Abstract
Background
Previous studies have reported a protective effect of obesity compared with normal body mass index (BMI) in patients undergoing percutaneous coronary intervention (PCI). However, it is unclear whether this effect extends to the extremely obese. In this large multicenter registry‐based study, we sought to examine the relationship between BMI and long‐term clinical outcomes following PCI, and in particular to evaluate the association between extreme obesity and long‐term survival after PCI.
Methods and Results
This cohort study included 25 413 patients who underwent PCI between January 1, 2005 and June 30, 2017, who were prospectively enrolled in the Melbourne Interventional Group registry. Patients were stratified by World Health Organization–defined BMI categories. The primary end point was National Death Index–linked mortality. The median length of follow‐up was 4.4 years (interquartile range 2.0‐7.6 years). Of the study cohort, 24.8% had normal BMI (18.5‐24.9 kg/m2), and 3.3% were extremely obese (BMI ≥40 kg/m2). Patients with greater degrees of obesity were younger and included a higher proportion of diabetics (P<0.001). After adjustment for age and comorbidities, a J‐shaped association was observed between different BMI categories and adjusted hazard ratio (HR) for long‐term mortality (normal BMI, HR 1.00 [ref]; overweight, HR 0.85, 95% CI 0.78‐0.93, P<0.001; mild obesity, HR 0.85, 95% CI 0.76‐0.94, P=0.002; moderate obesity, HR 0.95, 95% CI 0.80‐1.12, P=0.54; extreme obesity HR 1.33, 95% CI 1.07‐1.65, P=0.01).
Conclusions
An obesity paradox is still apparent in contemporary practice, with elevated BMI up to 35 kg/m2 associated with reduced long‐term mortality after PCI. However, this protective effect appears not to extend to patients with extreme obesity.
Keywords: long‐term outcome, obesity, percutaneous coronary intervention
Subject Categories: Obesity, Percutaneous Coronary Intervention, Mortality/Survival
Clinical Perspective
What Is New?
This study shows that the obesity paradox persists in contemporary percutaneous coronary intervention practice, whereby overweight and obese patients have better post–percutaneous coronary intervention long‐term survival than those with normal body mass index.
However, our study demonstrates that this protective effect does not extend to patients with extreme obesity.
What Are the Clinical Implications?
Our study demonstrates that there is a threshold effect to the obesity paradox, which is important for clinicians to recognize when risk‐stratifying patients.
We also show that patients with normal body mass index are less likely to receive appropriate secondary prevention therapy compared with their higher body mass index counterparts.
More attention needs to be paid to reducing this treatment gap in clinical practice, which may help improve outcomes in patients with normal body mass index.
Introduction
Obesity is a growing health concern worldwide, particularly in developed countries, where there has been an unprecedented rise in the proportion of overweight and obese individuals in the population.1, 2 Obesity is associated with numerous adverse health outcomes including coronary artery disease, stroke, heart failure, and diabetes mellitus and has also been linked to higher rates of mortality.3, 4 Despite this, several studies in the past have described an “obesity paradox” whereby obesity appears to confer a protective effect compared with normal body mass index (BMI), in a variety of medical conditions.5, 6, 7, 8, 9 This was also described in the setting of percutaneous coronary intervention (PCI), where overweight and obese patients were shown to have lower rates of short‐term mortality compared with normal‐BMI individuals.10, 11, 12 A meta‐analysis of over 200 000 patients with myocardial infarction also reported that obese patients have a 30% to 40% lower mortality compared with individuals with normal BMI over a 1‐ to 3‐year follow‐up period.13 However, more recent studies in patients in the contemporary era of PCI have produced conflicting results.14, 15, 16, 17, 18 In particular, despite extreme obesity (BMI≥40 kg/m2) increasing in prevalence among patients undergoing PCI, few studies have examined long‐term clinical outcomes in this group.19, 20 Studies examining in‐hospital mortality of patients undergoing PCI have suggested that although lesser degrees of obesity may be protective, this effect does not extend to patients with extreme obesity.12, 19 However, very few earlier studies have assessed mortality rates beyond 12 months in patients with extreme obesity undergoing PCI for both stable angina and acute coronary syndromes.
In this study we therefore sought to determine whether an obesity paradox persists in contemporary PCI practice over long‐term follow‐up and, in particular, to further evaluate the association between extreme obesity and long‐term clinical outcomes after PCI.
Methods
Due to the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Ms Angela Brennan of Monash University at angela.brennan@monash.edu.
This was a cohort study of patients undergoing PCI between January 1, 2005 and June 30, 2017 inclusive, enrolled prospectively in the MIG (Melbourne Interventional Group) registry. All consecutive adult patients undergoing PCI were eligible for inclusion. We excluded patients in whom height and/or weight was not recorded at the time of PCI, and therefore BMI could not be calculated. Patients who could not be considered for linkage to the Australian NDI (National Death Index) mortality database due to incomplete case information at the time the registry data were sent for linkage were also excluded (n=267).
For all patients included in this study, BMI was calculated by dividing weight (in kilograms) by the square of the height (in meters). Patients were classified into the following 6 groups by their BMI as per the World Health Organization Classification System: underweight (BMI<18.5 kg/m2), normal weight (BMI 18.5‐24.9 kg/m2), overweight (BMI 25‐29.9 kg/m2), class I obese (BMI 30‐34.9 kg/m2), class II obese (BMI 35‐39.9 kg/m2), and class III obese (BMI≥40 kg/m2).21 However, due to the very small sample size in the underweight group (n=232), which is likely to make comparisons with the other groups imprecise, these patients were excluded in deriving our final study cohort.
The MIG registry is a multicenter Australian PCI registry that collects data from 6 participating hospitals, 4 of which are located in metropolitan Melbourne, and 2 hospitals are located in large regional centers.22 Baseline demographic, clinical, procedural, and in‐hospital outcome data are prospectively recorded on case‐report forms using standardized definitions for all fields (Table S1). Relevant information for 30‐day outcomes was obtained through telephone follow‐up with further review of medical records performed in patients who reported any events.23 In addition, mortality data were obtained by linkage to the Australian NDI, a database housed at the Australian Institute of Health and Welfare that contains records of all deaths occurring in Australia since 1980. The censoring date for linkage with the NDI in this study was August 1, 2017. Successful matching of patients through this linkage process was achieved in 99.0% of all patients in the study cohort. The MIG registry has an “opt‐out” consent process as previously described and has been granted ethics approval by the ethics committee at The Alfred Hospital (approval number 92/04) as well as by committees at each participating hospital.22, 23
Baseline and procedural characteristics, as well as in‐hospital and 30‐day outcomes, were compared among the groups. The primary end point was NDI‐linked long‐term mortality. Secondary end points included death (all‐cause mortality and cardiac mortality), myocardial infarction, target vessel revascularization and major adverse cardiovascular events at 30‐day follow‐up. Major adverse cardiovascular events were defined as a composite of death, myocardial infarction, and target vessel revascularization. Major bleeding was defined as a fall in hemoglobin by >3.0 g/dL and/or requiring transfusion. Use of antiplatelet therapy, β‐blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, and cholesterol‐lowering therapies (statins, fibrates, and ezetimibe) at 30 days after the index PCI was also compared among the groups. Prescription of postdischarge medications was at the discretion of the treating physician according to contemporary guidelines.
