Abstract
DCs and epithelial cell‐derived thymic stromal lymphopoietin (TSLP) have pivotal roles in allergic inflammation. TSLP stimulates myeloid DCs to express OX40‐ligand (OX40L) and CCL17, which trigger and maintain Th2 cell responses. We have previously shown that statins, which are HMG‐CoA reductase inhibitors, have the ability to suppress type I IFN production by plasmacytoid DCs. Here, we extended our previous work to examine the immunomodulatory effect of statins on allergic responses, particularly the TSLP‐dependent Th2 pathway induced by myeloid DCs. We found that treatment of TSLP‐stimulated DCs with either pitavastatin or simvastatin suppressed both the DC‐mediated inflammatory Th2 cell differentiation and CRTH2+CD4+ memory Th2 cell expansion and also repressed the expressions of OX40L and CCL17 by DCs. These inhibitory effects of statins were mimicked by treatment with either a geranylgeranyl‐transferase inhibitor or Rho‐kinase inhibitor and were counteracted by the addition of mevalonate, suggesting that statins induce geranylgeranylated Rho inactivation through a mevalonate‐dependent pathway. We also found that statins inhibited the expressions of phosphorylated STA6 and NF‐κB‐p50 in TSLP‐stimulated DCs. This study identified a specific ability of statins to control DC‐mediated Th2 responses, suggesting their therapeutic potential for treating allergic diseases.
Keywords: CCL17, myeloid DCs, OX40L, statin, thymic stromal lymphopoietin (TSLP)
A cellular target in the cascade of allergic inflammation by which statins act as an inhibitor of allergy. Statins inhibit OX40L and CCL17 inductions from DCs through a blockade of NF‐κB p50 and STAT6 activation, leading to an inhibitory effect on DC‐mediated Th2 responses in the upstream phase of the immunological allergic cascade.
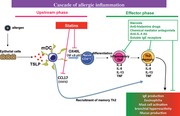
Introduction
Recently, the prevalence of allergic disease, such as atopic dermatitis, asthma, and allergic rhinitis, has increased in developed countries 1. These allergic disorders are characterized by inflammatory immune responses known as the T helper type 2 (Th2) responses in the allergic cellular cascade 2, 3, 4. Th2 cell‐derived cytokines, such as IL‐4, IL‐5, and IL‐13, induce an immunological cascade assembled by B cells, eosinophils, and mast cells, leading to increased IgE production, eosinophilia, and mucus hypersecretion 5, 6, 7. Accumulating evidence has indicated that DCs play an important role in the induction of inflammatory Th2 cells 8. Blood CD11c+ myeloid DCs (mDCs) are the direct precursors of epithelial Langerhans cells 9.
Epithelial cell‐derived thymic stromal lymphopoietin (TSLP) is a key cytokine that signals between epithelial cells and mDCs at one interface of allergic inflammation 10, 11. TSLP is highly produced by epithelial cells of the skin, lungs, and gastrointestinal tract in pathogenic conditions such as atopic dermatitis, bronchial asthma, and food allergy 12. TSLP‐stimulated mDCs can induce naïve CD4+ T cells to differentiate into inflammatory Th2 cells that produce IL‐4, IL‐5, IL‐13, and TNF‐α, but not IL‐10 13, 14. One molecule responsible for this differentiation into inflammatory Th2 cells is OX40‐ligand (OX40L), which is expressed on the cell surface of TSLP‐stimulated mDCs 15. OX40–OX40L is known to be involved in various inflammatory diseases, such as asthma and atopic dermatitis 16, 17, 18. Additionally, TSLP equips mDCs with the capacity to produce TARC/CCL17 19, which functions as a chemoattractant for memory Th2 cells, making them into the principal cells responsible for the maintenance of chronic allergic inflammation and the relapse of allergic inflammation upon re‐exposure to allergens 20, 21.
DC‐derived OX40L also plays a role in this process by contributing to the homeostatic expansion of allergen‐specific Th2 memory cells 19. These findings suggest that TSLP plays a critical role in the generation and maintenance of Th2 responses via activating DCs at the inflammatory sites, and that, notably, OX40L and CCL17 are key DC‐derived molecular components for triggering and maintaining allergic inflammatory cascades.
Statins, which are inhibitors of 3‐hydroxy‐3‐methylglutaryl‐CoA (HMG‐CoA) reductase, are usually prescribed to treat hypercholesterolemia. Recently, many studies have shown that statins have pleiotropic effects, including anti‐inflammatory or immunomodulatory effects, as demonstrated by the reduced rates of graft rejection in statin‐treated patients after heart transplantation 22, the beneficial effects of statins in autoimmune encephalomyelitis or MS 23, 24, the reduced leukocyte recruitment and edema formation induced by statins in animal models of acute inflammation 25, and the delayed disease progression induced by statins in the NZB × NZW murine model of systemic lupus erythematosus (SLE) 26. Recent studies have revealed that most of these effects are mediated via inhibiting the synthesis of isoprenoid intermediates, such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which are important lipid attachments for the intracellular signaling molecules Ras and Rho (small GTPases), in the mevalonate pathway 27. We previously showed that statins have the potential to repress type I IFN production by plasmacytoid DCs 28 and therefore can be utilized as therapeutic agents to shut off the IFN‐mediated pathogenic spiral observed in some autoimmune diseases, e.g., SLE. Although statins have been reported to be useful in the treatment of allergic diseases such as asthma and atopic dermatitis 29, 30, 31, the immunological mechanism by which statins act on DCs remain largely unknown. Therefore, in the present study, we investigated the immunomodulatory effects of statins on human mDC‐mediated Th2 responses.
Results
Effects of statins on the viability and maturation of human mDCs
Statins are known to have cytotoxic effects at high concentrations 32. Therefore, we first determined the viability of mDCs (isolated from blood PBMCs as shown in Supporting Information Fig. 1) in response to various concentrations of pitavastatin and simvastatin (1−100 µM) in the presence of TSLP by a trypan blue dye‐exclusion tests (Fig. 1A) and annexin‐V staining (data not shown). Although the highest tested concentration (100 µM) of both statins showed a cytotoxic effect on mDCs, statin concentrations of 1−10 µM did not affect the mDC survival. We next investigated the effect of statins on mDC maturation by measuring the expression of CD40, CD80, CD83, CD86, HLA‐DR, and programmed death ligand 1, known to be induced by the culture with TSLP or TLR‐ligand stimuli 15, 33. MFI of these markers remained unchanged in the presence of 1−10 µM of pitavastatin or simvastatin (Fig. 1B), indicating that the maturation of mDCs is not influenced by statins at these concentrations.
Figure 1.
