Abstract
Aim
Lusutrombopag is approved for the treatment of thrombocytopenia in chronic liver disease patients undergoing invasive procedures. This real‐world surveillance assesses the safety and effectiveness of lusutrombopag in Japan.
Methods
This ongoing, multicenter, prospective, real‐world surveillance is collecting data from case report forms between October 2016 and May 2021. Interim data up to September 2018 were used to evaluate safety (adverse events and adverse drug reactions [ADRs]) and effectiveness (proportion of patients avoiding preoperative platelet transfusion and change in platelet count from baseline).
Results
The safety analysis set included 331 patients. The mean baseline platelet count was 46.2 ± 13.7 × 109/L. Of 377 invasive procedures, radiofrequency ablation (110 procedures, 29.2%) was the most frequent. The mean time from starting lusutrombopag treatment to invasive procedure was 12.3 days. Incidences of serious adverse events and ADRs were 8.76% and 3.32%, respectively. Six cases (1.81%) of portal vein thrombosis were considered serious adverse events; of these, four cases (1.21%) were classified as serious ADRs. Of 300 patients who underwent an invasive procedure (excluding those with platelet transfusion refractoriness), 282 (94.0%) avoided preoperative platelet transfusion. In patients with platelet measurements before and after lusutrombopag administration who did not undergo platelet transfusion, the mean maximum change in platelet count from baseline was 41.7 ± 31.4 × 109/L (range, −6 to 276; n = 286). All patients receiving second (n = 20) and third (n = 1) treatments avoided preoperative platelet transfusion without developing any ADRs.
Conclusions
This real‐world surveillance further supports the safety and effectiveness of lusutrombopag in patients with chronic liver disease undergoing invasive procedures.
Keywords: Japan, liver diseases, lusutrombopag, postmarketing, product surveillance, thrombocytopenia
Introduction
Several liver conditions can lead to chronic liver disease (CLD), and might progress to cirrhosis or hepatocellular carcinoma. In Japan, causes of liver cirrhosis include chronic infection with hepatitis C (60.9%) or hepatitis B (13.9%) viruses, alcohol consumption (13.6%), primary biliary cirrhosis (2.4%), non‐alcoholic fatty liver disease/non‐alcoholic steatohepatitis (2.1%), and autoimmune hepatitis (1.9%).1 Thrombocytopenia, which is defined as moderate or severe if platelet levels are ≥50 to <100 × 109/L or < 50 × 109/L, respectively,2 is a common hematological disorder in patients with CLD.3
Patients with CLD might require invasive diagnostic or therapeutic procedures, and a high risk of procedure‐related bleeding complications has been reported among thrombocytopenic patients with platelet counts <75 × 109/L.4 Decreased production of thrombopoietin, which regulates both platelet production and maturation, is one of the main mechanisms of thrombocytopenia in patients with CLD.5 Additional mechanisms include splenic sequestration of platelets and increased destruction by means of shear stress, immune‐mediated responses (both autoimmune‐ and infection‐related), increased fibrinolysis, and bacterial translocation associated with endotoxemia.5
Lusutrombopag is a chemically synthesized, orally active, small‐molecule human thrombopoietin receptor agonist developed by Shionogi & Co., Ltd. (Osaka, Japan).6 It activates the signal transduction pathway similar to endogenous thrombopoietin and induces platelet production. Lusutrombopag was approved in Japan (2015) and the USA (2018) for the treatment of thrombocytopenia in adult patients with CLD who are scheduled to undergo a procedure.6, 7 In the European Union, the approved indication (2019) is the treatment of severe thrombocytopenia in adult patients with CLD undergoing invasive procedures.8
Data from multicenter, randomized, double‐blind, placebo‐controlled, clinical trials showed that lusutrombopag significantly reduced the proportion of patients with CLD who required platelet transfusion before invasive procedures (P < 0.01 vs. placebo).7, 8, 9 In the lusutrombopag treatment group of the phase 3 L‐PLUS 1 trial, the median platelet count increased to ≥50 × 109/L after 5 days, and the mean time to reach maximum platelet count was 13.4 days.7 The follow‐up L‐PLUS 2 study confirmed the efficacy of lusutrombopag, with the median platelet count increasing to ≥50 × 109/L after 6 days, and maximum platelet count being reached around day 12.8
In Japan, postmarketing surveillance is carried out by manufacturing companies and serves an important role in ensuring the appropriate use of newly approved drugs, and in confirming their safety and effectiveness in real‐world clinical practice.10 Thus, Shionogi is carrying out ongoing postmarketing surveillance of the safety and effectiveness of lusutrombopag in Japan for the treatment of thrombocytopenia in patients with CLD undergoing invasive procedures in routine clinical practice. This postmarketing surveillance was initiated in October 2016, and will be completed in May 2021. The current manuscript presents the results of interim postmarketing surveillance data up to 27 September 2018.
Methods
Study design and treatment
This surveillance was carried out in accordance with the Japanese regulatory requirements stipulated in the Good Post‐Marketing Study Practice. According to exemptions under the Good Post‐Marketing Study Practice ordinance by the Ministry of Health, Labor, and Welfare, institutional review board approval and informed consent were not required. However, the postmarketing surveillance protocol is consistent with the principles of the Declaration of Helsinki. This surveillance was registered with the Japan Pharmaceutical Information Center (identifier: JapicCTI‐163 432).
The planned survey period is from October 2016 to May 2021, and the enrollment period is from October 2016 to September 2020. The target number of patients is 1000. Patients with CLD and thrombocytopenia receiving lusutrombopag who are registered centrally during the surveillance period and scheduled for an invasive procedure are being enrolled in this postmarketing surveillance at approximately 250 sites in Japan. The observation period is 2 months, starting from the first administration of lusutrombopag. If additional treatment cycle(s) are carried out within 6 months of starting the first treatment, the observation period is extended for another 2 months from the start of each additional treatment cycle.
