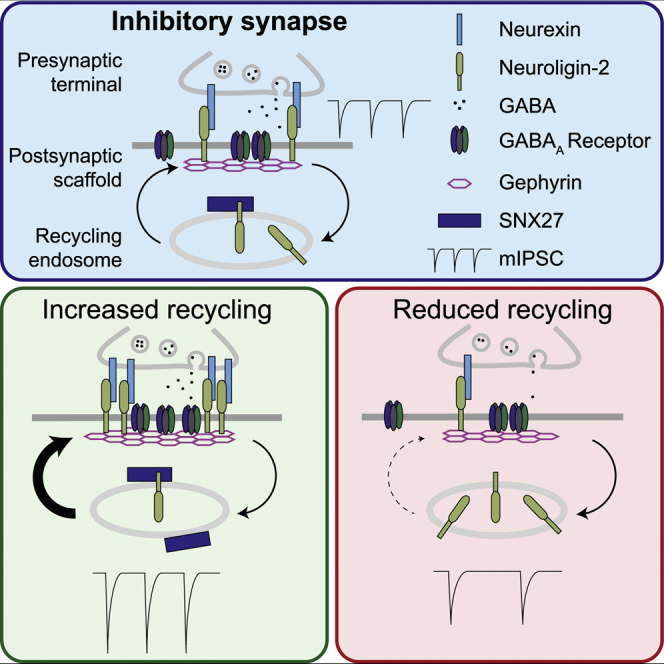
Summary
GABAA receptors mediate fast inhibitory transmission in the brain, and their number can be rapidly up- or downregulated to alter synaptic strength. Neuroligin-2 plays a critical role in the stabilization of synaptic GABAA receptors and the development and maintenance of inhibitory synapses. To date, little is known about how the amount of neuroligin-2 at the synapse is regulated and whether neuroligin-2 trafficking affects inhibitory signaling. Here, we show that neuroligin-2, when internalized to endosomes, co-localizes with SNX27, a brain-enriched cargo-adaptor protein that facilitates membrane protein recycling. Direct interaction between the PDZ domain of SNX27 and PDZ-binding motif in neuroligin-2 enables membrane retrieval of neuroligin-2, thus enhancing synaptic neuroligin-2 clusters. Furthermore, SNX27 knockdown has the opposite effect. SNX27-mediated up- and downregulation of neuroligin-2 surface levels affects inhibitory synapse composition and signaling strength. Taken together, we show a role for SNX27-mediated recycling of neuroligin-2 in maintenance and signaling of the GABAergic synapse.
Keywords: neuroligin, NLGN2, endocytosis, neuronal plasticity, E/I balance, GABA, retromer, SNX27, inhibitory signaling
Graphical Abstract
Highlights
-
•
Neuroligin-2 internalizes to endosomes and interacts with SNX27 and retromer
-
•
Direct interaction between neuroligin-2 and SNX27 mediates neuroligin-2 recycling
-
•
Modulation of synaptic neuroligin-2 levels affects inhibitory synapse composition
-
•
SNX27-mediated recycling of neuroligin-2 regulates inhibitory signaling strength
To maintain healthy brain function, neurons tightly regulate the balance between excitatory and inhibitory synaptic signaling. Neuroligin-2 is a postsynaptic adhesion protein important for inhibitory synapse function. In this article, Halff et al. describe how the protein SNX27 modulates synaptic levels of neuroligin-2, thereby regulating the strength of inhibitory signaling.
Introduction
Synaptic inhibition is crucial for the correct operation of the brain by controlling the balance between excitation and inhibition of neurons (E/I balance). The major class of inhibitory synapses in the CNS contain gamma-aminobutyric acid (GABA) type A receptors (GABAARs). The postsynaptic adhesion molecule neuroligin-2 (NL2) plays a key role in the development and function of the GABAergic synapse and ensures its correct positioning opposite presynaptic terminals through interacting with presynaptic neurexins (Bemben et al., 2015, Nguyen et al., 2016, Südhof, 2017). NL2, by activating collybistin, drives clustering of a postsynaptic gephyrin scaffold, which stabilizes GABAARs at the synapse (Poulopoulos et al., 2009, Tyagarajan and Fritschy, 2014). To maintain the E/I balance in response to activity, synaptic signaling strength can be rapidly adapted through changing the number of receptors in the postsynaptic domain. This process may involve receptor endocytosis, followed either by recycling and reinsertion into the membrane, or lysosomal degradation for long-term downmodulation (Luscher et al., 2011, Arancibia-Cárcamo et al., 2009). A disruption in these mechanisms can lead to neurological disorders, including epilepsy and autism spectrum disorders (Smith and Kittler, 2010, Bozzi et al., 2018). Whereas the pathways involved in GABAAR endocytosis, degradation, and recycling have extensively been studied (Smith et al., 2012, Smith et al., 2014, Twelvetrees et al., 2010, Heisler et al., 2011, Eckel et al., 2015, Jacob et al., 2008), the molecular mechanisms that regulate NL2 trafficking and the amount of NL2 in synapses remain less well understood.
The brain-enriched protein sorting nexin 27 (SNX27) is an important endosome-cargo adaptor that, through association with retromer (a trimeric complex comprising VPS26, VPS29, and VPS35) and the retromer-associated Wiskott-Aldrich syndrome protein and SCAR homolog (WASH) complex, rescues cargo protein from lysosomal degradation and mediates their retrieval to the plasma membrane (Steinberg et al., 2013, Temkin et al., 2011, Gallon and Cullen, 2015). Among the SNX family of proteins, SNX27 uniquely contains a PDZ (postsynaptic density protein, Drosophila disc large tumor suppressor, and zonula occludens-1 protein) domain, through which it interacts with protein cargo containing a C-terminal PDZ-binding motif (Lunn et al., 2007). SNX27 cargo includes proteins involved in neuronal signaling, such as the β2 adrenergic receptor (Lauffer et al., 2010), AMPA receptors (Loo et al., 2014, Hussain et al., 2014, Temkin et al., 2017), and potassium channels (Lunn et al., 2007). Deficiencies in SNX27 function have been associated with Down syndrome (Wang et al., 2013) and epilepsy (Damseh et al., 2015).
A proteomics study identified NL2 as putative cargo for SNX27 (Steinberg et al., 2013); however, the functional implications of this interaction remain unclear. Here, we investigate trafficking of NL2 and show that SNX27 mediates plasma membrane retrieval of NL2. Through regulating NL2 surface availability, SNX27 function modulates inhibitory synapse composition and, ultimately, contributes to the regulation of inhibitory signaling.
Results
NL2 Internalizes to Recycling Endosomes and Interacts with SNX27 and Retromer
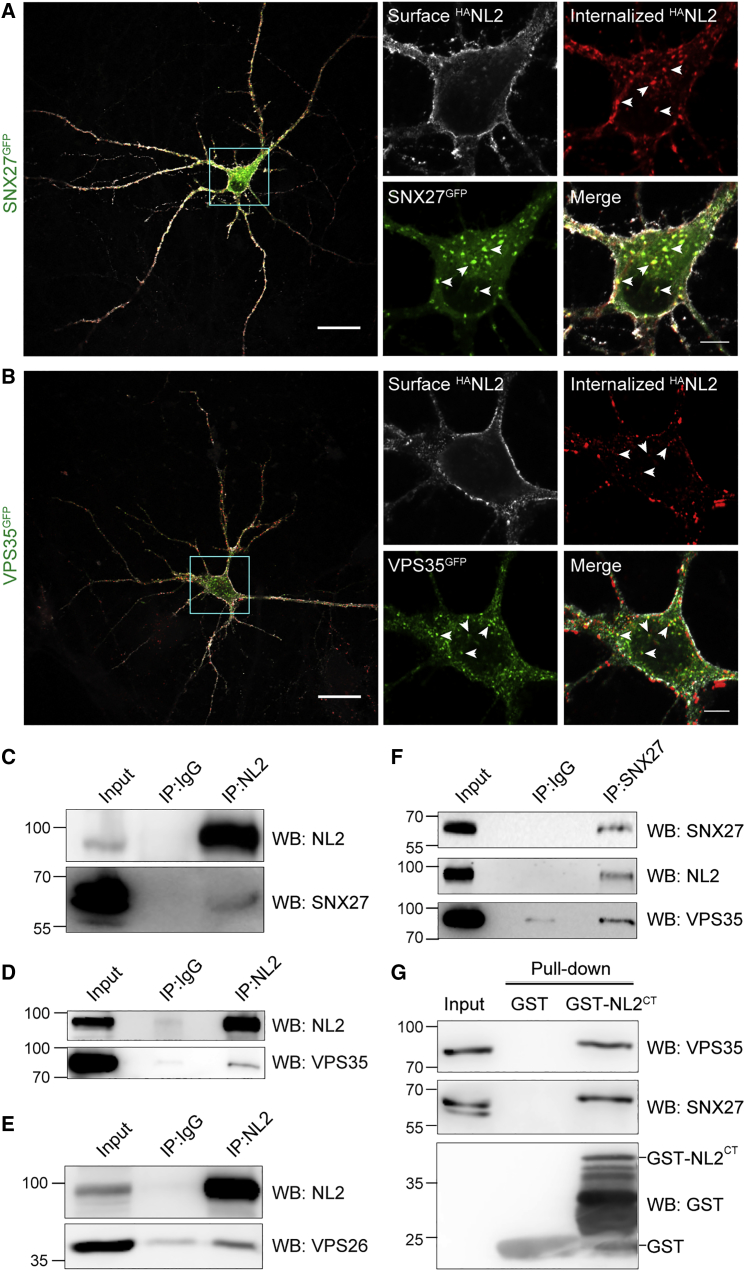
We hypothesized that surface levels of NL2 are regulated through endocytosis and recycling. To investigate trafficking of NL2, we used antibody feeding to follow the internalization of NL2 containing an HA tag at its extracellular N terminus (HANL2). COS-7 cells were co-transfected with HANL2 and dsRed-tagged endocytic markers Rab5 or Rab11. HANL2 readily internalizes and co-localizes with both Rab5dsRed-positive early endosomes and Rab11dsRed-positive recycling endosomes (Figures S1A and S1B), indicating that it may be targeted for recycling. To verify a potential role for the recycling protein SNX27 in NL2 trafficking, we performed antibody feeding in HeLa cells and hippocampal neurons co-expressing HANL2 and GFP-tagged SNX27 (SNX27GFP). Internalized HANL2 co-localizes with SNX27GFP-positive puncta in both cell types (Figures 1A, S1C, and S1D). SNX27 associates with retromer to mediate cargo recycling (Gallon and Cullen, 2015). Accordingly, we find that internalized HANL2 also co-localizes with GFP-tagged retromer component VPS35 (VPS35GFP) in both HeLa cells and neurons (Figures 1B, S1E, and S1F), in agreement with a previous interaction study (Kang et al., 2014).
