeded onto μ-slide well (Ibidi, Martinsried, Germany) precoated with vitronectin (see above) together with purified human IgG (final concentration 20 μg/ml), mab (final concentration 20 μg/ml) and or cRGD (final concentration 20 μg/ml) for 16 h at 37 °C, 5% CO2. After washings with 300 μl ice-cold PBS pH 7.4 (PAN, Aidenbach, Germany), cells were fixed with 300 μl 4% paraformaldehyde (PFA; Sigma) for 15 min and incubated with 1 μg/ml blue fluorescing dye (Hoechst 33342; Thermo Scientific, Rockford, IL, USA) for 3 min at RT. Stained chromatin DNA condensation and fragmentation was analysed using confocal microscopy with 60x magnification (Nikon Eclipse TE2000-E, Tokyo, Japan).
2.7. Acridine Orange/Ethidium Bromide staining (AO/EB)
A549 cells (1 × 105) were seeded in 24 well plate. Cells were incubated in CO2 incubator with 37 °C temperature and 5% CO2 then treated with 100 μg/ml (final concentration) of EEOS re-incubate for 24 h. Cells were fixed with 1 ml of 4% parafomaldehyde for 15–20 min in room temperature. Cells were washed with PBS 1x for three times. Cells were stained with Hoechst 10 μg/ml and Acridine Orange/Ethidium Bromide (AO/EB) 10 μg/ml on each well and then incubated for 15 min in humid chamber with 37 °C temperature. Cells were washed with PBS 1x then the cells were ready to be visualised by fluorescence inverted microscope.
2.8. Reactive oxygen species (ROS) analysis
80% of confluent A549 cells in T75 flask were harvested with 3 ml Trypsin-EDTA 0.25% then incubated for 3 min in the incubator at 37 °C temperature with 5% CO2. After the cells were detached from the flask, growth medium was added to stop the trypsin. Cells suspension then transferred into 15 ml centrifuge tube and centrifuge on 1600 rpm for 5 min. The supernatant was aspirated and the cells pellet were mixed in 5 ml growth medium. Cells were counted and 2.5 × 105 cells/0.5 ml transferred into FACS round tube. The cells were centrifuged on 1600 rpm for 5 min. The supernatant was aspirated, the cell pellet was resuspended using DCFDA 1x buffer +10% FBS. DCFDA 20 μM was added into cells suspension, then incubated for 45 min in the dark room maintaing 37 °C temperature and 5% CO2. After incubation, cells were treated with terbutyl hydroperoxide (TBHP) 250 μM (positive control), DCFDA 20 μM (negative control), DMSO 10%, and Ocimum sanctum extract 100 μg/ml for 4 h. ROS levels were measured using flow cytometer.
2.9. ELISA for caspase 9, caspase 3 and Bcl-2
The activity level of caspase 9, caspase 3, and Bcl-2 measured by colorimetric assay kit (Abcam, Cambridge, MA, USA) according to the manufacturer's protocol. Briefly, the cells were collected and resuspended in lysis buffer containing 50 mmol/L Hepes, pH 7.4, 0.1% CHAPS, 1 mmol/L DTT, 0.1 mmol/L EDTA and 0.1% Triton X-100. Following incubation for 30 min on ice, cell lysate was centrifuged at 11000 g for 10 min at 4 °C, and the protein concentration in the supernatants was measured using the Bradford method. The supernatants were incubated with reaction buffer containing 2 mmol/L Ac-DEVD-AFC for caspase-3 and LEHD-AFC for caspase-9 (Abcam, Cambridge, MA, USA) in a caspase assay buffer at 37 °C with 10 mmol/L DTT for 30 min. Caspase activity was determined by measuring the absorbance at 405 nm (Infinite Pro 200, Tecan, Mannedorf, Switzerland). terbutyl hydroperoxide (TBHP) 250 μM was run as positive control. In the same way, 50 μL of the sample were put on the 96 well, followed by adding 50 μL of the Bcl-2 antibody cocktail to each well. Incubate for 1 h at room temperature on a plate shaker set to 400 rpm. Wash each well for three times, after the last wash add 100 μL of TMB Substrate, incubate for 10 min. After last incubation put 100 μL of stop solution to each well. The expression of Bcl-2 was record on the OD at 450 nm. In all experiment, to correct the nonspecific binding, backgrounds (negative control) were run for cell lysate sample in parallel without the sampel. Cutoff values were determined as the mean signal of negative control (n = 10) plus three standard deviations (SDs).
2.10. FOX assay
FOX reagent were composed of 100 uM xylenol orange (Merck, Darmstadt, Germany), 250 uM ammonium ferrous sulfate (Sinopharm Chemical Reagent, Shanghai, China), 90% methanol (Merck, Darmstadt, Germany), 4 mM BHT (Acros Organics, New Jersey, USA) and 25 mM H2SO4 (Merck, Darmstadt, Germany) Halliwell and Whiteman (2004) and Rhee (2010) [29,30]. 50 ul sample were added to 550 ul FOX reagent. The samples were incubate at room temperature for 30 min then read immediately at 460 nm.
2.11. ELISA superoxide dismutase (SOD2)
The assay is conducted using ELISA Superoxide Dismutase (SOD 2) kit (Fine Test, Wuhan, China). The plate were washed 2 times before adding standard, sample, and control (zero) wells. 100 ul standard or sample was added to each well and incubate for 90 min at 37 °C. Aspirate and wash the plates 2 times. 100 ul Biotin-labeled SOD 2 antibody working solution was added to each well and incubated for 60 min at 37 °C. Aspirate and wash the plates 3 times. 100 ul SABC working solution was added to each well and incubated for 30 min at 37 °C. Aspirate and wash the plates 5 times. 90 ul TMB substrate was added and incubated 15–30 min at 37 °C. 50 ul stop solution was added, and read the plate at 450 nm immediately.
2.12. ELISA glutathione peroxidase (GPx)
The assay is conducted using ELISA Glutathione Peroxidase 1 (GPX1) kit (Fine Test, Wuhan, China). The plate were washed 2 times before adding standard, sample, and control (zero) wells. 100 ul standard or sample was added to each well and incubate for 90 min at 37 °C. Aspirate and wash the plates 2 times. 100 ul Biotin-labeled GPX1 antibody working solution was added to each well and incubated for 60 min at 37 °C. Aspirate and wash the plates 3 times. 100 ul SABC working solution was added to each well and incubated for 30 min at 37 °C. Aspirate and wash the plates 5 times. 90 ul TMB substrate was added and incubated 15–30 min at 37 °C. 50 ul stop solution was added, and read the plate at 450 nm immediately.