Data are collected from physicians using specific survey case report forms, and include demographic and clinical data, comorbidities and medical history, type of invasive procedures carried out, whether platelet transfusion was administered during the observation period, concomitant therapies, examination result of portal vein thrombosis, adverse events (AEs), and clinical laboratory tests (including platelet count).
According to product labeling in Japan, lusutrombopag is indicated for the treatment of thrombocytopenia in patients with CLD undergoing an invasive procedure, other than laparotomy, thoracotomy, open heart surgery, craniotomy, or organ excision.6, 7 The recommended oral dosage of lusutrombopag is 3 mg, once daily for 7 days. Lusutrombopag administration should begin approximately 8–13 days before the scheduled date of the invasive procedure, and platelets should be monitored closely in Japan.6, 7 For the purposes of this surveillance, an additional treatment cycle was defined as readministration of lusutrombopag between day 10 and 6 months from the start date of the first treatment.
Safety
AEs occurring during and after treatment with lusutrombopag were recorded. Serious AEs and adverse drug reactions (ADRs) were evaluated by cycle of treatment (first, second, or third). An ADR was defined as a reaction for which a causal relationship between the drug and the occurrence is suspected by either a reporting physician or the sponsor, according to the International Conference of Harmonization Tripartite Guideline E2D version 4. ADRs of special interest, namely thrombosis‐ and thromboembolism‐related events, were also evaluated. AEs were coded using the Medical Dictionary for Regulatory Activities/Japanese version 21.0, and classified by system organ class and preferred term.
Effectiveness
The effectiveness measure was the proportion of patients who did not require preoperative platelet transfusion, and the change in platelet count by baseline platelet count and by cycle of treatment (first, second, or third).
Statistical analysis
It was estimated that 10.8% of cirrhosis patients (Child–Pugh class A and B) would experience hepatofugal portal vein blood flow or have a history/presence of thrombosis or thromboembolism.11 This surveillance aimed to enroll 1000 patients in order to detect a ≥3‐fold increase in the incidence of thrombosis‐related AEs in such patients taking lusutrombopag based on clinical trial data with a one‐sided significance level of 2.5% and 80% power.
The safety analysis set was defined as all patients with a data‐fixed initial administration surveillance form or retreatment surveillance form, except those considered unsuitable for the safety evaluation (i.e., patients with registration violations, cases of data duplication, and patients from institutions that did not agree to publish data). The effectiveness analysis set was defined as all patients included in the safety analysis set minus exclusions (i.e. patients with off‐label use, unapproved dosage, or Child–Pugh class C [an unapproved population in Japan]).
Descriptive statistics were used for baseline demographic and clinical characteristics, with n (%) for categorical variables and mean ± standard deviation for continuous variables. The significance level was set at 5% (two‐tailed). The statistical software used was SAS Ver. 9.1 (SAS Institute, Cary, NC, USA).
Results
Patients
The first patient was enrolled on 27 October 2016, and the data cut‐off date for this interim analysis was 27 September 2018. Data from 338 patients were analyzed, and among them, 20 patients received two cycles of lusutrombopag, and one patient received three cycles of lusutrombopag. Patient disposition is shown in Figure 1.
Figure 1.
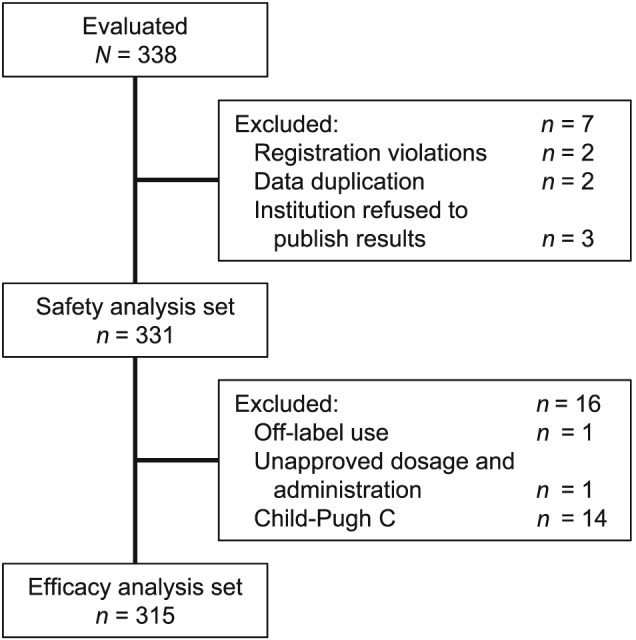
Patient disposition.
The safety analysis set included 331 patients. Seven patients were excluded for the following reasons: registration violations (n = 2), data duplication (n = 2), and institutional refusal to publish results (n = 3). A subset of 315 patients was included in the effectiveness analysis set. A total of 16 patients were excluded from the effectiveness analysis set for the following reasons: off‐label use (one patient without CLD), unapproved dosage/administration (one patient treated with lusutrombopag for >7 days), and use in an unapproved population (14 patients with Child–Pugh class C liver disease).
In the safety analysis set, most patients were men (n = 208, 62.8%), aged <80 years (n = 303, 91.5%), and had liver cirrhosis (n = 312, 94.3%; Table 1). The mean baseline platelet count was 46.2 ± 13.7 × 109/L (range, 15–110 × 109/L; n = 328). The proportion of patients with baseline platelet counts <30, 30–<50, and ≥ 50 × 109/L were 7.9%, 59.2%, and 32.0%, respectively; platelet count was unknown in three patients (0.9%). The proportion of patients who had current or a history of thrombosis/thromboembolism was 10.9% (Table 1).
Table 1.