Figure 1.
Internalized NL2 Co-localizes and Interacts with SNX27 and Retromer
(A and B) Confocal images of antibody feeding in hippocampal neurons co-expressing HANL2 with either SNX27GFP (A) or VPS35GFP (B). Arrowheads show examples of co-localization. Scale bars, 25 μm (whole cell) and 5 μm (soma).
(C–F) Western blots of co-immunoprecipitation from rat brain lysate via endogenous NL2 (C–E) showing interaction with endogenous SNX27 (C), VPS35 (D), and VPS26 (E) or via endogenous SNX27 (F) showing interaction with endogenous NL2 and VPS35. IP, immunoprecipitation. Numbers on the left indicate molecular weight in kDa.
(G) Western blot of GST pull-down from rat brain lysate.
See also Figure S1.
Next, we verified interaction between NL2 and SNX27/retromer at endogenous protein levels. Co-immunoprecipitation experiments from rat brain lysate demonstrate specific interaction of NL2 with SNX27 as well as retromer components VPS35 and VPS26 (Figures 1C–1F and S1G). To test whether this interaction involves the intracellular domain of NL2, we fused residue 699–835 to GST (GST-NL2CT; Figure S1H) and performed a GST-fusion protein pull-down in brain lysate. Both SNX27 and VPS35 interacted with GST-NL2CT but not with GST alone (Figure 1G), confirming direct interaction between SNX27/retromer and the intracellular domain of NL2.
Taken together, these data show that NL2 can be endocytosed and, once internalized, co-localizes with SNX27 and retromer. Direct interaction between NL2 and SNX27/retromer suggests a role for this complex in trafficking of NL2.
SNX27 Mediates Recycling of NL2 through PDZ-Ligand Interaction
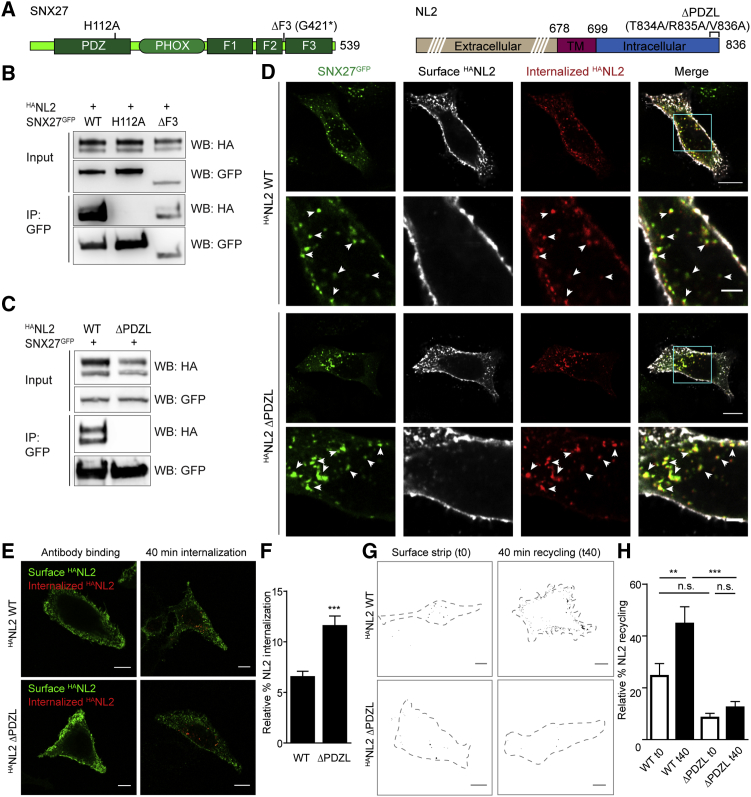
To test whether trafficking of NL2 involves its C-terminal PDZ-binding motif (or PDZ-ligand), we generated a mutant of HANL2 lacking this motif (HANL2ΔPDZL), as well as a point mutation in the SNX27 PDZ domain that abolishes interaction with PDZ-binding motifs, SNX27GFPH112A (Hussain et al., 2014) (Figure 2A). We also created a non-related deletion mutant of SNX27 lacking the third FERM domain (SNX27GFPΔF3); removal of this domain impedes recycling through loss of interaction with the WASH complex (Lee et al., 2016). These mutations in SNX27 did not affect its endosomal targeting, as shown by their vesicular staining and co-localization with endosomal marker EEA1 (early endosome antigen 1; Figure S2A). Co-immunoprecipitation in COS-7 cells expressing wild-type (WT) and mutant HANL2 and SNX27GFP revealed that HANL2, while not pulled down by GFP alone (Figure S2B), interacted with both SNX27GFPWT and SNX27GFPΔF3. The single point mutation in the SNX27 PDZ domain, however, completely abolished interaction with HANL2 (Figure 2B). Likewise, mutation of the PDZ-ligand in NL2 led to a complete loss of NL2-SNX27 interaction (Figure 2C). Thus, NL2 interacts with SNX27 via PDZ-ligand interaction.
Figure 2.
SNX27 Regulates Recycling of NL2 via PDZ-Ligand Interaction
(A) Schematic representation of SNX27 (left) and NL2 (right), indicating the positions of mutations generated for this study. F1-3, FERM1-3; PDZL, PDZ-ligand; TM, transmembrane.
(B and C) Western blots of co-immunoprecipitation from COS-7 cells co-expressing either WT HANL2 with WT or mutant SNX27GFP (B) or WT SNX27GFP with WT or mutant HANL2 (C). SNX27GFP was pulled down using GFP-Trap beads. IP, immunoprecipitation.
(D) Confocal images of antibody feeding in HeLa cells co-expressing HANL2 WT or ΔPDZL with SNX27GFP. Scale bars, 10 μm (whole cell) and 3 μm (zooms). Arrowheads show examples of co-localization.
(E) Confocal images of antibody feeding in HeLa cells expressing HANL2 WT or ΔPDZL. Scale bar, 10 μm.
(F) Quantification of relative HANL2 internalization in HeLa cells (n = 16; unpaired two-tailed t test).
(G) Confocal images of antibody recycling in HeLa cells expressing HANL2 WT or ΔPDZL. To enable better visualization of extracellular HANL2 upon recycling, only the channel representing surface labeling is shown after thresholding and binarizing. Dashed lines indicate the outlines of the cell. Scale bar, 10 μm.
(H) Quantification of relative HANL2 recycling in HeLa cells (n = 27; one-way ANOVA with Bonferroni’s correction).
Values are mean ± SEM; n.s., non-significant. ∗∗p < 0.01 and ∗∗∗p < 0.001. See also Figure S2.
Next, we investigated how NL2-SNX27 interaction directs trafficking of NL2. HANL2ΔPDZL, like HANL2WT, internalized to SNX27GFP-containing vesicles in both HeLa cells and neurons (Figures 2D and S2C–S2E), suggesting that endocytosis and initial sorting of NL2 are independent of SNX27. To characterize HANL2 internalization at endogenous levels of SNX27, we performed antibody feeding on HeLa cells overexpressing HANL2WT or HANL2ΔPDZL, but not SNX27GFP, and quantified the ratio between internal and total fluorescence as a relative measure for internalization (Figures 2E and 2F). We found a larger fraction of HANL2ΔPDZL to be localized intracellularly compared with HANL2WT, suggesting either increased internalization or decreased recycling of HANL2ΔPDZL. To quantify recycling, we allowed internalization of antibody-labeled HANL2, subsequently stripped the remaining surface-bound HA antibody, and then allowed for recycling of internalized antibody-labeled HANL2 (Figure 2G). This revealed that whereas HANL2WT re-appears at the membrane, HANL2ΔPDZL is impaired in its ability to recycle (Figure 2H). These results suggest that association between NL2 and SNX27 directs recycling of NL2.
