Abstract
The association between estrogen‐containing oral contraceptives and history of pregnancies with disease severity in women with polycystic liver disease (PLD) is unclear. We performed a cross‐sectional cohort study to assess this association by selecting female patients with PLD of which imaging was available prior to any liver volume‐reducing therapy. Patients received a questionnaire to collect detailed information on estrogen use and pregnancies. Preplanned subgroup analyses were performed on premenopausal and postmenopausal patients. The questionnaire was returned by 287 of 360 selected patients (80%). There was no significant association between estrogen‐containing oral contraceptives and height‐adjusted total liver volume (hTLV) in the total group (P = 0.06) and postmenopausal subgroup (P = 0.7). By contrast, each year of exposure corresponds with a 1.45% higher hTLV (P = 0.02) in the premenopausal subgroup, equivalent to a 15.5% higher hTLV for every 10 years of use. Pregnancy duration was not associated with hTLV. In conclusion, patients with PLD should avoid exogenous estrogens.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Female patients with polycystic liver disease (PLD) are more at risk to develop severe hepatomegaly compared with men. It is thought that exposure to estrogen‐containing oral contraceptives and a history of pregnancies influence the disease course in PLD. However, the evidence in literature is contradicting.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ We assessed whether estrogen‐containing oral contraceptives and/or pregnancies are associated with PLD severity expressed as height‐adjusted total liver volume (hTLV) menopausal and postmenopausal patients.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ In this large cross‐sectional cohort study, we demonstrate that use of estrogen‐containing oral contraceptives worsens PLD severity in premenopausal patients. Every year of exposure correlates with a 1.45% higher hTLV, which corresponds to a 15.5% higher hTLV for every 10 years of use, compared with unexposed women.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ With these findings, we legitimize our current counseling advice in the outpatient clinic with evidence and underline the importance to avoid estrogen use in patients with PLD. Clinicians should advise their patients to use nonhormonal contraceptives.
Polycystic liver disease (PLD) is a progressive genetic disorder with a time‐dependent increase of number and size of hepatic cysts.1 In some cases, this leads to hepatomegaly, pressure‐related symptoms, and reduced quality of life.2, 3 PLD occurs in isolated form as autosomal dominant polycystic liver disease (ADPLD) or as a secondary manifestation in patients with autosomal dominant polycystic kidney disease (ADPKD). Risk factors for disease severity are ADPKD, age, and gender.4 PLD is particularly penetrant in women, and >80% of patients from large cohort studies are women.5 Endogenous estrogen production decreases after the menopause, which is defined as the time in a woman's life when menstruation periods cease permanently,6 and literature suggests that liver volume increases in premenopausal patients but stabilizes postmenopausally.4, 7 These observations have led to the hypothesis that estrogen must be regarded as a trophic factor for PLD.
The concept that PLD severity is related to exposure to estrogen, either through use of estrogen‐containing oral contraceptives or pregnancies, stems from a number of studies. The most robust evidence comes from a prospective study in 19 postmenopausal patients with ADPKD, which showed that hormonal replacement therapy with estrogens was associated with a significant increase in total liver volume compared with controls.8 Based on this study, counseling advice in the outpatient clinic includes discouragement of exogenous estrogen therapy. However, it is unclear whether these data also hold for the population of premenopausal patients with PLD, as recently published cohort studies suggested that exposure to estrogen is not correlated with total liver volume.9, 10
These data raise the question whether the advice to discontinue estrogen‐containing oral contraceptives in premenopausal patients with PLD is valid. Through the International PLD Registry, we were able to explore this controversy in a large cohort covering all stages of PLD.4, 11 The goal of this cross‐sectional study was to assess whether estrogen‐containing oral contraceptives and/or pregnancies are associated with PLD severity at first presentation in premenopausal and postmenopausal patients. Our primary aim was to determine the correlation between cumulative exposure to estrogen‐containing oral contraceptives in years and disease severity expressed as height‐adjusted total liver volume (hTLV). Our secondary aim was to determine the correlation between cumulative months of pregnancy and hTLV.
Results
We identified 360 women who met the inclusion criteria for our study. Of all selected patients, a total of 287 patients (80%) returned the female hormonal status questionnaire (Radboudumc: 222, UK Leuven: 65) and were, therefore, eligible for analysis.