Baseline demographic and clinical characteristics (safety analysis set)
| Items | Items | Category | No. patients | Component ratio (%) |
|---|---|---|---|---|
| Total no. patients | 331 | 100.0 | ||
| Patient background factor | Sex | Male | 208 | 62.8 |
| Female | 123 | 37.2 | ||
| Age (years) | Mean (SD) | 68.3 (9.0) | ||
| <40 | 2 | 0.6 | ||
| 40–<50 | 8 | 2.4 | ||
| 50–<60 | 41 | 12.4 | ||
| 60–<70 | 132 | 39.9 | ||
| 70–<80 | 120 | 36.3 | ||
| ≥80 | 28 | 8.5 | ||
| Inpatient/outpatient | Inpatient | 33 | 10.0 | |
| Outpatient | 298 | 90.0 | ||
| Baseline platelet count (×109/L) | <30 | 26 | 7.9 | |
| 30–<40 | 74 | 22.4 | ||
| 40–<50 | 122 | 36.9 | ||
| 50–<60 | 57 | 17.2 | ||
| 60–<70 | 32 | 9.7 | ||
| ≥70 | 17 | 5.1 | ||
| Unknown | 3 | 0.9 | ||
| No. patients | 328 | |||
| Mean (SD) | 46.2 (13.7) | |||
| Chronic liver impairment | Chronic hepatitis | 21 | 6.3 | |
| Cirrhosis | 312 | 94.3 | ||
| Unknown | 1 | 0.3 | ||
| Ascites (for cases with cirrhosis) | No | 224 | 71.8 | |
| Yes | 88 | 28.2 | ||
| Hepatic encephalopathy (for cases with cirrhosis) | No | 291 | 93.3 | |
| Yes | 21 | 6.7 | ||
| Child–Pugh class (for cases with cirrhosis) | A | 142 | 45.5 | |
| B | 155 | 49.7 | ||
| C | 14 | 4.5 | ||
| Unknown | 1 | 0.3 | ||
| History of splenectomy | No | 330 | 99.7 | |
| Yes | 1 | 0.3 | ||
| Platelet transfusion refractoriness | No | 71 | 21.5 | |
| Yes | 6 | 1.8 | ||
| Unknown | 254 | 76.7 | ||
| Presence of thrombosis or thromboembolism (including past history) | No | 295 | 89.1 | |
| Yes | 36 | 10.9 | ||
| Treatment factor | Duration of treatment (days) | <7 | 25 | 7.6 |
| 7 | 305 | 92.1 | ||
| >7 | 1 | 0.3 | ||
| Retreatment | No | 310 | 93.7 | |
| 1 | 20 | 6.0 | ||
| 2 | 1 | 0.3 | ||
| ≥3 | 0 | 0.0 | ||
Component ratio (%) = number of patients / total number of patients × 100.
Survey on chronic liver impairment deals with patients with liver impairment.
Survey on patients with/without ascites, hepatic encephalopathy, and Child–Pugh class is applied to patients with a chronic liver impairment who have hepatic cirrhosis.
Drug cessation period is excluded from the duration of treatment.
Patients might have both chronic hepatitis and cirrhosis.
SD, standard deviation.
There were 377 invasive procedures in 331 patients in the safety analysis set. The most frequent procedures were radiofrequency ablation (110 procedures, 29.2%), transarterial chemoembolization (59 procedures, 15.6%), and endoscopic injection sclerotherapy (49 procedures, 13.0%; Table 2). The mean time from starting lusutrombopag treatment to the invasive procedure was 12.3 days.
Table 2.
Invasive procedures carried out (safety analysis set)
| Invasive procedure | No. procedures carried out | (%) |
|---|---|---|
| Total | 377 | 100.0 |
| Radiofrequency ablation | 110 | 29.2 |
| Hepatic artery chemotherapy embolization (TACE) | 59 | 15.6 |
| Endoscopic injection sclerotherapy | 49 | 13.0 |
| Endoscopic variceal ligation | 40 | 10.6 |
| Hepatic artery embolization | 18 | 4.8 |
| Percutaneous needle biopsy | 18 | 4.8 |
| Tooth extraction | 13 | 3.4 |
| Hepatic artery chemotherapy | 10 | 2.7 |
| Endoscopic submucosal dissection | 8 | 2.1 |
| Endoscopic mucosal resection | 6 | 1.6 |
| Percutaneous ethanol injection therapy | 5 | 1.3 |
| Partial splenic embolization | 5 | 1.3 |
| Endoscopic resection of polyp (polypectomy) | 4 | 1.1 |
| Argon plasma coagulation | 3 | 0.8 |
| Endoscopic papillotomy | 3 | 0.8 |
| Angiography | 3 | 0.8 |
| Various types of puncture (including aspiration of abscess) | 2 | 0.5 |
| Balloon‐occluded retrograde transvenous obliteration | 2 | 0.5 |
| Placement of access port | 2 | 0.5 |
| Cataract surgery | 2 | 0.5 |
| Laparoscopic microwave coagulation therapy | 1 | 0.3 |
| Laparoscopic inguinal hernia repair | 1 | 0.3 |
| Vascular embolization | 1 | 0.3 |
| Endoscopic esophageal/gastric varices consolidation therapy | 1 | 0.3 |
| Papillotomy | 1 | 0.3 |
| Right femoral head replacement | 1 | 0.3 |
| Lumbar posterior decompression | 1 | 0.3 |
| Total hip replacement | 1 | 0.3 |
| Placement of central venous embedded catheter for injection | 1 | 0.3 |
| Denver shunt creation | 1 | 0.3 |
| Shunt creation in left arm | 1 | 0.3 |
| Endoscopic retrograde cholangiopancreatography | 1 | 0.3 |
| Cell‐free and concentrated ascites reinfusion therapy | 1 | 0.3 |
| Upper gastrointestinal endoscopy | 1 | 0.3 |
| Gastric biopsy (endoscopic) | 1 | 0.3 |
% = number of invasive procedures / total number of invasive procedures × 100.
TACE, transarterial chemoembolization.
Safety
In total, 41 serious AEs occurred in 29 patients (8.76%), including hepatic failure (n = 3, 0.91%), hepatic encephalopathy (n = 2, 0.60%), ascites (n = 2, 0.60%), and thrombosis (portal vein thrombosis [n = 6, 1.81%]; splenic vein thrombosis [n = 1, 0.30%]; Table 3). No thromboembolism events were reported.