Continuous variables are expressed as mean±SD and were compared using a Kruskal‐Wallis equality‐of‐populations rank test. Categorical data are expressed as numbers and percentages and compared using the Pearson chi‐squared test or Fisher exact test as appropriate. The Kaplan‐Meier method was used to estimate post‐PCI survival rates, and the log‐rank test was used for survival comparisons. Cox proportional hazard modeling was used to identify independent predictors of the primary end point of NDI‐linked long‐term mortality. In this model in addition to BMI group, 28 other clinically relevant variables such as sex, cardiovascular risk factors including diabetes mellitus, hypertension, and renal impairment, history of previous myocardial infarction and/or previous stroke, disease extent on angiography, and type of stent used were considered. Aside from the BMI group, only variables with a P<0.10 on univariate analysis that were not collinear were entered into a stepwise backward selection modeling process for multivariable assessment. Complete case analysis was performed for purposes of multivariable modeling (ie, patients with missing values were excluded). The proportion of missing variables was <1% for all variables except smoking status (1.6%), estimated glomerular filtration rate (3.9%), family history of coronary artery disease (4.5%), 30‐day medications (7.3%), and left ventricular ejection fraction (11.7%).
All statistical analyses were performed using Stata 13.1 software (StataCorp LP, College Station, TX). P<0.05 was considered to be statistically significant.
Results
In total, 25 413 patients were included in this analysis. Of these, 6305 (24.6%) were in the normal BMI category, 10 608 (41.4%) were overweight, 5780 (22.5%) had mild (class I) obesity, 1874 (7.3%) had moderate (class II) obesity, and 846 (3.3%) had extreme (class III) obesity. The mean age of the whole study cohort was 64.2±12.0 years, and 23.0% were female.
Baseline Characteristics
Table 1 shows the baseline characteristics of the study cohort stratified by BMI groups. With increasing BMI, patients were younger and had more cardiovascular risk factors such as diabetes mellitus (all P<0.001). The proportion of women was highest at both extremes of BMI and lowest in the overweight group. With increasing BMI, the proportion of patients who presented with non–ST‐elevation acute coronary syndromes increased, whereas the proportion of patients who presented with ST‐elevation myocardial infarction, out‐of‐hospital cardiac arrest, and cardiogenic shock decreased (P≤0.001).
Table 1.
Baseline Characteristics
| BMI 18.5 to 24.9 kg/m2 | BMI 25.0 to 29.9 kg/m2 | BMI 30.0 to 34.9 kg/m2 | BMI 35.0 to 39.9 kg/m2 | BMI ≥40 kg/m2 | P for Trend | |
|---|---|---|---|---|---|---|
| N (%) | 6305 (24.6) | 10 608 (41.4) | 5780 (22.5) | 1874 (7.3) | 846 (3.3) | |
| Mean age±SD, y | 67.0±12.4 | 64.4±11.8 | 62.7±11.6 | 61.0±10.7 | 59.2±10.7 | <0.001 |
| Age >80 years | 966 (15.3) | 944 (8.9) | 340 (5.9) | 56 (3.0) | 19 (2.3) | <0.001 |
| Female | 1664 (26.4) | 1967 (18.5) | 1281 (22.2) | 586 (31.3) | 354 (41.8) | <0.001 |
| Diabetes mellitus | 1022 (16.2) | 2419 (22.8) | 1735 (30.0) | 784 (41.8) | 381 (45.0) | <0.001 |
| Hypertension | 3731 (59.2) | 6848 (64.6) | 4233 (73.3) | 1475 (78.8) | 675 (79.8) | <0.001 |
| Dyslipidemia | 3836 (61.0) | 7081 (66.9) | 4140 (71.6) | 1385 (74.0) | 611 (72.4) | <0.001 |
| Current or past smoker | 4016 (64.7) | 6975 (66.7) | 4001 (70.5) | 1262 (68.4) | 585 (70.1) | <0.001 |
| Family history of coronary artery disease | 2096 (34.7) | 3939 (38.9) | 2236 (40.6) | 726 (40.7) | 370 (46.1) | <0.001 |
| eGFR >60 mL/min per 1.73 m2 | 4594 (75.9) | 8019 (79.0) | 4346 (77.7) | 1400 (77.7) | 602 (74.1) | 0.694 |
| eGFR 30 to 60 mL/min per 1.73 m2 | 1242 (20.5) | 1864 (18.4) | 1108 (19.8) | 355 (19.7) | 181 (22.3) | |
| eGFR <30 mL/min per 1.73 m2 | 216 (3.6) | 262 (2.6) | 143 (2.6) | 48 (2.7) | 30 (3.7) | |
| Chronic obstructive pulmonary disease | 505 (8.0) | 545 (5.1) | 341 (5.9) | 109 (5.8) | 53 (6.3) | <0.001 |
| Obstructive sleep apnea | 98 (1.6) | 305 (2.9) | 366 (6.3) | 245 (13.1) | 175 (20.7) | <0.001 |
| Peripheral vascular disease | 439 (7.0) | 547 (5.2) | 347 (6.0) | 109 (5.8) | 36 (4.3) | 0.007 |
| Previous stroke | 427 (6.8) | 547 (5.2) | 320 (5.5) | 108 (5.8) | 51 (6.0) | 0.072 |
| Previous myocardial infarction | 1563 (24.8) | 2717 (25.6) | 1600 (27.7) | 535 (28.6) | 236 (28.0) | <0.001 |
| Previous percutaneous coronary intervention | 1543 (24.5) | 2734 (25.8) | 1610 (27.9) | 533 (28.4) | 227 (26.8) | <0.001 |
| Previous coronary artery bypass graft surgery | 457 (7.3) | 880 (8.3) | 488 (8.4) | 179 (9.6) | 49 (5.8) | 0.107 |
| Clinical presentation | ||||||
| Stable angina | 1832 (29.1) | 3597 (33.9) | 2075 (35.9) | 708 (37.8) | 264 (31.2) | <0.001 |
| Unstable angina | 513 (8.1) | 870 (8.2) | 448 (7.8) | 163 (8.7) | 66 (7.8) | 0.856 |
| NSTEMI | 1778 (28.2) | 2821 (26.6) | 1700 (29.4) | 569 (30.4) | 291 (34.4) | <0.001 |
| STEMI | 2180 (34.6) | 3318 (31.3) | 1554 (26.9) | 434 (23.2) | 224 (26.5) | <0.001 |
| Cardiogenic shock | 257 (4.1) | 286 (2.7) | 145 (2.5) | 32 (1.7) | 21 (2.5) | <0.001 |
| Out‐of‐hospital cardiac arrest | 200 (3.2) | 315 (3.0) | 148 (2.6) | 38 (2.0) | 18 (2.1) | 0.001 |
| Mean LV ejection fraction±SD | 52.2±11.0 | 52.8±10.4 | 53.2±9.9 | 53.4±9.8 | 53.5±9.9 | <0.001 |
| LV ejection fraction <30% | 130 (2.3) | 157 (1.7) | 62 (1.2) | 22 (1.4) | 8 (1.1) | <0.001 |
| LV ejection fraction 30% to 45% | 1295 (2.3) | 157 (1.7) | 62 (1.2) | 22 (1.4) | 8 (1.1) | |
| LV ejection fraction >45% | 4236 (74.8) | 7318 (77.8) | 4001 (79.5) | 1289 (80.9) | 598 (81.7) | |
Data expressed as mean±SD or numbers (%).BMI indicates body mass index; eGFR, estimated glomerular filtration rate; LV, left ventricular; NSTEMI, non–ST‐elevation myocardial infarction; STEMI, ST‐elevation myocardial infarction.