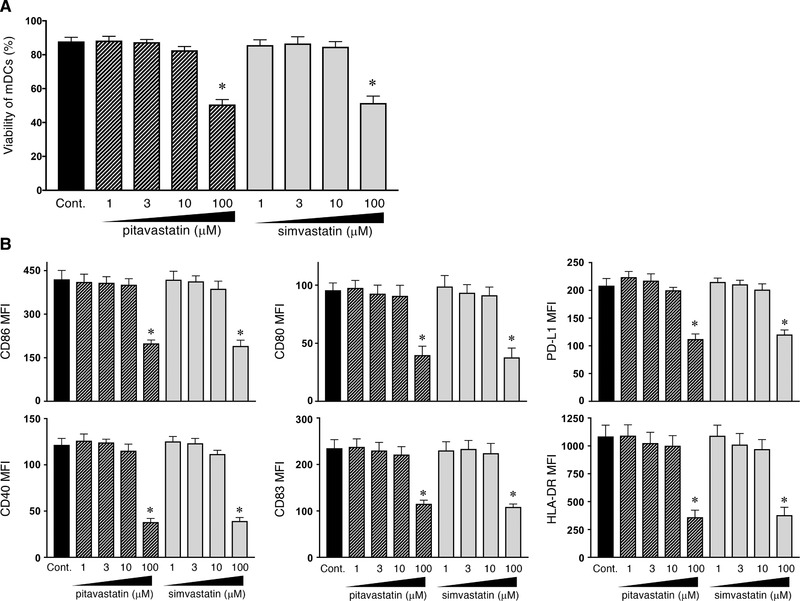
Survival and maturation of TSLP‐stimulated mDCs in the presence of statins. Human mDCs were incubated for 24 h with TSLP and the indicated concentrations of statins. (A) After culture, viable cells were measured by a trypan blue dye‐exclusion test. (B) After culture, the expression of CD40, CD80, CD83, CD86, HLA‐DR, and programmed death ligand 1 on mDCs was analyzed by flow cytometry. Data indicate the MFI, which was calculated by the subtraction of the MFI for the isotype control‐treated cells from the MFI for the cells treated with the indicated mAb. One set of experiment was performed by DCs from one donor, and data are shown as the mean ± SEM of six independent donors (A and B). Statistical significance was determined using paired Wilcoxon signed‐rank test (*p < 0.05), and the listed p‐values refer to the comparison between the data obtained without pitavastatin or simvastatin (as a control) and those obtained with each concentration of pitavastatin or simvastatin.
Treatment of DCs with statins suppresses the Th2 induction that is mediated by TSLP‐stimulated DCs
Because statins appear to possess the inhibitory effects for symptoms of allergic diseases 29, 31, 34 as well as against inflammatory responses in preclinical studies 35, we next examined the inhibitory effects of pitavastatin and simvastatin on the DC‐mediated inflammatory Th2 response that triggers the upstream immune cascade associated with allergic responses. Naïve CD4+ T cells were cocultured for 7 days with allogeneic mDCs that had been pretreated with TSLP, TSLP + pitavastatin, or TSLP + simvastatin, and the cytokine production by the primed CD4+ T cells was then examined. We found TLR7/8‐ligand R848‐stimulated mDCs induced the differentiation of T cells that produced high levels of IFN‐γ, TNF‐α, and IL‐10, but not of IL‐4, IL‐5, and IL‐13, indicating a dominant Th1 cell response (Fig. 2A), as previously reported 36. After the coculture with TSLP‐stimulated mDCs, naïve CD4+ T cells were differentiated into the T cells that produced high levels of IL‐4, IL‐5, IL‐13, and TNF‐α, but not IFN‐γ and IL‐10 (Fig. 2A). This cytokine profile is consistent with an inflammatory Th2 cell response; the addition of anti‐OX40L mAb into the DC−T cell cultures inhibited this inflammatory Th2 cell differentiation, as previously described 15, indicating that DC‐derived OX40L is responsible for inflammatory Th2 cell induction. Notably, addition of statins into mDCs precultured with TSLP also inhibited the production of IL‐4, IL‐5, IL‐13, and TNF‐α but promoted the production of IL‐10 by the generated T cells (Fig. 2A).
Figure 2.
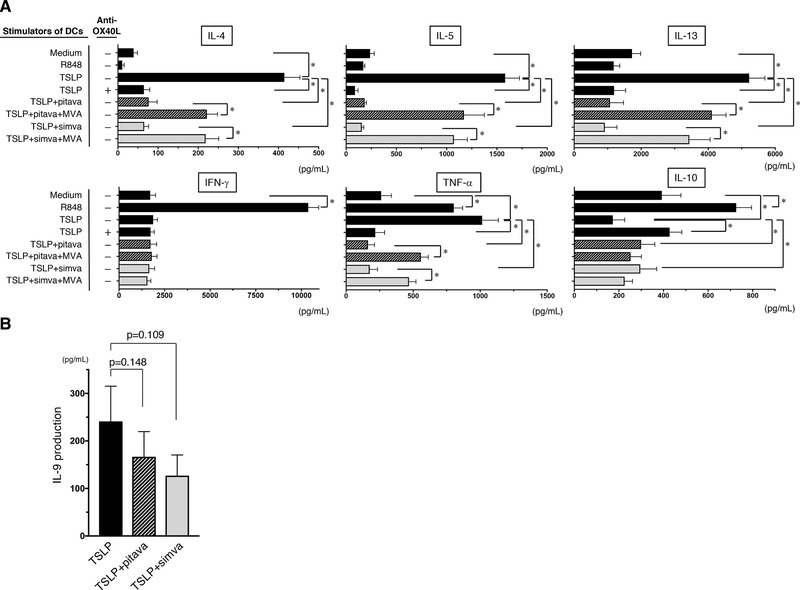
Effect of statin treatment on the induction of inflammatory Th2 response by TSLP‐stimulated DCs. (A) Naïve CD4+ T cells were cocultured for 7 days with DCs that had been pretreated with medium alone, R848, TSLP, TSLP + 10 µM pitavastatin, TSLP + 10 µM pitavastatin + 100 µM MVA, TSLP + 10 µM simvastatin, or TSLP + 10 µM simvastatin + 100 µM MVA for 24 h. In some cases, anti‐OX40L mAb was added into the DC−T cell cultures. Cytokine production by the primed CD4+ T cells was assessed by measuring the levels in the supernatants. (B) Naïve CD4+ T cells were cocultured for 7 days with DCs that had been pretreated with TSLP, TSLP + 10 µM pitavastatin, and TSLP + 10 µM simvastatin. IL‐9 production by the primed CD4+ T cells was assessed by measuring the levels in the supernatants. (A and B) One set of experiment was performed by DCs from one donor and T cells from another allogenic donor. Data are the mean ± SEM of six (A) and eight (B) independent experiments. Statistical significance was determined using paired Wilcoxon signed‐rank test (*p < 0.05).
Because statins inhibit the synthesis of mevalonate (mevalonic acid, MVA), the metabolite downstream of HMG‐CoA (Fig. 3), MVA is the limiting step in the effect of HMG‐CoA reductase. To investigate whether the modulatory effects of statins are mediated by their actions as HMG‐CoA reductase inhibitors, we added MVA to the mDC preculture along with the statins. The suppressive effect of statins on the differentiation of inflammatory Th2 cells was neutralized by the simultaneous addition of MVA to the mDC preculture (Fig. 2A). The level of IFN‐γ secreted by T cells primed with TSLP‐stimulated mDCs was lower than that from T cells primed with R848‐stimulated mDCs, and the IFN‐γ levels were unchanged by the presence of statins in the DC preculture. This could be attributable to the scarce production of IL‐12 by TSLP‐stimulated mDCs 14, 15. Our findings suggest that statins have the potential to suppress the upstream response in the immune cascade of allergy.
Figure 3.
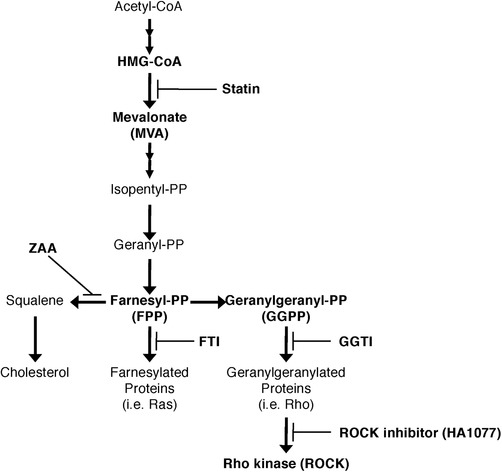
Schematic of the mevalonate pathway, showing the sites of action of statins and other inhibitors. Statins inhibit the conversion of 3‐hydroxy‐3‐methylglutaryl‐CoA (HMG‐CoA) to mevalonate and thus inhibit the downstream synthesis of not only cholesterol, but also isoprenoid intermediates, such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which regulate posttranslational modifications of the small GTPase Ras and Rho families. Zaragozic acid A (ZAA), farnesyl transferase inhibitor FTI‐277, and geranylgeranyl transferase inhibitor GGTI‐298 block the synthesis pathways that split off from FPP in the mevalonate pathway. HA1077 blocks the pathway of Rho kinase (ROCK).