Table 3.
Incidence of serious adverse events
| Postmarketing surveillance cumulative total | |
|---|---|
| No. institutions | 109 |
| No. patients investigated | 331 |
| No. patients with SAEs | 29 |
| No. SAEs | 41 |
| Percentage of patients with SAEs | 8.76% |
| Type of AE | n | % |
|---|---|---|
| Infections and infestations | 2 | (0.60) |
| Liver abscess† | 1 (1) | (0.30) |
| Peritonitis bacterial† | 1 (1) | (0.30) |
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 2 | (0.60) |
| Myelodysplastic syndrome† | 1 | (0.30) |
| Hepatocellular carcinoma† | 1 (1) | (0.30) |
| Blood and lymphatic system disorders | 3 | (0.91) |
| Anemia† | 1 (1) | (0.30) |
| Pancytopenia† | 1 | (0.30) |
| Splenic vein thrombosis† | 1 (1) | (0.30) |
| Nervous system disorders | 3 | (0.91) |
| Altered state of consciousness† | 1 (1) | (0.30) |
| Hepatic encephalopathy† | 2 (2) | (0.60) |
| Respiratory, thoracic and mediastinal disorders | 1 | (0.30) |
| Hemothorax† | 1 (1) | (0.30) |
| Gastrointestinal disorders | 6 | (1.81) |
| Ascites† | 2 (2) | (0.60) |
| Hematemesis† | 1 (1) | (0.30) |
| Esophageal variceal hemorrhage† | 1 (1) | (0.30) |
| Intra‐abdominal hemorrhage† | 2 (2) | (0.60) |
| Hepatobiliary disorders | 11 | (3.32) |
| Cholecystitis† | 1 (1) | (0.30) |
| Hepatic cirrhosis† | 1 (1) | (0.30) |
| Hepatic failure† | 3 (3) | (0.91) |
| Liver disorder | 1 (1) | (0.30) |
| Portal vein thrombosis | 6 (2) | (1.81) |
| Renal and urinary disorders | 2 | (0.60) |
| Myoglobinuria† | 1 (1) | (0.30) |
| Acute kidney injury† | 1 (1) | (0.30) |
| General disorders and administration site conditions | 2 | (0.60) |
| Pyrexia | 2 (1) | (0.60) |
| Investigations | 4 | (1.21) |
| Alanine aminotransferase increased† | 1 (1) | (0.30) |
| Aspartate aminotransferase increased† | 1 (1) | (0.30) |
| Blood bilirubin increased† | 1 (1) | (0.30) |
| Protein total decreased† | 2 (2) | (0.60) |
| White blood cell count decreased† | 1 (1) | (0.30) |
| Injury, poisoning, and procedural complications | 1 | (0.30) |
| Arterial injury† | 1 (1) | (0.30) |
Adverse events unexpected from those listed in “precautions for use” section on the package insert of lusutrombopag. Alanine transaminase increased, aspartate transaminase increased, and white blood cell count decreased are considered unexpected, as they are not listed in the “Clinically significant adverse reactions” section, although listed in the “Other adverse reactions” section.
() Number of cases in which a causal relationship between the product and adverse event was considered unlikely.
Japanese translation of Medical Dictionary for Regulatory Activities version 21.0.
AE, adverse event; SAE, serious adverse event.
Five patients had fatal outcomes: two with liver failure, and one each with bacterial peritonitis, acute renal failure, and intraperitoneal hemorrhage. No causal relationship was established between lusutrombopag and any of these events. For all events resulting in death, the cause was considered to be an underlying disease or a complication. Specifically, in one patient who experienced liver failure, this was thought to be related to the transcatheter arterial chemoembolization and radiofrequency ablation procedures.
A total of 14 ADRs, reactions for which a causal relationship between the drug and the occurrence was suspected, occurred in 11 patients (3.32%), including portal vein thrombosis (n = 4, 1.21%), increased alanine aminotransferase (n = 2, 0.60%), and increased aspartate aminotransferase (n = 2, 0.60%; Table 4). In the 21 patients who received a second or third treatment cycle, no ADRs were reported during the retreatment observational period.
Table 4.
Incidence of adverse drug reactions
| Postmarketing surveillance cumulative total | |
|---|---|
| No. institutions | 109 |
| No. patients investigated | 331 |
| No. patients with ADRs | 11 |
| No. ADRs | 14 |
| Incidence of ADRs | 3.32% |
| Type of ADR, n (%) | |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 1 (0.30) |
| Myelodysplastic syndrome† | 1 (0.30) |
| Blood and lymphatic system disorders | 1 (0.30) |
| Pancytopenia† | 1 (0.30) |
| Nervous system disorders | 1 (0.30) |
| Parosmia† | 1 (0.30) |
| Gastrointestinal disorders | 1 (0.30) |
| Diarrhea† | 1 (0.30) |
| Hepatobiliary disorders | 4 (1.21) |
| Portal vein thrombosis | 4 (1.21) |
| Skin and subcutaneous tissue disorders | 1 (0.30) |
| Pruritus† | 1 (0.30) |
| General disorders and administration site conditions | 1 (0.30) |
| Pyrexia | 1 (0.30) |
| Investigations | 2 (0.60) |
| Alanine aminotransferase increased | 2 (0.60) |
| Aspartate aminotransferase increased | 2 (0.60) |
Adverse drug reactions (ADRs) and infections unexpected from those listed in “Precautions for use” section on the package insert of lusutrombopag.
Japanese translation of Medical Dictionary for Regulatory Activities version 21.0.