Baseline characteristics
Baseline characteristics for the whole group are presented in Table 1. Mean age at first available imaging was 50.2 years. Median hTLV was 2,095 mL/m (interquartile range: 1,419–3,015), and patients were classified as mild (30%), moderate (48%), and severe PLD (21%). Baseline characteristics for premenopausal and postmenopausal subgroups are presented in Table 2. Patients with imaging in the premenopausal phase were taller and more frequently had been breastfed compared with women who were included after menopause. Premenopausal women had significantly larger liver volumes. We were unable to use regularity of menstruation cycle as a potential confounder, due to missing data and unclear provided answers.
Table 1.
Baseline characteristics
| Characteristic | Patients, n = 287 |
|---|---|
| Age, years | 50.2 ± 9.3 |
| Height, cm | 167.8 ± 7.0 |
| Center | |
| Radboud University Medical Center – n (%) | 222 (77) |
| University Hospital Leuven – n (%) | 65 (23) |
| Diagnosis | |
| ADPKD, n (%) | 181 (63) |
| ADPLD, n (%) | 106 (37) |
| TLV, mL | 3,514a (2,400–4,971) |
| hTLV, mL/m | 2,095a (1,419–3,015) |
| Severity classification:b | |
| Mild (<1,600 mL/m), n (%) | 87 (30) |
| Moderate (1,600–3,200 mL/m), n (%) | 139 (48) |
| Severe (>3,200 mL/m) – no. (%) | 61 (21) |
| Ln hTLV, mL/m | 7.66 ± 0.53 |
| Age at menarche, year | 13.2 ± 1.5 |
| Pregnancies, yes (%) | 236 (82) |
| No. of pregnancies | 2.6 ± 1.3 |
| No. of months pregnant | 20.5 ± 8.6 |
| Breastfeeding, yes (%) | 165 (70) |
| No. of months breastfeeding | 10.1 ± 9.1 |
| Fertility treatment, yes (%) | 15 (5.2) |
| Estrogen‐containing contraception, yes (%) | 268 (93) |
| Estrogen‐containing contraception, year | 14.1 ± 8.6 |
| Progestin‐only oral contraceptives, yes (%) | 13 (5) |
| IUD, yes (%) | 65 (23) |
| Menopausal status | |
| Premenopausal, n (%) | 114 (40) |
| Postmenopausal, n (%) | 115 (40) |
| Age at menopause | 50.1 ± 4.5 |
| Uncertain/unknown, n (%) | 22 (8) |
| Hysterectomy/oophorectomy before scan, n (%) | 36 (13) |
ADPKD, autosomal dominant polycystic kidney disease; ADPLD, autosomal dominant polycystic liver disease; hTLV, height‐adjusted total liver volume; IUD, intrauterine device; Ln, natural logarithm; TLV, total liver volume. Plus–minus values are means ± SD.
aNonparametric values expressed as median (interquartile range (IQR)). bSeverity classification is based on hTLV (mL/m).
Table 2.
Characteristics for menopausal status subgroups
| Characteristic | Premenopausal (n = 149) | Postmenopausal (n = 138) | P value |
|---|---|---|---|
| Age, year | 43.4 ± 6.4 | 57.5 ± 5.3 | <0.001 |
| Height, cm | 169.2 ± 6.8 | 166.4 ± 6.9 | 0.001 |
| Center | |||
| Radboud University Medical Center, n (%) | 119 (80) | 103 (75) | 0.29b |
| University Hospital Leuven, n (%) | 30 (20) | 35 (25) | |
| Diagnosis | |||
| ADPKD, n (%) | 96 (64) | 85 (62) | 0.62b |
| ADPLD, n (%) | 53 (36) | 53 (38) | |
| TLV, mL | 3,968 (2,741–5,881) | 3,047 (2,144–4,614) | 0.001a |
| hTLV, mL/m | 2,340 (1,616–3,467) | 1,807 (1,329–2,755) | 0.002a |
| Severity classification:c | 0.006b | ||
| Mild (<1,600), no. (%) | 37 (25) | 50 (36) | – |
| Moderate (1,600–3,200), no. (%) | 70 (47) | 69 (50) | – |
| Severe (>3,200), no. (%) | 42 (28) | 19 (14) | – |
| Ln hTLV, mL/m | 7.76 ± 0.55 | 7.56 ± 0.50 | 0.002 |
| Age at menarche, year | 13.0 ± 1.5 | 13.4 ± 1.5 | 0.071 |
| Pregnancies, yes (%) | 125 (85) | 111 (80) | 0.37 |
| No. of pregnancies | 2.6 ± 1.4 | 2.6 ± 1.2 | 0.86 |
| No. of months pregnant | 20.6 ± 9.1 | 20.4 ± 8.1 | 0.83 |
| Breastfeeding, yes (%) | 95 (76) | 70 (64) | 0.030 |
| No. of months breastfeeding | 11.5 ± 10.2 | 8.4 ± 7.2 | 0.034 |
| Fertility treatment, yes (%) | 7 (4.7) | 8 (5.8) | 0.72 |
| Estrogen‐containing contraception, yes (%) | 140 (94) | 128 (93) | 0.68 |
| Estrogen‐containing contraception, year | 13.3 ± 7.2 | 14.9 ± 9.9 | 0.121 |
ADPKD, autosomal dominant polycystic kidney disease; ADPLD, autosomal dominant polycystic liver disease; hTLV, height adjusted total liver volume; Ln, natural logarithm; TLV, total liver volume. Plus–minus values are means ± SD and tested with independent samples t‐test.