2.13. Qualitative assay of phenolic and flavonoid by Thin Layer Chromatography
The qualitative assay of phenolic was determined by putting 50 mg sample into test tube and extracted by using vortex for 3 min and 1 ml ethanol as solvent. Ethanol phase was aspirated then 20 ul sample was spotted on silica gel plate (Merck, Darmstadt, Germany) including gallic acid (Sigma Aldrich, St. Louis, USA) as comparation. The plate was put on saturated chamber (CAMAG, Muttenz, Switzerland) with mobile phase, the mix solution of 9.5 ml methanol (Merck, Darmstadt, Germany) and 0.5 ml formic acid 10% (Merck, Darmstadt, Germany), then elute until the border. The plate was dry off, observed under UV light (Sankyo Denki, Kanagawa, Japan), and spray using ferri chloride reagent (Merck, Darmstadt, Germany). Subsequently, the same methode was run for the flavonoid, with the modification of the comparation (quercetin) and the mobile phase, the mix solution of 6.0 ml n-hexane (Merck, Darmstadt, Germany), 4.0 ml ethyl acetate (Merck, Darmstadt, Germany), and 0.1 ml formic acid (Merck, Darmstadt, Germany).
2.14. Quantitative estimation of polyphenols
The total phenolic contents were determined using Folin-Ciocalteu reagent (FCR) (Merck, Darmstadt, Germany) where as gallic acid (Sigma Aldrich, St. Louis, USA) was used as a standard antioxidant. 50 mg sample were added to 0,5 ml FCR and diluted in 7,5 ml aquabidest, followed by 10 min incubation in room temperature. 1,5 ml Na2CO3 20% were added, then diluted with aquabidest until 10 ml. The solution were diluted 25 times and transferred to the cuvette, the absorbance were read at 760 nm using spectrophotometer (Shimadzu, Nagoya, Japan). Total phenolic was expressed in % gallic acid equivalent/gram extract (% b/b).
2.15. Quantitative estimation of flavonoids
The flavonoid content was determined using quercetin (Sigma Aldrich, St. Louis, USA) as a standard antioxidant. 0,3 ml 5% natrium nitrite was added to 0,05 g sample. After 5 min. 0,6 ml aluminium chloride 10% were added and incubate for 5 min followed by 2 ml NaOH 1M addition and aquadest until 10 ml with volumetric flask. The solution was diluted 50 times and transferred into cuvette. The absorbance was recorded in spectrophotometer (Shimadzu, Nagoya, Japan) at 510 nm. The total flavonoid content was expressed in % quercetin equivalent/gram extract (% b/b).
3. Results
To address the beneficial use and the role of EEOS in the lung adenocarcinoma, we investigated in the in vitro A549 cell culture model system by the treatment of A549 cells with different concentrations of OS. Cells adhesion to the extracellular matrix is a crucial part to guarantee the cells growth, survival, and communication. To analyze the ability of EEOS-inducing A 549 cells detachment we performed adhesion assay.
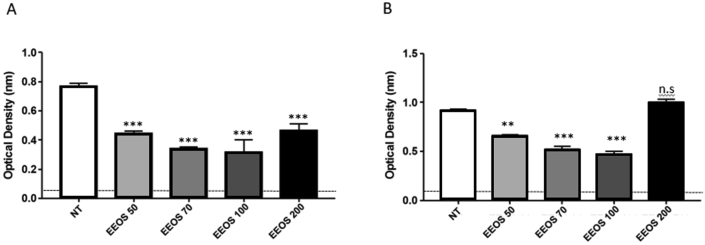
3.1. EEOS induces detachment of A549 cells into extracellular matrix
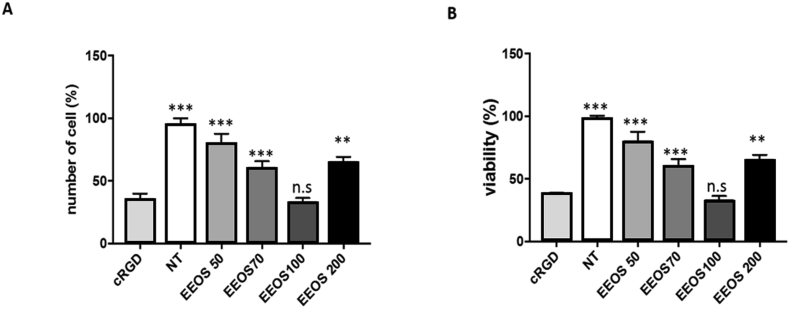
A549 cells (3 × 105 cells/ml) were cultured on vitronectin coated culture dishes and treated for 18 h with different concentrations of EEOS (50 ug/ml, 70 ug/ml, 100 ug/ml). cRGD peptide (20 ug/ml) treated A549 cells were used as a control. These cultures were subjected to adhesion assay and interestingly, EEOS induces cell detachment in a dose dependent manner in A549 cells. The optimal concentrations of EEOS with a dose of 100 ug/ml showed enormous cell detachment in comparison to the other concentrations. All of our experimental groups that were treated with EEOS (50 ug/ml, 70 ug/ml, 100 ug/ml) inhibited the binding of A549 onto immobilized vitronectin (Fig. 1A). Interestingly, the higher concentration of EEOS 200 ug/ml reduced the ability to inhibit gel attachment in comparison to the lower concentration of 70 ug/ml.
Fig. 1.
EEOS induced detachment of A549 into extracellular matrix (A) and decreased the viability of A549 cells significantly (B). Adhesion assay (A) performed by added the cells in the microtiter wells pre-coated with vitronectin. After washing, adherent cells were stained with crystal violet and measured in an ELISA reader. MTT assay (B) performed by incubating the cells in the presence of 50, 70, 100 or 200 ug/ml EEOS. After 48 h treatments, the cells were incubated with MTT 5 mg/ml for 4 h) at 37 °C. The viable cell number determined by measuring the absorbance at 570 nm using a microplate reader. Statistical analysis was performed by one-way ANOVA followed by Bonferroni's post-hoc test; n.s. = not significant. cRGD was used as a positive control anoikis.
3.2. EEOS decreases significantly the viability of A549 cells
In addition to adhesion assay, using this model we also performed the viability assay. Similarly, the cells were treated with EEOS (50–200 μg/mL) and the cell viability was assessed by the MTT. EEOS significantly exhibited cytotoxic effect in human A549 cells, demonstrated with %-mean viability decrement in a concentration dependent manner (Fig. 1B) compared with the untreated control. In addition, EEOS at 100 μg/mL induced most severe cytotoxicity in A549 cells, which was almost comparable to its positive control cRGD, while non-treated cells did not show any significant cytotoxicity as a negative control. Interestingly, the treatment of A549 cells with 200 μg/mL EEOS demonstrate less but still still exhibiting significant cytotoxic effect in A549 cancer cells.
