Abstract
Background
Community pharmacies are an easily accessible and cost‐effective platform for delivering health care worldwide, and the range of services provided has undergone rapid expansion in recent years. Thus, in addition to dispensing medication, pharmacy workers within community pharmacies now give advice on a range of health‐promoting behaviours that aim to improve health and to optimise the management of long‐term conditions. However, it remains uncertain whether these health‐promotion interventions can change the professional practice of pharmacy workers, improve health behaviours and outcomes for pharmacy users and have the potential to address health inequalities.
Objectives
To assess the effectiveness and safety of health‐promotion interventions to change community pharmacy workers' professional practice and improve outcomes for users of community pharmacies.
Search methods
We searched MEDLINE, Embase, CENTRAL, six other databases and two trials registers to 6 February 2018. We also conducted reference checking, citation searches and contacted study authors to identify any additional studies.
Selection criteria
We included randomised trials of health‐promotion interventions in community pharmacies targeted at, or delivered by, pharmacy workers that aimed to improve the health‐related behaviour of people attending the pharmacy compared to no treatment, or usual treatment received in the community pharmacy. We excluded interventions where there was no interaction between pharmacy workers and pharmacy users, and those that focused on medication use only.
Data collection and analysis
We used standard procedures recommended by Cochrane and the Effective Practice and Organisation of Care review group for both data collection and analysis. We compared intervention to no intervention or to usual treatment using standardised mean differences (SMD) and 95% confidence intervals (95% CI) (higher scores represent better outcomes for pharmacy user health‐related behaviour and quality of life, and lower scores represent better outcomes for clinical outcomes, costs and adverse events). Interpretation of effect sizes (SMD) was in line with Cochrane recommendations.
Main results
We included 57 randomised trials with 16,220 participants, described in 83 reports. Forty‐nine studies were conducted in high‐income countries, and eight in middle‐income countries. We found no studies that had been conducted in low‐income countries. Most interventions were educational, or incorporated skills training. Interventions were directed at pharmacy workers (n = 8), pharmacy users (n = 13), or both (n = 36). The clinical areas most frequently studied were diabetes, hypertension, asthma, and modification of cardiovascular risk. Duration of follow‐up of interventions was often unclear. Only five studies gave details about the theoretical basis for the intervention, and studies did not provide sufficient data to comment on health inequalities.
The most common sources of bias were lack of protection against contamination ‐ mainly in individually randomised studies ‐ and inadequate blinding of participants. The certainty of the evidence for all outcomes was moderate. We downgraded the certainty because of the heterogeneity across studies and evidence of potential publication bias.
Professional practice outcomes
We conducted a narrative analysis for pharmacy worker behaviour due to high heterogeneity in the results. Health‐promotion interventions probably improve pharmacy workers' behaviour (2944 participants; 9 studies; moderate‐certainty evidence) when compared to no intervention. These studies typically assessed behaviour using a simulated patient (mystery shopper) methodology.
Pharmacy user outcomes
Health‐promotion interventions probably lead to a slight improvement in health‐related behaviours of pharmacy users when compared to usual treatment (SMD 0.43, 95% CI 0.14 to 0.72; I2 = 89%; 10 trials; 2138 participants; moderate‐certainty evidence). These interventions probably also lead to a slight improvement in intermediate clinical outcomes, such as levels of cholesterol or glycated haemoglobin, for pharmacy users (SMD ‐0.43, 95% CI ‐0.65 to ‐0.21; I2 = 90%; 20 trials; 3971 participants; moderate‐certainty evidence).
We identified no studies that evaluated the impact of health‐promotion interventions on event‐based clinical outcomes, such as stroke or myocardial infarction, or the psychological well‐being of pharmacy users.
Health‐promotion interventions probably lead to a slight improvement in quality of life for pharmacy users (SMD 0.29, 95% CI 0.08 to 0.50; I2= 82%; 10 trials, 2687 participants; moderate‐certainty evidence).
Adverse events
No studies reported adverse events for either pharmacy workers or pharmacy users.
Costs
We found that health‐promotion interventions are likely to be cost‐effective, based on moderate‐certainty evidence from five of seven studies that reported an economic evaluation.
Authors' conclusions
Health‐promotion interventions in the community pharmacy context probably improve pharmacy workers' behaviour and probably have a slight beneficial effect on health‐related behaviour, intermediate clinical outcomes, and quality of life for pharmacy users.
Such interventions are likely to be cost‐effective and the effects are seen across a range of clinical conditions and health‐related behaviours. Nevertheless the magnitude of the effects varies between conditions, and more effective interventions might be developed if greater consideration were given to the theoretical basis of the intervention and mechanisms for effecting behaviour change.
Plain language summary
Can community pharmacy interventions help improve pharmacy workers' skills and pharmacy users' health outcomes through health promotion?
What is the aim of this review?
We aimed to find out whether interventions that support people to change health behaviours, and are delivered in community pharmacies, can change the way that pharmacy workers interact with pharmacy users and can improve health outcomes for those users.
Key messages
Community pharmacies and their workers may have an important part to play in health promotion, and probably improve the health outcomes of pharmacy users slightly, at an acceptable cost and with no evidence of harm (adverse events may or may not have occurred, this is unclear as no adverse effects were reported by the studies).
What was studied in the review?
Community pharmacies are an easy place for many people to access healthcare advice. In the past this advice was limited to how best to take medicines, but, increasingly, community pharmacy workers are carrying out other activities, such as giving advice on healthy eating and management of long‐term conditions. While some community pharmacy workers may offer the sale of products without a strong evidence‐base, the professional guidance issued to pharmacists has attempted to reduce these transactions, and has placed more emphasis on developing evidence‐based public health services. Many people find health‐related lifestyle and self‐management behaviours difficult. Pharmacies may be convenient for people to use, but it is important to understand whether health‐promoting activities delivered in pharmacies are worthwhile and effective, so that those responsible for commissioning health care can decide whether it is worth spending resources to support them.
What are the main results of the review?
We identified 57 studies with a total of 16,220 participants that investigated the effects of health‐promotion activities compared to normal treatment or no treatment. These were conducted across the world, 49 of them in high‐income countries and eight in middle‐income countries. Most studies (36/57) targeted both pharmacy workers and pharmacy users; eight were directed at pharmacy workers only, and 13 at pharmacy users only. The health areas most frequently studied were diabetes, hypertension, asthma and reduction of cardiovascular risk. The studies varied in quality. Some studies did not take enough precautions to stop the participants who should have received either no treatment or usual treatment (i.e. the control group) receiving parts of the intervention.
We found that pharmacy workers may be able to change their behaviour, for example improve their communication skills, to help people to manage their health conditions more effectively.
Overall these studies probably show a slight beneficial effect on pharmacy users' health‐related behaviour, intermediate clinical outcomes (e.g. levels of cholesterol or glycated haemoglobin) and quality of life. No studies reported measuring pharmacy users' clinical events such as heart attacks or stroke. There was also no evidence of harm reported in any of the studies, but no studies reported measuring adverse events. Five out of seven studies that measured costs showed that health promotion delivered by pharmacy workers was cost effective.
These findings suggest that community pharmacy workers can probably slightly improve pharmacy users' health outcomes at a reasonable cost. The variety of studies includes different countries, conditions, interventions and outcomes, and suggests there is great interest in using the community pharmacy setting for workers to promote health‐related behaviours. However, in order to make future studies easier to compare, there is a need for greater use of thorough, systematic approaches in the description of these interventions, use of a standardised set of outcomes, and for new studies to build on prior work.
How up to date is this review?
We searched for studies that had been published up to February 2018.
Summary of findings
Summary of findings for the main comparison. Health‐promotion interventions within community pharmacy compared to usual treatment: effects on professional practice and health outcomes.
| Do health‐promotion interventions improve professional practice of community pharmacy workers and improve health outcomes for community pharmacy users? | ||||
| Patient or population: community pharmacy workers (examples pharmacists, counter assistants etc), community pharmacy users Setting: community pharmacy ‐ the majority of community pharmacies were in urban settings in high‐income countries Intervention: a health‐promotion intervention delivered to pharmacy workers or users within community pharmacy commonly consisting of education and skills training Comparison: no treatment or usual treatment received within the community pharmacy | ||||
| Outcomes | Effect of intervention (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Pharmacy worker behaviour1 | Six of nine studies reported improvement in pharmacy worker behaviour, one study found no benefit, while two had mixed results | 2944 (9 RTs) | ⊕⊕⊕⊝ MODERATE2 | |
| Pharmacy user health‐related behaviour3 (Higher scores indicate a better outcome) |
The mean score in the intervention group was 0.43 SD higher (0.14 higher to 0.72 higher) | 2138 (10 RTs) | ⊕⊕⊕⊝ MODERATE2,4 | A SMD of 0.43 represents a small improvement in pharmacy user health‐related behaviour, according to Cohen's rule of thumb (Higgins 2011b). |
| Pharmacy user intermediate clinical outcomes e.g. cholesterol, glycated haemoglobin5 (Lower scores indicate a better outcome) |
The mean score in the intervention group was 0.43 SD lower (0.65 lower to 0.21 lower) | 3971 (20 RTs) | ⊕⊕⊕⊝ MODERATE2,4 | A SMD of 0.43 represents a small difference between groups with greater benefit in the intervention group, according to Cohen's rule of thumb Higgins 2011b |
| Pharmacy user event‐based clinical outcomes e.g. stroke, myocardial infarction | No studies reported this outcome. | (0 studies) | ‐ | |
| Pharmacy user quality of life6 (Higher scores indicate better quality of life) |
The mean score in the intervention group was 0.29 SD higher (0.08 higher to 0.5 higher) | 2687 (10 RTs) | ⊕⊕⊕⊝ MODERATE2,4 | A SMD of 0.29 higher represents a small difference between groups with greater benefit in the intervention group according to Cohen's rule of thumb (Higgins 2011b). |
| Adverse events | No studies reported this outcome | (0 studies) | ‐ | |
| Costs | Five of seven studies found the intervention to be cost‐effective. | (7 RTs) | ⊕⊕⊕⊝ MODERATE2 | |
| CI: confidence interval; RT: randomised trial; SD: standard deviation; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||
1Includes communication/consultation skills, referral to smoking quit line, demonstration of inhaler technique.
2Downgraded by one level for inconsistency (due to substantial heterogeneity in studies).
3Includes medication adherence (n = 3); inhaler technique (n = 4), alcohol consumption (n = 1), diabetes self‐management (n = 1), activity impairment (n = 1).
4Asymmetric funnel plots ‐ considered insufficient to require further downgrading.
5Includes asthma control (n = 8), blood glucose (n = 5), systolic blood pressure (n = 5), low‐density lipoprotein (n = 2).
6Includes generic quality of life (n = 5), asthma quality of life (n = 5), diabetes quality of life (n = 1).
Background
Description of the condition
Pharmacists are the third largest regulated healthcare professional group in the world (Chan 2006), with community pharmacy the most common discipline represented. Community pharmacies are an easily accessible platform for delivering healthcare worldwide (DOH 2005; WHO 1998). For example, in England there are over 11,500 community pharmacies, with approximately 89% of the population able to access one within a 20‐minutes walk (Todd 2014). In Australia, over 90% of the population visit a pharmacist during the course of a year (Benrimoj 2004). Pharmacies are more densely distributed in areas of high deprivation – a so‐called ‘positive pharmacy care law’ ‐ where better access to pharmacy care is available to those with greatest deprivation (Todd 2014). In low‐ and middle‐income countries, but also increasingly in high‐income countries, pharmacies are often seen as the first place to call for advice on symptoms and for early diagnosis of illness (Smith 2009).
The role of the pharmacist has undergone rapid expansion in recent years (Blouin 2017; Mossialos 2015; WHO 2006). For example, in addition to dispensing and medication‐linked services, pharmacy workers are now required to give advice on public‐health priorities, including modification of health behaviour to minimise risk of disease and to promote a healthy lifestyle in pharmacy users (DOH 2005;Public Health England 2017). Smoking cessation was one of the first behaviour‐change roles to be delivered in community pharmacies (Anderson 2007), and now others, such as promotion of general healthy lifestyle behaviours, increasing uptake of screening and giving sexual health advice, have been added (Blouin 2017; NICE 2018; RSPH 2016). To address the needs of this changing role and to maintain high professional standards, international guidance for good pharmacy practice has been published which outlines health promotion as one of six components that contribute to the health improvement of the individuals who access community pharmacy services (WHO 2011).
The evidence base that underpins these wider health‐promotion responsibilities has not yet been collated to determine effective methods of changing professional practice, or evaluation of the health gains that could result from these changes. Research evidence suggests that whilst pharmacy workers and their users hold positive attitudes to pharmacist involvement in public‐health activities, pharmacist confidence in delivering the services is currently low, and additional training needs are perceived (Eades 2011; Lindsey 2017; Weir 2019).
Systematic reviews examining behaviour‐change interventions delivered in community pharmacies have begun to emerge by clinical topic (Brown 2016; Garcia‐Cardenas 2013; Sabater 2016; Soprovich 2019); but do not provide a comprehensive overview of the role of community pharmacy in health promotion. In addition, some reviews have included small numbers of poor quality studies (Gordon 2011; Sinclair 2004; Watson 2006), which limits conclusions regarding the effectiveness of these services (RSPH 2016). Thus a broad overview of studies of health‐promotion interventions in community pharmacies is needed to inform current pharmacy practice and to identify areas for future research.
Description of the intervention
The World Health Organization (WHO) defines health promotion as "the process of enabling people to increase control over, and to improve, their health". The idea of health promotion has expanded beyond a focus on individual behaviour towards a wide range of social and environmental interventions (WHO 2009). Interventions that target a specific aspect of lifestyle ‐ such as smoking ‐ or that address wider aspects of clinical management ‐ such as obesity or type 2 diabetes mellitus ‐ therefore fall within this definition.
Interventions to support these broad health‐promotion and behaviour‐change tasks may be directed at pharmacy workers, pharmacy users (who may or may not be patients), or at both groups. The types of intervention vary from educational programmes (Sarayani 2012), to specific training that is targeted at behaviour change, such as motivational interviewing (Brackett 2015). Other interventions target management of medical conditions, for example blood pressure monitoring (Fikri‐Benbrahim 2012), or managing asthma (Armour 2007). These types of interventions go beyond the traditional remit of community pharmacy workers, which has conventionally focused on the preparation, dispensing and management of medicines.
Previous Cochrane Reviews have examined non‐dispensing services in pharmacies (De Barra 2018; Nkansah 2010; Pande 2013), however, these have still had a strong focus on medications, including medication reviews or stopping medications, and did not focus solely on community pharmacy. To avoid overlap with this previous work, we have excluded any purely medication‐related interventions in this review, including those focused primarily at promoting medication adherence.
How the intervention might work
The way in which health‐promotion and behaviour‐change interventions work within the community pharmacy setting is likely to be dependent on the theoretical basis for the intervention (Michie 2010), and the behaviour‐change techniques used (Michie 2008). For example, interventions may aim to increase self‐efficacy (perceived confidence) in performing a behaviour that promotes health, or examine ways of overcoming barriers to performing that behaviour. The behavioural theory underpinning interventions and the mechanisms by which community pharmacy interventions might work have not previously been studied in detail. However, an understanding of the mechanisms by which health‐behaviour change is achieved in successful community pharmacy interventions, and the behaviour‐change theories used, is important for designing more effective interventions, both for existing clinical areas and to support the expansion of the future role of the community pharmacy.
This review sought to identify which underpinning theories and theoretical constructs are most effective in achieving health‐behaviour change when interventions are delivered in a community pharmacy setting. We aimed to identify generic approaches that could be used to inform development of any health‐promotion intervention delivered in a community pharmacy setting.
Many interventions involve training community pharmacists or pharmacy workers, however, evidence is sparse regarding the best methods of training to achieve health‐behaviour change. Even if pharmacists and pharmacy workers can be trained effectively and can deliver the intervention with fidelity, there still remains the question of whether pharmacy users follow the advice given and whether this results in meaningful improvements in health and well‐being. There are no previous comprehensive reviews of the effectiveness of community pharmacy workers as agents for health‐behaviour change (Anderson 2003). It is important, therefore, to consider the complete pathway from intervention to effects on health outcomes. Hence we examined study outcomes related to both the professional behaviour of pharmacy workers and to health‐related behaviour and outcomes in their users.
Why it is important to do this review
This review is important because community pharmacists and their teams are increasingly taking on health‐promotion activities as part of their rapidly expanding role in the delivery of health care and public‐health services (Blouin 2017; Mossialos 2013). Much of this change has been driven by need for cost efficiencies in the health system, and the need to reduce health inequalities (Crombie 2005), which is predicted to continue in many countries.
Objectives
To assess the effectiveness and safety of health‐promotion interventions to change community pharmacy workers' professional practice and improve outcomes for users of community pharmacies.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised trials (RTs) and cluster‐randomised trials (cluster‐RTs) (EPOC 2017a). Cluster‐RTs were only eligible if there were at least two intervention sites and two control sites. Publication status of study (full text, unpublished data) was not a bar to inclusion, unless there was insufficient data, for example, regarding intervention content. For this reason, we excluded abstracts that were not supported with further information (Chandler 2013).
Types of participants
Participants in the review were pharmacy workers and users of community pharmacies (defined as regulated pharmacy outlets outside secondary healthcare), under the direction of a pharmacist. We included interventions directed at any worker within the community pharmacy, including pharmacists and other workers such as pharmacy technicians and assistants. We excluded studies where participants were seen in a hospital or non‐community‐based pharmacy, e.g. an outpatient clinic. We included studies that had mixed settings only if the majority of participants took part in the community pharmacy setting, or if the community pharmacy subset was analysed independently. Similarly, where the intervention was multidisciplinary we included studies only if the majority of the intervention was delivered in community pharmacy, or the community pharmacy aspect of the intervention was evaluated separately, for example, change in community pharmacists' behaviour.
Types of interventions
We included any health‐promotion intervention targeted at, or delivered by, community pharmacy workers (including pharmacists, counter assistants etc.) which aimed to improve health behaviours of individuals attending the community pharmacy.
We excluded studies where the intervention was solely focused on medication. This included those interventions that were concerned only with prescription of medication, medication review, or those that focused on promoting adherence to medication. We included interventions where medication management was a single component of an intervention and other behavioural aspects (e.g. diet or exercise) were also targeted.
We excluded studies in which interventions did not involve active interaction between pharmacy workers and their users (e.g. displays of leaflets/posters on lifestyle in the pharmacy).
We have described interventions in terms of:
mode of delivery (e.g. video/DVD, one‐to‐one or group‐based or web‐based sessions);
agent delivering the intervention (e.g. pharmacist, pharmacy assistant);
setting (e.g. on site in pharmacy); duration (including length and number of sessions and period over which the intervention was delivered);
content (e.g. smoking cessation, lifestyle recommendations, condition management).
We also documented the intervention fidelity (i.e. the degree to which the intervention was delivered as intended), where this was assessed. Where necessary, we contacted authors of studies to obtain additional details of interventions and training of pharmacy workers.
Types of outcome measures
We present the results that were assessed closest to the end of the intervention but only after the intervention was finished.
Primary outcomes
To assess the effects of community pharmacy interventions on health promotion delivered by pharmacy workers, we looked at three categories of outcomes:
-
Professional practice outcomes were primarily behavioural and included:
uptake of intervention by pharmacy worker, adherence to the intervention (e.g. number of pharmacy users asked about smoking status);
pharmacy worker behaviour (e.g. correct demonstration of inhaler technique).
-
Pharmacy user outcomes included assessment of:
health‐related behaviour (e.g. smoking, exercise, inhaler technique);
-
health status including:
intermediate clinical outcomes (e.g. cholesterol, glycated haemoglobin);
event‐based clinical outcomes (e.g. stroke, myocardial infarction);
psychological well‐being (e.g. anxiety and depression); and
quality of life.
Adverse events included any effect defined as adverse by the included studies, either at the professional or user level.
In line with Cochrane Effective Practice and Organisation of Care Group (EPOC) recommendations (EPOC 2017a), we included only those studies where at least one outcome was assessed using an objective or validated tool, such as a validated questionnaire. For assessment of pharmacy workers, we considered simulated patients (mystery shoppers) to be an objective measurement tool, and trials using them to be eligible for inclusion (Watson 2006; Xu 2012).
Secondary outcomes
We included costs, as reported by the studies, as a secondary outcome. This included direct and indirect healthcare costs, including scheduled and unscheduled visits to other healthcare providers (healthcare utilisation) and cost‐effectiveness.
Search methods for identification of studies
Electronic searches
The EPOC Cochrane Information Specialist wrote the search strategies in consultation with the review authors. We searched the Cochrane Database of Systematic Reviews and the Database of Abstracts of Reviews of Effects (DARE) for related systematic reviews, and searched the following databases for primary studies on 6 February 2018:
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 1) in the Cochrane Library;
Health Technology Assessment Database (DARE; 2016; Issue 4) in the Cochrane Library;
NHS Economic Evaluation Database (NHSEED; 2015, Issue 2) in the Cochrane Library;
MEDLINE Ovid (including Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Versions) (1946 to 31 January 2018);
EMBASE Ovid (1974 to 5 February 2018);
PsycINFO Ovid (1967 to January Week 5 2018).
The finalised search strategies are provided in Appendix 1. We tested the MEDLINE strategy by screening selected citations for relevance, and validated it using a selection of exemplar papers on the topic of this review. We modified the MEDLINE strategy for other databases using appropriate syntax and vocabulary for those databases. We applied no limits regarding date or language.
Searching other resources
We searched the grey literature to identify studies that were not indexed in the databases listed above. We searched the following sources on 6 February 2018:
Open Grey (www.opengrey.eu);
ProQuest Dissertations & Theses Global (including COS Conference Papers Index);
ProQuest Dissertations & Theses: UK & Ireland.
Trial Registries
We searched the following trial registries on 6 February 2018:
International Clinical Trials Registry Platform (ICTRP), Word Health Organization (WHO) (www.who.int/ictrp/en);
ClinicalTrials.gov, US National Institutes of Health (NIH) (clinicaltrials.gov).
We also:
reviewed reference lists of all included studies, relevant systematic reviews, primary studies and other publications;
contacted authors of relevant studies or reviews to clarify reported information and to seek unpublished results and data;
conducted cited reference searches for all included studies in citations indexes.
Data collection and analysis
Selection of studies
We imported results of each search into a reference management software package (Endnote 2013). One review author removed duplicates and screened titles and abstracts for obvious irrelevance to the review (e.g. not an intervention study). A second review author completed sequential 10% checks of titles and abstracts until we achieved an inter‐rater reliability of 0.75 or greater (excellent agreement) (Orwin 1994). The emphasis was on over‐inclusion at this stage. We then retrieved potentially relevant papers and two review authors independently screened all of these against the inclusion criteria. We resolved any disagreements through discussion, referring where necessary to a third review author for arbitration. Where such arbitration was necessary and a study was excluded, we added it to the Characteristics of excluded studies table, and gave reasons for its exclusion. We collated multiple reports for the same study, so that each study ‐ rather than each report ‐ was the unit of interest.
We have documented the full screening process in a PRISMA flow‐chart Figure 1.
1.
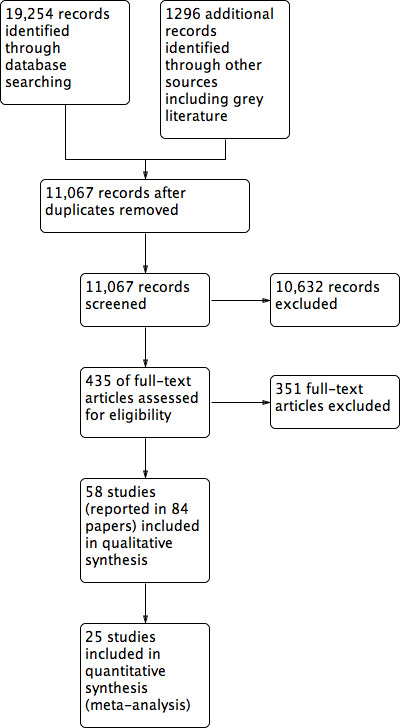
Study flow diagram.
Data extraction and management
We extracted data from eligible studies using a tailored extraction form based on the generic EPOC data collection checklist (EPOC 2017b), and included the following data.
Study details: author; year; research question; country where research was carried out; inclusion and exclusion criteria; study design (randomised trial (RT), cluster randomised trial (cluster‐RT); recruitment method (e.g. self‐referral, advertisement); description of usual care.
Intervention details: intervention target (pharmacy workers, or pharmacy users, or both); behavioural target (smoking, diet, exercise, etc.); health condition targeted; intervention description (mode of delivery; theoretical basis as reported by study authors; and theoretical constructs targeted, as coded by mapping interventions to the Theoretical Domains Framework (TDF) (Cane 2012).
Pharmacy worker details: number; age; socioeconomic status; ethnicity; gender; time since qualification.
Pharmacy user details: number; age; socioeconomic status; ethnicity; gender; time since diagnosis (where applicable).
Quality criteria (in line with EPOC recommendations) (EPOC 2017c).
Results of primary and secondary outcomes.
Two review authors independently extracted all key information (inclusion criteria, e.g. design, participants, interventions and outcomes, quality criteria and results) from each included paper. As mentioned previously, we resolved any errors or disagreements through discussion, with recourse to a third review author for arbitration (RW), and discussion among the full author group where necessary. EK entered data into Review Manager 5.3 software (RevMan 2014), while a second review author checked the data entry (LS, CR).
Assessment of risk of bias in included studies
We assessed the risk of bias of the studies using Cochrane's 'Risk of bias' assessment tool (Higgins 2011a), and following the EPOC 'suggested risk of bias criteria for EPOC reviews’ (EPOC 2017c). There are nine standard criteria for all RTs:
Was the allocation sequence adequately generated?
Was the allocation adequately concealed?
Were baseline outcome measurements similar?
Were baseline characteristics similar?
Was the study adequately protected against contamination?
Were incomplete outcome data adequately addressed?
Was knowledge of the allocated interventions adequately prevented during the study?
Was the study free from selective outcome reporting?
Was the study free from other risks of bias?
We scored each study as being at low, high or unclear (if not specified in the paper) risk of bias. For some studies it may not have been possible to blind participants to the intervention, e.g. an exercise intervention, but we still recorded this aspect in the quality assessment. Two review authors assessed each study's risk of bias, compared results, and resolved discrepancies by discussion and by recourse to a third review author when necessary. We measured inter‐rater agreement using Cohen’s kappa coefficient (Uebersax 1987). We have presented results in both a ‘Risk of bias’ table Figure 2, and graphically Figure 3. The authors of the current review were also authors of one included study (Madurasinghe 2017). AT was not an author of the study, and, therefore, screened it for inclusion, and extracted and checked all its data.
2.
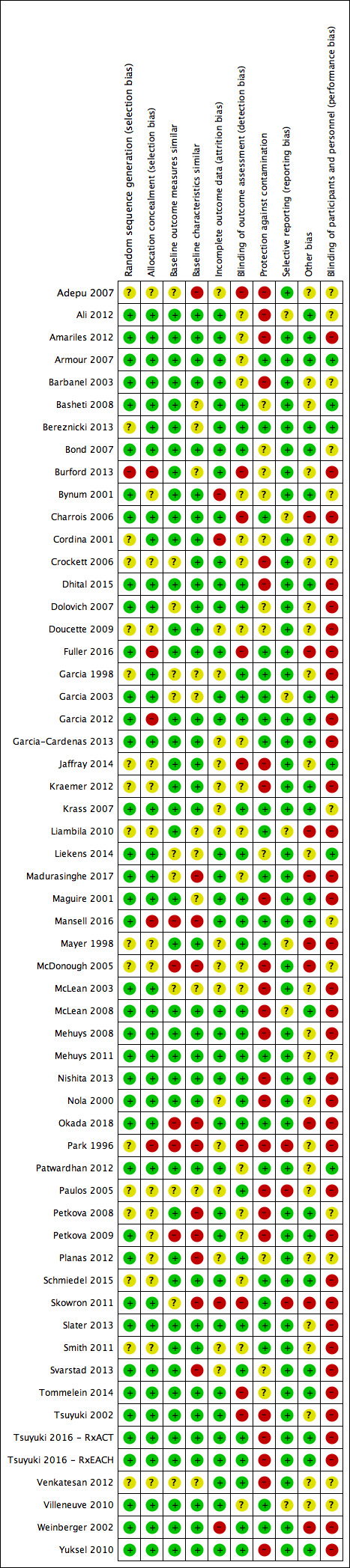
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
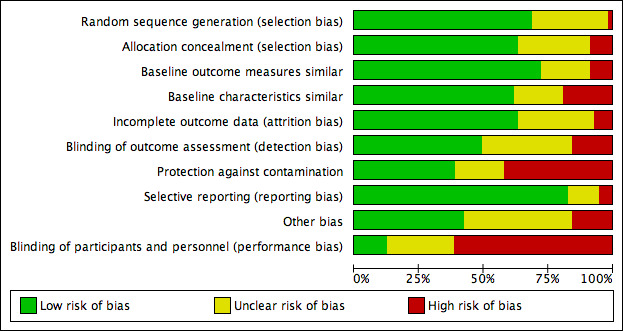
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
For continuous data we estimated treatment effect sizes as standardised mean differences (SMDs) for each outcome, or weighted mean differences where studies had a common outcome measure. We treated the available data as continuous unless there was a defensible cut‐point available, in which case we considered the data to be dichotomous. We gave preference to final value scores over change scores where both were presented, although analysis with both results is presented where there were sufficient studies for both analyses.
Unit of analysis issues
Where cluster‐RTs were included, we considered whether any unit of analysis errors had been made in the original analysis. Where we identified such errors, we performed a re‐analysis using information on the size or number of clusters and the value of the intra‐cluster correlation coefficient (ICC) where the information was available, or we excluded the study from analysis if necessary.
Dealing with missing data
When a study was missing data, we contacted the study authors and requested the additional data. After this, if data were still missing, we calculated standard deviations for changes, where possible. When there was insufficient information available to calculate the standard deviations, we imputed missing standard deviations for changes from baseline using other available information (e.g. correlation coefficients) (Higgins 2011b). If it was not possible to impute data, we did not include the study in the analysis and we noted its absence.
For dichotomous data, where possible we derived missing treatment estimates and standard errors from the number of participants included or randomised, and from the numbers of individuals with and without the outcomes of interest. We used confidence intervals (CI) to derive missing standard error estimates.
Assessment of heterogeneity
Given the diverse nature of behavioural interventions, we anticipated some heterogeneity between studies. We assessed this both qualitatively (e.g. examining intervention characteristics, study populations, context, etc.) and quantitatively. We inspected forest plots visually for poorly overlapping CIs for the results of individual studies. We also discussed possible reasons for heterogeneity and considered this in interpretation of results.
We assessed the extent of statistical heterogeneity formally using the Cochran Q statistic and corresponding Chi² and I² statistics. This latter statistic describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) (Higgins 2003); the significance threshold is set at P < 0.05.