SNX27-Mediated Recycling of NL2 Enhances Inhibitory Signaling
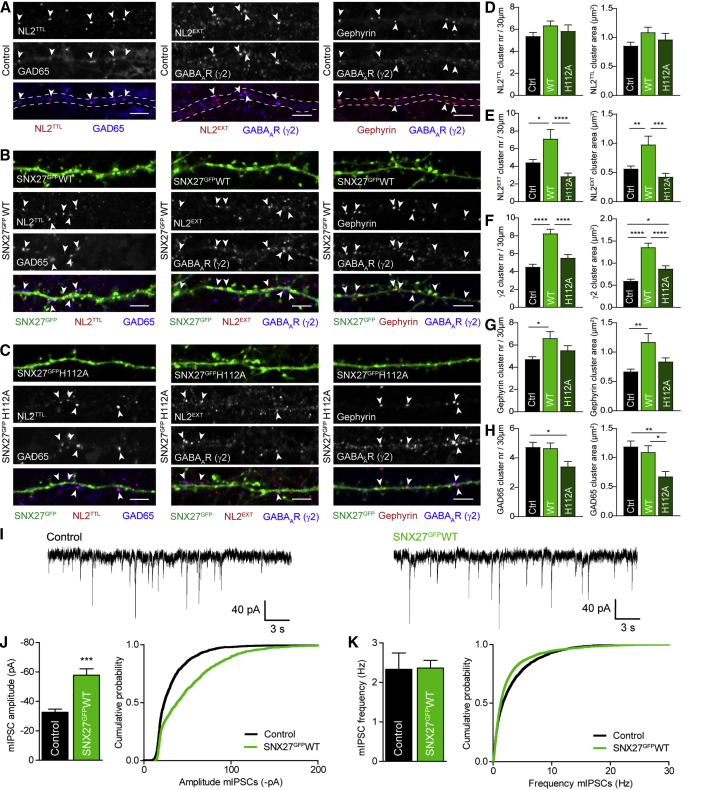
To investigate whether SNX27 also mediates recycling of endogenous NL2 in neurons, we transfected hippocampal cultures with either SNX27GFPWT or the mutant SNX27GFPH112A and assessed the effects on inhibitory synapse composition and signaling in comparison with mock-transfected cells. Both overexpressed SNX27GFP constructs are widely distributed on endosomes throughout the cell (Figure S3A). We analyzed the number and size of inhibitory synapses by immunostaining for NL2, the presynaptic marker GAD65, and postsynaptic markers gephyrin or the GABAAR γ2 subunit (Figures 3A–3C). To distinguish between extracellular (synaptic) NL2 and total NL2, we separately stained with two different NL2 antibodies: one that detects an extracellular epitope and therefore, when incubated without permeabilization, only labels synaptic NL2 (NL2EXT) versus one that labels the intracellular domain of NL2 and thus, after permeabilization, detects the total pool of NL2 (NL2TTL). Overexpression of SNX27GFPWT, but not SNX27GFPH112A, led to a striking increase in the number and size of extracellular NL2 puncta, while total NL2 remained unaffected (Figures 3D and 3E). This suggests that overexpression of WT SNX27 results in NL2 redistribution, favoring synaptic localization of NL2, consistent with a role for SNX27 in NL2 recycling. The non-binding H112A mutant, however, did not facilitate recruitment of NL2 to the membrane but, in contrast, trended toward a decrease in synaptic NL2 clusters.
Figure 3.
Overexpression of SNX27GFPWT but Not SNX27GFPH112A Increases Postsynaptic Clusters and Inhibitory Signaling
(A–C) Confocal images of 30 μm dendritic sections of hippocampal neurons, mock transfected (control) (A) or overexpressing SNX27GFPWT (B) or SNX27GFPH112A (C). Neurons were stained for GAD65, total NL2 (NL2TTL), synaptic NL2 (NL2EXT), gephyrin, or the GABAAR γ2 subunit. Arrowheads show synaptic clusters. Dashed lines indicate the dendritic outline for mock-transfected cells (A). Scale bar, 4 μm.
(D–H) Quantification of cluster number (left) and area (right) in hippocampal neurons either mock transfected (Ctrl) or overexpressing SNX27GFPWT (WT) or SNX27GFPH112A (H112A). Quantified are NL2TTL (D) (n = 21, 19, and 19), NL2EXT (E) (n = 24, 23, and 24), γ2 (F) (n = 35, 46, and 52), gephyrin (G) (n = 25, 34, and 26), and GAD65 (H) (n = 21, 20, and 17).
(I) Representative traces of mIPSC patch-clamp recordings from hippocampal cultures, mock transfected (Control) or overexpressing SNX27GFPWT.
(J and K) Pooled data (left) and cumulative probability plot (right) of mIPSCs amplitude (J) and frequency (K) (n = 13 and 22).
Values are mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001, one-way ANOVA with Bonferroni’s correction (D–H) or unpaired two-tailed t test (J and K). See also Figure S3.
Simultaneously with increased surface NL2EXT, an increase in the number of synaptic GABAARs and gephyrin cluster number and size was observed with WT but not H112A SNX27GFP overexpression (Figures 3F and 3G). Presynaptic terminals remained unaffected by SNX27GFPWT overexpression but appeared to be destabilized by SNX27GFPH112A (Figure 3H). Likewise, the number of presynaptic GAD65 clusters overlapping with postsynaptic γ2 clusters was not affected by SNX27GFPWT overexpression but reduced by overexpression of SNX27GFPH112A (Figure S3B). We postulate that this is related to the apparent reduction of synaptic NL2 in this condition. These data show that SNX27 enhances NL2 membrane retrieval, which in turn enables recruitment of gephyrin and GABAARs to the synapse.
To test the functional relevance of enhanced surface NL2 and GABAARs on inhibitory signaling, we performed whole-cell patch-clamp recordings of miniature inhibitory postsynaptic currents (mIPSCs) from hippocampal cultures overexpressing SNX27GFPWT in comparison with mock-transfected cells (Figures 3I–3K, S3C, and S3D). Overexpression of SNX27GFPWT resulted in an increase in mIPSC amplitude (Figure 3J) and corresponding total charge transfer (Figure S3C), which correlates with an increase in synaptic GABAARs (Figure 3F). The frequency of mIPSCs remained unaffected (Figure 3K). In a separate control experiment we recorded mIPSCs from hippocampal neurons overexpressing SNX27GFPH112A in comparison with mock-transfected cells (Figures S3E–S3I) and found no significant changes in mIPSC amplitude and frequency (Figures S3F and S3G), suggesting that the small increase in GABAAR cluster size upon SNX27GFPH112A overexpression (Figure 3F) has no functional consequence.
Taken together, these data show that SNX27-mediated retrieval of internalized NL2 to the synapse leads to enhanced recruitment of gephyrin and GABAARs, and increased inhibitory signaling.
Reduced NL2 Recycling Interferes with Inhibitory Synaptic Function
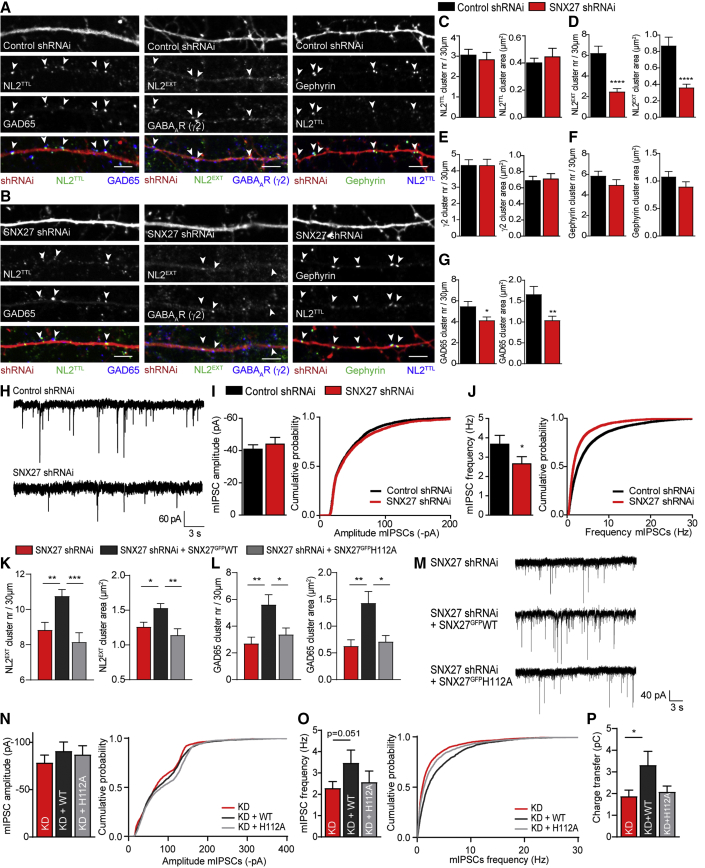
Next, we transfected hippocampal cultures with SNX27-specific short hairpin RNAi (shRNAi) and confirmed efficient knockdown (KD) in comparison with control shRNAi (Figures S4A and S4B). We then analyzed the impact of interfering with NL2 recycling on the number and size of inhibitory synaptic clusters (Figures 4A–4G). Whereas total levels of NL2 were unaffected, we found a 60% decrease in synaptic NL2 clusters (Figures 4C and 4D), indicating that SNX27 KD results in redistribution of NL2 to intracellular pools. Interestingly, the number and size of GABAAR and gephyrin clusters remained unchanged (Figures 4E and 4F), but we observed a significant reduction in presynaptic GAD65 clusters (Figure 4G) and, consequently, in overlapping GAD65/γ2 clusters (Figure S4C). mIPSC recordings from hippocampal cultures expressing control or SNX27-specific shRNAi (Figures 4H–4J, S4D, and S4E) showed no change in mIPSC amplitude (Figure 4I), which concurs with a lack of change in surface GABAARs. In contrast, mIPSC frequency was significantly decreased (Figure 4J), consistent with a decrease in presynaptic terminals (Figure 4G).
Figure 4.
SNX27 Knockdown Decreases Synaptic NL2 and Disrupts Inhibitory Signaling
(A and B) Confocal images of 30 μm dendritic sections of hippocampal neurons, overexpressing control shRNAi (A) or SNX27-specific shRNAi (B). Neurons were stained as in Figures 3A–3C. Arrowheads show synaptic clusters. Scale bar, 4 μm.
(C–G) Quantification of cluster number (left) and area (right) in hippocampal neurons transfected as in (A) and (B). Quantified are NL2TTL (C) (n = 19 and 17; cluster number, unpaired two-tailed t test; cluster area, Mann-Whitney test), NL2EXT (D) (n = 36 and 37; Mann-Whitney tests), γ2 (E) (n = 20 and 19; unpaired two-tailed t tests), gephyrin (F) (n = 19 and 17; Mann-Whitney tests), and GAD65 (G) (n = 27; cluster number, Mann-Whitney test; cluster area, unpaired two-tailed t test).
(H) Representative traces of mIPSC patch-clamp recordings from hippocampal cultures overexpressing control or SNX27-specific shRNAi.
(I and J) Pooled data (left) and cumulative probability (right) of mIPSC amplitude (I) and frequency (J) (n = 23 and 20; unpaired one-tailed t tests).