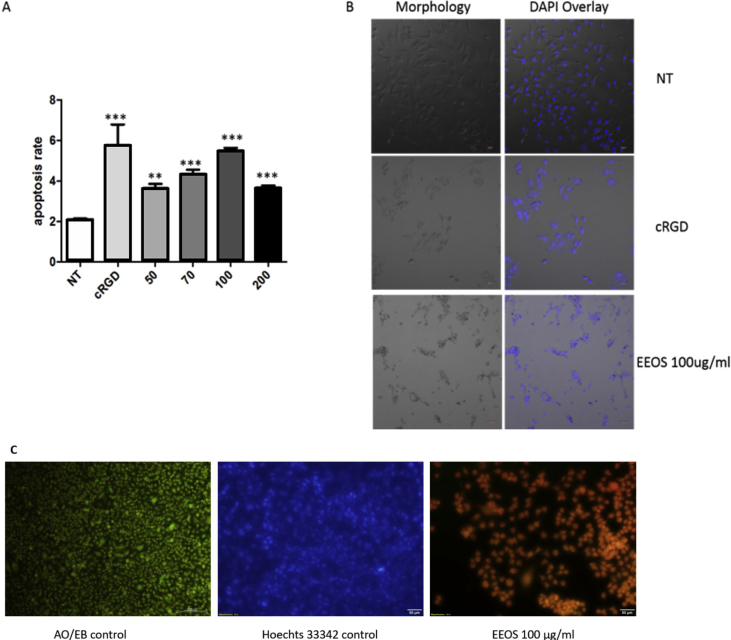
3.3. EEOS treatment induced apoptosis and promote ROS production in A549 cells
To demonstrate the anticancer effect of EEOS in A549 cels, we performed an apoptosis assay by using caspase-Glo 3/7. Indeed, this experiment showed that the EEOS treatment induced apoptosis in A549 cells in comparison to the untreated cells. Further, the cRGD used as a positive control (Fig. 2A and B). Since the concentration of EEOS 200 ug/ml did not show the results with adhesion and viability assay, therefore, this concentration was not included in this experiment. In addition, DNA fragmentation analysis by DAPI staining also showed significant cell shrinkage and DNA fragmentation in the 100 ug/ml EEOS treated cells in comparison to the control cells (Fig. 2B). Those findings are further confirmed by Hoechst 33342 and acridine orang/ethidium bromide (AO/EB) staining (Fig. 2C). It's found that only less cells exhibit blue fluorescence of Hoechst 33342 on EEOS treatment which means the apoptosis rate of EEOS treatment group is higher than control group. Acridine orange/ethidium bromide staining also can be seen qualitatively that AO/EB control cells exhibit green colours. Green colour cells represents normal, viable and non-apoptotic cells. On the other hand, EEOS treatment group exhibit orange fluorescent that represent apoptotic cells. It is known that disruption of cell adhesion into extracellular matrix results in detachment-induced apoptosis, termed anoikis, which is associated with increased intracellular ROS level.
Fig. 2.
EEOS induced apoptosis in A549 cells. (A) Caspase-Glo 3/7 assay was performed by coated the vitronectin on 96 well plate for 8 h, then aliquots of A549 suspensions were incubated with EEOS and cRGD then seeded into vitronection coated wells for 16 h. 10 μl of Caspase-Glo 3/7 reagents were then added and luminescence was measured using a microtiter reader. (B) DAPI staining of A549 (NT, cRGD and 100 ug/ml EEOS) were performed using confocal microscopy with 60x magnification. (C) Acridine Orange-Ethidium Bromide (AO/EB) Staining. 105 A549 cells were seeded in 24-well plate. Cells were treated with 100 μg/ml (final concentration) of EEOS then incubate for 24 h, fixed with 1 ml of 4% parafomaldehyde for 15–20 min in RT. Cells were washed by PBS 1x, stained with Hoechst and AO/EB (10 μg/ml), incubated for 15 min. Cells were visualised by fluorescence inverted microscope. Green fluorescent represents viable cells, meanwhile orange fluorescent represents apoptotic cells.
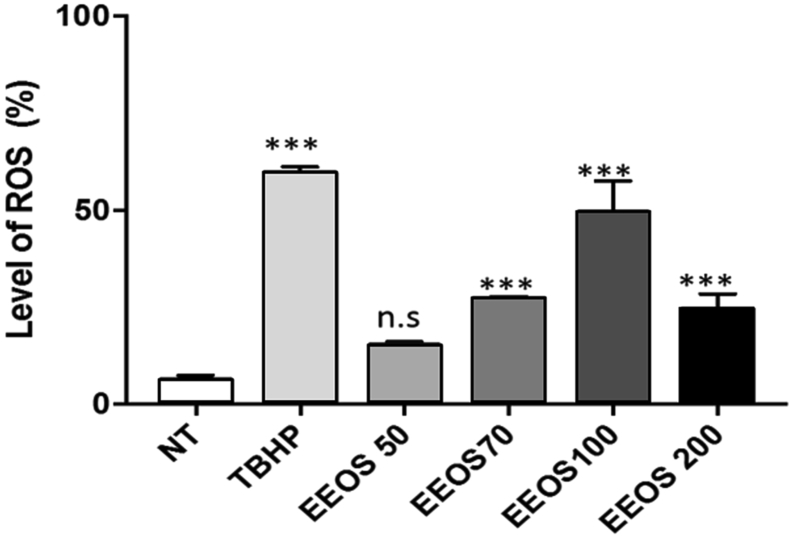
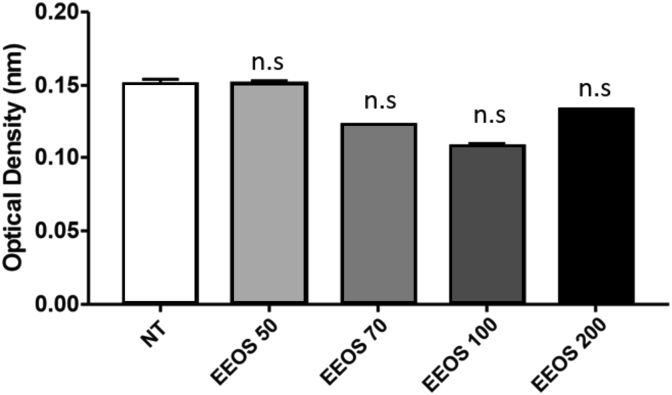
To analyze whether EEOS can also trigger A549 anoikis, the generation of intracellular ROS induced by EEOS was measured by oxidation of DCFDA using flow cytometry. As shown in Fig. 3, ROS production was increased in a dose-dependent manner and optimum was at 100 ug/ml in EEOS treated cells as well as in the control group treated with trybutil hydroperoxide (TBHP). To exclude the involvement of cell culture medium components interactions with EEOS in inducing the cytotoxic effect, we performed the Fox Assay. Our results showed clearly that, there was no difference or very low effect in the hydrogen peroxide concentrations in the both medium non treated and treatment (NT vs EEOS 50 μg/mL vs EEOS 70 μg/mL vs EEOS 100 μg/mL vs 200 μg/mL: 0.1513 vs 0.1510 vs 0.1222 vs 0.1092 vs 0.1321) (Fig. 4).