Assessment of reporting biases
To test for publication bias we drew funnel plots, if more than 10 studies were identified, and where standard errors and a unitary measure of effect were available (Higgins 2011a). For any given outcome we inspected funnel plots visually for asymmetry.
Data synthesis
We have provided details of all included studies in a Characteristics of included studies table, irrespective of whether the measured outcome data were reported in a useable way.
For the main analysis, we split outcomes into those that examined the effect on pharmacy workers and those that examined the effect on pharmacy users.
Firstly, we considered the suitability of studies for meta‐analysis. If there was considerable evidence of heterogeneity, such that meta‐analysis might be misleading, we reported a narrative synthesis of studies, and presented descriptive and summary data of interventions.
Where meta‐analysis was deemed appropriate, given the likely heterogeneity in terms of intervention, setting, and population, we adopted the more conservative random‐effects model. If an outcome was measured at different times in the same study, we selected the first value after the end of the intervention period. When there were related outcomes from the same study, we used the outcome most consistent across studies (e.g. SF‐36 above condition‐specific measures) or the most clinically rigorous measure (for asthma this was: severity or asthma control as measured by (for example) the asthma control questionnaire, followed by forced expiratory volume in one second, followed by peak expiratory flow; for diabetes this was: HbA1c followed by plasma blood glucose; for hypertension this was: systolic blood pressure followed by diastolic blood pressure; for lipids this was: low density lipoproteins followed by cholesterol). In this way we pooled only a single effect size for each study. We used Review Manager 5.3 software to collate data and perform calculations.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses in RevMan 5 for different patient behaviours, clinical conditions, and generic versus specific quality of life measures, where there were sufficient studies for this to be meaningful.
We also planned to consider whether there were different effects from studies conducted within low‐ and middle‐income countries (LMICs) compared with high‐income countries (HMICs) as classified by the (World Bank Group 2009). We also planned to examine whether people from particular ethnic groups and those at extremes of adverse health behaviour (e.g. heavy smokers) were more likely to respond to pharmacy‐based interventions. If there were sufficient studies we also planned to explore whether theory‐based interventions were more effective than those not based on theory, and whether a financial incentive influences effectiveness. Unfortunately there were insufficient studies for these planned sub‐group analyses to be conducted.
Meta regression
We planned to perform a meta‐regression where there was an adequate amount of data, using Stata 12.1. This was to consider which features of interventions were more likely to be successful, and to examine effects of intervention delivery (e.g. single brief consultation, several brief consultations plus follow‐up telephone contact etc.).
Sensitivity analysis
We conducted sensitivity analyses by excluding studies that we assessed as being at high risk of bias. This involved undertaking the meta‐analysis twice, with and without the studies in question.
Summary of findings
We prepared Table 1 for health‐promotion interventions delivered within the community pharmacy compared to no intervention or usual care. We used the Grading of Recommendations and Assessment Development and Evaluation (GRADE) approach to evaluate our confidence in the findings (GRADE 2013). Table 1 includes the seven most important outcomes for both community pharmacy workers and community pharmacy users. LS and RW assessed all outcomes for importance in line with EPOC recommendations (EPOC 2017d), and were in agreement. They assessed the certainty of the evidence independently, using standard procedures and resolving discrepancies by consultation with ST. We selected pharmacy workers' behaviour and pharmacy users' health‐related behaviours, intermediate clinical outcomes (e.g. cholesterol, glycated haemoglobin), event‐based clinical outcomes, quality of life, adverse events and costs for inclusion in Table 1.
Results
Description of studies
Studies are described in the Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables.
Results of the search
The search is summarised in Figure 1 and yielded 20,550 citations, including 1296 from the grey literature. Following removal of duplicates, we screened 11,067 studies and assessed 435 full text papers. We excluded 352 papers, as they did not meet inclusion criteria. We did not categorise papers by individual reasons for exclusion, as many papers had multiple reasons for exclusion, and any categorisation would have misrepresented the situation. We included a total of 57 studies, which were reported in 83 papers. We identified five further studies as ongoing (Davis 2016; Ekers 2017; Michiels 2017; Porteous 2013; Spadaro 2010).
Included studies
Location
Although the included studies were conducted worldwide, none were undertaken in low‐income countries (as defined by the World Bank) (World Bank Group 2009). Three studies were conducted in low‐middle income countries, including India (Adepu 2007; Venkatesan 2012), and Kenya (Liambila 2010); five were conducted in high‐middle income countries, specifically Peru (Garcia 1998; Garcia 2003; Garcia 2012), and Bulgaria (Petkova 2008; Petkova 2009). The remaining 49 studies were conducted in high‐income countries, including Australia (9 studies), Belgium (4 studies), Germany (1 study), Malta (1 study), Poland (1 study), Spain (2 studies), Chile (1 study), Japan (1 study), the UK (7 studies), the USA (11 studies), and Canada (11 studies). Twenty nine studies were conducted in urban settings; thirteen studies did not report the type of setting i.e. rural or urban. It was not possible to determine whether interventions reached lower‐socioeconomic status populations, as this was poorly described.
Participants
Overall, the studies involved a total of 16,220 participants. Twenty‐seven studies were cluster‐RTs, while all the others were simple randomised trials. We excluded four of the cluster‐RTs from entry into meta‐analysis, as their analysis did not adequately account for clustering effects (Krass 2007; Mehuys 2011; Skowron 2011; Smith 2011). The majority of studies compared intervention to usual treatment, although eight studies compared the intervention to no treatment. These eight studies all had interventions which primarily targeted the community pharmacy worker (Dolovich 2007; Garcia 1998; Garcia 2003; Garcia 2012; Liambila 2010; Liekens 2014; Mayer 1998; Patwardhan 2012).
Conditions
Most studies (47 of 57) were directed towards secondary prevention of conditions, including allergic rhinitis (Smith 2011), arthritis (Petkova 2009), asthma (13 studies), chronic obstructive pulmonary disease (Tommelein 2014), cardiovascular disease (Bond 2007), depression (Crockett 2006; Liekens 2014), type 2 diabetes (10 studies), dyslipidaemia (Nola 2000; Paulos 2005; Tsuyuki 2016 ‐ RxACT; Villeneuve 2010), hypertension (Okada 2018; Park 1996; Skowron 2011; Svarstad 2013), low back pain (Slater 2013), osteoporosis (McDonough 2005); skin cancer (Mayer 1998), and insomnia (Fuller 2016). In approximately half of these conditions the intervention was described as being focused on the pharmacy user, whilst the other half mentioned some degree of training for the community pharmacy workers.
Six studies focused specifically on prevention of either diabetes (Schmiedel 2015), osteoporosis (Yuksel 2010), or cardiovascular risk factors (Amariles 2012; McLean 2008; Tsuyuki 2002; Tsuyuki 2016 ‐ RxEACH). A further nine studies targeted lifestyle behaviours including smoking (Burford 2013; Maguire 2001; Patwardhan 2012; Madurasinghe 2017), illicit drug use (Jaffray 2014), family planning (Liambila 2010), and sexually transmitted infection prevention (Garcia 1998; Garcia 2003; Garcia 2012). All of these lifestyle interventions, with the exception of Burford 2013, targeted behaviour change through intervening at the pharmacy worker level, for example by improving knowledge or skills.
Interventions
Most interventions were educational or incorporated skills training, for example asthma interventions typically trained pharmacy users in inhaler technique. Interventions directed at the community pharmacy workers typically consisted of group workshops supported by written materials for self‐directed learning. Training ranged from a single session to sessions held over several weeks (Mayer 1998). In a number of instances the training involved interactive exercises, such as role‐play, which are important for the development of skills (Bond 2007; Garcia 1998; Garcia‐Cardenas 2013; Krass 2007; Liekens 2014; Madurasinghe 2017; Petkova 2009; Svarstad 2013). Typically training was face to face, although other methods were used occasionally, for example video‐conferencing (Crockett 2006), videotape‐based training (Mayer 1998), or online training (Tsuyuki 2016 ‐ RxEACH). Face‐to‐face delivery was also most common for user‐directed interventions. Usually, this involved direct face‐to‐face communication with the community pharmacy worker.
The duration of follow‐up was often unclear. Several studies reported assessment at what appeared to be a long‐term follow‐up (e.g. 12 months), however, this was often the length of the delivery period of the intervention. For this reason, we present the first set of results after the end of the intervention.
Funding
The majority of studies (34 of 57) were funded by grants from national funding bodies, charities, or institutional funds. Five studies were funded by industry and a further five by a combination of public and industry funding. Eight studies did not report their funding source.
Theory in interventions
Only five studies reported whether the intervention was based on a specific theoretical approach. Svarstad 2013 based intervention development on Svarstad and Bultman's Health Collaboration Model and Roger's Diffusion of Innovation Model (Rogers 2003; Svarsted 2000). Jaffray 2014 and Nishita 2013 trained pharmacy workers in motivational interviewing. Although motivational interviewing is not underpinned by any specific theory, it is a recognised approach to behaviour change (Miller 2012). Smith 2011 reported a 'goal setting self‐management study' which, although not specified, appeared to draw on Social Cognitive Theory (Bandura 1986). A summary of how many interventions addressed each theoretical domain, as coded using the theoretical domains framework (Cane 2012), is reported with the Characteristics of included studies. Most commonly community pharmacy workers were trained to increase knowledge and skills, and frequently the intervention added some form of object to the environment, which could be as simple as having information leaflets to distribute. Pharmacy users were typically provided with information, and, particularly in interventions for asthma, were taught skills such as inhaler technique. Behavioural regulation approaches, such as self‐monitoring, were used in 19 interventions. Of note, few interventions addressed the theoretical domains of beliefs about capabilities and consequences, or intentions and emotions.
Excluded studies
In total, we excluded 352 studies. Studies where consensus was not immediate were discussed amongst the team and are presented in the Characteristics of excluded studies table. We excluded studies for four reasons, namely:
not being conducted in a community pharmacy setting;
inappropriate design;
an intervention that did not fit our inclusion criteria;
no validated or appropriate outcome.
Often, there were multiple reasons for the exclusion of a study, however, in the table we report only the first reason of the four given above to optimise efficiency in screening. When we excluded studies on the basis of intervention, it was usually because they targeted medication adherence without a wider behavioural focus. There was some debate as to whether disease management interventions ‐ particularly those related to cardiovascular risk (i.e. hypertension, dyslipidaemia) ‐ should be included or excluded, as many of these were medication focused but also mentioned lifestyle‐behaviour change. The extent to which lifestyle advice drew on behaviour‐change principles was difficult to determine fully from descriptions; we included these studies, but evaluated them with this point in mind.
Risk of bias in included studies
We assessed risk of bias, and provide a summary table and graph of risk of bias in Figure 2 and Figure 3, respectively. The most common sources of bias were lack of protection against contamination, mainly in individually randomised studies, and inadequate blinding of pharmacy users and pharmacy workers.
Allocation
We included 27 cluster‐RTs, which used the community pharmacy as the unit of randomisation. The remaining 30 studies used the pharmacy user as the unit of randomisation. Most individual‐level RT studies conducted randomisation in a robust way and conserved allocation concealment. In cluster‐RTs, allocation concealment at the pharmacy level was frequently conserved, but for individuals it was typically more complex (Eldridge 2012), and frequently was not clear.
Blinding
Due to the nature of the interventions, it was often not possible to blind providers (pharmacy workers) and recipients (pharmacy users). This is a common difficulty for interventions of a behavioural nature (Friedberg 2010), although risk can be minimised by the use of independent blinded assessors, which was done in some of the more robust studies (e.g. Amariles 2012; Bereznicki 2013; Liekens 2014; Svarstad 2013). Additionally, the use of objective outcomes ‐ for example those used for intermediate clinical outcomes, such as HbA1c, or blood pressure ‐ can help to minimise detection bias.
Incomplete outcome data
Some level of attrition was common in many studies, most commonly amongst pharmacy users, but also at the pharmacy level in some cases. While a number of studies reported how missing data were managed, this was unclear or not described in approximately half the studies. Therefore, attrition bias is a potential threat to the generalisability of the findings of this review.
Selective reporting
Examination of funnel plots for the main outcomes suggested possible publication bias for pharmacy users' intermediate clinical outcomes and quality of life (Figure 4; Figure 5; Figure 6).
4.
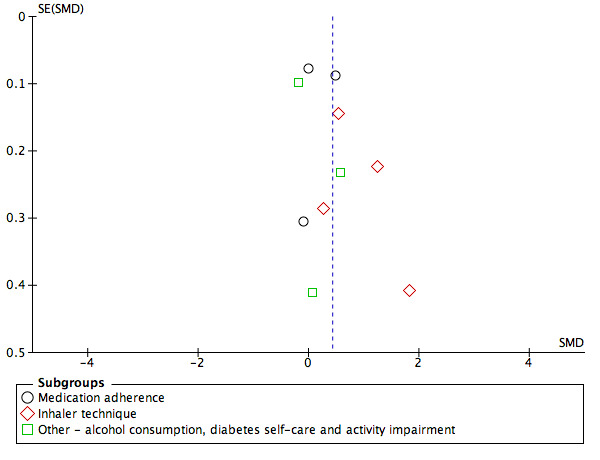
Funnel plot of comparison: 1 Usual treatment versus Health‐promotion intervention, outcome: 1.1 Pharmacy user health‐related behaviour.
5.
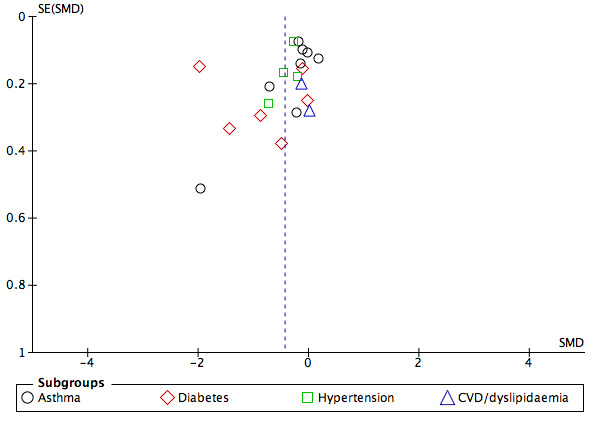
Funnel plot of comparison: 1. Health‐promotion intervention versus Usual treatment outcome: Analysis 1.2 Pharmacy user intermediate clinical outcomes (final value scores)
6.
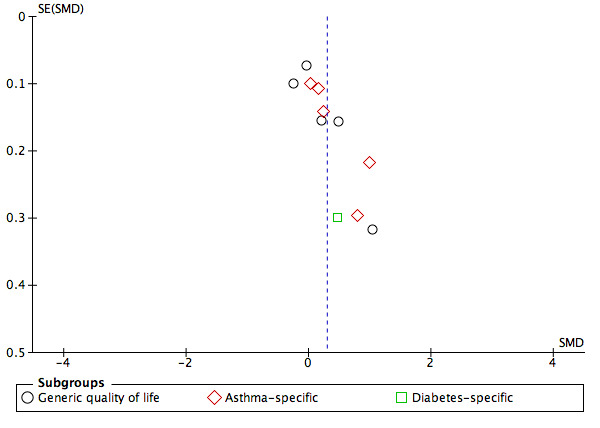
Funnel plot of comparison: 1. Usual treatment versus Health‐promotion intervention outcome: Analysis 1.4 Pharmacy user quality of life
Other potential sources of bias
An important potential bias in the included studies was the possibility of contamination between intervention and control groups (see Figure 2). We judged this to be at high risk where randomisation occurred at the level of pharmacy user within the pharmacy, because it can be difficult for a pharmacy worker not to implement skills that have been learned, which risks contamination of the control participants.
Effects of interventions
See: Table 1
Table 1 presents an overview of the effectiveness of interventions; we have used GRADE to indicate the certainty of the evidence. For all outcomes GRADE scores were downgraded to moderate, primarily due to the high heterogeneity present within the studies and evidence of potential publication bias.
Primary outcomes
1. Professional practice outcomes
Fourteen of the 57 studies reported the proportion of pharmacies or pharmacy workers participating in the study. Some studies were conducted in just one or two pharmacies, and others selected pharmacies with specific characteristics. Those studies that reported the proportion of pharmacy workers who consented to take part in the study compared to those invited to participate, reported relatively low figures, for example, 26% in the Basheti 2008 study, and 33% in the Armour 2007 study.
Nine studies assessed pharmacy worker outcomes and compared these to no intervention controls. All nine studies assessed the outcome of pharmacy worker behaviour. Eight of the studies were set in urban pharmacies. Seven studies assessed behaviour using a simulated patient model (Dolovich 2007; Garcia 1998; Garcia 2003; Garcia 2012; Liambila 2010; Liekens 2014; Mayer 1998). The behaviours measured by simulated patients ranged from communication skills ‐ using validated measures such as the Roter Interaction Analysis (Dolovich 2007; Liekens 2014) ‐ to noting behaviours such as recommending use of condoms (Garcia 2012). Patwardhan 2012 used an objective measure of behaviour, namely referrals to a smoking quit line following smoking cessation training. The Basheti 2008 study assessed maintenance of pharmacy workers' ability to demonstrate asthma inhaler technique two years post training. Two further studies, Jaffray 2014 and Nishita 2013, assessed behaviour as an assessment of fidelity to training but only in the intervention group, not in the control group, so these data were not included in our analysis.
Six of the studies reported improvement in community pharmacy worker behaviour (Basheti 2008; Dolovich 2007; Garcia 2003; Garcia 2012; Mayer 1998; Patwardhan 2012), while one showed no benefits (Liambila 2010), and two had mixed results (Garcia 1998; Liekens 2014). The Dolovich 2007 study indicated a positive effect on both verbal and non verbal communication skills. The Liekens 2014 study showed improved pharmacy worker counselling for depression, and the intervention used in Mayer 1998 improved counselling to avoid ultra‐violet radiation (i.e. sunlight). Sexual health counselling was improved in the Garcia 2003 and Garcia 2012 studies at six‐ and 12‐month follow‐up, respectively. The Patwardhan 2012 study showed significant improvement in demonstration of inhaler behaviour post‐intervention, and the Basheti 2008 study showed maintenance of pharmacy workers' ability to demonstrate correct asthma inhaler technique two years after training. In contrast, interventions in the Liambila 2010 and Garcia 1998 studies produced mixed results for sexual health management.
Due to the heterogeneity of the behaviours measured and the methods used in the studies, we did not consider meta‐analysis to be appropriate. We downgraded the certainty of evidence one level, to moderate, because of the high heterogeneity of studies (GRADE 2013).
2. Pharmacy user outcomes
2.1 Pharmacy user health‐related behaviour e.g. smoking, exercise, inhaler technique
Health‐related behaviour of pharmacy users was measured in 28 studies (summarised in Table 2). Twelve studies measured medication adherence (please note that this was not the primary target of the intervention, or the trial would have been excluded). Adherence was measured through prescription data, or validated adherence measures such as the medication adherence rating scale (MARS) (Thompson 2000). Seven studies measured inhaler technique within the asthma population specifically. Lifestyle behaviours that were assessed included smoking (Burford 2013; Maguire 2001; Madurasinghe 2017), alcohol consumption (Dhital 2015), diabetes self‐care (Doucette 2009; Mansell 2016), physical activity (Okada 2018; Schmiedel 2015), and activity impairment (Slater 2013).
1. Studies included and excluded from meta‐analysis of behavioural outcome.
| Adherence | Inhaler technique | Other behaviours | |
| Studies included in the meta‐analysis and outcome measure used |
|
|
|
| Studies excluded from the meta‐analysis with reasons for exclusion |
|
|
|
Ten studies provided suitable data for meta‐analysis of overall health‐related behaviour of pharmacy users (Analysis 1.1). Overall meta‐analysis of health‐related behaviour of community pharmacy users suggested a probable slight improvement relative to control (SMD 0.43, 95% CI 0.14 to 0.72; I2 = 89%; 10 trials, 2138 participants; moderate‐certainty evidence). Inhaler technique was probably improved (SMD 0.92, 95% CI 0.35 to 1.48; I2 = 82%; 4 trials; 384 participants; moderate‐certainty evidence), but interventions showed little or no effect on medication adherence (SMD 0.17, 95% CI ‐0.23 to 0.57; I2 = 89%; 3 trials, 1245 participants; moderate‐certainty evidence), or other behaviours (SMD 0.14, 95% CI 0.‐41 to 0.68; I2 = 78%; 3 trials, 509 participants; moderate‐certainty evidence). We downgraded the certainty of evidence one level to moderate to take into account the high heterogeneity of studies as indicated by high I2values.
1.1. Analysis.
Comparison 1 Community pharmacy user health‐promotion intervention versus usual treatment, Outcome 1 Health‐related behaviour.
2.1.2 Intermediate clinical outcomes, e.g. cholesterol, HbA1c
Most studies (35 of 57) included some level of intermediate clinical outcome. The ones measured most consistently were in asthma (9 studies), diabetes (10 studies) and cardiovascular risk (hypertension (8 studies) and dyslipidaemia (4 studies)). We prioritised measures of glycaemic control (e.g. HbA1c) and asthma control (e.g. asthma control test) as the most clinically appropriate for diabetes and asthma, respectively. See Table 3 for an overview of studies. For blood pressure control, most studies presented results for both systolic and diastolic blood pressure. Since we could enter only one value per study into the meta‐analysis, we prioritised systolic blood pressure, as recommended by Strandberg 2003. Similarly, the dyslipidaemia studies reported a range of measures, including total cholesterol, high density lipoproteins (HDL) and low‐density lipoproteins (LDL). On the basis of the recognised clinical importance of these measures (Silverman 2016), we decided to include LDL values in the meta‐analysis.
2. Studies included and excluded from meta‐analysis of intermediate clinical outcomes.
| Asthma | Diabetes | CVD/hypertension | Other conditions | |
| Studies included in the meta‐analysis with outcome measure used |
|
|
||
| Studies excluded from the meta‐analysis, with reasons for exclusion |
|
|
|
|
Abbreviations
ACQ: Asthma Control Questionnaire; FEV: forced expiratory volume ; HbA1c: glycosylated haemoglobin; LDL: low‐density lipoprotein; PEF: peak expiratory flow; SBP: systolic blood pressure
Meta‐analyses for intermediate clinical outcomes, including sub‐group analysis by condition are shown in Analysis 1.2. Health‐promotion interventions probably improve intermediate clinical outcomes slightly in pharmacy users (SMD ‐0.43, 95% CI ‐0.65 to ‐0.21; I2 = 90%; 20 trials, 3971 participants; moderate‐certainty evidence). These findings were also replicated when mean change rather than final scores were used (SMD ‐0.27, 95% CI ‐0.38 to ‐0.17; I2 = 0%; 7 trials, 1413 participants; moderate‐certainty evidence; Analysis 1.3).
1.2. Analysis.
Comparison 1 Community pharmacy user health‐promotion intervention versus usual treatment, Outcome 2 Intermediate clinical outcomes (final value scores).
1.3. Analysis.
Comparison 1 Community pharmacy user health‐promotion intervention versus usual treatment, Outcome 3 Intermediate clinical outcome (mean change scores).
Sub‐group analyses separated by condition suggested that interventions probably improve blood pressure in hypertension (SMD ‐0.34, 95% CI ‐0.49 to ‐0.18; I2 = 18%; 4 trials; 1050 participants; moderate‐certainty evidence; Analysis 1.2). Interventions probably improve blood glucose levels in diabetes (SMD ‐0.81, 95% CI ‐1.60 to ‐0.02; I2 = 96%; 6 trials; 651 participants; moderate‐certainty evidence; Analysis 1.2), though the high level of heterogeneity is important here. Interventions probably made little or no difference for asthma control (SMD ‐0.20, 95% CI ‐0.40 to ‐0.00; I2 = 75%; 8 trials, 2220 participants; moderate‐certainty evidence; Analysis 1.2) or for cardiovascular risk (SMD ‐0.08, 95% CI ‐0.40 to 0.24; I2 = 0%; 2 trials, 150 participants; moderate‐certainty evidence; Analysis 1.2). I2 values were higher for diabetes and asthma than hypertension or cardiovascular risk. This is likely to reflect the greater similarity in outcome measurement and intervention which was more medication focused in the hypertension and cardiovascular risk than asthma and diabetes.
We assessed the certainty of evidence as moderate after downgrading to take into account the high heterogeneity of studies and unclear distribution (possible publication bias) in funnel plots (GRADE 2013).
2.1.3 Event‐based clinical outcomes, e.g. stroke, myocardial infarction (MI)
No study measured event‐based clinical outcomes such as mortality, stroke or MI.
2.1.4 Psychological well‐being, e.g. anxiety and depression
Two studies; Crockett 2006 and Fuller 2016, measured psychological well‐being. Crockett 2006 employed an intervention targeted at depression and measured distress using the K10 (Kessler 2003), but did not report benefits. Fuller 2016 used the DASS‐21 to measure depression, anxiety, and stress (Norton 2007), and reported improvement in the intervention group compared to controls on some but not all scales. Given the different ways of combining and calculating psychological well‐being in each of these studies it was not considered appropriate to conduct meta‐analysis, however, overall it appears that psychological well‐being was neither improved nor negatively affected by such interventions.
2.1.5 Quality of life
Quality of life was measured in 28 studies as reported Table 4. Fourteen studies used a generic measure, most commonly the SF‐36 (Ware 1992), or EQ‐5D (Herdman 2011). One study used both a generic and an illness‐specific quality of life measure, and 14 studies used an illness‐specific measure only. We meta‐analysed 10 studies Analysis 1.4. As participants should only be included once in the meta‐analysis the diabetes specific quality of life scores from Ali 2012 were not included in the overall analysis. Overall, interventions probably improve quality of life slightly (SMD 0.29, 95% CI 0.08 to 0.50; I2 = 84%; 10 trials, 2687 participants; moderate‐certainty evidence). For quality of life measured by a generic tool the interventions may make little or no difference (SMD 0.21, 95% CI ‐0.10 to 0.52; I2 = 86%; 5 trials, 1567 participants; low‐certainty evidence). Importantly, however, several studies using a generic quality of life measure were not included in the meta‐analysis as data were reported on multiple sub‐scales (e.g. Bond 2007, Cordina 2001). For illness specific quality of life there is probably a slight improvement in favour of the intervention groups (SMD 0.38, 95% CI 0.11 to 0.66; I2 = 77%; 6 trials; 1166 participants; moderate‐certainty evidence).
3. Studies included and excluded from meta‐analysis of quality of life.
| Generic | Asthma specific | Diabetes specific | Other illness specific | |
| Studies included in the meta‐analysis and outcome measure used |
|
|||
| Studies excluded from the meta‐analysis and reasons fpr exclusion |
|
|
|
|
Abbreviations
BPI: Back Pain Index; EQ‐5D: Euroqol quality of life measure; MAP: Maudsley Addiction Profile; RQLQ: Rhinitis Quality of Life Questionnaire; SF‐36: Short Form‐36;
1.4. Analysis.
Comparison 1 Community pharmacy user health‐promotion intervention versus usual treatment, Outcome 4 Quality of life.
3. Adverse events
No adverse events were reported in any of the studies.
Secondary outcomes
Costs
Seven studies conducted a costs analysis (Armour 2007; Bond 2007; Burford 2013, Garcia 1998; McLean 2003; Svarstad 2013; Tsuyuki 2002). Five of these found the intervention to be cost‐effective relative to usual care, even when accounting for costs of intervention. We did not include these in a meta‐analysis because of the heterogeneity of methods used, but the consistency of finding is important. Further studies measured healthcare utilisation, most commonly general practitioner visits or hospitalisation (Ali 2012; Charrois 2006; Cordina 2001; Mehuys 2008; Petkova 2008; Petkova 2009; Tommelein 2014; Villeneuve 2010; Weinberger 2002), however, these presented mixed findings about whether the intervention group showed improvement relative to controls.
We were not able to conduct the planned sub‐group meta‐analysis of low‐ and middle‐income countries versus high‐income countries, as there was not sufficient homogeneity of outcomes or consistency in reporting across these groups. From visual assessment it does appear that, in general, studies from lower‐ to middle‐income countries had a higher risk of bias than those in high‐income countries. We could not analyse groups at the extremes of health behaviour or cultural/ethnic groups as these data were not reported adequately.
Sensitivity analyses
We repeated meta‐analysis for pharmacy user health‐related behaviour, intermediate clinical outcomes and quality of life while omitting outliers or any trial with a high risk of bias (e.g. Park 1996 ), however, this did not significantly change the outcomes of analyses.
Publication bias
Examination of funnel plots suggested that there was potential publication bias in the community pharmacy user outcomes of quality of life and intermediate clinical outcomes. For these outcomes, fewer smaller non‐significant studies were published than small positive studies, however, this effect was not seen for larger studies which contributed greater weight to meta‐analysis.
Discussion
Summary of main results
The findings of this review suggest that the community pharmacy is potentially a helpful setting in which to offer behavioural and health‐promotion interventions. There is evidence to suggest that such interventions probably slightly improve pharmacy user health‐related behaviour, intermediate clinical outcomes ‐ particularly for diabetes and hypertension ‐ and quality of life. Importantly, there is also some indication that these interventions may be cost effective. Although these findings were consistent across conditions and outcomes, it is important to note that there was considerable heterogeneity, as indicated by high I2 between studies in terms of intervention content and delivery, outcomes measured, and follow‐up periods, so we do not have complete certainty in our findings. Nonetheless, the evidence from this review agrees with the current drive in healthcare provision, both within the UK (NICE 2018), and internationally (Blouin 2017), to extend the role of the community pharmacy.
In addition to the probable slight effects on pharmacy user outcomes, there is evidence that the professional practice of pharmacy workers is probably influenced positively by the interventions. It is of note, however, that only a minority of studies evaluated pharmacy worker behaviour. Descriptions of the pharmacy worker interventions were often reported more briefly than those of the pharmacy user interventions. Many studies were poor at reporting use of theory. This has important implications for replicability of studies, and also the maximisation of benefits and refinement of interventions. We did not find any studies that measured harms from these interventions. Although adverse effects on pharmacy worker and pharmacy user health‐related behaviour, intermediate clinical, quality of life, and cost outcomes were not indicated, more subtle harms ‐ such as disruption to traditional pharmacological services due to a misdirection in the pharmacy workers' time ‐ is possible, and should be investigated in future studies.
Overall completeness and applicability of evidence
This review used stringent inclusion criteria to ensure that only studies of robust quality were included. This led to the exclusion of a significant number of studies from the review, particularly with respect to certain outcomes ‐ for example, lifestyle interventions such as smoking cessation. Smoking cessation interventions are a common health‐promotion service for community pharmacies to offer, but trials exploring the effectiveness of these interventions were included less frequently in this review than we anticipated because of our requirement for an objective, clinically verified outcome measure, for example, cotinine levels. The requirement for an objective outcome measure was less of a problem for intermediate clinical outcomes, which typically were objective measurements (e.g. HbA1c). The overall finding of effectiveness for this outcome has more weight for generalisability given the number of studies across different countries and different conditions that contributed to it. This must, however, be balanced against the considerable heterogeneity of trials that makes it difficult to conclude whether specific types, or content, of interventions are more beneficial. It is also important to consider that standardized mean difference (SMD) scores were used in Analysis 1.1 and Analysis 1.2 where the constructs of pharmacy user behaviour and pharmacy user intermediate clinical outcomes were measured across different conditions and different sub‐groups e.g. of behaviour. As a result findings may be driven by difference in one sub‐group (for example inhaler technique Analysis 1.1) but not in others. In addition it is not clear to what extent these findings persist after the intervention period since we used the first measurement after intervention completion.