aMann–Whitney U‐for nonparametric values expressed as median (interquartile range (IQR)). bFisher's exact test. cSeverity classification is based on hTLV (mL/m).
Nonresponder analysis
Baseline characteristics of those who returned the questionnaire were not different compared with nonresponders.
Estrogen‐containing oral contraceptives
Patients who were exposed to estrogen‐containing oral contraceptives had a higher natural logarithmic (Ln) hTLV, although this association did not reach statistical significance in an unadjusted linear regression analysis (B = 0.0061; P = 0.09; Figure 1 a and Table 3). Menopausal status was the only confounder that altered the effect of estrogen‐containing oral contraceptives on Ln hTLV by >10%. After adjustment for this confounder, the correlation coefficient was 0.0068 (P = 0.06). Based on our hypothesis that disease progression differs before and after menopause, we repeated our analysis for both subgroups independently.
Figure 1.
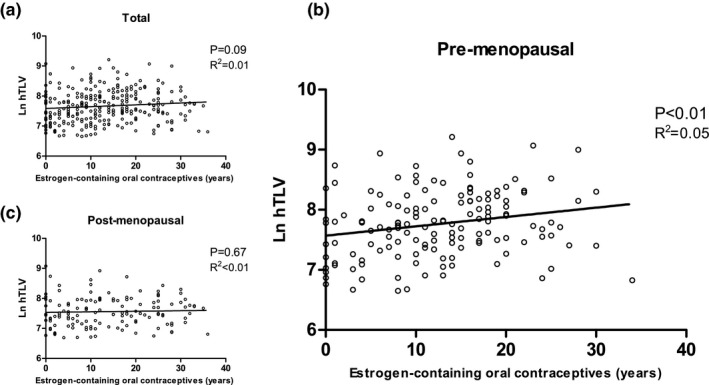
Unadjusted linear regression analysis. Scatter plots and unadjusted (univariable) regression lines for estrogen‐containing oral contraceptives (years of use). (a) Shows the total group. (b) Shows the premenopausal. (c) Shows the postmenopausal subgroups. All data points represent individual patients. Natural logarithmic (Ln) transformed height‐adjusted total liver volume (hTLV) is presented on the y‐axis. P value and R 2 of the regression analysis are shown.
Table 3.
Multiple linear regression analysis (Ln hTLV)
| Estrogen‐containing oral contraceptives | N | B | %/Year | P value |
|---|---|---|---|---|
| Total group | ||||
| Univariable | 281 | 0.0061 | 0.61% | 0.09 |
| Adjusteda | 281 | 0.0068 | 0.68% | 0.06 |
| Premenopausal | ||||
| Univariable | 146 | 0.0156 | 1.57% | <0.01 |
| Adjustedb | 142 | 0.0144 | 1.45% | 0.02 |
| Sensitivity analysisb | 111 | 0.0143 | 1.44% | 0.05 |
| Postmenopausal | ||||
| Univariable | 135 | 0.0018 | 0.18% | 0.67 |
| Adjustedc | 131 | 0.0017 | 0.17% | 0.70 |
| Sensitivity analysisd | 109 | −0.0012 | −0.12% | 0.80 |
| Pregnancy | N | B | %/Month | P value |
|---|---|---|---|---|
| Total group | ||||
| Univariable | 287 | −0.0024 | −0.24% | 0.40 |
| Adjustede | 285 | −0.0011 | −0.11% | 0.69 |
| Premenopausal | ||||
| Univariable | 149 | −0.0029 | −0.29% | 0.48 |
| Adjustedf | 149 | −0.0026 | −0.26% | 0.54 |
| Postmenopausal | ||||
| Univariable | 138 | −0.0027 | −0.26% | 0.50 |
| Adjustedg | 138 | −0.0011 | −0.10% | 0.80 |
hTLV, height adjusted total liver volume; Ln, natural logarithm.