Procedual characteristics are shown in Table 2. As BMI increased, there was more radial access and less femoral access use (P<0.001). There were no significant differences in extent of coronary artery disease or lesion complexity by BMI group. Drug‐eluting stents were more frequently implanted in the higher BMI groups (P<0.001). Procedural complications such as coronary dissection and perforation were overall infrequent, but less common in the higher BMI groups (both P<0.04), although the overall proportion of unsuccessful PCIs was similar across the BMI groups (P=0.440). There was also a reduction in the proportion of patients with severe left ventricular systolic dysfunction (left ventricular ejection fraction <30%) at the time of PCI, with increasing BMI (P<0.001).
Table 2.
Procedural Characteristics
| BMI 18.5 to 24.9 kg/m2 | BMI 25.0 to 29.9 kg/m2 | BMI 30.0 to 34.9 kg/m2 | BMI 35.0 to 39.9 kg/m2 | BMI ≥40 kg/m2 | P for Trend | |
|---|---|---|---|---|---|---|
| Lesion characteristics | ||||||
| Multivessel disease | 3712 (59.0) | 6248 (59.0) | 3383 (58.6) | 1085 (58.1) | 461 (54.6) | 0.054 |
| Left main lesion | 89 (1.2) | 135 (1.1) | 76 (1.1) | 22 (1.0) | 7 (0.7) | 0.243 |
| Chronic total occlusion lesion | 229 (3.1) | 480 (3.8) | 269 (4.0) | 98 (4.5) | 37 (3.7) | 0.003 |
| ACC/AHA type B2/C lesion | 4221 (56.2) | 7075 (56.2) | 3804 (56.1) | 1304 (59.2) | 566 (56.3) | 0.206 |
| Procedural details | ||||||
| Radial access | 1434 (22.7) | 2675 (25.2) | 1594 (27.6) | 549 (29.3) | 290 (34.3) | <0.001 |
| Femoral access | 4871 (77.3) | 7932 (74.8) | 4186 (72.4) | 1325 (70.7) | 556 (65.7) | |
| Arterial access closure device used | 645 (10.2) | 1123 (10.6) | 587 (10.2) | 223 (11.9) | 121 (14.3) | 0.004 |
| Balloon angioplasty only | 411 (6.5) | 657 (6.2) | 410 (7.1) | 130 (6.9) | 56 (6.6) | 0.001 |
| Bare metal stent | 2471 (39.2) | 4035 (38.0) | 2089 (36.1) | 628 (33.5) | 308 (36.4) | |
| Drug‐eluting stent | 3423 (54.3) | 5916 (55.8) | 3281 (56.8)) | 1116 (59.6) | 482 (57.0) | |
| Intra‐aortic balloon pump use | 136 (2.2) | 177 (1.7) | 82 (1.4) | 17 (0.9) | 9 (1.1) | <0.001 |
| Thrombectomy device used | 520 (8.0) | 884 (8.0) | 406 (6.8) | 105 (5.4) | 49 (5.4) | <0.001 |
| Glycoprotein IIb/IIIa inhibitors | 1854 (29.4) | 2991 (28.2) | 1441 (25.0) | 431 (23.0) | 197 (23.3) | <0.001 |
| Complications | ||||||
| Dissection | 339 (4.5) | 533 (4.2) | 265 (3.9) | 84 (3.8) | 32 (3.2) | 0.004 |
| Perforation | 24 (0.3) | 39 (0.3) | 12 (0.2) | 5 (0.2) | 0 (0.0) | 0.011 |
| Transient/persistent no‐reflow | 280 (3.9) | 350 (2.9) | 200 (3.1) | 58 (2.7) | 32 (3.3) | 0.019 |
| Unsuccessful PCI | 334 (5.3) | 514 (4.9) | 310 (5.4) | 102 (5.4) | 48 (5.7) | 0.440 |
Data expressed as mean±SD, or numbers (%).ACC/AHA indicates American College of Cardiology/American Heart Association; BMI, body mass index; PCI, percutaneous coronary intervention.
Clinical Outcomes
In‐hospital and 30‐day outcomes are shown in Table 3. A 30‐day follow‐up was completed in 99.6% of the study cohort. There was a J‐shaped association between BMI and both in‐hospital and 30‐day mortality, with a steady fall in mortality from the normal BMI group to the moderate obesity group, followed by a substantial rise in mortality in the extreme obesity group (P<0.001). A similar pattern of association was also seen with in‐hospital and 30‐day major adverse cardiovascular events. With increasing BMI, there was a significant stepwise reduction in in‐hospital bleeding (P<0.001). There were no significant differences in 30‐day readmission rates across the BMI groups.
Table 3.