(K and L) Quantification of cluster number (left) and area (right) in hippocampal neurons transfected with SNX27-specific shRNAi alone (red) or combined with RNAi-resistant SNX27GFPWT (black) or SNX27GFPH112A (gray). Quantified are NL2EXT (K) (n = 22, 23, and 19; one-way ANOVA with Bonferroni’s correction) and GAD65 (L) (n = 23, 22, and 28; Kruskal-Wallis test with Dunn’s correction). See Figures S4I and S4J for quantification of γ2 and gephyrin.
(M) Representative traces of mIPSC patch-clamp recordings from hippocampal cultures overexpressing SNX27-specific shRNAi alone (KD) or combined with RNAi-resistant SNX27GFPWT (KD+WT) or SNX27GFPH112A (KD+H112A).
(N and O) Pooled data (left) and cumulative probability (right) of mIPSC amplitude (N) and frequency (O) (n = 14, 14, and 13; unpaired one-tailed t tests).
(P) Quantification of mIPSC charge transfer (unpaired one-tailed t tests).
Values are mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. See also Figure S4.
To confirm that the reduction in synaptic NL2 clusters, GAD65 clusters, and mIPSC frequency is specific to the loss of SNX27, we performed KD and rescue experiments using RNAi-resistant SNX27GFPWT or SNX27GFPH112A and quantified synaptic clusters using immunostaining (Figures S4F–S4H) and inhibitory signaling by recording mIPSCs (Figures 4M–4P and S4L) as before. KD combined with SNX27GFPWT, but not SNX27GFPH112A, restored the number and size of synaptic NL2 clusters as well as GAD65 clusters (Figures 4K and 4L). We observed an increase in γ2 cluster area, reminiscent of the effect of SNX27 overexpression without KD, but γ2 cluster number, as well as gephyrin clusters and GAD65/γ2 overlap remained unaffected (Figures S4I–S4K). The amplitude of mIPSCs was unaffected by SNX27 KD, and likewise co-expression with rescue constructs did not alter the mIPSC amplitude (Figure 4N). However, we did observe a trend toward a rescue of mIPSC frequency upon WT but not H112A SNX27GFP co-expression (p = 0.051; Figure 4O). Moreover, the total charge transfer, a parameter that reflects both the amplitude and frequency of miniature synaptic events, significantly increased upon rescue with WT but not H112A SNX27GFP (Figure 4P), in agreement with the increased γ2 and GAD65 clusters in this condition.
Thus, we show that SNX27 KD leads to a loss of NL2 from the synapse, synaptic destabilization, and decreased inhibitory signaling. These effects can be rescued by reintroducing SNX27GFPWT. All data taken together, we provide evidence that SNX27 regulates surface availability of NL2 and, as a result, the stability and composition as well as signaling strength of the inhibitory synapse.
Discussion
Neuroligins are well known for their role in regulating the development, function, and plasticity of synapses (Bemben et al., 2015, Varoqueaux et al., 2006). The mechanisms that direct the trafficking, and thereby alter synaptic levels, of neuroligins themselves, however, remain less well understood. In this study we investigated how surface levels of neuroligin-2 (NL2) are regulated and how this affects inhibitory signaling. We show that NL2 can be endocytosed and subsequently co-localizes and interacts with recycling protein SNX27 and retromer. SNX27 enables membrane retrieval of internalized NL2 through direct PDZ-ligand interaction. Finally, we show that SNX27-mediated modulation of NL2 surface availability contributes to the regulation of inhibitory synapse composition and signaling strength.
Our data support the following molecular model (Figure S4M). SNX27 activity enhances retrieval of NL2 to the synapse and consequently positively modulates postsynaptic gephyrin and GABAAR clustering, thereby enhancing inhibitory currents, consistent with the well-known role of NL2 in synapse maintenance (Varoqueaux et al., 2006). We speculate that this effect may be through the stabilization of GABAARs that were already present at extra-synaptic sites. Considering that GABAAR subunits do not possess a PDZ-binding motif and do not interact with SNX27 directly (Wang et al., 2013), and that SNX27 KD does not decrease synaptic GABAARs levels (Figure 4; Wang et al., 2013), we speculate that endocytosis and recycling of NL2 and GABAARs involve separate mechanisms. We furthermore propose that the reduced inhibitory signaling observed upon SNX27 KD and loss of NL2 at the synapse is due to reduced transsynaptic interactions, particularly between NL2 and neurexin (Dean et al., 2003, Shipman and Nicoll, 2012), consequently destabilizing the presynaptic terminal. Likewise, NL2 KD reduces presynaptic vGAT clusters (Chih et al., 2005).
The SNX27/retromer/WASH nexus has been characterized as machinery that rescues internalized cargo from lysosomal degradation (Lee et al., 2016). Indeed, while our manuscript was in revision, another study demonstrated a role for SNX27 in rescuing NL2 from lysosomal degradation (Binda et al., 2019), although that study did not assess its effect on inhibitory transmission. Using surface protein biotinylation, they demonstrate reduced NL2 surface levels upon SNX27 KD, consistent with our immunostaining data showing reduced synaptic NL2 upon SNX27 shRNAi (Figure 4D). Whereas they also report reduced total NL2 protein levels and gephyrin cluster numbers upon SNX27 KD, we found no change in total NL2 or gephyrin clusters in this condition (Figures 4C and 4F). A possible explanation for the difference is that we transfected and analyzed the neurons at a younger age (day in vitro [DIV] 7 and DIV 13, respectively). We speculate that at the neuronal age and in the time frame of our experiments, impaired NL2 recycling leads to a redistribution of NL2 from the surface to internal storage, whereas enhanced degradation only occurs at a later time point. In a similar way, the β1-adrenergic receptor was shown to be retained at the trans-Golgi network and protected from degradation upon internalization (Koliwer et al., 2015). In comparison with the slower process of degradation and de novo synthesis, membrane protein internalization and recycling enables more rapid modulation of protein surface levels, on a timescale of seconds, to respond to changes in the E/I balance (Luscher et al., 2011). Our data are thus consistent with a model in which internalized NL2 is stored in a “ready-to-go” intracellular pool, enabling rapid SNX27-mediated membrane reinsertion and upregulation of inhibitory signaling.
A major question yet to be answered is which physiological signals prompt enhanced endocytosis of NL2 and intracellular retainment, as well as the reverse mechanism leading to increased NL2 insertion by SNX27. It is tempting to speculate that these processes are determined by neuronal activity and thus that the modulation of synaptic NL2 levels plays a role in maintaining the E/I balance. In a recent study, Kang et al. (2014) identified a range of protein interactors of NL2, including regulators of endocytosis, trafficking, and phosphorylation. Although the functional roles of these proteins in modulating surface levels of NL2 remain to be characterized, this work and ours together are suggestive of a role for directed trafficking of NL2 in synaptic signaling.
Possible mechanisms that direct NL2 internalization versus recycling could include phosphorylation of the residues adjacent to its PDZ-ligand, as phosphorylation near these motifs has been shown to determine binding affinity between the SNX27 PDZ domain and its ligand (Clairfeuille et al., 2016). PDZ domain containing proteins play a dual role in synaptic function: on one hand, scaffold proteins such as PSD95 and other members of the MAGUK family stabilize transmembrane proteins at the postsynaptic domain via PDZ interaction (Meyer et al., 2004); NL2 itself is stabilized at the synapse by S-SCAM (Sumita et al., 2007). On the other hand, PDZ-ligand interactions are essential for trafficking in neuronal function (Bauch et al., 2014, Koliwer et al., 2015). Thus, regulation of affinity between a PDZ domain and its ligand may play an important role in modulating surface availability of synaptic proteins. Our data suggest that the PDZ-ligand is not required for de novo membrane insertion of NL2, as HANL2ΔPDZL still localizes to the cell membrane (Figure 2D) and interfering with SNX27-mediated NL2 recycling reduces but does not abolish inhibitory synapse formation (Figure 4). Accordingly, previous studies showed that the PDZ-ligand is dispensable for the synaptogenic properties of neuroligins (Shipman et al., 2011, Li et al., 2017).
Previously characterized SNX27 cargo includes G-protein-coupled receptors (Lauffer et al., 2010, Lin et al., 2015) and excitatory synaptic receptors (Loo et al., 2014, Hussain et al., 2014). Our study now provides evidence that SNX27 plays a role in regulating inhibitory signaling. Importantly, the PDZ-binding motif is conserved in other neuroligins, and SNX27 also interacts with and regulates protein levels of NL1 and NL3 (Binda et al., 2019). Specifically altered trafficking of NL3 may affect inhibitory synapse function (Davenport et al., 2019, Nguyen et al., 2016) and have an additional impact on inhibitory synapse modulation as assessed in our study. Future studies could include SNX27 KD in a neuroligin-null background combined with rescue of individual neuroligins to dissect specific effects of each neuroligin family member. Disrupted SNX27-dependent trafficking of both inhibitory and excitatory synaptic cargo may contribute to altered E/I balance in SNX27-associated neurological diseases, including Down syndrome and epilepsy (Wang et al., 2013, Damseh et al., 2015). Given that epilepsy has been widely ascribed to impaired synaptic inhibition (Gouzer et al., 2014, González, 2013), it would be interesting to investigate whether disrupted NL2 recycling coincides with an increased chance of epileptiform activity.