Included confounders are for amenopausal status (yes/no/unclear); bdiagnosis (autosomal dominant polycystic kidney disease (ADPKD)/autosomal dominant polycystic liver disease (ADPLD)), breastfeeding duration (in months), fertility treatment (yes/no); ccenter (Radboud/Leuven), age of menarche (in years), breastfeeding duration (in months), hysterectomy (yes/no), intrauterine device (IUD) use (yes/no); dsee (c), excluding hysterectomy (yes/no); ediagnosis (ADPKD/ADPLD), age at scan (in years), fertility treatment (yes/no), hysterectomy (yes/no); fdiagnosis (ADPKD/ADPLD), age at scan (in years), hysterectomy (yes/no); gcenter (Radboud/Leuven), diagnosis (ADPKD/ADPLD), IUD use (yes/no).
Premenopausal
Unadjusted linear regression analysis showed an association (B = 0.0156; P < 0.01) between estrogen‐containing oral contraceptives and Ln hTLV in premenopausal patients (Figure 1 b). Diagnosis, duration of breastfeeding, and fertility treatment were identified as confounders as they changed the correlation coefficient by >10%. After adjustment for these confounders, the association between use of estrogen‐containing oral contraceptives and Ln hTLV remained significant (B = 0.0144; P = 0.02; Table 3). After back‐transforming, this association can be interpreted as an estimated 1.45% higher hTLV per year of estrogen‐containing oral contraceptive use compared with women who never used estrogen‐containing oral contraceptives. We performed a sensitivity analysis using the same model, in which we excluded patients with imputed menopausal status. In 111 patients, the correlation coefficient remained unchanged (B = 0.0143; P = 0.05; 1.44% higher hTLV per year of estrogen‐containing oral contraceptive use).
When dividing all premenopausal patients into disease severity groups, total years of estrogen‐containing oral contraceptives use was significantly higher in the severe group when compared with the mild group (P = 0.001) and significantly higher in the moderate group when compared with the mild group (P = 0.02). There was no significant difference between the severe and moderate groups (P = 0.17; Figure 2 a).
Figure 2.
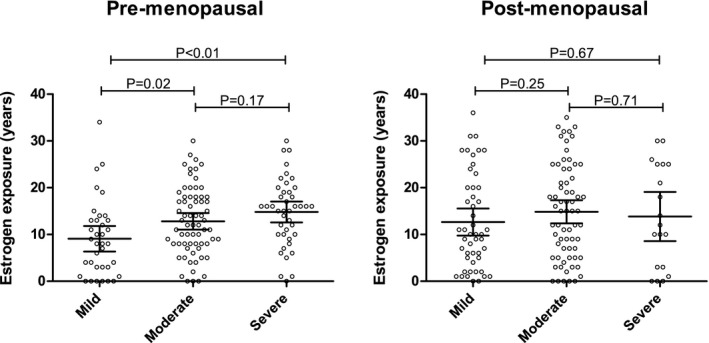
Categorical analysis. Categorical analysis for premenopausal (a) and postmenopausal subgroups (b). Estrogen‐containing oral contraceptives use (years) on the y‐axis and disease severity: mild (height‐adjusted total liver volume (hTLV) < 1,600 mL/m), moderate (hTLV 1,600–3,200 mL/m), and severe (>3,200 mL/m) on the x‐axis. All data points represent individual patients. Mean and 95% confidence interval are superimposed.
Postmenopausal
In postmenopausal women, cumulative exposure to estrogen‐containing oral contraceptives was not correlated with Ln hTLV (B = 0.0018; P = 0.67; Figure 1 c). Center, age at menarche, breastfeeding duration, hysterectomy, and intrauterine device (IUD) use were confounders as they changed the correlation coefficient by >10%. After adjustment for these confounders, the association was not different (B = 0.0017; P = 0.70). Sensitivity analysis, excluding patients with imputed menopausal status, did not change results (B = −0.0012; P = 0.80; Table 3).
There were no differences in estrogen‐containing oral contraceptives use among disease severity groups in postmenopausal women (Figure 2 b).
Pregnancy
Pregnancy duration
Duration of pregnancy (in months) was not correlated with Ln hTLV in the univariable analysis (B = −0.0024; P = 0.40) or after adjustment for the confounders diagnosis, age, fertility treatment, and hysterectomy (B = −0.0011; P = 0.69). Similar results were found in a subgroup analysis as no correlation was found in premenopausal (B = −0.0026; P = 0.54) or postmenopausal women (B = −0.0011; P = 0.80; Table 3).