Th9 cells are closely associated with Th2 cells and play pleiotropic and pathogenic roles in allergic inflammation 37. Also TSLP‐stimulated mDCs can induce the differentiation of Th9 cells 38. We here found that TSLP‐stimulated mDCs can instruct naïve CD4+ T cells into T cells producing IL‐9, while addition of statins into DC culture moderately but not significantly reduced the IL‐9 production by the primed T cells (Fig. 2B).
Statins inhibit maintenance of CRTH2+CD4+ Th2 memory cells induced by TSLP‐stimulated mDCs
CRTH2+CD4+ Th2 memory cells are important in the maintenance of Th2‐mediated atopic dermatitis, and TSLP‐stimulated mDCs induce the expansion of CRTH2+CD4+ cells through OX40L expression 19, 39, 40. Therefore, we next investigated whether statins are able to inhibit the expansion of CRTH2+CD4+ Th2 memory cells and the Th2 phenotype of CRTH2 cells maintained by TSLP‐stimulated mDCs. Purified CRTH2+CD4+ Th2 cells were cocultured for 7 days with allogeneic mDCs that had been pretreated with TSLP, TSLP + pitavastatin, or TSLP + pitavastatin + MVA. The resulting cell expansion and Th2 cytokine expression of the primed CRTH2+CD4+ Th2 cells were analyzed. We found that TSLP‐stimulated mDCs induced a robust expansion of CRTH2+CD4+ Th2 cells, with a sixfold increase in the total number of T cells compared with polyclonal stimulation with anti‐CD3 and anti‐CD28 antibodies, in agreement with findings from a previous report 19. In contrast, the addition of anti‐OX40L mAb into the DC−T cell cultures inhibited the expansion of CRTH2+CD4+ Th2 cells (Fig. 4A), indicating that the expansion of these memory cells requires for DC‐derived OX40L. Notably, mDCs precultured with TSLP + pitavastatin failed to induce CRTH2+CD4+ Th2 cell expansions, whereas the suppressive effect of pitavastatin was counteracted by the addition of MVA to the mDC preculture. Furthermore, we found that production of Th2 cytokine IL‐4, IL‐5, and IL‐13 from CRTH2+CD4+ Th2 cells induced by TSLP‐stimulated mDCs was significantly decreased by preculture with pitavastatin on the DCs, as well as the addition of anti‐OX40L mAb into the DC−T cell cultures (Fig. 4B). This suppressive effect of pitavastatin was also counteracted by the addition of MVA. Thus, the statin has the ability to quantitatively and qualitatively suppress the maintenance of the Th2 response induced by TSLP.
Figure 4.
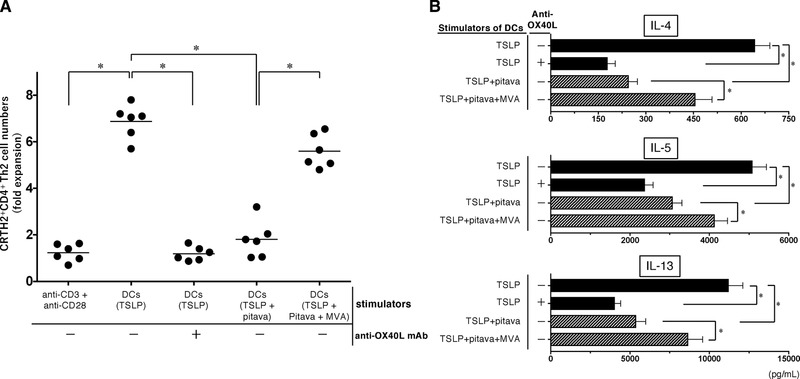
Effect of statin treatment on maintenance of CRTH2+CD4+ Th2 memory cell induced by TSLP‐stimulated DCs. CRTH2+CD4+ T cells were cultured for 7 days with immobilized anti‐CD3 plus soluble anti‐CD28, or with allogeneic mDCs that had been pretreated with TSLP, TSLP + 10 µM pitavastatin, or TSLP + 10 µM pitavastatin + 100 µM MVA for 24 h. In some cases, anti‐OX40L mAb was added into the DC−T cell cultures. After culture, the cell numbers were measured via a trypan blue dye‐exclusion test (A). Cytokine production by the primed CRTH2+CD4+ T cells was assessed by measuring the levels in the supernatants (B). One set of experiment was performed by DCs from one donor and T cells from another allogenic donor, and data are the mean ± SEM of six independent experiments (A and B). Statistical significance was determined using paired Wilcoxon signed‐rank test (*p < 0.05).
These findings suggest that statins are able to suppress not only the induction but also the maintenance of the Th2 responses that stem from TSLP.
Statins inhibit TSLP‐induced OX40L expression by mDCs
In the DC‐mediated allergic response, OX40L represents a key molecule in the induction of inflammatory Th2 responses 15, 41, 42, 43 and is responsible for Th2 memory cell expansion 19, 44. Therefore, we next examined whether statins can repress the TSLP‐induced OX40L upregulation on mDCs. As shown in Figure 5A and B, in agreement with the previous works 14, 15, TSLP induced upregulation of OX40L expression on mDCs. Notably, pitavastatin and simvastatin each significantly suppressed the expression of OX40L by TSLP‐stimulated mDCs (Fig. 5A; simvastatin data are not shown). We also found that the statin‐induced suppression of OX40L expression was counteracted by the simultaneous addition of MVA.
Figure 5.
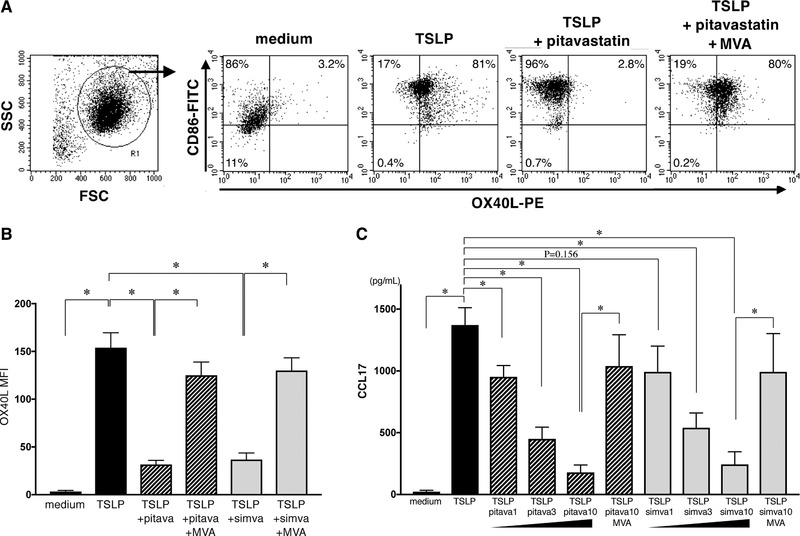
Effect of statins on OX40L and CCL17 expression by TSLP‐stimulated DCs. (A−C) mDCs were treated with medium alone, TSLP, TSLP + 10 µM pitavastatin, TSLP + 10 µM pitavastatin + 100 µM MVA, TSLP + 10 µM simvastatin, or TSLP + 10 µM simvastatin + 100 µM MVA. OX40L expression on these mDCs was analyzed by flow cytometry after 48 h of culture (A and B), and the CCL17 secretion in supernatants after 24 h of mDC culture was analyzed by an ELISA (C). The staining profiles of anti‐OX40L mAb and isotype‐matched control are indicated by shaded and open areas, respectively, and results of a representative experiment are shown in six independent experiments (A). Data indicate the MFI, which was calculated by the subtraction of the MFI for the isotype control‐treated cells from the MFI for the cells treated with anti‐OX40L mAb (B). One set of experiment was performed by DCs from one donor, and data are shown as the mean ± SEM of six independent donors (B and C). Statistical significance was determined using paired Wilcoxon signed‐rank test (*p < 0.05).