All in all, we have shown that SNX27-mediated modulation of synaptic NL2 levels is an important mechanism in the regulation of inhibitory signaling.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit IgG control | ThermoFisher Scientific | Cat# 10500C; RRID: AB_2532981 |
| Mouse IgG control | ThermoFisher Scientific | Cat# 10400C; RRID: AB_2532980 |
| Mouse-anti-EEA1 | BD Transduction Labs | Cat# 610457; Clone# 14/EEA1; RRID: AB_397830 |
| Guinea Pig-anti-GABAAR-ɣ2 | Synaptic Systems | Cat# 224 004; RRID: AB_10594245 |
| Mouse-anti-GAD65 (supernatant) | Neuromab | Cat# 73-508; Clone#L127/12; RRID: AB_2756510 |
| Mouse-anti-Gephyrin | Synaptic Systems | Cat# 147 011; Clone# mAb7a; RRID: AB_887717 |
| Rabbit-anti-GFP | SantaCruz | Cat# sc-8334; RRID: AB_641123 |
| Rat-anti-GFP | Nacalai Tesque | Cat# 04404-84; RRID: AB_10013361 |
| Mouse-anti-GST (supernatant) | Neuromab | Cat# 75-148; clone# N100/13; RRID: AB_10671817 |
| Mouse-anti-HA-tag | Produced and purified in house from hybridoma cells. Vendor: James Trimmer, UC Davis | Clone# 12CA5; RRID: AB_2532070 |
| Rabbit-anti-Neuroligin2 | Alomone Labs | Cat# ANR-036; RRID: AB_2341007 |
| Rabbit-anti-Neuroligin2 | Synaptic Systems | Cat# 129-202; RRID: AB_993011 |
| Mouse-anti-SNX27 | Abcam | Cat# ab77799; RRID: AB_10673818 |
| Rabbit-anti-SNX27 | Atlas antibodies | Cat# HPA045816 (discontinued) |
| Rabbit-anti-VPS26 | Abcam | Cat# ab181352; RRID: AB_2665924 |
| Rabbit-anti-VPS35 | Abcam | Cat# ab97545; RRID: AB_10696107 |
| Rabbit-anti-VPS35 | Atlas antibodies | Cat# HPA040802; RRID: AB_2677142 |
| Goat-anti-rabbit IgG (H+L), HRP | Jackson ImmunoResearch | Cat# 111-035-003; RRID: AB_2313567 |
| Goat-anti-mouse IgG (H+L), HRP | Jackson ImmunoResearch | Cat# 115-035-003; RRID: AB_10015289 |
| Goat anti-mouse IgG (light chain specific), HRP | Jackson ImmunoResearch | Cat# 115-035-174; RRID: AB_2338512 |
| Goat-anti-mouse AlexaFluor 405 | ThermoFisher Scientific | Cat# A-31553; RRID: AB_221604 |
| Donkey-anti-mouse AlexaFluor 488 | Jackson ImmunoResearch | Cat# 715-545-151; RRID: AB_2341099 |
| Donkey-anti-rabbit AlexaFluor 488 | ThermoFisher Scientific | Cat# A-21206; RRID: AB_2535792 |
| Donkey-anti-rat AlexaFluor 488 | ThermoFisher Scientific | Cat# A-21208; RRID: AB_141709 |
| Goat-anti-mouse AlexaFluor 555 | ThermoFisher Scientific | Cat# A-21424; RRID: AB_141780 |
| Goat-anti-rabbit AlexaFluor 555 | ThermoFisher Scientific | Cat# A-21430; RRID: AB_2535851 |
| Goat-anti-guinea pig AlexaFluor 647 | ThermoFisher Scientific | Cat# A-21450; RRID: AB_2535867 |
| Donkey-anti-mouse AlexaFluor 647 | ThermoFisher Scientific | Cat# A-31571; RRID: AB_162542 |
| Donkey-anti-rabbit AlexaFluor 647 | ThermoFisher Scientific | Cat# A-31573; RRID: AB_2536183 |
| Bacterial and Virus Strains | ||
| One Shot TOP10 Chemically Competent E.coli | Invitrogen | Cat# C404010 |
| BL21(DE3) One Shot Chemically Competent E.Coli | Invitrogen | Cat# C600003 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Hank’s Buffered Salt Solution (HBSS) | GIBCO | Cat# 14180046 |
| 1M HEPES buffer | GIBCO | Cat# 15630080 |
| Minimal Essential Medium (MEM) | GIBCO | Cat# 31095029 |
| Heat inactivated Horse Serum (HRS) | GIBCO | Cat# 26050088 |
| Sodium pyruvate | GIBCO | Cat# 11360070 |
| Glucose | GIBCO | Cat# A2494001 |
| Neurobasal medium | GIBCO | Cat# 21103049 |
| B-27 | GIBCO | Cat# 17504044 |
| GlutaMAX | GIBCO | Cat# 35050061 |
| DMEM (high glucose) | GIBCO | Cat# 41965039 |
| Fetal Bovine Serum | GIBCO | Cat# 10082147 |
| Penicillin/Streptomycin | GIBCO | Cat# 15140122 |
| 2.5% Trypsin | GIBCO | Cat# 15090046 |
| DNase | Sigma-Aldrich | Cat# DN-25 |
| Poly-L-lysine (PLL) | Sigma-Aldrich | Cat# P6282-5MG |
| Lipofectamine-2000 | Invitrogen | Cat# 11668027 |
| CsCl | Sigma-Aldrich | Cat# C4036 |
| QX314 Br | Sigma-Aldrich | Cat# L5783 |
| NBQX | Abcam | Cat# ab120046 |
| APV | Abcam | Cat# ab120003 |
| Tetrodotoxin citrate (TTX) | Tocris | Cat# 1078 |
| IPTG | Melford | Cat# 367-93-1 |
| PMSF | AppliChem | Cat# A0999,0025 |
| Antipain | Peptide | Cat# 4062 |
| Pepstatin | Peptide | Cat# 4397 |
| Leupeptin | Peptide | Cat# 4041 |
| Glutathione Sepharose 4B | GE Healthcare | Cat# 17075601 |
| Protein A Sepharose | Generon | Cat# PC-A25 |
| GFP-Trap | Chromotek | Cat# gta-100 |
| Luminate Crescendo Western HRP substrate | Milipore | Cat# WBLUR0500 |
| ProLong Gold antifade reagent | Invitrogen | Cat# P36930 |
| Critical Commercial Assays | ||
| BioRad protein assay | BioRad | Cat# PI-23225 |
| Gateway™ LR Clonase™ Enzyme Mix | ThermoFisher Scientific | Cat# 11791019 |
| In-Fusion® HD Cloning Plus | Takara | Cat# 638909 |
| Experimental Models: Cell Lines | ||
| COS-7 | ATCC | Cat# CRL-1651; RRID: CVCL_0224 |
| HeLa | ATCC | Cat# CRM-CCL-2; RRID: CVCL_0030 |
| Experimental Models: Organisms/Strains | ||
| Wild-type Sprague-Dawley rats | Charles River | N/A |
| Oligonucleotides | ||
| Subcloning mSNX27 forward primer: gatctcgagctcaagctt atggcggacgaggacgg |
This paper | N/A |
| Subcloning mSNX27 reverse primer: catggtggcgaccggtgg ggtggccacatccctctg |
This paper | N/A |
| Subcloning mSNX27-ΔF3 reverse primer: catggtggcgacc ggtgggccctcgcaggtccttag |
This paper | N/A |
| Mutagenesis to create RNAi-resistant mSNX27, forward primer: gggcagctggagaaccaagtgatcgcattcgaatgggatga gatgc |
Hussain et al., 2014 | N/A |
| Mutagenesis to create RNAi-resistant mSNX27, reverse primer: gcatctcatcccattcgaatgcgatcacttggttctccagctgccc | Hussain et al., 2014 | N/A |
| Mutagenesis to create mSNX27-H112A, forward primer: gagggggcgacagccaagcaggtggtgg | This paper | N/A |
| Mutagenesis to create mSNX27-H112A, reverse primer: ccaccacctgcttggctgtcgccccctc | This paper | N/A |
| Subcloning GST-NL2CT, forward primer: catcatggatccta caagcgggaccggcgcc |
This paper | N/A |
| Subcloning GST-NL2CT, reverse primer: catcatctcgagcta tacccgagtggtggagtg |
This paper | N/A |
| Recombinant DNA | ||
| pNICE-NL2(-) | Peter Scheiffele (Chih et al., 2006) | Cat# Addgene 15246 |
| pNICE-NL2(-)-ΔPDZL | This paper | N/A |
| pCMV6-Entry-mSNX27 | OriGene | Cat# MR218832 |
| pEGFP-N1-mSNX27 | This paper | N/A |
| pEGFP-N1-mSNX27-ΔF3 | This paper | N/A |
| pEGFP-N1-mSNX27-H112A | This paper | N/A |
| pEGFP-N1-mSNX27 RNAi-resistant | This paper | N/A |
| pEGFP-N1-mSNX27-H112A RNAi-resistant | This paper | N/A |
| pDONR223-VPS35 | Lynda Chin (Scott et al., 2009) | Cat# Addgene 21689 |
| pDEST-eGFP-C1-VPS35 | This paper | N/A |
| pDsRed-Rab5 | Richard Pagano (Sharma et al., 2003) | Cat# Addgene 13050 |
| pDsRed-Rab11 | Richard Pagano (Choudhury et al., 2002) | Cat# Addgene 12679 |
| pEGFP-N1 | Clontech | Cat# 6085-1 |
| pDEST-eGFP-C1 | Robin Shaw (Hong et al., 2010) | Cat# Addgene 31796 |
| pGEX4T3 | GE Healthcare | Cat# 28954552 |
| pGEX4T3-NL2CT | This paper | N/A |
| pSuper-dsRed control | Kumiko Ui-Tei (Nishi et al., 2013) | Cat# Addgene 42053 |
| pSuper-dsRed SNX27 shRNAi | This paper, following method as published (Hussain et al., 2014) | N/A |
| Software and Algorithms | ||
| Fiji/ImageJ | National Institutes of Health | https://imagej.net/Welcome RRID: SCR_003070 |
| Metamorph | Molecular Devices | N/A |
| ZEN LSM | Zeiss | N/A |
| GraphPad Prism | GraphPad Software | N/A |
| Clampfit | Molecular Devices | N/A |
Lead Contact and Materials Availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Josef T. Kittler (j.kittler@ucl.ac.uk). Plasmids generated in this study are available from the Lead Contact upon request.
Experimental Model and Subject Details
Animals
All procedures for the care and treatment of animals were in accordance with the Animals (Scientific Procedures) Act 1986, and had full Home Office ethical approval. Animals were maintained under controlled conditions (temperature 20 ± 2°C; 12h light-dark cycle), were housed in conventional cages and had not been subject to previous procedures. Food and water were provided ad libitum. Wild-type E18 Sprague-Dawley rats were generated as a result of wild-type breeding; embryos of either sex were used for generating primary neuronal cultures.
