CRITICALLY ILL PATIENTS IN THE INTENSIVE CARE UNIT (ICU) REQUIRE urgent and complex interventions that expose them to twice as many medications as the number encountered on general medical wards.1 Opioids have been the mainstay of pain control and sedation in the ICU, despite substantial adverse consequences that continue to plague their use.2 Long-term opioid use leads to tolerance (i.e., less susceptibility to the effects of the opioid, which can result in a need for higher and more frequent doses to achieve the same analgesic effect), physical dependence, and opioid-withdrawal symptoms during weaning and contributes to the development of chronic pain later and opioid-induced hyperalgesia (a paradoxical hypersensitivity to pain).3–5 Opioid tolerance can be seen during all types of critical illnesses; the magnitude, however, seems exaggerated in patients who have had major trauma (e.g., burn injury), in patients requiring prolonged mechanical ventilation, and in pediatric patients.6–8 The development of tolerance is due in part to the large doses needed to control pain in these critically ill patients. However, the inflammatory response to opioids themselves, seen in patients in the medical ICU and those in the surgical ICU, plays an important role in tolerance. This review describes the indications for opioid therapy in patients in the ICU, opioid signal transduction during short-term and long-term use, the role of inflammation and opioid-mediated innate immune responses in tolerance, and current and potential mitigation strategies for opioid tolerance. Sedative–anxiolytic drugs, which are adjuncts to analgesia, are not within the scope of this review.
TISSUE AND SPINAL CORD RESPONSES TO INJURY
Most patients in the ICU have some form of tissue injury that causes local and often systemic inflammatory responses. These responses launch a cascade of events, including release of proinflammatory substances and activation of spinal cord N-methyl-d-aspartate (NMDA) receptors (Fig. 1).9 Concomitantly, endogenous antinociceptive mechanisms also become operative. Centrally, the inhibitory opioidergic, serotonergic, and noradrenergic pathways are activated, which can reduce nociception. Leukocytes released at the injury site secrete endogenous opioid peptides that interact with the injury-induced opioid receptors that are up-regulated along nerve terminals and reduce pain.10 However, injury-induced reduction of inhibitory control over pain by means of glycine and γ-aminobutyric acid receptors enhances central sensitization.11 These local and central changes lead to exaggerated basal and procedural pain, referred to as hyperalgesia (exaggerated responses to painful stimuli such as a pinprick) and allodynia (pain responses to nonpainful stimuli such as touch).9,10 These changes are consistent with the body’s need to produce essential warning signs and withdrawal responses during nociception.
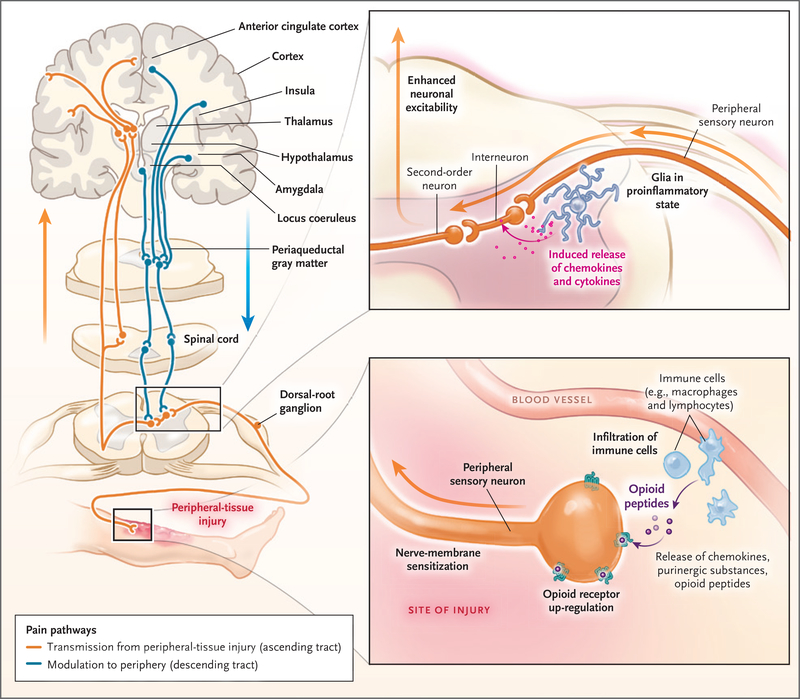
Figure 1. Sites of Action of Opioids and Effects of Injury on Modulation of Nociception.
Sites of action of opioids for pain relief include the brain (cortex, thalamus, hypothalamus, locus coeruleus, amygdala, and periaqueductal gray matter), spinal cord, and peripheral-nerve membrane. Transmission of pain sensation (nociception) from the peripheral-tissue injury to the central nervous system occurs through the ascending spinothalamic tract to the thalamus and then to the somatosensory cortex (orange). Descending inhibitory tracts (blue) from the brain and other regions, including the rostroventral medulla, modulate nociception. Nociception can be amplified by dorsal-root ganglia and changes in the dorsal horn of the spinal cord (top inset). The afferent neurons are sensitized by the sprouting of new axons around the cell bodies of dorsal-root ganglia, as well as by infiltrating macrophages, which release inflammatory substances. Neuron projections from dorsal-root ganglia to the dorsal horn amplify the pain by the release of other pro-nociceptive mediators (e.g., calcitonin gene–related peptide), activation of N-methyl-d-aspartate receptors, and the increase in glutamate levels. Second-order neurons transmit these signals upstream to the brain (orange). Injury to tissues (bottom inset) results in local and often systemic inflammatory responses, which prime the peripheral sensory neurons and dorsal-root ganglia to exaggerated nociception by up-regulation or modulation of ligand-gated and voltage-gated ion channels. Mu-opioid receptors are newly expressed throughout the nerve membrane. Extravasated circulating leukocytes (e.g., macrophages and lymphocytes) release proinflammatory mediators, further sensitizing the neurons to pain. These leukocytes also release antinociceptive endogenous opioid peptides, which bind to the up-regulated opioid receptors on the nerve, attenuating pain.