Statins inhibit TSLP‐induced CCL17 secretion by mDCs
TSLP‐stimulated mDCs secrete the Th2 cell‐attracting chemokine, CCL17 (TARC) 19, which contributes to the migration of memory Th2 cells at the inflammatory sites and is a clinical biomarker of atopic dermatitis 45; thus, they are largely responsible for chronic allergic inflammation. We next examined the effect of statins on the capacity of TSLP‐stimulated mDCs to secrete CCL17. Both pitavastatin and simvastatin significantly inhibited the CCL17 secretion from TSLP‐stimulated mDCs in a dose‐dependent manner (only 1 µM simvastatin showed a trend; Fig. 5C). We also found that the reduced CCL17 secretion induced by statins was recovered by the addition of MVA. This counter‐effect of MVA to statins confirms that the inhibitory effect of statins can be attributed to their role as an HMG‐CoA reductase inhibitor in the mevalonate cascade. These findings suggest that statins functioning as HMG‐CoA reductase inhibitors act as potent inhibitors of both OX40L and CCL17 expressions by TSLP‐stimulated mDCs.
Statins inhibit the activation of NF‐κB p50 and STAT6
Arima et al. previously demonstrated that predominant p50 activation in the NF‐κB pathway triggered by TSLP is the determinant for OX40L upregulation, while STAT6 activation by TSLP is the responsible mechanism for inducing CCL17 production in mDCs 46. CCL17 expression has been observed to be positively regulated by STAT6 in epithelial cells, macrophages, and T cells 47. To assess whether statins modulate these processes in their inhibition of OX40L and CCL17 expressions, we next examined the intranuclear activation of NF‐κB p50 and STAT6 in mDCs in the presence or absence of TSLP, pitavastatin, and MVA.
The results from flow cytometry using Phosflow revealed that intranuclear pSTAT6 and NF‐κB p50 were each upregulated after 3 h of stimulation with TSLP (Fig. 6A and B). Virtualized intranuclear pSTAT6 of mDCs was brighter in cells subjected to TSLP stimulation than in cells cultured with control medium according to a single cell‐analysis by ImageStream X mark II (Fig. 6C). We also found that the TSLP‐driven induction of pSTAT6 and NF‐κB p50 were each inhibited by the addition of 10 µM pitavastatin. Furthermore, MVA counteracted the effect of this statin. Hence, pitavastatin suppresses TSLP‐induced CCL17 secretion through the inhibition of STAT6 phosphorylation and suppresses TSLP‐induced OX40L expression through the inhibition of NF‐κB p50 activation.
Figure 6.
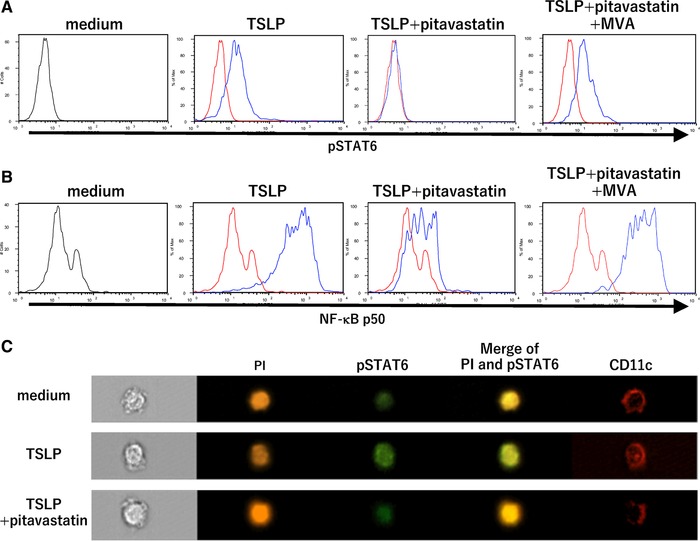
Effect of statin treatment on the TSLP‐induced activation of STAT6 and NF‐κB p50. (A−C) mDCs were preliminarily treated with medium alone for 6 h to induce the upregulation of the TSLP receptor, and then TSLP, TSLP + 10 µM pitavastatin, or TSLP + 10 µM pitavastatin + 100 µM MVA was added to the DC culture. After 3 h, DCs were immediately fixed using a Cytofix/Cetoperm kit (BD biosciences) and stained with anti‐pSTAT6 mAbs (A) and anti‐NF‐κB p50 mAbs (B). The staining profiles produced by the indicated stimuli are shown by blue lines and those produced by medium alone as a control are shown by red lines (A and B). DCs were visualized at the single cell level via immunofluorescence with anti‐pSTAT6 mAbs (Alexa 488, green), nuclei staining with PI (orange), and surface staining with anti‐CD11c mAb (APC, red; C). One set of experiment was performed by DCs from one donor. Similar results were observed in four independent experiments, and the results of a representative experiment are shown.
Statin‐induced suppression of OX40L and CCL17 can be attributed to inhibition of GGPP/ROCK pathway
In the cholesterol biosynthesis, statins can inhibit the synthesis of isoprenoids (FPP and GGPP) and the resultant Ras and Rho GTPases, which are responsible for the pleiotropic effects of statins 27, 32, 48 (Fig. 3). To assess the mevalonate pathway targets of statins during their inhibition of mDC expression of Th2‐related molecules, we compared the effects of the squalene synthetase inhibitor ZAA, the farnesyl transferase inhibitor FTI‐277, the geranylgeranyl transferase inhibitor GGTI‐298, and the ROCK inhibitor HA1077 with that of statins in the culture of mDCs with TSLP. We found that both GGTI‐298 and HA1077, but neither ZAA nor FTI‐277, mimicked the effect of statins on the TSLP‐stimulated mDCs in regard to their inhibition of OX40L expression (Fig. 7A and B) and CCL17 secretion (Fig. 7C). These findings suggest that statins inhibit inflammatory Th2 responses through a blockade of the GGPP‐ROCK branch in the mevalonate pathway.
Figure 7.
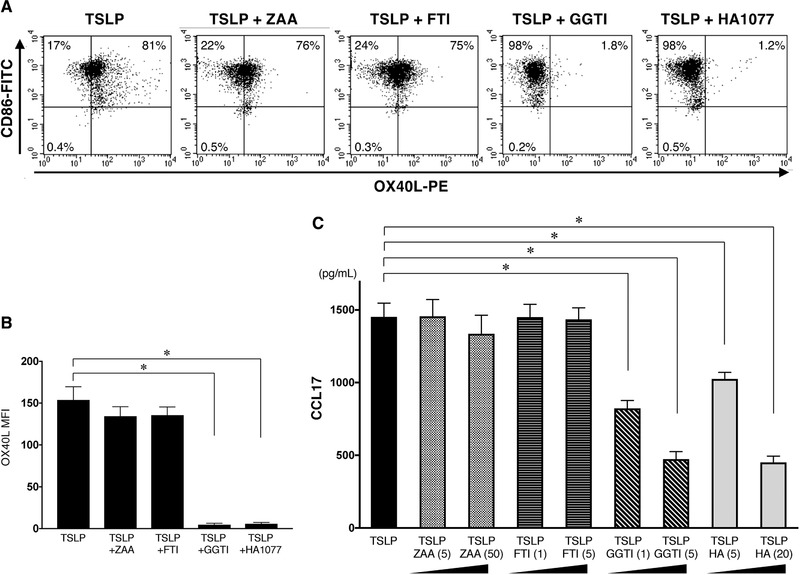
Inhibitors of GGPP/ROCK pathway mimic the effect of statins on the TSLP‐mediated OX40L and CCL17 expression by mDCs. (A and B) mDCs were treated with TSLP in the presence or absence of 5 µM ZAA, 5 µM FTI‐277, 5 µM GGTI‐298, or 20 µM HA1077 (HA). The OX40L expression on mDCs was analyzed by flow cytometry after 48 h of culture. The staining profiles of anti‐OX40L mAb and isotype‐matched control are indicated by shaded and open areas, respectively (A). Data indicate the MFI, which was calculated by the subtraction of the MFI for the isotype control‐treated cells from the MFI for the cells treated with anti‐OX40L mAb (B). One set of experiment was performed by DCs from one donor. The results of a representative experiment are shown (A) and data are shown as the mean ± SEM of six independent donors (B). (C) mDCs were treated with TSLP in the presence or absence of 5 µM or 50 µM ZAA, 1 µM or 5 µM FTI‐277, 1 µM or 5 µM GGTI‐298, or 5 µM or 20 µM HA1077 (HA). After 24 h, the concentrations of CCL17 in the culture supernatants were measured by ELISA assay. One set of experiment was performed by DCs from one donor, and data are shown as the mean ± SEM of six independent donors. Statistical significance was determined using paired Wilcoxon signed‐rank test (*p < 0.05).