Fig. 3.
EEOS promoted ROS production in A549 cells. 80% confluency of A549 in T75 flask were harvested with trypsin-EDTA 0,25%. Cells were counted and 2.5 × 105 cells/0.5 ml transferred into FACS round tube. The cells were centrifuged on 1600 rpm for 5 min. The supernatant was aspirated and the pellet was resuspended using DCFDA 1x buffer +10% FBS. After incubation, cells were treated as follows. Cell only, TBHP 250 μM (positive control), DCFDA 20 μM (negative control), DMSO 10%, Ocimum sanctum extract 100 μg/ml. Cells were incubated for 4 h on 37 °C temperature and 5% CO2. ROS levels were measured using flow cytometer.
Fig. 4.
EEOS treatment promoted oxidative stress in A549 cells. FOX assay was performed to answer that the cytotoxicity induced by EEOS is due to ROS generation. The cells were collected and resuspended in lysis buffer. 50 ul cell lysate were added to 550 ul FOX reagent. The samples were incubate at room temperature for 30 min then read immediately at 460 nm.
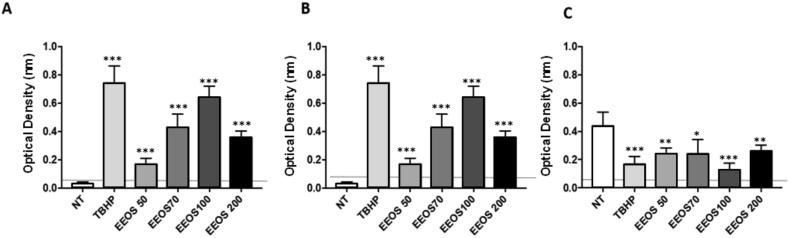
3.4. EEOS regulated apoptosis correlation to proteins in A549 cells
Since increased ROS levels can disrupt the mitochondrial membrane potential thereby activating the apoptosis signaling pathway by involving the differential activity of different enzymes namely caspases. Therefore, in this study, pro-apoptotic and anti-apoptotic activities were analyzed in EEOS treated A549 cells. Interestingly, EEOS-treatment derived ROS might disrupt the mitochondrial membrane potential and activated caspase 9 and caspase 3 as shown in Figs. 5A and B. This increased activity might promote the cleaved caspase-mediated PARP cleavage in A549 cells. In addition, EEOS-treatment in A549 cells decreased the activity of anti-apoptotic protein Bcl-2 in a concentration dependent manner (Fig. 5C). These data imply that EEOS treatment in A549 cells increases the ratio of Bax protein suggesting the activation of apoptotic pathway than survival or anti-apoptotic signaling mechanism (Fig. 5). These data suggest that EEOS induces apoptosis via activation of caspases and inhibition of anti-apoptotic protein in A549 cells.
Fig. 5.
EEOS regulated apoptosis correlation to proteins in A549 cells. (A) Caspase 9 (B) Caspase 3 and (C) Bcl-2. The activity level of Caspase 9, Caspase 3, and Bcl-2 measured by colorimetric assay kit. The cells were collected and resuspended in lysis buffer. The cell lysate was centrifuged at 11000 g for 10 min at 4 °C, and the protein concentration in the supernatants was measured using the Bradford method. The supernatants were incubated with reaction buffer containing 2 mmol/L Ac-DEVD-AFC for caspase-3 and LEHD-AFC for caspase-9 (Abcam) in a caspase assay buffer at 37 °C with 10 mmol/L DTT for 30 min. Caspase activity was determined by measuring the absorbance at 405 nm. Statistical analysis was performed by one-way ANOVA followed by Bonferroni's post-hoc test; n.s. = not significant.
3.5. Oxidative stress induce by EEOS decrease the activity of antioxidant enzyme
The main antioxidant defense enzymes that contribute in reducing the oxidative stress are superoxide dismutase (SOD) and glutathione peroxidase (GPx). Since the EEOS induced apoptosis and promote ROS production in A549 cells, we performed an ELISA assay to describe the activity of SOD and GPx. Our results demonstrated that, SOD2 activity was gradually decreased, however at the concentration of 200 μg/mL there was slightly increased level was detected (Fig. 6A). In addition, we observed a gradual decrease of GPx-activity during the treatment of cancer cells during increased EEOS concentration (from 50 ug/ml to 100 ug/ml) (Fig. 6B).
Fig. 6.
EEOS suppressed antioxidant enzyme activity, (A) SOD and (B) GPx. Enzyme-linked immunosorbent assay was performed to describe SOD and GPX activity. The cells were collected and resuspended in lysis buffer. The cell lysate were tested using SOD and GPx ELISA kit. SOD and GPx activity were determined by measuring the absorbance at 450 nm.
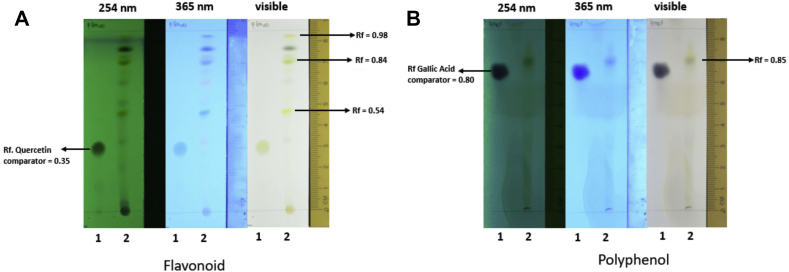
3.6. The EEOS contain abundant polyphenol and flavonoid
The experimental analysis on the TLC found that on the EEOS contain abundant polyphenol (12.50 % b/b) and flavonoid (28.22%b/b) (Fig. 7).
Fig. 7.
The ingredient of EEOS based on Thin Layer Chromatography. (A) Flavonoid (Rf: 0,54; 0,84; 0,98) and (B) phenolic compound (Rf: 0,85) are positively contained in EEOS. The qualitative assay of phenolic was determined by putting 50 mg sample into test tube and extracted by using vortex and ethanol as solvent. Ethanol phase was aspirated then sample was spotted on silica gel plate including gallic acid as standard. The plate was put on saturated chamber with mobile phase then elute until the border. The plate was dry off, observed under UV light and spray using ferri chloride reagent. Subsequently, the same methode was run for the flavonoid with quercetin as standard.
4. Discussion
Recently, several species of genus Ocimum are already in use as a herbal medicine not only in Asia but also in Europe and North America [31]. Some evidences indicate that Ocimum extracts possess antitumor effects [11, 12, 13, 14]. Moreover, Ocimum sanctum combining with Azadirachta indica was reported to have a synergistic chemopreventive effects on chemical-induced gastric carcinogenesis [32]. These studies illustrate the importance of OS as an anti-oxidant, anti-angiogenic, and anti-proliferative. Over the years, the herbal usage has been increased many folds and the conventional medical usage also showed the positive results [8]. Therefore, we hypothesized that EEOS might exhibit beneficial effects in lung adenocarcinoma. However, there is no information about the mechanisms of the anticancer effect from ethanolic extract Ocimum sanctum (EEOS) in lung adenocarcinoma cells. In the present study, we investigated to understand the anticancer effects of EEOS in A549 cell culture model system.