INDICATIONS FOR OPIOID USE AND CONSEQUENCES OF INADEQUATE ANALGESIA
Moderate-to-severe pain, which generally accompanies critical illness, is often distressing and frequently underrecognized.12 The underlying illness or surgery, placement of penetrating invasive tubes or catheters, and other routine intensive care procedures are recognized sources of pain.13 Patients in the ICU often cannot communicate about their pain because of the combined effects of endotracheal intubation, sedation, neuromuscular blockers, altered mental status, physical restraints, and other disease-related complications. Therefore, it is imperative for caregivers to assess pain severity reliably through the use of standardized pain-assessment tools validated for use in the ICU.14 Although opioids represent the primary pharmacologic therapy for moderate-to-severe pain, there are numerous other indications for opioid use, including sedation (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Analgesia-first sedation (also known as analgosedation) is a strategy for managing pain and discomfort that relies on analgesic agents first, before sedatives such as benzodiazepines. Analgesia-first sedation results in improved outcomes, including fewer days on a ventilator, as compared with combined analgesic–sedative regimens,15,16 and has been recommended in clinical practice guidelines for the ICU.2
Unrelieved pain affects physiological and psychological function and is associated with both short- and long-term consequences, most of which are due to exacerbation of the stress responses induced by catecholamines, glucocorticoids, and antidiuretic-hormone release.17,18 Stress activation of the hypothalamic–pituitary–adrenal axis and the renin–angiotensin–aldosterone axis can lead to fluid retention, generalized edema, and hypertension. Other adverse consequences of stress include impaired tissue oxygenation, wound healing, and immunity17,18; increased myocardial and total oxygen consumption and muscle catabolism; and neuroinflammatory priming.19–22 Unrelieved pain has psychological consequences, including anxiety, depression, impaired sleep, and demoralization, and is a risk factor for subsequent post-traumatic stress disorder.22,23 Both patients and family members report pain as the most stressful experience during their time in the ICU and after discharge.24 Some patients, particularly those who have undergone major surgery, have persistent pain after discharge from the ICU that contributes to a reduced quality of life.25 Risk factors for the development of persistent pain are poorly controlled, high-intensity, acute pain; preoperative pain or anxiety; long-term opioid use; a relatively long ICU stay; and major surgery.26
SIDE EFFECTS OF OPIOID THERAPY
Side effects of opioid therapy are categorized as either peripheral (e.g., constipation, urinary retention, and bronchospasm) or central (e.g., oversedation, respiratory depression, hypotension, nausea, truncal rigidity, and cough suppression). Opioid-induced vasodilatation and hypotension can increase fluid requirements after trauma.27 In contrast, vasodilatation can be beneficial during disease-, anxiety-, and pain-induced hypertension. The respiratory and cough-suppressive effects can also be beneficial in the ICU setting (Table S1 in the Supplementary Appendix). Other detrimental effects that are often overlooked include inappropriate immune modulation through neuroendocrine pathways or direct effects through receptors that are present on immunocytes.28,29 That opioids impair immune function has aroused concern during the care of patients with cancer in the ICU,30 but the role of opioids in cancer recurrence is far from clear.31 The negative effect of intolerable pain on immune function is well documented; however, the more immediate concern is the alleviation of pain and suffering and avoidance of their deleterious consequences.31
Opioids can contribute to delirium, poor sleep quality, and unintended sedation; this is particularly true in patients in the ICU, because of altered drug clearance, concomitant drug therapy, and central metabolic dysfunction. The strategy of analgesia-first sedation in trauma and burn populations in the ICU has been associated with a reduced risk of delirium,32 whereas opioids in combination with benzodiazepines, particularly in the elderly, have been associated with an increased risk of delirium.33 Conversely, complete lack of sedation can result in more cases of delirium than sedation with daily interruption,34 although the lack of sedation may simply result in more cases of delirium being identified.
PHARMACOKINETIC COMPONENTS OF OPIOID TOLERANCE
Pharmacokinetic studies of opioid use during critical illness are limited. Autoinduction of cytochrome P-450 enzyme and enhanced drug clearance do not occur with long-term opioid use and therefore cannot explain dose escalation (i.e., the need to increase the dose to maintain equipotent analgesic effects).35 However, cytochrome P-450 inducers increase clearance of some drugs (e.g., methadone), resulting in subtherapeutic plasma levels, which may be misinterpreted as pharmacodynamic tolerance (i.e., tolerance due to changes in sites of action).36 Similarly, during the hyperdynamic phase of trauma and compensated sepsis, the enhanced elimination kinetics of “flow dependent” drugs (e.g., fentanyl and morphine) could cause dose escalation.37
Inflammation increases the expression of α1-acid glycoprotein, an acute-phase reactant protein, which binds some drugs. Since methadone has a high affinity for α1-acid glycoprotein, there is a decreased free fraction of methadone in the plasma.36,38 Despite minimal binding of fentanyl and morphine to α1-acid glycoprotein,36,38 tolerance still occurs. Thus, increased glycoprotein binding contributes minimally to opioid dose escalation. The P-glycoprotein transporter that is present in brain capillaries controls drug efflux from the central nervous system. Long-term administration of oxycodone, morphine, and alfentanil, but not methadone, up-regulates P-glycoprotein expression, causing decreased drug penetration in the central nervous system and attenuated analgesia.36 Similarly, tumor necrosis factor α increases expression and activity of P-glycoprotein.39 Together, these observations imply that critical illness–related cytokine release and opioid administration may tighten the permeability of the P-glycoprotein–controlled blood–brain barrier, reducing the efficacy of some opioids.
PHARMACODYNAMIC COMPONENTS OF OPIOID TOLERANCE
METABOLITE CONTRIBUTIONS
Opioid metabolism can result in metabolites that enhance or antagonize the analgesic effect or have no pharmacologic effect. In the case of morphine, the parent drug is active, although its metabolites have contrasting effects: normorphine is inactive, and morphine-6-glucuronide is more potent than morphine, whereas morphine-3-glucuronide is considered to have hyperalgesic effects that oppose the analgesic effects of morphine and of morphine-6-glucuronide.40 During renal failure or dose escalation of morphine (or hydromorphone), as seen in the ICU, markedly increased morphine-3-glucuronide levels can counteract the analgesic potency of morphine and morphine-6-glucuronide.40 The hyperalgesic effects of morphine-3-glucuronide are simultaneously opioid receptor–dependent and opioid receptor–independent, as shown in a study involving knockout mice41 and studies involving naloxone.28 The receptor-independent effects are mediated by activation of both microglia toll-like receptors and NMDA receptors.28 The magnitude of the contribution of morphine-3-glucuronide to a deficiency of analgesia is controversial.
OPIOID-RECEPTOR SIGNALING DURING SHORT-TERM AND LONG-TERM OPIOID USE
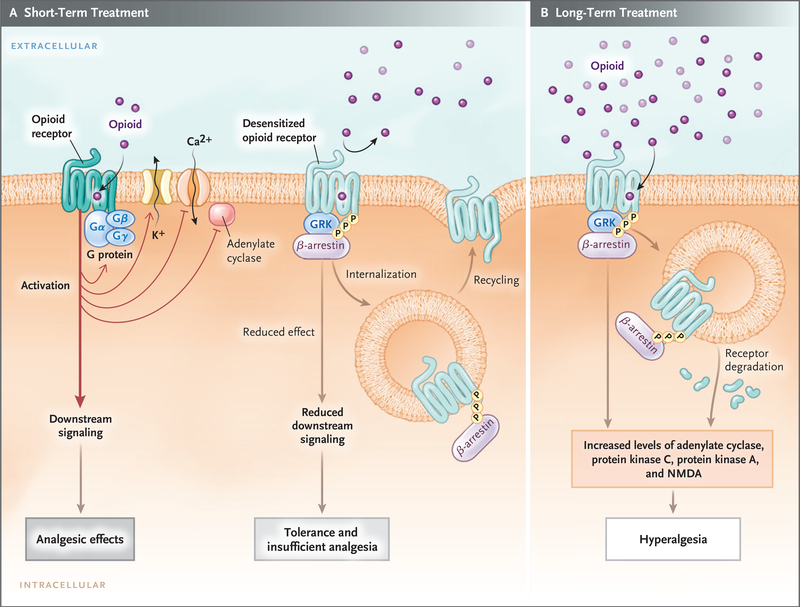
Most clinically used opioids act through muopioid receptors, which belong to the G-protein–coupled receptors family, and transmit downstream signals through heterotrimetric Gαβγ-proteins. When an opioid binds to the mu-opioid receptor, the receptor-associated Gαβγ-protein dissociates into Gα and Gβγ subunits. Concomitantly, the mu-opioid receptor becomes phosphorylated by G-protein–coupled receptor kinase,3 which recruits β-arrestin protein and binds it to the receptor, sometimes leading to receptor internalization (Fig. 2). These processes lead to desensitization (conversion from a responsive-receptor state to a decreased-signaling state), which partly explains acute tolerance.42 The desensitized receptors recover over time (minutes to hours, depending on the agonist) after the stimulus has been withdrawn, and the endocytosed receptors are recycled to the plasma membrane in a resensitized state.