Discussion
It has been reported that atorvastatin improved cough in patients with bronchiectasis 29 and that statins reduce airway inflammation in asthmatics 31. Indeed, statin‐users achieved better asthma control compared with non‐users 34. These observations suggest that statins could be a potential anti‐inflammation treatment and are beneficial for patients with asthma. However, the cellular and molecular immunoregulatory mechanisms of statins have not been elucidated. In the present study, we clarified a cellular target and intracellular mechanism by which statins are useful for treating allergic diseases and act as an inhibitor of allergic inflammation.
Historically, the drugs for treating allergies have been focused on the associated effector cells, such as T cells, mast cells, and eosinophils 6, 7. These drugs include corticosteroids, chemical mediator‐antagonists, anti‐IgE antibodies 49, and soluble IL‐4 receptor α‐chains 50, which basically target the effector cells or factors of allergy. Recently, it has been reported that DCs play a critical role in the upstream phase of allergy 51, 52, 53, and TSLP is extremely important in this process as a trigger of the allergic inflammation cascade 10. mDCs that have been stimulated with TSLP can initiate the development and expansion of inflammatory Th2 cells through the expression of OX40L and can recruit memory Th2 cells through the CCL17 secretion 15, 19. Thus, OX40L and CCL17 are key molecules in triggering and maintaining the inflammatory Th2 response in the allergic cascade and could be new targets for the treatment of allergic diseases 54, 55, 56. Although there is evidence indicating that statins have anti‐inflammatory effects on macrophages or monocyte‐derived DCs 57, 58, there are no studies describing their effects on blood mDCs. In this context, our results showing that statins exert a cholesterol‐independent inhibitory effect on DC‐mediated Th2 responses and on OX40L/CCL17 inductions from DCs through a blockade of NF‐κB p50 and STAT6 activation confirm that statins could be useful in the treatment of allergic disorders by targeting DCs.
The polarization of Th9 cells requires the cytokines both IL‐4 and TGF‐β 59. However, a common consensus has not been reached regarding the involvement of OX40L in Th9 differentiation. OX40L leads to an increase in Th9 differentiation from naïve CD4+ T cells 60, 61. In contrast, Froidure et al. reported the TSLP‐driven development of Th9 cells does not depend on OX40L 38. From our findings as statins inhibited OX40L expression on DCs and moderately suppressed IL‐9 induction from generated T cells, OX40L appears to be involved in Th9 polarization. Since addition of statins on DC culture simultaneously inhibit T cell‐derived IL‐4 in the T cell differentiation, it is possible that Th9 differentiation is suppressed through the IL‐4 downregulation.
It has been demonstrated that the molecular mechanism underlying the immunomodulatory effect of statins is largely due to the inhibition of Rho/ROCK 62. Additionally, our results showing that both GGTI‐298 and ROCK inhibitor mimicked the inhibitory function of statins suggest that statins exhibit their immunomodulatory effect on DCs by targeting geranylgeranylated Rho and the Rho kinase pathway as illustrated in Figure 3. Our result is consistent with those of recent studies showing that ROCK inhibitors improve asthma symptoms by suppressing airway hyperresponsiveness and attenuating allergic airway inflammation 63, 64, and that a ROCK inhibitor reduces mucous secretion and downregulates the levels of IL‐4 and IL‐13 in OVA‐challenged mice by regulating STAT6 and NF‐κB 65.
In conclusion, our study may provide insights into the possibility of applying statins as an option for the supportive treatment of allergic diseases. Statins work as an inhibitor of the DC‐mediated Th2 response to induce allergic inflammation regardless of TSLP functions. Based on the new concept of targeting the upstream phase rather than the effector phase of the immunological allergic cascade, our results confirm the curative effect of statins and also indicate that a new strategy of targeting to OX40L and CCL17 may be plausible for the treatment of allergic diseases.
Materials and methods
Media and reagents
RPMI‐1640 supplemented with 2 mM l‐glutamine, 100 U/mL penicillin, 100 ng/mL streptomycin, and 2% heat‐inactivated human AB serum was used for cell cultures throughout the experiments. TSLP (R&D Systems) and R848 (Invivogen) were used at 15 ng/mL and 1 µg/mL, respectively. Pitavastatin (Kowa), simvastatin (Calbiochem), HA1077 (Calbiochem), and mevalonate (Sigma) were each dissolved in anhydrous ethanol. FPP (Calbiochem) and GGPP (Calbiochem) were each dissolved in methanol. FTI‐277(Calbiochem) and geranylgeranyl transferase inhibitor 298 (GGTI‐298) (Calbiochem) were each dissolved in DMSO. Zaragozic acid A (ZAA; Squalestatin) (Sigma) was dissolved in distilled water. Ethanol, methanol, DMSO, and distilled water were each diluted as vehicle controls.
Isolation and culture of blood DCs
All studies involving human samples were performed following institutional review board approved protocols at Kansai Medical University through which informed consent was obtained. All subjects gave written informed consent in accordance with the recommendations of the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki.
Human peripheral blood DC subsets (mDCs and plasmacytoid DCs) and T cells were isolated from PBMCs of total ten healthy adult donors without allergy (Supporting Information Table SI), as described previously 36, 66. Briefly, PBMCs from normal healthy donors were isolated by Ficoll Hypaque density gradient centrifugation, and the DC‐enriched PBMCs were isolated from the total PBMCs by immunobeads‐selection methods (CD3‐ and CD14‐beads negative selection and subsequent CD4‐beads positive selection). The CD11c+/lineage−/BDCA4−/CD4+ fraction (considered to be the mDCs) was sorted using a FACS Aria® (BD Biosciences) using PE‐labeled anti‐CD304 (AD5‐17F6: BDCA‐4; Miltenyi Biotec), allophycocyanin (APC)‐labeled anti‐CD11c (B‐ly6: BD Biosciences), a mixture of FITC‐labeled monoclonal antibodies (mAbs) against lineage markers (CD3 [M2AB: Exalpha], CD14 [M5E2: BD Biosciences], CD15 [M5E2: BD Biosciences], CD16 [J5511: Exalpha], CD19 [HIB19: BD Biosciences], and CD56 [NCAM16.2: BD Biosciences]), and PE‐Cy5.5‐labeled anti‐CD4 (RPA‐T4: BD Biosciences; Supporting Information Fig. S1). Purified mDCs were seeded at a density of 5 × 104 cells per 200 µL of medium in flat‐bottomed 96‐well plates.
