Abstract
Axonal degeneration is an active, highly controlled process that contributes to beneficial processes, such as developmental pruning, but also to neurodegeneration. In glaucoma, ocular hypertension leads to vision loss by killing the output neurons of the retina, the retinal ganglion cells (RGC). Multiple processes have been proposed to contribute to and/or mediate axonal injury in glaucoma including: neuroinflammation, loss of neurotropic factors, dysregulation of the neurovascular unit, and disruption of the axonal cytoskeleton. While the inciting injury to RGCs in glaucoma is complex and potentially heterogeneous, axonal injury is ultimately thought to be the key insult that drives glaucomatous neurodegeneration. Glaucomatous neurodegeneration is a complex process, with multiple molecular signals contributing to RGC somal loss and axonal degeneration. Furthermore, the propagation of the axonal injury signal is complex with injury triggering programs of degeneration in both the somal and axonal compartment. Further complicating this process is the involvement of multiple cell types that are known to participate in the process of axonal and neuronal degeneration after glaucomatous injury. Here, we review the axonal signaling that occurs after injury and the molecular signaling programs currently known to be important for somal and axonal degeneration after glaucoma-relevant axonal injuries.
Keywords: apoptosis, axon, axonopathy, dendritic remodeling, optic neuropathy, intraocular pressure, neuroprotection, synaptic loss, neurodegeneration, retinal ganglion cell, axonal degeneration
Glaucomatous neurodegeneration is a complex process with numerous signaling pathways implicated in the death of retinal ganglion cells (RGCs) and their axons. Glaucoma is frequently associated with elevated intraocular pressure (IOP) and increased age (exceptions being normal tension glaucoma and congenital glaucoma, respectively, Gordon et al., 2002; Tielsch, 1996). Study of the molecular signaling pathways important for glaucomatous neurodegeneration has thus focused on understanding the molecular injury response to ocular hypertension, the focus of this review. As the molecular etiology of glaucoma is complex and heterogeneous, this suggests glaucoma might have multiple inciting causes which may differ between patients and that pro-degenerative signaling may even differ between RGCs within a patient. Over the last several decades research efforts have thus focused on identifying the molecular signaling pathway(s) that lead from glaucomatous insult to RGC death. While these efforts have not defined the critical molecular pathway responsible for RGC death in glaucoma, they have resulted in important insights into glaucomatous neurodegeneration. Axonal injury has been defined as the key early insult in glaucoma that ultimately leads to apoptotic RGC death. Importantly, these efforts led to the hypothesis that glaucomatous neurodegeneration is compartmentalized, with distinct molecular signals that contribute to axonal degeneration and somal degeneration. In this review, we will summarize what has been discovered regarding the cell signaling pathways controlling axonal injury signaling, somal apoptosis, and axonal degeneration with special focus on the pathways specifically important after ocular hypertensive injury.
1. Historical perspectives
The condition glaukos was first described in ancient Greek writings (Leffler et al., 2015b). Glaukos was used to describe eyes in which the pupil of the eye appeared to take on a hazy green/gray hue. The condition likely represented what is considered a cataract today, although the pupil in closed-angle glaucoma may take on a green hue. The first description of the disease that we now recognize as glaucoma occurred in the 17th century. Englishman Dr. Richard Banister described a correlation between a palpable hardness of the eye and blindness in 1622 (Leffler et al., 2015a). In the 19th century, the Scottish ophthalmologist Dr. William MacKenzie first described both an acute and chronic disease with hardness of the eye (Mackenzie, 1830). He also suggested that lowering IOP might be a treatment for glaucoma; “it is not unreasonable to conclude that occasionally puncturing the sclerotic and choroid might prove serviceable, by relieving the pressure of the accumulated fluid on the retina” (Mackenzie, 1830).
It was not until the 1970s, however, that animal models were used to interrogate the relationship between elevated intraocular pressure, axonal degeneration, and retinal ganglion cell death. In seminal studies completed in non-human primates, moderate increases in IOP (performed with manometry) led to proportional partial obstruction of axonal transport in the lamina cribrosa region of the eye and limited transport of retinal proteins to the lateral geniculate nucleus (Anderson and Hendrickson, 1974; Minckler et al., 1977; Quigley and Addicks, 1980; Quigley and Anderson, 1977; Radius and Anderson, 1981). The lamina cribrosa is a specialized structure comprised of a network of collagen fibers covered with astrocytes in the primate optic nerve head (Anderson, 1969; Kiumehr et al., 2012). RGC axon fibers from the retinal nerve fiber layer pass through pores in the collagen thereby exiting the posterior eye, and coalesce to form the myelinated optic nerve. When IOP was increased within 25 mmHg of mean blood pressure, axonal transport was blocked at the lamina cribrosa (Anderson and Hendrickson, 1974). On histologic exam, axons within the lamina region were dilated and there was an accumulation of mitochondria and other cytoplasmic organelles. Prolonged elevation of IOP led to optic disc cupping and degeneration of RGCs (Quigley and Addicks, 1980). Similar experiments were conducted to evaluate the effect of elevated intracranial pressure (ICP) on axonal transport because elevated ICP is known to contribute to the pathogenesis of some optic neuropathies (Binder et al., 2004; Markey et al., 2016). Transient elevations of ICP did not alter rapid axoplasmic transport at the optic nerve head in non-human primates, suggesting that pathogenic changes in glaucomatous injury are specific to IOP elevation and not due to global elevation of ICP (Anderson and Hendrickson, 1977). Together these results implicated the optic nerve head, specifically the lamina cribrosa, in the pathogenesis of glaucomatous injury and suggest even modest elevation of IOP can selectively alter axonal function prior to RGC death.
Additional studies of human visual outcomes in glaucoma patients and of post-mortem glaucomatous eyes have shown ocular hypertension induced axonal injury in the region of the optic nerve head and lamina cribrosa. Classically, the clinical visual field defect observed in patients with glaucoma is the arcuate scotoma (Su et al., 2013). This pattern recapitulates the path of RGC axons as they travel across the retina to exit the eye at the optic nerve head. Post-mortem evaluation of the degree of damage in the optic nerve head correlated with previous visual field testing and clinical evaluation of the optic disc (Quigley and Green, 1979). Pathologic changes were also observed at the level of the lamina cribrosa in eyes of glaucoma patients (Quigley and Addicks, 1981; Quigley et al., 1981). Furthermore, when glaucomatous eyes were categorized by the severity of previous visual field defects, compression at the lamina cribrosa and loss of RGC axons was observed on histopathology even in patients without visual field loss (Quigley et al., 1983). These findings support previous work done in non-human primates that axonal changes at the lamina cribrosa are an early, important component of the glaucomatous disease process.
The era of molecular genetics provided further evidence that axonal injury at the lamina cribrosa is the critical event triggering ocular hypertension induced glaucomatous neurodegeneration (Howell et al., 2013; Inman et al., 2006; Jakobs et al., 2005; Schlamp et al., 2006). In DBA/2J mice, a model of age-related ocular hypertension, a structure analogous to the primate lamina cribrosa exists, which has been termed the glial lamina (Howell et al., 2007). The glial lamina is formed by a network of astrocytes without collagen components. Despite the structural differences between mouse and human, the earliest axonal damage in glaucomatous injury in the mouse appears to occur in the glial lamina (Howell et al., 2007; Jakobs et al., 2005; Sun et al., 2009). In DBA/2J mice, damaged axon segments are first apparent in the glial lamina suggesting this is an area of early injury (Howell et al., 2007). Wedge-shaped sectors of axonal loss were observed radiating from the optic nerve head, suggesting that damage to axon bundles occurred at the level of lamina cribrosa (Jakobs et al., 2005; Sun et al., 2009). The identification of the lamina region as the location of this critical, early insult in glaucomatous neurodegeneration, has allowed for investigation into the molecular signals governing glaucomatous neurodegeneration (Whitmore et al., 2005).
2. Compartmentalization of axonal degeneration and somal apoptosis in glaucoma
In the early 2000s, a theory was postulated suggesting axonal degeneration, rather than neuronal death, might be a primary driver of disease progression for some neurodegenerative conditions (Raff et al., 2002; Whitmore et al., 2005). Furthermore, work suggested axons have unique molecular programs of degeneration, independent from the molecular programs driving neuronal (somal) apoptosis (Deckwerth and Johnson, 1994; Finn et al., 2000). Using a wide variety of animal models of glaucoma, researchers have begun to identify and order the unique and overlapping molecules important for the somal and axonal programs of neurodegeneration in glaucomatous injury.
Evidence from both animal models and human post-mortem studies suggest RGC somas undergo apoptosis after glaucomatous injury (Maes et al., 2017; McKinnon, 1997). In an effort to determine molecular events that contribute to the pathogenesis of glaucomatous injury, BAX, a known pro-apoptotic member of the B-cell lymphoma/leukemia 2 (Bcl2) family, was tested in glaucoma-relevant injuries. Bax deficiency provided complete protection to RGC somas in the DBA/2J ocular hypertensive mouse and after optic nerve crush (Figure 1, Isenmann et al., 1999; Li et al., 2000; Libby et al., 2005b; Semaan et al., 2010). Bax deficiency delayed glaucomatous axonal degeneration in DBA/2J mice and protected the proximal component of the RGC axon (that is, the axon segment from the soma to the lamina, Howell et al., 2007; Libby et al., 2005b). Interestingly, it is possible that Bax has a second non-canonical role outside of RGC somal apoptosis directly in the axon. BAX is known to have a role in axonal degeneration, at least in cultured neurons (Nikolaev et al., 2009). In DBA/2J mice, Bax deficiency delayed axonal degeneration in some eyes, though Bax deficiency did not provide long-term protection for RGC axons distal to the lamina (Bax deficient DBA/2J mice had similar levels of glaucomatous optic nerve damage as control mice despite having complete somal protection, Howell et al., 2007; Libby et al., 2005b). Thus, it is unclear if BAX has a direct role in the axon in adult RGCs and further studies are needed to directly test BAX’s role in axonal degeneration in RGCs. Thus, axonal degeneration does not require BAX or caspase 3 activation (Finn et al., 2000; Libby et al., 2005b; Maes et al., 2017; Nickells, 2007; Whitmore et al., 2003). Together these data suggest that molecular signaling events critical for somal apoptosis are independent of those necessary for distal axonal degeneration in glaucoma. At the time when Bax deficiency was shown to protect RGC somas from axonal injury, however, little was known about the processes controlling axonal degeneration.
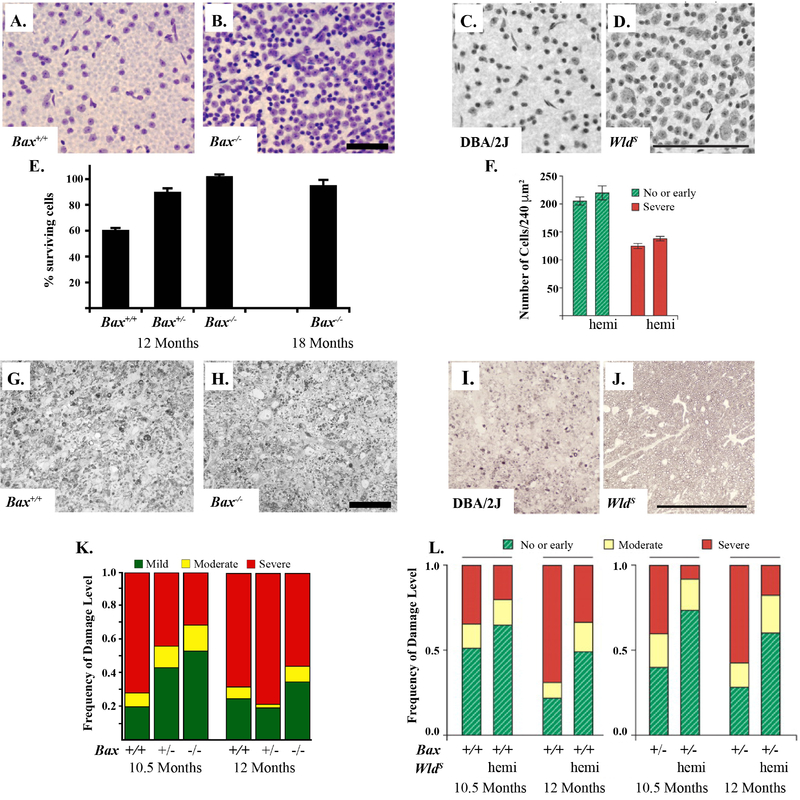
Figure 1: BAX deficiency protects RGC somas from glaucomatous damage and the WldS allele protects both somas and axons.
BAX deficiency protects RGC somas in the DBA/2J model of ocular hypertension. The corresponding retinas of severely glaucomatous optic nerves (95% or more axonal degeneration) from BAX deficient mice (Bax−/−) had significantly more RGCs as compared to wildtype animals (Bax+/+, A and B, Nissel-stained with cresyl violet, Scale bar = 50 μm). BAX deficient animals have significantly increased RGCs at baseline (BAX is required for normal developmental RGC apoptosis) and thus quantification is presented as percent surviving RGCs (retinas from corresponding optic nerves with severely glaucomatous retinas as compared to retinas with corresponding optic nerves with no glaucomatous damage from the same genotype, E). Optic nerve cross-sections stained with p-phenylenediamine (PPD) demonstrate glial scarring and loss of axons in both wildtype and BAX deficient animals (G and H, Scale bar = 50 μm). BAX deficiency does not prevent glaucomatous axonal degeneration (K). The WldS allele protects both axons and somas from glaucomatous damage. By rescuing approximately 50% of optic nerves from severe glaucoma, the WldS allele also protected RGCs in the corresponding retinas of these nerves (C- severe glaucoma wildtype DBA/2J and D- no/early glaucoma with WldS allele, Scale bar = 100 μm). In retinas with corresponding severe nerves, the WldS allele did not protect RGCs somas (F). As compared with wildtype animals, optic nerves of animals carrying the WldS allele (hemi) had less glaucomatous damage as compared to wildtype animals. Optic nerve cross-sections stained with PPD demonstrate glial scarring and loss of axons in the wildtype glaucomatous DBA/2J nerves but not in those carrying the WldS allele (I and J, Scale bar = 100 μm). BAX heterozygotes (Bax+/−) were not significantly different from wildtype DBA/2J animals (L, Figure adapted from Howell et al., 2007; Libby et al., 2005b).
In the late 1990s and early 2000s, axonal degeneration was beginning to be understood as a unique molecular process. A spontaneous murine mutation, WldS (Wallerian degeneration slow), encodes a fusion protein of UBE4B and NMNAT1 (Coleman et al., 1998; Mack et al., 2001). This gain of function mutation was found to delay leucocyte invasion after injury and prolong the survival of injured axons in a variety of neurodegenerative disorders, and thus has been used to test the role of axonal degeneration in disease (Ferri et al., 2003; Gillingwater et al., 2004; Lunn et al., 1989; Samsam et al., 2003). The WldS allele lessened axonal degeneration after optic nerve crush (young animals) and age-related ocular hypertension (Figure 1, Beirowski et al., 2008; Fernandes et al., 2014; Fernandes et al., 2018; Howell et al., 2007). Importantly, in the ocular hypertensive DBA/2J mouse, WldS expression significantly lessened glaucomatous neurodegeneration. In fact, WldS expression appeared to both protect RGCs from dendritic remodeling and preserve their function (Harder et al., 2017; Howell et al., 2007). The protection of components in both the somal and axonal compartments by WldS further implicates axonal degeneration as a key driver of glaucomatous neurodegeneration. However, despite the protection from axonal degeneration, WldS did not prevent RGC somal degeneration after axonal injury (Howell et al., 2007). Together these findings suggest axonal degeneration is a key early pathogenic event in glaucomatous neurodegeneration and support the idea of compartmentalization of the molecular mechanisms that contribute to RGC death in glaucoma (Figure 2). The specific molecular signals responsible for the protection conferred by the gain of function WldS allele, however, remain unknown.
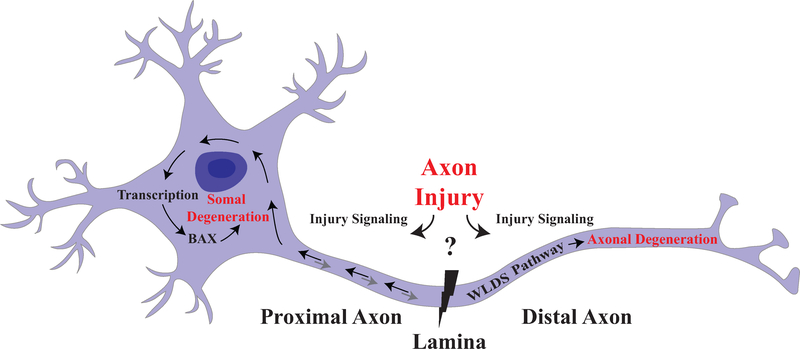
Figure 2: Compartmentalization of axonal degeneration and somal apoptosis.
Schematic of axonal injury leading to both somal apoptosis and axonal degeneration. Inciting extrinsic factors lead to injury at the glial lamina after ocular hypertensive injury. Axonal injury signaling in the proximal and distal axon leads to degeneration of both somal and axonal compartment. Somal degeneration is known to occur through transcription and subsequent BAX-dependent apoptosis while the WldS allele is known to protect the axonal compartment from degeneration. Furthermore, anterograde signaling from the somal compartment contributes to axonal degeneration suggesting that while axonal injury is a major driver of pro-degenerative signals, there are interactions between the two compartments (Simon et al., 2016).