Clinical Outcomes
| BMI 18.5 to 24.9 kg/m2 | BMI 25.0 to 29.9 kg/m2 | BMI 30.0 to 34.9 kg/m2 | BMI 35.0 to 39.9 kg/m2 | BMI ≥40 kg/m2 | P for Trend | |
|---|---|---|---|---|---|---|
| In‐hospital outcomes | ||||||
| Death | 142 (2.3) | 175 (1.6) | 74 (1.3) | 20 (1.1) | 16 (1.9) | <0.001 |
| Cardiac death | 113 (1.8) | 144 (1.4) | 58 (1.0) | 18 (1.0) | 11 (1.3) | 0.799 |
| Periprocedural myocardial infarction | 76 (1.2) | 113 (1.1) | 60 (1.0) | 20 (1.1) | 6 (0.7) | 0.208 |
| Heart failure | 263 (4.2) | 357 (3.4) | 188 (3.3) | 52 (2.8) | 38 (4.5) | 0.041 |
| Acute kidney injury | 111 (1.8) | 191 (1.8) | 87 (1.5) | 21 (1.1) | 23 (2.7) | 0.591 |
| Major bleeding | 208 (3.3) | 196 (1.9) | 107 (1.9) | 24 (1.3) | 10 (1.2) | <0.001 |
| Stroke | 24 (0.4) | 22 (0.2) | 22 (0.4) | 4 (0.2) | 1 (0.1) | 0.380 |
| Target vessel revascularization | 77 (1.2) | 115 (1.1) | 69 (1.2) | 19 (1.0) | 11 (1.3) | 0.931 |
| MACE | 265 (4.2) | 374 (3.5) | 181 (3.1) | 58 (3.1) | 31 (3.7) | 0.007 |
| 30‐day outcomes | ||||||
| Death | 177 (2.8) | 211 (2.0) | 95 (1.7) | 24 (1.3) | 21 (2.5) | <0.001 |
| Cardiac death | 133 (2.1) | 161 (1.5) | 71 (1.2) | 21 (1.1) | 13 (1.5) | 0.774 |
| Myocardial infarction | 129 (2.1) | 175 (1.7) | 104 (1.8) | 30 (1.6) | 8 (1.0) | 0.045 |
| Stroke | 38 (0.6) | 33 (0.3) | 25 (0.4) | 8 (0.4) | 1 (0.1) | 0.084 |
| Target vessel revascularization | 146 (2.3) | 234 (2.2) | 133 (2.3) | 52 (2.8) | 20 (2.4) | 0.452 |
| Any readmission | 751 (12.3) | 1084 (10.5) | 653 (11.6) | 219 (12.0) | 93 (11.3) | 0.610 |
| MACE | 375 (6.0) | 527 (5.0) | 276 (4.8) | 88 (4.7) | 42 (5.0) | 0.008 |
| NDI‐linked mortality | ||||||
| No. of deaths | 1195 (19.2) | 1423 (13.5) | 751 (13.1) | 225 (12.2) | 118 (14.1) | <0.001 |
| Median follow‐up time (IQR), y | 4.4 (2.0‐7.5) | 4.5 (2.0‐7.7) | 4.4 (2.0‐7.6) | 4.1 (2.0‐7.0) | 3.9 (1.5‐6.9) | 0.047 |
Data expressed as median (IQR) or numbers (%). BMI indicates body mass index; IQR, interquartile range; MACE, major adverse cardiovascular events; NDI, national death index.
All‐cause mortality data beyond 30 days were obtained using linkage with the NDI database. Median length of follow‐up was 4.4 years (IQR 2.0‐7.6 years) overall and similar in all the groups (P=0.047). Patients with moderate obesity had the lowest mortality rate (12.2%), whereas patients both with normal BMI and extreme obesity were found to have a higher mortality rate (19.2% and 14.1% respectively). The Kaplan‐Meier survival curves for the 5 BMI groups are shown in Figure 1, and they confirm that patients with extreme obesity had significantly lower long‐term survival, compared to the other groups (log rank P<0.001). Using Cox‐proportional hazards modelling with the normal BMI group as the reference category, a J‐shaped association between BMI and adjusted hazard ratio for NDI‐linked long‐term mortality was observed (Figure 2). The adjusted hazard ratio (HR) was highest for patients with extreme obesity (HR 1.33, 95% CI 1.07‐1.65). Being overweight and having mild obesity both appeared to be protective for long‐term NDI‐linked mortality, with the latter group having the lowest adjusted hazard ratio. The 3 strongest independent predictors of NDI‐linked long‐term mortality were stage 4 to 5 chronic kidney disease, cardiogenic shock, and severe left ventricular systolic dysfunction (HR 3.46, 2.98 and 2.50 respectively; all P<0.001) (Table 4).
Figure 1.
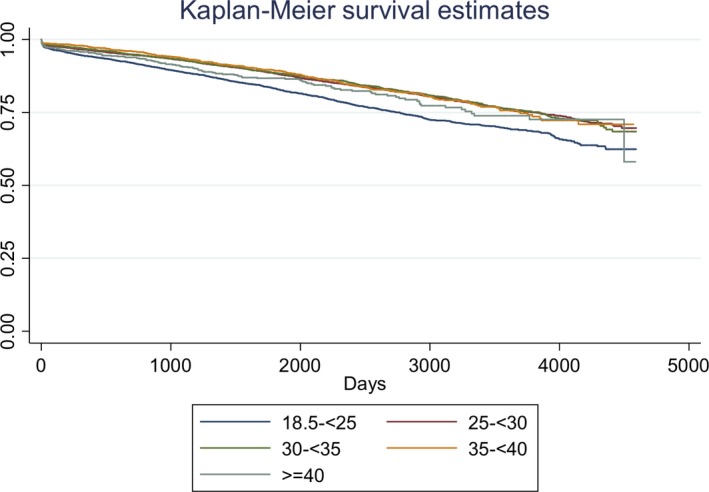
Kaplan‐Meier curves of long‐term survival by body mass index group.
Figure 2.
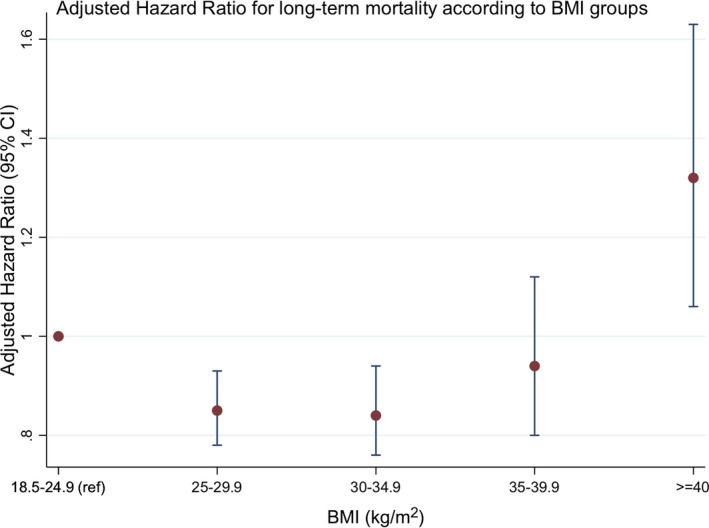
Adjusted hazard ratios for NDI‐linked mortality according to body mass index groups. BMI indicates body mass index; NDI, National Death Index.
Table 4.