Purification of naïve CD4+ and CRTH2+CD4+ memory T cell subsets
Naïve CD4+ T cells were isolated using a naïve CD4+ T cell isolation kit II (Miltenyi Biotec) according to the manufacturer's instructions, reaching >94 % purity; this was confirmed by PE‐labeled anti‐CD45RA (HI100: BD Biosciences), FITC‐labeled CD3, and PE‐Cy5.5‐labeled anti‐CD4 staining. CRTH2+ CD4+ memory T cells were isolated using a CD294 (CRTH2) MicroBead Kit (Miltenyi Biotec) according to the manufacturer's instructions, followed by cell sorting using an FACS Aria® using PE‐labeled anti‐CD294 (BM‐16: Miltenyi Biotec) and PE‐Cy5.5‐labeled anti‐CD4.
Analysis of DCs
mDCs were stained with FITC‐labeled anti‐CD40 (5C3: BD Biosciences), FITC‐labeled anti‐CD80 (2D10: BioLegend), FITC‐labeled anti‐CD83 (HB15a: Beckman Coulter), FITC‐labeled anti‐CD86 (2331: BD Biosciences), FITC‐labeled anti‐CD274 (programmed death ligand 1) (MIH2: BioLegend), or PE‐labeled anti‐HLA‐DR (TU36: BD Biosciences) after 24 h of culture and with PE‐labeled anti‐OX40L (ANC10G1: Ancell) after 48 h of culture, then analyzed using a FACS Calibur (BD Biosciences). Isotype‐matched mAbs (R&D Systems) were used as negative controls. Viable cells were counted in triplicate using trypan‐blue exclusion to identify dead cells and were also evaluated as annexin V‐negative fractions using an annexin V‐FITC Apoptosis Detection kit (Sigma–Aldrich) after 24 h of culture. The production of CCL17 in the culture supernatants after 24 h was determined by ELISA (R&D Systems). To evaluate intracellular phosphorylated (p) STAT6 and NF‐κB‐p50, mDCs were preliminarily cultured for 6 h with medium alone to induce upregulation of the TSLP receptor, and then stimulated with TSLP ± statins for 3 h, after which the cells were immediately fixed using a Cytofix/Cytoperm kit (BD biosciences) and stained using Alexa 488‐labeled anti‐NF‐κB‐p50 (4D1: BioLegend) and Alexa 488‐labeled or PE‐labeled anti‐phospho‐stat6 (18/P‐Stat6: BD Biosciences). The stained cells were then analyzed using a FACSCalibur and visualized by ImageStream X mark II (Merck Millipore). In some experiments, PI was used for nuclei staining.
DC–T cell coculture
After 24 h of culture under different conditions, CD11c+ mDCs were collected and subsequently cocultured with 2 × 104 freshly purified allogenic naïve CD4+ T cells (DC‐to‐T cell ratio, 1:4) or 1 × 104 freshly purified allogeneic CRTH2+CD4+ Th2 memory cells (DC‐to‐T cell ratio, 1:2) in 96‐well round‐bottom culture plates. In some experiments, anti‐OX40L mAb (ik‐5: 50 µg/mL) was used 15. Mouse IgG2a and goat IgG (R&D Systems), and PBS were used as controls.
Analyses of T‐cell cytokine production
After 7 days of DC−T cell coculture, the primed CD4+ T cells were collected and washed. For the detection of cytokine production in the culture supernatants, the T cells were re‐stimulated for 24 h with immobilized anti‐CD3 (OKT3, 5 µg/mL) and soluble anti‐CD28 (1 µg/mL) at a concentration of 106 cells/mL. The levels of IL‐4, IL‐5, IL‐9, IL‐10, IL‐13, TNF‐α, and IFN‐γ were each measured by ELISA (R&D Systems) and CBA (BD Biosciences).
Analyses of T‐cell expansion
Using freshly isolated CRTH2+CD4+ Th2 memory cells (purity, >99%), and 2 × 104 T cells were stimulated for 7 days with immobilized anti‐CD3 (OKT3, 5 µg/ml) and soluble anti‐CD28 (1 µg/ml) or with DCs that had been precultured under one of several conditions. The cultured T cells were then collected and resuspended in an EDTA‐containing medium to dissociate the clusters. Viable cells were counted by trypan blue exclusion of the dead cells.
Statistical analysis
Data were analyzed using Wilcoxon signed‐rank test (nonparametric test), and p‐values of < 0.05 were considered statistically significant. Data analysis was carried out using GraphPad Prism (GraphPad Software).
Conflict of interest
The authors do not have any conflicts of interest to report in this work.
Abbreviations
- FPP
farnesyl pyrophosphate
- GGPP
geranylgeranyl pyrophosphate
- HMG‐CoA
3‐hydroxy‐3‐methylglutaryl‐CoA
- mDC
myeloid DC
- MVA
mevalonic acid
- Ox40‐L
OX40‐ligand
- SLE
systemic lupus erythematosus
- Th2
T helper type 2
- TSLP
thymic stromal lymphopoietin
Supporting information
Supplementary Figure S1. Isolation of blood mDCs. Blood mDCs were detected and isolated as CD11c‐positive and BDCA‐4‐negative population in the lineage (CD3, CD14, CD15, CD16, CD19, and CD56)‐negative and CD4‐positive fraction in DC‐enriched PBMCs after immunobeads‐selection methods (CD3‐ and CD14‐beads negative selection and subsequent CD4‐beads positive selection) from the total PBMCs. A representative FACS analysis to detect mDCs in PBMCs from ten healthy donors was shown. Indicated numbers are the percentages of gated fraction. FSC; forward scatter, SSC; side scatter.
Supplementary Table SI Characteristics of donors for isolation of mDCs or T cells
Acknowledgments
The authors thank Katie Oakley, PhD, from Edanz Group (http://www.edanzediting.com/ac) for editing a draft of this manuscript. This study was supported in part by a grant KAKENHI (Grants‐in‐Aid for Scientific Research) (Grant Number JP17K09963).