We showed that the EEOS derived from the leaf of Ocimum sanctum is dose dependent (100 ug/ml). Based on our experiments, the adhesion and viability assays revealed that EEOS promoted cell detachment and decreased the cell viability at 100 ug/ml (Fig. 1A and B). It has been documented that cell adhesion to extracellular matrix (ECM) is essential for cell proliferation and survival [33]. Inhibition of cell adhesion that disrupt the ligation between αvβ3 on the cell surface and vitronectin lead to cell anoikis [34] and subsequently to cell apoptosis [35, 36, 37, 38]. In this experiments, we use Cyclic synthetic peptides containing the arginine-glycine-aspartate motif (cRGD) as a positive control to promote cell detachment and cell death. The cRGD targeted for integrin αvβ3, integrin αvβ3 plays a significant role in tumor angiogenesis and is a receptor for the extracellular matrix proteins with the expose of RGD tripeptide sequence. The αvβ3 integrin expressed on the tumoral endothelial cells as well as on the tumor cells [39, 40]. It has been shown the cRGD able to inhibit adhesion of various cells to several extracellular matrix proteins including fibronectin, vitronectin, von Willbrand factor, fibrinogen, thrombospondin and osteopontin. cRGD will strongly block cell attachment to the extracellular matrix and lead to cell death [26, 41]. Kim and colleagues also reported the inhibitory effect of ethanol leaf extract of O. sanctum extract on lung metastasis using the mouse LLC cells. Ethanol leaf extract of O. sanctum extract prevented cell adhesion and invasion of LLC cells to extracellular matrix (ECM) [42].
Furthermore, to analyze its role in apoptosis, we performed an apoptotic assay. Interestingly, we found that the EEOS induced apoptosis in A549 cells (Fig. 2), in agreement to the results, our ROS analysis in the presence of EEOS also showed a significant increase in ROS (Fig. 3). In line with these results, cell detachment is normally associated with the activation of mitochondria to produce reactive oxygen species (ROS). It is well known that ROS can initiate and regulate the transcription and activation of different mediators which culminate in the common mechanism of cell damage via apoptosis caspase pathway [43]. In addition, ROS is known to cause oxidative modification of DNA, proteins, lipids and cellular small molecules [44]. Increased ROS levels are thought to constitute an essential step in apoptotic induction. Apoptosis or programmed cell death has two well known pathways, extrinsic and intrinsic pathways. In the case of the intrinsic pathway, a release of cytochrome C from mitochondria results in binding to Apaf-1 and subsequently leads to activation of procaspase-9 and following caspase-3. Activated caspase-3 exerts as the key executioner of apoptosis to induce the cleavage and inactivation of key cellular protein [43, 45]. In the present study, EEOS treatment demonstrated the increasing of caspase-3 expression level (Fig. 5), meanwhile this substance depressed the activity of anti-apoptotic protein Bcl-2. There is an evidence that early event in apoptosis is occur by the disruption of the mitochondrial membrane potential.
Apoptosis also contributes to morphological changes of some features inside the cells. The morphological features of apoptosis consist of chromatin condensation, nuclear fragmentation, an increase cell density and the formation of cytoplasmic blebs. The nucleus acquires an irregular shape, nucleolar size increases and its granules become enlarged and spread out [46]. The final stage of apoptosis is characterized by DNA degradation and cell damage into dense membrane-surrounded fragments (apoptotic bodies). Some apoptotic bodies contain fragments of the nucleus, while others contain only cytoplasm [47]. Therefore, nuclear condensation can be used to determined apoptotic cells from healthy cells or necrotic cells. Dyes that bind to DNA, such as Hoechst 33342, can be used to observe nuclear condensation (Fig. 6). Cells that have died by apoptosis will generally display condensed DNA and fragmented nuclei, meanwhile the healthy and necrotic cells do not [48]. Dual AO/EB fluorescent staining also detect basic morphological changes in apoptotic cells and necrotic cells on A549 treated with EEOS (Fig. 6). Liu et al. 2015 proposed that AO penetrated normal and early apoptotic cells with intact membranes, fluorescing green when bound to DNA. EB only entered cells with damages membranes, such as late apoptotic and dead cells, emitting orange-red fluorescence when bound to concentrated DNA fragments or apoptotic bodies [49].
To strengthen our results, we performed the antioxidant enzymes activities for SOD2 and GPx. SOD drives the reaction of dismutation of superoxide into hydrogen peroxide. The SOD2 or also known as manganese-dependent superoxide dismutase (MnSOD) is mainly localized in the mitochondria [50, 51] and the first detoxification enzyme. However, MnSOD overexpression is common in tumors and contribute to therapy resistance [52]. From the results we can see the gradually decreasing level of SOD2 meanwhile on the concentration EEOS at 200 μg/mL there is slightly increasing level of SOD2. Since mitochondria are the major source of reactive oxygen species, decreasing their detoxifying ability by means blocking SOD2 activity in cancer might contribute to induce apoptosis [50]. Plumbagin proved to efficiently induce apoptosis in effect in prostate cancer cell lines, partially through decreasing SOD2 expression [53]. Phenethyl isothiocyanate was found to inhibit expression of SOD2 in LN229 glioma cell line, weakening cellular antioxidant defense and causing apoptosis [54]. Suppression of SOD2 enzymatic activity by apigenin in combination with ROS-inducing paclitaxel was found to sensitize HeLa cells to apoptosis and allowed to lower paclitaxel doses [55]. Apigenin is one of chemical constituent which presents in Ocimum sanctum extract. It had been reported that apigenin could form stabel complexation with metal ions in vitro [56] and decrease SOD activity by blocking SOD from assemble with its cofactors. It has been addressed that apigenin was a potential inducer of intracellular oxidative stress [57].