Figure 2. Opioid-Receptor Signaling during Short-Term Therapy and Long-Term Therapy.
In short-term treatment, the binding of an opioid to its receptor (Panel A) causes downstream G-protein–coupled receptors, composed of Gαβγ subunits, to dissociate into Gα and Gβγ subunits. The dissociated G-protein subunits inhibit voltage-gated calcium channels by means of reduced transmitter release, activate inward-rectifying potassium channels (causing hyperpolarization of the membrane), and inhibit downstream adenylate cyclase enzymes, decreasing cyclic adenosine monophosphate levels. These events reduce excitability and nociception and result in analgesic effects. When an opioid binds to its receptor, it becomes an immediate substrate for phosphorylation by G-protein–coupled receptor kinase (GRK), which leads to recruitment and binding of β-arrestin protein to the receptor. This results in desensitization and sometimes endocytosis of the receptor; each of these events decreases the responses to opioids, inducing tolerance and insufficient analgesia. Opioid-receptor signaling terminates when the opioid is displaced from the receptor. After the stimulus (i.e., the agonist) is withdrawn, the desensitized receptor recovers over time (minutes to hours, depending on the agonist), Gα rebinds to Gβγ and once again forms Gαβγ, and the endocytosed receptor is reexpressed on the plasma membrane in a resensitized state. In long-term treatment (Panel B), escalating doses of opioids and concomitant persistent activation of the receptor lead to aggravation of the tolerance by receptor-dependent and receptor-independent intracellular signaling changes, which include up-regulation of the antiopioid (pro-nociceptive) signaling pathways. The sustained β-arrestin binding to the receptor often leads to internalization, degradation, and down-regulation of membrane receptor number, further decreasing response to opioids. Receptor down-regulation occurs with some opioids (e.g., fentanyl) but not others (e.g., morphine). Phosphorylation by other kinases (e.g., protein kinases A and C), increased adenylate cyclase activity (with increased cyclic adenosine monophosphate levels), activation of N-methyl-d-aspartate (NMDA) receptor, and down-regulation of glutamate receptors (increased glutamate levels) are all implicated in the imbalance between pro-nociceptive and antinociceptive pathways, which results in attenuated analgesic effects, aggravated pain behaviors, increased tolerance, and opioid-induced hyperalgesia.
Long-term opioid use leads to exaggerated opioid tolerance, which is characterized by escalating dose requirements to maintain analgesia, and subsequently contributes to opioid-induced hyperalgesia. Tolerance to the analgesic effects of opioids (and euphoria) develops faster than tolerance to respiratory depression, which explains the increased risk of hypoventilation with dose escalation during tolerance. Both duration and dose appear to affect the development of tolerance; infusions induce tolerance faster than intermittent therapy.43 The potent opioid remifentanil induces tolerance more quickly than the less potent meperidine. Persons with substanceuse disorder who are receiving maintenance therapy with methadone or buprenorphine are observed to have opioid-induced hyperalgesia, which is absent in those who are not receiving opioids.44
Prominent signaling changes develop during the continued presence of exogenous or endogenous ligands because the central nervous system has intrinsic mechanisms to prevent overstimulation or understimulation. The typical response of G-protein–coupled receptors to chronic agonists is receptor internalization and down-regulation together with intracellular signaling changes, leading to decreased analgesia.45,46 Additional cellular adaptations during long-term opioid use include induction of systems that attenuate analgesic effects. These systems elicit adaptive responses to persistent opioid-induced inhibitory downstream signaling.45 The adaptations encompass the activation of NMDA receptors, down-regulation of glutamate transporter, conversion from a state of decreased to a state of increased adenylate cyclase activity3 (Fig. 2), and increased transduction through other nociception channels.3,4 The formation of opioid-receptor heterodimers that bind opioids has also been implicated in opioid-induced hyperalgesia.47 Thus, activation of the analgesia-attenuating system at multiple sites during long-term opioid therapy leads to an imbalance between pro-nociceptive and antinociceptive pathways, resulting in reduction of analgesia, increased tolerance, and opioid-induced hyperalgesia.
INNATE IMMUNE RESPONSES IN THE CENTRAL NERVOUS SYSTEM
Injury- or inflammation-related pain can become aggravated or long-lasting, features that cannot be explained by neuronal activation alone. In the central nervous system, the glia (astroglia and microglia) play a major role in central sensitization.3,28 Persistent activation of the dorsal horn of the spinal cord by the injury-induced barrage of nociceptive input and the associated release of damage-associated molecular patterns (DAMPs) activate glia, which release inflammatory mediators that enhance the excitability of adjacent neurons (Fig. 3).48 Although acute stress results in stress-induced analgesia, persistent sympathetic overactivity leads to stress-induced hyperalgesia.49 Repetitive stress can also lead to central and peripheral leukocyte priming and release of inflammatory mediators,21,22,28 causing exaggerated pain behaviors.50,51 The stress-induced catecholamine surge releases immunocytes, including phenotypical inflammatory M1 monocytes (as opposed to antiinflammatory M2 monocytes). This release compounds inflammatory-mediator responses.52 Stress-associated glucocorticoid release can function as DAMPs, promoting activation of the glia.22,53 Superimposition of bacterial inflammation and release of pathogen-associated molecular patterns further augment leukocyte-related toll-like receptor activation and cytokine release, which can lead to nociceptor sensitization.54 Systemic inflammatory diseases can also lead to neuroinflammation,55,56 with selective breakdown of the blood–brain barrier to inflammatory M1 monocytes, which further exaggerate neuroinflammation, modulating both mood and nociception.20–22
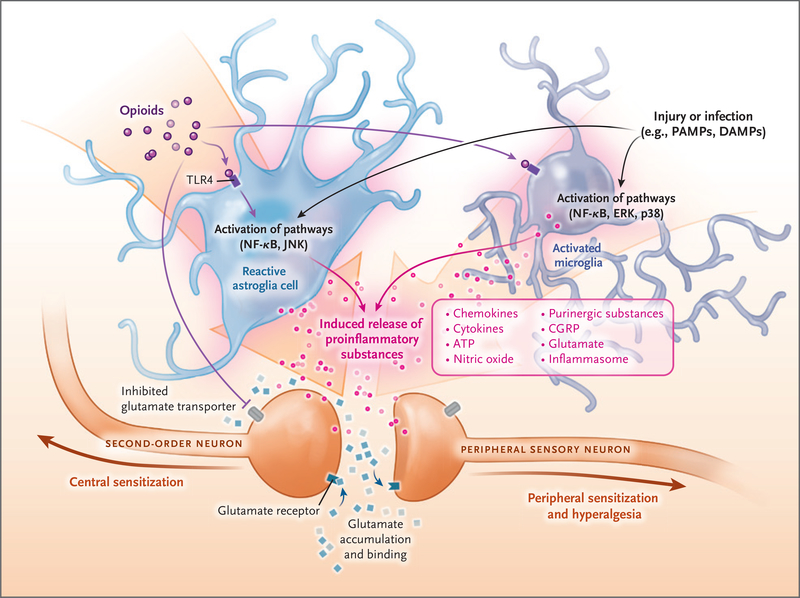
Figure 3. Cross Talk between Neuronal and Non-Neuronal Cells during Injury and Inflammation.