Primary Hippocampal Culture and transfection
Cultures of hippocampal neurons were prepared from E18 WT Sprague-Dawley rat embryos of either sex as previously described (Muir and Kittler, 2014, Davenport et al., 2019). In brief, rat hippocampi were dissected from embryonic brains in ice-cold HBSS (GIBCO) supplemented with 10mM HEPES. Dissected hippocampi were incubated in the presence of 0.25% trypsin and 5 Units/ml DNase for 15 mins at 37°C, washed twice in HBSS with HEPES, and triturated to a single cell suspension in attachment media (MEM (GIBCO) containing 10% horse serum, 10 mM sodium pyruvate, and 0.6% glucose) using a fire-polished glass pasteur pipette. Dissociated cells were plated on poly-L-lysine coated coverslips in attachment media at a density of 5x105 cells/6 cm dish. After 6h serum-containing medium was replaced with Neurobasal medium (GIBCO) containing 2% B-27 (GIBCO), 2 mM glutaMAX (GIBCO), 100 U/ml penicillin and 100 μg/ml streptomycin. Cultures were maintained at 37°C in humidified atmosphere with 5% CO2. Neurons were transfected using Lipofectamine 2000 (Invitrogen) at DIV10 for antibody feeding, or at DIV7 for cluster analysis and electrophysiology, and maintained until DIV13-14.
COS-7 and HeLa Cell culture and transfection
COS-7 and HeLa cells (ATCC) were maintained in DMEM (GIBCO), supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin, at 37°C in humidified atmosphere with 5% CO2. Cells were transfected 24h before further processing using the Amaxa Nucleofector® device (Lonza) following the manufacturer’s protocol.
Bacterial cell culture
OneShot TOP10 E.coli (used for standard cloning) and BL21(DE3) OneShot E.Coli (used for recombinant production of GST protein) were cultured in Luria broth medium according to standard protocols.
Method Details
DNA Constructs
Murine SNX27 in pCMV6-Entry vector was purchased from Origene (United States) and subcloned into pEGFP-N1 (Clontech) via the HindIII and AgeI sites using In-Fusion cloning (Clontech) and removal of the Stop codon (forward primer gatctcgagctcaagcttatggcggacgaggacgg; reverse primer catggtggcgaccggtggggtggccacatccctctg). The ΔF3 mutant was created via the same method, using a reverse primer that eliminates the C-terminal region (sequence: catggtggcgaccggtgggccctcgcaggtccttag). The H112A mutant and RNAi-resistant constructs of SNX27 WT and H112A were created using the Quickchange method (Agilent) (WT forward primer: gggcagctggagaaccaagtgatcgcattcgaatgggatgagatgc; reverse primer: gcatctcatcccattcgaatgcgatcacttggttctccagctgccc. H112A forward primer: gagggggcgacagccaagcaggtggtgg; reverse primer: ccaccacctgcttggctgtcgccccctc.).
HA-tagged murine NL2 lacking the splice A insert (pNICE-NL2(-)) was a gift from Peter Scheiffele (Addgene plasmid #15246; Chih et al., 2006). HANL2-ΔPDZL was generated using this NL2 cDNA as a template, where subsequent mutagenesis was performed by DNAExpress (Canada). To create GST-NL2CT, a PCR product of the intracellular domain flanked by BamHI and XhoI restriction sites was created (forward primer catcatggatcctacaagcgggaccggcgcc; reverse primer catcatctcgagctatacccgagtggtggagtg) and subcloned into pGEX4T3 (GE Healthcare).
pDONR223-VPS35 was a gift from Lynda Chin (Addgene plasmid #21689; Scott et al., 2009). VPS35 was subcloned into pDEST-eGFP-C1 (Addgene plasmid #31796; Hong et al., 2010) using Gateway LR Clonase II (ThermoFisher). Rab5-dsRed and Rab11-dsRed were obtained from Addgene (plasmid #13050 and #12679, respectively; Sharma et al., 2003, Choudhury et al., 2002). pSuper-dsRed was a gift from Kumiko Ui-Tei (Addgene plasmid #42053; Nishi et al., 2013). A pSuper vector containing SNX27 shRNAi was created as previously described (Hussain et al., 2014). The shRNAi sequence targets a sequence that is identical in rat and murine SNX27. This vector simultaneously expresses dsRed to enable identification of transfected cells.
Preparation of GSTfusion protein
GST fusion proteins were produced as described (Kittler et al., 2000). In brief, BL21 E.Coli containing empty pGEX4T3 or pGEX4T3-NL2CT were grown in Luria Broth until an OD600 of 0.5-0.6. Protein production was induced by addition of 1mM IPTG, after which cells were grown for an additional 3h. Cells were harvested by centrifugation for 30 min at 4°C, washed in buffer containing 50 mM Tris pH 8.0, 25% sucrose, 10 mM EDTA, and pelleted by centrifugation for 30 mins at 4°C. Cells were then lysed by sonication in buffer containing 1% Triton X-100, 10 mM Tris pH 7.4, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, and antipain, pepstatin and leupeptin at 10 μg/ml. Lysates were further incubated for 30 mins upon addition of 12.5 mM HEPES pH 7.6, 75 mM KCl, 125 mM EDTA, 12.5% glycerol, and then spun for 1h at 12,000xg and 4°C. GST protein was purified by adding Glutathione Sepharose 4B beads (GE Healthcare) and incubating for 2h at 4°C. Beads were washed and stored at 4°C in buffer containing 20 mM HEPES pH 7.6, 100 mM KCl, 0.2M EDTA, 20% glycerol, 1 mM DTT, and 1 mM PMSF.
GST pull-down and co-immunoprecipitation
Rat brain lysate was obtained from adult female WT Sprague-Dawley rats. A whole brain, excluding the cerebellum, was homogenized on ice in lysis buffer (50 mM HEPES pH 7.5, 0.5% Triton X-100, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF with antipain, pepstatin and leupeptin at 10 μg/ml) and then left to rotate for 1h at 4°C. Membranes were pelleted by ultracentrifugation at 38,000xg for 40 mins at 4°C. Protein content of the supernatant was assayed by BioRad protein assay. For co-immunoprecipitations from brain lysate, 4 mg of brain lysate was incubated overnight at 4°C with rotation with 1 μg Rabbit IgG (ThermoFisher, 10500C) or Rabbit-anti-NL2 (Alomone Labs, ANR-036), or with 5 μg Mouse IgG (ThermoFisher, 10400C) or Mouse-anti-SNX27 (Abcam, ab77799). Complexes were precipitated with 15 μL of 50% protein A Sepharose bead slurry (Generon) for 2h at 4°C. For GSTpull-downs, 4 mg of brain lysate was incubated with 30 μg of GST or GST-NL2CT fusion protein attached to Glutathione Sepharose 4B beads (GE Healthcare) for 2h at 4°C with rotation. For co-immunoprecipitations from COS-7 cells, transfected cells were lysed on a dish in lysis buffer by scraping. Cell lysates were left to rotate at 4°C for 1h. Membranes were pelleted by centrifugation at 14,000xg for 10 mins at 4°C. Supernatants were then incubated with 6 μL of a 50% GFP-Trap bead slurry (Chromotek) for 2h at 4°C. All beads were washed 3 times in lysis buffer before resuspending in sample buffer, and then analyzed by SDS-PAGE and western blotting.
Western blotting
Protein samples were separated by standard Laemli SDS-PAGE on 9% Tris-Glycine gels and transferred onto nitrocellulose membrane (GE Healthcare Bio-Sciences). Membranes were blocked for 1h in milk (PBS, 0.1% Tween, 4% milk), and then incubated overnight at 4°C with shaking in primary antibodies diluted in milk (1:1000 Rabbit-anti-Neuroligin2 (Synaptic Systems 129-202), 1:100 Rabbit-anti-VPS35 (Atlas antibodies, HPA040802, Figure 1G), 1:500 Rabbit-anti-VPS35 (Abcam, ab97545, Figures 1D and 1F), 1:2000 Rabbit-anti-VPS26 (Abcam, ab181352), 1:100 Rabbit-anti-SNX27 (Atlas antibodies, HPA045816, Figures 1C and 1G), 1:100 Mouse-anti-SNX27 (Abcam, ab77799, Figure 1F), 1:200 Mouse-anti-HA-tag (supernatant, clone 12CA5, produced in house), 1:500 Mouse-anti-GST (Neuromab, N100/13) or 1:500 Rabbit-anti-GFP (SantaCruz, sc-8334)). Blots were then incubated with the appropriate HRP-conjugated secondary antibody for 1h at room temperature and developed using Luminate Crescendo Western HRP substrate (Milipore). Signal was detected using an ImageQuant LAS4000 mini (GE Life Sciences).
Immunocytochemistry
For regular immunostaining, COS-7 and HeLa cells were fixed 24h post transfection, and cultured neurons were fixed at DIV13-14, for 7 mins in 4% PFA (PBS, 4% paraformaldehdye, 4% sucrose, pH 7). For surface labeling, cells were incubated in block solution (PBS, 10% horse serum, 0.5% BSA) for 10 mins, and then stained for 1h at RT with primary antibody diluted in block solution (1:100 Rabbit-anti-NL2 (Alomone Labs, ANR-036), 1:500 Guinea Pig-anti-γ2 (Synaptic Systems, 224-004)). Cells were then permeabilised in block solution supplemented with 0.2% Triton X-100, and incubated for 1h at RT with the appropriate primary antibodies (1:500 Rabbit-anti-Neuroligin2 (Synaptic Systems, 129-203), 1:500 mouse-anti-gephyrin (Synaptic Systems 147-011, clone mAb7a), 1:100 Mouse-anti-GAD65 (supernatant, Neuromab, L127/12), 1:1000 Rat-anti-GFP (Nacalai Tesque, 04404-84), 1:500 Mouse-anti-EEA1 (BD Transduction Labs, clone 14/EEA1)), followed by 45-60 mins incubation with the appropriate Alexa-fluorophore conjugated secondary antibody (goat-anti-mouse Alexa405 or donkey-anti-mouse Alexa488 or goat-anti-mouse Alexa555; donkey-anti-rabbit Alexa488 or goat-anti-rabbit Alexa555 or donkey-anti-rabbit Alexa-647; goat-anti-guinea pig Alexa647; donkey-anti-rat Alexa488). Finally, coverslips were mounted onto glass slides using ProLong Gold antifade reagent (Invitrogen).