3. Axon injury signaling in glaucomatous injury
Though many events extrinsic to RGCs have been proposed to activate the molecular changes within RGCs that lead to RGC death in glaucoma, we still do not understand the early, necessary pathologic events within RGCs that lead to axonal degeneration and somal apoptosis. Thus, much work has been done to determine the intrinsic molecular signaling events that occur as a local axonal injury response signal.
The progression from early, focal injury to RGC axons to distinct compartmental degenerative processes in glaucoma suggests axonal stress signaling pathways operate along the somal-axonal axis (Howell et al., 2013; Larhammar et al., 2017; Whitmore et al., 2005; Yang et al., 2015). As discussed above, axonal injury at the level of the lamina cribrosa is thought to be the inciting event of glaucomatous neurodegeneration. Axon injury is thought to be the critical early injury to RGCs in glaucoma, and axon injury dependent axonal degeneration can be prevented with manipulation of molecular signaling (WldS). Thus, determining the specific mechanisms of the molecular signaling events that govern axonal degeneration is essential for both understanding and intervening in the glaucomatous disease process. A challenge of research efforts is understanding the specific molecular programs that control somal and axonal degeneration, and also determining potential interactions between these two molecular signaling compartments. Given the somal protection afforded by BAX deficiency and the axonal protection afforded by the WldS allele after glaucoma relevant injuries, many groups have focused on determining and ordering the upstream signaling events required for somal and axonal degeneration. To understand glaucomatous neurodegeneration, it is critical that we define the molecular event(s) controlling axonal injury signaling since it is likely that axonal injury signaling is an important early event within RGCs after a glaucomatous insult.
Identifying the local axonal signaling pathways that detect and respond to damage is an important step in understanding glaucoma and other axonopathies. The mitogen-activated protein kinase (MAPK) family plays an integral role in signal transduction in response to changes in the cellular environment by integrating diverse signals arising from cellular stressors. Multiple lines of evidence support c-Jun N-terminal kinases (JNK) signaling, members of the MAPK family, as an important event in glaucomatous axonal injury signaling. Retrograde transport of JNKs in peripheral nerve axons following axonal injury is known to alter somal transcription of known injury response molecules (Lindwall and Kanje, 2005). Glaucoma relevant axonal insults, such as distortion of the RGC axonal cytoskeleton, have been shown to activate JNK (Huang et al., 2011; Muller et al., 2006). JNK can also be activated by neurotrophic deprivation and extrinsic pro-inflammatory signaling, particularly by TNF and IL-1, both of which have been implicated as deleterious signals in the optic nerve head (Eilers et al., 2001; Howell et al., 2011a; Howell et al., 2011b; Saklatvala et al., 1996; Tezel et al., 2004). Importantly, JNK signaling is known to be activated in RGCs after many glaucoma relevant injuries (e.g. axonal injury and ocular hypertension) and pJNK is present in RGCs in human glaucoma patients (Fernandes et al., 2012; Isenmann and Bahr, 1997; Kwong and Caprioli, 2006; Levkovitch-Verbin et al., 2005; Lukas et al., 2009; Munemasa et al., 2006; Pelzel et al., 2010; Roth et al., 2003; Tezel et al., 2003). Several studies have shown JNK inhibitors lessen or delay RGC death after glaucoma relevant insults (Liu et al., 2011a; Sun et al., 2011; Tezel et al., 2004; Yang et al., 2008). Interestingly, JNK is also known to regulate axonal degeneration (Larhammar et al., 2017; Miller et al., 2009; Xiong and Collins, 2012; Yang et al., 2015). These findings highlight the functional importance of JNK signaling in axonal degeneration and RGC death in glaucoma.
Activated JNK is detected in three cellular compartments after axonal injury: the RGC soma, the axonal segments proximal to the site of injury, and axonal segments distal to the site of injury (Fernandes et al., 2014; Lukas et al., 2009). JNK is required for axonal degeneration after axonal injury in some systems, and pharmacologic and genetic inhibition of JNK signaling delayed axonal degeneration in multiple models of axonal injury (Barrientos et al., 2011; Miller et al., 2009; Simon et al., 2016; Yang et al., 2015; Yoshimura et al., 2011). Glaucomatous neurodegeneration may differ from other experimentally induced axonal injuries – in the magnitude of insult and kinetics of RGC cell loss, or extrinsic triggers (e.g. glial signaling) versus intrinsic triggers (e.g. cytoskeleton disruption in optic nerve crush) – thus the role of molecules thought to be important to glaucomatous neurodegeneration must be tested in an ocular hypertensive model (Libby et al., 2005b). Recent studies have tested the role of JNK-signaling in age-related ocular hypertension induced axonal injury and RGC death. Combined deficiency of Jnk2 and Jnk3 did not provide protection to RGCs somas in ocular hypertensive DBA/2J mice (Harder et al., 2018). The finding is in contrast to after optic nerve crush, in which combined deficiency of Jnk2/3 protected RGC somas from axonal injury (Fernandes et al., 2012). As JUN activation can occur via JNK1, JNK2, or JNK3, these data suggest a role for JNK1 in RGC somal protection after ocular hypertensive injury. Interestingly, sole deficiency of Jnk2 increased both RGC death and axonal degeneration in ocular hypertension, which suggests JNK2 might play a pro-survival role after axonal injury (Harder et al., 2018). These studies were completed in a complete Jnk2/3 knockout and, as JNK is widely expressed, determining the cell-specific role of pro-survival signaling in RGCs or another other glaucoma-relevant cell types will be important for understanding the early axonal signaling critical for subsequent somal degeneration in ocular hypertension. The pro-survival function of JNK is contrary to the current thinking developed by many groups: that inhibiting JNK signaling would be neuroprotective in an ocular hypertensive environment (Fernandes et al., 2012; Fernandes et al., 2014; Liu et al., 2011a; Sun et al., 2011; Syc-Mazurek et al., 2017a; Syc-Mazurek et al., 2017b; Tezel et al., 2004; Welsbie et al., 2013; Yang et al., 2015; Yang et al., 2008). This result stresses the need to identify specific pathogenic signaling mechanisms activated by long term ocular hypertensive stress in RGC somas and axons so only deleterious JNK signaling may be therapeutically targeted in glaucoma patients. Unfortunately, knocking out all three JNK isoforms in RGCs is likely to create new pathologies since JNK signaling controls many cellular activities and has physiological functions in neurons (Yamasaki et al., 2012). There are different pools of JNKs within neurons, some of which mediate physiological processes while others are involved in stress signaling (pathological pools). Determining how these distinct pools contribute to pathogenic signaling in sustained ocular hypertension will be important future experiments for understanding glaucomatous injury signaling.
Upstream of JNK-JUN signaling are the MAP2Ks, MKK4 and MKK7, which are the only known molecules to activate the JNKs (Jeffrey et al., 2007; Tournier et al., 2001). As compared to other members of MAPK signaling, less is known about the role of MKK4 and MKK7 in glaucomatous neurodegeneration, although both molecules have been shown to be expressed after axonal injury and contribute to axonal degeneration. Genetic knockdown of Mkk4 and Mkk7 together provided moderate protection to injured sensory neurons and axotomized dorsal root ganglia explants (Walker et al., 2017; Yang et al., 2015). In the central nervous system, MKK7-JNK dependent axonal transport has been suggested to be necessary for neuronal maintenance in the adult spinal cord (Yamasaki et al., 2017). After optic nerve crush, MKK4 phosphorylation was detected to peak within one hour of injury (Yang et al., 2015). Consistent with this finding, JNK inhibitors are protective only when applied within 3 hours of axonal injury in DRG explants, prior to the onset of axonal fragmentation (Miller et al., 2009). Together these data suggest MKK4/7-JNK signaling is part of the early axonal signaling response proposed to occur just prior to or as the axon commits to programmed degeneration (Gerdts et al., 2016). In single MKK4 or MKK7 deficient mice, JNK activation occurs in the optic nerve head and pJUN is evident in the RGCs after optic nerve crush, suggesting these MAP2Ks play redundant roles activating downstream JNK signaling (Syc-Mazurek et al., 2018). MKK4 and MKK7 have yet to be tested independently or together in a model of ocular hypertension.
Upstream of JNK and the MAP2Ks, are the MAP3Ks, most notably the dual leucine zipper kinase (DLK) and leucine-zipper bearing kinase (LZK), that are expressed in axons and contribute to axonal and somal degeneration after glaucomatous related injuries (Farley and Watkins, 2018; Fernandes et al., 2014; Gerdts et al., 2016; Hirai et al., 2005; Tedeschi and Bradke, 2013; Valakh et al., 2015; Watkins et al., 2013; Welsbie et al., 2017; Welsbie et al., 2013). The MAP3Ks often control the activation of different physiological and pathological pools of JNK (Chen et al., 2002; Craig et al., 2008). Of the MAP3Ks, DLK has been shown to be critical for pathological activation of JNK but does not appear to have a role in regulating the physiological pool of JNK (Ghosh et al., 2011). Genetic and pharmacologic manipulation of DLK in Drosophilia (DLK ortholog Wallenda) and mouse has been shown to afford axonal protection in some injury paradigms such as in DRG neurons and after nerve growth factor withdrawal, however, this protection does not appear to be sustained or as robust as the protection afforded by WldS (Ghosh et al., 2011; Miller et al., 2009; Shin et al., 2012a). In contrast, however, DLK overexpression has also been shown to be protective of Drosophilia motoneuron axons (Xiong and Collins, 2012). LZK, like DLK, is a MAP3K that cooperates with DLK to induce downstream signaling through MKK4 and MKK7 and subsequently the JNKs after axonal injury in RGCs (Chen et al., 2016; Itoh et al., 2014; Welsbie et al., 2017). Knockdown of LZK has been shown to synergize with knockdown of DLK to protect RGCs after optic nerve crush and a protein kinase inhibitor against both LZK and DLK promoted human RGC survival in vivo (Welsbie et al., 2017). These results highlight the important of determining the necessary molecular requirements for axonal degeneration in RGCs in ocular hypertension models as the molecular requirements for axonal degeneration likely vary between neuronal subtypes and mechanisms of axonal injury.
After optic nerve crush injury, Dlk deficiency decreased the JNK somal pool, attenuated somal JUN accumulation (the canonical target of the JNKs), and ultimately provided protection to RGC somas. DLK deficiency did not, however, attenuate activation of axonal JNK and also did not protect RGC axons after optic nerve crush injury (Fernandes et al., 2014; Watkins et al., 2013; Welsbie et al., 2013). DLK is known to be transported via retrograde axonal signaling after axonal injury and retrograde transport of activated transcription factors is reduced in DLK deficient mice after axonal injury (Holland et al., 2016; Shin et al., 2012a; Xiong et al., 2010). Further highlighting the complexity of DLK-JNK signaling in early axonal injury, is that JNK-dependent phosphorylation of DLK is important for the stabilization of DLK levels (Huntwork-Rodriguez et al., 2013). These data suggest different pools of JNK serve distinct purposes after axonal injury, such that phosphorylated JNK in the axon stabilizes axonal DLK levels in a JUN-independent manner whereby retrograde transport of DLK activates somal JNK as part of the somal molecular program (Fernandes et al., 2014; Ghosh et al., 2011). In addition to JNK-mediated DLK signaling, JNK activity is able to positively regulate LZK levels, JNK-dependent signaling targets, and other axonal maintenance microtubule binding proteins, such as SCG10 (Huntwork-Rodriguez et al., 2013; Tararuk et al., 2006; Welsbie et al., 2013). Loss of axonal SCG10 may promote axonal degeneration via autophagy or deficiencies in axonal transport (SCG10 is replenished from the soma via anterograde transport, Shin et al., 2012b). Determining both the levels of feedback regulation within the molecular signals responsible for axonal degeneration and the specific roles of individual MAP3K members is necessary because JNK and DLK signaling appear to be important in regulating axon injury signaling and the subsequent somal response.
Ultimately, axonal injury signaling likely contributes to molecular events in both the somal and axonal compartment. As such, identifying the first upstream triggers of axonal injury will be important for ordering injury signaling, and may provide a target for therapeutic intervention. Furthermore, signaling molecules may play important roles at multiple times in multiple compartments during the pathological response after axon injury (i.e. JNK in both initial axonal injury signaling but also in the somal molecular degeneration program). Identifying and ordering compartment specific roles of pro-degenerative signaling molecules will provide greater biologic understanding of the progression of pathogenic signaling in glaucoma.
4. Axon injury induced somal apoptosis
Somal death by apoptosis is a hallmark of many neurodegenerative diseases, including glaucoma (Almasieh et al., 2012; Maes et al., 2017; Mattson, 2000; Nickells, 1999; Qu et al., 2010; Quigley, 1999; Thomas et al., 2017). In multiple glaucoma relevant injuries, including optic nerve crush and age-related ocular hypertension, somal death has been found to be BAX dependent (as discussed above). The convergence of somal apoptosis pathways to a central point—BAX activation—provides a powerful starting point for unraveling the signaling pathways leading to glaucomatous neurodegeneration. Thus, efforts to link somal apoptosis to axonal injury signaling have focused on signaling families known to activate BAX. Proposed intrinsic molecular signaling programs upstream of BAX include other members of the Bcl2 family, mitogen-activated protein kinase family (MAPK), and endoplasmic reticulum (ER) stress signaling (Figure 3, Hu, 2016; Kim and Choi, 2010; Libby et al., 2005b; Maes et al., 2017).
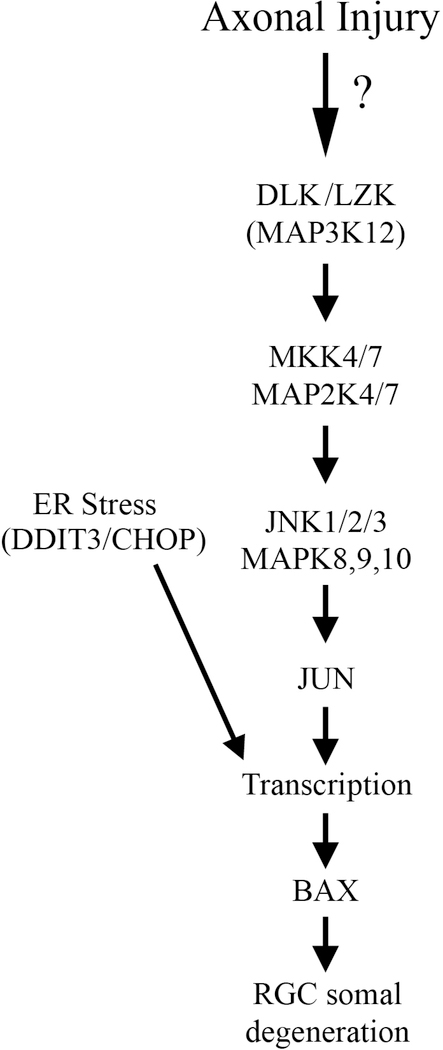
Figure 3: Somal apoptotic signaling in glaucomatous neurodegeneration.
Diagram of the molecules that contribute to somal degeneration in glaucoma-relevant axonal injury. The mitogen activated protein kinase family and endoplasmic reticulum are important for pro-apoptotic signaling in glaucomatous neurodegeneration. MAPK3 kinases, DLK and LZK, activate the MAP2Ks, MKK4 and MKK7, which in turn activate the MAPKs, JNK1–3 and their canonical target JUN. The transcription factors JUN and DDIT3/CHOP lead to transcriptional changes that ultimate culminate in BAX activation and RGC apoptosis (Figure adapted from Fernandes et al., 2018).
4.1. Bcl2 Family of ayoytotic regulators
The Bcl2 family of apoptotic regulators are powerful mediators of neuronal death. In fact, many cell death and survival pathways exert their effect by regulating the activity of the Bcl2 family. By removing one of the executors of the Bcl2 family, BAX, the Bcl2 family has been shown to be necessary for retinal ganglion cell (RGC) death in glaucoma (Libby et al., 2005b). An important next step toward defining the key molecular and genetic players in glaucoma is to identify the members of the Bcl2 family that regulate BAX activation in RGCs. The Bcl2 family is composed of both pro-survival and pro-death components. The integration of the numerous cell signaling pathways active in a sick cell by the Bcl2 family is critical in determining whether a cell survives an insult. There is evidence that both pro-survival and pro-death pathways are active in glaucoma, suggesting the interaction of Bcl2 pro-survival and pro-death pathways is important in determining if an RGC can withstand a glaucomatous insult.