There were insufficient data to conduct a number of our planned subgroup analysis, including whether outcomes varied according to cultural or ethnic group, and the level of health behaviour or theory used, and these remain important questions to answer. Additionally, it was uncommon for studies to report socio‐economic status of participants, so the extent to which these interventions reached into populations that are more difficult to access could not be ascertained.
Our original intention was to categorise interventions according to the behaviour‐change techniques used, but this was not possible due to the insufficiency of intervention descriptions. In addition, the way in which pharmacy workers were trained to deliver the intervention was poorly reported. We did conduct a higher level coding of interventions according to the theoretical domains framework (see Characteristics of included studies), which suggested that although studies did frequently involve knowledge and some basic behavioural regulation approaches, they commonly did not explore the more complex elements needed for behaviour change, such as addressing beliefs and emotions. The studies rarely reported being driven by a theoretical model; this is now recommended for development of complex interventions (Craig 2008), and should apply to the development of future community pharmacy health‐promotion interventions.
One issue that is important to consider is the extent to which the studies included in this review were representative of the general community pharmacy population. Studies rarely reported their organisational structure or issues, such as the culture within the practice, which would have aided interpretation of results. In addition, many studies conducted trials in a relatively low number of community pharmacies, which were often close to the research base. When a larger number of pharmacy sites were recruited, there was variable uptake, often with considerable dropout (e.g. 26% of pharmacists in Skowron 2011), although this was not always the case (e.g. 0% dropout in Slater 2013). The importance of this issue was highlighted in Garcia 2012, which reported that over a three‐year study period 29% of enrolled pharmacies closed, and the turnover of staff was remarkably high, with 81% of the staff base changing jobs during the study period. This is an important issue to consider when training staff, and suggests that, if interventions are to be supported in the long term, regular and ongoing pharmacy worker training events should be organised.
Certainty of evidence
GRADE assessment suggested that there was moderate certainty for the outcomes evaluated, and, therefore, that the research presented is a good indication of the probable effect. We downgraded the certainty from high to moderate because of the considerable heterogeneity in the studies, and the indication of a level of publication bias. Although our methodology excluded very poor quality designs by virtue of only including randomised trials, there was still a wide range in the quality of the studies included. The inclusion of cluster‐randomised trials in this review minimised selection bias and protected against contamination (Gums 2016), and so was a strength, as was the overall total number of participants included (16,315).
Where participants were individually randomised within pharmacies, this led to a high risk of contamination bias and was a weakness of such studies. Study quality was also threatened in a number of studies due to poor blinding regarding study group. This blinding is particularly difficult to achieve for behavioural interventions, where it is clear there is a change in practice, however, it can be managed by a choice of objective outcomes or by having outcome assessors who are blinded to study group.
Poor reporting of outcomes, or the use of non validated tools, occurred and led to a number of studies being excluded from the meta‐analysis (see Table 3, Table 2, Table 4), which suggests that the results might need to be treated with some caution. Additionally, poor descriptions of some interventions was a significant limitation of many of the studies. The difficulty of reporting behavioural interventions in sufficient detail is a well recognised problem that reporting frameworks, including TiDieR (Hoffmann 2014), and Wider (Albrecht 2013), have aimed to address. These frameworks were not readily reported for any of the trials included in this review, but should be included in future trials (Steed 2017). This will become more feasible with the increase of online supplementary data and open access journals. A final limiting factor concerning the trials was the minimal assessment of fidelity of interventions, which was reported explicitly only by Svarstad 2013, and Nishita 2013. According to Borrelli 2011, this should be assessed at five levels (i.e. study design, training, delivery, receipt, and enactment), but even the most common aspect of fidelity (delivery) that has been measured in other reviews was poorly measured or reported in these trials (Walton 2017).
Potential biases in the review process
We minimised biases in the review processes by having duplicate screening for full text and extraction, and ensuring reliability of title and abstract screening by using duplicate screening until an excellent level of accuracy was achieved. For several studies, however, we had difficulty in deciding whether to included them or not, usually because the intervention content had not been reported clearly enough. To ensure consistency and minimise bias, two review authors reviewed all studies and, where there was disagreement, sought discussion with a third author. The study team also met to agree issues such as structure of analysis; and to classify which studies focused on pharmacy workers, pharmacy users or both; and whether the intervention could be considered to be behavioural, was purely medication focused, or involved interaction.
One area of deliberation concerned interventions that were primarily managing disease through altering or promoting medication adherence, but also included some lifestyle advice; this was particularly common in hypertension and dyslipidaemia trials. Typically the extent of lifestyle advice was not well categorised, and this may have caused bias through inclusion of studies that were primarily medication focused. However, we conducted meta‐analysis using subgroups with different conditions, and our findings were generally consistent across these subgroups.
The study searches were conducted in February 2018, this may mean some studies have been published since the last search date which could impact on the reliability of the findings.
Agreements and disagreements with other studies or reviews
The findings of this review largely agree with other recent systematic reviews that have looked at the community pharmacy as a context for the delivery of non‐pharmacological interventions. Buss 2018 examined a range of clinical services in community pharmacy and concluded that these led to "improved asthma control, detection of diabetes and cardiovascular risk factors, reduction in smoking rates and weight, and identification of drug‐related problems". Brown 2016, which evaluated community pharmacy interventions focused on lifestyle behaviours that included smoking cessation, weight management or alcohol use, concluded that smoking cessation services delivered in this context were both effective and cost‐effective. Weight management interventions also appeared feasible, but there were insufficient data to permit conclusions to be drawn regarding their effectiveness.
Several reviews have examined community pharmacy‐led management of long‐term conditions. Their role in control of blood pressure was reported in a review by Cheema 2014, which concluded that community pharmacy‐led interventions can significantly reduce both systolic and diastolic blood pressure. Similarly positive effects for diabetes care have been reported in two other reviews that focused on foot care for individuals with type 2 diabetes (Deters 2018; Soprovich 2019). The Garcia‐Cardenas 2016 review reported potential benefit for asthma.
However, the Cochrane Review, De Barra 2018, found less clear results, stating it was unclear whether pharmacist services reduced the percentage of patients with glycated haemoglobin levels outside the target range, although it suggested that such services may reduce the percentage of patients whose blood pressure lies outside the target range. The authors concluded there was probably little or no difference in hospital attendance or admissions, adverse drug effects, and mortality, although there was a possibility of improvement in physical functioning. It is worth noting that the De Barra 2018 review was not specific to community pharmacies as it included pharmacists working in hospital outpatient departments, and those attached to primary care practices. The interventions also included those targeted at improving health through use or stopping of medication and excluded health‐promotion interventions. These are important differences that may account for the differences in results between this review and the others previously mentioned, including our review.
A further point of similarity encountered by our review and other reviews is the difficulties surrounding the level of description of interventions and how to code for theory and behaviour‐change techniques. Scott 2016 similarly called for a higher level of detail to be provided for descriptions of interventions. Finally, a review of reviews in the community pharmacy context concluded that there were insufficient data to assess the impact of public health interventions in this context on health inequalities (Thomson 2019).
Authors' conclusions
Implications for practice.
Community pharmacy interventions probably slightly improve pharmacy users' intermediate clinical, behavioural, and quality of life outcomes, and are also cost‐effective, so community pharmacies may be considered as another option for patients in terms of accessing public health services and health promotion. Additionally the potential ‘reach’ of the community pharmacy network ‐ especially in deprived communities (Todd 2014) ‐ means that they could offer a platform to people who might not be able to access other public health services. Community pharmacy staff are often more accessible than other healthcare professionals such as General Practitioners (Todd 2014b), and so may have the opportunity to reduce health inequalities.
Implications for research.
This review supports further study of the development of community pharmacy health and health‐promotion services. To date there is insufficient evidence to be clear about the reach of these interventions and whether they are moderated by socio‐economic status; this is an area that would benefit from clarification through future research, as these interventions have the potential to be an effective means of reducing health inequalities. Additional high‐quality studies across countries with different income levels, in different settings ‐ such as rural and urban ‐ or in different populations (for example people who do not speak English), would also be helpful.
Additionally many of the interventions investigated to date are complex in nature and require targeting the pharmacy team and pharmacy environment as a whole (Steed 2017), as well as pharmacy users. Interventions would benefit from being based upon appropriate theory and using recent approaches to intervention development (O'Cathain 2019). Interventions would also benefit from being described more clearly, as this would improve both examination and replicability (Scott 2016). Description of both pharmacy‐worker and user‐level interventions should follow the guidelines for description of complex behavioural interventions (Albrecht 2013; Hoffmann 2014). There is also a requirement for greater assessment of fidelity at both the intervention delivery and receipt level.
History
Protocol first published: Issue 7, 2014 Review first published: Issue 12, 2019
| Date | Event | Description |
|---|---|---|
| 18 August 2014 | Amended | Change to author's name |
Acknowledgements
We thank Michelle Fiander for development of the search strategy and conduct of the original search and Paul Miller for the updated search in 2018. A team of several researchers were involved in data extraction including Val Khan and Andrea Takeda, and Emma Konstantiou (EK) contributed significantly to data input. Katrina Kassavou had a significant role at the early stages of the project including design of the data extraction sheet, and Glenn Poon provided considerable administrative support. We are grateful to the whole team for their input and support and referees and reviewers for their recommendations.
Appendices
Appendix 1. Search Strategies
MEDLINE (OVID)
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily, Ovid MEDLINE and Versions(R) <1946 to January 31, 2018>
| No. | Search terms |
| 1 | community pharmacy services/ |
| 2 | ((pharmacy or pharmacist? or pharmacies) adj2 (community or communities)).ti,ab,kf. |
| 3 | ((pharmacy or pharmacist? or pharmacies) adj2 intervention?).ti,ab,kf. |
| 4 | pharmaceutical care.ti,ab,kf. |
| 5 | (community or communities).ti,ab,kf. |
| 6 | 4 and 5 |
| 7 | or/1‐3,6 |
| 8 | exp randomized controlled trial/ |
| 9 | controlled clinical trial.pt. |
| 10 | randomi#ed.ti,ab. |
| 11 | placebo.ab. |
| 12 | randomly.ti,ab. |
| 13 | Clinical Trials as topic.sh. |
| 14 | trial.ti. |
| 15 | or/8‐14 |
| 16 | exp animals/ not humans/ |
| 17 | 15 not 16 |
| 18 | 7 and 17 |
Embase (OVID)
Embase <1974 to 2018 February 05>
| No. | Search terms |
| 1 | ((pharmacy or pharmacist? or pharmacies) adj2 (community or communities)).ti,ab,kw. |
| 2 | ((pharmacy or pharmacist? or pharmacies) adj2 intervention?).ti,ab,kw. |
| 3 | pharmaceutical care.ti,ab,kw. |
| 4 | (community or communities).ti,ab,kw. |
| 5 | 3 and 4 |
| 6 | or/1‐2,5 |
| 7 | random*.ti,ab. |
| 8 | factorial*.ti,ab. |
| 9 | (crossover* or cross over*).ti,ab. |
| 10 | ((doubl* or singl*) adj blind*).ti,ab. |
| 11 | (assign* or allocat* or volunteer* or placebo*).ti,ab. |
| 12 | crossover procedure/ |
| 13 | single blind procedure/ |
| 14 | randomized controlled trial/ |
| 15 | double blind procedure/ |
| 16 | or/7‐15 |
| 17 | exp animal/ not human/ |
| 18 | 16 not 17 |
| 19 | 6 and 18 |
The Cochrane Library
| No. | Search terms |
| #1 | [mh "community pharmacy services"] |
| #2 | ((pharmacy or pharmacist? or pharmacies) near/2 (community or communities)):ti,ab |
| #3 | ((pharmacy or pharmacist? or pharmacies) near/2 intervention?):ti,ab |
| #4 | (pharmaceutical next care):ti,ab |
| #5 | (community or communities):ti,ab |
| #6 | #4 and #5 |
| #7 | {or #1‐#3, #6} |
PsycINFO (OVID)
PsycINFO <1967 to January Week 5 2018>
| No. | Search terms |
| 1 | ((pharmacy or pharmacist? or pharmacies) adj2 (community or communities)).ti,ab,hw. |
| 2 | ((pharmacy or pharmacist? or pharmacies) adj2 intervention?).ti,ab,hw. |
| 3 | pharmaceutical care.ti,ab,hw. |
| 4 | (community or communities).ti,ab,hw. |
| 5 | 3 and 4 |
| 6 | or/1‐2,5 |
| 7 | exp clinical trial/ |
| 8 | random*.ti,ab. |
| 9 | ((clinical or control*) adj3 trial*).ti,ab. |
| 10 | ((singl* or doubl* or trebl* or tripl*) adj5 (blind* or mask*)).ti,ab. |
| 11 | (volunteer* or control group or controls).ti,ab. |
| 12 | placebo/ or placebo*.ti,ab. |
| 13 | or/7‐12 |
| 14 | 6 and 13 |
COS Conference Papers Index
ProQuest Dissertations & Theses: UK & Ireland
ProQuest Dissertations & Theses Global
| No. | Search terms |
| 1 | TI,AB((pharmacy or pharmacist? or pharmacies) NEAR/2 (community or communities)) OR TI,AB((pharmacy or pharmacist? or pharmacies) NEAR/2 intervention?) OR (TI,AB(pharmaceutical care) AND TI,AB(community OR communities)) |
ClinicalTrials.gov
community pharmacy OR community pharmacist
WHO International Clinical Trials Registry Platform (ICTRP)
community pharmacy OR community pharmacist
OpenGrey
((communit* NEAR/2 pharmac*) OR (intervention* NEAR/2 phramac*))
Data and analyses
Comparison 1. Community pharmacy user health‐promotion intervention versus usual treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Health‐related behaviour | 10 | 2138 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.14, 0.72] |
| 1.1 Medication adherence | 3 | 1245 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.23, 0.57] |
| 1.2 Inhaler technique | 4 | 384 | Std. Mean Difference (IV, Random, 95% CI) | 0.92 [0.35, 1.48] |
| 1.3 Other ‐ alcohol consumption, diabetes self‐care and activity impairment | 3 | 509 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.41, 0.68] |
| 2 Intermediate clinical outcomes (final value scores) | 20 | 3971 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.65, ‐0.21] |
| 2.1 Asthma | 8 | 2120 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.40, ‐0.00] |
| 2.2 Diabetes | 6 | 651 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.60, ‐0.02] |
| 2.3 Hypertension | 4 | 1050 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.49, ‐0.18] |
| 2.4 CVD/dyslipidaemia | 2 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.40, 0.24] |
| 3 Intermediate clinical outcome (mean change scores) | 7 | 1413 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.38, ‐0.17] |
| 3.1 Asthma | 2 | 467 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.32, 0.04] |
| 3.2 Diabetes | 2 | 133 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.64, 0.05] |
| 3.3 Hypertension | 1 | 546 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.53, ‐0.19] |
| 3.4 Lipids | 2 | 267 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.67, ‐0.00] |
| 4 Quality of life | 10 | 2733 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.10, 0.50] |
| 4.1 Generic quality of life | 5 | 1567 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.10, 0.52] |
| 4.2 Asthma‐specific | 5 | 1120 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.08, 0.67] |
| 4.3 Diabetes‐specific | 1 | 46 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.11, 1.06] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adepu 2007.
| Methods | Design: RT Groups: intervention group (pharmacist counselling); control group (waiting list control) |
|
| Participants | Pharmacies: 2 Pharmacy workers: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy users: 70 people with type 2 diabetes
Setting: urban Country: India |
|
| Interventions |
Pharmacy worker‐directed intervention: not reported Pharmacy worker control: it was unclear whether one pharmacy site acted as a control and the other as the intervention, or whether pharmacists across both sites delivered both counselling to patients receiving the intervention and no treatment to controls ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received counselling and an information leaflet about their disease, diet and lifestyle modifications.
Pharmacy user control: waiting list |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: JS Mahavidyapeetha, Mysore |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline outcome measures similar | Unclear risk | Not reported |
| Baseline characteristics similar | High risk | In the intervention group a higher % of men had a greater range of duration of illness |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was reported, but it was unclear whether this was accounted for in the analysis. Quote: "Out of 70 patients, two expired, four were hospitalized and four did not respond." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Protection against contamination | High risk | Patients randomised within pharmacy |
| Selective reporting (reporting bias) | Low risk | All 3 outcomes mentioned in the Methods were reported. |
| Other bias | Unclear risk | Not clear whether the 2 participating pharmacies were representative of this area. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
Ali 2012.
| Methods | Design: RT Groups: intervention (diabetes education); control (usual care) |
|
| Participants | Pharmacies: 2 Pharmacy workers: pharmacists 3 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharrmacy users: 48 people with type 2 diabetes
Setting: unsure Country: UK |
|
| Interventions |
Pharmacy worker‐directed intervention: 8‐hour training programme involving workshop sessions with a consultant diabetologist and diabetes specialist nurse TDF: knowledge Pharmacy worker control: it appears the same pharmacists delivered both control and intervention treatments ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received a programme of education about diabetes, its treatment and associated cardiovascular risk factors.
Pharmacy user control: usual care |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none reported Funding source: Department of Health, UK; Merck Sharp, Dohme Ltd |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation conducted by a computer‐generated randomised list. |
| Allocation concealment (selection bias) | Low risk | List held by the researcher at the School of Pharmacy, eliminating the potential influence of pharmacists on the randomisation. |
| Baseline outcome measures similar | Low risk | No difference in primary outcomes, some secondary outcomes not used in current analysis were significantly different |
| Baseline characteristics similar | Low risk | No differences |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Protection against contamination | High risk | Control and intervention participants randomised within same pharmacy |
| Selective reporting (reporting bias) | Unclear risk | Some selective reporting; assessed questionnaires at 5 months but did not report, data on medication use not included, but no significant differences reported. |
| Other bias | Low risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
Amariles 2012.
| Methods | Design: RT Groups: intervention (pharmaceutical care for CVD); control (usual care) |
|
| Participants | Pharmacies: not reported Pharmacy workers: 60 community pharmacists invited 40 (66.7%) of whom participated ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy users: 714 patients with a prescription for at least 1 drug indicated for CVD or CV risk factors
Setting: urban Country: Spain |
|
| Interventions |
Pharmacy worker‐directed intervention: 8‐hour training‐lectures on CVD, CV risk factors, cardiovascular prevention and intervention TDF: knowledge, environment context and resources Pharmacy worker control: it appears the same pharmacists delivered both control and intervention treatments ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: the Dader method ‐ patients received verbal and written information regarding CV prevention
Pharmacy user control: usual treatment and written information on CV risk |
|
| Outcomes |
Pharmacy workers: not assessed. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy users:
|
|
| Notes | Study/intervention name: Effectiveness of Dader method for pharmaceutical care on control of blood pressure and total cholesterol in outpatients with cardiovascular disease or cardiovascular risk (EMDADER‐CV ) study Funding source: Roche Diagnostics and Stada Laboratory (Spain) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Blinded |
| Baseline outcome measures similar | Low risk | No significant differences |
| Baseline characteristics similar | Low risk | No significant differences |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Accounted for |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported in paper EMDADER‐CV (Efecto del Método Dáder de Seguimiento Farmacoterapéutico en el riesgo cardiovascular de pacientes con factores de riesgo o enfermedad cardiovascular [Effectiveness of Dader Method for Pharmaceutical Care on Control of Blood Pressure and Total Cholesterol in Outpatients with Cardiovascular Disease or Cardiovascular Risk) |
| Protection against contamination | High risk | Intervention and controls in same pharmacy |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Low risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants |
Armour 2007.
| Methods | Design: cluster‐RT Groups: intervention (asthma management); control (usual care) |
|
| Participants | Pharmacies: invitations to 174 pharmacies, 57 participated (29 intervention and 28 control) Pharmacy workers: mean number of pharmacists on duty: intervention 2.0 (SD 0.8); control 1.9 (SD 0.7)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy users: 396 patients with asthma
Setting: rural and urban Country: Australia |
|
| Interventions |
Pharmacy worker‐directed intervention: intervention pharmacists received an asthma education manual and were trained on risk assessment, pathophysiology, medications, the National Asthma Campaign (NAC) 6‐step asthma management plan, patient education, goal setting, adherence assessment, spirometry and the Pharmacy Asthma Care Program (PACP) protocol. Renumeration per patient
Pharmacy worker control: trained on risk assessment, spirometry and the control protocol during a 1‐day workshop. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received education on asthma, assessment, and optimisation of drug therapy by the pharmacist, and referral to a respiratory therapist and/or physician as needed.
Pharmacy user control: usual care |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name:Pharmacy Asthma Care Program (PACP) Funding source: Australia Department of Health and Aging. Gordois 2007 paper reported economic outcomes. Gordois 2007, Saini 2004 (cited under Armour 2007) also report on this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was accomplished through an internet randomisation service provided by the Epidemiology Coordinating and Research (EPICORE) Centre, and the Centre for Community Pharmacy Research and Interdisciplinary Strategies (COMPRIS) at the University of Alberta. Randomisation was stratified by centre. |
| Allocation concealment (selection bias) | Low risk | Centralised service, see above |
| Baseline outcome measures similar | Low risk | Difference in level of control, but accounted for in analysis |
| Baseline characteristics similar | Low risk | Although differences in smoking, lung disease, and brief medication questionnaire, these were controlled for in the analyses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sensitivity analysis conducted |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Protection against contamination | Low risk | Cluster‐RT |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Low risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Pharmacists not informed regarding allocation to groups |
Barbanel 2003.
| Methods | Design: RT Groups: intervention (asthma self‐management); control (usual care) |
|
| Participants | Pharmacies: 1 Pharmacy worker: 1 pharmacist ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy users: 24 patients with asthma
Setting: urban Country: UK |
|
| Interventions |
Pharmacy worker‐directed intervention: a single pharmacist acting as the study intervention attended a 3‐day multidisciplinary course on asthma care and self‐management.
Pharmacy worker control: it appears the same pharmacist delivered both control and intervention treatments ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: self‐management advice on asthma
Pharmacy user control: usual care, no input from pharmacist |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using sealed envelopes |
| Allocation concealment (selection bias) | Low risk | As above |
| Baseline outcome measures similar | Low risk | No differences |
| Baseline characteristics similar | Low risk | No differences |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nothing noted |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Protection against contamination | High risk | intervention and control patients from same pharmacy |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Unclear risk | Possible recruitment bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
Basheti 2008.
| Methods | Design: cluster‐RT Groups: intervention (asthma inhaler technique); control (usual care) |
|
| Participants | Pharmacies: not reported Pharmacy worker: 31 pharmacists (16 intervention; 15 control) of 120 invited ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 97 patients with asthma
Setting: urban Country: Australia |
|
| Interventions |
Pharmacy worker‐directed intervention: pharmacists received general information about asthma, inhaled medications, and peak flow meter technique. They were also trained to assess and teach correct Turbuhaler and Diskus inhaler techniques, asthma management etc. They were reassessed at the end of the workshop and 2 years after.
Pharmacy worker control: pharmacists received general information about asthma, inhaled medications, and peak flow meter technique. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients' inhaler technique was assessed and then they were educated using a specialised ‘‘Show and Tell’’ inhaler technique counselling service, going through each step on a checklist to describe and demonstrate correct use; had an inhaler technique label placed on their inhaler, which highlighted incorrect steps
Pharmacy user control: wait list ‐ inhaler technique assessed and then inhaler technique counselling provided at end of study. |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: Faculty of Pharmacy, Univeristy of Sydney; placebo inhalers by AstraZeneca and GlaxoSmithKline Basheti 2007 and Basheti 2009 (both cited under Basheti 2008) also report on this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Pharmacists were allocated randomly by computer‐generated list to Active or Control groups." |
| Allocation concealment (selection bias) | Low risk | By computer |
| Baseline outcome measures similar | Low risk | Analysis accounted for baseline |
| Baseline characteristics similar | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal dropouts |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "We blinded pharmacists and patients by teaching both groups how to educate patients in correct peak flow meter technique." |
| Protection against contamination | Unclear risk | Unclear, as intervention and control pharmacists could work in same pharmacy |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Unclear risk | Not noted ‐ possible recruitment bias of patients ‐ every second asthma patient |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "pharmacists ... blinded to true nature of intervention" |
Bereznicki 2013.
| Methods | Design: cluster‐RT Groups: intervention (asthma education delivered either face to face or by mail); control (usual care) |
|
| Participants | Pharmacies: 71 Pharmacy worker: at least 1 per pharmacy ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy users: 1483 patients with asthma
Setting: unclear Country: Australia (South Australia, Tasmania and Victoria) |
|
| Interventions |
Pharmacy worker‐directed intervention: education sessions for all participating pharmacists ‐ overview of asthma management in Australia; outline of project’s objectives and methods; demonstration of the data‐mining software. For pharmacists unable to attend an education session, a personalised one‐to‐one visit was arranged. Renumeration for training and AUD 200 per pharmacy
Pharmacy worker control: received the same education as above. Randomization at pharmacy user level ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received educational material and a referral to their GP for an asthma management review either by mail or a face‐to‐face intervention
Pharmacy user control: usual treatment, no intervention pack |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: Australian Government Department of Health and Aging Bereznicki 2008, 2011 (cited under Bereznicki 2013) also reported on this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pharmacies randomly assigned to deliver first type of pharmacist‐initiated intervention – mailed or face‐to‐face, then alternate allocation for remaining pharmacies. First patients within pharmacies randomly allocated to receive intervention or control then alternately allocation. |
| Allocation concealment (selection bias) | Low risk | Software allocated |
| Baseline outcome measures similar | Low risk | No significant differences |
| Baseline characteristics similar | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers mentioned in paper |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patient receiving the greatest number of relievers was randomly assigned to the intervention or control group, with subsequent patients being alternately assigned to the control or intervention group." |
| Protection against contamination | Low risk | Each pharmacy only performed one type of intervention |
| Selective reporting (reporting bias) | Low risk | Nothing noted |
| Other bias | Low risk | Nothing noted |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Pharmacists were blinded to the control patients’ identities until the end of the 12‐month post‐intervention period, with the intention that control patients would receive no intervention other than the pharmacists’ usual care until after the post‐intervention period." |
Bond 2007.
| Methods | Design: RT Groups: intervention (medicines management for cardiovascular disease); control (usual care) |
|
| Participants | Pharmacies: 9 Pharmacy workers: 62 pharmacists ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 1493 patients with CHD
Setting: mixed Country: UK |
|
| Interventions |
Pharmacy worker‐directed intervention: pharmacists received training on medicines management, identification of essential information from GP patient records, facilitation of independent studying, communication skills, and action learning.
Pharmacy worker control: it appears the same pharmacists delivered both control and intervention treatments ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: consultations on medicines management delivered, included assessments of the following: therapy, medication compliance, lifestyle and social support
Pharmacy user control: usual treatment from GP and community pharmacist |
|
| Outcomes |
Pharmacy worker: no valid measures ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: community pharmacy‐led medicines management (MEDMAN) Funding source: Department of Health England and Wales Jaffray 2007 and Scott 2007 (cited under Bond 2007) also reported on this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized in a ratio of 2:1, intervention to control group. This was done independently of the research team using a password protected computer programme in permuted blocks stratified by practice." |
| Allocation concealment (selection bias) | Low risk | Quote: "Audit clerks performing data extraction were blind to the randomization status of participants, as were the researchers conducting the statistical analyses." |
| Baseline outcome measures similar | Low risk | Quote: "No substantial differences in the baseline characteristics of the study groups" |
| Baseline characteristics similar | Low risk | Quote: "No substantial differences in the baseline characteristics of the study groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Missing data was tested, and adjusted for" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Data extraction and analysis were blind," |
| Protection against contamination | Unclear risk | Unclear if possible contamination |
| Selective reporting (reporting bias) | Low risk | Nothing noted |
| Other bias | Low risk | Nothing noted |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients could not be blind to trial intervention because of its nature. Community pharmacists were not informed which control patients had nominated their pharmacy." |
Burford 2013.
| Methods | Design: RT Groups: intervention (photo‐aging smoking cessation); control group (usual care) |
|
| Participants | Pharmacies: 8 Pharmacy worker: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy users: 160 smokers
Setting: urban Country: Perth, Australia |
|
| Interventions |
Pharmacy worker‐directed intervention: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: standard 2‐minute smoking cessation advice from the pharmacist plus participants were digitally photo‐aged so they could preview images of themselves as a lifelong smoker and as a nonsmoker, and were invited to view the age‐processed images, received smoking cessation advice, and were screened for body dysmorphia.
Pharmacy user control: standard 2‐minute smoking cessation advice from the pharmacist. |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation by researcher on alternate weeks |
| Allocation concealment (selection bias) | High risk | Quote: "Allocation to groups was not performed as eligible participants were recruited, but according to the treatment being used at the pharmacy during that week." |
| Baseline outcome measures similar | Low risk | Quote: "there were no significant differences between the control and intervention group on demographic or smoking dependence at baseline" |
| Baseline characteristics similar | Unclear risk | There were differences between groups for concern about physical appearance, and the belief that facial wrinkles are associated with smoking. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants lost |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Because of the nature of the intervention, the participants and researcher could not be blinded to the study group." |
| Protection against contamination | Unclear risk | Allocation to group dependent on week to avoid contamination, but unclear if successful |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to have been reported. |
| Other bias | Unclear risk | Low numbers of control groups self report quit status was verified with objective carbon monoxide measurement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Because of the nature of the intervention, the participants and researcher could not be blinded to the study group." |
Bynum 2001.
| Methods | Design: RT Groups: intervention (telepharmacy counselling); control group (usual care) |
|
| Participants | Pharmacies: not reported Pharmacy worker: 2 pharmacists ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 49 asthma patients
Setting: rural Country: USA |
|
| Interventions |
Pharmacy worker‐directed intervention: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: pharmacists used interactive compressed video (telepharmacy) to teach metered dose inhaler (MDI) technique to a rural, adolescent asthma population in junior high and high schools.