Multi‐Variable Cox‐Proportional Hazards Modeling for NDI‐Linked Mortality
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| eGFR | |||
| eGFR >60 mL/min per 1.73 m2 | 1 (ref) | ||
| eGFR 30 to 60 mL/min per 1.73 m2 | 1.45 | 1.33 to 1.58 | <0.001 |
| eGFR <30 mL/min per 1.73 m2 | 3.46 | 3.03 to 3.95 | <0.001 |
| Cardiogenic shock | 2.98 | 2.57 to 3.44 | <0.001 |
| Left ventricular ejection fraction | |||
| Left ventricular ejection fraction >45% | 1 (ref) | ||
| Left ventricular ejection fraction 30% to 45% | 1.57 | 1.44 to 1.70 | <0.001 |
| Left ventricular ejection fraction <30% | 2.50 | 2.12 to 2.94 | <0.001 |
| Chronic obstructive airways disease | 2.11 | 1.90 to 2.34 | <0.001 |
| Out‐of‐hospital cardiac arrest | 1.76 | 1.47 to 2.10 | <0.001 |
| BMI category | |||
| BMI 18.5 to 24.9 kg/m2 | 1 (ref) | ||
| BMI 25.0 to 29.9 kg/m2 | 0.85 | 0.78 to 0.93 | <0.001 |
| BMI 30.0 to 34.9 kg/m2 | 0.85 | 0.76 to 0.94 | 0.002 |
| BMI 35.0 to 39.9 kg/m2 | 0.95 | 0.80 to 1.12 | 0.543 |
| BMI ≥40.0 kg/m2 | 1.33 | 1.07 to 1.65 | 0.010 |
| Diabetes mellitus | 1.45 | 1.34 to 1.57 | <0.001 |
| Peripheral vascular disease | 1.44 | 1.29 to 1.60 | <0.001 |
| Obstructive sleep apnea | 1.39 | 1.19 to 1.63 | <0.001 |
| Previous coronary artery bypass graft surgery | 1.37 | 1.17 to 1.60 | <0.001 |
| Previous stroke | 1.35 | 1.21 to 1.51 | <0.001 |
| Left main disease | 1.31 | 1.04 to 1.64 | 0.023 |
| Multivessel disease | 1.25 | 1.16 to 1.36 | <0.001 |
| Previous myocardial infarction | 1.19 | 1.10 to 1.30 | <0.001 |
| Hypertension | 1.11 | 1.01 to 1.22 | 0.034 |
| Age (per year increase) | 1.06 | 1.05 to 1.06 | <0.001 |
| Drug‐eluting stent use | 0.79 | 0.73 to 0.85 | <0.001 |
BMI indicates body mass index; eGFR, estimated glomerular filtration rate; NDI, National Death Index.
Secondary Prevention Therapy
At 30 days post PCI, there were no significant differences in use of aspirin, a second antiplatelet agent, or statins across the BMI groups (all P>0.05) (Table 5). However, patients with normal BMI were significantly less likely to receive a β‐blocker or angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker compared with the other BMI groups (P=0.003 and P<0.001 respectively).
Table 5.
Medication Use at 30‐Day Follow‐Up
| BMI 18.5 to 24.9 kg/m2 | BMI 25.0 to 29.9 kg/m2 | BMI 30.0 to 34.9 kg/m2 | BMI 35.0 to 39.9 kg/m2 | BMI ≥40 kg/m2 | P for Trend | |
|---|---|---|---|---|---|---|
| Aspirin | 5688 (97.7) | 9690 (97.4) | 5299 (97.6) | 1733 (98.1) | 758 (96.3) | 0.628 |
| Clopidogrel/prasugrel/ticagrelor | 5592 (96.1) | 9590 (96.4) | 5188 (95.5) | 1688 (95.6) | 751 (95.6) | 0.045 |
| β‐Blocker | 4494 (77.7) | 7827 (79.2) | 4295 (80.0) | 1410 (80.5) | 617 (79.4) | 0.003 |
| ACEi/ARB | 4340 (75.0) | 7792 (78.8) | 4424 (82.2) | 1491 (85.1) | 647 (83.3) | <0.001 |
| Statin | 5450 (94.2) | 9403 (95.1) | 5096 (94.5) | 1673 (95.3) | 747 (95.5) | 0.119 |
Data expressed as numbers (%). ACEi indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; BMI, body mass index.
Discussion
In this large, multicenter study evaluating the relationship between BMI and long‐term mortality in patients undergoing PCI, we observed a J‐shaped association between different BMI groups and adjusted mortality risk, with patients at the extremes of BMI experiencing the highest risk. Although an obesity paradox was present with underweight patients having the highest mortality out of all of the groups, it only extended as far as patients with mild obesity. Therefore, patients with extreme obesity remain at significantly increased risk of long‐term mortality compared with their healthy weight and less obese counterparts.
The results of our study provide important additional insights to the literature regarding outcomes after PCI in patients with varying BMI. Our results are in accordance with several large studies that have demonstrated a similar association between in‐hospital mortality and BMI group.12, 17, 19 A feature of our study is that very few earlier studies have assessed mortality rates beyond 12 months in extremely obese patients undergoing PCI for both stable angina and acute coronary syndromes. Holroyd et al evaluated mortality up to 5 years after PCI in over 300 000 patients and also found that patients who were overweight and obese (BMI >30 kg/m2) had reduced mortality risk up to 5 years compared with those with normal BMI.16 However, the authors did not further subdivide obese patients further into degrees of obesity, and therefore no conclusions can be made as to whether extreme obesity remains protective. Interestingly, a subgroup analysis on 15 603 patients who underwent PCI and were enrolled in the Canadian APPROACH registry with a median follow‐up of 46 months showed that whereas underweight patients had the highest adjusted mortality risk and moderate obesity was protective, those with extreme obesity had very similar adjusted mortality risk to their normal weight counterparts.20 It is however difficult to make comparisons with our study to understand reasons behind this difference in outcomes as baseline or procedural characteristics of the PCI subgroup were not presented separately, and medication use data were only available for 12% of the whole cohort (including those not treated with PCI). However, a similar neutral effect of severe obesity (defined as BMI ≥35 kg/m2) compared with normal weight on cardiovascular mortality risk after percutaneous or surgical revascularization was also seen in a recent meta‐analysis by Sharma et al, suggesting that further large studies in this area are required.24
Several possible mechanisms for the obesity paradox have been postulated. In accordance with previous studies, our data show that there was a linear relationship between BMI and the prevalence of comorbidities such as diabetes mellitus, hypertension, and dyslipidemia. However, patients with higher BMI may be more likely to have been screened earlier and aggressively treated for these cardiovascular risk factors, thereby leading to better long‐term outcomes despite obesity.25 Overweight and mild‐to‐moderately obese patients were also less likely to present with cardiogenic shock and post–out‐of‐hospital cardiac arrest, factors that are usually associated with poorer outcomes.26, 27 Similar to other studies, in‐hospital major bleeding complications were also lower in overweight and obese patients, which is likely at least in part due to the increased use of radial access in these patients.16 Excess dosing of anticoagulant and antiplatelet drugs is also potentially less likely to occur in more obese patients, which may also reduce their bleeding risk. Bleeding has been shown to be independently associated with worse short‐ and long‐term mortality and therefore may explain our results to some extent.28