Bcl2 family members upstream of BAX activation integrate the pro-survival and pro-death signals and regulate RGC viability by controlling BAX activation (Maes et al., 2017). BH3 only molecules include a subset of 8 pro-death Bcl2 family members, such as BID, BIM, and BBC3 (PUMA), that are canonically required for BAX activation and also molecules that mediate viability, such as BCL2 and BCL2L1 (BCXL, Maes et al., 2017; Youle and Strasser, 2008). Studies completed in models of tropic factor withdrawal-induced axon injury (a model of injury that also leads to BAX-dependent somal death) have demonstrated that BBC3 was upregulated in tropic factor deprived neurons and that somal BBC3 played an integrative role driving axonal degeneration through anterograde pro-apoptotic signaling (Ambacher et al., 2012; Nikolaev et al., 2009; Simon et al., 2016). In the retina, BBC3 was important for developmental RGCs apoptosis, however, deficiency of BBC3 did not protect RGCs after optic nerve crush in adult mice (Harder and Libby, 2011). Another Bcl2 family member, BIM, was expressed in RGCs after optic nerve injury (Harder et al., 2012b; Napankangas et al., 2003). Deficiency of BIM delayed RGC death after axonal injury, but BIM deficiency did not promote long-term RGC survival and combined BIM, BID, and BBC3 deficiency did not phenocopy BAX deficiency (Harder et al., 2012b; Harder and Libby, 2011; McKernan and Cotter, 2007). In DBA/2J mice, BIM deficiency reduced axonal damage at 12 months of age, however, deficiency in BIM also delayed IOP elevation (Harder and Libby, 2013). BIM, unlike BAX, did not prevent RGC death in severely injured glaucomatous nerves, suggesting that BIM is not an important activator of BAX in glaucomatous injury (Harder et al., 2012b). The molecule BID serves to link extrinsic and intrinsic signaling (Qu et al., 2010). Ocular hypertension leads to BID cleavage and Bid deficiency protects other cell types from apoptosis (Huang et al., 2005; Yin et al., 1999). After optic nerve crush, deficiency of BID did not protect RGC somas, however, Bid deficiency has not been tested in a model of ocular hypertension (Harder and Libby, 2013).
In addition to pro-apoptotic signaling molecules, the Bcl2 family also has 6 anti-apoptotic members, such BCL2 and BCL2L1 (BCLXL, Youle and Strasser, 2008). BCL2 overexpression protected RGCs after hypertension induced axonal injury, suggesting that as in other models, pro-survival BCL2 family members can antagonize pro-death signals in RGCs after injury (Bonfanti et al., 1996; Burne et al., 1996; Cenni et al., 1996; Chierzi et al., 1999; Ji et al., 2005). BCL2 overexpression, however, did not protect the distal axon from degeneration after axotomy which is consistent with the role of Bcl-2 family proteins acting in the somal compartment but not in axonal molecular signaling (Burne et al., 1996). BCL2, however, has not been tested in a model of ocular hypertension. Overexpression of BCLL1 has been shown to protect RGCs after injury while deficiency of BCLL1 led to decreased RGCs survival after axonal injury, which is consistent with its presumed role as a pro-survival signal (Harder et al., 2012a; Kretz et al., 2004; Liu et al., 2001; Malik et al., 2005). This finding may represent the compartmentalization of molecular signals in axonal degeneration and somal death whereby the molecular signaling pathways that control somal death are the same as those responsible for degeneration of the axon segment proximal to the site of injury (Howell et al., 2007).
The involvement of the Bcl2 family in glaucomatous injury is complex as members play both pro-apoptotic and pro-survival roles. While Bcl2 family members are clearly important for somal degeneration after glaucoma relevant injures, currently, there is no evidence that they have a role in axonal degeneration in glaucoma. BAX and BIM have been shown to not contribute to axonal degeneration signaling in ocular hypertensive mice. BBC3 has been suggested to be involved in axonal degeneration in other systems, but, to date, the role of BBC3 has not been tested in a model of ocular hypertension. Furthermore, though the pro-survival molecules BCL2 and BCL2L1 protected RGCs in acute models of axonal injury, they have not been tested in models of ocular hypertension. Future work will be needed to critically test these molecules in models of ocular hypertension in order to determine if these molecules contribute to the molecular programs governing glaucomatous neurodegeneration.
4.2. Pro-apoptotic signaling
Bcl2 family members are regulated by a variety of upstream signaling pathways (Maes et al., 2017; Youle and Strasser, 2008). Identifying the pathways regulating Bcl2 family members after a glaucomatous injury is a key step toward understanding glaucomatous neurodegeneration. Importantly, these pathways will likely be a link between axonal injury and RGC death and may help identify the earliest molecular changes within RGCs after axonal injury. Numerous studies have implicated both MAPK and ER stress signaling in glaucomatous neurodegeneration (Hu, 2016; Lei and Davis, 2003). Components of MAPK signaling, such as JUN-JNK signaling, and ER stress signaling are known to regulate members of the Bcl-2 family and be key drivers of apoptosis after a variety of injuries (Kim and Choi, 2010; Puthalakath et al., 2007; Tabas and Ron, 2011).
JUN, the canonical target of the JNKs, is a member of the activator protein-1 (AP1) transcription factor family, and homodimerizes or heterodimerizes with members of the Fos or activator transcription factor (ATF) families after phosphorylation by the JNKs (Shaulian and Karin, 2002). JUN signaling is elevated in RGCs after glaucoma-relevant insults such as optic nerve crush, excitotoxicity, and IOP elevation (Fernandes et al., 2012; Fernandes et al., 2013; Isenmann and Bahr, 1997; Levkovitch-Verbin et al., 2005; Munemasa et al., 2006). Furthermore, genetic manipulation (JNK and JUN deficiency in the retina) and pharmacologic manipulation (JNK inhibitors) attenuated RGC death after optic nerve crush (Figure 4, Fernandes et al., 2012; Fernandes et al., 2013; Syc-Mazurek et al., 2017b; Tezel et al., 2004). Together, these findings implicate JNK-JUN signaling as a critical pathway in the pathogenesis of somal apoptosis in glaucomatous injury. Jun deficiency did not prevent ocular hypertension induced RGC axonal degeneration, however, it did significantly lessen somal loss (approximately 70% of RGC somas remained in retinas with severely degenerated optic nerves, Syc-Mazurek et al., 2017a). This result was similar to what was observed in Bax deficient mice, again highlighting the compartmentalized nature of RGC degeneration and importance of axonal degeneration after ocular hypertensive insult. Since JUN is a transcription factor, it is highly likely to drive the transcription of pro-death molecules and/or represses pro-survival molecules after axonal injury. Importantly, transcriptional changes are thought to integrate pro-survival and pro-degenerative signals in the cell body that then contribute to axonal degeneration programs via anterograde signaling (Simon et al., 2016). Unfortunately, to date there are only a few direct targets of JUN identified. One such downstream target is Atf3, another member of the AP-1 family of transcription factors, that has been suggested to be involved in glaucomatous neurodegeneration (Guo et al., 2009; Guo et al., 2011; Hunt et al., 2004; Ueno et al., 2018; Yang et al., 2007). Similar to Bim deficiency, however, Atf3 deficiency only lessened RGC loss at the earliest time points after axonal injury but did not provide sustained protection to RGCs (Fernandes et al., 2013). JUN signaling has also been suggested to regulate BBC3 in other injury models (Akhter et al., 2015; Simon et al., 2016). Future studies to identify the key downstream JUN-dependent pro-death transcriptional events linking JUN activation to BAX activation in ocular hypertensive eyes will be important for ordering the downstream sequence of events in glaucomatous injury.
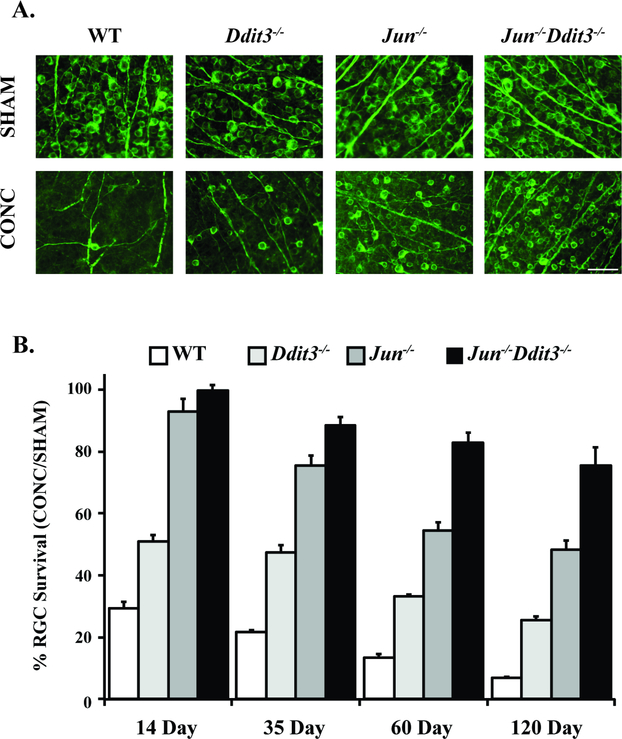
Figure 4: Somal apoptosis is controlled by JUN and DDIT (CHOP).
The majority of somal apopotic signaling in RGCs is controlled by the transcription factors JUN, a member of MAPK signaling, and DDIT (CHOP), a key mediator of endoplasmic reticulum stress. Animals deficient in both of these molecules had sustained robust protection after optic nerve crush injury (CONC) as compared to animals individually deficient in JUN or DDIT. Representative retinal flat mount images with TUJ-1 staining, a marker of RGCs, (A) and quantification of TUJ-1+ cells (B) are presented. RGC survival significantly differed (P<0.01) between DDIT3, JUN, and dual DDIT3/JUN deficient animals at all time points with the exception of between JUN and dual DDIT3/JUN at the 14 day time point which was nonsignificant. Data are presented as the percent of RGCs surviving in the CONC animals relative to sham animals (Scale bar = 50 um, Figure from Syc-Mazurek et al., 2017b).
Regulation of JUN activation by other MAPK signaling molecules has been studied after axonal injury. After optic nerve crush injury, JNK2 and JNK3 controled JUN activation and JUN-dependent RGC somal degeneration (Fernandes et al., 2012). Deficiency of JUN protected RGCs both after optic nerve crush injury and from ocular hypertensive induced RGC death in DBA/2J mice (Fernandes et al., 2012; Syc-Mazurek et al., 2017a). As combined deficiency of JNK2/3 did not provide protection after ocular hypertensive injury as discussed above, these data suggest a novel role for JNK1 within RGCs in response to ocular hypertensive injury. This is consistent with results showing that JNK1 and JNK3 are important for RGC axonal degeneration after optic nerve crush injury (Yang et al., 2015). Together these data suggest JNK signaling has unique roles in RGC somal and axonal degeneration and these roles are likely dependent on the individual pools of JNK.
Upstream of JNK signaling, MKK4 and MKK7 are the only two MAP2Ks that are known to activate the JNKs (Davis, 2000; Minden and Karin, 1997). Single deficiency of MKK4 or MKK7 provided mild but significant protection to RGCs after optic nerve crush. This protection, however, was lower than that seen with DLK, JNK2/3, or JUN deficiency suggesting MKK4 and MKK7 might play overlapping and redundant roles in programmed somal death (Fernandes et al., 2012; Fernandes et al., 2014; Syc-Mazurek et al., 2017a; Syc-Mazurek et al., 2018). In addition to JNK-JUN signaling, MKK4 is upstream of p38, which has been shown to be expressed in RGCs after axotomy and in human glaucomatous eyes (Kikuchi et al., 2000; Tezel et al., 2003). Daily topical pharmacologic inhibition of p38 protected RGC axons after acute ocular hypertensive injury, whereas a single early application (within 3 hours) of a p38 inhibitor after axotomy in DRG cultures did not protect axons (Dapper et al., 2013; Miller et al., 2009). Since inhibiting both MKK4 and MKK7 would stop all physiological JNK activity (and p38 activity), inhibiting MAPK signaling at the level of MAP2Ks is not a viable treatment option (Brancho et al., 2003; Davis, 2000; Jeffrey et al., 2007; Minden and Karin, 1997). Thus, determining the upstream activators of MKK4 and MKK7 at the level of the MAP3Ks will be important for determining important differences in the molecular signaling pathways involved in or required for RGC death after axonal injury and ocular hypertensive injury as previously discussed. Further targeted study of MKK4 and MKK7 in an age-related model of ocular hypertension will be needed to parse the activation and the contributions of these molecules and their downstream targets to glaucomatous injury signaling.
Endoplasmic reticulum (ER) stress signaling, like MAPK signaling, contributes to neurodegeneration and plays a role in the pathogenesis of glaucoma and other retinal diseases (Hu, 2016; Lindholm et al., 2006; Peters et al., 2015; Scheper and Hoozemans, 2015; Zode et al., 2014). In other systems, expression of a component of ER stress signaling, C/EBP homologous protein (CHOP/GADD153; official gene name, DNA damage inducible transcript 3, Ddit3) led to decreased levels of anti-apoptotic molecules (BCL2, McCullough et al., 2001) and increased levels of pro-apoptotic molecules (BAX, BIM, Fu et al., 2010; Puthalakath et al., 2007). PERK-eIF2-DDIT3 signaling has been implicated in multiple models of axon injury induced RGC death. eIF2 was found to be enriched in the earliest stages of DBA/2J ocular hypertensive injury, and DDIT3 signaling was elevated in RGCs after glaucoma-relevant insults such as optic nerve crush, retinal ischemia, and intraocular pressure elevation (Doh et al., 2010; Fernandes et al., 2013; Hu et al., 2012; Nashine et al., 2015; Williams et al., 2017b; Yang et al., 2013b). Not only does glaucoma relevant injury lead to activation of DDIT3 expression, but reduction of ER stress via genetic manipulation and pharmacologic inhibition of DDIT3 has been shown to provide moderate protection to RGCs. Ddit3 deficiency reduced the number of dying RGCs and increased RGC survival after optic nerve injury and acute elevation of intraocular pressure (Hu et al., 2012). Furthermore, Ddit3 deficiency promoted moderate axonal survival after optic nerve crush and intraocular pressure elevation, but did not prevent functional axonal degeneration changes after optic nerve crush injury (Figure 4, Syc-Mazurek et al., 2017b; Yang et al., 2016). Such findings suggest the unfolded protein response and ER stress contribute to the somal molecular signaling cascade in glaucomatous injury. Determining the contributions of ER stress signaling to RGC death and axonal degeneration is further complicated by the fact that components of ER stress signaling, such as overexpression of XBP-1 can be protective to RGCs and their axons (Hu, 2016; Hu et al., 2012). Future studies will be needed to further parse the contributions of the pro-survival vs pro-apoptotic pathways of the ER stress response to both the axonal and somal compartments. Such study will also need to investigate how the magnitude and duration of an axonal injury might alter the molecular response to injury, and thus the balance from survival to somal and axonal degeneration.
Going forward it will be important to determine the integration and interconnection between molecular signaling pathways important for somal apoptosis. Dlk has been proposed to regulate Ddit3 expression after axonal injury in addition to its role as a MAP3K kinase (Larhammar et al., 2017). Unbiased gene expression profiling after optic nerve crush demonstrated Dlk deficiency reduced DDIT3 expression after injury (Watkins et al., 2013). These results suggest DLK might be upstream of both Jun and Ddit3, thus making Dlk a master regulator of RGC death after axonal injury. After optic nerve crush, however, combined JUN/DDIT3 deficiency provided greater protection to RGCs than DLK deficiency (Syc-Mazurek et al., 2017b). Instead, LZK (discussed above) or cooperative signaling of both LZK and DLK might serve to integrate pro-degenerative molecular signaling programs after injury. Identification of such a master regulator, if one exists, might inform how somal and axonal degeneration patterns are interconnected.
5. Program of axonal degeneration in glaucomatous injury
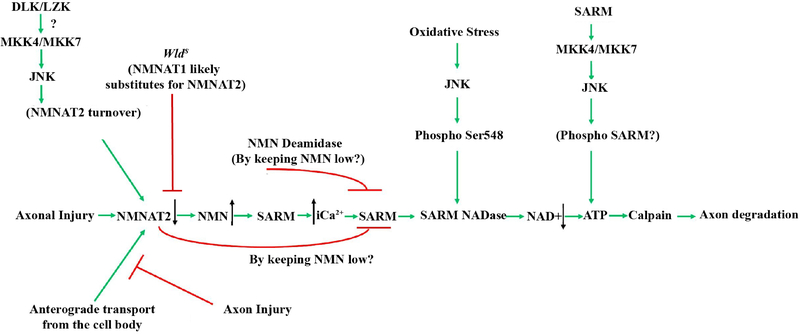
The cell biology of axonal injury signaling and subsequent axonal degeneration, almost entirely unknown a decade ago, is becoming better understood. Studies have demonstrated that even in the same neuronal cell type, axon degeneration can proceed via different mechanisms, depending on the injury (Chen et al., 2012; Miller et al., 2009; Schoenmann et al., 2010). Not only do the molecular mechanisms of axonal degeneration differ across injury type, but the mechanisms governing axonal pruning and developmental axonal degeneration are distinct from injury-induced axon degeneration in the adult (Cusack et al., 2013; Hoopfer et al., 2006). Furthermore, the molecular regulation of axonal degeneration might be inherently different across different neuronal subtypes. These observations highlight the necessity of testing a molecule’s role in adult RGC axon degeneration following glaucoma relevant insults in order to define the axonal degeneration signaling cascade responsible for axonal degeneration in glaucoma. After axonal injury to RGCs, there appears to be a molecular cascade that drives axonal injury in the distal portion of the axon (Figure 5). Note, the degeneration of the proximal axonal segment appears to be secondary to somal apoptosis and thus the proximal segment is not an appropriate read out for axonal degeneration (Fernandes et al., 2014; Howell et al., 2007). While the axonal compartment is molecularly distinct from the somal compartment in terms of degeneration, there may be some overlapping components and/or signaling processes. Work done with the WldS allele showed the expression of this allele was important in RGC axon degeneration after glaucoma relevant injuries (Fernandes et al., 2018; Harder et al., 2017; Howell et al., 2007). Thus, much work has focused on determining the molecular signaling responsible for the protection provided by the WldS allele.
Figure 5: Axonal degeneration signaling in glaucomatous neurodegeneration.