Pharmacy user control: had telepharmacy contact, but not counselling until after study |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Funding source: grant from the Office for the Advancement of Telehealth in the Department of Health Resources and Services Administration Study/intervention name: none given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number chart |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Baseline outcome measures similar | Low risk | Reported in text as non significant |
| Baseline characteristics similar | Low risk | Reported in text as non significant |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some loss to follow‐up and no reporting of correction for missing data |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Protection against contamination | Unclear risk | Not clear if control had access to intervention pharmacists |
| Selective reporting (reporting bias) | Low risk | Seemed to report all planned outcomes. |
| Other bias | Low risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear whether pharmacist assessors were aware of grouping |
Charrois 2006.
| Methods | Design: RT Groups: intervention (asthma management); control group (usual care) |
|
| Participants | Pharmacies: 5 Pharmacy workers: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 70 patients with asthma
Setting: rural Country: Canada |
|
| Interventions |
Pharmacy worker‐directed intervention: pharmacist trained in an interactive, activity and case‐based program which focused on patient assessment, patient interviewing and communication skills
Pharmacy worker control: the same pharmacists delivered care to both intervention and control groups ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received education on asthma, assessment, and optimisation of drug therapy, with focus on a written asthma plan
Pharmacy user control: wait list with asthma education and advice as needed, as well as referral to respiratory therapist |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: Better Respiratory Education and Asthma Treatment in Hinton and Edson study (BREATHE) Funding source: Canadian Institues of Health Research Charrois 2004 (cited under Charrois 2006) also referred to this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was accomplished through an Internet randomization service provided by the Epidemiology Coordinating and Research (EPICORE) Centre and the Centre for Community Pharmacy Research and Interdisciplinary Strategies (COMPRIS) at the University of Alberta. Randomization was stratified by centre." |
| Allocation concealment (selection bias) | Low risk | Centralised service, see above |
| Baseline outcome measures similar | Low risk | No differences for main outcomes |
| Baseline characteristics similar | Low risk | Differences for range characteristics ‐ text reported that this was controlled for in analyses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Last value of ACQ carried forward where missing |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It is possible that assessors were not blinded |
| Protection against contamination | Low risk | Cluster randomised |
| Selective reporting (reporting bias) | Unclear risk | Not noted |
| Other bias | High risk | Quote: “The sites did not apply the intervention uniformly. According to case report forms received, follow‐up was poor, few asthma management recommendations were made, and one‐quarter of patients in the intervention group never received a written action plan, [which was] the focus of the intervention. The follow‐up completed at each site varied, with some sites having less than 30% follow‐up at the time of the 6‐month visit. The low rate of follow‐up leads us to believe that the application of the intervention was also minimal at these sites.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Caregivers/pharmacists involved in the study were not blinded. |
Cordina 2001.
| Methods | Design: cluster‐RT Groups: intervention (asthma education and monitoring); control (routine dispensing services) |
|
| Participants | Pharmacies: 22 (intervention 11; control 11) Pharmacy worker: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 152 asthma patients
Setting: both urban and rural Country: Malta |
|
| Interventions |
Pharmacy worker‐directed intervention: A manual was prepared in the form of a self‐study program with 2 sections: Section 1 dealt with the pathophysiology of asthma and its treatment, including standard intervention instructions; Section 2 provided details of outcome measures and data collection instruments to be used in the study.
Pharmacy worker control: only attended second evening and received section 2 of manual ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received verbal counselling, an educational video, an information leaflet, and subsequent monitoring with reinforcement.
Pharmacy user control: patients were given their prescribed drugs and informed of the dosage regimen, but received no other assistance. |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Low risk | Cluster randomised |
| Baseline outcome measures similar | Low risk | Some differences, but adjusted for in analysis. |
| Baseline characteristics similar | Low risk | Some differences, but adjusted for in analysis. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout and unclear how this was adjusted for. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Protection against contamination | Unclear risk | Randomisation by pharmacists, but patients came from same asthma clinic. |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Unclear risk | Differences in groups at baseline and attrition may have had significant effect. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
Crockett 2006.
| Methods | Design: cluster‐RT Groups: intervention (depression management); control (usual care) |
|
| Participants | Pharmacies: 32 Pharmacy worker: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 106 patients with depression
Setting: rural Country: Australia |
|
| Interventions |
Pharmacy worker‐directed intervention: intervention pharmacists were given video‐conference training on the nature and management of depression and were asked to dispense medication with extra advice and support.
Pharmacy worker control: usual care ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: education on depression management; patient’s psychological well‐being monitoring, attitudes towards taking antidepressants, adherence and patient satisfaction with service.
Follow‐up: 3 months Pharmacy user control: usual care |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: grant from the Rural and Remote Pharmacy Infrastructe Grants scheme |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but no specific method mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Baseline outcome measures similar | Unclear risk | Baseline differences unclear |
| Baseline characteristics similar | Low risk | No differences in the characteristics reported, but reported adjustment in analyses for baseline differences |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nothing noted |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Protection against contamination | High risk | Randomisation at pharmacist level but four of control pharmacies were delivering similar intervention |
| Selective reporting (reporting bias) | Low risk | Nothing noted. |
| Other bias | Unclear risk | Assessments may have impacted outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
Dhital 2015.
| Methods | Design: RT Groups: intervention (brief motivational interviewing alcohol intervention); control (leaflet only) |
|
| Participants | Pharmacies: 16 Pharmacy worker: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 407 (205 intervention; 202 control)
Setting:urban Country: London, UK |
|
| Interventions |
Pharmacy worker‐directed intervention: Training in motivational and problem solving approach
Pharmacy worker control: it appears the same pharmacists saw both intervention and control groups. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: structured 10‐minute discussion about drinking based on motivational interviewing, also gave participants 'Units and You' booklet and unit/calorie calculator wheel and alcohol services leaflet
Pharmacy user control: given a leaflet called "Alcohol: The Basics" |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: Pharmacy Practice Research Trust, Royal Pharmaceutical Society of Great Britain, and the Harold and Marjorie Moss Charitable Trust |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed numbered envelopes, monitored for tampering |
| Allocation concealment (selection bias) | Low risk | Pharmacists not involved in research data collection and allocation after consent |
| Baseline outcome measures similar | Low risk | Did not differ between the two groups |
| Baseline characteristics similar | Low risk | No significant differences |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sensitivity analysis and adjustment for attrition |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researcher blinded to allocation status |
| Protection against contamination | High risk | Both control and intervention participants within one pharmacy |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias obvious |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Relevant personnel were blinded to randomisation status throughout the trial, but participants not blinded. |
Dolovich 2007.
| Methods | Design: RT Groups: intervention (Asthma Education Program (AEP); control (usual care‐delayed AEP) |
|
| Participants | Pharmacies: not reported Pharmacy workers: 64 of 160 approached (40%)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: not targeted Setting: urban Country: Canada |
|
| Interventions |
Pharmacy worker‐directed intervention: volunteer community pharmacists received an asthma education program (AEP)
Pharmacy worker control: delayed AEP ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: not targeted |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: Merck Frosst Canada Inc, and in‐kind contribution from Agro Health Associates Inc, and the Centre for Evaluation of Medicines |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Concealed |
| Baseline outcome measures similar | Unclear risk | Only assessed post workshop |
| Baseline characteristics similar | Low risk | No reported differences |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, no patterns identified |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to assignment of the pharmacists to intervention or control groups. |
| Protection against contamination | Unclear risk | Unclear if there was interaction between sites |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Unclear risk | Possible selection bias ‐ pharmacists volunteers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded to group allocation |
Doucette 2009.
| Methods | Design: RT Groups: intervention (extended diabetes care); control (usual care) |
|
| Participants | Pharmacies: 7 Pharmacy workers: 9 pharmacists ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 78 people with type 2 diabetes
Setting: unclear Country: USA |
|
| Interventions |
Pharmacy worker‐directed intervention: participating pharmacists received training in diabetes management and study protocol. Both self‐study and live programs included discussion of mock cases. Skills training in monitoring blood pressure, using a blood glucose meter, filling an insulin syringe, and administering an insulin injection
Pharmacy worker control: not applicable as control patients seen by other primary care providers ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients had already received 2 diabetes education sessions, then received extended diabetes care, discussed medications, clinical goals, and self‐care activities; pharmacists recommended medication changes to physicians when appropriate.
Pharmacy user control: usual diabetes care from their primary care provider |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐. Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: a grant from the Community Pharmacy Federation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear, no information provided on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Unclear, not stated |
| Baseline outcome measures similar | Low risk | No differences |
| Baseline characteristics similar | Low risk | Minimal differences |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No intention‐to‐treat analysis. Some patients did not present for final data collection, and 2 intervention patients did not meet pharmacist. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Protection against contamination | Unclear risk | Recruitment at patient level, from same centre, unclear if patients attended different pharmacies |
| Selective reporting (reporting bias) | Low risk | Paper seemed to report all relevant outcomes. |
| Other bias | Unclear risk | Nothing noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
Fuller 2016.
| Methods | Design: cluster‐RT Groups: intervention (Modified Brief Behavioural Intervention Insomnia (MBBTi)); control (usual care + information leaflet) |
|
| Participants | Pharmacies: 12 (7 intervention; 5 control) Pharmacy workers: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 56 insomniacs (22 intervention; 34 control)
Setting: unclear Country: New South Wales, Australia |
|
| Interventions |
Pharmacy worker‐directed intervention: training on sleep and sleep management through interactive lectures, case study discussions, role play plus manual with details of sleep and MBBTi
Pharmacy worker control: control group manual provided detailed background information on sleep and sleep health, insomnia and its impact, models of insomnia and general insomnia treatment (pharmacological and sleep hygiene methods). ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: standardised education + sleep restriction and/or stimulus control, goal setting, sleep diaries
Pharmacy user control: usual care and information sheets on insomnia if needed |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Funding source: Scholarship Faculty Pharmacy, University of Sydney and CIRUS (Centre for Integrated Research into the Understanding of Sleep) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used RAND function in Excel for simple randomisation |
| Allocation concealment (selection bias) | High risk | Incomplete allocation concealment |
| Baseline outcome measures similar | Low risk | MBBTi and control patients were similar at baseline |
| Baseline characteristics similar | Low risk | No significant differences between groups in any of the demographics |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Alternative analysis performed to allow for all available data to be used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Pharmacists undertook data collection, but did not score key outcomes at follow‐up. |
| Protection against contamination | Low risk | Cluster randomised |
| Selective reporting (reporting bias) | Low risk | Not apparent |
| Other bias | High risk | Cluster effects not taken into account for all key outcomes other than the ISI. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pharmacists were aware of participants' groups. |
Garcia 1998.
| Methods | Design: cluster‐RT Groups: intervention group (education on sexually transmitted disease (STD) recognition, management, and prevention counselling); control group (usual care) |
|
| Participants | Pharmacies: 168 Pharmacy workers: average 1.7 (range 1 to 7) workers per pharmacy
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: not reported Setting: urban Country: Peru |
|
| Interventions |
Pharmacy worker‐directed intervention: pharmacists and pharmacist technicians received education on STD recognition, management, and prevention counselling, and were visited by standardised simulated patients.
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: not reported |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: not assessed |
|
| Notes | Study/intervention name: none given Funding source: Fogarty International Center grant NIAID Center for AIDS Research Grant NIH grant, USAID |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but method not mentioned |
| Allocation concealment (selection bias) | Low risk | By pharmacy |
| Baseline outcome measures similar | Unclear risk | Overall scores reported, but not comparison between control and intervention |
| Baseline characteristics similar | Unclear risk | Overall scores reported, but not comparison between control and intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported loss to follow‐up, but unclear how this was adjusted for |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Simulated patients were blinded |
| Protection against contamination | Low risk | Pharmacies cluster randomised |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Only intervention group were offered training |
Garcia 2003.
| Methods | Design: cluster‐RT Groups: intervention group (management and prevention of STDs); control group (1‐day seminar on management of diarrhoea) |
|
| Participants | Pharmacies: the 24 districts in Lima that had the lowest socio‐economic status were selected and matched in 12 pairs; 7 pairs of districts were chosen randomly to participate in 2 phases reported separately by study:
Pharmacy workers: 2223 workers in intervention group participated in at least one seminar, 1872 (84.2%) attended all seminars. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: not reported Setting: rural Country: Peru |
|
| Interventions |
Pharmacy worker‐directed intervention: pharmacies received education on STD recognition, management, and prevention counselling and were visited by standardised simulated patients.
Pharmacy worker control: invited to a seminar on diarrhoea management ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: not reported |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: not assessed |
|
| Notes | Study/intervention name: none given Funding source: Wellcome Trust‐Burroughs Wellcome Fund Infectious Disease Initiative Pilot study for 2012 studies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised with table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Adequately concealed ‐ data extraction form |
| Baseline outcome measures similar | Unclear risk | Preintervention assessments not conducted |
| Baseline characteristics similar | Unclear risk | Preinterventions assessments not conducted |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal attrition |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Standardised simulated patients were blinded to the nature of the training, the randomisation procedure, and the status of districts or pharmacies as intervention or controls. |
| Protection against contamination | Low risk | Cluster randomised |
| Selective reporting (reporting bias) | Unclear risk | Findings were presented for the 4 outcomes, but only for selection of pharmacies |
| Other bias | Low risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Control pharmacies offered training in diarrhoea to maintain blinding |
Garcia 2012.
| Methods | Design: cluster‐RT (by city; n = 20) Groups: intervention group (management and prevention of STDs); control group (standard care) |
|
| Participants | Pharmacies: 773 Pharmacy workers: 2292 Median age: 34.6 years % female: 62% ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: targeted through pharmacies (data available from 12930 young adults) Setting: urban Country: Peru |
|
| Interventions |
Pharmacy worker‐directed intervention: the intervention comprised 4 modalities:
Pharmacy worker control: usual treatment ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: not reported |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: Peru‐PREVEN Funding Source: Wellcome Trust and Burroughs Wellcome Fund, National Institues of Health, Centre for AIDS Research, CIPRA, and USAID‐Peru. Garcia 2012 (cited under Garcia 2012) also reported on this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | High risk | Participants in outcome surveys were recruited after city randomisation, precluding allocation concealment |
| Baseline outcome measures similar | Low risk | Adjusted for in analysis |
| Baseline characteristics similar | Low risk | Adjusted for in analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data replaced with classification of negative composite endpoint |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | US and UK investigators masked until testing complete |
| Protection against contamination | Low risk | Cluster randomised |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported |
| Other bias | Low risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Fieldworkers and Peruvian study team could not be masked |
Garcia‐Cardenas 2013.
| Methods | Design: cluster‐RT Groups: intervention group (management of asthma); control group (standard care) |
|
| Participants | Pharmacies: 51 (29 intervention; 22 control) Pharmacy workers: 33 pharmacists in intervention group; 32 pharmacists in control group ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 346 patients with asthma (186 intervention; 160 control)
Setting: all pharmacies in Malaga and Madrid (urban and rural) Country: Spain |
|
| Interventions |
Pharmacy worker‐directed intervention: 33 pharmacists allocated to the intervention group attended a 1‐day workshop. They were trained to provide education on asthma control, medication adherence and inhaler technique and received the Spanish Guide for Asthma Management (GEMA 2009).
Pharmacy worker control: received instructions by phone about study protocol and monitored through 2 visits to the pharmacy ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: asthma self‐management
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: The AFasma Study Funding Source: AstraZeneca Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Computer generated |
| Baseline outcome measures similar | Low risk | Differences adjusted for in analyses |
| Baseline characteristics similar | Low risk | Differences adjusted for in analyses |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how missing data were managed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear if assessor blinded |
| Protection against contamination | Low risk | Cluster randomised |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control group pharmacies were asked not to change care, which suggests that they had knowledge of groups. |
Jaffray 2014.
| Methods | Design: cluster‐RT Groups: intervention group (motivational interviewing to improve methadone outcomes); control group (standard practice) |
|
| Participants | Pharmacies: 76 of 87 approached were recruited Pharmacy workers: 84 pharmacists of 95 contacted were recruited ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 542 methadone patients (295 intervention; 247 control)
Setting: Tayside, Ayrshire, Fort Valley, Lanarkshire, Grampian, Fife Country: Scotland, UK |
|
| Interventions |
Pharmacy worker‐directed intervention: training in motivational interviewing during 4 sessions, the first 2 sessions emphasised techniques and discussion, and subsequent sessions allowed practice of skills. The intervention was also supported by a resource pack.
Pharmacy worker control: usual practice ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: motivational interviewing offered at sessions over 6 months
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Funding source: Chief Scientist Office, Edinburgh, Scotland, UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but method unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear method for randomisation |
| Baseline outcome measures similar | Low risk | Any differences adjusted for in analyses |
| Baseline characteristics similar | Low risk | Any differences adjusted for in analyses |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat with imputation for missing values but only for certain variables, others excluded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor aware of groupings |
| Protection against contamination | High risk | Although cluster randomised, report considerable movement of patients between pharmacies. |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Unclear risk | Only missing estimates were made for treatment satisfaction, physical and psychological scores. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients blinded |
Kraemer 2012.
| Methods | Design: RT Groups: intervention group (pharmacist counselling); control group (printed materials) |
|
| Participants | Pharmacies: not reported Pharmacy worker: 22 pharmacists trained ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 67 patients with type 1 or type 2 diabetes mellitus (with insurance from participating employer)
Setting: urban Country: USA |
|
| Interventions |
Pharmacy worker‐directed intervention: pharmacists had to demonstrate evidence of prior certification as a diabetes educator or complete 16 hours of online training. All also had to complete 14 hours of didactic and case‐based workshops with emphasis on patient education and empowerment, clinical intervention techniques, documentation and billing.
Pharmacy worker control: it appears the same pharmacists saw both intervention and control groups. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: received waiver of patient out‐of‐pocket expenses (e.g. co‐payments and/or co‐insurance) for specified medications and physician visits, plus multiple scheduled education appointments with a pharmacist over a 12‐month period.
Pharmacy user control: printed education materials and the same financial benefits as the intervention group |
|
| Outcomes |
Pharmacy worker: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: the EMPOWER study Funding source: partial funding for this project was received from the Community Pharmacy Foundation, Sanofi‐Avetis, and Lane County Pharmacists Association. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but methods were not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Baseline outcome measures similar | Low risk | Any differences accounted for in analyses |
| Baseline characteristics similar | Low risk | Any differences accounted for in analyses |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear how missing data were managed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear whether the research team conducting follow‐up assessments were blind to group. |
| Protection against contamination | High risk | Participants were known to discuss the study between themselves, so that blinding was broken and risk of contamination was possible. |
| Selective reporting (reporting bias) | Low risk | No issues noted. Four parameters (cholesterol‐to‐HDL ratio, weight, waist circumference, and BMI) were not shown due to lack of changes from baseline and difference between groups. |
| Other bias | Low risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | On self‐report 54% of control group indicated they were controls while 75% of intervention group were unclear on their grouping. At least some participants discussed the study between themselves, hence blinding was possibly broken. |
Krass 2007.
| Methods | Design: cluster‐RT Groups: intervention group (education on diabetes, its management, medicines); control group (standard care) |
|
| Participants | Pharmacies: 56 Pharmacy workers: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 289 patients with type 2 diabetes 289
Setting: both rural and urban Country: Australia |
|
| Interventions |
Pharmacy worker‐directed intervention: all intervention pharmacists received a diabetes education manual for self‐directed learning and also attended a 2‐day workshop that consisted of lectures, role playing, training on monitors. Pharmacists received reimbursement for patients who completed all study visits.
Pharmacy worker control: training on study procedures and payment for every patient that completed both baseline and follow‐up assessments ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients were given a blood glucose meter, instructed to use it daily. Measurements discussed at each visit to identify other interventions to support patient care (adherence support, medication review, diabetes self‐management, lifestyle information etc.)
Pharmacy user control: usual care |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: the Pharmacy Diabetes Care Program NB This appears to be the same research group as Armour 2004, but this is a more complex study with a separate population. Funding source: Australian Government Department of Health and Aging |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The pharmacies identified were located within 300 km of the participating universities. The percentage of pharmacies in each local government area (LGA) was then calculated. To obtain a random stratified sample of 60 pharmacies, the corresponding percentage per LGA was calculated and the required number within each stratum was randomly chosen using Excel. |
| Allocation concealment (selection bias) | Low risk | As above |
| Baseline outcome measures similar | Low risk | Any differences controlled for in analyses |
| Baseline characteristics similar | Low risk | Any differences controlled for in analyses |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comparison was made between completers and non‐completers, but unclear how accounted for in analyses |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcomes |
| Protection against contamination | Low risk | Cluster‐RT |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Low risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear whether intervention group participants were aware of grouping |
Liambila 2010.
| Methods | Design: cluster‐RT Groups: intervention (training on use of emergency contraceptives); control (standard care) |
|
| Participants | Pharmacies: 20 selected from 98 Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: not reported Setting: urban Country: Nairobi, Kenya |
|
| Interventions |
Pharmacy worker‐directed intervention: updating the private pharmacy providers on appropriate use of emergency contraceptives, how best to dispense it to users, family planning methods, referral for sexually transmitted infection (STI)/HIV testing and counselling. In‐pharmacy information for pharmacy workers and users
Pharmacy worker control: usual practice ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: advice on emergency contraceptives, family planning and STI management.
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: not assessed |
|
| Notes | Study/intervention name: none given Funding source: William and Flora Hewlett Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but method not specified |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Baseline outcome measures similar | Low risk | No differences |
| Baseline characteristics similar | Unclear risk | Little information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on missing data |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear if mystery clients were aware of groupings |
| Protection against contamination | Low risk | Control and intervention in separate geographical areas |
| Selective reporting (reporting bias) | Unclear risk | Nothing noted |
| Other bias | High risk | Not all baseline assessments completed before study commenced |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention participants must have had awareness of grouping due to materials. |
Liekens 2014.
| Methods | Design: cluster‐RT Groups: intervention group (pharmacists received communication skills training related to depression); control group (no intervention) |
|
| Participants | Pharmacies: 40 Pharmacy worker: 21 in intervention group; 19 in control group ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: not assessed Setting: urban Country: Belgium |
|
| Interventions |
Pharmacy worker‐directed intervention: pharmacists received communication skills training related to depression including role playing with a simulated patient and feedback on their counselling skills.
Pharmacy worker control: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: not targeted |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: not assessed |
|
| Notes | Study/intervention name: none given Funding source: no external funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly ordered list of pharmacy codes |
| Allocation concealment (selection bias) | Low risk | Researchers blinded to the identity of pharmacies allocated to study groups |
| Baseline outcome measures similar | Unclear risk | No baseline assessment |
| Baseline characteristics similar | Unclear risk | No baseline assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported attrition |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The researchers were blind to the identity of the pharmacies allocated to the study groups." and "The mystery shoppers (MS) were blind to the pharmacy assignment to training or control groups." |
| Protection against contamination | Unclear risk | All pharmacists from same pharmacy chain |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Unclear risk | Quote: "limitations of the current study include variable exposure to the intervention as several pharmacists did not complete the role play at the end of the training day. This may have diminished the impact of the intervention for them in terms of patient counselling skills." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The researchers were blind to the identity of the pharmacies allocated to the study groups." and "The mystery shoppers (MS) were blind to the pharmacy assignment to training or control groups." |
Madurasinghe 2017.
| Methods | Design: cluster‐RT Groups: intervention group (pharmacy workers trained in smoking cessation); control (no treatment) |
|
| Participants | Pharmacies: 12 (7 intervention; 5 control) Pharmacy worker: pharmacists and counter assistants Pharmacy user: 621 (302 intervention; 319 control)
Setting: urban Country: UK |
|
| Interventions |
Pharmacy worker‐directed intervention: training in communication and behaviour change skills
Pharmacy worker control: no training ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: optimised smoking cessation programme
Pharmacy user control: Usual care |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: Smoking Treatment Optimisation in Pharmacy (STOP) Funding source: National Institute of Health Research,UK Steed 2017 (cited under Madurasinghe 2017) also refers to this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation generated using Stata 12 software. |
| Allocation concealment (selection bias) | Low risk | Independent statistician generated and administered randomisation list. |
| Baseline outcome measures similar | Unclear risk | Not reported |
| Baseline characteristics similar | High risk | Differences in age and % female |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Protection against contamination | Low risk | Cluster randomised |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Only 12 of 54 pharmacies participated, no comparison with those who were not recruited |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pharmacy workers were aware of intervention arm. |
Maguire 2001.
| Methods | Design: RT Groups: intervention group (Pharmacists Action on Smoking (PAS)); control group (usual care) |
|
| Participants | Pharmacies: 124 (1 pharmacist per site) Pharmacy workers: 124 pharmacists (100 in Northern Ireland, 24 in London) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 484 smokers
Setting: urban Country: UK |
|
| Interventions |
Pharmacy worker‐directed intervention: before study, pharmacists were sent the pharmacy‐based smoking cessation (PAS model) documentation and literature review and were asked to study it. Attended workshops on epidemiology, smoking statistics, the use of nicotine replacement therapy, the cycle of change model and the PAS model. Researchers visited the pharmacists to provide support and to address any queries they had in implementing the model.
Pharmacy worker control: it appears the same pharmacists saw both intervention and control groups. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received counselling using PAS approach
Pharmacy user control: usual care including the provision of nicotine replacement therapy as appropriate |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: Pharmacist Action on Smoking (PAS) Funding source: Medical Research Council and Northern Ireland Department of Health and Social Services Also informed by Maguire 1996 (additional reference under Maguire 2001) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a sealed envelope technique |
| Allocation concealment (selection bias) | Low risk | Blind to allocation |
| Baseline outcome measures similar | Low risk | All participants were smokers at baseline. |
| Baseline characteristics similar | Unclear risk | Differences between groups not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Where data were missing for participants, the participants were assumed to still be smoking. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcome measure |
| Protection against contamination | High risk | Within pharmacy randomisation |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Low risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pharmacy workers aware of group |
Mansell 2016.
| Methods | Design: cluster‐RT Groups: intervention (self‐monitoring of blood glucose (SMBG)); control (usual care) |
|
| Participants | Pharmacies: 9 (7 intervention; 2 control) Pharmacy worker: 9 (1 pharmacist per pharmacy) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 36 (26 intervention; 10 control)
Setting: mainly rural Country: Canada |
|
| Interventions |
Pharmacy worker‐directed intervention: provided education on SMBG, the recent Canadian Diabetes Association (now renamed 'Diabetes Canada') recommendations and the study glucose meter
Pharmacy worker control: no additional information ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: recommendations for SMBG and behaviours to change
Pharmacy user control: usual care |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: unrestricted research grant from Sanofi, Canada |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | High risk | Blinding not possible |
| Baseline outcome measures similar | High risk | Considerable missing data for baseline BP |
| Baseline characteristics similar | High risk | Difference diabetes in age between groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition accounted for |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data analyst was blind to treatment allocations |
| Protection against contamination | Low risk | Cluster design |
| Selective reporting (reporting bias) | Low risk | Not apparent |
| Other bias | Low risk | Not apparent |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Aware of groups |
Mayer 1998.
| Methods | Design: cluster‐RT Groups: intervention group (pharmacist training in skin cancer prevention); control group (usual care) |
|
| Participants | Pharmacies 54 (out of 88 sites) Pharmacy worker: pharmacists 147 (out of 178 invited)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: not targeted Setting: unclear Country: USA |
|
| Interventions |
Pharmacy worker‐directed intervention: training was provided to pharmacists about how to reduce ultraviolet radiation exposure and use sun protection of 15 or higher. A videotape and accompanying print materials were used for 3 weeks, then pharmacists received weekly written feedback on skin cancer prevention counselling performance, plus incentives for the "winning" performance for a further 3 weeks. The 23‐minute videotape contained didactic information about skin cancer prevention, a model ("Ask, Advise, and Assist") to help pharmacists give brief counselling to their patients,and 6 brief scenes showing pharmacist‐patient interactions.
Pharmacy worker control: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: not directly targeted |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: not assessed |
|
| Notes | Study/intervention name: Project SUNWISE Funding source ‐ Grant AR 43025 from the National Institue of Arthritis and Musculskeletal and Skin Diseases (NIAMS), videotape by Glaxo Wellcome Mayer 1998 (cited under Mayer 1998) also refers to this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | No information in trial report |
| Baseline outcome measures similar | Low risk | Although differences at baseline were reported, these were controlled for in analyses |
| Baseline characteristics similar | Low risk | No significant differences apparent |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Across all observations, 138 pharmacists were observed. Of these, 33 were observed at pretest only, 25 were observed at post‐test only, and 80 were observed at both times. Intervention site pharmacists 71; control site pharmacists 67 Not clear how missing data from pre/post‐test were handled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Simulated patients (mystery shoppers) blind to study groups |
| Protection against contamination | Low risk | Cluster randomisation |
| Selective reporting (reporting bias) | Unclear risk | Not noted |
| Other bias | High risk | There appeared to be some discrepancy in figures reported between publications. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Confederates were blinded to pharmacy study conditions, but pharmacists were aware of intervention group. |
McDonough 2005.
| Methods | Design: cluster‐RT Groups: intervention group (identification of glucocorticoid‐induced osteoporosis risk); control group (usual care) |
|
| Participants | Pharmacies 15 (8 intervention; 7 control) Pharmacy worker: pharmacists ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: patients believed to be at high risk for‐developing osteoporosis 96 (70 intervention; 26 control)
Setting: both urban and rural Country: USA |
|
| Interventions |
Pharmacy worker‐directed intervention: pharmacists received classroom education/training on the pathophysiology and management of glucocorticoid‐induced osteoporosis, and were given a packet of articles for independent study.