In addition, we perform an ELISA assay for glutathione peroxidase (GPx). The GPx is a enzymatic scavenger of proxides (H2O2), thus H2O2 can be detoxified by GPx and converted into H2O [58, 59]. The clinical importance of GPx has been underlined by a number of studies. Increasing level of GPx can protect the cells against hydrogen peroxide induce cell death or apoptotic, meanwhile depletion or silencing GPx may suppresses cell cancer growth [60]. In addition, lower GPx activity is predisposed to impaired antioxidant protection, which leads to oxidative damage to the cancer cells [61]. Forgione and colleagues [62] had previously hypothesized that GPX deficiency directly induces an increase in vascular oxidative stress, with attendant endothelial dysfunction. Based on our GPx experimental results, we observe gradual decrement of GPx-activity during the treatment of cancer cells with increased EEOS concentration (from 50 ug/ml to 100 ug/ml). These results indicated that specific EEOS concentration presumably induced the increases of the lipid peroxidation, which therefore prevent it's enzymatic activity, and prevent the cytoprotective effect of cancer cells. Interestingly, the GPx-activity-inhibition effect was abolished by the treatment of high dose EEOS (200 ug/ml), To answer the phenomenon that the EEOS at 200 ug/ml is always less effective than lower concentrations, we did an experiment by Thin Layer Chomatography (TLC) to analyse the ingredient of the EEOS. Based on the TLC results the EEOS contain abundant polyphenol (12.50 % b/b) and flavonoid (28.22%b/b) on the EEOS (Fig. 7). Our data is in line with the other research before, which is mention the EEOS contained many substances consisting of apigenin, luteolin, apigenin-7-O-β-D-rutinopyranoside, luteolin-7-O- β-D-glucopyranoside, vicenin-2, vitexin, isovitexin, orientin, isoorientin, aesculetin, aesculin, chlorogenic acid, eugenol, and caffeic acid [63, 64, 65]. Phenol and flavonoids have gained importance as anti-cancer agents and have shown great potential as cytotoxic anti-cancer agents promoting apoptosis in cancer cells [66]. Danciu et al. [67] reported the increase in anticancer activity was correlated with the increase in amount of polyphenol compounds. Moreover, the results from many biomedical research teams indicated that various kinds of flavonoids can promote apoptosis in various cancer cells [68, 69, 70]. Presumably, some substances such as eugenol induced general protective effects [64, 71, 72], and in contrary, some substance such as flavonoid and phenol is well known as specific cancer cells inhibiting agents, both in different opposite effective dose dependent functionality, and could causes different physiological mechanism within different doses. It is likely that this reason causes EEOS to be less effective at a concentration of 200 ug/ml due to the work imbalance of the active substance. Thus it is important to separate the active substance from EEOS to be able to optimize the cytotoxicity function of EEOS.
In conclusion, the present study demonstrates the significant evidence of EEOS treatment in altering the viability of lung adenocarcinoma A549 cells through a synergy of induction of apoptotic signaling via intrinsic-mitochondria pathway. Further research is needed to isolate the phytochemicals that are responsible for cancer cell apoptosis.
5. Conclusion
Taking together, our results showed that EEOS provide anticancer activity by decreasing cell proliferation and viability, increasing intracellular ROS, interfere the activity of SOD and GPx thus promoting apoptosis in A549 cells. The results derived from our investigation confirmed the therapeutic potency of EEOS.
Declarations
Author contribution statement
Hevi Wihadmadyatami, Dwi Liliek Kusindarta: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Srikanth Karnati: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Puspa Hening: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yudy Tjahjono: Analyzed and interpreted the data; Wrote the paper.
Rizal, Fitriana Maharjanti: Performed the experiments; Analyzed and interpreted the data.
Teguh Triyono, Supriatno: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by a research grant from Directorate Research and Community Service, Directorate General of Higher Education, Ministry of Research, Technology, and Higher Education of the Republic of Indonesia (PDUPT UGM Grant number: 60/UN1/DIT-LIT/LT/2018).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to thank Made Bagus Auriva Mataram, M.Sc. and Mrs. Istini for the excellent technical assistance.
References
- 1.Alberg A.J., Brock M.V., Ford J.G., Samet J.M., Spivack S.D. Epidemiology of lung cancer. Chest. 2013;143:e1S–e29S. doi: 10.1378/chest.12-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridge C.A., McErlean A.M., Ginsberg M.S. Epidemiology of lung cancer. Semin. Interv. Radiol. 2013;30:93–98. doi: 10.1055/s-0033-1342949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahal Z., El Nemr S., Sinjab A., Chami H., Tfayli A., Kadara H. Smoking and lung cancer: a geo-regional perspective. Front. Oncol. 2017;7:1–7. doi: 10.3389/fonc.2017.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behera D D. New approach to the treatment of lung cancer : the molecular targeted therapy. Indian J. Chest Dis. Allied Sci. 2007;49(3):149–158. [Google Scholar]
- 5.Yin S.Y., Wei W.C., Jian F.Y., Yang N.S. Therapeutic applications of herbal medicines for cancer patients. Evid. Based Complement Altern. Med. ECAM. 2013;302426:1–15. doi: 10.1155/2013/302426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenwell M., Rahman P.K.S.M. Medicinal plants: their use in anticancer treatment. Int. J. Pharm. Sci. Res. 2015;6:4103–4112. doi: 10.13040/IJPSR.0975-8232.6(10).4103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thapliyal A., Khar R.K., Chandra A. Overview of cancer and medicinal herbs used for cancer therapy. Asian J. Pharm. 2018;1:1–8. [Google Scholar]
- 8.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:1–10. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotecha R., Takami A., Espinoza J.L. Dietary phytochemicals and cancer chemoprevention: a review of the clinical evidence. Oncotarget. 2016;7:52517–52529. doi: 10.18632/oncotarget.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levi M.S., Borne R.F., Williamson J.S. A review of cancer chemopreventive agents. Curr. Med. Chem. 2001;8:1349–1362. doi: 10.2174/0929867013372229. [DOI] [PubMed] [Google Scholar]
- 11.Chen H.M., Lee M.J., Kuo C.Y., Tsai P.L., Liu J.Y., Kao S.H. Ocimum gratissimum aqueous extract induces apoptotic signalling in lung adenocarcinoma cell A549. Evid. Based Complement Altern. Med. ECAM. 2011:1–7. doi: 10.1155/2011/739093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Flora S., Ferguson L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. 2005;591:8–15. doi: 10.1016/j.mrfmmm.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T. Chemoṕrevention of human cancer: biology and therapy. Crit. Rev. Oncol. Hematol. 1997;25:139–174. doi: 10.1016/s1040-8428(97)00232-1. [DOI] [PubMed] [Google Scholar]