In our study we also found that increased BMI up to the level of moderate obesity was associated with an increased use of guideline‐based medical therapy, in particular β‐blockers, renin‐angiotensin‐system blockers, and statins. Previous studies have shown that increased use of evidence‐based cardiovascular medications is associated with lower long‐term mortality after PCI.29 Nonpharmacological measures such as smoking cessation, dietary counseling, and cardiac rehabilitation referral have been shown to be employed more frequently in overweight and obese patients as well, which could also account for the improved outcomes.30, 31
With an increase in the proportion of overweight and obese individuals in the general population as well as in those undergoing PCI, it has also been proposed that the worse prognosis observed in patients with normal BMI may be due to the effect of residual confounding.17, 19 Given that 67% of the Australian population are overweight or obese, even having a normal BMI may potentially reflect the presence of unmeasured serious comorbidities that carry substantial mortality hazard.32 Previous studies have indeed shown that patients with low BMI have higher rates of noncardiac mortality.33, 34 In our study we also observed an inverse relationship between BMI and the presence of comorbidities such as chronic obstructive pulmonary disease and peripheral vascular disease. However, we were unable to account for the prevalence of serious conditions such as cancer, dementia, malnutrition, and overall measures of frailty, which could explain the higher mortality in patients even with normal BMI.35
Finally, there is also evidence that adipose tissue may itself have potentially cardioprotective effects by producing hormones such as leptin and adiponectin.36 These hormones have anti‐inflammatory and antiapoptotic properties and might reduce infarct size.37, 38 Obesity‐inducing high‐fat diets in rats have also been shown to be cardioprotective.39 Obesity may also be protective against malnutrition following a major cardiac event or procedure.40 However, the increase in mortality seen in patients with extreme, class III obesity suggests that there is likely a threshold effect. Therefore, as BMI increases to over 40 kg/m2, the protective effects of milder degrees of obesity may be abrogated by the deleterious effects of extreme obesity including alterations in cardiac structure and function, potentiation of an inflammatory and prothrombotic state, and increased noncardiovascular mortality.41, 42, 43 This may explain why the obesity paradox did not extend to the extremely obese in several studies including ours.17, 44
Limitations
Our study has several limitations. First, due to the retrospective design of this study, we were unable to account for all potential confounding factors such as socioeconomic status, noncardiac comorbidities such as cancer, as well as measures of frailty, which can all potentially affect post‐PCI short‐ and long‐term mortality. Second, BMI measured at the time of PCI might not necessarily reflect BMI at the time of linkage with the NDI, which was on average 4 years after the index PCI procedure. It is also not known how dynamic weight changes might impact clinical outcomes among patients whose weight had changed between the index PCI and the time of NDI linkage. Third, we did not capture measures of central adiposity such as waist circumference and waist‐to‐hip ratio, which have been shown to be better predictors of cardiovascular outcomes than BMI alone.45, 46 However, BMI is the measurement used and endorsed by the World Health Organization to classify obesity worldwide given its simple and easily quantifiable nature, and it was therefore chosen for this study. Finally, we did not have data on the use of guideline‐recommended secondary prevention therapy beyond 30 days after PCI, which might also have explained some of the differences in mortality among BMI groups.40
Conclusions
In conclusion, there remains an obesity paradox with regard to long‐term mortality in patients undergoing PCI in contemporary practice, with mildly obese patients having the lowest adjusted mortality hazard. However, this protective effect does not extend to patients with extreme obesity. Factors behind this phenomenon are likely multifactorial and require further mechanistic and epidemiological studies.
Sources of Funding
Dr Biswas is supported by scholarships from the National Heart Foundation (NHF) of Australia (reference no. 101518), National Health and Medical Research Council of Australia (NHMRC) Cardiovascular Centre of Research Excellence in Cardiovascular Outcomes Improvement (CRE‐COI), and the Australian Government Research Training Program. Dr Noaman is supported by a scholarship from the NHMRC CRE‐COI. Professor Duffy's work is supported by a NHMRC grant (reference no. 1111170). Professor Reid is supported by a NHMRC Principal Research Fellowship (reference no. 11136372). Associate Professor Stub is supported by a NHF Future Leader Fellowship (reference no. 101908) and a Viertel Foundation Clinical Investigator award. Associate Professor Chan is supported by the Alfred Hospital Research Trust and the Harold Cora Brennan Benevolent Trust. The Melbourne Interventional Group acknowledges funding from Abbott, Astra‐Zeneca, Medtronic, MSD, Pfizer, Servier, and The Medicines Company. These companies do not have access to data and do not have the right to review manuscripts or abstracts before publication. Medtronic also assisted in defraying the publication cost of this article with an unrestricted educational grant.
Disclosures
None.
Supporting information
Table S1. Data Dictionary for Variables in Melbourne Interventional Group Registry
Acknowledgments
We thank the Steering Committee and all the investigators and data managers at the institutions that participate in the MIG registry. The MIG Steering Committee consisted of Professor Chris Reid, Associate Professor Andrew Ajani, Professor Stephen Duffy, Associate Professor David Clark, Dr Melanie Freeman, Dr Chin Hiew, Associate Professor Ernesto Oqueli, and Ms Angela Brennan. The following investigators, data managers, and institutions participated in the MIG Database: at Alfred Hospital, S. J. Duffy, J. A. Shaw, A. Walton, A. Dart, A. Broughton, C. Keighley, C. Hengel, K. H. Peter, D. Stub, W. Chan, M. Freilich, N. Htun, R. Prakash, S. Biswas, and L. Selkrig; at Austin Hospital, D. J. Clark, O. Farouque, M. Horrigan, J. Johns, L. Oliver, J. Brennan, R. Chan, G. Proimos, T. Dortimer, B. Chan, R. Huq, D. Fernando, M. Yudi, L. Brown, A. Al‐Fiadh, J. Ramchand, and S. Picardo; at Ballarat Base Hospital, E. Oqueli, A. Sharma, N. Ryan, and C. Barry; at Box Hill Hospital, M. Freeman, J. Cooke, L. Roberts, J. Chandrasekhar, A. Teh, M. Rowe, G. Proimos, Y. Cheong, C. Goods, D. Fernando, L. Marceddo, K. Soon, and D. Natarajan; at Monash University, C. Reid, N. Andrianopoulos, A. L. Brennan, D. Dinh, and B. P. Yan; at Royal Melbourne Hospital, A. E. Ajani, R. Warren, D. Eccleston, J. Lefkovits, R. Iyer, R. Gurvitch, W. Wilson, M. Brooks, and L. P. Dawson; at University Hospital Geelong, C. Hiew, M. Sebastian, T. Yip, M. Mok, C. Jaworski, A. Hutchison, B. McDonald, R. Pavletich, and N. Herbert.