Pharmacy worker control: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received education; an educational pamphlet about the risks ‐ including behavioural risks ‐ of glucocorticoid‐induced osteoporosis; and pharmacists monitored the patients’ medical therapy, to identify and address medicine‐related problems
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: an unrestricted educational grant from Merck and Co, and by the Center for Improving Medication Use in the Community at the University of Iowa |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Baseline outcome measures similar | High risk | Significant difference by group for alcohol and bisphosphonate therapy at baseline. |
| Baseline characteristics similar | High risk | Significant difference by group for postmenopausal status at baseline. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts reported, but unclear how missing data were managed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment done by web‐based survey, but completed in pharmacy. |
| Protection against contamination | High risk | Both groups of pharmacists received education on glucocorticoid induced osteoporosis. |
| Selective reporting (reporting bias) | Low risk | Nothing noted |
| Other bias | High risk | Insufficient sample size to detect an effect, all pharmacists participated in research and trained in monitoring drug therapies. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is likely that pharmacists were aware of grouping. |
McLean 2003.
| Methods | Design: cluster‐RT (paired by geographic similarity) Groups: intervention group (enhanced pharmaceutical care); control group (usual care) |
|
| Participants | Pharmacies: 27 Pharmacy worker: 33 pharmacists, all of whom had prior training in the pharmaceutical care of asthma ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy users: 405 (191 enhanced care; 214 usual care) Setting: unclear Country: British Columbia, Canada |
|
| Interventions |
Pharmacy worker‐directed intervention: nothing additional to their pre study training in asthma ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: tailored education to patient's readiness to change (intervention only begun once someone in contemplation and strategies applied when in preparation). Taught correct inhaler technique, peak flow monitoring and self‐management skills, and enhanced pharmaceutical care
Pharmacy user control: taught inhaler technique and provided a minimum of 9 months usual care after which enhanced care was offered |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: the BC Community Pharmacy Asthma Study Funding source: Health Transition Fund, Health Canada, and Glaxo‐Smith‐Kline for educational materials and diaries |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By coin toss, central randomisation |
| Allocation concealment (selection bias) | Low risk | By coin toss, central randomisation |
| Baseline outcome measures similar | Unclear risk | No specific test of baseline similarity, although mean change was used in analysis. |
| Baseline characteristics similar | Unclear risk | No specific test of baseline similarity |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Considerable dropout, although numbers reported it was unclear whether this was corrected for. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear whether it was the pharmacist or an independent individual who conducted assessment interviews. |
| Protection against contamination | High risk | 11 'grand‐fathered' pharmacists appear to have offered both enhanced and usual care. |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Unclear risk | Although central randomisation used, there were different patient allocation methods which complicated study design. Usual care was also received from highly trained pharmacists which may not be reflective of all practice. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pharmacists aware of groupings |
McLean 2008.
| Methods | Design: RT Groups: intervention (pharmaceutical care); control (usual care) |
|
| Participants | Pharmacies: 14 Pharmacy workers: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 227 (115 intervention; 112 control)
Setting: unclear Country: Alberta, Canada |
|
| Interventions |
Pharmacy worker‐directed intervention: pharmacist training using a combination of an online learning program and a case‐based learning session ‐ both based on the Canadian Hypertension Education Program (CHEP) guidelines (www.hypertension.ca)
Pharmacy worker control: it appears that pharmacists treated both intervention and control groups. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: education about high blood pressure, diabetes and consequences, a focus on potential lifestyle changes, a BP wallet card, fax to GP
Pharmacy user control: a BP wallet card, a pamphlet on diabetes, general diabetes advice, a 12‐week follow‐up call and usual care |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: Study of Cardiovascular Risk Intervention by Pharmacists‐Hypertension (SCRIP‐HTN) Funding source ‐ grants from the Canadian Diabetes Association, Heart and Stroke Foundation of Canada, Canadian Council of Cardiovascular Nurses, Alberta Heritage Foundation for Medical Research, and Merck Frosst Canada Ltd McLean 2006 and Houle 2012 (cited under McLean 2008) also refer to this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed centrally to preserve allocation concealment using a computer‐generated sequence over a secure internet service at the Epidemiology Coordinating and Research (EPICORE) Centre. |
| Allocation concealment (selection bias) | Low risk | As above |
| Baseline outcome measures similar | Low risk | No reported differences |
| Baseline characteristics similar | Low risk | No reported differences |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up similar in both arms |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective measures, although assessors not blinded |
| Protection against contamination | High risk | Randomisation at patient level |
| Selective reporting (reporting bias) | Unclear risk | Not clear |
| Other bias | Low risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients and pharmacists were not blind to group. |
Mehuys 2008.
| Methods | Design: RT Groups: intervention group (asthma self‐management); control group (usual care) |
|
| Participants | Pharmacies: 66 Pharmacy worker: pharmacists ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 201 patients with asthma (107 intervention; 94 control)
Setting: urban Country: Belgium |
|
| Interventions |
Pharmacy worker‐directed intervention: a training session about asthma (pathophysiology), its non‐pharmacological and pharmacological treatment (Global Initiative for Asthma (GINA) guidelines), and about the use of the study protocol.
Pharmacy worker control: it appears that pharmacists saw both control and intervention participants. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: intervention focused on ensuring correct use of drug therapy including inhaler use and good adherence
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: funded by Ghent Unviersity |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Predetermined by the investigators based on a randomisation table. Serially numbered, closed envelopes were made for each participating pharmacy. The envelope with the lowest number was opened by the pharmacist upon inclusion of a new patient. |
| Allocation concealment (selection bias) | Low risk | See above |
| Baseline outcome measures similar | Low risk | Baseline variables used as covariates in the analyses |
| Baseline characteristics similar | Low risk | No significant differences |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Linear mixed model used with maximum‐likelihood method to handle missing data |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessment at 6 months |
| Protection against contamination | High risk | Randomisation at patient level, not pharmacy level |
| Selective reporting (reporting bias) | Low risk | Nothing noted |
| Other bias | Unclear risk | Potential selection bias, as only regular clients recruited |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pharmacists aware of patients' groups. |
Mehuys 2011.
| Methods | Design: cluster‐RT Groups: intervention group (self‐management of type 2 diabetes); control group (usual care) |
|
| Participants | Pharmacies: 66 Pharmacy worker: pharmacists ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 288 patients with type 2 diabetes (153 intervention; 135 control)
Setting: urban Country: Belgium |
|
| Interventions |
Pharmacy worker‐directed intervention: a training session about type 2 diabetes (pathophysiology), its non‐pharmacological and pharmacological management (current guidelines), and the study protocol
Pharmacy worker control: only received training on the study protocol ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received protocol‐defined intervention at start of the study and at each prescription refill visit (for hypoglycaemic medication) during the course of the study; received education on disease and medication management, lifestyle and annual reviews.
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: Ghent University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence of allocation to control or intervention group was predetermined by the investigators based on randomisation table generated using SPSS. |
| Allocation concealment (selection bias) | Low risk | Sequence of allocation predetermined by randomisation table. |
| Baseline outcome measures similar | Low risk | No differences between groups |
| Baseline characteristics similar | Low risk | No differences between groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal attrition |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective primary outcome |
| Protection against contamination | Low risk | Randomisation at pharmacy level |
| Selective reporting (reporting bias) | Low risk | Nothing noted |
| Other bias | Unclear risk | Potential selection bias, as only regular clients recruited. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear whether participants or pharmacists were aware of grouping, but likely. |
Nishita 2013.
| Methods | Design: RT Groups: intervention group (diabetes life‐coaching and pharmacist counselling); control group (usual care) |
|
| Participants | Pharmacies: 5 Pharmacy worker: 5 licensed pharmacists ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 190 patients with diabetes
Setting: urban Country: USA |
|
| Interventions |
Pharmacy worker‐directed intervention: pharmacists received a structured training which covered the 8 behaviours key to motivational interviewing. Additional training was provided on medication management, diabetes education, and diet and exercise support. Life coaches also had project training and diabetes education.
Pharmacy worker control: no training ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: pharmacists supported patients in setting and achieving lifestyle goals using motivational interviewing techniques. Patients also had access to life coaches where conversations could focus on lifestyle changes, diabetes health‐related behaviours or employment. In addition, participants were provided with access to additional intervention components that included nutrition and diabetes counselling, diabetes education materials a fitness club membership, and reimbursement for diabetes‐related medical expenses.
Pharmacy user control: no treatment |
|
| Outcomes |
Pharmacy worker: not accessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: Hawai‘i Demonstration to Maintain Independence and Employment (Hawai‘i DMIE) Funding source: Centers for Medicare and Medicaid Services |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised blocked design 2:1 allocation intervention:control, sealed envelopes |
| Allocation concealment (selection bias) | Low risk | As above |
| Baseline outcome measures similar | Low risk | No significant differences reported at baseline |
| Baseline characteristics similar | Low risk | No significant differences reported at baseline |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Multiple imputation to manage missing data |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective primary outcome |
| Protection against contamination | High risk | Randomisation at pharmacy user level |
| Selective reporting (reporting bias) | Low risk | Nothing noted |
| Other bias | Low risk | Nothing noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pharmacy workers must have been aware of group allocation. |
Nola 2000.
| Methods | Design: RT Groups: intervention group (lipid management program); control group (usual care) |
|
| Participants | Pharmacies: not targeted Pharmacy worker: not targeted ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 51 patients at risk of coronary artery disease (25 intervention; 26 control)
Setting: urban Country: USA |
|
| Interventions |
Pharmacy worker‐directed intervention: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received the lipid management program: diet and exercise evaluation and instruction, monitoring of cholesterol levels, monitoring of drug therapy, collaboration with physicians, education.
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: Pharmacia‐Upjohn Corporation and education grant from Novartiz and Bristol‐Myers Squibb |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomization schedule was developed using a computer‐generated list of random numbers" |
| Allocation concealment (selection bias) | Low risk | As above |
| Baseline outcome measures similar | Low risk | No significant differences between groups at baseline |
| Baseline characteristics similar | Low risk | No significant differences between groups at baseline |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear how missing data managed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information on blinding but objective outcomes |
| Protection against contamination | High risk | In‐pharmacy randomisation |
| Selective reporting (reporting bias) | Low risk | Nothing noted |
| Other bias | Unclear risk | Possible that seasonal fluctuations influenced outcomes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pharmacists must have been aware of group allocation. |
Okada 2018.
| Methods | Design: cluster‐RT Groups: intervention group (lifestyle support for blood pressure); control (usual care) |
|
| Participants | Pharmacies: 73 (37 intervention; 36 control) Pharmacy worker: pharmacist ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 125 hypertensive patients (64 intervention; 61 control)
Setting: unclear Country: Japan |
|
| Interventions |
Pharmacy worker‐directed intervention: training in motivational interviewing‐based communication
Pharmacy worker control: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: home blood pressure monitoring, healthy lifestyle advice using motivational interviewing and brochures
Pharmacy user control: provided with home blood pressure monitor and basic explanation of medications |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: COMmunity Pharmacists ASSist for Blood Pressure (COMPASS‐BP) Funding source: KAKENHI Grant in Aid |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Baseline outcome measures similar | High risk | Intervention group had lower blood pressure at baseline |
| Baseline characteristics similar | High risk | Differences on several measurements at baseline |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Final analysis used carry forward method to address missing data |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was blinded |
| Protection against contamination | Low risk | Cluster design |
| Selective reporting (reporting bias) | Low risk | Not apparent |
| Other bias | High risk | Only patients who had adhered sufficiently to a strict 2‐week run‐in monitoring period were recruited so the sample may not be representative of less motivated individuals. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Group not blinded |
Park 1996.
| Methods | Design: RT Groups: intervention group (Pharmaceutical Scare for Hypertension); control group (usual care) |
|
| Participants | Pharmacies: 2 Pharmacy worker: pharmacy resident ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 53 hypertensive patients (27 intervention; 26 control)
Setting: urban Country: USA |
|
| Interventions |
Pharmacy worker‐directed intervention: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received counselling on lifestyle modifications that would help them manage their condition, and especially their medical treatment.
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but method not specified. Furthermore, article stated "the randomization was not balanced. Far more control patients (26.9%) than study patients (17.4%) had controlled blood pressure, and more study patients (13%) had stage III hypertension at baseline randomization." |
| Allocation concealment (selection bias) | High risk | Patients were not aware, but the pharmacy residents were aware of the allocation. |
| Baseline outcome measures similar | High risk | Reported that at baseline there were differences in blood pressure and severity of hypertension |
| Baseline characteristics similar | High risk | Reported that at baseline there were differences in blood pressure and severity of hypertension |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts reported, but not clear how managed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Protection against contamination | High risk | Randomisation at patient level |
| Selective reporting (reporting bias) | High risk | Whilst significance for outcomes was reported, this was only shown in graphs. |
| Other bias | Unclear risk | Study sample size may have been underpowered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A limitation of this study was that it was a Single‐blinded study. The pharmacy residents were aware of patient assignment into two different treatment groups, which could have introduced bias." |
Patwardhan 2012.
| Methods | Design: cluster‐RT Groups: intervention group (tobacco cessation counselling); control group (usual care) |
|
| Participants | Pharmacies: 16 Pharmacy worker: 32 pharmacists, 48 technicians
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: not assessed Setting: urban Country: USA |
|
| Interventions |
Pharmacy worker‐directed intervention: Ask‐Advise‐Refer (AAR) tobacco cessation counselling through 30 minutes on‐site training, recommendations for integrating AAR in pharmacy work flow, a cessation poster and a support visit that drew on social cognitive theory. Also given the same materials as the control group.
Pharmacy worker control: received quit line cards (a card with the telephone number to access free behavioural support), an informational presentation about the quit line and its services, and enrolment in a free service called Fax‐to‐Quit (FTQ). FTQ enabled pharmacies to refer tobacco users proactively to the quit line by faxing a signed consent form that allowed the quit line to call users back directly to initiate cessation treatment. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: AAR tobacco cessation counselling, given quit line cards which have the telephone number of a quit line which provided free counseling and free medication
Pharmacy user control: received quit line cards and FTQ |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: not assessed |
|
| Notes | Study/intervention name: none given Funding: Clinical and Translational Science Award, NIH and Wisconsin Department of Health Services and Sonderegger Research Centre Patwardhan 2009 and Patwardhan 2010 (cited under Patwardhan 2012) also refer to the same intervention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Researchers blinded to study goal |
| Baseline outcome measures similar | Low risk | No differences reported |
| Baseline characteristics similar | Low risk | No differences reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected from objective records |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who collected data |
| Protection against contamination | Low risk | Cluster design |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Outcome measures, except for quit line records, were self‐report by pharmacists |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Pharmacy staff not informed of the existence of 2 groups and therefore blind |
Paulos 2005.
| Methods | Design: RT Groups: intervention group (pharmaceutical care for dyslipidaemia); control group ('normal counselling') |
|
| Participants | Pharmacies: 1 Pharmacy worker: pharmacists ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 42 patients with dyslipidaemia (23 intervention; 19 control)
Setting: unclear Country: Chile |
|
| Interventions |
Pharmacy worker‐directed intervention: not described ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received education on the role of cholesterol in illness and health, explaining risk factors associated with cardiovascular disease, and providing education/counselling regarding medication.
Pharmacy user control: usual treatment (2 interviews) |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: not reported although Roche Diagnostics provided Accutrend GCT device and strips. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but specific method not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Baseline outcome measures similar | Unclear risk | Not reported |
| Baseline characteristics similar | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but objective primary outcome |
| Protection against contamination | High risk | Randomisation at client level |
| Selective reporting (reporting bias) | High risk | No full reporting of all outcomes at all time points |
| Other bias | Unclear risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
Petkova 2008.
| Methods | Design: RT Groups: intervention group (asthma self‐management); control group (usual care) |
|
| Participants | Pharmacies: 10 (those pharmacies with the highest number of asthma patients) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 50 (22 intervention; 28 control)
Setting: urban, Sofia Country: Bulgaria |
|
| Interventions |
Pharmacy worker‐directed intervention: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: education program with information on asthma, medication, inhalers, drug reactions, exacerbation and control of asthma attacks and smoking cessation
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Assigned based on principle of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No detail provided |
| Baseline outcome measures similar | Low risk | No differences on outcomes |
| Baseline characteristics similar | High risk | Differences between groups at the start of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent attrition on main outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if assessor blind to group |
| Protection against contamination | High risk | Pharmacies offer both intervention and control |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Low risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if participants aware of grouping |
Petkova 2009.
| Methods | Design: RT Groups: intervention (arthritis management); control (usual care) |
|
| Participants | Pharmacies: not reported Pharmacy worker: not targeted ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 90 (45 intervention; 45 control)
Setting: urban, Sofia Country: Bulgaria |
|
| Interventions |
Pharmacy worker‐directed intervention: 3‐day intensive training. Review of disease, pain management, risks, exercise, joint protection, role‐play
Pharmacy worker control: it appears the same pharmacist delivered intervention and control treatment. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: education program with information on arthritis, heat‐cold therapy, physical training, pain management, self‐study leaflets
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random number generator |
| Allocation concealment (selection bias) | Unclear risk | Used random number generator |
| Baseline outcome measures similar | High risk | There were differences between the intervention and control groups at baseline for a variety of variables which were not controlled for in analysis. |
| Baseline characteristics similar | High risk | Differences in age and healthcare use, without evidence that these were controlled for in analyses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal attrition |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if assessor knew patient grouping |
| Protection against contamination | High risk | Randomisation at patient level |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pharmacists must have been aware of group allocation |
Planas 2012.
| Methods | Design: RT Groups: intervention group (diabetes management); control group (standard care) |
|
| Participants | Pharmacies: 5 Pharmacy worker: pharmacists ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 65 patients with diabetes and hypertension (38 intervention; 27 control)
Setting: urban Country: USA |
|
| Interventions |
Pharmacy worker‐directed intervention: training on diabetes management, including the most recent treatment guidelines for diabetes, hypertension and dyslipidaemia, and on study procedures. Compensated by pharmacy chain
Pharmacy worker control: it appears the same pharmacists delivered treatment to both intervention and control groups. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patient education and diabetes management services
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: American Society of Health System Pharcists Research and Education Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by previously generated random number list |
| Allocation concealment (selection bias) | Unclear risk | Unclear if allocation was concealed |
| Baseline outcome measures similar | Low risk | No differences reported |
| Baseline characteristics similar | High risk | Difference in BMI |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported that used carry forward of missing data, but some exclusions if the 3 month visit was not attended, also significant dropout |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Variable use of objective primary outcome |
| Protection against contamination | Unclear risk | Individuals were allowed to choose what intervention to visit, it is possible that pharmacies offered both intervention and control |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Unclear risk | All pharmacies belonged to the same chain, which is a cause of potential bias. Each participant had to attend the initial 3 month visit to be included in analyses. Participants who dropped out of the study before the 3 month period were excluded from analyses because no effect of intervention on the outcome measures could be determined. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned ‐ not blinded |
Schmiedel 2015.
| Methods | Design: cluster‐RT Groups: intervention group (diabetes prevention); control group (no treatment) |
|
| Participants | Pharmacies: 22 (11 intervention; 11 control) Pharmacy worker: pharmacists ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 1092 (565 intervention; 575 control)
Setting: unclear Country: Germany (Bavaria) |
|
| Interventions |
Pharmacy worker‐directed intervention: half day training on behaviour change + 1 day on how to conduct the trial
Pharmacy worker control: 1 day training on how to conduct the trial ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: 3 individual counselling sessions and 5 group lectures covering diabetes and lifestyle issues and personalised goals. Provided written information on healthy diet and physical activity
Pharmacy user control: assessed and informed about their health status but no further counselling. |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: GLICEMIA (this is the program name not an acronym) Funding source: Dr August and Dr Anni Lesmuller‐Stiftung Foundation, the Bavarian State Ministry of Public Health and Care Services (through the funding and health promotion initiative Gesund Leben Bayern), the Bavarian State Corporate Health Insurers, and the funding initiative for prevention (Forderinitiative Pravention eV). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline outcome measures similar | Low risk | No significant differences |
| Baseline characteristics similar | Low risk | No significant differences |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data imputed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Protection against contamination | Low risk | Multi‐centre cluster‐RT |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
Skowron 2011.
| Methods | Design: cluster‐RT Groups: intervention group (pharmaceutical care for hypertension); control group (standard care) |
|
| Participants | Pharmacies: 55 (28 intervention; 27 control) Pharmacy worker: 95 pharmacists (44 intervention; 51 control) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 193 patients with hypertension (70 intervention; 123 control)
Setting: urban, Krakow Country: Poland |
|
| Interventions |
Pharmacy worker‐directed intervention: training on detection, classification and monitoring of drug‐related problems, pathophysiology of hypertension, risk factors and life‐style factors influencing the disease, and rules of pharmacotherapy of hypertension
Pharmacy worker control: wait list; received the same training as the intervention group after final study visit ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received pharmaceutical care and were educated about pathophysiology, risk factors, treatment and style of life with hypertension, as well as blood pressure measurement, and self‐measurement of blood pressure.
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: no specific grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization of community pharmacies to control and study group was done by generation of random numbers by computer software." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization of community pharmacies to control and study group was done by generation of random numbers by computer software." |
| Baseline outcome measures similar | Unclear risk | Differences in baseline for education, age and place of residence. Unclear if this was accounted for in the analysis. |
| Baseline characteristics similar | High risk | Differences in education, age and residence |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant number of dropouts from both groups |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information on blinding |
| Protection against contamination | Low risk | Randomisation by pharmacies |
| Selective reporting (reporting bias) | High risk | No numerical reporting of quality of life |
| Other bias | High risk | High number of control pharmacies withdrew |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information about blinding provided |
Slater 2013.
| Methods | Design: cluster‐RT Groups:
|
|
| Participants | Pharmacy worker: 35 pharmacies (11 in group 1; 11 in group 2; 13 in control) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
Setting: urban Country: Australia |
|
| Interventions |
Pharmacy worker‐directed intervention: pharmacist staff allocated to the pamphlet + education intervention were provided with specific training: pretrial workshops by the study team, during which pharmacists were instructed about the key pamphlet messages to reinforce and were advised about the necessity of delivering these messages strictly in accordance with the pamphlet content.
Pharmacy worker control: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: Intervention group 1 (pamphlet + education): in addition to usual care, participants received verbal reinforcement of the pamphlet’s content from a trained pharmacy staff member Intervention group 2 (pamphlet only): in addition to usual care, participants were provided with the pamphlet, but without further specific reinforcement of pamphlet content.
Pharmacy user control: no pamphlet at the time of the trial |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: grant by Department of Health, Government of Western Australia and Curtin University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Pharmacies from within each SEIFA block were then randomised (simultaneously)" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation of pharmacies was concealed from the PSWA [Pharmaceutical Society of Western Australia] and the investigator (KW) [Kim Watkins] who provided access to the clusters." |
| Baseline outcome measures similar | Low risk | Analyses adjusted for baseline scores |
| Baseline characteristics similar | Low risk | Analyses adjusted for baseline scores |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used likelihood‐based estimation procedure for missing data |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blinding to group allocation included primary investigators, outcome assessors and the statistician." |
| Protection against contamination | Low risk | Randomisation by pharmacists |
| Selective reporting (reporting bias) | Low risk | Nothing noted |
| Other bias | Unclear risk | Possible selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Pharmacy staff and consumers were un‐blinded." |
Smith 2011.
| Methods | Design: cluster‐RT Groups: intervention group (goal setting for allergic rhinitis); control group (standard pharmacy care) |
|
| Participants | Pharmacies: 20 (8 intervention; 12 control) Pharmacy workers: 38 (22 clinicians; 16 non‐clinicians) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 150 patients with intermittent allergic rhinitis (IAR) (77 intervention; 77 control)
Setting: urban Country: Australia |
|
| Interventions |
Pharmacy worker‐directed intervention: workshop covering the pathophysiology of allergic rhinitis (AR), the current 'Allergic Rhinitis and its Impact on Asthma' (ARIA) guidelines and pharmacotherapy relating to specific AR symptoms. Also training in self‐management theory, goal setting and up‐skilling in patient counselling
Pharmacy worker control: only received the workshop covering the pathophysiology of AR, the current ARIA guidelines and pharmacotherapy relating to specific AR symptoms. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received an informational brochure and received a goals card titled ‘‘My Goals and Treatment Card’’ where two goals were stated: ‘‘Eliminate/minimise hay fever symptoms’’ and ‘‘Avoid/minimise hay fever triggers’’ to record what they experienced. Individually tailored strategies were developed from these data collaboratively between the participant and the pharmacist or assistant, and entered onto the goals card.
Pharmacy user control: usual treatment and a take‐home brochure on AR at follow‐up visit |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: Pharmacy Allergic Rhinitis Intervention Study (PARIS) Funding source: funded by the Australian Government Department of Health and Ageing as part of the Fourth Community Pharmacy Agreement Research & Development Program managed by the Pharmacy Guild of Australia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but method not specified |
| Allocation concealment (selection bias) | Unclear risk | Randomised, but method not specified |
| Baseline outcome measures similar | Low risk | No significant differences between groups |
| Baseline characteristics similar | Low risk | No significant differences between groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding |
| Protection against contamination | Low risk | Randomisation by pharmacists |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Only a small group of pharmacies |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Presumably pharmacy workers knew grouping |
Svarstad 2013.
| Methods | Design: cluster‐RT Groups: intervention group (hypertension intervention); control group (usual care ) |
|
| Participants | Pharmacies 28 (14 intervention; 14 control) Pharmacy worker: pharmacist ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 576 black patients with hypertension
Setting: urban Country: USA |
|
| Interventions |
Pharmacy worker‐directed intervention: 7 hours of continuing education through an interactive workshop that included a lecture, slides, handouts, a demonstration and practice, role play case studies, and break‐out discussions. One additional hour of self‐study, clinical guidelines summary, clinical tools e.g. BP monitoring equipment. Also BP clinic hours established.
Pharmacy worker control: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients were sent brochures and received an intervention using scheduled visits, Brief Medication Questionnaires (BMQs), and new toolkits including a pill organiser, BP tracker, pedometer, and tips and goals
Pharmacy user control: received patient information only, including a 14‐page guide for lowering BP, pamphlet about hypertension in black people, and cards showing their BP at baseline and follow‐up interviews, and instructions on when to seek immediate medical care for high BP. |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: Team Education and Adherence Monitoring (TEAM) Funding source: National Heart, Lung and Blood Institute (NHLBI) #R01HL78580 Svarstad 2009 and Shireman 2016 also refer to this study (cited under Svarstad 2013). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer software for randomisation |
| Allocation concealment (selection bias) | Low risk | Computer software for randomisation |
| Baseline outcome measures similar | Low risk | No differences |
| Baseline characteristics similar | High risk | Difference between groups for physical activity |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how managed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded data collectors |
| Protection against contamination | Unclear risk | Randomisation by pharmacy |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Low risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clients aware of grouping |
Tommelein 2014.
| Methods | Design: RT Groups: intervention (pharmaceutical care); control (usual care) |
|
| Participants | Pharmacies: 22 (11 intervention; 11 control) Pharmacy workers: 170 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 734 chronic obstructive pulmonary disease (COPD) patients (371 intervention; 363 control)
Setting: unclear Country: Beligum |
|
| Interventions |
Pharmacy worker‐directed intervention: training session addressing pathophysiology of COPD and its nonpharmacologic and pharmacological treatment
Pharmacy worker control: not clear whether control pharmacists also received the same training session as intervention pharmacists ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: counselling sessions, addressing basic knowledge of COPD, inhalation technique and self‐management and lifestyle issues
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: Pharmaceutical care of patients with COPD (PHARMACOP) Funding source: Ghent University, Liège University and GlaxoSmithKline (grant protocol number 114684) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based system |
| Allocation concealment (selection bias) | Low risk | Central system |
| Baseline outcome measures similar | Low risk | Similar |
| Baseline characteristics similar | Low risk | Similar |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis accounted for missing data |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Pharmacists assessed inhalation techniques |
| Protection against contamination | Unclear risk | Possible due to design |
| Selective reporting (reporting bias) | Low risk | Not apparent |
| Other bias | Low risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pharmacists not blinded, but patients were not told of group assignment |
Tsuyuki 2002.
| Methods | Design: RT Groups: intervention group (cholesterol risk management); control group (usual care) |
|
| Participants | Pharmacies: 54 Pharmacy workers: pharmacists ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 675 patients with high risk of vascular events (344 intervention; 331 control)
Setting: both urban and rural Country: Alberta and Saskatchewan, Canada |
|
| Interventions |
Pharmacy worker‐directed intervention: training sessions to review the management of heart disease risk factors, especially hyperlipidaemia
Pharmacy worker control: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received a brochure; pharmacists completed a physician contact form that listed the patient's risk factors, medications and any recommendations; patients were encouraged to contact physician; and also received education about cardiovascular risk factors to reinforce adherence
Pharmacy user control: patients given a copy of the same brochure and general advice only |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: the Study of Cardiovascular Risk Intervention by Pharmacists (SCRIP) Funding source: University of Alberta Hospital Foundation, Merck Frossst Canada Inc, SCRIP study Tsuyuki 1999, Simpson 2001, and Simpson 2004 (cited under Tsuyuki 2002) also refer to this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation stratified by pharmacy, computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Block size for randomisation was not revealed to ensure allocation concealment |
| Baseline outcome measures similar | Low risk | Baseline scores controlled for in analyses |
| Baseline characteristics similar | Low risk | Baseline scores controlled for in analyses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition, balanced across groups |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessed by pharmacist |
| Protection against contamination | High risk | Patient‐level of randomisation |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Unclear risk | Pharmacies were highly selected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded study |
Tsuyuki 2016 ‐ RxACT.
| Methods | Design: RT Groups: intervention (dyslipidaemia care); control (usual care + pamphlet on CV risk) |
|
| Participants | Pharmacies: 14 Pharmacy workers: 22 (intervention 11; control 11) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: uncontrolled dyslipidaemia 99 (intervention 49; control 50)
Setting: unclear Country: Alberta, Canada |
|
| Interventions |
Pharmacy worker‐directed intervention: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: identification, assessment, care plan development, education/counselling on CV risk, medications and health behaviour. Also prescribing/titration of lipid‐lowering medications and close follow‐up.
Pharmacy user control: usual care, lipid results and a pamphlet on CVD |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: RxACT (no expansion of this name provided) Funding source: AstraZeneca grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Via a secure website |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Baseline outcome measures similar | Low risk | Similar |
| Baseline characteristics similar | Low risk | Similar |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Last value carried forward for missing data |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcome |
| Protection against contamination | High risk | Randomisation at patient level |
| Selective reporting (reporting bias) | Low risk | Not apparent |
| Other bias | Low risk | None noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible |
Tsuyuki 2016 ‐ RxEACH.
| Methods | Design: RT Groups: intervention (CV risk assessment and education); control (usual care) |
|
| Participants | Pharmacies: 56 Pharmacy worker: 723 (370 intervention; 353 control) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: high risk for CVD 723
Setting: unclear Country: Alberta, Canada |
|
| Interventions |
Pharmacy worker‐directed intervention: online modules on case finding, CVD and risk factors, communicating risk, lifestyle behaviours
Pharmacy worker control: it appears all pharmacists had training and saw both intervention and control patients. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: medication therapy management assessment, and education including lifestyle
Pharmacy user control: usual care with no specific intervention |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: Alberta Vascular Risk Reduction Community Pharmacy Project (RxEACH) Funding source: Alberta Health, Merck Canada funds for educational materials Al Hamarneh 2017 and Al Hamarneh 2018 (cited under Tsuyuki 2016 ‐ RxEACH) also refer to this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Baseline outcome measures similar | Low risk | Similar |
| Baseline characteristics similar | Low risk | Similar |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Accounted for in analysis |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcome |
| Protection against contamination | High risk | Pharmacists delivered both intervention and control |
| Selective reporting (reporting bias) | Low risk | Not apparent |
| Other bias | Low risk | None detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pharmacists not blinded |
Venkatesan 2012.
| Methods | Design: RT Groups: intervention group (pharmaceutical care model for diabetes); control group (usual care) |
|
| Participants | Pharmacies: 2 Pharmacy worker: pharmacist ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 39 patients with type 2 diabetes (19 intervention; 20 control)
Setting: rural Country: Tamil Nadu, India |
|
| Interventions |
Pharmacy worker‐directed intervention: not targeted ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received diabetic medication counselling, printer educational material and instructions on dietary regulation, exercise and lifestyle modifications.