Schematic of the molecules thought to contribute to glaucomatous neurodegeneration. Multiple possible mechanisms are thought to lead to decreased levels of NMNAT after injury. Axonal injury interrupts the anterograde transport of cytoplasmic NMNAT2 from the cell body and activation of JNK signaling leads to NMNAT2 turnover. The axonal protection afforded by animals carrying the WldS allele is thought to occur through NMNAT1 substituting for NMNAT2. Decreased levels of NMNAT2 can lead to accumulation of nicotinamide (NMN) which has been shown to be pro-degenerative at certain levels in other systems (Di Stefano et al., 2015). Nicotinamide is also a precursor of NAD+ and oral administration of nicotinamide or increasing expression of Nmnat1 (an enzyme that produces NAD+) in ocular hypertensive animals is protective from glaucomatous damage (Williams et al., 2017b). Decreased NMNAT2 activity subsequently leads to activation of SARM NADase activity likely via an increase in intracellular calcium, and additional work will be needed to determine the exact sequence of events in glaucomatous neurodegeneration given the potential complexity of NMN signaling. JNK can also activate SARM NADase activity via phosphorylation of SARM. Ultimately these events lead to deceased NAD+ levels leading to decreased ATP (which can also occur through SARM-JNK signaling), calpain activation and axon degradation. Reprinted from Biochemical Pharmacology, 161 (2019), Michael Carty and Andrew G. Bowie, SARM: from immune regulator to cell executioner, 52–62, 2019 with permission from Elsevier (Carty and Bowie, 2019).
WldS has been shown to protect axons from degeneration and lessen glaucomatous optic nerve damage and RGC loss in DBA/2J mice (Coleman, 2005; Coleman et al., 1998; Harder et al., 2017; Howell et al., 2007; Lunn et al., 1989; Mack et al., 2001; Wang et al., 2001). WldS expression may also prevent the loss of RGC function as determined by pattern ERGs (Howell et al., 2007). In an attempt to understand the protection of RGC function, but also failure to protect some eyes from glaucoma, further analysis of WldS mice was performed to determine if there might be an additive effect with multiple copies of the WldS allele on DBA/2J glaucoma (Harder et al., 2017). Adding two copies of WldS did not increase protection, suggesting the level of WldS was not rate limiting. Importantly, ~25% of eyes from animals carrying two copies of the allele had some signs of severe glaucomatous damage at 12 months, indicating either WldS was not completely capable of preventing activation of the axonal degeneration pathway or alternatively there is another activated pathway that cannot be inhibited by WldS expression. WldS expression also did not prevent the entry of blood derived cells or glial activation in the optic nerve head, both of which have been proposed to be involved in RGC injury in glaucoma, but did prevent loss of axonal transport in protected DBA/2J animals (Harder et al., 2017; Howell et al., 2011a; Howell et al., 2012b; Johnson and Morrison, 2009). Of note, WldS is a gain-of-function mutation that generates a novel molecule and thus, the WldS experiments do not reveal the specific molecular pathway controlling axonal degeneration in wildtype animals. However, WldS experiments do show that lessening the activation of axonal degeneration pathways can prevent glaucomatous neurodegeneration regardless of the presence of potential extrinsic insults (e.g. glial activation and monocyte infiltration, Harder et al., 2017). Determining the critical events within a normal RGC for axonal degeneration after glaucomatous injury will be essential for the development of targeted interventions for glaucoma.
5.1. SARM1
SARM1 (sterile alpha and TIR motif containing protein 1), a toll-like receptor adaptor protein, was identified in a screen for molecules capable of suppressing Wallerian degeneration after axotomy (Osterloh et al., 2012). Activation of SARM1 was sufficient to cause axonal degeneration in neurons and deficiency in SARM1 has been shown to protect both axons and neuronal cells from various axonal injuries (Fernandes et al., 2018; Geisler et al., 2016; Gerdts et al., 2015; Gerdts et al., 2016; Henninger et al., 2016; Kim et al., 2007; Turkiew et al., 2017). In the retina, SARM1 levels were elevated after injury to RGCs (Massoll et al., 2013; Yang et al., 2015). After optic nerve crush, axonal degeneration was significantly slowed in SARM1 deficient animals which is consistent with SARM1’s known role in axonal degeneration (Fernandes et al., 2018). However, after direct optic nerve crush injury, SARM1 deficiency did not protect RGCs somas (Fernandes et al., 2018), despite SARM1 having a known role in somal degeneration (sarmoptosis, Summers et al., 2014). Interestingly, this is similar to what was observed in animals carrying the WldS allele where the presence WldS allele only affected axonal but not somal degeneration in RGCs after optic nerve crush injury, despite a known role in both processes. Thus, given the known role of WldS in lessening glaucomatous neurodegeneration, it will be important to test if manipulating SARM1’s function can lessen RGC axonal and somal degeneration in glaucoma. If SARM1 is shown to have a direct role in axonal degeneration in glaucoma, it will be an important result since it will identify an endogenous RGC axonal protein as critical for glaucomatous neurodegeneration.
Given the importance of SARM1 in the axonal degeneration program, recent studies have sought to determine if SARM1 might serve as either an upstream or downstream regulator of MAPK signaling activation (reviewed in Geden and Deshmukh, 2016). Interestingly, many of the molecular pathways SARM1 is thought to regulate have been implicated in glaucomatous neurodegeneration. SARM1 activation was found to be necessary for activation of MAPK signaling and subsequent axonal degeneration after optic nerve crush while inhibition of MAPK signaling prevented SARM1-dependent alterations in dendritic arbor morphology (Chen et al., 2011; Yang et al., 2015). In cultured neurons, the immune response to distal axotomy occurred in a SARM1 and JNK-JUN dependent manner (Wang et al., 2018). These data suggest SARM1 activation may represent an early upstream event in axonal degeneration. Other studies have suggested SARM1 might act as a downstream effector of MAPK signaling. MAPK signaling has been shown to degrade nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2), which can inhibit SARM1 activation and phosphorylation and occurred in a JNK-dependent manner after oxidative stress (Essuman et al., 2017; Gerdts et al., 2015; Murata et al., 2018). Specifically, combined knockdown of MKK4 and MKK7 decreased depletion of nicotinamide adenine dinucleotide (NAD+) in a SARM1-independent manner in peripheral nerves after axotomy (Walker et al., 2017). In RGCs, SARM1 deficiency did not alter JNK signaling in the proximal segment after axonal injury, which suggests SARM1 is downstream of MAPK signaling after optic nerve crush injury (Fernandes et al., 2018). These contrasting findings may be a result of different injury models and different neuronal subtypes and further demonstrate the need to test molecules of interest in glaucoma models, including ocular hypertensive models.
5.2. NMNAT enzymes, NAD+, and energy metabolism
The NMNAT enzymes consist of three isoforms (NMNAT1–3) that are responsible for the last step in the synthesis of NAD+ from nicotinamide mononucleotide (NMN) and ATP (Ali et al., 2013; Berger et al., 2005). As previously discussed, the spontaneous murine mutation WldS is a fusion of NMNAT1 and UBE4B and axonal degeneration is delayed in DBA/2J mice carrying this allele (Beirowski et al., 2008; Coleman and Freeman, 2010; Conforti et al., 2014; Geden and Deshmukh, 2016; Howell et al., 2007; Mack et al., 2001; Sasaki, 2018). The major axonal protective component of the WldS allele appears to be NMNAT1 (Araki et al., 2004). NMNAT1 overexpression conferred protection from axonal degeneration in some experimental paradigms, including elevated intraocular pressure injury, but other studies have shown NMNAT1 overexpression does not phenocopy the axonal protection observed in animals with the WldS allele after axonal injury (Avery et al., 2012; Conforti et al., 2007; Sasaki et al., 2009; Vohra et al., 2010; Zhu et al., 2013). Decreased levels of NMNAT2 might serve as a trigger for axonal degeneration and thus the proposed mechanism for the axonal protection conferred by the WldS allele is that NMNAT1 (nuclear isoform) substitutes for NMNAT2 (cytoplasmic isoform, Gilley and Coleman, 2010). NMNAT2 is the most labile isoform and NMNAT2 levels decline quickly after axonal injury (prior to the onset of appreciable axonal degeneration, Gilley and Coleman, 2010). Additional studies have shown that delaying degradation/increasing levels of NMNAT2 also protected axons from degeneration after injury and that inhibition of MAPK signaling, specifically MKK4 and MKK7, increased levels of NMNAT2 after injury (Babetto et al., 2013; Milde et al., 2013; Walker et al., 2017; Xiong et al., 2012).
In glaucomatous injury, disturbances in metabolism and mitochondrial function have been identified as an early change in animal models of ocular hypertension (Baltan et al., 2010; Inman and Harun-Or-Rashid, 2017; Williams et al., 2017b). ATP reserves were decreased in high IOP groups in DBA/2J mice which suggests metabolic dysfunction is detectable even prior to structural changes in the optic nerve (Baltan et al., 2010). Mitochondria migration to the axons was found to be partially dependent on DLK and inhibition of JNK translocation to the mitochondria reduced neurotoxicity in in-vitro models of oxidative stress (Chambers et al., 2013; Han et al., 2016). These results suggest that DLK-JNK signaling might contribute in multiple ways to axon injury response signaling. Mitochondrial dysfunction was also an early change in RGCs in chronic ocular hypertension, and the formation of abnormal mitochondria may be prevented by oral supplementation with nicotinamide, a precursor to NAD+ (Williams et al., 2017b). NAD+ is an abundant coenzyme important for cellular metabolism present in all cells. Levels of NAD+ and its reduced form NADH decrease during the aging process and thus augmenting NAD+ levels might serve as protective for age-associated neurodegenerative conditions (Verdin, 2015; Williams et al., 2017b). In aged ocular hypertensive mice, augmenting NAD either through oral supplementation of nicotinamide, or overexpression of NMNAT also prevented axonal injury in DBA/2J ocular hypertensive mice (Williams et al., 2018; Williams et al., 2017b). Furthermore, administration of nicotinamide to animals carrying the WldS allele provided greater protection than WldS or nicotinamide alone (Williams et al., 2017a). Nicotinamide has also been shown to decrease pJNK elevation and synaptic loss in other animal models of neurodegeneration suggesting NMNAT- NAD signaling might serve as an early modulator of other pro-degenerative molecular programs (Yao et al., 2017). The exact molecular mechanism for the axonal protection afforded by WldS remains unknown, however, studies have identified SARM1 and PHR1, a ubiquitin ligase, as potential upstream mediators of NMNAT activity. After axonal injury, SARM1 directly depleted NAD+, a pro-axonal survival molecule, and SARM1-dependent NADase activity contributed to axonal degeneration after injury (Essuman et al., 2017; Gerdts et al., 2015). Alterations in calcium buffering, mitochondrial motility, and oxidative state have also been identified as downstream of WldS and NMNAT dependent changes after axonal injury (Avery et al., 2012; Babetto et al., 2013; Fang et al., 2012; O’Donnell et al., 2013; Sasaki et al., 2016). Though the presence of the WldS allele provided significant protection to axons after injury, it did not provide complete protection to RGC axons in DBA/2J mice (Howell et al., 2007). Thus, future efforts must identify not only the molecular mechanisms responsible for the protection afforded by the WldS allele, but also potential WldS independent mechanisms that contribute to the program of axonal degeneration.
5.3. Calcium and calpain signaling
Calcium signaling and calcium dependent activation of calpains (cysteine proteases) have also been identified as pro-degenerative events after glaucoma relevant axonal injuries (Huang et al., 2010; Yang et al., 2013a). Calcium/calpain dependent signaling contributes to proteolysis of axonal neurofilaments and subsequent degeneration of the axonal cytoskeleton (George et al., 1995; Ma et al., 2013; Villegas et al., 2014). Intracellular calcium has also been found to be increased in RGCs in a rat model of ocular hypertension and in cultured human lamina cribrosa cells after oxidative stress which may lead to differentiation of fibroblasts and increased fibrosis at the level of the ONH (Irnaten et al., 2018; Niittykoski et al., 2010). Levels of the endogenous inhibitor of the calpains, calpastatin, decreased after optic nerve transection and inhibition of calpain activation lessened axonal degeneration in the same model (Yang et al., 2013a; Zhang et al., 2016). Calpains are also know to interact with Bcl-2 family members. Calpain dependent cleavage of BID and BAX was sufficient to induce cytochrome c release and apoptosis in other systems (Chen et al., 2001; Gil-Parrado et al., 2002; Toyota et al., 2003). Efforts to identify downstream targets of the calpains, such as CRMP2 (Zhang et al., 2016), and test these molecules in glaucoma-relevant axonal injury, specifically in age-related ocular hypertension, will be important for study of the molecular mechanisms that govern glaucomatous neurodegeneration of the axonal compartment.
5.4. Other molecules involved in axon degeneration
Numerous molecules have been shown to be involved in axon degeneration in other systems but have not yet been implicated in glaucomatous neurodegeneration. Given how little we know about the molecular mechanism of axonal degeneration in RGCs after a glaucomatous injury, however, we must consider all molecular pathways that are involved in axonal degeneration in any cell. Other molecules implicated in axonal degeneration include: mTOR, Pebbled/ Ras-responsive element binding protein 1(RREB1), IκB kinase (IKK) and glycogen synthase kinase 3 (GSK3, Farley et al., 2018; Gerdts et al., 2011; Terenzio et al., 2018). mTOR has been shown to be upregulated in axons after injury, important for local translation of proteins and retrograde signaling of transcription factors, and may contribute to neuronal survival after axonal injury (Terenzio et al., 2018). Pebbled/RREB1 has been proposed to contribute to activation of axonal injury signaling in Drosophila (Farley et al., 2018). GSK3 and IKK were identified as molecules required for rapid axonal fragmentation in vitro (Gerdts et al., 2011). Inhibition of GSK3 and IKK delayed axonal degeneration after axotomy in DRG explants. Furthermore, GSK3 has been implicated in the regulation of axonal autophagy in Wallerian degeneration (Wakatsuki et al., 2017). In addition to reducing the number of dying RGCs, Ddit3 deficiency also increased axonal survival after intraocular pressure injury (Hu, 2016; Hu et al., 2012). These molecules and others identified as important for axonal degeneration should be critically tested in age-related models of glaucomatous axon injury. Additional molecules important for axonal degeneration are likely to be identified in future studies. Identification of such molecules, especially those involved in early axonal injury signaling, may lead to a better understanding of the initiating signal in glaucomatous injury.
6. Synaptic and dendritic changes in glaucomatous injury
In addition to somal and axonal programmed signaling after axonal injury, other compartments such as synaptic and dendritic changes contribute to pathologic signaling after glaucomatous injury (recently extensively reviewed in Agostinone and Di Polo, 2015; Lawlor et al., 2018; Liu et al., 2011b). Though not the focus of this review, these changes will be briefly summarized here as these changes represent an important third component in glaucomatous neurodegeneration. Additional research is still needed to best understand how molecular signaling along the somal-axonal axis might contribute to synaptic or dendritic changes, or if unique molecules contribute to this compartment. It has been suggested that synaptic and dendritic changes occur early in the glaucomatous process and thus understanding these changes might serve to understand the early changes that occur within glaucomatous injury. Remodeling of the dendritic arbor and synaptic loss precedes RGC death after ocular hypertensive injury and optic nerve crush injury (Berry et al., 2015; Jakobs et al., 2005). In primate models of glaucoma, changes including dendritic thickening, dendritic shortening, and loss of dendritic field area were observed at the lateral geniculate nucleus (LGN, Gupta et al., 2007; Ly et al., 2011). Loss of LGN volume has also been observed using magnetic resonance imaging and histopathology techniques in patients with primary open angle glaucoma (Gupta et al., 2006; Lee et al., 2014). In the superior colliculus, synaptic structures in the mouse persisted after failure of axonal transport which proceeded in a distal to proximal pattern in models of ocular hypertension (Crish et al., 2010). There is also evidence that glaucomatous changes including metabolic changes and retinotopic reorganization extend as far in the brain as primary visual cortex in human glaucoma patients (Duncan et al., 2007; Gupta and Yucel, 2003). Furthermore, dendritic pruning, RGC synaptic loss, and axonal retraction occur in the retina following ocular hypertension injury in mouse models (Ou et al., 2016; Risner et al., 2018). Dendrite retraction and remodeling was prevented in ocular hypertensive animals carrying the WldS allele (Figure 6, Harder et al., 2017). The WldS allele, however, prevents axonal degeneration, and thus in this model it is difficult to determine if dendritic changes occur early during axonal injury signaling or are prevented by the preservation of axonal integrity by the WldS allele. In short, further studies will be important to determine if these synaptic events occur prior to, contiguous with, or are a sequala of axonal injury signaling. In order to determine the potential sequence of events, manipulation to delay another process (e.g. synaptic remodeling) will be needed to determine if such manipulation affects pathological responses in other compartments (e.g. axonal degeneration). Activated microglia and the complement system are both known to be involved in synaptic elimination and have been implicated in glaucomatous neurodegeneration (Howell et al., 2011a; Howell et al., 2014; Rathnasamy et al., 2018; Stephan et al., 2012; Stevens et al., 2007). C1q was expressed in microglia in the optic nerve head and localized to the inner plexiform layer in aged DBA/2J prior to the appreciable loss of optic nerve axons and RGCs. Animals deficient in C1q also had decreased axonal degeneration and RGC loss in the DBA/2J model suggesting that irregular synaptic clearance might contribute to glaucomatous neurodegeneration (Howell et al., 2011a; Stevens et al., 2007). Additional studies must be done to further characterize synaptic and dendritic changes in glaucomatous neurodegeneration at both the level of the retina and LGN. Such study should also seek to identify and define the molecular signaling programs that control this compartment of glaucomatous injury. Determining and subsequently ordering the unique and potentially overlapping molecular programs of the axonal, somal, and synaptic/dendritic compartment will be necessary for determining the pathologic signaling processes that contribute to glaucomatous neurodegeneration.