The peer review history for this article is available at https://publons.com/publon/10.1002/eji.201847992
References
- 1. Devereux, G. , The increase in the prevalence of asthma and allergy: food for thought. Nat. Rev. Immunol. 2006. 6: 869–874. [DOI] [PubMed] [Google Scholar]
- 2. Mosmann, T. R. and Coffman, R. L. , TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989. 7: 145–173. [DOI] [PubMed] [Google Scholar]
- 3. Abbas, A. K. , Murphy, K. M. and Sher, A. , Functional diversity of helper T lymphocytes. Nature 1996. 383: 787–793. [DOI] [PubMed] [Google Scholar]
- 4. Constant, S. L. and Bottomly, K. , Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 1997. 15: 297–322. [DOI] [PubMed] [Google Scholar]
- 5. Renauld, J. C. , New insights into the role of cytokines in asthma. J. Clin. Pathol. 2001. 54: 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Busse, W. W. and Lemanske, R. F. , Asthma. N. Engl. J. Med. 2001. 344: 350–362. [DOI] [PubMed] [Google Scholar]
- 7. Holgate, S. T. , Science, medicine, and the future. Allergic disorders. BMJ 2000. 320: 231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pulendran, B. , Tang, H. and Manicassamy, S. , Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat. Immunol. 2010. 11: 647–655. [DOI] [PubMed] [Google Scholar]
- 9. Ito, T. , Inaba, M. , Inaba, K. , Toki, J. , Sogo, S. , Iguchi, T. , Adachi, Y. et al., A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J. Immunol. 1999. 163: 1409–1419. [PubMed] [Google Scholar]
- 10. Liu, Y. J. , Soumelis, V. , Watanabe, N. , Ito, T. , Wang, Y. H. , Malefyt, R. E. W. , Omori, M. et al., TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 2007. 25: 193–219. [DOI] [PubMed] [Google Scholar]
- 11. Ziegler, S. F. and Artis, D. , Sensing the outside world: TSLP regulates barrier immunity. Nat. Immunol. 2010. 11: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allakhverdi, Z. , Comeau, M. R. , Jessup, H. K. , Yoon, B. R. , Brewer, A. , Chartier, S. , Paquette, N. et al., Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007. 204: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reche, P. A. , Soumelis, V. , Gorman, D. M. , Clifford, T. , Liu M. R., Travis, M. , Zurawski, S. M. et al., Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 2001. 167: 336–343. [DOI] [PubMed] [Google Scholar]
- 14. Soumelis, V. , Reche, P. A. , Kanzler, H. , Yuan, W. , Edward, G. , Homey, B. , Gilliet, M. et al., Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002. 3: 673–680. [DOI] [PubMed] [Google Scholar]
- 15. Ito, T. , Wang, Y. H. , Duramad, O. , Hori, T. , Delespesse, G. J. , Watanabe, N. , Qin, F. X. et al., TSLP‐activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005. 202: 1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sugamura, K. , Ishii, N. and Weinberg, A. D. , Therapeutic targeting of the effector T‐cell co‐stimulatory molecule OX40. Nat. Rev. Immunol. 2004. 4: 420–431. [DOI] [PubMed] [Google Scholar]
- 17. Ilves, T. and Harvima, I. T. , OX40 ligand and OX40 are increased in atopic dermatitis lesions but do not correlate with clinical severity. J. Eur. Acad. Dermatol. Venereol. 2013. 27: e197‐205. [DOI] [PubMed] [Google Scholar]
- 18. Arestides, R. S. , He, H. , Westlake, R. M. , Chen, A. I. , Sharpe, A. H. , Perkins, D. L. and Finn, P. W. , Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur. J. Immunol. 2002. 32: 2874–2880. [DOI] [PubMed] [Google Scholar]
- 19. Wang, Y. H. , Ito, T. , Homey, B. , Watanabe, N. , Martin, R. , Barnes, C. J. , McIntyre, B. W. et al., Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin‐activated dendritic cells. Immunity 2006. 24: 827–838. [DOI] [PubMed] [Google Scholar]
- 20. Prescott, S. L. , Macaubas, C. , Smallacombe, T. , Holt, B. J. , Sly, P. D. and Holt, P. G. , Development of allergen‐specific T‐cell memory in atopic and normal children. Lancet 1999. 353: 196–200. [DOI] [PubMed] [Google Scholar]
- 21. Mojtabavi, N. , Dekan, G. , Stingl, G. and Epstein, M. M. , Long‐lived Th2 memory in experimental allergic asthma. J. Immunol. 2002. 169: 4788–4796. [DOI] [PubMed] [Google Scholar]
- 22. Kobashigawa, J. A. , Katznelson, S. , Laks, H. , Johnson, J. A. , Yeatman, L. , Wang, X. M. , Chia, D. et al., Effect of pravastatin on outcomes after cardiac transplantation. N. Engl. J. Med. 1995. 333: 621–627. [DOI] [PubMed] [Google Scholar]
- 23. Youssef, S. , Stüve, O. , Patarroyo, J. C. , Ruiz, P. J. , Radosevich, J. L. , Hur, E. M. , Bravo, M. et al., The HMG‐CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002. 420: 78–84. [DOI] [PubMed] [Google Scholar]
- 24. Vollmer, T. , Key, L. , Durkalski, V. , Tyor, W. , Corboy, J. , Markovic‐Plese, S. , Preiningerova, J. et al., Oral simvastatin treatment in relapsing‐remitting multiple sclerosis. Lancet 2004. 363: 1607–1608. [DOI] [PubMed] [Google Scholar]
- 25. Sparrow, C. P. , Burton, C. A. , Hernandez, M. , Mundt, S. , Hassing, H. , Patel, S. , Rosa, R. et al., Simvastatin has anti‐inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler. Thromb. Vasc. Biol. 2001. 21: 115–121. [DOI] [PubMed] [Google Scholar]
- 26. Lawman, S. , Mauri, C. , Jury, E. C. , Cook, H. T. and Ehrenstein, M. R. , Atorvastatin inhibits autoreactive B cell activation and delays lupus development in New Zealand black/white F1 mice. J. Immunol. 2004. 173: 7641–7646. [DOI] [PubMed] [Google Scholar]
- 27. Goldstein, J. L. and Brown, M. S. , Regulation of the mevalonate pathway. Nature 1990. 343: 425–430. [DOI] [PubMed] [Google Scholar]
- 28. Amuro, H. , Ito, T. , Miyamoto, R. , Sugimoto, H. , Torii, Y. , Son, Y. , Nakamichi, N. et al., Statins, inhibitors of 3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase, function as inhibitors of cellular and molecular components involved in type I interferon production. Arthritis Rheum. 2010. 62: 2073–2085. [DOI] [PubMed] [Google Scholar]
- 29. Mandal, P. , Chalmers, J. D. , Graham, C. , Harley, C. , Sidhu, M. K. , Doherty, C. , Govan, J. W. et al., Atorvastatin as a stable treatment in bronchiectasis: a randomised controlled trial. Lancet Respir. Med. 2014. 2: 455–463. [DOI] [PubMed] [Google Scholar]
- 30. Egesi, A. , Sun, G. , Khachemoune, A. and Rashid, R. M. , Statins in skin: research and rediscovery, from psoriasis to sclerosis. J. Drugs Dermatol. 2010. 9: 921–927. [PubMed] [Google Scholar]
- 31. Yuan, C. , Zhou, L. , Cheng, J. , Zhang, J. , Teng, Y. , Huang, M. , Adcock, I. M. et al., Statins as potential therapeutic drug for asthma? Respir. Res. 2012. 13: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nagashima, T. , Okazaki, H. , Yudoh, K. , Matsuno, H. and Minota, S. , Apoptosis of rheumatoid synovial cells by statins through the blocking of protein geranylgeranylation: a potential therapeutic approach to rheumatoid arthritis. Arthritis Rheum. 2006. 54: 579–586. [DOI] [PubMed] [Google Scholar]
- 33. Moret, F. M. , van der Wurff‐Jacobs, K. M. , Bijlsma, J. W. , Lafeber, F. P. and van Roon, J. A. , Synovial T cell hyporesponsiveness to myeloid dendritic cells is reversed by preventing PD‐1/PD‐L1 interactions. Arthritis Res. Ther. 2014. 16: 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeki, A. A. , Oldham, J. , Wilson, M. , Fortenko, O. , Goyal, V. , Last, M. , Last, A. et al., Statin use and asthma control in patients with severe asthma. BMJ Open 2013. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bhattacharjee, D. , Chogtu, B. and Magazine, R. , Statins in Asthma: Potential Beneficial Effects and Limitations. Pulm Med 2015. 2015: 835204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Torii, Y. , Ito, T. , Amakawa, R. , Sugimoto, H. , Amuro, H. , Tanijiri, T. , Katashiba, Y. et al., Imidazoquinoline acts as immune adjuvant for functional alteration of thymic stromal lymphopoietin‐mediated allergic T cell response. J. Immunol. 2008. 181: 5340–5349. [DOI] [PubMed] [Google Scholar]