- 14.Clark J., You M. Chemoprevention of lung cancer by tea. Mol. Nutr. Food Res. 2006;50:144–151. doi: 10.1002/mnfr.200500135. [DOI] [PubMed] [Google Scholar]
- 15.Kusindarta D.L., Wihadmadyatami H., Haryanto A. Ocimum sanctum Linn. stimulate the expression of choline acetyltransferase on the human cerebral microvascular endothelial cells. Vet. World. 2016;9:1348–1354. doi: 10.14202/vetworld.2016.1348-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S.K., Prakash J., Srivastava S. Validation of traditional claim of Tulsi, Ocimum sanctum Linn. as a medicinal plant. Indian J. Exp. Biol. 2002;40:765–773. [PubMed] [Google Scholar]
- 17.Hening P., Mataram M.B.A., Wijayanti N., Kusindarta D.L., Wihadmadyatami H. The neuroprotective effect of Ocimum sanctum Linn. ethanolic extract on human embryonic kidney-293 cells as in vitro model of neurodegenerative disease. Vet. World. 2018;11:1237–1243. doi: 10.14202/vetworld.2018.1237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusindarta D.L., Wihadmadyatami H., Jadi A.R., Karnati S., Lochnit G., Hening P., dkk Ethanolic extract Ocimum sanctum . Enhances cognitive ability from young adulthood to middle aged mediated by increasing choline acetyl transferase activity in rat model. Res. Vet. Sci. 2017;118:431–438. doi: 10.1016/j.rvsc.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Magesh V., Lee J.C., Ahn K.S., Lee H.J., Lee E.O. Ocimum sanctum induces apoptosis in A549 lung cancer cells and suppresses the in vivo growth of Lewis lung carcinoma cells. Phytother Res. PTR. 2009;23:1385–1391. doi: 10.1002/ptr.2784. [DOI] [PubMed] [Google Scholar]
- 20.Nangia-Makker P., Tait L., Shekhar M.P.V., Palomino E., Hogan V., Piechocki M.P. Inhibition of breast tumor growth and angiogenesis by a medicinal herb: Ocimum gratissimum. Int. J. Cancer. 2007;121:884–894. doi: 10.1002/ijc.22733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma M., Agrawal S.K., Sharma P.R., Chadha B.S., Khosla M.K., Saxena A.K. Cytotoxic and apoptotic activity of essential oil from Ocimumviride towards COLO 205 cells. Food Chem. Toxicol. 2010;48:336–344. doi: 10.1016/j.fct.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Prakash J., Gupta S.K. Chemopreventive activity of Ocimum sanctum seed oil. J. Ethnopharmacol. 2000;72:29–34. doi: 10.1016/s0378-8741(00)00194-x. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee S., Prashar R., Kumar A., Rao A.R. Modulatory influence of alcoholic extract of Ocimum leaves on carcinogen-metabolizing enzyme activities and reduced glutathione levels in mouse. Nutr. Cancer. 1996;25:205–217. doi: 10.1080/01635589609514443. [DOI] [PubMed] [Google Scholar]
- 24.Gang D.R., Beuerle T., Ullmann P., Werck-Reichhart D., Pichersky E. Differential production of meta hydroxylated phenylpropanoids in sweet basil peltate glandular trichomes and leaves is controlled by the activities of specific acyltransferases and hydroxylases. Plant Physiol. 2002;130:1536–1544. doi: 10.1104/pp.007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karthikeyan K., Ravichandran P., Govindasamy S. Chemopreventive effect of Ocimum sanctum on DMBA-induced hamster buccal pouch carcinogenesis. Oral Oncol. 1999;35:112–119. doi: 10.1016/s1368-8375(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 26.Santoso S., Wihadmadyatami H., Bakchoul T., Werth S., Al-Fakhri N., Bein G. Antiendothelial αvβ3 antibodies are a major cause of intracranial bleeding in fetal/neonatal alloimmune thrombocytopenia. Arterioscler. Thromb. Vasc. Biol. 2016;36:1517–1524. doi: 10.1161/ATVBAHA.116.307281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wihadmadyatami H., Röder L., Berghöfer H., Bein G., Heidinger K., Sachs U.J. Immunisation against αIIbβ3 and αvβ3 in a type 1 variant of Glanzmann’s thrombasthenia caused by a missense mutation Gly540Asp on β3. Thromb. Haemost. 2016;116:262–271. doi: 10.1160/TH15-12-0982. [DOI] [PubMed] [Google Scholar]
- 28.Wihadmadyatami H. 2016. The role of antibodies against endothelial cells in immune mediated thrombocytopenia.http://geb.uni-giessen.de/geb/volltexte/2016/12268/ GEB-IDN/12268. Available at: [Google Scholar]
- 29.Halliwell B., Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br. J. Pharmacol. 2004;142(2):231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhee S.G., Chang T.S., Jeong W., Kang D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol. Cells. 2010;29(6):539–549. doi: 10.1007/s10059-010-0082-3. [DOI] [PubMed] [Google Scholar]
- 31.Shasany A. The holy basil (Ocimum sanctum L.) and its genome. Indian J. Hist. Sci. 2016;26:51. [Google Scholar]
- 32.Manikandan P., Vidjaya Letchoumy P., Prathiba D., Nagini S. Combinatorial chemopreventive effect of Azadirachta indica and Ocimum sanctum on oxidant-antioxidant status, cell proliferation, apoptosis and angiogenesis in a rat forestomach carcinogenesis model. Singap. Med. J. 2008;49:814–822. [PubMed] [Google Scholar]
- 33.Li F., Zhang Y., Wu C. Integrin-linked kinase is localized to cell-matrix focal adhesions but not cell-cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J. Cell Sci. 1999;112:4589–4599. doi: 10.1242/jcs.112.24.4589. [DOI] [PubMed] [Google Scholar]
- 34.Erdreich-Epstein A., Tran L.B., Cox O.T., Huang E.Y., Laug W.E., Shimada H. Endothelial apoptosis induced by inhibition of integrins αvβ3 and αvβ5 involves ceramide metabolic pathways. Blood. 2005;105:4353–4361. doi: 10.1182/blood-2004-08-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brassard D.L., Maxwell E., Malkowski M., Nagabhushan T.L., Kumar C.C., Armstrong L. Integrin αvβ3-mediated activation of apoptosis. Exp. Cell Res. 1999;251:33–45. doi: 10.1006/excr.1999.4559. [DOI] [PubMed] [Google Scholar]
- 36.Brooks P.C., Montgomery A.M., Rosenfeld M., Reisfeld R.A., Hu T., Klier G. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 37.Eliceiri B.P., Cheresh D.A. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J. Clin. Investig. 1999;103:1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montgomery A.M., Reisfeld R.A., Cheresh D.A. Integrin alpha v beta 3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8856–8860. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S. Radiolabeled cyclic RGD peptides as integrin r v 3 -targeted Radiotracers. Bioconjug. Chem. 2009;20:2199–2213. doi: 10.1021/bc900167c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danhier F., Breton A Le. RGD based strategies to target avb3 integrin in cancer therapy and diagnosis. Mol. Pharm. 2012;9:2961–2973. doi: 10.1021/mp3002733. [DOI] [PubMed] [Google Scholar]