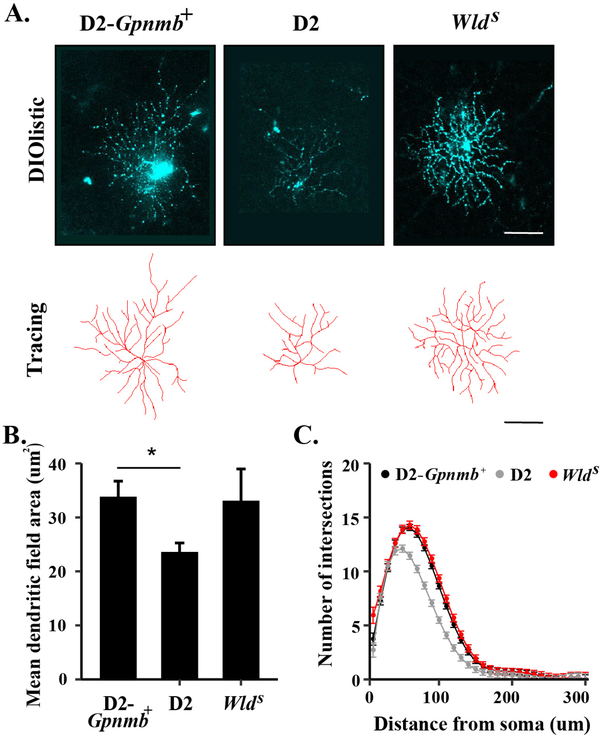
Figure 6: The WldS allele prevents ocular hypertensive induced dendritic remodeling.
Retinal cells were labeled using DiO/Dil bullets in 9 month D2-Gpnmb+, wildtype DBA/2J (D2), and DBA/2J animals carrying the WldS allele and RGCs were subsequently selected for analysis (A). While there was a significant decrease (*) in mean dendritic field area in RGCs from wildtype D2 animals as compared to D2-Gpnmb+ animals, there was no difference between mean field area (B) or number of intersections (C, as determined by Sholl analysis) between RGCs from D2-Gpnmb+ animals and those DBA/2J animals carrying the WldS allele. These results demonstrate that presence of the WldS allele prevents ocular hypertension induced dendritic changes (Figure from Harder et al., 2017).
7. Conclusions and future directions
As study of glaucomatous neurodegeneration has advanced, multiple cell-types and signaling molecules have been shown to contribute to disease initiation and progression (Figure 7). Due to extensive research in glaucoma patients and animal models of glaucoma there is much known about glaucomatous neurodegeneration at the physiological and cellular levels (Calkins, 2012; Howell et al., 2008; Howell et al., 2012a; Li et al., 2000; Mansouri et al., 2011; Morgan, 2012; Nickells et al., 2012; Quigley, 2012). The lamina cribrosa (glial lamina in the rodent) has been identified as an early, critical site of glaucomatous damage, axonal injury signaling and axonal degeneration pathways have been shown to be important for RGC death in glaucoma, intrinsic signaling has been shown to be important for RGC somal apoptosis, and extrinsic signaling events may be early changes in glaucomatous neurodegeneration. Despite this knowledge, we still lack a molecular understanding of the early pathological events that occur within RGC axons and how these events trigger RGC death. Identifying these molecular pathways will define targets for therapeutic intervention.
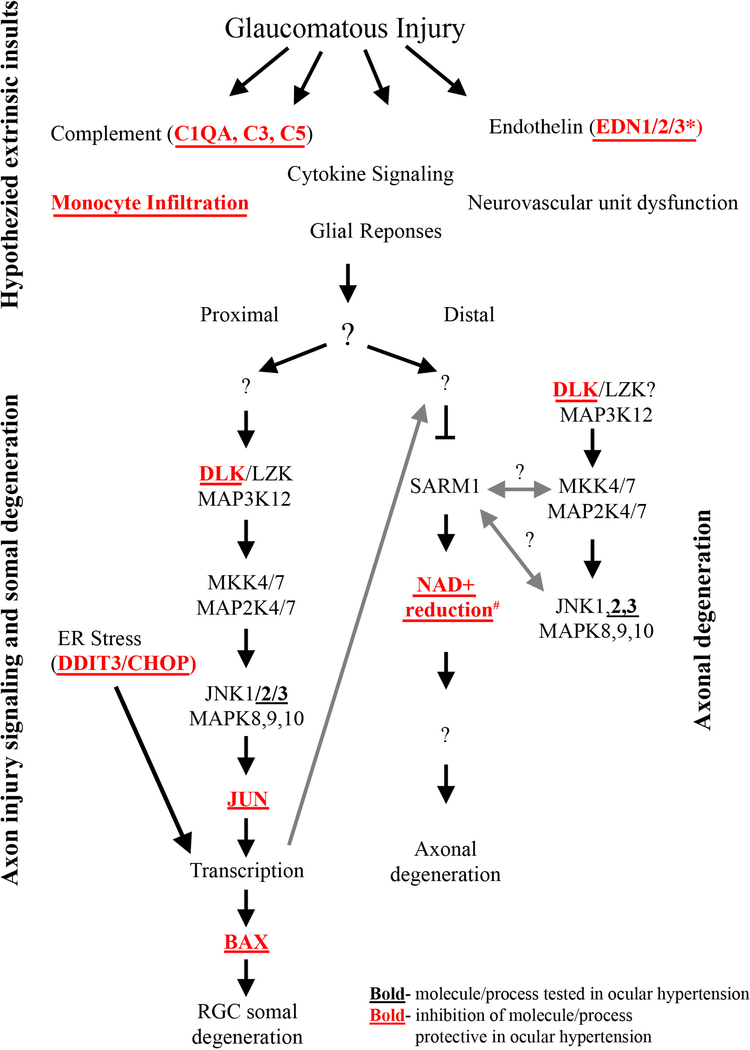
Figure 7: Molecular program of somal apoptosis and axonal degeneration after axonal injury.
Diagram of the molecules that contribute to somal and axonal degeneration in glaucoma-relevant axonal injury. Molecules in bold have been tested in ocular hypertension and deficiency of the molecules/processes in bold with red font have been shown to be protective for somal apoptosis in ocular hypertension. Question marks indicate areas currently unknown or areas where research has shown potential for bidirectional signaling. Multiple extrinsic events are thought to be important for early changes in glaucomatous neurodegeneration, but the ordering of these early changes and the possible interactions between these early changes is still poorly understood. * The role of endothelin was tested using a pan endothelin antagonist (Howell et al., 2011a). # Inhibition of the reduction of NAD+ has been tested in models of ocular hypertension with oral administration of nicotinamide, an NAD+ precursor, and using gene therapy to increase expression of Nmnat1 (Williams et al., 2017b). Anterograde transcriptional changes from the somal compartment are also thought to influence axonal degeneration signaling (large gray arrow, components of figure adapted from Fernandes et al., 2018).
An important challenge of glaucoma research is to decipher whether the molecular changes that occur in glaucomatous eyes are harmful, protective, or inconsequential. To obtain a clearer understanding of the deleterious signaling pathways involved in glaucomatous neurodegeneration, we must identify both the critical events in the degenerative process and define the relationship(s) between these critical events. Using animal models of glaucoma, the molecular processes of ocular hypertension induced somal and axonal degeneration have been determined to be distinct molecular programs. The progression from early, focal injury to RGC axons to distinct compartmental degeneration processes in glaucoma suggests that axonal stress signaling pathways operate along the somal-axonal axis. The fundamental signals that link axonal damage to the degeneration processes remain incompletely defined. Important questions to address include: (i) Does axonal stress activate different signaling pathways proximally and distally to cause somal and axonal degeneration, respectively? (ii) How does degeneration of one compartment affect (and potentially activate) the degeneration cascade of the other cellular compartments? (iii) Is axonal degeneration driven by an active death signal or passively initiated by the depletion of axonal survival/maintenance factors? (iv) How might other mechanisms of cell death (e.g. autophagy, necroptosis, parthanatos, mitoptosis) contribute to signaling along the somal-axonal axis? (v) How might different patterns of axonal degeneration (anterograde degeneration vs retrograde die-back of axons) alter axonal-somal signaling after injury? (vi) How does glaucomatous axonal-somal signaling lead to changes within the synaptic/dendritic compartment and at the level of the LGN? It will also be important to understand how pro-degenerative somal-axonal signaling might vary between patients and even within RGCs within a patient given the heterogeneity and complexity of molecular signals in glaucoma. By carefully characterizing axon-damage signaling events initiated in RGC axons at the lamina, the somal responses to axonal injury, and responses in the axon segment distal to the insult, we can begin to answer these questions and define the degenerative cascade(s) that control compartmentalized destruction of both the RGC soma and axon.
An important step for answering the above questions is to understand the earliest molecular events that drive axonal dysfunction in RGC axons after an ocular hypertensive insult. Several years ago, it was shown that a mutation that protects axons from degeneration, the Wallerian degeneration slow mutation (WldS), lessened the incidence of eyes with glaucomatous optic nerve damage and RGC loss in DBA/2J mice (Howell et al., 2007). It is important to note that WldS is a gain-of-function mutation and thus, the WldS experiments do not reveal the specific molecular pathway controlling axonal degeneration. However, the WldS experiments do show that lessening the activation of axonal degeneration pathways can prevent glaucomatous neurodegeneration regardless of the presence of potentially extrinsic insults (e.g. glial activation and monocyte infiltration, Harder et al., 2017). WldS signaling, however, was originally found to delay the infiltration of immune cells in the peripheral nervous system after injury (Lunn et al., 1989). Study of axonal injury signaling after ocular hypertension might thus be complicated by the intersection of intrinsic molecular signaling with extrinsic inflammatory signaling. However, RGCs transduced with virally delivered NMNAT1, which is critical for WldS function, significantly reduced glaucomatous damage in DBA/2J ocular hypertension suggesting WldS may have a direct role in RGCs. While study of WldS molecular signaling offers an intriguing mechanism for study of these intersections, further study of WldS signaling must be interpreted within this framework.
The cell biology of axonal injury signaling, almost entirely unknown a decade ago, is becoming better understood. Work in this field has shown that different kinds of axonal insults trigger degeneration via distinct mechanisms even in the same neuronal cell type (Chen et al., 2012; Miller et al., 2009; Schoenmann et al., 2010). Additionally, developmental axon degeneration that occurs during axon pruning differs from injury-induced axon degeneration (Cusack et al., 2013; Hoopfer et al., 2006). Further, there could be inherent differences in the requirement of specific molecules in regulating axon degeneration across different neuronal subtypes. These observations highlight the necessity of testing molecules in adult RGC axon degeneration following glaucoma relevant insults. To this end, many groups have been working to define the axonal degeneration pathway in adult RGCs after glaucoma relevant insults. Determining this pathway will likely define the key pathogenic events within RGCs that trigger RGC dysfunction and death in glaucoma. While to date, there is no clear understanding of the molecular pathways controlling this, recent work has suggested that NAD+ depletion in RGC axons after an ocular hypertensive injury is a major driver of RGC degeneration in glaucoma (Williams et al., 2017b). This result is consistent with the growing literature that shows nicotinamide metabolism being important for maintaining axonal integrity in numerous systems. Over the next few years it will be important to define how NAD+ levels are regulated in RGCs, how NAD+ maintains axon integrity, and what molecular events lead to NAD+ depletion in RGCs. Answering these questions will provide fundamental knowledge about the initial, intrinsic events driving vision loss in glaucoma.
Though not the focus of this review, determining the inciting signals and thereby earliest changes in the glaucomatous disease process might serve to identify a unifying initial signal for both the somal and axonal degeneration program (Figure 7). It is not well understood if there is a single inciting factor or a combination of multiple factors that ultimately leads to RGC injury and glaucomatous neurodegeneration. Pro-degenerative extrinsic signaling from other glaucoma relevant cell-types, including astrocytes, microglia, and monocytes have all been suggested to be important early mediators of glaucomatous neurodegeneration (Bosco et al., 2011; Cooper et al., 2018; Hernandez, 2000; Liddelow et al., 2017; Nadal-Nicolas et al., 2017; Neufeld, 1999; Rathnasamy et al., 2018; Soto and Howell, 2014; Sun et al., 2017; Tezel, 2013). Furthermore, signaling pathways such as the complement system and endothelin system have been suggested to contribute to early extrinsic signaling in glaucomatous injury (Howell et al., 2011a; Howell et al., 2014; Prasanna et al., 2002; Stevens et al., 2007). In addition to astrocyte signaling and microglia activation, entry of infiltrating blood derived cells may have a role in early pro-degenerative signaling (Chen et al., 2018; Gramlich et al., 2015; Howell et al., 2012c). Manipulation of some of these early changes have been shown to be partially protective for ocular hypertension induced RGC death in animal models (e.g. monocyte infiltration, complement activation, and endothelin signaling; Howell et al., 2014; Williams et al., 2019). Further complicating understanding of the glaucomatous process is that extrinsic signals may be produced and then subsequently act upon multiple glaucoma relevant cell-types (RGCs, astrocytes, microglia, Müller glia, vasculature, myeloid cells, etc). It will be necessary to determine the individual pro-survival or pro-degenerative role of specific components of the RGC extrinsic signaling and how these roles might change depending on cell type. Given many glaucoma-relevant cell types are hypothesized to contribute to pro-degenerative extrinsic signaling, it will be necessary to understand the potential interactions between glaucoma-relevant cell types and understand how RGC extrinsic signaling may lead to axonal injury and intrinsic degenerative signaling. It will be necessary to define the extrinsic signaling pathways activated after a glaucomatous injury and determine if these extrinsic signaling pathways are critical drivers of axonal degeneration. Such investigation may determine where in the glaucomatous molecular cascade is (are) the point of no return—presumably a point where RGC intrinsic pathogenic signaling pathways have been initiated. Identifying modifiable signaling pathways upstream of this point(s) may serve to halt or slow the initiation of a degenerative process. Finally, once these early factors are identified, it will be necessary to how these early signals activate axonal injury signaling that leads to axonal and somal degeneration.
Furthermore, it will be important to investigate how axonal injury signaling, somal apoptosis, and axonal degeneration occur in the setting of an individual’s genome and their environment throughout their lifespan. As a largely age-related disease with a stochastic regional disease progression, it is also unknown how specific predisposing factors might lead to a regional injury. Additional work should also focus on determining what genetic factors affecting the retina contribute to a patient’s likelihood of developing glaucomatous neurodegeneration and conversely, what genetic factors may be protective. While ocular hypertension is one of the most common risk factors for development of glaucomatous pathology and progressive visual impairment, in some individuals, optic nerve pathology and visual field loss may occur in the absence of an IOP insult. Additional work must be completed to understand the intersection of complex genetic susceptibilities and multiple molecular signaling pathways important for glaucoma (Danford et al., 2017).
While distinct molecular pathways execute degeneration of different compartments, the specific critical molecular events that control RGC somal and axonal degeneration after a glaucomatous injury are largely undefined (Almasieh et al., 2012; Calkins, 2012; Farley and Watkins, 2018; Howell et al., 2013; Libby et al., 2005a). Furthermore, there are multiple important outstanding questions about how axonal injury drives RGC death in glaucoma. For instance, we do not know how a local axonal injury response activates somal and axonal degenerative programs, how somal and axonal degeneration pathways are interconnected, or where in the overall degeneration cascade RGCs irreversibly lose the ability to function. Further complicating understanding of this complex program is that feedback exists at multiple levels during the transduction of a pro-death signal. Recent work also highlights the important of testing a specific molecule of interest in the specific neuronal cell and injury mechanism of interest. Understanding the level and context of molecular signaling will be important as multiple molecules implicated in pro-death axonal degeneration signaling may also have protective roles in only specific experimental contexts (DLK, JNK2, Harder et al., 2018; Xiong and Collins, 2012). In glaucoma, it will be necessary to test specific molecules suggested to be important in glaucomatous neurodegeneration in a model of ocular hypertension, ideally an age-related model of ocular hypertension. Such study will hopefully parse the specific molecules necessary for somal and axonal degeneration in glaucoma and also allow for molecular ordering to determine the molecular programs for glaucomatous neurodegeneration. Thus, while much work has been completed to study the sequence of molecular events from initiation of glaucomatous injury to subsequent axonal degeneration and somal apoptosis, there are still multiple key components of pro-degenerative signaling that will need to be determined in future studies.
Highlights.
Axonal injury drives compartmentalized glaucomatous neurodegeneration
Numerous signaling pathways are important for glaucomatous neurodegeneration
Distinct molecular pathways execute degeneration of somal and axonal compartments
Molecules suggested important for glaucoma must be tested in ocular hypertension SBS wrote 75% of the manuscript and RTL wrote 25% of the manuscript. Both authors contributed to revision of the manuscript.