Parity
In an unadjusted analysis, nulliparous women (n = 50) had a 3.19% higher hTLV compared with parous women (n = 236; B = 0.0314; P = 0.71). After adjustment for center, diagnosis, age at scan, estrogen‐containing oral contraceptive use, fertility treatment, and hysterectomy, the difference was 4.97% (B = 0.0485; P = 0.56).
Discussion
The main finding of this study is that a history of exposure to estrogen‐containing oral contraceptives worsens PLD severity in premenopausal women. Every year of exposure correlates with a 1.45% higher hTLV, which corresponds to a 15.5% higher hTLV for every 10 years of use compared with unexposed women. This association was absent in postmenopausal patients with PLD. PLD severity was independent of total duration and number of pregnancies.
The effect of estrogen and pregnancies on PLD has been the subject of several studies. A prospective study (n = 19) found that 1 year of (postmenopausal) estrogen exposure resulted in a liver volume increase by 7% (SD: 12%). By contrast, liver volume decreased by 2% (SD: 8%) in controls (P < 0.03).8 There was no association between pregnancy and PLD severity in a cross‐sectional analysis of 265 female patients with ADPKD.9 In a subgroup of 69 nulliparous women, no independent effect of estrogen use was found.9 Another cross‐sectional cohort study in 199 patients with ADPKD found that nulliparous women had similar liver volume compared with patients with a history of pregnancy; the effect of estrogen exposure could not be analyzed because of missing data.10
In contrast to these studies, we established a firm association between the use of estrogen‐containing oral contraceptives and liver volume in premenopausal women. The most important difference between our study and the aforementioned cohorts is that we have been able to include a broad spectrum of PLD severity, whereas other cohorts predominantly included patients with mild PLD.9, 10 Another strength of our study is that we collected high‐quality data on estrogen exposure by directly querying patients using a questionnaire. Compared with studies that retrieved data from patient charts, we were, therefore, able to minimize missing data and to determine the total exposure duration of estrogen‐containing contraceptive use as accurately as possible. Furthermore, we obtained a high response rate, as 80% returned our questionnaire. There were no differences in baseline characteristics between responders and nonresponders to the questionnaire, which minimizes response bias.
The association between estrogen‐containing oral contraceptive use and hTLV was not detected in postmenopausal patients. This subgroup is relatively less affected, as the postmenopausal patients had lower hTLV at the time of first imaging in our hospitals (Table 2). There are epidemiological data to suggest that liver volume stabilizes or decreases after menopause.8 Thus, it is possible that postmenopausal patients in our study already had reduction in hTLV, which might cause dilution of the effect size of estrogen on liver volume. We were unable to explore the role of the interval period after discontinuation of estrogen‐containing oral contraceptives due to the absence of longitudinal data.
Total months of pregnancy were not associated with hTLV in this cohort. This was unexpected, as endogenous estrogen levels increase considerably during pregnancy.12 In addition, some patients remarked that they had experienced increased growth due to pregnancy. However, several studies report a prolonged reduction in endogenous estrogen exposure of up to 9 years after pregnancy, independent of having breastfed.12, 13, 14 This might result in a similar or even lower lifetime estrogen exposure in parous women compared with their nulliparous counterparts, thereby negating the potential proliferative effect of pregnancy in cross‐sectional and long‐term studies.
Our data raise the question as to how estrogen influences cyst growth. The genes implicated in PLD encode proteins that act in concert to ensure quality control and proper folding of glycoproteins (e.g., polycystin‐1). Experimental studies suggest that polycystin‐1 expression is decreased in PLD. Together with PRKCSH or SEC63 inactivation, it provides the trigger for cyst formation.15 Estrogen affects expression of many genes and a comprehensive DNA microarray analysis found that PRKCSH is suppressed more than twofold after exposure to estrogen, contributing to an altered genetic balance.16 In a murine model, estrogens stimulated proliferation of cholangiocytes, the cell type that covers the inner lining of hepatic cysts.17 These data are consistent with the notion that estrogens possess an inherent trophic effect for cholangiocytes. Recent discovery of a G protein–coupled estrogen receptor that uses signaling pathways, which are also implicated in PLD (i.e., cyclic AMP and calcium), further support a role of estrogens in cyst formation.18 The important role of the various genetic backgrounds implicated in PLD and their potential effect on disease severity could not be explored in this study, as mutation analysis was not performed standardly.