Non-neuronal cells (e.g., astroglia and microglia) can modify pain perception through the production and release of pro-nociceptive m ediators. Opioids, injury, cancer, chemotherapy, stress, and other causes of sterile or microbial inflammation can induce the release of damage-associated molecular pathogens (DAMPs) and pathogen-associated molecular patterns (PAMPs). DAMPs and PAMPs cause inflammasome release, which leads to the transition of microglia to an active state and astroglia to a reactive state. The “switched on” glia release inflammatory substances through activation of toll-like receptors (TLRs) and their downstream signaling proteins (Jun N-terminal kinase [JNK], nuclear factor κB [NF-κB], extracellular signal-regulated kinase [ERK], and p38 mitogen-activated protein kinase [p38]). Peripheral macrophages infiltrate the central nervous system because of selective breakdown of the blood–brain barrier, and they contribute to the inflammatory responses. The released proinflammatory substances (inflammasomes, ATP, and calcitonin gene–related peptide [CGRP]) sensitize the pre- and postsynaptic central neurons, leading to a vicious cycle characterized by the need for more opioids and more sensitization and more glia inflammation. The end result is a marked exaggeration of nociception, severe opioid tolerance, peripheral and central sensitization, and opioid-induced hyperalgesia.
Opioids, even in the absence of systemic inflammation, cause neuroinflammation by activating toll-like receptors in glia and other immune cells that permeate the blood–brain barrier.3,28,52 The cytokine release from activated immune cells leads to exaggerated nociception; antagonism of toll-like receptors or their knockout in mice abrogates the hyperalgesia.28,46 Similarly, specific antagonists of putative inflammatory mediators (e.g., interleukin-1β) attenuate hyperalgesia. Other factors that contribute to central sensitization include age, sex, and concomitant inflammatory conditions (e.g., those caused by cancer and chemotherapy). Notably, greater opioid tolerance seems to develop in pediatric patients7,8; this may be related to less inhibition in the dorsal horn and more facilitation by the rostroventral medulla than in adults.57 Thus, there are multiple factors (e.g., inflammation, infection, stress, and use of opioids) that can lead to activation of the glia in patients in the ICU and can exaggerate pain behaviors, tolerance, and opioid-induced hyperalgesia, creating a vicious cycle of dose escalation and worsening pain (Figs. 3 and 4).50,51 Thus, tolerance appears to reflect a desensitization of receptor-mediated antinociceptive pathways, whereas opioid-induced hyperalgesia involves induction of pro-nociceptive glial–neuronal pathways. Clinical observations confirm that hyperalgesia in persons with critical illnesses can be more profound than hyperalgesia in the general population.6,7 Further understanding of this phenomenon should help explain why simple and routine procedures can cause pain in patients in the ICU and will allow for more empathic and better care of patients in the ICU.
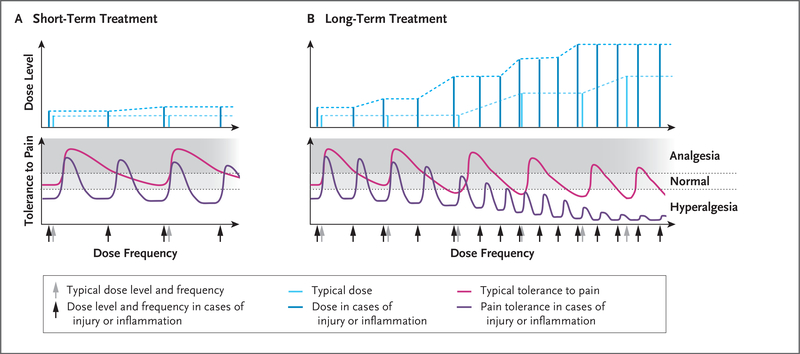
Figure 4. Short-Term and Long-Term Opioid Therapy and Effects of Inflammation or Injury on Pain Threshold.
In Panel A, short-term opioid administration (light blue) provides sufficient analgesia with no or minimal opioid tolerance (pink). In cases of inflammation or injury, as compared with uninjured states, the analgesic potency of the opioid (i.e., the threshold for pain) is decreased, resulting in hyperalgesia; short-term administration of higher doses (dark blue) provides sufficient analgesia but for a shorter duration (purple), requiring more frequent doses (dark blue). Panel B shows that in an uninjured patient with long-term exposure to opioids, the analgesic potency decreases (pink) and the duration of opioid-induced analgesia also decreases with each dose, requiring an increase in dose frequency. Long-term opioid administration will result in induction of antinociceptive mechanisms, resulting in hyperalgesia; even higher doses of opioids (light blue) do not restore complete analgesia (pink). During opioid-induced hyperalgesia in an injured patient, exaggerated pain sensitivity occurs at the injured and uninjured areas. Any cause of systemic inflammation or neuroinflammation (e.g., infection, cancer, diabetes, stress, or chemotherapy) decreases the analgesic potency and the duration of analgesic effects and leads to earlier development of opioid-induced hyperalgesia; even high doses (dark blue) result in minimal analgesia (purple) because of decreased analgesic potency.
STRATEGIES TO MITIGATE OPIOID TOLERANCE AND OPIOID-INDUCED HYPERALGESIA
Strategies for mitigating opioid tolerance and opioid-induced hyperalgesia include reducing the dose of analgesics and the duration of treatment by interrupting infusions of sedative or analgesic agents daily or modulating infusions on the basis of analgesic assessment and sedation scores, by using multimodal analgesic agents (nerve blocks and nonopioid analgesics), and by rotating analgesic agents sequentially (Table 1, and Tables S2 and S3 in the Supplementary Appendix). Patients who have not previously received opioids usually have a good response to opioid analgesics, whereas those with prolonged exposure (illicit or prescribed) may have opioid tolerance on admission to the ICU, confounding therapy.44,58 Four case scenarios are discussed in Appendix S1 and Table S4 in the Supplementary Appendix.
Table 1.
Strategies for Mitigating Opioid Tolerance or Opioid-Induced Hyperalgesia.*
| Appropriate use of opioids |
| Use of valid assessment scales of pain before and during administration of the analgesic drug |
| Use of intermittent opioid therapy (oral or intravenous) rather than continuous infusions, when possible |
| Opioid rotation |
| Use of remifentanil for short-term analgesia (because of potent induction of opioid-induced hyperalgesia), except when rapid offset of effect is required, as in evaluation of head injury |
| Minimal use of benzodiazepines (because of delirium and potential opioid-induced hyperalgesia associated with long-term use) |
| Avoidance of excessive dose escalation; supplementation of opioid with nonopioid analgesics |
| Addition of methadone to attenuate or delay opioid tolerance |
| Coadministration of nonopioid analgesics as rescue therapy during procedures or to potentiate the effects of opioids |
| N-methyl-d-aspartate receptor antagonists (ketamine) |
| α2-Adrenergic receptor agonists (clonidine or dexmedetomidine) |
| Gabapentinoids (gabapentin or pregabalin) |
| Continuous administration of nerve blocks by means of a catheter |
| Neuraxial: thoracic or lumber epidural blocks for thoracic, abdominal, or bilateral leg analgesia |
| Regional: brachial plexus block for arm analgesia; femoral or obturator block or both, with or without sciatic nerve block for lower-limb analgesia |
| Local: paravertebral block for rib fractures or chest-tube–associated pain; transversus abdominis block for lower abdominal surgery |
| Prevention or reversal of opioid-induced hyperalgesia and opioid-withdrawal symptoms |
| Tapering of opioid dose when pain score goal is achieved (10–20% dose reduction every 1–4 days) |
| Use of valid withdrawal assessment scales |
| Use of adjuncts to opioids (ketamine, dexmedetomidine, or gabapentinoids [gabapentin or pregabalin]) |
| Use of methadone |
| Reduction of inflammation |
| Scheduled acetaminophen therapy |
| Short-term use of ketorolac† |
The nonopioid strategies that are listed are usually used in combination with opioids; dosing regimens and routes of drug administration are provided in Tables S2 and S3 in the Supplementary Appendix.