Acknowledgements
This work was supported by EY018606 (RTL), EY027701 (RTL), and Research to Prevent Blindness (an unrestricted grant to the Department of Ophthalmology at the University of Rochester Medical Center). The funding agencies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Footnotes
Declarations of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agostinone J, Di Polo A, 2015. Retinal ganglion cell dendrite pathology and synapse loss: Implications for glaucoma. Prog Brain Res 220, 199–216. [DOI] [PubMed] [Google Scholar]
- Akhter R, Sanphui P, Das H, Saha P, Biswas SC, 2015. The regulation of p53 up-regulated modulator of apoptosis by JNK/c-Jun pathway in beta-amyloid-induced neuron death. J Neurochem 134, 1091–1103. [DOI] [PubMed] [Google Scholar]
- Ali YO, Li-Kroeger D, Bellen HJ, Zhai RG, Lu HC, 2013. NMNATs, evolutionarily conserved neuronal maintenance factors. Trends Neurosci 36, 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A, 2012. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res 31, 152–181. [DOI] [PubMed] [Google Scholar]
- Ambacher KK, Pitzul KB, Karajgikar M, Hamilton A, Ferguson SS, Cregan SP, 2012. The JNK- and AKT/GSK3beta- signaling pathways converge to regulate Puma induction and neuronal apoptosis induced by trophic factor deprivation. PLoS One 7, e46885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DR, 1969. Ultrastructure of human and monkey lamina cribrosa and optic nerve head. Arch Ophthalmol 82, 800–814. [DOI] [PubMed] [Google Scholar]
- Anderson DR, Hendrickson A, 1974. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol 13, 771–783. [PubMed] [Google Scholar]
- Anderson DR, Hendrickson AE, 1977. Failure of increased intracranial pressure to affect rapid axonal transport at the optic nerve head. Invest Ophthalmol Vis Sci 16, 423–426. [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J, 2004. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305, 1010–1013. [DOI] [PubMed] [Google Scholar]
- Avery MA, Rooney TM, Pandya JD, Wishart TM, Gillingwater TH, Geddes JW, Sullivan PG, Freeman MR, 2012. WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering. Curr Biol 22, 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babetto E, Beirowski B, Russler EV, Milbrandt J, DiAntonio A, 2013. The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction. Cell Rep 3, 1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltan S, Inman DM, Danilov CA, Morrison RS, Calkins DJ, Horner PJ, 2010. Metabolic vulnerability disposes retinal ganglion cell axons to dysfunction in a model of glaucomatous degeneration. J Neurosci 30, 5644–5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos SA, Martinez NW, Yoo S, Jara JS, Zamorano S, Hetz C, Twiss JL, Alvarez J, Court FA, 2011. Axonal degeneration is mediated by the mitochondrial permeability transition pore. J Neurosci 31, 966–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Babetto E, Coleman MP, Martin KR, 2008. The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur J Neurosci 28, 1166–1179. [DOI] [PubMed] [Google Scholar]
- Berger F, Lau C, Dahlmann M, Ziegler M, 2005. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem 280, 36334–36341. [DOI] [PubMed] [Google Scholar]
- Berry RH, Qu J, John SW, Howell GR, Jakobs TC, 2015. Synapse Loss and Dendrite Remodeling in a Mouse Model of Glaucoma. PLoS One 10, e0144341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Horton JC, Lawton MT, McDermott MW, 2004. Idiopathic intracranial hypertension. Neurosurgery 54, 538–551; discussion 551–532. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Strettoi E, Chierzi S, Cenni MC, Liu XH, Martinou JC, Maffei L, Rabacchi SA, 1996. Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. J Neurosci 16, 4186–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Steele MR, Vetter ML, 2011. Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol 519, 599–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancho D, Tanaka N, Jaeschke A, Ventura JJ, Kelkar N, Tanaka Y, Kyuuma M, Takeshita T, Flavell RA, Davis RJ, 2003. Mechanism of p38 MAP kinase activation in vivo. Genes Dev 17, 1969–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne JF, Staple JK, Raff MC, 1996. Glial cells are increased proportionally in transgenic optic nerves with increased numbers of axons. J Neurosci 16, 2064–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins DJ, 2012. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res 31, 702–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty M, Bowie AG, 2019. SARM: From immune regulator to cell executioner. Biochem Pharmacol 161, 52–62. [DOI] [PubMed] [Google Scholar]
- Cenni MC, Bonfanti L, Martinou JC, Ratto GM, Strettoi E, Maffei L, 1996. Long-term survival of retinal ganglion cells following optic nerve section in adult bcl-2 transgenic mice. Eur J Neurosci 8, 1735–1745. [DOI] [PubMed] [Google Scholar]
- Chambers JW, Howard S, LoGrasso PV, 2013. Blocking c-Jun N-terminal kinase (JNK) translocation to the mitochondria prevents 6-hydroxydopamine-induced toxicity in vitro and in vivo. J Biol Chem 288, 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Lin CW, Chang CY, Jiang ST, Hsueh YP, 2011. Sarm1, a negative regulator of innate immunity, interacts with syndecan-2 and regulates neuronal morphology. J Cell Biol 193, 769–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Cho KS, Vu THK, Shen CH, Kaur M, Chen G, Mathew R, McHam ML, Fazelat A, Lashkari K, Au NPB, Tse JKY, Li Y, Yu H, Yang L, Stein-Streilein J, Ma CHE, Woolf CJ, Whary MT, Jager MJ, Fox JG, Chen J, Chen DF, 2018. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat Commun 9, 3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Geoffroy CG, Wong HN, Tress O, Nguyen MT, Holzman LB, Jin Y, Zheng B, 2016. Leucine Zipper-bearing Kinase promotes axon growth in mammalian central nervous system neurons. Sci Rep 6, 31482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA, 2001. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem 276, 30724–30728. [DOI] [PubMed] [Google Scholar]
- Chen M, Maloney JA, Kallop DY, Atwal JK, Tam SJ, Baer K, Kissel H, Kaminker JS, Lewcock JW, Weimer RM, Watts RJ, 2012. Spatially coordinated kinase signaling regulates local axon degeneration. J Neurosci 32, 13439–13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, White MA, Cobb MH, 2002. Stimulus-specific requirements for MAP3 kinases in activating the JNK pathway. J Biol Chem 277, 49105–49110. [DOI] [PubMed] [Google Scholar]
- Chierzi S, Strettoi E, Cenni MC, Maffei L, 1999. Optic nerve crush: axonal responses in wild-type and bcl-2 transgenic mice. J Neurosci 19, 8367–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M, 2005. Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci 6, 889–898. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Conforti L, Buckmaster EA, Tarlton A, Ewing RM, Brown MC, Lyon MF, Perry VH, 1998. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proc Natl Acad Sci U S A 95, 9985–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Freeman MR, 2010. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci 33, 245–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Fang G, Beirowski B, Wang MS, Sorci L, Asress S, Adalbert R, Silva A, Bridge K, Huang XP, Magni G, Glass JD, Coleman MP, 2007. NAD(+) and axon degeneration revisited: Nmnat1 cannot substitute for Wld(S) to delay Wallerian degeneration. Cell Death Differ 14, 116–127. [DOI] [PubMed] [Google Scholar]
- Conforti L, Gilley J, Coleman MP, 2014. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci 15, 394–409. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Collyer JW, Calkins DJ, 2018. Astrocyte remodeling without gliosis precedes optic nerve Axonopathy. Acta Neuropathol Commun 6, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EA, Stevens MV, Vaillancourt RR, Camenisch TD, 2008. MAP3Ks as central regulators of cell fate during development. Dev Dyn 237, 3102–3114. [DOI] [PubMed] [Google Scholar]
- Crish SD, Sappington RM, Inman DM, Horner PJ, Calkins DJ, 2010. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci U S A 107, 5196–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack CL, Swahari V, Hampton Henley W, Michael Ramsey J, Deshmukh M, 2013. Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nat Commun 4, 1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danford ID, Verkuil LD, Choi DJ, Collins DW, Gudiseva HV, Uyhazi KE, Lau MK, Kanu LN, Grant GR, Chavali VRM, O’Brien JM, 2017. Characterizing the “POAGome”: A bioinformatics-driven approach to primary open-angle glaucoma. Prog Retin Eye Res 58, 89–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapper JD, Crish SD, Pang IH, Calkins DJ, 2013. Proximal inhibition of p38 MAPK stress signaling prevents distal axonopathy. Neurobiol Dis 59, 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ, 2000. Signal transduction by the JNK group of MAP kinases. Cell 103, 239–252. [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Johnson EM Jr., 1994. Neurites can remain viable after destruction of the neuronal soma by programmed cell death (apoptosis). Dev Biol 165, 63–72. [DOI] [PubMed] [Google Scholar]
- Di Stefano M, Nascimento-Ferreira I, Orsomando G, Mori V, Gilley J, Brown R, Janeckova L, Vargas ME, Worrell LA, Loreto A, Tickle J, Patrick J, Webster JR, Marangoni M, Carpi FM, Pucciarelli S, Rossi F, Meng W, Sagasti A, Ribchester RR, Magni G, Coleman MP, Conforti L, 2015. A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ 22, 731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doh SH, Kim JH, Lee KM, Park HY, Park CK, 2010. Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Res 1308, 158–166. [DOI] [PubMed] [Google Scholar]
- Duncan RO, Sample PA, Weinreb RN, Bowd C, Zangwill LM, 2007. Retinotopic organization of primary visual cortex in glaucoma: Comparing fMRI measurements of cortical function with visual field loss. Prog Retin Eye Res 26, 38–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers A, Whitfield J, Shah B, Spadoni C, Desmond H, Ham J, 2001. Direct inhibition of c-Jun N-terminal kinase in sympathetic neurones prevents c-jun promoter activation and NGF withdrawal-induced death. J Neurochem 76, 1439–1454. [DOI] [PubMed] [Google Scholar]
- Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, Milbrandt J, 2017. The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD(+) Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron 93, 1334–1343 e1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Soares L, Teng X, Geary M, Bonini NM, 2012. A novel Drosophila model of nerve injury reveals an essential role of Nmnat in maintaining axonal integrity. Curr Biol 22, 590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley JE, Burdett TC, Barria R, Neukomm LJ, Kenna KP, Landers JE, Freeman MR, 2018. Transcription factor Pebbled/RREB1 regulates injury-induced axon degeneration. Proc Natl Acad Sci U S A 115, 1358–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley MM, Watkins TA, 2018. Intrinsic Neuronal Stress Response Pathways in Injury and Disease. Annu Rev Pathol 13, 93–116. [DOI] [PubMed] [Google Scholar]
- Fernandes KA, Harder JM, Fornarola LB, Freeman RS, Clark AF, Pang IH, John SW, Libby RT, 2012. JNK2 and JNK3 are major regulators of axonal injury-induced retinal ganglion cell death. Neurobiol Dis 46, 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes KA, Harder JM, John SW, Shrager P, Libby RT, 2014. DLK-dependent signaling is important for somal but not axonal degeneration of retinal ganglion cells following axonal injury. Neurobiol Dis 69, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes KA, Harder JM, Kim J, Libby RT, 2013. JUN regulates early transcriptional responses to axonal injury in retinal ganglion cells. Exp Eye Res 112, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes KA, Mitchell KL, Patel A, Marola OJ, Shrager P, Zack DJ, Libby RT, Welsbie DS, 2018. Role of SARM1 and DR6 in retinal ganglion cell axonal and somal degeneration following axonal injury. Exp Eye Res 171, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri A, Sanes JR, Coleman MP, Cunningham JM, Kato AC, 2003. Inhibiting axon degeneration and synapse loss attenuates apoptosis and disease progression in a mouse model of motoneuron disease. Curr Biol 13, 669–673. [DOI] [PubMed] [Google Scholar]
- Finn JT, Weil M, Archer F, Siman R, Srinivasan A, Raff MC, 2000. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J Neurosci 20, 1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu HY, Okada K, Liao Y, Tsukamoto O, Isomura T, Asai M, Sawada T, Okuda K, Asano Y, Sanada S, Asanuma H, Asakura M, Takashima S, Komuro I, Kitakaze M, Minamino T, 2010. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation 122, 361–369. [DOI] [PubMed] [Google Scholar]
- Geden MJ, Deshmukh M, 2016. Axon degeneration: context defines distinct pathways. Curr Opin Neurobiol 39, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Doan RA, Strickland A, Huang X, Milbrandt J, DiAntonio A, 2016. Prevention of vincristine-induced peripheral neuropathy by genetic deletion of SARM1 in mice. Brain 139, 3092–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EB, Glass JD, Griffin JW, 1995. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J Neurosci 15, 6445–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J, 2015. SARM1 activation triggers axon degeneration locally via NAD(+) destruction. Science 348, 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, Sasaki Y, Vohra B, Marasa J, Milbrandt J, 2011. Image-based screening identifies novel roles for IkappaB kinase and glycogen synthase kinase 3 in axonal degeneration. J Biol Chem 286, 28011–28018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, Summers DW, Milbrandt J, DiAntonio A, 2016. Axon Self-Destruction: New Links among SARM1, MAPKs, and NAD+ Metabolism. Neuron 89, 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AS, Wang B, Pozniak CD, Chen M, Watts RJ, Lewcock JW, 2011. DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J Cell Biol 194, 751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Parrado S, Fernandez-Montalvan A, Assfalg-Machleidt I, Popp O, Bestvater F, Holloschi A, Knoch TA, Auerswald EA, Welsh K, Reed JC, Fritz H, Fuentes-Prior P, Spiess E, Salvesen GS, Machleidt W, 2002. Ionomycin-activated calpain triggers apoptosis. A probable role for Bcl-2 family members. J Biol Chem 277, 27217–27226. [DOI] [PubMed] [Google Scholar]
- Gilley J, Coleman MP, 2010. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol 8, e1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingwater TH, Haley JE, Ribchester RR, Horsburgh K, 2004. Neuroprotection after transient global cerebral ischemia in Wld(s) mutant mice. J Cereb Blood Flow Metab 24, 62–66. [DOI] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK 2nd, Wilson MR, Kass MA, 2002. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 120, 714–720; discussion 829–730. [DOI] [PubMed] [Google Scholar]
- Gramlich OW, Ding QJ, Zhu W, Cook A, Anderson MG, Kuehn MH, 2015. Adoptive transfer of immune cells from glaucomatous mice provokes retinal ganglion cell loss in recipients. Acta Neuropathol Commun 3, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Johnson E, Cepurna W, Jia L, Dyck J, Morrison JC, 2009. Does elevated intraocular pressure reduce retinal TRKB-mediated survival signaling in experimental glaucoma? Exp Eye Res 89, 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Johnson EC, Cepurna WO, Dyck JA, Doser T, Morrison JC, 2011. Early gene expression changes in the retinal ganglion cell layer of a rat glaucoma model. Invest Ophthalmol Vis Sci 52, 1460–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Ang LC, Noel de Tilly L, Bidaisee L, Yucel YH, 2006. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol 90, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Ly T, Zhang Q, Kaufman PL, Weinreb RN, Yucel YH, 2007. Chronic ocular hypertension induces dendrite pathology in the lateral geniculate nucleus of the brain. Exp Eye Res 84, 176–184. [DOI] [PubMed] [Google Scholar]
- Gupta N, Yucel YH, 2003. Brain changes in glaucoma. Eur J Ophthalmol 13 Suppl 3, S32–35. [DOI] [PubMed] [Google Scholar]
- Han SM, Baig HS, Hammarlund M, 2016. Mitochondria Localize to Injured Axons to Support Regeneration. Neuron 92, 1308–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Braine CE, Williams PA, Zhu X, MacNicoll KH, Sousa GL, Buchanan RA, Smith RS, Libby RT, Howell GR, John SWM, 2017. Early immune responses are independent of RGC dysfunction in glaucoma with complement component C3 being protective. Proc Natl Acad Sci U S A 114, E3839–E3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Ding Q, Fernandes KA, Cherry JD, Gan L, Libby RT, 2012a. BCL2L1 (BCL-X) promotes survival of adult and developing retinal ganglion cells. Mol Cell Neurosci 51, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Fernandes KA, Libby RT, 2012b. The Bcl-2 family member BIM has multiple glaucoma-relevant functions in DBA/2J mice. Sci Rep 2, 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Libby RT, 2011. BBC3 (PUMA) regulates developmental apoptosis but not axonal injury induced death in the retina. Mol Neurodegener 6, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Libby RT, 2013. Deficiency in Bim, Bid and Bbc3 (Puma) do not prevent axonal injury induced death. Cell Death Differ 20, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Williams PA, Soto I, Foxworth NE, Fernandes KA, Freeburg NF, Libby RT, John SWM, 2018. Jnk2 deficiency increases the rate of glaucomatous neurodegeneration in ocular hypertensive DBA/2J mice. Cell Death Dis 9, 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninger N, Bouley J, Sikoglu EM, An J, Moore CM, King JA, Bowser R, Freeman MR, Brown RH Jr., 2016. Attenuated traumatic axonal injury and improved functional outcome after traumatic brain injury in mice lacking Sarm1. Brain 139, 1094–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez MR, 2000. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res 19, 297–321. [DOI] [PubMed] [Google Scholar]