Antibody feeding was performed as described (Arancibia-Cárcamo et al., 2009), and where necessary the protocol was adapted. Transfected COS-7 cells and HeLa cells were incubated for 15 mins at 12°C with 1:50 Mouse-anti-HA in serum-free DMEM supplemented with 10 mM HEPES pH 7.6. Unbound antibody was removed by washing in serum-free DMEM, before internalisation for 40 mins at 37°C. Where appropriate to visualize recycling, surface-bound antibody was stripped using acid wash (150 mM NaCl, 50 mM HAc), and internalised protein was allowed to recycle back to the membrane for 40 mins at 37°C. Antibody feeding in neurons (DIV13-14) was performed by adding 1:50 Mouse-anti-HA directly to neuronal maintenance medium and allowing internalisation for 40 mins at 37°C. Neurons were washed twice in medium before fixation and blocking. To distinguish between surface and internal HANL2, fixed cells were first incubated with 1:300 donkey-anti-mouse Alexa647 in block solution (PBS, 10% horse serum, 0.5% BSA) without detergent. Cells were then permeabilised in block solution supplemented with 0.2% Triton X-100 for 10 mins and incubated for 1h at RT with 1:1000 Rat-anti-GFP (Nacalai Tesque, 04404-84) to visualize SNX27 where applicable. Cells were then stained for 45 mins with 1:500 donkey-anti-rat Alexa488 and goat-anti-Mouse-Alexa-555, and finally mounted using ProLong Gold antifade reagent (Invitrogen).
Image acquisition and analysis
Confocal images were acquired on a Zeiss LSM700 upright confocal microscope using a 63x oil objective (NA: 1.4) and digitally captured using ZEN LSM software (version 2.3; Zeiss), with excitation at 405 nm for Alexa-Fluor405, 488 nm for GFP and Alexa-Fluor488, 555 nm for Alexa-Fluor555, and 647 nm for Alexa-Fluor647 conjugated secondary antibodies. Pinholes were set to 1 Airy unit creating an optical slice of 0.57 μm. For cultured neurons, a whole-cell stack was captured using a 0.5x zoom. Per neuron 3 sections of dendrite, ∼50 μm from the soma, were imaged with a 3.4x zoom (equating to 30 μm length of dendrite). For antibody feeding, settings were adjusted to ensure channels were not saturated. Acquisition settings and laser power were kept constant within experiments.
Cluster analysis was performed as described (Smith et al., 2014, Davenport et al., 2017). In brief, a suitable threshold was selected for each channel using Metamorph software (version 7.8; Molecular Devices) and applied to all images within the same dataset. Clusters with an intensity above this threshold and size of at least 0.05 μm2 were quantified. Quantification was performed on 5-8 cells per experiment.
For quantification of antibody feeding, images of surface and internal staining were thresholded to generate a mask, which was then used to measure total intensity of surface and internal signal in the original image. The ratio between internal and total (surface plus internal) intensity was calculated as a relative measure for internalisation; a relative measure for recycling was obtained by calculating the ratio between surface and total (surface plus internal) intensity.
Pearson’s correlation coefficient for co-localization, R, was determined using the co-localization tool Coloc2 in ImageJ. For each image, R was calculated for the original image as well as after randomization of pixels in one of the channels to quantify specificity of the co-localization.
Electrophysiology
Whole-cell voltage-clamp recording was performed on transfected cultured hippocampal neurons at DIV13-14 as described (Eckel et al., 2015, Smith et al., 2014, Davenport et al., 2017, Davenport et al., 2019). Neurons were held at −70 mV. Patch electrodes (4-5 MΩ) were filled with an internal solution containing (in mM): 120 CsCl, 5 QX314 Br, 8 NaCl, 0.2 MgCl2, 10 HEPES, 2 EGTA, 2 MgATP and 0.3 Na3GTP. The osmolarity and pH were adjusted to 300 mOsm/L and 7.2 respectively. The external artificial cerebro–spinal fluid (ACSF) solution consisted of the following (in mM): 125 NaCl, 25 NaHCO3, 2.5 KCl, 2 MgCl2, 1.25 NaH2PO4, 2 CaCl2, and 25 glucose (pH 7.4, 320 mOsm). This solution was supplemented with NBQX (20 μM), APV (50 μM) and TTX (1 μM) to isolate mIPSCs. All recordings were performed at room temperature (22-25°C). Series resistance (typically 10–20 mOhms) was monitored throughout the experiment. The access resistance, monitored throughout the experiments, was < 20 MΩ and results were discarded if it changed by more than 20%. Miniature events and their kinetics were analyzed using template-based event detection in Clampfit (version 10.5; Molecular Devices).
Quantification and Statistical Analysis
For all quantified experiments the experimenters were blind to the condition of the sample analyzed, with the exception of mock transfected neurons (Figures 3 and S3). All experiments were performed at least 3 times from independent cell preparations and transfections, unless stated otherwise in the figure legend. Repeats for experiments are given in the figure legends as N-numbers and refer to number of cells of the respective conditions unless stated otherwise. Values are given as mean ± standard error of the mean (SEM). Error bars represent SEM. Statistical analysis was performed in GraphPad Prism (version 8; GraphPad Software, CA, USA) or Microsoft Excel. All data was tested for normal distribution with D’Agostino & Pearson test to determine the use of parametric (Student’s t test, one-way ANOVA) or non-parametric (Mann-Whitney, Kruskal-Wallis) tests. When p < 0.05, appropriate post hoc tests were carried out in analyses with multiple comparisons and are stated in the figure legends.
Data and Code Availability
Datasets generated for this study are available from the corresponding author upon reasonable request. This study did not generate new code. A custom-made script using pre-existing tools in ImageJ to quantify antibody feeding experiments will be made available upon request.
Acknowledgments
This work was supported by grants to J.T.K. from the BBSRC (BB/S017496/1), the Medical Research Council ( MR/N025644/1). E.F.H. received funding from the European Union’s Horizon 2020 research and innovation program under Marie Skłodowska-Curie Actions grant agreement UbiGABA 661733.
Author Contributions
Conceptualization, Methodology, and Funding Acquisition, E.F.H. and J.T.K.; Investigation, E.F.H., B.R.S., and F.L.; Formal Analysis, E.F.H. and B.R.S.; Resources, J.T.K.; Writing – Original Draft and Visualization, E.F.H. and J.T.K.; Writing – Review & Editing, E.F.H., B.R.S., F.L., and J.T.K.
Declaration of Interests
The authors declare no competing interests.
Published: November 26, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2019.10.096.
Supplemental Information
References
- Arancibia-Cárcamo I.L., Yuen E.Y., Muir J., Lumb M.J., Michels G., Saliba R.S., Smart T.G., Yan Z., Kittler J.T., Moss S.J. Ubiquitin-dependent lysosomal targeting of GABA(A) receptors regulates neuronal inhibition. Proc. Natl. Acad. Sci. U S A. 2009;106:17552–17557. doi: 10.1073/pnas.0905502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauch C., Koliwer J., Buck F., Hönck H.H., Kreienkamp H.J. Subcellular sorting of the G-protein coupled mouse somatostatin receptor 5 by a network of PDZ-domain containing proteins. PLoS ONE. 2014;9:e88529. doi: 10.1371/journal.pone.0088529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemben M.A., Shipman S.L., Nicoll R.A., Roche K.W. The cellular and molecular landscape of neuroligins. Trends Neurosci. 2015;38:496–505. doi: 10.1016/j.tins.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda C.S., Nakamura Y., Henley J.M., Wilkinson K.A. Sorting nexin 27 rescues neuroligin 2 from lysosomal degradation to control inhibitory synapse number. Biochem. J. 2019;476:293–306. doi: 10.1042/BCJ20180504. [DOI] [PubMed] [Google Scholar]
- Bozzi Y., Provenzano G., Casarosa S. Neurobiological bases of autism-epilepsy comorbidity: a focus on excitation/inhibition imbalance. Eur. J. Neurosci. 2018;47:534–548. doi: 10.1111/ejn.13595. [DOI] [PubMed] [Google Scholar]
- Chih B., Engelman H., Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chih B., Gollan L., Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Choudhury A., Dominguez M., Puri V., Sharma D.K., Narita K., Wheatley C.L., Marks D.L., Pagano R.E. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J. Clin. Invest. 2002;109:1541–1550. doi: 10.1172/JCI15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clairfeuille T., Mas C., Chan A.S., Yang Z., Tello-Lafoz M., Chandra M., Widagdo J., Kerr M.C., Paul B., Mérida I. A molecular code for endosomal recycling of phosphorylated cargos by the SNX27-retromer complex. Nat. Struct. Mol. Biol. 2016;23:921–932. doi: 10.1038/nsmb.3290. [DOI] [PubMed] [Google Scholar]
- Damseh N., Danson C.M., Al-Ashhab M., Abu-Libdeh B., Gallon M., Sharma K., Yaacov B., Coulthard E., Caldwell M.A., Edvardson S. A defect in the retromer accessory protein, SNX27, manifests by infantile myoclonic epilepsy and neurodegeneration. Neurogenetics. 2015;16:215–221. doi: 10.1007/s10048-015-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport E.C., Pendolino V., Kontou G., McGee T.P., Sheehan D.F., López-Doménech G., Farrant M., Kittler J.T. An essential role for the tetraspanin LHFPL4 in the cell-type-specific targeting and clustering of synaptic GABAA receptors. Cell Rep. 2017;21:70–83. doi: 10.1016/j.celrep.2017.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport E.C., Szulc B.R., Drew J., Taylor J., Morgan T., Higgs N.F., Lopez-Domenech G., Kittler J.T. Autism and schizophrenia-associated CYFIP1 regulates the balance of synaptic excitation and inhibition. Cell Rep. 2019;26:2037–2051.e6. doi: 10.1016/j.celrep.2019.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Scholl F.G., Choih J., DeMaria S., Berger J., Isacoff E., Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat. Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel R., Szulc B., Walker M.C., Kittler J.T. Activation of calcineurin underlies altered trafficking of α2 subunit containing GABAA receptors during prolonged epileptiform activity. Neuropharmacology. 2015;88:82–90. doi: 10.1016/j.neuropharm.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallon M., Cullen P.J. Retromer and sorting nexins in endosomal sorting. Biochem. Soc. Trans. 2015;43:33–47. doi: 10.1042/BST20140290. [DOI] [PubMed] [Google Scholar]