- 41.Verrier S., Pallu S., Bareille R., Jonczyk A., Meyer J., Dard M. Function of linear and cyclic RGD-containing peptides in osteoprogenitor cells adhesion process. Biomaterials. 2002;23:585–596. doi: 10.1016/s0142-9612(01)00145-4. [DOI] [PubMed] [Google Scholar]
- 42.Kim S.C., Magesh V., Jeong S.J., Lee H.J., Ahn K.S., Lee H.J. Ethanol extract of Ocimum sanctum exerts anti-metastatic activity through inactivation of matrix metalloproteinase-9 and enhancement of anti-oxidant enzymes. Food Chem. Toxicol. Int J Publ. Br. Ind. Biol. Res. Assoc. 2010;48:1478–1482. doi: 10.1016/j.fct.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Apoptosis S. Elmore. A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C., Youle R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horký M., Kotala V., Anton M., Wesierska-Gadek J. Nucleolus and apoptosis. Ann. N. Y. Acad. Sci. 2002;973:258–264. doi: 10.1111/j.1749-6632.2002.tb04645.x. [DOI] [PubMed] [Google Scholar]
- 47.Kalinichenko S.G., Matveeva N.Y. Morphological characteristics of apoptosis and its significance in neurogenesis. Neurosci. Behav. Physiol. 2008;38:333–344. doi: 10.1007/s11055-008-0046-7. [DOI] [PubMed] [Google Scholar]
- 48.Crowley L.C., Marfell B.J., Waterhouse N.J. Analyzing cell death by nuclear staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016;9 doi: 10.1101/pdb.prot087205. [DOI] [PubMed] [Google Scholar]
- 49.Ribble D., Goldstein N.B., Norris D.A., Shellman Y.G. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005;5:12. doi: 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sznarkowska A., Kostecka A., Meller K., Bielawski K.P. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget. 2017;8:15996–16016. doi: 10.18632/oncotarget.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karnati S., Lüers G., Pfreimer S., Baumgart-Vogt E. Mammalian SOD2 is exclusively located in mitochondria and not present in peroxisomes. Histochem. Cell Biol. 2013;140:105–117. doi: 10.1007/s00418-013-1099-4. [DOI] [PubMed] [Google Scholar]
- 52.Buettner G.R. Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anti Cancer Agents Med. Chem. 2011;11:341–346. doi: 10.2174/187152011795677544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powolny A.A., Singh S.V. Plumbagin-induced apoptosis in human prostate cancer cells is associated with modulation of cellular redox status and generation of reactive oxygen species. Pharm. Res. 2008;25:2171–2180. doi: 10.1007/s11095-008-9533-3. [DOI] [PubMed] [Google Scholar]
- 54.Su J.-C., Lin K., Wang Y., Sui S.-H., Gao Z.-Y., Wang Z.-G. In vitro studies of phenethyl isothiocyanate against the growth of LN229 human glioma cells. Int. J. Clin. Exp. Pathol. 2015;8:4269–4276. [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Y., Xin Y., Diao Y., Lu C., Fu J., Luo L. Synergistic effects of apigenin and paclitaxel on apoptosis of cancer cells. Sarkar FH. PLoS One. 2011;6 doi: 10.1371/journal.pone.0029169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J., Wang J., Brodbelt J.S. Characterization of flavonoids by aluminum complexation and collisionally activated dissociation. J. Mass Spectrom. 2005;40:350–363. doi: 10.1002/jms.793. [DOI] [PubMed] [Google Scholar]
- 57.Miyoshi N., Naniwa K., Yamada T., Osawa T., Nakamura Y. Dietary flavonoid apigenin is a potential inducer of intracellular oxidative stress: the role in the interruptive apoptotic signal. Arch. Biochem. Biophys. 2007;466:274–282. doi: 10.1016/j.abb.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 58.Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018;54:287–293. [Google Scholar]
- 60.Chen Z., Hu T., Zhu S., Mukaisho K., El-Rifai W., Peng D.-F. Glutathione peroxidase 7 suppresses cancer cell growth and is hypermethylated in gastric cancer. Oncotarget. 2017;8:54345–54356. doi: 10.18632/oncotarget.17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simioni C., Zauli G., Martelli A.M., Vitale M., Sacchetti G., Gonelli A. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018;9:17181–17198. doi: 10.18632/oncotarget.24729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forgione M.A., Weiss N., Heydrick S., Cap A., Klings E.S., Bierl C. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am. J. Physiol. Cell Physiol. 2002;282:H1255–H1261. doi: 10.1152/ajpheart.00598.2001. [DOI] [PubMed] [Google Scholar]
- 63.Venuprasad M.P., Kandikattu H.K., Khanum F. Neuroprotective effects of hydroalcoholic extract of Ocimum sanctum against H2O2 induced neuronal cell damage in SH-SY5Y cells via its antioxidative defence mechanism. Neurochem. Res. 2013;38:2190–2200. doi: 10.1007/s11064-013-1128-7. [DOI] [PubMed] [Google Scholar]
- 64.Kusindarta D.L., Wihadmadyatami H., Haryanto A. The analysis of hippocampus neuronal density ( CA1 and CA3 ) after Ocimum sanctum ethanolic extract treatment on the young adulthood and middle age rat model. Vet. World. 2018;11:135–140. doi: 10.14202/vetworld.2018.135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venuprasad M.P., Kumar Kandikattu H., Razack S., Khanum F. Phytochemical analysis of Ocimum gratissimum by LC-ESI-MS/MS and its antioxidant and anxiolytic effects. South Afr. J. Bot. 2014;92:151–158. [Google Scholar]
- 66.Abotaleb M., Samuel S.M., Varghese E., Varghese S., Kubatka P., Liskova A. Flavonoids in cancer and apoptosis. Cancers. 2019;11 doi: 10.3390/cancers11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danciu C., Vlaia L., Fetea F., Hancianu M., Coricovac D.E., Ciurlea S.A. Evaluation of phenolic profile, antioxidant and anticancer potential of two main representants of Zingiberaceae family against B164A5 murine melanoma cells. Biol. Res. 2015;48:1. doi: 10.1186/0717-6287-48-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mishra D.K., Wu Y., Sarkissyan M., Sarkissyan S., Chen Z., Shang X. Vitamin D receptor gene polymorphisms and prognosis of breast cancer among african-American and hispanic women. Ling MT. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brusselmans K., De Schrijver E., Heyns W., Verhoeven G., Swinnen J.V. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int. J. Cancer. 2003;106:856–862. doi: 10.1002/ijc.11317. [DOI] [PubMed] [Google Scholar]
- 70.Brusselmans K., Vrolix R., Verhoeven G., Swinnen J.V. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005;280:5636–5645. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- 71.Wie M.B., Won M.H., Lee K.H., Shin J.H., Lee J.C., Suh H.W. Eugenol protects neuronal cells from excitotoxic and oxidative injury in primary cortical cultures. Neurosci. Lett. 1997;225:93–96. doi: 10.1016/s0304-3940(97)00195-x. [DOI] [PubMed] [Google Scholar]
- 72.Bezerra D.P., Militão G.C.G., De Morais M.C., De Sousa D.P. The dual antioxidant/prooxidant effect of eugenol and its action in cancer development and treatment. Nutrients. 2017;9:1–15. doi: 10.3390/nu9121367. [DOI] [PMC free article] [PubMed] [Google Scholar]