- Hirai S, Kawaguchi A, Suenaga J, Ono M, Cui DF, Ohno S, 2005. Expression of MUK/DLK/ZPK, an activator of the JNK pathway, in the nervous systems of the developing mouse embryo. Gene Expr Patterns 5, 517–523. [DOI] [PubMed] [Google Scholar]
- Holland SM, Collura KM, Ketschek A, Noma K, Ferguson TA, Jin Y, Gallo G, Thomas GM, 2016. Palmitoylation controls DLK localization, interactions and activity to ensure effective axonal injury signaling. Proc Natl Acad Sci U S A 113, 763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopfer ED, McLaughlin T, Watts RJ, Schuldiner O, O’Leary DD, Luo L, 2006. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron 50, 883–895. [DOI] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, Barbay JM, Marchant JK, Mahesh N, Porciatti V, Whitmore AV, Masland RH, John SW, 2007. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol 179, 1523–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Libby RT, John SW, 2008. Mouse genetic models: an ideal system for understanding glaucomatous neurodegeneration and neuroprotection. Prog. Brain Res. 173, 303–321. [DOI] [PubMed] [Google Scholar]
- Howell GR, Macalinao DG, Sousa GL, Walden M, Soto I, Kneeland SC, Barbay JM, King BL, Marchant JK, Hibbs M, Stevens B, Barres BA, Clark AF, Libby RT, John SW, 2011a. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest 121, 1429–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, MacNicoll KH, Braine CE, Soto I, Macalinao DG, Sousa GL, John SW, 2014. Combinatorial targeting of early pathways profoundly inhibits neurodegeneration in a mouse model of glaucoma. Neurobiol Dis 71, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Libby RT, John SW, 2012a. Intrinsic axonal degeneration pathways are critical for glaucomatous damage. Exp. Neurol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Libby RT, John SW, 2013. Intrinsic axonal degeneration pathways are critical for glaucomatous damage. Exp Neurol 246, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, Caddle LB, MacNicoll KH, Barbay JM, Anderson MG, Smith RS, Clark AF, Libby RT, John SW, 2012b. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J. Clin. Invest In the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, Caddle LB, MacNicoll KH, Barbay JM, Porciatti V, Anderson MG, Smith RS, Clark AF, Libby RT, John SW, 2012c. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Invest 122, 1246–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Walton DO, King BL, Libby RT, John SW, 2011b. Datgan, a reusable software system for facile interrogation and visualization of complex transcription profiling data. BMC Genomics 12, 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, 2016. Axon injury induced endoplasmic reticulum stress and neurodegeneration. Neural Regen Res 11, 1557–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Park KK, Yang L, Wei X, Yang Q, Cho KS, Thielen P, Lee AH, Cartoni R, Glimcher LH, Chen DF, He Z, 2012. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron 73, 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Dobberfuhl A, Filippopoulos T, Ingelsson M, Fileta JB, Poulin NR, Grosskreutz CL, 2005. Transcriptional up-regulation and activation of initiating caspases in experimental glaucoma. Am J Pathol 167, 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Fileta J, Rawe I, Qu J, Grosskreutz CL, 2010. Calpain activation in experimental glaucoma. Invest Ophthalmol Vis Sci 51, 3049–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Kong W, Zhou Y, Gregori G, 2011. Distortion of axonal cytoskeleton: an early sign of glaucomatous damage. Invest Ophthalmol Vis Sci 52, 2879–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D, Hossain-Ibrahim K, Mason MR, Coffin RS, Lieberman AR, Winterbottom J, Anderson PN, 2004. ATF3 upregulation in glia during Wallerian degeneration: differential expression in peripheral nerves and CNS white matter. BMC Neurosci 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntwork-Rodriguez S, Wang B, Watkins T, Ghosh AS, Pozniak CD, Bustos D, Newton K, Kirkpatrick DS, Lewcock JW, 2013. JNK-mediated phosphorylation of DLK suppresses its ubiquitination to promote neuronal apoptosis. J Cell Biol 202, 747–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman DM, Harun-Or-Rashid M, 2017. Metabolic Vulnerability in the Neurodegenerative Disease Glaucoma. Front Neurosci 11, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman DM, Sappington RM, Horner PJ, Calkins DJ, 2006. Quantitative correlation of optic nerve pathology with ocular pressure and corneal thickness in the DBA/2 mouse model of glaucoma. Invest Ophthalmol Vis Sci 47, 986–996. [DOI] [PubMed] [Google Scholar]
- Irnaten M, Zhdanov A, Brennan D, Crotty T, Clark A, Papkovsky D, O’Brien C, 2018. Activation of the NFAT-Calcium Signaling Pathway in Human Lamina Cribrosa Cells in Glaucoma. Invest Ophthalmol Vis Sci 59, 831–842. [DOI] [PubMed] [Google Scholar]
- Isenmann S, Bahr M, 1997. Expression of c-Jun protein in degenerating retinal ganglion cells after optic nerve lesion in the rat. Exp Neurol 147, 28–36. [DOI] [PubMed] [Google Scholar]
- Isenmann S, Engel S, Gillardon F, Bahr M, 1999. Bax antisense oligonucleotides reduce axotomy-induced retinal ganglion cell death in vivo by reduction of Bax protein expression. Cell Death Differ 6, 673–682. [DOI] [PubMed] [Google Scholar]
- Itoh T, Horiuchi M, Ikeda RH Jr., Xu J, Bannerman P, Pleasure D, Penninger JM, Tournier C, Itoh A, 2014. ZPK/DLK and MKK4 form the critical gateway to axotomy-induced motoneuron death in neonates. J Neurosci 34, 10729–10742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs TC, Libby RT, Ben Y, John SW, Masland RH, 2005. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol 171, 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey KL, Camps M, Rommel C, Mackay CR, 2007. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov 6, 391–403. [DOI] [PubMed] [Google Scholar]
- Ji J, Chang P, Pennesi ME, Yang Z, Zhang J, Li D, Wu SM, Gross RL, 2005. Effects of elevated intraocular pressure on mouse retinal ganglion cells. Vision Res 45, 169–179. [DOI] [PubMed] [Google Scholar]
- Johnson EC, Morrison JC, 2009. Friend or foe? Resolving the impact of glial responses in glaucoma. J Glaucoma 18, 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M, Tenneti L, Lipton SA, 2000. Role of p38 mitogen-activated protein kinase in axotomy-induced apoptosis of rat retinal ganglion cells. J Neurosci 20, 5037–5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EK, Choi EJ, 2010. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 1802, 396–405. [DOI] [PubMed] [Google Scholar]
- Kim Y, Zhou P, Qian L, Chuang JZ, Lee J, Li C, Iadecola C, Nathan C, Ding A, 2007. MyD88–5 links mitochondria, microtubules, and JNK3 in neurons and regulates neuronal survival. J Exp Med 204, 2063–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiumehr S, Park SC, Syril D, Teng CC, Tello C, Liebmann JM, Ritch R, 2012. In vivo evaluation of focal lamina cribrosa defects in glaucoma. Arch Ophthalmol 130, 552–559. [DOI] [PubMed] [Google Scholar]
- Kretz A, Kugler S, Happold C, Bahr M, Isenmann S, 2004. Excess Bcl-XL increases the intrinsic growth potential of adult CNS neurons in vitro. Mol Cell Neurosci 26, 63–74. [DOI] [PubMed] [Google Scholar]
- Kwong JM, Caprioli J, 2006. Expression of phosphorylated c-Jun N-terminal protein kinase (JNK) in experimental glaucoma in rats. Exp Eye Res 82, 576–582. [DOI] [PubMed] [Google Scholar]
- Larhammar M, Huntwork-Rodriguez S, Jiang Z, Solanoy H, Sengupta Ghosh A, Wang B, Kaminker JS, Huang K, Eastham-Anderson J, Siu M, Modrusan Z, Farley MM, Tessier-Lavigne M, Lewcock JW, Watkins TA, 2017. Dual leucine zipper kinase-dependent PERK activation contributes to neuronal degeneration following insult. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor M, Danesh-Meyer H, Levin LA, Davagnanam I, De Vita E, Plant GT, 2018. Glaucoma and the brain: Trans-synaptic degeneration, structural change, and implications for neuroprotection. Surv Ophthalmol 63, 296–306. [DOI] [PubMed] [Google Scholar]
- Lee JY, Jeong HJ, Lee JH, Kim YJ, Kim EY, Kim YY, Ryu T, Cho ZH, Kim YB, 2014. An investigation of lateral geniculate nucleus volume in patients with primary open-angle glaucoma using 7 tesla magnetic resonance imaging. Invest Ophthalmol Vis Sci 55, 3468–3476. [DOI] [PubMed] [Google Scholar]
- Leffler CT, Schwartz SG, Giliberti FM, Young MT, Bermudez D, 2015a. What was Glaucoma Called Before the 20th Century? Ophthalmol Eye Dis 7, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler CT, Schwartz SG, Hadi TM, Salman A, Vasuki V, 2015b. The early history of glaucoma: the glaucous eye (800 BC to 1050 AD). Clin Ophthalmol 9, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Davis RJ, 2003. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A 100, 2432–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Quigley HA, Martin KR, Harizman N, Valenta DF, Pease ME, Melamed S, 2005. The transcription factor c-jun is activated in retinal ganglion cells in experimental rat glaucoma. Exp Eye Res 80, 663–670. [DOI] [PubMed] [Google Scholar]
- Li Y, Schlamp CL, Poulsen KP, Nickells RW, 2000. Bax-dependent and independent pathways of retinal ganglion cell death induced by different damaging stimuli. Exp Eye Res 71, 209–213. [DOI] [PubMed] [Google Scholar]
- Libby RT, Gould DB, Anderson MG, John SW, 2005a. Complex genetics of glaucoma susceptibility. Annu Rev Genomics Hum Genet 6, 15–44. [DOI] [PubMed] [Google Scholar]
- Libby RT, Li Y, Savinova OV, Barter J, Smith RS, Nickells RW, John SW, 2005b. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet 1, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA, 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L, 2006. ER stress and neurodegenerative diseases. Cell Death Differ 13, 385–392. [DOI] [PubMed] [Google Scholar]
- Lindwall C, Kanje M, 2005. Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Mol Cell Neurosci 29, 269–282. [DOI] [PubMed] [Google Scholar]
- Liu H, Sun H, Liu C, 2011a. Interference of the apoptotic signaling pathway in RGC stress response by SP600125 in moderate ocular hypertensive rats. Chin J Physiol 54, 124–132. [PubMed] [Google Scholar]
- Liu M, Duggan J, Salt TE, Cordeiro MF, 2011b. Dendritic changes in visual pathways in glaucoma and other neurodegenerative conditions. Exp Eye Res 92, 244–250. [DOI] [PubMed] [Google Scholar]
- Liu XH, Collier RJ, Youle RJ, 2001. Inhibition of axotomy-induced neuronal apoptosis by extracellular delivery of a Bcl-XL fusion protein. J Biol Chem 276, 46326–46332. [DOI] [PubMed] [Google Scholar]
- Lukas TJ, Wang AL, Yuan M, Neufeld AH, 2009. Early cellular signaling responses to axonal injury. Cell Commun Signal 7, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S, 1989. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur J Neurosci 1, 27–33. [DOI] [PubMed] [Google Scholar]
- Ly T, Gupta N, Weinreb RN, Kaufman PL, Yucel YH, 2011. Dendrite plasticity in the lateral geniculate nucleus in primate glaucoma. Vision Res 51, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Ferguson TA, Schoch KM, Li J, Qian Y, Shofer FS, Saatman KE, Neumar RW, 2013. Calpains mediate axonal cytoskeleton disintegration during Wallerian degeneration. Neurobiol Dis 56, 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, Thomson D, Gillingwater T, Court F, Conforti L, Fernando FS, Tarlton A, Andressen C, Addicks K, Magni G, Ribchester RR, Perry VH, Coleman MP, 2001. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci 4, 1199–1206. [DOI] [PubMed] [Google Scholar]
- Mackenzie W, 1830. A practical treatise on the diseases of the eye. Longman, Rees, Orme, Brown & Green London. [Google Scholar]
- Maes ME, Schlamp CL, Nickells RW, 2017. BAX to basics: How the BCL2 gene family controls the death of retinal ganglion cells. Prog Retin Eye Res 57, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik JM, Shevtsova Z, Bahr M, Kugler S, 2005. Long-term in vivo inhibition of CNS neurodegeneration by Bcl-XL gene transfer. Mol Ther 11, 373–381. [DOI] [PubMed] [Google Scholar]
- Mansouri K, Leite MT, Medeiros FA, Leung CK, Weinreb RN, 2011. Assessment of rates of structural change in glaucoma using imaging technologies. Eye (Lond) 25, 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey KA, Mollan SP, Jensen RH, Sinclair AJ, 2016. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. Lancet Neurol 15, 78–91. [DOI] [PubMed] [Google Scholar]
- Massoll C, Mando W, Chintala SK, 2013. Excitotoxicity upregulates SARM1 protein expression and promotes Wallerian-like degeneration of retinal ganglion cells and their axons. Invest Ophthalmol Vis Sci 54, 2771–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, 2000. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 1, 120–129. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ, 2001. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21, 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan DP, Cotter TG, 2007. A Critical role for Bim in retinal ganglion cell death. J Neurochem 102, 922–930. [DOI] [PubMed] [Google Scholar]
- McKinnon SJ, 1997. Glaucoma, apoptosis, and neuroprotection. Curr Opin Ophthalmol 8, 28–37. [DOI] [PubMed] [Google Scholar]
- Milde S, Gilley J, Coleman MP, 2013. Subcellular localization determines the stability and axon protective capacity of axon survival factor Nmnat2. PLoS Biol 11, e1001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A, 2009. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci 12, 387–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minckler DS, Bunt AH, Johanson GW, 1977. Orthograde and retrograde axoplasmic transport during acute ocular hypertension in the monkey. Invest Ophthalmol Vis Sci 16, 426–441. [PubMed] [Google Scholar]
- Minden A, Karin M, 1997. Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta 1333, F85–104. [DOI] [PubMed] [Google Scholar]
- Morgan JE, 2012. Retina ganglion cell degeneration in glaucoma: an opportunity missed? A review. Clin Experiment Ophthalmol 40, 364–368. [DOI] [PubMed] [Google Scholar]
- Muller GJ, Geist MA, Veng LM, Willesen MG, Johansen FF, Leist M, Vaudano E, 2006. A role for mixed lineage kinases in granule cell apoptosis induced by cytoskeletal disruption. J Neurochem 96, 1242–1252. [DOI] [PubMed] [Google Scholar]
- Munemasa Y, Ohtani-Kaneko R, Kitaoka Y, Kumai T, Kitaoka Y, Hayashi Y, Watanabe M, Takeda H, Hirata K, Ueno S, 2006. Pro-apoptotic role of c-Jun in NMDA-induced neurotoxicity in the rat retina. J Neurosci Res 83, 907–918. [DOI] [PubMed] [Google Scholar]
- Murata H, Khine CC, Nishikawa A, Yamamoto KI, Kinoshita R, Sakaguchi M, 2018. JNK-mediated phosphorylation of SARM1 regulates NAD(+) cleavage activity to inhibit mitochondrial respiration. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal-Nicolas FM, Jimenez-Lopez M, Salinas-Navarro M, Sobrado-Calvo P, Vidal-Sanz M, Agudo-Barriuso M, 2017. Microglial dynamics after axotomy-induced retinal ganglion cell death. J Neuroinflammation 14, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napankangas U, Lindqvist N, Lindholm D, Hallbook F, 2003. Rat retinal ganglion cells upregulate the pro-apoptotic BH3-only protein Bim after optic nerve transection. Brain Res Mol Brain Res 120, 30–37. [DOI] [PubMed] [Google Scholar]
- Nashine S, Liu Y, Kim BJ, Clark AF, Pang IH, 2015. Role of C/EBP homologous protein in retinal ganglion cell death after ischemia/reperfusion injury. Invest Ophthalmol Vis Sci 56, 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld AH, 1999. Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma. Arch Ophthalmol 117, 1050–1056. [DOI] [PubMed] [Google Scholar]
- Nickells RW, 1999. Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Surv Ophthalmol 43 Suppl 1, S151–161. [DOI] [PubMed] [Google Scholar]
- Nickells RW, 2007. From ocular hypertension to ganglion cell death: a theoretical sequence of events leading to glaucoma. Can J Ophthalmol 42, 278–287. [PubMed] [Google Scholar]
- Nickells RW, Howell GR, Soto I, John SW, 2012. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu. Rev. Neurosci 35, 153–179. [DOI] [PubMed] [Google Scholar]
- Niittykoski M, Kalesnykas G, Larsson KP, Kaarniranta K, Akerman KE, Uusitalo H, 2010. Altered calcium signaling in an experimental model of glaucoma. Invest Ophthalmol Vis Sci 51, 6387–6393. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M, 2009. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature 457, 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- O’Donnell KC, Vargas ME, Sagasti A, 2013. WldS and PGC-1alpha regulate mitochondrial transport and oxidation state after axonal injury. J Neurosci 33, 14778–14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, MacDonald JM, Ziegenfuss JS, Milde S, Hou YJ, Nathan C, Ding A, Brown RH Jr., Conforti L, Coleman M, Tessier-Lavigne M, Zuchner S, Freeman MR, 2012. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science 337, 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Jo RE, Ullian EM, Wong RO, Della Santina L, 2016. Selective Vulnerability of Specific Retinal Ganglion Cell Types and Synapses after Transient Ocular Hypertension. J Neurosci 36, 9240–9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzel HR, Schlamp CL, Nickells RW, 2010. Histone H4 deacetylation plays a critical role in early gene silencing during neuronal apoptosis. BMC Neurosci 11, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JC, Bhattacharya S, Clark AF, Zode GS, 2015. Increased Endoplasmic Reticulum Stress in Human Glaucomatous Trabecular Meshwork Cells and Tissues. Invest Ophthalmol Vis Sci 56, 3860–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanna G, Krishnamoorthy R, Clark AF, Wordinger RJ, Yorio T, 2002. Human optic nerve head astrocytes as a target for endothelin-1. Invest Ophthalmol Vis Sci 43, 2704–2713. [PubMed] [Google Scholar]
- Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A, 2007. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129, 1337–1349. [DOI] [PubMed] [Google Scholar]