- González M.I. The possible role of GABAA receptors and gephyrin in epileptogenesis. Front. Cell. Neurosci. 2013;7:113. doi: 10.3389/fncel.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzer G., Specht C.G., Allain L., Shinoe T., Triller A. Benzodiazepine-dependent stabilization of GABA(A) receptors at synapses. Mol. Cell. Neurosci. 2014;63:101–113. doi: 10.1016/j.mcn.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Heisler F.F., Loebrich S., Pechmann Y., Maier N., Zivkovic A.R., Tokito M., Hausrat T.J., Schweizer M., Bähring R., Holzbaur E.L. Muskelin regulates actin filament- and microtubule-based GABA(A) receptor transport in neurons. Neuron. 2011;70:66–81. doi: 10.1016/j.neuron.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T.T., Smyth J.W., Gao D., Chu K.Y., Vogan J.M., Fong T.S., Jensen B.C., Colecraft H.M., Shaw R.M. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 2010;8:e1000312. doi: 10.1371/journal.pbio.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain N.K., Diering G.H., Sole J., Anggono V., Huganir R.L. Sorting Nexin 27 regulates basal and activity-dependent trafficking of AMPARs. Proc. Natl. Acad. Sci. U S A. 2014;111:11840–11845. doi: 10.1073/pnas.1412415111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob T.C., Moss S.J., Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y., Ge Y., Cassidy R.M., Lam V., Luo L., Moon K.M., Lewis R., Molday R.S., Wong R.O., Foster L.J., Craig A.M. A combined transgenic proteomic analysis and regulated trafficking of neuroligin-2. J. Biol. Chem. 2014;289:29350–29364. doi: 10.1074/jbc.M114.549279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler J.T., Delmas P., Jovanovic J.N., Brown D.A., Smart T.G., Moss S.J. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J. Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliwer J., Park M., Bauch C., von Zastrow M., Kreienkamp H.J. The Golgi-associated PDZ domain protein PIST/GOPC stabilizes the β1-adrenergic receptor in intracellular compartments after internalization. J. Biol. Chem. 2015;290:6120–6129. doi: 10.1074/jbc.M114.605725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer B.E., Melero C., Temkin P., Lei C., Hong W., Kortemme T., von Zastrow M. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J. Cell Biol. 2010;190:565–574. doi: 10.1083/jcb.201004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Chang J., Blackstone C. FAM21 directs SNX27-retromer cargoes to the plasma membrane by preventing transport to the Golgi apparatus. Nat. Commun. 2016;7:10939. doi: 10.1038/ncomms10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Han W., Pelkey K.A., Duan J., Mao X., Wang Y.X., Craig M.T., Dong L., Petralia R.S., McBain C.J., Lu W. Molecular dissection of neuroligin 2 and Slitrk3 reveals an essential framework for GABAergic synapse development. Neuron. 2017;96:808–826.e8. doi: 10.1016/j.neuron.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.B., Lai C.Y., Hsieh M.C., Wang H.H., Cheng J.K., Chau Y.P., Chen G.D., Peng H.Y. VPS26A-SNX27 interaction-dependent mGluR5 recycling in dorsal horn neurons mediates neuropathic pain in rats. J. Neurosci. 2015;35:14943–14955. doi: 10.1523/JNEUROSCI.2587-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo L.S., Tang N., Al-Haddawi M., Dawe G.S., Hong W. A role for sorting nexin 27 in AMPA receptor trafficking. Nat. Commun. 2014;5:3176. doi: 10.1038/ncomms4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn M.L., Nassirpour R., Arrabit C., Tan J., McLeod I., Arias C.M., Sawchenko P.E., Yates J.R., 3rd, Slesinger P.A. A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction. Nat. Neurosci. 2007;10:1249–1259. doi: 10.1038/nn1953. [DOI] [PubMed] [Google Scholar]
- Luscher B., Fuchs T., Kilpatrick C.L. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G., Varoqueaux F., Neeb A., Oschlies M., Brose N. The complexity of PDZ domain-mediated interactions at glutamatergic synapses: a case study on neuroligin. Neuropharmacology. 2004;47:724–733. doi: 10.1016/j.neuropharm.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Muir J., Kittler J.T. Plasticity of GABAA receptor diffusion dynamics at the axon initial segment. Front. Cell. Neurosci. 2014;8:151. doi: 10.3389/fncel.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q.A., Horn M.E., Nicoll R.A. Distinct roles for extracellular and intracellular domains in neuroligin function at inhibitory synapses. eLife. 2016;5:e19236. doi: 10.7554/eLife.19236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi K., Nishi A., Nagasawa T., Ui-Tei K. Human TNRC6A is an Argonaute-navigator protein for microRNA-mediated gene silencing in the nucleus. RNA. 2013;19:17–35. doi: 10.1261/rna.034769.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulopoulos A., Aramuni G., Meyer G., Soykan T., Hoon M., Papadopoulos T., Zhang M., Paarmann I., Fuchs C., Harvey K. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63:628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Scott K.L., Kabbarah O., Liang M.C., Ivanova E., Anagnostou V., Wu J., Dhakal S., Wu M., Chen S., Feinberg T. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature. 2009;459:1085–1090. doi: 10.1038/nature08109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D.K., Choudhury A., Singh R.D., Wheatley C.L., Marks D.L., Pagano R.E. Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 2003;278:7564–7572. doi: 10.1074/jbc.M210457200. [DOI] [PubMed] [Google Scholar]
- Shipman S.L., Nicoll R.A. Dimerization of postsynaptic neuroligin drives synaptic assembly via transsynaptic clustering of neurexin. Proc. Natl. Acad. Sci. U S A. 2012;109:19432–19437. doi: 10.1073/pnas.1217633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman S.L., Schnell E., Hirai T., Chen B.S., Roche K.W., Nicoll R.A. Functional dependence of neuroligin on a new non-PDZ intracellular domain. Nat. Neurosci. 2011;14:718–726. doi: 10.1038/nn.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.R., Kittler J.T. The cell biology of synaptic inhibition in health and disease. Curr. Opin. Neurobiol. 2010;20:550–556. doi: 10.1016/j.conb.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Smith K.R., Muir J., Rao Y., Browarski M., Gruenig M.C., Sheehan D.F., Haucke V., Kittler J.T. Stabilization of GABA(A) receptors at endocytic zones is mediated by an AP2 binding motif within the GABA(A) receptor β3 subunit. J. Neurosci. 2012;32:2485–2498. doi: 10.1523/JNEUROSCI.1622-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.R., Davenport E.C., Wei J., Li X., Pathania M., Vaccaro V., Yan Z., Kittler J.T. GIT1 and βPIX are essential for GABA(A) receptor synaptic stability and inhibitory neurotransmission. Cell Rep. 2014;9:298–310. doi: 10.1016/j.celrep.2014.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg F., Gallon M., Winfield M., Thomas E.C., Bell A.J., Heesom K.J., Tavaré J.M., Cullen P.J. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell Biol. 2013;15:461–471. doi: 10.1038/ncb2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T.C. Synaptic neurexin complexes: a molecular code for the logic of neural circuits. Cell. 2017;171:745–769. doi: 10.1016/j.cell.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumita K., Sato Y., Iida J., Kawata A., Hamano M., Hirabayashi S., Ohno K., Peles E., Hata Y. Synaptic scaffolding molecule (S-SCAM) membrane-associated guanylate kinase with inverted organization (MAGI)-2 is associated with cell adhesion molecules at inhibitory synapses in rat hippocampal neurons. J. Neurochem. 2007;100:154–166. doi: 10.1111/j.1471-4159.2006.04170.x. [DOI] [PubMed] [Google Scholar]
- Temkin P., Lauffer B., Jäger S., Cimermancic P., Krogan N.J., von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat. Cell Biol. 2011;13:715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin P., Morishita W., Goswami D., Arendt K., Chen L., Malenka R. The retromer supports AMPA receptor trafficking during LTP. Neuron. 2017;94:74–82.e5. doi: 10.1016/j.neuron.2017.03.020. [DOI] [PubMed] [Google Scholar]
- Twelvetrees A.E., Yuen E.Y., Arancibia-Carcamo I.L., MacAskill A.F., Rostaing P., Lumb M.J., Humbert S., Triller A., Saudou F., Yan Z., Kittler J.T. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagarajan S.K., Fritschy J.M. Gephyrin: a master regulator of neuronal function? Nat. Rev. Neurosci. 2014;15:141–156. doi: 10.1038/nrn3670. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F., Aramuni G., Rawson R.L., Mohrmann R., Missler M., Gottmann K., Zhang W., Südhof T.C., Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhao Y., Zhang X., Badie H., Zhou Y., Mu Y., Loo L.S., Cai L., Thompson R.C., Yang B. Loss of sorting nexin 27 contributes to excitatory synaptic dysfunction by modulating glutamate receptor recycling in Down’s syndrome. Nat. Med. 2013;19:473–480. doi: 10.1038/nm.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets generated for this study are available from the corresponding author upon reasonable request. This study did not generate new code. A custom-made script using pre-existing tools in ImageJ to quantify antibody feeding experiments will be made available upon request.