Other nonsteroidal antiinflammatory drugs (e.g., ibuprofen) have limited use in the intensive care unit because of cardiovascular, nephrotoxic, and gastrointestinal side effects.
DAILY INTERRUPTION OF SEDATIVE INFUSIONS
Interrupted infusions of analgesics and sedatives, as compared with uninterrupted infusions, allow patients to be more awake (without iatrogenic coma), yield improved assessment and treatment of pain with fewer days on a ventilator, lessen psychological distress, and do not increase the incidence of cardiovascular and other outcomes.59–62 Conversion to intermittent bolus therapy or patient-controlled analgesia should be instituted as early as possible. Prolonged coadministration of a benzodiazepine and morphine was shown to exacerbate opioid tolerance63; therefore, reducing benzodiazepine use may mitigate tolerance and delirium. Alternative nonanalgesic drugs could be used for sedation and potentiation of opioid effects.
NEURAXIAL AND NON-NEURAXIAL ANALGESIA
Neuraxial (thoracic or lumbar epidural) analgesic techniques provide effective analgesia while reducing opioid exposure and tolerance, pulmonary morbidity, duration of mechanical ventilation, and the incidence of postoperative ileus. They also render patients more awake and able to adhere to physical therapy regimens.64,65 Indications for epidurals include thoracic or major abdominal surgery, chest wall trauma, or pulmonary contusion. Despite possible contraindications in critically ill patients (e.g., administration of anticoagulant therapy), epidurals can be placed safely before — and continued during — anticoagulant administration. Other regional or non-neuraxial analgesic techniques with catheters are as effective as epidural administration of analgesics in selected patients. Non-neural blocks include paravertebral block for rib fractures or after thoracotomy, as well as transversus abdominis block for surgery of the lower abdomen.65 Ultrasound-guided nerve blocks have been safely performed even in patients in the ICU who have had clinically significant coagulopathy.66
OPIOID ROTATION
Opioid rotation (i.e., administration of a different opioid to control pain) generally mitigates tolerance. A single gene encodes the mu-opioid receptor, but during gene expression some exons are excluded from the final messenger RNA. Consequently, several receptor subtypes can coexist (e.g., mu1 and mu2) owing to alternative exon splicing. One receptor subtype may undergo desensitization to one opioid, leaving other subtypes available for a new opioid; hence, the rationale for opioid rotation for the restoration of analgesic efficacy and decreased doses.67 During opioid rotation, the dose of the new opioid is derived empirically because opioid conversion tables (used for calculations of opioid equivalence) may be inapplicable in a patient in whom tolerance has developed, particularly during the coadministration of nonopioid adjuvants (e.g., dexmedetomidine or ketamine).67 Another explanation for the efficacy of drug rotation during morphine and hydromorphone therapy involves the properties of the morphine-3-glucuronide metabolite, which counteract the analgesic effects of morphine and morphine-6-glucuronide.40 Replacing morphine and hydromorphone with another opioid that has inactive metabolites (e.g., fentanyl or methadone) will decrease morphine-3-glucuronide levels, allowing more effective analgesia by the second drug.
MULTIMODAL ANALGESIA
In multimodal pain management, multiple nonopioid drugs can be used in combination with or in place of opioids to target various nociceptive pathways in the peripheral or central nervous system.68,69 This approach produces additive and even synergistic effects of the analgesic agents and reduces the adverse effects of opioids. Only the common nonopioid analgesics available for pain control are discussed here (Table 1, and Table S3 in the Supplementary Appendix).69
Ketamine induces analgesia largely by blocking the NMDA receptors, but it also has modulatory roles by means of cholinergic, aminergic, and opioid systems.70 Since NMDA-receptor hyperactivity underlies a key mechanism of opioid tolerance and opioid-induced hyperalgesia, ketamine effectively ameliorates these conditions.70 Additional advantages include opioid sparing, minimal respiratory depression without psychotomimetic effects (at lower levels),69,70 and the antidepressant effects of ketamine metabolites.71
Clonidine, an α2-adrenergic agonist, has modest analgesic, sedative, antihypertensive, and opioid-sparing effects and has also been used to mitigate opioid-induced hyperalgesia and withdrawal symptoms during opioid weaning.72 Dexmedetomidine is more potent and has a shorter half-life than clonidine, and doses can easily be adjusted according to the response.69
The gabapentinoids gabapentin and pregabalin are commonly used adjuncts for multimodal analgesia. The involvement of multiple sites of action is probably responsible for their superior pain relief.69 Common dose-related side effects include sedation, dizziness, and confusion, and their opioid potentiation can cause respiratory arrest.73
OTHER NONOPIOID DRUGS
Acetaminophen is a weak analgesic but is commonly incorporated in multimodal analgesic strategies for opioid sparing because it blocks the central production of prostaglandins.74 Parenteral acetaminophen causes hypotension. Doses are reduced or not administered during hepatic dysfunction, malnutrition, or dehydration. The traditional nonsteroidal antiinflammatory drugs (e.g., ibuprofen), which are nonselective cyclooxygenase inhibitors, are not conventionally used in the ICU because of their side effects. Ketorolac is effective as an opioid-sparing agent, and short-term ketorolac use does not increase postoperative bleeding.75
FUTURE DIRECTIONS FOR PREVENTION AND TREATMENT OF OPIOID TOLERANCE
The basic and translational development of forthcoming opioid-receptor–targeted and nonopioid-receptor–targeted pain therapies unrelated to patients in the ICU has been recently reviewed.76 Several approaches presented in that review show promise for mitigating tolerance and hyperalgesia in the ICU. We will highlight three approaches.
MODULATION OF IMMUNE RESPONSES
Studies have suggested that inflammasome and toll-like receptor activation mediated by DAMPs critically affects the glial response to both tissue injury and opioids.3,28,48 Toll-like receptor activation can be regulated by racemic naloxone or a stereoselective naloxone isomer, (+)-naloxone.28 For example, dextromorphine exerts a stereoselective action over levomorphine in the activation of glia; opioid-receptor agonists, including morphine, activate toll-like receptors but are antagonized by (+)-naloxone.28 Thus, (−)-naloxone and (+)-naloxone could be used to differentiate between the role of an opioid in analgesia and activation of toll-like receptors, making it possible to lessen the inflammatory responses to tissue injury and opioid exposure without antagonizing opioid-induced analgesia. Specific inflammasome inhibitors may also be used to modulate gliamediated exaggerated nociception and opioid tolerance, without broadly inhibiting immunity.77
The neuronal α7 nicotinic acetylcholine receptor agonists have been shown to attenuate microglia activation52,78 and to have protective effects on inflammation induced by critical illnesses.79,80 Moreover, α7 nicotinic acetylcholine receptor activation attenuates inflammatory and neuropathic pain and opioid-induced hyperalgesia in rodent models.78,79,81 Therefore, α7 nicotinic acetylcholine receptor agonists could be useful in the reduction of both critical illness–related inflammation and pain.