Of six patients who experienced serious portal vein thrombosis, four events were reported in four patients as ADRs. The onset for three of these was between day 8 and day 14, and the fourth occurred on day 36 after initiation of lusutrombopag. Three thrombotic events occurred after the invasive procedure and one occurred before the procedure. Portal vein thrombosis was identified by ultrasonography and computed tomography in two patients, and by computed tomography in the other two patients. One patient was treated with warfarin potassium, one was treated with human antithrombin III and danaparoid sodium, and the other two patients were not treated. The outcome of the thrombotic event was “not resolved” in one patient who did not receive treatment, and was “resolving” in three patients. Three patients had baseline platelet counts <50 × 109/L, and one patient had a baseline platelet count ≥50 × 109/L. Platelet counts on the day of thrombosis onset were 35–72 × 109/L, and the maximum change in platelet count from baseline was 15–41 × 109/L, indicating that there was not an excessive increase in platelet count. Of 36 patients who had current or a history of thrombosis/thromboembolism, only one patient developed portal vein thrombosis (aggravation of portal vein thrombosis). No thrombotic events were observed in the 14 patients with Child–Pugh class C (a population in which lusutrombopag is not approved in Japan).
Three patients with baseline platelet counts <50 × 109/L and one patient with a baseline platelet count ≥50 × 109/L reached a platelet count ≥200 × 109/L. Portal vein thrombosis occurred in one patient; however, the event was considered to be not related to lusutrombopag.
Effectiveness
Of 300 patients who underwent an invasive procedure, except for those with platelet transfusion refractoriness, 282 patients (94.0%) underwent invasive procedures without platelet transfusion, and 185 of 199 (93.0%) of those with a baseline platelet count <50 × 109/L underwent invasive procedures without platelet transfusion (Table 5).
Table 5.
Proportions of patients not requiring preoperative platelet transfusion
| Items | Category | No. cases† | No. effective cases‡ | Rate of effective cases (%) |
|---|---|---|---|---|
| All patients | 300 | 282 | 94.0 | |
| Platelet count at the beginning of treatment (×109/L) | <30 | 22 | 19 | 86.4 |
| 30–<40 | 65 | 57 | 87.7 | |
| 40–<50 | 112 | 109 | 97.3 | |
| 50–<60 | 52 | 51 | 98.1 | |
| 60–<70 | 29 | 27 | 93.1 | |
| ≥70 | 17 | 17 | 100.0 | |
| Unknown | 3 | 2 | 66.7 | |
| Child–Pugh classification (For cases with cirrhosis) | A | 139 | 131 | 94.2 |
| B | 143 | 133 | 93.0 | |
| C§ | 0 | 0 | – | |
| Unknown | 1 | 1 | 100.0 | |
| Splenectomy | No | 299 | 281 | 94.0 |
| Yes | 1 | 1 | 100.0 | |
| Platelet transfusion refractoriness | No | 66 | 59 | 89.4 |
| Yes | 0 | 0 | – | |
| Unknown | 234 | 223 | 95.3 | |
| Retreatment | None | 281 | 263 | 93.6 |
| 1 | 18 | 18 | 100.0 | |
| 2 | 1 | 1 | 100.0 | |
| ≥3 | 0 | 0 | – |
Number of patients who underwent an invasive procedure, except for those with platelet transfusion refractoriness.
Number of patients who did not require platelet transfusion before an initial invasive procedure.
Not included in the effectiveness analysis, because this is an unapproved indication for lusutrombopag in Japan.
The time course and magnitude of changes in platelet counts over time were similar among patient subgroups stratified by baseline platelet count (Fig. 2). In patients with platelet measurements before and after lusutrombopag administration who did not undergo platelet transfusion, the mean maximum platelet count was 88.7 ± 35.1 × 109/L (range 25–352; n = 286), and the mean maximum change in platelet count from baseline was 41.7 ± 31.4 × 109/L (range −6 to 276; n = 286). The time course and magnitude of changes in platelet count with/without platelet transfusion after lusutrombopag administration are shown in Figure 3. Platelet count was consistently lower in patients who underwent platelet transfusion after lusutrombopag administration than in those without platelet transfusion. There was no significant increase in platelet count in patients with platelet transfusion. Although the number of patients retreated with lusutrombopag was small, the time course and change in platelet count from baseline were similar regardless of treatment cycle (Figure 4).
Figure 2.
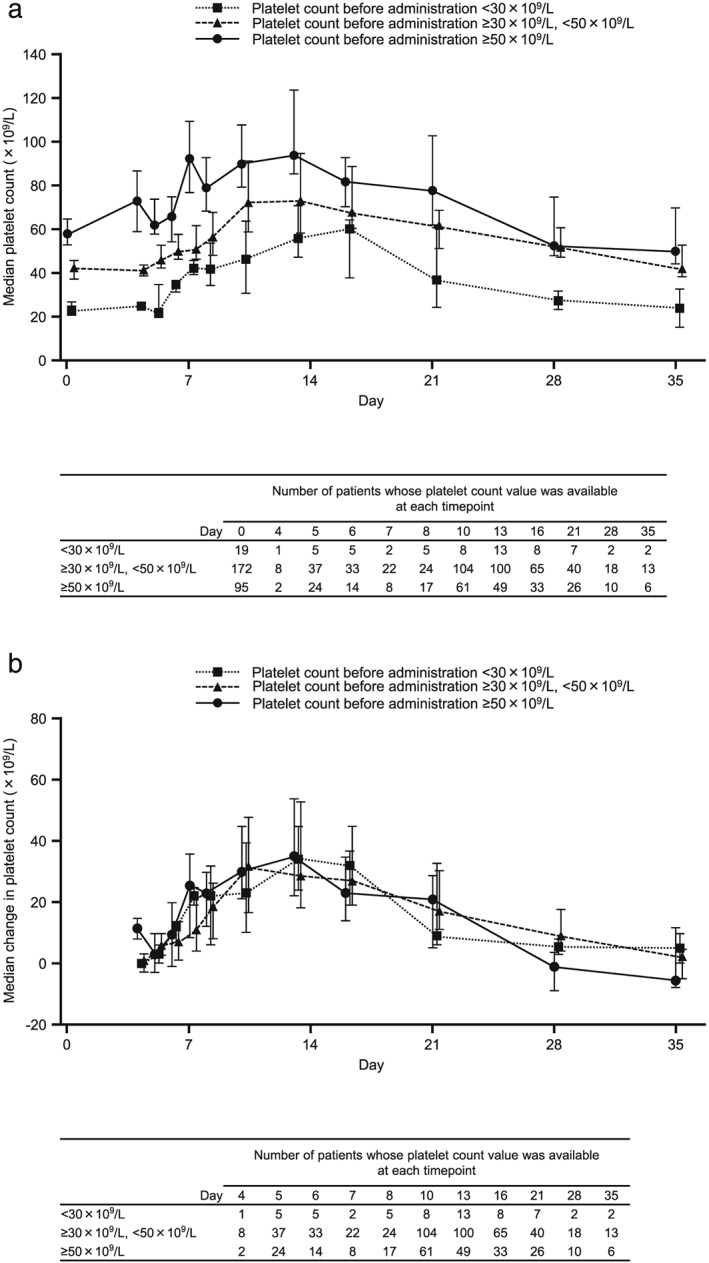
(a) Median and (b) median change in platelet count by baseline platelet count. Data for day 0 are for platelet count before lusutrombopag administration. Error bars represent the 25th and 75th percentiles.