- 37. Soroosh, P. and Doherty, T. A. , Th9 and allergic disease. Immunology 2009. 127: 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Froidure, A. , Shen, C. , Gras, D. , Van Snick, J. , Chanez, P. and Pilette, C. , Myeloid dendritic cells are primed in allergic asthma for thymic stromal lymphopoietin‐mediated induction of Th2 and Th9 responses. Allergy 2014. 69: 1068–1076. [DOI] [PubMed] [Google Scholar]
- 39. Kapsenberg, M. , Tweaking of memory T helper 2 cells by TSLP. Immunity 2006. 24: 673–675. [DOI] [PubMed] [Google Scholar]
- 40. Wang, Y. H. , Angkasekwinai, P. , Lu, N. , Voo, K. S. , Arima, K. , Hanabuchi, S. , Hippe, A. et al., IL‐25 augments type 2 immune responses by enhancing the expansion and functions of TSLP‐DC‐activated Th2 memory cells. J. Exp. Med. 2007. 204: 1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen, A. I. , McAdam, A. J. , Buhlmann, J. E. , Scott, S. , Lupher, M. L. , Greenfield, E. A. , Baum, P. R. et al., Ox40‐ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity 1999. 11: 689–698. [DOI] [PubMed] [Google Scholar]
- 42. Murata, K. , Ishii, N. , Takano, H. , Miura, S. , Ndhlovu, L. C. , Nose, M. , Noda, T. et al., Impairment of antigen‐presenting cell function in mice lacking expression of OX40 ligand. J. Exp. Med. 2000. 191: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu, Y. J. , Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell‐mediated allergic inflammation. J. Allergy Clin. Immunol. 2007. 120: 238–244; quiz 245‐236. [DOI] [PubMed] [Google Scholar]
- 44. Salek‐Ardakani, S. , Song, J. , Halteman, B. S. , Jember, A. G. , Akiba, H. , Yagita, H. and Croft, M. , OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J. Exp. Med. 2003. 198: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kataoka, Y. , Thymus and activation‐regulated chemokine as a clinical biomarker in atopic dermatitis. J. Dermatol. 2014. 41: 221–229. [DOI] [PubMed] [Google Scholar]
- 46. Arima, K. , Watanabe, N. , Hanabuchi, S. , Chang, M. , Sun, S. C. and Liu, Y. J. , Distinct signal codes generate dendritic cell functional plasticity. Sci Signal 2010. 3: ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fulkerson, P. C. , Zimmermann, N. , Hassman, L. M. , Finkelman, F. D. and Rothenberg, M. E. , Pulmonary chemokine expression is coordinately regulated by STAT1, STAT6, and IFN‐gamma. J. Immunol. 2004. 173: 7565–7574. [DOI] [PubMed] [Google Scholar]
- 48. Wang, C. Y. , Liu, P. Y. and Liao, J. K. , Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol. Med. 2008. 14: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Singh, J. and Kraft, M. , Anti‐IgE and other antibody targets in asthma. Handb. Exp. Pharmacol. 2008: 257–288. [DOI] [PubMed] [Google Scholar]
- 50. Borish, L. C. , Nelson, H. S. , Corren, J. , Bensch, G. , Busse, W. W. , Whitmore, J. B. , Agosti, J. M. et al., Efficacy of soluble IL‐4 receptor for the treatment of adults with asthma. J. Allergy Clin. Immunol. 2001. 107: 963–970. [DOI] [PubMed] [Google Scholar]
- 51. Holt, P. G. , Macrophage: dendritic cell interaction in regulation of the IgE response in asthma. Clin. Exp. Allergy 1993. 23: 4–6. [DOI] [PubMed] [Google Scholar]
- 52. Lambrecht, B. N. , De Veerman, M. , Coyle, A. J. , Gutierrez‐Ramos, J. C. , Thielemans, K. and Pauwels, R. A. , Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J. Clin. Invest. 2000. 106: 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hammad, H. , Lambrecht, B. N. , Pochard, P. , Gosset, P. , Marquillies, P. , Tonnel, A. B. and Pestel, J. , Monocyte‐derived dendritic cells induce a house dust mite‐specific Th2 allergic inflammation in the lung of humanized SCID mice: involvement of CCR7. J. Immunol. 2002. 169: 1524–1534. [DOI] [PubMed] [Google Scholar]
- 54. Gauvreau, G. M. , Boulet, L. P. , Cockcroft, D. W. , FitzGerald, J. M. , Mayers, I. , Carlsten, C. , Laviolette, M. et al., OX40L blockade and allergen‐induced airway responses in subjects with mild asthma. Clin. Exp. Allergy 2014. 44: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang, Y. H. and Liu, Y. J. , OX40‐OX40L interactions: a promising therapeutic target for allergic diseases? J. Clin. Invest. 2007. 117: 3655–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abboud, D. and Hanson, J. , Chemokine neutralization as an innovative therapeutic strategy for atopic dermatitis. Drug Discov. Today 2017. 22: 702–711. [DOI] [PubMed] [Google Scholar]
- 57. Liang, S. L. , Liu, H. and Zhou, A. , Lovastatin‐induced apoptosis in macrophages through the Rac1/Cdc42/JNK pathway. J. Immunol. 2006. 177: 651–656. [DOI] [PubMed] [Google Scholar]
- 58. Yilmaz, A. , Reiss, C. , Weng, A. , Cicha, I. , Stumpf, C. , Steinkasserer, A. , Daniel, W. G. et al., Differential effects of statins on relevant functions of human monocyte‐derived dendritic cells. J. Leukoc. Biol. 2006. 79: 529–538. [DOI] [PubMed] [Google Scholar]
- 59. Veldhoen, M. , Uyttenhove, C. , van Snick, J. , Helmby, H. , Westendorf, A. , Buer, J. , Martin, B. et al., Transforming growth factor‐beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9‐producing subset. Nat. Immunol. 2008. 9: 1341–1346. [DOI] [PubMed] [Google Scholar]
- 60. Xiao, X. , Balasubramanian, S. , Liu, W. , Chu, X. , Wang, H. , Taparowsky, E. J. , Fu, Y. X. et al., OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nat. Immunol. 2012. 13: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Murakami‐Satsutani, N. , Ito, T. , Nakanishi, T. , Inagaki, N. , Tanaka, A. , Vien, P. T. , Kibata, K. et al., IL‐33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol. Int. 2014. 63: 443–455. [DOI] [PubMed] [Google Scholar]
- 62. Greenwood, J. , Steinman, L. and Zamvil, S. S. , Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat. Rev. Immunol. 2006. 6: 358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Henry, P. J. , Mann, T. S. and Goldie, R. G. , A rho kinase inhibitor, Y‐27632 inhibits pulmonary eosinophilia, bronchoconstriction and airways hyperresponsiveness in allergic mice. Pulm. Pharmacol. Ther. 2005. 18: 67–74. [DOI] [PubMed] [Google Scholar]
- 64. Taki, F. , Kume, H. , Kobayashi, T. , Ohta, H. , Aratake, H. and Shimokata, K. , Effects of Rho‐kinase inactivation on eosinophilia and hyper‐reactivity in murine airways by allergen challenges. Clin. Exp. Allergy 2007. 37: 599–607. [DOI] [PubMed] [Google Scholar]
- 65. Xie, T. , Luo, G. , Zhang, Y. , Wang, X. , Wu, M. and Li, G. , Rho‐kinase inhibitor fasudil reduces allergic airway inflammation and mucus hypersecretion by regulating STAT6 and NFκB. Clin. Exp. Allergy 2015. 45: 1812–1822. [DOI] [PubMed] [Google Scholar]
- 66. Ito, T. , Kanzler, H. , Duramad, O. , Cao, W. and Liu, Y. J. , Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood 2006. 107: 2423–2431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Isolation of blood mDCs. Blood mDCs were detected and isolated as CD11c‐positive and BDCA‐4‐negative population in the lineage (CD3, CD14, CD15, CD16, CD19, and CD56)‐negative and CD4‐positive fraction in DC‐enriched PBMCs after immunobeads‐selection methods (CD3‐ and CD14‐beads negative selection and subsequent CD4‐beads positive selection) from the total PBMCs. A representative FACS analysis to detect mDCs in PBMCs from ten healthy donors was shown. Indicated numbers are the percentages of gated fraction. FSC; forward scatter, SSC; side scatter.
Supplementary Table SI Characteristics of donors for isolation of mDCs or T cells