(J Am Heart Assoc. 2019;8:e012860 DOI: 10.1161/JAHA.119.012860.)
References
- 1. World Health Organization . Obesity and Overweight. Fact sheet. 2018. Available at: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed May 3, 2018.
- 2. National Heart Foundation of Australia . Overweight and obesity statistics. 2015. Available at: https://www.heartfoundation.org.au/about-us/what-we-do/heart-disease-in-australia/overweight-and-obesity-statistics. Accessed October 3, 2017.
- 3. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard‐Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. [DOI] [PubMed] [Google Scholar]
- 4. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26‐year follow‐up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. [DOI] [PubMed] [Google Scholar]
- 5. Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F, Krumholz HM. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. [DOI] [PubMed] [Google Scholar]
- 6. Prabhakar G, Haan CK, Peterson ED, Coombs LP, Cruzzavala JL, Murray GF. The risks of moderate and extreme obesity for coronary artery bypass grafting outcomes: a study from the Society of Thoracic Surgeons’ database. Ann Thorac Surg. 2002; 74:1125–1130; discussion 1130–1121. [DOI] [PubMed] [Google Scholar]
- 7. Bhaskaran K, Dos‐Santos‐Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause‐specific mortality: a population‐based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 2018; 6:944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lancefield T, Clark DJ, Andrianopoulos N, Brennan AL, Reid CM, Johns J, Freeman M, Charter K, Duffy SJ, Ajani AE, Proietto J, Farouque O, Registry MIG. Is there an obesity paradox after percutaneous coronary intervention in the contemporary era? An analysis from a multicenter Australian registry. JACC Cardiovasc Interv. 2010;3:660–668. [DOI] [PubMed] [Google Scholar]
- 9. McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, Lane S, Lee DY, Kaper M, McKean M, Beckermann KE, Rubinstein SM, Rooney I, Musib L, Budha N, Hsu J, Nowicki TS, Avila A, Haas T, Puligandla M, Lee S, Fang S, Wargo JA, Gershenwald JE, Lee JE, Hwu P, Chapman PB, Sosman JA, Schadendorf D, Grob JJ, Flaherty KT, Walker D, Yan Y, McKenna E, Legos JJ, Carlino MS, Ribas A, Kirkwood JM, Long GV, Johnson DB, Menzies AM, Davies MA. Association of body‐mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19:310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ellis SG, Elliott J, Horrigan M, Raymond RE, Howell G. Low‐normal or excessive body mass index: newly identified and powerful risk factors for death and other complications with percutaneous coronary intervention. Am J Cardiol. 1996;78:642–646. [DOI] [PubMed] [Google Scholar]
- 11. Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, Ahmed LM, Kent KM, Pichard AD, Suddath WO, Satler LF, Lindsay J Jr. The impact of obesity on the short‐term and long‐term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39:578–584. [DOI] [PubMed] [Google Scholar]
- 12. Minutello RM, Chou ET, Hong MK, Bergman G, Parikh M, Iacovone F, Wong SC. Impact of body mass index on in‐hospital outcomes following percutaneous coronary intervention (report from the New York State Angioplasty Registry). Am J Cardiol. 2004;93:1229–1232. [DOI] [PubMed] [Google Scholar]
- 13. Niedziela J, Hudzik B, Niedziela N, Gasior M, Gierlotka M, Wasilewski J, Myrda K, Lekston A, Polonski L, Rozentryt P. The obesity paradox in acute coronary syndrome: a meta‐analysis. Eur J Epidemiol. 2014;29:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Payvar S, Kim S, Rao SV, Krone R, Neely M, Paladugu N, Daggubati R. In‐hospital outcomes of percutaneous coronary interventions in extremely obese and normal‐weight patients: findings from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2013;62:692–696. [DOI] [PubMed] [Google Scholar]
- 15. Neeland IJ, Das SR, Simon DN, Diercks DB, Alexander KP, Wang TY, de Lemos JA. The obesity paradox, extreme obesity, and long‐term outcomes in older adults with ST‐segment elevation myocardial infarction: results from the NCDR. Eur Heart J Qual Care Clin Outcomes. 2017;3:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holroyd EW, Sirker A, Kwok CS, Kontopantelis E, Ludman PF, De Belder MA, Butler R, Cotton J, Zaman A, Mamas MA. The relationship of body mass index to percutaneous coronary intervention outcomes: does the obesity paradox exist in contemporary percutaneous coronary intervention cohorts? Insights from the British Cardiovascular Intervention Society Registry. JACC Cardiovasc Interv. 2017;10:1283–1292. [DOI] [PubMed] [Google Scholar]
- 17. Das SR, Alexander KP, Chen AY, Powell‐Wiley TM, Diercks DB, Peterson ED, Roe MT, de Lemos JA. Impact of body weight and extreme obesity on the presentation, treatment, and in‐hospital outcomes of 50,149 patients with ST‐segment elevation myocardial infarction: results From the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2011;58:2642–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akin I, Tölg R, Hochadel M, Bergmann MW, Khattab AA, Schneider S, Senges J, Kuck K‐H, Richardt G, Nienaber CA. No evidence of “Obesity Paradox” after treatment with drug‐eluting stents in a routine clinical practice: results from the prospective multicenter German DES.DE (German Drug‐Eluting Stent) Registry. JACC Cardiovasc Interv. 2012; 5:162–169. [DOI] [PubMed] [Google Scholar]
- 19. Buschur ME, Smith D, Share D, Campbell W, Mattichak S, Sharma M, Gurm HS. The burgeoning epidemic of morbid obesity in patients undergoing percutaneous coronary intervention: insight from the Blue Cross Blue Shield of Michigan cardiovascular consortium. J Am Coll Cardiol. 2013;62:685–691. [DOI] [PubMed] [Google Scholar]
- 20. Oreopoulos A, Padwal R, Norris CM, Mullen JC, Pretorius V, Kalantar‐Zadeh K. Effect of obesity on short‐ and long‐term mortality postcoronary revascularization: a meta‐analysis. Obesity (Silver Spring). 2008;16:442–450. [DOI] [PubMed] [Google Scholar]
- 21. World Health Organization . Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation on Obesity. WHO Technical Series. 2000. [PubMed]
- 22. Ajani AE, Szto G, Duffy SJ, Eccleston D, Clark DJ, Lefkovits J, Chew DP, Warren R, Black A, New G, Walton A, Lew R, Shaw J, Horrigan M, Sebastian M, Yan BP, Brennan A, Meehan A, Reid C, Krum H. The foundation and launch of the Melbourne Interventional Group: a collaborative interventional cardiology project. Heart Lung Circ. 2006;15:44–47. [DOI] [PubMed] [Google Scholar]