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker: not targeted ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: Tamil Nadu Pharmaceutical Sciences Welfare Trust, Chennai, India |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but method not specified |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Baseline outcome measures similar | Unclear risk | Not clear if any differences were significant |
| Baseline characteristics similar | Unclear risk | Not clear if any differences were significant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear if blinded, but objective outcome |
| Protection against contamination | High risk | Randomisation at level of patient |
| Selective reporting (reporting bias) | Low risk | Objective outcomes presented in text |
| Other bias | Unclear risk | Low power |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information about blinding |
Villeneuve 2010.
| Methods | Design: cluster‐RT Groups: intervention group (collaborative dyslipidaemia management); control group (usual care) |
|
| Participants | Pharmacies: 15 (from 148 eligible) Personnel: 77 physicians; 108 pharmacists
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 225 patients with dyslipidaemia (108 intervention; 117 control)
Setting: unclear Country: Canada |
|
| Interventions |
Pharmacy worker‐directed intervention: pharmacists in the collaborative care group attended a 1‐day training workshop. During this workshop, formal lectures, role‐playing and interactive exercises were used to present the Canadian treatment recommendations, guidance about the pharmacotherapy, information about the treatment protocol, and communication strategies for optimising adherence + a 2‐hour gathering to discuss the intervention after 1 month.
Pharmacy worker control: no additional training ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received counselling and a treatment plan, which included lifestyle changes and pharmacotherapy.
Pharmacy user control: usual treatment |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: Trial to Evaluate an Ambulatory primary care Management program for patients with dyslipidemia (TEAM) Funding: funded by a grant from the Canadian Institutes of Health Research (grant number 200409MCT‐133732‐RCT) and unrestricted research grants from AstraZeneca Canada Inc, Merck Frosst Canada Ltd and Pfizer Canada Inc. Villeneuve 2009 and Villeneuve 2007 (cited under Villeneuve 2010) also refer to the same study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We stratified the randomization by type of medical clinic and number of physicians per cluster. We also blocked the clusters, with two or four clusters per block and balanced randomization within each block' |
| Allocation concealment (selection bias) | Low risk | Quote: "We stratified the randomization by type of medical clinic and number of physicians per cluster. We also blocked the clusters, with two or four clusters per block and balanced randomization within each block' |
| Baseline outcome measures similar | Low risk | Adjustment for baseline in analyses |
| Baseline characteristics similar | Low risk | Adjustment for baseline in analyses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used last value carried forward approach |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on blinding |
| Protection against contamination | Low risk | Randomisation by pharmacy |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Only 15 of 148 clusters eligible and agreed to participate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding |
Weinberger 2002.
| Methods | Design: cluster‐RT Groups:
|
|
| Participants | Pharmacies: 36 (12 pharmacies per group) Pharmacy worker: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 1113 patients with asthma or COPD (447 PCP; 363 PFMCG; 303 UCCG)
Setting: urban Country: Indianapolis, USA |
|
| Interventions |
Pharmacy worker‐directed intervention: included an overview of pharmaceutical care, orientation to study, interpretation and use of data, measuring PEF, resources.
Pharmacy worker control: pharmacists received 4‐hour training, but were excluded from PCP ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received individualised handouts based on problems associated with specific clinical data stored on the computer
Pharmacy user control: usual care |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: none given Funding source: Agency for Healthcare Research and Quality and the Health Services Research and Development Service, Department of Veteran Affairs (grant 5 R01 HS09083) Weinberger 2001 (cited under Weinberger 2002) also refers to the same study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random number chart |
| Allocation concealment (selection bias) | Low risk | Quote: "interviewers, blinded to study group assignment, obtained informed consent and conducted baseline interviews. After completing the interview, the laptop computer used to administer interviews revealed the patient’s study group assignment.” At that time, interviewers distributed peak flow meters as appropriate. |
| Baseline outcome measures similar | Low risk | Differences between groups controlled for in analyses |
| Baseline characteristics similar | Low risk | Differences between groups controlled for in analyses |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts had worse breathing problems at 12 months |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers blinded |
| Protection against contamination | Low risk | Cluster randomised |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | High risk | Fidelity may have been low, as pharmacists only implemented protocol approximately 50% of the time |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pharmacists aware of groupings |
Yuksel 2010.
| Methods | Design: RT Groups: intervention group (osteoporosis risk management); control group (usual care) |
|
| Participants | Pharmacy worker: not targeted ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user: 262 patients with osteoporosis (129 intervention; 133 control)
Setting: unclear Country: Canada |
|
| Interventions |
Pharmacy worker‐directed intervention: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients received tailored education program on aspects of osteoporosis; including risk factors, bone mineral density testing, lifestyle measures, calcium and vitamin D intake, and medications and written information, and discussion of heel ultrasound
Pharmacy user control: usual treatment and information provided by pharmacy |
|
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
|
| Notes | Study/intervention name: OSTEOPHARM (no expansion of acronym provided). By a grant from the Institute of Health Economics (Edmonton) and Faculty Start Up Grant to Nesé Yuksel from the Faculty of Pharmacy and Pharmaceutical Sciences (University of Alberta) Yuksel 2006 (cited under Yuksel 2010) also refers to this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Internet randomisation |
| Allocation concealment (selection bias) | Low risk | Internet randomisation |
| Baseline outcome measures similar | Low risk | Similar |
| Baseline characteristics similar | Low risk | Similar ‐ control group had higher family history of osteoporosis but unlikely to change result |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used intention‐to‐treat analysis; similar dropout in both groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded |
| Protection against contamination | High risk | Both groups were based in the same pharmacies |
| Selective reporting (reporting bias) | Low risk | Not noted |
| Other bias | Low risk | Not noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
BMI: body‐mass index; BP: blood pressure; cluster‐RT: cluster randomised trial; COPD: chronic obstructive pulmonary disease; CV: cardiovascular; CVD: cardiovascular disease; DBP: diastolic blood pressure; EQ‐5D: Euroqol Measure of quality of life; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; GP: general practitioner (family doctor); HbA1c: glycosylated haemoglobin; HCU: health care utilisation; HDL: high‐density lipoprotein; HDL‐C: high‐density lipoprotein cholesterol; LDL: low‐density lipoprotein; LDL‐C: low‐density lipoprotein cholesterol; NHS: National Health Service; PEF: peak expiratory flow; PEFR: peak expiratory flow rate; RT: randomised trial; SBP: systolic blood pressure; SD: standard deviation; SF‐12: short form‐12; STD: sexually transmitted disease; TC: total cholesterol; TDF: theoretical domains framework; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahrens 2003 | Compared 2 active intervention groups |
| Aleo 2014 | Inappropriate intervention |
| Ammari 2013 | Compared 2 active intervention groups. Control not randomised or representative |
| Anderson 1995 | Inappropriate design, retrospective controlled study |
| Anderson 2003 | Inappropriate design |
| Armour 2004 | Inappropriate design |
| Armour 2013 | Compared 2 active intervention groups; 3 versus 4 counselling sessions |
| Basheti 2005 | Compared 3 active intervention groups ‐ 3 forms of verbal counselling for turboinhaler device |
| Bauld 2009 | Compared 2 active intervention groups ‐ 1:1 versus group smoking cessation |
| Bernsten 2001 | Inappropriate intervention |
| Bock 2010 | Compared 2 intervention groups; control group not concurrent or randomised |
| Butt 2016 | Not community pharmacy |
| Chabot 2003 | Inappropriate design |
| Chalker 2002 | Unclear intervention ‐ focus primarily on medication, no valid outcomes |
| Cody 1998 | Not community pharmacy |
| Correr 2009 | Inappropriate intervention |
| Crawford 2013 | No validated outcomes, only process‐level outcomes (pharmacy support for service) |
| De Vera 2014 | Inappropriate intervention |
| de Vries 2010 | Inappropriate intervention |
| Denig 2003 | Inappropriate intervention |
| DeRemer 2008 | Inappropriate design |
| DiDonato 2013 | Inappropriate design |
| Ditusa 2001 | Not community pharmacy |
| Ekedahl 2008 | Inappropriate intervention |
| Fera 2008 | Inappropriate design |
| Fikri‐Benbrahim 2012 | Inappropriate design |
| Fornos 2006 | Inappropriate intervention |
| Fuller 2007 | Inappropriate outcomes |
| Garcao 2002 | Inappropriate intervention |
| Goeree 2013 | Inappropriate intervention |
| Gorgas 2012 | Inappropriate intervention |
| Grainger‐Rousseau 1997 | Inppropriate intervention |
| Green 2008 | Not community pharmacy |
| Haga 2017 | Inappropriate intervention |
| Herborg 2001 | Inappropriate design |
| Kaczorowski 2008 | Not community pharmacy |
| Karwalajtys 2009 | Inapproriate intervention |
| Kradjan 1999 | Inappropriate outcome |
| Krass 2011 | Comparison of 2 active interventions (6 months versus 12 months) and no control group |
| Kritikos 2007 | Inappropriate design |
| Kumar, 2009 | Not community pharmacy |
| Lalonde 2008 ‐ PRoFIL | Inappropriate intervention |
| Lugo de Ortellado 2007 | Inappropriate intervention |
| Manfrin 2015 | Inappropriate intervention |
| Mangiapane 2005 | Inappropriate design |
| Marra 2012 | The intervention included both education from a pharmacist, exercise from a physiotherapist and referral to a self‐management programme. It was not possible to identify the contribution of the pharmacist's intervention. |
| Marrero 2006 | Inappropriate intervention |
| Meijer 2005 | No validated outcome |
| Michie 2014 | No validated outcomes |
| Michiels 2017a | Inappropriate intervention |
| Noor 2016 | Inappropriate design |
| O'Dwyer 2016 | Inappropriate intervention |
| Obarcanin 2015 | Not community pharmacy |
| Olivera 2016 | Inappropriate intervention |
| Phimarn 2017 | Inappropriate design ‐ comparison of 2 interventions |
| Podhipak 1993 | Inappropriate intervention |
| Prokhorov 2010 | 2 active interventions (smoking cessation counselling versus skin cancer prevention counselling); only process outcomes and not validated. |
| Ratanajamit 2002 | Inappropriate intervention |
| Rickles 2006 | Inappropriate intervention |
| Rouleau 2007 | Unable to retrieve |
| Rubio‐Valera 2009 | Inappropriate intervention |
| Saini 2008 | Inappropriate design |
| Saji 2012 | Inappropriate setting (clinic and pharmacy) |
| Santos 2010 | Unable to retrieve |
| Sarayani 2012 | 3 active interventions (3 different intervention formats) and only process outcomes |
| Sarkadi 2004 | Inappropriate outcomes |
| Sinclair 1998 | No objective outcomes, only self‐reported smoking status provided |
| Sperandio 2012 | Inappropriate intervention |
| Stergachis 2002 | Not community pharmacy (< 50% community pharmacy) |
| Suppapitiporn 2005 | Not community pharmacy |
| Taskila 2012 | Compared 2 active interventions |
| Thavorn 2008 | Not community pharmacy |
| Tobari 2010 | Not community pharmacy |
| Tsuyuki 2015 | Not clear whether it was community pharmacy |
| Tumwikirize 2004 | Inappropriate intervention |
| Usami 2009 | Unable to retrieve |
| Van de Steeg‐van 2011 | Inappropriate intervention |
| Viens 2007 | Inappropriate intervention |
| Wang 2013 | Inappropriate intervention |
| Watson 2002 | Inappropriate design |
| Westrick 2016 | Comparison of 2 interventions, no control group |
| Wilson 2004 | Inappropriate intervention/design |
| Young 2012 | Setting not clearly community pharmacy |
Characteristics of ongoing studies [ordered by study ID]
Davis 2016.
| Trial name or title | Effectiveness of a pharmacist‐driven intervention in COPD (EPIC) |
| Methods | Design: cluster‐RT Groups: intervention group (enhanced care for management of COPD); control group (usual care) |
| Participants | Pharmacies: 20 (10 intervention; 10 control) Pharmacy workers: pharmacists ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy users: 140 patients with COPD Setting: unclear Country: Canada |
| Interventions |
Pharmacy worker‐directed intervention: refresher COPD management training, and how to deliver the intervention Pharmacy worker control: training on study protocols ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: 7 elements:
Pharmacy user control: usual care and a COPD education pamphlet |
| Outcomes |
Pharmacy worker: not targeted ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
| Starting date | May 2016 |
| Contact information | emdavis@mun.ca |
| Notes | Funding: Health Research Foundation: Canada |
Ekers 2017.
| Trial name or title | Community pHarmaciEs Mood Intervention STudy (CHEMIST) |
| Methods | Design: pilot RT Groups: intervention group (enhanced support for depression); control group (usual care) |
| Participants | Pharmacies: 7 Pharmacy users: 130 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy users: patients with sub‐threshold depression Setting: unclear Country: UK |
| Interventions |
Pharmacy worker‐directed intervention: none specific but pharmacists must have experience of extended role or training to Royal Society of Public Health standard (Understanding Health Improvement Level 2). ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: behavioural activation focused self‐help support; proactive follow‐up; symptom monitoring; and decision supported signposting
Pharmacy user control: usual care |
| Outcomes |
Pharmacy worker: not targeted ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
| Starting date | 13 June 2016 |
| Contact information | liz.littlewood@york.ac.uk; david.ekers@york.ac.uk |
| Notes | Funding NIHR |
Michiels 2017.
| Trial name or title | Impact of a Community Pharmacy‐Based Information Program on Type 2 Diabetic Patients’ Adherence to Their Oral Treatment: (Iphodia) |
| Methods | Design: cluster‐RT Groups: intervention group (diabetes management); control group (usual care) |
| Participants | Pharmacies: 182 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy users: 800 patients with type 2 diabetes (required from sample size calculation) Setting: unclear Country: France |
| Interventions |
Pharmacy worker‐directed intervention: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: thematic information on diabetes, namely diet for diabetics, monitoring drug treatment and the complications of diabetes
Pharmacy user control: usual care |
| Outcomes |
Pharmacy worker: unclear ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
| Starting date | 1 March 2014 |
| Contact information | Dr Yves Michiels |
| Notes | Funding source: MSG, France |
Porteous 2013.
| Trial name or title | Help for Hayfever |
| Methods | Design: pilot cluster‐RT Groups: intervention group (hay fever management); control group (usual care) |
| Participants | Pharmacies:12 Pharmacy workers: at least one pharmacist and pharmacy assistant per pharmacy ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy users: 144 patients with allergic rhinitis Setting: unclear Country: Scotland |
| Interventions |
Pharmacy worker‐directed intervention: 3‐hour training workshop in self‐management theory, the use of goal‐setting as a behaviour‐change technique ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: setting and achieving goals that aim to avoid/minimise triggers for, and eliminate/minimise symptoms of allergic rhinitis, including problem solving
Pharmacy user control: usual care |
| Outcomes |
Pharmacy worker: uptake ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
| Starting date | April 2012 |
| Contact information | t.porteous@abdn.ac.uk |
| Notes | Funded by the Chief Scientist Office of the Scottish Government. |
Spadaro 2010.
| Trial name or title | GIFT (the Genetic Informatics Trial of Warfarin to Prevent Deep Vein Thrombosis trial) |
| Methods | Design: RT Groups: intervention group (community pharmacy follow‐up); control group (usual care) |
| Participants | Pharmacy worker: not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy users: 220 patients with heart failure Setting: unclear Country: Italy |
| Interventions |
Pharmacy worker‐directed intervention: informed about epidemiological relevance of heart failure and therapeutic management
Pharmacy worker control: no intervention ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user‐directed intervention: patients and relatives receive education in hospital, then community pharmacy follow‐up
Pharmacy user control: usual care |
| Outcomes |
Pharmacy worker: not assessed ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Pharmacy user:
|
| Starting date | October 2010 |
| Contact information | Francesca Spadaro |
| Notes | Funding source: unclear |
Abbreviations
COPD: chronic obstructive pulmonary disease; EQ‐5D: EuroQol measure of quality of life; HbA1c: glycosylated haemoglobin; QALY: quality adjusted life year; SF‐12: Short Form‐12
Differences between protocol and review
Due to the large number of studies that we retrieved and a desire to include only data of the highest quality and utmost relevance to investigate the research question, we made a number of amendments to the review compared to what was stated in the protocol. With regard to inclusion criteria, we decided to exclude studies that compared two or more active interventions without the inclusion of a comparable control group, and to include only randomised controlled studies.
Finally, we decided not to collect data on process variables. This was in response to the level of data available, and the finding that there was high heterogeneity between process outcomes and measures, which meant that synthesis of these data would be unlikely to yield any clear findings.
We did not report data on the behaviour‐change techniques of the interventions due to limited resources and poor descriptions in the study reports.
Contributions of authors
Authors LS, RW, and AT have contributed to writing the manuscript.
LS, RS, EE, and CR assisted in data searches and conducted data extraction of studies.
RW assisted in development of the 'Risk of bias' summary tables.
VM provided statistical overview of the analyses.
CR reviewed all quality assessments of studies.
ST and CS provided senior level of guidance and support.
All authors have commented on the manuscript and provided expert advice on the review.
This project was supported by the National Institute for Health Research (NIHR), Department of Health, UK. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Sources of support
Internal sources
No sources of support supplied
External sources
NIHR Programme grant RP‐PG‐0609‐10181, UK.
Declarations of interest
LS: is in receipt of grant funding for various projects from the UK National Institutes of Health Research, but has no known conflicts of interest for the current publication
RS: none known
AT: none known
VM: none known
CR: none known
EE: none known
CS: none known
ST: is in receipt of grant funding for various projects from the UK National Institutes of Health Research and (in part) supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) North Thames at Bart’s Health NHS Trust but has no known conflicts of interest for the current publication.
RW: is in receipt of grant funding for various projects from the UK National Institutes of Health Research. This review was funded by a programme grant for smoking cessation from the National Institute of Health Research in the UK. RW has received consultancy fees from TTS Pharma and holds shares in this company. He has received royalties from a patent on genetic indicators of tobacco consumption. The Cochrane Funding Arbiter has looked closely into whether his patent constitutes a conflict of interest in this instance, and has decided that it does not.
The authors of the current review were also authors of one included study (Madurasinghe 2017). AT, who was not an author of the study therefore screened for inclusion, extracted, and checked all the data for this study.
New
References
References to studies included in this review
Adepu 2007 {published data only}
- Adepu R, Rasheed A, Nagavi B. Effect of patient counseling on quality of life in type‐2 diabetes mellitus patients in two selected South Indian community pharmacies: a study. Indian Journal of Pharmaceutical Sciences 2007;69:519‐24. [Google Scholar]
Ali 2012 {published data only}
- Ali M, Schifano F, Robinson P, Phillips G, Doherty L, Melnick P, et al. Impact of community pharmacy diabetes monitoring and education programme on diabetes management: a randomized controlled study. Diabetic Medicine 2012;29(9):e326‐e33. [DOI] [PubMed] [Google Scholar]
Amariles 2012 {published data only}
- Amariles P, Sabater‐Hernandez D, Garcia‐Jimenez E, Rodriguez‐Chamorro MA, Prats‐Mas R, Marin‐Magan F, et al. Effectiveness of Dader method for pharmaceutical care on control of blood pressure and total cholesterol in outpatients with cardiovascular disease or cardiovascular risk: EMDADER‐CV randomized controlled trial. Journal of Managed Care Pharmacy 2012;18(4):311‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharmaceutical Care Research Group. Pharmacotherapy follow‐up: the Dader method (3rd revision: 2005). Pharmacy Practice 2006;4(1):44‐53. [Google Scholar]
Armour 2007 {published data only}
- Armour C, Bosnic‐Anticevich S, Brillant M, Burton D, Emmerton L, Krass I, et al. Pharmacy Asthma Care Program (PACP) improves outcomes for patients in the community. Thorax 2007;62(6):496‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordois A, Armour C, Brillant M, Bosni‐Anticevich S, Burton D, Emmerton L, et al. Cost‐effectiveness analysis of a pharmacy asthma care program in Australia. Disease Management Health Oucomes 2007;15(6):387‐96. [Google Scholar]
- Saini B, Krass I, Armour C. Development, implementation, and evaluation of a community pharmacy‐based asthma care model. Annals of Pharmacotherapy 2004;38(11):1854‐60. [DOI] [PubMed] [Google Scholar]
Barbanel 2003 {published data only}
- Barbanel D, Eldridge S, Griffiths C. Can a self‐management programme delivered by a community pharmacist improve asthma control? A randomised trial. Thorax 2003;58(10):851‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basheti 2008 {published data only}
- Basheti IA, Armour CL, Bosnic‐Anticevich SZ, Reddel HK. Evaluation of a novel educational strategy including inhaler‐based reminder labels, to improve asthma inhaler technique. Patient Education and Counseling 2008;72:26‐33. [DOI] [PubMed] [Google Scholar]
- Basheti IA, Armour CL, Reddel HK, Bosnic‐Anticevich SZ. Long‐term maintenance of pharmacists' inhaler technique demonstration skills. American Journal of Pharmaceutical Education 2009;73(2):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basheti IA, Reddel H, Armour CL, Bosnic‐Antichevich S. Improved asthma outcomes with a simple inhaler technique intervention by community pharmacists. Journal of Allergy and Clinical Immunology 2007;119(6):1537‐8. [DOI] [PubMed] [Google Scholar]
Bereznicki 2013 {published data only}
- Bereznicki BJ, Peterson G, Jackson S, Walters EH, George J, Stewart K, et al. Uptake and effectiveness of a community pharmacy intervention programme to improve asthma management. Journal of Clinical Pharmacy & Therapeutics 2013;38(3):212‐8. [DOI] [PubMed] [Google Scholar]
- Bereznicki BJ, Peterson GM, Jackson SL, Walters EH, Gee PR. The sustainability of a community pharmacy intervention to improve the quality use of asthma medication. Journal of Clinical Pharmaceutical Therapy 2011;36:144‐51. [DOI] [PubMed] [Google Scholar]
- Bereznicki BJ, Peterson GM, Jacson SL, Walters EH, Fitzmaurice KD, Gee PR. Data‐mining of medication records to improve asthma management. Medical Journal of Australia 2008;189:21‐5. [DOI] [PubMed] [Google Scholar]
Bond 2007 {published data only}
- Bond C, on behalf of The Community Pharmacy Medicines Management Project Evaluation Team. The MEDMAN study: a randomized controlled trial of community pharmacy‐led medicines management for patients with coronary heart disease. Family Practice 2007;24(2):189‐200. [DOI] [PubMed] [Google Scholar]
- Jaffray M, Krska A, Lee AJ, Bond CM. The MEDMAN project: evaluation of the medicines management training for community pharmacists. Pharmacy Education 2007;7(3):207‐14. [Google Scholar]
- Scott A, Tinelli M, Bond C. Costs of a community pharmacist‐led medicines management service for patients with coronary heart disease in England: healthcare system and patient perspectives. Pharmacoeconomics 2007;25(5):3970411. [DOI] [PubMed] [Google Scholar]
Burford 2013 {published data only}
- Burford O, Jiwa M, Carter O, Parsons R, Hendrie D. Internet‐based photoaging within Australian pharmacies to promote smoking cessation: randomized controlled trial. Journal of Medical Internet Research 2013;15(3):e64. [DOI: 10.2196/jmir.2337] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bynum 2001 {published data only}
- Bynum A, Hopkins D, Thomas A, Copeland N, Irwin C. The effect of telepharmacy counseling on metered‐dose inhaler technique among adolescents with asthma in rural Arkansas. Telemedicine Journal and E‐health 2001;7(3):207‐17. [DOI] [PubMed] [Google Scholar]
Charrois 2006 {published data only}
- Charrois T, Newman S, Sin D, Senthilselvan A, Tsuyuki RT. Improving asthma symptom control in rural communities: the design of the Better Respiratory Education and Asthma Treatment in Hinton and Edson study. Controlled Clinical Trials 2004;25:502‐14. [DOI] [PubMed] [Google Scholar]
- Charrois TL, Newman SC, Senthilselvan A, Tsuyuki RT. Improving asthma control in the rural setting: the BREATHE (Better Respiratory Education and Asthma Treatment in Hinton and Edson) study. Canadian Pharmacists Journal 2006;139(4):44‐50. [Google Scholar]
Cordina 2001 {published data only}
- Cordina M, McElnay JC, Hughes CM. Assessment of a community pharmacy‐based program for patients with asthma. Pharmacotherapy 2001;21(10):1196‐203. [DOI] [PubMed] [Google Scholar]
Crockett 2006 {published data only}
- Crockett J, Taylor S, Grabham A, Stanford P. Patient outcomes following an intervention involving community pharmacists in the management of depression. Australian Journal of Rural Health 2006;14(6):263‐9. [DOI] [PubMed] [Google Scholar]
Dhital 2015 {published data only}
- Dhital R, Norman I, Whittlesea C, Murrells T, McCambridge J. Effectiveness of alcohol brief intervention delivered by community pharmacists: study protocol of a two‐arm randomised controlled trial. BMC Public Health 2013;13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhital R, Norman I, Whittlesea C, Murrells T, McCambridge J. The effectiveness of brief alcohol interventions delivered by community pharmacists: randomized controlled trial. Addiction 2015;110:1586‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk A, MacNeil V, Dhital R, Whittlesea C, Norman I, McCambridge J. Qualitative process study of community pharmacist brief alcohol intervention effectiveness trial: can research participation effects explain a null finding?. Drug & Alcohol Dependence 2016;161:36‐41. [DOI] [PubMed] [Google Scholar]
Dolovich 2007 {published data only}
- Dolovich L, Sabharwal M, Agro K, Foster G, Lee A, McCarthy L, et al. The effect of pharmacist education on asthma treatment plans for simulated patients. Pharmacy World & Science 2007;29(3):228‐39. [DOI] [PubMed] [Google Scholar]
Doucette 2009 {published data only}
- Doucette WR, Witry MJ, Farris KB, McDonough RP. Community pharmacist‐provided extended diabetes care. Annals of Pharmacotherapy 2009;43(5):882‐9. [DOI] [PubMed] [Google Scholar]
Fuller 2016 {published data only}
- Fuller JM, Wong KK, Hoyos C, Krass I, Saini B. Dispensing good sleep health behaviours not pills ‐ a cluster‐randomized controlled trial to test the feasibility and efficacy of pharmacist‐provided brief behavioural treatment for insomnia. Journal of Sleep Research 2016;25:104‐15. [DOI] [PubMed] [Google Scholar]
Garcia 1998 {published data only}
- Garcia PJ, Gotuzzo E, Hughes JP, Holmes KK. Syndromic management of STDs in pharmacies: evaluation and randomised intervention trial. Sexually Transmitted Infections 1998;74 Suppl 1:S153‐8. [PubMed] [Google Scholar]
Garcia 2003 {published data only}
- Garcia P, Hughes J, Carcamo C, Holmes KK. Training pharmacy workers in recognition, management, and prevention of STDs: district‐randomized controlled trial. Bulletin of the World Health Organization 2003;81(11):806‐14. [PMC free article] [PubMed] [Google Scholar]
Garcia 2012 {published data only}
- Garcia JP, Holmes KK, Carcamo CP, Garnett GP, Hughes JP, Campos PE, et al. Prevention of sexually transmitted infections in urban communities (Peru PREVEN): a multi‐component community‐randomised controlled trial. Lancet 2012;379:1120‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia PJ, Carcamo CP, Garnett GP, Campos PE, Holmes KK. Improved STD syndrome management by a network of clinicians and pharmacy workers in Peru: the PREVEN Network. PLOS ONE 2012;7(10):e47750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia‐Cardenas 2013 {published data only}
- Garcia‐Caredenas V, Sabater‐Hernandez D, Kenny P, Martínez‐Martínez F, Faus MJ, Benrimoj SI. Effect of a pharmacist intervention on asthma control. A cluster randomised trial. Respiratory Medicine 2013;107(9):1346‐55. [DOI] [PubMed] [Google Scholar]
Jaffray 2014 {published data only}
- Jaffray M, Matheson C, Bond C, Lee AJ, McLernon DJ, Johnstone A, et al. Does training in motivational interviewing for community pharmacists improve outcomes for methadone patients? A cluster randomised controlled trial.. International Journal of Pharmacy Practice 2014;22(1):4‐12. [DOI] [PubMed] [Google Scholar]
Kraemer 2012 {published data only}
- Kraemer DF, Kradjan WA, Bianco TM, Low JA. A randomized study to assess the impact of pharmacist counseling of employer‐based health plan beneficiaries with diabetes: the EMPOWER study. Journal of Pharmacy Practice 2012;25(2):169‐79. [DOI] [PubMed] [Google Scholar]
Krass 2007 {published data only}