This study comes with several limitations. First, the cross‐sectional nature of this study complicates drawing firm conclusions on the true effect size of estrogen‐containing oral contraceptives use on PLD severity. However, because of the growing body of evidence and the already widely accepted management advice, it is very unlikely that a prospective controlled trial will be conducted in the future. Second, we were not able to analyze the effect of different types of estrogen‐containing oral contraceptives or dosage because of missing data and recall bias. Most patients reported used combined oral contraceptives (estrogen and progestin); therefore, we cannot exclude a potential effect of progestins on liver volume. Third, we could not exclude selection bias, as these findings are solely based on patients from national referral centers in which imaging was performed. Despite this limitation, we were able to collect data from a large, clinically representative cohort.
In conclusion, the use of estrogen‐containing oral contraceptives is associated with a higher liver volume in premenopausal patients with PLD. With these findings, we legitimize our current counseling advice in the outpatient clinic with evidence and underline the importance to avoid estrogen use in patients with PLD. Clinicians should advise their patients to use nonhormonal contraceptives. We advise clinicians to counsel daughters of patients with PLD to avoid exogenous estrogen exposure until it has been confirmed that they did not inherit the disease.
Methods
Study population and design
The International PLD Registry consists of patients with PLD defined as having >10 liver cysts.4, 11 For this study, patients from two referral centers (Radboud University Medical Center (Nijmegen, The Netherlands) and University Hospital Leuven (Leuven, Belgium)) were evaluated for inclusion. Patients were enrolled based on the following inclusion criteria: (i) female gender, (ii) computed tomography (CT) or magnetic resonance imaging was performed, (iii) imaging was performed prior to any liver volume‐reducing therapy (aspiration sclerotherapy, fenestration, hepatic resection, liver transplantation, or treatment with somatostatin analogues), (iv) hTLV was measured and available in the dataset, (v) age between 18 and 75 years (a maximum age was chosen to minimize recall bias), and (vi) contact information was available. For all patients, the first available type of imaging on which liver volume was measured was used for analysis.
Data collection
Data were extracted from the International PLD registry: age at imaging, diagnosis (ADPKD or ADPLD), total liver volume, and height. Data on the female hormonal history were collected using a specific adapted questionnaire.19 All selected patients received a questionnaire and a reminder 2 months later, if needed. Women were asked about age at menarche, regularity of menstruation cycle, exposure to estrogen‐containing oral contraceptives (including temporary stops), use of non–estrogen‐containing contraceptives (e.g., IUD), pregnancies and miscarriage, menopausal status and last menstruation, hormonal replacement therapy, and history of hysterectomy and/or oophorectomy.
Based on this dataset, we calculated the total years of exposure to estrogen‐containing oral contraceptives and the total months that patients were pregnant. For each live‐born child, pregnancy duration of 9 months was assumed. In case a miscarriage was reported without information on duration of pregnancy, we assumed pregnancy duration of 3 months as 80% of miscarriages occur in the first 12 weeks.19 To determine whether patients were premenopausal or postmenopausal at the time of imaging we used the age at last menstruation. Postmenopausal status was defined as the time after 12 consecutive months without menstruation. In some cases, the menopausal status was uncertain because the patient underwent hysterectomy, used an IUD or hormonal replacement therapy, or because data on the last menstruation were missing. In these cases, we imputed their menopausal status for our subgroup analyses. For this, we used the average age of postmenopausal status (52 years) to classify them as premenopausal or postmenopausal accordingly.20
Measurement of liver volume
All imaging was performed from July 2000 until May 2018 as part of regular clinical care or because of participation in a clinical trial. Liver volume was measured by segmentation technique tracing the circumference of the liver. Volumetry on CT scans in the Netherlands was performed with Pinnacle3 version 8.0 (Philips, Eindhoven, The Netherlands).21, 22 For CT scans in Belgium, we used Volume (Siemens, Erlangen, Germany).23, 24 The volume on magnetic resonance imaging scans was measured with Analyze 11 software (AnalyzeDirect).25 Previous studies have investigated interobserver variability (−0.2 ± 2%) and agreement between different software programs.22, 24 We divided liver volume by height in meters to obtain hTLV. Patients were classified in disease severity groups, as mild (hTLV < 1,600 mL/m), moderate (hTLV 1,600–3,200 mL/m), or severe (hTLV > 3,200 mL/m) PLD.26
Statistical analyses
Descriptive variables were expressed as mean (±SD) or median (interquartile range). Differences in baseline characteristics between premenopausal and postmenopausal women were tested with independent t‐tests for normally distributed data or Mann–Whitney U‐test for nonnormally distributed data. χ2 tests were used to test differences between categorical variables.