- Qu J, Wang D, Grosskreutz CL, 2010. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp Eye Res 91, 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, 1999. Neuronal death in glaucoma. Prog Retin Eye Res 18, 39–57. [DOI] [PubMed] [Google Scholar]
- Quigley HA, 2012. Clinical trials for glaucoma neuroprotection are not impossible. Curr Opin Ophthalmol 23, 144–154. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Addicks EM, 1980. Chronic experimental glaucoma in primates. II. Effect of extended intraocular pressure elevation on optic nerve head and axonal transport. Invest Ophthalmol Vis Sci 19, 137–152. [PubMed] [Google Scholar]
- Quigley HA, Addicks EM, 1981. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol 99, 137–143. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Addicks EM, Green WR, Maumenee AE, 1981. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol 99, 635–649. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Anderson DR, 1977. Distribution of axonal transport blockade by acute intraocular pressure elevation in the primate optic nerve head. Invest Ophthalmol Vis Sci 16, 640–644. [PubMed] [Google Scholar]
- Quigley HA, Green WR, 1979. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology 86, 1803–1830. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Hohman RM, Addicks EM, Massof RW, Green WR, 1983. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol 95, 673–691. [DOI] [PubMed] [Google Scholar]
- Radius RL, Anderson DR, 1981. Rapid axonal transport in primate optic nerve. Distribution of pressure-induced interruption. Arch Ophthalmol 99, 650–654. [DOI] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT, 2002. Axonal self-destruction and neurodegeneration. Science 296, 868–871. [DOI] [PubMed] [Google Scholar]
- Rathnasamy G, Foulds WS, Ling EA, Kaur C, 2018. Retinal microglia - A key player in healthy and diseased retina. Prog Neurobiol. [DOI] [PubMed] [Google Scholar]
- Risner ML, Pasini S, Cooper ML, Lambert WS, Calkins DJ, 2018. Axogenic mechanism enhances retinal ganglion cell excitability during early progression in glaucoma. Proc Natl Acad Sci U S A 115, E2393–E2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Shaikh AR, Hennelly MM, Li Q, Bindokas V, Graham CE, 2003. Mitogen-activated protein kinases and retinal ischemia. Invest Ophthalmol Vis Sci 44, 5383–5395. [DOI] [PubMed] [Google Scholar]
- Saklatvala J, Davis W, Guesdon F, 1996. Interleukin 1 (IL1) and tumour necrosis factor (TNF) signal transduction. Philos Trans R Soc Lond B Biol Sci 351, 151–157. [DOI] [PubMed] [Google Scholar]
- Samsam M, Mi W, Wessig C, Zielasek J, Toyka KV, Coleman MP, Martini R, 2003. The Wlds mutation delays robust loss of motor and sensory axons in a genetic model for myelin-related axonopathy. J Neurosci 23, 2833–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, 2018. Metabolic aspects of neuronal degeneration: From a NAD(+) point of view. Neurosci Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Nakagawa T, Mao X, DiAntonio A, Milbrandt J, 2016. NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD(+) depletion. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Baloh RH, Milbrandt J, 2009. Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. J Neurosci 29, 6526–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper W, Hoozemans JJ, 2015. The unfolded protein response in neurodegenerative diseases: a neuropathological perspective. Acta Neuropathol 130, 315–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW, 2006. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci 7, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmann Z, Assa-Kunik E, Tiomny S, Minis A, Haklai-Topper L, Arama E, Yaron A, 2010. Axonal degeneration is regulated by the apoptotic machinery or a NAD+-sensitive pathway in insects and mammals. J. Neurosci 30, 6375–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaan SJ, Li Y, Nickells RW, 2010. A single nucleotide polymorphism in the Bax gene promoter affects transcription and influences retinal ganglion cell death. ASN Neuro 2, e00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E, Karin M, 2002. AP-1 as a regulator of cell life and death. Nat Cell Biol 4, E131–136. [DOI] [PubMed] [Google Scholar]
- Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A, 2012a. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron 74, 1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, Miller BR, Babetto E, Cho Y, Sasaki Y, Qayum S, Russler EV, Cavalli V, Milbrandt J, DiAntonio A, 2012b. SCG10 is a JNK target in the axonal degeneration pathway. Proc Natl Acad Sci U S A 109, E3696–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DJ, Pitts J, Hertz NT, Yang J, Yamagishi Y, Olsen O, Tesic Mark M, Molina H, Tessier-Lavigne M, 2016. Axon Degeneration Gated by Retrograde Activation of Somatic Pro-apoptotic Signaling. Cell 164, 1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto I, Howell GR, 2014. The complex role of neuroinflammation in glaucoma. Cold Spring Harb Perspect Med 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B, 2012. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 35, 369–389. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA, 2007. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178. [DOI] [PubMed] [Google Scholar]
- Su D, Park SC, Simonson JL, Liebmann JM, Ritch R, 2013. Progression pattern of initial parafoveal scotomas in glaucoma. Ophthalmology 120, 520–527. [DOI] [PubMed] [Google Scholar]
- Summers DW, DiAntonio A, Milbrandt J, 2014. Mitochondrial dysfunction induces Sarm1-dependent cell death in sensory neurons. J Neurosci 34, 9338–9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Lye-Barthel M, Masland RH, Jakobs TC, 2009. The morphology and spatial arrangement of astrocytes in the optic nerve head of the mouse. J Comp Neurol 516, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Moore S, Jakobs TC, 2017. Optic nerve astrocyte reactivity protects function in experimental glaucoma and other nerve injuries. J Exp Med 214, 1411–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Wang Y, Pang IH, Shen J, Tang X, Li Y, Liu C, Li B, 2011. Protective effect of a JNK inhibitor against retinal ganglion cell loss induced by acute moderate ocular hypertension. Mol Vis 17, 864–875. [PMC free article] [PubMed] [Google Scholar]
- Syc-Mazurek SB, Fernandes KA, Libby RT, 2017a. JUN is important for ocular hypertension-induced retinal ganglion cell degeneration. Cell Death Dis 8, e2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syc-Mazurek SB, Fernandes KA, Wilson MP, Shrager P, Libby RT, 2017b. Together JUN and DDIT3 (CHOP) control retinal ganglion cell death after axonal injury. Mol Neurodegener 12, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syc-Mazurek SB, Rausch RL, Fernandes KA, Wilson MP, Libby RT, 2018. Mkk4 and Mkk7 are important for retinal development and axonal injury-induced retinal ganglion cell death. Cell Death Dis 9, 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Ron D, 2011. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13, 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tararuk T, Ostman N, Li W, Bjorkblom B, Padzik A, Zdrojewska J, Hongisto V, Herdegen T, Konopka W, Courtney MJ, Coffey ET, 2006. JNK1 phosphorylation of SCG10 determines microtubule dynamics and axodendritic length. J Cell Biol 173, 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi A, Bradke F, 2013. The DLK signalling pathway--a double-edged sword in neural development and regeneration. EMBO Rep 14, 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenzio M, Koley S, Samra N, Rishal I, Zhao Q, Sahoo PK, Urisman A, Marvaldi L, Oses-Prieto JA, Forester C, Gomes C, Kalinski AL, Di Pizio A, Doron-Mandel E, Perry RB, Koppel I, Twiss JL, Burlingame AL, Fainzilber M, 2018. Locally translated mTOR controls axonal local translation in nerve injury. Science 359, 1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, 2013. Immune regulation toward immunomodulation for neuroprotection in glaucoma. Curr Opin Pharmacol 13, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Chauhan BC, LeBlanc RP, Wax MB, 2003. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Ophthalmol Vis Sci 44, 3025–3033. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X, Yang J, Wax MB, 2004. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res 996, 202–212. [DOI] [PubMed] [Google Scholar]
- Thomas CN, Berry M, Logan A, Blanch RJ, Ahmed Z, 2017. Caspases in retinal ganglion cell death and axon regeneration. Cell Death Discov 3, 17032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielsch JM, 1996. The epidemiology and control of open angle glaucoma: a population-based perspective. Annu Rev Public Health 17, 121–136. [DOI] [PubMed] [Google Scholar]
- Tournier C, Dong C, Turner TK, Jones SN, Flavell RA, Davis RJ, 2001. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev 15, 1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyota H, Yanase N, Yoshimoto T, Moriyama M, Sudo T, Mizuguchi J, 2003. Calpain-induced Bax-cleavage product is a more potent inducer of apoptotic cell death than wild-type Bax. Cancer Lett 189, 221–230. [DOI] [PubMed] [Google Scholar]
- Turkiew E, Falconer D, Reed N, Hoke A, 2017. Deletion of Sarm1 gene is neuroprotective in two models of peripheral neuropathy. J Peripher Nerv Syst 22, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Yoneshige A, Koriyama Y, Hagiyama M, Shimomura Y, Ito A, 2018. Early Gene Expression Profile in Retinal Ganglion Cell Layer After Optic Nerve Crush in Mice. Invest Ophthalmol Vis Sci 59, 370–380. [DOI] [PubMed] [Google Scholar]
- Valakh V, Frey E, Babetto E, Walker LJ, DiAntonio A, 2015. Cytoskeletal disruption activates the DLK/JNK pathway, which promotes axonal regeneration and mimics a preconditioning injury. Neurobiol Dis 77, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E, 2015. NAD(+) in aging, metabolism, and neurodegeneration. Science 350, 1208–1213. [DOI] [PubMed] [Google Scholar]
- Villegas R, Martinez NW, Lillo J, Pihan P, Hernandez D, Twiss JL, Court FA, 2014. Calcium release from intra-axonal endoplasmic reticulum leads to axon degeneration through mitochondrial dysfunction. J Neurosci 34, 7179–7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra BP, Sasaki Y, Miller BR, Chang J, DiAntonio A, Milbrandt J, 2010. Amyloid precursor protein cleavage-dependent and -independent axonal degeneration programs share a common nicotinamide mononucleotide adenylyltransferase 1-sensitive pathway. J Neurosci 30, 13729–13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatsuki S, Tokunaga S, Shibata M, Araki T, 2017. GSK3B-mediated phosphorylation of MCL1 regulates axonal autophagy to promote Wallerian degeneration. J Cell Biol 216, 477–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LJ, Summers DW, Sasaki Y, Brace EJ, Milbrandt J, DiAntonio A, 2017. MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MS, Fang G, Culver DG, Davis AA, Rich MM, Glass JD, 2001. The WldS protein protects against axonal degeneration: a model of gene therapy for peripheral neuropathy. Ann. Neurol 50, 773–779. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang S, Liu T, Wang H, Liu K, Wang Q, Zeng W, 2018. Sarm1/Myd88–5 Regulates Neuronal Intrinsic Immune Response to Traumatic Axonal Injuries. Cell Rep 23, 716–724. [DOI] [PubMed] [Google Scholar]
- Watkins TA, Wang B, Huntwork-Rodriguez S, Yang J, Jiang Z, Eastham-Anderson J, Modrusan Z, Kaminker JS, Tessier-Lavigne M, Lewcock JW, 2013. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc Natl Acad Sci U S A 110, 4039–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsbie DS, Mitchell KL, Jaskula-Ranga V, Sluch VM, Yang Z, Kim J, Buehler E, Patel A, Martin SE, Zhang PW, Ge Y, Duan Y, Fuller J, Kim BJ, Hamed E, Chamling X, Lei L, Fraser IDC, Ronai ZA, Berlinicke CA, Zack DJ, 2017. Enhanced Functional Genomic Screening Identifies Novel Mediators of Dual Leucine Zipper Kinase-Dependent Injury Signaling in Neurons. Neuron 94, 1142–1154 e1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsbie DS, Yang Z, Ge Y, Mitchell KL, Zhou X, Martin SE, Berlinicke CA, Hackler L Jr., Fuller J, Fu J, Cao LH, Han B, Auld D, Xue T, Hirai S, Germain L, Simard-Bisson C, Blouin R, Nguyen JV, Davis CH, Enke RA, Boye SL, Merbs SL, Marsh-Armstrong N, Hauswirth WW, DiAntonio A, Nickells RW, Inglese J, Hanes J, Yau KW, Quigley HA, Zack DJ, 2013. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc Natl Acad Sci U S A 110, 4045–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore AV, Libby RT, John SW, 2005. Glaucoma: thinking in new ways-a role for autonomous axonal self-destruction and other compartmentalised processes? Prog Retin Eye Res 24, 639–662. [DOI] [PubMed] [Google Scholar]
- Whitmore AV, Lindsten T, Raff MC, Thompson CB, 2003. The proapoptotic proteins Bax and Bak are not involved in Wallerian degeneration. Cell Death Differ 10, 260–261. [DOI] [PubMed] [Google Scholar]
- Williams PA, Braine CE, Kizhatil K, Foxworth NE, Tolman NG, Harder JM, Scott RA, Sousa GL, Panitch A, Howell GR, John SWM, 2019. Inhibition of monocyte-like cell extravasation protects from neurodegeneration in DBA/2J glaucoma. Mol Neurodegener 14, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Harder JM, Cardozo BH, Foxworth NE, John SWM, 2018. Nicotinamide treatment robustly protects from inherited mouse glaucoma. Commun Integr Biol 11, e1356956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Harder JM, Foxworth NE, Cardozo BH, Cochran KE, John SWM, 2017a. Nicotinamide and WLD(S) Act Together to Prevent Neurodegeneration in Glaucoma. Front Neurosci 11, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Harder JM, Foxworth NE, Cochran KE, Philip VM, Porciatti V, Smithies O, John SW, 2017b. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 355, 756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Collins CA, 2012. A conditioning lesion protects axons from degeneration via the Wallenda/DLK MAP kinase signaling cascade. J Neurosci 32, 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Hao Y, Sun K, Li J, Li X, Mishra B, Soppina P, Wu C, Hume RI, Collins CA, 2012. The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS Biol 10, e1001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Wang X, Ewanek R, Bhat P, Diantonio A, Collins CA, 2010. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol 191, 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T, Deki-Arima N, Kaneko A, Miyamura N, Iwatsuki M, Matsuoka M, Fujimori-Tonou N, Okamoto-Uchida Y, Hirayama J, Marth JD, Yamanashi Y, Kawasaki H, Yamanaka K, Penninger JM, Shibata S, Nishina H, 2017. Age-dependent motor dysfunction due to neuron-specific disruption of stress-activated protein kinase MKK7. Sci Rep 7, 7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T, Kawasaki H, Nishina H, 2012. Diverse Roles of JNK and MKK Pathways in the Brain. Journal of Signal Transduction 2012, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weimer RM, Kallop D, Olsen O, Wu Z, Renier N, Uryu K, Tessier-Lavigne M, 2013a. Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin. Neuron 80, 1175–1189. [DOI] [PubMed] [Google Scholar]
- Yang J, Wu Z, Renier N, Simon DJ, Uryu K, Park DS, Greer PA, Tournier C, Davis RJ, Tessier-Lavigne M, 2015. Pathological axonal death through a MAPK cascade that triggers a local energy deficit. Cell 160, 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Li S, Miao L, Huang H, Liang F, Teng X, Xu L, Wang Q, Xiao W, Ridder WH 3rd, Ferguson TA, Chen DF, Kaufman RJ, Hu Y, 2016. Rescue of Glaucomatous Neurodegeneration by Differentially Modulating Neuronal Endoplasmic Reticulum Stress Molecules. J Neurosci 36, 5891–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wu L, Wang D, Li Y, Dou H, Tso MO, Ma Z, 2013b. Role of endoplasmic reticulum stress in the loss of retinal ganglion cells in diabetic retinopathy. Neural Regen Res 8, 3148–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Luo C, Cai J, Pierce WM, Tezel G, 2008. Phosphorylation-dependent interaction with 14–3-3 in the regulation of bad trafficking in retinal ganglion cells. Invest Ophthalmol Vis Sci 49, 2483–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Quigley HA, Pease ME, Yang Y, Qian J, Valenta D, Zack DJ, 2007. Changes in gene expression in experimental glaucoma and optic nerve transection: the equilibrium between protective and detrimental mechanisms. Invest Ophthalmol Vis Sci 48, 5539–5548. [DOI] [PubMed] [Google Scholar]
- Yao Z, Yang W, Gao Z, Jia P, 2017. Nicotinamide mononucleotide inhibits JNK activation to reverse Alzheimer disease. Neurosci Lett 647, 133–140. [DOI] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ, 1999. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400, 886–891. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Ueno M, Lee S, Nakamura Y, Sato A, Yoshimura K, Kishima H, Yoshimine T, Yamashita T, 2011. c-Jun N-terminal kinase induces axonal degeneration and limits motor recovery after spinal cord injury in mice. Neurosci Res 71, 266–277. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A, 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9, 47–59. [DOI] [PubMed] [Google Scholar]
- Zhang JN, Michel U, Lenz C, Friedel CC, Koster S, d’Hedouville Z, Tonges L, Urlaub H, Bahr M, Lingor P, Koch JC, 2016. Calpain-mediated cleavage of collapsin response mediator protein-2 drives acute axonal degeneration. Sci Rep 6, 37050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Sasaki Y, Milbrandt J, Gidday JM, 2013. Protection of mouse retinal ganglion cell axons and soma from glaucomatous and ischemic injury by cytoplasmic overexpression of Nmnat1. Invest Ophthalmol Vis Sci 54, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zode GS, Sharma AB, Lin X, Searby CC, Bugge K, Kim GH, Clark AF, Sheffield VC, 2014. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J Clin Invest 124, 1956–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]