Figure 3.
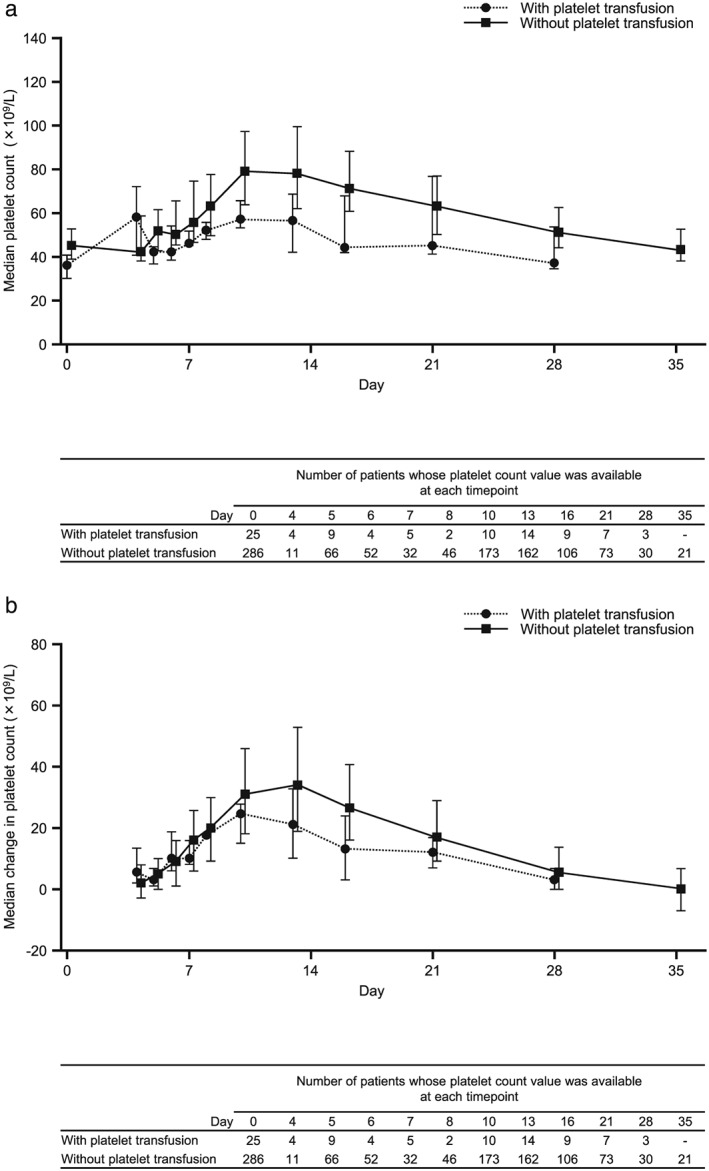
(a) Median and (b) median change in platelet count with/without platelet transfusion after lusutrombopag administration. Data for day 0 are for platelet count before lusutrombopag administration.
Error bars represent 25th and 75th percentiles.
Figure 4.
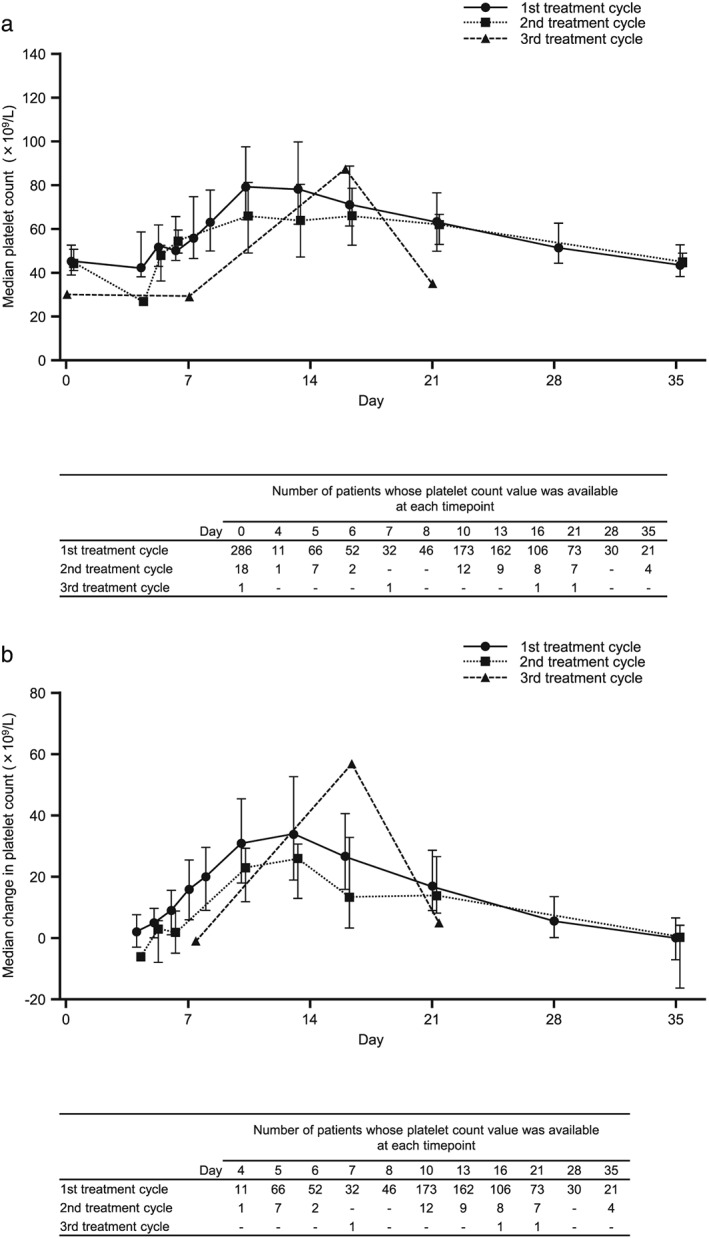
(a) Median and (b) median change in platelet count by cycle of treatment. Data for day 0 are for platelet count before lusutrombopag administration. Error bars represent 25th and 75th percentiles.