We assessed the association between the total exposure of estrogen‐containing oral contraceptive in years (main independent factor) and logarithmic transformed hTLV (dependent factor) using multiple linear regression models. Ln transformation of hTLV was used because of a nonnormal distribution of hTLV. For ease of interpretation we back‐transformed the unstandardized coefficients from all final models by exponentiation. Several potential confounders were defined based on an assumed association on both hTLV and the use of estrogen‐containing oral contraception (treatment center, diagnosis (ADPKD/ADPLD), age at imaging, age at menarche, duration of pregnancy in months, duration of breastfeeding in months, fertility treatment (yes/no), hysterectomy (yes/no), menopausal status (yes/no/unclear), use of progestin‐only contraceptives (yes/no), and use of IUD (yes/no)). Each potential confounder was added separately to our regression model. Confounders that had a relevant impact on the association, defined as >10% difference of the unstandardized coefficient (B) for the main independent factor, were included in our final model. In case of high collinearity (variance inflation factor > 5) between confounders, the most significant confounder was selected.
It is hypothesized that liver volume increases in fertile women and stabilizes or even decreases after menopause.7, 8 Therefore, we a priori decided to repeat our model for the premenopausal and postmenopausal subgroup separately. Again, potential confounders were added to our regression model for each subgroup separately. Furthermore, we performed a sensitivity analysis in which we excluded all patients for which we had to impute their menopausal status based on average age at menopause (n = 58).
The same method was used to determine the association between total months of pregnancy and Ln hTLV (secondary outcome) as well as between nulliparous/parous women and Ln hTLV.
In addition, we performed a post hoc categorical analysis on disease severity groups based on hTLV. Mean duration of estrogen‐containing oral contraceptive use was compared between groups and tested for statistical significance using an independent samples t‐test.
Statistical analyses were performed with SPSS Statistics version 25 (IBM, Armonk, NY), and statistical significance was defined as P values < 0.05, based on two‐sided testing.
Ethical considerations
Concerning ethical approval of the PLD registry and thereby these analyses, formal evaluation was waived by the Institute Review Board Committee on Research Involving Human Subjects Arnhem‐Nijmegen given the retrospective character of the data collection in the PLD Registry. The study was conducted in accordance with good clinical practice guidelines and the code of conduct for medical research (http://www.federa.org). Patients were informed about the intended use of the questionnaire in an accompanying information letter. All patient data were entered in the database codified.4 All questionnaires were coded before processing.
Funding
No funding was received for this work.
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
R.M.M.v.A. and L.H.P.B. wrote the manuscript. R.M.M.v.A., L.H.P.B., T.J.G.N., F.N., and J.P.H.D. designed the research. R.M.M.v.A., L.H.P.B., L.K., and F.N. performed the research. R.M.M.v.A., L.H.P.B., T.J.G.N., and W.K. analyzed the data.
Supporting information
Supplementary File. Translation of female sex hormones questionnaire.
References
- 1. Bae, K.T. et al Magnetic resonance imaging evaluation of hepatic cysts in early autosomal‐dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin. J. Am. Soc. Nephrol. 1, 64–69 (2006). [DOI] [PubMed] [Google Scholar]
- 2. Wijnands, T.F. et al Evaluating health‐related quality of life in patients with polycystic liver disease and determining the impact of symptoms and liver volume. Liver Int. 34, 1578–1583 (2014). [DOI] [PubMed] [Google Scholar]
- 3. Neijenhuis, M.K. , Kievit, W. , Verheesen, S.M. , D'Agnolo, H.M. , Gevers, T.J. & Drenth, J.P. Impact of liver volume on polycystic liver disease‐related symptoms and quality of life. United European Gastroenterol. J. 6, 81–88 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Aerts, R.M.M. et al Severity in polycystic liver disease is associated with aetiology and female gender: results of the International PLD Registry. Liver Int. 39, 575–582 (2019). [DOI] [PubMed] [Google Scholar]
- 5. van Aerts, R.M.M. , van de Laarschot, L.F.M. , Banales, J.M. & Drenth, J.P.H. Clinical management of polycystic liver disease. J. Hepatol. 68, 827–837 (2018). [DOI] [PubMed] [Google Scholar]