- Krass I, Armour CL, Mitchell B, Brillant M, Dienaar R, Hughes J, et al. The Pharmacy Diabetes Care Program: assessment of a community pharmacy diabetes service model in Australia. Diabetic Medicine 2007;24(6):677‐83. [DOI] [PubMed] [Google Scholar]
Liambila 2010 {published data only}
- Liambila W, Obare F, Keesbury J. Can private pharmacy providers offer comprehensive reproductive health services to users of emergency contraceptives? Evidence from Nairobi, Kenya. Patient Education and Counseling 2010;81(3):368‐73. [DOI] [PubMed] [Google Scholar]
Liekens 2014 {published data only}
- Liekens S, Vandael E, Roter D, Larson S, Smits T, Laekeman G, et al. Impact of training on pharmacists' counseling of patients starting antidepressant therapy. Patient Education and Counseling 2014;94(1):110‐5. [DOI] [PubMed] [Google Scholar]
Madurasinghe 2017 {published data only}
- Madurasinghe VW, Sohanpal R, James W, Steed L, Eldridge S, Taylor S, et al. Smoking treatment optimisation in pharmacies (STOP): a cluster randomised pilot trial of a training intervention. Pilot & Feasibility Studies 2017;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed L, Sohanpal R, James WY, Rivas C, Jumbe S, Chater A, et al. Equipping community pharmacy workers as agents for health behaviour change: developing and testing a theory‐based smoking cessation intervention. BMJ Open 2017;7(8):e015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maguire 2001 {published data only}
- Maguire T. Pharmaceutical care ‐ a realistic pharmaceutical service. Pharmacy Today 1996;7:20‐4. [Google Scholar]
- Maguire TA, McElnay JC, Drummond A. A randomized controlled trial of a smoking cessation intervention based in community pharmacies. Addiction 2001;96(2):325‐31. [DOI] [PubMed] [Google Scholar]
Mansell 2016 {published data only}
- Mansell K, Evans C, Tran D, Sevany S. The association between self‐monitoring of blood glucose, hemoglobin A1C and testing patterns in community pharmacies: results of a pilot study. Canadian Pharmacists Journal 2016;149:28‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayer 1998 {published data only}
- Mayer JA, Eckhardt L, Stepanski BM, Sallis JF, Elder JP, Slymen DJ, et al. Promoting skin cancer prevention counseling by pharmacists. American Journal of Public Health 1998;88(7):1096‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JA, Slymen DJ, Eckhardt L, Rosenberg C, Stepanski BM, Creech L, et al. Skin cancer prevention counseling by pharmacists: specific outcomes of an intervention trial. Cancer Detection & Prevention 1998;22(4):367‐75. [DOI] [PubMed] [Google Scholar]
McDonough 2005 {published data only}
- McDonough RP, Doucette WR, Kumbera P, Klepser DG. An evaluation of managing and educating patients on the risk of glucocorticoid‐induced osteoporosis. Value in Health 2005;8(1):24‐31. [DOI] [PubMed] [Google Scholar]
McLean 2003 {published data only}
- McLean W, Gillis J, Waller R. The BC Community Pharmacy Asthma Study: A study of clinical, economic and holistic outcomes influenced by an asthma care protocol provided by specially trained community pharmacists in British Columbia. Canadian Respiratory Journal 2003;10(4):195‐202. [DOI] [PubMed] [Google Scholar]
McLean 2008 {published data only}
- Houle SK, Chuck AW, McAlister FA, Tsuyuki RT. Effect of a pharmacist‐managed hypertension program on health system costs: an evaluation of the Study of Cardiovascular Risk Intervention by Pharmacists‐Hypertension (SCRIP‐HTN). Pharmacotherapy 2012;32(6):527‐37. [DOI] [PubMed] [Google Scholar]
- McLean D, McAlistair F, Johnson J. A randomized trial of the effect of community pharmacist and nurse care on improving blood pressure management in patients with diabetes mellitus: study of cardiovascular risk intervention by pharmacists–hypertension (SCRIP‐HTN). Archives of Internal Medicine 2008;168(21):2355‐61. [DOI] [PubMed] [Google Scholar]
- McLean DL, McAlister FA, Johnson JA, King DM, Jones CA, Tsuyuki RT. Improving blood pressure management in patients with diabetes: the design of the SCRIP‐HTN study. Canadian Pharmaceutical Journal 2006;139(4):36‐9. [Google Scholar]
Mehuys 2008 {published data only}
- Mehuys E, Bortel L, Bolle L, Tongelen I, Annemans L, Remon JP, et al. Effectiveness of pharmacist intervention for asthma control improvement. European Respiratory Journal 2008;31(4):790‐9. [DOI] [PubMed] [Google Scholar]
Mehuys 2011 {published data only}
- Mehuys E, Bortel L, Bolle L, Tongelen I, Annemans L, Remon JP, et al. Effectiveness of a community pharmacist intervention in diabetes care: a randomized controlled trial. Journal of Clinical Pharmacy & Therapeutics 2011;36(5):602‐13. [DOI] [PubMed] [Google Scholar]
Nishita 2013 {published data only}
- Nishita C, Cardazone G, Uehara DL, Tom T. Empowered diabetes management: life coaching and pharmacist counseling for employed adults with diabetes. Health Education & Behavior 2013;40(5):581‐91. [DOI] [PubMed] [Google Scholar]
Nola 2000 {published data only}
- Nola KM, Gourley DR, Portner TS, Gourley GK, Solomon DK, Elam M, et al. Clinical and humanistic outcomes of a lipid management program in the community pharmacy setting. Journal of the American Pharmaceutical Association 2000;40(2):166‐73. [DOI] [PubMed] [Google Scholar]
Okada 2018 {published data only}
- Okada H, Onda M, Shoji M, Sakane N, Nakagawa Y, Sozu T, et al. Effects of lifestyle advice provided by pharmacists on blood pressure: the COMmunity Pharmacists ASSist for Blood Pressure (COMPASS‐BP) randomized trial. Bioscience Trends 2018;11:632‐9. [DOI] [PubMed] [Google Scholar]
Park 1996 {published data only}
- Park JJ, Kelly P, Carter BL, Burgess PP. Comprehensive pharmaceutical care in the chain setting: drug therapy monitoring and counseling by pharmacists contributed to improved blood pressure control in study patients. Journal of the American Pharmaceutical Association 1996;NS36(7):443‐51. [DOI] [PubMed] [Google Scholar]
Patwardhan 2012 {published data only}
- Patwardhan PD, Chewning BA. Ask, advise and refer: hypothesis generation to promote a brief tobacco‐cessation intervention in community pharmacies. International Journal of Pharmacy Practice 2009;17(4):221‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan PD, Chewning BA. Effectiveness of intervention to implement tobacco cessation counseling in community chain pharmacies. Journal of the American Pharmacists Association : JAPhA 2012;52(4):507‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan PD, Chewning BA. Tobacco users' perceptions of a brief tobacco cessation intervention in community pharmacies. Journal of the American Pharmacists Association: JAPhA 2010;50(5):568‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paulos 2005 {published data only}
- Paulós CP, Nygren CE, Celedón C, Cárcamo CA. Impact of a pharmaceutical care program in a community pharmacy on patients with dyslipidemia. Annals of Pharmacotherapy 2005;39(5):939‐43. [DOI] [PubMed] [Google Scholar]
Petkova 2008 {published data only}
- Petkova VB. Pharmaceutical care for asthma patients: a community pharmacy based pilot project. Allergy and Asthma Proceedings 2008;29(1):55‐61. [DOI] [PubMed] [Google Scholar]
Petkova 2009 {published data only}
- Petkova VB. Education for arthritis patient: a community pharmacy based pilot project. Pharmacy Practice (Granada) 2009;7(2):88‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Planas 2012 {published data only}
- Planas LG, Crosby KM, Farmer KC, Harrison DL. Evaluation of a diabetes management program using selected HEDIS measures. Journal of the American Pharmaceutical Association 2012;52(6):e130‐8. [DOI] [PubMed] [Google Scholar]
Schmiedel 2015 {published data only}
- Schmiedel K, Mayr A, Fiesler C, Schlager H, Friedland K. Effects of the lifestyle intervention program GLICEMIA in people at risk for type 2 diabetes: a cluster‐randomized controlled trial. Diabetes Care 2015;38:937‐9. [DOI] [PubMed] [Google Scholar]
Skowron 2011 {published data only}
- Skowron A, Polak S, Brandys J. The impact of pharmaceutical care on patients with hypertension and their pharmacists. Pharmacy Practice (Granada) 2011;9(2):110‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slater 2013 {published data only}
- Slater H, Briggs AM, Watkins K, Chua J, Smith AJ. Translating evidence for low back pain management into a consumer‐focused resource for use in community pharmacies: a cluster‐randomised controlled trial.. PLOS One 2013;8(8):e71918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2011 {published data only}
- Smith L, Nguyen T, Seeto C, Saini B, Brown L. The role of non‐clinicians in a goal setting model for the management of allergic rhinitis in community pharmacy settings. Patient Education and Counseling 2011;85(2):26‐32. [DOI] [PubMed] [Google Scholar]
Svarstad 2013 {published data only}
- Shireman TI, Svarstad BL. Cost‐effectiveness of Wisconsin TEAM model for improving adherence and hypertension control in black patients. Journal of the American Pharmacists Association: JAPhA 2016;56(4):389‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svarstad BL, Kotchen JM, Shireman TI, Brown RL, Crawford SY, Mount JK, et al. Improving refill adherence and hypertension control in black patients: Wisconsin TEAM trial. Journal of the American Pharmacists Association : JAPhA 2013;53(5):520‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svarsted BL, Morley Kotchen J, Shireman TI, Crawford SY, Palmer PA, Vivian EM, et al. The Team Education and Adherence Monitoring (TEAM) trial: pharmacy interventions to improve hypertensive control in blacks. Circulation. Cardiovascular Quality and Outcomes 2009;2(3):264‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tommelein 2014 {published data only}
- Tommelein E, Mehuys E, Hees T, Adriaens E, Bortel L, Christiaens T, et al. Effectiveness of pharmaceutical care for patients with chronic obstructive pulmonary disease (PHARMACOP): a randomized controlled trial. British Journal of Clinical Pharmacology 2014;77:756‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsuyuki 2002 {published data only}
- Simpson SH, Johnson JA, Biggs RS, Tsuyuki RT, Scrip I. Greater effect of enhanced pharmacist care on cholesterol management in patients with diabetes mellitus: a planned subgroup analysis of the Study of Cardiovascular Risk Intervention by Pharmacists (SCRIP).. Pharmacotherapy 2004;24(3):389‐94. [DOI] [PubMed] [Google Scholar]
- Simpson SH, Johnson JA, Tsuyuki RT. Economic impact of community pharmacist intervention in cholesterol risk management: an evaluation of the study of cardiovascular risk intervention by pharmacists. Pharmacotherapy 2001;21(5):627‐35. [DOI] [PubMed] [Google Scholar]
- Tsuyuki RT, Johnson JA, Teo KK, Simpson SH, Ackman ML, Biggs RS, et al. A randomized trial of the effect of community pharmacist intervention on cholesterol risk management: the Study of Cardiovascular Risk Intervention by Pharmacists (SCRIP). Archives of Internal Medicine 2002;27(162):1149‐55. [DOI] [PubMed] [Google Scholar]
- Tsuyuki RT, Olson KL, Dubyk AM, Schindel TJ, Johnson JA. Effect of community pharmacist intervention on cholesterol levels in patients at high risk of cardiovascular events: the Second Study of Cardiovascular Risk Intervention by Pharmacists (SCRIP‐plus). American Journal of Medicine 2004;116(2):130‐3. [DOI] [PubMed] [Google Scholar]
- Tsuyuki RT1, Johnson JA, Teo KK, Ackman ML, Biggs RS, Cave A, Chang WC, Dzavik V, Farris KB, Galvin D, Semchuk W, Simpson SH, Taylor JG. Study of Cardiovascular Risk Intervention by Pharmacists (SCRIP) a randomized trial design of the effect of a community pharmacist intervention program on serum cholesterol risk.. Ann Pharmacother 1999;33(9):909‐910. [DOI] [PubMed] [Google Scholar]
Tsuyuki 2016 ‐ RxACT {published data only}
- Tsuyuki RT, Rosenthal M, Pearson GJ. A randomized trial of a community‐based approach to dyslipidemia management: pharmacist prescribing to achieve cholesterol targets (RxACT Study). Canadian Pharmacists Journal 2016;149:283‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyuki RT, Rosenthal M, Pearson GJ. Improving dyslipidemia management in the community: a randomized trial of pharmacist prescribing, the RxACT study. Canadian Journal of Cardiology 2014;30(10 Suppl 1):S118‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsuyuki 2016 ‐ RxEACH {published data only}
- Al Hamarneh YN, Hemmelgarn BR, Hassan I, Jones CA, Tsuyuki RT. The effectiveness of pharmacist interventions on cardiovascular risk in adult patients with type 2 diabetes: the multicentre randomized controlled RxEACH trial. Canadian Journal of Diabetes 2017;41(6):580‐6. [DOI] [PubMed] [Google Scholar]
- Al Hamarneh YN, Tsuyuki RT, Jones CA, Manns B, Tonelli M, Scott‐Douglass N, et al. Effectiveness of pharmacist interventions on cardiovascular risk in patients with CKD: a subgroup analysis of the randomized controlled RxEACH trial. American Journal of Kidney Diseases 2018;71(1):42‐51. [DOI] [PubMed] [Google Scholar]
- Tsuyuki RT, Al Hamarneh YN, Jones CA, Hemmelgarn BR. The effectiveness of pharmacist interventions on cardiovascular risk: the multicenter randomized controlled RxEACH trial. Journal of the American College of Cardiology 2016;67:2846‐54. [DOI] [PubMed] [Google Scholar]
Venkatesan 2012 {published data only}
- Venkatesan R, Devi AS, Parasuraman S, Sriram S. Role of community pharmacists in improving knowledge and glycemic control of type 2 diabetes. Perspectives in Clinical Research 2012;3(1):26‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Villeneuve 2010 {published data only}
- Villeneuve J, Genest J, Blais L, Vanier MC, Lamarre D, Fredette M, et al. A cluster randomized controlled Trial to Evaluate an Ambulatory primary care Management program for patients with dyslipidemia: the TEAM study. Canadian Medical Association Journal 2010;182(5):447‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve J, Lamarre D, Lussier MT. Physician‐pharmacist collaborative care for dyslipidemia patients: knowledge and skills of community pharmacists. Journal of Continuing Education in the Health Professions 2009;29:201‐8. [DOI] [PubMed] [Google Scholar]
- Villeneuve J, Lamarre D, Vanier MC. How to help patients manage their dyslipidemia: a primary care physician‐pharmacist team intervention. Canadian Pharmaceutical Journal 2007;140:300‐5. [Google Scholar]
Weinberger 2002 {published data only}
- Weinberger M, Murray MD, Marrero DG, Brewer N, Lykens M, Harris LE, Tierney WM. Pharmaceutical care program for patients with reactive airways disease.. Am J Health Syst Pharm. 2001;58(9):791‐6. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Murray MD, Marrero DG, Brewer N, Lykens M, Harris LE, et al. Effectiveness of pharmacist care for patients with reactive airways disease: a randomized controlled trial. JAMA 2002;288(13):1594‐602. [DOI] [PubMed] [Google Scholar]
Yuksel 2010 {published data only}
- Yuksel N, Majumdar SR, Biggs C, Tsuyuki RT. Community pharmacist‐initiated screening program for osteoporosis: randomized controlled trial. Osteoporosis International 2010;21(3):391‐8. [DOI] [PubMed] [Google Scholar]
- Yuksel N, Majumdar SR, Biggs C, Tsuyuki RT. Design of a randomized trial of a community pharmacist initiated screening and intervention program for osteoporosis. Canadian Pharmaceutical Journal 2006;139(2):50‐1. [Google Scholar]
References to studies excluded from this review
Ahrens 2003 {published data only}
- Ahrens RA, Hower M, Best AM. Effects of Weight Reduction Interventions by Community Pharmacists. J Am Pharm Assoc 2003;43:583‐9. [DOI] [PubMed] [Google Scholar]
Aleo 2014 {published data only}
- Aleo CL, Murchison AP, Dai Y, Hark LA, Mayro EL, Collymore B, et al. Improving eye care follow‐up adherence in diabetic patients with ocular abnormalities: the effectiveness of patient contracts in a free, pharmacy‐based eye screening. Public Health 2015;129:996‐9. [DOI] [PubMed] [Google Scholar]
- Aleo CL, Murchison AP, Hark LA, Dai Y, Mayro E, Leiby B, et al. Improving eye care follow‐up adherence in patients with diabetes: the effectiveness of patient contracts in a community‐based eye screening. Investigative Ophthalmology and Visual Science 2014;55 (13):6094. [Google Scholar]
Ammari 2013 {published data only}
- Ammari WG, Chrystyn H. Optimizing the inhalation flow and technique through metered dose inhalers of asthmatic adults and children attending a community pharmacy. Journal of Asthma 2013;50(5):505‐13. [DOI] [PubMed] [Google Scholar]
Anderson 1995 {published data only}
- Anderson C. A controlled study of the effect of a health promotion training scheme on pharmacists' advice about smoking cessation. Journal of Social and Administrative Pharmacy 1995;12(3):115‐24. [Google Scholar]
Anderson 2003 {published data only}
- Anderson C. Pharmacists' role in asthma. Pharmacy in practice 2003;16:300. [Google Scholar]
Armour 2004 {published data only}
- Armour CL, Taylor SJ, Hourihan F, Smith C, Krass I. Implementation and evaluation of Australian pharmacists' diabetes care services. Journal of the American Pharmacists Association: JAPhA 2004;44(4):455‐66. [DOI] [PubMed] [Google Scholar]
Armour 2013 {published data only}
- Armour CL, Reddel HK, LeMay KS, Saini B, Smith LD, Bosnic‐Anticevich SZ, et al. Feasibility and effectiveness of an evidence‐based asthma service in Australian community pharmacies: a pragmatic cluster randomized trial. Journal of Asthma 2013;50(3):302‐9. [DOI] [PubMed] [Google Scholar]
Basheti 2005 {published data only}
- Basheti IA, Reddel HK, Armour CL, Bosnic‐Anticevich SZ. Counseling about turbuhaler technique: needs assessment and effective strategies for community pharmacists. Respiratory Care 2005;50(5):617‐23. [PubMed] [Google Scholar]
Bauld 2009 {published data only}
- Bauld L, Chesterman J, Ferguson J, Judge K. A comparison of the effectiveness of group‐based and pharmacy‐led smoking cessation treatment in Glasgow. Addiction 2009;104(2):308‐16. [DOI] [PubMed] [Google Scholar]
Bernsten 2001 {published data only}
- Bernsten C, Bjorkman I, Caramona M, Crealey G, Frokjaer F, Grundberger E, et al. Improving the well‐being of elderly patients via community pharmacy‐based provision of pharmaceutical care: a multi‐centre study in seven European countries. Drugs and Aging 2001;18(1):63‐77. [DOI] [PubMed] [Google Scholar]
Bock 2010 {published data only}
- Bock BC, Hudmon KS, Christian J, Graham AL, Bock FR. A tailored intervention to support pharmacy‐based counseling for smoking cessation. Nicotine & Tobacco Research 2010;12(3):217‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butt 2016 {published data only}
- Butt M, Mhd Ali A, Bakry MM, Mustafa N. Impact of a pharmacist led diabetes mellitus intervention on HbA1c, medication adherence and quality of life: a randomised controlled study. Saudi Pharmaceutical Journal 2016;24:40‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chabot 2003 {published data only}
- Chabot I, Moisan J, Gregoire JP, Milot A. Pharmacist intervention program for control of hypertension. Annals of Pharmacotherapy 2003;37(9):1186‐93. [DOI] [PubMed] [Google Scholar]
Chalker 2002 {published data only}
- Chalker J, Chuc NT, Falkenberg T, Tomson G. Private pharmacies in Hanoi, Vietnam: a randomized trial of a 2‐year multi‐component intervention on knowledge and stated practice regarding ARI, STD and antibiotic/steroid requests. Tropical Medicine & International Health 2002;7(9):803‐10. [DOI] [PubMed] [Google Scholar]
- Chuc NT, Larsson M, Do NT, Diwan VK, Tomson GB, Falkenberg T. Improving private pharmacy practice: a multi‐intervention experiment in Hanoi, Vietnam. Journal of Clinical Epidemiology 2002;55:1148‐55. [DOI] [PubMed] [Google Scholar]
Cody 1998 {published data only}
- Cody M, McCombs JS, Parker JP. The Kaiser Permanente/USC Patient Consultation Study: change in quality of life. American Journal of Health‐system Pharmacy 1998;55:2615‐20. [DOI] [PubMed] [Google Scholar]
Correr 2009 {published data only}
- Correr CJ, Pontarolo R, Wiens A, Rossignoli P, Melchiors AC, Radominski R, et al. Economic evaluation of pharmacotherapeutic follow‐up in type 2 diabetes mellitus patients in community pharmacies. Arquivos Brasileiros de Endocrinologia e Metabologia 2009;53(7):825‐33. [DOI] [PubMed] [Google Scholar]
Crawford 2013 {published data only}
- Crawford ND, Amesty S, Rivera AV, Harripersaud K, Turner A, Fuller CM. Community impact of pharmacy‐randomized intervention to improve access to syringes and services for injection drug users. Health Education & Behavior 2014;41:397‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford ND, Amesty S, Rivera AV, Harripersaud K, Turner A, Fuller CM. Randomized, community‐based pharmacy intervention to expand services beyond sale of sterile syringes to injection drug users in pharmacies in New York City. American Journal of Public Health 2013;103(9):1579‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Denig 2003 {published data only}
- Denig P, Onnes B, Haaijer‐Ruskamp FM. Improvement remains forthcoming. Effect of a peer review programme about the treatment of asthma on quality of life in patients [Verbetering Blijft nog uit. Effect vam eem FTO‐programma over asthma op kwaliteit van leven bij patienten]. Pharmceutisch Weekblad 2003;138:1276‐81. [Google Scholar]
DeRemer 2008 {published data only}
- DeRemer CE, VanLandingham J, Carswell J, Killough D. Pharmacy outreach education program in local community. American Journal of Pharmaceutical Education 2008;72(4):94. [PMC free article] [PubMed] [Google Scholar]
De Vera 2014 {published data only}
- Vera MA, Sadatsafavi M, Tsao NW, Lynd LD, Lester R, Gastonguay L, et al. Empowering pharmacists in asthma management through interactive SMS (EmPhAsIS): study protocol for a randomized controlled trial. Trials 2014;15:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Vries 2010 {published data only}
- Vries TW, Berg PB, Duiverman EJ, Jong‐van den Berg LT. Effect of a minimal pharmacy intervention on improvement of adherence to asthma guidelines. Archives of Disease in Childhood 2010;95(4):302‐4. [DOI] [PubMed] [Google Scholar]
DiDonato 2013 {published data only}
- DiDonato KL, May JR, Lindsey CC. Impact of wellness coaching and monitoring services provided in a community pharmacy. Journal of the American Pharmacists Association: JAPhA 2013;53(1):14‐21. [DOI] [PubMed] [Google Scholar]
Ditusa 2001 {published data only}
- Ditusa L, Luzier AB, Brady PG, Reinhardt RM, Snyder BD. A pharmacy‐based approach to cholesterol management. American Journal of Managed Care 2001;7(10):973‐9. [PubMed] [Google Scholar]
Ekedahl 2008 {published data only}
- Ekedahl A, Oskarsson V, Sundberg B, Gustafsson V, Lundberg T, Gullberg B. Impact of postal and telephone reminders on pick‐up rates of unclaimed e‐prescriptions. Pharmacy World & Science 2008;30(5):503‐8. [DOI] [PubMed] [Google Scholar]
Fera 2008 {published data only}
- Fera T, Bluml BM, Ellis WM. Diabetes Ten City Challenge: final economic and clinical results. Journal of the American Pharmacists Association : JAPhA 2009;49(3):383‐91. [DOI] [PubMed] [Google Scholar]
- Fera T, Bluml BM, Ellis WM, Schaller CW, Garrett DG. The Diabetes Ten City Challenge: interim clinical and humanistic outcomes of a multi‐site community pharmacy diabetes care program. Journal of the American Pharmacists Association: JAPhA 2008;48(2):181‐90. [DOI] [PubMed] [Google Scholar]
Fikri‐Benbrahim 2012 {published data only}
- Fikri‐Benbrahim N, Faus MJ, Martinez‐Martinez F, Alsina DG, Sabater‐Hernandez D. Effect of a pharmacist intervention in Spanish community pharmacies on blood pressure control in hypertensive patients. American Journal of Health‐System Pharmacy 2012;69(15):1311‐8. [DOI] [PubMed] [Google Scholar]
Fornos 2006 {published data only}
- Fornos JA, Andres NF, Andres JC, Guerra MM, Egea B. A pharmacotherapy follow‐up program in patients with type‐2 diabetes in community pharmacies in Spain. Pharmacy World & Science 2006;28(2):65‐72. [DOI] [PubMed] [Google Scholar]
Fuller 2007 {published data only}
- Fuller CM, Galea S, Caceres W, Blaney S, Sisco S, Vlahov D. Multilevel community‐based intervention to increase access to sterile syringes among injection drug users through pharmacy sales in New York City. American Journal of Public Health 2007;97(1):117‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcao 2002 {published data only}
- Garcao JA, Cabrita J. Evaluation of a pharmaceutical care program for hypertensive patients in rural Portugal. Journal of the American Pharmaceutical Association 2002;42(6):858‐64. [DOI] [PubMed] [Google Scholar]
Goeree 2013 {published data only}
- Goeree R, Keyserlingk C, Burke N, He J, Kaczorowski J, Chambers L, et al. Economic appraisal of a community‐wide cardiovascular health awareness program. Value in Health 2013;16(1):39‐45. [DOI] [PubMed] [Google Scholar]
Gorgas 2012 {published data only}
- Gorgas Torner MQ, Paez VF, Camos RJ, Puig CE, Jolonch SP, Homs PE, et al. Integrated pharmaceutical care programme in patients with chronic diseases. Farmacia Hospitalaria 2012;36(4):229‐39. [DOI] [PubMed] [Google Scholar]
Grainger‐Rousseau 1997 {published data only}
- Grainger‐Rousseau TJ, Miralles MA, Hepler CD, Segal R, Doty RE, Ben‐Joseph R. Therapeutic outcomes monitoring: application of pharmaceutical care guidelines to community pharmacy. Journal of the American Pharmaceutical Association 1997;NS37(6):647‐61. [DOI] [PubMed] [Google Scholar]
Green 2008 {published data only}
- Green BB, Anderson ML, Ralston JD, Catz S, Fishman PA, Cook AJ. Patient ability and willingness to participate in a web‐based intervention to improve hypertension control. Journal of Medical Internet Research 2011;13(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BB, Anderson ML, Ralston JD, Catz SL, Cook AJ. Blood pressure 1 year after completion of web‐based pharmacist care. JAMA Internal Medicine 2013;173(13):1250‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, et al. Effectiveness of home blood pressure monitoring, web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA 2008;299(24):2857‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haga 2017 {published data only}
- Haga SB, Moaddeb J, Mills R, Voora D. Assessing feasibility of delivering pharmacogenetic testing in a community pharmacy setting. Pharmacogenomics 2017;18:327‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herborg 2001 {published data only}
- Herborg H, Soendergaard B, Froekjaer B, Fonnesbaek L, Jorgensen T, Hepler CD, et al. Improving drug therapy for patients with asthma ‐ part 1: patient outcomes. Journal of the American Pharmaceutical Association 2001;41(4):539‐50. [DOI] [PubMed] [Google Scholar]
- Herborg H, Soendergaard B, Jorgensen T, Fonnesbaek L, Hepler CD, Holst H, et al. Improving drug therapy for patients with asthma ‐ part 2: use of antiasthma medications. Journal of the American Pharmaceutical Association 2001;41(4):551‐9. [DOI] [PubMed] [Google Scholar]
Kaczorowski 2008 {published data only}
- Kaczorowski J, Chambers LW, Dolovich L, Paterson JM, Karwalajtys T, Gierman T, et al. Improving cardiovascular health at population level: 39 community cluster randomised trial of Cardiovascular Health Awareness Program (CHAP). BMJ 2011;342:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski J, Chambers LW, Karwalajtys T, Dolovich L, Farrell B, McDonough B, et al. Cardiovascular Health Awareness Program (CHAP): a community cluster‐randomised trial among elderly Canadians. Preventive Medicine 2008;46(6):537‐44. [DOI] [PubMed] [Google Scholar]
Karwalajtys 2009 {published data only}
- Karwalajtys T, Kaczorowski J, Hutchison B, Myers MG, Sullivan SM, Chambers LW, et al. Blood pressure variability and prevalence of hypertension using automated readings from multiple visits to a pharmacy‐based community‐wide programme. Journal of Human Hypertension 2009;23(9):585‐9. [DOI] [PubMed] [Google Scholar]
Kradjan 1999 {published data only}
- Kradjan WA, Schulz R, Christensen DB, Stergachis A, Sullivan S, Fullerton DS, et al. Patients' perceived benefit from and satisfaction with asthma‐related pharmacy services. Journal of the American Pharmaceutical Association (Washington,D.C. : 1996) 1999;39(5):658‐66. [DOI] [PubMed] [Google Scholar]
Krass 2011 {published data only}
- Krass I. Optimising pharmacy support in type 2 diabetes. Australian Journal of Pharmacy 2010;91(1086):39‐40. [Google Scholar]
- Krass I, Mitchell B, Song YJ, Stewart K, Peterson G, Hughes J, et al. Diabetes medication assistance service stage 1: impact and sustainability of glycaemic and lipids control in patients with type 2 diabetes. Diabetic Medicine 2011;28(8):987‐93. [DOI] [PubMed] [Google Scholar]
Kritikos 2007 {published data only}
- Kritikos V, Armour CL, Bosnic‐Anticevich SZ. Interactive small‐group asthma education in the community pharmacy setting: a pilot study. Journal of Asthma 2007;44(1):57‐64. [DOI] [PubMed] [Google Scholar]
Kumar, 2009 {published data only}
- Kumar A, Adepu R, Parthasarathi G, Mahesh PA. Impact of community pharmacist provided patient education in asthma patients on treatment outcomes ‐ a study. Indian Journal of Pharmaceutical Education and Research 2009;43:125‐33. [Google Scholar]
Lalonde 2008 ‐ PRoFIL {published data only}
- Beaunoyer S, Dupuis S, Dumoulin‐Charette A, Mouchbahani M, Daigneault AM, Lord A, et al. The impact of ProFiL program on the progression of chronic kidney disease (CKD) and its risk factors: an interim analysis. Journal of Population Therapeutics and Clinical Pharmacology 2014;21(1):e126‐7. [Google Scholar]
- Lalonde L, Letendre S, Clement V, Lord A, Bell R, Mouchbahani M. ProFiL, a training‐and‐communication network program in nephrology for community pharmacists: Impact on knowledge, clinical competences, quality of medication use and clinical variables. International Journal of Clinical Pharmacy 2015;37:423‐4. [Google Scholar]
- Lalonde L, Normandeau M, Lamarre D, Lord A, Berbiche D, Corneille L, et al. Evaluation of a training and communication‐network nephrology program for community pharmacists. Pharmacy World & Science 2008;30(6):924‐33. [DOI] [PubMed] [Google Scholar]
- Lalonde L, Quintana‐Barcena P, Lord A, Bell R, Clement V, Daigneault AM, et al. Community pharmacist training‐and‐communication network and drug‐related problems in patients with CKD: a multicenter, cluster‐randomized, controlled trial. American Journal of Kidney Diseases 2017;70:386‐96. [DOI] [PubMed] [Google Scholar]
Lugo de Ortellado 2007 {published data only}
- Lugo de Ortellado G, Bittner MR, Chavez H, Perez S. Implementation of a pharmaceutical care program for the detection of hypertension and drug therapy to be followed up in community pharmacies [Implementacion de un programa de atencion farmaceutica en farmacias comunitarias pas la deteccion de la hipertension arterial y su seguimiento farmacoterpeutico]. Latin American Journal of Pharmacy 2007;26:590‐5. [Google Scholar]
Manfrin 2015 {published data only}
- Manfrin A, Thomas T, Krska J. Randomised evaluation of the Italian medicines use review provided by community pharmacists using asthma as a model (RE I‐MUR). BMC Health Services Research 2015;15:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfrin A, Thomas T, Krska J. Symptom control and adherence are major issues for asthmatic patients: can they be improved and are they linked?. Pharmacoepidemiology and Drug Safety 2016;25(S2):18‐9. [Google Scholar]
- Manfrin A, Tinelli M, Thomas T, Krska J. A cluster randomised control trial to evaluate the effectiveness and cost‐effectiveness of the Italian medicines use review (I‐MUR) for asthma patients. BMC Health Services Research 2017;17:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mangiapane 2005 {published data only}