- 23. Chan W, Clark DJ, Ajani AE, Yap CH, Andrianopoulos N, Brennan AL, Dinh DT, Shardey GC, Smith JA, Reid CM, Duffy SJ. Progress towards a National Cardiac Procedure Database–development of the Australasian Society of Cardiac and Thoracic Surgeons (ASCTS) and Melbourne Interventional Group (MIG) registries. Heart Lung Circ. 2011;20:10–18. [DOI] [PubMed] [Google Scholar]
- 24. Sharma A, Vallakati A, Einstein AJ, Lavie CJ, Arbab‐Zadeh A, Lopez‐Jimenez F, Mukherjee D, Lichstein E. Relationship of body mass index with total mortality, cardiovascular mortality, and myocardial infarction after coronary revascularization: evidence from a meta‐analysis. Mayo Clin Proc. 2014;89:1080–1100. [DOI] [PubMed] [Google Scholar]
- 25. Molenaar EA, Hwang SJ, Vasan RS, Grobbee DE, Meigs JB, D'Agostino RB Sr, Levy D, Fox CS. Burden and rates of treatment and control of cardiovascular disease risk factors in obesity: the Framingham Heart Study. Diabetes Care. 2008;31:1367–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kunadian V, Qiu W, Ludman P, Redwood S, Curzen N, Stables R, Gunn J, Gershlick A; National Institute for Cardiovascular Outcomes Research . Outcomes in patients with cardiogenic shock following percutaneous coronary intervention in the contemporary era: an analysis from the BCIS database (British Cardiovascular Intervention Society). JACC Cardiovasc Interv. 2014; 7:1374–1385. [DOI] [PubMed] [Google Scholar]
- 27. Lim HS, Stub D, Ajani AE, Andrianopoulos N, Reid CM, Charter K, Black A, Smith K, New G, Chan W, Lim CC, Farouque O, Shaw J, Brennan A, Duffy SJ, Clark DJ. Survival in patients with myocardial infarction complicated by out‐of‐hospital cardiac arrest undergoing emergency percutaneous coronary intervention. Int J Cardiol. 2013;166:425–430. [DOI] [PubMed] [Google Scholar]
- 28. Kwok CS, Khan MA, Rao SV, Kinnaird T, Sperrin M, Buchan I, de Belder MA, Ludman PF, Nolan J, Loke YK, Mamas MA. Access and non‐access site bleeding after percutaneous coronary intervention and risk of subsequent mortality and major adverse cardiovascular events: systematic review and meta‐analysis. Circ Cardiovasc Interv. 2015; 8:pii: e001645. [DOI] [PubMed] [Google Scholar]
- 29. Jaber WA, Lennon RJ, Mathew V, Holmes DR Jr, Lerman A, Rihal CS. Application of evidence‐based medical therapy is associated with improved outcomes after percutaneous coronary intervention and is a valid quality indicator. J Am Coll Cardiol. 2005;46:1473–1478. [DOI] [PubMed] [Google Scholar]
- 30. Mongraw‐Chaffin ML, Peters SAE, Huxley RR, Woodward M. The sex‐specific association between BMI and coronary heart disease: a systematic review and meta‐analysis of 95 cohorts with 1.2 million participants. Lancet Diabetes Endocrinol. 2015; 3:437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diercks DB, Roe MT, Mulgund J, Pollack CV Jr, Kirk JD, Gibler WB, Ohman EM, Smith SC Jr, Boden WE, Peterson ED. The obesity paradox in non‐ST‐segment elevation acute coronary syndromes: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association Guidelines Quality Improvement Initiative. Am Heart J. 2006;152:140–148. [DOI] [PubMed] [Google Scholar]
- 32. Australian Bureau of Statistics . National Health Survey First results, Australia 2017‐2018. ABS Catalogue No. 4364.0.55.001. 2018. Available at: https://www.ausstats.abs.gov.au/ausstats/subscriber.nsf/0/4B3976684C09F43FCA258399001CE630/$File/4364.0.55.001%20-%20national%20health%20survey,%20first%20results,%202017-18.pdf. Accessed June 15, 2019.
- 33. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW Jr. Body‐mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999; 341:1097–1105. [DOI] [PubMed] [Google Scholar]
- 34. Tremblay A, Bandi V. Impact of body mass index on outcomes following critical care. Chest. 2003;123:1202–1207. [DOI] [PubMed] [Google Scholar]
- 35. Wada H, Dohi T, Miyauchi K, Doi S, Naito R, Konishi H, Tsuboi S, Ogita M, Kasai T, Hassan A, Okazaki S, Isoda K, Suwa S, Daida H. Prognostic impact of the geriatric nutritional risk index on long‐term outcomes in patients who underwent percutaneous coronary intervention. Am J Cardiol. 2017; 119:1740–1745. [DOI] [PubMed] [Google Scholar]
- 36. Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–2971. [DOI] [PubMed] [Google Scholar]
- 37. Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. [DOI] [PubMed] [Google Scholar]
- 38. Smith CC, Mocanu MM, Davidson SM, Wynne AM, Simpkin JC, Yellon DM. Leptin, the obesity‐associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol. 2006;149:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Salie R, Huisamen B, Lochner A. High carbohydrate and high fat diets protect the heart against ischaemia/reperfusion injury. Cardiovasc Diabetol. 2014;13:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bundhun PK, Li N, Chen MH. Does an obesity paradox really exist after cardiovascular intervention? A systematic review and meta‐analysis of randomized controlled trials and observational studies. Medicine (Baltimore). 2015;94:e1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. [DOI] [PubMed] [Google Scholar]
- 42. De Pergola G, Pannacciulli N. Coagulation and fibrinolysis abnormalities in obesity. J Endocrinol Invest. 2002;25:899–904. [DOI] [PubMed] [Google Scholar]
- 43. Cottam DR, Mattar SG, Barinas‐Mitchell E, Eid G, Kuller L, Kelley DE, Schauer PR. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg. 2004;14:589–600. [DOI] [PubMed] [Google Scholar]
- 44. Angeras O, Albertsson P, Karason K, Ramunddal T, Matejka G, James S, Lagerqvist B, Rosengren A, Omerovic E. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J. 2013;34:345–353. [DOI] [PubMed] [Google Scholar]
- 45. Myint PK, Kwok CS, Luben RN, Wareham NJ, Khaw KT. Body fat percentage, body mass index and waist‐to‐hip ratio as predictors of mortality and cardiovascular disease. Heart. 2014;100:1613–1619. [DOI] [PubMed] [Google Scholar]
- 46. Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjonneland A, Halkjaer J, Jensen MK, Stegger J, Clavel‐Chapelon F, Boutron‐Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PH, May AM, Bueno‐de‐Mesquita HB, van Duijnhoven FJ, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quiros JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Data Dictionary for Variables in Melbourne Interventional Group Registry