CANNABINOIDS
The role of endocannabinoids in anxiety, stress, and pain is well documented,82,83 but the usefulness of exogenous cannabinoids in stress-induced hyperalgesia and opioid-sparing in patients in the ICU is unknown. In addition, cannabinoids regulate inflammatory responses in preclinical models.82 The analgesic efficacy of cannabinoids, their interactions (additive or synergistic) with opioids, and their abuse potential when combined with opioids for pain control in patients in the ICU are topics for future study.
BUPRENORPHINE WITH OR WITHOUT NALOXONE AND METHADONE
Buprenorphine, an opioid analgesic agent, is currently used (with or without naloxone) as replacement therapy in persons with substance-use disorders and for the treatment of chronic pain.84 Although it is a weak analgesic for patients who do not have opioid dependency, it has shown promise in patients with opioid dependency because it reverses opioid-induced hyperalgesia.84 Opioid-induced hyperalgesia is partially related to increased interaction of dynorphins with the kappa-opioid receptor; buprenorphine blocks the binding of dynorphins to the kappa-opioid receptor and attenuates opioid-induced hyperalgesia.85,86 Furthermore, buprenorphine, although a partial opioid agonist, has high affinity for opioid receptors and thereby blocks other opioids from activating the same receptors. Although few studies have directly compared the analgesic effects of buprenorphine and methadone, buprenorphine may be superior in cases of renal failure because of extrarenal excretion.87 Thus, pragmatic clinical protocols are needed to guide the use of buprenorphine and naloxone in patients in the ICU who are progressing toward opioid tolerance and for those already receiving buprenorphine or naloxone therapy before admission.
Methadone, another opioid analgesic, mitigates opioid-induced hyperalgesia by inhibiting NMDA receptors and serotonin-reuptake activity and blocking adenylate cyclase overactivity, which is partly responsible for the withdrawal symptoms.36,88 The major downside of methadone is its variable metabolism, intra- and interindividually. During opioid rotation or opioid weaning, methadone doses must be adjusted to maintain sufficient alleviation of symptoms while avoiding side effects.88–90 Oversedation and prolongation of the cardiac QT interval can occur with cytochrome P-450 inhibitors or low magnesium levels. Methadone is used cautiously in the ICU because of its unpredictable half-life and cardiac toxicity.91,92 Methadone is a racemic mixture of two stereoisomers (l-methadone and d-methadone), with l-methadone being 8 to 50 times as potent as d-methadone36; therefore, in patients in the ICU who do not have a response to other opioids, the usefulness of l-methadone or methadone, which produces analgesia through multiple sites of action that differ in potency, is not clear.36,88,90
OTHER DRUGS AND CONSIDERATIONS
Lidocaine, β-adrenoceptor antagonists, magnesium, tricyclic antidepressants, and other drugs have been used as analgesic adjuncts to decrease opioid doses. Their long-term safety and efficacy in the ICU have not been well established in randomized, controlled trials. Selective serotonin-reuptake inhibitors and serotonin–norepinephrine reuptake inhibitors have antinociceptive effects, but mortality is higher among patients who use them before ICU admission than among patients who do not.93 Antipsychotic agents are currently used in the ICU as adjuncts to opioids, but their use has not been studied systematically. Multiple drugs are often administered simultaneously, and in cases of organ dysfunction, the altered drug disposition increases the risk of additive, synergistic, or antagonistic drug interactions, underscoring the importance of reviewing all medications.94 Hepatic disease mildly affects the dispositions of morphine and fentanyl, and their elimination depends on hepatic blood flow; therefore, the drugs are useful in hepatic dysfunction but not in low-flow states.95 Remifentanil, although rapidly cleared even in cases of organ dysfunction, quickly induces tolerance and opioid-induced hyperalgesia.91
CONCLUSIONS
There are many indications for opioid use in persons with critical illnesses. However, long-term opioid use has detrimental effects, including analgesic tolerance, which drives dose escalation and leads to opioid-induced hyperalgesia. Pain management (analgesia) in patients in the ICU, who are more vulnerable than the general population to both exaggerated tolerance and the deleterious effects of opioids, has been a challenge for decades, and methods to mitigate the risks associated with opioid administration are unresolved. The etiologic factors (i.e., cell types and receptors, sex, extremes of age, and the underlying inflammation- and opioid-induced immune responses) that contribute to these maladaptive responses have not been well characterized and pose a barrier to improving analgesic therapy in cases of critical illness. A more nuanced understanding of the way critical illness and inflammation affect the body’s response to opioids could lead to tremendous reduction in morbidity among critically ill patients. Furthermore, the translational application of pain therapies targeted to opioid receptors and those targeted to nonopioid receptors is currently being studied in the general population and provides a road map for strategies to mitigate opioid tolerance in persons with critical illnesses.
Supplementary Material
Acknowledgments
We thank Ethan Sanford, M.D., Derek Hursey, Pharm.D., Melissa Gorman, M.S.N., C.C.R.N.-K., and M. Trejeeve Martyn, M.D., for their comments and critiques on an earlier version of the manuscript.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Cullen DJ, Sweitzer BJ, Bates DW, Burdick E, Edmondson A, Leape LL. Preventable adverse drug events in hospitalized patients: a comparative study of intensive care and general care units. Crit Care Med 1997; 25: 1289–97. [DOI] [PubMed] [Google Scholar]
- 2.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013; 41: 263–306. [DOI] [PubMed] [Google Scholar]
- 3.Roeckel LA, Le Coz GM, Gavériaux-Ruff C, Simonin F. Opioid-induced hyperalgesia: cellular and molecular mechanisms. Neuroscience 2016; 338:1 60–82. [DOI] [PubMed] [Google Scholar]
- 4.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain 2008; 24: 479–96. [DOI] [PubMed] [Google Scholar]
- 5.Puntillo KA, Naidu R. Chronic pain disorders after critical illness and ICU-acquired opioid dependence: two clinical conundra. Curr Opin Crit Care 2016; 22: 506–12. [DOI] [PubMed] [Google Scholar]
- 6.Bittner EA, Shank E, Woodson L, Martyn JA. Acute and perioperative care of the burn-injured patient. Anesthesiology 2015; 122:4 48–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand KJ, Willson DF, Berger J, et al. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics 2010; 125(5): e1208–e1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Mitchell J, Moriyama K, et al. Age-dependent morphine tolerance development in the rat. Anesth Analg 2005; 100: 1733–9. [DOI] [PubMed] [Google Scholar]
- 9.Mendell JR, Sahenk Z. Painful sensory neuropathy. N Engl J Med 2003;3 48: 1243–55. [DOI] [PubMed] [Google Scholar]
- 10.Stein C Opioid receptors. Annu Rev Med 2016;67:4 33–51. [DOI] [PubMed] [Google Scholar]
- 11.Meisner JG, Marsh AD, Marsh DR. Loss of GABAergic interneurons in laminae I-III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J Neurotrauma 2010; 27: 729–37. [DOI] [PubMed] [Google Scholar]