Discussion
This ongoing postmarketing surveillance provides interim data on the safety and effectiveness of lusutrombopag in Japanese patients with CLD undergoing invasive procedures in routine clinical practice. To the best of our knowledge, our surveillance is the largest to date involving the use of lusutrombopag in this setting, and patients will continue to be enrolled in the surveillance program until September 2020.
In this interim analysis, 32.0% of patients had a baseline platelet count ≥50 × 109/L, which is notably different from clinical trials of lusutrombopag, in which patients with a baseline platelet count <50 × 109/L were supposed to be enrolled according to the protocol.7, 8, 9 Under real‐world conditions, platelet target levels might be set at ≥50 × 109/L depending on the individual needs of the patient and the procedures being carried out; thus, we would expect a proportion of patients to be administered lusutrombopag in some clinical practice situations despite having a platelet count ≥50 × 109/L. In this surveillance, only one patient with a baseline platelet count ≥50 × 109/L experienced a thrombus‐related AE. Furthermore, 10.9% of patients in our surveillance population had a current thrombosis/thromboembolism or a history of thrombosis/thromboembolism, although such patients were excluded in the prior clinical trials.7, 8, 9 Of patients who had a current thrombosis/thromboembolism or a history of thrombosis/thromboembolism, only one developed portal vein thrombosis (aggravation of portal vein thrombosis). Therefore, the surveillance data provide important information on outcomes in patients for whom clinical trial results provide no guidance on the usefulness of therapy.
Overall rates of serious AEs and ADRs, respectively, in the surveillance data, were 8.76% (including six cases [1.81%] of portal vein thrombosis) and 3.32% (including four cases [1.21%] of portal vein thrombosis). Most patients in the postmarketing surveillance population had cirrhosis (94.3%), and some could have had pre‐existing thrombosis. It is also well known that patients with cirrhosis are at an increased risk of spontaneous or procedurally‐related portal vein thrombosis.12, 13 Thus, the portal vein thrombosis cases identified during the postmarketing surveillance might not necessarily be related to lusutrombopag treatment. In fact, three of four ADRs occurred after invasive procedures, such as radiofrequency ablation, and it is possible that the procedure itself played a role in thrombus occurrence. In addition, there was no excessive increase in platelet count at the time of these events. It is possible that patients who experienced thrombosis were in a hypercoagulable state; however, because markers, such as anti‐thrombin and protein C, were not measured in this surveillance, this is difficult to assess. Nevertheless, it is important to monitor the platelet count closely and to be vigilant for any occurrence of thrombosis/thromboembolism.
In our surveillance, the proportion of patients who did not require preoperative platelet transfusion was high (94.0%). Improvements in platelet count were similar across a range of different baseline platelet count values, with a mean maximum change from baseline of 41.7 ± 31.4 × 109/L. All 21 patients who received a second or third round of treatment with lusutrombopag avoided platelet transfusion before their invasive procedure. Contrary to the protocols of the clinical trials of lusutrombopag,7, 8, 9 26 patients did not receive platelet transfusion, despite having a platelet count <50 × 109/L before the procedure. This was based on a decision by their physician, as per routine clinical practice.
In patients treated with 3‐mg lusutrombopag in clinical trials carried out both in Japan7 and globally,8 ADR rates of 8.3% and 5.6% were observed. The respective proportions of patients who did not require platelet transfusion were 79.2% and 64.8%. In this surveillance, the ADR rate was 3.32%, and the proportion of patients not requiring platelet transfusion was 94.0%. Given the variability across the clinical studies and this surveillance, such as differences in study design and patient enrollment criteria, it is difficult to directly compare the present surveillance results with those of the L‐PLUS studies. Nevertheless, despite the differences in characteristics between patients enrolled in the clinical trials compared with the present surveillance, it is reassuring that the present results confirm the safety and effectiveness of lusutrombopag, and further validate the results of the clinical trials.7, 8, 9 These interim results are also consistent with other recent reports of the safety and effectiveness of lusutrombopag in both treatment‐naïve and retreatment patients.14, 15, 16
Lusutrombopag is currently the only thrombopoietin receptor agonist with available postmarketing surveillance data for treatment of patients with CLD and thrombocytopenia undergoing invasive procedures. Another agent, avatrombopag, was approved in the USA in May 2018, but no real‐world surveillance data have yet been reported.17, 18
The main strength of the present postmarketing surveillance is the fact that data were collected from patients with a variety of baseline characteristics. Thus, the results of this surveillance will allow a more thorough assessment of the safety and effectiveness of lusutrombopag in real‐world clinical practice. However, definitive conclusions cannot be made from this interim analysis and will need to wait until final data become available.
The main limitations of this surveillance are those inherent to the observational study design. For example, not all data points were collected at the same time for all patients, and there was no control arm. Furthermore, the fact that this surveillance was carried out only in Japan means that the findings might have limited generalizability to other ethnic populations.