- 6. Collaborative Group on Hormonal Factors in Breast Cancer . Menarche, menopause, and breast cancer risk: individual participant meta‐analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 13, 1141–1151 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gevers, T.J. et al Young women with polycystic liver disease respond best to somatostatin analogues: a pooled analysis of individual patient data. Gastroenterology 145, 357–365.e2 (2013). [DOI] [PubMed] [Google Scholar]
- 8. Sherstha, R. et al Postmenopausal estrogen therapy selectively stimulates hepatic enlargement in women with autosomal dominant polycystic kidney disease. Hepatology 26, 1282–1286 (1997). [DOI] [PubMed] [Google Scholar]
- 9. Hogan, M.C. et al Liver involvement in early autosomal‐dominant polycystic kidney disease. Clin. Gastroenterol. Hepatol. 13, 155–164.e6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chebib, F.T. et al Effect of genotype on the severity and volume progression of polycystic liver disease in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 31, 952–960 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D'Agnolo, H.M. , Kievit, W. , Andrade, R.J. , Karlsen, T.H. , Wedemeyer, H. & Drenth, J.P. Creating an effective clinical registry for rare diseases. United European Gastroenterol. J. 4, 333–338 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toriola, A.T. et al Determinants of maternal sex steroids during the first half of pregnancy. Obstet. Gynecol. 118, 1029–1036 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barrett, E.S. , Parlett, L.E. , Windham, G.C. & Swan, S.H. Differences in ovarian hormones in relation to parity and time since last birth. Fertil. Steril. 101, 1773–1780.e1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernstein, L. , Pike, M.C. , Ross, R.K. , Judd, H.L. , Brown, J.B. & Henderson, B.E. Estrogen and sex hormone‐binding globulin levels in nulliparous and parous women. J. Natl. Cancer Inst. 74, 741–745 (1985). [PubMed] [Google Scholar]
- 15. van de Laarschot, L.F.M. & Drenth, J.P.H. Genetics and mechanisms of hepatic cystogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 1864(4 Pt B), 1491–1497 (2018). [DOI] [PubMed] [Google Scholar]
- 16. Terasaka, S. et al Using a customized DNA microarray for expression profiling of the estrogen‐responsive genes to evaluate estrogen activity among natural estrogens and industrial chemicals. Environ. Health Perspect. 112, 773–781 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alvaro, D. et al Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology 119, 1681–1691 (2000). [DOI] [PubMed] [Google Scholar]
- 18. Nilsson, B.O. , Olde, B. & Leeb‐Lundberg, L.M. G protein‐coupled oestrogen receptor 1 (GPER1)/GPR30: a new player in cardiovascular and metabolic oestrogenic signalling. Br. J. Pharmacol. 163, 1131–1139 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mul, K. , Horlings, C.G.C. , Voermans, N.C. , Schreuder, T.H.A. & van Engelen, B.G.M. Lifetime endogenous estrogen exposure and disease severity in female patients with facioscapulohumeral muscular dystrophy. Neuromuscul. Disord. 28, 508–511 (2018). [DOI] [PubMed] [Google Scholar]
- 20. Yarde, F. et al Prenatal famine, birthweight, reproductive performance and age at menopause: the Dutch hunger winter families study. Hum. Reprod. 28, 3328–3336 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Keimpema, L. et al Lanreotide reduces the volume of polycystic liver: a randomized, double‐blind, placebo‐controlled trial. Gastroenterology 137, 1661–1668.e1–e2 (2009). [DOI] [PubMed] [Google Scholar]
- 22. D'Agnolo, H.M. et al Ursodeoxycholic acid in advanced polycystic liver disease: a phase 2 multicenter randomized controlled trial. J. Hepatol. 65, 601–607 (2016). [DOI] [PubMed] [Google Scholar]
- 23. Temmerman, F. et al Lanreotide reduces liver volume, but might not improve muscle wasting or weight loss, in patients with symptomatic polycystic liver disease. Clin. Gastroenterol. Hepatol. 13, 2353–2359.e1 (2015). [DOI] [PubMed] [Google Scholar]
- 24. Temmerman, F. et al Safety and efficacy of different lanreotide doses in the treatment of polycystic liver disease: pooled analysis of individual patient data. Aliment. Pharmacol. Ther. 38, 397–406 (2013). [DOI] [PubMed] [Google Scholar]
- 25. Meijer, E. et al Rationale and design of the DIPAK 1 study: a randomized controlled clinical trial assessing the efficacy of lanreotide to Halt disease progression in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 63, 446–455 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim, H. et al Clinical correlates of mass effect in autosomal dominant polycystic kidney disease. PLoS One 10, e0144526 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File. Translation of female sex hormones questionnaire.