- 12.Chanques G, Sebbane M, Barbotte E, Viel E, Eledjam JJ, Jaber S. A prospective study of pain at rest: incidence and characteristics of an unrecognized symptom in surgical and trauma versus medical intensive care unit patients. Anesthesiology 2007; 107: 858–60. [DOI] [PubMed] [Google Scholar]
- 13.Puntillo KA, Max A, Timsit JF, et al. Determinants of procedural pain intensity in the intensive care unit: the Europain study. Am J Respir Crit Care Med 2014;1 89: 39–47. [DOI] [PubMed] [Google Scholar]
- 14.Chanques G, Pohlman A, Kress JP, et al. Psychometric comparison of three behavioural scales for the assessment of pain in critically ill patients unable to self-report. Crit Care 2014; 18: R160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faust AC, Rajan P, Sheperd LA, Alvarez CA, McCorstin P, Doebele RL. Impact of an analgesia-based sedation protocol on mechanically ventilated patients in a medical intensive care unit. Anesth Analg 2016; 123: 903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devabhakthuni S, Armahizer MJ, Dasta JF, Kane-Gill SL. Analgosedation: a paradigm shift in intensive care unit sedation practice. Ann Pharmacother 2012; 46:5 30–40. [DOI] [PubMed] [Google Scholar]
- 17.Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain 2008; 9: 122–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tennant F The physiologic effects of pain on the endocrine system. Pain Ther 2013;2 : 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN. The surgically induced stress response. JPEN J Parenter Enteral Nutr 2013; 37:Suppl: 21S–29S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wohleb ES, McKim DB, Sheridan JF, Godbout JP. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci 2015; 8: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fonken LK, Weber MD, Daut RA, et al. Stress-induced neuroinflammatory priming is time of day dependent. Psychoneuroendocrinology 2016; 66:8 2–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson BJ, Mikkelsen ME. Stress-ing the brain: the immune system, hypothalamic-pituitary-adrenal axis, and psychiatric symptoms in acute respiratory distress syndrome survivors. Ann Am Thorac Soc 2017; 14:8 39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain 2013;1 4: 1539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novaes MA, Knobel E, Bork AM, Pavão OF, Nogueira-Martins LA, Ferraz MB. Stressors in ICU: perception of the patient, relatives and health care team. Intensive Care Med 1999; 25: 1421–6. [DOI] [PubMed] [Google Scholar]
- 25.Baumbach P, Götz T, Günther A, Weiss T, Meissner W. Prevalence and characteristics of chronic intensive care-related pain: the role of severe sepsis and septic shock. Crit Care Med 2016;4 4: 1129–37. [DOI] [PubMed] [Google Scholar]
- 26.Jiang X, Orton M, Feng R, et al. Chronic opioid usage in surgical patients in a large academic center. Ann Surg 2017; 265:7 22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wibbenmeyer L, Sevier A, Liao J, et al. The impact of opioid administration on resuscitation volumes in thermally injured patients. J Burn Care Res 2010; 31: 48–56. [DOI] [PubMed] [Google Scholar]
- 28.Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev 2011; 63:7 72–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gudin JA, Laitman A, Nalamachu S. Opioid related endocrinopathy. Pain Med 2015; 16: Suppl 1: S9–S15. [DOI] [PubMed] [Google Scholar]
- 30.Carmona-Bayonas A, Jiménez-Fonseca P, Castañón E, et al. Chronic opioid therapy in long-term cancer survivors. Clin Transl Oncol 2017; 19: 236–50. [DOI] [PubMed] [Google Scholar]
- 31.Juneja R. Opioids and cancer recur-rence. Curr Opin Support Palliat Care 2014;8 : 91–101. [DOI] [PubMed] [Google Scholar]
- 32.Pandharipande P, Cotton BA, Shin-tani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma 2008; 65:3 4–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pisani MA, Murphy TE, Araujo KL, Van Ness PH. Factors associated with persistent delirium after intensive care unit admission in an older medical patient population. J Crit Care 2010; 25(3): 540.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet 2010;3 75: 475–80. [DOI] [PubMed] [Google Scholar]
- 35.Schaller SJ, Alam SM, Mao J, et al. Pharmacokinetics cannot explain the increased effective dose requirement for morphine and midazolam in rats during their extended administration alone or in combination. J Pharm Pharmacol 2017; 69: 82–8. [DOI] [PubMed] [Google Scholar]
- 36.Kharasch ED. Current concepts in methadone metabolism and transport. Clin Pharmacol Drug Dev 2017; 6: 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han T, Harmatz JS, Greenblatt DJ, Martyn JA. Fentanyl clearance and volume of distribution are increased in patients with major burns. J Clin Pharmacol 2007; 47: 674–80. [DOI] [PubMed] [Google Scholar]
- 38.Bista SR, Haywood A, Hardy J, Lobb M, Tapuni A, Norris R. Protein binding of fentanyl and its metabolite nor-fentanyl in human plasma, albumin and α−1 acid glycoprotein. Xenobiotica 2015; 45: 207–12. [DOI] [PubMed] [Google Scholar]
- 39.Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol 2007; 71: 667–75. [DOI] [PubMed] [Google Scholar]
- 40.Smith MT. Neuroexcitatory effects of morphine and hydromorphone: evidence implicating the 3-glucuronide metabolites. Clin Exp Pharmacol Physiol 2000; 27: 524–8. [DOI] [PubMed] [Google Scholar]
- 41.Roeckel LA, Utard V, Reiss D, et al. Morphine-induced hyperalgesia involves mu opioid receptors and the metabolite morphine-3-glucuronide. Sci Rep 2017; 7: 10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porreca F, Cowan A, Raffa RB, Tal-larida RJ. Estimation in vivo of the receptor constants of morphine in naive and morphine-tolerant rats. Life Sci 1982;3 1: 2355–8. [DOI] [PubMed] [Google Scholar]
- 43.Dumas EO, Pollack GM. Opioid toler-ance development: a pharmacokinetic/pharmacodynamic perspective. AAPS J 2008; 10:5 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend 2001; 63: 139–46. [DOI] [PubMed] [Google Scholar]
- 45.Cahill CM, Walwyn W, Taylor AMW, Pradhan AAA, Evans CJ. Allostatic mechanisms of opioid tolerance beyond desensitization and down-regulation. Trends Pharmacol Sci 2016; 37: 963–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waxman AR, Arout C, Caldwell M, Dahan A, Kest B. Acute and chronic fentanyl administration causes hyperalgesia independently of opioid receptor activity in mice. Neurosci Lett 2009; 462: 68–72. [DOI] [PubMed] [Google Scholar]
- 47.Costantino CM, Gomes I, Stockton SD, Lim MP, Devi LA. Opioid receptor heteromers in analgesia. Expert Rev Mol Med 2012; 14: e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science 2016; 354: 572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donello JE, Guan Y, Tian M, et al. A peripheral adrenoceptor-mediated sympathetic mechanism can transform stress-induced analgesia into hyperalgesia. Anesthesiology 2011; 114:1 403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Goeij M, van Eijk LT, Vanelderen P, et al. Systemic inflammation decreases pain threshold in humans in vivo. PLoS One 2013; 8(12):e 84159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nyland JE, McLean SA, Averitt DL. Prior stress exposure increases pain behaviors in a rat model of full thickness thermal injury. Burns 2015; 41: 1796–804. [DOI] [PubMed] [Google Scholar]
- 52.Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 2017; 46: 927–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frank MG, Watkins LR, Maier SF. Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav Immun 2013; 33:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiu IM, Heesters BA, Ghasemlou N, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013; 501:5 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fusco M, Skaper SD, Coaccioli S, Var-rassi G, Paladini A. Degenerative joint diseases and neuroinflammation. Pain Pract 2017; 17:5 22–32. [DOI] [PubMed] [Google Scholar]