- Mangiapane S, Schulz M, Muhlig S, Ihle P, Schubert I, Waldmann HC. Community pharmacy‐based pharmaceutical care for asthma patients. Annals of Pharmacotherapy 2005;39(11):1817‐22. [DOI] [PubMed] [Google Scholar]
Marra 2012 {published data only}
- Marra CA, Cibere J, Grubisic M, Grindrod KA, Gastonguay L, Thomas JM, et al. Pharmacist‐initiated intervention trial in osteoarthritis: a multidisciplinary intervention for knee osteoarthritis. Arthritis Care & Research 2012;64(12):1837‐45. [DOI] [PubMed] [Google Scholar]
Marrero 2006 {published data only}
- Marrero W, Hernandez L, Garcia R, Gutirrez L. Immunization program against influenza for adults 65 years or older at a community pharmacy in Puerto Rico [Programa de Immunizacion contra la Influenza para adultos de 65 anos o mas en una farmacia de comunidad en Puerto Rico]. Puerto Rico Health Sciences Journal 2006;25(1):35‐42. [PubMed] [Google Scholar]
Meijer 2005 {published data only}
- Meijer WM, Smit DJ, Jurgens RA, Jon‐van den Berg LT. Improved periconceptional use of folic acid after patient education in pharmacies: promising results of a pilot study in the Netherlands. International Journal of Pharmacy Practice 2005;13:47‐51. [Google Scholar]
Michie 2014 {published data only}
- Michie L, Cameron S T, Glasier A, Larke N, Muir A, Lorimer A. Pharmacy‐based interventions for initiating effective contraception following the use of emergency contraception: a pilot study. Contraception 2014;90:447‐53. [DOI] [PubMed] [Google Scholar]
Michiels 2017a {published data only}
- Michiels Y, Tilleul P, Mechin H, Hammes F. Impact of a community pharmacy‐based information protocol on multiple sclerosis patients' adherence to treatment with dimethyl fumarate: TECPHIE, a randomized study vs usual practice. Multiple Sclerosis Journal 2017;23(3 Suppl 1):922. [Google Scholar]
Noor 2016 {published data only}
- Noor ZM, Smith AJ, Smith SS, Nissen LM. A feasibility study: use of actigraph to monitor and follow‐up sleep/wake patterns in individuals attending community pharmacy with sleeping disorders. Journal of pharmacy & bioallied sciences 2016;8:173‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor ZM, Smith AJ, Smith SS, Nissen LM. A study protocol: a community pharmacy‐based intervention for improving the management of sleep disorders in the community settings. BMC Health Services Research 2014;14:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Dwyer 2016 {published data only}
- O'Dwyer SM, MacHale E, Sulaiman I, Holmes M, Hughes C, D'Arcy S, et al. The effect of providing feedback on inhaler technique and adherence from an electronic audio recording device, INCA, in a community pharmacy setting: study protocol for a randomised controlled trial. Trials 2016;17:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Obarcanin 2015 {published data only}
- Obarcanin E, Kruger M, Muller P, Nemitz V, Schwender H, Hasanbegovic S, et al. Pharmaceutical care of adolescents with diabetes mellitus type 1: the DIADEMA study, a randomized controlled trial. International Journal of Clinical Pharmacy 2015;37:790‐8. [DOI] [PubMed] [Google Scholar]
Olivera 2016 {published data only}
- Olivera CM, Vianna EO, Bonizio RC, Menezes MB, Ferraz E, Cetlin AA, et al. Asthma self‐management model: randomized controlled trial. Health Education Research 2016;31:639‐52. [DOI] [PubMed] [Google Scholar]
Phimarn 2017 {published data only}
- Phimarn W, Paktipat P, Pansiri K, Klabklang P, Duangjanchot P, Tongkul A. Effect of weight control counselling in overweight and obese young adults. Indian Journal of Pharmaceutical Sciences 2017;79:35‐41. [Google Scholar]
- Phimarn W, Pianchana P, Limpikanchakovit P, Suranart K, Supapanichsakul S, Narkgoen A, et al. Thai community pharmacist involvement in weight management in primary care to improve patient's outcomes. International Journal of Clinical Pharmacy 2013;35(6):1208‐17. [DOI] [PubMed] [Google Scholar]
Podhipak 1993 {published data only}
- Podhipak A, Varavithya W, Punyaratabandhu P, Vathanophas K, Sangchai R. Impact of an educational program on the treatment practices of diarrheal diseases among pharmacists and drugsellers. Southeast Asian Journal of Tropical Medicine and Public Health 1993;24(1):32‐9. [PubMed] [Google Scholar]
Prokhorov 2010 {published data only}
- Prokhorov AV, Humon KS, Marani S, Foxhall L, Ford KH, Luca NS, et al. Engaging physicians and pharmacists in providing smoking cessation counseling. Archives of Internal Medicine 2010;170(18):1640‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratanajamit 2002 {published data only}
- Ratanajamit C, Chongsuvivatwong V, Geater AF. A randomized controlled educational intervention on emergency contraception among drugstore personnel in southern Thailand. Journal of the American Medical Womens Association 2002;57(4):196‐9. [PubMed] [Google Scholar]
Rickles 2006 {published data only}
- Rickles NM, Svarstad BL, Statz‐Paynter JL, Taylor LV, Kobak KA. Improving patient feedback about and outcomes with antidepressant treatment: a study in eight community pharmacies. Journal of the American Pharmacists Association: JAPhA 2006;46(1):25‐32. [DOI] [PubMed] [Google Scholar]
Rouleau 2007 {published data only}
- Rouleau R, Beauchesne MF, Laurier C. Impact of a continuing education program on community pharmacists' interventions and asthma medication use: a pilot study. Annals of Pharmacotherapy 2007;41(4):574‐80. [DOI] [PubMed] [Google Scholar]
Rubio‐Valera 2009 {published data only}
- Rubio‐Valera M, March PM, Fernandez A, Penarrubia‐Maria MT, Trave P, Lopez Del HY, et al. Evaluation of a pharmacist intervention on patients initiating pharmacological treatment for depression: a randomized controlled superiority trial. European Neuropsychopharmacology 2013;23(9):1057‐66. [DOI] [PubMed] [Google Scholar]
- Rubio‐Valera M, Serrano‐Blanco A, Trave P, Penarrubia‐Maria MT, Ruiz M, Pujol MM. Community pharmacist intervention in depressed primary care patients (PRODEFAR study): randomized controlled trial protocol. BMC Public Health 2009;9:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saini 2008 {published data only}
- Saini B, Filipovska J, Bosnic‐Anticevich S, Taylor S, Krass I, Armour C. An evaluation of a community pharmacy‐based rural asthma management service. Australian Journal of Rural Health 2008;16(2):100‐8. [DOI] [PubMed] [Google Scholar]
- Saini B, Krass I, Armour C. Development, implementation, and evaluation of a community pharmacy‐based asthma care model. Annals of Pharmacotherapy 2004;38(11):1954‐60. [DOI] [PubMed] [Google Scholar]
Saji 2012 {published data only}
- Saji M, Jiju AJ, Sundaran S. Study on the impact of patient counseling on the quality of life and pulmonary function of asthmatic patients. International Journal of Pharmacy and Pharmaceutical Sciences 2012;4:300‐4. [Google Scholar]
Santos 2010 {published data only}
- Santos DO, Martins MC, Cipriano SL, Pinto RM, Cukier A, Stelmach R. Pharmaceutical care for patients with persistent asthma: assessment of treatment compliance and use of inhaled medications. Jornal Brasileiro De Pneumologia: Publicacao Oficial Da Sociedade Brasileira De Pneumologia E Tisilogia 2010;36(1):14‐22. [DOI] [PubMed] [Google Scholar]
Sarayani 2012 {published data only}
- Sarayani A, Rashidian A, Gholami K, Torkamandi H, Javadi M. Efficacy of continuing education in improving pharmacists' competencies for providing weight management service: three‐arm randomized controlled trial. Journal of Continuing Education in the Health Professions 2012;32(3):163‐73. [DOI] [PubMed] [Google Scholar]
Sarkadi 2004 {published data only}
- Sarkadi A, Rosenqvist U. Experience‐based group education in type 2 diabetes: a randomised controlled trial. Patient Education & Counseling 2004;53(3):291‐8. [DOI] [PubMed] [Google Scholar]
- Sarkadi A, Veg A, Rosenqvist U. The influence of participant's self‐perceived role on metabolic outcomes in a diabetes group education program. Patient Education & Counseling 2005;58(2):137‐45. [DOI] [PubMed] [Google Scholar]
Sinclair 1998 {published data only}
- Sinclair HK, Bond CM, Lennox AS, Silcock J, Winfield AJ, Donnan PT. Training pharmacists and pharmacy assistants in the stage‐of‐change model of smoking cessation: a randomised controlled trial in Scotland. Tobacco Control 1998;7(3):253‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sperandio 2012 {published data only}
- Sperandio da Silva GM, Chambela MC, Sousa AS, Sangenis LH, Xavier SS, Costa AR, et al. Impact of pharmaceutical care on the quality of life of patients with Chagas disease and heart failure: randomized clinical trial. Trials 2012;13:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stergachis 2002 {published data only}
- Stergachis A, Gardner JS, Anderson MT, Sullivan SD. Improving pediatric asthma outcomes in the community setting: does pharmaceutical care make a difference?. Journal of the American Pharmaceutical Association 2002;42(5):743‐52. [DOI] [PubMed] [Google Scholar]
Suppapitiporn 2005 {published data only}
- Suppapitiporn S, Chindavijak B, Onsanit S. Effect of diabetes drug counseling by pharmacist, diabetic disease booklet and special medication containers on glycemic control of type 2 diabetes mellitus: a randomized controlled trial. Journal of the Medical Association of Thailand 2005;88 Suppl 4:S134‐41. [PubMed] [Google Scholar]
Taskila 2012 {published data only}
- Taskila T, Macaskill S, Coleman T, Etter JF, Patel M, Clarke S, Bridson R, Aveyard P. A randomised trial of nicotine assisted reduction to stop in pharmacies ‐ the RedPharm study. BMC Public Health 2012;12(12):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thavorn 2008 {published data only}
- Thavorn K, Chaiyakunapruk N. A cost‐effectiveness analysis of a community pharmacist‐based smoking cessation programme in Thailand. Tobacco Control 2008;17(3):177‐82. [DOI] [PubMed] [Google Scholar]
Tobari 2010 {published data only}
- Tobari H, Arimoto T, Shimojo N, Yhara K, Noda H, Yamagishi K, et al. Physician‐pharmacist cooperation program for blood pressure control in patients with hypertension: a randomized‐controlled trial. American Journal of Hypertension 2010;10:1144‐52. [DOI] [PubMed] [Google Scholar]
Tsuyuki 2015 {published data only}
- Tsuyuki RT, Houle SK, Charrois TL, Kolber MR, Rosenthal MM, Lewanczuk R, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta clinical trial in optimizing hypertension (RxACTION). Circulation 2015;132:93‐100. [DOI] [PubMed] [Google Scholar]
Tumwikirize 2004 {published data only}
- Tumwikirize WA, Ekwaru PJ, Mohammed K, Ogwal‐Okeng JW, Aupont O. Impact of a face‐to‐face educational intervention on improving the management of acute respiratory infections in private pharmacies and drug shops in Uganda. East African Medical Journal 2004;Suppl:S25‐32. [PubMed] [Google Scholar]
Usami 2009 {published data only}
- Usami T, Hashiguchi M, Kouhara T, Ishii A, Nagata T, Mochizuki M. Impact of community pharmacists advocating immunization on influenza vaccination rates among the elderly. Yakugaku Zasshi ‐ Journal of the Pharmaceutical Society of Japan 2009;129(9):1063‐8. [DOI] [PubMed] [Google Scholar]
Van de Steeg‐van 2011 {published data only}
- Steeg‐van Gompel CH, Wensing M, Smet PA. Implementation of a pharmacist‐led intervention to enhance statin prescribing for secondary prevention in primary care: a cluster randomized trial. European Journal of Preventive Cardiology 2012;19(2):169‐76. [DOI] [PubMed] [Google Scholar]
- Steeg‐van Gompel CH, Wensing M, Smet PA. Implementation of patient education at first and second dispensing of statins in Dutch community pharmacies: the sequel of a cluster randomized trial. BMC Health Services Research 2011;11:313, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg‐van Gompel CH, Wensing M, Smet PA. Implementation of patient education at first and second dispensing of statins in Dutch community pharmacies: the sequel of a cluster randomized trial. Pharmaceutisch Weekblad 2012;147:115‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viens 2007 {published data only}
- Viens C, Leclerc G, Moisan S, Lebeau A. Assess the impact of the health education program prescription drugs: yes...no....maybe [Efficacite d'un programme d'eduction des aines a la sante]. Canadian Journal of Public Health 2007;98(4):301‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2013 {published data only}
- Wang J, Ford LJ, Wingate L, Uroza SF, Jaber N, Smith CT, et al. Effect of pharmacist intervention on herpes zoster vaccination in community pharmacies. Journal of the American Pharmacists Association: JAPhA 2013;53(1):46‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 2002 {published data only}
- Watson MC, Bond CM, Grimshaw JM, Mollison J, Ludbrook A, Walker AE. Educational strategies to promote evidence‐based community pharmacy practice: a cluster randomized controlled trial (RCT). Family Practice 2002;19(5):529‐36. [DOI] [PubMed] [Google Scholar]
Westrick 2016 {published data only}
- Westrick SC, Owen J, Hagel H, Owensby JK, Lertpichitkul T. Impact of the RxVaccinate program for pharmacy‐based pneumococcal immunization: a cluster‐randomized controlled trial. Journal of the American Pharmacists Association: JAPhA 2016;56:29‐36.e1. [DOI] [PubMed] [Google Scholar]
Wilson 2004 {published data only}
- Wilson SJ, MacLellan E, Cox JL, Meek W, Monette K, Morash T, et al. A pilot study evaluating the feasibility of monitoring oral anticoagulant therapy with point of care testing in a community pharmacy. Canadian Journal of Hospital Pharmacy 2004;57:158‐64. [Google Scholar]
Young 2012 {published data only}
- Young HN, Havican SN, Griesbach S, Thorpe JM, Chewning BA, Sorkness CA. Patient and phaRmacist telephonic encounters (PARTE) in an underserved rural patient population with asthma: results of a pilot study. Telemedicine Journal and E‐health 2012;18(6):427‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Davis 2016 {published data only}
- Davis E, Marra C, Gamble JM, Farrell J, Lockyer J, FitzGerald JM, et al. Effectiveness of a pharmacist‐driven intervention in COPD (EPIC): study protocol for a randomized controlled trial. Trials 2016;17:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekers 2017 {published data only}
- Ekers D, Littlewood L. Community pharmacies mood intervention study (CHEMIST). www.isrctn.com/ISRCTN11290592. [DOI: 10.1186/ISRCTN11290592] [DOI]
Michiels 2017 {published data only}
- Michiels Y, Bugnon O, Chicoye A, Verges B, Moisan C, Mechin H, et al. Impact of a community pharmacy‐based information program on type 2 diabetic patients' adherence to their oral treatment: IPhODia, a cluster randomized study vs usual practice. International Journal of Clinical Pharmacy 2016;38(5):1342. [Google Scholar]
- Michiels Y, Bugnon O, Chicoye A, Verges B, Moisan C, Mechin H, et al. Impact of a community pharmacy‐based information program on type 2 diabetic patients' adherence to their oral treatment: IPhODia, a cluster randomized study vs usual practice. Value in Health 2016;19(7):A675‐6. [Google Scholar]
Porteous 2013 {published data only}
- Porteous T, Wyke S, Smith S, Bond C, Francis J, Lee AJ, et al. 'Help for Hay Fever', a goal‐focused intervention for people with intermittent allergic rhinitis, delivered in Scottish community pharmacies: study protocol for a pilot cluster randomized controlled trial. Trials 2013;14:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spadaro 2010 {published data only}
- Spadaro F, Falzone R, Bastiani E, Ferri M, Roni R, et al. Towards an involvement of pharmacies in the integrated management of patients with heart failure. The protocol of the GIFT project. Giornale Italiano di Farmacia Clinica 2010;24(2):88‐98. [Google Scholar]
Additional references
Albrecht 2013
- Albrecht L, Archibald M, Arseneau D, Scott SD. Development of a checklist to assess the quality of reporting of knowledge translation interventions using the Workgroup for Intervention Development and Evaluation Research (WIDER) recommendations. Implementation Science 2013;8(52):1748‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2007
- Anderson S. Community pharmacy and public health in Great Britain, 1936 to 2006: how a phoenix rose from the ashes. Journal of Epidemiology and Community Health 2007;61:844‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bandura 1986
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Prentice‐Hall, 1986. [Google Scholar]
Benrimoj 2004
- Benrimoj SI, Frommer MS. Community pharmacy in Australia. Australian Health Review 2004;28(2):238‐46. [DOI] [PubMed] [Google Scholar]
Blouin 2017
- Blouin RA, Adams ML. The role of the pharmacist in health care: expanding and evolving. North Carolina Medical Journal 2017;78:165‐7. [DOI] [PubMed] [Google Scholar]
Borrelli 2011
- Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. Journal of Public Health Dentistry 2011;71:S51‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brackett 2015
- Brackett A, Butler M, Chapman L. Using motivational interviewing in the community pharmacy to increase adult immunization readiness: a pilot evaluation. Journal of the American Pharmacists Association 2015;55(2):182‐6. [DOI] [PubMed] [Google Scholar]
Brown 2016
- Brown TJ, Todd A, O'Malley C, Moore HJ, Husband AK, Bambra C, et al. Community pharmacy‐delivered interventions for public health priorities: a systematic review of interventions for alcohol reduction, smoking cessation and weight management, including meta‐analysis for smoking cessation. BMJ Open 2016;6:e009828. [10.1136/bmjopen‐2015‐ 009828] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buss 2018
- Buss V, Shield A, Kosari S, Naunton M. The impact of clinical services provided by community pharmacies on the Australian healthcare system: a review of the literature. Journal of Pharmaceutical Policy and Practice 2018;11:22. [Google Scholar]
Cane 2012
- Cane J, O'Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation. Implementation Science 2012;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2006
- Chan XH, Wuliji T. Global pharmacy workforces and migration report: a call to action. Available at www.fip.org/files/fip/publications/PharmacyWorkforceMigration.pdf 2006.
Chandler 2013
- Chandler J, Churchill R, Higgins J, Lasserson T, Tovey D. Methodological standards for the conduct of new Cochrane intervention reviews. www.editorial‐unit.cochrane.org/sites/editorial‐unit.cochrane.org/files/uploads/MECIR_conduct_standards%202.3%2002122013.pdf (accessed 5 March 2014); Vol. version 2.3.
Cheema 2014
- Cheema E, Sutliffe P, Singer DR. The impact of interventions by pharmacists in community pharmacies on control of hypertension: a systematic review and meta‐analysis of randomized controlled trials. British Journal of Clinical Pharmacology 2014;78(6):1238‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crombie 2005
- Crombie I, Irvine L, Elliott L, Wallace H. Closing the health inequalities gap: an international perspective. World Health Organisation Europe 2005.
De Barra 2018
- Barra M, Scott CL, Scott NW, Johnston M, Bruin M, Nkansah N, et al. Pharmacist services for non‐hospitalised patients. Cochrane Database of Systematic Reviews 2018, Issue 9. [DOI: 10.1002/14651858.CD013102] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deters 2018
- Deters MA, Laven A, Castejon A, Doucette WR, Ev LS, Krass I, et al. Effective interventions for diabetes patients by community pharmacists: a meta‐analysis of pharmaceutical care components. Annals of Pharmacotherapy 2018;52(2):198‐211. [DOI] [PubMed] [Google Scholar]
DOH 2005
- Department of Health. Implementing the new Community Pharmacy Contractual Framework (draft). webarchive.nationalarchives.gov.uk/20130123204454/. London, 2005.
Eades 2011
- Eades CE, Ferguson JS, O'Carroll RE. Public health in community pharmacy: a systematic review of pharmacist and consumer views. BMC Public Health 2011;11:582‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eldridge 2012
- Eldridge S, Kerry S. A Practical Guide to Cluster Randomized Trials in Health Services Research. Chichester: Wiley, 2013. [Google Scholar]
Endnote 2013 [Computer program]
- Endnote. EndNote Collect, Collaborate, Create from Anywhere x6. Thomson Reuters, 2013.
EPOC 2017a
- Cochrane Effective Practice and Organisation of Care Review Group (EPOC). What study designs should be included in an EPOC review and what should they be called?. available at epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources‐forauthors accessed 2 December 2017.
EPOC 2017b
- Cochrane Effective Practice and Organisation of Care Review Group (EPOC). Good practice data extraction form. available at epoc.cochrane.org/resources/epoc‐resources‐review‐authors#conducting accessed 2 December 2017.
EPOC 2017c
- Cochrane Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors 2017: epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources‐for‐authors (accessed 17 May 2019).
EPOC 2017d
- Cochrane Effective Practice, Organisation of Care (EPOC). EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. EPOC Resources for review authors 2017: epoc.cochrane.org/resources/epoc‐resources‐review‐authors (accessed 29 September 2018).
Friedberg 2010
- Friedberg JP, Lipsitz SR, Natarajan S. Challenges and recommendations for blinding in behavioral interventions illustrated using a case study of a behavioral intervention to lower blood pressure. Patient Education and Counselling 2010;78:5‐11. [DOI] [PubMed] [Google Scholar]
Garcia‐Cardenas 2016
- Garcia‐Cardenas V, Armour C, Benrimoj SI, Martinez‐Martinez F, Rotta I, Fernandez‐Llimos F. Pharmacists' interventions on clinical asthma outcomes: a systematic review. European Respiratory Journal 2016;47(4):1134‐43. [DOI] [PubMed] [Google Scholar]
Gordon 2011
- Gordon J, Watson M, Avenell A. Lightening the load? A systematic review of community based weight management interventions. Obesity Reviews 2011;12(11):897‐911. [DOI] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html. Cochrane.
Gums 2016
- Gums T, Carter B, Foster E. Cluster randomized trials for pharmacy practice research. International Journal of Clinical Pharmacy 2016;38(3):607‐14. [DOI] [PubMed] [Google Scholar]
Herdman 2011
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Quality of Life Research 2011;20(10):1727‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343(9):d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Hoffmann 2014
- Hoffmann, TC, Gasziou PP, Milne R, Moher D, Barbour V, Johnston M, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:1687. [DOI] [PubMed] [Google Scholar]
Kessler 2003
- Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, et al. Screening for serious mental illness in the general population. Archives of General Psychiatry 2003;60(2):184‐9. [DOI] [PubMed] [Google Scholar]
Lindsey 2017
- Lindsey L, Husband A, Steed L, Todd A. Helpful advice and hidden expertize: pharmacy users' experiences of community pharmacy accessibility. Journal of Public Health (Oxford, England) 2017;39(3):609‐15. [DOI] [PubMed] [Google Scholar]
Michie 2008
- Michie S. What works and how? Designing more effective interventions needs answers to both questions. Addiction 2008;103(6):886‐7. [Google Scholar]
Michie 2010
- Michie S, Prestwich A. Are interventions theory‐based? Development of a theory coding scheme. Health Psychology 2010;29(1):1‐8. [DOI] [PubMed] [Google Scholar]
Miller 2012
- Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd Edition. New York (NY): Guildford Press, 2012. [Google Scholar]
Mossialos 2013
- Mossialos E, Naci H, Courtin E. Expanding the role of community pharmacists: policymaking in the absence of policy‐relevant evidence?. Health Policy 2013;111(2):135‐48. [DOI] [PubMed] [Google Scholar]
Mossialos 2015
- Mossialos E, Courtin E, Naci H, Benrimoj S, Bouvy M, Farris K, et al. From "retailers" to health care providers: transforming the role of community pharmacists in chronic disease management.. Health Policy 2015;119(5):628‐39. [DOI] [PubMed] [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence. Community pharmacies: promoting health and well‐being [NICE guideline (NG102)]. Available at www.nice.org.uk/guidance/indevelopment/gid‐ng10008 2018.
Nkansah 2010
- Nkansah N, Mostovetsky O, Yu C, Chheng T, Beney J, Bond CM, et al. Effect of outpatient pharmacists' non‐dispensing roles on patient outcomes and prescribing patterns. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD000336.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Norton 2007
- Norton PJ. Depression Anxiety and Stress Scales (DASS‐21): psychometric analysis across four racial groups. Anxiety Stress Coping 2007;20(3):253‐65. [DOI] [PubMed] [Google Scholar]
O'Cathain 2019
- O'Cathain A, Croot L, Sworn K, Duncan E, Rousseau N, Turner K, et al. Taxonomy of approaches to developing interventions to improve health: a systematic methods overview. Pilot and Feasibility Studies 2019;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orwin 1994
- Orwin RG. Evaluating coding decisions. In: Cooper H, Hedges LV editor(s). The Handbook of Research Synthesis. New York: Russel Sage Foundation, 1994:177‐203. [Google Scholar]
Pande 2013
- Pande S, Hiller JE, Nkansah N, Bero L. The effect of pharmacist‐provided non‐dispensing services on patient outcomes, health service utilisation and costs in low‐ and middle‐income countries. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD010398] [DOI] [PMC free article] [PubMed] [Google Scholar]
Public Health England 2017
- Public Health England. Pharmacy: A Way Forward For Public Health. Opportunities For Action Through Pharmacy For Public Health. Available at assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/643520/Pharmacy_a_way_forward_for_public_health.pdf 2017.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rogers 2003
- Rogers E. Diffusion of Innovation. Diffusion of Innovation. 5th Edition. New York (NY): Simon and Schuster, 2003. [Google Scholar]
RSPH 2016
- Royal Society Public Health. Building Capacity: realising the potential of community pharmacy assets for improving the public’s health. Public Health England 2016.
Sabater 2016
- Sabater‐Hernández D, Sabater‐Galindo M, Fernandez‐Llimos F, Rotta I, Hossain LN, Durks D, et al. A systematic review of evidence‐based community pharmacy services aimed at the prevention of cardiovascular disease. Journal of Managed Care & Specialty Pharmacy 2016;22(6):699‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 2016
- Scott C, Barra M, Johnston M, Bruin M, Scott N, Bond C, et al. Changing patient behaviour in pharmacy interventions: what are the active ingredients?. International Journal of Pharmacy Practice 2016;24(S2):25‐6. [Google Scholar]
Silverman 2016
- Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. Association between lowering LDL‐C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta‐analysis. JAMA 2016;12:1289‐97. [DOI] [PubMed] [Google Scholar]
Sinclair 2004
- Sinclair HK, Bond CM, Stead LF. Community pharmacy personnel interventions for smoking cessation. Cochrane Database of Systematic Reviews 200, Issue 1. [DOI: 10.1002/14651858.CD003698.pub2] [DOI] [PubMed] [Google Scholar]
Smith 2009
- Smith F. The quality of private pharmacy services in low and middle‐income countries: a systematic review. Pharmacy World & Science 2009;31(3):351‐61. [DOI] [PubMed] [Google Scholar]
Soprovich 2019
- Soprovich AL, Sharma V, Tjosvold L, Eurich DT, Johnson JA. Systematic review of community pharmacy‐based and pharmacist‐led foot care interventions for adults with type 2 diabetes. Canadian Pharmaceutical Journal 2019;152(2):109‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steed 2017
- Steed L, Sohanpal R, James WY, Rivas C, Jumbe S, Chater A, et al. Equipping community pharmacy workers as agents for health behaviour change: developing and testing a theory‐based smoking cessation intervention. BMJ Open 2017;7(8):e015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strandberg 2003
- Strandberg TE, Pitkala K. What is the most important component of blood pressure: systolic, diastolic or pulse pressure?. Current Opinion In Internal Medicine 2003;2:312‐6. [DOI] [PubMed] [Google Scholar]
Svarsted 2000
- Svarstad BL, Bultman DC. The patient: behavioral determinants. In: Gennaro AR editor(s). Remington: The Science and Practice of Pharmacy. 20th Edition. Baltimore (MD): Lippincott Williams & Wilkins, 2000:1948–56. [Google Scholar]
Thompson 2000
- Thompson K, Kulkarni J, Sergejew AA. Reliabitility and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophrenia Research 2000;42(3):241‐7. [DOI] [PubMed] [Google Scholar]
Thomson 2019
- Thomson K, Hillier‐Brown F, Walton N, Bilaj M, Bambra C, Todd A. The effects of community pharmacy delivered public health interventions on population health and health inequalities: a review of reviews. Preventative Medicine 2019;19(39):127. [DOI] [PubMed] [Google Scholar]
Todd 2014
- Todd A, Copeland A, Husband A, Kasim A, Bambra C. The positive pharmacy care law: an area‐level analysis of the relationship between community pharmacy distribution, urbanity and social deprivation in England. BMJ Open 2014;4(8):e005764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Todd 2014b
- Todd A, Copeland A, Husband A, Kasim A, Bambra C. Access all areas? An area‐level analysis of accessibility to general practice and community pharmacy services in England by urbanity and social deprivation. BMJ Open 2014;5(5):e007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uebersax 1987
- Uebersax JS. Diversity of decision‐making models and the measurement of interrater agreement. Psychological Bulletin 1987;101(1):140‐6. [Google Scholar]
Walton 2017
- Walton H, Spector A, Tombor I, Michie S. Measures of fidelity of delivery of, and engagement with, complex, face‐to‐face health behaviour change interventions: a systematic review of measure quality. British Journal of Health Psychology 2017;22(4):872‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Watson 2006
- Watson MC, Blenkinsopp A. The feasibility of providing community pharmacy based services for alcohol misuse: a literature review. International Journal of Pharmacy Practice 2009;17(4):199‐205. [PubMed] [Google Scholar]
Weir 2019
- Weir NM, Newham R, Dunlop E, Bennie M. Factors influencing national implementation of innovations within community pharmacy: a systematic review applying the Consolidated Framework for Implementation Research. Implementation Science 2019;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1998
- World Health Organization. The role of the pharmacist in self‐care and self‐medication. Available at apps.who.int/medicinedocs/en/d/Jwhozip32e/ 1998.
WHO 2006
- World Health Organization. New tool to enhance role of pharmacists in health care. Available at www.who.int/mediacentre/news/new/2006/nw05/en/ 2006.
WHO 2009
- World Health Organization. Milestones in Health Promotion: Statements from Global Conferences. Available at www.who.int/healthpromotion/Milestones_Health_Promotion_05022010.pdf 2009.
WHO 2011
- World Health Organization. Joint FIP/WHO Guidelines on Good Pharmacy Practice: Standards for Quality of Pharmacy Services [WHO Technical Report Series, No. 961, 2011]. Available at www.who.int/medicines/areas/quality_safety/quality_assurance/FIPWHOGuidelinesGoodPharmacyPracticeTRS961Annex8.pdf (accessed 2nd March 2014) 2011.
World Bank Group 2009
- World Bank Group. Country and Lending Groups. Available at datahelpdesk.worldbank.org/knowledgebase/articles/906519‐world‐bank‐country‐and‐lending‐groups (accessed 18 July 2018).
Xu 2012
- Xu T, Almedia Neto AC, Moles RJ. A systematic review of simulated‐patient methods in community pharmacy to assess the provision of non‐prescription medicines. International Journal of Pharmacy Practice 2012;20(5):307‐19. [DOI] [PubMed] [Google Scholar]