The interim results of this real‐world surveillance did not show any unexpected safety signals related to lusutrombopag, and most of the treated patients did not require platelet transfusion before a planned invasive procedure. Therefore, lusutrombopag appears to be a safe and effective treatment option for patients with CLD and thrombocytopenia undergoing invasive procedures. We expect to further expand the present results with those of the final analysis of the postmarketing surveillance data.
Author contributions
CS conceived and designed the study, was involved in the acquisition and analysis of data, and drafted the manuscript. RS conceived and designed the study and drafted the manuscript. RB, BC, and MY drafted the manuscript. All authors were involved in data interpretation, critical revision of the manuscript, and final approval of the manuscript to be published.
Declaration of interest
NA has acted as a consultant and advisory board member for Merck, Gilead, Echosens, Ligand, and Shionogi; has been employed and worked as a Chief Medical Officer and received directorships, travel grants, and intellectual property rights from Spring Bank Pharmaceuticals; worked as a Director for TRIO Healthcare; and owns stock or has stock options from Spring Bank Pharmaceuticals, Allurion, and TRIO. MI received advisory fees from Shionogi. RS, CS and her spouse, RB, BC, and MY are employees of the study sponsor (Shionogi and Shionogi Group Companies).
Acknowledgments
The authors are grateful to all participating physicians for their cooperation in this PMS. This surveillance was funded by Shionogi & Co., Ltd. The authors thank Michelle Belanger, MD, and Keyra Martinez Dunn, MD, of Edanz Medical Writing for providing medical writing support, which was funded by Shionogi & Co., Ltd. through EMC K.K. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). The authors also thank Toshimitsu Ochiai and Eri Sakai, employees of Shionogi & Co., Ltd., for their assistance with the statistical analysis.
Sasaki, R. , Shiino, C. , Imawari, M. , Bentley, R. , Cai, B. , Yoshida, M. , and Afdhal, N. (2019) Safety and effectiveness of lusutrombopag in Japanese chronic liver disease patients with thrombocytopenia undergoing invasive procedures: Interim results of a postmarketing surveillance. Hepatol Res, 49: 1169–1181. 10.1111/hepr.13392.
References
- 1. Michitaka K, Nishiguchi S, Aoyagi Y et al Etiology of liver cirrhosis in Japan: a nationwide survey. J Gastroenterol 2010; 45: 86–94. [DOI] [PubMed] [Google Scholar]
- 2. Hayashi H, Beppu T, Shirabe K, Maehara Y, Baba H. Management of thrombocytopenia due to liver cirrhosis: a review. World J Gastroenterol 2014; 20: 2595–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peck‐Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int 2017; 3: 778–793. [DOI] [PubMed] [Google Scholar]
- 4. Giannini EG, Greco A, Marenco S, Andorno E, Valente U, Savarino V. Incidence of bleeding following invasive procedures in patients with thrombocytopenia and advanced liver disease. Clin Gastroenterol Hepatol 2010; 8: 899–902. [DOI] [PubMed] [Google Scholar]
- 5. Mitchell O, Feldman DM, Diakow M, Sigal SH. The pathophysiology of thrombocytopenia in chronic liver disease. Hepat Med 2016; 8: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim ES. Lusutrombopag: First Global Approval. Drugs 2016; 76: 155–158. [DOI] [PubMed] [Google Scholar]
- 7. Hidaka H, Kurosaki M, Tanaka H et al Lusutrombopag Reduces Need for Platelet Transfusion in Patients With Thrombocytopenia Undergoing Invasive Procedures. Clin Gastroenterol Hepatol 2018; 17: 1192–1200. 10.1016/j.cgh.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 8. Peck‐Radosavljevic M, Simon K, Iacobellis A et al Lusutrombopag for the treatment of thrombocytopenia in patients with chronic liver disease undergoing invasive procedures (L‐PLUS 2). Hepatology 2019. 10.1002/hep.30561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tateishi R, Seike M, Kudo M et al A randomized controlled trial of lusutrombopag in Japanese patients with chronic liver disease undergoing radiofrequency ablation. J Gastroenterol 2019; 54: 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maeda K, Katashima R, Ishizawa K, Yanagawa H. Japanese Physicians' Views on Drug Post‐Marketing Surveillance. J Clin Med Res 2015; 7: 956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. von Herbay A, Frieling T, Häussinger D. Color Doppler sonographic evaluation of spontaneous portosystemic shunts and inversion of portal venous flow in patients with cirrhosis. J Clin Ultrasound 2000; 28: 332–339. [DOI] [PubMed] [Google Scholar]
- 12. Matsutani S, Fukuzawa T, Watanabe Y et al Pathophysiological view of the management of portal vein thrombosis [In Japanese]. Kan tan sui 2010; 61: 259–268. [Google Scholar]
- 13. Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther 2010; 31: 366–374. [DOI] [PubMed] [Google Scholar]
- 14. Ishikawa T, Okoshi M, Tomiyoshi K et al Efficacy and safety of repeated use of lusutrombopag prior to radiofrequency ablation in patients with recurrent hepatocellular carcinoma and thrombocytopenia. Hepatol Res 2019; 49: 590–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takada H, Kurosaki M, Nakanishi H et al Real‐life experience of lusutrombopag for cirrhotic patients with low platelet counts being prepared for invasive procedures. PLoS ONE 2019; 14: e0211122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uojima H, Arase Y, Itokawa N et al Relationship between response to lusutrombopag and splenic volume. World J Gastroenterol 2018; 24: 5271–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Terrault N, Chen YC, Izumi N et al Avatrombopag Before Procedures Reduces Need for Platelet Transfusion in Patients With Chronic Liver Disease and Thrombocytopenia. Gastroenterology 2018; 155: 705–718. [DOI] [PubMed] [Google Scholar]
- 18. Shirley M. Avatrombopag: First Global Approval. Drugs 2018; 78: 1163–1168. [DOI] [PubMed] [Google Scholar]