- 56.Gatson JW, Liu MM, Rivera-Chavez FA, Minei JP, Wolf SE. Serum levels of neurofilament-H are elevated in patients suffering from severe burns. J Burn Care Res 2015; 36:5 45–50. [DOI] [PubMed] [Google Scholar]
- 57.Verriotis M, Chang P, Fitzgerald M, Fabrizi L. The development of the nociceptive brain. Neuroscience 2016; 338: 207–19. [DOI] [PubMed] [Google Scholar]
- 58.Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care 2011; 39: 804–23. [DOI] [PubMed] [Google Scholar]
- 59.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000; 342: 1471–7. [DOI] [PubMed] [Google Scholar]
- 60.Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med 2003; 168: 1457–61. [DOI] [PubMed] [Google Scholar]
- 61.Nassar APJ, Park M. Sedation proto-cols versus daily sedation interruption: a systematic review and meta-analysis. Rev Bras Ter Intensiva 2016; 28: 444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shehabi Y The golden hours of ICU sedation: the clock is ticking. Crit Care Med 2018; 46:4 90–1. [DOI] [PubMed] [Google Scholar]
- 63.Song L, Wang S, Zuo Y, Chen L, Martyn JA, Mao J. Midazolam exacerbates morphine tolerance and morphine-induced hyperactive behaviors in young rats with burn injury. Brain Res 2014; 1564: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Capdevila M, Ramin S, Capdevila X. Regional anesthesia and analgesia after surgery in ICU. Curr Opin Crit Care 2017; 23: 430–9. [DOI] [PubMed] [Google Scholar]
- 65.De Pinto M, Dagal A, O’Donnell B, Stogicza A, Chiu S, Edwards WT. Regional anesthesia for management of acute pain in the intensive care unit. Int J Crit Illn Inj Sci 2015; 5: 138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wiebalck A, Grau T. Ultrasound im-aging techniques for regional blocks in intensive care patients. Crit Care Med 2007;3 5: Suppl:S 268–S274. [DOI] [PubMed] [Google Scholar]
- 67.Webster LR, Fine PG. Review and cri-tique of opioid rotation practices and associated risks of toxicity. Pain Med 2012; 13:5 62–70. [DOI] [PubMed] [Google Scholar]
- 68.Kohler M, Chiu F, Gelber KM, Webb CA, Weyker PD. Pain management in critically ill patients: a review of multimodal treatment options. Pain Manag 2016;6 : 591–602. [DOI] [PubMed] [Google Scholar]
- 69.Kumar K, Kirksey MA, Duong S, Wu CL. A review of opioid-sparing modalities in perioperative pain management: methods to decrease opioid use postoperatively. Anesth Analg 2017;1 25: 1749–60. [DOI] [PubMed] [Google Scholar]
- 70.Mion G, Villevieille T. Ketamine phar-macology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther 2013; 19: 370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Costi S, Van Dam NT, Murrough JW. Current status of ketamine and related therapies for mood and anxiety disorders. Curr Behav Neurosci Rep 2015; 2: 216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang JG, Belley-Coté E, Burry L, et al. Clonidine for sedation in the critically ill: a systematic review and meta-analysis. Crit Care 2017; 21: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quintero GC. Review about gabapen-tin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017;9 : 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McDaid C, Maund E, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery: a systematic review. Health Technol Assess 2010;1 4: 1–153, iii-iv. [DOI] [PubMed] [Google Scholar]
- 75.Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg 2017; 152:6 91–7. [DOI] [PubMed] [Google Scholar]
- 76.Knezevic NN, Yekkirala A, Yaksh TL. Basic/translational development of forthcoming opioid- and nonopioid-targeted pain therapeutics. Anesth Analg 2017; 125: 1714–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boriushkin E, Wang JJ, Li J, Bhatta M, Zhang SX. p58(IPK) Suppresses NLRP3 inflammasome activation and IL-1β production via inhibition of PKR in macrophages. Sci Rep 2016; 6: 25013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bagdas D, Gurun MS, Flood P, Papke RL, Damaj MI. New insights on neuronal nicotinic acetylcholine receptors as targets for pain and inflammation: a focus on α7 Opioid Tolerance in Critical Illness nAChRs. Curr Neuropharmacol 2018;1 6: 415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ren C, Tong YL, Li JC, Lu ZQ, Yao YM. The protective effect of alpha 7 nicotinic acetylcholine receptor activation on critical illness and its mechanism. Int J Biol Sci 2017; 13: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chavan SS, Tracey KJ. Essential neuroscience in immunology. J Immunol 2017; 198: 3389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abbas M, Rahman S. Effects of alpha-7 nicotinic acetylcholine receptor positive allosteric modulator on lipopolysaccharide-induced neuroinflammatory pain in mice. Eur J Pharmacol 2016; 783: 85–91. [DOI] [PubMed] [Google Scholar]
- 82.Ueda M, Iwasaki H, Wang S, et al. Cannabinoid receptor type 1 antagonist, AM251, attenuates mechanical allodynia and thermal hyperalgesia after burn injury. Anesthesiology 2014; 121:1 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Woodhams SG, Chapman V, Finn DP, Hohmann AG, Neugebauer V. The cannabinoid system and pain. Neuropharmacology 2017; 124: 105–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.CheY, CheL, Ma J. Buprenorphine-naloxone therapy in pain management. Anesthesiology 2014; 120: 1262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vanderah TW, Gardell LR, Burgess SE, et al. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci 2000; 20: 7074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horan P, Taylor J, Yamamura HI, Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther 1992; 260:1 237–43. [PubMed] [Google Scholar]
- 87.Davis MP. Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. J Support Oncol 2012; 10:2 09–19. [DOI] [PubMed] [Google Scholar]
- 88.Elefritz JL, Murphy CV, Papadimos TJ, Lyaker MR. Methadone analgesia in the critically ill. J Crit Care 2016; 34: 84–8. [DOI] [PubMed] [Google Scholar]
- 89.McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict 2010; 19: 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moody DE. Metabolic and toxicological considerations of the opioid replacement therapy and analgesic drugs: methadone and buprenorphine. Expert Opin Drug Metab Toxicol 2013; 9: 675–97. [DOI] [PubMed] [Google Scholar]
- 91.Hayhurst CJ, Durieux ME. Differential opioid tolerance and opioid-induced hyperalgesia: a clinical reality. Anesthesiology 2016;1 24: 483–8. [DOI] [PubMed] [Google Scholar]
- 92.Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MC. QTc interval screening in methadone treatment. Ann Intern Med 2009;1 50: 387–95. [DOI] [PubMed] [Google Scholar]
- 93.Ghassemi M, Marshall J, Singh N, Stone DJ, Celi LA. Leveraging a critical care database: selective serotonin reuptake inhibitor use prior to ICU admission is associated with increased hospital mortality. Chest 2014; 145: 745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feng XQ, Zhu LL, Zhou Q. Opioid analgesics-related pharmacokinetic drug interactions: from the perspectives of evidence based on randomized controlled trials and clinical risk management. J Pain Res 2017; 10: 1225–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi L, Ferrell BA, Vasilevskis EE, et al. Population pharmacokinetics of fentanyl in the critically ill. Crit Care Med 2016; 44: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.